Abstract
Objective
The level of neutralising capacity against Omicron BA.1 and BA.2 after third COVID-19 vaccination in patients on paused or continuous methotrexate (MTX) therapy is unclear.
Methods
In this observational cohort study, neutralising serum activity against SARS-CoV-2 wild-type (Wu01) and variant of concern Omicron BA.1 and BA.2 were assessed by pseudovirus neutralisation assay before, 4 and 12 weeks after mRNA booster immunisation in 50 rheumatic patients on MTX, 26 of whom paused the medication. 44 non-immunosuppressed persons (NIP) served as control group.
Results
While the neutralising serum activity against SARS-CoV-2 Wu01 and Omicron variants increased 67–73 fold in the NIP after booster vaccination, the serum activity in patients receiving MTX increased only 20–23 fold. Patients who continued MTX treatment during vaccination had significantly lower neutralisation against all variants at weeks 4 and 12 compared with patients who paused MTX and the control group, except for BA.2 at week 12. Patients who paused MTX reached comparably high neutralising capacities as NIP, except for Wu01 at week 12. The duration of the MTX pause after—not before—was associated with a significantly higher neutralisation capacity against all three variants, with an optimal duration at 10 days after vaccination.
Conclusion
Patients pausing MTX after COVID-19 booster showed a similar vaccine response to NIP. Patients who continued MTX demonstrated an impaired response indicating a potentially beneficial second booster vaccination. Our data also suggest that a 1 week MTX break is sufficient if the last administration of MTX occurs 1–3 days before vaccination.
Keywords: Methotrexate, COVID-19, Vaccination, Arthritis, Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Holding methotrexate (MTX) has shown to increase immunogenicity after COVID-19 vaccination.
No previous studies have investigated the neutralizing capacity against Wuhan (Wu01) and Omicron after COVID-19 mRNA-booster vaccination in an MTX cohort using the pseudovirus neutralization assay.
WHAT THIS STUDY ADDS
Pausing MTX after an mRNA booster significantly improves the neutralising capacity against Wu01 and the Omicron sublineages to a level comparable to individuals without immunosuppressive medication.
An MTX pause of at least 10 days after third vaccination is necessary for optimal vaccination response.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
MTX should be paused for each booster vaccination.
Our data suggest that pausing MTX once is sufficient if the last dose occurs 1–3 days before vaccination.
Introduction
SARS-CoV-2 has caused at least 520 million confirmed infections and 6.25 million deaths worldwide by June 2022.1 Over time, naturally occurring mutations alter the genome of SARS-CoV-2. If the evolved virus variants show increased transmissibility and/or virulence, disease severity and escape from humoral immunity, they are designated as variants of concern (VOC). One of these VOCs, which is globally prevalent in early 2022, is the Omicron variant and its sublineages BA.1 and BA.2.1 It displays an unusually high number of mutations in the receptor-binding (RBD) or N-terminal domain of the viral spike (S) protein. Some of these mutations were already identified in other VOCs and are associated with increased susceptibility and escape from neutralising antibody responses.2 Fortunately, the T cell reactivity against the Omicron variant is not reduced after basic immunisation3 and booster vaccination with wild-type spike mRNA induces robust levels of neutralising serum activity against the Omicron variant.4–6 Thus, these vaccines continue to provide protection against severe disease.7 8
Various immunosuppressants reduce the immune response after COVID-19 vaccination.9–11 Methotrexate (MTX) is the most commonly prescribed disease-modifying antirheumatic drug in the world.12 MTX reduces the humoral vaccination response and CD8+T cell activation after second vaccination against COVID-19.13 14 Pausing MTX therapy 10 or 14 days after both vaccinations of the basic immunisation against COVID-19 significantly improves the production of neutralising antibodies.15 16 A recently published study found that a 2-week interruption of MTX after the booster vaccination increases the antibody responses against the S1 RBD of the wildtype about two fold.17 In another study MTX patients showed no reduction in vaccine antibodies against the SARS-CoV-2 Wu01, as all 269 patients paused therapy for 2 weeks after booster with CoronaVac vaccine (Sinovac Biotech).18 The effect of continued MTX on neutralisation activity, especially regarding the Omicron variant, remains to be elucidated.
International and national authorities and commissions worldwide have recommended a fourth COVID-19 vaccination for immunocompromised patients.19–22 To date, there are no data to support this recommendation for MTX patients in the context of new variants. The aim of this work was to compare the neutralisation against Omicron BA.1 and BA.2 in patients with paused and continuous MTX therapy after COVID-19 booster vaccination with that of non-immunosuppressed individuals.
Methods
Study design and participants
This is the continuation of our recently published subanalysis of the VACCIMMUN study, which investigated the factors influencing the humoral immune response of a COVID-19 basic immunisation in MTX patients.15 Blood samples were collected under identical inclusion and exclusion criteria from MTX patients and NIP shortly before, 4 and 12 weeks after an mRNA booster vaccination in the period from July 2021 to March 2022. Samples from individuals who had a COVID-19 infection prior to one of the blood collections were excluded. The patients provided information regarding medical history including COVID-19 vaccination status and/or infection and immunosuppressive therapy directly.
