Abstract
Bacteroides thetaiotaomicron uses starch as a source of carbon and energy. Early steps in the pathway of starch utilization, such as starch binding and starch hydrolysis, are encoded by sus genes, which have been characterized previously. The sus structural genes are expressed only if cells are grown in medium containing maltose or higher oligomers of glucose. Regulation of the sus structural genes is mediated by SusR, an activator that is encoded by a gene located next to the sus structural genes. A strain with a disruption in susR cannot grow on starch but can still grow on maltose and maltotriose. A search for transposon-generated mutants that could not grow on maltose and maltotriose unexpectedly located a gene, designated malR, which regulates expression of an α-glucosidase not controlled by SusR. Although a disruption in susR did not affect expression of the malR controlled gene, a disruption in malR reduced expression of the sus structural genes. Thus, MalR appears to participate with SusR in regulation of the sus genes. Results of transcriptional fusion assays and reverse transcription-PCR experiments showed that malR is expressed constitutively. Moreover, multiple copies of malR provided on a plasmid (5 to 10 copies per cell) more than doubled the amount of α-glucosidase activity in cell extracts. Our results demonstrate that the starch utilization system of B. thetaiotaomicron is controlled on at least two levels by the regulatory proteins SusR and MalR.
Bacteroides thetaiotaomicron and other human colonic Bacteroides species utilize a variety of polysaccharides as a source of carbon and energy (15). This trait may be important for their survival in the human colon because polysaccharides are the main form of carbohydrate available to colon bacteria. The starch utilization system of B. thetaiotaomicron is the best studied of the Bacteroides polysaccharide utilization systems. Previously, a cluster of starch utilization genes, designated sus genes, was identified and characterized (4, 5, 13, 14). This cluster contains seven structural genes (susA to susG), most of which encode proteins that mediate the initial steps in starch utilization, such as starch binding and starch hydrolysis (13, 14). These genes are organized into two transcriptional units (5, 14), one containing susA and one containing susB to susG.
Expression of the structural genes is regulated at the transcriptional level by maltose and higher oligomers of starch. Regulation is mediated by SusR, a protein encoded by a gene that is located upstream of susA. Unlike the structural genes, susR is constitutively expressed (5). Since multiple copies of susR in trans increased sus gene expression and since a disruption in susR abolished expression of susA-susG, it appeared that SusR alone was responsible for controlling expression of the structural genes. In this report, we show that there is at least one other regulatory gene that participates in control of sus gene expression.
No other starch utilization genes were found in the region of the sus gene cluster, but it was clear that B. thetaiotaomicron must have other starch utilization genes. For one thing, disruption of susB, which encodes an α-glucosidase, did not eliminate all of the α-glucosidase activity in cell extracts. For another, the susR disruption mutant still grew as well as wild type on maltose and maltotriose even though it could not grow on higher oligomers. Thus, other maltose utilization genes must be located elsewhere on the chromosome. We report here that although a search for mutants unable to grow on maltose and maltotriose failed to locate the second α-glucosidase gene or any other genes encoding maltose utilization proteins, it did unexpectedly yield a second regulatory gene, malR, that controls expression of the sus genes, as well as expression of the second α-glucosidase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All Escherichia coli strains used in this study were grown in Luria-Bertani (LB) broth or on LB agar at 37°C. B. thetaiotaomicron 5482, transposon-generated derivatives, and some singly or doubly disrupted mutants used in this study have been described previously (1, 4, 14).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Description and/or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5αMCR | RecA− Gns | 8 |
| S17-1 | RecA− Gns | IncP RP4 inserted into the chromosome (19) |
| HB101 | RecA− Gns Strr | 11 |
| B. thetaiotaomicron | ||
| BT5482 | Wild type, Gnr (G2–G7)+ starch+ | Anaerobe Laboratory, Virginia Polytechnic Institute, Blacksburg, Va. |
| BT4009 | Tcr Emr Gnr (G2-G7)+ starch+ | B. thetaiotaomicron mutant with CTn7853 element carrying Tcr (tetQ) and Emr (ermG) genes; known as Tcr Emr 7853 (3) |
| BTMAL | Tcr Emr Gnr G2+ G3− starch− | Tn4351-generated mutant of BTΩsusR (this study) |
| Ms-1 | Emr Gnr G2+ G3+ starch− | Tn4351-generated mutant of strain BT5482 (1); displays BTΩsusR phenotype since susR is disrupted |
| BTΩsusR | Tcr Gnr G2+ G3+ starch− | B. thetaiotaomicron mutant with a susR disruption created by a suicide vector pBT-1 containing 185-bp ClaI-BstYI internal susR; known as susR::pBT-1 (5) |
| BTΩmalR | Emr Gnr G2+ G3+ starch+ | B. thetaiotaomicron mutant with a malR disruption created by a suicide vector pGERM containing a PCR-generated 0.33-kbp internal fragment of malR (this study) |
