Abstract
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has inflicted immense damage to countries, economies and societies worldwide. Authorized COVID-19 vaccines based on different platforms have been widely inoculated in adults, showing up to 100% immunogenicity with significant efficacy in preventing SARS-CoV-2 infections and the occurrence of severe COVID-19. It has also greatly slowed the evolution of SARS-CoV-2 variants, as shown in clinical trials and real-world evidence. However, the total dosage of COVID-19 vaccines for children is much smaller than that for adults due to limitations from parental concern of vaccine safety, presenting a potential obstacle in ending the COVID-19 pandemic. SARS-CoV-2 not only increases the risk of severe multisystem inflammatory syndrome (MIS-C) in children, but also negatively affects children's psychology and academics, indirectly hindering the maintenance and progress of normal social order. Therefore, this article examines the clinical manifestations of children infected with SARS-CoV-2, the status of vaccination against COVID-19 in children, vaccination-related adverse events, and the unique immune mechanisms of children. In particular, the necessity and challenges of vaccinating children against SARS-CoV-2 were highlighted from the perspectives of society and family. In summary, parental hesitancy is unnecessary as adverse events after COVID-19 vaccination have been proven to be infrequent, comprise of mild symptoms, and have a good prognosis.
Keywords: SARS-CoV-2, COVID-19, Children, Vaccine, Adverse event, MIS-C, Omicron
1. Introduction
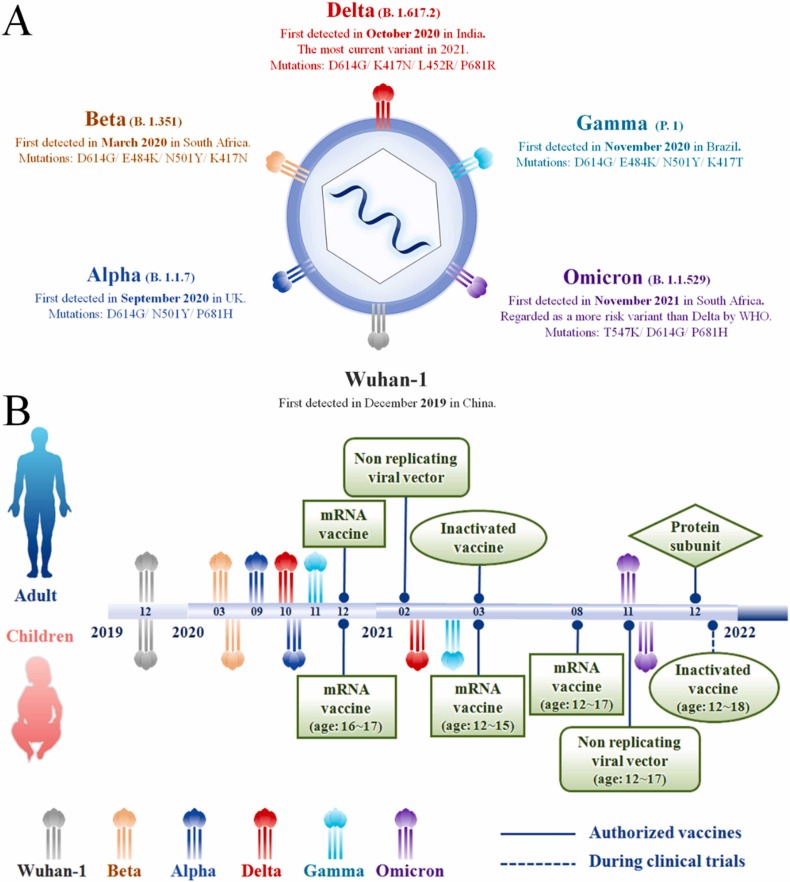
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first emerged at the end of 2019, causing the coronavirus disease 2019 (COVID-19) which has resulted in more than 406 million infected cases and nearly 5.8 million deaths worldwide as of February 10, 2022 [1], [2], [3], [4]. Since the first outbreak of the COVID-19 pandemic, SARS-COV-2 has evolved into thirteen variants from the original D614G lineage (Wuhan-1), including five variants of concern (VOCs) ( Fig. 1A) and eight variants of interest (VOIs), namely Epsilon (B.1.427 and B.1.429), Zeta (P.2), Eta (B.1.525), Theta (P.3), Iota (B.1.526), Kappa (B.1.617.1), Lambda (C.37) and Mu (B.1.621) [5], [6], [7], [8]. Successively, Wuhan-1, Beta (B.1.351), Delta (B.1.617.2), and Omicron (B.1.1.529) have led four waves of global pandemics, and have displayed increasing transmissibility and reduced sensitivity to immune mechanisms [9], [10], [11], [12], [13], [14], [15], [16]. Therefore, the emergence of SARS-CoV-2 VOCs presents a challenge in controlling the spread of SARS-CoV-2 infection and in establishing immunity among the community.
