Abstract
Background
The cardiopulmonary haemodynamic profile observed during exercise may identify patients with early-stage pulmonary vascular and primary cardiac diseases, and is used clinically to inform prognosis. However, a standardised approach to interpreting haemodynamic parameters is lacking.
Methods
We performed a systematic literature search according to PRISMA guidelines to identify parameters that may be diagnostic for an abnormal haemodynamic response to exercise and offer optimal prognostic and differential-diagnostic value. We performed random-effects meta-analyses of the normal values and report effect sizes as weighted mean±sd. Results of diagnostic and prognostic studies are reported descriptively.
Results
We identified 45 eligible studies with a total of 5598 subjects. The mean pulmonary arterial pressure (mPAP)/cardiac output (CO) slope, pulmonary arterial wedge pressure (PAWP)/CO slope and peak cardiac index (or CO) provided the most consistent prognostic haemodynamic parameters during exercise. The best cut-offs for survival and cardiovascular events were a mPAP/CO slope >3 Wood units (WU) and PAWP/CO slope >2 WU. A PAWP/CO slope cut-off >2 WU best differentiated pre- from post-capillary causes of PAP elevation during exercise. Upper limits of normal (defined as mean+2sd) for the mPAP/CO and PAWP/CO slopes were strongly age-dependent and ranged in 30–70-year-old healthy subjects from 1.6 to 3.3 WU and 0.6 to 1.8 WU, respectively.
Conclusion
An increased mPAP/CO slope during exercise is associated with impaired survival and an independent, prognostically relevant cut-off >3 WU has been validated. A PAWP/CO slope >2 WU may be suitable for the differentiation between pre- and post-capillary causes of PAP increase during exercise.
Short abstract
Elevated mPAP/CO slope is associated with impaired survival and an independent, prognostically relevant cut-off >3 WU has been validated. A PAWP/CO slope >2 WU may be suitable for identifying post-capillary causes of PAP increase during exercise. https://bit.ly/3tgVVMB
Introduction
Cardiopulmonary haemodynamic parameters during exercise have been investigated since the introduction of right heart catheterisation (RHC) into clinical practice [1]. The relevance of exercise haemodynamic parameters per se to diagnosing pulmonary circulatory disorders, which include a constellation of highly morbid pulmonary hypertension (PH) subtypes encountered commonly in cardiovascular medicine practice, was considered by the World Health Organization (WHO) meeting on cor pulmonale in 1960 [2] and at the first WHO Pulmonary Hypertension congress in 1973 [3]. The first expert consensus definition of exercise PH focused on mean pulmonary artery pressure (mPAP) >30 mmHg at peak physical activity [4]. This definition was also used by the National Institutes of Health (NIH) registry in the USA to collect data on patients diagnosed as having primary PH [5]. However, this approach did not consider the effect of age and workload on global haemodynamic response to exercise, and, therefore, did not distinguish normal from clinically relevant patient profiles. This has been pointed out in a systematic literature review that analysed the haemodynamic data of almost 1200 healthy subjects at rest and during exercise [1]. As a consequence, the term exercise PH has been abandoned from the haemodynamic definition of PH in the latest PH guidelines [6–8]. A standardised definition of exercise PH is still lacking, despite accumulating data indicating that exercise cardiopulmonary haemodynamic parameters offer a critical opportunity for timely PH diagnosis, optimised risk stratification and appropriate management strategies.
To address these issues, a European Respiratory Society (ERS) Task Force [9] and a consecutive Clinical Research Collaboration (PEX-NET) [10] have been assembled. In addition to this large collaborative effort, a significant number of studies have been initiated in the last 10 years by individual centres investigating exercise haemodynamic parameters in healthy subjects and various patient populations. Novel haemodynamic variables addressing the pressure–flow relationship during exercise, such as the mPAP/cardiac output (CO), pulmonary arterial wedge pressure (PAWP)/CO and trans-pulmonary gradient (TPG)/CO slopes have been introduced in order to appropriately define exercise PH [11]. Two potential haemodynamic definitions for exercise PH have been suggested [12–16], each acknowledging that pulmonary pressure is strongly dependent on changes in pulmonary blood flow provoked by exercise. According to the ERS Task Force, the preliminary definition of exercise PH is mPAP >30 mmHg and total pulmonary resistance (TPR) >3 Wood units (WU) at peak exercise [9, 15]; an alternative suggested definition is to use a threshold of an mPAP/CO slope >3 WU [13, 16]. With minor differences, both definitions imply that, in patients with exercise PH, mPAP increases steeply in relation to pulmonary blood flow during exercise.
Based on the above considerations, we had three aims in this systematic literature review and meta-analysis: 1) to assess the thresholds of normal exercise haemodynamic parameters based on RHC investigations in healthy individuals, focusing mainly on the pressure-flow relationship during exercise; 2) to assess the prognostic value of cardiopulmonary haemodynamic parameters during exercise; and 3) to assess the differential-diagnostic value of exercise haemodynamic parameters for the distinction between pre- and post-capillary causes of PAP increase.
Methods
To address these questions, we performed three independent systematic literature analyses: one for normal values (i.e. to diagnose an abnormal haemodynamic reaction to exercise), one for prognostic values and one for differential-diagnostic values of cardiopulmonary haemodynamic parameters during exercise. We searched for English-language, peer-reviewed original publications (we only included original manuscripts with original data) that assessed cardiopulmonary haemodynamic parameters during exercise by using RHC. Two independent researchers (K.Z. and G.K.) evaluated study eligibility and quality independently. The same researchers performed data extraction using standardised data collection sheets. Disagreements were resolved by consensus.
Of note, we included studies from 1945 for the prognostic and differential-diagnostic questions, because these questions have not been addressed systematically. For the normal values we included studies after 2003, because this question has been addressed in a previous systematic review, which included studies between 1947 and 2003 [1]. An additional reason was that more-recent studies used modern diagnostic tools to exclude any relevant comorbidities in the included healthy volunteers.
For normal values, studies were included if pulmonary haemodynamic parameters during exercise were assessed by RHC, with at least one valid measurement at rest as well as during exercise; if they provided at least mPAP and CO at rest and during exercise; and if they included at least one group of subjects that was described as being healthy. Here, we identified studies that included healthy volunteers (“healthy subjects”) and studies that included subjects presenting with mild to moderate dyspnoea on effort and undergoing RHC due to clinical reasons who were claimed to be healthy by the authors (“healthy patients”). These patients had normal resting haemodynamic parameters and a clinical work-up did not provide an explanation for their symptoms. In the main analysis, we only included the data from symptom-free healthy volunteers.
In the final analysis of normal values, haemodynamic parameters were estimated separately for studies in the supine and upright positions for both healthy subjects and healthy patients. Upper limits of normal (ULN) were calculated as mean+2sd. Slopes (mPAP/CO slope=(mPAPmax–mPAPrest)/(COmax–COrest); PAWP/CO slope=(PAWPmax–PAWPrest)/(COmax–COrest); TPG/CO slope=(TPGmax–TPGrest)/(COmax–COrest)) were only calculated when measurements at rest and during exercise were performed in the same body position. The meta-analysis was computed with a random-effects model, thus assuming a degree of between-study heterogeneity. We further conducted three separate moderator analyses: 1) we compared estimates of haemodynamic parameters at rest versus during exercise; 2) we compared estimates of haemodynamic parameters in healthy subjects versus healthy patients; and 3) we tested whether age had an effect on the haemodynamic parameters. Moderator analyses 1 and 2 were conducted with categorical moderator variables, while age was included as a continuous covariate in the meta-regression. Given the limited number of studies in each condition, we decided to follow a conservative strategy for the estimation of the parameters by applying the Knapp–Hartung correction [17–19] to the meta-analysis with and without moderators. This correction returns the meta-analytic findings with robust standard errors and broader confidence intervals, which in turn enable a more conservative interpretation of results. To illustrate how age influenced the mPAP/CO and PAWP/CO slopes, we provided age-adjusted estimates for the minimum, mean and maximum mean age values in the group of included studies under consideration. We calculated values for the mPAP/CO slope with the equation estimate= −0.2719+0.0386×age, R2=0.93, and for the PAWP/CO slope with estimate= −0.5805+0.0293×age, R2=1.00. R2 indicates the amount of heterogeneity accounted for. The results of prognostic and differential-diagnostic studies were summarised with descriptive statistics.
Detailed additional description of data sources, search strategy, study selection, data preparation and stratification of the data are available in the supplementary material.
Results
Normal pulmonary haemodynamic parameters and the diagnosis of abnormal haemodynamic response to exercise
We identified 11 studies that included 250 symptom-free volunteers in whom major comorbidities had been excluded with state-of-the-art methods. RHC was performed in six of 11 studies (119 subjects) in the supine position and in five of 11 studies (131 subjects) in the upright position. A detailed overview of the studies is provided in supplementary table S1a, b.