| BTΩsusRΩmalR | Tcr Emr Gnr G2+ G3− starch− | BTΩsusR with a malR disruption created by a suicide vector pGERM containing a PCR-generated 0.33-kbp internal fragment of malR (this study) |
| BTΩsusB | Tcr Gnr starch− | B. thetaiotaomicron mutant with a susB disruption created by a suicide vector pBT-1 containing a PCR-generated 0.61-kbp internal fragment of susB (16) |
| BTΩsusB1 | Emr Gnr starch− | B. thetaiotaomicron mutant with a susB disruption created by suicide vector pNJR-6 containing a 0.57-kbp internal fragment of susB; known as susB1::pNJR6 (4) |
| BTΩsusBΩmalR | Tcr Emr Gnr starch− | BTΩsusB with a malR disruption created by a suicide vector pGERM containing a PCR-generated 0.33-kbp internal fragment of malR (this study) |
| BTΩmalRGUS | Emr Gnr G2+ G3+ starch+ | B. thetaiotaomicron mutant with a GUS fusion created by suicide vector pΩMALRGUS (this study) |
| Plasmids | ||
| pBT-1 | Knr Tcr | RSF1010-based suicide vector used to make insertional disruptions in Bacteroides spp. (21) |
| pGERM | Apr Emr | pUC19-based suicide vector (expressing ermG) used to make insertional disruptions in Bacteroides spp. (18) |
| pMALR | Apr Tcr Tcr | PCR-generated 1.2-kbp DNA fragment containing entire malR and its promoter region cloned into pT-COW (this study) |
| pMALRGUS | Apr Em | PCR-generated 0.82-kbp DNA fragment containing malR promoter region cloned into pMJF-2 (this study) |
| pΩMALRGUS | Apr Emr | pCQW-1 containing a PCR-generated 0.33-kbp internal fragment of malR (this study) |
| pT-COW | Apr Tcr Tcr | pVAL-based shuttle vector with tetQ (7) |
| pMJF-2 | Apr Emr | pFD160-based shuttle vector containing a promoterless GUS gene (6) |
| pCQW-1 | Apr Emr | pUC19-based suicide vector containing a promoterless GUS gene (6) |
Abbreviations: G2, maltose; G3, maltotriose; G7, maltoheptaose; Tc, tetracycline; Em, erythromycin; Gn, gentamicin; Ap, ampicillin; Kn, kanamycin; Cm, chloramphenicol; Str, streptomycin. Underlined antibiotic resistances are expressed only in E. coli. Other resistances are expressed only in Bacteroides spp.
Bacteroides strains were grown initially in a prereduced Trypticase-yeast extract-glucose (TYG) medium. For the characterization of Bacteroides strains, cells were transferred to a defined minimal medium (9) containing glucose, maltose, maltotriose, amylopectin, or dextran (0.3% [wt/vol]), respectively, as a sole carbohydrate source. Antibiotic concentrations used in this study were as follows: ampicillin, 50 μg/ml; chloramphenicol at 20 μg/ml (E. coli) or at 15 μg/ml (B. thetaiotaomicron); erythromycin, 10 μg/ml; gentamicin, 200 μg/ml; and tetracycline at 10 μg/ml (E. coli), at 3 μg/ml for selection after conjugation and measurement of growth rates, and otherwise at 1 μg/ml (B. thetaiotaomicron), unless it is mentioned specifically.
DNA methods.
Isolation of plasmids was done by using a Wizard Plus DNA purification system (Promega Corp.). Dephosphorylation reactions and restriction digests were performed in accordance with the manufacturer's instructions (Bethesda Research Laboratories [Bethesda, Md.] or New England BioLabs [Beverly, Mass.]). Transformation of E. coli DH5αMCR was done by the method of Lederberg and Cohen (10). Conjugations, where constructs generated in E. coli were transferred to Bacteroides recipients, were performed as described by Shoemaker et al. (17). Insertional and replicative shuttle vectors were mobilized from E. coli donors to Bacteroides recipients by transfer genes of RP4 integrated in the chromosome of S17-1 (19). Southern blotting was done as described by Maniatis et al. (11) except that a Renaissance Detection Kit (DuPont-NEN) was used for detection of the bound DNA probe.
Chemicals.
Glucose, maltose, maltotriose, amylopectin, dextran, phenylmethylsulfonyl fluoride, p-nitrophenyl-α-d-glucopyranoside, were purchased from Sigma Corp. 4-Nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene was purchased from Boehringer Mannheim Biochemicals.
Isolation of a B. thetaiotaomicron mutant deficient in maltose and maltotriose utilization.
To isolate a mutant of B. thetaiotaomicron that was deficient in maltose and maltotriose utilization, transposon mutagenesis was done by introducing the Bacteroides transposon Tn4351 into two different hosts: B. thetaiotaomicron 5482 (wild type) and the susR disruption mutant B. thetaiotaomicron ΩsusR (BTΩsusR). In the mutant strain, BTΩsusR, the susR gene had been disrupted by a single crossover insertion of the suicide vector, pBT-1, into which an internal segment of susR had been cloned (5). The selectable marker on pBT-1 was a tetracycline resistance gene, tetQ, so that the selectable marker on Tn4351, the erythromycin resistance gene ermF, could be used. Tn4351 was introduced into the wild-type strain or the BTΩsusR mutant by conjugation, by using as a donor E. coli HB101 containing Tn4351 on the self-transmissible IncP plasmid, R751 (2, 12). Transconjugants harboring Tn4351 insertions were selected by growth on TYG agar plates containing erythromycin (10 μg/ml) and gentamicin (200 μg/ml). The gentamicin selection eliminated the E. coli donors. Tetracycline (3 μg/ml) was also included in the medium to ensure retention of the pBT-1 insertion in BTΩsusR. Transconjugants were screened for growth on maltose-defined medium agar plates.
Cloning of DNA adjacent to the Tn4351 insertion in B. thetaiotaomicron MAL (BTMAL).
