Highlights
-
•
Duration, type and timing of TKI remains controversial for Ph+ ALL.
-
•
Pre-transplant dasatinib/imatinib led to favorable outcomes among Ph+ ALL patients.
-
•
Type of pre-transplant TKI did not impact overall or event-free survival.
-
•
Attaining complete molecular response pre-transplant improved event-free survival.
Keywords: Philadelphia chromosome, Acute lymphoblastic Leukemia, Stem cell transplantation, Complete molecular response, Survival
Abstract
Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) has been associated with a worse prognosis compared to Ph negative ALL. Tyrosine kinase inhibitor (TKI) therapy has led to an improvement in response rates and survival, thus becoming a critical component of therapy. We performed a retrospective cohort study of Ph+ ALL patients treated at the University of Michigan who received TKI therapy pre- and post-allogeneic hematopoietic stem cell transplant (HSCT) from April 2007 to November 2019. The study included 40 patients with Ph+ ALL (47.5% female) with a median age of 54 (24-69) years. Median event-free survival (EFS) was not reached, with a 5-year EFS of 61%. Median overall survival (OS) was not reached, with a 5-year OS of 71%. There was no difference in 2-year EFS or OS for patients on pre-transplant imatinib or dasatinib (p = 0.16, 0.09, respectively), though definitive conclusions are challenging as post-transplant TKI therapy was variable. The incidence of any grade acute graft-versus-host disease (GVHD) was 62.5% (25/40) and any grade chronic GVHD was 77.5% (31/40). Complete molecular remission (CMR) was achieved in 57.5% of patients pre-transplant with no significant difference when stratified by induction TKI (p = 1). Achievement of CMR pre-HSCT showed a trend towards improved 2-year EFS (p=0.0198) but did not significantly change 2-year OS (p = 1). Patients receiving 1st and 2nd generation TKIs pre- and post-HSCT seem to have favorable outcomes, although type of TKI (pre-HSCT) did not significantly impact EFS or OS. In addition, attaining a CMR pre-transplant improved EFS, but did not change OS.
1. Introduction
Acute lymphoblastic leukemia (ALL) is defined as the presence of ≥20% lymphoblasts in the bone marrow [1]. It typically has a bimodal distribution, primarily affecting young children or adults older than 50 years [2]. In adults with ALL, typically 20-30% of cases have positive Philadelphia chromosome (Ph) [3]. Presence of the Philadelphia chromosome denotes a reciprocal translocation between the long arms of chromosomes 9 and 22 [4]. This cytogenetic aberration not only impacts prognosis and treatment, but its incidence increases with age, up to 50% in adults older than 50 years [4], [5], [6], [7].
Historically, Ph positive ALL patients had a worse 5-year overall survival (OS) rate compared to Ph negative ALL (25% vs 41%, respectively) [8]. Prior to the advent of tyrosine kinase inhibitor (TKI) therapy, 5-year OS rates among adults with disease were ≤ 10-20% [9, 10]. The most significant development in Ph positive ALL management has been the addition of TKIs to front-line therapy. A large retrospective analysis (n = 473) from the European Group for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party analyzed outcomes with TKIs in de novo Ph positive ALL patients in first complete remission (CR1) with a 5-year follow up. TKIs given pre-allogeneic hematopoietic stem cell transplant (HSCT) during induction/consolidation were associated with better overall survival (47% vs 38%) and lower incidence of relapse (33% vs 50%) when compared to no TKIs pre-transplant [11]. Thus, the current standard of care is concurrent chemotherapy with a TKI in patients who are fit enough for intensive therapy [1, [12], [13], [14], [15]]. This is typically followed by allogeneic HSCT for those who are medically eligible and have attained CR1 [16], [17], [18].
Recently, however, there has been controversy about whether HSCT is necessary in all patients, especially in those who attain a deep molecular response after induction therapy [19]. Patients achieving complete molecular response (CMR) with hyper-CVAD and ponatinib alone had a favorable 4-year overall survival (OS) of 66% despite not receiving HSCT after CR1 [20]. Similarly, patients achieving CMR with the combination of dasatinib and steroids had improved 30-month disease-free survival (75%) compared to those without CMR (44%), further raising the question of whether transplant is necessary in all patients with Ph positive ALL [21].
