Abstract
microRNAs are regulatory RNAs that silence specific mRNA by binding to it, inducing translational repression. Over the recent decades since the discovery of RNA interference, the field of microRNA therapeutics has expanded tremendously. The role of miRNAs in disease development has attracted researchers to investigate their potential in therapeutics. In lung cancer, multiple miRNAs are deregulated, and their involvement is observed in cell proliferation, immunomodulation, angiogenesis, and epithelial-mesenchymal transition. Thus, synthetic oligonucleotides are developed to downregulate the overexpressed miRNA or to upregulate the repressed miRNA. However, their clinical efficiency is limited due to the requirement for an effective delivery strategy. Advances in the current understanding of nanotechnology, biomaterial science, and disease molecular pathology have increased the chances of overcoming the limitations of miRNA-based therapy. This review enlists downregulated and upregulated miRNAs in lung cancer. This review also highlights the major contributions to miRNA-based therapeutics for lung cancer and strategies to overcome endosomal barriers. It also attempts to understand the nuances between current advancements in delivery methods, advantages, disadvantages, and practical issues for the large-scale development of miRNA-based therapeutics.
Keywords: AntagomiR, AgomiR, RNA interference, Lung cancer, miRNA-based therapeutics
Highlights
-
•
Multiple miRNAs are deregulated in lung cancer, and they are involved in tumor progression.
-
•
Synthetic oligonucleotides downregulate the overexpressed miRNA or to upregulate the repressed miRNA.
-
•
This review also highlights the major contributions to miRNA-based therapeutics for lung cancer.
-
•
It also attempts to understand the nuances between current advancements in delivery methods, advantages, disadvantages, and practical issues for the large-scale development of miRNA-based therapeutics.
Abbreviations
- %
Percentage
- 2D
Two dimension
- 3D
Three dimension
- AGO2
Argonaute 2
- ALI
Air Lung Interface
- Alix
Asparagine-Linked Glycosylation 2 interacting protein X
- ALK
Anaplastic lymphoma kinase
- ALT
Alanine aminotransferase
- ANKRD46
Ankyrin Repeat Domain 46
- Apaf-1
Apoptotic protease activating factor 1
- AST
Aspartate aminotransferase
- Bax
BCL2 Associated X protein
- BCL
B-cell lymphoma 2
- BRAF
Serine/threonine-protein kinase B-Raf
- BUN
Blood urea nitrogen
- CCNE-1
Cyclin E
- CD31
Cluster of differentiation 31
- CD44
Cluster of differentiation 44
- CD80
Cluster of differentiation 80
- CD81
Cluster of differentiation 81
- CDH13
Cadherin-13
- CDH1
Cadherin-1
- CDK-6
Cell division protein kinase 6
- CDKN2A
Cyclin-dependent kinase inhibitor 2A
- c-MET
Tyrosine-protein kinase Met
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DDAB
Dimethyl dioctadecyl ammonium bromide
- DDAH1
Dimethylarginine Dimethylaminohydrolase 1
- DET
Diethylenetriamine
- DGCR8
DiGeorge syndrome critical region gene 8
- DICER
A type of endoribonuclease
- DISC
Death-inducing signalling complex
- DNMT3B
DNA Methyltransferase 3 Beta
- DOTAP
1,2-dioleoyl-3-(trimethylammonium) propane
- DOTMA
(N-[1-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium chloride)
- DROSHA
A class 2 ribonuclease III enzyme
- DSDAP
1,2-distearoyl-3-dimethylammonium-propane
- DSPE
1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine
- EGFR
Epithelial Growth Factor Receptor
- FADD
Fas-associated Death Domain
- FDA
Food and Drug Administration
- FHIT
Fragile histidine triad protein
- GESS
Genome-wide Enrichment of Seed Sequence
- GMNN
Geminin DNA replication inhibitor
- HDAC1
Histone deacetylase1
- HDGF
Hepatoma derived Growth Factor
- HMGA2
High Mobility Group A2, a transcription factor
- HSPC
l-α-phosphatidylcholine, hydrogenated soy
- HUVEC
Human Umbilical Vein Endothelial Cells
- IFNγ
Interferon γ
- IgG
Immunoglobulin G
- IL-1β
Interleukin 1β
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- IL-12p40
Interleukin 12p40
- IL-1α
Interleukin 1α
- IL-2
Interleukin 2
- IL-6
Interleukin 6
- KEAP1
Kelch-like ECH-associated protein 1
- Kras
Kirsten rat sarcoma virus protein
- LCSCs
Lung Cancer Stem Cells
- LNPs
Lipid nanoparticles
- LPH
Liposome polycation-hyaluronic acid
- MCL1
Myeloid leukemia cell differentiation protein
- MDM-2
Mouse Double Minute 8
- MEK
Mitogen-activated protein kinase
- miRNA
microRNA
- MPM
Malignant Pleural Mesothelioma
- MRX-34
Liposome-mediated miR-34a
- mtDNMT
Mitochondrial DNA Methyltransferase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- mt-TET1-3
Mitochondrial ten-eleven-translocase enzyme
- MUC1
Mucin 1
- NGO
Nano graphene oxide
- NLE
Neutral lipid emulsion
- NSCLC
Non-small cell lung cancer
- PACT
Protein kinase RNA (PKR) activator
- p-AKT
Phosphorylated protein kinase
- PAMAM
Polyamidoamine
- PARP
ADP-ribose polymerase
- PCNA
Proliferating cell nuclear antigen
- PD-1
Programmed cell death 1
- PD-4
Programmed cell death 4
- PDCD4
Programmed cell death protein 4
- PDI
Polydispersity Index
- PD-L1
Programmed cell death ligand 1
- PDMS
Polydimethylsiloxane
- PED
Phosphoprotein enriched in diabetes
- PEG
Polyethylene glycol
- PEI
Polyethyleneimine
- p-ERK
Extracellular signal-regulated kinase 1/2
- PGA-co-PDL
(Poly (glycerol adipate-co-ω-pentadecalactone)
- PLGA
Poly (D, l-lactide-co-glycolide)
- PLLA
Poly l-lactic acid polymer
- Pri-miRNA
Primary microRNA
- PTEN
Phosphatase and tensin homolog
- PUMA
p53 upregulated modulator of apoptosis
- QA-CL
Quaternary amine-cationic lipids
- qRT-PCR
Real-Time Quantitative Reverse Transcription
- RA
Retinoic acid
- Ran-GTP
RAs-related nuclear protein-guanosine triphosphate
- RASSF1A
Ras Association Domain Family Member 1A
- RECK
Reversion-inducing-cysteine-rich protein with kazal motifs
- RET
Rearranged during transfection
- RGD
Arginine-glycine-aspartic acid peptide
- RISC
RNA-Induced Silencing Complex
- RNA
Ribonucleic acid
- RNAi
RNA interference
- ROS-1
Receptor tyrosine kinase
- SCID
Severe combined immunodeficiency
- SCLC
Small cell lung cancer
- SELEX
Systematic Evolution of Ligands through Exponential Enrichment
- SIRT
Sirtuin
- SLN
Solid lipid nanoparticle
- SOX-4
SRY-Box Transcription Factor 4
- SPC
Soybean phosphatidylcholine
- STK-11
Serine/threonine kinase 11
- TA-CL
Tertiary amine-cationic lipids
- TEM
Transmission Electron Microscope
- TGF-β
Transforming growth factor β
- TIMP3
Tissue inhibitor of metalloproteinase 3
- TKI
Tyrosine Kinase Inhibitor
- TNFγ
Tumor necrosis factor γ
- TRBP
Transactivation response (TAR) RNA binding protein
- TSG101
Tumor susceptibility gene 101
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UTR
Untranslated regions
- VEGF
Vascular Endothelial Growth factor
- VEGFR
Vascular Endothelial Growth Factor Receptor
- vitE
Vitamin E
- WWOX
WW Domain Containing Oxidoreductase
- YAP-1
Yes-associated protein 1
- ZEB
Zinc finger E-box-binding homeobox
1. Introduction
Lung cancer is one of the highest morbidity and mortality-causing cancer worldwide. According to the global survey conducted by GLOBOCAN 2020, around 11.4% of diagnosed cancer will occur due to lung cancer, and 18.0% of cancer death might occur due to lung cancer [1]. In the USA alone, 2.36 lakhs new lung cancer cases were estimated, and 1.30 lakhs estimated deaths from lung cancer in 2022 [2]. Lung cancer is majorly classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The 5-year survival rate based on the SEER (Surveillance, Epidemiology, and End Results) observation for NSCLC is 25% and for SCLC is 7%, respectively [3].
The clinical studies on lung adenocarcinoma tissues indicate a high incidence of genetic alternations in TP53 (46%), KRAS (Kirsten rat sarcoma virus protein) (33%), KEAP1 (Kelch-like ECH associated protein-1) (17%), STK11 (Serine/threonine kinase 11) (17%), EGFR (Epithelial Growth Factor Receptor) (14%), MET (Tyrosine-protein kinase Met) (7%), etc [4]. Thus, clinicians worldwide are treating lung cancer using the drugs such as monoclonal antibodies and small molecule inhibitors, which target these molecular alternations. However, not all drugs provide a fruitful result even by using FDA (Food and Drug Administration)-approved drugs. Many of the drugs often show severe adverse effects, inability to penetrate deep into the solid tumors, lack of specificity, etc. These are some of the general issues. In the case of monoclonal antibodies, they face problems like penetrability to the tumor site; there are some late-phase failures of monoclonal antibody therapies such as bavituxumab, onartuzumab, and tremelimumab for NSCLC were reported [5]. For instance, bevacizumab treated patients who suffered clinically significant bleeding issues. The same issue was even seen in combination therapy of bevacizumab with carboplatin and paclitaxel [6]. Thus, inferring that these VEGFR (Vascular Endothelial Growth Factor Receptor) targeting antibodies might cause bystander effects on the normal healthy blood vessels, thereby causing bleeding problems [7]. Ipilimumab treatment showed grade 3 or more immune-related adverse effects on 5–25% of patients in clinical trials [8]. Another significant molecular signature in lung cancer is EGFR overexpression or EGFR mutation. Many EGFR inhibitors are being developed and used for treatment. However, some EGFR inhibitors have failed to provide a complete response even if there are high expression levels of EGFR on the lung tumor cells. These are due to the inefficiency of inhibitors in targeting the receptor, and some tumors are intrinsically resistant towards inhibitors, or due to the presence of mutant EGFR, etc [9]. Thus, there is a requirement to design a novel method for targeting lung tumor cells to overcome the problems mentioned above. In short, a novel drug candidate should have high penetration capability, should not generate adverse effects, should not be affected by mutant variants, etc. The miRNA-based therapy could bypass the limitations as mentioned earlier and might act as an alternative strategy for treating lung cancer.
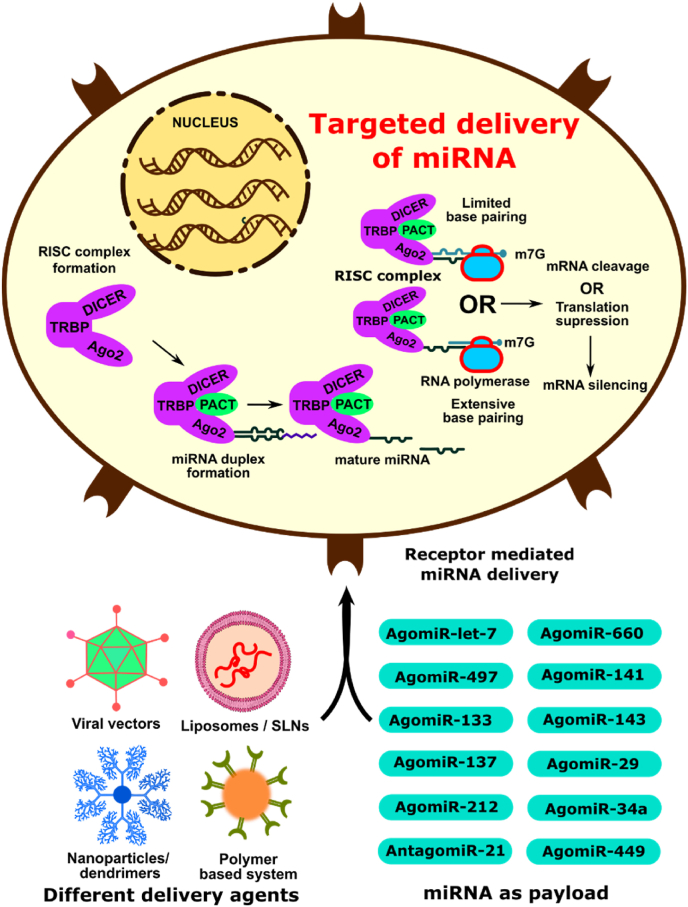
The miRNAs are a class of 19–25 nucleotides chain of single-stranded non-coding ribonucleic acids that regulates gene expression. Only 3–4% of human genes code for miRNA, and this 3–4% of miRNA regulates one-third of the genes in the human genome. This emphasizes the potential of miRNA in regulating protein expression. A single miRNA can bind to as many as 100 different mRNAs and regulate their corresponding protein expression. Thus, miRNA can target many different proteins, unlike siRNA and shRNA. Thus, this “one hit, multiple target” biological effect is one of the therapeutically interesting concepts in miRNA-based therapy. If a tumor condition intervenes in miRNA function, one could externally correct the levels of miRNA expression either by providing agomiR or antagomiR [10].
miRNA-based therapies can be categorized into two major types: (a) miRNA suppression therapy, in which miRNA inhibitors, also known as miRNA antagomiRs or miRNA masks or miRNA sponges, could be used to downregulate the corresponding endogenous miRNA levels (Fig. 1). AntagomiR is used for overexpressed miRNA in tumor cells, thereby will limit the silencing of tumor suppression proteins. Thus, antagomiR-based therapies are known as RNA suppression therapy. (b) miRNA replacement therapy, in which miRNA mimics or miRNA agomiRs or miRNA precursors (pre-miRNA) or miRNA-expressing plasmids could be used to induce the upregulation of miRNA, which leads to posttranscriptional repression. AgomiR is designed for under-expressed miRNA in the tumor cells, and these agomiRs will increase the presence of under-expressed miRNA, thereby will inhibit the translation of the oncoproteins. Thus, agomiR-based miRNA therapy is known as RNA replacement therapy [11].