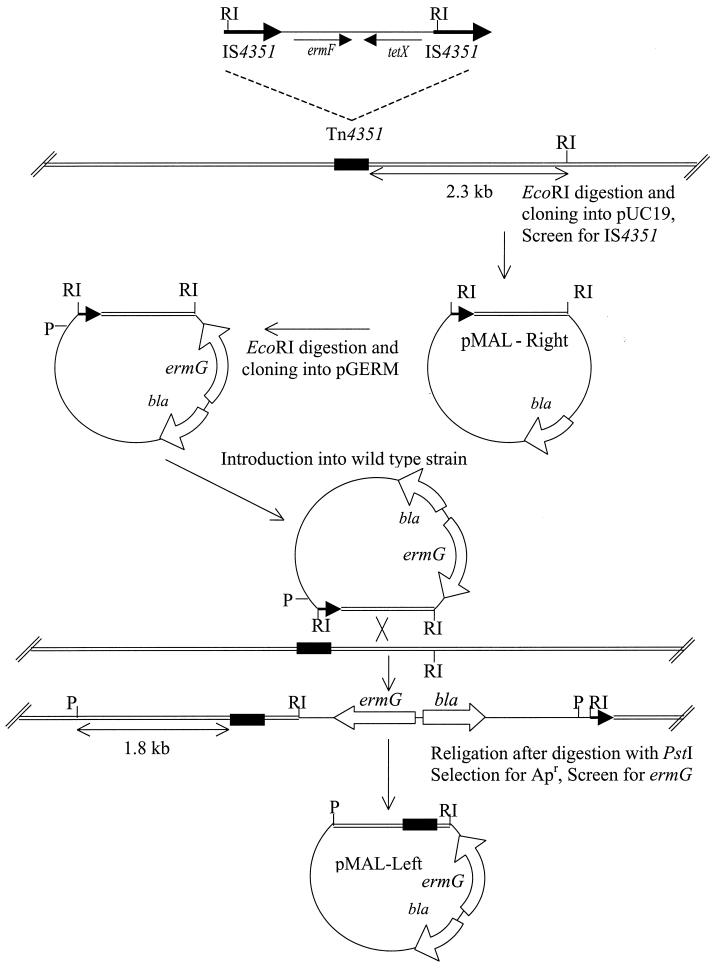
The strategy used to clone DNA adjacent to the Tn4351 insertion is shown in Fig. 1. Chromosomal DNA was isolated from the mutant and digested with EcoRI. EcoRI cuts Tn4351 near one end of each of the directly repeated insertion sequence (IS) elements that flank Tn4351 but nowhere else in the transposon. The EcoRI fragments were ligated into the EcoRI site of pUC19. E. coli DH5α MCR transformants were plated onto Luria agar containing ampicillin (100 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg/ml). White colonies on X-Gal plates were screened for the Tn4351 junction fragment by colony hybridization, by using a probe that contained IS element DNA. To clone the other chromosomal junction, we first recloned the EcoRI fragment containing IS DNA into pGERM. This clone was transferred to wild-type B. thetaiotaomicron to create a single crossover insertion in the cloned region. Chromosomal DNA from the resulting strain was digested with PstI, religated, and then transformed into E. coli with selection for ampicillin. PstI cuts only once in pGERM and not at all in the IS element. Thus, ampicillin-resistant transformants should contain chromosomal DNA adjacent to the insertion site on both sides of the transposon insertion.
FIG. 1.
Cloning of pMAL-Right and pMAL-Left to sequence the region where Tn4351 is inserted in BTMAL. The position of the transposon insertion in BTMAL is indicated by the black rectangle. The heavy horizontal arrows in Tn4351 indicate the direct repeat insertion sequence (IS4351). Tn4351 carries an erythromycin resistance gene (ermF), which is expressed only in Bacteroides strains, and a tetracycline resistance gene (tetX), which is expressed only in aerobically grown E. coli strains. pGERM, a suicide vector in Bacteroides, has a different erythromycin resistance gene (ermG). The double lines (=) indicate Bacteroides chromosomal DNA. Abbreviations: RI, EcoRI; P, PstI
The clones were sequenced by the University of Illinois Biotechnology Automated Sequencing Facility (University of Illinois Biotechnology Center, Urbana). The BLAST network service was used to search for proteins in the databases that have homology with the open reading frames (ORFs) of the sequenced DNA.
Enzyme assays.
α-Glucosidase activity and amylase activity in sonically disrupted cell extracts were measured by determining the rate of hydrolysis of p-nitrophenyl-α-d-glucopyranoside and 4-nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene, respectively. α-Glucosidase activity was measured as described by Smith and Salyers (20). Amylase activity was measured with 2 mM 4-nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene in potassium phosphate buffer (20 mM, pH 6.5) at 37°C (Boehringer Mannheim Biochemicals). The protein concentration in each extract was measured by using the Bio-Rad DC protein kit. The Km values of the α-glucosidases were calculated from a Lineweaver-Burke plot. BTΩsusB was used to determine the Km of the second α-glucosidase, and BTΩmalR was used to determine the Km of the susB-encoded α-glucosidase. β-Glucuronidase (GUS) assays, used to monitor expression of uidA (GUS) fusions, were done as described by Feldhaus et al. (6).
malR gene expression.
To provide malR in trans on a multicopy plasmid, malR was amplified by PCR with primers TCAAAGTACTGGATCCCGAAATGACC, which lies ca. 300 bp upstream of the first start codon in the ORF, and TATATTGACAGGATCCATGTACTTGT, which lies ca. 150 bp downstream of the first stop codon in the ORF. This product (ca. 1.2 kb) was cloned into pT-COW, which has a copy number in Bacteroides of 5 to 10 (22). The resulting vector was called pMALR.