MTX patients were asked at week 4 post booster vaccination whether MTX was paused and for how long. Instructions to continue or withhold MTX were not given in this study but observed as part of it.
Laboratory analyses
SARS-CoV-2 pseudovirus constructs
The nucleotide sequence of expression plasmids encoding all SARS-CoV-2 spike proteins was codon-optimised. The SARS-CoV-2 pseudovirus expressing the Wu01 spike protein (EPI_ISL_40671) was generated using expression plasmids that include a C-terminal deletion of 21 cytoplasmatic amino acids to achieve enhanced pseudovirus titers. Expression plasmids encoding the spike proteins of Omicron sublineage (BA.1 and BA.2) were generated by assembly and cloning of codon-optimised overlapping gene fragments (Thermo Fisher) into the pCDNA3.1/V5-HisTOPO vector (Thermo Fisher) using the NEBuilder Hifi DNA Assembly Kit (New England Biolabs). Expression plasmids for the Omicron sublineage included the following amino acid changes relative to Wu01:
Lineage B.1.1.529, sublineage BA.1: A67V, Δ69–70, T95I, G142D, Δ143–145, N211I, Δ212, ins215EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F.
Lineage B.1.1.529, sublineage BA.2: T19I, Δ24–26, A27S, A67V, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K (online supplemental file 1).
rmdopen-2022-002639supp001.pdf (313.9KB, pdf)
Sequences of all expression plasmids were verified by Sanger Sequencing.
SARS-CoV-2 pseudovirus neutralisation assays
SARS-CoV-2 pseudoviruses were generated by co-transfection of individual plasmids encoding HIV Tat, HIV Gag/Pol, HIV Rev, luciferase followed by an IRES and ZsGreen, and the SARS-CoV-2 spike protein (Wu01, BA.1 and BA.2) in adherent HEK 293 T cells using the FuGENE 6 Transfection Reagent (Promega). Cell culture supernatants containing pseudovirus particles were harvested 48–72 hour after transfection, centrifuged, filtered using a 0.45 µm filter, and stored at −80°C till use. Titration of the pseudoviruses was performed by infection of HEK293T cells expressing human ACE223 at 37°C and 5% CO2. After an incubation period of 48 hours, luciferase activity was determined by addition of luciferin/lysis buffer (10 mM MgCl2, 0.3 mM ATP, 0.5 mM Coenzyme A, 17 mM IGEPAL (all Sigma-Aldrich) and 1 mM D-Luciferin (GoldBio) in Tris-HCL) using a microplate reader (Berthold). For neutralisation assays, a virus dilution with a relative luminescence unit (RLU) of approximately 1000-fold in infected cells versus non-infected cells was selected.
Before usage, serum samples of study participants were inactivated at 56°C for 40 min. For determination of the serum neutralising activity, threefold serial dilutions of samples (starting dilution at 1:10) in cell culture medium were coincubated with pseudovirus supernatants for 1 hour at 37°C and 293T-ACE2 cells were added. After incubation for 48 hours at 37°C and 5% CO2, luciferase activity was determined using the luciferin/lysis buffer. The background RLUs of non-infected cells was subtracted and the 50% inhibitory serum dilution (ID50) that resulted in a 50% reduction of signal compared with the virus-infected untreated control was determined using a non-linear fit model to plot an agonist versus normalised dose response curve with variable slope using the least squares fitting method in GraphPad Prism V.7.0 (GraphPad). All serum samples were tested in duplicates.
Statistical analysis
Descriptive statistics included mean with SD, geometric mean with 95% CI and absolute and relative frequencies. The unpaired t-test with Welch’s correction was performed to compare continuously distributed variables and the binomial test for parts of a whole for binary data in table 1 and table 2.
Table 1.
Characteristics of patients and controls
| Characteristics | MTX n=50 | NIP n=44 | P value |
| Age, mean (SD) | 61.68 (12.4) | 61.9 (21.5) | 0.946 |
| Female, n (%) | 40 (80) | 29 (65.9) | 0.339 |
| BMI mean, (SD) | 25.7 (4.0) | 25.77 (4.5) | 0.927 |
| Third vaccination | |||
| BNT162b2, n (%) | 38 (76) | 44 (100) | 0.032 |
| mRNA-1273, n (%) | 12 (24) | 0 (0) | |
| Time between blood sampling and third vaccination | |||
| Days between first visit and booster (pre booster), mean (range) | 10 (0–30) | 7 (0–36) | 0.089 |
| Days between booster and second visit (week 4), mean (range) | 31 (25–53) | 27 (17–42) | 0.001 |
| Days between booster and third visit (week 12), mean (range) | 88 (76–129) | 97 (78–124) | 0.009 |
| Rheumatic diagnosis | |||
| Rheumatoid arthritis, n (%) | 36 (72) | / | |
| Psoriatic arthritis, n (%) | 7 (14) | / | |
| Other, n (%)* | 7 (14) | / | |
| Medication | |||
| MTX-mono, n (%) | 15 (30) | / | |
| MTX+prednisolone, n (%) | 12 (24) | / | |
| MTX+TNF-α-inhibitor, n (%) | 11 (22) | / | |
| MTX+TNF-α-inhibitor+prednisolone, n (%) | 6 (12) | / | |
| MTX+other, n (%)† | 6 (12) | / | |
| MTX-dose in mg/week, mean (SD) | 12.5 (4.2) | / | |
| Prednisolone in mg/day, mean (SD) | 3.8 (3.3) | / | |
| MTX regimen | |||
| MTX continued, n (%) | 24 (48) | / | |
| MTX pause, n (%) | 26 (52) | / | |
| Duration of hold (days), mean (SD) | 19.9 (7.7) | / | |
| Duration of hold before vaccine (days), mean (SD) | 7.6 (3.9) | / | |
| Duration of hold after vaccine (days), mean (SD) | 12.4 (7.1) | / | |
*Takayasu arteritis, 2 × axial spondylarthritis (ankylosing spondylitis), dermatomyositis/polymyositis, polymyalgia rheumatica, blistering dermatitis, systemic sclerosis.
