Graphical abstract

Keywords: Hypertrophic cardiomyopathy, Cardiac imaging, 3D printing, Virtual reality, Medical education
Highlights
-
•
Echocardiography and CMR are used for diagnosis of HCM.
-
•
Learning echocardiography requires advanced and repetitive training.
-
•
A 3D model can enhance understanding of cardiac anatomy and pathophysiology.
Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disease characterized by concentric left ventricular hypertrophy with preserved or increased ejection fraction.1 Disease progression is highly variable. While some patients remain asymptomatic for years, others rapidly progress toward end-stage heart failure and sudden cardiac death.2 Patients with end-stage HCM characterized by progressive left ventricular dysfunction and restrictive ventricular filling are candidates for orthotopic heart transplantation (OHT).3 Echocardiography and cardiovascular magnetic resonance (CMR) are used to establish the diagnosis and during follow-up for disease progression.2 While echocardiography is a cost-effective and accessible diagnostic tool, learning echocardiography requires advanced and repetitive training.4 Conventional educational strategies for teaching echocardiography rely on idealized cardiac models5 or models computed from in vivo imaging techniques.6 While these models are anatomically correct, they lack variability in the surface anatomy, internal structures, and pathology a learner would expect to see between human hearts.7 As an initial step toward being able to augment our real-time understanding of the anatomic and pathophysiologic features of end-stage HCM beyond transthoracic echocardiography (TTE) and contrast-enhanced CMR, we describe a novel approach using microcomputed tomography (μCT). This modality provides a much higher resolution, of both the internal and surface cardiac anatomy, to create a three-dimensional (3D) printed model and virtual reality scene.
The overarching aim of our work is to develop an integrated, immersive, and interactive educational platform that uses multimodal digital tools to enhance the knowledge and understanding of cardiac anatomy, physiology, and pathophysiology for medical students, residents, fellows, and faculty across multiple medical and bioengineering disciplines.
Case Presentation
A 12-year-old female patient presented to the Emergency Department with chest pain and palpitations while running. Transthoracic echocardiography confirmed a diagnosis of HCM. Following rapid disease progression managed medically with metoprolol, intravenous milrinone, and an automatic implantable cardioverter-defibrillator (ICD), the patient underwent a successful OHT. Prior to OHT, the electrocardiogram showed biventricular hypertrophy with strain pattern, T-wave inversion throughout (Figure 1), and ischemic ST segment changes during exercise stress testing. Transthoracic echocardiography showed significant biventricular hypertrophy with posterior and septal wall thickness of 22 and 32 mm, respectively (Figure 2, Videos 1-3). Other findings included a midcavitary gradient of 17 mm Hg (Figure 3), restrictive filling pattern, and visually estimated left ventricular ejection fraction of 60% to 65%. Cardiovascular magnetic resonance confirmed the diagnosis of HCM (Figure 4). Right heart cardiac catheterization demonstrated a cardiac index of 2.22 L/min/m2, a mixed venous oxygen saturation of 64%, and a pulmonary capillary wedge pressure of 20 mm Hg, consistent with diastolic dysfunction and increased left atrial pressure.
Figure 1.
Twelve-lead electrocardiogram showing right atrial enlargement, elevated QRS voltages associated with left ventricular hypertrophy, inverted T waves in all precordial leads (V1-V6), and ST-segment depression in septal leads (V1-V3).
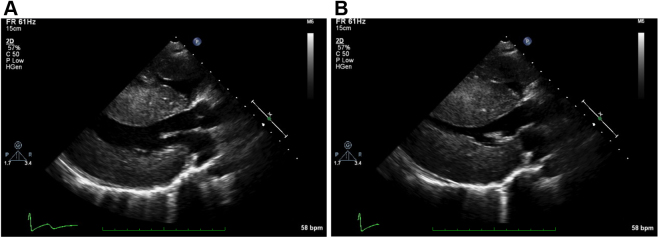
Figure 2.