To create a malR-uidA (GUS) fusion for studies of malR expression, a DNA segment including the promoter region of malR was first amplified by PCR with primers TCAAAGTACTGGATCCCGAAATGACC and AATCAGCGATGGATCCAGACGTCCAC, a sequence which lies ca. 210 bp upstream of the first stop codon in the ORF, and then cloned into pMJF-2, a shuttle vector which has the GUS gene clomed downstream of a multiple cloning site. The resulting vector was called pMALRGUS.
To monitor malR expression in the chromosome, a DNA segment containing an internal portion of the malR gene was amplified by PCR, by using the forward primer CCTCTCATTTGAGGATCCTCATTACC and the reverse primer AATCAGCGATGGATCCAGACGTCCAC, and then cloned into pCQW-1, a GUS suicide vector (6). This vector was called pΩMALRGUS. The plasmids were transferred to Bacteroides strains by conjugation.
Reverse transcription-PCR (RT-PCR) was also used to examine the expression of malR. B. thetaiotaomicron was grown on the defined medium containing 0.3% (wt/vol) glucose or maltose as a sole carbon source. A portion (5 ml) of the culture was harvested at an optical density at 650 nm of 0.3. A Qiagen RNeasy kit (Qiagen, Chatsworth, Calif.) was used for the isolation of total RNA from cells. To prevent DNA contamination, the RNA was treated with Recombinant RNasin RNase Inhibitor and RQ1 RNase-free DNase (Promega, Madison, Wis.). Superscript II RNase− reverse transcriptase (Gibco-BRL) was used for the synthesis of cDNA from the isolated RNA.
Nucleotide sequence accession number.
The nucleotide sequence of malR region (Fig. 2A) has been deposited in GenBank under accession number AF391102.
FIG. 2.
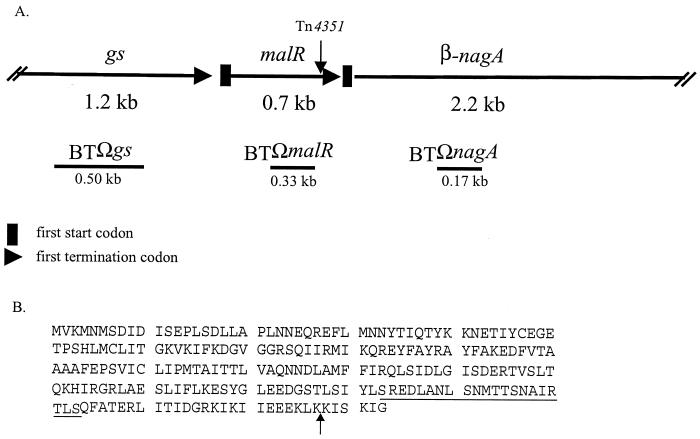
(A) Map showing the relative locations of ORFs in the malR gene area. The position of the transposon insertion in mutant BTMAL is indicated by the vertical arrow above the map. DNA segments used to make insertional disruptions are shown as horizontal lines under the map and marked with an “Ω.” The sizes of the DNA fragments used to make the disruptions are also indicated under the lines. gs, putative glutamine synthetase gene; malR, putative regulatory protein gene; β-nagA, putative β-N-acetylglucosaminidase gene. (B) Deduced amino acid sequence of malR. A possible carboxy-terminal helix-turn-helix motif is underlined. The vertical arrow indicates the transposon insertion site in the transposon-generated mutant, BTMAL.
RESULTS
Isolation of a mutant with reduced ability to grow on maltose and maltotriose.
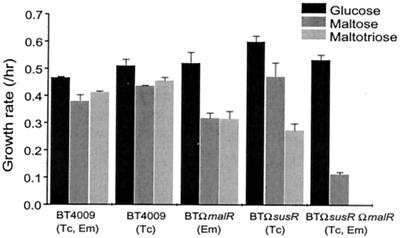
A screen of thousands of transposon-generated mutants of wild-type B. thetaiotaomicron was done to find mutants that had lost the ability to utilize maltose or maltotriose. No such mutants were found. A possible explanation for the failure to find such mutants was that the sus genes were contributing to the utilization of maltose and maltotriose, and this redundancy with the other maltose or maltotriose utilization genes made it impossible for a single transposon insertion to abolish maltose utilization. Accordingly, a mutant with a disruption in susR (BTΩsusR) was used as the background for transposon mutagenesis. This mutant did not produce any of the Sus structural proteins. Over 15,000 transposon-generated mutants were screened for maltose and maltotriose utilization. One mutant was found that was deficient in the ability to grow on maltose and maltotriose. The mutant was named BTMAL. The growth rate of BTMAL on glucose (0.45 h−1) was the same as that of the parent strain, BTΩsusR, but BTMAL had one-fourth the growth rate of the parent strain on maltose (0.11 h−1) and did not grow at all on maltotriose.
The DNA sequence of a 4.1-kb chromosomal DNA segment, in which the transposon had inserted, was analyzed. There were three possible ORFs in this segment. The transposon had disrupted a small orf in the middle of the segment (Fig. 2A). This small orf, which was 699 bp in size, was designated malR. The deduced amino acid sequence of the MalR protein had a low amino acid sequence homology (21 to 26% identity, 45 to 47% similarity) to transcriptional regulators of the Crp/Fnr family from Bacillus subtilis, Pseudomonas spp., and Aquifex aeolicus. A possible helix-turn-helix DNA-binding motif was found in the carboxy terminus (Fig. 2B). Tn4351 inserted 18 bp upstream of the 3′ end of malR.
