Abstract
Microbially-induced calcium carbonate precipitation (MICP) is a bio-cementation process that can improve the engineering properties of granular soils through the precipitation of calcium carbonate (CaCO3) minerals on soil particle surfaces and contacts. The technology has advanced rapidly as an environmentally conscious soil improvement method, however, our understanding of the effect of changes in field-representative environmental conditions on the physical and chemical properties of resulting precipitates has remained limited. An improved understanding of the effect of subsurface geochemical and soil conditions on process reaction kinetics and the morphology and mineralogy of bio-cementation may be critical towards enabling successful field-scale deployment of the technology and improving our understanding of the long-term chemical permanence of bio-cemented soils in different environments. In this study, thirty-five batch experiments were performed to specifically investigate the influence of seawater ions and varying soil materials on the mineralogy, morphology, and reaction kinetics of ureolytic bio-cementation. During experiments, differences in reaction kinetics were quantified to identify conditions inhibiting CaCO3 precipitation and ureolysis. Following experiments, scanning electron microscopy, x-ray diffraction, and chemical composition analyses were employed to quantify differences in mineralogical compositions and material morphology. Ions present in seawater and variations in soil materials were shown to significantly influence ureolytic activity and precipitate mineralogy and morphology, however, calcite remained the predominant CaCO3 polymorph in all experiments with relative percentages exceeding 80% by mass in all precipitates.
Subject terms: Civil engineering, Applied microbiology, Biogeochemistry, Natural hazards
Introduction
Commercially available ground improvement methods oftentimes rely on high mechanical energy and/or energy-intensive materials, such as portland cement, to improve the engineering properties of soils1–5. Recently the emerging field of bio-mediated soil improvement has demonstrated the potential of biogeochemical processes to enable comparable engineering improvements with significant reductions in detrimental environmental impacts6–9. Microbially-induced calcium carbonate precipitation (MICP) is one such technology, which involves the precipitation of calcium carbonate (CaCO3) minerals on soil particle surfaces and contacts following the biologically mediated hydrolysis of urea in the presence of soluble calcium (Ca2+)10–12. MICP has the ability to transform the engineering properties of soils by increasing initial shear stiffness, strength, and thermal conductivity, while reducing soil hydraulic conductivity and porosity13–20. The bio-mediated process can address a diverse range of engineering applications including liquefaction mitigation, slope stability improvement, subsurface flow manipulation, construction material development, and contaminant immobilization8,12,21–24. Despite many recent advances in the technology including improved characterization of engineering behaviors14,25–28 and successful demonstration at meter-scale29–36, our collective understanding of the impact of environmental conditions on the bio-cementation process and the physical and chemical properties of bio-cemented soils including mineralogy, crystal morphology, chemical composition, and solubility has remained limited.
Although bio-cementation is commonly referred to as consisting of exclusively calcite, CaCO3 can exist as different mineral polymorphs each with varying atomic structures, morphologies, and physicochemical properties37. The mineralogy of generated CaCO3 will be a critical factor governing the long-term chemical permanence of bio-cementation once employed at field-scale as CaCO3 mineral polymorphs have solubilities that can span orders of magnitude. For example, at 1 atm and 25 °C, calcite has the lowest polymorph solubility product (Ksp) of 10−8.48, however, aragonite (10−8.34), vaterite (10−7.91), ikaite (10−6.62), and amorphous calcite (10−6.40) each have solubilities that are considerably higher, despite all minerals being CaCO338–40. Thus, an improved understanding of the influence of chemical conditions during MICP, including those imposed by treatment solution compositions and environmental conditions, on the mineralogy and morphology of generated bio-cementation may have important implications towards evaluating and optimizing the long-term permanence and engineering performance of bio-cemented soils. Past researchers have considered an extensive range of different chemical reagents and concentrations in applied bio-cementation treatment solutions, however, almost all treatment solutions have included supplied urea (for microbial CO32− production), soluble Ca2+ (for CaCO3 precipitation), and microbial nutrients and substrates (to maintain and/or increase ureolytic cell densities). Occasionally, other reactants have also been supplied to enable control of reaction rates (e.g., NH4Cl), microbial enrichment (e.g., NaOH, NH4Cl, sodium acetate), and/or alter other environmental conditions (e.g., NaHCO3)29,41–47. While continued optimization of treatment solution compositions was to be expected as the technology has matured, large variations in applied treatment solutions continue to persist even between the most recent studies suggesting that researchers have yet to reach firm conclusions regarding the effect of treatment solution compositions and environmental conditions on the bio-cementation process8,20.
Although changes in treatment solution compositions afford the opportunity to directly influence chemical conditions during CaCO3 precipitation, environmental conditions including subsurface groundwater chemistry and in situ soil mineralogy present unavoidable site-specific factors governed by application location. An improved understanding of how these conditions may alter reaction kinetics and generated mineral products will have important practical implications, guiding employed treatment solution compositions, allowing for the identification of favorable application locations, and enabling improved assessment of long-term material permanence. A multitude of environmental conditions may be present at any given site including differences in available cations, anions, solution pH, microorganisms, and other factors, however, a clear opportunity exists to apply the MICP process to marine and brackish environments wherein elevated total alkalinity and calcium concentrations may permit improved permanence48. Past studies have examined the effect of seawater on MICP-treated soils with most focusing on investigating differences in achieved mechanical properties. Mortenson et al.44 found that higher shear wave velocities could be achieved when MICP was performed in seawater due to its higher total alkalinity and calcium concentrations. Cheng et al.49 found that higher unconfined compressive strengths could be obtained for the same CaCO3 content when seawater was used to prepare treatment solutions, however, permeability reductions remained similar to samples treated with freshwater at comparable cementation levels. Similarly, Yu and Rong50 observed increases in the unconfined compressive strength of cemented sand blocks when seawater-based solutions were employed. In contrast, Miftah et al.51 found that seawater had minimal effects on the strength, CaCO3 content, and mineralogy of precipitates obtained using enzyme-induced calcium carbonate precipitation (EICP) in a beach sand material. Although the majority of these studies suggest that the presence of seawater may have beneficial effects on the mechanical improvements afforded by MICP, such studies have provided limited insights regarding which specific ions in seawater may influence microbial activity and precipitation kinetics and how the mineralogy and composition of generated precipitates may shift as a function these differences.
Recent investigations have also expanded the range of soil materials considered suitable for the MICP process beyond the poorly-graded sand materials for which the majority of past studies have been performed. These investigations have involved various natural sand mixtures36,52–54, clay minerals55,56, peaty soils57,58, and mine wastes59–61, however, almost all of these studies have involved differences in treatment solutions, preparation methods, and application and testing procedures, thereby rendering the effect of changes in soil mineralogy difficult to isolate from other experimental variables. Most these studies have also solely investigated changes in macroscale engineering behaviors (i.e., peak shear strength, shear wave velocity) which would not be expected to resolve important differences in precipitate microstructure and composition nor provide insights regarding changes in reaction behaviors. In order to more effectively understand the effect of specific environmental factors on the bio-cementation process, systematic experimentation must be performed which minimizes differences in biological, chemical, and physical factors.
Although not yet fully explored for bio-cementation, abiotic CaCO3 synthesis experiments completed under more controlled conditions suggest that even small changes in solution chemistry can dramatically alter precipitate formation including the presence of various ions found in seawater (e.g., SO42−, Na+, Mg2+) and associated with common soil minerals. For example, Berner62, Zhang and Dawe63, and Meldrum and Hyde64 observed large changes in CaCO3 precipitation kinetics and precipitate morphology when trace concentrations of magnesium (Mg2+) were present. Busenberg and Plummer65 found that sulfate (SO42−) concentrations in seawater (≈ 28 mM) significantly inhibited CaCO3 precipitation rates and that both SO42− and Na+ were incorporated within CaCO3 when precipitation occurred in artificial seawater. The presence of manganese (Mn2+), strontium (Sr2+), and phosphate (PO43−) have also been identified as important factors influencing CaCO3 precipitation66. Furthermore, differences in mineral surface chemistry have also long been recognized by researchers as having a significant impact on carbonate mineral precipitation including influencing the particular mineral polymorphs formed as well as crystal nucleation and rates of formation67. For example, Liu et al.68 found that the presence of clay minerals can influence abiotic CaCO3 and dolomite (CaMg(CO3)2) formation, with illite and montmorillonite clay minerals accelerating carbonate mineral formation, but kaolinite exhibiting more minimal effects on such reactions. Although such studies have yielded important insights regarding the effect of soil minerals and solution chemistry on abiotic mineral formation, the addition of biological catalysts during MICP may present further complications, thereby altering mineral formation in manners that are not thermodynamically predictable69,70. As the technology advances towards field-scale implementation, an improved understanding of the consequences of site-specific conditions will be increasingly important.
In this study, thirty-five small-scale batch experiments were conducted to investigate the effect of field-representative environmental conditions on the mineralogy, morphology, and reaction kinetics of ureolytic bio-cementation using augmented Sporosarcina pasteurii (S. pasteurii) bacteria. Experiments were completed in five different series and explored the effect of varying concentrations of artificial seawater, differences in Mg2+, Sr2+, SO42−, and Na+ ion additions, and variations in soil materials on the MICP process and resulting CaCO3 precipitates. Results from these experiments provide new insights regarding the effect of soil materials and seawater ions on the reaction kinetics, morphology, and mineralogy of ureolytic bio-cementation, relevant towards improving our understanding of process deployment and material longevity when applied to different geochemical environments.
Materials and methods
Batch experiments
All experiments were conducted in 100 mm diameter, 15 mm deep, flat bottom glass petri dishes (Corning Inc.) which included 5.3 g of oven-dried soil and 45 mL of treatment solutions. The performed batch experiments provided several advantages relative to soil columns including: (1) permitting homogenous solution conditions to be achieved at the start of experiments, (2) eliminating the potential for changes in reaction kinetics and precipitation formation due to uncontrolled differences in cell attachment, reactions during injections, hydraulic conductivity differences, and other physical phenomena, and (3) minimizing the influence of solution sampling events on specimen saturation. In order to simulate field-representative treatment processes wherein soils are either first augmented or stimulated to achieve sufficient ureolytic activity and then subsequently bio-cemented12,29,36,41,43,47, dry soil masses were directly mixed with S. pasteurii cell suspensions (≈ 1 mL) prior to all experiments. After mixing, soil and cell suspensions were allowed to equilibrate at 1.6 °C for a minimum of 12 h prior to introducing treatment solutions in order to promote attachment of cells onto soil particle surfaces following other augmented studies12,29,47,71 while limiting microbial activity in the absence of supplied nutrients. Prepared treatment solutions (≈ 44 mL) were mixed with augmented soil mixtures after the equilibration period, vortexed at 800 rpm for 10 s, placed within plates, and then covered with parafilm to minimize solution-air interactions and the potential for evaporation. All experiments were allowed to react for up to 30 h at a constant temperature of 23 °C to allow ureolysis and precipitation reactions to achieve completion in most experiments. All experiments contained no supplied nutrients intended to mitigate the potential for cell growth and aerobic respiration during experiments as well as eliminate the presence of excess proteins and amino acids, which may have altered precipitate formation. Although efforts were made to minimize the potential for biological contamination, included soil materials were not strictly sterilized as all experiments lacked nutrients and were designed to minimize the potential for microbial growth. After all retention periods, remaining solutions were drained, collected, and frozen and bio-cemented soils were rinsed twice with absolute ethanol to remove soluble reaction byproducts and then oven-dried at 110 °C for 48 h. After drying, all bio-cemented soil specimens were stored in a vacuum desiccator at room temperature and a relative humidity of less than 10% and were stored for no more than 2 weeks before completing material analyses intended to mitigate the potential for subsequent mineralogical changes. Similar mineral preservation processes have been used successfully in other past studies involving CaCO3 minerals72,73.
Experimental series
Thirty-five batch experiments (E1–E35) were completed in five different experimental series to examine the effect of changes in seawater ions and differences in soil materials on the bio-cementation process and resulting precipitates. Table 1 summarizes all batch experiments including experiment name, experimental series, soil material, treatment solution composition, augmented cell densities measured via OD600 measurements (direct measurements of cell densities), augmented cell densities estimated from observed urea hydrolysis activity and a PHREEQC kinetic model (urea hydrolysis activity based estimates of cell densities), the ratio between PHREEQC-estimated (activity based estimates) and OD600-measured cell densities (direct measurements), and the ratio between PHREEQC-estimated cell densities for experiments (activity-based estimates for experiments) and their respective controls for each series (activity-based estimates for respective control specimens). Experiments were augmented with cells at the same cell density using the same batch of growth media for each respective series, in order to minimize biological differences between experiments. For all experimental series, similar control experiments (E1, E4, E13, E16, E21, E25) were also repeated which contained only urea, Ca2+, augmented S. pasteurii cells, and Ottawa F-65 sand, in order to allow for comparison of reaction kinetics and precipitates between experiments while controlling for unavoidable variations in augmented cell activities. Experimental series 1 (E1–E3) examined bio-cementation in artificial seawater mixtures prepared at three different concentrations by volume. Experimental series 2 (E4–E11) examined bio-cementation in the presence of discrete Mg2+, Sr2+, and SO42− ion additions in order to further investigate the effect of specific seawater ion concentrations on the MICP process at concentrations between 50 and 200% that present in seawater. Experimental series 3 (E12–E20) examined the effect of discrete seawater ion additions on urea hydrolysis alone in experiments which contained minimal supplied Ca2+ (0 or 10 mM) and no significant precipitation. Experimental series 4 (E21–E24) further examined bio-cementation in the presence of Na+ ion additions intended to assess the effect of ionic strength increases relevant to earlier seawater experiments. Lastly, experimental series 5 (E25–E35) explored bio-cementation in the presence of eleven different soils of varying mineralogy.
Table 1.