Two-dimensional TTE parasternal long-axis view showing severe biventricular and septal hypertrophy during (A) diastole and (B) systole.
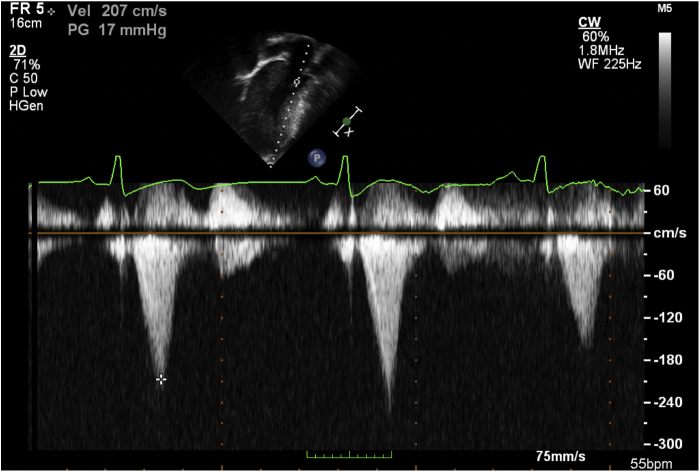
Figure 3.
Two-dimensional TTE apical 4-chamber view with continuous-wave Doppler across the left ventricular outflow tract showing a 17 mm Hg gradient.
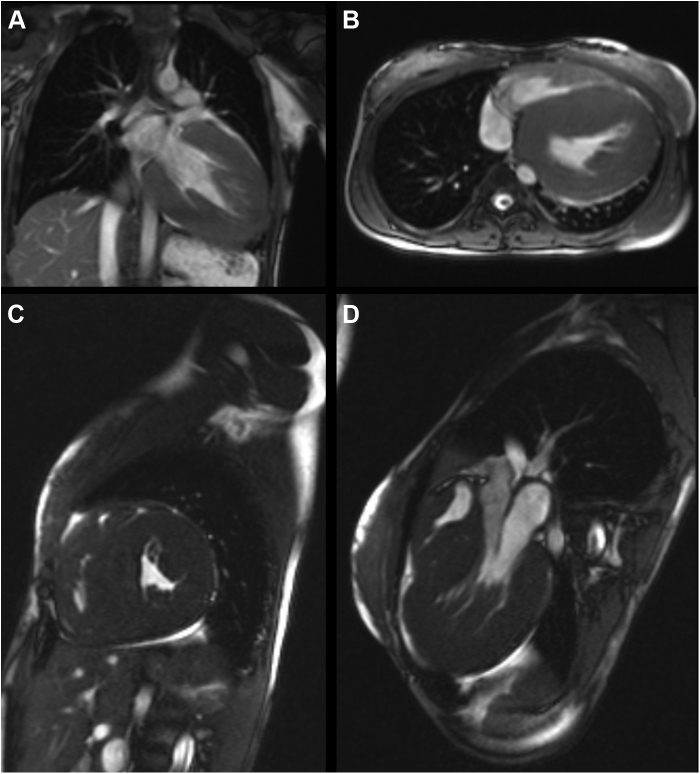
Figure 4.
Cardiovascular magnetic resonance showing severe free-wall and septal left ventricular hypertrophy in (A) coronal, (B) axial, (C) sagittal, and (D) oblique long-axis displays.
Following the patient’s OHT, the heart was donated to the Visible Heart Laboratory at the University of Minnesota for further imaging and characterization. Immediately upon receipt of the diseased heart, it was formalin fixed. A μCT was performed (X3000 CT, NorthStar Imaging, Rogers, MN) with 91 μm resolution (Figure 5). The μCT imaging data sets were imported into Mimics Innovation Suite (Materialise NV, Leuven, Belgium) to create a 3D computational model that was subsequently sliced to represent the standard imaging views recommended by the American Society of Echocardiography (Figure 6). The model was 3D printed, and a virtual reality application was developed that allowed for dynamic cutting of cardiac anatomy in real time (Figure 7).
Figure 5.
