Abstract
Some patients with schizophrenia have severe cognitive impairment and functional deficits that require long-term institutional care. The patterns of brain-behavior alterations in these individuals, and their differences from patients living successfully in the community, remain poorly understood. Previous cognition-based studies for stratifying schizophrenia patients highlight the importance of subcortical structures in the context of illness heterogeneity. In the present study, subcortical volumes from 96 institutionalized patients with long-term schizophrenia were evaluated using cluster analysis to test for heterogeneity. These data were compared to those from two groups of community-dwelling individuals with schizophrenia for comparison purposes, including 68 long-term ill and 126 first-episode individuals. A total of 290 demographically matched healthy participants were included as normative references at a 1:1 ratio for each patient sample. A subtype of institutionalized patients was identified based on their pattern of subcortical alterations. Using a machine learning algorithm developed to discriminate the two groups of institutionalized patients, all three patient samples were found to have similar rates of patients assigned to the two subtypes (approximately 50% each). In institutionalized patients, only the subtype with the identified pattern of subcortical alterations had greater neocortical and cognitive abnormalities than those in the similarity classified community-dwelling patients with long-term illness. Thus, for the subtype of patients with a distinctive pattern of subcortical alterations, when the distinct pattern of subcortical alterations is present and particularly severe, it is associated with cognitive impairments that may contribute to persistent disability and institutionalization.
Subject terms: Diagnostic markers, Schizophrenia
Introduction
A significant number of individuals living with schizophrenia require long-term institutional care [1]. These patients typically show more pronounced cognitive impairment [2], which in many samples has been related to abnormal brain volumes [3–5]. Moreover, both cognitive function and brain volume alterations have been associated with poor functional outcomes [6, 7]. Characterizing brain-behavior profiles in institutionalized individuals may facilitate understanding their severe illness course and potentially discover biomarkers helpful in identifying such individuals for novel cognition-targeted treatments.
At present, it remains unclear whether there is meaningful neurobiological heterogeneity in severely affected institutionalized individuals with schizophrenia. This is a crucial issue, as evaluation of group-level differences may obscure distinct biological processes relevant for different individuals. Previously in schizophrenia studies, discrete heterogeneity has been identified in psychosis symptoms [8], cognitive function [9], molecular biological profiles such as in the brain transcriptome [10], peripheral immune factors, and growth factors [11], as well as in neuroanatomic features [12–14].
Studies resolving heterogeneity in schizophrenia have emphasized the importance of subcortical volumes, which in turn have been linked to cognitive deficits [15–19]. These observations are consistent with many demonstrations that subcortical structures, including the basal ganglia, thalamus, and hippocampus, play well-established roles in schizophrenia and cognitive function [20–22].
In this study, we tested for heterogeneity of subcortical brain volumes in institutionalized patients with schizophrenia using cluster analysis. Cognitive function, as an external validator, was compared between identified subtypes. In addition to delineating patterns of subcortical heterogeneity in the institutionalized patients, we aimed to examine whether similar heterogeneity would exist in community-dwelling individuals. To address this issue, we trained a brain-based classifier in the institutionalized sample utilizing a machine learning algorithm applied to subcortical brain features and subtype labels identified in clustering. This classifier was then applied to two data sets comprised of community-dwelling individuals with schizophrenia (early and later course of illness groups) to determine the proportion of individuals in these samples with the distinct subcortical neuroanatomic patterns identified in the institutionalized individuals. In addition to our primary focus on subcortical features, we performed cluster analyses based on cortical brain volumes in secondary analyses for comparison purposes.
We hypothesized that (1) some institutionalized patients would be classified into a subtype with distinct subcortical and cognitive deficits, and (2) community-dwelling patients would be different from institutionalized patients in terms of the pattern or severity of the identified brain-behavior pattern.
Materials and methods
Participants
Three independent samples were used in this study (N = 580). In a subtype discovery sample, 96 institutionalized patients with schizophrenia and 96 demographically matched healthy controls were included from a single site in China to identify subtypes of patients defined by subcortical volumes. These patients were institutionalized (the total length of institutionalization: 1451.72 ± 890.84 days, the length of current hospitalization: 656.04 ± 901.08 days, lifetime number of hospitalizations: 6.82 ± 6.28). Generally, they were maintained in the hospital because of persistent and severe functional disability rather than persistent acute positive symptoms.
Two samples of community-dwelling patients with schizophrenia and demographically matched healthy controls were recruited for comparison purposes. First, 68 community-dwelling individuals with long-term schizophrenia and 68 controls from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium were examined [23, 24]. Also, 126 community-dwelling drug-naïve individuals with first-episode schizophrenia (FES) and 126 controls were included from a single site in China. The B-SNIP and FES participants were living in the community (except for brief hospitalization for the FES participants). Patients in the subtype discovery set of institutionalized patients were marginally older than the community-dwelling individuals with long-term illness (Supplementary Table S2 in Supplementary Materials). In all samples, patient diagnosis was determined using the Structured Clinical Interview for DSM-IV (SCID), and psychopathology was evaluated using the Positive and Negative Syndrome Scale (PANSS) [25]. All institutionalized patients and community-dwelling individuals with long-term illness had an illness duration greater than 5 years, and FES patients had an illness duration of fewer than 2 years. All institutionalized patients were receiving ongoing atypical antipsychotic treatment.
