Abstract
Objectives: This meta-analysis aimed to assess the effectiveness and safety of Chinese herbal medicine (CHM) in treating chronic fatigue syndrome (CFS).
Methods: Nine electronic databases were searched from inception to May 2022. Two reviewers screened studies, extracted the data, and assessed the risk of bias independently. The meta-analysis was performed using the Stata 12.0 software.
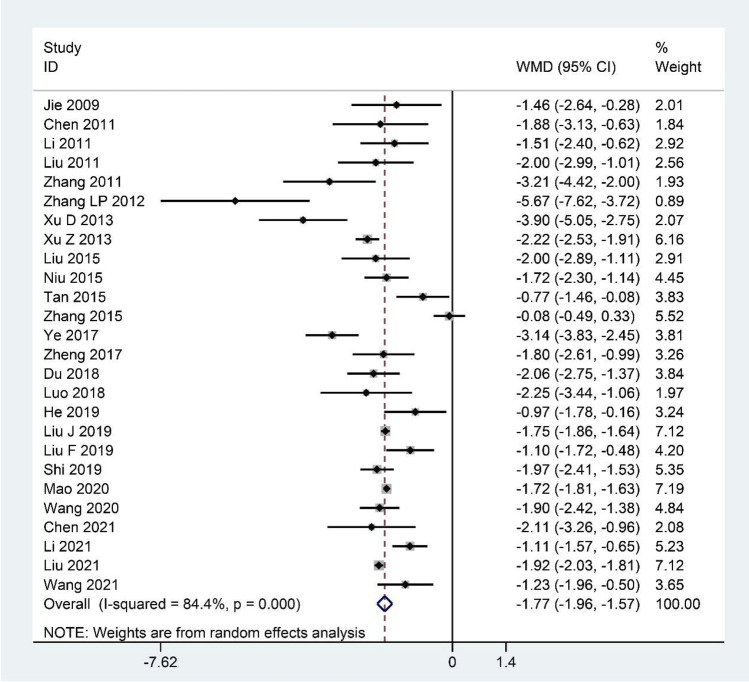
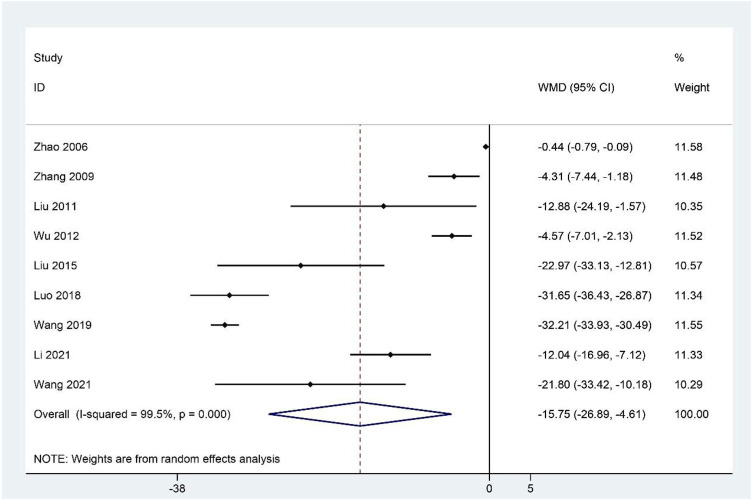
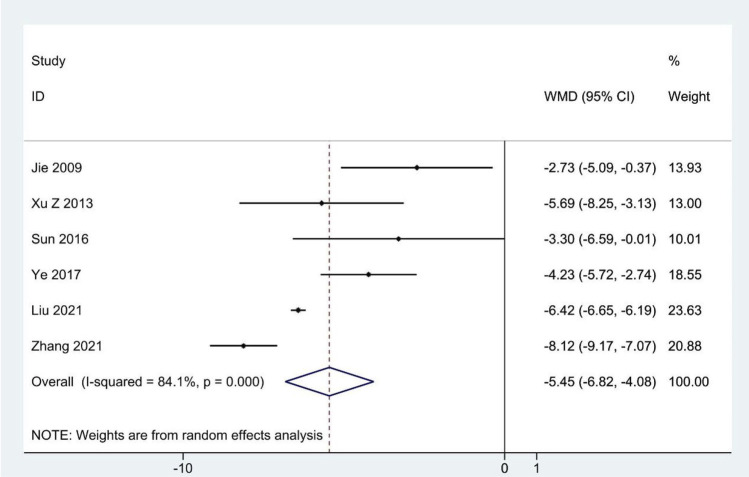
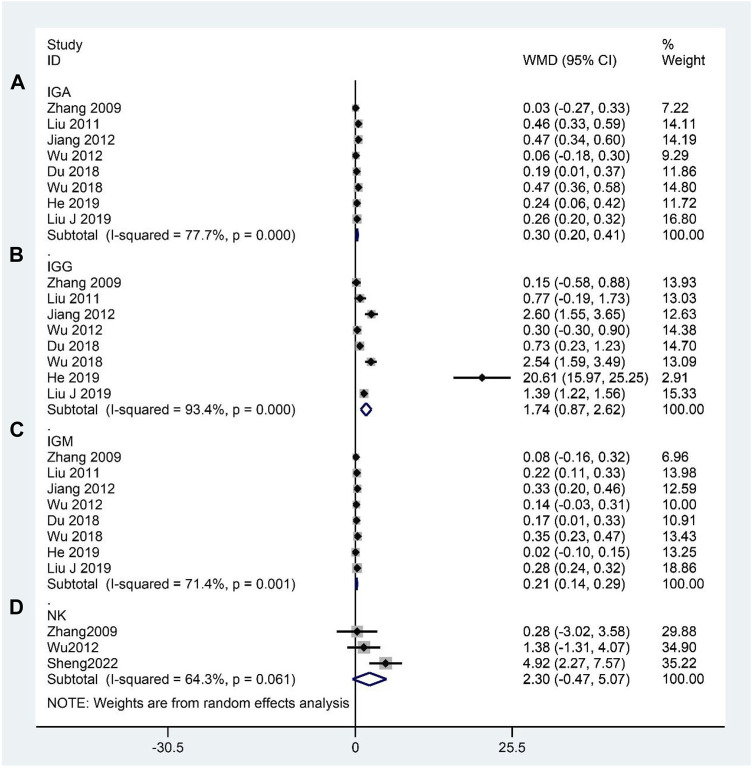
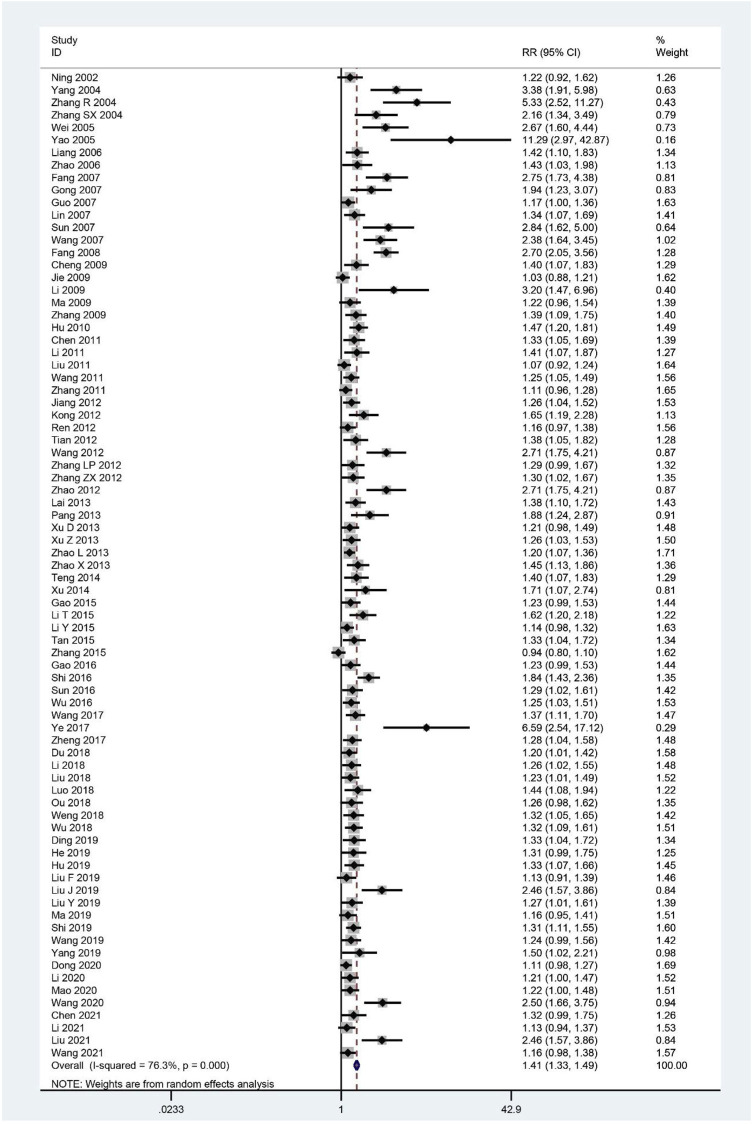
Results: Eighty-four RCTs that explored the efficacy of 69 kinds of Chinese herbal formulas with various dosage forms (decoction, granule, oral liquid, pill, ointment, capsule, and herbal porridge), involving 6,944 participants were identified. This meta-analysis showed that the application of CHM for CFS can decrease Fatigue Scale scores (WMD: –1.77; 95%CI: –1.96 to –1.57; p < 0.001), Fatigue Assessment Instrument scores (WMD: –15.75; 95%CI: –26.89 to –4.61; p < 0.01), Self-Rating Scale of mental state scores (WMD: –9.72; 95%CI:–12.26 to –7.18; p < 0.001), Self-Rating Anxiety Scale scores (WMD: –7.07; 95%CI: –9.96 to –4.19; p < 0.001), Self-Rating Depression Scale scores (WMD: –5.45; 95%CI: –6.82 to –4.08; p < 0.001), and clinical symptom scores (WMD: –5.37; 95%CI: –6.13 to –4.60; p < 0.001) and improve IGA (WMD: 0.30; 95%CI: 0.20–0.41; p < 0.001), IGG (WMD: 1.74; 95%CI: 0.87–2.62; p < 0.001), IGM (WMD: 0.21; 95%CI: 0.14–0.29; p < 0.001), and the effective rate (RR = 1.41; 95%CI: 1.33–1.49; p < 0.001). However, natural killer cell levels did not change significantly. The included studies did not report any serious adverse events. In addition, the methodology quality of the included RCTs was generally not high.
Conclusion: Our study showed that CHM seems to be effective and safe in the treatment of CFS. However, given the poor quality of reports from these studies, the results should be interpreted cautiously. More international multi-centered, double-blinded, well-designed, randomized controlled trials are needed in future research.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022319680], identifier [CRD42022319680].
Keywords: herbal medicine, chronic fatigue syndrome, treatment, systematic review, meta-analysis
Introduction
Chronic fatigue syndrome (CFS) is a medically unexplained and debilitating mental and physical condition characterized by persistent fatigue (lasting for at least 6 months) and several other symptoms, including sleep disorders, lengthy malaise after exertion, sore throat, muscle pain, multi-joint pain, tender lymph nodes, headache, impairment of concentration or short-term memory, anxiety, and depression, which lead to severe disability and suffering in patients. Studies have shown that the prevalence of CFS is 0.006%–3% in the general population (Cleare et al., 2015), and 836,000–2.5 million people suffer from CFS in the US alone (Clayton, 2015). In addition, a meta-analysis showed that the overall incidence of CFS is 0.77% and 0.76% in Korea and Japan, respectively (Lim and Son, 2021). If there is no effective treatment, CFS will cause a decline in multi-system function and cause systemic diseases such as immune system, circulatory system, nervous system, digestive system, and visceral dysfunction, thus posing a serious threat to human health.
Although the cause of CFS remains uncertain, popular hypotheses include triggers (viral infections, physical trauma, physical and mental stress, vaccinations, and environmental toxins), microbiome disruption, dysregulated immune response, chronic low-grade inflammation, neuroendocrine abnormalities, oxidative stress, metabolic dysfunction, mitochondrial dysfunction, and genetic predisposition (Brinth et al., 2019; Gregorowski et al., 2019; Noor et al., 2021). These factors can also interact to promote the occurrence and development of CFS. Some studies have suggested that infectious triggers can trigger systemic inflammation by activating the antiviral immune response (Kennedy et al., 2010; Maes et al., 2012; Glassford, 2017; Cortes Rivera et al., 2019). The composition of gut microbes is altered in CFS patients, which might lead to increased intestinal permeability that allows bacterial translocation into the bloodstream, thus increasing systemic inflammation (Deumer et al., 2021). The hypothalamic-pituitary-adrenal (HPA) axis is impaired in patients with CFS, which may result in neuroendocrine abnormalities and metabolic and inflammatory changes (Deumer et al., 2021). In addition, genetic predisposition is associated with autoimmunity (Deumer et al., 2021).
Currently, the treatment of CFS remains suboptimal because there is a lack of an adequate understanding of the mechanisms and etiology of the disease. Current recommendations for the treatment of CFS include cognitive behavioral therapy (CBT), graded exercise therapy (GET), western conventional medicine (WCM), complementary or alternative medicine, and nutritional support therapy. CBT challenges patients’ thoughts to relieve patients’ psychological stress, and this may provide short-term benefits but does not permanently reduce symptoms (Fernie et al., 2016; Geraghty and Blease, 2018). Exercise therapy, including aerobic exercises (e.g., walking, jogging, swimming, and cycling) and anaerobic exercises (e.g., strength and stability exercises), could improve physical function and reduce fatigue (Marques et al., 2015; Larun et al., 2017). However, some patients have expressed disappointment with GET because it can interfere with the outcome of alternative treatments and may indirectly exacerbate symptoms in patients (Goudsmit and Howes, 2017; Geraghty and Blease, 2019). Western conventional medicines such as immune modulators, antivirals, antidepressants, antibiotics, and medications to treat specific symptoms that are used for treating CFS have insufficient evidence for their efficacy and may cause serious adverse effects (Mücke et al., 2015; Smith et al., 2015; Yang et al., 2017). In addition, alternative medicine (e.g., meditation and relaxation response, warm baths, massages, stretching, acupuncture, hydrotherapy, chiropractic, yoga, and Tai Chi), nutritional support therapy, transcutaneous electrical nerve stimulation, physiotherapy, and nerve blocks have all been proposed, but the evidence regarding these treatments is limited and their efficacy is uncertain (Bested and Marshall, 2015; Noor et al., 2021).
Chinese herbal medicine (CHM) has been widely used to treat CFS in China and other parts of the world, such as South Korea and Japan (Wang et al., 2014; Joung et al., 2019; Shin et al., 2021). First, according to the dialectical treatment theory of traditional Chinese medicine, specific formulas consisting of different Chinese herbs are used to treat CFS patients with different symptoms. Such treatment tailored to the patient’s specific needs is urgently needed given the obvious heterogeneity in CFS symptoms. The pathogenesis of CFS in traditional Chinese medicine is the deficiency of qi, blood, and yin and yang, accompanied by the stagnation of qi, fire, phlegm, and blood. The treatment is focused on tonifying deficiencies and relieving bruising. CHM such as Panax ginseng C.A.Mey., Codonopsis pilosula (Franch.) Nannf., and Astragalus mongholicus Bunge can nourish deficiency and improve fatigue and lengthy malaise after exertion in CFS patients, whereas Bupleurum falcatum L. and Citrus × aurantium L., among others, can resolve stagnation and relieve pain, insomnia, swollen lymph nodes, and other symptoms. Therefore, CHM can not only improve the main symptoms, but also relieve the accompanying symptoms in CFS. Second, modern pharmacological research has demonstrated that the modern use of CHM in treating CFS mainly focuses on adjusting immune dysfunction, acting as an antioxidant, improving the energy metabolism disorder, and regulating abnormal activity in the HPA axis (Chen et al., 2010; Chi et al., 2016). Buzhong Yiqi decoction, Kuibi decoction, Danggui Buxue decoction, Young Yum pill, and Renshen Yangrong decoction can regulate the immune function of patients with CFS and relieve fatigue symptoms (Ogawa et al., 1992; Shin et al., 2004; Chen et al., 2010; Yin et al., 2021; Miao et al., 2022). Ginsenoside, Jujube polysaccharide conjugate, Quercetin, Withania somnifera (L.) Dunal, Hypericum perforatum L., and Ginkgo biloba L. can be antioxidants (Logan and Wong, 2001; Singh et al., 2002; Chi et al., 2015). Schisandra Chinensis Polysaccharide (SCP), HEP2-a extracted from Epimedium brevicornum Maxim., can improve energy metabolism and can regulate the abnormal activity of the HPA axis (Chi et al., 2016; Chi et al., 2017). Additionally, multiple randomized controlled trials (RCTs) have reported that CHM significantly improves fatigue, insomnia, and other concomitant symptoms; reduces negative emotions such as anxiety and depression; and clearly improves treatment effectiveness and quality of life compared to exercise therapy and alternative therapy (Wang et al., 2011; Kong, 2012; Wang, 2021). Systematic reviews and meta-analyses comparing CHM with western medicine also confirmed the above views (Peng et al., 2013; Wang et al., 2014). These studies demonstrate the remarkable efficacy and comprehensiveness of CHM for CFS, which is consistent with treatment guidelines emphasizing a holistic, patient-centered approach that considers the patient’s physical, mental, and social well-being (Baker and Shaw, 2007). Finally, CHM has no serious side effects and is relatively safe to treat CFS.
A previous meta-analysis and another systematic review indicated the beneficial role of CHM as a complementary approach for CFS (Peng et al., 2013; Wang et al., 2014). However, those studies were limited in terms of sample size and outcome indicators because the systematic review only assessed 10 RCTs (including 919 patients), and the meta-analysis of 11 RCTs (including 1,049 patients) only assessed clinical efficacy rates and lacked sufficient evidence. In addition, nearly 50 new trials assessing the effects of CHM for CFS have been published since the previous systematic reviews and meta-analyses were published. Therefore, we conducted a larger systematic review and meta-analysis including more outcome indicators (FS-14, FAI, SCL-90, SAS, SDS, clinical symptom scores, IGA, IGG, IGM, NK cell levels, effective rate, and adverse events) to provide a comprehensive update of previously published studies and stronger evidence for the effectiveness of CHM for CFS.
Methods
Protocol and registration
This meta-analysis was reported in compliance with the PRISMA statement, and the protocol was registered on PROSPERO (CRD42022319680). [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022319680]. The full details of the protocol are available on request.
Search strategy
Electronic databases including PubMed, Embase, Cochrane Library, Web of Science, the Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese VIP Database, the US Clinical Trials Registry, and the Chinese Clinical Trials Registry were systematically searched from their inception to May 2022. There was no restriction on language. The search terms used included “Fatigue Syndrome, Chronic”, “CFS”, “Chronic Fatigue Syndrome”, “Myalgic Encephalomyelitis”, “ME”, “Encephalomyelitis, Myalgic”, “Chronic Fatigue Disorder”, “Fatigue Disorder, Chronic”, “Systemic Exertion Intolerance Disease”, “Chinese herbal medicine”, “Chinese traditional”, “Oriental traditional”, “traditional Chinese medicine”, “traditional Chinese medicinal materials”, “Chinese herb”, “herbal medicine”, “herbal”, “decoction”, “tang”, “pill”, “wan”, “powder”, “formula”, “granule”, “capsule”, “particles”, “ointment”, “prescription”, “receipt”, “placebo”, “random controlled trial”, “random”, and “RCT”. The full details of the search strategy are available (Additional file 1). In addition, we performed manual searches in the reference lists of previously published systematic reviews and meta-analyses on the subject to further look for potentially eligible studies. The search was conducted independently by two authors (YZ and WS).
Eligibility criteria
Types of studies
RCTs assessing the efficacy and safety of CHM in the treatment of CFS were included in our review. We only extracted data from the CHM and control groups when we found relevant studies with three treatment groups.
Types of participants
Trials of participants over the age of 16 were included regardless of gender, culture, or setting. CFS was diagnosed using the Center for Disease Control criteria (1987, 1994, or 1998), the Guiding Principles for Clinical Research of New Chinese Medicines (2002), Chinese medicine internal disease diagnosis and treatment routines, the clinical research guidelines for new Chinese medicines for CFS, Chinese internal medicine diagnoses, or the diagnostic efficacy criteria for Chinese medical evidence. All patients had the primary symptom of unexplained fatigue that lasted at least 6 months accompanied by four or more of the following symptoms: unrefreshing sleep, lengthy malaise after exertion, impairment of concentration or short-term memory, sore throat, tender lymph nodes, multi-joint pain, and headaches.
Types of interventions
The formulations of CHM were included. CHM is defined as medicinal raw materials derived from medicinal plants, minerals, and animal sources, according to the Chinese Pharmacopoeia edited in 2020 (Chinese Pharmacopoeia Commission, 2020). A formulation of CHM is usually made up of two or more herbs to produce a synergistic effect on specific illnesses. These materials are prescribed by doctors based on the individual characteristics of the patient according to the dialectical treatment theory of traditional Chinese medicine (Xiong et al., 2019; Chinese Pharmacopoeia Commission, 2020).
Participants were treated with CHM alone or combined with WCM, GET, or health guidance. We did not place any limits on the formulation of CHM or the duration of treatment, but CHM was required to be taken orally. We did not include experiments combining Chinese herbal medicine with other traditional Chinese medicine treatments.
Types of controls
Patients in the control group used WCM, GET, health guidance, or placebo, with no limit on the duration of treatment. We did not include experiments combining any Chinese medicine therapy.
Types of outcome measures
The primary outcome measures were Fatigue Scale (FS-14) and Fatigue Assessment Instrument (FAI) scores. The secondary outcome measures were Self-Rating Scale of mental state (SCL-90) scores, Self-Rating Anxiety Scale (SAS) scores, Self-Rating Depression Scale (SDS) scores, clinical symptom scores, immunological indicators (IGA, IGG, IGM, and NK cell levels), effective rate, and adverse events.
The clinical symptom scores are used to assess the severity of fatigue. The main symptoms and other symptoms of CFS are scored according to their severity, and a higher cumulative score of all symptoms indicates more severe fatigue symptoms. The effective rate is a measure to assess clinical efficacy. It is assessed at the end of treatment using four grades: clinical cure (the patients’ clinical symptoms were basically cured, and they could live and work normally), markedly effective (the cure rate for major and concomitant clinical symptoms up to 2/3), effective (the cure rate for major and concomitant clinical symptoms is 1/3 to 2/3), and invalid (the cure rate for major and concomitant clinical symptoms <1/3).
Study selection
Study selection was performed independently by two authors (YZ and FJ) according to the inclusion criteria. After eliminating duplicates, they independently scanned the title/abstract and full text to identify eligible studies. Any disagreements were settled by a discussion with a third evaluator (WS).
Data extraction
Two investigators (YZ and XW) independently reviewed and extracted the following information: general information (first author, year of publication, region, and types), characteristics of the participants (sample size, age, gender, and course of disease), details of the intervention and the comparison (type of intervention and duration), and outcomes. Any discrepancies were resolved by discussions or adjudication by a third reviewer (YP).
Quality assessment
The risk of bias in the included studies was evaluated independently by two authors (XW and FJ) using the Cochrane collaboration tool with the following seven domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Each domain can be classified as “low-risk,” “high-risk,” or “uncertain risk.” Any differences were resolved by discussion with a third evaluator.