Many studies have shown improvement in overall survival when TKI therapy is combined with conventional chemotherapy [12], [13], [14]. However, relapse and recurrence of disease remain prevalent among Ph positive ALL patients, in part due to development of resistance to earlier generation TKIs [22]. Dasatinib is a second-generation TKI and is a dual inhibitor of both BCR-ABL and Src-family kinases (SRK), the latter of which is a common etiology for imatinib resistance [23]. In vitro, dasatinib is 325 times more potent in inhibiting wild-type BCR-ABL cells compared to imatinib [24]. In addition to its superior resistance profile, dasatinib has also been shown to penetrate the blood brain barrier in the setting of CNS leukemia [25]. When comparing the combination of induction chemotherapy with imatinib or dasatinib, there was not a significant difference in disease-free survival (DFS) or OS at follow-up, though sub-group analysis of 53 patients suggested dasatinib had better DFS and OS among the patients receiving allogeneic-HSCT [26]. Thus, with limited prospective data comparing first and second generation TKIs, the optimal choice of TKI for patients with Ph positive ALL remains unknown.
While the beneficial role of TKIs pre-transplant has been well established, the use of maintenance TKI therapy post-allogeneic stem cell transplant remains poorly defined. Relapse post-HSCT is the most frequent cause of treatment failure, thus raising the possibility that maintenance therapy could be beneficial [27]. A few small, single institution studies have shown improved outcomes with the use of maintenance TKIs post-allogeneic stem cell transplant, though the optimal duration and choice of TKI remain unknown [28], [29], [30]. Retrospective data from MD Anderson Cancer Center has shown that patients who achieved and maintained CMR both before and 3 months following HSCT had higher 2-year progression-free survival (PFS) while on prophylactic TKI therapy post-transplant when compared to no prophylactic TKI therapy (94.5% vs 75%). Additionally, patients who completed 2 years of maintenance TKI therapy post-transplant were found to have a lower risk of relapse (HR 0.12, p = 0.045) compared to those not on maintenance therapy [29].
Recent studies have illustrated the prognostic impact of achieving CMR. It has been shown that achievement of CMR at 3 months led to longer median OS and relapse-free survival among Ph positive ALL patients getting induction chemotherapy and TKI, without HSCT [31]. What remains controversial, however, is whether or not achieving a CMR prior to allogeneic stem cell transplant improves outcomes or if additional therapy should be given to patients who achieve complete hematologic response without CMR in order to achieve a CMR prior to allogeneic stem cell transplant.
To further address these questions, we conducted a single center retrospective study of patients with Ph positive ALL who received tyrosine kinase inhibitors pre and post allogeneic stem cell transplant at the University of Michigan.
2. Materials and methods
2.1. Data collection
A retrospective cohort study was conducted at the University of Michigan. Patients included had Ph positive ALL defined according to pathology review of bone marrow and/or peripheral blood in addition to a BCR/ABL1 rearrangement identified by FISH, karyotype and/or qPCR [32]. Those who received an allogeneic HSCT were selected for analysis. Patients were identified with use of the HeMe CaRe Hematologic Malignancies database and were diagnosed between 2007-2019. Patients with CML blast crisis, concomitant leukemias (such as CLL), mixed lineage leukemia, and those who lacked pre- or post-transplant data for 3 months were excluded from our analysis. Details regarding demographics, cancer cytogenomics, induction and consolidation chemotherapy, remission, allogeneic HSCT characteristics, post-transplant TKI use and duration, and safety outcomes (acute/chronic GVHD, grade 3 GVHD) were extracted from clinical records within the electronic medical records. The study was approved by the Institutional Review Board (IRB) at the University of Michigan Hospital (HUM00153261).
2.2. Chemotherapy regimens
Chemotherapy regimens included hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), rituximab with hyper-CVAD (R-hyper CVAD), steroids alone, modified Berlin‐Frankfurt‐Münster protocol (BFM), vincristine, rituximab, dexamethasone (EWALL), or rituximab, cyclophosphamide, daunorubicin, peg-asparaginase, vincristine (R-LARSON). Prior to allogeneic stem cell transplant, patients either received imatinib (400-800mg/day) or dasatinib (70-140mg/day) along with induction chemotherapy followed by consolidation if necessary prior to transplant.