Summary of all batch experiments, treatment solution compositions, and augmented cell measurements and estimations.
| Batch experiments | Treatment solution composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen name | Experimental series | Soil material | Urea (mM) | Ca2+ (mM) | Mg2+ (mM) | SO42− (mM) | Sr2+ (mM) | Na+ (mM) | Cl− (mM) | K+ (mM) | B3+ (mM) | CO32− mM) |
| E1_0% ASW | (1) ASW | Ottawa F-65 sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E2_50% ASW | (1) ASW | Ottawa F-65 sand | 250 | 255 | 27 | 14 | 0.05 | 242 | 782 | 2. 5 | 0.015 | 1.5 |
| E3_100% ASW | (1) ASW | Ottawa F-65 sand | 250 | 260 | 54 | 27 | 0. 10 | 481 | 1051 | 5 | 0. 03 | 3 |
| E4_0 mM Mg2+/0 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E5_27 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | 27 | – | – | 3 | 557 | – | – | – |
| E6_54 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | 54 | – | – | 3 | 611 | – | – | – |
| E7_108 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | 108 | – | – | 3 | 719 | – | – | – |
| E8_0.055 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | – | – | 0. 055 | 3 | 504 | – | – | – |
| E9_0.11 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | – | – | 0. 11 | 3 | 504 | – | – | – |
| E10_0.22 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | – | – | 0. 22 | 3 | 504 | – | – | – |
| E11_14 mM SO42− | (2) ASW ions | Ottawa F-65 sand | 250 | 250 | – | 14 | 31 | 504 | – | – | – | |
| E12_100% ASW | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | 10 | 54 | 27 | 0. 10 | 481 | 539 | 5 | 0. 03 | 3 |
| E13_0 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | – | – | – | 3 | 3 | – | – | – |
| E14_14 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | – | 14 | – | 31 | 3 | – | – | – |
| E15_28 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | – | 28 | – | 59 | 3 | – | – | – |
| E16_0 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | – | – | – | 3 | 3 | – | – | – |
| E17_54 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | 54 | – | – | 3 | 111 | – | – | – |
| E18_54 mM Mg2+ + 10 mM Ca2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | 10 | 54 | – | – | 3 | 131 | – | – | – |
| E19_108 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | – | 108 | – | – | 3 | 219 | – | – | – |
| E20_108 mM Mg2+ + 10 mM Ca2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 250 | 10 | 108 | – | – | 3 | 239 | – | – | – |
| E21_0 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E22_10 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 250 | 250 | – | – | – | 13 | 513 | – | – | – |
| E23_100 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 250 | 250 | – | – | – | 103 | 603 | – | – | – |
| E24_1000 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 250 | 250 | – | – | – | 1003 | 1503 | – | – | – |
| E25_Ottawa sand | (5) Soil variations | Ottawa F-65 sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E26_Fraser river sand | (5) Soil variations | Fraser river sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E27_Concrete sand | (5) Soil variations | Concrete sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E28_Covelo sand | (5) Soil variations | Covelo sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E29_Delta sand | (5) Soil variations | Delta sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E30_Monterey sand | (5) Soil variations | Monterey sand | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E31_Feldspar | (5) Soil variations | Feldspar | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E32_Olivine | (5) Soil variations | Olivine | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E33_Mica | (5) Soil variations | Mica | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E34_Kaolinite | (5) Soil variations | Kaolinite | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| E35_Montmorillonite | (5) Soil variations | Montmorillonite | 250 | 250 | – | – | – | 3 | 503 | – | – | – |
| Batch experiments | Augmented cells | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen name | Experimental series | Soil material | OD600-based direct measurement of cell density (cells/mL) | PHREEQC activity-based estimate of cell density (cells/mL) | Ratio of PHREEQC activity-based estimated cell density to OD600-based direct measure of cell density | Ratio of PHREEQC activity-based estimated cell density for experiment to PHREEQC activity-based estimated cell density of control | ||||||
| E1_0% ASW | (1) ASW | Ottawa F-65 sand | 7.6E+07 | 1.3E+08 | 171% | 100% (control) | ||||||
| E2_50% ASW | (1) ASW | Ottawa F-65 sand | 7.6E+07 | 1.2E+08 | 158% | 92% | ||||||
| E3_100% ASW | (1) ASW | Ottawa F-65 sand | 7.6E+07 | 6.0E+07 | 79% | 46% | ||||||
| E4_0 mM Mg2+/0 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 1.0E+08 | 140% | 100% (control) | ||||||
| E5_27 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 5.5E+07 | 77% | 55% | ||||||
| E6_54 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 5.0E+07 | 70% | 50% | ||||||
| E7_108 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 4.5E+07 | 63% | 45% | ||||||
| E8_0.055 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 9.0E+07 | 126% | 90% | ||||||
| E9_0.11 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 8.5E+07 | 119% | 85% | ||||||
| E10_0.22 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 8.0E+07 | 112% | 80% | ||||||
| E11_14 mM SO42− | (2) ASW ions | Ottawa F-65 sand | 7.1E+07 | 8.5E+07 | 119% | 85% | ||||||
| E12_100% ASW | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 2.0E+07 | 30% | 44% | ||||||
| E13_0 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 4.5E+07 | 68% | 100% (control) | ||||||
| E14_14 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 4.5E+07 | 68% | 100% | ||||||
| E15_28 mM SO42− | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 4.5E+07 | 68% | 100% | ||||||
| E16_0 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 4.5E+07 | 68% | 100% (control) | ||||||
| E17_54 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 2.5E+07 | 38% | 56% | ||||||
| E18_54 mM Mg2+ + 10 mM Ca2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 2.8E+07 | 42% | 61% | ||||||
| E19_108 mM Mg2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 1.5E+07 | 23% | 33% | ||||||
| E20_108 mM Mg2+ + 10 mM Ca2+ | (3) ASW ions (ureolysis only) | Ottawa F-65 sand | 6.6E+07 | 1.5E+07 | 23% | 33% | ||||||
| E21_0 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 8.0E+07 | 1.1E+08 | 131% | 100% (control) | ||||||
| E22_10 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 8.0E+07 | 1.1E+08 | 137% | 105% | ||||||
| E23_100 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 8.0E+07 | 1.1E+08 | 131% | 100% | ||||||
| E24_1000 mM Na+ | (4) Sodium variations | Ottawa F-65 sand | 8.0E+07 | 5.5E+07 | 69% | 52% | ||||||
| E25_Ottawa sand | (5) Soil variations | Ottawa F-65 sand | 8.2E+07 | 1.3E+08 | 159% | 100% (control) | ||||||
| E26_Fraser river sand | (5) Soil variations | Fraser river sand | 8.2E+07 | 7.5E+06 | 9% | 6% | ||||||
| E27_Concrete sand | (5) Soil variations | Concrete sand | 8.2E+07 | 8.0E+07 | 98% | 62% | ||||||
| E28_Covelo sand | (5) Soil variations | Covelo sand | 8.2E+07 | 4.5E+07 | 55% | 35% | ||||||
| E29_Delta sand | (5) Soil variations | Delta sand | 8.2E+07 | 7.3E+07 | 89% | 56% | ||||||
| E30_Monterey sand | (5) Soil variations | Monterey sand | 8.2E+07 | 1.1E+08 | 135% | 85% | ||||||
| E31_Feldspar | (5) Soil variations | Feldspar | 8.2E+07 | 1.1E+08 | 129% | 81% | ||||||
| E32_Olivine | (5) Soil variations | Olivine | 8.2E+07 | 1.0E+08 | 126% | 79% | ||||||
| E33_Mica | (5) Soil variations | Mica | 8.2E+07 | 1.7E+08 | 208% | 131% | ||||||
| E34_Kaolinite | (5) Soil variations | Kaolinite | 8.2E+07 | 1.7E+08 | 202% | 127% | ||||||
| E35_Montmorillonite | (5) Soil variations | Montmorillonite | 8.2E+07 | 1.1E+08 | 135% | 85% | ||||||
Soil materials
All batch experiments were performed using Ottawa F-65 Sand with the exception of the soil varied experiments (experimental series 5), which involved ten additional soils. Ottawa F-65 Sand was selected for the majority of the batch experiments performed in this study due to its chemically inert quartz mineralogy, near uniform grain size distribution, low fines content, and extensive characterizations in past bio-cementation and other geotechnical studies25,27,74–76. Ottawa F-65 Sand has a D10 of 0.13 mm, a D30 of 0.18 mm, a D60 of 0.23 mm, no fines75, and classifies as a poorly-graded sand (SP) following ASTM D2487-1777. In experimental series 5, other soil materials were present including other poorly-graded natural and processed sands (Fraser River Sand, Covelo Sand, Delta Sand, Concrete Sand, Monterey Sand), low and high plasticity phyllosilicate minerals (mica, kaolinite, montmorillonite), and other common soil minerals (feldspar, olivine). Table 2 presents the material sources, USCS classifications, average particle sizes (D50), fines contents, and mineralogical composition of all soils used in this study as determined by XRD analyses.
Table 2.
Summary of all tested soil materials.
| Soil material | Source | USCS | D50 (mm) | Fines content (%) | XRD Characterization | |
|---|---|---|---|---|---|---|
| Primary minerals | Other detectable minerals (> 2%) | |||||
| Ottawa sand | Commerical (US Silica Inc.) | SP | 0.2 | – | Quartz (~ 100%) | – |
| Fraser river sand | Field Sample (Delta, BC, Canada) | SM | 0.2 | 15.6 | Quartz (~ 85%) | Albite (~ 15%) |
| Concrete sand | Teichert Aggregates (Woodland, CA, USA) | SP | 1.2 | 1.1 | Quartz (~ 75%) | Albite (~ 25%) |
| Covelo sand | DenBeste Supply (Ukiah, CA, USA) | SP | 1.2 | 1.6 | Quartz (~ 87%) | Albite (~ 13%) |
| Delta sand | NorCal Aggregates (Petaluma, CA, USA) | SP | 0.3 | 1.3 | Quartz (~ 58%) | Albite (~ 42%) |
| Monterey sand | Cemex Inc. (Marina, CA, USA) | SP | 1.4 | – | Quartz (~ 47%) | Microcline (~ 42%), Albite (~ 11%) |
| Feldspar | Laguna Clay (City of Industry, CA, USA) | SP | 4.0 | – | Microcline (~ 78%) | Albite (~ 12%), Quartz (~ 10%) |
| Olivine | Laguna Clay (City of Industry, CA, USA) | SP | 2.4 | – | Forsterite (~ 90%) | Fayalite (~ 10%) |
| Mica | Laguna Clay (City of Industry, CA, USA) | SP | 2.6 | – | Lepidolite (~ 100%) | – |
| Kaolinite | Commerical (Sigma Adrich) | ML | < 200 µm | = 100 | Kaolinite (~ 100%) | – |
| Montmorillonite | Commerical (Sigma Adrich) | CH | < 200 µm | = 100 | Montmorillonite (~ 96%) | Quartz (~ 4%) |
In order to characterize the chemical composition of tested soils, cation exchange capacity (CEC) and exchangeable cation measurements were completed on all soil materials using a process similar to U.S. EPA Method 908078. During measurements, 10 g of dry soil and 50 mL of a 1 M NH4Cl solution were added to a plastic syringe and equilibrated for 12 h. Soil solutions were then extracted, collected, and exchangeable cations were characterized using ICP-MS. The remaining extracted soil samples were agitated in absolute ethanol for 6 h, decanted to remove ammonium (NH4+) ions that may have remained in free solution, and 50 mL of 1 M KCl solution was added to all samples and allowed to equilibrate for 12 h to encourage replacement of sorbed NH4+. Soil solutions were then extracted and NH4+ concentrations in the extracted solution were quantified using a salicylate colorimetric assay and used to calculate soil CEC values. Soil CEC values reflect the capacity of negatively charged soil surfaces to sorb cations and it was hypothesized that CEC differences between tested soils could affect ion exchange at soil surfaces and CaCO3 precipitation. Supplemental Table S1 presents both CEC values and exchangeable cations quantified for all soil materials used in this study.
Cell culture preparation
Sporosarcina pasteurii (ATCC 11859) cell suspensions were prepared for batch experiments using frozen stock cultures and ATCC 1376 growth media (15.74 g/L tris base, 20 g/L yeast extract, 10 g/L ammonium sulfate, pH-adjusted to 9.0). All growth media volumes were autoclaved at 121 °C for 24 min, cooled to room temperature (23 °C), and inoculated with a S. pasteurii frozen stock culture. S. pasteurii stock cultures were prepared from freeze-dried cell pellets using ATCC 1376 growth media that was incubated, stabilized in 25% (v/v) glycerol, and stored at − 80 °C until use. Following inoculation, growth media volumes were incubated for 48 h at 30 °C using a double-orbital shaker at 175 rpm prior to harvesting cells. Concentrated S. pasteurii cell pellet suspensions were obtained by centrifuging ≈ 45 mL volumes of incubated growth media in a conical tube for 10 min at 1972 g, discarding the supernatant, rinsing the remaining cells using ≈ 45 mL of sterile isotonic saline solution (154 mM NaCl), mixing thoroughly, and repeating centrifuging and rinsing steps until the discarded supernatant appeared clear (≈ 2–3 rinse sequences). The cell rinsing process was performed both to concentrate cells and to remove growth factors present in ATCC 1376 growth media, which could have resulted in the growth of augmented cells during batch experiments, thereby impacting reaction progression and precipitation events. Final cell suspensions were prepared by adding 10 mL of sterile isotonic saline to rinsed cells and mixing thoroughly. The optical densities of these final cell suspensions were measured using a microplate spectrophotometer (Biotek Inc.) at a wavelength of 600 nm (OD600) and values typically ranged between 1.5 and 1.6, indicative of between 2.7 and 3.3 × 109 cells/mL based upon a lab-specific total direct cell count to OD600 correlation (Supplemental Figure S1). Once diluted in cementation solutions, all experiments had estimated S. pasteurii cell densities between 6.6 and 8.2 × 107 cells/mL, similar to augmented cell densities used in other bio-cementation experiments25,29,79,80.
