Abstract
Escherichia coli generates about 14 μM hydrogen peroxide (H2O2) per s when it grows exponentially in glucose medium. The steady-state intracellular concentration of H2O2 depends on the rates at which this H2O2 is dissipated by scavenging enzymes and by efflux from the cell. The rates of H2O2 degradation by the two major scavenging enzymes, alkyl hydroperoxide reductase and catalase, were quantified. In order to estimate the rate of efflux, the permeability coefficient of membranes for H2O2 was determined. The coefficient is 1.6 × 10−3 cm/s, indicating that permeability is substantial but not unlimited. These data allowed internal H2O2 fluxes and concentrations to be calculated. Under these growth conditions, Ahp scavenges the majority of the endogenous H2O2, with a small fraction degraded by catalase and virtually none persisting long enough to penetrate the membrane and exit the cell. The robust scavenging activity maintains the H2O2 concentration inside glucose-grown cells at <10−7 M, substantially below the level (10−6 M) at which toxicity is evident. When extracellular H2O2 is present, its flux into the cell can be rapid, but the internal concentration may still be an order of magnitude lower than that outside. The presence of such gradients was confirmed in experiments that revealed different degrees of oxidative stress in cocultured scavenger-deficient mutants. The limited permeability of membranes to H2O2 rationalizes the compartmentalization of scavenging systems and predicts that bacteria that excrete redox-cycling drugs do not experience the same H2O2 dose that they impose on their competitors.
Molecular oxygen chemically oxidizes redox enzymes in all aerobic organisms, generating a flux of H2O2 that can potentially damage the cell. The propensity of enzymes for autooxidation depends on their electronic and physical structures, and so the rate of H2O2 production presumably varies among organisms, depending on the types and abundance of these enzymes (12). Escherichia coli generates about 14 μM H2O2 per s when it grows aerobically on glucose (16).
This H2O2 can potentially damage enzymes by oxidizing sulfhydryl and iron-sulfur moieties, and on conversion to a hydroxyl radical it can produce mutagenic and lethal lesions (18). Therefore, microbes typically contain multiple catalases and/or peroxidases. Alkyl hydroperoxide reductase is the primary scavenger of endogenous H2O2 in E. coli (16). Catalase contributes little when H2O2 levels are low, but it becomes the more effective scavenger when H2O2 levels are high or, presumably, when the absence of a carbon source depletes the cell of the NADH necessary for Ahp activity. Mutants that lacked both of these scavengers accumulated 2 μM H2O2 and grew poorly. This, then, represents a toxic dose of H2O2, and it is clear that in the absence of scavengers, sufficient H2O2 is generated by metabolic sources to achieve it.
The concentration of H2O2 inside any cellular compartment depends on the rates of its influx and/or endogenous formation, balanced against the rates of scavenging and efflux. In some discussions the transit of H2O2 across membranes is stipulated to be so rapid that endogenous H2O2 effluxes from the cell before Ahp or catalase can scavenge it. If so, the only role of these enzymes would be to detoxify the environment once H2O2 had accumulated in it and reentered cells. For this reason it has been proposed that H2O2 scavenging is a communal activity (11).
A troubling consequence of such a situation would be that very dilute bacteria, having little collective scavenging activity, would be unable to detoxify an H2O2-containing environment enough to make it habitable. This situation could arise when bacteria enter new environments. It may also occur when pathogens are challenged with H2O2 inside a phagolysosome; the fact that catalase is not required for full virulence has been explained in this way (4).
However, there are problems with the idea that the intracellular H2O2 concentration is equivalent to that outside the cell. First, isolated cells can clearly survive the micromolar amount of H2O2 that exists in growth media (16), even though this is a toxic dose that inhibits scavengerless mutants. This result argues that endogenous scavengers are sufficiently active and permeability is sufficiently poor that they can lower the H2O2 concentration in the cell well below that of the environment. Second, catalase is compartmentalized in the lysosomes of eukaryotic cells, a site of H2O2 production. It seems reasonable to assume that this localization ensures that H2O2 is scavenged before it can exit and toxify the cell; the amount of catalase in the lysosome would be inadequate for this effect if H2O2 efflux were truly unlimited. More recently, it has been shown that some bacteria also compartmentalize catalases in their periplasms (2, 3, 9, 17), an effort that would be wasted if H2O2 rapidly equilibrated between that compartment and the cytoplasm.
This study was undertaken to measure the activities of E. coli Ahp and catalase and the permeability coefficient of H2O2. With this information in hand, intracellular concentrations of H2O2 can be predicted, and the steepness and physiological significance of transmembrane concentration gradients can be appraised. We find that scavenging processes are more rapid than the flow of H2O2 across membranes. As a consequence, E. coli reduces endogenous H2O2 to submicromolar levels, and these cells can continue to grow when environmental H2O2 concentrations exceed what can be tolerated internally.
MATERIALS AND METHODS
Chemicals, enzymes, and growth conditions are described in the accompanying report (16). When indicated, minimal glucose medium included Casamino Acids (CAA) at 0.2% (minimal glucose CAA medium) or l-amino acids at 0.5 mM (minimal glucose 20 AA medium). Tryptophan was added when CAA was used. The strains used in this study were derived from E. coli K-12 (Table 1); isogenic strains were used in all experiments.
TABLE 1.