The amino acid sequences of the ORFs upstream and downstream of malR had significant sequence homology to a glutamine synthetase from Bacteroides fragilis and a putative β-N-acetylhexosaminidase from Porphyromonas gingivalis, respectively. Thus, they seemed unlikely to be involved in maltose utilization. Nonetheless, single crossover disruptions were constructed in each of the three ORFs in BTΩsusR. The only disruption that had the same phenotype as the mutant was the disruption in malR (BTΩsusRΩmalR; Fig. 3). Hence, malR alone among these ORFs was responsible for the reduced maltose utilization phenotype (Fig. 3). A disruption of malR in the wild-type background (BTΩmalR) reduced somewhat the rate of growth on both maltose and maltotriose, but the bacteria were still able to grow on these substrates (Fig. 3). This result supports the hypothesis that maltotriose and maltose are utilized via the sus system, as well as by the mal system.
FIG. 3.
Growth rates of various mutants on glucose, maltose, and maltotriose. The medium used was defined medium that contained glucose, maltose, and maltotriose, and the results are indicated by the three shaded bars in each set from left to right, respectively. The concentrations of antibiotics used in this experiment were 3 μg/ml for tetracycline (Tc) and 10 μg/ml for erythromycin (Em). These measurements were done in triplicate; the range of values is indicated by the error bars. B. thetaiotaomicron BT4009 was used as the wild-type control because it contains a single copy of tetQ and ermF. Thus, either tetracycline or erythromycin or both (Tc, Em) can be added to the medium used to grow both the control and the mutant strains. This eliminates the slight differences in growth rate that can sometimes occur due to the presence of antibiotics in the medium (note the difference between “Tc” columns versus the “Tc, Em” columns). The selectable marker used to create the BTΩmalR strain was ermF, and the marker used to create the BTΩsusR strain was tetQ. The double mutant BTΩsusRΩmalR contained both resistance genes.
The malR gene controls expression of the second α-glucosidase.
α-Glucosidase and amylase activities in the various mutant strains were measured. Amylase activity was used as an indicator of the sus gene expression because the only amylases produced by B. thetaiotaomicron are encoded by susA and susG (16). Thus, the activity of these proteins is a good indicator of the activity of proteins encoded in this region. As expected, BTΩsusR had no detectable amylase activity and had lower α-glucosidase activity than the wild type (Table 2). The α-glucosidase activity remaining in extracts from the BTΩsusR mutant did not come from SusB; when we disrupted susB, the level of α-glucosidase activity was similar to that of BTΩsusR. When malR in BTΩsusR was disrupted to create BTΩsusRΩmalR, virtually all of the α-glucosidase activity disappeared. This was also true of BTΩsusBΩmalR. Hence, malR controls expression of the second α-glucosidase.
TABLE 2.
α-Glucosidase and amylase activities of whole-cell extracts of strainsa
| Strain | Growth substrate (carbon source) | Activityb (U/g of cell protein)
|
|
|---|---|---|---|
| α-Glucosidase | Amylase | ||
| BT5482 | Glucose | 2.2c | <0.6 |
| Maltose | 60.3 | 49.1 | |
| BTΩsusR | Glucose | <0.6 | <0.6 |
| Maltose | 7.4 | <0.6 | |
| BTΩsusB | Glucose | ND | ND |
| Maltose | 6.1 | 84.9 | |
| BTΩsusRΩmalR | Glucose | <0.6 | <0.6 |
| Maltose | 1.5 | <0.6 | |
| BTΩmalR | Glucose | <0.6 | <0.6 |
| Maltose | 14.0 | 5.7 | |
| BTΩsusBΩmalR | Glucose | ND | ND |
| Maltose | <0.6 | 4.5 | |
For this assay, strains were grown on minimal medium containing glucose or maltose (0.3%).
The substrates used to detect α-glucosidase and amylase activities are p-nitrophenyl-α-d-glucopyranoside and 4-nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene, respectively. One unit of activity was defined as one micromole of p-nitrophenol liberated per minute. This experiment was conducted in duplicate, and the variation between replicates was <10%. ND, not determined.
There was some α-glucosidase activity from wild type on glucose, so there was a basal level expression of α-glucosidase on glucose.
Comparison of properties of the two α-glucosidases.
Cell extracts from BTΩmalR and BTΩsusB were used to assay SusB and the second α-glucosidase, respectively. The Km values of the two α-glucosidases were similar: 132 μM for SusB and 103 μM for the second α-glucosidase. Also, the cellular location of these two α-glucosidase were the same. SusB partitioned to the inner membrane fraction but could be eluted by washing the membrane with 0.5 M NaCl (4). This was also the case for the second α-glucosidase; about two-thirds of the α-glucosidase activity partitioned with the membrane fraction. There was, however, a difference in the stability of the two enzymes in cell extracts. The second α-glucosidase was not stable even at 4°C. The enzyme activity that was detectable in a cell extract from BTΩsusB after 14 h at 4°C was just 33% of the initial activity. In contrast, 87% of the original activity was detectable in an extract from BTΩmalR that had been stored for 14 h at 4°C.
malR regulates expression of sus genes.