Treatment solutions
All treatment solutions were prepared using urea (Fisher Scientific Inc., > 99.2% assay), calcium chloride dihydrate (Fisher Scientific Inc., > 99.0% assay), and deionized water. Solutions contained 250 mM equimolar concentrations of urea and Ca2+ in all experiments with the exception of experimental series 3, which contained 250 mM urea, but minimal added Ca2+ (0 mM or 10 mM). Treatment solutions used for all controls were similar to solutions used in many past studies36,45,81 and contained only urea and Ca2+ to minimize potential effects from other additives. Since other solution compositions were not examined, however, the results of this study may be specific to these formulations. All other chemical constituents were added to solutions using dry reagents immediately prior to experiments including magnesium chloride hexahydrate (Fisher Scientific Inc., > 99.0% assay), strontium chloride hexahydrate (MP Biomedicals LLC, > 99.0% assay), sodium chloride (Fisher Scientific Inc., > 99.0% assay), and sodium sulfate (Fisher Scientific Inc., > 99.0% assay). Artificial seawater (ASW) solutions were prepared using S9983 dried sea salts (Millipore Sigma) wherein 100% ASW solutions contained 478 mM Na+, 536 mM Cl−, 54 mM Mg2+, 27 mM SO42−, 10 mM Ca2+, 5 mM K+, 3 mM CO32−, 0.1 mM Sr2+, and 0.03 mM B3+, which was consistent with average concentrations expected in natural seawater82. All treatment solutions were prepared more concentrated in order to achieve targeted concentrations after dilution with augmented soils, which contained ≈ 1 mL solution volumes from cell inoculants.
Aqueous solution sampling
Small-volume aqueous solution samples (≈ 120 µL) were collected from all experiments at various times during reactions to monitor changes in solution urea and Ca2+ concentrations reflective of urea hydrolysis activity and CaCO3 precipitation. Sampling intervals varied between experiments due to differences in achieved ureolytic activities but included the collection of at least six samples over the first 10 h of experiments during which substantial reactant concentration changes were expected. In order to minimize the impact of sampling events on experimental behaviors, the total volume sampled for all experiments never exceeded 3.5% of the total solution volume (< 1.6 mL). All solution samples were collected near the center of plates using a pipette with disposable polypropylene tips. After collection, aqueous samples were pipetted into a 2.0 mL conical tube with a 0.22-µm cellulose acetate filter (Corning Inc.) and were centrifuged at 1318 g for a minimum of 30 s to remove solids. After filtration, 80 µL of filtered solution samples were added to 300 µL of a 1 M hydrochloric acid (Fisher Scientific) and samples were mixed thoroughly using a vortexer. Acid stabilization of samples was intended to mitigate potential volatilization of ammonia present within samples and the potential for continued ureolytic activity and CaCO3 precipitation reactions following sampling. All aqueous samples were frozen immediately after stabilization until thawing for subsequent urea and Ca2+ analyses.
Aqueous chemical measurements
Measurements of aqueous urea and Ca2+ in time were performed for all samples obtained from batch experiments. Aqueous urea measurements were performed using a colorimetric assay modified from Knorst et al.83 wherein a colorimetric reagent consisting of 4% (w/v) p-Dimethylaminobenzaldehyde and 19% (v/v) HCl in absolute ethanol was added to dilute sample volumes. Absorbance values were measured at 422 nm using a microplate spectrophotometer and were compared to calibration curve relationships to determine sample concentrations. Aqueous Ca2+ measurements were completed using a QuantiChrom DICA-500 calcium assay kit (BioAssay Systems) with a phenolsulphonephthalein-based colorimetric dye. In this process, samples were first diluted in ultrapure water (< 7.2 × 10−9 mM Ca2+) to achieve concentrations within the linear range of the assay (< 5.0 mM Ca2+). 200 µL of a colorimetric reagent was then added to samples and absorbances were measured after 10 min at a wavelength of 612 nm. Supplemental Figure S2 provides example calibration curves for both assays used in this study.
PHREEQC biogeochemical modeling
PHREEQC84, an open-source batch reaction aqueous geochemical code, was used to estimate cell densities for all experiments from observed urea degradation activity. In the PHREEQC model, microbial urea hydrolysis rates (ureolytic rates) were modeled using the cell-normalized Michaelis–Menten ureolytic kinetic expression presented in [Eq. 1] wherein ρcell is the S. pasteurii cell density in cells/L, Km is the half-saturation coefficient in [mM urea] and Vmax cell is the cell-normalized maximal velocity in [mM urea cell−1 h−1]85. In all models, Km and Vmax cell were assumed to be 305 mM urea and 1.057 × 10−9 mmol urea cell−1 h−1, respectively, following whole cell ureolytic parameters reported by Graddy et al.86 for S. pasteurii ATCC 11859.
| 1 |
In order to match modeled rates to experimentally observed urea degradation activity, S. pasteurii cell densities (ρcell) in the models were varied. Recognizing that changes in microbial activity may occur during reactions due to a number of factors including cell death and encapsulation43,87–89, modeled trends were used to quantitatively estimate changes in ureolytic activity resulting from inhibitory ion concentrations and/or changes in cell viability during reaction periods. Since the primary motivation for calibration of the model to experimentally observed urea degradation data was to quantify ureolytic rates, CaCO3 precipitation was assumed to be an equilibrium reaction and all other kinetically controlled reactions were ignored. Cell density estimates from the PHREEQC model were compared to known augmented cell densities measured using OD600 measurements to evaluate consistency between activity-based estimates and direct cell density measurements. Initial urea degradation rates were also determined for experiments using the cell-normalized Michaelis–Menten ureolytic kinetic model, estimated cell densities, and the known initial urea concentration of 250 mM.
X-ray diffraction analyses
X-ray diffraction (XRD) analyses were performed to characterize the mineralogy of CaCO3 precipitates from batch experiments, identify soil minerals, and detect the presence of any other non-CaCO3 minerals that may have formed. Characterization of CaCO3 precipitates specifically focused on detecting and quantifying the presence of calcite, vaterite, and aragonite, all of which are crystalline CaCO3 polymorphs. All XRD analyses were performed using a Bruker D8 Discover X-ray powder diffractometer with a IμS 2-D powder microfocus source, a high-efficiency Cu anode, and a Pilatus 100 k large-area 2-D detector. Scans were performed using a phi rotation of 45 s for 2θ values from 10.6° to 99.4° at increments of 0.02°. Samples were prepared from absolute ethanol-rinsed precipitates obtained from batch experiments and were ground into a fine powder using an agate mortar and pestle prior to XRD analyses in order to obtain particle sizes between ≈ 10 and 50 µm following recommendations by Pecharsky and Zavalij90. Semi-quantitative weight percentage analysis (S-Q analysis) of the obtained diffraction patterns were performed to estimate relative quantities (by mass) of the different CaCO3 mineral phases present using Diffrac.EVA XRD software (version 4.3) and the ICDD PDF2, ICDD PDF4, and COD reference diffraction databases91. S-Q analyses involve the fitting of measured diffraction patterns using integrated diffraction peak intensities for known crystalline minerals, in order to determine the presence of minerals and their relative fractions by mass. Relative CaCO3 percentages were determined from S-Q analyses for aragonite, vaterite, and calcite minerals, specifically, and had an estimated error of ± 5% by mass which was determined from independent characterizations of select specimens using Fourier transform infrared spectroscopy (FTIR). Table 3 summarizes S-Q analysis results for all experiments. Since x-ray diffraction requires the knowledge of known refraction angles for crystalline atomic structures, the performed XRD analyses were unable to reliably quantify amorphous CaCO3.
Table 3.
Summary of X-ray diffraction (XRD) S-Q analysis results for all precipitates.
| Specimen name | Experimental series | Parent soil | Relative % of CaCO3 (By Mass)* | Other detected minerals (in order of decreasing abundance) | ||
|---|---|---|---|---|---|---|
| % Calcite | % Vaterite | % Aragonite | ||||
| E1_0% ASW | (1) ASW | Ottawa F-65 sand | 85 | 9 | 6 | Quartz |
| E2_50% ASW | (1) ASW | Ottawa F-65 sand | 81 | 10 | 9 | Quartz |
| E3_100% ASW | (1) ASW | Ottawa F-65 sand | 80 | 7 | 13 | Quartz |
| E4_0 mM Mg2+/0 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 86 | 8 | 6 | Quartz |
| E5_27 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 85 | 9 | 6 | Quartz |
| E6_54 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 90 | 5 | 5 | Quartz |
| E7_108 mM Mg2+ | (2) ASW ions | Ottawa F-65 sand | 81 | 11 | 8 | Quartz, Magnesian Calcite |
| E8_0.055 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 88 | 6 | 6 | Quartz |
| E9_0.11 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 86 | 8 | 6 | Quartz |
| E10_0.22 mM Sr2+ | (2) ASW ions | Ottawa F-65 sand | 85 | 9 | 6 | Quartz |
| E11_14 mM SO42− | (2) ASW ions | Ottawa F-65 sand | 85 | 10 | 5 | Quartz |
| E25_Ottawa sand | (5) Soil variations | Ottawa F-65 sand | 85 | 10 | 5 | Quartz |
| E26_Fraser river sand | (5) Soil variations | Fraser river sand | 86 | 7 | 7 | Quartz, Albite |
| E27_Concrete sand | (5) Soil variations | Concrete sand | 92 | 5 | 3 | Quartz, Albite |
| E28_Covelo sand | (5) Soil variations | Covelo sand | 91 | 5 | 4 | Quartz, Albite |
| E29_Delta sand | (5) Soil variations | Delta sand | 89 | 7 | 4 | Quartz, Albite |
| E30_Monterey sand | (5) Soil variations | Monterey sand | 86 | 8 | 6 | Quartz, Microcline, Albite |
| E31_Feldspar | (5) Soil variations | Feldspar | 87 | 7 | 6 | Microcline, Albite, Quartz |
| E32_Olivine | (5) Soil variations | Olivine | 93 | 4 | 3 | Forsterite, Fayalite |
| E33_Mica | (5) Soil variations | Mica | 88 | 9 | 3 | Lepidolite |
| E34_Kaolinite | (5) Soil variations | Kaolinite | 86 | 10 | 4 | Kaolinite |
| E35_Montmorillonite | (5) Soil variations | Montmorillonite | 80 | 14 | 6 | Montmorillonite, Quartz |
*XRD S-Q analyses have an estimated error of ± 5% by mass following other independent analyses.
Scanning electron microscope imaging
Scanning electron microscope (SEM) imaging was completed to examine the morphology of CaCO3 precipitates including the relative size, shape, and distribution of CaCO3 crystals within specimens. SEM imaging was performed using a FEI XL830 dual-beam focused ion beam scanning electron microscope using acceleration voltages between 1 and 5 kV and magnifications between 200x and 1000x. Prior to imaging, precipitates were oven-dried at 110 °C for at least 2 days, subsampled, mounted to imaging pedestals using carbon tape, and sputter-coated using a 60%/40% Au/Pd alloy target for 60 s at a deposition rate of 13 nm/min in an argon gas chamber. Sputter-coating was completed to increase the conductivity of samples, minimize charging effects, and improve image resolution92.
Precipitate chemical composition analyses
For select experiments, CaCO3 precipitates were dissolved in dilute acid solutions to further examine their chemical composition. Prior to the acid dissolution process, dry precipitate subsamples (≈ 1 g) were rinsed and decanted three times using absolute ethanol to remove soluble ions, subsamples were oven-dried at 110 °C for at least 2 days, and 0.5-g subsamples were added to 25 mL of a 15 mM hydrochloric acid solution to induce dissolution. Samples were allowed to equilibrate for 72 h, during which samples were mechanically agitated once every 24 h. Resulting aqueous solutions, which contained ions from dissolved precipitates, were filtered using 0.45-µm syringe filters to remove solid particulate and select ion concentrations were quantified using ICP-MS measurements. Supplemental Table S2 provides a full summary of aqueous ion concentrations present in dissolution solutions. Ca2+ concentrations in solution samples ranged between 3.8 mM and 5.5 mM and were consistent with concentrations observed in similar dissolution experiments93. Ratios between various ion concentrations and Ca2+ concentrations were examined to further evaluate incorporation and substitution of Ca2+ ions in precipitates.
Results and discussion
Artificial seawater experiments
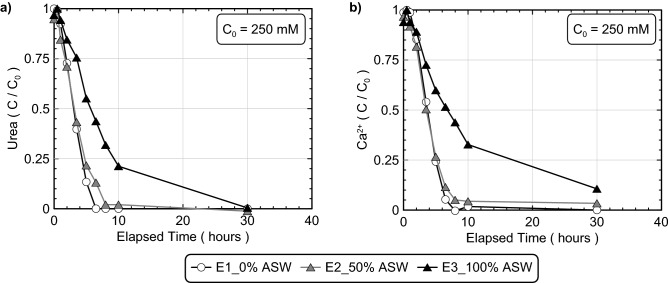
Figure 1 presents normalized concentrations of urea and Ca2+ in time for artificial seawater experiments (experimental series 1). All experiments contained S. pasteurii cells at a cell density of 7.6 × 107 cells/mL and 250 mM urea and Ca2+ to initiate bio-cementation in a deionized water-based solution with varying concentrations of artificial seawater (ASW) by volume, representative of freshwater (0% ASW), brackish (50% ASW), and seawater (100% ASW) geochemical environments. As shown in Fig. 1a, both the 0% and 50% ASW experiments showed similar urea degradation behaviors, however, the 100% ASW experiment had an initial urea degradation rate that was nearly ≈ 50% lower than that of the 0% ASW control specimen. This activity reduction likely resulted from the higher ionic strength and cation (i.e., Na+, Mg2+, SO42−, Ca2+, K+, Sr2+, B3+) concentrations present in seawater, which have been previously shown to interfere with intercellular diffusion and enzyme activity across a broad range of bacterial species94,95 as well as inhibit ureolysis in select indigenous ureolytic microorganisms96. When considering Ca2+ concentrations in time shown in Fig. 1b, significantly slower precipitation rates were also observed in the 100% ASW experiment, similar to urea degradation trends. The agreement between urea and Ca2+ concentration trends in time suggested that CaCO3 precipitation rates were limited by urea hydrolysis activity, with CaCO3 precipitation occurring exceedingly fast following the availability of CO32− from urea degradation.