Statistical analysis
Statistical analysis was performed using the Stata software (version 12.0; StataCorp, College Station, TX). The weighted mean difference (WMD) for continuous variables and the risk ratio (RR) for dichotomous data with 95% confidence intervals (Cl) were used. Heterogeneity was assessed by the Q test and the I2 statistic. When p ≥ 0.10 and I2 ≤ 50%, the fixed-effect model was used; otherwise, the random effects model was used. p ≤ 0.05 was considered statistically significant. The publication bias was assessed by funnel plots and Egger’s test if the number of trials was sufficient. When heterogeneity was detected, the sensitivity analysis was conducted to assess the stability of the results by excluding individual studies one by one. Subgroup analysis was performed to explore the sources of heterogeneity according to treatment method (CHM vs. WCM, CHM plus WCM vs. WCM, CHM vs. GET, CHM plus GET vs. GET, CHM vs. health guidance, and CHM vs. placebo) and duration of the intervention (≤30 days vs. 31–60 days vs. > 60 days) based on different treatment methods.
Results
Literature search
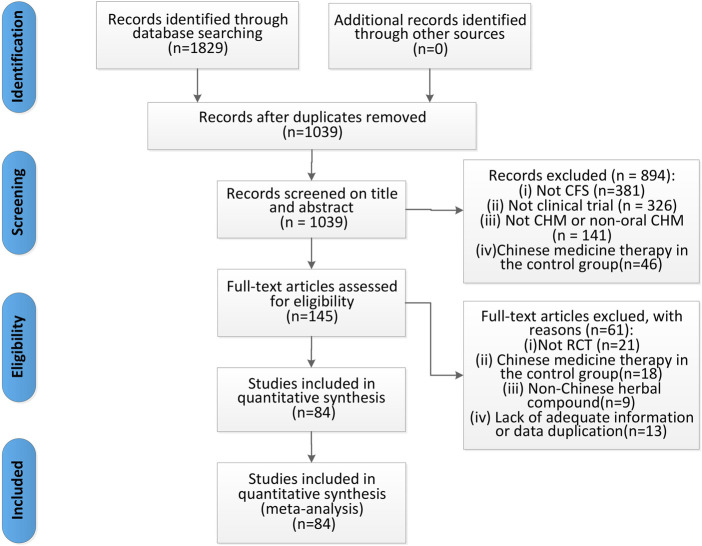
We identified 1,829 articles in the original screening. After eliminating duplicates, 1,039 remained, 894 of which were excluded because they did not meet the inclusion criteria after scanning the titles and abstracts. Moreover, we reviewed the full text of the remaining 145 articles and deleted 61 articles due to the following reasons: 1) non-RCTs, 2) Chinese medicine therapy used in the control group, 3) non-Chinese herbal compounds used, 4) published using repeated data, and 5) missing data. Finally, 84 articles were included in the meta-analysis (Figure 1).
FIGURE 1.
Flow diagram of the studies selection process.
Study characteristics and quality assessment
A total of 84 RCTs were included, published from 2002 to 2022, and all studies were conducted in China. The sample sizes in the studies varied from 38 to 230 patients, with a total sample size of 3,552 patients in the treatment groups and 3,392 patients in the control groups. The duration of diseases lasted from 0.5 to 24.27 years. Of the 84 studies, five trials (Li, 2009; Liu J. et al., 2019; Wang, 2020; Liu et al., 2021; Sheng et al., 2022) compared CHM with placebo, whereas comparisons of CHM alone vs. WCM were performed in 63 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Jiang, 2012; Tian and Wang, 2012; Wang, 2012; Wu et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Li, 2015; Li and Zao, 2015; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Shi and Wu, 2016; Wu et al., 2016; Du, 2018; Luo, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Liu F. et al., 2019; Liu Y. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Chen, 2021; Zhang, 2021). CHM plus WCM vs. WCM was compared in 12 studies (Guo et al., 2007; Jie and Wang, 2009; Ren and Yu, 2012; Xu and Wang, 2013; Gao and Pang, 2015; Gao and Pang, 2016; Sun et al., 2016; Wang, 2017; Zheng et al., 2017; Li et al., 2018; Liu and Cai, 2018; Li et al., 2021); CHM vs. GET was compared in one study (Wang, 2021); CHM plus GET vs. GET was compared in two studies (Wang et al., 2011; Kong, 2012); and CHM vs. health guidance was compared in one study (Ye, 2017). The course of treatment ranged from 7 to 120 days. In the outcome indicators, 26 studies (Jie and Wang, 2009; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang L. P. et al., 2012; Xu et al., 2013; Xu and Wang, 2013; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Ye, 2017; Zheng et al., 2017; Du, 2018; Luo, 2018; Liu F. et al., 2019; Liu J. et al., 2019; He, 2019; Shi, 2019; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reported FS-14 scores; nine studies (Zhao et al., 2006; Zhang et al., 2009; Liu et al., 2011; Wu et al., 2012; Liu et al., 2015; Luo, 2018; Wang, 2019; Li et al., 2021; Wang, 2021) reported FAI scores; five studies (Zhang et al., 2009; Zhang Z. X. et al., 2012; Wu et al., 2012; Niu et al., 2015; Sheng et al., 2022) reported SCL-90 scores; seven studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Yang, 2019; Liu et al., 2021; Zhang, 2021) reported SAS scores; six studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Liu et al., 2021; Zhang, 2021) reported SDS scores; 24 studies (Zhang et al., 2004; Yao and Qiu, 2005; Fang et al., 2007; Wang et al., 2007; Fang et al., 2008; Li, 2009; Hu et al., 2010; Wang, 2012; Zhao, 2012; Li, 2015; Li and Zao, 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Liu J. et al., 2019; He, 2019; Hu, 2019; Shi, 2019; Dong, 2020; Wang, 2020; Liu et al., 2021) reported clinical symptom scores; eight studies (Zhang et al., 2009; Liu et al., 2011; Jiang, 2012; Wu et al., 2012; Du, 2018; Wu et al., 2018; Liu J. et al., 2019; He, 2019) reported the level of IGA, IGG, and IGM; three studies (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) reported the NK cell levels; and 79 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Li, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Wang et al., 2011; Zhang et al., 2011; Jiang, 2012; Kong, 2012; Ren and Yu, 2012; Tian and Wang, 2012; Wang, 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Xu and Wang, 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Gao and Pang, 2015; Li and Zao, 2015; Li, 2015; Tan et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Shi and Wu, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Li et al., 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Luo, 2018; Ding, 2019; He, 2019; Hu, 2019; Liu F. et al., 2019; Liu J. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reported effective rate. The occurrence of adverse effects was reported in 14 studies (Liang, 2006; Gong, 2007; Lin, 2007; Wang et al., 2007; Jie and Wang, 2009; Li, 2009; Li et al., 2011; Xu and Wang, 2013; Zhang et al., 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Li et al., 2018; Liu and Cai, 2018). The basic characteristics of the included studies are summarized in Table 1, and components of CHM used in the included studies are presented in Table 2.
TABLE 1.
Characteristics of the included studies.
| Study | Region | Types | Sample size (TG/CG) | Age (Y) | Gender (M/F) | Course of disease | Interventions | Duration (days) | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TG | CG | TG | CG | TG | CG | TG | CG | ||||||
| Ning and Li (2002) | China | RCT | 43 (23/20) | 42 | 41 | 7/16 | 5/15 | 0.5–5 years | 0.5–5 years | Sijunzi decoction (1 package, bid) | Oryzanol + antipsychotic + vitamin | 30 | ⑪ |
| Yang et al. (2004) | China | RCT | 72 (38/34) | 36.4 | 36.4 | NR | NR | 2.5 years | 2.5 years | Buzhong Yiqi decoction and Xiaochaihu decoction (qd) | ATP (2 tablets, tid) | 30 | ⑪ |
| Zhang et al. (2004) | China | RCT | 100 (60/40) | 36.2 ± 7.82 | 34.92 ± 10.28 | 26/34 | 18/22 | 0.5–5 years | 0.5–5 years | Self-designed Shenqi Fuyuan decoction (200 ml, bid) | Oryzanol + multivitamin | 30 | ⑥⑪ |
| Zhang and Zhou (2004) | China | RCT | 68 (40/28) | 37 | 37 | NR | NR | NR | NR | Buzhong Yiqi decoction | Vitamins B, B1, B6 + oryzanol + estazolam + ibuprofen sustained release capsule | 42 | ⑪ |
| Wei (2005) | China | RCT | 72 (37/35) | 29.5 | 28.9 | 12/25 | 10/25 | 2.5 years | 2.4 years | Xiaochaihu decoction (1 package, bid) | Vitamin C (bid) + vitamin B (bid) + diclofenac sodium (25 mg) | 21 | ⑪ |
| Yao and Qiu (2005) | China | RCT | 56 (31/25) | 36.2 ± 7.82 | 35.92 ± 10.28 | 10/21 | 10/15 | 0.6–5 years | 0.6–5 years | Self-designed Xianshen decoction (200 ml, bid) | Centrum vitaminamin A–Z (1 granule, qd) | 30 | ⑥⑪ |
| Liang (2006) | China | RCT | 121 (63/58) | 37.2 | 35.3 | 36/27 | 34/24 | 0.58–6 years | 0.67–8 years | Shengmai pulvis and Xuefu Zhuyu decoction (200 ml, bid) | Vitamin C (0.1 g, tid) + vitamin B (0.2 g, tid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 28 | ⑪⑫ |
| Zhao et al. (2006) | China | RCT | 58 (30/28) | 44 | 43 | 11/19 | 11/17 | 1.8 a | 1.6 a | Yiqi Yangyin Jichu prescription (tid) | ATP (40 mg, tid) + vitamin C (0.2 g, tid) | 90 | ②⑪ |
| Fang et al. (2007) | China | RCT | 70 (35/35) | 42.15 ± 9.31 | 42.15 ± 9.31 | NR | NR | 16.58 ± 7.69 years | 16.58 ± 7.69 years | Xiaopiling granule (20 g, tid) | Oryzanol (20 mg, tid) | 28 | ⑥⑪ |
| Gong (2007) | China | RCT | 58 (30/28) | 36.3 | 36.8 | 10/20 | 12/16 | 0.5–5 years | 0.5–5 years | Guipi decoction (200 ml, bid) | ATP (40 mg, tid) | 45 | ⑪⑫ |
| Guo et al. (2007) | China | RCT | 120 (60/60) | 51.07 ± 7.57 | 49.75 ± 7.78 | 29/31 | 32/28 | 2.63 ± 1.16 years | 2.65 ± 1.39 years | Qi and blood proral solution (1 package, 10 ml, tid) + oryzanol (20 mg, tid) | Oryzanol (20 mg, tid) | 30 | ⑪ |
| Lin (2007) | China | RCT | 88 (50/38) | 28–60 | 25–65 | 15/35 | 21/17 | 1–6 years | 0.7–5 years | Shenling Baizhu powder (60–90 g, bid/tid) | Oryzanol + vitamin B1 + vitamin B6 + deanxit + amino acid | 56 | ⑪⑫ |
| Sun et al. (2007) | China | RCT | 64 (34/30) | 35.8 | 35.8 | NR | NR | 2.2 a | 2.2 a | Shuyu decoction (200 ml, bid) | Vitamin C (0.1 g, tid) + vitamin B (0.2 g, tid) + oryzanol (20 mg, tid) | 40 | ⑪ |
| Wang et al. (2007) | China | RCT | 105 (53/52) | 41.37 ± 8.52 | 41.37 ± 8.52 | NR | NR | 17.51 ± 6.39 m | 17.51 ± 6.39 m | Fuzheng Jieyu prescription (1 package, bid) | Oryzanol (20 mg, tid) + ATP (40 mg, tid) | 28 | ⑥⑪⑫ |
| Fang et al. (2008) | China | RCT | 230 (120/110) | 40 | 40 | NR | NR | 2.5 years | 2.5 years | Fufang Shenqi ointment (10 g, bid) | ATP (3 tablets, tid) + vitamin C (2 tablets, tid) | 60 | ⑥⑪ |
| Cheng (2009) | China | RCT | 60 (30/30) | NR | NR | 13/17 | 12/18 | 0.5–2 years | 0.4–2 years | Bainian Le oral liquid (15 ml, bid) | Vitamin B solution (15 ml, bid) | 14 | ⑪ |
| Jie and Wang (2009) | China | RCT | 70 (35/35) | 34.55 ± 7.45 | 33.95 ± 6.54 | 14/21 | 16/19 | 14.50 ± 4.50 m | 14.20 ± 4.15 m | Xiaoyao pill (24 pills, tid) + Paroxetine (20–40 mg/d) | Paroxetine (20–40 mg/d) | 56 | ①④⑤⑪⑫ |
| Li (2009) | China | RCT | 38 (19/19) | 32.75 | 31.45 | 6/13 | 12/7 | 0.5–10 years | 0.5–10 years | Anti-fatigue no. 2 decoction granule (tid) | Placebo (tid) | 21 | ⑥⑪⑫ |
| Ma (2009) | China | RCT | 118 (78/40) | 52.5 | 50.2 | 35/43 | 18/22 | NR | NR | Guipi decoction (150 ml, tid) | Vitamin (2 tablets, tid) + oryzanol (20 mg, tid) + estazolam (1–2 mg, qn) + nimesulide (0.1 g, bid) | 30 | ⑪ |
| Zhang et al. (2009) | China | RCT | 75 (40/35) | 38.63 ± 11.49 | 38.66 ± 10.94 | 19/21 | 14/21 | 10.95 ± 3.73 m | 10.80 ± 2.95 m | Lixu Jieyu prescription (200 ml, bid) | Vitaeamphor (10 mg, bid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 90 | ②③⑦⑧⑨⑩⑪ |
| Hu et al. (2010) | China | RCT | 120 (60/60) | 41.23 | 41.23 | NR | NR | NR | NR | Buqi Tongluo prescription (1 package, tid) | Vitamin B complex (5 ml, tid) | NR | ⑥⑪ |
| Chen et al. (2011) | China | RCT | 80 (40/40) | 44.7 ± 5.6 | 47.4 ± 3.4 | 16/24 | 18/22 | 0.5–4 years | 0.5–3.5 years | Qixue Liangxu prescription + Ganyu Pixu prescription + Ganshen Kuixu prescription | Multidimensional tablet (10 mg, bid) + meloxicam (1 tablet, qd) + estazolam tablet (1 mg, qd) + flupentixton melitoxin (1 tablet, bid) | 90 | ①⑪ |
| Li et al. (2011) | China | RCT | 71 (36/35) | 38.36 ± 7.16 | 39.22 ± 6.85 | 16/20 | 17/18 | 0.5–3 years | 0.6–3 years | Chaihu Shugan pulvis and Guipi decoction (1 package, bid) | Oryzanol (20 mg, tid) + ATP (20 mg, tid) | 56 | ①⑪⑫ |
| Liu et al. (2011) | China | RCT | 80 (42/38) | 42.3 ± 10.6 | 41.2 ± 9.5 | 20/22 | 19/19 | 0.5–3 years | 0.67–3 years | Shugan Yangxue prescription (1 package, bid) | Vitamin C (0.1 g) + vitamin B (0.2 g) + ATP (20 mg) + oryzanol (20 mg, tid) | 56 | ①②⑦⑧⑨⑪ |
| Wang et al. (2011) | China | RCT | 96 (48/48) | 39 | 38.5 | 27/21 | 25/23 | 5 years | 5 years | Yiqi Ziyin Buyang prescription (300 ml, bid) + GET | GET | 60 | ⑪ |
| Zhang et al. (2011) | China | RCT | 64 (32/32) | 37.97 ± 10.35 | 38.66 ± 11.03 | 15/17 | 14/18 | 11.30 ± 4.73 m | 10.98 ± 4.26 m | Zhenqi Jiepi decoction (100 ml, bid) | Gold theragran (1 tablet, tid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 60 | ①⑪ |
| Jiang (2012) | China | RCT | 70 (35/35) | 37 | 38 | 17/18 | 18/17 | 0.67 years | 1.67 years | Buzhong Yiqi decoction and Guipi decoction (1 package, bid) | Vitamin C + vitamin B complex + oryzanol | 56 | ⑦⑧⑨⑪ |
| Kong (2012) | China | RCT | 60 (30/30) | NR | NR | NR | NR | 0.5–3 years | 0.5–3 years | Self-designed anti-fatigue decoction (200 ml, tid) + GET | GET | 45 | ⑪ |
| Ren and Yu. (2012) | China | RCT | 80 (40/40) | 42 | 44 | 22/18 | 24/16 | 5 a | 5.6 a | Self-designed Zhongyao Buxu decoction (1 package) + oryzanol (2 tablets, tid) | Oryzanol (2 tablets, tid) | 21 | ⑪ |
| Tian and Wang. (2012) | China | RCT | 64 (32/32) | 28–60 | 25–65 | 15/17 | 21/11 | 1–6 years | 0.6–3 years | Buzhong Yiqi decoction (1 package, bid) | Oryzanol + vitamin B1 + vitamin B6 + deanxit + amino acid | 56 | ⑪ |
| Wang. (2012) | China | RCT | 84 (42/42) | 40.65 ± 12.25 | 40.65 ± 12.25 | NR | NR | 2.14士1.07 years | 2.14士1.07 years | Buzhong Yiqi decoction and Xiaochaihu decoction | ATP (2 tablets, tid) | 28 | ⑥⑪ |
| Wu et al. (2012) | China | RCT | 120 (60/60) | NR | NR | 24/36 | 22/38 | ≥6 m | ≥6 m | Lixu Jieyu prescription (150 ml, bid) | Vitaeamphor (1 tablet, tid) + ATP (20 mg, bid) + oryzanol (20 mg, tid) | 84 | ②③⑦⑧⑨⑩ |
| Zhang et al. (2012a) | China | RCT | 66 (33/33) | 38.74 ± 11.39 | 39.45 ± 10.97 | NR | NR | 10.94 ± 3.72 years | 10.81 ± 2.97 years | Lixu Jieyu prescription (200 ml, bid) | Vitaeamphor (10 mg, bid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 84 | ③⑪ |
| Zhang et al. (2012b) | China | RCT | 60 (30/30) | 37.16+-9.93 | 37.77+-11.48 | 13/17 | 12/18 | 12.52 ± 5.18 m | 13.35 ± 5.17 m | Yaoyao Xiaopi prescription (100 ml, bid) | Gold theragran (1 tablet, tid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 60 | ①⑪ |
| Zhao. (2012) | China | RCT | 84 (42/42) | 40.65 ± 12.25 | 40.65 ± 12.25 | NR | NR | 2.14 ± 1.07 years | 2.14 ± 1.07 years | Buzhong Yiqi decoction and Xiaochaihu decoction | ATP (2 tablets, tid) | NR | ⑥⑪ |
| Lai and Lei. (2013) | China | RCT | 68 (34/34) | 46.8 | 47.6 | 15/19 | 14/20 | 0.6–3 years | 0.5–3.5 years | Baiyu Jianpi decoction (200 ml, bid) | Oryzanol (20 mg, tid) + ATP (20 mg, tid) | 56 | ⑪ |
| Pang and Liu. (2013) | China | RCT | 60 (32/28) | 28–53 | 24–55 | 9/23 | 11/17 | 0.5–4 years | 0.58- y | Shengmai pulvis and Xiaoyao pulvis | Vitamin C (0.2 g, tid) + vitamin Bco (2 tablets, tid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 28 | ⑪ |
| Xu et al. (2013) | China | RCT | 68 (40/28) | 33.24 ± 1.56 | 30.24 ± 1.28 | 22/18 | 16/12 | 24.24 ± 4.30 m | 22.20 ± 3.24 m | Jiawei Naoxin Kang (100 ml, bid) | ATP (1 tablet, bid) | 10 | ①⑪ |
| Xu and Wang. (2013) | China | RCT | 84 (42/42) | 35.29 ± 6.18 | 34.87 ± 7.08 | 17/25 | 19/23 | 15.06 ± 4.80 m | 14.75 ± 5.02 m | Chaihu Jia Longgu Muli decoction (200 ml, bid) + paroxetine (20–40 mg/d) | Paroxetine (20–40 mg/d) | NR | ①④⑤⑪⑫ |
| Zhao (2013) | China | RCT | 176 (88/88) | 52.5 | 50.2 | 35/53 | 29/59 | ≥6 m | ≥6 m | Self-designed Baihe Yangxin Jianpi decoction (175 ml, bid) | Vitamina (NR) + oryzanol (NR) + vitamin B (NR) | NR | ⑪ |
| Zhao et al. (2013) | China | RCT | 90 (45/45) | 36.5 | 35.6 | NR | NR | 9.35 ± 2.13 m | 9.05 ± 3.13 m | Compound of Fufangteng mixture (15 ml, bid) | Vitaeamphor (10 mg, bid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 90 | ⑪ |
| Teng et al. (2014) | China | RCT | 60 (30/30) | 43 | 43 | 12/18 | 11/19 | 2.4 years | 2.7 years | Buzhong JiePi decoction (1 package, bid) | Oryzanol (10 mg, tid) + vitamin B1 tablet (10 mg, tid) | 56 | ⑪ |
| Xu (2014) | China | RCT | 63 (32/31) | NR | NR | 18/14 | 16/15 | NR | NR | Qingshu Yiqi decoction (1 package) | Oryzanol diazepam tablet (NR) + poly methamphetamine tablet (NR) | 7 | ⑪ |
| Gao and Pang. (2015) | China | RCT | 70 (35/35) | 32.8 ± 10.5 | 33.6 ± 12.7 | 12/23 | 15/20 | 0.75–4 years | 0.7–4.2 years | Wendan decoction and Sini decoction (1 dose/d, bid) + fluoxetine hydrochloride capsules (20–40 mg, qod) | Fluoxetine hydrochloride capsule (20–40 mg, qod) | 28 | ⑪ |
| Li and Zao. (2015) | China | RCT | 74 (37/37) | 55.3 ± 6.2 | 54.7 ± 6.9 | NR | NR | ≥6 m | ≥6 m | Jianpi Wenshen Shugan prescription (1 dose/d, tid) | Multivitamin tablet (1 tablet, tid) + oryzanol (1 tablet, tid) | 30 | ⑥⑪ |
| Liu et al. (2015) | China | RCT | 100 (51/49) | 43.2 ± 12.6 | 42.6 ± 10.5 | 20/31 | 19/30 | 0.5–3 years | 0.8–3 years | Shugan Yangxue decoction (1 dose/d, bid) | Vitamin C (0.1 g, tid) + vitamin B (0.2 g, tid) + ATP (20 mg, tid) + oryzanol (20 mg, tid) | 42 | ①② |
| Li (2015) | China | RCT | 68 (34/34) | 41.3 ± 2.3 | 42.3 ± 2.4 | 18/16 | 19/15 | 2.4 ± 1.1 y | 2.5 ± 1.2 years | Buzhong Yiqi decoction and Xiaochaihu decoction (1 dose/d, bid) | ATP (2 tablets, tid) | 28 | ⑥⑪ |
| Niu et al. (2015) | China | RCT | 132 (66/66) | 44.18 ± 8.66 | 46.34 ± 9.39 | 26/40 | 22/44 | ≥6 m | ≥6 m | Bushen Shugan decoction (1 dose/d, bid) | ATP (20 mg, bid) + oryzanol (20 mg, tid) | 56 | ①③ |
| Tan et al. (2015) | China | RCT | 60 (30/30) | 35.6 ± 9.7 | 35.0 ± 10.4 | 14/16 | 13/17 | 1.4 ± 0.7 years | 1.1 ± 0.5 years | Sini decoction and Wulin powder (1 dose/d, 100 ml, bid) | Vitamin B1 tablet (10 mg, tid) + vitamin B6 tablet (20 mg, tid) + oryzanol tablet (20 mg, tid) | 28 | ①⑪ |
| Zhang et al. (2015) | China | RCT | 172 (88/84) | 32 ± 6.38 | 33 ± 7.26 | 34/54 | 31/53 | NR | NR | Wenzhen Yunqi prescription (1 package, bid) | Deanxit (2 tablets, bid) | NR | ①⑪⑫ |
| Gao and Pang. (2016) | China | RCT | 70 (35/35) | 32.8 ± 10.5 | 33.6 ± 12.7 | 12/23 | 15/20 | 0.75–4 years | 0.7–4.2 years | Wendan decoction and Sini powder (1 dose/d, 150 ml, bid) + fluoxetine hydrochloride capsule (20–40 mg, qod) | Fluoxetine hydrochloride capsule (20–40 mg, qod) | 28 | ⑪ |