E. coli strains
| Strain | Genotype | Source or reference |
|---|---|---|
| MG1655 | F−, wild type | E. coli Genetic Stock Center |
| JI364 | Δ(katG17::Tn10)1 (Tets) | 16 |
| JI367 | As JI364 plus katE12::Tn10 | 16 |
| JI372 | Δ(katE12::Tn10)1 ΔahpCF′ kan::′ahpF | 16 |
| JI377 | As JI367 plus ΔahpCF′ kan::′ahpF | 16 |
| MC4100 | araD139 Δ(argF-lac)169 λ−flhD5301 fruA25 relA1 rpsL150 rbsR22 deoC1 | E. coli Genetic Stock Center |
| MC4100 Δahp | As MC4100 plus ΔahpCF′ kan::′ahpF | Gisela Storz |
| GS022 | As MC4100 plus λRS45 Φ(katG::lacZ) | Gisela Storz |
| LC70 | As GS022 plus ΔahpCF′ kan::′ahpF | 16 |
H2O2 scavenging by whole cells and cell extracts.
H2O2 was detected throughout this study using the Amplex red/horseradish peroxidase detection method (16). To compare the ability of whole cells and cell extracts to scavenge 1.5 μM H2O2, cultures were grown overnight in Luria broth (LB), diluted to 0.01 optical density unit (OD) at 600 nm in fresh LB, and grown for ca. six generations. Cells were then pelleted and washed twice with phosphate-buffered saline (PBS). Half the cells were resuspended in PBS (pH 7.3) at 1/20 the original culture volume, and half the cells were resuspended at 1/20 the original volume in 50 mM potassium phosphate (KPi, pH 7.8). The whole cells were then diluted to an OD of 0.030 in 5 ml of PBS, and H2O2 was then added to a final concentration of 1.5 μM. At various time intervals, 0.45 ml was removed, and residual H2O2 was quantitated by the Amplex red/horseradish peroxidase assay. The cells that had been suspended in 50 mM KPi were lysed by sonication, and cell debris was removed by centrifugation at 13,000 × g for 20 min. Cell extract was diluted, by the same ratio as the whole cells, into 5 ml of PBS, producing a cell extract that represented 0.030 OD cells. H2O2 was then added, and at timed intervals the remaining H2O2 was measured.
Hydroperoxidase I (HPI) denotes the catalase encoded by the katG gene and is amply expressed during exponential growth. To determine the ability of HPI to scavenge different H2O2 concentrations, cells were grown and assayed as above. However, in these studies cells were diluted to 0.1 OD prior to H2O2 exposure. When high concentrations of H2O2 were used, samples were diluted in PBS prior to H2O2 measurement.
To define an Ahp dose-response curve in vivo, strain JI367 was grown to the exponential phase as described above, using minimal glucose 20 AA medium. Cells were diluted in that medium to 0.1 OD and exposed to H2O2 at 37°C.
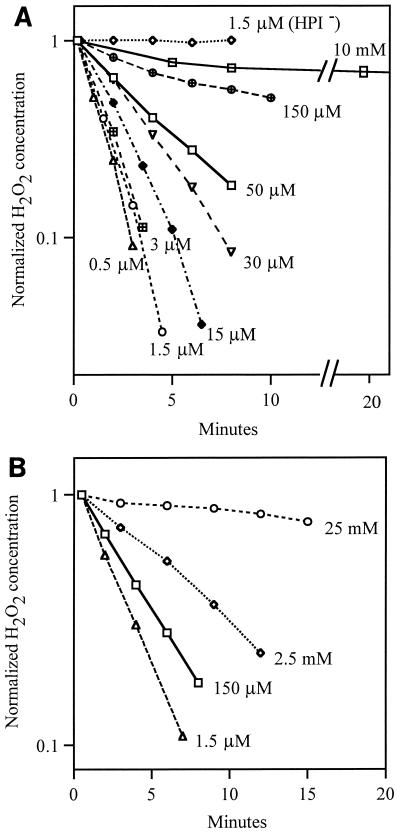
First-order rate constants for in vitro catalase activity were determined by semilogarithmic plots of substrate versus time (Fig. 1). In order to obtain rate constants that describe the intracellular activity, the rates that were observed in vitro were multiplied by the difference in HPI concentration between the assay solution and the intracellular environment. We used the relation that 1 ml of 1.0 OD bacteria contains 0.47 μl of cytosolic volume (7). Because this same relation is incorporated into calculations of endogenous H2O2 production and transmembrane H2O2 flux, the error in it (estimated to be about 20%) does not affect the calculations of intracellular H2O2 concentrations or of the relative fluxes through Ahp, HPI, and the cell membrane.
FIG. 1.
Decomposition of H2O2 by HPI in vitro and in vivo. (A) First-order decomposition of H2O2 by extracts from wild-type (MG1655) and HPI− cells (JI364). (B) Kinetics of decomposition of H2O2 by whole Ahp− HPII− HPI+ (JI372) cells suspended at 0.1 OD.
Mixed-culture experiments.