2.3. Response definitions
Remission definitions were defined according to the Center for International Blood and Marrow Transplant Research (CIBMTR) response criteria [33]. Complete remission/complete remission with incomplete count recovery (CR/CRi) was defined as < 5% blasts in the bone marrow, no evidence of extramedullary disease, and transfusion independence. An absolute neutrophil count (ANC) ≥ 1000 K/uL and platelet count ≥100,000 K/uL was required for achieving a CR, but CR/CRi were viewed as equivalent for the purposes of our analysis. Complete cytogenetic remission was defined as normal karyotype on cytogenetic studies performed on a sample obtained during bone marrow biopsy. CMR was defined as an undetectable level of BCR/ABL1 transcripts from quantitative reverse transcription polymerase chain reaction (RT-PCR) performed on either serum or an aspirate from bone marrow with a sensitivity of 10−4. Relapse was defined as presence of ≥ 5% blasts in bone marrow or peripheral blood, presence of extramedullary disease, or BCR/ABL1 on RT-PCR > 0.1% (derived from molecular relapse definition for CML).
2.4. Transplant regimens
Patients included in our analysis received donated stem cells from multiple sources including matched related donors, matched unrelated donors, haploidentical donors, and cord blood. Conditioning regimens included cyclophosphamide with total body irradiation (CyTBI), busulfan with fludarabine alone (FluBu) or with rituximab (R-FluBu), fludarabine with melphalan (FluMel), fludarabine, cyclophosphamide with TBI (FluCy-TBI), fludarabine with TBI alone (Flu-TBI) or with rituximab (R-FluTBI), and etoposide with TBI. Donor stem cells were obtained from bone marrow or peripheral blood. Our institutional practice is to initiate TKI therapy post-transplant based on blood counts, performance status, and anticipated tolerance between 45 and 100 days post-transplant. TKI therapy is continued for at least 2 years if tolerated and assuming a continued CMR throughout their therapy.
3. Statistical analysis
Descriptive statistics, such as median and range, were calculated for continuous variables while frequencies were calculated for categorical variables. All data were analyzed using SPSS software, version 25.0 (SPSS, Inc., Chicago, IL). Means (standard deviation) were reported for parametric data and medians (range) were reported for non-parametric data. Dichotomous variables were analyzed utilizing Fisher's exact test or Pearson's Chi-square test, where appropriate. Continuous, normally distributed variables were compared using a 2-tailed Student's t-test. Non-normally distributed continuous variables were compared using a Mann–Whitney U test. An alpha-level of 0.05 was considered statistically significant. Time to event analyses for OS and EFS were assessed via the Kaplan–Meier method and compared with a log-rank test. OS was defined as time from diagnosis of Ph-positive ALL until patient death or date of last follow-up (November 4, 2019). Event-free survival (EFS) was defined as time from diagnosis of Ph positive ALL to relapse, death, or date of last follow-up following CR1.
4. Results
4.1. Demographics
Seventy-one patients with Ph positive ALL were identified at the University of Michigan between 2007 and 2019. Of the 71 patients eligible for analysis, 40 patients had allogeneic HSCT. Nineteen of those patients (47.5%) were female. The median age at diagnosis was 54 years (range 24-69), and 10 patients (25%) were 60 years or older. Patient demographics were similar and summarized in Table 1.
Table 1.
Patient and transplant characteristics.
