Abstract
Objectives
This meta-analysis investigated the use of fluvoxamine for the treatment of nonhospitalized patients with COVID-19.
Methods
PubMed, Web of Science, Ovid medline, Embase, Scopus, Cochrane Library databases, and ClinicalTrials.gov were searched for studies published before June 25, 2022. Only clinical studies that compared the efficacy and safety of fluvoxamine with other alternatives or placebos in the treatment of nonhospitalized patients with COVID-19 were included.
Results
Four studies with 1814 patients, of whom 912 received fluvoxamine, were included in this study. Compared with the control group receiving placebo or no therapy, the study group receiving fluvoxamine demonstrated a lower risk of hospitalization and emergency department (ED) visits (odds ratio [OR], 0.59; 95 % CI, 0.44–0.79; I2 = 26 %). In addition, the rate of hospitalization remained significantly lower in patients who received fluvoxamine than in the control group (OR, 0.69; 95 % CI, 0.51–0.94; I2 = 36 %). Although the study group demonstrated a lower risk of requirement of mechanical ventilation and intensive care unit admission, and mortality than the control group, these differences were nonsignificant. Finally, fluvoxamine use was associated with a similar risk of adverse events as that observed in the control group.
Conclusion
Fluvoxamine can be safely used in nonhospitalized patients with COVID-19 and can reduce the hospitalization rate or ED visits in these patients.
Keywords: COVID-19, Emergency department, Fluvoxamine, Hospitalization, SARS-CoV-2
Introduction
Since the end of 2019, when the first outbreak of COVID-19 caused by SARS-CoV-2 occurred in Wuhan, China, more than 545 million COVID-19 cases have been confirmed, with more than 6 million deaths as of July 1, 2021 [1], [2]. The clinical spectrum of COVID-19 ranges from asymptomatic infection to acute respiratory distress syndrome or critical illness [3], [4]. Although> 80 % of patients with COVID-19 present with asymptomatic or mild disease with favorable clinical outcome, some may progress to severe illness and require emergency department (ED) visits or hospitalization, particularly those with older age, obesity, cardiovascular diseases, chronic lung disease, diabetes, and immunocompromised status [3]. Therefore, prevention of disease progression, which contributes to ED visits or hospitalization, in patients with mild or moderate COVID-19 is a critical topic.
However, knowledge on effective agents for the treatment of nonhospitalized patients with mild to moderate COVID-19 is limited. Neutralizing monoclonal antibodies, which can interact with the surface spike glycoprotein of SARS-CoV-2, thereby preventing viral attachment and infectivity, show promise in lowering the incidence of COVID-19-related hospitalization and mortality and accelerating viral load decline [5], [6], [7], [8]. However, neutralizing monoclonal antibodies are costly and some of them were not effective in the management of omicron variant. Therefore, drug repurposing is necessary to discover readily available, safe, and inexpensive drugs that can help manage mild COVID-19.
A recent observational, multicenter, retrospective cohort study that enrolled 7230 adults hospitalized for COVID-19, in which 345 patients (4.8 %) received an antidepressant within 48 h of hospital admission, reported that antidepressant use may be associated with lower risk of mortality or intubation in patients hospitalized for COVID-19 [9]. Fluvoxamine is a selective serotonin reuptake inhibitor and a strong agonist for theσ-1 receptor, which helps control inflammation [10]. In addition to its anti-inflammatory effect, fluvoxamine may exhibit an antiviral effect and ameliorate cytokine response through various mechanisms, including by enhancing mast cell degranulation, interfering in endolysosomal viral trafficking, and increasing melatonin level [10]. Furthermore, fluvoxamine can inhibit acid sphingomyelinase activity, the formation of ceramide-enriched membrane domain, and attenuates SARS-CoV-2 cell entry [11], [12]. Moreover, fluvoxamine can act as a potent sigma-1 receptor agonist that may decrease SARS-CoV-2 replication and subsequent endoplasmic reticulum stress and inflammation [12], [13]. Thus, fluvoxamine was repurposed as a potential agent against SARS-CoV-2 infection [14]. Moreover, several clinical studies, including randomized controlled trials (RCTs), have demonstrated the benefit of fluvoxamine in the treatment of nonhospitalized patients with COVID-19 [15], [16], [17]. Therefore, we conducted this systematic review and meta-analysis of clinical studies to provide robust and up-to-date evidence of the clinical efficacy and safety of fluvoxamine for patients with COVID-19 treated as outpatients.