If malR regulates only the expression of the second α-glucosidase, BTΩmalR should still have full SusB and amylase (SusA and SusG) activity. That is, the α-glucosidase activity of the malR disruption strain (BTΩmalR) should be ca. 53 U/g of cell protein, the value of the activity in BT5482 extracts minus the activity in BTΩsusR extracts, and the amylase activity should be 49 to 50 U/g of cell protein. However, the α-glucosidase activity in BTΩmalR extracts was only 14 U/g cell protein, one-fourth of the expected value (Table 2). Moreover, the amylase activity in BTΩmalR extracts was much lower than in extracts from wild type. Thus, malR seems to be necessary for full expression of the sus genes. To make sure that the α-glucosidase of BTΩmalR was due to SusB, we disrupted susB in BTΩmalR to create BTΩsusBΩmalR. BTΩsusBΩmalR has no detectable α-glucosidase activity. Thus, the α-glucosidase activity in extracts from BTΩmalR came from SusB. The α-glucosidase specific activity in BTΩsusR extracts was almost the same as in BTΩsusB extracts, so SusR does not control the expression of the α-glucosidase controlled by MalR.
Interestingly, the amylase activity of BTΩsusB was higher than that of wild type. We measured the amylase activities of several mutants with disruptions in genes downstream of susB such as susC, susE, or susG and amylase activities of those strains were almost the same as that of wild type (data not shown). Thus, only the disruption in susB increased amylase activity from SusA. This increased amylase activity could be due to the fact that maltose, the inducer of sus gene expression, was not broken down as rapidly in the cell. Higher levels of maltose could make SusR and/or MalR better able to activate gene expression.
The malR disruption in BTΩsusB abolished virtually all of the α-glucosidase in the cell extract, the double-disruption strain, BTΩsusBΩmalR, yet the mutant still grew slowly on maltose (0.11 h−1). Thus, there might be the third α-glucosidase in BT5482 that is not detected by our enzyme assay.
The growth rate of BTΩmalR on the starch amylopectin was measured. Even though the malR gene disruption lowered the α-glucosidase and amylase activities, the growth rate of BTΩmalR (0.39 h−1) on amylopectin was only slightly lower than that of the wild type (0.49 h−1).
Effect of providing malR in trans.
The data from disruption mutants indicated that malR had its own promoter, because disruptions in the adjacent ORFs had no effect on maltose utilization. Accordingly, malR plus ca. 300 bp of upstream DNA was cloned to produce pMALR and was introduced into wild type and BTΩmalR. The plasmid in which malR was cloned has a copy number of ca. 5 to 10 copies per cell (22). When pMALR was present in BTΩmalR, α-glucosidase and amylase activities were twofold higher than those of BTΩmalR (Table 3), so malR in trans complemented the disrupted chromosomal malR and increased expression of starch utilization genes. This same effect was seen when pMalR was introduced into the wild-type strain (Table 3). These results showed that MalR is not an α-glucosidase but is a regulatory protein that regulates positively genes encoding α-glucosidase, amylase genes (susA and susG) and presumably susC to susF as well. The growth rate of BT5482(pMALR) on amylopectin was 0.61 h−1, a value slightly higher than that of a control strain, BT5482(pT-COW), which contained only the vector into which malR was cloned (0.52 h−1).
TABLE 3.
α-Glucosidase and amylase activities of whole-cell extracts of strainsa
| Strain | Genotype | Growth substrate (carbon source) | Activityb (U/g of cell protein)
|
|
|---|---|---|---|---|
| α-Glucosidase | Amylase | |||
| BT5482 | One copy of malR in the chromosome | Glucose | 2.2 | <0.6 |
| Maltose | 60.3 | 49.1 | ||
| BT5482(pMALR) | One chromosomal malR and malR in trans | Glucose | 1.8 | <0.6 |
| Maltose | 148.5 | 88.1 | ||
| BTΩmalR(pMALR) | Disrupted chromosomal malR and malR in trans | Glucose | 1.8 | <0.6 |
| Maltose | 126.4 | 78.1 | ||
| Ms-1(pMALR) | Disrupted chromosomal susR and malR in trans | Glucose | ND | ND |
| Maltose | 6.3 | <0.6 | ||
| BTΩsusB1(pMALR) | Disrupted chromosomal susB and malR in trans | Glucose | ND | ND |
| Maltose | 3.7 | 80.0 | ||
For this assay, strains were grown on minimal medium containing glucose or maltose (0.3%).
The substrates used to detect α-glucosidase and amylase activities are p-nitrophenyl-α-d-glucopyranoside and 4-nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene, respectively. One unit of activity was defined as one micromole of p-nitrophenol liberated per minute. This experiment was conducted in duplicate, and the variation between replicates was <10%. ND, not determined.
To determine whether malR exerted its effect on expression of the sus genes in the absence of SusR, pMALR was transferred into Ms-1, a mutant strain of B. thetaiotaomicron strain that has a transposon insertion in susR. In this background multiple copies of malR in trans did not restore expression of the sus genes (Table 3).
pMALR was transferred into BTΩsusB, a susB disruption strain, to determine whether multiple copies of malR in trans increased the activity of the second α-glucosidase. The α-glucosidase activity in BTΩsusB(pMALR) did not increase compared to BTΩsusB. Thus, excess MalR does not have the same effect on the expression of the second α-glucosidase as it does on sus gene expression. The amylase activity of BTΩsusB(pMALR) was almost the same as that of BTΩsusB. Thus, multiple copies of malR in BTΩsusB(pMALR) did not increase amylase activity further.
malR is expressed constitutively, and its expression is not autoregulated.
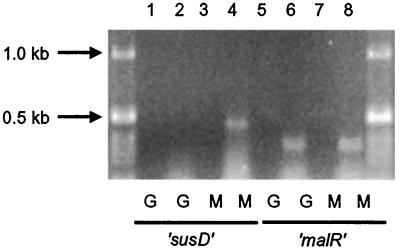
RT-PCR was used to determine whether malR is expressed constitutively or is induced by maltose. Expression of susD was used as a control. RT-PCR detected susD mRNA only when cells were grown on maltose (Fig. 4). In contrast, malR mRNA was detected in cells grown on glucose as well as in cells grown on maltose (Fig. 4). Thus, malR is expressed constitutively.