†2x Leflunomide, IL-12/IL-23-Inhibitor, prednisolone/immunglobulins, hydroxychloroquine, IL-17-Inhibitor.
BMI, body mass index; MTX, methotrexate; NIP, non-immunosuppressed persons.
Table 2.
Characteristics of patients on continuous MTX and on MTX hold
| Characteristics | MTX n=24 | MTX pause n=26 | P value |
| Age, mean (SD) | 63.13 (13.9) | 60.35 (11.0) | 0.439 |
| Female, n (%) | 19 (79) | 21 (81) | 0.795 |
| BMI mean, (SD) | 26.1 (3.9) | 25.31 (4.1) | 0.489 |
| Rheumatic diagnosis | 0.855 | ||
| Rheumatic arthritis, n (%) | 17 (71) | 19 (73) | |
| Psoriatic arthritis, n (%) | 4 (17) | 3 (12) | |
| Other, n (%)* | 3 (12) | 4 (15) | |
| Medication | 0.441 | ||
| MTX-mono, n (%) | 7 (29) | 8 (31) | |
| MTX-prednisolone, n (%) | 7 (29) | 5 (19) | |
| MTX-TNF-α-inhibitor, n (%) | 5 (21) | 6 (23) | |
| MTX-TNF-α-inhibitor-prednisolone, n (%) | 4 (17) | 2 (8) | |
| MTX-other, n (%)† | 1 (4) | 5 (19) | |
| MTX-dose in mg/week, mean (SD) | 13.44 (4.2) | 11.63 (4.0) | 0.128 |
| Prednisolone in mg/d, mean (SD) | 4.50 (4.3) | 3.25 (1.8) | 0.423 |
| 3.Vaccination | 0.647 | ||
| BNT162b2, n (%) | 19 (79) | 19 (73) | |
| mRNA-1273, n (%) | 5 (21) | 7 (27) | |
| MTX regimen | |||
| MTX-dose in mg/week, mean (SD) | 13.44 (4.2) | 11.63 (4.0) | 0.128 |
| Duration of hold (days), mean (SD) | / | 19.9 (7.7) | |
| Duration of hold before vaccine (days), mean (SD) | / | 7.6 (3.9) | |
| Duration of hold after vaccine (days), mean (SD) | / | 12.4 (7.1) |
*MTX: Takayasu arteritis, blistering dermatitis, systemic sclerosis; MTX pause: 2 × axial spondylarthritis (ankylosing spondylitis), dermatomyositis/polymyositis, polymyalgia rheumatica.
†MTX: IL-12/IL-23-inhibitor; MTX pause: 2 × leflunomide, prednisolone/immunglobulins, hydroxychloroquine, IL-17-inhibitor.
BMI, body mass index; MTX, methotrexate.
Neutralising antibody levels were not normally distributed and therefore differences between defined groups (eg, MTX vs NIP or MTX pause vs non-pause) were analysed using the Mann-Whitney U test (MWUT).
By using generalised linear mixed regression analysis, we determined the association of time before vaccination (TBV) and time after vaccination (TAV) with the neutralising capacity. Non-parametric regression analysis was performed to model the association between immunisation status and days of MTX pausing. The non-parametric changepoint estimator proposed by Huh and Carriere24 was applied in order to estimate a meaningful cut-off for days of MTX pausing.
GraphPad Prism V.9.3.0 and STATA V.12.1 were used for all statistical analyses.
Patient and public involvement
There was no patient or public involvement in the designing of this study.
Results
Patient characteristics
Of 65 people on MTX therapy with blood samples taken 4 weeks after booster vaccination 15 had to be excluded due to unacceptable immunosuppressive comedication known to significantly decrease vaccination response, such as rituximab.9 One participant had to be excluded from the study 12 weeks after vaccination due to a COVID-19 infection.