Figure 1.
Normalized concentrations of (a) urea and (b) Ca2+ in time for 0%, 50%, and 100% ASW experiments. All experiments included the addition of 250 mM urea, 250 mM Ca2+, S. pasteurii cells at 7.6 × 107 cells/mL, and varying ASW additions.
Figure 2 presents results from S-Q analyses completed on XRD diffraction patterns obtained from the ASW precipitate samples. As shown, calcite was the dominant mineral phase in all experiments (> 80%), with minor decreases in calcite relative percentages (≈ 5%) and minor increases in aragonite relative percentages as ASW was increased from 0 to 100% by volume. In the 100% ASW experiment, XRD diffraction patterns corresponding to calcite exhibited a small 2θ shift of ≈ 0.2° consistent with the reference signal for magnesian calcite, a mineral wherein Mg2+ is partially substituted for Ca2+ within the crystalline structure of calcite97. Although magnesite (MgCO3) was expected to form in ASW specimens due to its relatively low solubility (Ksp = 10−8.03), no magnesite peaks were detected in any of the obtained diffraction patterns for the ASW experiments98. Since calcite (Ksp = 10−8.48) has a lower solubility than magnesite, it remains possible that some magnesite may have precipitated during the treatment process but dissolved near the end of reactions due to reductions in surrounding aqueous CO32− activities98.
Figure 2.

Relative CaCO3 mineral content percentages for 0%, 50%, and 100% ASW experiments determined from S-Q analysis of XRD diffraction patterns.
Although XRD analyses indicated that minimal changes in precipitate mineralogy occurred with changes in ASW concentrations, SEM images of the ASW experiments (Fig. 3) exhibited more pronounced changes in precipitate morphology. As shown in Fig. 3a, the 0% ASW control specimen exhibited almost exclusively rhombohedral crystal morphologies characteristic of calcite, however, the 50% ASW specimen (Fig. 3b) included spherical vaterite-like morphologies that were interspersed with rhombohedral crystal forms. Many of the rhombohedral crystal forms present in the 50% ASW specimen also exhibited duller, more well-rounded edges in comparison to the sharper, more well-defined crystalline structures observed in the 0% ASW control. In the 100% ASW specimen, morphological differences were even more pronounced (Fig. 3c,d), with increases in the abundance more well-rounded rhombohedral crystals and crystal surfaces which appeared to have a rougher appearance in comparison to the smoother crystal faces found in the 0% ASW control.
Figure 3.
SEM images of precipitates from (a) 0% ASW, (b) 50% ASW, and (c, d) 100% ASW experiments.
Discrete seawater ion experiments
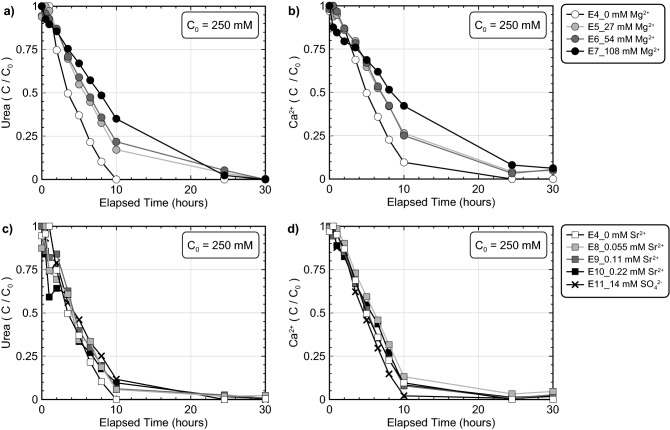
Although the previous experiments demonstrated that seawater ions could influence bio-cementation morphology and reaction kinetics, it remained unclear which specific ions were responsible for these differences. A series of experiments were therefore performed which included varying concentrations of Mg2+, Sr2+, and SO42− in order to further investigate the effect of discrete seawater ions additions (experiment series 2). Experiments were designed to examine the effect of ions at concentrations corresponding to ≈ 50%, ≈ 100%, and ≈ 200% of their expected concentrations in seawater, with the exception of SO42−, which could not be prepared at concentrations exceeding 14 mM (≈ 50% ASW) due to the immediate precipitation of gypsum (CaSO4) in cementation solutions. All experiments contained S. pasteurii cells at a cell density of 7.1 × 107 cells/mL, 250 mM urea and Ca2+ to initiate bio-cementation, and varying concentrations of ASW ions. Figure 4 presents normalized concentrations of urea and Ca2+ in time for all discrete seawater ion experiments including those with Mg2+ variations (Fig. 4a,b) and SO42− and Sr2+ variations (Fig. 4c,d). As shown, increases in Mg2+ concentrations resulted in progressive inhibition of ureolytic rates (Fig. 4a) with a ≈ 15% decrease in initial ureolytic rates observed in the 27 mM and 54 mM experiments and a larger ≈ 45% decrease in initial ureolytic rate observed in the 108 mM Mg2+ experiment. In contrast, when Sr2+ and SO42− were present at concentrations up to 0.22 mM and 14 mM, respectively, no substantial differences in ureolytic rates were observed (Fig. 4c). Despite the presence of additional cations, urea and Ca2+ trends in time were again similar with slightly elevated Ca2+ concentrations observed in the Mg2+ varied experiments (Fig. 4b). Although minimal inhibition of CaCO3 precipitation was observed in the 14 mM SO42− experiment, greater inhibition of precipitation may have been observed if higher SO42− concentrations near 24 mM were present, as noted by Busenberg and Plummer65.
Figure 4.
Normalized concentrations of (a, c) urea and (b, d) Ca2+ in time for (a, b) Mg2+ varied experiments and (c, d) Sr2+ and SO4−2 varied experiments. All experiments included the addition of 250 mM urea, 250 mM Ca2+, S. pasteurii cells at 7.1 × 107 cells/mL, and varying Mg2+, Sr2+, or SO42− additions.
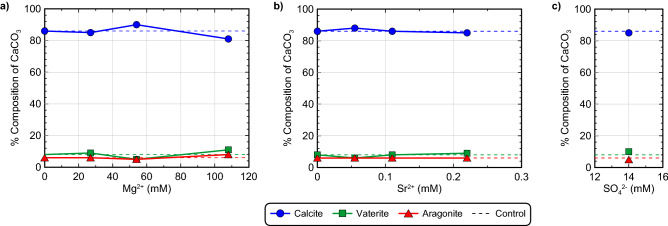
Figure 5 presents the results of S-Q analyses completed on the XRD diffraction patterns obtained from precipitate samples for all discrete seawater ion experiments. Again, calcite was the predominant mineral phase with relative percentages exceeding 81% in all experiments. At the highest Mg2+ concentration considered (108 mM), a slight decrease in the relative percentage of calcite (≈ 5%) was observed with a corresponding increase in vaterite and aragonite (Fig. 5a). Similar to the previous ASW experiments, XRD diffraction patterns again indicated the presence of magnesian calcite in Mg2+ amended experiments, although its relative abundance was sufficiently small that it could not be reliably quantified (< 2% by mass). No other Mg2+ containing minerals (e.g., MgCO3) were detected in precipitates. When Sr2+ concentrations were increased and 14 mM SO42− was added, no substantial deviations in mineralogical compositions were observed from the control specimen (Fig. 5b,c). No other non-CaCO3 mineral phases were detected in the obtained precipitates.
Figure 5.
Relative CaCO3 mineral content percentages for (a) Mg2+ varied experiments, (b) Sr2+ varied experiments, and the (c) 14 mM SO42− experiment determined from S-Q analysis of XRD diffraction patterns.
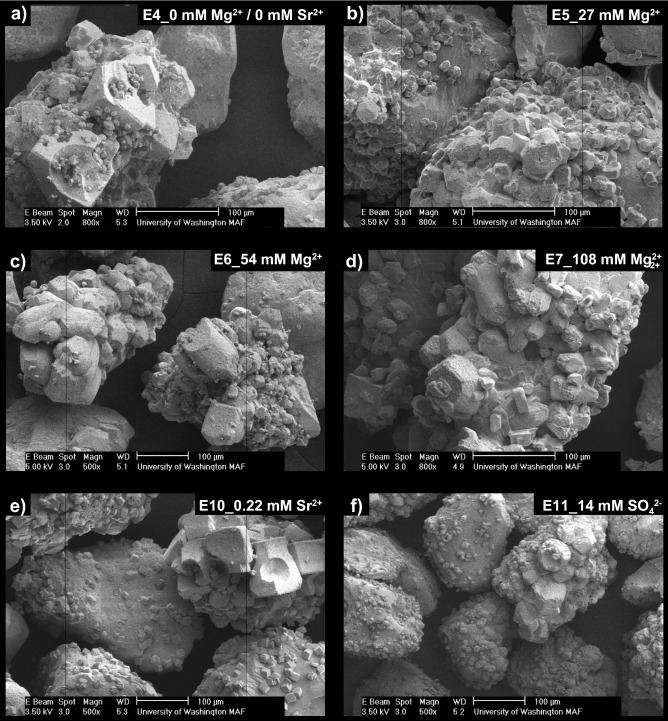
SEM images of precipitates again revealed more dramatic differences between experiments (Fig. 6). In the deionized water control (Fig. 6a), rhombohedral crystals were observed that were consistent with calcite and morphologies found in the previous control (Fig. 3a). Small spherical voids in rhombohedral crystals, consistent with the shape of vaterite crystals, were also occasionally observed in the control. Similar morphologies have been observed in previous abiotic studies and have been attributed to the dissolution and re-precipitation of vaterite via Ostwald ripening99. In this process, some vaterite crystals that are precipitated initially under highly supersaturated conditions, dissolve to form lower solubility calcite crystals near the end of reactions, thereby leaving behind calcite crystals with vaterite-like “casts” or impressions100. In the 27 mM Mg2+ experiment, crystal morphologies were further modified and were more similar to dodecahedrons (Fig. 6b) which also displayed a greater degree of anisotropy when compared to the control. Similar crystal forms were also observed at higher Mg2+ concentrations (Fig. 6c,d), with crystal surfaces becoming rougher and crystal edges becoming duller in appearance as Mg2+ concentrations increased. Crystal morphologies resembling cylinders were also prominent in the 108 mM Mg2+ experiment (Fig. 6c,d). Interestingly, morphologies in the Mg2+ experiments differed from those previously observed in the ASW experiments with spherical forms consistent with vaterite being largely absent. When considering the effect of Sr2+ and SO42− additions on precipitate morphology, both the highest concentration 0.22 mM Sr2+ and 14 mM SO42− specimens had well-defined rhombohedral crystals, which did not substantially differ from the control experiment (Fig. 6e,f).
Figure 6.
SEM images of precipitates from the (a) 0 mM Mg2+/0 mM Sr2+, (b) 27 mM Mg2+, (c) 54 mM Mg2+, (d) 108 mM Mg2+, (e) 0.22 mM Sr2+ and (f) 14 mM SO42− experiments.
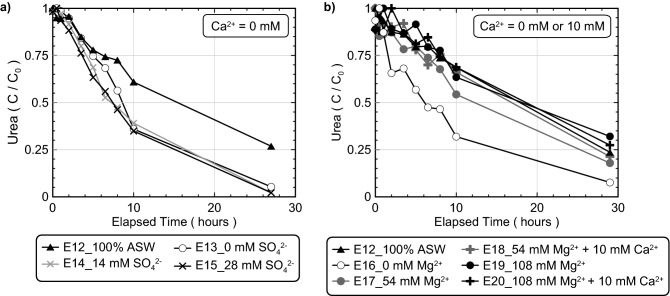
Discrete seawater ion experiments (ureolysis)
While earlier experiments evaluated the effect of discrete seawater ions on bio-cementation morphology, mineralogy, and reaction kinetics, the primary mechanisms responsible for ureolytic inhibition the high Mg2+ and 100% ASW experiments remained unclear. It was hypothesized that the higher Mg2+ concentrations supplied in these experiments may have interfered with cellular and enzymatic activity directly or resulted in the formation of additional precipitates during the reaction period that could have inhibited ureolytic activity via cell encapsulation. In addition, while Sr2+ and SO42− additions were shown to have minimal effects on process kinetics, the effect of SO42− on ureolytic activity was not explored at concentrations present in 100% ASW due to solution preparation limitations. In order to better isolate the effect of SO42− and Mg2+ additions on ureolytic activity alone, a series of experiments were performed containing minimal Ca2+ intended to eliminate CaCO3 precipitation and its related effects on process kinetics (experimental series 3). Figure 7 presents normalized concentrations of urea in time for all experiments containing minimal Ca2+ including those with SO42− and ASW (Fig. 7a) and Mg2+ and ASW (Fig. 7b). As shown in Fig. 7a, SO42− concentrations comparable to that present in 100% ASW (≈ 28 mM) had no detectable effects on urea hydrolysis activity. In contrast, urea hydrolysis in 100% ASW was again significantly slower than the deionized water controls (E13, E16) despite containing minimal Ca2+ (10 mM) when compared to the earlier 100% ASW experiment that was augmented with 250 mM Ca2+. Figure 7b presents normalized concentrations of urea in time for Mg2+ varied experiments containing either 10 mM Ca2+ or no added Ca2+ (0 mM) along with 100% ASW and deionized water control experiments. The addition of 10 mM Ca2+ was included in select experiments to match the Ca2+ present in 100% ASW. For some specimens, Ca2+ additions were also entirely removed to mitigate any potential inhibitory effects related to CaCO3 precipitation. As shown, for all experiments containing more than 54 mM Mg2+, ureolytic activity was significantly inhibited when compared to the control regardless of the supplied Ca2+ concentration. When comparing ureolytic activity in the 54 mM Mg2+ with 10 mM Ca2+ experiment to the 100% ASW specimen, however, ureolytic rates were nearly identical, suggesting that the inhibition of ureolysis observed in 100% ASW may primarily result from the presence of 54 mM Mg2+.