Methods
Search strategy
We searched the PubMed, Web of Science, Ovid medline, Embase, Scopus, Cochrane Library databases and ClinicalTrials.gov for relevant articles from their inception to June 25, 2022. The following search terms were used: “COVID-19,” “coronavirus infections,” “coronavirus,” “corona infection,” “SARS-CoV-2,” “fluvoxamine,” and “luvox.” This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [18]. The protocol of the systematic review and meta-analysis was registered at PROSPERO (CRD42021289764).
Inclusion and exclusion criteria
Only clinical studies that assessed the clinical efficacy of fluvoxamine in the treatment of nonhospitalized patients with COVID-19 were included. We also manually searched for additional eligible articles from the reference lists of relevant articles. Studies were included if they met the following criteria: (1) included nonhospitalized patients with confirmed COVID-19; (2) used fluvoxamine as intervention; (3) used placebo or alternative agents as comparator; (4) was an RCT or observational cohort study; and (5) reported of clinical efficacy as a study outcome.
The exclusion criteria were as following: (1) nonhuman studies, reviews, or meta-analyses; (2) studies without adequate data for outcome analysis; and (3) poster or conference abstracts.
Data extraction
Two authors (SHL and CMC) independently screened and identified articles to avoid bias. A third author (LCL) was consulted in cases of disagreement over the same publication and made the final decision. The following data were extracted separately by 2 authors (CCL and SPC) from each included study: year of publication, study design, fluvoxamine regimen, clinical outcomes, and risk of adverse events (AEs). If the extracted data were inconsistent, a third author (LCL) was consulted. The primary outcome was risk of hospitalization or ED visits. Secondary outcomes were requirement of mechanical ventilation (MV) and intensive care unit (ICU) admission, risk of mortality, and risk of AEs.
Statistical analysis
We used the RoB 2.0 [19] to assess the quality of included studies and risk of bias and Review Manager using random (version 5.3; Nordic Cochrane Centre, Copenhagen, Denmark) for statistical analysis. The degree of heterogeneity was evaluated using Q statistics generated from the χ2 test, and the I 2 measure was used to assess statistical heterogeneity. Heterogeneity was defined as significant when P < .10 or I 2> 50 %. A fixed-effects model was applied for homogeneous data, and a random-effects model was applied for heterogeneous data. We calculated pooled odds ratios (ORs) and 95 % CIs for analysis of outcomes of interests.
Results
Study selection
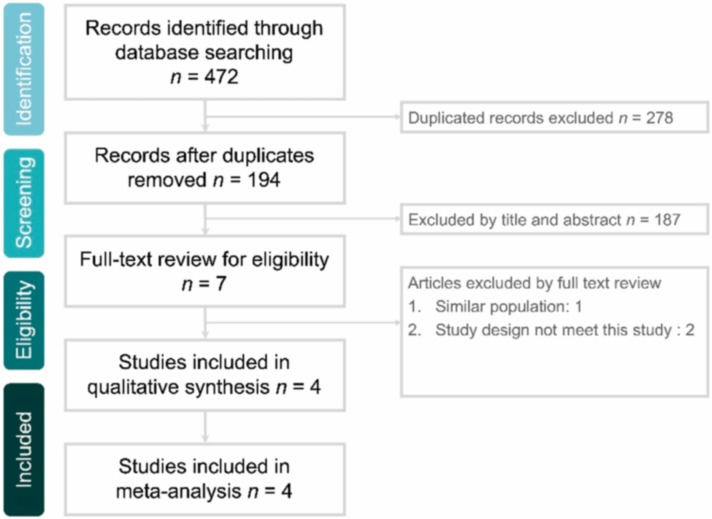
The online database search yielded 472 studies, of which 278 duplicate studies were excluded. In total, 187 studies were excluded if they were deemed irrelevant after their titles and abstracts were screened or if their full text was unavailable. The full texts of the remaining 7 articles were screened, with 3 being excluded. Finally, four studies [15], [16], [17], [20] were included in this meta-analysis ( Fig. 1 and Appendix 1).