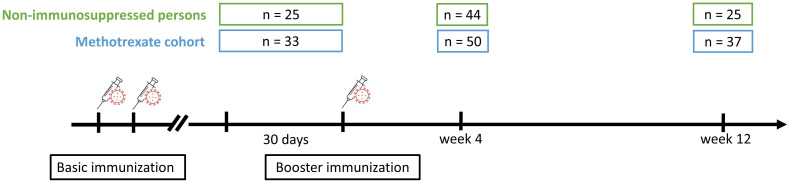
Neutralising serum activity of patients on MTX against SARS-CoV-2 variants Wuhan, Omicron BA.1, and BA.2 was compared with NIP before, 4 and 12 weeks after COVID-19 mRNA booster immunisation (figure 1).
Figure 1.
Observational study design around booster vaccination including number of participants at each visit.
There were no significant differences between the groups regarding age, gender and body mass index (table 1). However, both groups were significantly different regarding the vaccines administered, since 100% of NIP received the BNT162b2 vaccine while 24% of MTX patients were vaccinated with the mRNA-1273 vaccine. Detailed clinical characterisation of the MTX and NIP control cohort can be found in table 1.
Impaired SARS-CoV-2 neutralising activity in sera from MTX patients
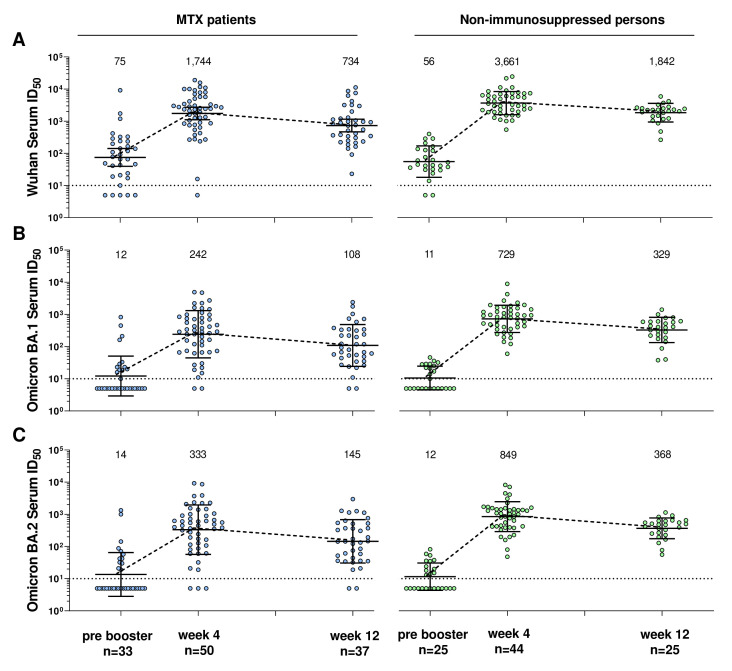
In the following, we compare serum neutralising capacity of MTX patients with NIP against different virus variants over the three visits. We discovered that the neutralising serum activity against the two SARS-CoV-2 variants Wu01 and Omicron (sublineages BA.1 and BA.2) was reduced in MTX patients compared with the control group (figure 2). Furthermore, the neutralising activity against the Omicron sublineages was significantly lower than against Wu01.
Figure 2.
Neutralising serum activity against SARS-CoV-2 Wu01 and Omicron variant (sublineages BA.1 and BA.2) in patients with methotrexate (left side) versus non-immunosuppressed persons (right side) before, 4 weeks and 12 weeks after mRNA-Booster vaccination. Fifty percent inhibitory serum dilutions (ID50s) were determined by pseudovirus neutralisation assays. Dot plots and numbers above the graph illustrate the geometric mean ID50 and error bars indicate the 95% CI. Dotted black lines display the lower limit of quantification (LLOQ) of the neutralisation assay (ID50 of 10). ID50s below the LLOQ (ID50=10) were assigned to half the LLOQ (ID50=5). MTX, methotrexate.
Impaired neutralisation activity against Wuhan variant in MTX patients
Serum neutralising activity against the Wu01 variant before booster vaccination was not significantly different between MTX patients and controls (p=0.424, MWUT, figure 2A).
While Wu01-neutralising serum titres in NIP increased 67-fold (to a geometric mean ID50 of 3,735) 4 weeks after vaccination, titres in MTX patients increased only 23-fold (to a geometric mean ID50 of 1,744). This resulted in a significantly lower neutralising serum activity against the Wu01 variant in the MTX patients 4 weeks after vaccination than in the controls (p=0.015, MWUT).
Serum ID50s of both groups decreased to about half from week 4 to week 12 (to a geometric mean ID50 of 734 for MTX patients and 1842 for NIP, respectively). At week 12, the Serum ID50s were also significantly lower for the MTX patients than for the NIPs (p=0.001, MWUT).
Impaired neutralisation activity against the Omicron BA.1 in MTX patients
Serum neutralising activity against Omicron BA.1 was not significantly different between MTX patients and controls before booster vaccination (p=0.657, MWUT, figure 2B).
