Abstract
Gap junctions (GJs), essential structures for cell-cell communication, are made of two hemichannels (commonly called connexons), one on each adjacent cell. Found in almost all cells, GJs play a pivotal role in many physiological and cellular processes, and have even been linked to the progression of diseases, such as cancer. Modulation of GJs is under investigation as a therapeutic strategy to kill tumor cells. Furthermore, GJs have also been studied for their key role in activating anti-cancer immunity and propagating radiation- and oxidative stress-induced cell death to neighboring cells, a process known as the bystander effect. While, gap junction (GJ)-based therapeutic strategies are being developed, one major challenge has been the paradoxical role of GJs in both tumor progression and suppression, based on GJ composition, cancer factors, and tumoral context. Therefore, understanding the mechanisms of action, regulation, and the dual characteristics of GJs in cancer is critical for developing effective therapeutics. In this review, we provide an overview of the current understanding of GJs structure, function, and paradoxical pro- and anti-tumoral role in cancer. We also discuss the treatment strategies to target these GJs properties for anti-cancer responses, via modulation of GJ function.
Keywords: Gap junctions, Anti-cancer immunity, Oxidative stress, Bystander effect
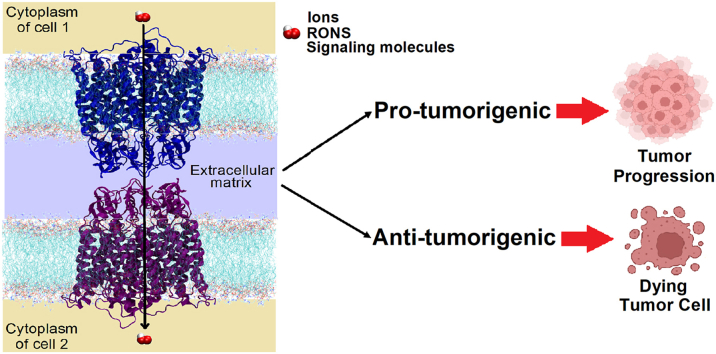
Graphical abstract
Graphical representation of the pro- and anti-tumorigenic properties of gap junction proteins in cancer cells.
Highlights
-
•
GJs can act as tumor suppressors and tumor promoters in cancer cells, depending on GJs, cancer, and tumor factors.
-
•
Inhibition of GJs can inhibit the pro-tumorigenic property of GJs.
-
•
Modulation of GJs can assist the activation of anti-cancer immunity, the propagation of cell death, and the oxidative stress-mediated cell death.
Abbreviations
- GJ
Gap junction
- Ca2+
Calcium ions
- Ag
Antigen
- DC
Dendritic cell
- CD8+
Cluster of differentiation 8+
- NK
Natural killer
- cGAMP
Cyclic guanosine monophosphate–adenosine monophosphate
- GrzmB
Granzyme B
- GJIC
Gap junction intercellular communication
- RONS
Reactive oxygen and nitrogen species
- PDT
Photodynamic therapy
- NTP
Non-thermal plasma
- Cx
Connexin
- TM
Transmembrane
- EL-1
Extracellular loop 1
- EL-2
Extracellular loop 2
- CL
Cytoplasmic loop
- NT
Amino terminus
- CT
Carboxyl terminus
- NAD+
Nicotinamide adenine dinucleotide
- ATP
Adenosine triphosphate
- MD
Molecular dynamics
- IP3
Inositol 1,4,5-trisphosphate
- cAMP
Cyclic adenosine monophosphate
- miR-125b
MicroRNA-125b
- Cx26-GJs
Cx26 proteins-composed GJs
- Cx32-GJs
Cx32 proteins-composed GJs
- SCC
Squamous cell carcinoma
- Cx43-GJs
Cx43 proteins-composed GJs
- LUAD
lung adenocarcinoma
- G1
Gap 1
- S
Synthesis
- G2
Gap 2
- HCC
Hepatocellular carcinoma
- EMT
Epithelial-to-mesenchymal transition
- KIRC
kidney renal clear cell carcinoma
- miRNAs
MicroRNAs
- αCT1
Alpha connexin carboxy-terminus 1
- IS
Immunological synapse
- APCs
Antigen presenting cells
- CTL
Cytotoxic T lymphocyte
1. Introduction
Gap junctions (GJs) are protein channels that enable direct intercellular communication (Fig. 1) [1], thus allowing cells to exchange signals and molecules directly from the inside of one cell to a neighboring cell. As such, they provide an essential way for the maintenance of physiological functions, e.g., cell growth, differentiation, homeostasis [2], angiogenesis [3], neural migration [4], and stem cell development [5]. Recently, the importance of GJs for disease induction and progression is becoming more appreciated, especially in the context of oncology, and is therefore seen as a novel target for therapy development. Studies into solid tumor cells revealed a lack of communication through GJs in certain tumor types, resulting in abnormal cell growth [[6], [7], [8]]. Moreover, restoration of gap junction intercellular communication (GJIC) in tumor cell lines reduced tumor growth and proliferation [[9], [10], [11]], suggesting that GJs have anti-tumorigenic properties. However, interestingly, GJIC was also able to facilitate the sharing of cancer cell metabolites with normal (healthy) cells, which in turn led to a gain of malignant properties in normal cells [12,13]. These reports suggest a pro-tumorigenic role of GJs properties. Hence, the current understanding of GJs in cancer cells is paradoxical, as GJs have both tumor-suppressing and promoting properties which depend on gap junction (GJ) type, cancer stage, and tumoral factors [14]. In fact, GJs are often reduced or lost completely in early cancer stages and upregulated in later stages and metastatic lesions, which contribute to tumor aggressiveness [14,15]. Therefore, therapeutic strategies to enhance GJs in early tumor development [[16], [17], [18]] or to inhibit them in advanced stages [[19], [20], [21]] have emerged.
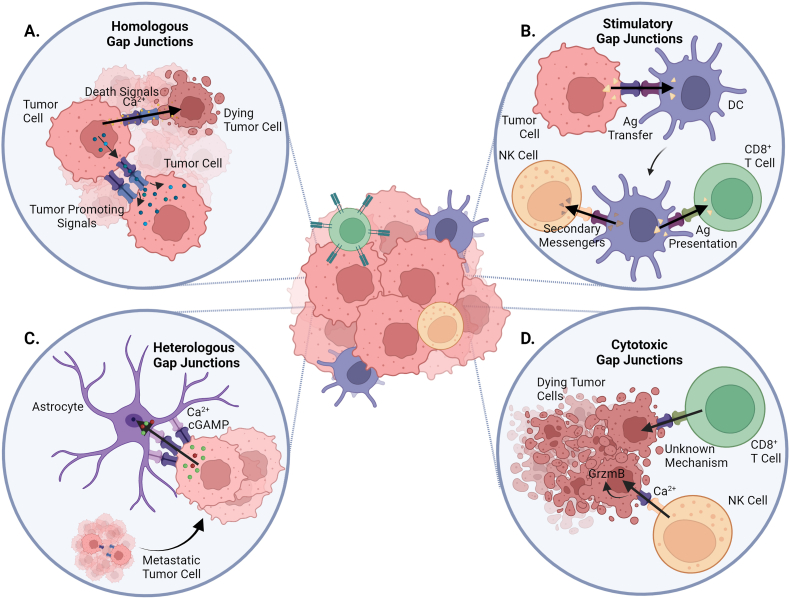
Fig. 1.
Schematic representation of GJs and effector functions between different cell types. GJs are key mediators of intercellular communication. Moreover, a previously underestimated role of GJs in alternative pathways for immune regulation and activation has been recently described (see section 6 for explanation). (A) Tumor cell transfers tumor promoting signals to another tumor cell via homologous GJs, increasing tumoral effects. Besides, homologous GJs also transfer death signals (Ca2+) between tumor cells, inducing tumor cell death. (B) Tumor cell transfers Ag peptides to DC via GJs, leading to antigen cross-presentation by DC. Further, DC presents Ag peptides and transfers secondary messengers to CD8+ T cell and NK cell, respectively, killing target cells by cytotoxic lymphocytes. (C) Metastatic tumor cell transfers Ca2+ and cGAMP to astrocyte via heterologous GJs, inducing further tumor spreading and therapy resistance. (D) NK cell transfers Ca2+ to tumor cells via Cx43 GJs, for induction of GrzmB-mediated cell death. A rise in the intracellular Ca2+ concentration in the target cell is needed for efficient killing by cytotoxic T lymphocytes or NK cells.
Peptides [17,22,23], antibodies [24,25], and chemotherapeutic agents [26,27] have been used to inhibit GJ functions in cancer cells, targeting the pro-tumorigenic properties of GJs. They have been proven useful to restore the sensitivity of cancer cells to cytotoxic drugs [28] and reduce tumor growth [24]. On the other hand, GJs have also been explored to kill cancerous cells by stimulating their anti-tumorigenic property. Strategies that leverage GJs ability to facilitate the transport of reactive oxygen and nitrogen species (RONS) between adjacent cells can cause cellular stress and death via oxidative damage to proteins and membrane lipids [[29], [30], [31]]. An increasing body of experimental evidence demonstrates that oxidative stress modulates channel gating (pore opening/closing) of GJs, and that in turn facilitates the traffic of RONS to the cell interior [32,33]. Therefore, oxidative stress-inducing therapies as novel anti-cancer modality for modulating GJs, are gaining attention, which include photodynamic therapy (PDT) [34,35] and non-thermal plasma (NTP) [36,37].
GJs have been shown to propagate oxidative stress-induced cell death [38,39], apoptotic cell death [40,41], and radiation-induced cell death [42,43] in cancer cells. This phenomenon is named the “bystander effect”, and refers to the transmission of responses from cells exposed to certain stimuli, to non-targeted neighboring or more distant cells by means of intercellular communication. Therefore, the development of therapeutic strategies to improve this propagation can contribute to tumor suppression in cancer cells. Furthermore, GJs are also able to transport antigenic peptides between cancer cells and dendritic cells (DCs), which supports activation and tumor-specific killing by cytotoxic lymphocytes [44,45]. Modulation of GJs is therefore also an emerging target in immunotherapeutic research.
However, a better understanding of GJ structures and their function are required to elucidate in which conditions GJs act as pro- or anti-tumorigenic agents. Here, computer simulations can be a powerful tool, to investigate the interaction mechanisms at the atomic and molecular level. Moreover, computer simulations can also provide insights into the structural and dynamic properties of GJs, which is sometimes inaccessible with experimental methods. For these simulations to accurately represent the physiological conditions, a detailed understanding of GJ structures is needed. In this way, we can design better GJ promoters or inhibitors for improved cancer treatment.
In this review, we provide an overview of the in-depth knowledge on the structure and functions of GJs, and cover the current understanding of their paradoxical pro- and anti-tumoral properties, together with the therapeutic opportunities of GJ function modulation.
2. GJs structure and composition
The first time that GJs were isolated and characterized by X-ray diffraction analysis was in 1972 [46], and since then, it is known that three families of GJs proteins make up this structure: innexins, pannexins, and connexins [47,48]. Of these three, the connexin (Cx) family, first described in 1974 [49], is the most abundant in vertebrate animals [50].
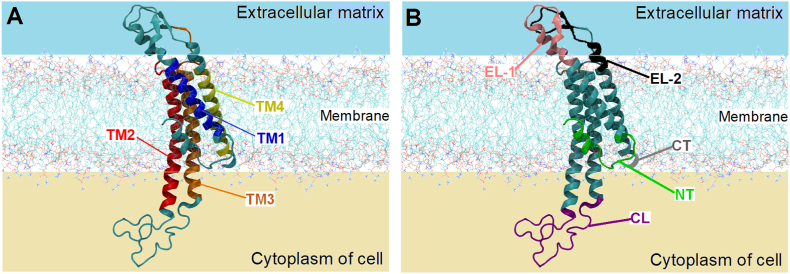
The human Cx protein family contains 21 members, named according to their relative molecular mass [51,52]. Each Cx is a transmembrane (TM) protein with four TM domains in the α-helical conformation (TM1 to TM4) (Fig. 2A). These domains are connected by two extracellular loops (EL-1 and EL-2) and one cytoplasmic loop (CL), containing an amino (NT) and carboxyl terminus (CT) in the cytoplasm (Fig. 2B) [53]. Whereas the size of the CT domain is the major determinant of Cx molecular mass, which can range from 23 to 62 kDa, the NT domain is of similar length in all Cxs (first 22–23 amino acids), with their first part present in an α-helical conformation. Although present on almost all human cells [54,55], Cx isotype expression is usually restricted to a certain organ, tissue, or cell type. However, certain Cx proteins, such as Cx43, are more ubiquitously expressed.