Fig. 1.
Illustration explaining various methods for delivering miRNA and the mechanism of action of a mature miRNA in silencing a targeted mRNA.
For instance, in the study conducted by Esposito et al., miRNA let-7g was selectively delivered using aptamers, and this miRNA had the capacity to inhibit the function of four different proteins by silencing the expression of HMGA2 (High Mobility Group A2, a transcription factor), N-Ras (a cell proliferation regulator), Bcl-XL (an anti-apoptotic protein), cyclin D1 (a G1 checkpoint regulator) by targeting their corresponding mRNAs [12]. This provides a new modality in miRNA replacement therapy [10]. The main challenge of miRNA therapeutics is the delivery of miRNAs into their site of action, which is the cytosol of target cells. Since they are not stable molecules, it needs to be protected from the nucleases, and delivery of miRNA is one of the crucial tasks to achieve. This review highlights different methods of delivering antagomiRs and agomiRs for lung cancer therapy.
1.1. Role of microRNAs in lung tumorigenesis
miRNA plays an essential role in regulating various proteins, including oncoproteins and tumor suppressor proteins. Dysregulation in the expression levels of these miRNAs can disturb homeostasis and could lead to tumorigenesis. Thus, based on the expression levels of miRNA and their function could be classified as tumor-promoting miRNAs or oncomiRs and tumor-suppressing miRNAs or anti-oncomiR. Usually, in tumor tissues, expression levels of oncomiRs would be higher, and tumor-suppressive miRNAs would be downregulated. For instance, miRNAs involved in lung inflammation, epithelial-mesenchymal transition (EMT), angiogenesis, migration, and cell proliferation will enhance tumorigenesis. miR-92a is an oncomiR that activates STAT3, a cytoplasmic transcription factor which is responsible for the transcription of various growth factors and cytokines. Due to the up-regulation of these transcription factors, STAT3 (which increases the expression levels of cyclin D1 to increase cell proliferation) and BCL-XL (an anti-apoptotic protein), cancer cell escapes from death. STAT3 also inhibits RECK (cysteine-rich protein with Kazal motifs), which is an MMP-2 (metalloproteinase-2) inhibitor that breaks extracellular matrix, which assists invasion in lung cancer cells [13]. miR-151a is overexpressed in metastatic NSCLC tissue. NSCLCs having overexpression of miR-151a stimulates EMT via TGF-β activation. miR-151a silences slug protein, E-cadherin, which are inhibitors of EMTs [14]. Some miRNAs have the ability to increase drug resistance which leads to the failure of chemotherapy. For instance, miR-223 induces doxorubicin resistance and cisplatin resistance, enhances EMT, and regulates autophagy in NSCLC cells [15]. Similar to miR-223, miR-155 confers therapy resistance. Overexpression of miR-155 was observed in the most aggressive and therapy-resistant tumors in various human cancer tissues [16]. miR-214 confers radiotherapy resistance by increasing the expression levels of p38MAPK [17]. miR-30a also radio-sensitizes NSCLCs by targeting activating transcription factor 1 (ATF1). miR-30a also inhibits ionizing radiation-induced G2/M cell cycle arrest in NSCLCs [18]. Zhao et al. induced lung cancer in non-cancerous 16-HBE cells (human bronchial epithelial cells) by carcinogen anti-benzo[a]pyrene-trans-7,8-dihydrodiol-9,10-epoxide (anti-BPDE), and these cells transformed into cancerous, malignant 16-HBE-T cells. These transformed cancerous cells were analyzed for miR-506, which is an anti-oncomiR. It was observed that transformed cells had downregulated miR-506 levels, and restoration of miR-506 reduced cell proliferation, cell cycle arrest in the G0-G1 phase, etc. This indirectly indicates that miR-506 is downregulated in lung cancer cells [19]. Wu and co-workers identified that miR-96 was responsible for inducing cisplatin resistance by downregulating the sterile α motif domain-containing (SAMD9). SAMD9 is a potent tumor suppressor protein that inhibits tumorigenesis [20]. These were some of the examples of miRNA that are involved in cell proliferation, invasion, migration, EMT, inducing drug resistance, etc. Thus, regulation of these abnormal levels of miRNAs either by the downregulation of oncomiRs or by the upregulation of anti-oncomiRs might be a successful strategy for treating lung cancer.
1.2. Challenges and potential solutions for miRNA-based therapy
Even though miRNA has great therapeutic potential, it has limitations such as maintaining stability and circulation period, endosomal escape, lack of specificity in delivery, off-target effects, etc. To increase the potency of miRNA-based therapy, these challenges need to be addressed. For instance, chemical modifications to the oligonucleotide sequence could enhance the stability and protect the oligonucleotides from enzymatic degradation. The lack of specificity could be improved by ligand-assisted receptor-targeted delivery, and endosomal escape can be improved by adding endosomolytic agents, etc [21]. These are some of the challenges that need to be addressed to design a reliable, safe, non-toxic miRNA candidate for successful delivery and for treating lung cancer. Thus, extensive optimization is required to finalize a potential miRNA, a prospective delivery agent, a highly specific targeting ligand, and an endosomolytic agent for its in vitro and preclinical evaluation. Thus, it is essential to understand various techniques of miRNA delivery, their advantages, and their limitations. This review summarizes potential miRNA-based therapy strategies for enthusiasts in the miRNA lung cancer therapeutics field. The scope of this review does not emphasize the use of commercial transfection agents as miRNA delivery since it may not be able to deliver target organ-specifically, lack scalability, and cost ineffectiveness. Based on the therapeutic action of miRNAs, they are categorized as RNA suppression therapy and RNA replacement therapy, which are summarized below (Fig. 2):
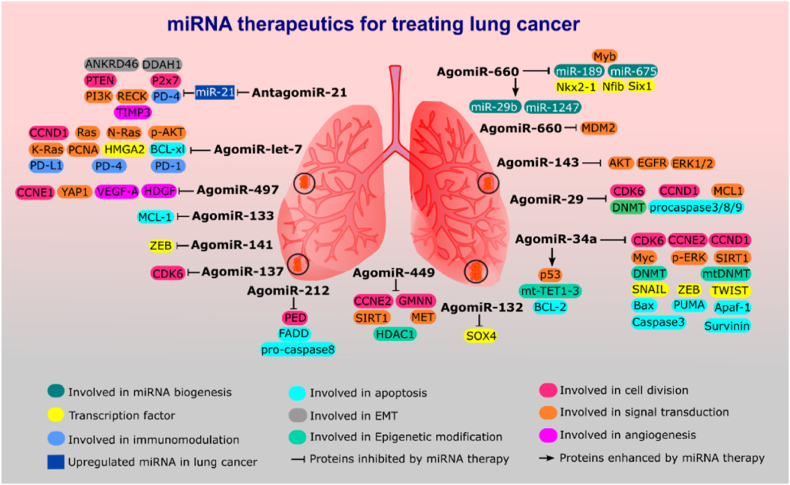
Fig. 2.
Illustrate highlights the miRNA-based therapeutics which can exert effective function in modulation of the various protein targets to hinder tumor growth, invasion, angiogenesis, and immune evasion.
2. RNA suppression therapy in lung cancer
AntagomiRs are anti-miRNAs that block the interaction between the RISC complex and the target mRNA, thereby preventing mRNA translation. For instance, miR-10b [22], miR-21 [23], miR-150 [24], miR-222 [25], miR-96 [20], miR-1290 [26], miR-499 [27], etc. are some of the overexpressed miRNAs in lung cancer. These miRNA-based therapies are differentiated based on their families, and they are enlisted in (Table 1).
Table 1.
Comprehensive information on microRNA suppression therapy for treating lung cancer.
| Sr No | Target miRNA | Delivery | Family | Targeting mRNA | In vitro study | Preclinical studies | Ref |
|---|---|---|---|---|---|---|---|
| AntagomiRs | |||||||
| 1. | AntagomiR-21 | Solid lipid nanoparticles | MicroRNA MIR21 family | miR-21 |
|
- | [28] |
| 2. | AntagomiR-21 | Qtsomes | MicroRNA MIR21 family | miR-21 |
|
- | [29] |
| 3. | AntagomiR-21 | NGO-PEG-dendrimer-antagomiR-21 | MicroRNA MIR21 family | miR-21 |
|
|
[23] |
| 4. | AntagomiR-21 | CaP-lipid/PLLA nanoparticle | MicroRNA MIR21 family | miR-21 |
|
– | [30] |
2.1. MicroRNA-21 family
miR-21 belongs to the microRNA-21 family, which is located on the 17th chromosome in humans, and it is encoded by the MIR21 gene. miR-21 is highly expressed in various solid tumors like lung cancer, breast cancer, colon cancer, gastric cancer, pancreatic cancer, etc. miR-21 is considered as an oncomiR (oncogenic miRNA) since it is involved in enhancing cell growth, metastasis and inhibits apoptosis by targeting Programmed Cell Death-4 (PD-4), Purinergic Receptor P2X7, and phosphoinositide 3-kinases (PI3Ks). In a study conducted by Tian and his team, miR-21 expression was analyzed in 204 pairs of samples of NSCLC patients, and the analysis inferred that higher expression of miR-21 showed a low prognosis and higher chances of metastasis [31]. Thus, it is essential to develop antagomiRs against miR-21 to reduce their expression levels.
Solid lipid nanoparticles (SLN) were used for systemic delivery of antagomir-21 [28]. Shi et al. used DDAB (cationic dimethyl dioctadecyl ammonium bromide) for the creation of a unilamellar vessel which mimics lysosomes. This mode of delivery was significantly more effective than lipofectamine-based delivery. The expression levels of miR-21 were compared with control cells, lipofectamine-antagomiR-21 and SLN-antagomiR-21 treated cells, and levels of miR-21 in SLN treated cells showed the least expression of miR-21 due to antagomiR-21 mediated silencing of miR-21 [28]. One of the major limitations is that the study claims that encapsulation efficacy was 83.42%; however, it is difficult to obtain reproducible encapsulation in the complex design of SLN. This drawback was reflected in the cell viability assay, which did not significantly reduce cell viability in antagomiR-21 treated A549 cells. Another drawback of this study was preclinical studies might have shed more information on the stability, internalization, and toxicity status of SLN-antagomiR-21 liposomes. However, the same group has performed a similar experiment using SLN and a combination of miR-34a and paclitaxel for treating lung cancer and performed detailed analysis on in vivo studies [32]. Anti-proliferation assay revealed that a combination of paclitaxel and miR-34a was found to be more potent than individual miR-34a or paclitaxel in B16F10-CD44+ cells. The tumor volume was drastically reduced in the combination treatment group in the subcutaneous lung cancer-induced mice model, indicating the synergistic effect reduces tumorigenesis. This study once again proves the capability of SLNs as a miRNA delivery vehicle.