Fig. 1.
Flow diagram of study selection.
Study characteristics
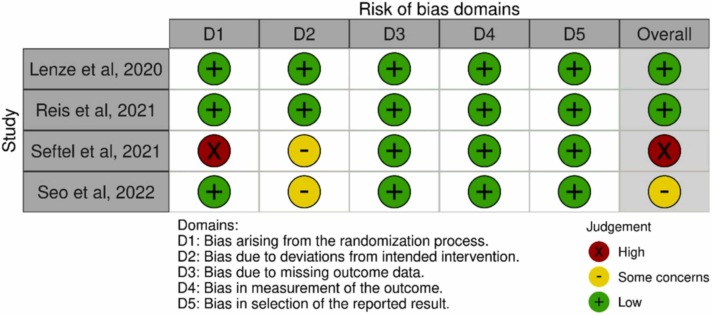
Three studies [15], [17], [20] were RCTs and one [16] was a prospective, nonrandomized cohort study, in which fluvoxamine was used at the patient’s discretion ( Table 1). Two [15], [16] studies were conducted in the United States, and each one in Brazil [17] and South Korea [20]. Although all studies included nonhospitalized patients with COVID-19, only one study [17] focused on patients at risk of progression to severe COVID-19. The fluvoxamine regimen differed between these studies. The three RCTs [15], [17], [20] used placebo as a comparator, whereas the prospective cohort study [16] used a non-fluvoxamine therapy as control. Overall, 1814 patients were included in these four studies, and 912 patients received fluvoxamine. The demographic characteristics of the included patients are summarized in Table 2. In the 2 RCTs, [15], [17] more than 50 % of the patients had obesity with a body mass index of ≥ 30 kg/m2. The included patients in two studies were unvaccinated, [15], [17], but the vaccine status was not applicable in two other studies [16], [20]. The distribution of race and ethnicity differed between the studies. Diabetes and hypertension were the 2 most common underlying diseases among the included patients (Table 2). For the risk of bias, two studies [16], [20] had a bias due to deviations from intended interventions, and one [16] has some concerns for multiple domains ( Fig. 2).
Table 1.
Characteristics of included studies.
| Study design | Study site | Study subjects | Vaccine status | Regimen of fluvoxamine | No of study patients |
||
|---|---|---|---|---|---|---|---|
| Study group | Control group | ||||||
| Lenze et al., 2020 | double-blind, placebo-controlled, randomized clinical trial | St Louis metropolitan area, US | Non-hospitalized adults with COVID-19 | Unvaccinated | 50- to 100-mg loading dose, then 100 mg thrice daily for 15 days | 80 | 72 |
| Reis et al., 2021 | placebo-controlled, randomized, adaptive platform trial | 11 clinical sites in Brazil | Non-hospitalized adults with COVID-19 and a known risk factor for progression to severe disease | Unvaccinated | 100 mg twice daily for 10 days | 741 | 756 |
| Seftel et al., 2021 | Prospective open-label cohort study | California, US | Non-hospitalized patients with COVID-19 | NA | 50- to 100-mg loading dose, then 50 mg twice daily for 14 days | 65 | 48 |
| Seo et al., 2022 | placebo-controlled, randomized trial | Seoul, Korea | Non-hospitalized adults with COVID-19 | NA | 100 mg twice daily for 10 days | 26 | 26 |
NA, not applicable.
Table 2.