For each of the three samples of individuals with schizophrenia, healthy controls were recruited from nearby communities via advertisement as the normative reference for brain-behavior patterns and excluded if they had a history of significant psychiatric illness identified using the non-patient SCID interview. All study participants with non-right handedness, a history of head injury, neurological illness, systemic disease, substance use, or magnetic resonance (MR) scanning contraindications were excluded from the study. Written informed consent was provided by all participants, and this study was approved by the local research ethics committee at all study sites. Details regarding the three samples are provided in Supplementary Methods.
Cognitive assessments
The Brief Assessment of Cognition in Schizophrenia (BACS) [26] was used to evaluate cognitive function in two of the three samples. BACS scores of community-dwelling individuals from the B-SNIP sample with long-term schizophrenia and corresponding controls were transformed into z-scores based on published norms [27]. The institutionalized sample was assessed with the Chinese version of the BACS [28], where raw test scores relative to controls rather normative data were used, especially for within-sample analyses, as a large normative sample of this test version is not currently available in a Mandarin-speaking population. Institutionalized patients had more severely impaired cognition than the community-dwelling individuals with long-term illness (Supplementary Table S3 in Supplementary Materials).
Acquisition and preprocessing of structural images
Three-dimensional T1-weighted images were acquired using 3-T MR scanners for all participants. Scanning parameters and quality assessment are reported in Supplementary Materials. All images were processed by FreeSurfer (https://surfer.nmr.mgh.harvard.edu) software version 6.0 with a ‘recon-all’ workflow [29]. Regional brain volumes (including cortical and subcortical volumes) and global brain features including cortical gray matter volume (GMV), subcortical GMV, total GMV, cerebral white matter volume (WMV), and total brain volume (TBV) were extracted for patient subtyping and comparisons. Euler number [30], a measure of the quality of cortical reconstruction in the FreeSurfer pipeline, was calculated (see Supplementary Methods).
Subtype identification in the institutionalized sample
K-means++ clustering [31] was used for subtyping institutionalized patients with schizophrenia (N = 96). In our primary cluster analysis (Fig. 1A), volumes of 14 subcortical structures (bilateral thalamus, caudate nucleus, putamen, globus pallidus, hippocampus, amygdala, and nucleus accumbens) were employed for patient subtyping without further dimensional reduction steps. Feature standardization steps, including removing variance related to age, sex, and intracranial volume (ICV) and standardizing them into z-scores, were implemented. During clustering, center initiation and the number of clusters were both randomly tuned to achieve better model performance. The Silhouette coefficient [32] was used to identify the optimal number of clusters. Methodological details of clustering and validation are presented in Supplementary Materials. To compare clustering results based on subcortical features with those via other features, we performed four supplementary cluster analyses based on regional cortical volumes, regional cortical and subcortical volumes, global cortical volumes, and global brain volumes (See Supplementary Materials).
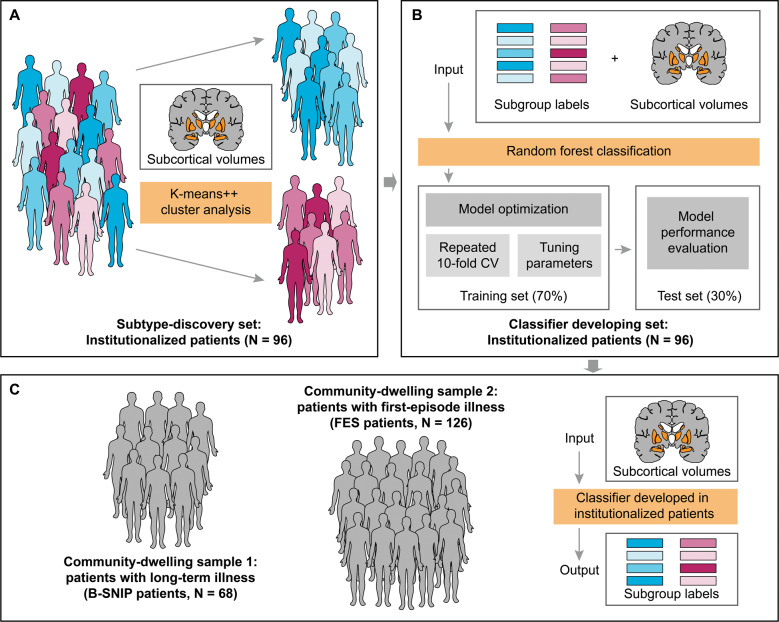
Fig. 1. Schema of subtype discovery, classifier development, and patient assignment.
Subtypes were identified using K-means ++ cluster analysis with regional subcortical volumes in institutionalized patients with schizophrenia (A). These institutionalized patients were subsequently used to develop a brain-based classifier with a random forest algorithm, and subgroup labels generated in cluster analysis and regional subcortical volumes applied in cluster analysis were as input of the classifier (B). The classifier developed in institutionalized patients was applied to classify community-dwelling schizophrenia individuals with long-term illness or first-episode illness (C). B-SNIP, the Bipolar-Schizophrenia Network on Intermediate Phenotypes consortium; CV, cross-validation; FES, first-episode schizophrenia; N, number of patients.
Classifier training, validation, and patient assignment
Institutionalized patients were randomly split into training and test sets at a ratio of 70%/30%. Subcortical volumes used in the primary cluster analysis, following the same feature standardization steps as the clustering, were employed to train a brain-based classifier to distinguish the two subgroups of institutionalized patients (Fig. 1B). Random forest algorithm [33], a supervised machine learning approach, was applied for classifier modeling. In the training set, 100-repetitions of 10-fold cross-validation and tuning parameters were applied to optimize the model. The prediction was first performed using the optimal model in the test set to evaluate classifier performance. In both community-dwelling samples, predictions were subsequently made with the optimal model developed for the institutionalized subgrouping (Fig. 1C). Detailed information of classification models and evaluation of feature importance is described in Supplementary Materials.