Fig. 2.
Schematic representation of a Cx43 protein in a lipid bilayer membrane. (A) The transmembrane domains TM1, TM2, TM3, and TM4 are represented in blue, red, orange and yellow colors, respectively. (B) The extracellular (EL-1 and EL-2) and cytoplasmic (CL) loops are represented in pink, black, and purple colors, respectively. The amino (NT) and carboxyl (CT) terminus are represented in green and gray colors, respectively. For the sake of clarity, only a small part of the CT domain is represented. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
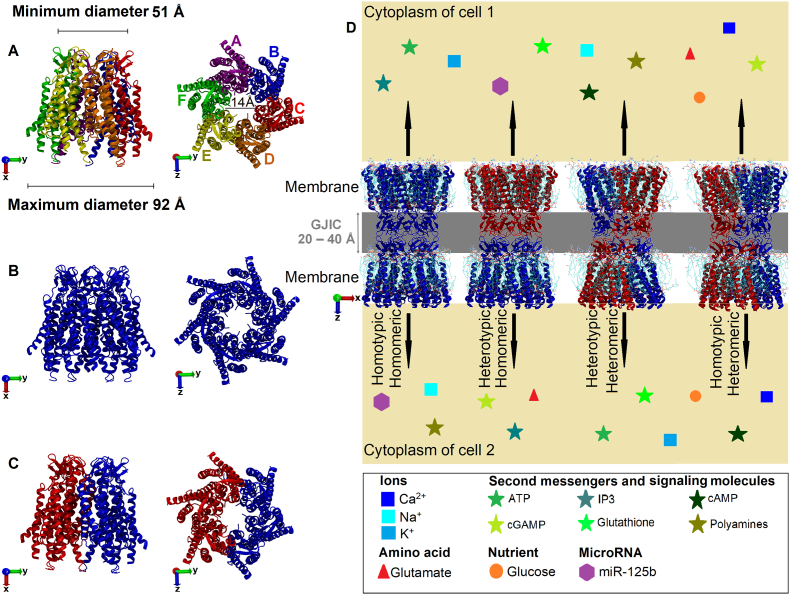
Cx proteins are able to come together to form a large diversity of strictly organized assemblies. For instance, six Cx protein monomers oligomerize to form a hemichannel usually referred to as “connexon” (Fig. 3A), i.e., a hydrophilic pore that enables the passage of molecules for direct cytoplasm-to-extracellular communication [51,56]. Connexons are normally closed, but when activated, they can release autocrine and paracrine signals, such as nicotinamide adenine dinucleotide (NAD+), glutamate, and adenosine triphosphate (ATP). These signals can affect cell proliferation and survival [57]. The NT domain of a Cx is critical for holding the connexon open, and the pore diameter can differ depending on the Cx they are composed of. For example, a connexon made up of Cx26 proteins has a pore diameter of ∼10 Å, as estimated by molecular dynamics (MD) simulations [58] and ∼14 Å by crystallography [59] (Fig. 3A). Likewise, a connexon made up of Cx46 or Cx50 proteins has a pore diameter of ∼14 Å, as visualized with single-particle cryo-electron microscopy [60].
Fig. 3.
Representations of the 3D structure of Cx26 proteins-composed connexons. The Cx26 structure can be obtained from the Protein Data Bank website (https://www.rcsb.org/) (accession no. 2ZW3). Side view (on the left) and top view (on the right). (A) Each color represents a Cx monomer. (B) Homomeric connexon. (C) Heteromeric connexon. (D) Schematic representation of GJIC (or only GJ) between Cx46 proteins-composed connexons (4 possibilities are shown). The Cx46 structure can be obtained from the Protein Data Bank website (https://www.rcsb.org/) (accession no. 6MHQ). Exchange of possible types of ions, amino acids, secondary messengers, cancer-associated signaling molecules, nutrients and microRNAs between two cells is illustrated as geometrical shapes of different colors. For simplicity only a few examples for each class are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Once at the plasma membrane, two opposing connexons from adjacent cells can interact with each other to form a GJ channel, facilitating GJIC. Depending on the type of GJIC, various biophysical properties will be impacted [61,62]. The GJIC can be either homomeric, i.e., when they are composed of Cxs within the same class (Fig. 3B), or heteromeric, i.e., when they are composed of Cxs from different classes (Fig. 3C). Thus, GJIC could be classified as (1) homotypic-homomeric, which consists of two identical connexons formed by only one class of Cx; (2) heterotypic-homomeric, which consists of different connexons, each one formed of the same class of Cx; (3) heterotypic-heteromeric, which consists of different connexons, each one formed with two or more non-symmetric isotypes of Cxs; (4) homotypic-heteromeric, which consists of identical connexons, both similar formed with two or more non-symmetric isotypes of Cxs (Fig. 3D).
It has already been reported that homotypic and heterotypic GJIC exhibit different electrical potentials between the inside and outside (Vi‒o) of the cells and different voltage differences between the interiors of communicating cells, known as the trans-junctional voltage (Vj). For example, homotypic Cx26 proteins-composed GJs (Cx26-GJs) are sensitive to both Vi‒o and Vj, while homotypic Cx32 proteins-composed GJs (Cx32-GJs) are sensitive to only Vi‒o [63]. Conversely, heterotypic Cx26/Cx32 proteins-composed GJs (Cx26/Cx32-GJs) are more sensitive to Vj than to Vi‒o [63]. These changes in electrical properties suggest that there are differences between the molecular conformations of connexons that depend on the Cx type-composed connexons. Interestingly, changes on GJs electrical properties have been linked to pathological conditions [64].
The GJ is stabilized by non-covalent interactions via H-bonds between EL-1 and EL-2 of their Cxs, which are responsible for docking processes [65,66], while the NT domain participates in the oligomerization, trafficking, and channel gating of Cxs [67,68]. The CL and CT domains are responsible for specific channel properties of different Cxs, including unitary conductance, pH and voltage dependence, and selective permeability to small molecules (<∼1.2 kDa) [69,70] (Fig. 3D). In addition, the CT domain plays a role in the phosphorylation of some Cxs (e.g. Cx43 and Cx47 proteins) [71,72] and regulates intercellular Ca2+ flow [73,74], cell growth, and cell mobility. Recently, Ray and Mehta demonstrated experimentally the importance of the two CT cysteine residues of Cx32 proteins in regulating the trafficking and stability of Cx32 proteins from the endoplasmic reticulum to the Golgi apparatus, and hence its ability to assemble into GJs [75].
Structural, electrical and dynamic properties of GJs channel can facilitate the exchange of various molecules such as RONS, ions, and different cytokines between adjacent cells [[29], [30], [31]] (Fig. 3D), but these properties can be altered during malignant cellular transformations.
3. Pro-tumorigenic properties of Cxs and GJs
An increasing body of experimental work has demonstrated the pro-tumorigenic properties of Cxs and GJs in normal and cancer cells (Fig. 1A). Several Cx subtypes (e.g. Cx26, Cx32, and Cx43) are linked to malignant transformation and tumor progression, mainly in the advanced cancer stages. Increased expression of Cx26 proteins was found in lung squamous cell carcinomas (SCC), which facilitated invasion and metastasis [76]. The authors found that Cx26-positive lung SCC cells were specifically located facing the tumor stroma or fibrous capsule, and the ratio of Cx26-positive over Cx26-negative cancer cells was significantly higher in metastatic lesions, compared to the corresponding primary tumor. Moreover, in these cancer cells, Cx26 proteins were preferentially localized on the plasma membrane, and could form Cx26-GJs between lung SCC cells and normal lung cells [76]. This heterologous communication between malignant and normal cells via GJs was also reported by others; Zhang et al. demonstrated dye transfer between lung cancer cells and normal lung fibroblasts via GJs formation. GJs formation allowed the sharing of metabolites to initiate metastasis, although coupling levels may need to exceed a certain threshold to allow propagation of signals over a sufficient distance to affect the behavior of a cell population [77]. Likewise, more in-depth studies detected coupling between melanoma and endothelial cells via homologous [78] and heterologous [79] Cx26-GJs, which contributed to the intravasation and extravasation of melanoma cells during the metastatic process [78,79]. Conversely, inhibition of Cx26 proteins rendered the tumor cells deficient in Cx26-GJs formation and reduced their metastatic potential [79].
Overexpression of Cx32 proteins in normal and metastatic breast cancer cells led to a more mesenchymal-like phenotype [80]. Adak and co-workers reported an increased migratory capacity of healthy breast cells, while mesenchymal markers, including vimentin, were further upregulated in the metastatic counterpart, thus presenting, for the first time, the metastasis-stimulating properties of the Cx32 protein in breast cancer [80]. Heterologous Cx43 protein-composed GJs (Cx43-GJs) have also been linked to the initiation of brain metastatic lesions from both melanoma and breast cancer. Depletion of Cx43 proteins or pharmacological blocking of the Cx43-GJ coupling inhibited brain colonization via blocking of tumor cell extravasation and blood vessel co-option, a non-angiogenic mechanism of tumor vascularization in which cancer cells utilize pre-existing blood vessels instead of inducing new blood vessel formation [81]. Taken together, these results suggest an important pro-tumoral role of Cxs in advanced cancer stages, and more specifically points at GJ formation between cancer and normal cells as an important facilitator of cancer cells growth, tumor progression and metastasis.
In addition to cancer stage, Aasen et al. dictate the importance of Cx isotype and histological tumor subtypes for pro-tumorigenic properties of GJs and patient prognosis [82]. The team compared the mRNA expression levels of different Cxs between healthy lung tissue and lung tumors, and found that Cx26, Cx30.3, and Cx30 proteins were upregulated in lung adenocarcinoma (LUAD) and lung SCC. However, Cx32 proteins were slightly upregulated in LUAD and downregulated in lung SCC, thus highlighting the importance of Cx isotype and cancer subtype-specific [82]. Yamasaki et al. also observed that in many tumor cells, Cxs were normally expressed but aberrantly localized, possibly due to a lack of an appropriate cell-cell recognition apparatus, and phosphorylation of Cxs [83]. Cx phosphorylation is described to influence GJs formation, connexon function, and GJs/Cxs degradation [84]. Thus, the pro-tumorigenic properties of Cxs do not only depend on the pathological overexpression of specific Cx isotypes and cancer stage, but also on tumor subtype and Cxs delocalization and phosphorylation.
It is critical to acknowledge that the role of Cxs and GJs in cancer development and progression is highly complex, nuanced, and far from being fully understood. A comprehensive view must be taken to develop strategies that modulate GJs during cancer metastasis, and in-depth studies are required to reveal under which conditions Cxs and GJs may act as tumor promoter.
4. Anti-tumorigenic properties of Cxs and GJs
Apart from the pro-tumorigenic properties, studies have demonstrated that Cxs and GJs can also have anti-tumorigenic properties in specific structural and tumoral context. Transfection of Cx30 proteins into rat glioma cell lines, which have lost their Cx expression, reduced tumor cell growth and proliferation [85]. Likewise, transfection of Cx26 proteins into human hepatoma cells [86] and breast cancer cells [87] reduced their malignant potential, by inhibiting dedifferentiation, suppressing cell proliferation and tumor growth, and inducing apoptosis [86,87]. Mesnil et al. reported that out of several transfected Cx subtypes (e.g. Cx26, Cx40, Cx43), only the Cx26 proteins inhibited tumor growth and proliferation in HeLa cells, both in vitro and in vivo [88]. Moreover, different Cx species or combinations of them are expressed in different tumor types, suggesting that Cxs have cancer type-specific roles. For example, Cx43 proteins had no effect on the proliferation of tumorigenic rat insulinoma cells [89], while they were able to reduce tumor growth and proliferation in human breast cancer tumors [90]. Taken together, these results suggest that Cxs can also act as tumor suppressors, dependent on Cx isotype, Cx expression levels, and cancer type.