While the geometric mean ID50s in NIP increased 68-fold 4 weeks after booster immunisation, the geometric mean ID50s in MTX patients increased only 20-fold. This resulted in a significantly lower serum neutralising activity against Omicron BA.1 in MTX patients 4 weeks after booster immunisation than in the controls (p<0.001, MWUT).
The geometric mean ID50s of both groups decreased to about half from week 4 to week 12. At week 12, serum neutralisation activity was also significantly lower in the MTX patients than in the NIP (p=0.001, MWUT).
Impaired neutralisation activity against the Omicron BA.2 in MTX patients
Serum neutralising activity against Omicron BA.2 was not significantly different between MTX patients and controls before booster vaccination (p=0.838, MWUT, figure 2C).
While the geometric mean ID50s in NIP increased 73-fold 4 weeks after booster immunisation, the geometric mean ID50s in MTX patients increased only 23-fold. This resulted in a significantly lower serum neutralising activity against Omicron BA.2 in the MTX patients 4 weeks after booster immunisation than in the controls (p<0.001, MWUT).
The geometric mean ID50s of both groups decreased to about half from week 4 to week 12. Also at week 12, BA.2 neutralising serum activity was significantly lower in the MTX patients than in the NIP (p=0.001, MWUT).
Comparison of neutralising activity of Wu01 against Omicron sublineages
The neutralising capacities against the BA.1 sublineage were on average lower by a factor of 6.75 (6.25, 7.20, 6.79) in the MTX cohort and by a factor of 5.23 (5.09, 5.02, 5.59) in the NIP compared with Wu01 across the three time points.
The neutralising capacities against the BA.2 sublineage were on average lower by a factor of 5.21 (5.36, 5.23, 5.06) in the MTX cohort and by a factor of 4.65 (4.66, 4.31, 5.00) in the NIP compared with Wu01 across the three time points.
Neutralising activity against Wu01 was significantly higher in both study groups compared with both Omicron sublineages at week 4 and week 12 (always p<0.001). However, there was no statistically significant difference between the Omicron sublineages BA.1 and BA.2 in both cohorts at any point in time (always p>0.05).
Impact of MTX discontinuation on neutralising capacities against SARS-CoV-2 variants
We and others have previously demonstrated that the humoral vaccination response after basic immunisation against COVID-19 can be improved by pausing MTX.15 16 Accordingly, we aimed to investigate whether this effect can also be observed 4 and/or 12 weeks after third vaccination and whether the patients who paused MTX achieved similarly high neutralising serum activity as untreated controls.
Of the 50 MTX patients whose neutralising activity against SARS-CoV-2 variants was examined 4 weeks after mRNA booster immunisation, 26 paused MTX and 24 did not. Detailed characteristics can be found in table 2.
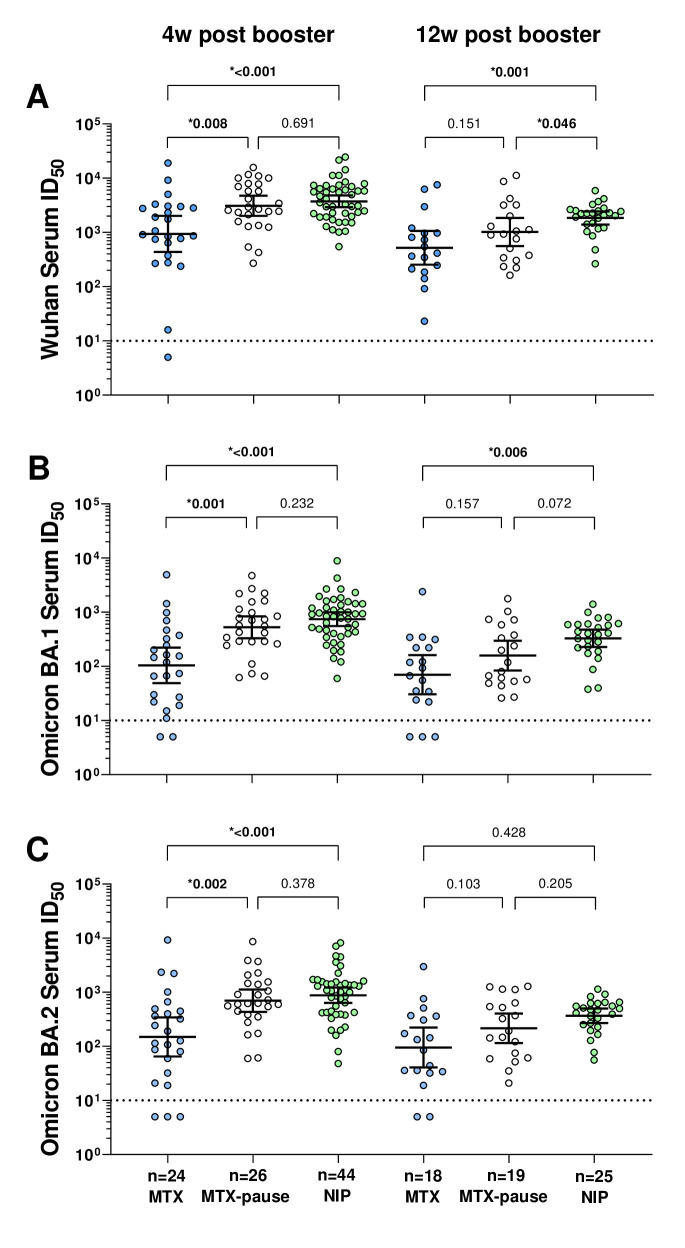
Serum neutralisation activity against the Wu01 variant at week 4 after booster vaccination (figure 3A) was significantly lower in patients continuously taking MTX than in patients pausing their medication (p=0.008, MWUT) or in NIP (p<0.001, MWUT). Patients on MTX pause achieved comparably high neutralising serum activity as controls. Interestingly, serum activity at week 12 was significantly lower in patients on MTX pause (p<0.001) and patients on continuous MTX (p=0.046) than in non-immunosuppressed controls.