Statistical analysis
Statistical analyses for this study were performed with R (version 4.0.2) [34]. Neuroanatomic and cognitive group comparisons were carried out using analysis of covariance (ANCOVA) with Tukey HSD post hoc tests, treating age, sex, ICV, and education level as covariates. False discovery rate (FDR) adjustment was applied to p-values generated in the primary analyses and post hoc tests for group differences in symptoms, cognitive function, regional and global brain volumes. Glass’s delta (Δ) effect sizes were calculated to characterize patient-control differences for neuroanatomic and cognitive profiles after removing variance related to covariates. To determine whether potential confounders would drive the patient subtyping and subsequent brain-behavior comparisons, sensitivity analyses for factors such as illness duration, ICV, and the Euler number were performed. In addition, we performed correlation analyses between neuroanatomic and behavioral features to characterize their relationships across samples. Detailed procedures of between-sample statistical tests and sensitivity analyses are described in Supplementary Materials.
Results
Two distinct subtypes defined by subcortical volumes in institutionalized patients
Institutionalized patients with schizophrenia were clustered into two subtypes based on subcortical volumes, and each subtype was composed of 48 patients (50.00%) (Supplementary Table S4).
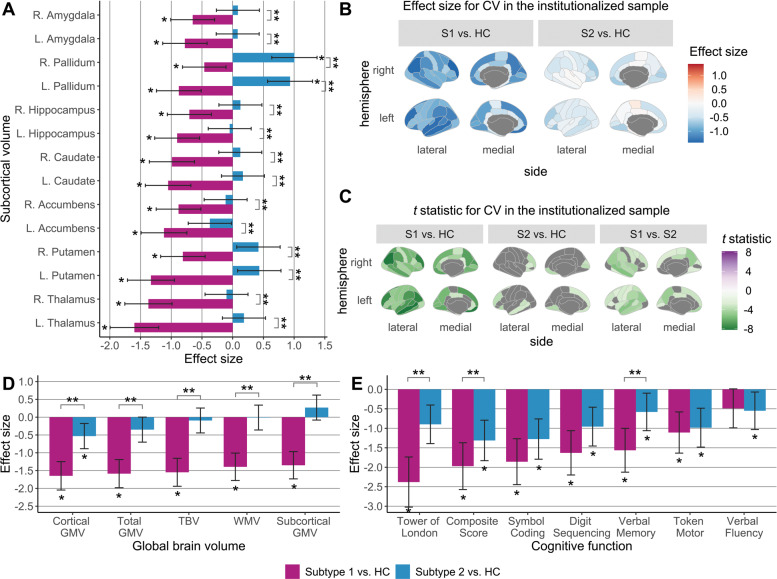
Subtype 1 of institutionalized patients was characterized as having widespread neuroanatomic and cognitive deficits relative to controls, affecting all subcortical (Δ ranged from −1.60 to −0.46, PFDR ranged from < 0.001 to 0.020) and multiple regional cortical volumes (Δ ranged from −1.40 to −0.18, PFDR ranged from < 0.001 to 0.047), BACS composite scores (Δ = −1.97, PFDR < 0.001) and all BACS subtest scores (Δ ranged from −2.38 and −1.11, PFDR < 0.001) except the verbal fluency test (Fig. 2).
Fig. 2. Between-group comparisons in brain-behavior profiles within the institutionalized sample.
ANCOVA and post hoc Tukey HSD tests were used to detect between-group differences in (A) subcortical volumes, (B, C) cortical volumes, (D) global brain volumes, and (E) cognitive function in two subtypes of institutionalized patients and demographically matched healthy controls. Age, sex, and ICV were included as covariates for brain volumes, and age, sex, and education level were covariates for BACS raw scores. In bar charts, significant patient-control and between-subtype differences, determined by FDR-corrected P-values generated in post hoc pairwise tests, are marked by one and two asterisks, respectively. Shading bars represent Glass’s delta (Δ) effect sizes, which were calculated after removing variance related to corresponding covariates and used to demonstrate patient-control differences for each subtype. Error bars mean 95% confidence interval of Δ. In cortical maps, only regions that survived FDR corrections are colored by t statistics from post hoc tests. CV Cortical volume; GMV Gray matter volume; HC Healthy controls; L the left hemisphere; R the right hemisphere; S1 Subtype 1 of institutionalized patients; S2 Subtype 2 of institutionalized patients; TBV Total brain volume; WMV White matter volume.
In secondary cluster analyses, subtypes identified based on cortical features did not significantly differ in cognitive function (Supplementary Table S13), highlighting the importance of subcortical alterations for identifying a subgroup of patients with pronounced cognitive disabilities.
Compared with controls, Subtype 2 of institutionalized patients displayed significantly increased volume in the bilateral pallidum (Δ ranged from 0.93 to 1.00, PFDR < 0.001) (see Fig. 2). At the same time, other subcortical brain measures in Subtype 2 did not show significant alterations relative to controls. Subtype 2 patients showed limited cortical deficits, mainly involving the default network, as well as cognitive impairments with medium-to-large effect sizes relative to controls.