| Shi and Wu. (2016) | China | RCT | 120 (60/60) | 45.25 ± 9.81 | 43.14 ± 8.35 | 22/38 | 25/35 | 1.20 ± 0.45 years | 1.15 ± 0.50 years | Suanzaoren decoction (1 dose/d) | Oryzanol (30 mg, tid) | 14 | ⑪ |
| Sun et al. (2016) | China | RCT | 80 (40/40) | 36.58 ± 5.48 | 36.87 ± 6.58 | NR | NR | 18.56 ± 6.45 m | 17.75 ± 5.92 m | Shugan Yiyang capsule (0.75 g, tid) + paroxetine hydrochloride tablets (20 mg, 1 dose/d) | Paroxetine hydrochloride tablet (20 mg, 1 dose/d) | NR | ④⑤⑥⑪⑫ |
| Wu et al. (2016) | China | RCT | 80 (42/38) | 40.15 ± 8.51 | 41.46 ± 7.94 | 28/14 | 25/13 | 1.32 ± 0.67 years | 1.28 ± 0.59 years | Xiaopi Yin (1 dose/d, 200 ml, tid) | Vitamin B6 (2 tablets, 1 dose/d) | 42 | ⑥⑪⑫ |
| Wang (2017) | China | RCT | 140 (70/70) | 42.47 ± 12.46 | 42.33 ± 17.40 | 38/32 | 39/31 | 12.63 ± 4.11 m | 12.78 ± 4.24 m | Bupi Yishen decoction (1 dose/d, bid) + ATP (60 mg, tid) + oryzanol (20 mg, tid) | ATP (60 mg, tid) + oryzanol (20 mg, tid) | 30 | ⑪ |
| Ye (2017) | China | RCT | 76 (37/39) | 40.19 ± 8.05 | 37.67 ± 7.30 | 12/25 | 9/30 | 13.46 ± 4.25 m | 15.13 ± 4.60 m | Shenxian congee (1 dose/d, qd) + health guidance | Health guidance | 56 | ①④⑤⑥⑪⑫ |
| Zheng et al. (2017) | China | RCT | 90 (45/45) | 35.8 ± 7.6 | 34.9 ± 8.1 | 21/24 | 18/27 | 15.4 ± 3.8 m | 16.2 ± 3.5 m | Shugan Jianpi Yishen prescription (1 dose/d, bid) + paroxetine hydrochloride tablet (20 mg, qd) | Paroxetine hydrochloride tablet (20 mg, qd) | 56 | ①⑪ |
| Du (2018) | China | RCT | 108 (54/54) | 40.59 ± 5.60 | 40.64 ± 5.81 | 26/29 | 26/28 | 2.08 ± 0.57 years | 2.10 ± 0.5 years | Self-designed Yishen Buxue ointment (150–200 ml, bid) | Vitamin C (0.1 g, tid) + vitamin B (0.2 g, tid) + oryzanol (20 mg, tid) + ATP (20 mg, tid) | 42 | ①⑥⑦⑧⑨⑪ |
| Li et al. (2018) | China | RCT | 60 (30/30) | 42.65 ± 8.42 | 42.12 ± 7.86 | 18/12 | 17/13 | 2.26 ± 0.67 years | 2.12 ± 0.76 years | Yiqi Yangxue Bupi Hegan prescription (150 ml, bid) + paroxetine hydrochloride tablet (20–40 mg, 1 dose/d) | Paroxetine hydrochloride tablet (20–40 mg, 1 dose/d) | 15 | ⑪⑫ |
| Liu and Cai. (2018) | China | RCT | 82 (41/41) | 34.65 ± 6.98 | 32.99 ± 6.47 | 17/24 | 15/26 | 14.24 ± 4.66 m | 16.01 ± 5.23 m | Bupiwei Xieyinhuo Shengyang decoction (1 dose/d, bid) + fluoxetine hydrochloride capsule (20 mg, qd) | Fluoxetine hydrochloride capsule (20 mg, qd) | 28 | ⑥⑪⑫ |
| Ou et al. (2018) | China | RCT | 80 (40/40) | 50.3 ± 11.35 | 49.8 ± 10.45 | 18/22 | 19/21 | 2–5 years | 2–6 years | Guipi decoction Jiawei (1 dose/d, bid) | Fluoxetine hydrochloride capsule (20–40 mg, qod) | 90 | ⑪ |
| Weng (2018) | China | RCT | 150 (75/75) | 40.9 ± 8.9 | 41.7 ± 9.2 | 28/47 | 26/49 | ≥6 m | ≥6 m | Liujunzi decoction | Oryzanol (10–20 mg, tid) + Vitamin B1 (20 mg, tid) | 90 | ⑪ |
| Wu et al. (2018) | China | RCT | 86 (43/43) | 39.7 ± 6.9 | 40.3 ± 7.5 | 16/27 | 18/25 | 0.5–5 years | 0.5–7 years | Guipi decoction (1 dose/d, bid) | Vitamin C + Vitamin B | 84 | ⑦⑧⑨⑪ |
| Luo (2018) | China | RCT | 56 (28/28) | 28.14 | 28.86 | 15/13 | 14/14 | NR | NR | Qingre Qushi prescription (1 package, bid) | Oryzanol tablet (2 tablets, tid) + multivitamin B tablet (2 tablets, tid) | 14 | ①②⑥⑪ |
| Ding (2019) | China | RCT | 60 (30/30) | 39.21 ± 1.25 | 41.15 ± 1.29 | 15/15 | 16/14 | NR | NR | Guipi decoction (1 dose/d, bid) | Vitamin C + vitamin B | 84 | ⑪ |
| He (2019) | China | RCT | 65 (33/32) | 33.84 ± 4.98 | 33.70 ± 4.02 | 9/23 | 8/22 | 12.66 ± 3.16 m | 12.57 ± 3.35 m | Shugan Jianpi Huoxue prescription (1 dose/d, bid) | Oryzanol (20 mg, tid) | 28 | ①⑥⑦⑧⑨⑪ |
| Hu (2019) | China | RCT | 66 (33/33) | 55.14 ± 1.26 | 55.11 ± 1.22 | 16/17 | 17/16 | 3.15 ± 1.14 years | 3.11 ± 1.11 years | Buzhong Yiqi and Xiaochaihu decoction (1 dose/d, bid) | ATP (2 tablets, tid) | NR | ⑥⑪ |
| Liu et al. (2019a) | China | RCT | 60 (30/30) | 42 | 42 | NR | NR | 1 year | 1 year | Jiawei Lingzhi pill (1 dose/d, bid) | Fluoxetine tablet (20 mg, qd) | 30 | ①⑪ |
| Liu et al. (2019b) | China | RCT | 72 (36/36) | NR | NR | 9/27 | 11/25 | 0.58–2 years | 0.58–2.2 years | Chaihu Guizhi decoction grain (1 package, bid) | placebo (1 package, bid) | 28 | ①⑥⑦⑧⑨⑪ |
| Liu et al. (2019c) | China | RCT | 60 (30/30) | 43.3 ± 12.6 | 42.9 ± 10.6 | 10/20 | 11/19 | 15.0 ± 5.6 m | 16.0 ± 6.3 m | Jianpi Yishen decoction (1 dose/d, bid) | Vitamin C (0.1 g, tid) + vitamin B (0.2 g, tid) + vitamin E (0.1 g, tid) | 42 | ⑪ |
| Ma et al. (2019) | China | RCT | 80 (40/40) | 45.2 | 43 | 12/28 | 10/30 | NR | NR | Self-designed Jiawei Erxian decoction (1 dose/d, bid) | Vitamin B1 (20 mg) + oryzanol (20 mg) + Bailemen (4 tablets, tid) | 60 | ⑪ |
| Shi (2019) | China | RCT | 160 (78/82) | 41.51 ± 9.347 | 40.55 ± 9.775 | 35/43 | 32/50 | 0.9–3 years | 0.7–2 years | Xiaoyao pulvis Jiawei (1 dose/d, bid) | Multivitamin B tablet (2 tablets, tid) + oryzanol (20 mg, tid) | 21 | ①⑥⑪ |
| Wang (2019) | China | RCT | 69 (35/34) | 34.67 | 35.34 | 18/17 | 19/15 | NR | NR | Sanren decoction and Sijunzi decoction (1 dose/d, bid) | oryzanol (20 mg, tid) + multivitamin B tablet (20 mg, tid) | 14 | ②⑪ |
| Yang (2019) | China | RCT | 40 (20/20) | 38.45 ± 5.36 | 39.12 ± 5.21 | 11/9 | 10/10 | 0.5–1.5 years | 0.5–1.5 years | Zuogui pill (9 g, bid) | Symptomatic treatment + anti-virus + improve immunity + anti-depression + psychotherapy | 120 | ④⑪ |
| Dong (2020) | China | RCT | 80 (40/40) | 37.68 ± 3.41 | 37.72 ± 3.34 | 26/14 | 23/17 | 1.24 ± 0.17 years | 1.21 ± 0.15 years | Qingshu Yiqi decoction grain (200 ml, bid) | Nuodikang capsule (2 tablets, tid) | 90 | ⑥⑪ |
| Li (2020) | China | RCT | 72 (36/36) | 37.82 ± 6.03 | 39.11 ± 5.94 | 19/17 | 21/15 | NR | NR | Buzhong Yiqi decoction and Xiaochaihu decoction | ATP (40 mg, tid) | 30 | ⑪ |
| Mao (2020) | China | RCT | 59 (30/29) | 39.58 ± 0.46 | 39.40 ± 0.37 | 17/13 | 18/11 | 1.26 ± 0.38 years | 1.37 ± 0.22 years | Yishen Tiaodu method (1 dose/d, qod) | Oryzanol (20 mg, tid) | 56 | ①⑪ |
| Wang (2020) | China | RCT | 90 (45/45) | 47.5 ± 7.3 | 48.1 ± 7.6 | 16/29 | 17/28 | 17.2 ± 3.5 m | 17.7 ± 3.8 m | Chaihu Guizhi decoction (1 dose/d, bid) | Placebo (12 g, bid) | 28 | ①⑥⑪ |
| Chen (2021) | China | RCT | 63 (33/30) | 33.8 ± 13.1 | 33.6 ± 13.2 | 15/18 | 14/16 | 18.51 ± 9.03 m | 16.32 ± 8.94 m | Xiaoyao powder (1 dose/d, bid) | Oryzanol (20 mg, tid) + vitamin B1 (20 mg, tid) + ATP (20 mg, tid) | 60 | ①⑪ |
| Li et al. (2021) | China | RCT | 79 (40/39) | 41.60 ± 9.29 | 39.51 ± 9.79 | 22/18 | 19/20 | 10.98 ± 3.03 m | 11.49 ± 3.60 m | Jiannao Yizhi ointment (bid) + oryzanol (20 mg, tid) + vitamin B1 (10 mg, tid) | Oryzanol (20 mg, tid) + vitamin B1 (10 mg, tid) | 56 | ①②⑪ |
| Liu et al. (2021) | China | RCT | 72 (36/36) | NR | NR | NR | NR | NR | NR | Chaihu Guizhi decoction grain (1 package, bid) | Placebo (1 package, bid) | 28 | ①④⑤⑥⑪ |
| Wang (2021) | China | RCT | 60 (30/30) | 40.06 ± 11.51 | 41.23 ± 8.47 | 10/20 | 12/18 | NR | NR | Shenling Baizhu powder (1 dose/d, bid) | GET | 30 | ①②⑪ |
| Zhang (2021) | China | RCT | 60 (30/30) | 45.2 ± 3.1 | 46.8 ± 3.4 | 14/16 | 16/14 | NR | NR | Jiawei Guizhi Xinjia decoction (1 dose/d, bid) | Fluoxetine hydrochloride capsule (20–60 mg, qd) | 84 | ④⑤ |
| Sheng et al. (2022) | China | RCT | 69 (35/34) | NR | NR | 16/19 | 14/20 | NR | NR | Wenshen Lipi prescription (1 package, bid) | Placebo (1 package, bid) | 28 | ③⑩ |
RCT: randomized controlled trial; TG: trial group; CG: control group; F: female; M: male; NR: not reported; ATP: adenosine triphosphate; Y: year; GET: graded exercise therapy; ①: Fatigue Scale scores; ②: Fatigue Assessment Instrument scores; ③: Self-Rating Scale of mental state scores; ④: Self-Rating Anxiety Scale scores; ⑤: Self-Rating Depression Scale scores; ⑥: clinical symptom scores; ⑦: Immunoglobulin A; ⑧: Immunoglobulin G; ⑨: Immunoglobulin M; ⑩: natural killer cell level; ⑪: effective rate; ⑫: adverse events.
TABLE 2.
Components of Chinese herbal medicine used in the included studies.
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| Ning and Li. (2002) | Sijunzi decoction | Codonopsis pilosula (Franch.) Nannf. 10 g, Atractylodes macrocephala Koidz. 12 g, Poria cocos (Schw.) Wolf 12 g, Glycyrrhiza glabra L. 6 g, Astragalus mongholicus Bunge 20 g, Acorusgramineus Aiton 10 g, Polygala tenuifolia Willd. 10 g, Dimocarpus Longan Lour. 10 g | Decoction |
| Yang et al. (2004) | Buzhong Yiqi decoction and Xiaochaihu decoction | Codonopsis pilosula (Franch.) Nannf. 25 g, Astragalus mongholicus Bunge 30 g, Bupleurum falcatum L. 15 g, Agrimonia Pilosa Ledeb 25 g, Atractylodes macrocephala Koidz. 20 g, Pinellia ternata (Thunb.) Makino 15 g, Poria cocos (Schw.) Wolf 20 g, Curcuma aromatica Salisb. 20 g, Platycodon grandiflorus (Jacq.) A.DC. 6 g, Citrus × aurantium L. 15 g, Scutellaria baicalensis Georgi 12 g, Glycyrrhiza glabra L. 12 g | Granule |
| Zhang et al. (2004) | Self-designed Shenqi Fuyuan decoction | Astragalus mongholicus Bunge, Panax ginseng C.A.Mey., Atractylodes macrocephala Koidz., Angelica sinensis (Oliv.) Diels, Actaea cimicifuga L., Bupleurum falcatum L., Citrus × aurantium L., Glycyrrhiza glabra L. | Decoction |
| Zhang and Zhou. (2004) | Buzhong Yiqi decoction | Panax ginseng C.A.Mey. 10 g, Astragalus mongholicus Bunge 12 g, Atractylodes macrocephala Koidz. 10 g, Poria cocos (Schw.) Wolf 10 g, Angelica sinensis (Oliv.) Diels 9 g, Polygala tenuifolia Willd. 9 g, Glycyrrhiza glabra L. 9 g, Bupleurum falcatum L. 9 g, Paeonia lactiflora Pall. 9 g, Spatholobus suberectus Dunn 12 g, Citrus × aurantium L. 10 g, Rehmannia glutinosa (Gaertn.) DC. 2 g | Decoction |
| Wei (2005) | Xiaochaihu decoction | Bupleurum falcatum L. 12 g, Scutellaria baicalensis Georgi 12 g, Pinellia ternata (Thunb.) Makino 12 g, Zingiber officinale Roscoe 10 g, Panax ginseng C.A.Mey. 10 g, Glycyrrhiza glabra L. 6 g, Ziziphus Jujuba Mill. 5 pieces | Decoction |
| Yao and Qiu. (2005) | Self-designed Xianshen decoction | Panax ginseng C.A.Mey. 10 g, Paeonia lactiflora Pall. 12 g, Agrimonia Pilosa Ledeb 30 g, Panax notoginseng (Burkill) F.H.Chen 6 g | Decoction |
| Liang (2006) | Shengmai pulvis and Xuefu Zhuyu decoction | Panax ginseng C.A.Mey. 12 g, Ophiopogon japonicus (Thunb.) Kergawl. 15 g, Schisandra chinensis (Turcz.) Baill. 10 g, Rehmannia glutinosa (Gaertn.) DC. 20 g, Paeonia lactiflora Pall. 15 g, Angelica sinensis (Oliv.) Diels 8 g, Conioselinum anthriscoides “Chuanxiong” 6 g, Prunus Persica (L.) Batsch 10 g, Carthamus tinctorius L. 10 g, Citrus × aurantium L. 8 g, Platycodon grandiflorus (Jacq.) A.DC. 10 g, Bupleurum falcatum L. 10 g, Achyranthes bidentata Blume 15 g, Curcuma aromatica Salisb. 20 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Zhao et al. (2006) | Yiqi Yangyin Jichu prescription | Astragalus mongholicus Bunge 20 g, Pseudostellaria Heterophylla (Miq.) Pax 10 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 10 g, Angelica sinensis (Oliv.) Diels 15 g, Paeonia lactiflora Pall. 20 g, Rehmannia glutinosa (Gaertn.) DC. 10 g, Ophiopogon japonicus (Thunb.) Kergawl. 15 g, Lycium chinense Mill. 15 g, Cornus Officinalis Siebold & Zucc. 20 g, Anemarrhena asphodeloides Bunge 10 g | Decoction |
| Fang et al. (2007) | Xiaopiling granule | Panax ginseng C.A.Mey., Astragalus mongholicus Bunge, Equus Asinus L., Ophiopogon japonicus (Thunb.) Kergawl., Dimocarpus Longan Lour., Angelica sinensis (Oliv.) Diels, Salvia miltiorrhiza Bunge, Ganoderma lucidum (Leyss. ex Fr.) Karst., Ziziphi Spinosae Semen, Poria cocos (Schw.) Wolf, Schisandra chinensis (Turcz.) Baill., Crataegus Pinnatifida Bunge, Spatholobus suberectus Dunn | Granule |
| Gong (2007) | Guipi decoction | Astragalus mongholicus Bunge, Codonopsis pilosula (Franch.) Nannf., Poria cocos (Schw.) Wolf, Atractylodes macrocephala Koidz., Polygala tenuifolia Willd., Dimocarpus Longan Lour., Dolomiaea Costus (Falc.) Kasana and A.K.Pandey, Agrimonia Pilosa Ledeb, Ziziphus Jujuba Mill., Ziziphi Spinosae Semen, Matricaria Chamomilla L., Strobilanthes Cusia (Nees) Kuntze, Glycyrrhiza glabra L. | Decoction |
| Guo et al. (2007) | Qi and Blood Proral Solution | Codonopsis pilosula (Franch.) Nannf., Astragalus mongholicus Bunge, Epimedium brevicornum Maxim., Atractylodes macrocephala Koidz., Rehmannia glutinosa (Gaertn.) DC., Lycium chinense Mill., Poria cocos (Schw.) Wolf, Curculigo Orchioidesgaertn., Paeonia lactiflora Pall., Angelica sinensis (Oliv.) Diels | Oral liquids |
| Lin (2007) | Shenling Baizhu powder | Pseudostellaria Heterophylla (Miq.) Pax 90 g, Poria cocos (Schw.) Wolf 90 g, Euryale Ferox Salisb. 90 g, Nelumbo Nuciferagaertn. 90 g, Lablab Purpureus Subsp. Purpureus 90 g, glycine Max (L.) Merr. 90 g, Lycium chinense Mill. 90 g, Polygonum multiflorum Thunb. 90 g, Dioscorea oppositifolia L. 150 g, Coix lacryma-jobi L. 60 g, Astragalus mongholicus Bunge 60 g, Paeonia lactiflora Pall. 40 g, Citrus × aurantium L. 25 g, Placenta Hominis 50 g, Ligustrum Lucidum W.T.Aiton 50 g, Cornus Officinalis Siebold & Zucc. 50 g, Oryza sativa L. 1250 g | Decoction |
| Sun et al. (2007) | Shuyu decoction | Lilium Lancifolium Thunb. 30 g, Anemarrhena asphodeloides Bunge 10 g, Triticum aestivum L. 30 g, Ziziphus Jujuba Mill. 30 g, Bupleurum falcatum L. 10 g, Paeonia lactiflora Pall. 20 g, Citrus × aurantium L. 10 g, Glycyrrhiza glabra L. 10 g, Albiziae Cortex 30 g, Curcuma aromatica Salisb. 15 g, Ziziphi Spinosae Semen 30 g, Codonopsis pilosula (Franch.) Nannf. 20 g, Atractylodes macrocephala Koidz. 10 g | Decoction |
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| Wang et al. (2007) | Fuzheng Jieyu prescription | Angelica sinensis (Oliv.) Diels 10 g, Conioselinum anthriscoides “Chuanxiong” 6 g, Paeonia lactiflora Pall. 12 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Panax ginseng C.A.Mey. 5 g, Atractylodes macrocephala Koidz. 10 g, Poria cocos (Schw.) Wolf 8 g, Glycyrrhiza glabra L. 5 g, Dioscorea oppositifolia L. 12 g, Lycium chinense Mill. 12 g, Cornus Officinalis Siebold & Zucc. 12 g, Achyranthes bidentata Blume 9 g, Cuscuta chinensis Lam. 12 g, Cervus nippon Temminck 12 g, Colla Carapacis et Plastri Testudinis 12 g, Citrus × aurantium L. 6 g, Bupleurum falcatum L. 6 g, Citrus × aurantium L. 9 g, Atractylodes lancea (Thunb.) DC. 6 g, Cyperus rotundus L. 6 g, Hyssopus officinalis L. 9 g, gardenia Jasminoides J.Ellis 6 g | Decoction |
| Fang et al. (2008) | Fufang Shenqi ointment | Panax ginseng C.A.Mey., Astragalus mongholicus Bunge, Atractylodes macrocephala Koidz., Poria cocos (Schw.) Wolf, Glycyrrhiza glabra L., Paeonia lactiflora Pall., Conioselinum anthriscoides “Chuanxiong,” Angelica sinensis (Oliv.) Diels, Rehmannia glutinosa (Gaertn.) DC., Curcuma aromatica Salisb., Bupleurum falcatum L. | Ointment |
| Cheng (2009) | Bainian Le oral liquid | Euonymus fortunei var. fortunei, Panax ginseng C.A.Mey., Astragalus mongholicus Bunge, Saccharum officinarum L.talis (L.) Franco | Oral liquids |
| Jie and Wang. (2009) | Xiaoyao pill | Bupleurum falcatum L., Angelica sinensis (Oliv.) Diels, Paeonia lactiflora Pall., Atractylodes macrocephala Koidz., Poria cocos (Schw.) Wolf | Pill |
| Li (2009) | Anti-fatigue no. 2 decoction granule | Astragalus mongholicus Bunge, Angelica sinensis (Oliv.) Diels, Platycladus orientalis (L.) Franco, Polygala tenuifolia Willd., Cyperus rotundus L | Granule |
| Ma (2009) | Guipi decoction | Panax ginseng C.A.Mey. 10 g, Poria cocos (Schw.) Wolf 20 g, Astragalus mongholicus Bunge 30 g, Neolitsea cassia (L.) Kosterm. 10 g, Atractylodes macrocephala Koidz. 15 g, Ziziphi Spinosae Semen 30 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 10 g, Angelica sinensis (Oliv.) Dielslog 10 g, Polygala tenuifolia Willd. 10 g, Actaea cimicifuga L. 8 g, Bupleurum falcatum L. 10 g | Decoction |
| Zhang et al. (2009) | Lixu Jieyu prescription | Astragalus mongholicus Bunge 30 g, Pueraria montana var. 30 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Salvia miltiorrhiza Bunge 10 g, Panax notoginseng (Burkill) F.H.Chen 15 g, Epimedium brevicornu Maxim. 10 g, Curcuma aromatica Salisb. 10 g, Acorus gramineus Aiton 10 g | Decoction |
| Hu et al. (2010) | Buqi Tongluo prescription | Astragalus mongholicus Bunge 30 g, Panax ginseng C.A.Mey. 5 g, Citrus × aurantium L. 15 g, Bupleurum falcatum L. 10 g, Pheretima vulgaris Chen 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g | Decoction |
| Chen et al. (2011) | 1. Qixue Liangxu prescription. 2. Ganyu Pixu prescription. 3. Ganshen Kuixu prescription | 1. Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala Koidz. 15 g, Astragalus mongholicus Bunge 15 g, Poria cocos (Schw.) Wolf 15 g, Angelica sinensis (Oliv.) Diels 15 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Paeonia lactiflora Pall. 15 g, Platycladus orientalis (L.) Franco 12 g, Poria cocos (Schw.) Wolf 12 g, Albiziae Cortex 15 g, Glycyrrhiza glabra L. 6 g. 2. Bupleurum falcatum L. 10 g, Citrus × aurantium L. 10 g, Cyperus rotundus L. 10 g, Angelica sinensis (Oliv.) Diels 15 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Paeonia lactiflora Pall. 15 g, Atractylodes macrocephala Koidz. 15 g, Dioscorea oppositifolia L. 15 g, Poria cocos (Schw.) Wolf 15 g, Poria cocos (Schw.) Wolf 12 g, Glycyrrhiza glabra L. 6 g. 3. Anemarrhena asphodeloides Bunge 9 g, Phellodendron amurense Rupr 9 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Dioscorea oppositifolia L. 15 g, Cornus Officinalis Siebold & Zucc. 15 g, Lycium chinense Mill. 15 g, Cuscuta chinensis Lam. 15 g, Achyranthes bidentata Blume 15 g, Trionyx sinensis Wiegmann 15 g, Salvia miltiorrhiza Bunge 30 g, Ziziphi Spinosae Semen 12 g, Reynoutria Multiflora (Thunb.) Moldenke 30 g | Decoction |
| Li et al. (2011) | Chaihu Shugan pulvis and Guipi decoction | Bupleurum falcatum L. 12 g, Citrus × aurantium L. 12 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Cyperus rotundus L. 12 g, Citrus medica L. 10 g, Astragalus mongholicus Bunge 15 g, Angelica sinensis (Oliv.) Diels 10 g, Panax ginseng C.A.Mey. 6 g, Atractylodes macrocephala Koidz. 10 g, Poria cocos (Schw.) Wolf 12 g, Polygala tenuifolia Willd. 15 g, Ziziphi Spinosae Semen 15 g, Glycyrrhiza glabra L. 10 g | Decoction |