Figure 7.
Normalized concentrations of urea in time for experiments with (a) varying SO42− and ASW concentrations and no added Ca2+ and (b) varying Mg2+ and ASW concentrations with minimal added Ca2+. All experiments included the addition of 250 mM urea, 0 mM or 10 mM Ca2+, S. pasteurii cells at 6.6 × 107 cells/mL, and varying SO42−, Mg2+, or ASW additions.
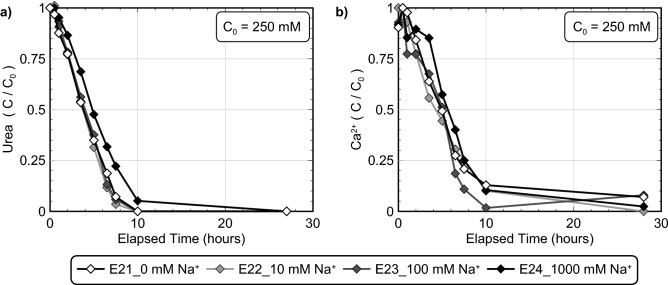
Sodium varied experiments
In the previous experiments which examined the effect of ASW and discrete seawater ion concentrations, the effect of corresponding ionic strength increases remained relatively unknown. A series of experiments were therefore performed to examine the effect of solution ionic strength differences as realized by NaCl additions (experiment series 4). NaCl additions were used in these experiments to examine ionic strength changes due to their frequent use as an inert electrolyte in biological experiments101. All experiments contained S. pasteurii cells at a cell density of 8.0 × 107 cells/mL, 250 mM urea and Ca2+ to initiate bio-cementation, and either 0 mM (I ≈ 753 mM), 10 mM (I ≈ 763 mM), 100 mM (I ≈ 853 mM), or 1000 mM Na+ (I ≈ 1753 mM). Figure 8 presents normalized concentrations of urea and Ca2+ in time for all Na+ varied experiments. As shown, no detectable changes in ureolytic rates were observed at Na+ concentrations up to 100 mM. In the 1000 mM Na+ experiment, which had an ionic strength nearly twice as large, however, initial ureolytic rates were reduced by ≈ 20% when compared to the control. Although ureolytic inhibition observed in the 100% ASW experiments were primarily attributed to the 54 mM Mg2+ concentrations, the ionic strength of the 100% ASW solutions were near 1458 mM when 250 mM Ca2+ was supplied. Thus, it is likely that some smaller fraction of the ureolytic inhibition observed in 100% ASW may be related to the higher ionic strength of these solutions. Figure 9 presents SEM images of precipitates from both the 0 mM and 1000 mM Na+ experiments. As shown, precipitates from both the control (Fig. 9a) and 1000 mM Na+ experiment (Fig. 9b) exhibited consistent rhombohedral calcite-like crystals and no other substantial differences in precipitate morphology, distribution, or size were observed as a function of Na+ variations.
Figure 8.
Normalized concentrations of (a) urea and (b) Ca2+ in time for Na+ varied experiments. All experiments included the addition of 250 mM urea, 250 mM Ca2+, S. pasteurii cells at 8.0 × 107 cells/mL and varying added Na+ concentrations.
Figure 9.
SEM images of precipitates from (a) 0 mM Na+ and (b) 1000 mM Na+ specimens.
Soil varied experiments
In order to investigate the effect of changes in soil materials, a series of experiments were performed using eleven different soils (experiment series 5). Figure 10 presents normalized concentrations of urea and Ca2+ in time for all soil varied experiments including relatively pure soil minerals (Fig. 10a,b) and natural and processed poorly-graded sands (Fig. 10c,d). All experiments contained S. pasteurii at a cell density of 8.2 × 107 cells/mL, identical treatment solutions containing 250 mM urea and Ca2+ to initiate bio-cementation, but different soils. As shown in Fig. 10a, minimal changes in ureolytic rates were observed between experiments containing different pure soil minerals with near full urea hydrolysis occurring after 10 h. Although unexpected, the kaolinite and mica specimens exhibited slightly faster initial ureolytic rates when compared to the quartz-based Ottawa F-65 Sand control, which may have resulted from the hydration of these minerals, local increases in urea concentrations, and small increases in ureolytic rates. In other experiments containing different poorly-graded sands (Fig. 10c), however, more significant variations in ureolytic rates were observed when compared to the control. In particular, the Fraser River Sand experiment exhibited strong inhibition of ureolysis within the first 10 h, with urea degradation ceasing entirely after only ≈ 35% reaction completion. Although not as pronounced, minor reductions in ureolytic rates were also observed in the Covelo, Delta, and Concrete Sand experiments. While all considered sands were primarily composed of quartz and feldspar minerals, significant differences in exchangeable cation concentrations were detected between these materials (Supplemental Table S1). Notably, the Fraser River Sand material contained the highest concentrations of exchangeable Al3+, Zn2+, and Fe3+ of all considered sands. In addition, all sands exhibiting detectable ureolytic inhibition (Covelo, Delta, Concrete, and Fraser River Sand) contained higher concentrations of exchangeable Mg2+ (60–239 µg/g soil) and barium (Ba+) (5–21 µg/g soil) when compared to Ottawa F-65 Sand and Monterey Sand, which had similar ureolytic activities and much lower Mg2+ and Ba+ concentrations (Mg2+ = < 0.2–31 µg/g soil, Ba+ = 0.2–0.3 µg/g soil). Although the observed ureolytic rate differences could not be definitively attributed to these specific ions, these results do suggest that differences in exchangeable soil ions may significantly influence the ureolytic activity of augmented bacteria and should be characterized when assessing the treatment feasibility of new soils. When comparing urea and Ca2+ trends in time from all soil varied experiments, similar behaviors were again observed, with the Fraser River Sand specimen achieving limited utilization of the supplied Ca2+ due to the inhibition of ureolysis.
Figure 10.
Normalized concentrations of (a, c) urea and (b, d) Ca2+ in time for (a, b) soil mineral varied experiments and (c, d) poorly-graded sand varied experiments. All experiments included the addition of 250 mM urea, 250 mM Ca2+, S. pasteurii cells at 8.2 × 107 cells/mL, and varying soil materials.
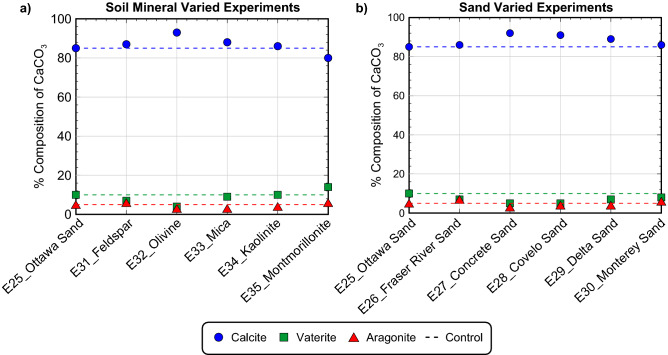
Figure 11 presents the results of S-Q analyses completed on the XRD diffraction patterns obtained from precipitate samples for all soil varied experiments. As shown, calcite was again found to be the dominant mineral phase with relative percentages exceeding 80%. The montmorillonite experiment had the lowest calcite relative percentage of 80% which was ≈ 5% less than the Ottawa F-65 Sand control as well as the highest relative percentage of vaterite at ≈ 14%. All other experiments had relative calcite percentages between ≈ 85% and 93%, vaterite percentages between ≈ 4% and 10%, and aragonite percentages between ≈ 3% and 6%, and were similar to the control. Collectively, these results suggested that changes in soil materials had minimal effects on the mineralogy of resulting precipitates, despite having more pronounced impacts on reaction kinetics.
Figure 11.
Relative CaCO3 mineral content percentages for (a) soil mineral varied experiments and (b) poorly-graded sand varied experiments determined from S-Q analysis of XRD diffraction patterns.
Figure 12 presents SEM images of precipitates from select soil varied experiments. As shown in Fig. 12a, precipitation in the Ottawa F-65 Sand control exhibited rhombohedral morphologies consistent with those expected for calcite and other control experiments. Although morphological differences between experiments were more difficult to assess given differences in soil particle sizes and geometries, precipitation in the montmorillonite experiment appeared to consist of rhombohedral crystals that were consistently smaller (diameter ≈ 5 µm) than those present in the control (diameter ≈ 10 to 50 µm) (Fig. 12b, c). CaCO3 crystals also appeared to form large clusters of loosely bound precipitates in the montmorillonite specimen, an outcome which was unexpected given the much larger specific surface area of this soil. Although XRD analyses suggested that slightly higher vaterite relative percentages were present in the montmorillonite experiment, clear morphological differences were not observed. In the kaolinite experiment, rhombohedral crystals consistent with calcite were interspersed with clay particles (Fig. 12d,e) and appeared to be larger than those in the montmorillonite specimen. This was consistent with earlier XRD analyses, which suggested that the mineralogical composition of the kaolinite experiment was similar to that of the control. Lastly, SEM images of the Fraser River Sand experiment (Fig. 12f) exhibited morphologies consistent with calcite with no discernable morphological differences when compared to the control. While this specimen exhibited significant inhibition of ureolysis, this outcome did not appear to significantly influence resulting CaCO3 precipitation mineralogy or morphology.
Figure 12.
SEM images of precipitates from (a) Ottawa F-65 Sand, (b, c) montmorillonite, (d, e) kaolinite, and (f) Fraser River Sand experiments.
Comparison of calcium utilization and urea degradation
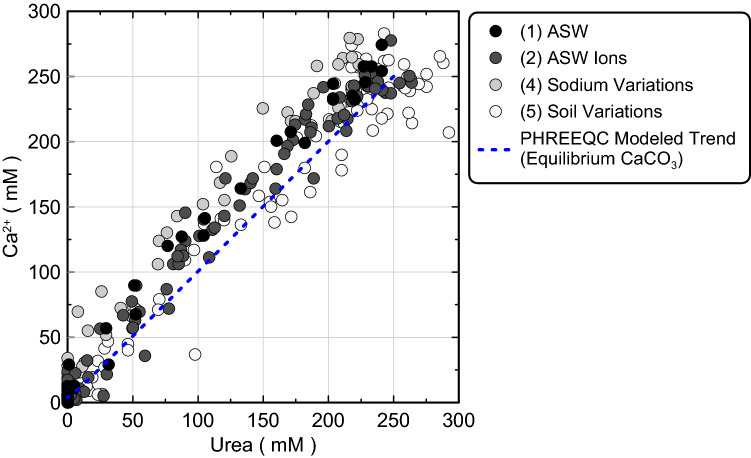
Figure 13 presents corresponding aqueous Ca2+ and urea concentration measurements for all experiments during bio-cementation as well as a PHREEQC modeled trend, which assumed CaCO3 precipitation to be an equilibrium reaction (immediate precipitation of CaCO3 upon supersaturation of solutions). As shown, all aqueous Ca2+ and urea concentrations had initial values near 250 mM and followed a near 1:1 slope as precipitation reactions proceeded toward completion. Ca2+ concentrations were generally between 0 and 40 mM higher than corresponding urea concentrations, however, suggesting that CaCO3 precipitation was limited by urea hydrolysis as expected. When comparing Ca2+ and urea concentration measurements to the PHREEQC modeled trend, measurements and modeled values exhibited good agreement suggesting that CaCO3 precipitation may occur relatively quickly following CO32− availability from urea hydrolysis with the kinetics of CaCO3 precipitation being reasonably approximated as an equilibrium reaction. Despite variations in soil materials and geochemical conditions between the performed experiments, no substantial differences in trends were observed. The agreement between Ca2+ and urea concentration measurements and the modeled equilibrium reaction trend is consistent with other recent reactive transport modeling efforts102,103, which have suggested that the kinetics of calcite precipitation can be well approximated as an equilibrium reaction. This outcome suggests that more other computationally intensive CaCO3 precipitation kinetic expressions such as those related to changes in mineral specific surface areas and saturation state104–107 used by previous researchers108–111 may be avoided when attempting to geochemically model MICP applications when conditions are similar to those considered in this study.
Figure 13.
Corresponding measurements of aqueous Ca2+ and urea concentrations from all experiments as well as a PHREEQC modeled trend which assumes CaCO3 precipitation to be an equilibrium reaction.
Comparison of OD600 and PHREEQC-based cell density estimates
For all experiments, augmented cell densities were quantified in two ways: (1) OD600 measurements with conversion to total cell densities using a OD600 to total direct cell count conversion, and (2) calibration of the cell-normalized Michaelis–Menten ureolytic kinetic expression (Eq. 1) to experimentally observed urea degradation behaviors using whole cell enzymatic parameters for S. pasteurii from Graddy et al.86 and PHREEQC batch reaction models. The kinetic model was calibrated to observed ureolytic activity: (1) to assess the ability of known augmented cell densities and whole cell enzymatic parameters to accurately estimate ureolytic activities across a broad range of experimental conditions and (2) to quantify differences in ureolytic activities between experiments as a function of environmental factors. In order to examine the former, the ratio of cell densities estimated using the PHREEQC model to known augmented cell densities from OD600 measurements were compared between all experiments (Table 1, Supplemental Figure S3). Significant discrepancies were observed between activity-based cell density estimates and known augmented cell densities with PHREEQC activity-based estimates ranging between 9 and 208% of known directly measured values. As expected, PHREEQC activity-based estimates substantially underestimated augmented cell densities for experiments exhibiting significant ureolytic inhibition including the 100% ASW (E03), high Mg2+ (E05–E07), and Fraser River Sand (E26) specimens. Surprisingly, considerable variations were also observed for control experiments wherein PHREEQC estimates varied between 68 and 171% of known augmented values. Although these results are specific to the whole cell enzymatic parameters86, experimental conditions, and cell preparation methods used in this study, results clearly demonstrated that augmented per cell activities vary significantly with differences in environmental conditions and should be carefully considered when attempting to model ureolytic activity for MICP field applications. From this perspective, direct measurements of ureolytic activity via urea concentration measurements or other means may afford superior insights when compared to the knowledge of augmented cell densities alone.