Figure 3.
Serum neutralising activity against Wu01, Omicron BA.1 and Omicron BA.2 4 and 12 weeks after mRNA booster vaccination in patients with and without MTX pause and non-immunosuppressed persons. The p values shown are based on the Mann-Whitney U test, which was used to analyse the differences between the groups. Fifty percent inhibitory serum dilutions (ID50s) were determined by pseudovirus neutralisation assays. Dot plots and numbers above the graph illustrate the geometric mean ID50 and error bars indicate the 95% CIs. Dotted black lines display the lower limit of quantification of the neutralisation assay (ID50 of 10). ID50s below the LLOQ (ID50=10) were assigned to half the LLOQ (ID50=5). LLOQ, lower limit of quantification; MTX, methotrexate; NIP, non-immunosuppressed persons.
Neutralisation against Omicron BA.1 at week 4 after booster vaccination (figure 3B) was significantly lower in patients taking continuous MTX in the MWUT (p<0.001) than in patients pausing MTX and in non-immunosuppressed controls (p<0.001). Again, patients with MTX pause achieved similar levels of serum neutralisation activity compared with controls at week 4. At week 12, only neutralising activity against BA.1 was significantly lower in patients taking continuous MTX than in NIP (p=0.006, MWUT), while there was no statistically significant difference in patients pausing MTX (p=0.072, MWUT).
The neutralising activity against Omicron BA.2 (figure 3C) was also significantly lower at week 4 in patients taking continuous MTX in MWUT than in patients pausing MTX (p=0.002) and in non-immunosuppressed NIP (p<0.001). Again, at week 4, the neutralising activity of paused MTX patients was not significantly different from that of controls. At week 12, the neutralising activity against BA.2 was not significantly lower than in the non-immunosuppressed controls, neither in patients taking continuous MTX nor in patients with MTX pause.
Optimal duration of the MTX break
As this was an observational study, patients paused MTX variably before and after vaccination. Therefore, we had the opportunity to determine an optimal pause duration through statistical analyses. Twenty-six patients changed their MTX schedule which resulted in an interval longer than 7 days around the vaccination, which was considered pausing MTX.
The time between last MTX intake and vaccination was considered as TBV and the time between vaccination and reintake of MTX as TAV.
We further analysed which of these time periods is most likely to determine antibody response. By using generalised linear mixed regression analysis, we found TAV to be highly significant for adequate neutralising capacity, while TBV was not significant (table 3).
Table 3.
Generalised linear mixed regression analysis to determine the effect of MTX pause on neutralising capacity
| β | 95% CI | P value | |
| Wuhan | |||
| TBV | 112.32 | −430.95 to 206.31 | 0.490 |
| TAV | 228.78 | 3.24 to 454.32 | 0.047 |
| TBV+TAV | 91.45 | −2.97 to 185.87 | 0.058 |
| BA.1 | |||
| TBV | −24.90 | −100.14 to 50.33 | 0.516 |
| TAV | 53.44 | 0.19 to 106.69 | 0.049 |
| TBV+TAV | 21.92 | −0.31 to 44.15 | 0.053 |
| BA.2 | |||
| TBV | 140.03 | −310.16 to 30.10 | 0.107 |
| TAV | 178.62 | 58.53 to 298.72 | 0.004 |
| TBV+TAV | 50.52 | −0.64 to 101.67 | 0.053 |
MTX, methotrexate; TAV, time after vaccination; TBV, time before vaccination.
The determination of the optimal cut-off for the TAV was not possible via ROC (receiver operating characteristic) analysis, as there were only few patients with a neutralising capacity below the cut-off of 10 serum ID50. Non-parametric regression analysis was used to model the relationship between TAV, and neutralising capacity and a cut-off of 10 days was estimated as the optimum.
Other potential influencing factors on neutralising capacity
Contrary to previous findings,15 16 there was no correlation between age and neutralising capacity in the MTX cohort for any of the viral variants 4 and 12 weeks after booster vaccination (each p>0.05). The NIP showed a correlation between age and neutralising capacity only for Wuhan at week 4 (p=0.037) and for Wuhan and Omicron BA.1 at week 12 (p=0.018 and p=0.017).