Demographic characteristics of patients.
| Lenze et al., 2020 |
Reis et al., 2021 |
Seftel et al., 2021 |
Seo et al., 2022 |
|||||
|---|---|---|---|---|---|---|---|---|
| Study group | Control group | Study group | Control group | Study group | Control group | Study group | Control group | |
| Age | 46 (35–58) | 45 (36–54) | 50 (39–56) | 49 (38–56) | 44 ± 15 | 43 ± 15 | 54 (44–60) | 52 (42–60) |
| Age ≥ 50 years | NA | NA | 327 (44) | 328 (43) | 22 (33) | 17 (35) | NA | NA |
| Body-mass index ≥ 30 kg/m2 | 43 (54) | 42 (58) | 376 (51) | 375 (50) | NA | NA | NA | NA |
| Male | 24 (30) | 19 (26) | 332 (45) | 303 (40) | 50 (59) | 35 (41) | 18 (70) | 13 (50) |
| Race/Ethnicity | ||||||||
| White | 56 (70) | 50 (69) | 6 (1) | 6 (1) | 3 (5) | 13 (27) | NA | NA |
| Black or African American | 18 (23) | 20 (28) | 5 (1) | 5 (1) | 1 (2) | 0 (0) | NA | NA |
| Latino | NA | NA | NA | NA | 61 (94) | 34 (71) | NA | NA |
| Asia | 3 (4) | 1 (1) | NA | NA | 0 (0) | 1 (2) | NA | NA |
| Mixed race | NA | NA | 709 (96) | 719 (95) | NA | NA | NA | NA |
| Comorbidity | ||||||||
| Diabetes | 9 (11) | 8 (11) | 129 (17) | 114 (15) | 11 (17) | 4 (8) | 3 (12) | 1 (4) |
| Hypertension | 15 (19) | 15 (21) | 106 (14) | 88 (12) | 11 (17) | 17 (35) | 8 (31) | 6 (23) |
| Lung disease | 17 (21) | 9 (13) | 18 (2) | 19 (3) | 2 (3) | 1 (2) | 2 (8) | 0 (0) |
| Cardiac disease | NA | NA | 9 (1) | 7 (1) | NA | NA | 0 (0) | 0 (0) |
| Chronic kidney disease | NA | NA | 2 (< 1) | 2 (<1) | NA | NA | 0 (0) | 0 (0) |
NA, not applicable.
Fig. 2.
Summary of the risk of bias in each domain.
Primary outcome
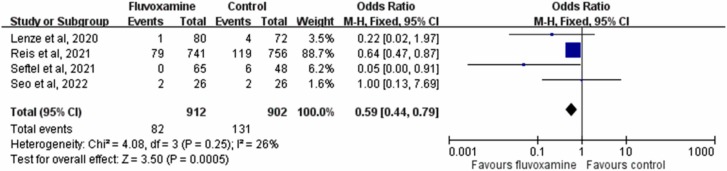
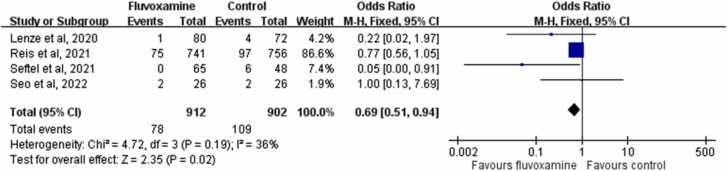
The rate of hospitalization or ED visits in patients who received fluvoxamine was only 8.8 % (82/912), which was much lower than that in controls who received placebo or no therapy (14.5 %, 131/912). A significant difference in the rate of hospitalization or ED visits was observed between patients who received fluvoxamine and those who received placebo (OR, 0.59; 95 % CI, 0.44–0.79; I 2 = 26 %, Fig. 3). This difference remained significant in the leave-one-out sensitivity test, in which individual studies were randomly excluded. When the results of the three RCTs were pooled [15], [17], [20], fluvoxamine was associated with a lower risk of hospitalization or ED visits than placebo (OR, 0.632; 95 % CI, 0.47–0.85; I 2 = 0 %). The rate of hospitalization remained significantly lower in patients who received fluvoxamine than in the control group in the pooled analysis of all included studies (OR, 0.69; 95 % CI, 0.51–0.94; I 2 = 36 %, Fig. 4).
Fig. 3.