Subtype 1 of institutionalized patients showed significantly decreased volumes relative to Subtype 2 in all subcortical (t ranged from −8.58 to −3.77, PFDR ranged from < 0.001 to 0.001) and multiple regional cortical volumes (t ranged from −5.34 to −2.56, PFDR ranged from < 0.001 to 0.045). Compared with Subtype 2 patients, Subtype 1 displayed significantly greater cognitive impairments in BACS composite scores (t = −2.72, PFDR = 0.048), verbal memory (t = −3.80, PFDR = 0.005), and Tower of London test scores (t = −3.36, PFDR = 0.011).
Sensitivity analyses for the institutionalized sample
Two subtypes of institutionalized patients displayed no significant differences in age, sex, education level, hospitalization length, the daily dose of antipsychotic medication, or psychotic symptoms (Table 1). Subtype 1 of institutionalized patients had significantly longer illness duration than Subtype 2 (t = 2.21, P = 0.029). When we re-performed the cluster analysis with prior removal of variance related to illness duration, similar brain-behavior patterns were found as in the primary clustering (Supplementary Fig. S3). Sensitivity analyses revealed that factors such as ICV, Euler number, and the daily dose of antipsychotics did not affect our subtyping findings in institutionalized patients (see Supplementary Materials).
Table 1.
Demographical and clinical comparisons in two identified subtypes of institutionalized patients with schizophrenia and healthy controls.
| Measure | Subtype 1 (N = 48) | Subtype 2 (N = 48) | HC (N = 96) | F/χ2/t | P/PFDR |
|---|---|---|---|---|---|
| Age (year, M ± SD) | 47.63 ± 7.59 | 45.33 ± 6.78 | 46.65 ± 7.53 | 1.17 | 0.312 |
| Sex (female: N/%) | 15 (31.25%) | 16 (33.33%) | 31 (32.29%) | 0.05 | 0.976 |
| Educational level (year, M ± SD) | 9.81 ± 2.65 | 10.02 ± 2.69 | 9.56 ± 3.24 | 0.40 | 0.672 |
| Illness duration (year, M ± SD) | 21.96 ± 8.22 | 18.08 ± 8.92 | – | 2.21 | 0.029* |
| Total length of institutionalization (day, M ± SD) | 1457.45 ± 982.71 | 1446.10 ± 801.21 | – | 0.06 | 0.951 |
| Length of current hospitalization (day, M ± SD) | 525.21 ± 900.12 | 786.87 ± 892.38 | – | −1.42 | 0.160 |
| Lifetime number of hospitalization (time, M ± SD) | 7.45 ± 5.74 | 6.21 ± 6.76 | – | 0.96 | 0.338 |
| CPZ equivalent (mg/day, M ± SD) | 484.30 ± 203.72 | 481.03 ± 206.63 | – | 0.08 | 0.938 |
| PANSS (M ± SD) | |||||
| Positive score | 11.16 ± 5.07 | 9.88 ± 3.45 | – | 1.41 | 0.357 |
| Negative score | 16.31 ± 5.07 | 16.23 ± 5.83 | – | 0.07 | 0.942 |
| General score | 26.87 ± 5.86 | 25.31 ± 5.13 | – | 1.36 | 0.357 |
| Total score | 54.33 ± 13.63 | 51.42 ± 12.48 | – | 1.07 | 0.381 |
CPZ equivalent, the daily dose of antipsychotics transformed into chlorpromazine equivalent; F F statistic in the analysis of variance (ANOVA); HC Healthy controls; M mean value; PANSS the Positive and Negative Syndrome Scale; PFDR False discovery rate (FDR) adjusted p-value; SD Standard deviation; t t-statistic in two-sample t-tests; χ2 the chi-squared statistic.
Age and education level were compared using ANOVA, and sex distributions were compared using the chi-squared test between two subtypes of patients and healthy controls. Between-subtype comparisons in illness duration, the daily dose of antipsychotics, and PANSS scores were performed with two-sample t-tests. P-values assessed PANSS differences were adjusted by FDR because of multiple comparisons. Asterisks demonstrate significant between-group differences.
Classifier training, validation, and patient assignment
Detailed descriptions of findings using the brain-based classifier are presented in Supplementary Materials. Using the 14 subcortical volumes and subtype labels identified in our cluster analysis, a brain-based classifier was trained and validated, achieving the average accuracy of 0.97 across 100-repetitions of 10-fold cross-validation in the training set and an accuracy of 1.00 (95% confidence interval = 0.88 ~ 1.00, P < 0.001), with a sensitivity of 1.00, and a specificity of 1.00 in the test set (the 30% of institutionalized cases held out from the training set). The area under the receiver operating characteristic (ROC) curve (AUC) was 1.00. Thalamic and basal ganglia volumes were the first few features in the contribution plot (Supplementary Fig. S7). Patients in each community-dwelling sample were assigned into two subgroups based on the brain-based classifier developed in the institutionalized sample. Their classification rates were not significantly different from the institutionalized sample (Supplementary Table S4).
Cross-sample statistical tests in brain-behavior profiles
We conducted statistical comparisons across the institutionalized, B-SNIP, and FES samples (Figs. 3–4, Supplementary Figs. S8, S9).