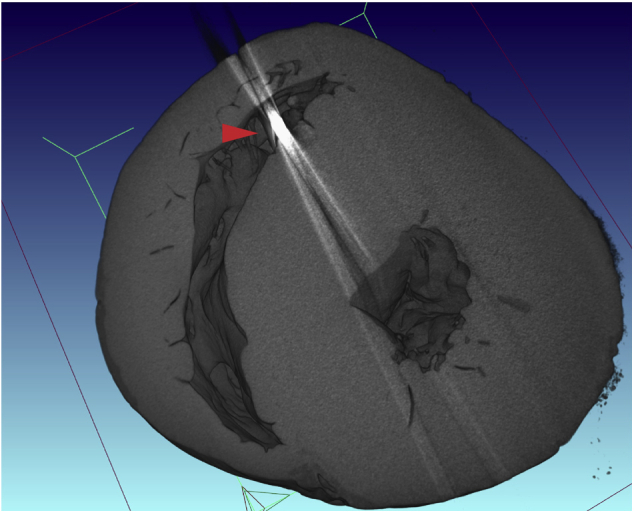
A μCT of the explanted heart in short-axis view showing biventricular hypertrophy and foreign material in the right ventricle corresponding to a right ventricular ICD lead (red arrow).
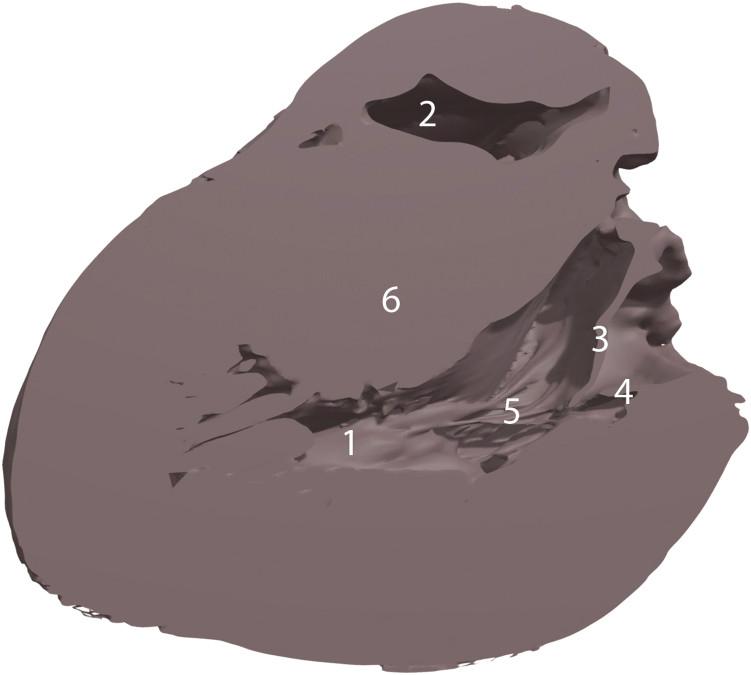
Figure 6.
Three-dimensional high-resolution computational model of the heart in the long-axis view with labels showing (1) posteromedial papillary muscle, (2) right ventricular cavity, (3) anterior mitral leaflet, (4) posterior mitral leaflet, (5) chordae tendinea, and (6) hypertrophied interventricular septum.
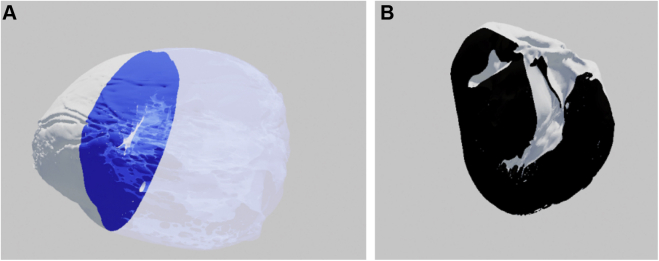
Figure 7.