In addition, MTX dose had no effect on neutralisation capacity in all MTX patients and in only the continuous MTX patients for any variant at any time point (each p>0.05).
Discussion
This is the first work demonstrating that MTX patients can also develop serum neutralisation activity against Omicron BA.1 and BA.2 after a COVID-19 mRNA booster immunisation. However, the extent depends largely on the pause of MTX. If patients continued to take MTX after vaccination, serum neutralisation activity was significantly reduced. In contrast, patients who paused MTX after booster vaccination exhibited neutralising capacities against all studied variants that were comparable to that of non-immunosuppressed individuals at week 4.
Before mRNA booster vaccination, MTX patients and controls had similar neutralising activity. The overall increase and differences of neutralising capacities against distinct virus variants after mRNA booster immunisation measured in our cohort were comparable to previous studies.4–6 The MTX patients exhibited a significantly lower increase than controls, resulting in markedly reduced neutralising serum activity against all variants. We further show that discontinuation of MTX reversed the drug-mediated attenuation of the vaccination response. These results reconfirm that MTX attenuates the humoral vaccination response and is consistent with observations made after COVID-19 immunisation15–17 and influenza vaccination.25
Although all patients on MTX pause had neutralising antibodies against all variants and at all time points, it should be noted that the levels at week 12 after the booster were slightly lower than in NIP. This effect was only weakly significant in Wu01. The lack of significance in the Omicron sublineages may be due to a combination of a small number of cases and a flatter increase in neutralising capacity against Omicron after booster vaccination.
Park et al taught us in influenza vaccination that the MTX pause after rather than before vaccination is important and that suspending MTX four times is no better for humoral vaccination success than omitting it twice.25 26 Recent randomised control trials have shown that the 2-week MTX pause improves the humoral immune response even after vaccination against COVID-19,16 17 but at the cost of an increased rate of disease flares.16 17 25 We have recently described that an MTX break of at least 10 days after basic COVID-19 immunisation is necessary to ensure vaccination success.15 In this present work, we again could not predefine a fixed MTX pause, which led to variable MTX intervals in our patients. This gave us the opportunity to calculate an optimal pause interval around the vaccination. As in our previous work,15 we found an MTX duration of at least 10 days necessary for optimal vaccination success, although this time we studied the third COVID-19 vaccination in an independent cohort with a different test system and different statistics. This finding is important and implies that a single MTX pause, which has not been studied so far, could be sufficient to reverse the MTX-mediated reduction in the humoral vaccination response. For example, if the last MTX dose is administered 1–3 days before vaccination and MTX is paused only once, then the next MTX application - assuming original weekday continuation - will take place 11–13 days after vaccination. If MTX can be paused only once instead of twice, a reduction of break-related disease flares is possible. Our considerations are highlighted by the fact that even Park et al recently started a trial investigating a 1 week MTX break after COVID-19 booster vaccination (NCT05313061).
This work has strengths and limitations. The strengths were a closely selected timeline for sample collection, a rigorous selection of MTX comedication,27 a follow-up over 12 weeks, similar age and sex distribution among MTX patients and controls, and use of virus neutralisation assays against Omicron BA.1 and BA.2, which is considered the gold standard for the determination of SARS-CoV-2 neutralising serum activity.23
Limitations include low number of cases, MTX pause recall bias in retrospective survey, and lack of systematic recording of disease activity and safety. Another weakness is that 24% of the MTX cohort were vaccinated with mRNA-1273, while the entire control group was vaccinated with BTN162b2. Our control group consisted of elderlies and healthcare workers who were vaccinated first in Germany and only with BNT162b2. However, percentage matching for both vaccines in both cohorts would have further increased the average neutralising capacity values in the controls, only making the differences larger and more significant compared with the MTX cohort. In addition, we did not investigate T-cell function in this cohort, which leaves questions regarding this important aspect of immunogenicity unanswered.
In summary, defining the aim of booster vaccinations as the induction of neutralising capacities against variants comparable to that of NIP, then there is a need for a fourth vaccination in patients that continued MTX during first booster vaccination. Further studies are needed to clarify whether a 1-week pause of MTX is not inferior to a 2-week pause.
Acknowledgments
We thank Veronika Scholz for her help in the digitalisation of the data. Also we thank Tanja Braun and Vera Höhne-Zimmer for their support in obtaining the ethics vote and for their organisational support.
Footnotes
Twitter: @BiesenRobert
EH and LG contributed equally.
Contributors: All authors contributed to the acquisition, analysis or interpretation of data and critical revision of the manuscript for important intellectual content. RB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RB is responsible for the overall content as the guarantor. LES, FNA, FK and RB were involved in the study design. Sample collection was done by EH and AtH. Experiments and data analysis were performed by LG, EA, EH and RB. EH, LG and RB were responsible for tables and figures. Data interpretation was done by all authors. Statistical analyses were done by EH, JK and RB. Writing of the manuscript were performed by EH, LG and RB. All authors were involved in critical proof reading of the manuscript.