In the supine position, resting weighted mean values for mPAP, PAWP and pulmonary vascular resistance (PVR) were 13.5±2.0 mmHg, 8.6±0.6 mmHg and 1.0±0.2 WU, respectively (table 1). Of all reported parameters, only systolic systemic arterial pressure and PVR were significantly influenced by age at rest. The ULN resting PVR ranged between 1.3 and 1.8 WU among 30–70-year-old healthy subjects. Corresponding values in the upright position are also provided in table 1. During exercise, mPAP and PAWP increased significantly in both positions (p<0.001). PVR showed a slight but nonsignificant decrease (supine: p=0.114; upright: p=0.05).
TABLE 1.
Resting and exercise cardiopulmonary haemodynamic parameters in healthy subjects in the supine and upright position
| Condition | Parameter (unit) | k | Estimate |
| Supine | |||
| Rest | mPAP (mmHg) | 8 | 13.5±2.0¶ |
| Rest | PAWP (mmHg) | 6 | 8.6±0.6¶ |
| Rest | PVR (WU) | 6 | 1.0±0.2#,+ |
| Rest | CO (L·min−1) | 8 | 5.6±0.5¶ |
| Rest | CI (L·min−1·m−2) | 8 | 2.9±0.2¶ |
| Rest | RAP (mmHg) | 5 | 6.1±1.5 |
| Rest | HR (bpm) | 8 | 63±3¶ |
| Rest | dSAP (mmHg) | 5 | 74±6¶ |
| Rest | sSAP (mmHg) | 5 | 129±10#,¶ |
| Rest | TPR (WU) | 8 | 2.4±0.5¶ |
| Exercise | mPAP (mmHg) | 8 | 29.2±5.3# |
| Exercise | PAWP (mmHg) | 6 | 17.8±3.7# |
| Exercise | PVR (WU) | 6 | 0.8±0.2 |
| Exercise | CO (L·min−1) | 8 | 16.0±2.0# |
| Exercise | CI (L·min−1·m−2) | 8 | 8.4±1.0# |
| Exercise | RAP (mmHg) | 4 | 8.6±2.0# |
| Exercise | HR (bpm) | 8 | 131±13 |
| Exercise | dSAP (mmHg) | 5 | 88±5 |
| Exercise | sSAP (mmHg) | 5 | 178±13# |
| Exercise | TPR (WU) | 8 | 1.8±0.5# |
| mPAP/CO slope (WU) | 8 | 1.5±0.6# | |
| PAWP/CO slope (WU) | 6 | 0.9±0.5# | |
| TPG/CO slope (WU) | 6 | 0.8±0.2 | |
| Upright | |||
| Rest | mPAP (mmHg) | 4 | 17.3±0.6¶ |
| Rest | PAWP (mmHg) | 4 | 10.5±1.7#,¶ |
| Rest | PVR (WU) | 4 | 1.4±0.2 |
| Rest | CO (L·min−1) | 4 | 4.7±0.3¶ |
| Rest | CI (L·min−1·m−2) | 4 | 2.6±0.2¶ |
| Rest | RAP (mmHg) | 4 | 6.0±0.9 |
| Rest | HR (bpm) | 4 | 68±10¶ |
| Rest | dSAP (mmHg) | 3 | 80±2 |
| Rest | sSAP (mmHg) | 3 | 129±1¶ |
| Rest | TPR (WU) | 4 | 3.6±0.3¶ |
| Exercise | mPAP (mmHg) | 8 | 27.6±4.3 |
| Exercise | PAWP (mmHg) | 7 | 16.5±3.4 |
| Exercise | PVR (WU) | 7 | 0.9±0.2 |
| Exercise | CO (L·min−1) | 8 | 14.7±3.6 |
| Exercise | CI (L·min−1·m−2) | 8 | 7.9±1.8 |
| Exercise | RAP (mmHg) | 7 | 8.4±2.0 |
| Exercise | HR (bpm) | 8 | 140±22# |
| Exercise | dSAP (mmHg) | 3 | 80±1 |
| Exercise | sSAP (mmHg) | 3 | 169±5 |
| Exercise | TPR (WU) | 8 | 2.0±0.5# |
| mPAP/CO slope (mmHg) | 4 | 1.3±0.2 | |
| PAWP/CO slope (mmHg) | 4 | 0.7±0.2# | |
| TPG/CO slope (mmHg) | 4 | 0.6±0.1 |
Data are presented as weighted mean±sd. Slopes were only calculated when rest and exercise measurements were performed in the same position; therefore, two studies were excluded for the calculation in the upright position (supine [20–25]; upright [26–30]). A more detailed description of all identified studies is provided in supplementary table S1a. mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; WU: Wood units; CO: cardiac output; CI: cardiac index; RAP: right atrial pressure; HR: heart rate; dSAP: diastolic systemic arterial pressure; sSAP: systolic systemic arterial pressure; TPR: total pulmonary resistance; TPG: trans-pulmonary gradient; ULN: upper limit of normal. #: significant influence of age (or age-dependency) (p<0.05); ¶: significant difference between rest and exercise conditions (p<0.05); +: ULN of PVR at rest in the supine position are 0.7±0.3 WU (ULN 1.3 WU) for ∼30-year-old subjects, 1.0±0.2 WU (ULN 1.3 WU) for ∼50-year-old subjects and 1.3±0.3 WU (ULN 1.8 WU) for ∼70-year-old subjects.
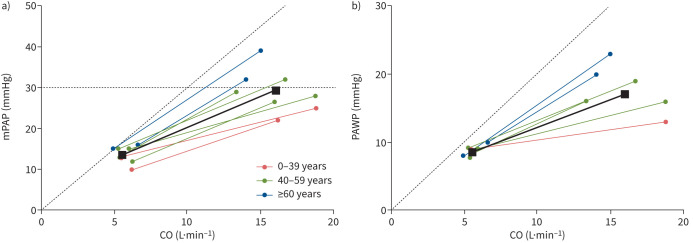
During supine exercise, older age was associated with a higher systolic systemic arterial pressure, mPAP, PAWP, TPR and right atrial pressure as well as with a higher mPAP/CO slope and PAWP/CO slope (table 1 and figure 1). The mPAP/CO slope was 0.8±0.4 WU (ULN 1.6 WU) in subjects aged ∼30 years (reflecting the minimum of reported mean age across the included studies), 1.6±0.2 WU (ULN 2.1 WU) in subjects aged ∼50 years (reflecting the mean of reported mean age across the included studies) and 2.4±0.5 WU (ULN 3.3 WU) in subjects aged ∼70 years (reflecting the maximum of reported mean age across the included studies). The PAWP/CO slope ranged from 0.3±0.2 WU (ULN 0.6 WU) in ∼30-year-old subjects to 1.4±0.2 WU (ULN 1.8 WU) in ∼70-year-old subjects (table 2). The TPG/CO slope was 0.8±0.2 WU (ULN 1.2 WU) and was not significantly affected by age. In the upright position, the influence of age on haemodynamic parameters was less pronounced (table 1). Multipoint mPAP/CO measurements during exercise were only available from a small number of studies (n=4) and were therefore not further analysed.
FIGURE 1.
a) Mean pulmonary arterial pressure (mPAP)/cardiac output (CO) slope (Wood units (WU) and b) pulmonary arterial wedge pressure (PAWP)/CO slope (WU) by age group in the supine position. Each line represents an individual study group or a subgroup according to stratification to age in one study (see figure 2 for details). Older subjects (blue line) had a steeper mPAP/CO and PAWP/CO slope and tended to have higher mPAP at rest. During exercise, older subjects reach higher mPAP and PAWP at lower CO values as than younger individuals. The solid black lines show the age-adjusted mean slopes (estimated by mean age across the included studies). Exercise values in healthy subjects did not exceed mPAP >30 mmHg in combination with exercise total pulmonary resistance >3 WU (dashed line in figure 1a).
TABLE 2.
Effect of age on exercise cardiopulmonary haemodynamic parameters and ULN for mPAP/CO slope and PAWP/CO slope in healthy subjects in the supine position
| Slope | Age (years) | Groups included (n) | Predicted value# (WU) | sd (WU) | ULN (WU) |
| mPAP/CO slope | 29 | 8 | 0.8 (0.5–1.27) | 0.4 | 1.6 |
| 39 | 8 | 1.2 (1.0–1.5) | 0.3 | 1.7 | |
| 49 | 8 | 1.6 (1.4–1.8) | 0.2 | 2.1 | |
| 59 | 8 | 2.0 (1.7–2.3) | 0.3 | 2.7 | |
| 69 | 8 | 2.4 (2.0–2.8) | 0.5 | 3.3 | |
| PAWP/CO slope | 29 | 6 | 0.3 (0.1–0.4) | 0.2 | 0.6 |
| 39 | 6 | 0.6 (0.4–0.7) | 0.1 | 0.8 | |
| 49 | 6 | 0.8 (0.7–1.0) | 0.1 | 1.0 | |
| 59 | 6 | 1.1 (1.0–1.3) | 0.1 | 1.4 | |
| 69 | 6 | 1.4 (1.2–1.7) | 0.2 | 1.8 |
ULN: upper limit of normal; mPAP: mean pulmonary arterial pressure; CO: cardiac output; PAWP: pulmonary arterial wedge pressure. #: mean (95% confidence interval).