Another interesting method of delivering antagomiR-21 that was explored by Yang and his group was using qtsomes [29]. Qtsomes are similar to liposomes made from a combination of quaternary and tertiary amine-based cationic lipids. This combination of quaternary and tertiary amines is required to generate weakly charged liposomes that could easily get internalized by the cancer cell and deliver the antagomiR-21. AntagomiR-21 encapsulated Qtsomes (referred to as Qtsomes) reduced the expression levels of miR-21 by 50.3 ± 2.1%, and upregulated tumor suppression proteins like PTEN (Phosphatase and tensin homolog), PDCD4 (Programmed cell death protein 4), RECK (Reversion-inducing-cysteine-rich protein with kazal motifs), TIMP3 (Tissue inhibitor of metalloproteinase 3) and migration regulators-ANKRD46 (Ankyrin Repeat Domain 46) and DDAH1 (Dimethylarginine Dimethylaminohydrolase 1). The preclinical studies confirmed the therapeutic dose of 1 mg/kg effectively showed a 16-fold tumor volume reduction. There was no significant variation in the spleen and liver weight in treated and untreated groups. Additional studies, such as western blotting analysis of whole mRNA profiling on the antagomiR-21 treated lung tumor tissues, could provide more information on the expression profiles of other proteins. Extensive studies on toxicology, pharmacokinetics, and pharmacodynamics might provide more insight into the safety of the treatment.
A novel method to deliver antagomiR-21 was developed by Wang and co-workers using PEG (Polyethylene glycol) functionalized nanographene oxide (NGO) conjugated with PAMAM (polyamidoamine) dendrimer [23]. The transfection efficiency was observed higher in this conjugate than in dendrimer alone or lipo2000. The probable reason is due to the higher electrostatic interaction between the negatively charged antagomir-21 and the positively charged amino groups on the dendrimer. This efficiency of antagomir-21 was evaluated by the increased expression levels for tumor suppressor proteins PTEN, which was otherwise inhibited by miR-21. Pre- and post-treatment, bioluminescence intensities were captured to study the changes in the subcutaneous lung tumor model, which showed that the group with Fluc-3xPS (lung cancer cells having code for firefly luciferase enzyme and 3 consecutive target sequences for miR-16) showed higher bioluminescence intensity than Fluc-control (plasmid having only firefly luciferase gene). This method of delivery could be promisable for delivering various RNAi; however, one of the major drawbacks of these nanoparticles is the unevenness of the antagomir-21 binding onto the NGO-PEG-dendrimer conjugate. Thus, it would severely affect the reproducibility of the conjugate in treating lung cancer cells. The study did not show a significant decrease in tumor volume post-treatment in the preclinical mice model. However, there was a decrease in the bioluminescence intensity post-treatment. Thus, it may be concluded that the antagomir-21 has the ability to inhibit the synthesis of miR-21 [23].
A similar study led by Sriram and his group to deliver antagomir-21 using calcium phosphate coated lipid/poly l-lactic acid polymer (PLLA) based nanoparticle along with chemotherapeutic drug doxorubicin for creating the synergistic effect in killing NSCLCs [30]. Calcium phosphate coating on the nanoparticle maintains stability and enhances the chance of endosomal escape for releasing antagomir-21 and doxorubicin into the cytoplasm. Release kinetics of antagomiR-21 and doxorubicin were studied using a fluorescence plate reader at pH 5 (mimicking intracellular microenvironment), 100% of antagomir-21 was released, and at pH 7 (mimicking systemic circulation), ∼70% of antagomir-21 was released in 48 h. There is a lack of specificity in the release of antagomir-21 since it could release miRNA even during systemic circulation. This might be one of the limitations of this nanoparticle. There was a decrease in the miR-21 concentration and an increase in the PTEN concentration after the antagomir-21 treatment. However, preclinical studies highlighting the toxicity of the nanoparticle might provide more information on its potential to be a novel drug candidate [30].
3. RNA replacement therapy in lung cancer
RNA replacement therapy includes using miRNA mimics that are synthetic double-stranded RNA that tends to simulate the endogenous downregulated miRNA. These miRNAs are known as agomiRs. Some of the examples of agomiRs in lung cancer are let-7, miR-29, miR-15/16, miR-34, miR-200, miR-212, miR-660, etc [[33], [34], [35], [36], [37], [38], [39]]. There are two methods for inducing mimics into the cellular system, i.e., by directly adding mature miRNA mimics or pre-miRNAs. Both these forms of miRNAs will lead to the suppression of targeted mRNA and these strategies are summarized in Table 2.
Table 2.
Comprehensively combines the studies that are developed using RNA replacement therapy in lung cancer.
| Sr No | Target miRNA | Delivery | Family | Targeting mRNA | In vitro study | Preclinical studies | Ref |
|---|---|---|---|---|---|---|---|
| AgomiRs | |||||||
| 1. | AgomiR let-7a | Liposomes | MicroRNA MIR let-7 family | Ras, N-Ras, and K-Ras |
|
– | [40] |
| 2. | AgomiR let-7g | GL21.T aptamer | MicroRNA MIR let-7 family | HMGA2, N-Ras, Bcl-XL, cyclin D1 |
|
|
[41] |
| 3. | AgomiR let-7b | Nanoassemblies | MicroRNA MIR let-7 family |
Ras, MYC, HMGA2, GAB2, and FN1 |
|
|
[42] |
| 4. | AgomiR let-7b | Aerosols of let-7b miR | MicroRNA MIR let-7 family | PD-L1, PD-1 |
|
|
[43] |
| 5. | AgomiR-497 | Exosome | MicroRNA MIR15/16 family | YAP-1, HDGF, CCNE-1, and VEGF-A |
|
– | [44] |
| 6. | AgomiR-29 | Lipoplexes | MicroRNA MIR29 family | CDK-6, DNMT3B, and MCL1 |
|
|
[45] |
| 7. | AgomiR-29 | MUC1 aptamer targeted polymer-based nanoparticle | MicroRNA MIR29 family | CDK-6, DNMT3B, and MCL1 |
|
– | [46] |
| 8. | miRIDIAN miR-29b | MUC1 aptamer targeted MAFMILHN nanoparticle | MicroRNA MIR29 family | DNMT3B |
|
|
[47] |
| 9. | AgomiR-29 | P103-PEI-RA/miR-29b and P123-PEI-RA/miR-29b | MicroRNA MIR29 family | CDK-6, DNMT3B, and MCL1 |
|
– | [48] |
| 10. | AgomiR-29 | PEN-miR-29a | MicroRNA MIR29 family | Caspase-3, caspase-8, caspase-9, PARP, and cyclin D1 |
|
– | [49] |
| 11. | AgomiR-34a | scFv GC4-Liposome-polycation-hyaluronic acid (LPH) nanoparticle | MicroRNA MIR34/449 family | c-Myc, MDM-2 and VEGF by siRNA survinin and p-ERK by miR-34a |
|
|
[50] |
| 12. | AgomiR-34a and miR-let-7 | Neutral lipid emulsion | MicroRNA MIR34/449 family | Kras, Ki-67 | – |
|
[51] |
| 13. | AgomiR-34a | 7C1-miR34a | MicroRNA MIR34/449 family | CCND1, SIRT1, CDK6, and CCNE2 |
|
|
[52] |
| 14. | AgomiR-34a | Multi-layered liposomes | MicroRNA MIR34/449 family | Ki-67, CC3, CDK6, SIRT1 |
|
|
[53] |
| 15. | AgomiR-34a | HA-PEI/HA-PEG encapsulation nanoparticle | MicroRNA MIR34/449 family | Bax, Apaf-1, PUMA, caspase3, Bcl-2, DNMT1, mtDNMT, and survivin |
|
– | [54] |
| 16. | AgomiR-34a | Folate targeted miRNA | MicroRNA MIR34/449 family | Bcl-2, Met, and Myc |
|
|
[55] |
| 17. | AgomiR-34a and paclitaxel | SLN | MicroRNA MIR34/449 family | CD44 |
|
|
[32] |
| 18. | AgomiR-34a | Lentivirus | MicroRNA MIR34/449 family | CD44 |
|
– | [56] |
| 19. | AgomiR-449a | Microbubble | MicroRNA MIR34/449 family | NOTCH |
|
|
[57] |
| 20. | AgomiR-212 | Liposomes | MicroRNA MIR132/212 family | PED |
|
– | [58] |
| 21. | AgomiR-200c | Nano-emulsion | MicroRNA MIR141/200 family | Nkx-2.1, Nfib, Myb, Six1 |
|
– | [59] |
| 22. | AgomiR-200c | Polymersomes | MicroRNA MIR141/200 family | Red cell hemolysis |
|
|
[60] |
| 23. | Pre-miR-133b | Lipoplexes | MicroRNA MIR133 family | MCL-1 |
|
|
[61] |
| 24. | pVAX plasmid containing miR-143 | Lipoplexes | MicroRNA MIR143 family | p-EGFR, p-AKT, p-ERK1/2 |
|
|
[62] |
| 25. | miR-660 | Cationic lipid nanoparticles | MicroRNA MIR188/532 family | MDM2 |
|
|
[63] |
| 26. | miR-320a | Gold nanoparticle | MicroRNA MIR320 family | Sp-1 |
|
|
[64] |
3.1. MicroRNA-let-7 family
Let-7 belongs to the let-7 family, and it is the second miRNA discovered in Caenorhabditis elegans. Let-7 miRNA is involved in the regulation of cell proliferation and regulation of the TGF-β and Wnt pathways. Let-7 miRNA functions as a tumor suppressor miRNA [65]. It is found that let-7 is downregulated in several tumors such as acute lymphoblastic leukemia, breast cancer, bronchioalveolar cancer, Burkitt lymphoma, colon cancer, bladder cancer, gastric cancer, kidney cancer, hepatocellular cancer, lung cancer, ovarian cancer, pancreatic cancer, prostate cancer, etc [66]. Thus, upregulation of let-7 miRNA is essential to regulate cell proliferation and migration in lung cancer.
Lee and co-workers synthesized lipid nanoparticles (LNPs) using DOTAP (1,2-dioleoyl-3-(trimethylammonium) propane)/cholesterol/DSPE-PEG (1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine) and coated ephrin-A1 on the surface of LNPs to target the ephrin-A2 receptor on the lung cancer cells. The Ephrin-A2 receptor is involved in cell adhesion and migration [40]. Transfection of let-7a miR reduced the cell proliferation and migration of A549 cells by 20–30%. The transfection of LNP-let-7a miR has significantly reduced the expression levels of onco-mRNAs of Ras, K-Ras, and N-Ras proteins. Let-7a miR was highly effective in attenuating colony formation and migration of MPM (Malignant Pleural Mesothelioma) cells and NSCLC cells. Encapsulation efficiency of let-7a miR ensured that there is a presence of miR on the LNP; however, there could be variation in the concentration of let-7a miR inside the LNP that could radically change the efficiency of the LNP in each dose. Toxicity studies on preclinical lung cancer models or MPM models would assist in analyzing the off-target toxicity of let-7a miR-based therapy [40].
Esposito and her team synthesized Axl targeting aptamers via systematic evolution of ligands through the exponential enrichment (SELEX) method [41]. GL21.T is the aptamer that binds to the Axl receptor (a tyrosine kinase receptor), mostly associated with invasive and metastatic cancer cells. To protect the aptamer-conjugate from nuclease degradation, the pyrimidines in the miR let-7g were modified to 2′-fluoropyrimidines. GL21.T-let-7g was analyzed on two different cell lines, i.e., A549 cells (Axl positive cells) and MCF-7 (Axl negative), to check the selectivity of the conjugate. let-7g miR in silenced HMGA2 (High Mobility Group A2, a transcription factor), N-Ras (a cell proliferation regulator), Bcl-XL (an anti-apoptotic protein), cyclin D1(G1 checkpoint protein). Gl21.T-let-7g miR conjugate has reduced the viability to 30–40% and migration ability to 60%. The conjugate was further analyzed on a preclinical subcutaneous lung cancer mice model, and the conjugate significantly reduced tumor burden. Thus, it also proves that the conjugate is selective in targeting the Axl receptor in vivo conditions. This study was the first example of generating a multifunctional aptamer conjugate where aptamer could target miRNA delivery and inhibit Axl receptor signaling. This study has great potential as an ideal candidate for miRNA delivery. Thus, there is a need to synthesize novel aptamers targeting different cancer types to enhance tiRNA delivery [41].