To monitor katG::lacZ expression in cells grown in mixed cultures, pure cultures were first grown overnight anaerobically in LB. Cultures were then diluted to 0.01 OD and grown to ≈0.1 OD anaerobically. These exponentially growing cultures were mixed at a 9:1 ratio of Ahp+ to Ahp− (MC4100:LC70 and GS022:MC4100 Δahp, respectively), where only one strain contained katG::lacZ. Both pure and mixed cultures were then aerated by vigorous shaking in room air. Once cultures reached 0.3 to 0.4 OD, an aliquot was removed from the mixed cultures, diluted, and plated on LB and LB plus kanamycin plates in order to determine the precise ratio of the two strains. The cultures were chilled on ice for 10 min, centrifuged, washed, and resuspended in 1/15 the culture volume in cold 50 mM KPi buffer (pH 7.0). Cells were lysed by sonication, and cell debris was removed by centrifugation at 13,000 × g for 20 min. Extracts were then assayed for β-galactosidase activity (16). To calculate the specific activity of the katG::lacZ strain within the mixed culture, the protein concentration of the extract was multiplied by the fraction of cells that contained the katG::lacZ allele. Cultures were grown and assayed in duplicate.
To determine the growth behavior of each strain in mixed cultures, pure cultures were first grown overnight anaerobically, diluted to 0.01 OD, and grown to ≈0.1 OD anaerobically in minimal glucose CAA medium. A mixed culture was then established by mixing strains at a 9:1 ratio (JI372 to JI377). Pure cultures and the mixed culture were then diluted into aerobic minimal glucose CAA medium that had been supplemented with 2 μM H2O2. The coculture was diluted to 0.0005 OD, and the pure cultures were diluted to the approximate OD of each strain in the mixed culture. Cultures were then grown aerobically, and the growth of both strains was monitored by intermittent dilution and plating on LB plates (to quantify total viable cells) and LB plus tetracycline (to quantify viable JI377).
RESULTS
Rationale.
The concentration of hydrogen peroxide inside bacterial cells is established by the balance between the processes that generate it and those that dissipate it.
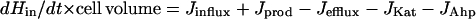 |
1 |
In this equation Hin denotes the intracellular concentration of H2O2. The fluxes (in moles per second) refer to influx into the cell from the external medium (Jinflux), endogenous H2O2 production (Jprod), efflux out of the cell (Jefflux), and H2O2 decomposition by catalase (JKat) and Ahp (JAhp). In this report J will refer to the flux per single cell.
The purpose of this study was to measure the rates of these processes, so that through equation 1 the relative fluxes and the steady-state concentration of H2O2 could be appraised. In the accompanying study, we determined Jprod for cells growing exponentially in glucose medium (16). The strategy here was to measure the activity of catalase in vitro and, by correction for its dilution, to obtain a rate constant that describes its activity in vivo.
The efflux rate depends on the permeability coefficient of the cell membrane, which can be determined by comparing the rates at which H2O2 is scavenged by catalase that is free in solution and catalase that is enclosed within cells. Finally, the rate constant for Ahp activity cannot be obtained by assay in extracts, because Ahp is unstable in vitro, but the constant can be inferred from the scavenging activity of cells that contain Ahp but lack catalase. With this information it is possible to predict levels of intracellular H2O2 and compare them to the levels that cause toxicity. One can also determine whether endogenous H2O2 is compartmentalized within the cell that forms it or whether intracellular and extracellular concentrations approach equilibrium.
HPI activity in vivo.
To obtain a term for JKat, catalase activity was assayed in vitro and extrapolated to the enzyme density found in vivo. The activity was primarily due to HPI, the OxyR-regulated enzyme that is encoded by katG, rather than HPII, the katE-encoded enzyme that is induced in stationary phase (13). Ahp was not active in these in vitro assays because NADH was not provided. Many catalases exhibit nonlinear kinetics when they are assayed with high (millimolar) concentrations of H2O2; the enzymes convert from a highly active form to a less active form during the first few minutes of the assay (15). HPI also gradually diminished in activity during exposure to millimolar H2O2 concentrations. However, it exhibited first-order behavior throughout the period of the assay when its activity was measured with micromolar concentrations of H2O2 (Fig. 1A). The inhibition by high concentrations of H2O2 is not an in vitro artifact, as high concentrations also inhibited in vivo in the Ahp− HPII− strain JI372. Larger amounts were needed to cause inhibition in vivo, because the intracellular concentrations are 10-fold less than the external concentrations (see Discussion).
Micromolar concentrations of H2O2 are likely to be more physiologically relevant, because these are the concentrations that are generated by aerobic metabolism (16), induce the OxyR regulon, and inhibit the growth of E. coli. Therefore, the rate constant that was determined at <15 μM in vitro was used in this study. The activity of the cell extracts can be extrapolated to intact cells, with the assumption that turnover numbers measured in vitro resemble those in vivo. These data were used to calculate an approximate first-order rate constant for the decomposition of micromolar concentrations of intracellular H2O2 by catalase (Materials and Methods). The deduced rate constant was 82.6 s−1 for a wild-type E. coli cell; therefore, JKat = [Hin] × 82.6 s−1 × cell volume.
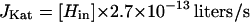 |
2 |
The amount of HPI can differ in other media and growth conditions.
The extracts of katG (HPI−) strains had <10% as much catalase activity as did the extracts of wild-type strains, indicating that HPI was responsible for the majority of the catalase activity under these growth conditions. That result was expected, because the cells had been repeatedly subcultured to dilute out stationary-phase proteins, including HPII (Materials and Methods). We observed previously that mutations that eliminate HPII did not affect the exponential-phase phenotypes even of strains lacking HPI and/or Ahp (16).