Patients with mild to moderate dyspnoea on exercise
In addition to the described 11 studies of healthy individuals, we identified nine studies with 303 subjects (194 subjects from six studies in the supine position and 109 subjects from three studies in the upright position) presenting with mild to moderate dyspnoea on exercise who were classed as “healthy patients” by the authors. This was based on normal resting haemodynamic parameters and the fact that clinical work-up had excluded obvious cardiovascular factors as an explanation of symptoms. When these subjects were compared to our healthy symptom-free volunteers, there were only slight haemodynamic differences at rest and exercise, and no significant differences in the mPAP/CO slope (healthy patients 1.7±0.8 WU versus healthy subjects 1.6±0.6 WU), PAWP/CO slope (healthy patients 0.8±0.4 WU versus healthy subjects 0.9±0.5 WU) and TPG/CO slope (healthy patients 0.8±0.3 versus healthy subjects 0.8±0.2 WU) either in the supine (data provided) or in the upright position. Of note, similar to healthy subjects, the mPAP/CO and PAWP/CO slopes of healthy patients were age-dependent in the supine position (supplementary tables S1b and S2).
Prognostic relevance of pulmonary haemodynamic parameters during exercise
We identified 18 studies with 3981 patients focusing on the prognostic relevance of cardiopulmonary haemodynamic parameters during exercise as assessed by RHC. In most of these studies, prognostic end-points were all-cause mortality alone or combined with heart failure-related hospitalisation. The studies were heterogeneous in size, with the number of subjects ranging from 27 to 1772 (median 71). Most studies (n=8) investigated patients with left heart disease (heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF), valvular heart disease or coronary artery disease) or pre-capillary PH (n=5). The remaining studies included patients with unexplained dyspnoea (n=2), chronic obstructive pulmonary disease (COPD) (n=2) and systemic sclerosis (n=1).
The following cardiopulmonary exercise parameters were most frequently reported to be significantly associated with prognosis in the identified studies: mPAP/CO slope, PAWP/CO slope, peak cardiac index (or CO), peak PVR, peak PAWP and the change in cardiac index, systolic pulmonary arterial pressure (sPAP) and heart rate from rest to peak exercise (table 3).
TABLE 3.
Overview of the identified studies for prognostic value and their main characteristics based on their underlying condition
| Publication | Subjects (n) | Age (years) # | Sex (M:F) | Main inclusion criteria | End-point | Exercise parameters predicting events |
| Exercise dyspnoea | ||||||
| Ho et al. 2020 [31] | 714 | 57±16 | 292:422 | Exercise dyspnoea; LVEF ≥50% |
All-cause mortality, HF-related hospitalisation |
mPAP/CO slope >3 WU, elevated TPG/CO slope and PAWP/CO slope |
| Eisman et al. 2018 [32] | 175 | 57±17 | 65:110 | Exercise dyspnoea; LVEF >50%, PAWP <15 mmHg |
HF-related hospitalisation, HF-related mortality, elevation of resting PAWP in follow-up RHC >15 mmHg |
PAWP/CO slope >2 WU |
| Dorfs et al. 2014 [33] | 355 | 61±11 | 120:235 | Exercise dyspnoea and suspected HFpEF | All-cause mortality | Steep PAWP increase (>25.5 mmHg·W−1·kg−1)¶ |
| Left heart disease | ||||||
| Dobarro et al. 2020 [34] | 33 | 74±8 | 30:3 | Moderate to severe aortic stenosis, <85 years | All-cause mortality, surgical aortic valve replacement, TAVI or planned intervention for AST | PaO2 at peak exercise |
| Huang et al. 2018 [35] | 104 | 61±12 | 39:65 | HFpEF (normal LVEF, no valvular heart disease) | All-cause mortality, HF-related hospitalisation |
PVR >1 WU at peak exercise |
| Rieth et al. 2017 [36] | 167 | 65±12 | 125:42 | HFrEF (LVEF ≤45%) | All-cause mortality, LuTX and/or HTX, heart assist device | Change in CO <1.154 L·min−1 and change in sPAP <17.5 mmHg |
| Lewis et al. 2011 [12] | 60 | 60±12 | 47:13 | HFrEF (LVEF <40%, NYHA II–IV) | All-cause mortality | mPAP/W slope >median (0.25 mmHg·W−1), steep increase in mPAP followed by a plateau pattern |
| Griffin et al. 1991 [37] | 49 | 63±11 | 39:10 | Congestive HF (symptoms >1 year) | HF-related mortality | PAWP at rest and exercise, peak stroke work index |
| Szlachicic et al. 1985 [38] | 27 | 56 | 27:0 | Congestive HF (clinically stable) | All-cause mortality | Peak CI |
| Gohlke et al. 1983 [39] | 1772 | 50±6 | 1595:177 | Coronary artery disease and normal or mildly impaired left ventricular function | All-cause mortality | Peak CO |
| Pulmonary arterial hypertension | ||||||
| Faure et al. 2020 [40] | 49 | 53±16 | 16:33 | PAH | All-cause mortality | Change in HR and sPAP |
| Tang et al. 2018 [41] | 140 | 33±11 | 39:101 | IPAH | LuTX and/or HTX, HF-related mortality |
Change in HR, peak work rate, PVR and CI |
| Hasler et al. 2016 [42] | 70 | 65 (50–73) | 27:43 | PAH+CTEPH | All-cause mortality, LuTX and/or HTX | Maximal workload, peak and change in CI and mPAP/CO |
| Chaouat et al. 2014 [43] | 55 | 54±16 | 25:30 | IPAH, heritable or anorexigen-associated PAH | All-cause mortality, LuTX and/or HTX | Peak CI, change in sPAP, change in CI |
| Blumberg et al. 2013 [44] | 36 | 54±15 | 15:21 | PAH+CTEPH (NYHA II–III) |
All-cause mortality, LuTX and/or HTX | mPAP/CO slope, peak CI¶ |
| Systemic sclerosis | ||||||
| Stamm et al. 2016 [45] | 72 | Range: 42–74 | 10:62 | SSc with exercise dyspnoea±reduced DLCO or FVC/DLCO >1.6 | All-cause mortality, LuTX and/or HTX | Peak mPAP, mPAP increase, mPAP/W increase¶ |
| COPD | ||||||
| Olsen et al. 1989 [46] | 29 | 64±5 | 29:0 | Lung resection due to airflow obstruction and lung mass | Postoperative death within 60 days or prolonged ventilation (>30 days) | Peak CI |
| Finlay et al. 1983 [47] | 74 | 59 | 60:14 | Clinically stable COPD, symptoms >3 years | All-cause mortality | Increase in mPAP+PVR during exercise |
Exercise protocol was ergometry for all studies. M: male; F: female; LVEF: left ventricular ejection fraction; HF: heart failure; mPAP: mean pulmonary arterial pressure; CO: cardiac output; TPG: trans-pulmonary pressure gradient; PAWP: pulmonary arterial wedge pressure; RHC: right heart catheterisation; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; W: Watts; TAVI: transcatheter aortic valve implantation; AST: aortic stenosis; PaO2: partial pressure of oxygen; PVR: pulmonary vascular resistance; LuTX: lung transplantation; HTX: heart transplantation; sPAP: systolic pulmonary arterial pressure; NYHA: New York Heart Association; CI: cardiac index; PAH: pulmonary arterial hypertension; IPAH: idiopathic pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; SSc; systemic sclerosis; DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity. #: data presented as mean±sd or mean (interquartile range), unless otherwise specified; ¶: only exercise and not resting pulmonary haemodynamic parameters predicted the end-point.
The mPAP/CO slope, a haemodynamic parameter that was suggested as a key parameter for the diagnosis of exercise PH [16], presented as a general prognostic marker across different conditions and was independently associated with survival in patients with exercise dyspnoea, pre-capillary PH, left heart disease and systemic sclerosis. In patients with exercise dyspnoea, the cut-off for increased mortality was 3 WU [31] and in systemic sclerosis it was 3.5 WU [48]. An elevated PAWP/CO slope was strongly associated with prognosis in subjects with exercise dyspnoea [31] and the best cut-off >2 WU was found in subjects with suspected or overt left heart disease [32]. Peak cardiac index (and CO) was also strongly associated with prognosis in several cohorts of patients with pre-capillary PH and left heart disease (table 3).