Interestingly, GJs in solid tumors are frequently decreased or missing in different cell populations within a tumor, suggesting that the loss of GJ coupling and may be another characteristic of malignant transformation. Jamakosmanovic and Loewenstein observed that the electrical coupling found in normal thyroid cells was lost in thyroid cancer [91]. This phenomenon was also observed in cancerous and non-cancerous epithelial cells [92]. These results suggest that inhibition of intercellular communication through GJs in cancer cells likely affect tissue growth and differentiation. Restoration of GJs have therefore, resulted in a decrease in tumor progression. Daniel-Wójcik et al. demonstrated a direct link between Cx43-GJ coupling intensity and cellular motility, a crucial aspect in cancer progression. An increase in Cx43 proteins at the cell-to-cell contact site and an enhancement of Cx43-GJs inhibited cancer cell motility in both prostate carcinoma and melanoma cells [93]. Another aspect to consider is that Cx-mediated tumor growth suppression can occur in a manner independent of GJ formation. Although Cx26, Cx40, and Cx43 protein-associated GJs increased in HeLa cells, only Cx26 proteins inhibited tumor growth and proliferation [88]. The authors observed that all three transfected Cxs communicated to a similar extent via GJ coupling, so the difference in their tumorigenicity may be related, at least in part, to the pattern of Cxs localization in the cells [88]. Altogether, these results demonstrated that GJs also have anti-tumorigenic properties, GJs-independent mechanisms of tumor suppression must be considered in order to find adequate therapeutic targets.
Tumor-suppressing properties are also described to be specific to Cx subtypes. Sirnes et al. demonstrated a mechanism by which Cx43 proteins specifically were able to reduce tumor growth and induce apoptosis in colon cancer cells. Cx43 proteins were found to be partly colocalized with ꞵ-catenin at the plasma membrane and inhibited Wnt signaling [94]. Wnt signaling has a key role in disease development and deregulation of the pathway connected to cancer and metastasis [95]. ꞵ-catenin is one of the proteins which regulate Wnt signaling [95], and thus inhibition of Wnt signaling by ꞵ-catenin targeting via Cx43 upregulation may suppress tumor development. Another mechanism by which Cxs and GJs may act as tumor suppressor has been proposed for the Cx37 subtype. Burt and co-workers reported that Cx37 protein expression suppressed cell proliferation in tumorigenic rat insulinoma cells by significantly extending the duration of some phases of the cell cycle (gap 1 (G1), synthesis (S), and gap 2 (G2)) and accumulating cells at the G1/S checkpoint [89]. Since cancer cells affect the normal interphase processes [96], extension of several cell cycle phases may slow down the progression of malignant cells. In addition, cell proliferation was also suppressed via the CT domain and pore-forming domains of Cx37 proteins, independently of connexon or GJ formation [97]. Therefore, Cx37 proteins can suppress tumor proliferation and growth by different mechanisms, such as affecting the interphase processes of the cell cycle and modulation of both the CT domain and pore-forming domains of Cx37 proteins. Lastly, the Cx32 protein also demonstrated tumoricidal effects in human gastric cancer cells, inhibiting cancer cell proliferation through G1 phase arrest and upregulation of p21 (Cip1) and p27 (Kip1) proteins, i.e., stoichiometric cyclin-dependent kinase inhibitors [98]. Yang et al. also reported that the Cx32 protein inhibits the highly-invasive malignant phenotype of hepatocellular carcinoma (HCC) both in vitro and in vivo by negatively regulating epithelial-to-mesenchymal transition (EMT), via downregulation of Snail signaling through the Wnt/ꞵ-catenin pathway [99]. EMT refers to the transformation to a more mesenchymal-like phenotypic, resulting in increased cellular motility and invasiveness. Since EMT plays a crucial role in liver cancer invasion and metastasis [100], negative regulation of EMT by Cx32 proteins is very appealing to inhibit cancer progression in HCC. Thus, Cx32 proteins can suppress cancer proliferation by affecting the G1 phase of cell cycle, upregulation of p21 and p27 proteins, and downregulation of the EMT.
Taken together, these findings support the growing concept that Cxs and GJs have several significant tumor-suppressive functions, but also highlight that the GJ-mediated tumor-suppressive function depends on Cx isotypes and cancer type. In fact, higher Cx expression levels are linked to a better cancer prognosis, while lower Cx expression levels correspond to a worse prognosis. This is summarized in Table 1 for various Cx up- and down regulations and various cancer types, based on available literature.
Table 1.
Relationship between Cx expression and cancer prognosis. Arrow direction corresponds to up- or down-regulation of Cxs. p-values correspond to the probability of obtaining that prognosis result.
| Connexin (gene) | Cancer type | Prognosis | p-value | Type of test |
|---|---|---|---|---|
| Cx26 (GJB2) ↓ | Breasta | Unfavorable | <1.00 × 10−4 | Mice |
| Gliomab | Unfavorable | 4.45 × 10−4 | Clinical | |
| Lungb | Unfavorable | 6.25 × 10−4 | Clinical | |
| Pancreaticb | Unfavorable | 2.90 × 10−4 | Clinical | |
| Renalb | Unfavorable | 2.53 × 10−6 | Clinical | |
| Cx31 (GJB3) ↓ | LUADb | Unfavorable | 3.54 × 10−8 | Clinical |
| Lungb | Unfavorable | 4.56 × 10−4 | Clinical | |
| Pancreaticb | Unfavorable | 7.56 × 10−5 | Clinical | |
| Cx32 (GJB1) ↑ | KIRCb | Favorable | 4.88 × 10−8 | Clinical |
| Renalb | Favorable | 2.85 × 10−6 | Clinical | |
| Lungc | Favorable | ≤5.00 × 10−2 | Mice | |
| Cx32 (GJB1) ↓ | HCCd | Unfavorable | <5.00 × 10−2 | Clinical |
| Intestinale | Unfavorable | 6.60 × 10−2 | Mice | |
| Liverf | Unfavorable | <5.00 × 10−2 | Mice | |
| Ovariang | Unfavorable | <5.00 × 10−2 | Clinical | |
| Cx43 (GJA1) ↑ | Bladderh | Favorable | 1.00 × 10−3 | Clinical |
| KIRCb | Favorable | 2.60 × 10−4 | Clinical | |
| Melanomai | Favorable | 5.00 × 10−2 | Mice | |
| Cx43 (GJA1) ↓ | Bone metastasesj | Unfavorable | 1.00 × 10−8 | Mice |
| Colorectalk | Unfavorable | <5.00 × 10−2 | Mice | |
| Gliomal | Unfavorable | Not available | Clinical | |
| LUADm | Unfavorable | 2.00 × 10−2 | Mice | |
| Lungn | Unfavorable | 3.50 × 10−2 | Clinical | |
| Stomachb | Unfavorable | 4.99 × 10−5 | Clinical | |
| Cx45 (GJC1) ↓ | Renalb | Unfavorable | 3.55 × 10−8 | Clinical |
| Urothelialb | Unfavorable | 7.83 × 10−4 | Clinical |
One of the mechanisms to explain why Cx expression and GJs are reduced in cancer cells is based on alterations in the activity of post-transcriptional factors of Cx-targeting microRNAs (miRNAs). Fukuda and co-workers reported for the first time the capability of miRNAs, produced by normal cells, to attenuate tumor progression after transfer to malignant cells through a Cx-dependent mechanism [115]. Another explanation for the depletion of Cxs in cancer cells is based on transcriptional and post-translational modifications, such as phosphorylation, acetylation and palmitoylation [116], and methylation [117]. It was demonstrated that many growth factors, oncogenes, and tumor-promoting chemicals were potent inducers of Cx phosphorylation, which is often associated with the inhibition of GJs [84].
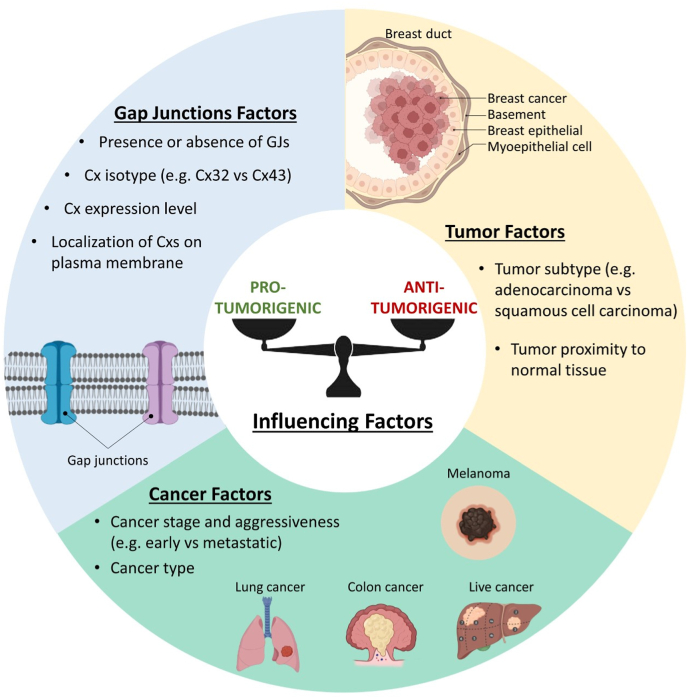
The mechanism underlying the tumor-suppressive functions of Cxs and GJs are highly complex, involving several properties intrinsic to Cxs proteins and other proteins surrounding it. Different electrical potential of GJIC, pore diameters in the connexons, and post-translational modifications of the Cxs conformations may also be responsible for changes in anti-tumorigenic properties of GJs. Such changes must be considered at both cellular and molecular levels for novel therapy development. To summarize, Cxs and GJs can act as pro- and anti-tumorigenic agents, depending on many factors such as GJs, cancer and tumor (Fig. 4).
Fig. 4.
Factors influencing the tumor-promoting and tumor-suppressing properties of Cxs and GJs.
5. Therapeutic strategies applied to Cxs and GJs
Novel combination strategies to restore GJs and Cx properties as tumor-suppressors in early cancer stages, or inhibit these structures in advanced stages when they display a tumor-supportive role have been studied to improve standard-of-care treatments such as chemotherapy and radiotherapy.
While chemotherapy is limited by drug toxicity and development of therapy resistance [118], novel multi-modal approaches to improve treatment response and tolerance are under investigation. Herein, stimulation of Cxs and GJs expression, via gene therapy, has been explored to potentialize anti-cancer drug activity in cancer cells. For instance, Cx43 gene therapy, in which the Cx43 gene is transfected into target cells to promote expression, has been demonstrated to increase cell sensitivity to several chemotherapeutics agents, in different cancer types [16,119,120]. In prostate cancer, the combination of Cx43-expressing plasmid DNA and the chemotherapeutic agent docetaxel had significantly stronger anti-cancer effects compared to docetaxel alone, both in vitro and in vivo [16]. Notably, transfection of Cx43 proteins into the cells without docetaxel neither inhibited tumor growth nor increased GJs. However, combination therapy of Cx43 protein upregulation and docetaxel significantly inhibited cell growth and induced apoptotic cell death by downregulation of Bcl-2 expression, a protein that regulates cell apoptosis; and upregulation of caspase-3 activity, a protein which induces apoptosis, compared to docetaxel alone [16]. Later, gene therapy in colorectal cancer showed that Cx43 protein upregulation improves sensitivity for the chemotherapeutic drug paclitaxel. Transfection of Cx43 into the cells increased GJ function and subsequently the mitotic arrest, tubulin polymerization, and apoptotic effects of paclitaxel, compared to cells treated with paclitaxel or Cx43 proteins alone [119]. Cell death was induced by activation of the caspase-3 apoptotic pathway [119]. More recently, similar results were found in breast cancer, in which Cx43 overexpression could attenuate EMT and improve the sensitivity of cancer cells to the chemotherapeutic drug tamoxifen [120]. EMT is an important event to confer tamoxifen resistance, and overexpression of Cx43 proteins was sufficient to inhibit TGF-β1-induced EMT activation, and to retard PI3K/Akt activation, a signaling pathway that plays a vital role in initiating EMT and drug resistance in different malignancies [120]. Altogether, these results show that enhancing the tumor-suppressive functions of Cx43 proteins and GJs has the potential to be combined with chemotherapeutic agents in order to overcome chemoresistance.
Due to the paradoxical anti- and pro-tumorigenic role of Cxs and GJs in cancer cells, therapeutic strategies to inhibit Cxs and GJs when they may act as tumor promoter have also emerged [20,21]. Considering that the CT domain of Cxs plays a pivotal role in the regulation of GJ function [22,121,122], manipulation of its secondary structure can help in the regulation of GJ function. For instance, peptides that mimic the CT domain of Cxs have been used to block GJs function [22,23,28]. An example of a clinically tested therapeutic peptide is the alpha connexin carboxy-terminus 1 (αCT1), a selective inhibitor of Cx43-GJs that mimics the CT domain of Cx43 proteins. Administration of αCT1 restored the sensitivity of resistant glioblastoma cells to temozolomide chemotherapy [28]. The combination of αCT1 and temozolomide induced autophagy and apoptosis in those tumor cells, through attenuation of AkT/MTOR activity, signaling pathway known to induce temozolomide-resistance [28]. Due to the tumor-sensitizing capacities, multiple cell-penetrating mimetic peptides targeting different Cx domains and Cx types are currently developed in an attempt to improve remaining shortcomings, like target specificity and selectivity [123]. Once these problems are overcome, Cx manipulation - and in specific Cx43 proteins - via mimetic peptides is a very promising combination strategy for tumoral management with clinical applications.