FIG. 4.
Detection of malR expression on glucose and maltose by using RT-PCR. Odd-numbered lanes contain negative controls in which the reaction was done without the RT step. The expression of the maltose-regulated susD gene was used as a control to assess whether we could detect regulated gene expression (lane 2, glucose [G]-grown cells; lane 4, maltose [M]-grown cells). The region amplified from susD was 0.5 kb in size. To check malR expression (lanes 6 and 8), a 0.4-kbp region of the mRNA was amplified. The 1-kb ladder (Gibco-BRL) is seen in the two outside lanes.
To confirm the RT-PCR data and to determine whether the expression of malR is autoregulated, a GUS fusion shuttle vector was used to monitor malR expression. A DNA segment that contained the malR promoter region and the 5′ end of malR cloned into pMJF-2 (pMALGUS) produced similar levels of GUS activity on glucose and maltose (Table 4). pMALR was transferred into BT5482(pMALRGUS) to create BT5482(pMALRGUS, pMALR). The two plasmids are compatible and have approximately the same copy number (5 to 10). Therefore, this arrangement should provide a similar level of MalR relative to the malR promoter, as is present in the wild type, but a higher level of MalR relative to the GUS fusion than in the strain that contained only the GUS fusion plasmid and one copy of malR in the chromosome. The GUS activity of this strain was almost the same as that of BT5482(pMALRGUS) (Table 4). The fact that expression from the malR promoter was not affected by different amounts of MalR in the cell suggests that the expression of malR is not autoregulated.
TABLE 4.
GUS specific activities of whole-cell extracts of strainsa
| Strain | Genotype | Growth substrate (carbon source) | GUS activity (U/mg of cell protein)b |
|---|---|---|---|
| BT5482 | One copy of malR in the chromosome | Glucose | <1.0 |
| Maltose | <1.0 | ||
| BT5482(pMALRGUS) | One copy of malR in the chromosome and GUS gene downstream of the promoter of malR in trans | Glucose | 33.6 |
| Maltose | 40.4 | ||
| Amylopectin | 41.9 | ||
| BT5482(pMALRGUS, pMALR) | One chromosomal malR, malR in trans, and GUS gene downstream of the promoter of malR in trans | Glucose | 39.5 |
| Maltose | 31.2 | ||
| BTΩmalRGUS | GUS gene downstream of the promoter of the chromosomal malR | Glucose | ND |
| Maltose | <1.0 | ||
| BTΩmalRGUS(pMALR) | GUS gene downstream of the promoter of chromosomal malR and malR in trans | Glucose | ND |
| Maltose | <1.0 |
For this assay, strains were grown on the defined medium plus glucose or maltose (0.3%).
Units are expressed in micromoles per minute. The substrate used to detect GUS activity is p-nitrophenyl glucuronide. This experiment was conducted in duplicate, and the variation between replicates was <10% except for BT5482(pMALRGUS, pMALR). BT5482(pMALRGUS, pMALR) produced a lot of capsule so the cell mass was much less than in other strains (ca. 10 to 20% of the wild-type level). The variation between replicates of this strain was 20 to 25%. ND, not determined.
DISCUSSION
Our results suggest that the genes controlled by susR and malR encode most or all of the proteins responsible for utilization of maltotriose. Disrupting both of these genes abolished growth on maltotriose and severely reduced growth on maltose. Proteins encoded by the sus genes aid in maltotriose utilization but do not contribute significantly to utilization of maltose because disruption of susR decreases growth on maltotriose but not growth on maltose. In contrast, disruption of malR decreases the rate of growth on both maltose and maltotriose. The malR gene was not linked genetically to the gene encoding the second α-glucosidase or to any other structural genes that may be under MalR control, so there is as yet little information about the genes MalR controls other than the sus genes. An attempt to purify the second α-glucosidase failed due to the instability of the enzyme (data not shown), so it was not possible to use N-terminal sequencing as a basis for designing a probe to locate the gene encoding it. The Km for this enzyme was virtually identical to that of SusB. In fact, the only difference between the two enzymes was stability in cell extracts. This raises the question of why there are two α-glucosidases, which appear to have redundant characteristics, and why one is associated with the sus system and one is not.
MalR appears to be a regulatory protein. Not only does it have amino acid similarity to known regulatory proteins, but its loss affects the activities of more than one protein. This latter observation supports the hypothesis that MalR controls the transcription of the genes encoding these proteins and probably other genes as well. The fact that the transcription of susB is affected by a malR disruption indicates that transcription of the other genes in the susB-susG operon would also be affected. An unexpected role of malR is its effect on sus gene expression. The sus genes were thought to be controlled only by susR, but results reported here show that disruption of malR reduced expression of the sus structural genes by 5- to 10-fold. Loss of SusR, however, did not seem to affect the expression of the α-glucosidase controlled by MalR. Since both susR and malR are expressed constitutively, it is likely that SusR and Mal R proteins interact with each other rather than one controlling the other's expression.