Recognition of left heart or pulmonary vascular disease based on exercise haemodynamic profiles
A total of 16 studies with 1367 patients investigated the cause of pulmonary pressure increase during exercise, mainly with the aims of recognising left heart or pulmonary vascular disease (PVD) and distinguishing between pre- and post-capillary causes of dyspnoea in patients with normal resting PAWP.
Haemodynamic parameters that identified left heart disease as the cause of dyspnoea or exercise limitation included peak PAWP with predefined cut-offs by the authors at 20 mmHg or 25 mmHg, and the PAWP/CO slope with a cut-off >2 WU. In contrast, an elevated TPG/CO slope or peak PVR may be suggestive for PVD in patients with exercise dyspnoea and systemic sclerosis (table 4).
TABLE 4.
Overview of the identified studies for diagnostic and differential-diagnostic value and their most relevant findings
| Publication | Subjects (n) | Age (years) # | Sex (M:F) | Patient cohort | Most relevant finding |
| Recognising LHD | |||||
| Goda et al. 2019 [50] | 71 | 67±11 | 15:56 | CTEPH | Patients with peak PAWP >20 mmHg (predefined) had larger left atrial volume index (40 versus 34 mL·m−2) than patients with peak PAWP ≤20 mmHg, suggesting LHD |
| Eisman et al. 2018 [32] | 175 | 57±17 | 65:110 | HFpEF+Dyspnoea+Controls | The ULN for PAWP/CO slope was 2 WU in controls; a ULN >2 was characteristic of HFpEF, related to lower exercise capacity, and may also identify HFpEF in patients with normal PAWP at rest |
| Maor et al. 2015¶ [51] | 63 | 60±20 | 18:45 | Dyspnoea | Patients with resting PAWP 12–15 mmHg were 4.5 times more likely to present with a steep PAWP increase during exercise as compared to patients with resting PAWP <12 mmHg |
| Andersen et al. 2015 [52] | 26 | 70±9 | 9:15 | HFpEF+Controls | 94% of patients with left ventricular diastolic dysfunction on echocardiography but 0% of controls had peak PAWP >25 mmHg during exercise A steep PAWP increase may uncover LHD |
| van Empel et al. 2014 [53] | 28 | 62±1 | – | HFpEF+Controls | HFpEF patients had higher PAWP at peak exercise than controls (32 versus 16 mmHg) |
| Borlaug et al. 2010 [54] | 55 | 56±15 | 17:38 | Dyspnoea | Exercise PAWP was used to classify patients with resting PAWP <15 mmHg as having HFpEF (PAWP at exercise ≥25 mmHg) or non-cardiac dyspnoea (PAWP at exercise <25 mmHg) PAWP and sPAP were strongly correlated during exercise |
| Yoshida et al. 1985 [55] | 40 | Range 26–71 | 38:2 | Coronary artery disease+Controls | dPAP/CO slope is steeper in patients with coronary artery disease and angina than in those without angina or in controls |
| Recognising PVD | |||||
| Nagel et al. 2019 [56] | 112 | 58±13 | 24:88 | SSc | SSc patients with resting mPAP 21–24 mmHg had higher peak PVR (2.7 versus 1.8 WU) and lower 6-min walking distance and peak cardiac index than patients with resting mPAP ≤20 mmHg, which may indicate early PVD |
| Gorter et al. 2018 [57] | 161 | 67±11 | 59:102 | HFpEF | Among HFpEF patients (resting PAWP ≥15 mmHg), combined post- and pre-capillary pH was associated with higher peak PVR (4.5 versus 1.9 WU) and lower peak pulmonary arterial compliance (1.4 versus 2.3 mL·mmHg−1) as compared to isolated post-capillary PH, suggesting the presence of PVD |
| Claessen et al. 2015 [20] | 36 | 62±12 | 27:9 | CTEPH+Controls | mPAP/CO slope was steeper in CTEPH patients after pulmonary endarterectomy than in controls and similar to those with unoperated CTEPH, suggesting the presence of residual PVD |
| Taylor et al. 2015 [58] | 39 | 57±9 | 32:7 | HF | At a given CO (∼4.5 L·min−1) during exercise, mPAP was greater in patients with HF and combined pre- and post-capillary PH, than in patients without PH and, to a lesser extent, than in patients with isolated post-capillary PH (∼55 versus ∼32 versus ∼45 mmHg, respectively) |
| Tolle et al. 2008 [59] | 109 | 55±15 | 40:69 | PAH+Controls | Exercise patterns differ between PAH patients and controls PAH presents with a strong initial increase of mPAP followed by a plateau, whereas a continuous moderate mPAP increase was characteristic in controls |
| Recognising LHD and PVD | |||||
| Bentley et al. 2020¶ [60] | 121 | 55 (range 50–60) | 61:60 | Dyspnoea+Controls | Pulse pressure/PAWP slope >2.5 (ULN in controls) uncovers a subgroup among subjects with a normal mPAP/CO slope (ULN in controls 3.2 WU) that is suggestive of an exaggerated pulmonary vascular to PAWP response and might indicate an abnormal PAP response, which is not driven by LHD ULN of the PAWP/CO slope in controls was 2.0 WU |
| Keusch et al. 2014¶ [61] | 101 | 61 (range 52–68) | 31:70 | Dyspnoea | Out of patients with exercise dyspnoea and resting PAP 20–24 mmHg, about the same number had either a steep PAWP or PVR increase, suggesting either post- or pre-capillary cause of mPAP elevation during exercise |
| Hager et al. 2013¶,+ [62] | 173 | 53±13 | 20:153 | SSc+Controls | Exercise may distinguish between pre-capillary (i.e. PVD, characterised by an increase in TPG and PVR during exercise) and post-capillary (i.e. mainly HFpEF, characterised by a steep PAWP/CO slope and no significant change in TPG during exercise) cause of exercise PH in SSc |
| Saggar et al. 2010¶ [63] | 57 | 50±13 | 12:45 | SSc | According to predefined criteria by the authors, SSc patients may reveal pre- or post-capillary causes of exercise PH The main characteristics of post-capillary exercise PH may be the relevant increase of PAWP at peak exercise, while the main characteristics of pre-capillary exercise PH may be an increased PVR and TPG at peak exercise |
M: male; F: female; LHD: left heart disease; CTEPH: chronic thromboembolic pulmonary hypertension; PAWP: pulmonary arterial wedge pressure; HFpEF: heart failure with preserved ejection fraction; CO: cardiac output; WU: Wood unit; ULN: upper limit of normal; sPAP: systolic pulmonary arterial pressure; dPAP: diastolic pulmonary arterial pressure; PVD: pulmonary vascular disease; SSc: systemic sclerosis; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; PH: pulmonary hypertension; PAH: pulmonary arterial hypertension; PAP; pulmonary arterial pressure; TPG: trans-pulmonary gradient. #: data presented as mean±sd or mean (interquartile range), unless otherwise specified; ¶: these studies provide data both for the recognition of LHD and PVD based on parameters of exercise haemodynamic parameters; +: in these studies the exercise protocol was arm lifting with weights, while in all other studies patients performed cycle-ergometry.
Discussion
Normal pulmonary haemodynamic parameters and the diagnosis of abnormal haemodynamic response to exercise
Cardiopulmonary haemodynamic parameters at rest and during exercise
The weighted means of resting haemodynamic variables (table 1) corresponded well to previously described normal values from systematic literature analyses and meta-analyses [1, 49, 64]. Of note, based on the provided values (mean±sd), it is likely that some individuals in the included studies had mPAP >20 mmHg, which is considered to be abnormal. In line with previous studies [1, 49], mPAP and PAWP increased significantly during exercise in both the supine and upright positions while PVR showed a trend for a moderate decrease during exercise. The mPAP/CO slope emerged as a simple and consistent variable characterising pulmonary haemodynamic changes during exercise.
We did not perform a direct comparison of data derived from the supine and upright position owing to the limited number of comparable studies and because the data in different positions were not available from the same subjects and the same studies. The effect of posture on cardiopulmonary haemodynamic parameters during exercise has been described previously [1, 49] considering only studies that tested the same subjects in both positions.
Of note, in this study, the variability of resting haemodynamic parameters (i.e. standard deviations) was smaller than in previous systematic reviews. This may be explained by the relative homogeneity of the subjects included in this analysis and the applied methodology (healthy volunteers without dyspnoea, relevant cardiopulmonary comorbidities excluded by modern diagnostic methods, more homogenous zero levels) as compared to previous systematic reviews.
The present review is solely based on studies providing haemodynamic data based on RHC. This decision was made to ensure the highest data quality to define thresholds for normal haemodynamic parameters during exercise, as well as prognostic and differential-diagnostic cut-offs. Non-invasive assessment of exercise haemodynamic parameters with echocardiography is of increasing clinical value; however, it is still considered to lack precision as compared to invasive haemodynamic measurements [9].