The use of Cxs-targeting antibodies has been another strategy to inhibit pathological GJ function and improve cancer therapies. Monoclonal antibodies to the EL-2 loop of Cx43 proteins (MAbE2Cx43) are intensively studied for human glioblastoma. Using a human glioblastoma rat model, MAbE2Cx43 monotherapy led to significant tumor reduction and prolonged animal survival, presumably via inhibition of specific functions of Cx43 proteins in the peritumoral zone [24]. Treatment with MAbE2Cx43 in combination with radiotherapy further inhibited tumor development and prolonged the median survival, likely due to the increase in blood-brain barrier permeability for antibodies after irradiation of the brain and inhibition of migration and/or signaling pathways [18]. Interestingly, MAbE2Cx43-temozolomide combination therapy attenuated the tumor-suppressive activity of both monotherapies. Since a portion of the cytotoxic drugs penetrate into the cell through connexon gating, MAbE2Cx43 binding and blocking of Cx43-GJ formation could affect the permeation of drugs like temozolomide into the cells [18]. These results highlight that combinatorial strategies using antibodies can be used to improve standard-of-care therapies like chemotherapy and radiation, however competitive inhibition of binding sites by MAbE2Cx43 needs to be circumvented to overcome antagonistic treatment effects.
Heterologous GJs established between cancer cells and healthy cell populations are reported to promote tumor spreading and treatment resistance, making them interesting targets for therapeutic intervention (Fig. 1C, see figure caption for more details) [26,124]. Chen et al. demonstrated that breast and lung cancer cells were able to establish Cx43-GJs with astrocytes, promoting brain metastasis. Once the heterologous GJs were formed, cancer cells transferred the secondary messenger cGAMP to the healthy brain cells, thereby triggering paracrine signaling to promote tumor growth and chemoresistance [26]. Two modulators of GJs (i.e. meclofenamate and tonabersat) broke this paracrine loop, shown by inhibiting dye transfer from astrocytes to cancer cells and brain metastases [26]. This result suggests a chemoprotective mechanism mediated by heterologous Cx43-GJs in advanced cancer stages, and inhibition of this interaction has therapeutic potential.
In addition to the role of GJs to improve chemotherapy, Vance and Wiley suggested that ionizing radiation destroys not only targeted cells but also cells that have not been directly irradiated (the bystander effect) [125], and this effect is partially regulated by GJs [42], prompting GJIC as an appealing therapeutic target in combinatorial strategies with radiotherapy [[126], [127], [128]]. Zhang et al. found that iodide-induced upregulation of Cx43 protein expression and Cx43-GJ activity in genetically-modified non-small cell lung cancer cells significantly increased the bystander tumoricidal effects generated by ionizing radiation, thereby enhancing tumor cell killing both in vitro and in vivo [43]. Furthermore, the authors suggested that iodide could also modulate a cascade of molecular pathways including RONS signaling through Cx43-GJs, to further sensitize non-small cell lung cancer cells to ionizing radiation and chemotherapies like paclitaxel [43]. In concordance, experimental evidence suggested that GJs enhance the intercellular propagation of “death signals”, thereby expanding therapeutical cytotoxicity (Fig. 1A) [[126], [127], [128]]. Krutovskikh et al. observed that GJs propagate and increase cell death in rat bladder carcinoma cells, a cellular model that is predisposed to spontaneous apoptosis upon achieving confluency, by spreading cell-killing signals initially generated by a single apoptotic cell into healthy (non-apoptotic) surrounding cells [40]. In depth studies with a neuropeptide (oleamide) that selectively restricted GJs permeability to Ca2+ ions showed that the spreading of cell death was not prevented upon administration while Lucifer yellow dye transfer was blocked, suggesting that Ca2+ ions were the most probable cell-killing signals spread through GJs [40].
In summary, therapies that modulate Cxs and GJs could be a promising anti-cancer strategy, especially in combination with other conventional treatments such as chemotherapy and radiotherapy. However, further delineation of the conditions in which Cxs and GJs can act as anti- or pro-tumorigenic agents; and treatment-intrinsic difficulties like target selectivity and competitive inhibition are important issues to solve in order to fully optimize and implement them as cancer treatment.
6. Cxs and GJs in immune activation and immunotherapy
Engagement of the patient's own immunity to recognize and eradicate malignant cells is a very promising anti-tumor strategy, which is highlighted by the prominent role of immunotherapy in the clinical management of cancer and development of new combination strategies. The formation of a stable immunological synapse (IS) enabling intercellular communication is one of the fundamental steps in the immune cell priming and activation process. This includes direct crosstalk between antigen presenting cells (APCs), and T cells and natural killer (NK) cells, or between target (e.g. malignant) cells with cytotoxic T lymphocytes (CTLs) and NK cells (Fig. 1B and D, see figure caption for more details) [129].
Several studies described a role of GJs in the antigenic peptide transfer and cross-presentation mechanism between target cells and APCs, whereby GJs are able to facilitate effective cell coupling and transport of antigenic peptides with lengths up to 16 amino acids when in extended formation (Fig. 1B, see figure caption for more details) [44,45]. Furthermore, functional GJs between DCs and cancer cells were reported in an ex vivo human melanoma model wherein antigen transfer between DCs led to activation and maturation of naive DCs, and subsequently specific CTLs engagement [10]. It was also found that GJs are required for DCs transfer secondary messengers to NK cells for subsequent NK cell activation, although the nature of these molecules is yet to unravel [130]. Mendoza-Naranjo et al. and others identified Cx43 proteins as the key Cx type mediating bidirectional GJs between DCs-DCs and DCs-T cells at the stimulatory IS, leading to antigen-dependent T cell activation, in both murine and human models (Fig. 1B, see figure caption for more details) [[131], [132], [133]]. It was found that efficient polarization of Cx43 proteins and subsequent functional Cx43-GJs in the cytotoxic IS between CTLs (or NK cells) and cancer cells are required for induction of granzyme B-mediated cell death in these target cells (Fig. 1D, see figure caption for more details) [134]. Further investigation into the underlying mechanisms revealed that Cx43 protein accumulation at different IS is antigen specific, time dependent, and requires an intact actin cytoskeleton. This process precedes a polarized Ca2+ influx, causing the granzyme B activity in the target cell via the NK cell/target cell lytic IS, while this mechanism is yet to be unraveled in the Cytotoxic T lymphocyte (CTL)-target cell synapse [130,135].
These data enlighten a previously underestimated role of GJs in alternative pathways for immune regulation and activation, and prompt these intercellular structures as potential targets for immunomodulating anti-cancer therapies. Illustrative of this potential is the recent finding that undifferentiated monocytes were able to elicit competent therapeutic CTL responses, solely when Cx43-GJs were established between tumor antigen-loaded monocytes and endogenous DCs in multiple in vivo mouse models [136]. In addition, a novel immunotherapeutic approach, based on immunogenic peptide release in the tumor microenvironment, pointed out that Cx43 protein overexpression and Cx43-GJs opening via post-translational modifications on target cells are required for the release of tumor-derived peptides and adequate anti-tumor responses in several model systems [137]. This research sheds light on the fact that besides mediating direct cell-cell contact, GJs have also a rather unexplored contribution in immunological processes. In addition, a role of other members of the Cx protein family cannot be ruled out, as research into this area is still very limited.
7. Oxidative stress on GJs as a cancer therapeutic strategy
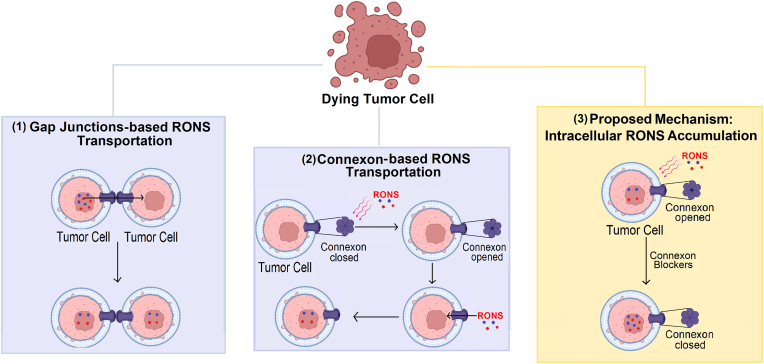
One of the major roles of GJs, is the exchange of ions and small molecules between the cytoplasm of adjacently connected cells [51,56]. In this way, GJs may mediate RONS transfer between adjacent cells to cause cell death via oxidative stress [[29], [30], [31]] (Fig. 5 (1)). RONS (e.g., H2O2, HO●, HO2●, O2●−, 1O2, ●NO) are products of normal cellular metabolism, generated within the mitochondria and cytoplasm. They are involved in cellular responses at physiological state [138], but elevated levels of RONS may lead to injurious oxidative stress; trigger damage to membrane lipids, proteins, and DNA; and ultimately can cause cell death [139]. When RONS permeate cell membranes, they can oxidize embedded proteins by direct reactions or indirect reactions with secondary products of oxidative stress, thus affecting membrane structure and dynamics. In particular, cysteine and methionine protein residues are more susceptible to oxidation, due to high reaction susceptibility of the sulfur group in those amino acids [140]. Oxidative modifications of proteins can change their physical and chemical properties, including conformation, structure, solubility, susceptibility to proteolysis, and enzyme activities. These modifications may represent the first step involved in mutagenesis and carcinogenesis [139]. On the other hand, protein oxidation has also been used as a therapeutic strategy to kill cancer cells, via inhibition of proliferative signaling pathways inside tumor cells [141] and reducing immunosuppressive signals on the surface of cancer cells [142].
Fig. 5.
Schematic representation of the effects of RONS-induced oxidative stress on GJs and connexons. (1) GJs are able to transport RONS between cells. (2) RONS induce connexon opening to allow the entry of RONS to the cell interior. (3) Proposed mechanism to increase the intracellular RONS accumulation: Connexon blockers induce connexon close state and increase the intracellular accumulation of RONS, by entrance of them through the membrane and by natural intracellular production (e.g., within the mitochondria and cytoplasm). Taken together, these events induce cell death.
Experimental reports have demonstrated that RONS can modulate GJs and connexons either via direct effects on channel gating (pore opening/closing) [32] or by affecting the intracellular trafficking of Cxs, impacting the number and stability of the GJ channel at the plasma membrane [143]. For instance, Ramachandran et al. demonstrated that H2O2-induced oxidative stress opens Cx43 proteins-composed connexons in fibroblastoid rat mammary tumor cell lines, via depolarization of the membrane potential. Consequently, the open connexons facilitated the entry of H2O2 in the cell interior to cause cell death, probably through apoptosis [32]. This result stresses an impressive property of the GJ channel under oxidation stress, where an open state can improve cancer cell death through a higher entrance of RONS to the cell interior (Fig. 5 (2)).
Lowering intracellular redox potential has also been suggested to increases the opening of Cx43 proteins-composed connexons. Retamal et al. transfected Cx43 proteins into HeLa cells to evaluate the effect of reducing agents on dye uptake and connexon opening, and found that the redox molecule dithiothreitol increases the rate of dye uptake at the resting redox potential in cells expressing Cx43 proteins. Moreover, dithiothreitol increased the open probability of connexons at positive potentials. They found that the opening of connexons is regulated by the intracellular redox potential, which may act through the cysteine residues in the CT domain of Cx43 proteins [144]. Connexons can also be opened in Ca2+ ions deficient medium and dephosphorylation of critical residues [145,146]. Ca2+ signaling is strongly intertwined with RONS signaling, and both play crucial roles in cell death; the entry of extracellular Ca2+ into the cell promotes RONS production [147,148]. Thus, molecules that increase intracellular Ca2+ may induce connexon activation and enhance intracellular RONS, increasing membrane permeability and eventually apoptotic cell death. Intracellular protein kinases can also affect the connexon opening probability [149,150], so small molecules that can trigger the activation of intracellular protein kinases could also indirectly modulate connexon activity. Hence, a potential approach in cancer therapy would be to enhance the connexon open state to improve oxidative stress-mediated cell death, though more studies focusing on the biophysical properties of potential connexon activators are necessary to improve their selectivity, solubility, permeability, and pharmacodynamics.