H2O2 scavenging can be limited by its penetration into the cell.
In the accompanying report we note that wild-type cells (containing both Ahp and HPI), katG mutants (containing only Ahp), and ahp mutants (containing only HPI) all degraded 1.5 μM extracellular H2O2 at similar rates (16). This observation is explicable in part because in the ahp mutant the OxyR protein was activated and induced the synthesis of HPI, to compensate for the lack of Ahp. However, given the likelihood that the kinetic behaviors of these enzymes differ, it seemed coincidental that at this particular H2O2 concentration the rates were essentially equivalent. A simplifying explanation might be that the rate-limiting step in scavenging was entry of the H2O2 into the cell. If all three strains contained enough scavengers to degrade >80% of the H2O2 before it exited, then the rates at which these cells scavenged H2O2 would be equivalent within the precision of our measurements. This logic also suggested that the permeability coefficient might be inferred from the rate at which the cells degraded H2O2.
In previous work we determined that 0.1 OD of E. coli grown in LB medium represents 1.45 × 107 cells/ml, with a cytoplasmic volume of 3.23 × 10−15 liters/cell (7). If LB-grown cells are modeled as approximate cylinders of length 3.7 μm and radius 0.53 μm, the surface area per cell is 1.41 × 10−7 cm2. These parameters were used in this study to deduce surface area and cytoplasmic volume from optical density, since cells were cultured under conditions in which growth rate and cell size approximated those in LB medium. For comparison, 0.1 OD of cells grown in minimal glucose medium (lacking amino acids) represents 8.41 × 107 cells/ml, with a volume of 6.9 × 10−16 liters/cell (7). Note that despite a large difference in cell size, there is <20% difference in cell volume per OD under these two growth conditions. Therefore, although discrepancies in cell size can arise from differences in culture growth rates, those differences will affect the absolute values of fluxes but will not introduce a substantial error in their relative values or in the steady-state concentrations of H2O2 that are derived from them.
The rate of entry of a substance that passively diffuses across the cell membrane should obey the relation
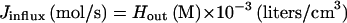 |
3 |
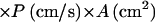 |
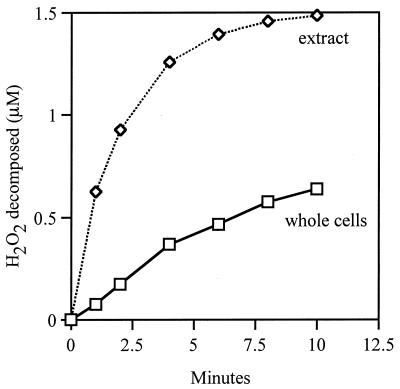
where Jinflux represents the influx into a single cell, Hout denotes the extracellular concentration of the substance, P is the permeability coefficient, and A is the surface area of the membrane. Ahp− cells, which have induced levels of catalase, at 0.030 OD degraded 1.5 μM H2O2 at a rate of 1.29 nM/s (Fig. 2). Therefore, Jin per cell was at least 3.0 × 10−19 mol/s. Applying the cell number, volume, and surface area parameters to equation 1, P is calculated to be >1.4 × 10−3 cm/s.
FIG. 2.
Decomposition of H2O2 by equivalent amounts of extracellular and intracellular HPI. Extract and whole cells of JI372 (ahpCF katE) were prepared as described in Materials and Methods, and the decomposition of 1.5 μM H2O2 was monitored.
Calculation of permeability coefficient.
The value of P can be determined more precisely by comparing the rates at which extracellular and intracellular catalases degrade H2O2 (Fig. 2). When a cell extract was used that contained the same amount of HPI as did 0.030 OD bacteria, the rate of H2O2 decomposition was 12.2 nM/s, or 9.4 times more rapid than with the equivalent amount of intact cells. Thus, it is immediately apparent that the finite permeability of the membrane limits the rate at which cells scavenge H2O2.
To calculate P, one must first determine the intracellular concentration of H2O2, denoted Hin, during the period of the measurement. The overall rate of extracellular H2O2 decomposition (dHout/dt) is equal to the rate of H2O2 decomposition by catalase minus the rate of endogenous H2O2 formation.
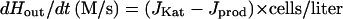 |
4 |
The catalase assay showed that in these Ahp− cells, JKat = 1.87 × 10−12 liters/s × Hin. Cells generate intracellular H2O2 at a rate of 3 μM/s under the conditions of the experiment (16), so Jprod = 9.69 × 10−21 mol/s. Since dHout/dt = 1.29 × 10−9 M/s and the cell density was 4.35 × 109 cells/liter, then Hin = 0.16 μM. Thus, when these Ahp− cells were exposed to 1.5 μM extracellular H2O2, the intracellular H2O2 concentration was only 11% as high.
The rate of scavenging is equal to the difference between H2O2 influx and efflux:
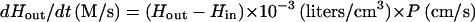 |
5 |
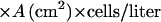 |
Substituting the same value (1.29 × 10−9 mol/s) for dHout/dt, 1.5 × 10−6 M for Hout, 1.64 × 10−7 M for Hin, 1.41 × 10−7 cm2 for the total cytoplasmic membrane area of one cell, and 4.35 × 109 cells/liter, one obtains:
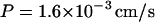 |
This value is slightly higher than the minimum value that was calculated above from the rate at which E. coli scavenges exogenous H2O2. The statistical error in this calculation, derived from small differences in measurements of scavenging rates, is <10%. We have assumed that the cytoplasmic membrane is not invaginated, which is supported by electron micrograph studies. H2O2 entry into the cell appears to occur by free diffusion, since the rate of scavenging by intracellular catalase shows no sign of saturation up to millimolar concentrations (16). That result also suggests that entry is not likely to be affected by any type of oxidative damage to the cell.