Age-dependency of the normal mPAP/CO slope
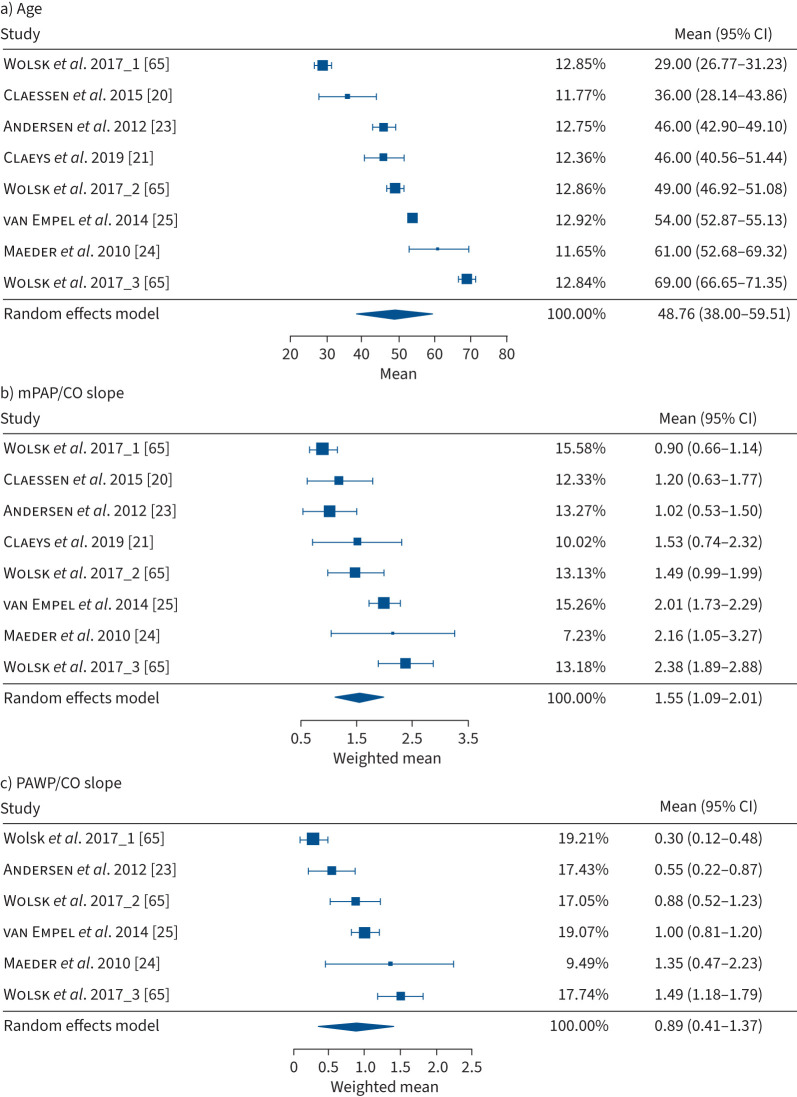
Based on the identified studies, the weighted mean of the mPAP/CO slope influenced by age and the ULN for 30–70-year-old subjects ranged from 1.6 to 3.3 WU. The age-dependency of the mPAP/CO slope was mainly driven by the age-dependency of the PAWP/CO slope while the TPG/CO slope was not significantly age-dependent. This might indicate the decline of the left ventricle's filling compliance during exercise as part of a physiological aging process [65], whereas the distensibility of the pulmonary vessels may remain largely unaffected by age. As shown in figure 2a, studies in healthy subjects with mean age >60 years were under-represented in the current meta-analysis, which was also the case in earlier physiological studies [1, 49]. Therefore, the haemodynamic values provided for older subjects may be less reliable.
FIGURE 2.
Forrest plots of the identified studies in healthy subjects in the supine position for a) mean age (years), b) mean pulmonary arterial pressure (mPAP)/cardiac output (CO) slope (Wood units (WU)) and c) pulmonary arterial wedge pressure (PAWP)/CO slope (WU). Estimates were computed using the Knapp–Hartung correction due to the low number of available studies. The study of Wolsk et al. [65] assessed different age groups that are separately displayed in the Forrest plot, showing the influence of age on cardiopulmonary haemodynamic parameters during exercise. Wolsk et al. 2017_1 provided the youngest age group (<40 years) and Wolsk et al. 2017_3 the oldest ( >60 years) [65].
Owing to the limited number of studies including multipoint mPAP/CO slopes and PAWP/CO slopes, it was not possible to analyse the curvilinearity of the slopes in healthy individuals within the framework of the present study. Previous investigations suggested an almost linear mPAP/CO slope, with an eventual gradual flattening at high levels of exercise [13, 48, 65–67]. This supports the use of the mPAP/CO slope as key in cardiopulmonary exercise haemodynamic parameters.
Prognostic relevance of pulmonary haemodynamic parameters during exercise
Prognostically relevant cut-offs in exercise dyspnoea: mPAP/CO >3 WU, PAWP/CO >2 WU
Two large studies aimed to provide prognostically relevant haemodynamic thresholds during exercise for a general population with dyspnoea on effort. Ho et al. [31] included 714 subjects and analysed the association between exercise PH and a combined end-point defined as all-cause mortality or cardiovascular hospitalisation. The authors defined exercise PH as an mPAP/CO slope >3 WU. The presence of exercise PH was associated with a 2-fold increased risk of an event. In addition, besides the mPAP/CO slope, both TPG/CO and PAWP/CO slopes were independently associated with prognosis [31].
In a second large study, Eisman et al. [32] included 110 patients with dyspnoea on exercise but normal PAWP and ejection fraction at rest. The authors defined the ULN PAWP/CO slope at 2 WU (1.2±0.4 WU), based on the haemodynamic values of a control group. In patients with dyspnoea, a PAWP/CO slope >2 WU was found in ∼40% of subjects and this was associated with adverse clinical outcomes, defined as cardiovascular death, hospitalisation due to heart failure or abnormal resting PAWP in a future RHC. As a consequence, a PAWP/CO slope >2 WU may be considered as a prognostically relevant marker in HFpEF subjects with normal resting PAWP and ejection fraction.
Prognostically relevant haemodynamic parameters in cardiopulmonary diseases
The prognostic relevance of cardiopulmonary haemodynamic parameters during exercise has also been assessed in patients with pre-capillary PH, left heart diseases and COPD. In pre-capillary PH, two haemodynamic variables appeared to have the strongest prognostic relevance. First, cardiac index at peak exercise or its change from rest to exercise was found to be of prognostic relevance in most of the studies [40–44]. Of note, an increase in cardiac index by >50% of its resting value [43] or ≥0.55 L·min−1·m−2 [42] was associated with a better prognosis. Second, similar to patients with exercise dyspnoea, in pre-capillary PH an elevated mPAP/CO slope was associated with poor survival [42, 44]. However, in patients with PH at rest, the range of the slopes was much higher: even in patients with better survival, the mPAP/CO slope was frequently >10 WU [42].
In patients with suspected or confirmed left heart disease, a steep increase of PAWP during exercise appeared as the single most important prognostic haemodynamic parameter. In patients with dyspnoea and suspected HFpEF, a steep increase in PAWP during exercise was strongly associated with mortality, even if haemodynamic parameters at rest were normal [33]. The best cut-off for a poor survival was >25.5 mmHg·W−1·kg−1, e.g. in a subject with a body weight of 75 kg, PAWP would increase above 25 mmHg at 75 W workload. Of note, pulmonary blood flow during exercise is dependent on workload, but with large individual variability [16]. Nevertheless, these results support the data of Eisman et al. [32] and the prognostic relevance of the PAWP/CO slope in patients with dyspnoea and at risk for HFpEF. In patients with established left heart disease, the mPAP/CO (or mPAP/workload) slope also appears to be of prognostic relevance. A steep initial increase of mPAP (0.41±0.16 mmHg·W−1) followed by a plateau was associated with severely impaired survival in patients with HFrEF as compared to subjects with a moderate, linear mPAP increase during exercise (0.28±0.12 mmHg·W−1) [12].
Two studies investigated the prognostic relevance of cardiopulmonary haemodynamic parameters during exercise in COPD. An increase in mPAP and PVR during exercise was associated with clinical deterioration [47], while a low peak cardiac index during exercise predicted poor results of lung surgery, defined as death or prolonged ventilation [46].