Three-dimensional model of the heart in (A) short-axis and (B) long-axis views as seen in virtual reality. The image plane in panel A is blue with a transparent silhouette of the heart on the right. The image plane in panel B is black and has no silhouette.
Discussion
Accurate and detailed characterization of the phenotypic changes associated with HCM is essential for appropriate prognostication and management.8,9 There are currently a number of subtypes of HCM, all associated with variable degrees of left ventricular hypertrophy.9 Echocardiography and magnetic resonance imaging are essential diagnostic tools for managing patients who present with HCM.10 Echocardiography has high sensitivity and specificity but is heavily operator dependent and requires a thorough knowledge of cardiovascular anatomy and pathophysiology.4 Educational simulators have recently emerged as popular tools to overcome these limitations but offer a limited number of pathological conditions and rely on a flat screen to display a highly complex 3D structure.11
A metanalysis demonstrated that 3D educational tools to visualize and learn anatomy and pathophysiology outperform two-dimensional methods.12 Therefore, we propose a novel approach to enhance the understanding of cardiac anatomy, pathophysiology, and disease progression in HCM. Furthermore, we propose an innovative workflow for clinicians and students to experience two-dimensional imaging in multiple 3D modalities. Indeed, leveraging data sets from μCT to create computational models that can be 3D printed or used to create virtual reality scenes allows clinicians the opportunity to interact more closely with a diseased heart. In the past, ex vivo μCT imaging has been used to generate high-resolution 3D models and define the anatomical features of congenital heart disease in fetuses.13
While providing adequate representation of cardiac surface anatomy, computational models developed from in vivo imaging using echocardiography, magnetic resonance imaging, and computed tomography are limited by the resolution required to segment the internal cardiac anatomy including atrioventricular valves and their corresponding subvalvular apparatuses in a way that accurately represents their details and variations.14 In using μCT, albeit ex vivo, we can generate computational models with logarithmically higher resolution and detail. In this instance, the authors acknowledge that, to date, we are unable to create these high-resolution images in vivo as we are unable to place humans in μCT machines, and as we have not animated these models, the myocardium and valves can only be visualized in a static position. We are currently working on developing high-fidelity tissue movement simulation using these images. However, we are encouraged by this proof of principle for several reasons: (1) We have demonstrated the feasibility of harvesting, fixing, and imaging an explanted human heart for the purpose of creating 3D prints and virtual scenes. (2) We can enhance the experience of clinicians and students caring for patients with heart disease by creating 3D prints and virtual scenes. (3) While HCM patients undergo gross anatomic changes that are easily seen using echocardiography and CMR, μCT-based computational modeling may offer meaningful advantages with cardiac lesions that are less obvious. These detailed 3D models can be used to provide insight beyond macroscopic anatomical variations, as they might aid in the understanding of hemodynamic repercussions secondary to the diseased cardiac geometry, although we acknowledge that additional educational research should be performed to confirm its value, and we are working toward that goal.
Conclusion
Herein, we report a novel approach for creating 3D printed models and virtual reality scenes using ex vivo imaging of an explanted heart of a female patient with HCM and massive concentric left ventricular hypertrophy. We consider this a foundational step toward developing a more comprehensive and multimodal approach for experiencing and understanding cardiac anatomy and pathophysiology.
Footnotes
Conflicts of Interest: None.
This study received an Academic Investment Program Grant from the University of Minnesota and MHealth, Minneapolis, Minnesota.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.06.005.
Supplementary Data
Two-dimensional TTE parasternal long-axis view demonstrating severe biventricular hypertrophy without significant valvular pathology.
Two-dimensional TTE parasternal short-axis view at the basal level demonstrating severe interventricular septal and papillary muscle hypertrophy and right ventricular free-wall hypertrophy and a right ventricular ICD lead.
Two-dimensional TTE apical 4-chamber view demonstrating severe biventricular hypertrophy and a right ventricular ICD lead.