A novel nano-assembly was formed using PEG5000, vitamin E (VitE), and diethylenetriamine (DET) to deliver paclitaxel and let-7b miRNA [42]. The endosomal escape mechanism of paclitaxel and let-7b miRNA are designed such that in the presence of cathepsin B (which is a lysosome protease) releases the contents from the nanoassemblies. Cellular internalization of let-7b was evaluated after 8 h of administration. The efficacy of let-7b by evaluating the diminished expression leaves of Kras, p-AKT (Phosphorylated protein kinase), and PCNA (Proliferating cell nuclear antigen). The preclinical evaluation confirmed that there is no toxic effect due to the presence of nano-assemblies [42]. Thus, it was also observed that paclitaxel and let-7b miRNA showed synergistic activity to decrease the tumor burden. Thus, this formulation can be used to deliver various miRNA therapeutics. It could be one of the potential candidates for delivering miRNAs.
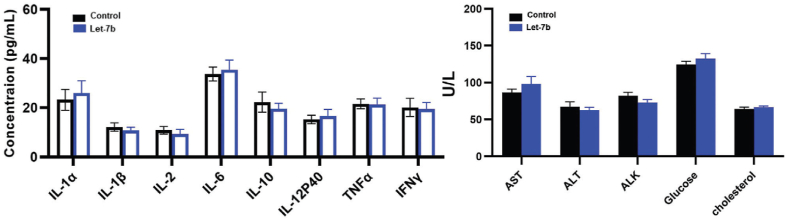
Another interesting study was conducted by Zhang et al., who developed an aerosol-based system for delivering let-7b miRNA [43]. They used a custom-made collision-type atomizer that could atomize let-7b miRNA. This strategy seems to be highly useful for cancer patients. Since there is minimum morbidity in the case of this treatment and thus, many patients will be encouraged to adopt this treatment. Another aspect of this treatment is that the aerosol will directly go to the site of the tumor location rather than the other injection strategies, thus minimizing the off-target effects, drug dilution effect, etc. Experimentally, it was proved that an aerosol-based system is a novel and potential delivery method to transfer miRNA in Benzo[a]pyrene-induced lung adenoma cancer model. Single-cell RNA sequencing on CD45+ tumor-infiltrating T cells isolated from aerosol-treated lung adenoma-induced cancer model revealed the ability of miRNA to silence the expression levels of PD-L1 and PD-1. Body weights, serum cytokines, and liver enzyme evaluation assays revealed that the aerosol-based let-7b miRNA delivery mechanism did not induce toxicity (Fig. 3) [43]. These immunomodulating effects in tumor-infiltrating T cells and anti-tumorigenic properties of aerosol-based let-7b miRNA delivery could be considered a great strategy in the new era of anti-cancer treatment. This study has much potential in translational research; however, tests such as the shelf life of let-7b miRNA in aerosol bulk production of miRNA-based aerosols are critical aspects that cannot be neglected while considering these therapeutic methods.
Fig. 3.
Potential toxicities of aerosolized Let-7b were assessed by examining serum cytokines and plasma levels of the liver enzymes (n = 6). Reproduced under copyright CC BY-NC 4.0 licence [43].
3.2. MicroRNA-15/16 family
miR-15, miR-16, and miR-497 belong to the miR15/16 family, and it is located at chromosome 13q14.3 [67]. miR15/16 family of miRNAs are downregulated in various solid tumors, which are tumor suppressor miRNAs. A study showed that the miR-16 expression was 22-fold downregulated in MPM tumors than in normal tissue [68]. Jeong et al. used exosomes from HEK293T cells and transfected miR-497 using lipofectamine [44]. The loading efficacy of the miR-497 was 22.4%. The dose evaluation study in A549 cells revealed 1 × 107 particles/μl as the optimum dosage. A cellular internalization study revealed that miR-497 was 25000 times higher in miR-497 loaded exosomes treated group than in control exosomes treated groups (miRNA unloaded exosomes) [44]. miR-497 loaded exosomes could inhibit 69.2% of migrant lung cancer cells. Further, Western blot analyses also confirmed a decrease in the concentration of migration-related proteins (YAP-1 (Yes-associated protein 1), HDGF (Hepatoma Derived Growth Factor), CCNE-1 (cyclin E), VEGF-A (Vascular Endothelial Growth Factor)). A microfluidic device was fabricated using PDMS (Polydimethylsiloxane) having three different channels, the first channel is for growing A549 cells mimicking tumor-affected lung portion, and the second channel was layered with collagen gel to mimic trans-epithelial membrane with pores for communicating between these two layers, and the third channel is for culturing HUVECs (Human Umbilical Vein Endothelial Cells) mimicking blood vessels. miR-497 loaded exosomes inhibit migration of A549 cells by downregulating VEGF-A and also inhibit migration in HUVEC cells in a gradient VEGF exposed environment, thus, inhibiting neo-angiogenesis. In the co-culturing system, inhibition of angiogenesis was visualized by structural changes like reduced tube number, tube area, and migration area. The key advantage of this study is that miR-497 could affect the tumor cells, influence nearby cells, and focus on creating an anti-tumorigenic microenvironment environment [44]. This work could be extended by creating a more lung tumor-like environment involving lung cancer stem cells and cancer-associated fibroblasts and by creating an air lung interface (ALI). Furthermore, an ALI-based system could also be utilized to study the impact of miR-497 on immune cells in the lung cancer microenvironment.
3.3. MicroRNA-29 family
miR-29 gene is located at two different locations, i.e., 1q32 and 7q32. miR-29 is downregulated in lung cancer, esophageal carcinoma, stomach cancer, hepatocellular carcinoma, cholangiocarcinoma, glioblastoma, neuroblastomas, osteoblastoma, rhabdomyosarcoma, ovarian cancer, breast cancer, lymphoblastic leukemia, nasopharyngeal carcinoma [69]. miR-29 is involved in DNA regulation by silencing DNMTs (DNA methyltransferase) [70]. Because upregulation of DNMTs leads to the silencing of various tumor suppressor genes involved in lung carcinogeneses, such as CDKN2A (cyclin-dependent kinase inhibitor 2A), CDH13 (cadherin-13), FHIT (fragile histidine triad protein), WWOX (WW Domain Containing Oxidoreductase), CDH1 (cadherin-1), and RASSF1A (Ras Association Domain Family Member 1) [70].
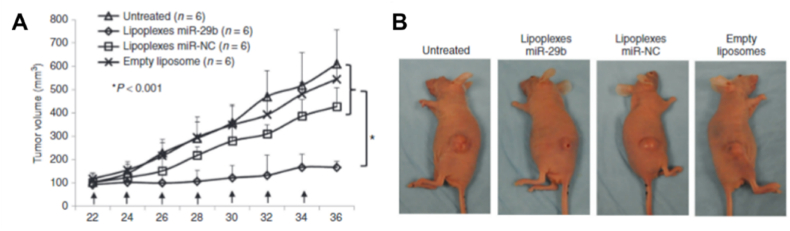
miRNA-29b is highly downregulated in 7/10 NSCLC tumor tissue samples. Wu and co-workers created lipoplexes to deliver miR-29b for treating lung cancer [45]. A549 cells were transfected with miR-29b using lipoplexes and NeoFX (commercial transfection agent). miR-29 expression was increased to 1230-fold and 98-fold in lipoplexes-miR-29b and NeoFX-miR-29b as delivery agents, respectively. The expression levels of CDK-6 (Cell division protein kinase 6), DNMT3B (DNA methyltransferase), and MCL1 (Myeloid leukemia cell differentiation protein 1) were significantly reduced in the miR-29 loaded lipoplexes. DNMT3B is a DNA methyltransferase enzyme that establishes new methylation patterns that could affect unmethylated CpG sites on gene promotes, eventually reprogramming the epigenome in lung cancer [71]. MCL1 is an anti-apoptotic gene [72]. A549 cells induced subcutaneous lung tumor model showed that there was a 69.3% tumor reduction volume post-miRNA-29b treatment (Fig. 4A and B). In vivo studies revealed tumor tissue having increased levels of miR-29b, reduction in tumor cell proliferation, and downregulation of CDK6, DNMT3B, and MCL1 [45]. This study highlights the use of miR-29b as an agomiR for treating lung cancer; however, some problems are associated with using lipoplexes as delivering agents, i.e., irregularity in the miRNA loading onto the system, which may impact the progress of the treatment.
Fig. 4.
Lipoplexes-miR-29b inhibit tumorigenicity of A549 cells in vivo. (A, B) Tumor growth in A549 cells induced nude mice. Reproduced under copyright CC BY-NC 3.0 licence [45].
Perepelyuk and her team also used miRNA-29b, like in the previous study; however, the mode of delivery of miRNA was using aptamer-targeted polymer-based nanoparticles [46]. In this study, a polymeric nanoparticle was synthesized using human immunoglobulin G (IgG) and poloxamer-188, which was coated using a MUC1 (Mucin 1) aptamer that targets the MUC1 protein. The MUC1 is a transmembrane protein that is highly upregulated in NSCLC (almost >80% in lung adenocarcinoma tissues). Thus, targeting MUC1 aptamer will specifically deliver miR-29b onto the target site of action. In vitro release studies showed that miR-29b is released better at pH 5, indicating endosomal escape of miRNA-29b. These nanoparticles showed a decrease in DNMT3B and MCL1 proteins compared to miR-29b transfected using lipofectamine 2000. However, the same group extended the work by using novel hybrid nanoparticles (MAFMILHN) and MUC1 aptamer [47]. MAFMILHN was prepared by mixing IgG and miRIDIAN miR-29b (miRIDIAN miRNA mimics are double-stranded RNA oligonucleotides designed to function as endogenous mature miRNAs) to form a hybrid nanoparticle. These nanoparticles were coated with poloxamer-188 for functionalizing MUC-1 aptamer. Like the previous study, these nanoparticles were also efficiently internalized in A549 cells. MAFMILHN showed downregulation of DNMT3B protein and a higher rate of apoptosis. Biodistribution studies in lung tumor-bearing SCID (Severe combined immunodeficiency) mice were performed. The results revealed that the lungs had the maximum deposition of miR-29b, thus indicating the specificity of MUC1 aptamers. Furthermore, a bioluminescence imaging assay was conducted to assess the tumor burden and tumor volume was also severely diminished in the MAFMILHN treated mice model. Thus, aptamer functionalized hybrid nanoparticles can potentially deliver miRNAs in NSCLC for downregulating oncogenes.
A multifunctional micellar nanosystem consisting of Pluronic P123/Pluronic P103 linked to Polyethyleneimine (PEI) was developed to deliver retinoic acid (RA) and miR-29b to treat NSCLC by Magalhães and her team [48]. Retinoic acid has a role in inhibiting cell proliferation via initiating RA-dependent apoptosis [73]. Cell viability assay confirmed that it is non-toxic to HEK293T (non-cancer cell line) while highly cytotoxic to A549 cells and H1299 cells, thus indicating specificity of delivery mechanism of miR-29b and RA. RA loaded miscelleplexes showed a much better reduction in tumor cell migration rather than miR-29b loaded miscelleplexes. Moreover, the expression levels of DNMTs (DNA methyltransferase) were significantly reduced in miR-29b loaded miscelleplexes treated tumor cells, indicating the post-translation repression of DNMTs by miR-29b. From these studies, it can be inferred that miR-29b and RA shows synergistic action in treating NSCLC cells. Furthermore, it was also elucidated that P103-PEI-RA/miR-29b miscelleplexes showed a stronger synergistic effect in treating NSCLC than P123-PEI-RA/miR-29b. Preclinical evaluation of these miscelleplexes might reveal the toxicological aspects. Further exploration might be useful in understanding other potential targets of miR-29b, which might have supported the anti-tumorigenic effect.
PEI derivative N-isopropylacrylamide-modified (PEN) was used as a carrier to deliver miR-29b by Xing et al. [49]. PEN-miR-29a induced cell apoptosis inhibits invasion and migration and induces cell cycle arrest in the G1 phase. It was also observed that apoptosis-inducing protein expression levels (procaspases-3/8/9, PARP) were severely diminished in PEN-miR-29a treated tumor cells, indicating that they are converted into active caspases-3/8/9 for initiation of apoptosis. PARP (ADP-ribose polymerase) is one of the caspase 3 cleavable enzymes that again confirm the initiation of the apoptosis pathway. Cell proliferation was decreased, which was indicated by decreased expression of cyclin D1 [49]. Thus, it can be concluded from the in vitro analysis that PEN-mediated miR-29a delivery could positively destroy tumor cells and could be a potential candidate for preclinical studies and to understand its biodistribution. However, this conjugate could be specifically tagged with some lung cancer cell-specific ligands to increase the efficacy and specificity of the conjugate.