Because systemic sclerosis (SSc) represents a significant risk for pulmonary arterial hypertension, changes in cardiopulmonary haemodynamic parameters during exercise may reveal early signs of PVD with potential clinical relevance. A recent study stratified SSc patients into subjects with PH at rest, with exercise PH and with normal haemodynamic parameters [45]. Survival was superior in patients with normal haemodynamic parameters as compared to the other groups, but it was not significantly different between resting and exercise PH. Haemodynamic variables including mPAP at peak exercise, mPAP increase during exercise and the mPAP/workload slope were predictors of transplant-free survival, while haemodynamic parameters at rest were not [45]. In a later study, PVR and CO at peak exercise and the mPAP/CO slope were predictors of long-term survival in SSc patients with no or mildly increased PAP, whereas resting haemodynamic parameters were not [48]. Of note, the best mPAP/CO cut-off to predict survival was 3.5 WU, which is very similar to the prognostic threshold in patients with exercise dyspnoea (3 WU).
Taken together, in patients with exercise dyspnoea or different cardiopulmonary conditions, the mPAP/CO slope, the PAWP/CO slope and peak cardiac index (or CO) appear to be the most robust prognostically relevant haemodynamic parameters during exercise.
Recognition of left heart disease or PVD based on exercise haemodynamic parameters
PAWP/CO slope >2 WU identifies a post-capillary cause of elevated PAP during exercise
According to the identified studies, an increased PAWP/CO slope with a cut-off >2 WU may be the most important indicator of a post-capillary cause for abnormal cardiopulmonary haemodynamic parameters during exercise. Nearly all patients with overt HFpEF and elevated resting PAWP (PAWP >15 mmHg) had a PAWP/CO slope far above this threshold [32], whereas in subjects with normal resting PAWP, a PAWP/CO slope >2.0 WU was associated with adverse cardiac outcomes [32].
In patients with normal resting PAWP, higher PAWP values during exercise were associated with increased left atrial area and volumes [51], highlighting the role of exercise haemodynamic parameters in uncovering latent left heart disease. Of note, peak PAWP ≥25 mmHg during exercise has been suggested to identify HFpEF in patients with exertional dyspnoea, normal ejection fraction and resting PAWP ≤15 mmHg [54]. This suggestion was also adopted in the current European Society of Cardiology diagnostic algorithm for HFpEF [68].
As compared to peak PAWP values, the PAWP/CO slope incorporates the level of increasing flow during exercise and may therefore be more suitable for describing an abnormal haemodynamic response to exercise than peak PAWP alone.
Haemodynamic patterns suggesting PVD
Some studies aimed to describe haemodynamic patterns during exercise that may be characteristic for pre-capillary pulmonary vascular involvement, despite normal or near normal resting haemodynamic parameters. The relevance of the mPAP/CO slope and pulmonary arterial compliance during exercise as potential markers of PVD was highlighted in a study comparing untreated chronic thromboembolic pulmonary hypertension (CTEPH) patients, CTEPH patients with normalised haemodynamic parameters after pulmonary endarterectomy and healthy controls. The mPAP/CO slope was steeper in post-pulmonary endarterectomy patients than in healthy controls, while pulmonary arterial compliance was similar to that in patients with untreated CTEPH, suggesting that such changes may indicate PVD [20].
In addition, increased PVR or TPG during exercise have been considered as suggestive for early PVD in patients with SSc [56, 62, 63]. However, currently no large prospective studies are available that could confirm that a certain haemodynamic pattern is significantly associated with the development of pulmonary arterial hypertension.
Potential definition of exercise PH
A flow-corrected, simple, reliable haemodynamic parameter with a single, prognostically relevant cut-off at the ULN would represent an optimal definition for exercise PH. However, mainly due to the strong age-dependency of most cardiopulmonary haemodynamic parameters during exercise and the limited number of available datasets in healthy older subjects, no parameter and cut-off appears to fulfil all these criteria. Haemodynamic parameters incorporating CO, such as the mPAP/CO slope, appropriately account for the impact of blood flow on mPAP as compared to the absolute value of maximal mPAP, which was previously used to define exercise PH. In addition, the mPAP/CO slope is a consistent variable to describe abnormalities of the pulmonary circulation during exercise and is independently associated with prognosis in patients with exercise dyspnoea and in several cardiovascular conditions. Based on these considerations, the mPAP/CO slope may be suitable to define exercise PH.
Limitations
We acknowledge several limitations of our study. We cannot exclude that some relevant studies were missed by our systematic search strategy. In addition, we included a limited number of studies per condition for the meta-regression models and therefore generalisation to a broader population of values may not be accurate. Our decision to consider only studies after 2003 for the analysis of normal values contributed to the limited number of included studies for this question. However, this approach was used to ensure the best possible quality of data, and the inclusion of 250 healthy subjects complying with state-of-the-art work-up for exclusion of comorbidities, examined by RHC, allowed for robust general conclusions. Referral bias may have influenced the available data, because invasive studies may not have been offered at all clinics. The estimation of age-dependency of the ULN for slopes in healthy older controls should be interpreted with caution because the number of these subjects in the dataset was small. Nevertheless, the results were consistent with a similar dataset of symptomatic patients with no pathological findings, suggesting robustness of the data. Age was included in the moderator analysis as an aggregated variable, underestimating the true variability of this parameter within each individual study. Some methodological details such as the zero reference point or the exact method for assessment of pulmonary pressures during exercise (end-expiratory versus averaging over several respiratory cycles) were not provided in all studies. However, it can be assumed that recent discussions and recommendations have reduced the heterogeneity as compared to previous studies. Further, study results were reported heterogeneously and therefore some statistical approximations and calculations had to be performed, as outlined in the supplementary material. These calculations may have introduced a degree of uncertainty in our data. It is unlikely, though, that they significantly influenced the major results of our analysis. Comparisons for sex and race have not been performed due to the limited number of studies that would have allowed such an analysis. Finally, beyond their description, a true direct comparison between haemodynamic indices for their prognostic or differential-diagnostic relevance is not possible based on the currently available data. We expect that this question may be addressed within the next years in a large, well-powered multicentre clinical registry study [10].
Conclusions
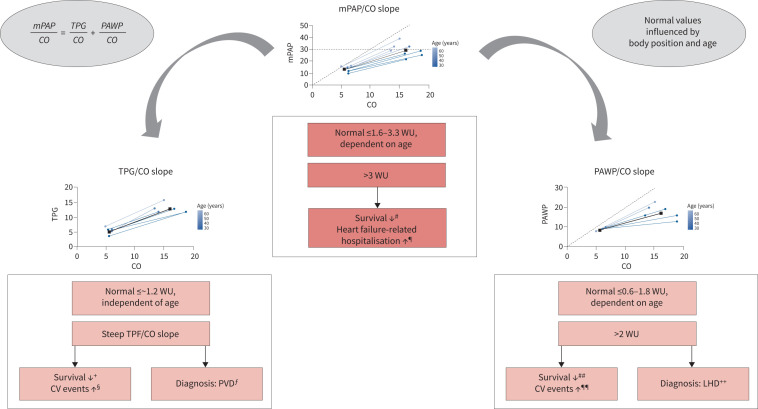
The mPAP/CO and PAWP/CO slopes appear to be the most valuable parameters to characterise pulmonary circulation during exercise. In contrast to the absolute values of mPAP, the mPAP/CO slope is largely unaffected by workload, but it is strongly age-dependent, its ULN ranging from 1.6 WU to 3.3 WU. An increased mPAP/CO slope is associated with impaired survival in different cardiopulmonary conditions, and an independent prognostic cut-off with mPAP/CO >3 WU has been validated in dyspnoea patients (figure 3).
FIGURE 3.
Mean pulmonary arterial pressure (mPAP)/cardiac output (CO), pulmonary arterial wedge pressure (PAWP)/CO and trans-pulmonary gradient (TPG)/CO slopes for the characterisation of pulmonary haemodynamic parameters during exercise. Abnormal pulmonary haemodynamic parameters during exercise may be defined by an increased mPAP/CO slope. This slope is strongly age-dependent and its upper limit of normal (ULN) (mean+2sd) ranges from 1.6 Wood units (WU) (in ∼30-year-old healthy subjects) to 3.3 WU (in ∼70-year-old healthy subjects) in the supine position (table 2). The ULN based on the weighted mean and sd of all healthy subjects included in this analysis was 2.7 WU in the supine position. An increased mPAP/CO slope with a cut-off above >3 WU is independently associated with poor survival and heart failure-related hospitalisations. The mPAP/CO slope corresponds to the sum of the TPG/CO slope and the PAWP/CO slope. Like the mPAP/CO slope, the PAWP/CO slope is also strongly age-dependent and its ULN ranges from 0.6 to 1.8 WU. An increased PAWP/CO slope with a cut-off >2 WU is associated with impaired survival and increased cardiovascular (CV) events and may be diagnostic for a post-capillary cause of PAP elevation during exercise. The ULN for the TPG/CO slope is 1.2 WU and age-independent. An increased TPG/CO slope is also associated with impaired survival and may be suggestive of pulmonary vascular disease (PVD). Studies reporting on the prognostic relevance of the mPAP/CO, TPG/CO and PAWP/CO slopes are indicated in the footnotes. LHD: left heart disease. #: for validating mPAP/CO >3 WU cut-off [31], and [12, 42, 44, 45, 48]; ¶: for validating mPAP/CO >3 WU cut-off [31]; +: [31, 48]; §: [31]; ƒ: [56, 57, 61–63]; ##: for validating PAWP/CO >2 WU cut-off [32], and [31, 33]; ¶¶: for validating PAWP/CO >2 WU cut-off [32], and [31]; ++: for validating PAWP/CO >2 WU cut-off [32], and [54].