| Total (N) | Imatinib | Dasatinib | P-value | |
|---|---|---|---|---|
| Patients | 40 | 17 | 23 | |
| Median age, years at diagnosis (range) | 54 (24 - 69) | 54 | 52 | |
| Ethnicity | ||||
| White | 38 | 17 | 21 | |
| Black | 2 | 0 | 2 | |
| Other Patient/Disease Characteristics | ||||
| Age ≥ 60 years | 10 | 3 | 7 | |
| Females | 19 | 5 | 14 | 0.0624 |
| CD20 Positive Status | 28 | 9 | 19 | 0.0329 |
| IKZF1 mutation* | 24 | 5 | 11 | 0.6214 |
| Induction regimen | ||||
| Hyper-CVAD +/- R | 29 | 17 | 12 | 0.008 |
| Steroids | 6 | 0 | 6 | 0.0295 |
| Modified BFM | 2 | 0 | 2 | 0.4987 |
| R-LARSON | 1 | 0 | 1 | 1 |
| EWALL | 2 | 0 | 2 | 0.4987 |
| Complete molecular response | 23 | 10 | 13 | 1 |
| Transplant Type | ||||
| MRD | 17 | 9 | 8 | |
| MUD | 12 | 3 | 9 | |
| Mismatched related | 1 | 1 | 0 | |
| Mismatched unrelated | 4 | 1 | 3 | |
| Haploidentical cord blood | 1 | 0 | 1 | |
| Haploidentical PBSC | 3 | 0 | 3 | |
| Cord blood | 2 | 2 | 0 | |
| GVHD | ||||
| Acute GVHD | 25 | 9 | 16 | 0.3355 |
| Chronic GVHD | 31 | 12 | 19 | 0.4561 |
| Grade 3 GVHD | 15 | 10 | 5 | 0.0235 |
*IKZF1 status only assessed in 24 patients
4.2. Transplant characteristics
Matched related donor transplants were performed in 17 patients (42.5%), mismatched related in 1 patient (2.5%), mismatched unrelated in 4 patients (10%), matched unrelated donor transplants in 12 patients (30%), cord blood transplants in 2 patients (5%), haploidentical peripheral blood stem cells in 3 patients (7.5%) and haploidentical unrelated with cord blood in 1 patient (2.5%).
4.3. Disease characteristics
Among patients who had cancer cytogenomic analysis (n=24), 16 patients (67%) had an Ikaros (IKZF1) mutation. Of patients with an Ikaros mutation, majority of them received pre-transplant dasatinib (69%). Of those who had cytogenetics on initial marrow (n = 38), 3 patients were hyperdiploid (51-65). Several patients (n = 12) had a complex karyotype (≥5 abnormalities) and majority of these patients received pre-transplant dasatinib (67%). Of 39 patients, 72% were CD20 positive.
4.4. Treatment regimens
Chemotherapy regimens varied across the cohort as seen in Table 1. Of the patients who received TKI therapy prior to transplant, 23 patients (57.5%) had dasatinib and 17 patients had imatinib (42.5%). Of the CD20 positive patients (72%), only 50% received rituximab.
4.5. Response rates
Prior to transplant, 40 patients (100%) achieved complete morphologic remission (CR or CRi), 38 patients (95%) achieved cytogenetic remission, and 23 patients (57.5%) achieved complete molecular remission. Median time to complete morphologic, cytogenetic, and molecular remission were 32, 38, and 99 days, respectively.
4.6. Survival analysis
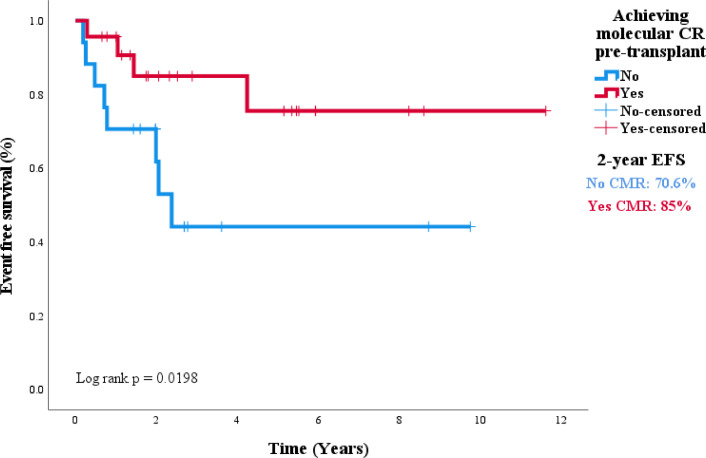
Median follow-up of this study was 1374 days. Median EFS and OS were not reached. The 5-year EFS was 61% (Supplemental Figure 1) and 5-year OS was 71% (Supplemental Figure 2). Thirty patients (75%) were still alive by the time to last follow-up. Of those who relapsed, 36% (4/11) had a CNS relapse and the remaining had molecular relapse or relapse in peripheral blood and/or bone marrow. Notably, 45% of the relapsed cases had mutations in either T315I (4) or E255K (1). When comparing patients who received imatinib vs. dasatinib with induction chemotherapy, 2-year EFS was 76% vs. 76%, respectively (p = 0.16) and 2-year OS was 94% vs 84%, respectively (p = 0.09) as seen in Fig. 1, Fig. 2. Patients with a complete molecular remission pre-transplant had 2-year EFS 85% vs 70.6% in those who did not (p = 0.0198). The 2-year OS was 88% for both those who achieved CMR pre-transplant and those who did not (p = 1) as seen in Fig. 3, Fig. 4. When looking at patients who attained CMR pre-transplant, there was no difference based on induction TKI therapy with imatinib vs dasatinib (p = 1).