Comparison of ureolytic activity
In order to better quantify differences in ureolytic activity between experiments under varying environmental conditions and respective control experiments, PHREEQC activity-based cell density estimates for experiments were also compared to the PHREEQC activity-based cell density estimates obtained for control experiments from the same experimental series (Supplemental Figure S4) to identify conditions which most significantly affected ureolytic activity. Although all control experiments had similar ureolytic activities, small variations in augmented cell densities and per cell activities between cell batches resulted in some differences between controls from different experimental series. When comparing activities, PHREEQC cell density estimates for all non-control experiments varied between 6 and 131% of their respective controls. Of these experiments, ureolytic inhibition was the most significant for the Fraser River Sand (6%) and 108 mM Mg2+ experiments (33%) with acceleration of ureolysis occurring in only the mica (131%) and montmorillonite (127%) experiments. Collectively, these results highlight five primary outcomes: (1) increases in ASW concentrations progressively decreased ureolytic activities, (2) increases in Mg2+ concentrations resulted in increased inhibition of ureolysis both with and without Ca2+ present, (3) SO42− and Sr2+ had no detectable effects on ureolysis at concentrations up to twice that present in seawater, (4) increases in ionic strength via NaCl additions resulted in no detectable effects on ureolysis at low concentrations (< 100 mM Na+), but detectable inhibition at higher concentrations (1000 mM Na+) which corresponded to an increase in ionic strength near one order of magnitude similar to that present in 100% ASW, and (5) variations in soil materials almost always resulted in decreased ureolytic activity relative to the Ottawa F-65 Sand control, which may be attributed to the presence of inhibitory exchangeable soil ions.
Precipitate composition analyses
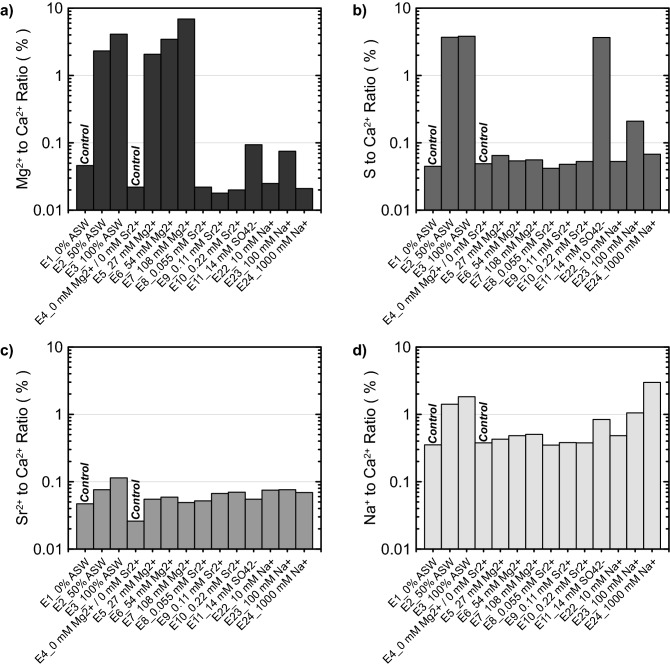
Precipitate composition analyses were completed for select experiments in order to further characterize the chemical composition of resulting mineral phases. Supplemental Table S2 presents a summary of all results and Fig. 14 presents results obtained from select experiments involving seawater ion additions (experimental series 1, 2, and 4) including comparisons of Mg2+-to-Ca2+ ion ratios (Fig. 14a), S-to-Ca2+ ion ratios (Fig. 14b), Sr2+-to-Ca2+ ion ratios (Fig. 14c), and Na+-to-Ca2+ ion ratios (Fig. 14d) within resulting precipitates. Ion concentrations were normalized by Ca2+ concentrations in order to examine the abundance of ions as a molar fraction of the total amount of CaCO3 dissolved following similar studies65. As shown in Fig. 14a, Mg2+-to-Ca2+ ratios were between 0.02% and 0.05% in control experiments, however, much higher Mg2+ abundances were observed in precipitates from the ASW and Mg2+ ion varied experiments. Both the 100% ASW and 54 mM Mg2+ experiments, which contained similar Mg2+ concentrations, had Mg2+-to-Ca2+ ratios between 2.5 and 4.1%. When Mg2+ concentrations were further increased to 108 mM, the highest Mg2+-to-Ca2+ ratio of 6.9% was observed. These values were consistent with earlier XRD analyses for the ASW and Mg2+ varied experiments, which suggested the presence of magnesian calcite through small diffraction peak shifts. Mg2+-to-Ca2+ ratios for all other experiments were less than 0.09%. Composition analyses also detected varying amounts of sulfur (S) in select precipitates. As shown in Fig. 14b, S-to-Ca2+ ratios between 0.04% and 0.05% were present in control experiments with much higher S-to-Ca2+ ratios between 3.7% and 3.8% measured in the 14 mM SO42−, 50% ASW, and 100% ASW experiments. Although XRD S-Q analyses for these experiments did not detect the presence of sulfate-based minerals (e.g., gypsum), these results suggest that some fraction of precipitates contained sulfur and may have been present at quantities below the threshold of detection (< ≈ 2% by mass) or amorphous in nature. When examining Sr2+-to-Ca2+ ratios, only small differences between experiments with and without added Sr2+ were detected with values near 0.05% in controls and the highest value of 0.11% detected in the 100% ASW experiment (Fig. 14c). Lastly, Na+-to-Ca2+ ratios were between 0.35% and 0.84% in all experiments without added ASW or Na+, however, these ratios progressively increased in both the Na+ added and ASW experiments. Na+-to-Ca2+ ratios were highest in the 1000 mM Na+ and 100% ASW experiments which had Na+-to-Ca2+ ratios of 3.0% and 1.8%, respectively (Fig. 14d). Collectively, these results suggest that Mg2+, S, and Na+ can be incorporated within precipitates when present in surrounding solutions. This is consistent with observations by Busenberg and Plummer65 wherein similar S-to-Ca2+ and Na+-to-Ca2+ ratios were measured in abiotic CaCO3 precipitates prepared in ASW. However, Busenberg and Plummer65 also found incorporation ratios to depend on the rate of crystal growth, suggesting that changes in ureolytic rates may have also impacted these ratios, if such differences had been considered.
Figure 14.
Comparison of (a) Mg2+ to Ca2+ ion ratios, (b) S to Ca2+ ion ratios, (c) Sr2+ to Ca2+ ion ratios, (d) Na+ to Ca2+ ion ratios obtained from precipitate composition analyses for select experiments.
Conclusions
A study was performed to investigate the effect of marine and brackish conditions and different soil materials on the mineralogy, morphology, and reaction kinetics of ureolytic bio-cementation mediated by augmented S. pasteurii bacteria. Thirty-five small-scale batch experiments were completed through five different experiment series and explored the effect of artificial seawater ions, discrete Mg2+, Sr2+, SO42−, and Na+ ion additions, and different poorly-graded sands and common soil minerals. During experiments, ureolysis and CaCO3 precipitation kinetics were assessed using direct measurements of aqueous urea and Ca2+ in time and generated precipitates were characterized using SEM imaging, x-ray diffraction, and precipitate composition analyses. A cell-normalized Michaelis–Menten kinetic model was calibrated to observed urea degradation behaviors using PHREEQC batch reaction models to further characterize the impact of the considered environmental conditions on ureolytic activity, compare activity-based cell density estimations to those measured directly via optical density measurements, and explored expected relationships between aqueous urea and Ca2+ concentrations during precipitation. From the results of this study, the following conclusions can be made:
Although differences in precipitate morphology and mineralogy were observed with changes in the examined geochemical conditions, in all experiments calcite was the predominant CaCO3 mineral polymorph with relative percentages exceeding 80% by mass. Factors found to decrease calcite and increase vaterite and aragonite relative percentages included increases in artificial seawater and supplied Mg2+ concentrations.
Artificial seawater experiments exhibited significant reductions in ureolysis and precipitation rates and exhibited distinct crystal morphologies, despite having similar mineralogical compositions as control experiments. Additional experiments containing minimal added Ca2+ (< 10 mM) further confirmed that the reaction inhibition observed in the 100% ASW experiment could be primarily attributed to the effect of Mg2+ on urea hydrolysis activity with some smaller fraction likely related to the higher ionic strength of seawater. Precipitate composition analyses further indicated that precipitates formed in artificial seawater solutions contained Mg2+, Sr2+, S, and Na+ substitutions. In experiments containing added SO42− and Sr2+, however, no significant differences in reaction kinetics or precipitate mineralogy or morphology were observed for the concentrations considered (Sr2+ < 0.22 mM, SO42− < 14 mM).
Aqueous urea and Ca2+ concentrations measured in time during all experiments were well approximated by modeled trends which assumed CaCO3 precipitation to be an equilibrium reaction. When geochemical conditions similar to those considered in this study are present, CaCO3 precipitation during MICP may be assumed to be an equilibrium reaction and eliminate the need for other computationally intensive kinetic expressions for CaCO3 precipitation to be incorporated in geochemical models.
Quantitative comparisons of activity-based cell density estimates between all experiments and their respective controls suggested that the most significant inhibition of ureolysis occurred in experiments containing 100% ASW, 108 mM Mg2+, and Fraser River Sand with ureolytic activities exceeding rates observed in the control experiments in only the montmorillonite and mica containing specimens. Moving forward, characterization of Mg2+ concentrations and other exchangeable ions in groundwater and field soils may be critical towards assessing potential inhibition of bio-cementation.
Comparisons of activity-based cell density estimates and direct measurements of augmented cell densities suggested that significant discrepancies existed between direct cell density measurements and observed bulk ureolytic activities for all experiments including controls. Moving forward, activity-based characterizations such as those afforded by direct urea concentration measurements will likely provide superior insights for field applications when compared to direct measurements of augmented cell densities such as those from OD600, total direct counts, or total protein measurements.
While the obtained results provide important insights regarding the influence of seawater ions and varying soil materials on the reaction kinetics and physicochemical properties of bio-cemented soils, further research is needed to more fully understand the effect of a variety of other important practical conditions including changes in treatment solution composition and biological treatment strategies.
Supplementary Information
Acknowledgements
Funding for this research work was provided by the National Science Foundation (ECI-1824647) and is greatly appreciated. Research collaboration made possible through the Engineering Research Center Program of the National Science Foundation under NSF Cooperative Agreement No. EEC-1449501 is also acknowledged. Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation. Presented SEM images and XRD analyses were made possible by the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington that is supported in part by the National Science Foundation Grant NNCI-1542101, the University of Washington, the Molecular Engineering & Science Institute, and the Clean Energy Institute. Research assistance from Liam Bradshaw, Scott Braswell, Charles M.R. Graddy, Minyong Lee, Dongsen Xue, and Samantha Young is also acknowledged and greatly appreciated.
Author contributions
R.J.B. and M.G.G. were involved in the design, performance, and analysis of all data achieved in the presented study. R.J.B. and M.G.G. co-wrote the first draft of the main manuscript text. M.G.G. prepared all figures. B.G.R.O. performed additional chemical measurements and analyses presented in this paper. All authors were involved in the discussion and analysis of the achieved results as well as editing of the final manuscript text and figures. M.G.G. supervised and designed all research activities.
Data availability
All data generated during the study are available from the corresponding author upon reasonable request. All data presented in the figures of this paper as well as experimental measurements be available through the NSF DesignSafe-CI Data Depot repository (https://www.designsafe-ci.org/data/browser/public/) under project number PRJ-3190.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21268-3.
References
- 1.DeJong JT, Mortensen BM, Martinez BC, Nelson DC. Bio-mediated soil improvement. Ecol. Eng. 2010;36(2):197–210. doi: 10.1016/j.ecoleng.2008.12.029. [DOI] [Google Scholar]
- 2.Pinske, M. A. Life Cycle Assessment of Ground Improvement Methods. M.S. thesis, University of California, Davis, CA (2011).
- 3.Mitchell JK, Kelly R. Addressing some current challenges in ground improvement. Proc. Inst. Civ. Eng. Ground Improv. 2013;166(3):127–137. doi: 10.1680/grim.12.00030. [DOI] [Google Scholar]
- 4.Raymond AJ, Pinkse MA, Kendall A, DeJong JT. Life-cycle assessment of ground improvement alternatives for the Treasure Island, California, redevelopment. Geotech. Front. 2017;2017:345–354. [Google Scholar]
- 5.Mohammed MA, Yunus NZM, Hezmi MA, Hasbollah DZA, Rashid ASA. Ground improvement and its role in carbon dioxide reduction: a review. Environ. Sci. Pollut. Res. 2021;28:8968–8988. doi: 10.1007/s11356-021-12392-0. [DOI] [PubMed] [Google Scholar]
- 6.Seagren, E. A., & Aydilek, A. H. Biomediated geomechanical processes. Environ. Microbiol. 2nd Ed., 319–348 (2010).