The PAWP/CO slope is strongly age-dependent and its ULN ranges between 0.6 WU and 1.8 WU. A PAWP/CO slope >2 WU is associated with adverse cardiovascular events and differentiates between pre- and post-capillary causes of exercise PH. These findings may contribute to the identification of early pulmonary vascular and early left heart disease and provide a basis for future therapeutic studies.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03181-2021.Supplement (901.6KB, pdf)
Shareable PDF
Footnotes
Author contributions: K. Zeder: study design and development, systematic literature analysis, data analysis and interpretation, writing the paper, final approval of the submitted version; C. Banfi and A. Berghold: statistical analysis, final approval of the submitted version; G. Steinrisser-Allex: systematic literature search, final approval of the submitted version; B.A. Maron, G.D. Lewis, M. Humbert: data analysis and interpretation, final approval of the submitted version; H. Olschewski: study design and development, data analysis and interpretation, final approval of the submitted version; G. Kovacs: study design and development, systematic literature analysis, data analysis and interpretation, writing the paper, final approval of the submitted version. All authors contributed to the writing and editing of the manuscript.
Conflict of interest: K. Zeder reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen, and support for attending meetings and/or travel from MSD and Ferrer. B.A. Maron reports grants from Actelion Pharmaceuticals, Tenax Therapeutics, Regeneron Pharmaceuticals, Deerfield Corporation and NIH Research, and discloses the following patents: U.S. Patent #9,605,047, PCT/US2020/066886, PCT/US2019/059890 and PCT/US2015/029672. A. Berghold reports participation on a data safety monitoring board or advisory board for Roche. H. Olschewski reports consulting fees from Actelion, Chiesi, AstraZeneca, GSK, Bayer, Inventiva, Boehringer, Ferrer, Janssen, Menarini, MSD and Novartis, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Springer and Medupdate, support for attending meetings and/or travel from Boehringer and Menarini, participation on a data safety monitoring board or advisory board for Aerovate, Bayer and Pfizer, receipt of equipment, materials, drugs, medical writing, gifts or other services from Algorithm Sciences, Boehringer and Inventiva, and is Deputy Director of the Ludwig Boltzmann Institute for Lung Vascular Research, Graz. The remaining authors disclose no potential conflicts of interest.
References
- 1.Kovacs G, Berghold A, Scheidl S, et al. . Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Expert Committee on Chronic Cor Pulmonale . Chronic Cor Pulmonale: Report of an Expert Committee [meeting held in Geneva from 10 to 15 October 1960]. 1961. Available from: https://apps.who.int/iris/handle/10665/40483
- 3.Hatano S, Strasser T, World Health Organization . Primary Pulmonary Hypertension: Report on a WHO Meeting, Geneva, 15–17 October 1973. 1975. Available from: https://apps.who.int/iris/handle/10665/39094
- 4.Galiè N, Torbicki A, Barst R, et al. . Guidelines on diagnosis and treatment of pulmonary arterial hypertension: The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004; 25: 2243–2278. doi: 10.1016/j.ehj.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Dantzker DR, Ayres SM, et al. . Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107: 216–223. doi: 10.7326/0003-4819-107-2-216 [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery JL, et al. . ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. doi: 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 8.Hoeper Marius M, Bogaard HJ, Robin C, et al. . Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. doi: 10.1016/j.jacc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 9.Kovacs G, Herve P, Barbera JA, et al. . An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017; 50: 1700578. doi: 10.1183/13993003.00578-2017 [DOI] [PubMed] [Google Scholar]
- 10.Kovacs G, Herve P, Olschewski H, et al. . The pulmonary haemodynamics during exercise - research network (PEX-NET) ERS Clinical Research Collaboration: investigating the prognostic relevance of exercise haemodynamics. Eur Respir J 2019; 53: 1900458. doi: 10.1183/13993003.00458-2019 [DOI] [PubMed] [Google Scholar]
- 11.Lewis GD, Bossone E, Naeije R, et al. . Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667 [DOI] [PubMed] [Google Scholar]
- 12.Lewis GD, Murphy RM, Shah RV, et al. . Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail 2011; 4: 276–U95. doi: 10.1161/CIRCHEARTFAILURE.110.959437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naeije R, Vanderpool R, Dhakal BP, et al. . Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. doi: 10.1164/rccm.201211-2090CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argiento P, Vanderpool RR, Mule M, et al. . Exercise stress echocardiography of the pulmonary circulation: limits of normal and sex differences. Chest 2012; 142: 1158–1165. doi: 10.1378/chest.12-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herve P, Lau EM, Sitbon O, et al. . Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J 2015; 46: 728–737. doi: 10.1183/09031936.00021915 [DOI] [PubMed] [Google Scholar]
- 16.Naeije R, Saggar R, Badesch D, et al. . Exercise-induced pulmonary hypertension: translating pathophysiological concepts into clinical practice. Chest 2018; 154: 10–15. doi: 10.1016/j.chest.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 17.Harrer M, Cuijpers P, Furukawa TA, et al. . Doing Meta-Analysis with R: A Hands-On Guide. Boca Raton, Chapman & Hall/CRC Press, 2021. [Google Scholar]
- 18.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22: 2693–2710. doi: 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 19.Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med 2001; 20: 1771–1782. doi: 10.1002/sim.791 [DOI] [PubMed] [Google Scholar]
- 20.Claessen G, La Gerche A, Dymarkowski S, et al. . Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc 2015; 4: e001602. doi: 10.1161/JAHA.114.001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claeys M, Claessen G, La Gerche A, et al. . Impaired cardiac reserve and abnormal vascular load limit exercise capacity in chronic thromboembolic disease. JACC Cardiovasc Imaging 2019; 12: 1444–1456. doi: 10.1016/j.jcmg.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 22.Wolsk E, Kaye D, Komtebedde J, et al. . Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail 2019; 7: 321–332. doi: 10.1016/j.jchf.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 23.Andersen MJ, Ersbøll M, Bro-Jeppesen J, et al. . Exercise hemodynamics in patients with and without diastolic dysfunction and preserved ejection fraction after myocardial infarction. Circ Heart Fail 2012; 5: 444–451. doi: 10.1161/CIRCHEARTFAILURE.112.967919 [DOI] [PubMed] [Google Scholar]
- 24.Maeder MT, Thompson BR, Rocca B-L, et al. . Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010; 56: 855–863. doi: 10.1016/j.jacc.2010.04.040 [DOI] [PubMed] [Google Scholar]
- 25.van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol 2014; 114: 131–135. doi: 10.1016/j.amjcard.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 26.Andersen MJ, Wolsk E, Bakkestrøm R, et al. . Hemodynamic response to rapid saline infusion compared with exercise in healthy participants aged 20–80 years. J Card Fail 2019; 25: 902–910. doi: 10.1016/j.cardfail.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 27.Esfandiari S, Wright SP, Goodman JM, et al. . Pulmonary artery wedge pressure relative to exercise work rate in older men and women. Med Sci Sports Exerc 2017; 49: 1297–1304. doi: 10.1249/MSS.0000000000001227 [DOI] [PubMed] [Google Scholar]
- 28.Wright SP, Esfandiari S, Gray T, et al. . The pulmonary artery wedge pressure response to sustained exercise is time-variant in healthy adults. Heart 2016; 102: 438–443. doi: 10.1136/heartjnl-2015-308592 [DOI] [PubMed] [Google Scholar]
- 29.Regensteiner JG, Bauer TA, Reusch JE, et al. . Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc 2009; 41: 977–984. doi: 10.1249/MSS.0b013e3181942051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lonsdorfer-Wolf E, Richards R, Doutreleau S, et al. . Pulmonary hemodynamics during a strenuous intermittent exercise in healthy subjects. Med Sci Sports Exerc 2003; 35: 1866–1874. doi: 10.1249/01.MSS.0000094181.07571.72 [DOI] [PubMed] [Google Scholar]