Funding: This work was supported by unconditional donations from Medac, Galapagos and Freunde und Förderer der Berliner Charité e.V. This work was further supported by grants from COVIM: NaFoUniMedCovid19 (FKZ: 01KX2021) (to LES, FKl and FKu), the Federal Institute for Drugs and Medical Devices (V-2021.3 / 1503_68403 / 2021-2022) (to FKu and LES) and the German Center for Infection Research (DZIF) (to FKl and LG), and the Deutsche Forschungsgemeinschaft (DFG) (CRC1310 to FKl and SFB-TR84 to LES).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to the study are included in the article. Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was ethically approved by the Regional Office for Health and Social Affairs Berlin, Germany (21/0098-IV E 13). Participants gave informed consent to participate in the study before taking part.
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L. Coronavirus pandemic (COVID-19). Our world in data. Available: https://ourworldindata.org/coronavirus [Accessed 5 March 2020].
- 2.Scott L, Hsiao N-Y, Moyo S, et al. Track omicron's spread with molecular data. Science 2021;374:1454–5. 10.1126/science.abn4543 [DOI] [PubMed] [Google Scholar]
- 3.Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant - PubMed. Available: https://pubmed.ncbi.nlm.nih.gov/35042228/ [Accessed 23 May 2022]. [DOI] [PMC free article] [PubMed]
- 4.Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 omicron variant. Nat Med 2022;28:477–80. 10.1038/s41591-021-01676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muik A, Lui BG, Wallisch A-K, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022;375:678–80. 10.1126/science.abn7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022;386:1088–91. 10.1056/NEJMc2119912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med 2022;386:494–6. 10.1056/NEJMc2119270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022;386:1532–46. 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. 10.1136/annrheumdis-2021-221244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albach FN, Burmester GR, Biesen R. Successful BNT162b2 booster vaccinations in a patient with rheumatoid arthritis and initially negative antibody response. Ann Rheum Dis 2021;80:1361–2. 10.1136/annrheumdis-2021-220834 [DOI] [PubMed] [Google Scholar]
- 11.Bitoun S, Henry J, Desjardins D, et al. Rituximab impairs B cell response but not T cell response to COVID-19 vaccine in autoimmune diseases. Arthritis Rheumatol 2022;74:927–33. 10.1002/art.42058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane S. Methotrexate - Drug Usage Statistics, ClinCalc DrugStats Database.. Available: https://clincalc.com/DrugStats/Drugs/Methotrexate [Accessed 23 May 2022].
- 13.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of standard and Third-Dose SARS-CoV-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol 2022;74:1321–32. 10.1002/art.42153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis 2022;81:881–8. 10.1136/annrheumdis-2021-221876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-Week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis 2022;81:889–97. 10.1136/annrheumdis-2021-221916 [DOI] [PubMed] [Google Scholar]
- 17.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med 2022;10:840–50. 10.1016/S2213-2600(22)00186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aikawa NE. Kupa L de VK, Medeiros-Ribeiro AC, et al. increment of immunogenicity after third dose of a homologous inactivated SARS-CoV-2 vaccine in a large population of patients with autoimmune rheumatic diseases. Ann Rheum Dis 2022. [DOI] [PubMed] [Google Scholar]
- 19.Nhs England and NHS improvement North West » advice for those who are severely immunosuppressed around COVID-19 vaccine doses.. Available: https://www.england.nhs.uk/north-west/covid-19-vaccination-information/advice-for-those-who-are-severely-immunosuppressed-around-covid-19-vaccine-doses/ [Accessed 23 May 2022].
- 20.RKI - Infektionskrankheiten A-Z - STIKO-Empfehlung zur COVID-19-Impfung.. Available: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Impfempfehlung-Zusfassung.html;jsessionid=CCB27383ED733194F303D97328155211.internet082?nn=2386228 [Accessed 23 May 2022].
- 21.COVID-19 Vaccination CDC. Centers for disease control and prevention, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html [Accessed 23 May 2022]. [PubMed]
- 22.COVID-19: Joint statement from ECDC and EMA on the administration of a fourth dose of mRNA vaccines . European centre for disease prevention and control., 2022. Available: https://www.ecdc.europa.eu/en/news-events/ema-ecdc-statement-fourth-covid-vaccine-dose [Accessed 23 May 2022].
- 23.Crawford KHD, Eguia R, Dingens AS, et al. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses 2020;12:E513. 10.3390/v12050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh J, Carriere KC. Estimation of regression functions with a discontinuity in a derivative with local polynomial %ts.
- 25.Park JK, Lee YJ, Shin K. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77. 10.1136/annrheumdis-2018-213222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017;76:1559–65. 10.1136/annrheumdis-2017-211128 [DOI] [PubMed] [Google Scholar]
- 27.Medeiros-Ribeiro AC, Bonfiglioli KR, Domiciano DS, et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis 2022;81:710–9. 10.1136/annrheumdis-2021-221735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002639supp001.pdf (313.9KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to the study are included in the article. Data are available on reasonable request.