An alternative possibility is that the second α-glucosidase or one of the other proteins encoded by MalR-controlled genes is not involved in catabolism of maltose but rather converts maltose to a derivative that is a more effective inducer than maltose itself. In this case, the drop in the expression of susA and susB in the malR disruption strain would be due to the fact that maltose is now the only inducer available to interact with SusR. This would explain why MalR seems to influence expression of the sus genes but SusR does not seem to influence expression of the MalR-controlled α-glucosidase. If SusR and MalR proteins formed a complex that increases transcription of the sus genes, one would expect this same complex to be responsible for expression of the MalR-controlled genes. The hypothesis that a MalR-controlled gene encodes an enzyme that makes a better inducer would also explain why expression of the sus genes is decreased in the malR disruption strain but does not fall to zero.
The results reported here suggest that the MalR regulon and the SusR regulon together account for all of the genes needed to grow on maltotriose and starch. The very low rate of growth of the BTΩsusBΩmalR strain on maltose could be due to uptake and breakdown of maltose by transporters and enzymes that normally handle a closely related substrate such as melibiose or lactose. Given that the mal and sus systems account for most or all of the utilization of small glucosides, the finding that the BTΩmalR strain still grew on starch confirms that the susA-susG gene products are solely responsible for the processing of starch. The fact that the malR disruption strain was able to grow on starch nearly as well as wild type was surprising since expression of the sus genes was decreased five- to ninefold in that strain. This observation can be explained by noting that B. thetaiotaomicron probably never encounters in nature concentrations of starch as high as those it encounters in laboratory medium. The bacteria may be optimized to operate with enzyme levels lower than those seen in bacteria growing on high concentrations of maltose. Whatever the explanation for the effect of the disruption in malR on sus gene expression, it is clear that the starch utilization pathway and the pathway controlled by MalR are linked at the metabolic and/or regulatory level.
ACKNOWLEDGMENT
This work was supported by grant number AI/GM 17876 from the National Institutes of Health.
REFERENCES
- 1.Anderson K L, Salyers A A. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989;171:3199–3204. doi: 10.1128/jb.171.6.3199-3204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chikami G K, Guiney D G, Schmidhauser T J, Helinski D R. Comparison of 10 IncP plasmid: homology in the regions involved in plasmid replication. J Bacteriol. 1985;162:656–660. doi: 10.1128/jb.162.2.656-660.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper A J, Shoemaker N B, Salyers A A. The erythromycin resistance gene from the Bacteroides conjugal transposon Tcr Emr 7853 is nearly identical to ermG from Bacillus sphaericus. Antimicrob Agents Chemother. 1996;39:797–805. doi: 10.1128/aac.40.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Elia J N, Salyers A A. Contribution of a neopullulanase, a pullulanase, and an α-glucosidase to growth of Bacteroides thetaiotaomicron on starch. J Bacteriol. 1996;178:7173–7179. doi: 10.1128/jb.178.24.7173-7179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Elia J N, Salyers A A. Effect of regulatory protein levels on utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1996;178:7180–7186. doi: 10.1128/jb.178.24.7180-7186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldhaus M J, Hwa V, Cheng Q, Salyers A A. Use of an Escherichia coli β-glucuronidase gene as a reporter gene for investigation of Bacteroides promoters. J Bacteriol. 1991;173:4540–4543. doi: 10.1128/jb.173.14.4540-4543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner R G, Russell J B, Willson D B, Wang G-R, Shoemaker N B. Use of a modified Bacteroides-Prevotella shuttle vector to transfer a reconstructed β-1,4-d-endoglucanase gene into Bacteroides uniformis and Prevotella ruminicola B14. Appl Environ Microbiol. 1996;62:196–202. doi: 10.1128/aem.62.1.196-202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D. Studies on the transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 9.Kotarski S F, Salyers A A. Isolation and characterization of outer membranes of B. thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984;158:102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lederberg E M, Cohen S M. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974;119:1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 12.Meyer R J, Shapiro J A. Genetic organization of the broad-host-range IncP-1 plasmid R751. J Bacteriol. 1980;143:1362–1373. doi: 10.1128/jb.143.3.1362-1373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves A R, D'Elia J N, Salyers A A. A Bacteroides thetaiotaomicron outer membrane protein that is essential for utilization of maltooligosaccharides and starch. J Bacteriol. 1996;178:823–830. doi: 10.1128/jb.178.3.823-830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves A R, Wang G-R, Salyers A A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1997;179:643–649. doi: 10.1128/jb.179.3.643-649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salyers A A, Vercellotti J R, West S H E, Wilkins T D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977;33:319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shipman J A, Cho K H, Siegel H A, Salyers A A. Physiological characterization of SusG: an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1999;181:7206–7211. doi: 10.1128/jb.181.23.7206-7211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoemaker N B, Getty C, Guthrie E P, Salyers A A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986;166:959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker N B, Wang G-R, Salyers A A. Multiple gene products and sequences required for the excision of the mobilizable integrated Bacteroides element NBU1. J Bacteriol. 2000;182:928–936. doi: 10.1128/jb.182.4.928-936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 20.Smith K A, Salyers A A. Characterization of a neopullulanase and an α-glucosidase from Bacteroides thetaiotaomicron 95-1. J Bacteriol. 1991;173:2962–2968. doi: 10.1128/jb.173.9.2962-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tancula E, Feldhaus M J, Bedzyk L A, Salyers A A. Location and characterization of genes involved in binding of starch to the surface of Bacteroides thetaiotaomicron. J Bacteriol. 1992;174:5609–5616. doi: 10.1128/jb.174.17.5609-5616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, J., G.-R. Wang, N. B. Shoemaker, and A. A. Salyers. Production of two proteins encoded by the Bacteroides mobilizable transposon, NBU1, correlates with the time-dependent accumulation of the excised NBU1 circular form. J. Bacteriol. 183:6335–6343. [DOI] [PMC free article] [PubMed]