- 31.Ho JE, Zern EK, Lau ES, et al. . Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol 2020; 75: 17–26. 10.1016/j.jacc.2019.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisman AS, Shah RV, Dhakal BP, et al. . Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail 2018; 11: e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorfs S, Zeh W, Hochholzer W, et al. . Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3103–3112. doi: 10.1093/eurheartj/ehu315 [DOI] [PubMed] [Google Scholar]
- 34.Dobarro D, Castrodeza-Calvo J, Varela-Falcón L, et al. . Exercise right heart catheterization predicts outcome in asymptomatic degenerative aortic stenosis. Rev Esp Cardiol (Engl Ed) 2020; 73: 457–462. doi: 10.1016/j.recesp.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 35.Huang W, Oliveira RKF, Lei H, et al. . Pulmonary vascular resistance during exercise predicts long-term outcomes in heart failure with preserved ejection fraction. J Card Fail 2018; 24: 169–176. doi: 10.1016/j.cardfail.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 36.Rieth A, Richter MJ, Gall H, et al. . Hemodynamic phenotyping based on exercise catheterization predicts outcome in patients with heart failure and reduced ejection fraction. J Heart Lung Transplant 2017; 36: 880–889. doi: 10.1016/j.healun.2017.02.022 [DOI] [PubMed] [Google Scholar]
- 37.Griffin BP, Shah PK, Ferguson J, et al. . Incremental prognostic value of exercise hemodynamic variables in chronic congestive heart failure secondary to coronary artery disease or to dilated cardiomyopathy. Am J Cardiol 1991; 67: 848–853. doi: 10.1016/0002-9149(91)90618-U [DOI] [PubMed] [Google Scholar]
- 38.Szlachicic J, Massie BM, Kramer BL, et al. . Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 1985; 55: 1037–1042. doi: 10.1016/0002-9149(85)90742-8 [DOI] [PubMed] [Google Scholar]
- 39.Gohlke H, Samek L, Betz P, et al. . Exercise testing provides additional prognostic information in angiographically defined subgroups of patients with coronary artery disease. Circulation 1983; 68: 979–985. doi: 10.1161/01.CIR.68.5.979 [DOI] [PubMed] [Google Scholar]
- 40.Faure M, Valentin S, Zysman M, et al. . Exercise hemodynamics in the prognosis of patients with pulmonary arterial hypertension. Eur Respir J 2020; 99: 678–685. 10.1159/000509144 [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, Yao L, Liu Z, et al. . Peak circulatory power is a strong prognostic factor in patients with idiopathic pulmonary arterial hypertension. Respir Med 2018; 135: 29–34. doi: 10.1016/j.rmed.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 42.Hasler ED, Müller-Mottet S, Furian M, et al. . Pressure-flow during exercise catheterization predicts survival in pulmonary hypertension. Chest 2016; 150: 57–67. doi: 10.1016/j.chest.2016.02.634 [DOI] [PubMed] [Google Scholar]
- 43.Chaouat A, Sitbon O, Mercy M, et al. . Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J 2014; 44: 704–713. doi: 10.1183/09031936.00153613 [DOI] [PubMed] [Google Scholar]
- 44.Blumberg FC, Arzt M, Lange T, et al. . Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail 2013; 15: 771–775. doi: 10.1093/eurjhf/hft044 [DOI] [PubMed] [Google Scholar]
- 45.Stamm A, Saxer S, Lichtblau M, et al. . Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur Respir J 2016; 48: 1658–1667. doi: 10.1183/13993003.00990-2016 [DOI] [PubMed] [Google Scholar]
- 46.Olsen GN, Weiman DS, Bolton JWR, et al. . Submaximal invasive exercise testing and quantitative lung scanning in the evaluation for tolerance of lung resection. Chest 1989; 95: 267–273. doi: 10.1378/chest.95.2.267 [DOI] [PubMed] [Google Scholar]
- 47.Finlay M, Middleton H, Peake M, et al. . Cardiac output, pulmonary hypertension, hypoxaemia and survival in patients with chronic obstructive airways disease. Eur J Respir Dis 1983; 64: 252–263. [PubMed] [Google Scholar]
- 48.Zeder K, Avian A, Bachmaier G, et al. . Exercise pulmonary resistances predict long-term survival in systemic sclerosis. Chest 2021; 159: 781–790. doi: 10.1016/j.chest.2020.08.2110 [DOI] [PubMed] [Google Scholar]
- 49.Kovacs G, Olschewski A, Berghold A, et al. . Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 2012; 39: 319–328. doi: 10.1183/09031936.00008611 [DOI] [PubMed] [Google Scholar]
- 50.Goda A, Takeuchi K, Kikuchi H, et al. . Etiology of exercise-induced pulmonary hypertension can be differentiated by echocardiography – insight from patients with chronic pulmonary thromboembolism with normal resting hemodynamics by balloon pulmonary angioplasty. Circ J 2019; 83: 2527–2536. doi: 10.1253/circj.CJ-19-0489 [DOI] [PubMed] [Google Scholar]
- 51.Maor E, Grossman Y, Balmor RG, et al. . Exercise haemodynamics may unmask the diagnosis of diastolic dysfunction among patients with pulmonary hypertension. Eur J Heart Fail 2015; 17: 151–158. doi: 10.1002/ejhf.198 [DOI] [PubMed] [Google Scholar]
- 52.Andersen MJ, Olson TP, Melenovsky V, et al. . Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015; 8: 41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731 [DOI] [PubMed] [Google Scholar]
- 53.van Empel VP, Mariani J, Borlaug BA, et al. . Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 2014; 3: e001293. doi: 10.1161/JAHA.114.001293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borlaug BA, Nishimura RA, Sorajja P, et al. . Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida A, Kadota K, Kambara H, et al. . Left ventricular responses to supine bicycle exercise assessed by radionuclide angiocardiography and a Swan-Ganz catheter. Jpn Circ J 1985; 49: 661–671. doi: 10.1253/jcj.49.661 [DOI] [PubMed] [Google Scholar]
- 56.Nagel C, Marra AM, Benjamin N, et al. . Reduced right ventricular output reserve in patients with systemic sclerosis and mildly elevated pulmonary artery pressure. Arthritis Rheumatol 2019; 71: 805–816. doi: 10.1002/art.40814 [DOI] [PubMed] [Google Scholar]
- 57.Gorter TM, Obokata M, Reddy YNV, et al. . Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018; 39: 2825–2835. doi: 10.1093/eurheartj/ehy331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor BJ, Smetana MR, Frantz RP, et al. . Submaximal exercise pulmonary gas exchange in left heart disease patients with different forms of pulmonary hypertension. J Card Fail 2015; 21: 647–655. doi: 10.1016/j.cardfail.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolle JJ, Waxman AB, Van Horn TL, et al. . Exercise-induced pulmonary arterial hypertension. Circulation 2008; 118: 2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bentley RF, Barker M, Esfandiari S, et al. . The relationship between pulmonary artery pulse pressure and pulmonary artery wedge pressure during exercise in health and in patients with suspected pulmonary hypertension. J Heart Lung Transplant 2020; 39: S18. doi: 10.1016/j.healun.2020.01.1143 [DOI] [Google Scholar]
- 61.Keusch S, Bucher A, Müller-Mottet S, et al. . Experience with exercise right heart catheterization in the diagnosis of pulmonary hypertension: a retrospective study. Multidiscip Respir Med 2014; 9: 51. doi: 10.1186/2049-6958-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hager WD, Collins I, Tate JP, et al. . Exercise during cardiac catheterization distinguishes between pulmonary and left ventricular causes of dyspnea in systemic sclerosis patients. Clin Respir J 2013; 7: 227–236. doi: 10.1111/j.1752-699X.2012.00310.x [DOI] [PubMed] [Google Scholar]
- 63.Saggar R, Khanna D, Furst DE, et al. . Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum 2010; 62: 3741–3750. doi: 10.1002/art.27695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esfandiari S, Wolsk E, Granton D, et al. . Pulmonary arterial wedge pressure at rest and during exercise in healthy adults: a systematic review and meta-analysis. J Card Fail 2019; 25: 114–122. doi: 10.1016/j.cardfail.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 65.Wolsk E, Bakkestrøm R, Thomsen JH, et al. . The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail 2017; 5: 337–346. doi: 10.1016/j.jchf.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 66.Godinas L, Lau EM, Chemla D, et al. . Diagnostic concordance of different criteria for exercise pulmonary hypertension in subjects with normal resting pulmonary arterial pressure. Eur Respir J 2016; 48: 254–257. doi: 10.1183/13993003.01678-2015 [DOI] [PubMed] [Google Scholar]
- 67.Lo Russo G, Riccardo M, Filippo T, et al. . Does PAP/CO ratio have a linear relationship? J Am Coll Cardiol 2020; 75: 2646–2646. doi: 10.1016/j.jacc.2020.02.071 [DOI] [PubMed] [Google Scholar]
- 68.Pieske B, Tschöpe C, de Boer RA, et al. . How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03181-2021.Supplement (901.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03181-2021.Shareable (334.4KB, pdf)