3.4. MicroRNA-34/449 family
miR-34 and miR-449 are the two different microRNAs belonging to the miR34/449 family. miRNA-34 is dysregulated in various cancers. This is the first miRNA to enter clinical trials. miR-34 is also a tumor suppressor miRNA that is downregulated. miR-34 represses epithelial-mesenchymal transcription factors like p53, zinc-finger transcription factor, SNAIL factor, ZEB transcription factor, TWIST transcription factors, etc. It is also involved in the inhibition of cell proliferation and enhances cancer cell apoptosis [74]. Daugaard and her colleagues investigated the expression levels of miR-34 in non-malignant and malignant NSCLC tissues, and the results concluded that malignant tissues exhibited low expression levels of miR-34 [75]. Thus, it is required to emphasize the upregulation of miR-34 for successful RNA replacement therapy. Similar to miR-34, miR-449 is also involved in regulating the cell cycle involving proteins such as MET, GMNN (Geminin DNA replication inhibitor), CCNE2, SIRT (sirtuin), HDAC1 (Histone deacetylase), etc. These are some of the direct targets of miR-449 [76].
A nanoparticle-based delivery system by combining liposome-polycation-hyaluronic acid (LPH) was conjugated with single-chain antibody fragment scFv GC4 [50]. This nanoparticle was intended for delivering siRNA targeting c-Myc, MDM-2 (Mouse Double Minute 8) and VEGF, and miRNA-34a in treating lung cancer. The GC4 scFv identifies a genetically conserved epitope in tumor cells, thereby increasing selectivity in delivering siRNA and miRNA. The efficacy of GC4-siRNA-nanoparticles was evaluated in a B19F10induced metastatic lung cancer model in C57BL/6 mice. GC4-targeted nanoparticles were able to reduce metastasis by 30% in B16F10 lung cancer as compared to the control group. This study was confirmed by the haematoxylin and eosin-stained tissue section, which showed reduced metastatic nodules in the lung tissue. Expression levels of survivin and p-ERK (phosphorylated-Extracellular signal-regulated kinase 1/2) were severely decreased in GC4-miR-34a-nanoparticles treated with B16F10 cells indicating their downregulation of tumor progression. TUNEL assay was performed to estimate the role of miR-34a, and the results suggested that miR-34a had triggered apoptosis. Furthermore, the authors evaluated the synergistic effect of siRNA and miR-34a, which indicated that metastatic nodules were significantly reduced in the presence of the siRNA and miR-34a co-formulation method. Toxicity studies were also conducted on tumor-induced mice by evaluating the levels of pro-inflammatory cytokines (IL-6, IL-12, TNF-γ) and certain liver enzymes like AST (Aspartate aminotransferase) and ALT (Alanine aminotransferase); and the results indicated that GC4 targeted nanoparticle formulation is safe and effective in delivering oligonucleotides [50]. Thus, these results indicated that co-delivering by GC4- nanoparticles could inhibit tumor growth and enhance the therapeutic outcome.
A neutral lipid emulsion (NLE) has been used to deliver let-7 and miR-34a for treating lung cancer [51]. Biodistribution studies were carried out by using NLE-miR-124. This study highlighted that maximum distribution was observed in the lung, liver, and followed by the kidney. Orthotopic lung tumor models were generated by endotracheal intubation of H460-luc lung tumor cells to analyze the efficacy of NLE-siRNA (for silencing luciferin). The tumor growth was monitored via luminescence imaging. After 48 h of treatment, there was a decrease in the luminescence intensity by >95%; however, in the negative group, the intensity was increased. This study deciphers that RNAi delivery led to cellular entry, and it can form a RISC complex and efficiently repress the target, i.e., luciferin. Furthermore, the efficacy of NLE-miR-34a and miR-let-7 was determined in the K-rasG12D autochthonous NSCLC mouse model. This model was created by intranasal administration of adenovirus expressing (Ad-cre) that could induce G12D mutation in K-ras protein. Mice treated with miR-NC had extensive diffuse hyperplasia and adenomas, while 4/5 mice treated with NLE-let-7b had a lower tumor burden. qRT-PCR studies inferred an increase in the expression of let-7b. Later, TUNEL assay and Ki-67 staining were performed to evaluate the apoptosis levels, and the results showed that there were poor apoptosis levels. It was concluded that lung tumors did not respond to the let-7b treatment. NLE-miR-34a treatment showed 60% tumor reduction. qRT-PCR analysis showed higher expression levels of miR-34a in lung tissues. TUNEL-positive cells were reduced to 20.2, while in the negative control, it was 51.5. Thus, it was concluded that K-ras-induced mice respond to NLE-miR-34a treatment [51]. Thus, this treatment strategy could be a potential candidate for further analysis to enter clinical trials. However, certain questions are unanswered in these experiments as it was unable to clarify the use of miR-124 to study biodistribution. Interestingly it was observed that there was a higher expression of let-7b; however, it cannot decrease the tumor burden.
Systemic delivery of miR34a via 7C1 polymer-based nanoparticle in autochthonous Kras p53 mutant lung cancer mice model was reported recently [52]. Like in the above-mentioned study, authors of this study have also conducted siRNA against luciferase (siLuc) based delivery in p53 deleted, Kras mutant mice model having reporter genes luciferase and tdTomato. 7C1-siLuc selectively silenced the luciferase signal. Later, 7C1-miR34a was delivered in the KP mice model, and the results showed a reduction in the expression of CCND1, SIRT1, CDK6, and CCNE2 proteins, and there was a 27-fold increase in the relative expression levels of miR-34a. There was a significant decline in the tumor burden after 4 weeks from the first injection. Furthermore, siRNA against Kras was also administered using 7C1 polymer, which showed great anti-tumor activity. This study also proves that miR-34a could be delivered safely by 7C1 nanoparticle formulation. Another study was conducted by Wang et al., who developed aptamer-conjugated dendrimers to deliver the plasmid-containing genes for miR-34a [77]. S6 aptamer is specifically targeted to recognize lung tumor cells and deliver pMiR-34a (plasmid expressing miR-34a). Later it was found that miR-34a had increased the expression of BCL-2 and p53. Cell viability was reduced to almost 40% due to the transfection of pMiR-34a. Although this study showed convincing results, however, plasmid transfection is a transient expression of miR-34a, and thus, there will be a requirement for multiple doses of treatment.
Gu and co-workers synthesized a multi-layered liposomes membrane consisting of core liposomes containing cisplatin, coated with miR-34a and siKras, a layer of poly l-arginine to enhance endosomal escape, and finally, a layer of hyaluronate having CD44 targeting IgG [53]. The combination of cisplatin, siKras, and miR-34a showed the highest reduction in the tumor burden in the KP lung adenocarcinoma orthotopic mouse model. Immunohistochemistry analysis revealed that biomarkers responsive for cell proliferation (Ki67), apoptosis (CC3), and downstream molecules of Kras pathway like phosphorylated-ERK, proteins of a miR-34a pathway like CDK6 and SIRT1 are significantly reduced. This study claims that the presence of a multi-modular system acts as a broad range of therapeutics. Thus, this system can target various oncogenic pathways; however, it can also be used as a tool for personalized medicine in the future by tuning the outer surface area.
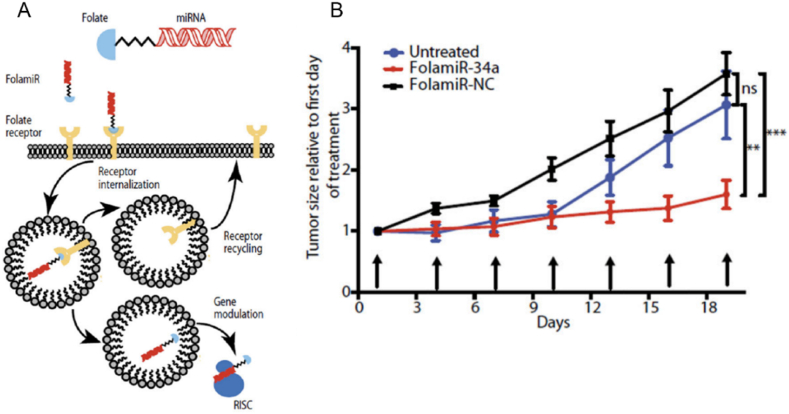
A novel miR-34a conjugate (FolamiR-34a) was developed using folate as a targeting ligand to conjugate miR-34a using click chemistry methods (Fig. 5A) [55]. FolamiR-34a targeted folate receptors that are overexpressed in the cancer cells, and this was evaluated in folate-positive MDA-MB-231 cells and folate-negative A549 cells. To assess the activity of FolamiR-34a, MDA-MB-231 cells were expressed with the miR-34 Renilla sensor, and in the presence of FolamiR-34a, the activity of Renilla was decreased since the genes were silenced by miR-34a. Furthermore, the efficiency of FolamiR-34a was evaluated in an immunocompetent aggressive Kras; p53 NSCLC mouse model, and there was a decrease in the tumor burden by 1.5-fold (Fig. 5B). The levels of miR-34a increased by 3-fold post-treatment, and levels of Bcl-2, Myc were reduced, indicating initiation of apoptosis. This study is highly significant since the conjugate targets tumor cells, such as folate overexpressing lung tumor cells. Other methods like lipoplexes-mediated, liposome-mediated, nanoparticles-mediated, and virus-mediated deliveries do not specifically aim at targeted delivery. Thus, this aspect of the FolamiR-34a acts as a potential candidate for further clinical trials.
Fig. 5.
(A) Illustration explaining the mechanism of action of FolamiR-34a (B) Quantitative analysis of tumor size post FolamiR-34a treatment, where arrows represent treatment intervals (0.8 mg/kg, 1 nmol intravenous injection). Reprinted with permission from Ref. [55] Copyright © 2017, The American Association for the Advancement of Science.
HA-PEI/HA-PEG encapsulated nanoparticles have been reported to deliver miR34a for targeting lung cancer by Trivedi and his colleagues [54]. Cellular uptake studies were evaluated in cisplatin-resistant A549 cells and cisplatin-sensitive A549 cells by using rhodamine labelled HA-PEI, and FITC labelled miR34a. The results inferred a 4-5-fold increase in the levels of miR34a in both A549 cells after 4 h of incubation. qRT-PCR analysis was done to analyze apoptosis genes like anti-apoptotic proteins (Bax (BCL2 Associated X protein), Apaf-1 (Apoptotic protease activating factor 1), PUMA (p53 upregulated modulator of apoptosis), caspase3) and pro-apoptotic proteins (Bcl-2, survivin). As expected, there was an increase in pro-apoptotic proteins, and a decline in anti-apoptotic proteins was observed in cisplatin sensitive as well as resistant A549 cell lines. miR-34a has altered the mitochondrial epigenetic enzymes like mtDNMT1 (Mitochondrial DNA Methyltransferase). The expression levels of DNMT1 (non-mitochondrial origin) declined by 15–20% in both the A549 cells and mtDNMT levels decreased by 30–40%. This was reflected in the increased expression levels of the mitochondrial ten-eleven-translocase enzyme (mt-TET1-3). mt-TET1-3 is involved in the methylation of DNA. This study could be a potential target for delivering nucleic acids for lung cancer. The novel development of this study was to focus on the contribution of miR-34a towards mtDNA epigenetic changes in lung cancer progression and development; further studies on the preclinical evaluation of such epigenetic transformation in a relevant tumor model could be of greater potential.
Another study was conducted using microbubbles as a novel miRNA-449a delivery system. miRNA-449a also belongs to the miRNA-34 family [57]. The ultrasonic dispersion technique was used for synthesizing cationic lipid microbubbles. miR-449a is severely downregulated in lung tumor tissue, and increasing the miR449a levels might inhibit epithelial-mesenchymal transition by regulating the NOTCH pathway. Microbubbles-mediated delivery of miR-449a promotes cell cycle arrest in the G2/M phase and induces apoptosis. Xenograft tumor model in mice has also confirmed the efficacy of miR-449a containing microbubbles and that there was a decrease in the weight and tumor volumes. Ki-67 staining also confirmed decreased levels of Ki-67 positive cells in the presence of miR-449a. Thus, indicating that miR-449a inhibits proliferation. In all the above studies, only lung cancer cells were analyzed with the corresponding miRNA-based therapeutic agent; however, Shi and co-workers attempted a different strategy to target lung cancer stem cells by isolating CD44 positive Lung Cancer Stem Cells (LCSCs) from three different cell lines, namely A549 cells, H460 cells and H1299 cells [56]. Isolated LCSCs were subjected to lentiviral vector-mediated overexpression of miR-34a. Interestingly, all the CD44 positive LCSCs from various origins showed decreased cell proliferation, migration, and clonogenic properties. CD44 negative cells showed an increase in tumor growth. It indicates that miR-34a acts as a negative regulator for CD44 expression in NSCLC cells. Thus, targeting LCSC is one of the novel and need-of-the-hour strategies for treating cancer.