The permeability coefficient that we have obtained is very similar to that of HO2⋅ (0.9 [±0.2] × 10−3 cm/s [10]), a molecule of similar size and polarity. Although it remains formally possible that H2O2 enters the cell passively through a pore, we think this unlikely, since the P for HO2⋅ was determined with artificial lipid vesicles that lacked pores. The value for H2O2 is slightly less than that of water (P = 2 × 10−3 to 4 × 10−3 cm/s) (5, 20), consistent with the larger size of H2O2.
From equation 4, the net flux of H2O2 into a single cell can be determined.
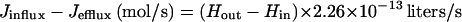 |
6 |
Ahp activity in vivo.
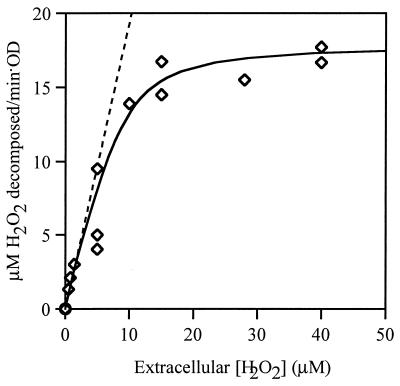
Ahp is the predominant scavenger of the low concentrations of H2O2 that are generated by endogenous metabolism. Unfortunately, its activity cannot be reliably assayed in cell extracts, because the AhpC and AhpF subunits dissociate easily (8, 14). Therefore we could quantify only the ability of intact Ahp+ HPI− cells to scavenge exogenous H2O2 (Fig. 3). Scavenging was saturated when the external H2O2 concentration exceeded 10 μM. At lower concentrations, the rate of scavenging (data points) was close to the rate at which H2O2 entered the cell, as predicted by the permeability coefficient (dashed line).
FIG. 3.
Decomposition of H2O2 by Ahp in vivo. Rates of decomposition were measured in JI367 (Ahp+ HPI− HPII−, diamonds). Solid line, curve predicted using JAhp = 2.1 × 10−18 mol/s (Hin) (Hin + 1.2 × 10−6 M). Note that the abscissa displays extracellular H2O2 concentrations; intracellular concentrations can be calculated from them by equation 8. Dashed line, the rate of H2O2 diffusion into the suspended cells, predicted from the permeability coefficient. Since cells can degrade H2O2 no faster than it penetrates them, this line represents the maximum possible rate of H2O2 decomposition.
These data indicate that the maximum JAhp inside a single cell, with saturating H2O2, is 2.1 × 10−18 mol/s. The saturation curve can be fit (Fig. 3, solid line) if JAhp is represented by a term with the Michaelis-Menten form:
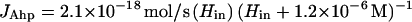 |
7 |
In this equation the value of the Km term was determined by its empirical fit to the data. We suspect that the turnover of Ahp is limited at higher concentrations of H2O2 by the rate of its reduction by NADH, so this value does not connote a binding constant for H2O2. Note that the flux is presented in equation 7 as a function of internal, not external, H2O2 concentration. Because intracellular Ahp scavenges subsaturating H2O2 approximately as fast as the H2O2 enters the cell, JAhp provides a lower limit for the true reaction rate when Hin is <5 μM. This restriction must be acknowledged in any application of this equation.
H2O2 homeostasis in E. coli
The intracellular steady-state concentration of H2O2 in growing cells is established by the relative rates of H2O2 influx, efflux, production, and scavenging by HPI and Ahp:
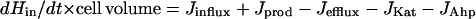 |
In growing cells H2O2 is produced metabolically at a rate of 14 μM/s (16), normalized to the intracellular volume, so that Jprod = 4.5 × 10−20 mol/s for a single cell. We do not know whether all of the H2O2 is formed inside the cytosolic membrane, but in vitro studies predict that at least a substantial fraction is intracellular. By substituting this value and the flux values from equations 2, 6, and 7 into equation 5 and combining terms for influx and efflux, one obtains:
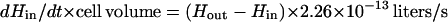 |
8 |
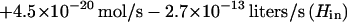 |
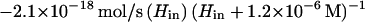 |
Under steady-state conditions, dHin/dt can be set to 0, and Hin can be calculated for any value of Hout. Conversely, for any value of Hin the fluxes through Ahp, HPI, and the membrane can be calculated. A significant caveat: because the activity of Ahp is indeterminate at low values of Hin, this equation may underestimate JAhp—and overestimate Hin—when the calculated value of Hin is <1.2 μM.
If H2O2 has not accumulated in the extracellular medium, then in equation 8, Hout = 0 and the internal steady-state concentration in a wild-type strain is predicted to be 20 nM. The true concentration will be lower if the equation understates the activity of Ahp. Equation 8 can also be solved for mutants which lack either catalase or Ahp by setting the corresponding terms equal to 0. While the absence of catalase would have little impact, raising H2O2 levels to 23 nM, the absence of Ahp would raise H2O2 to 100 nM (if catalase were not induced). In the absence of both enzymes, H2O2 would rise to 200 nM. Note that these calculations pertain only to the situation in which no H2O2 is present in the medium; as Ahp− HPI− cultures grow, H2O2 accumulates continuously.