An open state of connexons can also contribute to the release of RONS and/or the activation of other signaling pathways which have a protective mechanism against cell death [33,151,152]. For example, H2O2-induced oxidative stress opened Cx43 proteins-composed connexons in lens epithelial cells, mediating the exchange of oxidants and antioxidants in these cells undergoing oxidative stress [33]. These transporting activities facilitated a reduction of intracellular RONS accumulation and maintained the intracellular glutathione level, protecting lens against oxidative stress to prevent cataract formation during aging [33]. One therapeutic strategy to avoid this protective mechanism in cancer cells could be to design inhibitors that block connexons from opening during RONS-mediated oxidative stress, to increase intracellular accumulation of RONS (Fig. 5 (3)). In this way, monoclonal antibodies to the EL-2 loop of Cx43 proteins (173–208 amino acid residues) were developed, and they were demonstrated to block connexons from opening in glioma cells [153]. Moreover, these antibodies inhibited GJs formation, indicating that they react with target connexon solely [153]. Furthermore, it was shown that glioma cells presenting Cx43 proteins were more resistant to H2O2-induced oxidative stress, due to inhibition of caspase-3 activation; Cx43 proteins interacted with the upstream apoptosis signal-regulating kinase 1, known to mediate H2O2-induced apoptosis, providing a possible mechanism for the anti-apoptotic effect [151]. Interestingly, reducing the expression of Cx43 proteins with siRNA in cultured astrocytes sensitized these resistant cells to H2O2-mediated apoptosis, indicating that Cx43 proteins have an anti-apoptotic effect in normal astrocytes [151]. Thus, monoclonal antibody inhibitors of Cx43 proteins-composed connexon opening can be combined with oxidative stress-based cancer treatment, to improve cancer cell death. Therefore, the use of connexon blockers such as antibodies are also a promising therapeutic strategy during oxidative stress. However, further studies suggested that the use of antibodies should be treated carefully, as depending on the model, they may be considered anti- or pro-metastatic agents [[154], [155], [156]].
Considering the ability of GJs to enhance the intracellular accumulation of RONS, Wu et al. demonstrated that after PDT, the level of intracellular RONS was higher in HeLa cells with Cx32-GJs compared to those without. Hence, Cx32-GJs increased the efficacy of the treatment and this highlights the potential of GJs to transfer RONS to the cell interior [30] (Fig. 5 (1)). The same research group also observed that when Cx26 proteins were not expressed or if the Cx26-GJs were blocked, the phototoxicity of photofrin-mediated PDT in high-density cultures substantially reduced, emphasizing the importance of Cx26-GJs [157]. The GJs-mediated increase in PDT phototoxicity was associated with oxidative stress by RONS, Ca2+ ions, and lipid peroxide [157]. GJs have been shown to propagate localized oxidative insults in endothelial cells, while stimulating de-novo generation of RONS in bystander cells [38]. Interestingly, the oxidative insult propagated through GJs for many hours, over hundreds of microns from the primary photogeneration site [38]. These results highlight an impressive property of GJs to propagate oxidative stress-induced cell death.
Cell exposure to ionizing radiation may affect mitochondrial and membrane oxidases, leading to oxidative stress. Thus, Autsavapromporn et al. studied the involvement of oxidative stress and GJs in enhancing toxicity in α-particle-irradiated human fibroblast cells, and found that GJs were also able to propagate to neighboring cells the damaging effects of oxidative stress induced by α-particles [158]. Inhibition of GJs or downregulation of Cx43 proteins protected the cells against the toxic effects, suggesting that GJs contribute to propagate radiation-induced cell death [158]. Therefore, designing strategies to increase GJs in cancer cells could improve the extension of cell death to neighboring cells, enhancing the efficiency of PDT or any other treatment based on oxidative stress, such as irradiation and NTP.
Overall, oxidative stress has a crucial effect on the function of GJs by inducing connexon opening to allow the entrance of RONS to cause cell injury and death. Afterwards, the use of inhibitors/blockers of connexons opening can increase the accumulation of intracellular RONS during oxidative stress, to enhance cell damage. To summarize, the oxidative damage caused by RONS on GJs can be used as a therapeutic strategy to induce cancer cell death, but their effects are dependent on the treatment type and may vary among different cancer types.
A promisor therapeutic strategy based on oxidative stress to overcome the resistance of various cancer types to traditional treatments such as radiotherapy, chemotherapy, and surgery [159] is the NTP, a promising therapeutic strategy being explored as a cancer (immuno-)therapy. NTP is a partially ionized gas composed of neutral gas molecules, positive and negative ions, free electrons, excited species and radicals. Of major importance for biomedical applications, including cancer therapy, is the multitude of short-lived and persistent RONS generated by NTP [36]. The observed anti-cancer effects of these RONS have been attributed to the therapeutic response of NTP on cancer cells [160], with a particular emphasis on the short-lived species (e.g. HO●, O2●−, ●NO) [161].
Despite advances in understanding the effect of RONS on GJs, some relevant questions pertaining to NTP treatment effect remain open. For instance: 1) How can RONS be transported through GJs? 2) Can RONS chemically react with amino acids present in connexons and affect the function of GJs? One method to investigate these questions is through the use of computational simulations. Xu et al. demonstrated the possible interaction of HO● and HO2● with the NT domain of a Cx26 proteins-composed connexon via reactive MD simulations. They found that these radicals chemically react with the amino acids within the NT domain of Cx26 proteins, and can structurally damage them [162]. Considering the negative effects of Cxs and/or GJs upregulation in later cancer stages, structural damage induced by RONS can influence the effectiveness of GJs-mediated tumoricidal activities. In addition, Xu et al. also supported the hypothesis that NTP-generated RONS trigger the bystander effect based on GJs, highlighting again the potential role of GJs to propagate oxidative stress-mediated cell death [162]. However, additional studies will be necessary to elucidate the effects of connexon oxidation on GJs properties, as well as the mechanism of RONS transportation through GJs.
Bagati et al. reported additive-to-synergistic effects of a NTP combination therapy with the DNA-damaging agent tirapazamine in in vitro and in vivo metastatic melanoma cells, which underline the potential of NTP to improve cancer therapy via GJ modulation [163]. The authors observed that when high Cx26-GJs expression was induced in these cells, the combinatorial effects of NTP + tirapazamine therapy was augmented, spreading cell death. The presence of Cx26-GJs facilitated RONS cell penetration and signaling, while increased Cx26 protein expression and amplified tumoricidal activity [163]. Furthermore, they also observed an immune response through differential regulation of cytokines and chemokines, suggesting potential for this therapy to induce a cytotoxic immune response [163]. Using a modified non-thermal helium plasma torch, the same research group showed that Cx43-GJs also contributes to NTP-induced cell death in melanoma cells [164]. They observed a higher sensitivity of these cells to RONS and a 6-fold increase in cell death by apoptosis compared with human keratinocytes [164]. Moreover, they observed an increased area of cell death, likely due to the bystander effect of passing apoptotic signals between cells [164].
Therefore, NTP therapy, and specifically NTP-generated RONS, have a great potential impact on the function of GJs via oxidative stress. A full understanding of RONS-GJs interactions can help in the modulation of their protecting and damaging mechanisms under oxidative stress, to improve NTP-based cell death. Computer simulations can be a useful and powerful tool to provide insights into these mechanisms, as well as their effects on membrane properties and the function of GJs.
To summarize, modulation of GJs can help inhibit or improve the pro- and anti-tumorigenic properties of GJs. Peptides, antibodies, and chemotherapeutic agents can be used to inhibit the pro-tumorigenic property of GJs, restoring the sensitivity of cancer cells for chemotherapeutic drugs and reducing tumor growth. Oxidative stress and the role of GJs to mediate the propagation of cell death and activation of the immune system can improve the anti-tumorigenic property of GJs, increasing cancer cell death. But their effects are dependent on the treatment type and may vary among different cancer types. The pro- and anti-tumorigenic properties of GJs have been explored to increase cancer cell death not only in traditional treatments, such as chemotherapy and radiotherapy, but as well as in novel treatments, such as PDT and NTP.
8. Conclusion
Overall, clinical, experimental, and modeling work performed up to now highlight the profound impact of GJs in the context of tumor development and progression. These studies confirm emerging concepts that GJs have pro- and anti-tumorigenic properties in cancer cells, which depend on GJ composition, cancer factors, and tumoral context. Thus, modulation of GJs can be used to inhibit or improve the pro- and anti-tumorigenic properties of GJs for anti-cancer responses. Peptides, antibodies, and chemotherapeutic agents have shown to be very useful in inhibiting the pro-tumorigenic properties of GJs, restoring the sensitivity of cancer cells to chemotherapeutic drugs and reducing tumor growth. The role of GJs to mediate the transport of RONS between cells, the propagation of cell death, and the activation of the immune system, opens possibilities to modulate the function of GJs to improve the anti-tumorigenic property of GJs. Indeed, GJs have been advantageous for inducing cancer cell death via the transport of RONS to the cell interior and via the propagation of cell death induced by oxidative stress, apoptosis, and radiation.
The success of future cancer treatments may be improved by understanding the underlying mechanisms involving Cxs and the function of GJs in cancer cells, which if accurately determined would lead to better therapeutic targets and strategies for each specific treatment cases. Although significant progress has been made towards understanding these topics, challenges remain to be addressed, such as when Cxs and GJs are pro- and anti-tumorigenic, how cancer therapies can modulate these properties, how RONS are transported through GJs to mediate oxidative stress-induced cell death, and how GJs propagate cell death. Thus, additional studies are necessary to elucidate the underlying mechanisms of the pro- and anti-tumorigenic properties of GJs. This will contribute to designing better GJs inhibitors and/or activators to improve traditional cancer treatments, such as chemotherapy and radiotherapy, as well novel cancer treatments based on oxidative stress, such as PDT and NTP.
Conflicts of interest
None Declared.
Acknowledgments
We thank Coordination of Superior Level Staff Improvement (CAPES, Brazil) for the scholarship granted, and the Turing HPC infrastructure at the CalcUA core facility of the University of Antwerp, a division of the Flemish Supercomputer Center VSC, funded by the Hercules Foundation, the Flemish Government (department EWI) and the University of Antwerp, for providing the computational resources needed for running the simulations. This work was also funded in part by the funded by the Research Foundation - Flanders (FWO) and the Flemish Government. The FWO fellowships and grants that funded this work include: 12S9221N (Abraham Lin), G044420N (Abraham Lin and Annemie Bogaerts), and 1S67621N (Hanne Verswyvel). Fig. 1, Fig. 4, Fig. 5 were created in BioRender.com.
Data availability
Data will be made available on request.
