Abstract
Background
Several health authorities recommend a third (booster) vaccination to protect patients with rheumatic and musculoskeletal diseases from severe COVID-19. Methotrexate has been shown to reduce the efficacy of the first and second dose of SARS-CoV-2 mRNA vaccines. So far, it remains unknown how concomitant methotrexate affects the efficacy of a COVID-19 booster vaccination.
Methods
We compared the humoral immune response to SARS-CoV-2 vaccination in 136 patients with rheumatoid arthritis (RA) treated with methotrexate and/or biological or targeted synthetic (b/tsDMARDs). IgG targeting the receptor binding domain (RBD) of SARS-CoV-2 spike protein was measured at a median of 52.5 (range 2–147) days after a third dose of the SARS-CoV-2 mRNA vaccines BNT162b2 or mRNA-1273.
Results
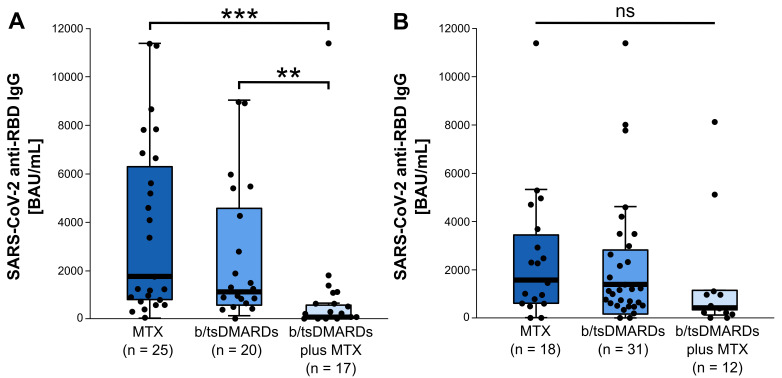
Anti-RBD IgG was significantly reduced in elderly patients receiving concomitant treatment with methotrexate as compared with elderly patients receiving monotherapy with b/tsDMARDs or methotrexate (64.8 (20.8, 600.3) binding antibody units per mL (BAU/mL) vs 1106.0 (526.3, 4965.2) BAU/mL vs 1743.8 (734.5, 6779.6) BAU/mL, median (IQR), p<0.001, Kruskal-Wallis test). In younger patients (< 64.5 years), concomitant methotrexate had no significant impact on the humoral immune response.
Conclusions
Concomitant methotrexate increases the risk of an insufficient humoral immune response to SARS-CoV-2 vaccination in elderly patients with RA. Pausing methotrexate during the third vaccination period may be considered for this group of patients.
Keywords: Rheumatoid Arthritis, COVID-19, Methotrexate
What is already known on this topic
Methotrexate (MTX) reduces the efficacy of the first and second dose of COVID-19 vaccines in elderly patients with rheumatoid arthritis (RA).
What this study adds
We show that concomitant MTX therapy reduces the efficacy of a third (booster) shot of SARS-CoV-2 mRNA vaccines in elderly but not younger patients with RA.
How this study might affect research, practice or policy
Discontinuation of concomitant MTX treatment during vaccination period might improve the efficacy of a third (booster) COVID-19 vaccine in elderly patients.
The SARS-CoV-2 mRNA vaccines BNT162b2 and mRNA-1273 provide efficient protection against severe COVID-19 infections for patients with rheumatoid arthritis (RA) or other rheumatic and musculoskeletal diseases (RMDs). However, some antirheumatic drugs have been negatively associated with vaccine immunogenicity, including rituximab and mycophenolate. Recently, it has been shown that methotrexate (MTX) reduces humoral vaccination response against SARS-CoV-2 in older but not younger patients with RA.1–3 A 2-week discontinuation of MTX treatment improves the anti-SARS-CoV-2 IgG response in patients with RA receiving the conventional vaccine Sinovac-CoronaVac and in patients with inflammatory diseases receiving a vaccine booster (mRNA or adenovirus platform).4 5 Moreover, holding MTX for at least 10 days after vaccination with the mRNA vaccines BNT162b2 or mRNA-1273 or with the adenoviral vector vaccine AZD1222 improves the antibody response in patients over 60 years of age.6 To protect patients with RMDs from severe COVID-19 infections, a third (booster) vaccination is recommended by several authorities.7 So far, it remains unknown if booster vaccinations with an mRNA vaccine are weakened by MTX. Of note, the efficiency of SARS-CoV-2 vaccines is not only determined by the humoral immune response, but also by cellular immune responses.8 Induction of SARS-CoV-2-specific B cells and T cells can be measured in patients with RA and were shown to be dependent on immunosuppressive treatment.9 10
In this retrospective analysis, we compared the humoral immune response against a third dose of the mRNA vaccines BNT162b2 or mRNA-1273 in 136 patients with RA treated with conventional synthetic DMARDs (disease-modifying anti-rheumatic drugs), biological DMARDs or targeted synthetic DMARDs. We compared the efficacy of the vaccination between patients with or without concomitant MTX therapy. Median age, disease duration, sex distribution, time between second and third SARS-CoV-2 vaccination and the time from third vaccination to blood draw for SARS-CoV-2 IgG serum-level analysis were similar between both groups. The patients’ characteristics are summarised in . Neither treatment modification at the time of SARS-CoV-2 vaccination nor prevaccination titers were assessed. We cannot exclude the possibility that some patients might have modified MTX treatment during vaccination phase without consulting their physician. However, such a bias would rather increase the difference between b/tsDMARD treatment with and without concomitant MTX during vaccination phase. All patients fulfilled the 2010 ACR/EULAR classification criteria. Patients’ blood samples were collected at a median of 52.5 (range 2–147) days after the third dose of mRNA vaccine.