| Liu et al. (2011) | Shugan Yangxue prescription | Bupleurum falcatum L. 10 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Citrus medica L. 10 g, Curcuma aromatica Salisb. 15 g, Paeonia lactiflora Pall. 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Angelica sinensis (Oliv.) Diels 10 g, Albiziae Cortex 20 g | Decoction |
| Wang et al. (2011) | Yiqi Ziyin Buyang prescription | 1. Tonifying qi: Codonopsis pilosula (Franch.) Nannf. 20 g, Astragalus mongholicus Bunge 30 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 12 g, Angelica sinensis (Oliv.) Diels 15 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Schisandra chinensis (Turcz.) Baill. 6 g, Citrus × aurantium L. 6 g, Glycyrrhiza glabra L. 3 g. 2. Nourishing the blood: Angelica sinensis (Oliv.) Diels 15 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Conioselinum anthriscoides “Chuanxiong” 12 g, Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan 12 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Astragalus mongholicus Bunge 15 g, Lycium chinense Mill. 12 g, Spatholobus suberectus Dunn 15 g, Glycyrrhiza glabra L. 3 g. 3. Nourishing Yin: Adenophora triphylla (Thunb.) A.DC. 12 g, Ophiopogon japonicus (Thunb.) Kergawl. 12 g, Polygonatum odoratum (Mill.) Druce 12 g, Rehmannia glutinosa (Gaertn.) | Decoction |
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| DC. 12 g, Pseudostellaria Heterophylla (Miq.) Pax 15 g, Schisandra chinensis (Turcz.) Baill. 6 g, Angelica sinensis (Oliv.) Diels 12 g, Glycyrrhiza glabra L. 3 g. 4. Tonifying yang: Rehmannia glutinosa (Gaertn.) DC. 15 g, Dioscorea oppositifolia L. 12 g, Cornus Officinalis Siebold & Zucc. 10 g, Lycium chinense Mill. 12 g, Angelica sinensis (Oliv.) Diels 12 g, Eucommia ulmoides Oliv. 12 g, Cuscuta chinensis Lam. 12 g, Cyperus rotundus L. 12 g, Neolitsea cassia (L.) Kosterm. 3 g | |||
| Zhang et al. (2011) | Zhenqi Jiepi decoction | Polygala fallax Hemsl 20 g, Ardisia gigantifolia Stapf 10 g, Astragalus mongholicus Bunge 30 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala Koidz. 10 g, Pueraria montana var. 30 g, Salvia miltiorrhiza Bunge 15 g, Epimedium sagittatum (Siebold & Zucc.) Maxim. 15 g | Decoction |
| Jiang. (2012) | Buzhong Yiqi decoction and Guipi decoction | Codonopsis pilosula (Franch.) Nannf. 20 g, Astragalus mongholicus Bunge 20 g, Dimocarpus Longan Lour. 12 g, Ziziphi Spinosae Semen 12 g, Atractylodes macrocephala Koidz. 9 g, Poria cocos (Schw.) Wolf 9 g, Angelica sinensis (Oliv.) Diels 9 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 6 g, Polygala tenuifolia Willd. 6 g, Citrus × aurantium L. 6 g, Actaea cimicifuga L. 6 g, Bupleurum falcatum L. 6 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Kong (2012) | Self-designed anti-fatigue decoction | Bupleurum falcatum L. 12 g, Astragalus mongholicus Bunge 25 g, Eleutherococcus Nodiflorus (Dunn) S.Y.Hu 18 g, Citrus × aurantium L. 12 g, Paeonia lactiflora Pall. 15 g, Angelica sinensis (Oliv.) Diels 15 g, Phyllolobium Chinense Fisch. 12 g, Cyperus rotundus L. 12 g, Lycium chinense Mill. 20 g, Epimedium sagittatum (Siebold & Zucc.) Maxim. 12 g, Atractylodes macrocephala Koidz. 15 g, Lonicera Japonica Thunb. 20 g | Decoction |
| Ren and Yu. (2012) | Self-designed Zhongyao Buxu decoction | Astragalus mongholicus Bunge 30 g, Angelica sinensis (Oliv.) Diels 20 g, Codonopsis pilosula (Franch.) Nannf. 20 g, Bupleurum falcatum L. 10 g, Citrus × aurantium L. 10 g, Schisandra chinensis (Turcz.) Baill. 10 g, Rehmannia glutinosa (Gaertn.) DC. 10 g, Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Tian and Wang. (2012) | Buzhong Yiqi decoction | Astragalus mongholicus Bunge 30 g, Codonopsis pilosula (Franch.) Nannf. 20 g, Atractylodes macrocephala Koidz. 20 g, Poria cocos (Schw.) Wolf 20 g, Angelica sinensis (Oliv.) Diels 15 g, Curcuma aromatica Salisb. 15 g, Citrus × aurantium L. 15 g, Actaea cimicifuga L.10 g, Bupleurum falcatum L. 15 g, Paeonia lactiflora Pall. 10 g, Neolitsea cassia (L.) Kosterm. 10 g, Ziziphus Jujuba Mill. 6 pieces, Ophiopogon japonicus (Thunb.) Kergawl. 15 g, Schisandra chinensis (Turcz.) Baill. 15 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Wang. (2012) | Buzhong Yiqi decoction and Xiaochaihu decoction | Codonopsis pilosula (Franch.) Nannf. 30 g, Astragalus mongholicus Bunge 30 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 15 g, Bupleurum falcatum L. 12 g, Agrimonia Pilosa Ledeb 20 g, Curcuma aromatica Salisb. 15 g, Pinellia ternata (Thunb.) Makino 12 g, Citrus × aurantium L. 10 g, Scutellaria baicalensis Georgi 10 g, Platycodon grandiflorus (Jacq.) A.DC. 5 g, Glycyrrhiza glabra L. 15 g | Decoction |
| Wu et al. (2012) | Lixu Jieyu prescription | Not reported | Decoction |
| Zhang et al. (2012a) | Lixu Jieyu prescription | Astragalus mongholicus Bunge, Pueraria montana var., Codonopsis pilosula (Franch.) Nannf., Salvia miltiorrhiza Bunge, Rhodiola crenulata (Hook.f. and Thomson) H.Ohba, Panax notoginseng (Burkill) F.H.Chen, Epimedium brevicornu Maxim., Curcuma aromatica Salisb., Acorusgramineus Aiton | Decoction |
| Zhang et al. (2012b) | Yaoyao Xiaopi prescription | Polygala fallax Hemsl 25 g, Radix fici simplicissimae 30 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala Koidz. 10 g, Salvia miltiorrhiza Bunge 15 g, Pueraria montana var. 15 g, Dimocarpus Longan Lour. 15 g | Decoction |
| Zhao. (2012) | Buzhong Yiqi decoction and Xiaochaihu decoction | Codonopsis pilosula (Franch.) Nannf. 30 g, Astragalus mongholicus Bunge 30 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 15 g, Bupleurum falcatum L. 12 g, Agrimonia Pilosa Ledeb 20 g, Curcuma aromatica Salisb. 15 g, Pinellia ternata (Thunb.) Makino 12 g, Citrus × aurantium L. 10 g, Scutellaria baicalensis Georgi 10 g, Platycodon grandiflorus (Jacq.) A.DC. 5 g, Glycyrrhiza glabra L. 15 g | Decoction |
| Lai and Lei. (2013) | Baiyu Jianpi decoction | Lilium Lancifolium Thunb. 15 g, Curcuma aromatica Salisb. 10 g, Bupleurum falcatum L. 10 g, Paeonia lactiflora Pall. 10 g, Astragalus mongholicus Bunge 10 g, Angelica sinensis (Oliv.) Diels 10 g, Cyperus rotundus L. 10 g, Citrus × aurantium L. 6 g, Atractylodes macrocephala Koidz. 10 g, Paeonia × Suffruticosa Andrews 10 g, Mentha Canadensis L. 6 g | Decoction |
| Pang and Liu. (2013) | Shengmai pulvis and Xiaoyao pulvis | Codonopsis pilosula (Franch.) Nannf. 20 g, Poria cocos (Schw.) Wolf 15 g, Atractylodes macrocephala Koidz. 15 g, Paeonia lactiflora Pall. 15 g, Angelica sinensis (Oliv.) Diels 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Albiziae Cortex 10 g, Bupleurum falcatum L. 10 g, Astragalus mongholicus Bunge 15 g, Ophiopogon japonicus (Thunb.) Ker Gawl. 10 g | Decoction |
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| Schisandra chinensis (Turcz.) Baill. 10 g, Glycyrrhiza glabra L. 6 g | |||
| Xu et al. (2013) | Jiawei Naoxin kang | Panax ginseng C.A.Mey. 20 g, Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan 20 g, Spatholobus suberectus Dunn 20 g, Polygonum multiflorum Thunb. 20 g, Conioselinum anthriscoides “Chuanxiong” 20 g, Angelica sinensis (Oliv.) Diels 15 g, Pheretima vulgaris Chen 15 g, Astragalus mongholicus Bunge 30 g, Lycopodium japonicum Thunb. 25 g | Decoction |
| Xu and Wang. (2013) | Chaihu Jia Longgu Muli decoction | Bupleurum falcatum L. 15 g, Scutellaria baicalensis Georgi 12 g, Pinellia ternata (Thunb.) Makino 10 g, Panax ginseng C.A.Mey. 10 g, Neolitsea cassia (L.) Kosterm. 6 g, Rheum Palmatum L. 6 g, Os Draconis 30 g, Ostrea gigas Thunberg 30 g, Succinum 3 g, Zingiber officinale Roscoe 5 g, Ziziphus Jujuba Mill. 6 pieces | Decoction |
| Zhao. (2013) | Self-designed Baihe Yangxin Jianpi decoction | Lilium Lancifolium Thunb. 30 g, Panax ginseng C.A.Mey. 10 g, Poria cocos (Schw.) Wolf 20 g, Astragalus mongholicus Bunge 30 g, Schisandra chinensis (Turcz.) Baill. 12 g, Dimocarpus Longan Lour. 12 g, Atractylodes macrocephala Koidz. 15 g, Ziziphi Spinosae Semen 30 g, Rehmannia glutinosa (Gaertn.) DC. 30 g, Cyperus rotundus L. 12 g, Eucommia ulmoides Oliv. 30 g, Albiziae Cortex 12 g | Decoction |
| Zhao et al. (2013) | Compound of Fufangteng Mixture | Euonymus fortunei var. fortunei, Astragalus mongholicus Bunge, Panax ginseng C.A.Mey | Oral liquids |
| Teng et al. (2014) | Buzhong Jiepi decoction | Codonopsis pilosula (Franch.) Nannf. 20–30 g, Astragalus mongholicus Bunge 20–30 g, Atractylodes macrocephala Koidz. 15 g, Glycyrrhiza glabra L. 9 g, Citrus × aurantium L. 9 g, Angelica sinensis (Oliv.) Diels 9 g, Actaea cimicifuga L. 5 g, Bupleurum falcatum L. 5 g, Pueraria montana var. lobata (Willd.) Maesen and S.M.Almeida ex Sanjappa & Predeep. 15 g, Os Draconis 30 g, Ostrea gigas Thunberg 30 g | Decoction |
| Xu. (2014) | Qingshu Yiqi decoction | Angelica sinensis (Oliv.) Diels 1 g, Astragalus mongholicus Bunge 30 g, Panax ginseng C.A.Mey. 10 g, Ophiopogon japonicus (Thunb.) Ker Gawl. 10 g, Schisandra chinensis (Turcz.) Baill. 9 g, Atractylodes lancea (Thunb.) DC. 9 g, Phellodendron amurense Rupr 10 g, Citrus × aurantium L. 10 g, Atractylodes macrocephala Koidz. 12 g, Alisma plantago-aquatica subsp. 9 g, Hyssopus officinalis L. 12 g, Pueraria montana var. lobata (Willd.) Maesen and S.M.Almeida ex Sanjappa & Predeep. 9 g, Actaea cimicifuga L. 9 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Gao and Pang. (2015) | Wendan decoction and Sini decoction | Pinellia ternata (Thunb.) Makino, Bambusa tuldoides Munro, Glycyrrhiza glabra L., Bupleurum falcatum L., Citrus × aurantium L., Paeonia lactiflora Pall., Zingiber officinale Roscoe, Citrus × aurantium L | Decoction |
| Li and Zao. (2015) | Jianpi Wenshen Shugan prescription | Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 15 g, Angelica sinensis (Oliv.) Diels 15 g, Conioselinum anthriscoides “Chuanxiong” 15 g, Paeonia lactiflora Pall. 15 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Curculigo Orchioides Gaertn. 5 g, Epimedium sagittatum (Siebold & Zucc.) Maxim. 5 g, Bupleurum falcatum L. 10 g, Coptis chinensis Franch 10 g | Decoction |
| Gardenia Jasminoides J.Ellis 10 g, Curcuma aromatica Salisb. 10 g, Zingiber officinale Roscoe 6 g, Glycyrrhiza glabra L. 6 g, Ziziphus Jujuba Mill. 6 pieces | |||
| Liu et al. (2015) | Shugan Yangxue decoction | Bupleurum falcatum L. 10 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Citrus medica L. 10 g, Curcuma aromatica Salisb. 15 g, Paeonia lactiflora Pall. 10 g, Conioselinum anthriscoides ‘Chuanxiong’ 10 g, Angelica sinensis (Oliv.) Diels 10 g, Albiziae Cortex 20 g | Decoction |
| Li. (2015) | Buzhong Yiqi decoction and Xiaochaihu decoction | Codonopsis pilosula (Franch.) Nannf. 25g, Astragalus mongholicus Bunge 30 g, Bupleurum falcatum L. 15 g, Agrimonia Pilosa Ledeb 25g, Atractylodes macrocephala Koidz. 20 g, Pinellia ternata (Thunb.) Makino 15 g, Poria cocos (Schw.) Wolf 20 g, Curcuma aromatica Salisb. 20 g, Platycodon grandiflorus (Jacq.) A.DC. 6 g, Citrus × aurantium L. 15 g, Scutellaria baicalensis Georgi 12 g, Glycyrrhiza glabra L. 12 g, Ziziphus Jujuba Mill. 12 g | Decoction |
| Niu et al. (2015) | Bushen Shugan decoction | Rehmannia glutinosa (Gaertn.) DC. 20 g, Lycium chinense Mill. 15 g, Rehmannia glutinosa (Gaertn.) DC. 20 g, Scrophularia ningpoensis Hemsl 15 g, Ophiopogon japonicus (Thunb.) Ker Gawl. 15 g, Angelica sinensis (Oliv.) Diels 12 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Bupleurum falcatum L. 10 g, Citrus × aurantium L. 10 g, Scutellaria baicalensis Georgi 12 g, Coptis chinensis Franch 6 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Tan et al. (2015) | Sini Decoction and Wulin powder | Cyperus rotundus L. 9 g, Zingiber officinale Roscoe 9 g, Glycyrrhiza glabra L. 9 g, Neolitsea cassia (L.) Kosterm. 10 g, Poria cocos (Schw.) Wolf 15 g, Polyporus umbellatus (Pers)Fr. 15 g, Atractylodes macrocephala Koidz. 10 g, Alisma plantago-aquatica subsp. 15 g, Plantago Asiatica L. 15 g, Bupleurum falcatum L. 9 g, Asarum Heterotropoides F.Schmidt 3 g, Brassica Juncea (L.) Czern. 6 g | Decoction |
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| Zhang et al. (2015) | Wenzhen Yunqi prescription | Astragalus mongholicus Bunge, Pueraria montana var. lobata (Willd.) Maesen and S.M.Almeida ex Sanjappa & Predeep., Curcuma aromatica Salisb., Acorus Gramineus Aiton, Actinolitum | Decoction |
| Gao and Pang. (2016) | Wendan decoction and Sini powder | Pinellia ternata (Thunb.) Makino 6 g, Bambusa tuldoides Munro 6 g, Glycyrrhiza glabra L. 6 g, Bupleurum falcatum L. 6 g, Citrus × aurantium L. 6 g, Paeonia lactiflora Pall. 6 g, Zingiber officinale Roscoe 12 g, Citrus × aurantium L. 9 g | Decoction |
| Shi and Wu. (2016) | Suanzaoren decoction | Ziziphi Spinosae Semen 15 g, Glycyrrhiza glabra L. 3 g, Anemarrhena asphodeloides Bunge 6 g, Poria cocos (Schw.) Wolf 6 g, Conioselinum anthriscoides “Chuanxiong” 6 g | Decoction |
| Sun et al. (2016) | Shugan Yiyang capsule | Bupleurum falcatum L., Tribulus Terrestris L., Aspongopus chinensis Dallas, Polistes mandarinus Saussure, Cnidium Monnieri (L.) Cusson, Cistanche Deserticola Ma, Cuscuta chinensis Lam, Schisandra chinensis (Turcz.) Baill., Gynochthodes Officinalis (F.C.How) Razafim. and B.Bremer, Polygala tenuifolia Willd., Acorus Gramineus Aiton, Pheretima vulgaris Chen, Whitmania pigra Whitman, Scolopendra subspinipes mutilans L. Koch | Capsule |
| Wu et al. (2016) | Xiaopi Yin | Panax ginseng C.A.Mey. 20 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 15 g, Cuscuta chinensis Lam. 10 g, Lycium chinense Mill. 10 g, Epimedium brevicornu Maxim. 10 g, Dioscorea oppositifolia L. 20 g, Psoralea corylifolia L. 10 g, Angelica sinensis (Oliv.) Diels 10 g, Alisma plantago-aquatica subsp. 6 g, Astragalus mongholicus Bunge 20 g, Matricaria Chamomilla L. 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Wang. (2017) | Bupi Yishen decoction | Panax ginseng C.A.Mey. 10 g, Atractylodes macrocephala Koidz. 30 g, Rhodiola Crenulata (Hook.F. and Thomson) H.Ohba 15 g, Poria cocos (Schw.) Wolf 12 g, Cuscuta chinensis Lam. 15 g, Psoralea corylifolia L. 20 g, Citrus × aurantium L. 12 g, Cornus Officinalis Siebold & Zucc. 15 g, Dioscorea oppositifolia L. 15 g, Pinellia ternata (Thunb.) Makino 10 g, Glycyrrhiza glabra L. 10 g, Bupleurum falcatum L. 10 g | Decoction |
| Ye. (2017) | Shenxian congee | Dioscorea oppositifolia L. 10 g, Euryale ferox Salisb. 10 g, Allium tuberosum Rottler ex Spreng. 10 g, Zea mays L. 50 g | Herbal porridge |
| Zheng et al. (2017) | Shugan Jianpi Yishen prescription | Astragalus mongholicus Bunge 30 g, Codonopsis pilosula (Franch.) Nannf. 12 g, Atractylodes macrocephala Koidz. 12 g, Anemarrhena asphodeloides Bunge 10 g, Citrus × aurantium L. 10 g, Bupleurum falcatum L. 10 g, Actaea cimicifuga L. 10 g, Paeonia lactiflora Pall. 10 g, Cuscuta chinensis Lam. 10 g, Epimedium sagittatum (Siebold & Zucc.) Maxim. 10 g, Lycium chinense Mill. 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Du (2018) | Self-designed Yishen Buxue ointment | Angelica sinensis (Oliv.) Diels 10 g, Rehmannia glutinosa (Gaertn.) DC. 15 g, Paeonia lactiflora Pall. 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Cuscuta chinensis Lam. 15 g, Epimedium sagittatum (Siebold & Zucc.) Maxim.12 g, Psoralea corylifolia L. 10 g, Lycium chinense Mill. 10 g | Decoction |
| Li et al. (2018) | Yiqi Yangxue Bupi Hegan prescription | Astragalus mongholicus Bunge 40 g, Atractylodes macrocephala Koidz. 15 g, Paeonia lactiflora Pall. 15 g, Poria cocos (Schw.) Wolf 15 g, Dioscorea oppositifolia L. 15 g, Panax ginseng C.A.Mey. 10 g, Rehmannia glutinosa (Gaertn.) DC. 10 g, Angelica sinensis (Oliv.) Diels 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Bupleurum falcatum L. 10 g, Cyperus rotundus L. 10 g, Corydalis yanhusuo (Y.H.Chou & Chun C.Hsu) W.T.Wang ex Z.Y.Su and C.Y.Wu 10 g, Poria cocos (Schw.) Wolf 10 g, Gardenia Jasminoides J.Ellis 10 g, Glycyrrhiza glabra L. 10 g | Decoction |
| Liu and Cai. (2018) | Bupiwei Xieyinhuo Shengyang decoction | Bupleurum falcatum L. 15 g, Astragalus mongholicus Bunge 10 g, Atractylodes lancea (Thunb.) DC. 10 g, Hansenia Weberbaueriana (Fedde Ex H.Wolff) Pimenov & Kljuykov 10 g, Glycyrrhiza glabra L. 10 g, Actaea cimicifuga L. 8 g, Panax ginseng C.A.Mey. 7 g, Scutellaria baicalensis Georgi 7 g, Coptis chinensis Franch 5 g | Decoction |
| Ou et al. (2018) | Guipi decoction | Astragalus mongholicus Bunge 30 g, Ziziphi Spinosae Semen 25 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Poria cocos (Schw.) Wolf 15 g, Dimocarpus Longan Lour. 15 g, Atractylodes macrocephala Koidz. 15 g, Polygala tenuifolia Willd. 15 g, Angelica sinensis (Oliv.) Diels 15 g, Glycyrrhiza glabra L. 10 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 7 g | Decoction |
| Weng (2018) | Liujunzi decoction | Atractylodes macrocephala Koidz. 9 g, Panax ginseng C.A.Mey. 9 g, Citrus × aurantium L. 3 g, Glycyrrhiza glabra L. 6 g, Poria cocos (Schw.) Wolf 9 g, Pinellia ternata (Thunb.) Makino 4.5 g | Decoction |
| Study | Prescription name | Ingredients of herb prescription | Preparation |
|---|---|---|---|
| Wu et al. (2018) | Guipi decoction | Codonopsis pilosula (Franch.) Nannf. 20 g, Astragalus mongholicus Bunge 20 g, Dimocarpus Longan Lour. 15 g, Ziziphi Spinosae Semen 15 g, Poria cocos (Schw.) Wolf 15 g, Atractylodes macrocephala Koidz. 10 g, Angelica sinensis (Oliv.) Diels 10 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Paeonia lactiflora Pall. 10 g, Bupleurum falcatum L. 10 g, Citrus × aurantium L. 10 g, Curcuma aromatica Salisb. 10 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 10 g, Polygala tenuifolia Willd. 10 g, Citrus × aurantium L. 10 g, Actaea cimicifuga L. 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Luo (2018) | Qingre Qushi prescription | Panax Quinquefolius L. 10 g, Morus alba L. 10 g, Prunus armeniaca L. 10 g, Pogostemon cablin (Blanco) Benth. 10 g, Atractylodes lancea (Thunb.) DC. 10 g, Magnolia officinalis var. biloba Rehder and E.H.Wilson 10 g, Citrus × aurantium L. 10 g, Artemisia Capillaris Thunb. 10 g, Coix lacryma-jobi L. 50 g, Coptis chinensis Franch 5 g, Zingiber officinale Roscoe 5 g, Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. 10 g, Tetrapanax papyrifer (Hook.) K.Koch 10 g, Glycyrrhiza glabra L. 5 g | Decoction |