Physiological evidence of transmembrane concentration gradients.
Equation 8 predicts that transmembrane flow is limited, so that intracellular and extracellular H2O2 concentrations can be substantially different. Two examples will be considered. First, because efflux is slowed by the limited permeability of the membrane, in an Ahp− strain the intracellular H2O2 should be elevated. Indeed, the OxyR regulon is activated; induction can be observed by use of a katG::lacZ fusion (16). Interestingly, when the Ahp− strain is cocultured with a scavenging-proficient strain, the fusion remains highly expressed (0.61 ± 0.04 U/mg) as it is in pure culture (0.69 ± 0.04 U/mg). However, when the converse experiment is performed—when the fusion is in the wild-type strain of the mixed culture—the fusion is not expressed (0.12 ± 0.02 U/mg compared to 0.05 ± 0.02 U/mg in pure wild-type cultures). The implication is that the H2O2 concentration inside the Ahp− strain is higher than that inside the wild-type strain, even when these strains are cocultured, due to the efficiency of H2O2 scavenging by Ahp inside the latter.
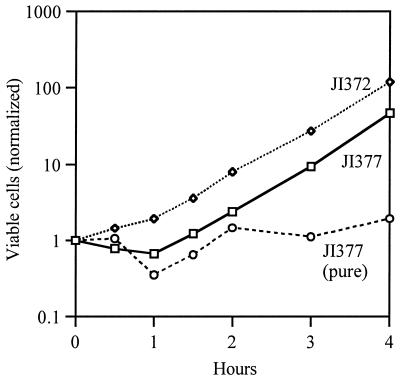
The existence of H2O2 gradients can also be demonstrated when H2O2 is provided exogenously and net flow is into the cell. In the experiment depicted in Fig. 4, dilute Ahp− Kat− and Ahp− Kat+ cells were cocultured in medium to which 2 μM H2O2 had been added. By calculation, the initial concentration of H2O2 inside the Ahp− Kat− strain should be 2.2 μM, while that inside the catalase-proficient strain (with sevenfold induction) should be 0.24 μM. We observed that the catalase-proficient cells grew immediately, while the catalase-deficient mutant did not; within 2 h the population had been 3.3-fold enriched in catalase-proficient cells. However, over time the Kat+ strain detoxified the extracellular medium, and the Ahp− Kat− strain resumed growth at the same rate as the Kat+ strain. This result illustrates nicely that membranes are semipermeable to H2O2: if the permeability coefficient were much higher, then H2O2 would have rapidly equilibrated across membranes, the concentrations inside both cell types would always have been equivalent, and the Kat+ cells would have had no initial growth advantage. Conversely, if the coefficient were much lower, then the Kat+ cells would have been much less efficient at detoxifying the medium and would not have enabled the growth of the Kat− cells.
FIG. 4.
Catalase-proficient cells have a growth advantage over catalase-deficient cells in a mixed culture. JI372 (ahpCF katE) and JI377 (ahpCF katE katG) were mixed at a 9:1 ratio of JI372 to JI377 and diluted into aerobic minimal glucose CAA medium containing an additional 2 μM H2O2. The number of viable cells of each strain was then monitored by intermittent dilution and plating on selective plates.
DISCUSSION
Quantification of intracellular H2O2 stress.
To characterize H2O2 stress in growing cells, it is necessary to quantify it. We have attempted to do so by measuring the rates at which H2O2 is generated and dissipated in wild-type E. coli. The rate of endogenous formation was determined in a connected study (16), and the fluxes through the three routes of dissipation, scavenging by Ahp and catalase and diffusion through the cell membrane, were measured here. The greatest surprise was that E. coli strives to keep H2O2 concentrations so low. It synthesizes enough Ahp that, in the absence of exogenous sources, H2O2 levels should rise no higher than 20 nM; when levels rise to 100 nM, E. coli increases the rates of Ahp and HPI synthesis by activating the OxyR regulon. Such vigilance may be warranted, since the 100 nM H2O2 that accumulates in Ahp− mutants is evidently sufficient to accelerate mutagenesis (6), and 2 μM causes substantial growth inhibition (16).
Gonzalez-Flecha and Demple estimated the intracellular levels of H2O2 to be 0.13 to 0.25 μM, based on its extracellular accumulation to these concentrations when the bacteria were resuspended in buffer (6). In contrast, our strains did not excrete measurable H2O2 due to the high activity of Ahp (16). It will be worth investigating whether the rates and routes of H2O2 dissipation change as cells grow to the higher densities used in their experiments.
Experimental support for quantitative estimates.
The H2O2 concentrations that are predicted by equation 6 have been directly measured only in the case of Ahp− Kat− cultures, which accumulate H2O2 in the extracellular medium. However, the predictions that derive from this equation are supported by a number of experimental observations. First, the 100 nM H2O2 that is predicted to accumulate in the Ahp− strain is within the 50 to 200 nM range, which activated OxyR effectively in vitro (1).