3.5. MicroRNA-132/212 family
miR-132 and miR-212 belong to the microRNA-132/212 family, and it is encoded by chromosome 17p13.3 [78]. miR-132 is significantly downregulated in lung cancer cells. miR-132 is the direct target of SOX4 (SRY-Box Transcription Factor 4) protein and can downregulate in the invasion. SOX4 protein is also involved in β-catenin/T-cell factor activity and acts as an agonist of the Wnt signaling pathway [79]. miR-212 is also involved in suppressing the invasion and migration of lung cancer cells by inhibiting USP9X-induced EMT. Hence, it is downregulated in lung cancer tissues. Thus, it is necessary to upregulate the expression of these miRNAs to reduce tumor growth [80].
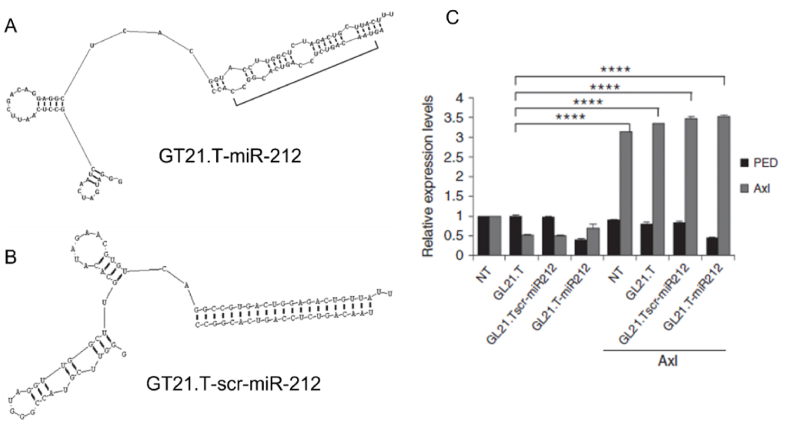
Iaboni and her colleagues synthesized a novel chimera molecule with an aptamer targeting the Axl and agomiR-212 (Fig. 6A and B) [58]. GL21.T is used as an aptamer to target the Axl receptor. miR-212 silences the PED (Phosphoprotein enriched in diabetes) protein inhibiting intrinsic and extrinsic apoptosis pathways. It competitively interacts with pro-caspase8 and FADD (Fas-associated Death Domain), inhibiting DISC (Death-inducing signalling complex) assembly. Overexpression of PED is observed in NSCLC cells. Thus, by silencing the PED expression via miR-212, apoptosis can be initiated in these NSCLC cells. They first prepared two sets of conjugates, i.e., GT21.T-miR-212 and GT21.T-scr-miR-212 (using scrambled RNA sequence) and evaluated them by treating on A549 Axl positive cells and Axl negative MCF-7 cells. It was experimentally proved that GT21.T-miR-212 could significantly downregulate the expression of PED, thereby regulating the TRAIL-induced cell death and activating caspase 8 for initiating apoptosis (Fig. 6C). The major limitation of this study was that the authors had not considered the toxicity levels due to the chimera conjugate [58]. Similar to this study, the same group has performed another set of experiments with miR-137. In this case, miR-137 and GT21.T aptamer was linked by a short dsRNA sequence with sticky ends that attaches aptamer at one end and miR-137 on the other end [81]. GT21.T-miR-137 showed an increase in the internalization rate by 62% in 2 h of incubation. GT21.T-miR-137 showed a reduction in cell proliferation, invasion, and migration in patient-derived NSCLC cells. Western blotting analysis showed an effective reduction of CDK6 protein by 40% in GT21.T-miR-137 treated Axl positive A549 cells. The efficacy of GT21.T-miR-137 was evaluated in the A549-induced lung cancer mice model, revealing that GT21.T-miR-137 showed a tremendous decrease in tumor volume. This study indicates that aptamer-mediated delivery of miRNA has higher potential in treating NSCLCs.
Fig. 6.
Schematic representation of the (A) GL21.T-miR-212 and (B) GL21.T-scr-miR-212 (C) Graph indicating 24 h transfection of Axl in A549 cells (Axl positive cells) treated with 300 nM of GL21.T-miR212, GL21.Tscr-miR212, or GL21.T for additional 48 h. PED and Axl levels were quantified by RT-qPCR. Reproduced under copyright CC BY-NC-ND 4.0 licence [58].
3.6. MicroRNA-141/200 family
miR-141, miR-200, miR-429 belongs to microRNA-141/200 family. miR200 acts as a potent tumor suppressor since it targets members of the ZEB (Zinc finger E-box-binding homeobox) family of translational repressors that drive EMT [82]. Thus, deficiency or downregulation of miR-200 could increase cell proliferation, invasion, migration, and angiogenesis in lung cancer tissues. Downregulation of miR-200 also enhances the NOTCH signaling pathway in cancer-associated fibroblasts, which further increases the severity of cancer. It is observed that the downregulation of miR-200 is coherent with the poor prognosis of lung adenocarcinoma [83]. Thus, targeting miR-200 could be a potential treatment strategy for targeting lung cancer. Similarly, Peng et al. also used a novel self-assembled amphiphilic polyphosphazenes (consists of amphiphilic [NP(PEG)0.3(EAB)1.7]n (PEEP) and weakly cationic [NP(PEG)0.5(DPA)1.5]n (PEDP)) polymersomes (Nano-ED) delivery system for miR-200c [60]. The results showed that nano-ED-200c inhibited 68% tumor reduction in A549 cells induced BALB/c nude mice. The authors have stated that this study was the first polymersomes-based system for delivering miRNA for therapy.
3.7. Miscellaneous microRNA family
Wu and co-workers synthesized pre-miR-133b containing lipoplexes for treating NSCLC [61]. There was a 20000 times increase in the expression of miR-133b compared to untreated A549 cells. Remarkably, lung cancer-induced mice treated with pre-miR-133b lipoplexes had a 52-fold increase in miR-133b. The study indicated that post-translational regulation of MCL-1 (one of the direct target mRNAs for miR-133b) was successfully silenced by 56.2%. This study showed the ability of lipoplexes in the successful delivery of miR-133b for treating lung cancer cells. Similar to this study, Jiang et al. worked on cationic lipoplexes (CL) for the transfection of miR-143 by plasmid pVAX [62]. miR-143 is known to suppress the phosphorylation of EGFR, AKT, and ERK1/2, eventually inhibiting cell proliferation and promoting apoptosis. Toxicological studies were carried out in mice treated with CL-pVAX-miR143, which indicated no significant change in the body weight, and levels of liver enzymes like ALT, AST, and BUN (Blood urea nitrogen); thus, indicating the complex was safe and non-toxic. Moro and his colleagues have also used cationic lipid nanoparticles to deliver miR-660, and its efficacy was evaluated in lung cancer patient-derived cells [63]. It was identified that miR-660 could induce cell cycle arrest in a p53-dependent manner via downregulating MDM-2. MDM-2 is a p53-dependent, p53-specific ubiquitin ligase that degrades p53. MDM2 inhibits the transcription factor p53, responsible for the transcription of various oncoproteins [84]. Preclinical studies also inferred a decrease in the tumor volume after injecting miR-660 containing lipid nanoparticles. Gold nanoparticles (Au NPs) are also gaining importance for delivering miRNA. Peng et al. have synthesized photothermally active gold nanorods, which were coated with RGD (Arginine-glycine-aspartic acid) peptides via PEI and miR-320a [64]. RGD peptide selectively binds to integrin that is overexpressed in lung cancer cells. Photothermally active Au-RGD-miR-320a induces photo-induced transfection of miR-320a in A549 cells. The transfection efficiency was almost 80% in the presence of laser 1.5 W/cm2 for 9 min. Au-RGD-miR-320a inhibits cell growth, and migration in A549 cells promotes apoptosis and induces DNA damage. Interestingly, this was not observed in scrambled RNA control, indicating that cell death occurred due to the transfection of miR-320a in A549 cells. Preclinical evaluation of the Au-RGD-miR-320a in lung cancer-induced tumor model was conducted; the results indicated that tumor volume decreased in laser-treated mice model was much higher than individual miRNA treatment, which deciphers that there was a requirement of laser treatment for the transfection of miR-320a onto the tumor tissues. Au-RGD-miR-320a possesses two-stage specificity, i.e., one using RGD peptide binding to overexpressed integrin-positive lung cancer cells and selectively releasing miR-320a in the laser-treated area of interest. Han et al. developed polyamidoamine (PAMAM) construct for delivering miR-23b, which is a tumor suppressor miRNA that regulates survivin, PTEN, MMP-9, and cyclin D1. In vitro studies in A549 cells indicated that miR-23b could reduce cell proliferation by 3–23.2%, initiate apoptosis and inhibit migration [85]. Similarly, various studies have been reported which indicate that using miRNA as a therapeutic molecule is beneficial and has a greater potential of being a valid candidate for clinical trials [85,86]. The success of miRNA-based therapies can be increased by turning them into personalized medicine platforms; therein, patients have suppression or overexpression of certain miRNA, and then their appropriate agomiR or antagomiR can be delivered.
4. microRNAs in clinical trials for lung cancer
The first FDA-approved non-coding siRNA-based therapeutics was patisiran for treating amyloidosis in 2018. After the successful case of the patisiran drug, various research laboratories, biotechnology companies, and pharmaceutical industries were interested in developing non-coding RNA-based therapeutics, especially miRNA [87]. Since microRNA-based therapy has higher attraction due to its “one hit, multiple target” strategy [10]. miRNA-based therapeutics are in different stages of clinical trials for many diseases like liver cancer, heart failure, T-cell lymphoma, Alpon syndrome, hepatitis C, mesothelioma, lymphoma, etc [10,87]. Unfortunately, to date, there is no FDA-approved miRNA lung cancer therapeutics. However, there are two miRNA therapeutic studies for treating lung cancer in clinical trials. The phase I and phase II study was conducted by Hong and co-workers to treat advanced solid tumors using liposome-mediated miR-34a (MRX-34) [88]. MRX-34 was injected into a 73-year-old patient who had metastatic small lung cancer. Unfortunately, the patient showed side effects and had symptoms like dyspnoea, chest pain, weakness, and bloody stool. The patient's condition worsened, the disease progressed to the liver, and the patient died due to multiple organ failures. This study got terminated due to toxicity [87]. The next candidate in the miRNA-based lung cancer therapeutics that successfully completed the phase I clinical trials was TargomiRs [89]. TargomiRs are miR-16 encapsulated in bacterial minicells, which are surface functionalized by bispecific EGFR antibodies for specifically targeting EGFR overexpressing MPM cells and NSCLCs [68]. TargomiR injections showed 5% partial response, 68% of the patients showed stable disease, and 27% showed progressive disease. Many miRNA delivery strategies are under research and in preclinical stages. It can be expected that much more novel miRNA delivery techniques and strategies could reach and successfully complete clinical trials in the future. Both these studies did not show satisfactory results in the patients. Thus, the selection of miRNA targeted delivery of miRNA and different strategies need to be devised to reduce the off-target effects of miRNA therapeutics.
5. Conclusion
miRNA-based therapeutics have gained interest during the past few decades, and their potential is endless. However, there are major challenges in miRNA therapeutics for treating lung cancer. The problems lie in the tissue-specific delivery to the lung cancer cells, maintaining the stability of miRNA against nucleases and other degradative enzymes, determining of effective dose, etc. Another major challenge is that miRNA could silence many different types of mRNA irrespective of its function, which leads to a risk that if injected, miRNA could silence some of the essential tumor suppressor genes or some housekeeping genes. This may lead to off-target effects of miRNA-based therapy. To resolve these issues, tumor tissue-specific delivery is one of the best solutions. Thus, researchers are trying to synthesize an ideal delivery tool that could deliver specifically to the site of action. There has been tremendous research on surface-modified lipoplexes having ligands or antibodies, ligands attached to miRNA, or aptamer attached to miRNA, tissue-specific receptor-expressing exosomes, surface-functionalized nanoparticles, etc. Using these strategies might assist in reducing the off-target effects of miRNA-based therapy. Various computational tools are available that could predict the potential targets of miRNA, and this information could enhance the knowledge of deciding the correct miRNA as a therapeutic agent. The future of this field lies in synthesizing an ideal delivery method and designing an in vivo tumor mimicking platform that could help in elucidating various information or assist in predicting off-target effects of miRNA.
Author contributions
D.M., drafted the manuscript, and R.L. revised and approved the manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Availability of data and materials
Not applicable.