| Ding (2019) | Guipi decoction | Astragalus mongholicus Bunge 30 g, Ziziphi Spinosae Semen 25 g, Poria cocos (Schw.) Wolf 15 g, Dimocarpus Longan Lour. 15 g, Polygala tenuifolia Willd. 15 g, Angelica sinensis (Oliv.) Diels 15 g, Atractylodes macrocephala Koidz. 15 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 7 g, Glycyrrhiza glabra L. 10 g | Decoction |
| He (2019) | Shugan Jianpi Huoxue prescription | Bupleurum falcatum L. 15 g, Cyperus rotundus L. 15 g, Codonopsis pilosula (Franch.) Nannf. 12 g, Atractylodes macrocephala Koidz. 12 g, Poria cocos (Schw.) Wolf 9 g, Eleutherococcus Senticosus (Rupr. and Maxim.) Maxim. 12 g, Agrimonia Pilosa Ledeb 20 g, Angelica sinensis (Oliv.) Diels 15 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Salvia miltiorrhiza Bunge 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Hu (2019) | Buzhong Yiqi and Xiaochaihu decoction | Codonopsis pilosula (Franch.) Nannf. 24 g, Astragalus mongholicus Bunge 29 g, Bupleurum falcatum L. 14 g, Agrimonia Pilosa Ledeb 24 g, Atractylodes macrocephala Koidz. 21 g, Pinellia ternata (Thunb.) Makino 14 g, Poria cocos (Schw.) Wolf 21 g, Curcuma aromatica Salisb. 21 g, Platycodon grandiflorus (Jacq.) A.DC. 7 g, Citrus × aurantium L. 14 g, Scutellaria baicalensis Georgi 14 g | Decoction |
| Liu et al. (2019a) | Jiawei Lingzhi pill | Polygonum multiflorum Thunb. 30 g, Colla Carapacis et Plastri Testudinis 12 g, Ganoderma lucidum (Leyss. ex Fr.) Karst. 10 g, Astragalus mongholicus Bunge 30 g, Panax notoginseng (Burkill) F.H.Chen 5 g, Acorus Gramineus Aiton 5 g, Polygala tenuifolia Willd. 10 g | Pill |
| Liu et al. (2019b) | Chaihu Guizhi decoction grain | Bupleurum falcatum L., Neolitsea cassia (L.) Kosterm., Codonopsis pilosula (Franch.) Nannf., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.) Makino, Paeonia lactiflora Pall., Glycyrrhiza glabra L., Zingiber officinale Roscoe, Ziziphus Jujuba Mill | Granule |
| Liu et al. (2019c) | Jianpi Yishen decoction | Cuscuta chinensis Lam. 10 g, Lycium chinense Mill. 15 g, Pseudostellaria Heterophylla (Miq.) Pax 15 g, Atractylodes macrocephala Koidz. 10 g, Poria cocos (Schw.) Wolf 10 g, Dioscorea oppositifolia L. 20 g, Citrus × aurantium L. 10 g, Cistanche Deserticola Ma 10 g, Psoralea corylifolia L. 10 g, Poria cocos (Schw.) Wolf 12 g, Polygala tenuifolia Willd. 12 g, Glycyrrhiza glabra L.6 g | Decoction |
| Ma et al. (2019) | Self-designed Jiawei Erxian decoction | Epimedium brevicornu Maxim. 15 g, Curculigo Orchioides Gaertn. 10 g, Gynochthodes Officinalis (F.C.How) Razafim. and B.Bremer 10 g, Astragalus mongholicus Bunge 30 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Angelica sinensis (Oliv.) Diels 10 g, Actaea cimicifuga L. Actaea heracleifolia (Kom.) J.Compton 6 g, Bupleurum falcatum L. 6 g, Phellodendron amurense Rupr 5 g, Anemarrhena asphodeloides Bunge 10 g | Decoction |
| Shi (2019) | Xiaoyao pulvis Jiawei | Angelica sinensis (Oliv.) Diels 10 g, Paeonia lactiflora Pall. 10 g, Bupleurum falcatum L. 6 g, Poria cocos (Schw.) Wolf 20 g, Atractylodes macrocephala Koidz. 10 g, Glycyrrhiza glabra L. 6 g, Mentha Canadensis L. 6 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Dioscorea oppositifolia L. 20 g | Decoction |
| Wang (2019) | Sanren decoction and Sijunzi decoction | Prunus armeniaca L. 10 g, Wurfbainia Vera (Blackw.) Skornick. and A.D.Poulsen 10 g, Coix lacryma-jobi L. 50 g, Pinellia ternata (Thunb.) Makino 10 g, Magnolia officinalis var. biloba Rehder and E.H.Wilson 10 g, Panax ginseng C.A.Mey. 10 g, Atractylodes macrocephala Koidz. 10 g, Poria cocos (Schw.) Wolf 20 g, Tetrapanax papyrifer (Hook.) K.Koch 10 g, Glycyrrhiza glabra L. 5 g | Decoction |
| Yang (2019) | Zuogui pill | Rehmannia glutinosa (Gaertn.) DC. 250 g, Dioscorea oppositifolia L. 120 g, Cornus Officinalis Siebold & Zucc. 90 g, Lycium chinense Mill. 20 g, Cervus nippon Temminck 120 g, Cuscuta chinensis Lam. 120 g, Eucommia ulmoides Oliv. 120 g, Angelica sinensis (Oliv.) Diels 90 g, Neolitsea cassia (L.) Kosterm. 60 g, Cyperus Rotundus L. 60 g | Pill |
| Dong (2020) | Qingshu Yiqi decoction grain | Glycyrrhiza glabra L. 6 g, Actaea cimicifuga L. 9 g, Schisandra chinensis (Turcz.) Baill. 9 g, Amomum villosum Lour. 10 g, Citrus × aurantium L. 10 g, Phellodendron amurense Rupr 12 g, Citrus × aurantium L. 12 g, Atractylodes lancea (Thunb.) DC. 15 g, Poria cocos (Schw.) Wolf 15 g, Alisma plantago-aquatica subsp. 15 g, Angelica sinensis (Oliv.) Diels 15 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala Koidz. 15 g, Mosla Chinensis Maxim. 15 g, Hyssopus officinalis L. 20 g, Astragalus mongholicus Bunge 30 g | Decoction |
| Li (2020) | Buzhong Yiqi decoction and Xiaochaihu decoction | Bupleurum falcatum L. 15 g, Atractylodes macrocephala Koidz. 15 g, Poria cocos (Schw.) Wolf 15 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Curcuma aromatica Salisb. 10 g, Glycyrrhiza glabra L. 12 g, Ziziphus Jujuba Mill. 5 pieces | Decoction |
| Mao (2020) | Yishen Tiaodu method | Dioscorea oppositifolia L. 10 g, Zea mays L. 50 g, Euryale Ferox Salisb. 10 g, Allium tuberosum Rottler ex Spreng. 10 g | Decoction |
| Wang (2020) | Chaihu Guizhi decoction | Bupleurum falcatum L. 12 g, Neolitsea cassia (L.) Kosterm. 9 g, Scutellaria baicalensis Georgi 9 g, Paeonia lactiflora Pall. 9 g, Codonopsis pilosula (Franch.) Nannf. 9 g, Pinellia ternata (Thunb.) Makino 9 g, Glycyrrhiza glabra L. 9 g, Ziziphus Jujuba Mill. 6 pieces, Zingiber officinale Roscoe 6 g | Decoction |
| Chen (2021) | Xiaoyao powder | Poria cocos (Schw.) Wolf 12 g, Paeonia lactiflora Pall. 12 g, Atractylodes macrocephala Koidz. 12 g, Angelica sinensis (Oliv.) Diels 10 g, Glycyrrhiza glabra L. 6 g, Bupleurum falcatum L. 6 g, Mentha Canadensis L. 5 g, Zingiber officinale Roscoe 3 g | Decoction |
| Li et al. (2021) | Jiannao Yizhi ointment | Astragalus mongholicus Bunge 30 g, Ziziphi Spinosae Semen 25 g, Poria cocos (Schw.) Wolf 15 g, Dimocarpus Longan Lour. 15 g, Polygala tenuifolia Willd. 15 g, Angelica sinensis (Oliv.) Diels 15 g, Atractylodes macrocephala Koidz. 15 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Dolomiaea Costus (Falc.) Kasana and A.K.Pandey 7 g, Glycyrrhiza glabra L. 10 g | Ointment |
| Liu et al. (2021) | Chaihu Guizhi decoction grain | Bupleurum falcatum L. 15 g, Cyperus rotundus L. 15 g, Codonopsis pilosula (Franch.) Nannf. 12 g, Atractylodes macrocephala Koidz. 12 g, Poria cocos (Schw.) Wolf 9 g, Eleutherococcus Senticosus (Rupr. and Maxim.) Maxim. 12 g, Agrimonia Pilosa Ledeb 20 g, Angelica sinensis (Oliv.) Diels 15 g, Conioselinum anthriscoides “Chuanxiong” 10 g, Salvia miltiorrhiza Bunge 10 g, Glycyrrhiza glabra L. 6 g | Decoction |
| Wang (2021) | Shenling Baizhu powder | Codonopsis pilosula (Franch.) Nannf. 24 g, Astragalus mongholicus Bunge 29 g, Bupleurum falcatum L. 14 g, Agrimonia Pilosa Ledeb 24 g, Atractylodes macrocephala Koidz. 21 g, Pinellia ternata (Thunb.) Makino 14 g, Poria cocos (Schw.) Wolf 21 g, Curcuma aromatica Salisb. 21 g, Platycodon grandiflorus (Jacq.) A.DC. 7 g, Citrus × aurantium L. 14 g, Scutellaria baicalensis Georgi 14 g | Decoction |
| Zhang (2021) | Jiawei Guizhi Xinjia decoction | Polygonum multiflorum Thunb. 30 g, Colla Carapacis et Plastri Testudinis 12 g, Ganoderma lucidum (Leyss. ex Fr.) Karst. 10 g, Astragalus mongholicus Bunge 30 g, Panax notoginseng (Burkill) F.H.Chen 5 g, Acorus Gramineus Aiton 5 g, Polygala tenuifolia Willd. 10 g | Decoction |
| Sheng et al. (2022) | Wenshen Lipi prescription | Bupleurum falcatum L., Neolitsea cassia (L.) Kosterm., Codonopsis pilosula (Franch.) Nannf., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.) Makino, Paeonia lactiflora Pall., Glycyrrhiza glabra L., Zingiber officinale Roscoe, Ziziphus Jujuba Mill. | Granule |
The quality assessment of the included studies is listed in Table 3. The Cochrane score ranged from 3 to 7, and three studies (Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) got 7 points; two studies (Li, 2009; Ye, 2017) got 6 points; two studies (Luo, 2018; Wang, 2019) got 5 points; 38 studies (Fang et al., 2007; Wang et al., 2007; Zhang et al., 2009; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Jiang, 2012; Ren and Yu, 2012; Wang, 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu and Wang, 2013; Zhao et al., 2013; Xu, 2014; Gao and Pang, 2015; Liu et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Zheng et al., 2017; Du, 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Liu Y. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Li et al., 2021; Wang, 2021; Zhang, 2021) got 4 points; and 39 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Ma, 2009; Hu et al., 2010; Chen et al., 2011; Wang et al., 2011; Kong, 2012; Tian and Wang, 2012; Wu et al., 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Xu et al., 2013; Zhao, 2013; Teng et al., 2014; Li and Zao, 2015; Li, 2015; Niu et al., 2015; Tan et al., 2015; Shi and Wu, 2016; Li et al., 2018; Wu et al., 2018; Liu F. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2020; Chen, 2021) got 3 points. All of the included studies reported random allocation, and 44 studies (Fang et al., 2007; Wang et al., 2007; Li, 2009; Zhang et al., 2009; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Jiang, 2012; Ren and Yu, 2012; Wang, 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu and Wang, 2013; Zhao et al., 2013; Xu, 2014; Gao and Pang, 2015; Liu et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Ou et al., 2018; Weng, 2018; Liu J. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Li et al., 2021; Liu et al., 2021; Wang, 2021; Zhang, 2021; Sheng et al., 2022) described the method of random sequence generation, whereas the remaining 40 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Ma, 2009; Hu et al., 2010; Chen et al., 2011; Wang et al., 2011; Kong, 2012; Tian and Wang, 2012; Wu et al., 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Xu et al., 2013; Zhao, 2013; Teng et al., 2014; Li and Zao, 2015; Li, 2015; Niu et al., 2015; Tan et al., 2015; Shi and Wu, 2016; Li et al., 2018; Wu et al., 2018; Liu F. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2020; Chen, 2021) provided no details. Five studies (Li, 2009; Ye, 2017; Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) mentioned concealment allocation. Three trials (Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) reported double blinding of patients and physicians, and eight trials (Li, 2009; Ye, 2017; Luo, 2018; Liu J. et al., 2019; Liu Y. et al., 2019; Wang, 2019; Liu et al., 2021; Sheng et al., 2022) described blinding of participants. All studies met the criterion of incomplete outcome data as drop-out data, or no drop-out patients were reported specifically. Pre-designed outcomes were reported in all studies, detecting a low risk of reporting bias, and other biases were not found in all included studies.
TABLE 3.
Risk of bias assessment of all included studies.
| Study |
Seven-item criteria |
|||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | Total | |
| Ning and Li. (2002) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Yang et al. (2004) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2004) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhang and Zhou. (2004) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Wei (2005) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Yao and Qiu. (2005) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Liang (2006) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhao et al. (2006) | − | ? | ? | ? | ? | ? | ? | 3? |
| Fang et al. (2007) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Gong. (2007) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Guo et al. (2007) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Lin (2007) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Sun et al. (2007) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Wang et al. (2007) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Fang et al. (2008) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Cheng (2009) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Jie and Wang (2009) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Li (2009) | ? | ? | ? | ? | ? | ? | ? | 6? |
| Ma (2009) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2009) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Hu et al. (2010) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Chen et al. (2011) | − | ? | ? | ? | ? | ? | ? | 3? |
| Li et al. (2011) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Liu et al. (2011) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wang et al. (2011) | − | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2011) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Jiang (2012) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Kong (2012) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Ren and Yu (2012) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Tian and Wang (2012) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Wang (2012) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wu et al. (2012) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2012a) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2012b) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhao (2012) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Lai and Lei (2013) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Pang and Liu (2013) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Xu et al. (2013) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Xu and Wang (2013) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Zhao (2013) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Zhao et al. (2013) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Teng et al. (2014) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Xu (2014) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Gao and Pang (2015) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Li and Zao (2015) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Liu et al. (2015) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Li (2015) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Niu et al. (2015) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Tan et al. (2015) | − | ? | ? | ? | ? | ? | ? | 3? |
| Zhang et al. (2015) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Gao and Pang (2016) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Shi and Wu (2016) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Sun et al. (2016) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wu et al. (2016) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wang (2017) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Ye (2017) | ? | ? | ? | ? | ? | ? | ? | 6? |
| Zheng et al. (2017) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Du (2018) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Li et al. (2018) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Liu and Cai (2018) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Ou et al. (2018) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Weng (2018) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wu et al. (2018) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Luo (2018) | ? | ? | ? | ? | ? | ? | ? | 5? |
| Ding (2019) | ? | ? | ? | ? | ? | ? | ? | 4? |
| He (2019) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Hu (2019) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Liu et al. (2019a) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Liu et al. (2019b) | ? | ? | ? | ? | ? | ? | ? | 7? |
| Liu et al. (2019c) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Ma et al. (2019) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Shi (2019) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Wang (2019) | ? | ? | ? | ? | ? | ? | ? | 5? |
| Yang (2019) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Dong (2020) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Li (2020) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Mao (2020) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Wang (2020) | ? | ? | ? | ? | ? | ? | ? | 3? |
| Chen (2021) | − | ? | ? | ? | ? | ? | ? | 3? |
| Li et al. (2021) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Liu et al. (2021) | ? | ? | ? | ? | ? | ? | ? | 7? |
| Wang (2021) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Zhang (2021) | ? | ? | ? | ? | ? | ? | ? | 4? |
| Sheng et al. (2022) | ? | ? | ? | ? | ? | ? | ? | 7? |
Results of meta-analysis
Primary outcomes
FS-14 scores
Pooled data from the 26 studies (Jie and Wang, 2009; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang L. P. et al., 2012; Xu et al., 2013; Xu and Wang, 2013; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Ye, 2017; Zheng et al., 2017; Du, 2018; Luo, 2018; Liu F. et al., 2019; Liu J. et al., 2019; He, 2019; Shi, 2019; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reporting the FS-14 scores showed that CHM clearly decreased the FS-14 scores as an adjuvant or monotherapy for CFS compared with the contrast group (WMD: –1.77; 95%CI: –1.96 to –1.57; p < 0.001; p for heterogeneity <0.001; I2 = 84.4%; Figure 2). The subgroup analysis showed similar results (Table 4).
FIGURE 2.
Forest plot for FS-14 scores.
TABLE 4.