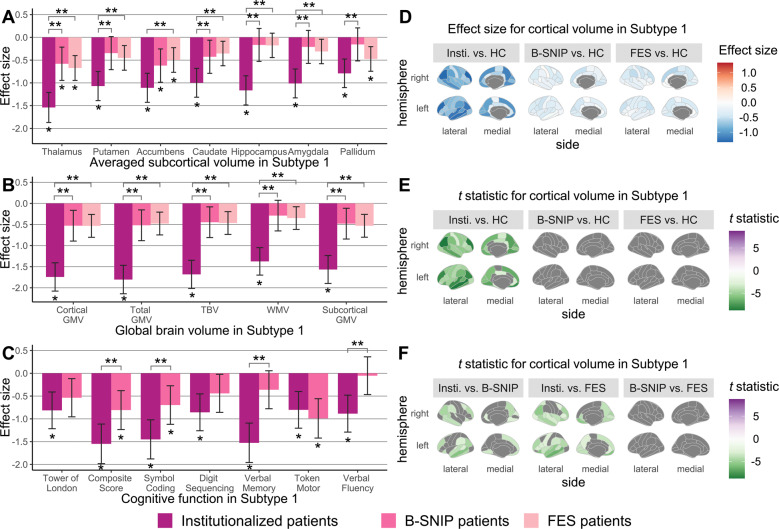
Fig. 3. Between-sample comparisons in brain-behavior profiles for Subtype 1.
ANCOVA and post hoc Tukey HSD tests were employed to test between-sample differences in (A) averaged subcortical volumes, (B) global brain volumes, (C) cognitive function, and (D–F) cortical volumes between Subtype 1 patients from different samples and the pooled healthy control group. Age, sex, and ICV were included as covariates for harmonized brain volumes, while education level was treated as the covariate for age- and sex-corrected BACS z-scores. In bar charts, significant patient-control and between-sample differences, determined by FDR-corrected P-values generated in post hoc pairwise tests, are marked by one and two asterisks, respectively. Shading bars represent Glass’s delta (Δ) effect sizes, which were calculated after removing variance related to covariates and used to demonstrate patient-control differences. Error bars mean 95% confidence interval of Δ. In cortical statistic maps, only regions that survived FDR corrections are colored by t statistics from post hoc tests. GMV Gray matter volume; HC Healthy controls; Insti. institutionalized patients; TBV Total brain volume; WMV White matter volume.
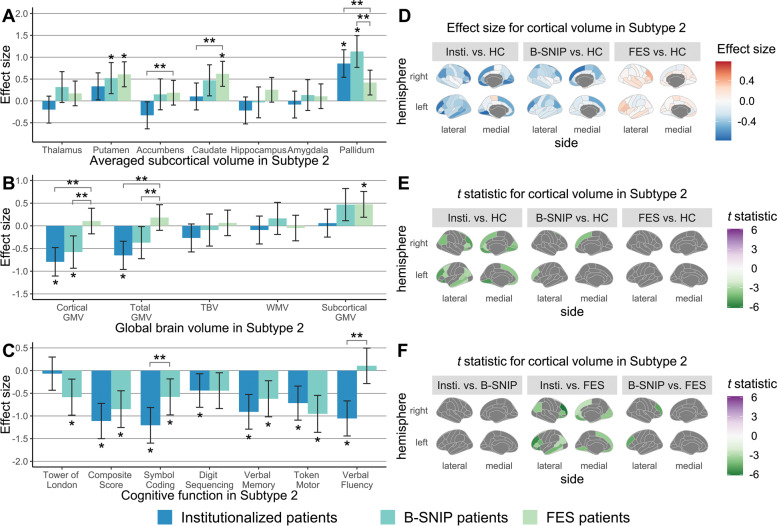
Fig. 4. Between-sample comparisons in brain-behavior profiles for Subtype 2.
ANCOVA and post hoc Tukey HSD tests were employed to test between-sample differences in (A) averaged subcortical volumes, (B) global brain volumes, (C) cognitive function, and (D–F) cortical volumes between Subtype 2 patients from different samples and the pooled healthy control group. Age, sex, and ICV were included as covariates for harmonized brain volumes, while education level was treated as the covariate for age- and sex-corrected BACS z-scores. In bar charts, significant patient-control and between-sample differences, determined by FDR-corrected P-values generated in post hoc pairwise tests, are marked by one and two asterisks, respectively. Shading bars represent Glass’s delta (Δ) effect sizes, which were calculated after removing variance related to covariates and used to demonstrate patient-control differences. Error bars mean 95% confidence interval of Δ. In cortical statistic maps, only regions that survived FDR corrections are colored by t statistics from post hoc tests. GMV Gray matter volume; HC Healthy controls; Insti. institutionalized patients; TBV Total brain volume; WMV White matter volume.
Key features of Subtype 1 institutionalized patients were more abnormal relative to controls than Subtype 1 individuals from the community-dwelling samples (Fig. 3). In neuroanatomic profiles, institutionalized and B-SNIP long-term ill patients in Subtype 2 did not display any significant differences but did share some common alterations such as increased pallidum volume (Fig. 4). However, institutionalized patients in Subtype 2 had significantly worse cognitive performance than Subtype 2 individuals of the B-SNIP sample (Fig. 4).
Discussion
This study identified two distinct subtypes of institutionalized patients with schizophrenia based on subcortical brain features. Subtype 1 of institutionalized patients demonstrated widespread and severe deficits in subcortical and neocortical brain volumes and in cognition. In contrast, subtypes identified based on cortical brain features did not significantly differ in cognition. According to a brain-based classifier developed in institutionalized patients, community-dwelling patients from both independent samples were also classified into two subgroups and in similar proportions as in the institutionalized patients. However, Subtype 1 neuroanatomic and (or) cognitive features in institutionalized patients were significantly more abnormal than in Subtype 1 individuals from the two community-dwelling samples. Regarding Subtype 2, increased pallidum volume was a consistent regional brain alteration for institutionalized and B-SNIP patients (i.e., community-dwelling individuals with long-term illness). Therefore, Subtype 1 institutionalized patients did not present with a novel form of illness in terms of subcortical features. Instead, when they did show that patterns as present in all schizophrenia groups, it was more severe and, in that case, was associated with particularly pronounced cognitive disability to the degree that likely contributed to their need for long-term inpatient care.