Fig. 1.
Event free survival stratified by TKI.
Fig. 2.
Overall survival stratified by TKI.
Fig. 3.
Event free survival stratified by achievement of CMR pre-transplant.
Fig. 4.
Overall survival stratified by achievement of CMR pre-transplant.
4.7. Post-transplant outcomes
Thirty-three patients (82.5%) were initiated on TKI therapy post-transplant. Timing of post-transplant TKI initiation varied based on patients’ blood counts, performance status and tolerance but ranged from 27 to 211 days. Those who did not receive post-transplant TKI were deemed ineligible due to relapse, death, development of mutations, renal failure, or insurance coverage. Median time to TKI initiation post-transplant was 76 days (range: 27-211 days). Median duration of TKI use was 514 days (1.4 years) and range was from 10-1547 days. Maintenance TKI post-HSCT was found at the following frequencies: dasatinib (72.7%), ponatinib (18.2%), and imatinib (9.1%).
The most common reason for TKI discontinuation was side effects including nausea/diarrhea/GI distress, pleural effusions, chest pain, cough, rash, cytopenias, renal failure, and elevated amylase/lipase. Other reasons included issues related to insurance coverage and molecular relapse with new ABL kinase domain mutations. Incidence of acute GVHD and chronic GVHD were 62.5% and 77.5%, respectively. Of those with GVHD, only 15 patients had grade 3 or higher GVHD (skin, GI, oral, lung, or overall). Of note, the rates of acute GVHD or chronic GVHD did not vary with choice of pre-transplant TKI (p = 0.3355, p = 0.4561). However, rates of grade 3 GVHD were higher in the imatinib arm (p = 0.0235).
5. Discussion
We report the results of our single institution retrospective study analyzing 40 Ph positive ALL patients treated with TKI-based therapy who received an allogeneic stem cell transplant between 2007-2019. Consistent with previous reports of improved outcomes for Ph positive ALL patients treated with combined chemotherapy with TKI and allogeneic stem cell transplant, we observed a 5-year EFS of 61% and 5-year OS of 71% [11], [12], [13]. In our cohort, patients received either imatinib (42.5%) or dasatinib (57.5%) with chemotherapy prior to allogeneic stem cell transplant and 82.5% of patients received a post-transplant TKI. Among our cohort, the most common induction regimen was TKI therapy and hyper-CVAD with or without rituximab. We found that the choice of pre-transplant TKI did not significantly impact EFS or OS (Figs. 1, 2). Post-transplant, patients received maintenance TKI with dasatinib (72.7%), ponatinib (18.2%), or imatinib (9.1%). The median duration of maintenance therapy was 1.4 years with a median time to initiation of 76 days (∼2.5 months) post allogeneic stem cell transplant.
In our analysis, 11 patients experienced relapse of their ALL with 36% of relapses occurring in the CNS, specifically leptomeningeal disease. Among patients with CNS relapse, 2 of 4 patients were adequately treated with CNS prophylaxis per guidelines while the other 50% were not. ABL kinase domain mutation testing showed that 45% of patients who relapsed had a mutation: 4 with T315I and 1 with E255K. All of the mutations that were detected occurred pre-transplant and those with T315I mutations were on dasatinib at the time and the patient with an E255K mutation was on imatinib. Of those who relapsed, 4 patients did not receive post-transplant TKI and of those who did, average duration of use was 717 days (range: 36–1547 days). These results further support the need for future prospective investigation of the optimal type and duration of TKI therapy post-transplant.