Fig. 1.
(A) The current variants of SARS-CoV-2; (B) A timeline depicting the distinction of SARS-CoV-2 variants and vaccines between adults and children.
Since the first wave of pandemic, a total of ten vaccines based on different platforms were authorized by the World Health Organization (WHO) listed under emergency use listing (EUL), including protein subunit vaccines (Novavax, COVOVAX), RNA vaccines (Pfizer-BioNTech, Moderna), non-replicating viral vectors (Janssen, Oxford/AstraZeneca, Covishield), and inactivated vaccines (Covaxin, Covilo, CoronaVac). The vaccines listed under EUL provide the fundamental impetus for the control of COVID-19 epidemic [17], [18], [19], [20]. Currently, 197 countries and regions have conducted widespread vaccinations, and the cumulative global dose has exceeded 10.32 billion doses, of which about 61.7% of the world population has received at least one dose (up to February 12, 2022) [21]. Vaccination against COVID-19 in adults showed considerable safety, high level of neutralizing antibody titers, and a strong immune effect against new variants of SARS-CoV-2, as shown by clinical trials and real-world evidence, such as achieving nearly 100% effectiveness in preventing the occurrence of severe cases and deaths [22], [23], [24], [25]. Therefore, COVID-19 vaccines have played an important role in controlling the spread of COVID-19 in adults in practical application.
However, the vaccination rate of children was much lower than that of adults, which may be closely related to the hesitancy of parents [26], [27]. The COVID-19 vaccine is worrying for some parents who have not vaccinated their children, and this concern stems from three main sources: (1) the lack of severe symptoms of COVID-19 in children [28], [29], [30]; (2) the adverse events after vaccination, such as fatigue, dizziness, injection pain and even myocarditis [31], [32], [33], [34], [35], [36]; (3) insufficient clinical data on the efficacy of each vaccine [37]. In fact, this hesitancy of parents is unnecessary, because more than 65% of the side effects caused by the COVID-19 vaccine in children is relieved or ceases within 1–3 days [38], [39], [40], [41]. Furthermore, clinical data proves that vaccines authorized for children have significantly shown strong efficacy in preventing infection and reducing the risk of morbidity of COVID-19 in children [42], [43], [44], [45], [46], [47], [48]. In addition, the innate immunity of children is not strong enough to block the invasion of new SARS-CoV-2 variants, which may be a key reason for the increase in the infection rate of children during the third and fourth waves of the COVID-19 pandemic [49], [50], [51], [52], [53]. Therefore, as an integral aspect of the societal transmission of SARS-CoV-2, children should be vaccinated as soon as possible to prevent a wider and more severe COVID-19 pandemic.
Children infected with SARS-CoV-2 display a series of unique characteristics in symptomatology, epidemiology and immune response mechanism compared to adults. Thus, deriving a set of exclusive vaccination strategies for children is necessary. In addition to establishing a systematic review of the efficacy of existing COVID-19 vaccines for children, the clinical manifestations of children infected with SARS-CoV-2, the status of vaccination against COVID-19 in children, vaccination-related adverse events, and the unique immune mechanisms of children are discussed in this article. Furthermore, the necessity and challenges of vaccinating children against SARS-CoV-2 from a social perspective is reviewed in order to provide a direction towards prevention strategies and effective countermeasures for children in the global post-pandemic era.
2. The clinical manifestation of SARS-CoV-2 infected children
The incidence of COVID-19 in children is lower than that in adults, which is related to the lower expression of angiotensin converting enzyme-2 (ACE2) in children [51], [54]. Pathophysiological analysis shows that ACE2, the primary target receptor for SARS-CoV-2, is less present in children and may be responsible for the low number of infections, mild symptoms, and low viral load in children with COVID-19 [55], [56], [57], [58], [59], [60]. However, children with stronger innate immunity than adults, are more susceptible to SARS-CoV-2-related severe multisystem inflammatory syndrome in children (MIS-C), an immune overexpression disease characterized by diarrhea, dizziness, arrhythmia and other multisystem symptoms [27], [59], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]. Severe MIS-C increases the risk of hospitalization and is the most common cause of death among children with COVID-19 [65], [72], [73]. Thus, the severe consequences for children infected with SARS-CoV-2 cannot be underestimated.