Forest plot of the comparison of hospitalization or emergency department visit rates between fluvoxamine and control.
Fig. 4.
Forest plot of the comparison of COVID-19-related hospitalization rates between fluvoxamine and control.
Secondary outcomes
Regarding the mortality risk, 17 of 912 patients who received fluvoxamine died compared with 26 of 902 patients in the control group. The mortality rate was lower in the study group than in the control group, but the difference did not reach statistical significance (OR, 0.66; 95 % CI, 0.36–1.21; I 2 = 0 %). Although the rate of MV or ICU admission requirement was lower in the study group than in the control group, the difference was nonsignificant (MV use: 2.9 % vs. 4.2 %; OR, 0.70; 95 % CI, 0.43–1.16; I 2 = 0 %; ICU admission: 0 % vs. 2.5 %; OR, 0.20; 95 % CI, 0.02–1.77; I 2 = 0 %). All these results remained unchanged in the pooled analysis of the three RCTs [15], [17], [20].
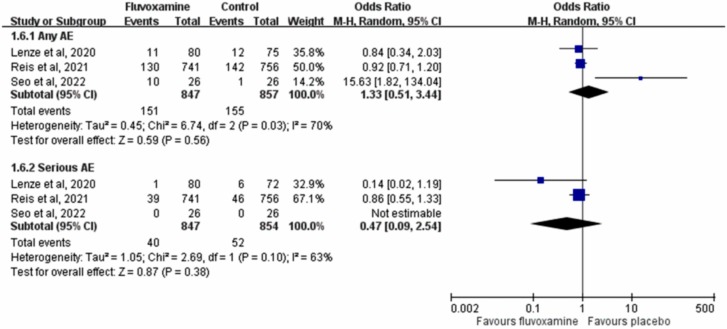
The pooled analysis of the 3 RCTs [15], [17], [20] revealed that the risks of any AE and serious AE were similar between fluvoxamine and placebo (any AE: OR, 1.33; 95 % CI, 0.51–3.44; I 2 = 70 %; serious AE: OR, 0.47; 95 % CI, 0.09–2.54; I 2 = 63 %, Fig. 5). Additionally, the risk of discontinuation of the study drug because of AE in patients receiving fluvoxamine was comparable with that in patients receiving placebo (OR, 2.60; 95 % CI, 0.45–15.11; I 2 = 65 %) in the pooled analysis of two RCTs [17], [20] with available data. In Seftel et al.’s study [16], no patient receiving fluvoxamine experienced serious AE or discontinued the study drug because of AE.
Fig. 5.
Forest plot of the comparison of the risk of adverse events (AEs) between fluvoxamine and control.
Discussion
In this meta-analysis, four studies [15], [16], [17], [20] including three RCTs were reviewed to compare the efficacy and safety of fluvoxamine in the treatment of nonhospitalized patients with COVID-19. Fist, we found that fluvoxamine can significantly reduce the risk of hospitalization or ED visits, which remained consistent in the leave-one-out sensitivity test and subgroup analysis of the three RCTs. In addition, fluvoxamine was associated with a significantly lower rate of COVID-19 related hospitalization than the comparators, Second, although we observed that those who used fluvoxamine had a requirement of MV and ICU admission, and mortality than those who received placebo or no therapy, these differences were nonsignificant; this may be attributable to the low number of events. Finally, we found no evidence that fluvoxamine was associated with a higher risk of AE compared with placebo or no therapy. Overall, based on the aforementioned findings, fluvoxamine appears to be an effective and safe agent to prevent hospitalization or ED visits in nonhospitalized patients with COVID-19. These findings were consistent with previous meta-analyses [21], [22] of only RCTs. However, the present study including both RCTs and observational studies. In addition, one of included RCTs was reported in 2022 and conducted in Asia [20]. Therefore, our findings are more updated and generalizable than previous meta-analyses [21], [22].