- 7.DeJong JT, Soga KS, Kavazanjian E, Burns S, van Paassen L, Al Qabany A, Aydilek A, Bang SS, Burbank M, Caslake L, Chen CY, Cheng X, Chu J, Ciurli S, Fauriel S, Filet AE, Hamdan N, Hata T, Inagaki Y, Jefferis S, Kuo M, Laloui L, Larrahondo J, Manning DAC, Martinez B, Montoya BM, Nelson DC, Palomino A, Renforth P, Santamarina JC, Seagren EA, Tanyu B, Tsesarsky M, Weaver T. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Geotechnique. 2013;63(4):287–301. doi: 10.1680/geot.SIP13.P.017. [DOI] [Google Scholar]
- 8.DeJong, J. T., Gomez, M. G., San Pablo, A. C., Graddy, C. M. R., Nelson, D. C., Lee, M., Ziotopoulou, K., Montoya, B., Kwon, T. H. State of the Art: MICP soil improvement and its application to liquefaction hazard mitigation. In Proceedings of the 20th International Conference on Soil Mechanics and Geotechnical Engineering, Sydney 2022. (2022).
- 9.DeJong, J. T., & Kavazanjian, E. in Geotechnical Fundamentals for Addressing New World Challenges 193–207 (Springer, Cham, 2019).
- 10.Ferris, F. G., Stehmeier, L. G., Kantzas, A., & Mourits, F. M. Bacteriogenic mineral plugging. J. Can. Petrol. Technol.36(09), (1997).
- 11.Stocks-Fischer S, Galinat JK, Bang SS. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999;31(11):1563–1571. doi: 10.1016/S0038-0717(99)00082-6. [DOI] [Google Scholar]
- 12.DeJong JT, Fritzges MB, Nüsslein K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006;132(11):1381–1392. doi: 10.1061/(ASCE)1090-0241(2006)132:11(1381). [DOI] [Google Scholar]
- 13.Phillips AJ, Gerlach R, Lauchnor E, Mitchell AC, Cunningham AB, Spangler L. Engineered applications of ureolytic biomineralization: A review. Biofouling. 2013;29:715–733. doi: 10.1080/08927014.2013.796550. [DOI] [PubMed] [Google Scholar]
- 14.Montoya BM, DeJong JT. Stress-strain ehaviour of sands cemented by microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2015;141(6):04015019. doi: 10.1061/(ASCE)GT.1943-5606.0001302. [DOI] [Google Scholar]
- 15.Gomez MG, DeJong JT, Anderson CM. Effect of bio-cementation on geophysical and cone penetration measurements in sands. Can. Geotech. J. 2018;55(11):1632–1646. doi: 10.1139/cgj-2017-0253. [DOI] [Google Scholar]
- 16.Xiao P, Liu H, Stuedlein AW, Evans TM, Xiao Y. Effect of relative density and biocementation on cyclic response of calcareous sand. Can. Geotech. J. 2019;56(12):1849–1862. doi: 10.1139/cgj-2018-0573. [DOI] [Google Scholar]
- 17.Liu L, Liu H, Stuedlein AW, Evans TM, Xiao Y. Strength, stiffness, and microstructure characteristics of biocemented calcareous sand. Can. Geotech. J. 2019;56(10):1502–1513. doi: 10.1139/cgj-2018-0007. [DOI] [Google Scholar]
- 18.Martinez A, Huang L, Gomez MG. Thermal conductivity of MICP-treated sands at varying degrees of saturation. Géotech. Lett. 2019;9(1):15–21. doi: 10.1680/jgele.18.00126. [DOI] [Google Scholar]
- 19.Jiang NJ, Tang CS, Hata T, Courcelles B, Dawoud O, Singh DN. Bio-mediated soil improvement: The way forward. Soil Use Manag. 2020;36(2):185–188. doi: 10.1111/sum.12571. [DOI] [Google Scholar]
- 20.Sharma M, Satyam N, Reddy KR. State of the art review of emerging and biogeotechnical methods for liquefaction mitigation in sands. J. Hazard. Toxic Radioact. Waste. 2021;25(1):03120002. doi: 10.1061/(ASCE)HZ.2153-5515.0000557. [DOI] [Google Scholar]
- 21.Fujita Y, Redden GD, Ingram JC, Cortez MM, Ferris FG, Smith RW. Strontium incorporation into calcite generated by bacterial ureolysis. Geochim. Cosmochim. Acta. 2004;68(15):3261–3270. doi: 10.1016/j.gca.2003.12.018. [DOI] [Google Scholar]
- 22.Montoya BM, DeJong JT, Boulanger RW. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique. 2013;63(4):302–312. doi: 10.1680/geot.SIP13.P.019. [DOI] [Google Scholar]
- 23.Minto JM, MacLachlan E, El Mountassir G, Lunn RJ. Rock fracture grouting with microbially induced carbonate precipitation. Water Resour. Res. 2016;52(11):8827–8844. doi: 10.1002/2016WR018884. [DOI] [Google Scholar]
- 24.Minto JM, Tan Q, Lunn RJ, El Mountassir G, Guo H, Cheng X. ‘Microbial mortar’-restoration of degraded marble structures with microbially induced carbonate precipitation. Constr. Build. Mater. 2018;180:44–54. doi: 10.1016/j.conbuildmat.2018.05.200. [DOI] [Google Scholar]
- 25.Darby KM, Hernandez GL, DeJong JT, Boulanger RW, Gomez MG, Wilson DW. Centrifuge model testing of liquefaction mitigation via microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2019;145(10):04019084. doi: 10.1061/(ASCE)GT.1943-5606.0002122. [DOI] [Google Scholar]
- 26.Zamani A, Montoya BM. Undrained cyclic response of silty sands improved by microbial induced calcium carbonate precipitation. Soil Dyn. Earthq. Eng. 2019;120:436–448. doi: 10.1016/j.soildyn.2019.01.010. [DOI] [Google Scholar]
- 27.Lee M, Gomez MG, El Kortbawi M, Ziotopoulou K. Effect of light biocementation on the liquefaction triggering and post-triggering behavior of loose sands. J. Geotech. Geoenviron. Eng. 2022;148(1):04021170. doi: 10.1061/(ASCE)GT.1943-5606.0002707. [DOI] [Google Scholar]
- 28.Xiao Y, He X, Wu W, Stuedlein AW, Evans TM, Chu J, et al. Kinetic biomineralization through microfluidic chip tests. Acta Geotech. 2021;13:3229–3237. doi: 10.1007/s11440-021-01205-w. [DOI] [Google Scholar]
- 29.Gomez MG, Anderson CM, Graddy CMR, DeJong JT, Nelson DC, Ginn TR. Large-scale comparison of bioaugmentation and biostimulation approaches for biocementation of sands. J. Geotech. Geoenviron. Eng. 2016 doi: 10.1061/(ASCE)GT.1943-5606.0001640. [DOI] [Google Scholar]
- 30.Gomez MG, Martinez BC, DeJong JT, Hunt CE, deVlaming LA, Major DW, Dworatzek SM. Field-scale bio-cementation tests to improve sands. Ground Improv. 2015;168(3):206–216. doi: 10.1680/grim.13.00052. [DOI] [Google Scholar]
- 31.Phillips AJ, Cunningham AB, Gerlach R, Hiebert R, Hwang C, Lomans BP, et al. Fracture sealing with microbially-induced calcium carbonate precipitation: A field study. Environ. Sci. Technol. 2016;50(7):4111–4117. doi: 10.1021/acs.est.5b05559. [DOI] [PubMed] [Google Scholar]
- 32.Saneiyan S, Ntarlagiannis D, Ohan J, Lee J, Colwell F, Burns S. Induced polarization as a monitoring tool for in-situ microbial induced carbonate precipitation (MICP) processes. Ecol. Eng. 2019;127:36–47. doi: 10.1016/j.ecoleng.2018.11.010. [DOI] [Google Scholar]
- 33.Chittoori BC, Pathak A, Burbank M, Islam MT. Application of bio-stimulated calcite precipitation to stabilize expansive soils: Field trials. In: Reston VA, editor. Geo-Congress 2020: Biogeotechnics. American Society of Civil Engineers; 2020. pp. 111–120. [Google Scholar]
- 34.Ghasemi P, Montoya BM. Field application of the microbially induced calcium carbonate precipitation on a coastal sandy slope. In: Reston VA, editor. Geo-Congress 2020: Biogeotechnics. American Society of Civil Engineers; 2020. pp. 141–149. [Google Scholar]
- 35.Terzis D, Laloui L, Dornberger S, Harran R. A full-scale application of slope stabilization via calcite bio-mineralization followed by long-term GIS surveillance. In: Reston VA, editor. Geo-Congress 2020: Biogeotechnics. American Society of Civil Engineers; 2020. pp. 65–73. [Google Scholar]
- 36.San Pablo AC, Lee M, Graddy CM, Kolbus CM, Khan M, Zamani A, et al. Meter-scale biocementation experiments to advance process control and reduce impacts: Examining spatial control, ammonium by-product removal, and chemical reductions. Journal of Geotechnical and Geoenvironmental Engineering. 2020;146(11):04020125. doi: 10.1061/(ASCE)GT.1943-5606.0002377. [DOI] [Google Scholar]
- 37.Sposito G. The chemistry of soils. Oxford University Press; 2008. [Google Scholar]
- 38.Plummer LN, Busenberg E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta. 1982;46(6):1011–1040. doi: 10.1016/0016-7037(82)90056-4. [DOI] [Google Scholar]
- 39.Gal JY, Fovet Y, Gache N. Mechanisms of scale formation and carbon dioxide partial pressure influence. Part I. Elaboration of an experimental method and a scaling model. Water Res. 2002;36(3):755–763. doi: 10.1016/S0043-1354(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 40.American Public Health Association (APHA), American Water Works Association (AWWA), & Water Environment Federation (WEF).Standard methods for the examination of water and wastewater 19th edn (Washington, DC, USA, 2005).
- 41.Gomez MG, Graddy CMR, DeJong JT, Nelson DC, Tsesarsky M. Stimulation of native microorganisms for biocementation in samples recovered from field-scale treatment depths. J. Geotech. Geoenviron. Eng. 2018 doi: 10.1061/(ASCE)GT.1943-5606.0001804. [DOI] [Google Scholar]
- 42.Whiffin VS, van Paassen LA, Harkes MP. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007;24(5):417–423. doi: 10.1080/01490450701436505. [DOI] [Google Scholar]
- 43.van Paassen, L. A. Biogrout: Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. thesis. Delft University of Technology, Delft (2009).
- 44.Mortensen BM, Haber MJ, DeJong JT, Caslake LF, Nelson DC. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011;111(2):338–349. doi: 10.1111/j.1365-2672.2011.05065.x. [DOI] [PubMed] [Google Scholar]
- 45.Al Qabany A, Soga K, Santamarina C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2012;138(8):992–1001. doi: 10.1061/(ASCE)GT.1943-5606.0000666. [DOI] [Google Scholar]
- 46.Chu J, Stabnikov V, Ivanov V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012;29(6):544–549. doi: 10.1080/01490451.2011.592929. [DOI] [Google Scholar]
- 47.Martinez BC, DeJong JT, Ginn TR, Montoya BM, Barkouki TH, Hunt C, et al. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 2013;139(4):587–598. doi: 10.1061/(ASCE)GT.1943-5606.0000787. [DOI] [Google Scholar]
- 48.Millero FJ, Feistel R, Wright DG, McDougall TJ. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Part I. 2008;55(1):50–72. doi: 10.1016/j.dsr.2007.10.001. [DOI] [Google Scholar]
- 49.Cheng, L., Shahin, M. A., Cord-Ruwisch, R., Addis, M., Hartanto, T., & Elms, C. Soil stabilisation by Microbial-Induced Calcite Precipitation (MICP): Investigation into some physical and environmental aspects. In: 7th International Congress on Environmental Geotechnics: ICEG2014 1105 (Engineers Australia, 2014).
- 50.Yu X, Rong H. Seawater based MICP cements two/one-phase cemented sand blocks. Appl. Ocean Res. 2022;118:102972. doi: 10.1016/j.apor.2021.102972. [DOI] [Google Scholar]
- 51.Miftah A, Khodadadi Tirkolaei H, Bilsel H. Biocementation of calcareous beach sand using enzymatic calcium carbonate precipitation. Curr. Comput. Aided Drug Des. 2020;10(10):888. [Google Scholar]
- 52.Gomez, M. G., Anderson, C. M., DeJong, J. T., Nelson, D. C., & Lau, X. Stimulating in situ soil bacteria for bio-cementation of sands. In Geo-Congress 2014 Technical Papers 1674–1682 (ASCE, Reston, VA, 2014)
- 53.van Paassen, L. A. Bio-mediated ground improvement: from laboratory experiment to pilot applications. Proc. Geo-Front. Adv. Geotech. Eng. 4099–108 (2011).
- 54.Riveros GA, Sadrekarimi A. Liquefaction resistance of Fraser River sand improved by a microbially-induced cementation. Soil Dyn. Earthq. Eng. 2020;131:106034. doi: 10.1016/j.soildyn.2020.106034. [DOI] [Google Scholar]
- 55.Cardoso R, Pires I, Duarte SO, Monteiro GA. Effects of clay's chemical interactions on biocementation. Appl. Clay Sci. 2018;156:96–103. doi: 10.1016/j.clay.2018.01.035. [DOI] [Google Scholar]
- 56.Sun X, Miao L, Chen R. Effects of different clay’s percentages on improvement of sand-clay mixtures with microbially induced calcite precipitation. Geomicrobiol. J. 2019;36(9):810–818. doi: 10.1080/01490451.2019.1631912. [DOI] [Google Scholar]
- 57.Safdar MU, Mavroulidou M, Gunn MJ, Garelick J, Payne I, Purchase D. Innovative methods of ground improvement for railway embankment peat fens foundation soil. Géotechnique. 2021;71(11):985–998. doi: 10.1680/jgeot.19.SiP.030. [DOI] [Google Scholar]
- 58.Gowthaman, S., Chen, M., Nakashima, K., Komatsu, S., & Kawasaki, S. Biocementation technology for stabilization/solidification of organic peat. In Low Carbon Stabilization and Solidification of Hazardous Wastes 49–64 (Elsevier, 2022).