Funding
Financial support from the Department of Biotechnology, New Delhi, for the DBT Ramalingaswami Re-entry Fellowship project (BT/RLF/Re-entry/44/2018) and Science and Engineering Research Board (SERB), New Delhi, for a Core Research Grant (CRG) (CRG/2020/001213), Board of Research in Nuclear Sciences (BRNS) 54/14/03/2022-BRNS/10207 and VIT SEED GRANT 2020–21, 2021–2022 are kindly acknowledged.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. A Cancer Journal for Clinicians. 2021;71:209–249. doi: 10.3322/CAAC.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Lung Cancer Survival Rates, American Cancer Society 2019. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html#references
- 4.Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 2017;7:193. doi: 10.3389/FONC.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun A., Benet L.Z. Late-stage failures of monoclonal antibody drugs: a retrospective case study analysis. Pharmacology. 2020;105:145–163. doi: 10.1159/000505379. [DOI] [PubMed] [Google Scholar]
- 6.A. Sandler, R. Gray, M.C. Perry, J. Brahmer, J.H. Schiller, A. Dowlati, R. Lilenbaum, D.H. Johnson, Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer, Http://Dx.Doi.Org/10.1056/NEJMoa061884. 355 (2009) 2542–2550. 10.1056/NEJMOA061884. [DOI] [PubMed]
- 7.Kurkjian C., Kim E.S. Risks and benefits with bevacizumab: evidence and clinical implications. Therapeutic Advances in Drug Safety. 2012;3:59. doi: 10.1177/2042098611430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fecher L.A., Agarwala S.S., Hodi F.S., Weber J.S. Ipilimumab and its toxicities: a multidisciplinary approach. Oncol. 2013;18:733. doi: 10.1634/THEONCOLOGIST.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wykosky J., Fenton T., Furnari F., Cavenee W.K. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin. J. Cancer. 2011;30:5. doi: 10.5732/CJC.010.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang V., Wu W. MicroRNA-based therapeutics for cancer. BioDrugs. 2009;23:15–23. doi: 10.2165/00063030-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y., Chen J., Huang Z. Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA. 2019:1–14. doi: 10.1186/S41544-019-0024-Y. 2019 1:1. 1. [DOI] [Google Scholar]
- 12.Liu Q., Zhang W., Chen S., Zhuang Z., Zhang Y., Jiang L., Lin J.S. SELEX tool: a novel and convenient gel-based diffusion method for monitoring of aptamer-target binding. J. Biol. Eng. 2020;14:1–13. doi: 10.1186/S13036-019-0223-Y/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H.Y., Chiang C.H., Hung W.C. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br. J. Cancer. 2013;109:731–738. doi: 10.1038/bjc.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugaard I., Sanders K.J., Idica A., Vittayarukskul K., Hamdorf M., Krog J.D., Chow R., Jury D., Hansen L.L., Hager H., Lamy P., Choi C.L., Agalliu D., Zisoulis D.G., Pedersen I.M. miR-151a induces partial EMT by regulating E-cadherin in NSCLC cells. Oncogenesis. 2017;6 doi: 10.1038/oncsis.2017.66. e366–e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Chen J., Zhang S., Zheng X., Xie S., Mao J., Cai Y., Lu X., Hu L., Shen J., Chai K., Chen W. MiR-223 regulates autophagy associated with cisplatin resistance by targeting FBXW7 in human non-small cell lung cancer. Cancer Cell Int. 2020;20:1–14. doi: 10.1186/s12935-020-01284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Roosbroeck K., Fanini F., Setoyama T., Ivan C., Rodriguez-Aguayo C., Fuentes-Mattei E., Xiao L., Vannini I., Redis R.S., D'Abundo L., Zhang X., Nicoloso M.S., Rossi S., Gonzalez-Villasana V., Rupaimoole R., Ferracin M., Morabito F., Neri A., Ruvolo P.P., Ruvolo V.R., Pecot C.V., Amadori D., Abruzzo L., Calin S., Wang X., You M.J., Ferrajoli A., Orlowski R., Plunkett W., Lichtenberg T.M., Davuluri R.V., Berindan-Neagoe I., Negrini M., Wistuba I.I., Kantarjian H.M., Sood A.K., Lopez-Berestein G., Keating M.J., Fabbri M., Calin G.A. Combining anti-miR-155 with chemotherapy for the treatment of lung cancers. Clin. Cancer Res. 2016;23:2891–2904. doi: 10.1158/1078-0432.CCR-16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salim H., Akbar N.S., Zong D., Vaculova A.H., Lewensohn R., Moshfegh A., Viktorsson K., Zhivotovsky B. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br. J. Cancer. 2012:1361–1373. doi: 10.1038/bjc.2012.382. 2012 107:8. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Sun W., Gong T., Chai Y., Wang J., Hui B., Li Y., Song L., Gao Y. MiR-30a radiosensitizes non-small cell lung cancer by targeting ATF1 that is involved in the phosphorylation of ATM. Oncol. Rep. 2017;37:1980–1988. doi: 10.3892/OR.2017.5448/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Liu H., Li Y., Wu J., Greenlee A.R., Yang C., Jiang Y. The role of miR-506 in transformed 16HBE cells induced by anti-benzo[a]pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Toxicol. Lett. 2011;205:320–326. doi: 10.1016/J.TOXLET.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Wu L., Pu X., Wang Q., Cao J., Xu F., Xu L., Li K. miR-96 induces cisplatin chemoresistance in non-small cell lung cancer cells by downregulating SAMD9. Oncol. Lett. 2016;11:945–952. doi: 10.3892/ol.2015.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segal M., Slack F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expet Opin. Drug Deliv. 2020;15:987–992. doi: 10.1080/17460441.2020.1765770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q., Ran R., Zhang L., Liu Y., Mei L., Zhang Z., Gao H., He Q. Simultaneous delivery of therapeutic antagomirs with paclitaxel for the management of metastatic tumors by a pH-responsive anti-microbial peptide-mediated liposomal delivery system. J. Contr. Release : Official Journal of the Controlled Release Society. 2015;197:208–218. doi: 10.1016/J.JCONREL.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang F., Zhang B., Zhou L., Shi Y., Li Z., Xia Y., Tian J. Imaging dendrimer-grafted graphene oxide mediated anti-miR-21 delivery with an activatable luciferase reporter. ACS Appl. Mater. Interfaces. 2016;8:9014–9021. doi: 10.1021/acsami.6b02662. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Zhao L., Li D., Yin Y., Zhang C.Y., Li J., Zhang Y. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein and Cell. 2013;4:932–941. doi: 10.1007/s13238-013-3092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindawi Role of miR-221/222 in tumor development and the underlying mechanism. https://www.hindawi.com/journals/jo/2019/7252013/ n.d.
- 26.Guz M., Jeleniewicz W., Cybulski M. An insight into miR-1290: an oncogenic miRNA with diagnostic potential. Int. J. Mol. Sci. 2022;23:1234. doi: 10.3390/ijms23031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He S., Li Z., Yu Y., Zeng Q., Cheng Y., Ji W., Xia W., Lu S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019;379:203–213. doi: 10.1016/j.yexcr.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Shi S.J., Zhong Z.R., Liu J., Zhang Z.R., Sun X., Gong T. Solid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cells. Pharmaceut. Res. 2012;29:97–109. doi: 10.1007/s11095-011-0514-6. [DOI] [PubMed] [Google Scholar]
- 29.Yung B.C., Li J., Zhang M., Cheng X., Li H., Yung E.M., Kang C., Cosby L.E., Liu Y., Teng L., Lee R.J. Lipid nanoparticles composed of quaternary amine-tertiary amine cationic lipid combination (QTsome) for therapeutic delivery of AntimiR-21 for lung cancer. Mol. Pharm. 2016;13:653–662. doi: 10.1021/acs.molpharmaceut.5b00878. [DOI] [PubMed] [Google Scholar]
- 30.Sriram V., Lee J.Y. Calcium phosphate-polymeric nanoparticle system for co-delivery of microRNA-21 inhibitor and doxorubicin. Colloids Surf. B Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112061. [DOI] [PubMed] [Google Scholar]
- 31.Tian L., Shan W., Zhang Y., Lv X., Li X., Wei C. Up-regulation of miR-21 expression predicate advanced clinicopathological features and poor prognosis in patients with non-small cell lung cancer. Pathol. Oncol. Res. 2015:161–167. doi: 10.1007/S12253-015-9979-7. 2015 22:1. 22. [DOI] [PubMed] [Google Scholar]
- 32.Shi S., Han L., Deng L., Zhang Y., Shen H., Gong T., Zhang Z., Sun X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Contr. Release. 2014;194:228–237. doi: 10.1016/j.jconrel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Koeppen K., Nymon A., Barnaby R., Bashor L., Li Z., Hampton T.H., Liefeld A.E., Kolling F.W., LaCroix I.S., Gerber S.A., Hogan D.A., Kasetty S., Nadell C.D., Stanton B.A. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2105370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad A., Maitah M.Y., Ginnebaugh K.R., Li Y., Bao B., Gadgeel S.M., Sarkar F.H. Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J. Hematol. Oncol. 2013;6:77. doi: 10.1186/1756-8722-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Liu C., Luo M., Zhang Z., Gong J., Li J., You L., Dong L., Su R., Lin H., Ma Y., Wang F., Wang Y., Chen J., Zhang J., Jia H., Kong Y., Yu J. Chemotherapy-induced miRNA-29c/Catenin-δ signaling suppresses metastasis in gastric cancer. Cancer Res. 2015;75:1332–1344. doi: 10.1158/0008-5472.CAN-14-0787. [DOI] [PubMed] [Google Scholar]
- 36.Farhan M., Malik A., Ullah M.F., Afaq S., Faisal M., Farooqi A.A., Biersack B., Schobert R., Ahmad A. Garcinol sensitizes NSCLC cells to standard therapies by regulating EMT-modulating miRNAs. Int. J. Mol. Sci. 2019;20:E800. doi: 10.3390/ijms20040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su H., Yu S., Sun F., Lin D., Liu P., Zhao L. LINC00342 induces metastasis of lung adenocarcinoma by targeting miR-15b/TPBG. Acta Biochim. Pol. 2022;69:291–297. doi: 10.18388/abp.2020_5697. [DOI] [PubMed] [Google Scholar]
- 38.Arora S., Singh P., Tabassum G., Dohare R., Syed M.A. miR-16-5p regulates aerobic glycolysis and tumorigenesis of NSCLC cells via LDH-A/lactate/NF-κB signaling. Life Sci. 2022;304 doi: 10.1016/j.lfs.2022.120722. [DOI] [PubMed] [Google Scholar]
- 39.Feng H., Ge F., Du L., Zhang Z., Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J. Cell Mol. Med. 2019;23:5282–5291. doi: 10.1111/jcmm.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H.Y., Mohammed K.A., Kaye F., Sharma P., Moudgil B.M., Clapp W.L., Nasreen N. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. Int. J. Nanomed. 2013;8:4481–4494. doi: 10.2147/IJN.S41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esposito C.L., Cerchia L., Catuogno S., De Vita G., Dassie J.P., Santamaria G., Swiderski P., Condorelli G., Giangrande P.H., De Franciscis V. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol. Ther. 2014;22:1151–1163. doi: 10.1038/mt.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai X., Fan W., Wang Y., Huang L., Jiang Y., Shi L., Mckinley D.A., Tan W., Tan C. Combined delivery of let-7b MicroRNA and paclitaxel via biodegradable nanoassemblies for the treatment of KRAS mutant cancer. Mol. Pharm. 2016;13:520–533. doi: 10.1021/acs.molpharmaceut.5b00756. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Pan J., Xiong D., Wang Y., Miller M.S., Sei S., Shoemaker R.H., Izzotti A., You M. Pulmonary aerosol delivery of let-7b microRNA confers a striking inhibitory effect on lung carcinogenesis through targeting the tumor immune microenvironment. Adv. Sci. 2021;8:1–11. doi: 10.1002/advs.202100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong K., Yu Y.J., You J.Y., Rhee W.J., Kim J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20:548–557. doi: 10.1039/c9lc00958b. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y., Crawford M., Mao Y., Lee R.J., Davis I.C., Elton T.S., Lee L.J., Nana-Sinkam S.P. Therapeutic delivery of MicroRNA-29b by cationic lipoplexes for lung cancer. Mol. Ther. Nucleic Acids. 2013;2:e84. doi: 10.1038/mtna.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perepelyuk M., Maher C., Lakshmikuttyamma A., Shoyele S.A. Aptamer-hybrid nanoparticle bioconjugate efficiently delivers miRNA-29b to non-small-cell lung cancer cells and inhibits growth by downregulating essential oncoproteins. Int. J. Nanomed. 2016;11:3533–3544. doi: 10.2147/IJN.S110488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perepelyuk M., Sacko K., Thangavel K., Shoyele S.A. Evaluation of MUC1-aptamer functionalized hybrid nanoparticles for targeted delivery of miRNA-29b to nonsmall cell lung cancer. Mol. Pharm. 2018;15:985–993. doi: 10.1021/acs.molpharmaceut.7b00900. [DOI] [PubMed] [Google Scholar]