In addition to nonhospitalized patients, one recent open label, prospective cohort trial with matched controls reported that adding fluvoxamine to the standard therapy for patients with COVID-19 in the ICU can have a positive impact on patient survival (hazard ratio, 0.58, 95 % CI, 0.36–0.94, P = .027). [23] According to our findings on the efficacy and safety of fluvoxamine for nonhospitalized patients with COVID-19, fluvoxamine may be of use during the COVID-19 pandemic. Crucially, this treatment is readily available and inexpensive, in contrast to the newly developed neutralizing monoclonal antibodies [24] and promising antiviral agent molnupiravir [25].
This study has several limitations. First, the analyses of the risk of AE were based on analysis of data that exhibited high heterogeneity (I 2>50 %). The heterogeneity could be a result of the small case and event numbers Second, the number of studies included and total number of patients in three of four included studies [15], [16], [20] were limited. By contrast, the TOGETHER randomized, platform clinical trial [17] was much larger than all the other three studies combined, and therefore, the results of this trial likely strongly affected the outcome of the present meta-analysis. However, we used the leave-one-out sensitivity test to assess the effect of individual studies and the results remained consistent. Finally, three trials excluded fully vaccinated individuals, therefore any estimates of absolute effect size would likely be an overestimate in vaccinated patients. Further study is warranted to assess the effect of fluvoxamine on the outcome of vaccinated patients with COVID-19.
In conclusion, fluvoxamine use can help reduce the risk of hospitalization or ED visits for nonhospitalized COVID-19 patients. Furthermore, this drug was found to be safe for use in COVID-19 treatment. However, the present evidence is insufficient to support recommending fluvoxamine in the treatment of nonhospitalized patients with COVID-19.
Funding
This study received no funding.
CRediT authorship contribution statement
Conception: LCL, CMC, CCL; Study design: LCL, SPC, SHL, CCL; Analysis and interpretation: CMC, SPC, and CCL; Drafted or written: LCL, CMC, CCL; Substantial revision or critical review: CCL; All authors have agreed on the journal to which the article will be submitted and have reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. In addition, all authors agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised on the accuracy or integrity of the published work.
Competing interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Acknowledgments
None.
Ethical approval
Not required.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.10.010.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data sharing not applicable – no new data generated.
References
- 1.World Health Organization. https://covid19.who.int/ Accessed on July 1, 2021.
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 4.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan M., Nirula A., Azizad M., Mocherla B., Gootlieb R.L., Chen P., et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385(15):1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P., Nirula A., Heller B., Gootlieb R.L., Boscia J., Morris J., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Gonzalez-Rojas Y., Juarez E., Casal M.C., Moya J., Falci D.R., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 9.Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.S., Neuraz A., et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26(9):5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 10.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharm. 2021;12 doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornhuber J., Hoertel N., Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2022;27(1):307–314. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto Y., Suzuki T., Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27(4):1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller J.K., Riederer P., Müller W.E. Neuropsychiatric drugs against COVID-19: What is the clinical evidence? Pharmacopsychiatry. 2022;55(1):7–15. doi: 10.1055/a-1717-2381. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto Y., Suzuki T., Hashimoto K. Old drug fluvoxamine, new hope for COVID-19. Eur Arch Psychiatry Clin Neurosci. 2022;272(1):161–163. doi: 10.1007/s00406-021-01326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2):ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Seo H., Kim H., Bae S., Park S., Chung H., Sung H.S., et al. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022;54(1):102–113. doi: 10.3947/ic.2021.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C.M., Harari O., Chernecki C., Thorlund K., Forrest J.I. Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials. Am J Trop Med Hyg. 2022;106(5):1315–1320. doi: 10.4269/ajtmh.21-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T.C., Vigod S., Bortolussi-Courval É., Hanula R., Boulware D.R., Lenze E.J., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calusic M., Marcec R., Luksa L., Jurkovis I., Kovac N., Mihaljevic S., et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharm. 2022;88(5):2065–2073. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeks E.D. Casirivimab/Imdevimab: first approval. Drugs. 2021;81(17):2047–2055. doi: 10.1007/s40265-021-01620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahase E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50 % in patients at risk, MSD reports. BMJ. 2021;375:n2422. doi: 10.1136/bmj.n2422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data sharing not applicable – no new data generated.