Subgroup analysis for outcomes.
| Subgroup | No. of studies | Effect size (95% CI) | p-value | Heterogeneity | p-value | |
|---|---|---|---|---|---|---|
| Subgroup analyses for FS-14 scores | ||||||
| CHM vs. WCM | 17 | WMD −1.84 (−2.23, −1.45) | <0.001 | 85.9 | <0.001 | |
| Duration of intervention ≤ 30 days | 5 | WMD −1.90 (−2.75, −1.05) | <0.001 | 85.0 | <0.001 | |
| 30 days < duration of intervention ≤ 60 days | 10 | WMD −1.97 (−2.36, −1.58) | <0.001 | 66.7 | <0.01 | |
| Duration of intervention > 60 days | 1 | WMD −1.88 (−3.13, −0.63) | <0.01 | — | — | |
| CHM plus WCM vs. WCM | 4 | WMD −1.67 (−2.34, −1.00) | <0.001 | 81.2 | <0.01 | |
| CHM vs. GET | 1 | WMD −1.23 (−1.96, −0.50) | <0.01 | — | — | |
| CHM vs. placebo | 3 | WMD −1.84 (−1.98, −1.70) | <0.001 | 54.8 | = 0.109 | |
| CHM vs. health guidance | 1 | WMD −3.14 (−3.83, −2.45) | <0.001 | — | — | |
| Subgroup analyses for FAI scores | ||||||
| CHM vs. WCM | 7 | WMD −15.49 (−28.39, −2.60) | = 0.019 | 99.6 | <0.001 | |
| Duration of intervention ≤ 30 days | 2 | WMD -32.15 (−33.76, −30.53) | <0.001 | 0 | = 0.829 | |
| 30 days < duration of intervention ≤ 60 days | 2 | WMD −18.24 (−28.11, −8.38) | <0.001 | 40.9 | = 0.193 | |
| Duration of intervention > 60 days | 3 | WMD −2.88 (−6.15, 0.38) | = 0.084 | 87.7 | <0.001 | |
| CHM plus WCM vs. WCM | 1 | WMD −12.04 (−16.96, −7.12) | <0.001 | — | — | |
| CHM vs. GET | 1 | WMD −21.80 (−33.42, −10.18) | <0.001 | — | — | |
| Subgroup analyses for SCL-90 scores | ||||||
| CHM vs. WCM | 4 | WMD −9.52 (−12.08, −6.97) | <0.001 | 0 | = 0.533 | |
| CHM vs. placebo | 1 | WMD −27.71 (−52.10, −3.32) | = 0.026 | — | — | |
| Subgroup analyses for SAS scores | ||||||
| CHM vs. WCM | 3 | WMD −9.14 (−17.31, −0.97) | = 0.028 | 95.4 | <0.001 | |
| CHM plus WCM vs. WCM | 2 | WMD −4.55 (−7.51, −1.58) | <0.01 | 68.3 | = 0.076 | |
| Duration of intervention ≤ 30 days | 1 | WMD −5.94 (−7.98, −3.90) | <0.001 | — | — | |
| 30 days < duration of intervention ≤ 60 days | 1 | WMD −2.90 (−5.56, −0.24) | = 0.033 | — | — | |
| CHM vs. placebo | 1 | WMD −6.77 (−7.08, −6.46) | <0.001 | — | — | |
| CHM vs. health guidance | 1 | WMD −6.55 (−8.84, −4.26) | <0.001 | — | — | |
| Subgroup analyses for SDS scores | ||||||
| CHM vs. WCM | 2 | WMD −5.97 (−10.67, −1.28) | = 0.013 | 86.6 | <0.01 | |
| CHM plus WCM vs. WCM | 2 | WMD −4.17 (−7.07, −1.27) | <0.01 | 64 | = 0.095 | |
| Duration of intervention ≤ 30 days | 1 | WMD −5.69 (−8.25, −3.13) | <0.001 | — | — | |
| 30 days < duration of intervention ≤ 60 days | 1 | WMD −2.73 (−5.09, −0.37) | = 0.023 | — | — | |
| CHM vs. placebo | 1 | WMD −6.42 (−6.65, −6.19) | <0.001 | — | — | |
| CHM vs. health guidance | 1 | WMD −4.23 (−5.72, −2.74) | <0.001 | — | — | |
| Clinical symptom scores | ||||||
| CHM vs. WCM | 18 | WMD −6.60 (−7.89, −5.31) | <0.001 | 96.9 | <0.001 | |
| Duration of intervention ≤ 30 days | 11 | WMD −8.40 (−11.00, −5.80) | <0.001 | 97.1 | <0.001 | |
| 30 days < duration of intervention ≤ 60 days | 3 | WMD −3.37 (−4.77, −1.96) | <0.001 | 80.5 | <0.01 | |
| Duration of intervention > 60 days | 1 | WMD −5.22 (−5.69, −4.75) | <0.001 | — | — | |
| CHM plus WCM vs. WCM | 2 | WMD −2.82 (−3.45, −2.20) | <0.001 | 47.3 | = 0.168 | |
| CHM vs. placebo | 3 | WMD −3.13 (−3.99, −2.27) | <0.001 | 95.2 | <0.001 | |
| CHM vs. health guidance | 1 | WMD −6.73 (−7.57, −5.89) | <0.001 | — | — | |
| Subgroup analyses for IGA | ||||||
| CHM vs. WCM | 7 | WMD 0.31 (0.18, 0.43) | <0.001 | 75.9 | <0.001 | |
| Duration of intervention ≤ 30 days | 1 | WMD 0.24 (0.06, 0.42) | <0.01 | — | — | |
| 30 days < duration of intervention ≤ 60 days | 3 | WMD 0.38 (0.23, 0.54) | <0.001 | 73.2 | = 0.024 | |
| Duration of intervention > 60 days | 3 | WMD 0.21 (−0.12, 0.53) | = 0.218 | 86.1 | <0.01 | |
| CHM vs. placebo | 1 | WMD 0.26 (0.20, 0.32) | <0.001 | — | — | |
| Subgroup analyses for IGG | ||||||
| CHM vs. WCM | 7 | WMD 2.21 (0.90, 3.51) | <0.01 | 94.0 | <0.001 | |
| Duration of intervention ≤ 30 days | 1 | WMD 20.61 (15.97, 25.25) | <0.001 | — | — | |
| 30 days < duration of intervention ≤ 60 days | 3 | WMD 1.30 (0.23, 2.38) | = 0.018 | 80.5 | <0.01 | |
| Duration of intervention > 60 days | 3 | WMD 0.95 (−0.36, 2.27) | = 0.154 | 89.1 | <0.001 | |
| CHM vs. placebo | 1 | WMD 1.39 (1.22, 1.56) | <0.001 | — | — | |
| Subgroup analyses for IGM | ||||||
| CHM vs. WCM | 7 | WMD 0.20 (0.10, 0.29) | <0.001 | 69.3 | <0.01 | |
| Duration of intervention ≤ 30 days | 1 | WMD 0.02 (−0.10, 0.15) | = 0.712 | — | — | |
| 30 days < duration of intervention ≤ 60 days | 3 | WMD 0.24 (0.16, 0.33) | <0.001 | 25.4 | = 0.262 | |
| Duration of intervention > 60 days | 3 | WMD 0.21 (0.03, 0.38) | = 0.019 | 68.1 | = 0.044 | |
| CHM vs. placebo | 1 | WMD 0.28 (0.24, 0.32) | <0.001 | — | — | |
| Subgroup analyses for NK cell | ||||||
| CHM vs. WCM | 2 | WMD 0.94 (−1.14, 3.03) | = 0.376 | 0 | = 0.613 | |
| CHM vs. Placebo | 1 | WMD 0.94 (−1.14, 3.03) | <0.001 | — | — | |
| Effective Rate | ||||||
| CHM vs. WCM | 59 | RR 1.43 (1.33, 1.52) | <0.001 | 76.6 | <0.001 | |
| Duration of intervention ≤ 30 days | 25 | RR 1.64 (1.43, 1.89) | <0.001 | 82.6 | <0.001 | |
| 30 days < duration of intervention ≤60 days | 19 | RR 1.38 (1.23, 1.54) | <0.001 | 77 | <0.001 | |
| Duration of intervention > 60 days | 11 | RR 1.28 (1.20, 1.37) | <0.001 | 0 | = 0.590 | |
| CHM plus WCM vs. WCM | 12 | RR 1.20 (1.13, 1.27) | <0.001 | 0 | = 0.762 | |
| Duration of intervention ≤ 30 days | 7 | RR 1.22 (1.14, 1.32) | <0.001 | 0 | = 0.918 | |
| 30 days < duration of intervention ≤ 60 days | 3 | RR 1.13 (0.99, 1.28) | = 0.062 | 31.1 | = 0.234 | |
| CHM vs. GET | 1 | RR 1.16 (0.98, 1.38) | = 0.093 | — | — | |
| CHM plus GET vs. GET | 2 | RR 1.39 (1.06, 1.83) | = 0.019 | 56.1 | = 0.131 | |
| CHM vs. placebo | 4 | RR 2.54 (2.00, 3.22) | <0.001 | 0 | = 0.943 | |
| CHM vs. health guidance | 1 | RR 6.59 (2.54, 17.12) | <0.001 | — | — | |
FS-14: Fatigue Scale; FAI: Fatigue Assessment Instrument; SCL-90: Self-Rating Scale of mental state; SAS: Self-Rating Anxiety Scale; SDS: Self-Rating Depression Scale; CHM: Chinese herbal medicine; WCM: western conventional medicine; GET: graded exercise therapy; WMD: weighted mean difference; RR: relative risk.
FAI scores
Meta-analysis of the nine studies (Zhao et al., 2006; Zhang et al., 2009; Liu et al., 2011; Wu et al., 2012; Liu et al., 2015; Luo, 2018; Wang, 2019; Li et al., 2021; Wang, 2021) reporting the FAI scores showed that the treatment group had significantly decreased FAI scores compared to the control group (WMD: –15.75; 95%CI: –26.89 to –4.61; p < 0.01; p for heterogeneity <0.001; I2 = 99.5%; Figure 3). Subgroup analysis revealed no significant difference (WMD: –2.88; 95%CI: –6.15 to 0.38; p = 0.084; p for heterogeneity <0.001; I2 = 87.7%) between CHM and WCM groups when the duration of intervention >60 days, whereas the relationship between the CHM treatment group and lower FAI scores remained constant in the other subgroups (Table 4).
FIGURE 3.
Forest plot for FAI scores.
Secondary outcomes
SCL-90 scores
The SCL-90 scores were reported in five studies (Zhang et al., 2009; Zhang Z. X. et al., 2012; Wu et al., 2012; Niu et al., 2015; Sheng et al., 2022). The pooled results suggested that SCL-90 scores were significantly lower in the CHM group compared to the contrast group (WMD: –9.72; 95%CI: –12.26 to –7.18; p < 0.001; p for heterogeneity = 0.366; I2 = 7.2%; Figure 4), and the subgroup analysis showed similar results (Table 4).
FIGURE 4.
Forest plot for SCL-90 scores.
SAS scores
Seven studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Yang, 2019; Liu et al., 2021; Zhang, 2021) reported the SAS scores, and meta-analysis indicated that CHM therapy clearly decreased SAS scores compared to the contrast group (WMD: –7.07; 95%CI: –9.96 to –4.19; p < 0.001; p for heterogeneity <0.001; I2 = 94.6%; Figure 5), and subgroup analysis showed that the results remained constant (Table 4).
FIGURE 5.
Forest plot for SAS scores.
SDS scores
Meta-analysis of six RCTs reporting the SDS scores (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Liu et al., 2021; Zhang, 2021) showed that the experimental group had significantly reduced SDS scores compared to the contrast group (WMD: –5.45; 95%CI: –6.82 to –4.08; p < 0.001; p for heterogeneity <0.001; I2 = 84.1%; Figure 6). Subgroup analysis showed that the results remained constant (Table 4).
FIGURE 6.
Forest plot for SDS scores.
Clinical symptom scores
The summary data of 24 studies (Zhang et al., 2004; Yao and Qiu, 2005; Fang et al., 2007; Wang et al., 2007; Fang et al., 2008; Li, 2009; Hu et al., 2010; Wang, 2012; Zhao, 2012; Li, 2015; Li and Zao, 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Liu J. et al., 2019; He, 2019; Hu, 2019; Shi, 2019; Dong, 2020; Wang, 2020; Liu et al., 2021) demonstrated that CHM, as an adjuvant or monotherapy, significantly decreased the clinical symptom scores compared with the control group (WMD: –5.37; 95%CI: –6.13 to –4.60; p < 0.001; p for heterogeneity <0.001; I2 = 96.6%; Figure 7). Subgroup analysis was performed, showing that the conclusion that CHM is relatively effective in treating CFS remained unchanged in each subgroup (Table 4).
FIGURE 7.
Forest plot for clinical symptom scores.
Immunological indicators
We identified eight RCTs that reported the IGA, IGG, and IGM levels (Zhang et al., 2009; Liu et al., 2011; Jiang, 2012; Wu et al., 2012; Du, 2018; Wu et al., 2018; Liu J. et al., 2019; He, 2019). Meta-analysis indicated that CHM significantly elevated IGA (WMD: 0.30; 95%CI: 0.20–0.41; p < 0.001; p for heterogeneity <0.001; I2 = 77.7%; Figure 8A); IGG (WMD: 1.74; 95%CI: 0.87–2.62; p < 0.001; p for heterogeneity <0.001; I2 = 93.4%; Figure 8B); and IGM (WMD: 0.21; 95%CI: 0.14–0.29; p < 0.001; p for heterogeneity <0.01; I2 = 71.4%; Figure 8C) compared to the contrast group. Three studies (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) reported the NK cell levels, and the meta-analysis indicated no significant difference between the experimental and control groups (WMD: 2.30; 95%CI: –0.47 to 5.07; p = 0.104; p for heterogeneity = 0.061; I2 = 64.3%; Figure 8D). Subgroup analyses of the IGA and IGG revealed no significant difference (WMD: 0.21; 95%CI: –0.12 to 0.53; p = 0.218; p for heterogeneity <0.01; I2 = 86.1%, WMD: 0.95; 95%CI: –0.36 to 2.27; p = 0.154; p for heterogeneity <0.001; I2 = 89.1%, respectively) between the CHM and WCM groups when the duration of intervention >60 days, and the subgroup analysis of the IGM showed no statistical significance (WMD: 0.02; 95%CI: –0.10 to 0.15; p = 0.712; no heterogeneity) between the CHM and WCM groups when the duration of intervention ≤30 days. The rest of the results indicated that the conclusion that CHM can elevate IGA, IGG, and IGM remained constant (Table 4). The subgroup analysis of the NK cell showed no statistical significance (WMD: 0.94; 95%CI: –1.14 to 3.03; p = 0.376; p for heterogeneity = 0.613; I2 = 0.0%) between the CHM and WCM groups, whereas one study comparing CHM with placebo showed that CHM significantly elevated the NK cell levels (WMD: 4.92; 95%CI: 2.27–7.57; p < 0.001; no heterogeneity) (Table 4).
FIGURE 8.
Forest plot for immunological indicators: (A) IGA, (B) IGG, (C) IGM, and (D) NK cell.
Effective rate
The effective rate was evaluated in 79 trials (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Li, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Wang et al., 2011; Zhang et al., 2011; Jiang, 2012; Kong, 2012; Ren and Yu, 2012; Tian and Wang, 2012; Wang, 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Xu and Wang, 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Gao and Pang, 2015; Li and Zao, 2015; Li, 2015; Tan et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Shi and Wu, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Li et al., 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Luo, 2018; Ding, 2019; He, 2019; Hu, 2019; Liu F. et al., 2019; Liu J. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021), and the pooled results showed that it was higher in the treatment group compared to the control group (RR = 1.41; 95%CI: 1.33–1.49; p < 0.001; p for heterogeneity <0.001; I2 = 76.3%; Figure 9). Subgroup analysis showed no significant difference (RR: 1.13; 95%CI: 0.99 to 1.28; p = 0.062; p for heterogeneity = 0.234; I2 = 31.1%) in CHM plus WCM compared with WCM when 30 days < intervention time ≤ 60 days, and only one study compared CHM and GET, showing similar results (RR: 1.16; 95%CI: 0.98–1.38; p = 0.093; no heterogeneity), whereas the rest of the results revealed that effectiveness of CHM for CFS remained constant (Table 4).
FIGURE 9.
Forest plot for effective rate.
Adverse events
Adverse events were reported in 14 studies (Liang, 2006; Gong, 2007; Lin, 2007; Wang et al., 2007; Jie and Wang, 2009; Li, 2009; Li et al., 2011; Xu and Wang, 2013; Zhang et al., 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Li et al., 2018; Liu and Cai, 2018), and two studies (Sun et al., 2007; Wang, 2017) reported that no adverse events occurred. The rest of the studies did not report the presence or absence of adverse events. The adverse events in the CHM group included mild nausea, dry mouth, indigestion, constipation, and fever. The majority of adverse events were mild, and serious adverse events or deaths were not found in the included studies, which suggests that CHM is relatively safe in patients with CFS.
Sensitivity analysis
We conducted a sensitivity analysis on FS-14, FAI, SAS, SDS, clinical symptom scores, IGA, IGG, IGM, NK cell levels, and effective rate. After we excluded each study one by one, the pooled WMD or RR for the rest of the RCTs did not change significantly, indicating that the result data were robust (Additional file 2).
Publication bias
The funnel plot showed a symmetric distribution of trials on either side of the funnel, and Egger’s test (p = 0.795) was consistent with the funnel plot, indicating that no significant publication bias existed in this meta-analysis (Additional file 3).
Description of the CHM
Our study evaluated 69 kinds of Chinese herbal formulas, including 54 decoctions, five granules, three oral liquids, three pills, two ointments, one capsule, and one herbal porridge. The most frequently used herbs in all formulations contained Chai Hu (Bupleurum falcatum L.); Gan Cao (Glycyrrhiza glabra L.); Bai Zhu (Atractylodes macrocephala Koidz.); Dang Gui [Angelica sinensis (Oliv.) Diels]; Huang Qi (Astragalus mongholicus Bunge); Dang Shen [Codonopsis pilosula (Franch.) Nannf.]; Bai Shao (Paeonia lactiflora Pall.); Fu Ling [Poria cocos (Schw.) Wolf]; Chen Pi (Citrus × Aurantium L.); Shu Di (Rehmannia glutinosa (Gaertn.) DC.); Chuan Xiong (Conioselinum anthriscoides “Chuanxiong”); Yu Jin (Curcuma aromatica Salisb.); Shan Yao (Dioscorea oppositifolia L.); Yuan Zhi (Polygala tenuifolia Willd.); Ban Xia [Pinellia ternata (Thunb.) Makino]; Gou QI (Lycium chinense Mill.); Da Zao (Ziziphus jujuba Mill.); Zhi Qiao (Citrus × Aurantium L.); Suanzaoren (Ziziphi Spinosae Semen); Huang Qin (Scutellaria baicalensis Georgi); Ren Shen (Panax ginseng C.A.Mey.); and Wu Wei Zi [Schisandra chinensis (Turcz.) Baill.] (Table 5).
TABLE 5.
Frequently used herbs in included studies.
| Chinese name | Accepted scientific name | English name | Family | Number of studies (%) |
|---|---|---|---|---|
| Gan Cao | Glycyrrhiza glabra L. | Liquorice root | Leguminosae | 53 (63%) |
| Bai Zhu | Atractylodes macrocephala Koidz | largehead atractylodes rhizome | Asteraceae | 50 (60%) |
| Huang Qi | Astragalus mongholicus Bunge | Milkvetch root | Leguminosae | 50 (60%) |
| Chai Hu | Bupleurum falcatum L. | Chinese thorowax root | Umbelliferae | 48 (57%) |
| Fu Ling | Poria cocos (Schw.) Wolf | Indian bread | Polyporaceae | 48 (57%) |
| Dang Gui | Angelica sinensis (Oliv.) Diels | Chinese angelica | Umbelliferae | 45 (54%) |
| Dang Shen | Codonopsis pilosula (Franch.) Nannf. | Tangshen | Campanulaceae | 37 (44%) |
| Bai Shao | Paeonia lactiflora Pall. | Debark peony root | Ranunculaceae | 30 (36%) |
| Chen Pi | Citrus × aurantium L. | Dried tangerine peel | Rutaceae | 25 (30%) |
| Ren Shen | Panax ginseng C.A.Mey | Ginseng | Araliaceae | 23 (27%) |
| Shu Di | Rehmannia glutinosa (Gaertn.) DC. | Prepared rehmannia root | Scrophulariaceae | 23 (27%) |
| Chuan Xiong | Conioselinum anthriscoides “Chuanxiong” | Chuanxiong | Umbelliferae | 20 (24%) |
| Yu Jin | Curcuma aromatica Salisb. | Turmeric root tuber | Zingiberaceae | 19 (23%) |
| Ban Xia | Pinellia ternata (Thunb.) Makino | Pinellia tuber | Araceae | 16 (19%) |
| Yuan Zhi | Polygala tenuifolia Willd | Milkwort root | Polygalaceae | 15 (18%) |
| Gou QI | Lycium chinense Mill. | Barbary wolfberry fruit | Solanaceae | 14 (17%) |
| Shan Yao | Dioscorea oppositifolia L. | Common yam rhizome | Dioscoreaceae | 13 (15%) |
| SuanZao Ren | Ziziphi Spinosae Semen | Spine date seed | Rhamnaceae | 13 (15%) |
| Zhi Qiao | Citrus × aurantium L. | Bitter orange | Rutaceae | 13 (15%) |
| Huang Qin | Scutellaria baicalensis Georgi | Baical skullcap root | Lamiaceae | 13 (15%) |
| Da Zao | Ziziphus Jujuba Mill. | Chinese date | Rhamnaceae | 11 (13%) |
| Wu Wei Zi | Schisandra chinensis (Turcz.) Baill. | Chinese magnoliavine fruit | Magnoliaceae | 11 (13%) |
Discussion
Medically unexplained chronic fatigue, including idiopathic chronic fatigue and CFS, is an unexplained adverse condition characterized by fatigue accompanied by behavioral, emotional, social, and cognitive imbalances. Approximately 10% of the general population suffers from chronic fatigue, which significantly reduces their quality of life and their ability to work. This is an important health care issue, presenting major challenges for its sufferers and health services. At present, a clear therapeutic approach is still lacking, but the use of CHM in patients with chronic fatigue is receiving increasing attention from physicians.
Summary of the evidence
A total of 84 RCTs, including 6,944 individuals, were identified for analysis. The findings demonstrated that CHM as adjuvant therapy or monotherapy for CFS could decrease the FS-14, FAI, SCL-90, SAS, SDS, and clinical symptom scores and improve IGA, IGG, IGM, and the effective rate.
Two internationally recognized scales were used to quantitatively assess fatigue. The FS-14 developed by Trudie Chalde et al. in 1993 consists of 14 items, each of which is a fatigue-related question, and it mainly reflects the changes in fatigue symptoms from two different perspectives (physical fatigue and mental fatigue), thus reflecting the real level of fatigue of patients in a more comprehensive way. The FAI includes 27 fatigue-related questions. Subjects rate each item based on their own performance over the previous 2 weeks, which can accurately and quantitatively evaluate the degree and characteristics of fatigue. In this study, CHM treatment significantly reduced FS-14 and FAI scores, indicating that it improved fatigue symptoms.
Patients with CFS commonly suffer from negative emotions such as anxiety, depression, paranoia, and obsessive-compulsive disorder. The degree of negative emotions is mainly assessed by professional mental status assessment scales such as SCL-90, SAS, and SDS. The present meta-analysis shows that CHM treatment can relatively improve negative emotions in patients with chronic fatigue.
The clinical effective rate and clinical symptom scores were used to evaluate the efficacy of CHM in the treatment of CFS because the severity of clinical symptoms is used to determine whether the disease is in remission. The clinical efficiency rate in patients treated with CHM alone or with CHM plus other treatments (e.g., WCM, GET, or health guidance) was 90% (2,961/3,308). The clinical efficiency rate in patients treated only with WCM, GET, health guidance, or placebo was 62% (1,956/3,149). Thus, CHM treatment clearly increased the efficiency rate and reduced the clinical symptom scores compared to WCM, GET, health guidance, or placebo, thus showing that CHM is effective to some extent for CFS.
Numerous studies have revealed that CFS is associated with immune system dysfunction (Matsuda et al., 2009; Guenther et al., 2015; Hornig et al., 2015; Montoya et al., 2017; Sotzny et al., 2018). Most CFS patients are prone to physical weakness and fatigue due to low immune function. Moreover, when the body tissue is in a state of fatigue for a long time, it will consume and destroy the immune system, which will eventually lead to low immune function. Immunoglobulins (IGA, IGG, and IGM) are important parts of humoral immunity. A study found that IGA, IGG, and IGM levels are significantly lower in patients with CFS than in healthy subjects (Hou et al., 2015). Our meta-analysis showed that the treatment group had elevated IGA, IGG, and IGM levels compared to the control group. In addition, immunological indicators also include NK cells and T lymphocyte subsets (CD4+, CD8+, and CD4+/CD8+). Hou’s study showed that NK cell activity, CD4+, and CD8+ were all significantly reduced in CFS patients (Hou et al., 2015). The results of our meta-analysis did not find any obvious effects of CHM on NK cell activity in CFS patients. However, three trials (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) suggested that CFS patients’ NK cell activity was higher in the CHM treatment group. We cannot reject the positive effect of CHM on the NK cell activity of CFS patients based on the negative results of this meta-analysis, which may be due to the lack of appropriate courses of treatment and limited sample sizes. Furthermore, a study showed that CHM dramatically improved NK cell activities, T cell proliferation, CD4 +/CD8 + ratio, and CD4 + counts in CFS rats, suggesting that CHM can improve the immune function of patients with CFS (Chi et al., 2015). Taken together, CHM may prevent CFS by modulating immune function, but further research is needed to confirm this.
Furthermore, only 14 studies referred to minor adverse reactions, and there were no serious adverse events, showing that CHM generally appears safe and effective for treating CFS. Thus, the present evidence supports that CHM can potentially be recommended for use in CFS patients.