References
- 1.Payton B.W., Bennett M.V., Pappas G.D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969;166:1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- 2.Mathias R.T., White T.W., Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010;90:179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto T., Usuda H., Tanaka T., Wada K., Shimaoka M. The functional implications of endothelial gap junctions and cellular mechanics in vascular angiogenesis. Cancers. 2019;11:237. doi: 10.3390/cancers11020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang G.Y., Cooper E.S., Waldo K., Kirby M.L., Gilula N.B., Lo C.W. Gap junction–mediated cell–cell communication modulates mouse neural crest migration. J. Cell Biol. 1998;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Q., Yue C., Chen A.C.H., Lee K.C., Fong S.W., Yeung W.S.B., Lee Y.L. Connexin 43 is involved in early differentiation of human embryonic stem cells. Differentiation. 2019;105:33–44. doi: 10.1016/j.diff.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R.G., Sheridan J.D. Junctions between cancer cells in culture: ultrastructure and permeability. Science. 1971;174:717–719. doi: 10.1126/science.174.4010.717. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki H., Krutovskikh V., Mesnil M., Tanaka T., Zaidan-Dagli M.L., Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C. R. Acad. des Sci. Paris, Sciences de la vie/Life Sciences. 1999;322:151–159. doi: 10.1016/s0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- 8.Aasen T., Hodgins M.B., Edward M., Graham S.V. The relationship between connexins, gap junctions, tissue architecture and tumour invasion, as studied in a novel in vitro model of HPV-16-associated cervical cancer progression. Oncogene. 2003;22:7969–7980. doi: 10.1038/sj.onc.1206709. [DOI] [PubMed] [Google Scholar]
- 9.Hirschi K.K., Xu C.E., Tsukamoto T., Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–870. [PubMed] [Google Scholar]
- 10.Zhang Z.Q., Zhang W.J., Wang N.Q., Bani-Yaghoub M., Lin Z.X., Naus C.C.G. Suppression of tumorigenicity of human lung carcinoma cells after transfection with connexin43. Carcinogenesis. 1998;19:1889–1894. doi: 10.1093/carcin/19.11.1889. [DOI] [PubMed] [Google Scholar]
- 11.Lahlou H., Fanjul M., Pradayrol L., Susini C., Pyronnet S. Restoration of functional gap junctions through internal ribosorne entry site-dependent synthesis of endogenous Connexins in density-inhibited cancer cells. Mol. Cell. Bio. 2005;25:4034–4045. doi: 10.1128/MCB.25.10.4034-4045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subak-Sharpe H., Bürk R.R., Pitts J.D. Metabolic co-operation between biochemically marked mammalian cells in tissue culture. J. Cell Sci. 1969;4:353–367. doi: 10.1242/jcs.4.2.353. [DOI] [PubMed] [Google Scholar]
- 13.Gilula N.B., Reeves O.R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- 14.Mesnil M., Crespin S., Avanzo J.L., Zaidan-Dagli M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta-Biom. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan E., Shao Q., Laird D.W. Connexins and gap junctions in mammary gland development and breast cancer progression. J. Membr. Biol. 2007;218:107–121. doi: 10.1007/s00232-007-9052-x. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima M., Hattori Y., Yoshizawa T., Maitani Y. Combination of non-viral connexin 43 gene therapy and docetaxel inhibits the growth of human prostate cancer in mice. Int. J. Oncol. 2007;30:225–231. [PubMed] [Google Scholar]
- 17.Alonso F., Domingos-Pereira S., Le Gal L., Derré L., Meda P., Jichlinski P., Nardelli-Haefliger D., Haefliger J.A. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget. 2016;7:14015–14028. doi: 10.18632/oncotarget.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusubalieva G.M., Baklaushev V.P., Gurina O.I., Zorkina Y.A., Gubskii I.L., Kobyakov G.L., Golanov A.V., Goryainov S.A., Gorlachev G.E., Konovalov A.N., Potapov A.A., Chekhonin V.P. Treatment of poorly differentiated glioma using a combination of monoclonal antibodies to extracellular connexin-43 fragment, temozolomide, and radiotherapy. Bull. Exp. Biol. Med. 2014;157:510–515. doi: 10.1007/s10517-014-2603-0. [DOI] [PubMed] [Google Scholar]
- 19.Kalimi G.H., Sirsat S.M. Phorbol ester tumor promoter affects the mouse epidermal gap junctions. Cancer Lett. 1984;22:343–350. doi: 10.1016/0304-3835(84)90173-3. [DOI] [PubMed] [Google Scholar]
- 20.Acosta M.L., Nor M.N.M., Guo C.X., Mugisho O.O., Coutinho F.P., Rupenthal I.D., Green C.R. Connexin therapeutics: blocking connexin hemichannel pores is distinct from blocking pannexin channels or gap junctions. Neural Regen. Res. 2021;16:482–488. doi: 10.4103/1673-5374.290097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.P Subedi Y., Altenberg G.A., Chang C.-W.T. Advances in the development of connexin hemichannel inhibitors selective toward Cx43. Future Med. Chem. 2021;13:379–392. doi: 10.4155/fmc-2020-0291. [DOI] [PubMed] [Google Scholar]
- 22.Bol M., Geyt C.V., Baert S., Decrock E., Wang N., De Bock M., Gadicherla A.K., Randon C., Evans W.H., Beele H., Cornelissen R., Leybaert L. Inhibiting connexin channels protects against cryopreservation-induced cell death in human blood vessels. Eur. J. Vasc. Endovasc. Surg. 2013;45:382–390. doi: 10.1016/j.ejvs.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Jaraíz-Rodríguez M., Tabernero M.D., González-Tablas M., Otero A., Orfao A., Medina J.M., Tabernero A. A short region of Connexin43 reduces human glioma stem cell migration, invasion, and survival through src, PTEN, and FAK. Stem Cell Rep. 2017;9:451–463. doi: 10.1016/j.stemcr.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusubalieva G.M., Baklaushev V.P., Gurina O.I., Gulyaev M.V., Pirogo Y.A., Chekhonin V.P. Antitumor effects of monoclonal antibodies to connexin 43 extracellular fragment in induced low-differentiated glioma. Bull. Exp. Biol. Med. 2012;153:163–169. doi: 10.1007/s10517-012-1667-y. [DOI] [PubMed] [Google Scholar]
- 25.De Meulenaere V., Bonte E., Verhoeven J., Okito J.-P.K., Pieters L., Vral A., De Wever O., Leybaert L., Goethals I., Vanhove C., Descamps B., Deblaere K. Adjuvant therapeutic potential of tonabersat in the standard treatment of glioblastoma: a preclinical F98 glioblastoma rat model study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q., Boire A., Jin X., Valiente M., Er E.E., Lopez-Soto A., Jacob L.S., Patwa R., Shah H., Xu K., Cross J.R., Massague J. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y.-P., Liu B., Wang Q., Yuan D.-D., Yang Y., Hong X.-T., Wang X.-D., Tao L. Propofol depresses the cytotoxicity of X-ray irradiation through inhibition of gap junctions. Anesth. Analg. 2011;112:1088–1095. doi: 10.1213/ANE.0b013e31820f288e. [DOI] [PubMed] [Google Scholar]
- 28.Murphy S.F., Varghese R.T., Lamouille S., Guo S., Pridham K.J., Kanabur P., Osimani A.M., Sharma S., Jourdan J., Rodgers C.M., Simonds G.R., Gourdie R.G., Sheng Z. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. J. Cancer Res. 2016;76:139–149. doi: 10.1158/0008-5472.CAN-15-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan D.B., Jones M., Garcia L.M., Noria S., del Nido P.J., McGowan F.X., Jr. Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler. Thromb. Vasc. Biol. 2003;23:1754–1760. doi: 10.1161/01.ATV.0000093546.10162.B2. [DOI] [PubMed] [Google Scholar]
- 30.Wu D.-P., Fan L., Xu C., Liu Z., Zhang Y., Liu L., Wang Q., Tao L. GJIC Enhances the phototoxicity of photofrin-mediated photodynamic treatment by the mechanisms related with ROS and Calcium pathways. J. Biophot. 2015;8:765–774. doi: 10.1002/jbio.201400131. [DOI] [PubMed] [Google Scholar]
- 31.Martins-Marques T., Rodriguez-Sinovas A., Girao H. Cellular crosstalk in cardioprotection: where and when do reactive oxygen species play a role? Free Radical Biol. Med. 2021;169:397–409. doi: 10.1016/j.freeradbiomed.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran S., Xie L.-H., John S.A., Subramaniam S., Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS One. 2007;8 doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan Y.-M., Du Y., Wu C.-G., Gu S., Jiangb J.X. Connexin hemichannels regulate redox potential via metabolite exchange and protect lens against cellular oxidative damage. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown S.B., Brown E.A., Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 35.Huang L., Zhao S.-J., Wu J.-S., Yu L., Singh N., Yang K., Lan M.-H., Wang P.-F., Kim J.S. Photodynamic therapy for hypoxic tumors: advances and perspectives. Coord. Chem. Rev. 2021;438 [Google Scholar]
- 36.Yan D., Sherman J.H., Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977–15995. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keidar M. first ed. Springer Nature; Switzerland: 2020. Plasma Cancer Therapy. [Google Scholar]
- 38.Feine I., Pinkas I., Salomon Y., Scherz A. Local oxidative stress expansion through endothelial cells – a key role for gap junction intercellular communication. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decrock E., Hoorelbeke D., Ramadan R., Delvaeye T., De Bock M., Wang N., Krysko D.V., Baatout S., Bultynck G., Aerts A., Vinken M., Leybaert L. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1099–1120. doi: 10.1016/j.bbamcr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Krutovskikh V.A., Piccoli C., Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene. 2002;21:1989–1999. doi: 10.1038/sj.onc.1205187. [DOI] [PubMed] [Google Scholar]
- 41.Decrock E., Krysko D.V., Vinken M., Kaczmarek A., Crispino G., Bol M., Wang N., De Bock M., De Vuyst E., Naus C.C., Rogiers V., Vandenabeele P., Erneux C., Mammano F., Bultynck G., Leybaert L. Transfer of IP₃ through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ. 2012;19:947–957. doi: 10.1038/cdd.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzam E.I., de Toledo S.M., Gooding T., Little J.B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 43.Zhang L., Sharma S., Hershman J.M., Brent G.A., Dubinett S.M., Huang M. Iodide sensitizes genetically modified non-small cell lung cancer cells to ionizing radiation. Cancer Gene Ther. 2006;13:74–81. doi: 10.1038/sj.cgt.7700875. [DOI] [PubMed] [Google Scholar]
- 44.Neijssen J., Herberts C., Drijfhout J.W., Reits E., Janssen L., Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 45.Pang B.-X., Neijssen J., Qiao X.-H., Janssen L., Janssen H., Lippuner C., Neefjes J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009;183:1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 46.Goodenough D.A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and X-ray diffraction. J. Cell Biol. 1972;54:646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen M.R., Saier M.H., Jr. Gap junctional proteins of animals: the innexin/pannexin superfamily. Prog. Biophys. Mol. Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scemes E., Spray D.C., Meda P., Connexins, pannexins, innexins Novel roles of “hemi-channels”. Pflügers Archiv. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodenough D.A. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J. Cell Biol. 1974;61:557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruciani V., Mikalsen S.-O. The vertebrate connexin family. Cell. Mol. Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodenough D.A., Paul D.L. Gap junctions. Cold Spring Harbor Perspect. Biol. 2009;1:1–19. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai D. Structural analysis of key gap junction domains — lessons from genome data and disease-linked mutants. Semin. Cell Dev. Biol. 2016;50:74–82. doi: 10.1016/j.semcdb.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Evans W.H. Cell communication across gap junctions: a historical perspective and current developments. Biochem. Soc. Trans. 2015;43:450–459. doi: 10.1042/BST20150056. [DOI] [PubMed] [Google Scholar]
- 54.Račkauskas M., Neverauskas V., Skeberdis V.A. Diversity and properties of connexin gap junction channels. Med. Lithuania. 2010;46:1–12. [PubMed] [Google Scholar]