Chand et al. [13] also identified two patient subtypes based on regional brain volumes in schizophrenia, including one subtype with widespread reductions in gray and white matter volumes and the other with increased volumes in the basal ganglia. Our identification of two patient subtypes is broadly consistent with this finding, especially regarding identifying a subgroup with increased rather than decreased basal ganglia features. In the broader context of ongoing efforts to characterize heterogeneity in individuals living with the schizophrenia syndrome [13, 18, 19, 35, 36], our work extends prior findings by identifying a subgroup defined by subcortical features that is associated with neurocognitive morbidity. Identifying the brain and behavioral features of Subgroup 1 adds to the growing knowledge of neurobiological heterogeneity associated with the disorder, the investigation of which has focused on higher functioning community-dwelling individuals [35, 36]. Since long-term hospitalization has been associated with persistent impairment in cognitive function and disability in schizophrenia, developing effective interventions targeting institutionalized patients could have considerable clinical benefits [2, 37–39]. Our findings raise the possibility that the identification of subgroups of individuals requiring institutional care might guide the application of novel pharmacological or cognitive remediation interventions [40, 41] to improve long-term clinical outcomes.
The particularly pronounced subcortical and cognitive deficits observed in Subtype 1 of institutionalized patients were significantly greater than in community-dwelling samples. The causes of these differences cannot be determined in our cross-sectional study but may be related to illness progression in some individuals, antipsychotic medication effects, or a distinct subpopulation of patients with these features from illness onset. While neuropsychological deficits are generally stable over the early course of illness in individuals with schizophrenia [42], over the longer-term illness course [43], neuroanatomic and neurocognitive alterations may be progressive in some individuals [44–46]. Longitudinal studies of parallel changes in neuroanatomic and behavioral characteristics with heterogeneity modeling are needed to determine whether our observations reflect abnormalities that evolve over the illness course. But perhaps the more immediate direction to follow up on our findings are detailed studies of brain function and chemistry in subcortical regions in this population, as these might yield novel mechanistic understanding and provide the rationale for targeted treatment development.
One important observation from our analyses was that the subtype defined by subcortical volume reductions had pronounced cognitive deficits, while this was not observed in subtypes identified by cortical brain features in the institutionalized patient group. This pattern was observed in the context of robust anatomic alterations in both cortical and subcortical features in that group. These findings highlight the importance of subcortical volume abnormalities for cognitive deficits in institutionalized patients with schizophrenia, consistent with findings in several cognition-based subtyping studies [18, 19]. Of note, we observed the importance of alterations in the thalamus and basal ganglia for distinguishing subgroups of institutionalized schizophrenia patients with severe cognitive deficits, consistent with the established roles of these brain regions in cognitive function [15, 20, 47, 48].
A somewhat unexpected finding in Subtype 2 of institutionalized patients was an increased volume of the pallidum, in contrast to other subcortical measurements where decreases were seen and to the significant reduction of pallidum in Subtype 1 individuals. This effect in pallidum may be an effect of antipsychotic treatment in some individuals. Specifically, increased volume in the pallidum has been reported in studies of antipsychotic-treated patients [49–52] and in meta-analyses [53, 54], but not in drug-naïve FES patients [55, 56] or never-treated long-term ill individuals [44]. Additionally, a randomized clinical trial [57] found that individuals with schizophrenia receiving antipsychotic treatment showed increased pallidum volumes, while patients treated with placebo did not. Further, a recent cross-sectional study in schizophrenia spectrum disorders demonstrated that responders to first-line antipsychotics showed larger after-treatment volume in the pallidum than patients who did not respond to treatment [58]. These findings suggest that institutionalized patients with greater subcortical and cognitive deficits may fail to show this effect in the basal ganglia that have been associated with positive treatment outcomes.
Moreover, increased pallidum volumes were also observed in Subgroup 2 of B-SNIP patients who were successfully living in the community. The mechanisms of increased pallidum volume remain to be studied, and future efforts that clarify antipsychotic effects on pallidum anatomy are needed to better understand their relation to cognitive deficits and treatment outcomes.
Some limitations need to be considered in interpreting the findings of the present study. First, our study design did not allow us to determine whether the features that defined the institutionalized patients were risk factors for poor illness course evident before illness onset or changes resulting from illness progression or treatment. Second, a replication sample is needed to confirm the specific features used for subtyping institutionalized individuals and the subtype classification itself. Third, a more thorough psychosocial and neuropsychiatric characterization of institutionalized patients in future studies might better establish the range of functional disabilities associated with the two subtypes of institutionalized individuals.
Conclusions
A subtype of institutionalized schizophrenia patients defined by subcortical anatomic features was identified. The subgroup (Subgroup 1) comprised of half of the institutionalized patient cohort, was associated with widespread deficits in cortical brain volumes and cognitive function. While two similar patient subgroups were identified in first-episode and long-term ill community-living individuals, the neuroanatomic and cognitive deficits of Subgroup 1 individuals were particularly pronounced in institutionalized patients, which may have contributed to their need for institutionalization. These findings add new insights into the heterogeneity of schizophrenia, particularly as pertains to those with the greatest persistent functional disability, and highlight the importance of subcortical abnormalities for the cognitive deficits in this patient population. Further, our findings offer promising biomarkers for identifying a distinctly impaired subgroup of schizophrenia patients who may benefit from novel cognition-targeted interventions to reduce severe functional disability.