Second, no H2O2 can be found in the supernatants of scavenging-proficient strains (16), consistent with the prediction that no more than 10% of the endogenous H2O2 should escape from the cell. Indeed, even if this much was excreted, cells could not substantially pollute their medium; a maximum of 20 nM could accumulate, since at this concentration the scavenging and excretion rates would be equivalent.
Third, according to equation 8, the H2O2 levels inside Ahp− Kat− cells should reach 2 μM by the time that 1.8 μM accumulates externally but only 0.2 μM if external catalase prevents the accumulation of H2O2 in the medium. In fact, external catalase relieves the growth deficits of these strains (16).
Finally, wild-type cells contain enough Ahp and catalase to create a substantial outside-to-inside H2O2 concentration gradient when H2O2 is added to the medium. For example, >10 μM external H2O2 must be added to raise intracellular levels to the 2 μM level, which substantially inhibited growth. This is consistent with the amounts of external H2O2 (ca. 30 μM) that are needed to block growth (unpublished data). Much higher amounts (>80 μM) should be necessary to inhibit OxyR-induced cells, which have about 10-fold higher levels of Ahp and catalase.
Compartmentalization of H2O2 sources and scavenging enzymes.
Because virtually no H2O2 escapes from E. coli, the stress is effectively contained within the cell. There is no obvious benefit to this, but it proves an expected principle: when highly active scavengers are compartmentalized inside organelles that generate H2O2, the H2O2 can be effectively contained. Thus, it is plausible that the catalase that is localized in peroxisomes and the peroxidases that are inside mitochondria can eliminate H2O2 before it can escape those compartments and threaten cytosolic enzymes and nuclear DNA. Were the permeability coefficient of H2O2 as high as, for example, that of molecular oxygen, transmembrane gradients could not be formed at any reasonable activity of the scavenging enzyme, and most H2O2 would spill out of these organelles.
Conversely, the cytoplasmic membrane limits the effectiveness with which E. coli scavenges extracellular H2O2. This refutes the suggestion (11) that its scavengers are communal defenses, serving the bacterial community as much as the individual cell in which they reside. For example, at moderate concentrations of H2O2 a 10-fold induction of Ahp will diminish the intracellular H2O2 concentration by 10-fold, but this induction will not accelerate the rate at which the medium is detoxified, since the uninduced cell already scavenges all the H2O2 that enters it. Equation 8 indicates that a 10-fold induction of catalase should increase the rate of medium detoxification (of high H2O2 concentrations) only twofold, and this result was observed (16 [Fig. 3, left panel]). To optimally serve the community, scavenger enzymes must be localized outside the cytosolic membrane.
Interestingly, pathogenic Legionella pneumophila, Pseudomonas syringae, E. coli, and Brucella abortus do compartmentalize catalases in their periplasms, in addition to expressing cytosolic enzymes (2, 3, 9, 17). Thus, it is possible that these organisms use catalase to act as a community in collectively detoxifying their habitat. An interesting alternative, however, is that these bacteria are as individualistic as any others but use a two-stage detoxification system (10) to avoid cytosolic stress from exogenous H2O2. If the rate at which H2O2 enters the periplasm is slow relative to the rate at which a periplasmic catalase scavenges it, the periplasmic H2O2 concentration will be lower than that outside the cell, and the rate of H2O2 influx into the cytosol will be proportionately diminished. In this situation the cytosol is more effectively protected from extracellular H2O2 if catalase is divided between periplasm and cytosol than if all of it is localized in the cytosol. Genetic studies should be useful in determining whether periplasmic superoxide dismutases protect periplasmic or cytosolic targets.
This situation has an interesting consequence for cell-cell warfare. When phagocytes attack bacterial cells, the extracellular concentration of H2O2 must be quite high in order to generate a killing intracellular dose because of the permeability barrier imposed by the cell membrane. The observation that HPI is not a virulence factor for Salmonella enterica serovar Typhimurium (4) may reflect the primacy of Ahp in scavenging. Alternatively, Vazquez-Torres et al. have reported that Salmonella disrupts the trafficking system that customarily directs NADPH oxidase to the phagolysosome (19). Although the oxidase can still be activated, the H2O2 it generates may not reach the bacteria, since the H2O2 would first have to diffuse through scavenger-filled compartments and then penetrate the phagolysosomal membrane. Compartmentalization in the phagolysosome might have the ironic effect of protecting the bacteria.
Some bacteria attack competitors with oxidants, but they use a tactic that, in an analogous way, spares them exposure to H2O2. They excrete redox-cycling drugs that, when ingested, generate crippling doses of superoxide and H2O2 inside the target cell. In this instance, the permeability barrier of the cytoplasmic membrane ensures that the aggressor cell is shielded from the H2O2 that is generated inside the other; while the concentration of H2O2 inside the attacked cell might be quite high, only a small fraction will escape, and the high catalase activity of the aggressor will ensure that it experiences very little H2O2 stress. Were membranes completely permeant to H2O2, it would be suicidal for bacteria to excrete these drugs into their own habitat.
Oxidative stress in experimental systems.