- 55.Oyamada M., Takebe K., Oyamada Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim. Biophys. Acta, Biomembr. 2013;1828:118–133. doi: 10.1016/j.bbamem.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 56.Sáez J.C., Berthoud V.M., Brañes M.C., Martínez A.D., Beyer E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 57.Bennett M.V., Contreras J.E., Bukauskas F.F., Saez J.C. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwon T., Harris A.L., Rossi A., Bargiello T.A. Molecular dynamics simulations of the Cx26 hemichannel: evaluation of structural models with Brownian dynamics. J. Gen. Physiol. 2011;138:475–493. doi: 10.1085/jgp.201110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maeda S., Nakagawa S., Suga M., Yamashita E., Oshima A., Fujiyoshi Y., Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 60.Myers J.B., Haddad B.G., O'Neill S.E., Chorev D.S., Yoshioka C.C., Robinson C.V., Zuckerman D.M., Reichow S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature. 2018;564:372–377. doi: 10.1038/s41586-018-0786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palacios-Prado N., Briggs S.W., Skeberdis V.A., Pranevicius M., Bennett M.V.L., Bukauskas F.F. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9897–9902. doi: 10.1073/pnas.1004552107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown T.R., Krogh-Madsen T., Christini D.J. Illuminating myocyte-fibroblast homotypic and heterotypic gap junction dynamics using dynamic clamp. Biophys. J. 2016;111:785–797. doi: 10.1016/j.bpj.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrio L.C., Suchyna T., Bargiello T., Xu L.X., Roginski R.S., Bennett M.V.L., Nicholson B.J. Gap-junctions formed by connexin-26 and connexin-32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fasciani I., Temperán A., Pérez-Atencio L.F., Escudero A., Martínez-Montero P., Molano J., Gómez-Hernández J.M., Paino C.L., González-Nieto D., Barrio L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013;75:479–490. doi: 10.1016/j.neuropharm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 65.Foote C.I., Zhou L., Zhu X., Nicholson B.J. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J. Cell Biol. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bai D., Yue B., Aoyama H. Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim. Biophys. Acta-Biom. 2018;1860:9–21. doi: 10.1016/j.bbamem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Purnick P.E.M., Benjamin D.C., Verselis V.K., Bargiello T.A., Dowd T.L. Structure of the amino terminus of a gap junction protein. Arch. Biochem. Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 68.Santos-Miranda A., Chen H., Chen R.C., Odoko-Ishimoto M., Aoyama H., Bai D. The amino terminal domain plays an important role in transjunctional voltage-dependent gating kinetics of Cx45 gap junctions. J. Mol. Cell. Cardiol. 2020;143:71–84. doi: 10.1016/j.yjmcc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Laird D.W. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alexander D.B., Goldberg G.S. Transfer of biologically important molecules between cells through gap junction channels. Curr. Med. Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 71.Sáez J.C., Martínez A.D., Brañes M.C., González H.E. Regulation of gap junctions by protein phosphorylation. Braz. J. Med. Biol. Res. 1998;31:593–600. doi: 10.1590/s0100-879x1998000500001. [DOI] [PubMed] [Google Scholar]
- 72.Liu J., Vitorin J.F.E., Weintraub S.T., Gu S., Shi Q., Burt J.M., Jiang J.X. Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J. Biol. Chem. 2011;286:16914–16928. doi: 10.1074/jbc.M111.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Q., Kopp R.F., Chen Y., Yang J.J., Roe M.W., Veenstra R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012;302:C1548–C1556. doi: 10.1152/ajpcell.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aseervatham J., Li X., Mitchell C.K., Lin Y.-P., Heidelberger R., O'Brien J. Calmodulin binding to connexin 35: specializations to function as an electrical synapse. Int. J. Mol. Sci. 2020;21:6346. doi: 10.3390/ijms21176346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray A., Mehta P.P. Cysteine residues in the C-terminal tail of connexin32 regulate its trafficking, Cell. Signalling. 2021;85 doi: 10.1016/j.cellsig.2021.110063. [DOI] [PubMed] [Google Scholar]
- 76.Ito A., Koma Y., Uchino K., Okada T., Ohbayashi C., Tsubota N., Okada M. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: significant correlation with poor prognosis. Cancer Lett. 2006;234:239–248. doi: 10.1016/j.canlet.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z.-Q., Hu Y., Wang B.-J., Lin Z.-X., Naus C.C.G., Nicholson B.J. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis. 2004;25:473–482. doi: 10.1093/carcin/bgh036. [DOI] [PubMed] [Google Scholar]
- 78.Saito-Katsuragi M., Asada H., Niizeki H., Katoh F., Masuzawa M., Tsutsumi M., Kuniyasu H., Ito A., Nojima H., Miyagawa S. Role for connexin 26 in metastasis of human malignant melanoma: communication between melanoma and endothelial cells via connexin 26. Cancer. 2007;110:1162–1172. doi: 10.1002/cncr.22894. [DOI] [PubMed] [Google Scholar]
- 79.Ito A., Katoh F., Kataoka T.R., Okada M., Tsubota N., Asada H., Yoshikawa K., Maeda S., Kitamura Y., Yamasaki H., Nojima H. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Investig. 2000;105:1189–1197. doi: 10.1172/JCI8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adak A., Unal Y.C., Yucel S., Vural Z., Turan F.B., Yalcin-Ozuysal O., Ozcivici E., Mese G. Connexin 32 induces pro-tumorigenic features in MCF10A normal breast cells and MDA-MB-231 metastatic breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2020.118851. [DOI] [PubMed] [Google Scholar]
- 81.Stoletov K., Strnadel J., Zardouzian E., Momiyama M., Park F.D., Kelber J.A., Pizzzo D.P., Hoffman R., VandenBerg S.R., Klemke R.L. Role of connexins in metastatic breast cancer and melanoma brain colonization. J. Cell Sci. 2013;126:904–913. doi: 10.1242/jcs.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aasen T., Sansano I., Montero M.Á., Romagosa C., Temprana-Salvador J., Martínez-Marti A., Moliné T., Hernández-Losa J., y Cajal S.R. Insight into the role and regulation of gap junction genes in lung cancer and identification of nuclear Cx43 as a putative biomarker of poor prognosis. Cancers. 2019;11:320. doi: 10.3390/cancers11030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamasaki H., Mesnil M., Omori Y., Mironov N., Krutovskikh V. Intercellular communication and carcinogenesis. Mutat. Res. 1995;333:181–188. doi: 10.1016/0027-5107(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 84.Lampe P.D., Lau A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Princen F., Robe P., Gros D., Jarry-Guichard T., Gielen J., Merville M.-P., Bours V. Rat gap junction connexin-30 inhibits proliferation of glioma cell lines. Carcinogenesis. 2001;22:507–513. doi: 10.1093/carcin/22.3.507. [DOI] [PubMed] [Google Scholar]
- 86.Muramatsu A., Iwai M., Morikawa T., Tanaka S., Mori T., Harada Y., Okanoue T. Influence of transfection with connexin 26 gene on malignant potential of human hepatoma cells. Carcinogenesis. 2002;23:351–358. doi: 10.1093/carcin/23.2.351. [DOI] [PubMed] [Google Scholar]
- 87.Lee H.-J., Rhee S.-K. Growth-suppressing activity of the transfected Cx26 on BICR-M1Rk breast cancer cell line. J. Microbiol. Biotechnol. 2011;21:21 477–482. doi: 10.4014/jmb.1012.12035. [DOI] [PubMed] [Google Scholar]
- 88.Mesnil M., Krutovskikh V., Piccoli C., Elfgang C., Traub O., Willecke K., Yamasaki H. Negative growth control of HeLa cells by connexin genes: connexin species specificity. J. Cancer Res. 1995;55:629–639. [PubMed] [Google Scholar]
- 89.Burt J.M., Nelson T.K., Simon A.M., Fang J.S. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am. J. Physiol. Cell Physiol. 2008;295:C1103–C1112. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banerjee D., Gakhar G., Madgwick D., Hurt A., Takemoto D., Nguyen T.A. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int. J. Cancer. 2010;127:839–848. doi: 10.1002/ijc.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jamakosmanovic A., Loewenstein W.R. Intercellular communication and tissue growth. III. Thyroid cancer. J. Cell Biol. 1968;38:556–561. doi: 10.1083/jcb.38.3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borek C., Higashino S., Loewenstein W.R. Intercellular communication and tissue growth: IV. Conductance of membrane junctions of normal and cancerous cells in culture. J. Membr. Biol. 1969;1:274–293. doi: 10.1007/BF01869786. [DOI] [PubMed] [Google Scholar]
- 93.Daniel-Wójcik A., Misztal K., Bechyne I., Sroka J., Miekus K., Madeja Z., Czyz J. Cell motility affects the intensity of gap junctional coupling in prostate carcinoma and melanoma cell populations. Int. J. Oncol. 2008;33:309–315. [PubMed] [Google Scholar]
- 94.Sirnes S., Bruun J., Kolberg M., Kjenseth A., Lind G.E., Svindland A., Brech A., Nesbakken A., Lothe R.A., Leithe E., Rivedal E. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int. J. Cancer. 2012;131:570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 95.Valenta T., Hausmann G., Basler K. The many faces and functions of β-catenin. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weinert T., Lydall D. Cell cycle checkpoints, genetic instability and cancer. Semin. Cancer Biol. 1993;4:129–140. [PubMed] [Google Scholar]
- 97.Nelson T.K., Sorgen P.L., Burt J.M. Carboxy terminus and pore-forming domain properties specific to Cx37 are necessary for Cx37-mediated suppression of insulinoma cell proliferation. Am. J. Physiol. Cell Physiol. 2013;305:C1246–C1256. doi: 10.1152/ajpcell.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jee H., Lee S.-H., Park J.-W., Lee B.-R., Nam K.-T., Kim D.-Y. Connexin32 inhibits gastric carcinogenesis through cell cycle arrest and altered expression of p21(Cip1) and p27(Kip1) BMB Rep. 2013;46:25–30. doi: 10.5483/BMBRep.2013.46.1.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Y., Zhang N., Zhu J., Hong X.T., Liu H., Ou Y.R., Su F., Wang R., Li Y.M., Wu Q. Downregulated connexin32 promotes EMT through the Wnt/β-catenin pathway by targeting Snail expression in hepatocellular carcinoma. Int. J. Oncol. 2017;50:1977–1988. doi: 10.3892/ijo.2017.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu X.-T., Dai Z., Song K., Zhang Z.-J., Zhou Z.-J., Zhou S.-L., Zhao Y.-M., Xiao Y.-S., Sun Q.-M., Ding Z.-B., Fan J. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int. J. Oncol. 2015;46:587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- 101.Stewart M.K., Bechberger J.F., Welch I., Naus C.C., Laird D.W. Cx26 knockout predisposes the mammary gland to primary mammary tumors in a DMBA-induced mouse model of breast cancer. Oncotarget. 2015;6:37185–37199. doi: 10.18632/oncotarget.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uhlen M., Zhang C., Lee S., Sjostedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z.-T., Edfors F., Sanli K., von Feilitzen K., Oksvold P., Lundberg E., Hober S., Nilsson P., Mattsson J., Schwenk J.M., Brunnstrom H., Glimelius B., Sjoblom T., Edqvist P.-H., Djureinovic D., Micke P., Lindskog C., Mardinoglu A., Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357:660. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 103.King T.J., Lampe P.D. The gap junction protein connexin 32 is a mouse lung tumor suppressor. J. Cancer Res. 2004;64:7191–7196. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y., Yao J.-H., Du Q.-Y., Zhou Y.-C., Yao T.-J., Wu Q., Liu J., Ou Y.-R. Connexin 32 downregulation is critical for chemoresistance in oxaliplatin-resistant HCC cells associated with EMT. Cancer Manag. Res. 2019;11:5133–5146. doi: 10.2147/CMAR.S203656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.King T.J., E Gurley K., Prunty J., Shin J.-L., Kemp C.J., Lampe P.D. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005;24:1718–1726. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- 106.Igarashi I., Makino T., Suzuki Y., Kai K., Teranishi M., Takasaki W., Furuhama K. Background lesions during a 24-month observation period in connexin 32-deficient mice. J. Vet. Med. Sci. 2013;75:207–210. doi: 10.1292/jvms.12-0280. [DOI] [PubMed] [Google Scholar]
- 107.Luo H., Wang X., Ge H., Zheng N., Peng F., Fu Y., Tao L., Wang Q. Inhibition of ubiquitin-specific protease 14 promotes connexin 32 internalization and counteracts cisplatin cytotoxicity in human ovarian cancer cells. Oncol. Rep. 2019;42:1237–1247. doi: 10.3892/or.2019.7232. [DOI] [PubMed] [Google Scholar]
- 108.Chi Q., Wang Z.-Y., Li H.-Y., Song D.-B., Xu H., Ma G., Wang Z.-M., Li X.-M. Tumor-suppressor microRNA-139-5p restrains bladder cancer cell line ECV-304 properties via targeting Connexin. Chin. Med. J. (Engl.) 2019;132:2354–2361. doi: 10.1097/CM9.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kou Y., Ji L.-Y., Wang H.-J., Wang W.-S., Zheng H.-M., Zou J., Liu L.-X., Qi X.-X., Liu Z.-Q., Du B.-Y., Lu L.-L. Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int. J. Cancer. 2017;141:1690–1703. doi: 10.1002/ijc.30872. [DOI] [PubMed] [Google Scholar]