Supplementary information
Author contributions
Conception: QZ, SL, QG, and JAS; Methodological development and statistical analysis: QZ, HC, WZ, and JAS; Data collection, acquisition, and interpretation: all authors; Manuscript draft: QZ, SL, JAS, and HC; Critical revisions of the manuscript and final approval of this version to be published: all authors
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82120108014 [to SL], 82071908 [to SL], 81761128023 [to QG], 81621003 [to QG], and 81901705 [to YX]), Chinese Academy of Medical Sciences (Project No. 2021-12M-C&T-A-022 [to SL]), Sichuan Science and Technology Program (Project No. 2021JDTD0002 [to SL]), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 [to SL] and ZYJC18020 [to SL]). This work was supported by the National Institute of Mental Health (NIMH) through its support of the Bipolar–Schizophrenia Network for Intermediate Phenotypes (Grant Nos. MH077851 [to CAT], MH078113 [to MSK], MH077945 [to GDP], MH096942 [to BAC], MH077862 [to JAS] and MH096957 [to ESG]). MSK is supported by research grants from the NIMH and the Bear and Natalia Foundations. EII is supported by a grant from the NIMH (Grant No. 1K23 MH102656). JAS is supported by the University of Cincinnati Schizophrenia Research Fund. SL, JAS, and YX acknowledge the support from Alexander von Humboldt Foundation, and SL acknowledges the support from Chang Jiang Scholars (Program No. T2019069).
Competing interests
WZ, SYL, and JAS consulted to VeraSci. CAT has served on the advisory board for drug development for Intra-Cellular Therapies, Inc., as an ad hoc consultant for Eli Lilly, Sunovion, Astellas, Pfizer, and Merck, has been a council member and unpaid volunteer for the National Alliance on Mental Illness, and served as deputy editor for the American Psychiatric Association. MSK has received research support from Sunovion and GlaxoSmithKline. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qiyong Gong, Email: qiyonggong@hmrrc.org.cn.
Su Lui, Email: lusuwcums@tom.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01300-w.
References
- 1.Wakuda T, Takei N. ‘Opening doors’ for long-term institutionalised patients with schizophrenia in Japan. Acta Psychiatr Scand. 2021;143:277–78. doi: 10.1111/acps.13269. [DOI] [PubMed] [Google Scholar]
- 2.Nemoto T, Niimura H, Ryu Y, Sakuma K, Mizuno M. Long-term course of cognitive function in chronically hospitalized patients with schizophrenia transitioning to community-based living. Schizophr Res. 2014;155:90–5. doi: 10.1016/j.schres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Kubota M, van Haren NE, Haijma SV, Schnack HG, Cahn W, Hulshoff Pol HE, et al. Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72:803–12. doi: 10.1001/jamapsychiatry.2015.0712. [DOI] [PubMed] [Google Scholar]
- 4.Nestor PG, Kubicki M, Nakamura M, Niznikiewicz M, Levitt JJ, Shenton ME, et al. Neuropsychological variability, symptoms, and brain imaging in chronic schizophrenia. Brain Imaging Behav. 2013;7:68–76. doi: 10.1007/s11682-012-9193-0. [DOI] [PubMed] [Google Scholar]
- 5.Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58:457–67. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212–19.. doi: 10.1016/j.neubiorev.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43:1329–47.. doi: 10.1093/schbul/sbx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie T, Zhang X, Tang X, Zhang H, Yu M, Gong G, et al. Mapping convergent and divergent cortical thinning patterns in patients with deficit and nondeficit schizophrenia. Schizophr Bull. 2019;45:211–21.. doi: 10.1093/schbul/sbx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quide Y. A Systematic Review of Studies Reporting Data-Driven Cognitive Subtypes across the Psychosis Spectrum. Neuropsychol Rev. 2020;30:446–60. [DOI] [PubMed]
- 10.Bowen EFW, Burgess JL, Granger R, Kleinman JE, Rhodes CH. DLPFC transcriptome defines two molecular subtypes of schizophrenia. Transl Psychiatry. 2019;9:147. doi: 10.1038/s41398-019-0472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz E, van Beveren NJ, Ramsey J, Leweke FM, Rothermundt M, Bogerts B, et al. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull. 2014;40:787–95. doi: 10.1093/schbul/sbt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 2020;143:1027–38.. doi: 10.1093/brain/awaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Y, Liao W, Long Z, Tao B, Zhao Q, Luo C, et al. Subtyping schizophrenia patients based on patterns of structural brain alterations. Schizophr Bull. 2022;48:241–50.. doi: 10.1093/schbul/sbab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Tsugawa S, Nakajima S, Plitman E, Chakravarty MM, Masuda F, et al. Thalamic and striato-pallidal volumes in schizophrenia patients and individuals at risk for psychosis: A multi-atlas segmentation study. Schizophr Res. 2020. 10.1016/j.schres.2020.04.016 [DOI] [PubMed]
- 16.Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep. 2018;8:1183. doi: 10.1038/s41598-017-18950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Subcortical association with memory performance in schizophrenia: a structural magnetic resonance imaging study. Transl Psychiatry. 2018;8:20. doi: 10.1038/s41398-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg D, Lenroot R, Jacomb I, Allen K, Bruggemann J, Wells R, et al. Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2016;73:1251–59. doi: 10.1001/jamapsychiatry.2016.2925. [DOI] [PubMed] [Google Scholar]
- 19.Van Rheenen TE, Cropley V, Zalesky A, Bousman C, Wells R, Bruggemann J, et al. Widespread volumetric reductions in schizophrenia and schizoaffective patients displaying compromised cognitive abilities. Schizophr Bull. 2018;44:560–74.. doi: 10.1093/schbul/sbx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beste C, Moll CKE, Potter-Nerger M, Munchau A. Striatal microstructure and its relevance for cognitive control. Trends Cogn Sci. 2018;22:747–51.. doi: 10.1016/j.tics.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37:5594–607.. doi: 10.1523/JNEUROSCI.0067-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41.. doi: 10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 23.Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–84. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–74. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 25.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 26.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102:108–15. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Wang LJ, Lin PY, Lee Y, Huang YC, Hsu ST, Hung CF, et al. Validation of the Chinese version of brief assessment of cognition in schizophrenia. Neuropsychiatr Dis Treat. 2016;12:2819–26.. doi: 10.2147/NDT.S118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–18.. doi: 10.1016/j.neuroimage.2017.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur D, Vassilvitskii S. k-means++: the advantages of careful seeding. Society for Industrial and Applied Mathematics: New Orleans, Louisiana; 2007.