References
- 1.Marian A.J., Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:749–770. doi: 10.1161/CIRCRESAHA.117.311059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron B.J. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. doi: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 3.Torres M.F., Perez-Villa F. Heart transplantation in patients with hypertrophic cardiomyopathy. Glob Cardiol Sci Pract. 2018;2018:32. doi: 10.21542/gcsp.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popescu B.A., Stefanidis A., Fox K.F., Cosyns B., Delgado V., Di Salvo G.D., et al. Training, competence, and quality improvement in echocardiography: the European Association of Cardiovascular Imaging Recommendations: update 2020 [published correction appears in Eur Heart J Cardiovasc Imaging 2021;22:187] Eur Heart J Cardiovasc Imaging. 2020;21:1305–1319. doi: 10.1093/ehjci/jeaa266. [DOI] [PubMed] [Google Scholar]
- 5.Maresky H.S., Oikonomou A., Ali I., Ditkofsky N., Pakkal M., Ballyk B. Virtual reality and cardiac anatomy: exploring immersive three-dimensional cardiac imaging, a pilot study in undergraduate medical anatomy education. Clin Anat. 2019;32:238–243. doi: 10.1002/ca.23292. [DOI] [PubMed] [Google Scholar]
- 6.James R.C., Monsky W.L., Jorgensen N.W., Seslar S.P. Virtual-reality guided versus fluoroscopy-guided transseptal puncture in a cardiac phantom. J Invasive Cardiol. 2020;32:76–81. doi: 10.25270/jic/19.00290. [DOI] [PubMed] [Google Scholar]
- 7.Meineri M., Qua-Hiansen J., Garijo J.M., Ansari B., Ruggeri G.M., Ender J., et al. Evaluation of a patient-specific, low-cost, 3-dimensional-printed transesophageal echocardiography human heart phantom. J Cardiothorac Vasc Anesth. 2021;35:208–215. doi: 10.1053/j.jvca.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Parato V.M., Antoncecchi V., Sozzi F., Marazia S., Zito A., Maiello M., et al. Echocardiographic diagnosis of the different phenotypes of hypertrophic cardiomyopathy. Cardiovasc Ultrasound. 2016;14:30. doi: 10.1186/s12947-016-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syed I.S., Ommen S.R., Breen J.F., Tajik A.J. Hypertrophic cardiomyopathy: identification of morphological subtypes by echocardiography and cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2008;1:377–379. doi: 10.1016/j.jcmg.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Ommen S.R., Mital S., Burke M.A., Day S.M., Deswal A., Elliott P., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Deng S., Wheeler G., Toussaint N., Munroe L., Bhattacharya S., Sajith G., et al. A virtual reality system for improved image-based planning of complex cardiac procedures. J Imaging. 2021;7:151. doi: 10.3390/jimaging7080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yammine K., Violato C. A meta-analysis of the educational effectiveness of three-dimensional visualization technologies in teaching anatomy. Anat Sci Educ. 2015;8:525–538. doi: 10.1002/ase.1510. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson J.C., Arthurs O.J., Ashworth M.T., Ramsey A.T., Mifsud W., Lombardi C.M., et al. Clinical utility of postmortem microcomputed tomography of the fetal heart: diagnostic imaging vs macroscopic dissection. Ultrasound Obstet Gynecol. 2016;47:58–64. doi: 10.1002/uog.15764. [DOI] [PubMed] [Google Scholar]
- 14.Ginty O., Moore J., Xia W., Bainbridge D., Peters T. In: Proceedings of SPIE, vol. 10135, Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling Bellingham. Webster R.J., Fei B., editors. SPIE; WA: March 3, 2017. Patient-specific indirectly 3D printed mitral valves for pre-operative surgical modelling. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE parasternal long-axis view demonstrating severe biventricular hypertrophy without significant valvular pathology.
Two-dimensional TTE parasternal short-axis view at the basal level demonstrating severe interventricular septal and papillary muscle hypertrophy and right ventricular free-wall hypertrophy and a right ventricular ICD lead.
Two-dimensional TTE apical 4-chamber view demonstrating severe biventricular hypertrophy and a right ventricular ICD lead.