Strengths and limitations
Our study included a large number of RCTs and large sample sizes (84 RCTs with 6,944 patients) and used more internationally recognized outcome measures to assess the effectiveness of CHM for CFS from different aspects. These outcome indicators included not only subjective indicators (FS-14, FAI, SCL-90, SAS, SDS, clinical symptom scores, and effective rate), but also the objective immune indicators IGA, IGG, IGM, NK cell levels, and adverse events. In addition, we included many new trials that were not included in the previous reviews and meta-analyses to provide a comprehensive update. Furthermore, the sensitivity analysis demonstrated that the results of the current meta-analysis are relatively robust, and we found no evidence of publication bias in this meta-analysis by funnel plot and Egger’s test.
Some limitations must be considered. First, although we included RCTs, some methodological limitations still existed in most studies. Specifically, 44 trials supplied sufficient information on the randomization process, only five RCTs described allocation concealment, only three trials reported double blinding of patients and physicians, and only eight trials described blinding of participants. These methodological flaws might generate bias, so our results should be interpreted cautiously. Second, there is significant clinical heterogeneity due to the variations in composition and dosage of CHM and different dosage forms of CHM (e.g., decoction, granule, oral liquid, pill, ointment, and capsule). Finally, all trials were conducted in China, which may limit the generalizability of the findings presented here. Therefore, further international multicenter RCTs are needed to popularize the results globally. Furthermore, we conducted subgroup analyses to explore the sources of heterogeneity based on the different intervention duration and measures. The result showed that the heterogeneity was lower after grouping according to the results of subgroup analyses, indicating that differences in intervention duration and measures may also be the underlying source of heterogeneity.
Implications for research
Based on the above limitations, some recommendations are suggested for further studies. First, further rigorously designed trials with high methodological quality are urgently needed. We advise designing and reporting RCTs of CFS strictly according to the CONSORT 2010 statement (Schulz et al., 2010) and the CONSORT Extension for Chinese Herbal Medicine Formulas 2017 (Cheng et al., 2017). Random sequence generation, allocation concealment, and blinding should all be strictly implemented in future studies. Second, an efficacy evaluation system in line with the characteristics of CHM should be set up, and sensitive and practical indicators of CHM should be explored. Third, adverse effects were not reported in many studies. Therefore, the presence or absence of adverse events should be reported in future studies based on the standard format of adverse reactions established by Bian et al. (2010), and clinical trials and studies with longer follow-up times should be conducted to confirm the long-term safety of CHM for CFS.
Implications for practice
The evidence available from our study suggested the effectiveness and safety of CHM therapy for CFS. The most commonly used herbs included Bupleurum falcatum L., Glycyrrhiza glabra L., Atractylodes macrocephala Koidz., Angelica sinensis (Oliv.) Diels, Astragalus mongholicus Bunge, Codonopsis pilosula (Franch.) Nannf., Paeonia lactiflora Pall., Poria cocos (Schw.) Wolf, Citrus × aurantium L., Rehmannia glutinosa (Gaertn.) DC., Conioselinum anthriscoides “Chuanxiong,” Curcuma aromatica Salisb., Dioscorea oppositifolia L., Polygala tenuifolia Willd., Pinellia ternata (Thunb.) Makino, Lycium chinense Mill., Ziziphus jujuba Mill., Citrus × aurantium L., Ziziphi Spinosae Semen, Scutellaria baicalensis Georgi, Panax ginseng C.A.Mey., and Schisandra chinensis (Turcz.) Baill. This list can facilitate further exploration of the therapeutic principles of these drugs for CFS in order to further develop herbal prescriptions to improve the efficacy and safety of the treatment of CFS. In addition, the efficacy of CHM depends on the accurate dialectical treatment, and the prescription of CHM should be based on the precise dialectical diagnosis of CFS. Thus, individualized herbal prescriptions can be implemented in future clinical practice by selecting appropriate drugs among the frequently used drugs.
The possible mechanisms of CHM for CFS are as follows. 1) Adjusting the immune dysfunction: a study found that Young Yum Pill, a proprietary herbal drug, could improve immune organ (thymus and spleen) indices, the mitogenic response of lymphocytes, and numbers of T-cell subsets (Yin et al., 2021). Buzhong Yiqi decoction, Kuibi decoction, and Danggui Buxue decoction significantly inhibit tumor necrosis factor-a, IL-6, IL-10, and transforming growth factor-b1 in CFS patients (Shin et al., 2004; Chen et al., 2010; Miao et al., 2022). Furthermore, Renshen Yangrong decoction can ameliorate lower NK cell activity, and extracts of Ginseng can also boost natural killer cell function and the cellular immunity of patients with CFS (Ogawa et al., 1992; See et al., 1997). 2) Antioxidant effects: superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are two major components of the antioxidative system, and their function is to detoxify reactive oxygen species. Danggui Buxue decoction, Ginsenoside, and Jujube polysaccharide conjugate could improve SOD and GSH-Px activities and decrease MDA levels (Chi et al., 2015; Miao et al., 2022). Additionally, Quercetin, Withania somnifera (L.) Dunal, Hypericum perforatum L., and Ginkgo biloba L. have also been reported to possess beneficial antioxidants for CFS (Logan and Wong., 2001; Singh et al., 2002). 3) Improving metabolic dysfunction: Chi et al.’s study confirmed that SCP treatment affects metabolic pathways, including the TCA cycle and alanine, aspartate, and glutamate metabolism (Chi et al., 2016). Danggui Buxue decoction might regulate serine, glycine, and threonine metabolism to improve energy supply and ameliorate the CFS-weakened immunity (Miao et al., 2022). In addition, HEP2-a increased the creatine level to improve the arginine and proline metabolism (Chi et al., 2017). 4) Regulating the abnormal activity of the HPA axis: Chi inferred that HEP2-a indirectly affected the HPA axis abnormality of CFS by increasing the noradrenaline level (Chi et al., 2017).
Conclusion
In conclusion, the current evidence suggests that CHM, either as adjuvant therapy or monotherapy, decreases FS-14, FAI, SCL-90, SAS, SDS, and clinical symptom scores and enhances IGA, IGG, IGM, and effective rate. However, NK cell levels did not change significantly. In addition, the included studies did not report serious adverse events, suggesting that CHM is relatively safe in patients with CFS. Our findings on commonly used CHM may help investigate their value and further clinical application for CFS. Our study suggests that CHM seems to be effective and safe in the treatment of CFS. However, given the poor quality of the included studies, more international multi-centered, double-blinded, placebo-controlled, well-designed clinical trials are needed in future research.
Acknowledgments
We would like to thank YZ and WS for their guidance, which have significantly improved the quality of this manuscript.
Author contributions
YZ and WS conceptualized the research question. FJ and YP participated in drafting and writing the review. YZ, FJ, WS, YP, and XW participated in the formulation of retrieval strategies, data acquisition, data analysis, and quality assessment. WS, QJ, and JX participated in the drawing of tables and figures. YZ and WS participated in the critical revision of the manuscript. All authors contributed to the research and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81973601), the TCM research projects of Heilongjiang Province (ZHY19-027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.958005/full#supplementary-material
References
- Baker R., Shaw E. J. (2007). Diagnosis and Management of Chronic Fatigue Syndrome or Myalgic Encephalomyelitis (Or Encephalopathy): Summary of NICE Guidance. BMJ 335 (7617), 446–448. 10.1136/bmj.39302.509005.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested A. C., Marshall L. M. (2015). Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Evidence-Based Approach to Diagnosis and Management by Clinicians. Rev. Environ. Health 30 (4), 223–249. 10.1515/reveh-2015-0026 [DOI] [PubMed] [Google Scholar]
- Bian Z. X., Tian H. Y., Gao L., Shang H. C., Wu T. X., Li Y. P., et al. (2010). Improving Reporting of Adverse Events and Adverse Drug Reactions Following Injections of Chinese Materia Medica. J. Evid. Based. Med. 3 (1), 5–10. 10.1111/j.1756-5391.2010.01055.x [DOI] [PubMed] [Google Scholar]
- Brinth L., Nielsen H., Varming K., Boonen S. E., Ebsen A., Fernández-Guerra P., et al. (2019). Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Ugeskr. Laeger 181 (24), V08180570. [PubMed] [Google Scholar]
- Chen G., Sheng Z. Y., Lu J. (2011). Clinical Observation on 40 Cases of Chronic Fatigue Syndrome Treated by Syndrome Differentiation. Jiangsu J. Tradit. Chin. Med. 43 (3), 46–47. [Google Scholar]
- Chen R., Moriya J., Yamakawa J., Takahashi T., Kanda T. (2010). Traditional Chinese Medicine for Chronic Fatigue Syndrome. Evid. Based. Complement. Altern. Med. 7 (1), 3–10. 10.1093/ecam/nen017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. N. (2021). Clinical Observation of Xiaoyao Powder in Treating 33 Cases of Chronic Fatigue Syndrome. Clin. Med. 13 (9), 140–141. 10.3969/j.issn.1674-7860.2021.09.05 [DOI] [Google Scholar]
- Cheng C. W., Wu T. X., Shang H. C., Li Y. P., Altman D. G., Moher D., et al. (2017). CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Traditional Chinese Version). Ann. Intern. Med. 167, W7–W20. 10.7326/IsTranslatedFrom_M17-2977_1 [DOI] [PubMed] [Google Scholar]
- Cheng S. H. (2009). Observation on the Efficacy of Fufangteng Mixture in the Treatment of Chronic Fatigue Syndrome. J. Liaoning Univ. Tradit. Chin. Med. 11 (8), 135. 10.13194/j.jlunivtcm.2009.08.137.chengshh.018 [DOI] [Google Scholar]
- Chi A., Kang C., Zhang Y., Tang L., Guo H., Li H., et al. (2015). Immunomodulating and Antioxidant Effects of Polysaccharide Conjugates from the Fruits of Ziziphus Jujube on Chronic Fatigue Syndrome Rats. Carbohydr. Polym. 122, 189–196. 10.1016/j.carbpol.2014.12.082 [DOI] [PubMed] [Google Scholar]
- Chi A., Shen Z., Zhu W., Sun Y., Kang Y., Guo F. (2017). Characterization of A Protein-Bound Polysaccharide from Herba Epimedii and its Metabolic Mechanism in Chronic Fatigue Syndrome. J. Ethnopharmacol. 203, 241–251. 10.1016/j.jep.2017.03.041 [DOI] [PubMed] [Google Scholar]
- Chi A., Zhang Y., Kang Y., Shen Z. (2016). Metabolic Mechanism of A Polysaccharide from Schisandra Chinensis to Relieve Chronic Fatigue Syndrome. Int. J. Biol. Macromol. 93, 322–332. 10.1016/j.ijbiomac.2016.08.042 [DOI] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission (2020). The 2020 Edition of Pharmacopoeia of the People’s Republic of China. Beijing, China: Chemical Industry Press. [Google Scholar]
- Clayton E. W. (2015). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An IOM Report on Redefining an Illness. JAMA 313 (11), 1101–1102. 10.1001/jama.2015.1346 [DOI] [PubMed] [Google Scholar]
- Cleare A. J., Reid S., Chalder T., Hotopf M., Wessely S. (2015). Chronic Fatigue Syndrome. BMJ Clin. Evid. 2015, 1101. [PMC free article] [PubMed] [Google Scholar]
- Cortes Rivera M., Mastronardi C., Silva-Aldana C. T., Arcos-Burgos M., Lidbury B. A. (2019). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagn. (Basel) 9 (3), 91. 10.3390/diagnostics9030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumer U. S., Varesi A., Floris V., Savioli G., Mantovani E., López-Carrasco P., et al. (2021). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 10 (20), 4786. 10.3390/jcm10204786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. M. (2019). Clinical Analysis of Guipi Decoction in Treating Chronic Fatigue Syndrome of Heart and Spleen Deficiency Type. Chin. Foreign. Med. Treat. 38 (32), 169–171. 10.16662/j.cnki.1674-0742.2019.32.169 [DOI] [Google Scholar]
- Dong L. L. (2020). Qingshu Yiqi Decoction in Treating Spleen Deficiency and Damp-Heat Type Clinical Observation of Chronic Fatigue Syndrome. China’s Naturop. 28 (20), 72–74. 10.19621/j.cnki.11-3555/r.2020.203 [DOI] [Google Scholar]
- Du Y. (2018). Clinical Observation on Self-Made Yishen Buxue Ointment in Treating Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 33 (22), 3295–3297. 10.3969/j.issn.10038914.2018.22.009 [DOI] [Google Scholar]
- Fang B., Wang J. Z., Zhang H. F. (2007). Clinical Observation on Chronic Fatigue Syndrome Treated with Anti-fatigue Granule. Chin. J. Integr. Tradit. West. Med. (Chin.) 16 (12), 1622–1623. [Google Scholar]
- Fang Y. Q., Ren Y. L., Wang G. T. (2008). Clinical Observation on Chronic Fatigue Syndrome Treated with Shenqi Ointment. Northwest Pharm. J. 23 (6), 389–390. [Google Scholar]
- Fernie B. A., Murphy G., Wells A., Nikčević A. V., Spada M. M. (2016). Treatment Outcome and Metacognitive Change in CBT and GET for Chronic Fatigue Syndrome. Behav. Cogn. Psychother. 44 (4), 397–409. 10.1017/S135246581500017X [DOI] [PubMed] [Google Scholar]
- Gao J., Pang M. (2016). Fatigue Syndrome with Liver-Depression and Spleen-Deficiency Type Treated by Wendan Decoction Combined with Sini Powder. Inf. Tradit. Chin. Med. 33 (1), 72–75. 10.19656/j.cnki.1002-2406.2016.01.023 [DOI] [Google Scholar]
- Gao J., Pang M. (2015). Treating Chronic Fatigue Syndrome by Draining the Liver and Strengthening the Spleen. Jilin J. Tradit. Chin. Med. 35 (10), 1022–1024. 10.13463/j.cnki.jlzyy.2015.10.015 [DOI] [Google Scholar]
- Geraghty K. J., Blease C. (2018). Cognitive Behavioural Therapy in the Treatment of Chronic Fatigue Syndrome: A Narrative Review on Efficacy and Informed Consent. J. Health Psychol. 23 (1), 127–138. 10.1177/1359105316667798 [DOI] [PubMed] [Google Scholar]
- Geraghty K. J., Blease C. (2019). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Biopsychosocial Model: A Review of Patient Harm and Distress in the Medical Encounter. Disabil. Rehabil. 41 (25), 3092–3102. 10.1080/09638288.2018.1481149 [DOI] [PubMed] [Google Scholar]
- Glassford J. A. (2017). The Neuroinflammatory Etiopathology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Physiol. 8, 88. 10.3389/fphys.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J. H. (2007). Clinical Observation on 30 Cases of Chronic Fatigue Syndrome Treated with Modified Guipi Decoction. Zhejiang J. Integr. Tradit. Chin. West. Med. 17 (10), 627–628. [Google Scholar]
- Goudsmit E., Howes S. (2017). Bias, Misleading Information and Lack of Respect for Alternative Views Have Distorted Perceptions of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and its Treatment. J. Health Psychol. 22 (9), 1159–1167. 10.1177/1359105317707216 [DOI] [PubMed] [Google Scholar]
- Gregorowski A., Simpson J., Segal T. Y. (2019). Child and Adolescent Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: Where Are We Now? Curr. Opin. Pediatr. 31 (4), 462–468. 10.1097/MOP.0000000000000777 [DOI] [PubMed] [Google Scholar]
- Guenther S., Loebel M., Mooslechner A. A., Knops M., Hanitsch L. G., Grabowski P., et al. (2015). Frequent IgG Subclass and Mannose Binding Lectin Deficiency in Patients with Chronic Fatigue Syndrome. Hum. Immunol. 76 (10), 729–735. 10.1016/j.humimm.2015.09.028 [DOI] [PubMed] [Google Scholar]
- Guo J. H., Hu B., Yao R. B., Zhao Z. M., Zhang Y. W., Zhao L. J., et al. (2007). A Clinical Research of the Efficacy of Nourishing Both Qi and Blood Peroral Solution in the Treatment of Chronic Fatigue Syndrome. Mil. Med. J. Southeast Chin. 10 (9), 325–327. [Google Scholar]
- He L. (2019). “Clinical Observation on Chronic Fatigue Syndrome Treated by Shugan Jianpi Huoxue Method,” Hebei, China: Hebei North University. [Google Scholar]
- Hornig M., Montoya J. G., Klimas N. G., Levine S., Felsenstein D., Bateman L., et al. (2015). Distinct Plasma Immune Signatures in ME/CFS Are Present Early in the Course of Illness. Sci. Adv. 1 (1), e1400121. 10.1126/sciadv.1400121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X. Y., Jia G. P., Tian L. Y., Gao F., Liu X. W. (2015). Discussion on the Pathogenesis of Chronic Fatigue Syndrome. Hebei Med. J. 37 (16), 2463. [Google Scholar]
- Hu B., Zhou Z. D., Zhang L. T., Qiu X. F. (2010). Clinical Observation of Buqi Tongluo Formula in the Treatment of Chronic Fatigue Syndrome. Hubei J. Tradit. Chin. Med. 32 (8), 45. [Google Scholar]
- Hu H. (2019). Clinical Effect of Buzhong Yiqi Decoction Combined with Xiaochaihu Decoction in the Treatment of Chronic Fatigue Syndrome with Liver Stagnation and Spleen Deficiency Syndrome. Integr. Tradit. Chin. West. Med. 6 (27), 106. [Google Scholar]
- Jiang Q. (2012). Observation on the Efficacy of Buzhong Yiqi Decoction and Guipi Decoction in the Treatment of Chronic Fatigue Syndrome. J. Beijing Univ. Chin. Med. 31 (2), 121–122. 10.16025/j.1674-1307.2012.02.016 [DOI] [Google Scholar]
- Jie R., Wang G. P. (2009). Comparative Study of Paroxetine in Combination with Xiaoyao Pill for the Treatment of Chronic Fatigue Syndrome. J. Clin. Psychosom. Dis. 15 (4), 307–313. 10.3969/jissn.1672-187X.2009.04.0307-03 [DOI] [Google Scholar]
- Joung J. Y., Lee J. S., Cho J. H., Lee D. S., Ahn Y. C., Son C. G. (2019). The Efficacy and Safety of Myelophil, an Ethanol Extract Mixture of Astragali Radix and Salviae Radix, for Chronic Fatigue Syndrome: A Randomized Clinical Trial. Front. Pharmacol. 10, 991. 10.3389/fphar.2019.00991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G., Khan F., Hill A., Underwood C., Belch J. J. (2010). Biochemical and Vascular Aspects of Pediatric Chronic Fatigue Syndrome. Arch. Pediatr. Adolesc. Med. 164 (9), 817–823. 10.1001/archpediatrics.2010.157 [DOI] [PubMed] [Google Scholar]
- Kong F. Y. (2012). Treatment of 30 Cases of Chronic Fatigue Syndrome with Anti-fatigue Decoction Combined with Exercise Therapy. Henan Tradit. Chin. Med. 32 (1), 69–70. 10.16367/j.issn.1003-5028.2012.01.029 [DOI] [Google Scholar]
- Lai J. Z., Lei L. M. (2013). Clinical Study of Baiyu Jianpi Decoction in the Treatment of Chronic Fatigue Syndrome. J. Chin. Med. 178 (28), 423–424. 10.16368/j.issn.1674-8999.2013.03.040 [DOI] [Google Scholar]
- Larun L., Brurberg K. G., Odgaard-Jensen J., Price J. R. (2017). Exercise Therapy for Chronic Fatigue Syndrome. Cochrane Database Syst. Rev. 4 (4), CD003200. 10.1002/14651858.CD003200.pub7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. D., Chen Z. L., Huang N. (2011). The Clinical Study of Soothing Liver and Activating Spleen in Treatment of Chronic Fatigue Syndrome. Liaoning J. Tradit. Chin. Med. 38 (10), 2037–2038. 10.13192/j.ljtcm.2011.10.120.lichd.034 [DOI] [Google Scholar]
- Li J., Chen X. D., Huang H. Q., Chen G. Z., Wang L., Zhang H., et al. (2021). Inorganic Nitrate Alleviates Irradiation-Induced Salivary Gland Damage by Inhibiting Pyroptosis. Free Radic. Biol. Med. 13 (17), 130–140. 10.1016/j.freeradbiomed.2021.08.227 [DOI] [PubMed] [Google Scholar]
- Li T., Zao Y. Q. (2015). Evaluation on Clinical Effect of Treatment of 37 Female Patients with Chronic Fatigue Syndrome by Chinese Medicines in Invigorating Spleen Warming Kidney and Smoothing. Clin. J. Tradit. Chin. Med. 7 (12), 98–101. 10.3969/j.issn.1674-7860.2015.12.049 [DOI] [Google Scholar]
- Li W., Xu X. F., Jia B., Xu G. J., Chen H. X., Zheng T. J., et al. (2018). Exploring the Efficacy of the Formula to Yiqi Yangxue Bupi Hegan Recipe in the Treatment of Chronic Fatigue Syndrome. Chin. J. Mod. Drug. Appl. 12 (15), 208–209. 10.14164/j.cnki.cn11-5581/r.2018.15.121 [DOI] [Google Scholar]
- Li X. (2009). “Clinical Study of Anti-fatigue No. 2 in the Treatment of Sub-healthy Chronic Fatigue of Liver Stagnation, Heart and Spleen Deficiency” (Beijing, China: Chinese Academy of Traditional Chinese Medicine; ). [Google Scholar]
- Li Y. (2020). Analysis of Therapeutic Effect and Quality of Life of Buzhong Yiqi Decoction Combined with Xiaochaihu Decoction on Chronic Fatigue Syndrome. Spec. Health 34, 86. [Google Scholar]