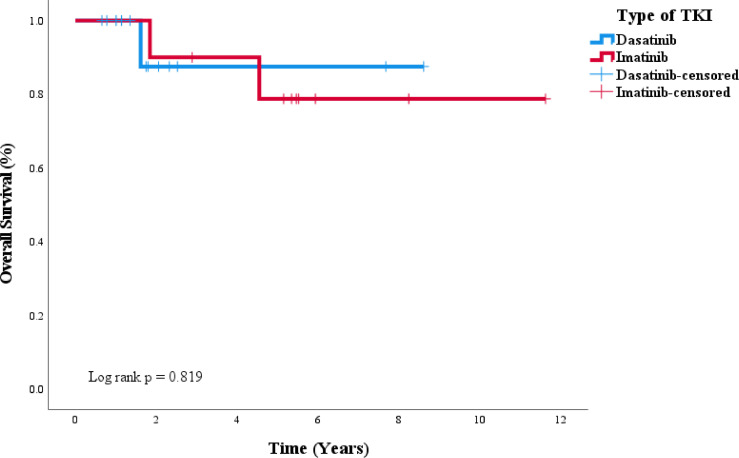
Our patients received a multitude of different conditioning regimens prior to transplant and most commonly received donor stem cells from a matched related donor. As discussed previously, it remains controversial as to whether or not achieving a CMR in addition to the requisite complete hematologic remission prior to allogeneic stem cell transplant portends better outcomes. In our study, patients who achieved CMR pre-transplant had improved EFS, but did not have a statistically significant improvement in OS. We suspect a statistical difference in OS was not seen as the study may have been underpowered to detect such a difference. Additionally, we recognize that pre-transplant and post-transplant TKI often varied as majority of patients received dasatinib post-transplant and this may have impacted survival outcomes as well. Among patients who attained CMR pre-transplant, we found no association when stratified by type of induction TKI (imatinib vs dasatinib), though high risk molecular mutations (IKZF1 and complex karyotypes) seemed to be more enriched in the dasatinib population. Due to the small numbers, we cannot provide meaningful statistical interpretations of how complex karyotype and Ikaros mutation play into the outcomes. In addition, further stratification of patients who achieved CMR pre-transplant by induction TKI therapy showed no difference in mortality outcomes (Fig. 5) (p = 0.819). This data supports our institutional practice of proceeding with allogeneic stem cell transplant in patients who achieve a complete remission (CR/CRi) even with persistent molecular evidence for disease. Lastly, we did not find that rates of acute or chronic GVHD differed between patients who received imatinib or dasatinib prior to transplant. However, rates of G3 or higher GVHD seemed to be more prevalent among patients who received imatinib pre-transplant.
Fig. 5.
Overall survival among patients achieving molecular CR pre-transplant stratified by TKI.
As mentioned previously, post-transplant TKI maintenance has been shown to be beneficial in a few small studies [28], [29], [30], however, in the absence of randomized controlled trials, it is challenging to discern optimal TKI and duration of maintenance. Our institutional practice is to utilize the same TKI used pre-transplant starting around day 60-100 following transplant, presuming quantitative PCR for BCL-ABL is negative. We typically continue treatment for at least 2 years if tolerated.
5.1. Conclusions
In conclusion, we report our experience treating Ph positive ALL patients with combination chemotherapy and TKI, allogeneic stem cell transplant, and maintenance TKI therapy post-transplant. We acknowledge the limitations of our study including its retrospective nature and the small, single institution cohort analyzed. In addition, the small patient population increases the risk of type II error in our statistical analyses and therefore limits the generalizability of our conclusions. Prospective, randomized studies with additional patients are needed to further investigate the optimal TKI therapy (type, dose, and duration) both pre- and post-transplant for Ph positive ALL patients as well as further attempts to stratify patient and disease factors that may be able to predict which patients may be able to defer transplant versus those that may need a more intensive approach.
Funding
This work was supported by the 5T32CA009357-39: Radhika Takiar and Charles Foucar.
Declaration of Competing Interest
The authors report no conflict of interest.
Acknowledgments
Special thanks to Ashley Crouch for assistance in database collection.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2022.100352.
Appendix. Supplementary materials
References
- 1.National Comprehensive Cancer Network. Acute Lymphoblastic Leukemia (Version 2.2020). http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed November 25th, 2020.