Antibody response to the receptor binding domain (RBD) of SARS-CoV-2 spike protein were determined with the SARS-CoV-2 IgG II Quant assay on the Alinity i (Abbott) and reported in binding antibody units per mL (BAU/mL). Anti-RBD were detectable in 132/136 (97.1%) patients with RA. Elderly patients with RA (≥64.5 years, median age in our cohort) with concomitant MTX treatment had a significantly lower serum level of anti-RBD SARS-CoV-2 IgG as compared with elderly patients receiving b/tsDMARD monotherapy or MTX monotherapy (64.8 (20.8, 600.3) BAU/mL vs 1106.0 (526.3, 4965.2) BAU/mL vs 1743.8 (734.5, 6779.6) BAU/mL, n=17, n=20 and n=25, respectively; median (IQR), p<0.001, Kruskal-Wallis test) (figure 1). We observed no differences in humoral anti-RBD response between elderly patients on MTX monotherapy and elderly patients treated with bDMARD or tsDMARD monotherapy. These results were dependent of age, as we did not observe any differences of anti-SARS-CoV-2 IgG serum levels between MTX monotherapy, b/tsDMARD with or without concomitant MTX within the younger patient subgroup (< 64.5 years, median). Interestingly, MTX monotherapy had no significant influence on the humoral anti-SARS-CoV-2 response when compared with patients with without any antirheumatic treatment (1743.8 (659.1, 5091.0) BAU/mL vs 1475.5 (727.1, 5228.4) BAU/mL, n=43 and n=10, respectively; median (IQR), p=0.982, Mann-Whitney U test). As shown previously, we observed a negative effect of rituximab on anti-RBD SARS-CoV-2 IgG serum levels compared with MTX monotherapy (114.7 (0.0, 1380.4) BAU/mL vs 1743.8 (659.1, 5091.0) BAU/mL, n=11 and n=43, respectively; median (IQR), p=0.009, Mann-Whitney U test) ().
Figure 1.
SARS-CoV-2 anti-spike protein receptor binding domain (RBD) IgG serum levels in patients with rheumatoid arthritis. (A) Elderly patients with rheumatoid arthritis (RA) (≥ 64.5 years, n=62) receiving b/ts DMARDs with concomitant MTX therapy show significantly lower SARS-CoV-2 anti RBD IgG serum levels (64.8 (20.8, 600.3) BAU/mL, n=17) compared with patients receiving MTX monotherapy (1743.8 (734.5, 6779.6) BAU/mL, n=25) or b/tsDMARSs monotherapy (1106.0 (526.3, 4965.2) BAU/mL, n=20) (median (IQR)) (p<0.001, Kruskal-Wallis test). (B) in the younger RA patient subgroup (< 64.5 years, n=61), no differences of SARS-CoV-2 anti-RBD IgG serum levels were found between MTX monotherapy (1566.9 (584.3, 3724.7) BAU/mL, n=18), b/tsDMARDs monotherapy (1385.9 (137.9, 2971.8) BAU/mL, n=31) and b/tsDMARDs with concomitant MTX (417.8 (326.1, 1141.8) BAU/mL, n=12) (median (IQR)) (p=0.334, Kruskal-Wallis test). Data are presented as box blots with horizontal bars representing the median. Pairwise comparison using the Dunn-Bonferroni approach, **p<0.01, ***p<0.001. As we also included patients with other treatment modalities, for example, leflunomide monotherapy and no therapy, which are not part of this figure, the number of patients is reduced (n=62, n=61, respectively) compared with the whole study cohort. BAU, binding antibody; bDMARDs, biological DMARDs; MTX, methotrexate; NS, not significant; tsDMARDS, targeted synthetic DMARDs (disease-modifying anti-rheumatic drugs).
To our knowledge, this is the first report showing that a concomitant MTX therapy reduces the efficacy of a booster shot of SARS-CoV-2 mRNA vaccines in elderly patients with RA. Moreover, we can confirm earlier results, showing that MTX reduces the humoral vaccination response against SARS-CoV-2 especially in older but not younger patients with RA.9 As suggested previously for the first and second shot, discontinuation of concomitant MTX treatment for about 2 weeks might help to improve vaccine immunogenicity of booster shots in this group of elderly patients, as well.
Footnotes
Contributors: DS, CtP, RLE, CB, JT and DMK collected and analysed clinical data and laboratory results. VDC analysed anti-SARS-CoV-2 IgG serum levels. All authors contributed to the discussion of results. DS and DMK drafted the manuscript. All authors read and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Feuchtenberger M, Kovacs MS, Eder A, et al. Methotrexate significantly reduces the humoral vaccination response against SARS-CoV-2 in older but not younger patients with rheumatoid arthritis. Rheumatol Int 2022;42:959–66. 10.1007/s00296-022-05123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. 10.1136/annrheumdis-2021-220503 [DOI] [PubMed] [Google Scholar]
- 4.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-Week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis 2022;81:889–97. 10.1136/annrheumdis-2021-221916 [DOI] [PubMed] [Google Scholar]
- 5.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med 2022;10:840–50. 10.1016/S2213-2600(22)00186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis 2022;81:881–8. 10.1136/annrheumdis-2021-221876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis 2022. doi: 10.1136/annrheumdis-2021-222006. [Epub ahead of print: 23 Feb 2022]. [DOI] [PubMed] [Google Scholar]
- 8.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021;184:861–80. 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picchianti-Diamanti A, Aiello A, Laganà B, et al. ImmunosuppressiveTherapies differently modulate Humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol 2021;12:740249. 10.3389/fimmu.2021.740249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrone L, Picchianti-Diamanti A, Sebastiani GD, et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis 2022;121:24–30. 10.1016/j.ijid.2022.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]