- 59.Montoya BM, Safavizadeh S, Gabr MA. Enhancement of coal ash compressibility parameters using microbial-induced carbonate precipitation. J. Geotech. Geoenviron. Eng. 2019;145(5):04019018. doi: 10.1061/(ASCE)GT.1943-5606.0002036. [DOI] [Google Scholar]
- 60.Liu Q, Montoya BM. Microbial-induced calcium carbonate precipitation to accelerate sedimentation of fine tailings. J. Geotech. Geoenviron. Eng. 2021;147(10):02821001. doi: 10.1061/(ASCE)GT.1943-5606.0002651. [DOI] [Google Scholar]
- 61.O’Toole, C., Liu, Q., Montoya, B. M., Kananizadeh, N., & Odle, W. The effect of microbial induced carbonate precipitation on fine-grained mine tailings. In Geo-Congress 2022 335–346 (2022).
- 62.Berner RA. The role of magnesium in the crystal growth of calcite and aragonite from sea water. Geochim. Cosmochim. Acta. 1975;39(4):489–494. doi: 10.1016/0016-7037(75)90102-7. [DOI] [Google Scholar]
- 63.Zhang Y, Dawe RA. Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem. Geol. 2000;163(1):129–138. doi: 10.1016/S0009-2541(99)00097-2. [DOI] [Google Scholar]
- 64.Meldrum FC, Hyde ST. Morphological influence of magnesium and organic additives on the precipitation of calcite. J. Cryst. Growth. 2001;231(4):544–558. doi: 10.1016/S0022-0248(01)01519-6. [DOI] [Google Scholar]
- 65.Busenberg E, Plummer LN. Kinetic and thermodynamic factors controlling the distribution of SO32− and Na+ in calcites and selected aragonites. Geochim. Cosmochim. Acta. 1985;49(3):713–725. doi: 10.1016/0016-7037(85)90166-8. [DOI] [Google Scholar]
- 66.Morse JW, Arvidson RS, Lüttge A. Calcium carbonate formation and dissolution. Chem. Rev. 2007;107(2):342–381. doi: 10.1021/cr050358j. [DOI] [PubMed] [Google Scholar]
- 67.Roberts JA, Kenward PA, Fowle DA, Goldstein RH, González LA, Moore DS. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. 2013;110(36):14540–14545. doi: 10.1073/pnas.1305403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu D, Xu Y, Papineau D, Yu N, Fan Q, Qiu X, Wang H. Experimental evidence for abiotic formation of low-temperature proto-dolomite facilitated by clay minerals. Geochim. Cosmochim. Acta. 2019;247:83–95. doi: 10.1016/j.gca.2018.12.036. [DOI] [Google Scholar]
- 69.Raz S, Hamilton PC, Wilt FH, Weiner S, Addadi L. The transient phase of amorphous calcium carbonate in sea urchin larval spicules: the involvement of proteins and magnesium ions in its formation and stabilization. Adv. Func. Mater. 2003;13(6):480–486. doi: 10.1002/adfm.200304285. [DOI] [Google Scholar]
- 70.Al-Sawalmih A, Li C, Siegel S, Fratzl P, Paris O. On the stability of amorphous minerals in lobster cuticle. Adv. Mater. 2009;21(40):4011–4015. doi: 10.1002/adma.200900295. [DOI] [Google Scholar]
- 71.Feng K, Montoya BM. Influence of confinement and cementation level on the behavior of microbial-induced calcite precipitated sands under monotonic drained loading. J. Geotech. Geoenviron. Eng. 2016;142(1):04015057. doi: 10.1061/(ASCE)GT.1943-5606.0001379. [DOI] [Google Scholar]
- 72.Albéric M, Bertinetti L, Zou Z, Fratzl P, Habraken W, Politi Y. The crystallization of amorphous calcium carbonate is kinetically governed by ion impurities and water. Adv. Sci. 2018;5(5):1701000. doi: 10.1002/advs.201701000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasanova I, Hasanova U, Gasimov E, Rzayev F, Hajiyev E, Eyvazova G, et al. PEG-assisted controlled precipitation of calcium hydroxide and calcium carbonate nanostructures for cement reinforcement. Mater. Chem. Phys. 2021;271:124865. doi: 10.1016/j.matchemphys.2021.124865. [DOI] [Google Scholar]
- 74.Ziotopoulou, K., Montgomery, J., Parra Bastidas, A. M., & Morales, B. Cyclic response of Ottawa F-65 Sand: Laboratory and numerical investigation. In: Proc. Geotechnical Earthquake Engineering and Soil Dynamics V (GEESDV 2018) June 10–13, 2018 (Austin, Texas, 2018).
- 75.Carey, T. J., Stone, N., & Kutter, B. L. Grain size analysis and maximum and minimum dry density testing of Ottawa F-65 sand for LEAP-UCD-2017. In: Model Tests and Numerical Simulations of Liquefaction and Lateral Spreading 31–44 (Springer, Cham, 2020).
- 76.El Ghoraiby, M., Park, H., & Manzari, M. T. Physical and mechanical properties of Ottawa F65 sand. In Model Tests and Numerical Simulations of Liquefaction and Lateral Spreading 45–67 (Springer, Cham, 2020).
- 77.ASTM. Standard Practice for Classification of Soils for Engineering Purpose. In ASTM D2487–17e1 (West Conshohocken, PA, 2017).
- 78.U.S. Environmental Protection Agency. Cation-exchange capacity of soils (ammonium acetate): Test methods for evaluating solid waste. In SW846, method 9080 (USEPA, Office of Solid Waste and Emergency Response, Washington, DC, 1986).
- 79.Wang Y, Soga K, DeJong JT, Kabla AJ. Effects of bacterial density on growth rate and characteristics of microbial-induced CaCO3 precipitates: Particle-scale experimental study. J. Geotech. Geoenviron. Eng. 2021;147(6):04021036. doi: 10.1061/(ASCE)GT.1943-5606.0002509. [DOI] [Google Scholar]
- 80.Tsesarsky M, Gat D, Ronen Z. Biological aspects of microbial-induced calcite precipitation. Environ. Geotech. 2016;5(2):69–78. doi: 10.1680/jenge.15.00070. [DOI] [Google Scholar]
- 81.Dawoud, O., Chen, C. Y., & Soga, K. Microbial induced calcite precipitation for geotechnical and environmental applications. In New Frontiers in Geotechnical Engineering 11–18 (ASCE, Reston, VA, 2014).
- 82.Pilson ME. An Introduction to the Chemistry of the Sea. Cambridge University Press; 2012. [Google Scholar]
- 83.Knorst MT, Neubert R, Wohlrab W. Analytical methods for measuring urea in pharmaceutical formulations. J. Pharm. Biomed. Anal. 1997;15(11):1627–1632. doi: 10.1016/S0731-7085(96)01978-4. [DOI] [PubMed] [Google Scholar]
- 84.Parkhurst, D. L., & Appelo, C. A. J. Description of input and examples for PHREEQC version 3: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations (No. 6-A43) (US Geological Survey, 2013).
- 85.Lauchnor EG, Topp DM, Parker AE, Gerlach R. Whole cell kinetics of ureolysis by S porosarcina pasteurii. J. Appl. Microbiol. 2015;118(6):1321–1332. doi: 10.1111/jam.12804. [DOI] [PubMed] [Google Scholar]
- 86.Graddy CM, Gomez MG, Kline LM, Morrill SR, DeJong JT, Nelson DC. Diversity of sporosarcina-like bacterial strains obtained from meter-scale augmented and stimulated biocementation experiments. Environ. Sci. Technol. 2018;52(7):3997–4005. doi: 10.1021/acs.est.7b04271. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Soga K, DeJong JT, Kabla AJ. A microfluidic chip and its use in characterising the particle-scale behaviour of microbial-induced calcium carbonate precipitation (MICP) Géotechnique. 2019;69(12):1086–1094. doi: 10.1680/jgeot.18.P.031. [DOI] [Google Scholar]
- 88.Zambare NM, Naser NY, Gerlach R, Chang CB. Mineralogy of microbially induced calcium carbonate precipitates formed using single cell drop-based microfluidics. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-73870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cuthbert MO, Riley MS, Handley-Sidhu S, Renshaw JC, Tobler DJ, Phoenix VR, Mackay R. Controls on the rate of ureolysis and the morphology of carbonate precipitated by S. Pasteurii biofilms and limits due to bacterial encapsulation. Ecol. Eng. 2012;41:32–40. doi: 10.1016/j.ecoleng.2012.01.008. [DOI] [Google Scholar]
- 90.Pecharsky, V. K., & Zavalij, P. Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials 2nd Version, 172. (2009).
- 91.Bruker.DIFFRAC.EVA: Software to Evaluate X-ray Diffraction Data Version 4.3 (2018).
- 92.Heu R, Shahbazmohamadi S, Yorston J, Capeder P. Target material selection for sputter coating of SEM samples. Microsc. Today. 2019;27(4):32–36. doi: 10.1017/S1551929519000610. [DOI] [Google Scholar]
- 93.Ribeiro, B. G. O., & Gomez, M. G. Investigating the dissolution behavior of calcium carbonate bio-cemented sands. In Geo-Congress 2022 385–395 (2022).
- 94.Martinac B, Saimi Y, Kung C. Ion channels in microbes. Physiol. Rev. 2008;88(4):1449–1490. doi: 10.1152/physrev.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood JM. Perspectives on: The response to osmotic challenges: Bacterial responses to osmotic challenges. J. Gen. Physiol. 2015;145(5):381. doi: 10.1085/jgp.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arias D, Cisternas LA, Rivas M. Biomineralization mediated by ureolytic bacteria applied to water treatment: A review. Curr. Comput. Aided Drug Des. 2017;7(11):345. [Google Scholar]
- 97.Loste E, Wilson RM, Seshadri R, Meldrum FC. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth. 2003;254(1–2):206–218. doi: 10.1016/S0022-0248(03)01153-9. [DOI] [Google Scholar]
- 98.Stumm, W., & Morgan, J. J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters Vol. 126 (Wiley, 2012).
- 99.Ratke, L., & Voorhees, P. W. Growth and Coarsening: Ostwald Ripening in Material Processing (Springer, 2013).
- 100.Rodriguez-Blanco JD, Shaw S, Benning LG. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale. 2010;3:265–271. doi: 10.1039/C0NR00589D. [DOI] [PubMed] [Google Scholar]
- 101.Otto K, Elwing H, Hermansson M. Effect of ionic strength on initial interactions of Escherichia coli with surfaces, studied on-line by a novel quartz crystal microbalance technique. J. Bacteriol. 1999;181(17):5210–5218. doi: 10.1128/JB.181.17.5210-5218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hommel J, Ebigbo A, Gerlach R, Cunningham AB, Helmig R, Class H. Finding a balance between accuracy and effort for modeling biomineralization. Energy Procedia. 2016;97:379–386. doi: 10.1016/j.egypro.2016.10.028. [DOI] [Google Scholar]
- 103.Nassar MK, Gurung D, Bastani M, Ginn TR, Shafei B, Gomez MG, et al. Large-scale experiments in microbially induced calcite precipitation (MICP): Reactive transport model development and prediction. Water Resour. Res. 2018;54(1):480–500. doi: 10.1002/2017WR021488. [DOI] [Google Scholar]
- 104.Plummer, L. N., Parkhurst, D. L., & Wigley, T. M. L. Critical review of the kinetics of calcite dissolution and precipitation (1979).
- 105.Zhong S, Mucci A. Calcite and aragonite precipitation from seawater solutions of various salinities: Precipitation rates and overgrowth compositions. Chem. Geol. 1989;78(3–4):283–299. doi: 10.1016/0009-2541(89)90064-8. [DOI] [Google Scholar]
- 106.Chou LEI, Garrels RM, Wollast R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 1989;78(3–4):269–282. doi: 10.1016/0009-2541(89)90063-6. [DOI] [Google Scholar]
- 107.Compton RG, Pritchard KL, Unwin PR. The dissolution of calcite in acid waters: Mass transport versus surface control. Freshw. Biol. 1989;22(2):285–288. doi: 10.1111/j.1365-2427.1989.tb01101.x. [DOI] [Google Scholar]
- 108.Ferris FG, Phoenix V, Fujita Y, Smith RW. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 C in artificial groundwater. Geochim. Cosmochim. Acta. 2004;68(8):1701–1710. doi: 10.1016/S0016-7037(03)00503-9. [DOI] [Google Scholar]
- 109.Barkouki TH, Martinez BC, Mortensen BM, Weathers TS, De Jong JD, Ginn TR, et al. Forward and inverse bio-geochemical modeling of microbially induced calcite precipitation in half-meter column experiments. Transp. Porous Media. 2011;90(1):23–39. doi: 10.1007/s11242-011-9804-z. [DOI] [Google Scholar]
- 110.Hommel J, Lauchnor E, Phillips A, Gerlach R, Cunningham AB, Helmig R, et al. A revised model for microbially induced calcite precipitation: Improvements and new insights based on recent experiments. Water Resour. Res. 2015;51(5):3695–3715. doi: 10.1002/2014WR016503. [DOI] [Google Scholar]
- 111.Ebigbo, A., Phillips, A., Gerlach, R., Helmig, R., Cunningham, A. B., Class, H., & Spangler, L. H. Darcy‐scale modeling of microbially induced carbonate mineral precipitation in sand columns. Water Resour. Res.48(7), (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during the study are available from the corresponding author upon reasonable request. All data presented in the figures of this paper as well as experimental measurements be available through the NSF DesignSafe-CI Data Depot repository (https://www.designsafe-ci.org/data/browser/public/) under project number PRJ-3190.