- 48.Magalhães M., Jorge J., Gonçalves A.C., Sarmento-Ribeiro A.B., Carvalho R., Figueiras A., Santos A.C., Veiga F. miR-29b and retinoic acid co-delivery: a promising tool to induce a synergistic antitumoral effect in non-small cell lung cancer cells. Drug Deliv. Transl. Res. 2020;10:1367–1380. doi: 10.1007/s13346-020-00768-7. [DOI] [PubMed] [Google Scholar]
- 49.Xing J., Jia J., Cong X., Liu Z., Li Q. N-Isopropylacrylamide-modified polyethylenimine-mediated miR-29a delivery to inhibit the proliferation and migration of lung cancer cells. Colloids Surf. B Biointerfaces. 2021;198 doi: 10.1016/j.colsurfb.2020.111463. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., Zhu X., Zhang X., Liu B., Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trang P., Wiggins J.F., Daige C.L., Cho C., Omotola M., Brown D., Weidhaas J.B., Bader A.G., Slack F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue W., Dahlman J.E., Tammela T., Khan O.F., Sood S., Dave A., Cai W., Chirino L.M., Yang G.R., Bronson R., Crowley D.G., Sahay G., Schroeder A., Langer R., Anderson D.G., Jacks T. vol. 111. 2014. Small RNA combination therapy for lung cancer; pp. 3553–3561. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu L., Deng Z.J., Roy S., Hammond P.T. A combination RNAi-chemotherapy layer-by-layer nanoparticle for systemic targeting of KRAS/P53 with cisplatin to treat non–small cell lung cancer. Clin. Cancer Res. 2017;23:7312–7323. doi: 10.1158/1078-0432.CCR-16-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trivedi M., Singh A., Talekar M., Pawar G., Shah P., Amiji M. MicroRNA-34a encapsulated in hyaluronic acid nanoparticles induces epigenetic changes with altered mitochondrial bioenergetics and apoptosis in non-small-cell lung cancer cells. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-02816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orellana E.A., Tenneti S., Rangasamy L., Lyle L.T., Low P.S., Kasinski A.L. FolamiRs: ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci. Transl. Med. 2017;9 doi: 10.1126/SCITRANSLMED.AAM9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y., Liu C., Liu X., Tang D.G., Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng L., Yuan S., Zhu L., Shangguan Z., Zhao R. Ultrasound-microbubbles-mediated microRNA-449a inhibits lung cancer cell growth via the regulation of notch1. OncoTargets Ther. 2019;12:7437–7450. doi: 10.2147/OTT.S217021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iaboni M., Russo V., Fontanella R., Roscigno G., Fiore D., Donnarumma E., Esposito C.L., Quintavalle C., Giangrande P.H., de Franciscis V., Condorelli G. Aptamer-miRNA-212 conjugate sensitizes NSCLC cells to TRAIL. Mol. Ther. Nucleic Acids. 2016;5:e289. doi: 10.1038/mtna.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D'Almeida O., Mothar O., Bondzie E.A., Lieumo Y., Tagne L., Gupta S., Volkert T., Levine S., Tagne J.B. Encapsulated miR-200c and Nkx2.1 in a nuclear/mitochondria transcriptional regulatory network of non-metastatic and metastatic lung cancer cells. BMC Cancer. 2019;19:1–24. doi: 10.1186/s12885-019-5337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng Y., Zhu X., Qiu L. Electroneutral composite polymersomes self-assembled by amphiphilic polyphosphazenes for effective miR-200c in vivo delivery to inhibit drug resistant lung cancer. Biomaterials. 2016;106:1–12. doi: 10.1016/j.biomaterials.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y., Crawford M., Yu B., Mao Y., Nana-Sinkam S.P., Lee L.J. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol. Pharm. 2011;8:1381–1389. doi: 10.1021/mp2002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang Q., Yuan Y., Gong Y., Luo X., Su X., Hu X., Zhu W. Therapeutic delivery of microRNA-143 by cationic lipoplexes for non-small cell lung cancer treatment in vivo. J. Cancer Res. Clin. Oncol. 2019;145:2951–2967. doi: 10.1007/s00432-019-03051-6. [DOI] [PubMed] [Google Scholar]
- 63.Moro M., Di D., Milione M., Centonze G., Bornaghi V., Borzi C., Gandellini P., Perri P., Pastorino U., Ponzoni M., Sozzi G., Fortunato O. Coated cationic lipid-nanoparticles entrapping miR-660 inhibit tumor growth in patient-derived xenografts lung cancer models. J. Contr. Release. 2019;308:44–56. doi: 10.1016/j.jconrel.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Peng J., Wang R., Sun W., Huang M., Wang R., Li Y., Wang P., Sun G., Xie S. Delivery of miR-320a-3p by gold nanoparticles combined with photothermal therapy for directly targeting Sp1 in lung cancer. Biomater. Sci. 2021;9:6528–6541. doi: 10.1039/d1bm01124c. [DOI] [PubMed] [Google Scholar]
- 65.Lee H., Han S., Kwon C.S., Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein & Cell. 2016;7:100. doi: 10.1007/S13238-015-0212-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barh D., Malhotra R., Ravi B., Sindhurani P. Microrna let-7: an emerging next-generation cancer therapeutic. Curr. Oncol. 2010;17:70. doi: 10.3747/CO.V17I1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aqeilan R.I., Calin G.A., Croce C.M. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2009:215–220. doi: 10.1038/cdd.2009.69. 2010 17:2. 17. [DOI] [PubMed] [Google Scholar]
- 68.Reid G., Pel M.E., Kirschner M.B., Cheng Y.Y., Mugridge N., Weiss J., Williams M., Wright C., Edelman J.J.B., Vallely M.P., McCaughan B.C., Klebe S., Brahmbhatt H., MacDiarmid J.A., van Zandwijk N. Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. 2013;24:3128–3135. doi: 10.1093/annonc/mdt412. [DOI] [PubMed] [Google Scholar]
- 69.Jiang H., Zhang G., Wu J.H., Jiang C.P. Diverse roles of miR-29 in cancer. Oncol. Rep. 2014;31:1509–1516. doi: 10.3892/OR.2014.3036/HTML. [DOI] [PubMed] [Google Scholar]
- 70.Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-Cymering C., Volinia S., Guler G., Morrison C.D., Chan K.K., Marcucci G., Calin G.A., Huebner K., Croce C.M. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA. 2007;104:15805–15810. doi: 10.1073/PNAS.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teneng I., Tellez C.S., Picchi M.A., Klinge D.M., Yingling C.M., Snider A.M., Liu Y., Belinsky S.A. Global identification of genes targeted by DNMT3b for epigenetic silencing in lung cancer. Oncogene. 2014:621–630. doi: 10.1038/onc.2013.580. 2014 34:5. 34. [DOI] [PubMed] [Google Scholar]
- 72.Munkhbaatar E., Dietzen M., Agrawal D., Anton M., Jesinghaus M., Boxberg M., Pfarr N., Bidola P., Uhrig S., Höckendorf U., Meinhardt A.L., Wahida A., Heid I., Braren R., Mishra R., Warth A., Muley T., Poh P.S.P., Wang X., Fröhling S., Steiger K., Slotta-Huspenina J., van Griensven M., Pfeiffer F., Lange S., Rad R., Spella M., Stathopoulos G.T., Ruland J., Bassermann F., Weichert W., Strasser A., Branca C., Heikenwalder M., Swanton C., McGranahan N., Jost P.J. MCL-1 gains occur with high frequency in lung adenocarcinoma and can be targeted therapeutically. Nat. Commun. 2020:1–13. doi: 10.1038/s41467-020-18372-1. 2020 11:1. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connolly R.M., Nguyen N.K., Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin. Cancer Res. 2013;19:1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L., Liao Y., Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/S13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daugaard I., Knudsen A., Kjeldsen T.E., Hager H., Hansen L.L. The association between miR-34 dysregulation and distant metastases formation in lung adenocarcinoma. Exp. Mol. Pathol. 2017;102:484–491. doi: 10.1016/J.YEXMP.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Bou Kheir T., Futoma-Kazmierczak E., Jacobsen A., Krogh A., Bardram L., Hother C., Grønbæk K., Federspiel B., Lund A.H., Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol. Cancer. 2011;10:1–12. doi: 10.1186/1476-4598-10-29/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Zhao X., Guo C., Ren D., Zhao Y., Xiao W., Jiao W. Aptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0139136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv Z.D., Yang D.X., Liu X.P., Jin L.Y., Wang X.G., Yang Z.C., Liu D., Zhao J.J., Kong B., Li F.N., Wang H.B. MiR-212-5p suppresses the epithelial-mesenchymal transition in triple-negative breast cancer by targeting Prrx2. Cell. Physiol. Biochem. 2017;44:1785–1795. doi: 10.1159/000485785. [DOI] [PubMed] [Google Scholar]
- 79.Li Y., Zu L., Wang Y., Wang M., Chen P., Zhou Q. miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. J. Thorac. Dis. 2015;7:1563. doi: 10.3978/J.ISSN.2072-1439.2015.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen W., Huang Y., Zhang S., Zheng X., Xie S., Mao J., Cai Y., Lu X., Hu L., Shen J., Dong Y., Chai K. MicroRNA-212 suppresses nonsmall lung cancer invasion and migration by regulating ubiquitin-specific protease-9. J. Cell. Biochem. 2019;120:6482–6489. doi: 10.1002/jcb.27939. [DOI] [PubMed] [Google Scholar]
- 81.Nuzzo S., Catuogno S., Capuozzo M., Fiorelli A., Swiderski P., Boccella S., de Nigris F., Esposito C.L. Axl-targeted delivery of the oncosuppressor miR-137 in non-small-cell lung cancer. Mol. Ther. Nucleic Acids. 2019;17:256–263. doi: 10.1016/j.omtn.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue B., Chuang C.H., Prosser H.M., Fuziwara C.S., Chan C., Sahasrabudhe N., Kühn M., Wu Y., Chen J., Biton A., Chen C., Wilkinson J.E., McManus M.T., Bradley A., Winslow M.M., Su B., He L. miR-200 deficiency promotes lung cancer metastasis by activating Notch signaling in cancer-associated fibroblasts. Genes Dev. 2021;35:1109–1122. doi: 10.1101/GAD.347344.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roybal J.D., Zang Y., Ahn Y.H., Yang Y., Gibbons D.L., Baird B.N., Alvarez C., Thilaganathan N., Liu D.D., Saintigny P., Heymach J.V., Creighton C.J., Kurie J.M. miR-200 inhibits lung adenocarcinoma cell invasion and metastasis by targeting flt1/VEGFR1. Mol. Cancer Res. 2011;9:25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moll U.M., Petrenko O. The MDM2-p53 interaction. Mol. Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 85.Han H., Li Q. Nucleobase-modified polyamidoamine-mediated mir-23b delivery to inhibit the proliferation and migration of lung cancer. Trans. Ann. Meet. Soc. Biomater. Ann. Int. Biomater. Symp. 2019;40:581. doi: 10.1039/C7BM00599G. [DOI] [PubMed] [Google Scholar]
- 86.Chiou G.Y., Cherng J.Y., Hsu H.S., Wang M.L., Tsai C.M., Lu K.H., Chien Y., Hung S.C., Chen Y.W., Wong C.I., Tseng L.M., Huang P.I., Yu C.C., Hsu W.H., Chiou S.H. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J. Contr. Release. 2012;159:240–250. doi: 10.1016/j.jconrel.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 87.Hanna J., Hossain G.S., Kocerha J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019;10:478. doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., Shin S., Becerra C.R., Falchook G., Stoudemire J., Martin D., Kelnar K., Peltier H., Bonato V., Bader A.G., Smith S., Kim S., O'Neill V., Beg M.S. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020:1630–1637. doi: 10.1038/s41416-020-0802-1. 2020 122:11. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Zandwijk N., Pavlakis N., Kao S.C., Linton A., Boyer M.J., Clarke S., Huynh Y., Chrzanowska A., Fulham M.J., Bailey D.L., Cooper W.A., Kritharides L., Ridley L., Pattison S.T., MacDiarmid J., Brahmbhatt H., Reid G. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–1396. doi: 10.1016/S1470-2045(17)30621-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.