- 110.Wang H., Tian L., Liu J., Goldstein A., Bado I., Zhang W.-J., Arenkiel B.R., Li Z.-H., Yang M., Dlo S.-Y., Zhao H., Rowley D.R., Wong S.T.C., Gugala Z., Zhang X.H.-F. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell. 2018;34:823–839. doi: 10.1016/j.ccell.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Wang W., Wu X., Li C., Huang Y., Zhou H., Cui Y. Resveratrol sensitizes colorectal cancer cells to cetuximab by connexin 43 upregulation-induced Akt inhibition. Front. Oncol. 2020;10:383. doi: 10.3389/fonc.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crespin S., Fromont G., Wager M., Levillain P., Cronier L., Monvoisin A., Defamie N., Mesnil M. Expression of a gap junction protein, connexin43, in a large panel of human gliomas: new insights. Cancer Med. 2016;5:1742–1752. doi: 10.1002/cam4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Oliveira K.D., Tedardi M.V., Cogliati B., Dagli M.L. Higher incidence of lung adenocarcinomas induced by DMBA in connexin 43 heterozygous knockout mice. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/618475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo M., Luo Y., Mao N., Huang G., Teng C., Wang H., Wu J., Liao X., Yang J. Cancer-associated fibroblasts Accelerate malignant progression of non-small cell lung cancer via connexin 43-formed unidirectional gap junctional intercellular communication. Cell. Physiol. Biochem. 2018;51:315–336. doi: 10.1159/000495232. [DOI] [PubMed] [Google Scholar]
- 115.Fukuda S., Akiyama M., Niki Y., Kawatsura R., Harada H., Nakahama K.-I. Inhibitory effects of miRNAs in astrocytes on C6 glioma progression via connexin 43. Mol. Cell. Biochem. 2021;476:2623–2632. doi: 10.1007/s11010-021-04118-0. [DOI] [PubMed] [Google Scholar]
- 116.Johnstone S.R., Billaud M., Lohman A.W., Taddeo E.P., Isakson B.E. Post-translational modifications in connexins and pannexins. J. Membr. Biol. 2012;245:319–332. doi: 10.1007/s00232-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fukumasu H., Avanzo J.L., Sanches D.S., Mennecier G., Mori C.M.C., Dagli M.L.Z. Higher susceptibility of spontaneous and NNK-induced lung neoplasms in connexin 43 deficient CD1 x AJ F1 mice: paradoxical expression of connexin 43 during lung carcinogenesis. Mol. Carcinog. 2013;52:497–506. doi: 10.1002/mc.21884. [DOI] [PubMed] [Google Scholar]
- 118.Lee S., Schmitt C.A. Chemotherapy response and resistance. Curr. Opin. Genet. Dev. 2003;13:90–96. doi: 10.1016/s0959-437x(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 119.Wang S., Zhang S., Zhao Z., Zhang C., Yang X., Wang Y. Connexin 43 enhances paclitaxel cytotoxicity in colorectal cancer cell lines. Exp. Ther. Med. 2017;14:1212–1218. doi: 10.3892/etm.2017.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu D.-P., Zhou Y., Hou L.-X., Zhu X.-X., Yi W., Yang S.-M., Lin T.-Y., Huang J.-L., Zhang B., Yin X.-X. Cx43 deficiency confers EMT-mediated tamoxifen resistance to breast cancer via c-Src/PI3K/Akt pathway. Int. J. Biol. Sci. 2021;17:2380–2398. doi: 10.7150/ijbs.55453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X., Berthoud V.M., Beyer E.C., Ebihara L. Functional role of the carboxyl terminal domain of human connexin 50 in gap junctional channels. J. Membr. Biol. 2002;186:101–112. doi: 10.1007/s00232-001-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li H., Spagnol G., Pontifex T.K., Burt J.M., Sorgen P.L. Chemical shift assignments of the connexin37 carboxyl terminal domain. Biomol. NMR Assign. 2017;11:137–141. doi: 10.1007/s12104-017-9735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caufriez A., Böck D., Martin C., Ballet S., Vinken M. Peptide-based targeting of connexins and pannexins for therapeutic purposes, Expert Opin. Drug Discov. 2020;15:1213–1222. doi: 10.1080/17460441.2020.1773787. [DOI] [PubMed] [Google Scholar]
- 124.Lin Q., Balasubramanian K., Fan D., Kim S.J., Guo L., Wang H., Bar-El M., Aldape K.D., Fidler I.J. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2010;12:748–754. doi: 10.1593/neo.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vance M.M., Wiley L.M. Gap junction intercellular communication mediates the competitive cell proliferation disadvantage of irradiated mouse preimplantation embryos in aggregation chimeras. J. Radiat. Res. 1999;152:544–551. [PubMed] [Google Scholar]
- 126.Mesnil M., Piccoli C., Yamasaki H. A tumor suppressor gene, Cx26, also mediates the bystander effect in HeLa cells. Cancer Res. 1997;57:2929–2932. [PubMed] [Google Scholar]
- 127.Wang Y.-W., Wang Q., Zhang S.-Z., Zhang Y., Tao L. Baicalein increases the cytotoxicity of cisplatin by enhancing gap junction intercellular communication. Mol. Med. Rep. 2014;10:515–521. doi: 10.3892/mmr.2014.2157. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y., Wang X.-Y., Wang Q., Ge H., Tao L. Propofol depresses cisplatin cytotoxicity via the inhibition of gap junctions. Mol. Med. Rep. 2016;13:4715–4720. doi: 10.3892/mmr.2016.5119. [DOI] [PubMed] [Google Scholar]
- 129.Dustin M.L., Long E.O. Cytotoxic immunological synapses. Immunol. Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tittarelli A., Mendoza-Naranjo A., Farías M., Guerrero I., Ihara F., Wennerberg E., Riquelme S., Gleisner A., Kalergis A., Lundqvist A., López M.N., Chambers B.J., Salazar-Onfray F. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J. Immunol. 2014;192:1313–1319. doi: 10.4049/jimmunol.1301297. [DOI] [PubMed] [Google Scholar]
- 131.Mendoza-Naranjo A., Saez P.J., Johansson C.C., Ramirez M., Mandakovic D., Pereda C., Lopez M.N., Kiessling R., Saez J.C., Salazar-Onfray F. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 2007;178:6949–6957. doi: 10.4049/jimmunol.178.11.6949. [DOI] [PubMed] [Google Scholar]
- 132.Mendoza-Naranjo A., Bouma G., Pereda C., Ramirez M., Webb K.F., Tittarelli A., Lopez M.N., Kalergis A.M., Thrasher A.J., Becker D.L., Salazar-Onfray F. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J. Immunol. 2011;187:3121–3132. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saccheri F., Pozzi C., Avogadri F., Barozzi S., Faretta M., Fusi P., Rescigno M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010;2:44ra57. doi: 10.1126/scitranslmed.3000739. [DOI] [PubMed] [Google Scholar]
- 134.Tittarelli A., Janji B., Van Moer K., Noman M.Z., Chouaib S. The selective degradation of synaptic connexin 43 protein by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J. Biol. Chem. 2015;290:23670–23679. doi: 10.1074/jbc.M115.651547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hofmann F., Navarrete M., Álvarez J., Guerrero I., Gleisner M.A., Tittarelli A., Salazar-Onfray F. Cx43-Gap junctions accumulate at the cytotoxic immunological synapse enabling cytotoxic T lymphocyte melanoma cell killing. Int. J. Mol. Sci. 2019;20:4509. doi: 10.3390/ijms20184509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang M.N., Nicholson L.T., Batich K.A., Swartz A.M., Kopin D., Wellford S., Prabhakar V.K., Woroniecka K., Nair S.K., Fecci P.E., Sampson J.H., Gunn M.D. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J. Clin. Invest. 2020;130:774–788. doi: 10.1172/JCI128267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melacarne A., Ferrari V., Tiraboschi L., Mishto M., Liepe J., Aralla M., Marconato L., Lizier M., Pozzi C., Zeira O., Penna G., Rescigno M. Identification of a class of non-conventional ER-stress-response-derived immunogenic peptides, Cell. For. Rep. 2021;36 doi: 10.1016/j.celrep.2021.109312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 139.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Stadtman E.R. Role of oxidant species in aging. Curr. Med. Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 141.Yusupov M., Privat-Maldonado A., Cordeiro R.M., Verswyvel H., Shaw P., Razzokov J., Smits E., Bogaerts A. Oxidative damage to hyaluronan–CD44 interactions as an underlying mechanism of action of oxidative stress-inducing cancer therapy. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin A., Razzokov J., Verswyvel H., Privat-Maldonado A., De Backer J., Yusupov M., De La Hoz E.C., Ponsaerts P., Smits E., Bogaerts A. Oxidation of innate immune checkpoint CD47 on cancer cells with non-thermal plasma. Cancers. 2021;13:579. doi: 10.3390/cancers13030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hutnik C.M.L., Pocrnich C.E., Liu H., Laird D.W., Shao Q. The protective effect of functional Connexin43 channels on a human epithelial cell line exposed to oxidative stress. Invest. Ophthalmol. Vis. Sci. 2008;49:800–806. doi: 10.1167/iovs.07-0717. [DOI] [PubMed] [Google Scholar]
- 144.Retamal M.A., Schalper K.A., Shoji K.F., Bennett M.V.L., Sáez J.C. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Vuyst E., Decrock E., Cabooter L., Dubyak G.R., Naus C.C., Evans W.H., Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Smet M.A.J., Lissoni A., Nezlobinsky T., Wang N., Dries E., Pérez-Hernández M., Lin X., Amoni M., Vervliet T., Witschas K., Rothenberg E., Bultynck G., Schulz R., Panfilov A.V., Delmar M., Sipido K.R., Leybaert L. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Invest. 2021;131 doi: 10.1172/JCI137752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan Y., Wei C.-L., Zhang W.-R., Cheng H.-P., Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 148.Gorlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bao X., Sung C.L., Reuss L., Altenberg G.A. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4919–4924. doi: 10.1073/pnas.0603154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alstrøm J.S., Hansen D.B., Nielsen M.S., MacAulay N. Isoform-specific phosphorylation-dependent regulation of connexin hemichannels. J. Neurophysiol. 2015;114:3014–3022. doi: 10.1152/jn.00575.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Giardina S.F., Mikami M., Goubaeva F., Yang J. Connexin 43 confers resistance to hydrogen peroxide-mediated apoptosis. Biochem. Biophys. Res. Commun. 2007;362:747–752. doi: 10.1016/j.bbrc.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kar R., Riquelme M.A., Werner S., Jiang J.X. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J. Bone Miner. Res. 2013;28:1611–1621. doi: 10.1002/jbmr.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baklaushev V.P., Gurina O.I., Yusubalieva G.M., Grinenko N.F., Cytrin E.B., Victorov I.V., Chekhonin V.P. Immunofluorescent analysis of connexin-43 using monoclonal antibodies to its extracellular domain. Bull. Exp. Biol. Med. 2009;148:725–730. doi: 10.1007/s10517-010-0802-x. [DOI] [PubMed] [Google Scholar]
- 154.Baklaushev V.P., Nukolova N.N., Khalansky A.S., Gurina O.I., Yusubalieva G.M., Grinenko N.P., Gubskiy I.L., Melnikov P.A., Kardashova K.S., Kabanov A.V., Chekhonin V.P. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT. Drug Deliv. 2015;22:276–285. doi: 10.3109/10717544.2013.876460. [DOI] [PubMed] [Google Scholar]
- 155.Bao B., Jiang J., Yanase T., Nishi Y., Morgan J.R. Connexon-mediated cell adhesion drives microtissue self-assembly. Faseb. J. 2011;25:255–264. doi: 10.1096/fj.10-155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou J.Z., Riquelme M.A., Gu S., Kar R., Gao X., Sun L., Jiang J.X. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35:5597–5607. doi: 10.1038/onc.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu D.-P., Lin T.-Y., Bai L.-R., Huang J.-L., Zhou Y., Zhou N., Zhong S.-L., Gao S., Yin X.-X. Enhanced phototoxicity of photodynamic treatment by Cx26-composed GJIC via ROS-, calcium- and lipid peroxide-mediated pathways. J. Biophot. 2017;10:1586–1596. doi: 10.1002/jbio.201600255. [DOI] [PubMed] [Google Scholar]
- 158.Autsavapromporn N., de Toledo S.M., Little J.B., Jay-Gerin J.-P., Harris A.L., Azzama E.I. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose a-particle-irradiated human cells. Radiat. Res. 2011;175:347–357. doi: 10.1667/RR2372.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Y.-P., Zheng C.-C., Huang Y.-N., He M.-L., Xu W.W., Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2020;2:315–340. doi: 10.1002/mco2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Keidar M., Shashurin A., Volotskova O., Stepp M.A., Srinivasan P., Sandler A., Trink B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas. 2013;20 [Google Scholar]
- 161.Lu X., Naidis G.V., Laroussi M., Reuter S., Graves D.B., Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Phys. Rep. 2016;630:1–84. [Google Scholar]
- 162.Xu R.-G., Chen Z., Keidar M., Leng Y. The impact of radicals in cold atmospheric plasma on the structural modification of gap junction: a reactive molecular dynamics study. Int. J. Smart & Nano Mat. 2019;10:144–155. [Google Scholar]
- 163.Bagati A., Hutcherson T.C., Koch Z., Pechette J., Dianat H., Higley C., Chiu L., Song Y., Shah J., Chazen E., Nicolais A., Casey P., Thompson K., Burke K., Nikiforov M.A., Zirnheld J., Zucker S.N. Novel combination therapy for melanoma induces apoptosis via a gap junction positive feedback mechanism. Oncotarget. 2020;11:3443–3458. doi: 10.18632/oncotarget.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zucker S.N., Zirnheld J., Bagati A., DiSanto T.M., Des Soye B., Wawrzyniak J.A., Etemadi K., Nikiforov M., Berezney R. Preferential induction of apoptotic cell death in melanoma cells as compared with normal keratinocytes using a non-thermal plasma torch. Cancer Biol. Ther. 2012;13:1299–1306. doi: 10.4161/cbt.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.