- Li Y. M. (2015). Treatment of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction and Xiaochaihu Decoction Clinical Observation on the Syndrome of Liver Depression and Spleen Deficiency. Asia-Pacific. Tradit. Med. 11 (17), 124–125. 10.11954/ytctyy.201517065 [DOI] [Google Scholar]
- Liang G. (2006). Clinical Observation of the Treatment of 63 Cases of Chronic Fatigue Syndrome by Using Shengmai Decoctionin Combination with Xuefu Zhuyu Decoction. Lishizhen Med. Mat. Med. Res. 17 (5), 801–802. [Google Scholar]
- Lim E. J., Son C. G. (2021). Prevalence of Chronic Fatigue Syndrome (CFS) in Korea and Japan: A Meta-Analysis. J. Clin. Med. 10 (15), 3204. 10.3390/jcm10153204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. (2007). Treatment of 50 Cases of Chronic Fatigue Syndrome with Modified Shenling Baizhu Powder. New J. Tradit. Chin. Med. 39 (3), 68. 10.13457/j.cnki.jncm.2007.03.047 [DOI] [Google Scholar]
- Liu F., Luo Q., Luo Y. H., Luo J. Y., Luo H. H., Deng Q. (2019a). Clinical Observation on 60 Cases of Chronic Fatigue Syndrome Treated by Jiawei Lingzhi Yishou Pill. Yunnan J. Tradit. Chin. Med. 40 (02), 34–35. 10.16254/j.cnki.53-1120/r.2019.02.012 [DOI] [Google Scholar]
- Liu G. X., Cai K. K. (2018). Observation on the Clinical Effect of Bupiwei Xieyinhuo Shengyang Decoction in the Treatment of Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 33 (19), 2827–2830. 10.3969/j.issn.1003-8914.2018.19.018 [DOI] [Google Scholar]
- Liu J., Hu Y. H., Ying R. J., Shen J., Sheng S. Y. (2019b). Chaihu Guizhi Decoction in the Treatment of Chronic Fatigue Syndrome of Liver-Depression and Spleen-Deficiency Type Clinical Efficacy and Effect on Immune Function of Patients. Lishizhen. Med. Mat. Med. Res. 30 (06), 1414–1416. 10.3969/j.issn.1008-0805.2019.06.044 [DOI] [Google Scholar]
- Liu J., Hu Y. H., Ying R. J., Sheng Z. Y. (2021). The Clinical Observation of Chaihu Guizhi Decoction in Treating Chronic Fatigue Syndrome and the Influence of Emotional Factors. World. J. Integr. Tradit. West. Med. 16 (10), 1908–1911. 10.13935/j.cnki.sjzx.211028 [DOI] [Google Scholar]
- Liu Y., Peng Y. Q., Ge X., Tong X. H., Yang T., Zhao M. C., et al. (2015). The Effect of the Method of Draining the Liver and Nourishing Blood on Fatigue in Patients with Chronic Fatigue Syndrome Degree and Quality of Survival. World. J. Integr. Tradit. West. Med. 10 (09), 1239–1241. 10.13935/j.cnki.sjzx.150918 [DOI] [Google Scholar]
- Liu Y., Peng Y. Q., Ge X., Yang T., Li Z. (2019c). Invigorating the Spleen and Nourishing the Kidney for Patients with Chronic Fatigue Syndrome Effects of Free Radical Metabolism. Beijing J. Tradit. Chin. Med. 38 (02), 140–142. 10.16025/j.1674-1307.2019.02.013 [DOI] [Google Scholar]
- Liu Y., Peng Y. Q., Ge X., Zhao M. C., Yang T., Zhang Y. (2011). Clinical Observation of Shugan Yangxue Formula in the Treatment of Chronic Fatigue Syndrome. Chin. J. Integr. Tradit. West. Med. (Chin.) 31 (02), 270–271. [Google Scholar]
- Logan A. C., Wong C. (2001). Chronic Fatigue Syndrome: Oxidative Stress and Dietary Modifications. Altern. Med. Rev. 6 (5), 450–459. [PubMed] [Google Scholar]
- Luo Y. (2018). “Clinical Observation to the Modified of Clearing Heat and Expelling Damp Decoction in Treating Chronic Fatigue Syndrome of Damp-Heat Syndrome,” Guangdong, China: Guangzhou University of Traditional Chinese Medicine. [Google Scholar]
- Ma G. X., Zhao W. H., Wang H. C., Pan L. H., Wang Y., Wang D. P., et al. (2019). Clinical Observation on Modified Erxian Decoction in Treating Chronic Fatigue Syndrome. Mod. Distance Educ. Chin. Med. 17 (02), 72–74. 10.3969/j.issn.1672-2779.2019.02.028 [DOI] [Google Scholar]
- Ma J. X. (2009). Treatment of 78 Cases of Chronic Fatigue Syndrome with Modified Guipi Decoction. World Health Dig. 6 (19), 23. [Google Scholar]
- Maes M., Twisk F. N., Kubera M., Ringel K. (2012). Evidence for Inflammation and Activation of Cell-Mediated Immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased Interleukin-1, Tumor Necrosis Factor-α, PMN-Elastase, Lysozyme and Neopterin. J. Affect. Disord. 136 (3), 933–939. 10.1016/j.jad.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Mao X. F. (2020). Clinical Discussion and Analysis of Yishen Tiaodu Method in Treating Chronic Fatigue Syndrome of Spleen and Kidney Yang Deficiency Type. World Latest Med. Inf. 20 (A0), 134–135. 10.3969/j.issn.1671-3141.2020.100.067 [DOI] [Google Scholar]
- Marques M. M., De Gucht V., Gouveia M. J., Leal I., Maes S. (2015). Differential Effects of Behavioral Interventions with A Graded Physical Activity Component in Patients Suffering from Chronic Fatigue (Syndrome): An Updated Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 40, 123–137. 10.1016/j.cpr.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Matsui T., Kataoka K., Fukada R., Fukuda S., Kuratsune H., et al. (2009). A Two-Year Follow-Up Study of Chronic Fatigue Syndrome Comorbid with Psychiatric Disorders. Psychiatry Clin. Neurosci. 63 (3), 365–373. 10.1111/j.1440-1819.2009.01954.x [DOI] [PubMed] [Google Scholar]
- Miao X., Li S., Xiao B., Yang J., Huang R. (2022). Metabolomics Study of the Effect of Danggui Buxue Tang on Rats with Chronic Fatigue Syndrome. Biomed. Chromatogr. 36 (7), e5379. 10.1002/bmc.5379 [DOI] [PubMed] [Google Scholar]
- Montoya J. G., Holmes T. H., Anderson J. N., Maecker H. T., Rosenberg-Hasson Y., Valencia I. J., et al. (2017). Cytokine Signature Associated with Disease Severity in Chronic Fatigue Syndrome Patients. Proc. Natl. Acad. Sci. U. S. A. 114 (34), E7150–E7158. 10.1073/pnas.1710519114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mücke M., MochamatCuhls H., Peuckmann-Post V., Minton O., Stone P., Radbruch L., et al. (2015). Pharmacological Treatments for Fatigue Associated with Palliative Care. Cochrane Database Syst. Rev. 2015 (5), CD006788. 10.1002/14651858.CD006788.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning T. C., Li Y. P. (2002). Treatment of 23 Cases of Chronic Fatigue Syndrome with Sijunzi Decoction. Res. Tradit. Chin. Med. 18 (04), 16–17. [Google Scholar]
- Niu Z. Z., Zhang X. P., Zhou H. I., Wang X. (2015). “The Observation of Curative Effect of Chronic Fatigue Syndrome Treated by Bushen Shugan Decoctoin,” in Proceedings of the Second Academic Annual Conference of the World Federation of Chinese Medicine Societies Committee on Traditional Chinese Medicine Health Management, 212–214. [Google Scholar]
- Noor N., Urits I., Degueure A., Rando L., Kata V., Cornett E. M., et al. (2021). A Comprehensive Update of the Current Understanding of Chronic Fatigue Syndrome. Anesth. Pain Med. 11 (3), e113629. 10.5812/aapm.113629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R., Toyama S., Matsumoto H. (1992). Chronic Fatigue Syndrome-Cases in the Kanebo Memorial Hospital. Nihon Rinsho. 50 (11), 2648–2652. [PubMed] [Google Scholar]
- Ou Y., Xiao L., Li J., Wang J. H., Liu W. H., Yang G. L. (2018). Clinical Study on the Treatment of Chronic Fatigue Syndrome with Deficiency of Both Heart and Spleen by Guipi Decoction. Inf. Tradit. Chin. Med. 35 (2), 87–90. 10.19656/j.cnki.1002-2406.180060 [DOI] [Google Scholar]
- Pang Y. H., Liu j. p. (2013). Therapeutic Effect of Shengmai Powder Plus Modified Xiaoyao Powder for Treatment of Chronic Fatigue Syndrome. J. Guangzhou Univ. Tradit. Chin. Med. 30 (3), 316–318. 10.13359/j.cnki.gzxbtcm.2013.03.008 [DOI] [Google Scholar]
- Peng W., Su J., Xu Q., Wang Q. J., Jiang X. J. (2013). Meta-Analysis of Clinical Effect of TCM Intervention on Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 28 (07), 1345–1349. [Google Scholar]
- Ren P., Yu X. K. (2012). Chronic Fatigue Syndrome 40 Cases Treated with Buxu Decoction and Rehabilitation Training. Henan Tradit. Chin. Med. 32 (10), 1321. 10.16367/j.issn.10035028.2012.10.068 [DOI] [Google Scholar]
- Schulz K. F., Altman D. G., Moher D. CONSORT Group (2010). CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 152 (11), 726–732. 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- See D. M., Broumand N., Sahl L., Tilles J. G. (1997). In Vitro Effects of Echinacea and Ginseng on Natural Killer and Antibody-dependent Cell Cytotoxicity in Healthy Subjects and Chronic Fatigue Syndrome or Acquired Immunodeficiency Syndrome Patients. Immunopharmacology 35 (3), 229–235. 10.1016/s0162-3109(96)00125-7 [DOI] [PubMed] [Google Scholar]
- Sheng S. Y., Shen j., Chen Y. Y., Hu Y. H., Liu J., Hu X. Y. (2022). Treatment of Chronic Fatigue Syndrome of Spleen-Kidney Yang Deficiency by Warming the Kidney and Regulating Fatigue Formula Clinical Efficacy Observation and Effect on Cellular Immunity. Chin. J. Tradit. Med. Sci. Technol. 29 (3), 228–230. [Google Scholar]
- Shi J., Zha W. (2019). Predicting Human Pharmacokinetics: Physiologically Based Pharmacokinetic Modeling and In Silico ADME Prediction in Early Drug Discovery. Eur. J. Drug Metab. Pharmacokinet. 4 (1), 135–137. 10.1007/s13318-018-0503-9 [DOI] [PubMed] [Google Scholar]
- Shi Z. H., Wu Y. F. (2016). Clinical Observation of Suanzaoren Decoction in the Treatment of Sub-healthy Sleep. Chin. J. Pract. Med. 11 (15), 196–197. 10.14163/j.cnki.11-5547/r.2016.15.143 [DOI] [Google Scholar]
- Shin H. Y., An N. H., Cha Y. J., Shin E. J., Shin T. Y., Baek S. H., et al. (2004). Effect of Kuibitang on Lipopolysaccharide-Induced Cytokine Production in Peripheral Blood Mononuclear Cells of Chronic Fatigue Syndrome Patients. J. Ethnopharmacol. 90 (2-3), 253–259. 10.1016/j.jep.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Shin S., Park S. J., Hwang M. (2021). Effectiveness A Herbal Medicine (Sipjeondaebo-Tang) on Adults with Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Integr. Med. Res. 10 (2), 100664. 10.1016/j.imr.2020.100664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Naidu P. S., Gupta S., Kulkarni S. K. (2002). Effect of Natural and Synthetic Antioxidants in A Mouse Model of Chronic Fatigue Syndrome. J. Med. Food 5 (4), 211–220. 10.1089/109662002763003366 [DOI] [PubMed] [Google Scholar]
- Smith M. E., Haney E., McDonagh M., Pappas M., Daeges M., Wasson N., et al. (2015). Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for A National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 162 (12), 841–850. 10.7326/M15-0114 [DOI] [PubMed] [Google Scholar]
- Sotzny F., Blanco J., Capelli E., Castro-Marrero J., Steiner S., Murovska M., et al. (2018). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Evidence for an Autoimmune Disease. Autoimmun. Rev. 17 (6), 601–609. 10.1016/j.autrev.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Sun H. J., Guo Y. J., Sun X. H., Guo F. C., Ma Y. (2016). Clinical Study on Treatment of Chronic Fatigue Syndrome by Shugan Yiyang Cap. J. Tradit. Chin. Med. 213 (31), 272–274. 10.16368/j.issn.1674-8999.2016.02.075 [DOI] [Google Scholar]
- Sun H. J., Zhen M. H., Yang X. W. (2007). The Effect of Shu Yu Formula on the Emotional State and Quality of Life of Patients with Chronic Fatigue Syndrome. Henan Tradit. Chin. Med. 27 (5), 30–32. 10.16367/j.issn.1003-5028.2007.05.018 [DOI] [Google Scholar]
- Tan D. H., Zhang R., Chen X. F., Wu X. H. (2015). Treatment of 30 Cases of Chronic Fatigue Syndrome by Dredging and Regulating Sanjiao Yuanqi. China J. Chin. Mat. Med. 2015, 1259–1261. [Google Scholar]
- Teng F. Y., Jiang Q. Y., Huang Y. N. (2014). Observation on the Curative Effect of Buzhong Jiepi Decoction in the Treatment of Chronic Fatigue Syndrome. Guid. J. Tradit. Chin. Med. Pharm. 20 (4), 108–109. 10.13862/j.cnki.cn43-1446/r.2014.04.047 [DOI] [Google Scholar]
- Tian H., Wang S. G. (2012). Treatment of 32 Cases of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction. Jiangxi Tradit. Chin. Med. 43 (08), 26–27. [Google Scholar]
- Wang C. (2019). “Clinical Observation of Sanren Decoction and Sijunzi Decoction in the Treatment of Chronic Fatigue Syndrome of Spleen Deficiency and Dampness Obstruction Syndrome,” Guangzhou, China: Guangzhou University of Chinese Medicine. [Google Scholar]
- Wang H. (2012). Clinical Study of Buzhong Yiqi Decoction and Xiaochaihu Decoction in Treatment of Chronic Fatigue Syndrome Liver Depression and Spleen Card. Asia-Pacific Tradit. Med. 8 (8), 67–68. [Google Scholar]
- Wang J. Q. (2020). Observation on the Clinical Effect of Chaihu Guizhi Decoction in the Treatment of Chronic Fatigue Syndrome of Liver Stagnation and Spleen Deficiency. Sci. Regimen. 23 (3), 146–147. [Google Scholar]
- Wang J. Z., Fang B., Zhang H. F., Wu C. Y. (2007). Clinical Study on the Treatment of Chronic Fatigue Syndrome with Fuzheng Jieyu Formula. Chin. J. Inf. Tradit. Chin. Med. 14 (9), 75–76. [Google Scholar]
- Wang M. X. (2021). “Based on the “Gas Monism” to Explore Mechanism of Chronic Fatigue Syndrome (Spleen Deficiency Syndrome) and the Clinical Curative Effect Observation.” Changchun, China: Changchun University of Chinese Medicine. [Google Scholar]
- Wang R. S. (2017). Randomized Parallel Controlled Study of Bupi Yishen Decoction Combined with Adenosine Triphosphate + Oryzanol in the Treatment of Chronic Fatigue Syndrome of Spleen-Kidney Yang Deficiency. Pract. Tradit. Chin. Intern. Med. 31 (2), 31–33. 10.13729/j.issn.1671-7813.2017.02.13 [DOI] [Google Scholar]
- Wang X. J., Zhang Y. R., Jian Q. R., He Y. X., Wang X. L., Lin L. (2011). Clinical Effective Observation on Treating Chronic Fatigue Syndrome in TCM. Clin. J. Chin. Med. 3 (2), 106–107. [Google Scholar]
- Wang Y. Y., Li X. X., Liu J. P., Luo H., Ma L. X., Alraek T. (2014). Traditional Chinese Medicine for Chronic Fatigue Syndrome: A Systematic Review of Randomized Clinical Trials. Complement. Ther. Med. 22 (4), 826–833. 10.1016/j.ctim.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Wei L. L. (2005). Clinical Observation of Xiaochaihu Decoction in the Treatment of Chronic Fatigue Syndrome. Chin. Arch. Tradit. Chin. Med. 23 (7), 1315–1316. 10.13193/j.archtcm.2005.07.157.weill.078 [DOI] [Google Scholar]
- Weng Y. N. (2018). Clinical Observation on the Therapeutic Effect of Liujunzi Decoction in Changxia Season in the Chronic Fatigue Syndrome Patients of Spleen Deficiency and Dampness Obstruction. Clin. Res. 235–238. 10.26914/c.cnkihy.2018.026256 [DOI] [Google Scholar]
- Wu J. D., Zhang X. Q., Zhang Y., Zhu A. S. (2018). Clinical Efficacy of Guipi Decoction in Treating Chronic Fatigue Syndrome with Deficiency of Heart and Spleen. Liaoning J. Tradit. Chin. Med. 45 (2), 305–306. 10.13192/j.issn.1000-1719.2018.02.027 [DOI] [Google Scholar]
- Wu L. L., Zhang Z. X., Zhang Y. (2012). Investigation of Lixu Jieyu Prescription in Treating 120 Cases of Chronic Fatigue Syndrome. Liaoning J. Tradit. Chin. Med. 39 (2), 283–284. 10.13192/j.ljtcm.2012.02.96.wull.035 [DOI] [Google Scholar]
- Wu X. J., Hu X. Z., Zhao H. B., Ma Q. L., Ma Z. G. (2016). Clinical Research on Xiaopi - Yin in the Treatment of Spleen - Kidney Deficiency Type Patients of Chronic Fatigue Syndrome. Ningxia Med. J. 38 (10), 922–924. 10.13621/j.1001-5949.2016.10.0922 [DOI] [Google Scholar]
- Xiong X., Yang X., Li X., Yue G., Xing Y., Cho W. C. (2019). Efficacy and Safety of Chinese Herbal Medicine for Patients with Postmenopausal Hypertension: A Systematic Review and Meta-Analysis. Pharmacol. Res. 141, 481–500. 10.1016/j.phrs.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Xu D., Dong Y. X., Yang X. Q. (2013). Observation of Curative Effect of Modified Naoxinkang in Treating 40 Cases of Chronic Fatigue Syndrome. J. Chang. Univ. Tradit. Chin. Med. 29 (02), 281–282. 10.13463/j.cnki.cczyy.2013.02.063 [DOI] [Google Scholar]
- Xu Y. C. (2014). Treatment of Chronic Fatigue Syndrome with Dongyuan Qingshu Yiqi Decoction Combined with Acupuncture. Chin. J. Clin. Res. 27 (4), 485–486. 10.13429/j.cnki.cjcr.2014.04.047 [DOI] [Google Scholar]
- Xu Z. H., Wang X. Z. (2013). Chaihu Jia Longgu Muli Decoction in the Treatment of 42 Cases of Chronic Fatigue Syndrome with Liver Depression and Spleen Deficiency. Henan Tradit. Chin. Med. 33 (6), 847–848. 10.16367/j.issn.1003-5028.2013.06.012 [DOI] [Google Scholar]
- Yang D. L. (2019). Clinical Effect of Zuogui Pill on Chronic Fatigue Syndrome of Kidney Deficiency Type. Health Everyone 22, 94–95. [Google Scholar]
- Yang S. H., Gao M., Yang X. W., Chen D. Q. (2004). Clinical Observation on Chronic Fatigue Syndrome Treated with Buzhong Yiqi Decoction and Xiaochaihu Decoction. J. Beijing Univ. Chin. Med. 27 (2), 87–89. [Google Scholar]
- Yang T. T., Wang L., Deng X. Y., Yu G. (2017). Pharmacological Treatments for Fatigue in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 380, 256–261. 10.1016/j.jns.2017.07.042 [DOI] [PubMed] [Google Scholar]
- Yao R. M., Qiu M. Y. (2005). Clinical Observation of Chronic Fatigue Syndrome Treated by Chinese Medicine in Hong Kong. Shanghai J. Tradit. Chin. Med. 39 (6), 12–13. 10.16305/j.1007-1334.2005.06.005 [DOI] [Google Scholar]
- Ye Y. (2017). “The Application Research of Immortal Porridge for Patients with Chronic Fatigue Syndrome Caused by Spleen and Kidney Yang Deficiency,” Chengdu, China: Chengdu University of Chinese Medicine. [Google Scholar]
- Yin C., Fu X., Chou J., Li J., Chen Y., Bai J., et al. (2021). A Proprietary Herbal Drug Young Yum Pill Ameliorates Chronic Fatigue Syndrome in Mice. Phytomedicine. 88, 153602. 10.1016/j.phymed.2021.153602 [DOI] [PubMed] [Google Scholar]
- Zhang J. Y. (2021). Analysis of the Curative Effect of Jiawei Guizhi Xinjia Decoction in the Treatment of Fatigue Syndrome. Heilongjiang J. Tradit. Chin. Med. 50 (06), 104–105. [Google Scholar]
- Zhang L. P., Dai B. S., Xiong T. X. (2012b). Analysis of Clinical Treatment on Chronic Fatigue Syndrome by Yao Medicine. Chin. J. Exp. Med. Formul. 18 (16), 311–313. 10.13422/j.cnki.syfjx.2012.16.006 [DOI] [Google Scholar]
- Zhang L. P., Xiong T. X., Su C. B. (2011). Effect of Zhenqi Jiepi with Yao Drug Recipe in Treating Patients with Chronic Fatigue Syndrome. J. Liaoning Univ. Tradit. Chin. Med. 13 (5), 177–178. 10.13194/j.jlunivtcm.2011.05.179.zhanglp.053 [DOI] [Google Scholar]
- Zhang R., Li J., Chen J., Zhang Z. J., Guo Z. H. (2004). Clinical Observation on the Treatment of Chronic Fatigue Syndrome with Shengqi Fuyuan Decoction. Chin. J. Inf. Tradit. Chin. Med. 11 (2), 148. [Google Scholar]
- Zhang S. X., Zhou W. (2004). Treatment of 40 Cases of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction. Chin. J. Prim. Med. 11 (5), 608. [Google Scholar]
- Zhang Z. X., Dong S. G., Huang Y., Zhang Y., Wu L. L. (2015). Clinical Observation on Chronic Fatigue Syndrome Depression and Anxiety by Wenzhen Yunqi Formula. Liaoning. J. Tradit. Chin. Med. 42 (12), 2346–2349. 10.13192/j.issn.1000-1719.2015.12.03 [DOI] [Google Scholar]
- Zhang Z. X., Wu L. L., Chen M., Zhang W. J., Qiu L. N., Xia X. (2009). Effect of Lixu Jieyu Recipe in Treating 75 Patients with Chronic Fatigue Syndrome. Chin. J. Integr. Tradit. West. Med. (Chin.) 29 (6), 501–505. [PubMed] [Google Scholar]
- Zhang Z. X., Zhang Y., Wang Y., Chen M., Wu L. L., Wang X. J., et al. (2012a). Effect of “Lixu Jieyu Recipe” on Negative Emotion, 5-HT and Cortisol of Patients with Chronic Fatigue Syndrome. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 26 (5), 38–40. 10.16306/j.1008-861x.2012.05.021 [DOI] [Google Scholar]
- Zhao D. F., Duan H. D., Zhao J. W. (2006). Curative Effect Observation of 30 Cases of Chronic Fatigue Syndrome Treated by Nourishing Qi and Nourishing Yin. Tianjin J. Tradit. Chin. Med. 23 (2), 126–127. [Google Scholar]
- Zhao L. (2013). Self-made Baihe Yangxin Jianpi Decoction in the Treatment of 88 Cases of Fatigue Syndrome (CFS). China Health Vis. 21 (2), 336–337. [Google Scholar]
- Zhao S. G. (2012). Clinical Study on Buzhong Yiqi Decoction Combined with Xiao Chaihu Decoction in the Treatment of Chronic Fatigue Syndrome with Liver Stagnation and Spleen Deficiency Syndrome. Psychol. Dr. 216, 355–356. 10.3969/j.issn.1007-8231.2012.05.408 [DOI] [Google Scholar]
- Zhao X., He X. W., Huang Q. (2013). Compound of Fufangteng Mixture on CFS with Blood Deficiency Type. Inf. Tradit. Chin. Med. 30 (04), 88–90. [Google Scholar]
- Zheng L. H., Mai J., Liu Y. X. (2017). Clinical Study of Shugan Jianpi Yishen Formula in Treating Chronic Fatigue Syndrome. Shandong J. Tradit. Chin. Med. 36 (10), 860–862. 10.16295/j.cnki.0257-358x.2017.10.01 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.