- 2.Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clinic. Proc. 2016;91(11):1645–1666. doi: 10.1016/j.mayocp.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, Kantarjian HM, Talpaz M, et al. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91(11):3995–4019. [PubMed] [Google Scholar]
- 4.Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98(7):1337–1354. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister T, Schwartz S, Bartram CR, et al. Patients' age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918–919. doi: 10.1182/blood-2008-04-149286. [DOI] [PubMed] [Google Scholar]
- 6.Larson RA. Management of acute lymphoblastic leukemia in older patients. Semin. Hematol. 2006;43(2):126–133. doi: 10.1053/j.seminhematol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Chiaretti S, Vitale A, Cazzaniga G, et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702–1710. doi: 10.3324/haematol.2012.080432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol. Oncol. Clin. North Am. 2000;14(6):1381–1396. doi: 10.1016/s0889-8588(05)70192-1. x-xi. [DOI] [PubMed] [Google Scholar]
- 10.Gleissner B, Gökbuget N, Bartram CR, et al. German multicenter trials of adult acute lymphoblastic leukemia study group. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5):1536–1543. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 11.Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100:392–399. doi: 10.3324/haematol.2014.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 13.Ravandi F, O'Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158–4164. doi: 10.1002/cncr.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu-Dumlao T, Kantarjian H, Thomas DA, et al. Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr. Oncol. Rep. 2012;14(5):387–394. doi: 10.1007/s11912-012-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 17.Snyder DS, Nademanee AP, O'Donnell MR, et al. Long-term follow-up of 23 patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with allogeneic bone marrow transplant in first complete remission. Leukemia. 1999;13(12):2053–2058. doi: 10.1038/sj.leu.2401589. [DOI] [PubMed] [Google Scholar]
- 18.Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litzow MR. Should anyone with Philadelphia chromosome-positive ALL who is negative for minimal residual disease receive a hematopoietic stem cell transplant in first remission? Best Pract. Res. Clin. Haematol. 2016;29(4):345–350. doi: 10.1016/j.beha.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour E, DerSarkissian M, Duh MS, et al. Efficacy of Ponatinib versus earlier generation tyrosine kinase inhibitors for front-line treatment of newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Clin. Lymphoma Myeloma Leuk. 2018;18(4):257–265. doi: 10.1016/j.clml.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Chiaretti S, Vitale A, Elia L, et al. Multicenter total therapy gimema LAL 1509 protocol for De Novo adult Ph+ acute lymphoblastic leukemia (ALL) patients. updated results and refined genetic-based prognostic stratification. Blood. 2015;126(23) [Google Scholar]
- 22.Leoni V, Biondi A. Tyrosine kinase inhibitors in BCR-ABL positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):295–299. doi: 10.3324/haematol.2015.124016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S. Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk. Lymphoma. 2008;49(1):19–26. doi: 10.1080/10428190701713689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 25.Porkka K, Koskenvesa P, Lundán T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- 26.Yu G, Chen F, Yin C, et al. Upfront treatment with the first and second-generation tyrosine kinase inhibitors in Ph-positive acute lymphoblastic leukemia. Oncotarget. 2017;8(63):107022–107032. doi: 10.18632/oncotarget.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassmann B, Pfeifer H, Stadler M, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106(2):458–463. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 28.Giebel S, Czyz A, Ottmann O, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A position statement of the acute leukemia working party of the European society for blood and marrow transplantation. Cancer. 2016;122(19):2941–2951. doi: 10.1002/cncr.30130. [DOI] [PubMed] [Google Scholar]
- 29.Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 2020;136(15):1786–1789. doi: 10.1182/blood.2019004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang FH, Ling YW, Zhai X, et al. The effect of imatinib therapy on the outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. 2013;18(3):151–157. doi: 10.1179/1607845412Y.0000000052. [DOI] [PubMed] [Google Scholar]
- 31.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoelzer D, Bassan R, Dombret H, et al. ESMO guidelines committee. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(suppl 5):v69–v82. doi: 10.1093/annonc/mdw025. [DOI] [PubMed] [Google Scholar]
- 33.Center for international blood & marrow transplant research. forms instruction manual. ALL Response Criteria http://www.cibmtr.org/manuals/fim/1/en/topic/all-response-criteria.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.