The speed with which E. coli scavenges H2O2 makes it difficult to do certain types of experiments, particularly to monitor the biochemical and physiological effects of the micromolar doses of H2O2 that E. coli is likely to confront in the real world. Even uninduced cells scavenge micromolar concentrations of exogenous H2O2 very quickly. In a culture of moderate density (0.1 OD), the half-life of H2O2 in the medium is only 3.5 min (see, e.g., Fig. 1); at 1.0 OD, it is 20 s. In our hands OxyR-inducing regimens which use micromolar concentrations of H2O2 are variably effective because the inducing dose is rapidly scavenged. Millimolar challenge doses of H2O2, on the other hand, are slowly degraded in cultures, both because Ahp is saturated and because HPI is less active at high substrate concentrations (Fig. 1). These doses provide more consistent results. Thus, in the past we and other experimenters have reluctantly resorted to challenging cells with higher, nonphysiological concentrations of H2O2. We anticipate that the availability of strains that cannot scavenge H2O2 will enable us to study the impact of more realistic doses.
A second experimental difficulty arises from the fact that growth media chemically generate H2O2 (16). From equation 8 it is apparent that when media contain more than 0.2 μM H2O2, the rate of H2O2 influx will exceed the rate of endogenous H2O2 production. Unless we specially prepared it, our glucose medium typically contained up to 10-fold more H2O2 than this, and at 37°C H2O2 continued to be formed at a rate of 0.04 μM/h. LB medium contains enough riboflavin that H2O2 is generated under room lights at a significant rate. Therefore, when cells are first inoculated into typical media, the primary oxidative insult is from H2O2 generated by the medium rather than H2O2 generated by metabolism. This will remain true until the bacteria scavenge sufficient H2O2 to diminish the external concentration to <0.2 μM. If kf represents the rate of H2O2 formation by medium autooxidation, ks represents the rate at which bacteria scavenge H2O2, and Hi and Ht represent the H2O2 concentration initially and at time t, respectively, then
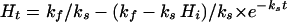 |
The value of ks is 0.119 h−1 for 0.001 OD E. coli, and kf = 0.04 μM/h in our glucose medium. By calculation, wild-type bacteria at 0.010 OD would require 2 h to diminish H2O2 from 2.0 to 0.2 μM; bacteria at 0.001 OD could never do so. Therefore, the major source of H2O2 stress with which dilute bacteria must contend is from their growth medium rather than from their metabolism. This fact may be important in experiments that measure aerobic mutation rates, in the isolation of mutants that generate little endogenous H2O2, or in attempts to isolate and culture bacteria that are acutely sensitive to H2O2 or do not rapidly scavenge it. The problem can be diminished by treating media with catalase.
ACKNOWLEDGMENTS
We thank Gigi Storz for strains used in this work and Jim Slauch for helpful discussions.
This study was supported by grant GM49640 from the National Institutes of Health.
REFERENCES
- 1.Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcriptional factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandyopadhyay P, Steinman H. Catalase-peroxidases of Legionella pneumophila: cloning of the katA gene and studies of KatA function. J Bacteriol. 2000;182:6679–6686. doi: 10.1128/jb.182.23.6679-6686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunder W, Schmidt H, Karch H. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:3305–3315. doi: 10.1099/13500872-142-11-3305. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier N, Libby S, Xu Y, Loewen P, Switala J, Guiney D, Fang F. DNA repair is more important than catalase for Salmonella virulence in mice. J Clin Investig. 1995;95:1047–1053. doi: 10.1172/JCI117750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fettiplace R. The influence of the lipid on the water permeability of artificial membranes. Biochim Biophys Acta. 1978;513:1–10. doi: 10.1016/0005-2736(78)90106-2. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Flecha B, Demple B. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imlay J A, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 8.Jacobson F S, Morgan R W, Christman M F, Ames B N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage: purification and properties. J Biol Chem. 1989;264:1488–1496. [PubMed] [Google Scholar]
- 9.Klotz M, Hutcheson S. Multiple periplasmic catalases in phytopathogenic strains of Pseudomonas syringae. Appl Environ Microbiol. 1992;58:2468–2473. doi: 10.1128/aem.58.8.2468-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korshunov S S, Imlay J A. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of gram-negative bacteria. 2001. Mol. Microbiol., in press. [DOI] [PubMed] [Google Scholar]
- 11.Ma M, Eaton J W. Multicellular oxidant defense in unicellular organisms. Proc Natl Acad Sci USA. 1992;89:7924–7928. doi: 10.1073/pnas.89.17.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messner K R, Imlay J A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 13.Mulvey M R, Switala J, Borys A, Loewen P C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990;172:6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds C M, Poole L B. Activity of one of two engineered heterodimers of AhpF, the NADH:peroxiredoxin oxidoreductase from Salmonella typhimurium, reveals intrasubunit electron transfer between domains. Biochemistry. 2001;40:3912–3919. doi: 10.1021/bi002766h. [DOI] [PubMed] [Google Scholar]
- 15.Rorth M, Jensen P K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967;139:171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- 16.Seaver L C, Imlay J A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha Z, Stabel T, Mayfield J. Brucella abortus catalase is a periplasmic protein lacking a standard signal sequence. J Bacteriol. 1994;176:7375–7377. doi: 10.1128/jb.176.23.7375-7377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storz G, Imlay J A. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden D W, Lucia S M, Dinauer M C, Mastoeni P, Fang F C. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 20.Xiang T-X, Anderson B. The relationship between permeant size and permeability in lipid bilayer membranes. J Membr Biol. 1994;140:111–122. doi: 10.1007/BF00232899. [DOI] [PubMed] [Google Scholar]