- 32.Rousseeuw PJ. Silhouettes - a graphical aid to the interpretation and validation of cluster-analysis. J Computational Appl Math. 1987;20:53–65. doi: 10.1016/0377-0427(87)90125-7. [DOI] [Google Scholar]
- 33.Breiman L. Random forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. 4.0.2 ed. R Foundation for Statistical Computing; 2020.
- 35.Hudgens-Haney ME, Clementz BA, Ivleva EI, Keshavan MS, Pearlson GD, Gershon ES, et al. Cognitive impairment and diminished neural responses constitute a biomarker signature of negative symptoms in psychosis. Schizophr Bull. 2020;46:1269–81.. doi: 10.1093/schbul/sbaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, et al. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82:26–39. doi: 10.1016/j.biopsych.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry. 1999;45:32–40. doi: 10.1016/S0006-3223(98)00273-X. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Harvey PD, Reichenberg A, Bowie CR, Patterson TL, Heaton RK. The course of neuropsychological performance and functional capacity in older patients with schizophrenia: influences of previous history of long-term institutional stay. Biol Psychiatry. 2010;67:933–9. doi: 10.1016/j.biopsych.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 41.Kambeitz-Ilankovic L, Betz LT, Dominke C, Haas SS, Subramaniam K, Fisher M, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci Biobehav Rev. 2019;107:828–45.. doi: 10.1016/j.neubiorev.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res. 2004;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 43.Islam MA, Habtewold TD, van Es FD, Quee PJ, van den Heuvel ER, Alizadeh BZ, et al. Long-term cognitive trajectories and heterogeneity in patients with schizophrenia and their unaffected siblings. Acta Psychiatr Scand. 2018;138:591–604. doi: 10.1111/acps.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Deng W, Yao L, Xiao Y, Li F, Liu J, et al. Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am J Psychiatry. 2015;172:995–1003. doi: 10.1176/appi.ajp.2015.14091108. [DOI] [PubMed] [Google Scholar]
- 45.Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020;25:3208–19.. doi: 10.1038/s41380-019-0509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fett AJ, Velthorst E, Reichenberg A, Ruggero CJ, Callahan JL, Fochtmann LJ, et al. Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the suffolk county mental health project. JAMA Psychiatry. 2019;77:387–96. doi: 10.1001/jamapsychiatry.2019.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pergola G, Danet L, Pitel AL, Carlesimo GA, Segobin S, Pariente J, et al. The regulatory role of the human mediodorsal thalamus. Trends Cogn Sci. 2018;22:1011–25.. doi: 10.1016/j.tics.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–63. doi: 10.1016/S0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Cao B, Yu R, Sim K. Basal ganglia volumetric changes in psychotic spectrum disorders. J Affect Disord. 2019;255:150–57.. doi: 10.1016/j.jad.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 50.Jorgensen KN, Nesvag R, Gunleiksrud S, Raballo A, Jonsson EG, Agartz I. First- and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266:451–60. doi: 10.1007/s00406-015-0650-9. [DOI] [PubMed] [Google Scholar]
- 51.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–7. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 52.Elkashef AM, Buchanan RW, Gellad F, Munson RC, Breier A. Basal ganglia pathology in schizophrenia and tardive dyskinesia: an MRI quantitative study. Am J Psychiatry. 1994;151:752–5. doi: 10.1176/ajp.151.5.752. [DOI] [PubMed] [Google Scholar]
- 53.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chua SE, Deng Y, Chen EY, Law CW, Chiu CP, Cheung C, et al. Early striatal hypertrophy in first-episode psychosis within 3 weeks of initiating antipsychotic drug treatment. Psychol Med. 2009;39:793–800. doi: 10.1017/S0033291708004212. [DOI] [PubMed] [Google Scholar]
- 56.Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chopra S, Fornito A, Francey SM, O'Donoghue B, Cropley V, Nelson B, et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study. Neuropsychopharmacology. 2021;46:1494–501. [DOI] [PMC free article] [PubMed]
- 58.Kim J, Plitman E, Iwata Y, Nakajima S, Mar W, Patel R, et al. Neuroanatomical profiles of treatment-resistance in patients with schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109839. doi: 10.1016/j.pnpbp.2019.109839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.