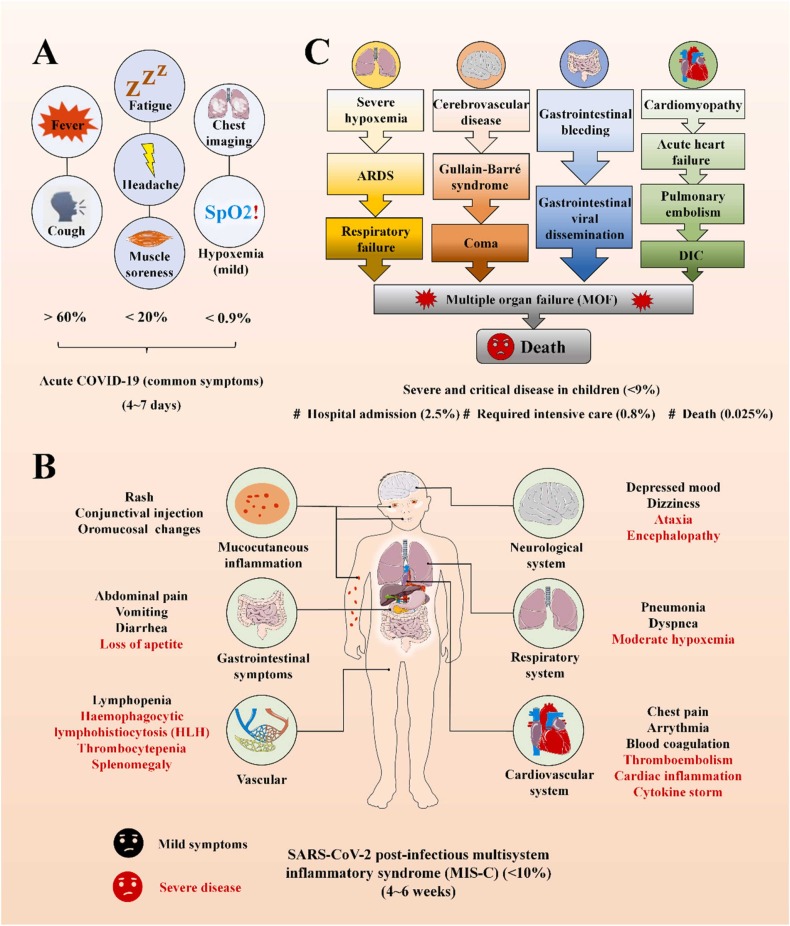
Anna Mania et al. (2021) evaluated clinical findings, laboratory parameters, and outcomes of a total of 332 children with COVID-19 who were split into three groups based on the severity of clinical symptoms, which includes mild to moderate COVID-19 (267 children), COVID-19 related pneumonia and those with oxygen therapy (OT) (60 children), and COVID-19 related pneumonia with intensive care required (ICU) (5 children) [74]. Combining the results of this study and observational studies in various countries, the infection of children with SARS-CoV-2 can be divided into three stages as follows ( Fig. 2) [62], [75]. Firstly, more than 80% of infections consisted of acute COVID-19 with mild or asymptomatic presentations, mainly occurring 4–6 days after infection, which indirectly suggests that the prevalence in children may be higher than reported (Fig. 2A) [76], [77], [78]. Secondly, less than 9% developed mild or moderate SARS-CoV-2 post-infectious MIS-C within 4–6 weeks of infection and inflammatory dysfunction of various degrees in multiple systems, such as mucocutaneous, gastrointestinal and cardiovascular, throughout the body (Fig. 2B) [72], [79], [80], [81], [82]. Thirdly, a small amount of patients with severe underlying diseases or moderate MIS-C without timely intervention, deteriorated and eventually progress to severe and critical disease, which included multiple organ failure (MOF) or death. Among all the above, about 2.5% were admitted to hospital, 0.8% required intensive care and the mortality rate was around 0.025% (Fig. 2C) [73], [83], [84], [85], [86], [87].
Fig. 2.
(A) Schematic represent the acute COVID-19 (common symptoms) caused by SARS-CoV-2. (B) Schematic represent the multisystem inflammatory syndrome (MIS-C) caused by SARS-CoV-2. (C) Schematic represent the severe critical disease in children.
3. Why children should be vaccinated against COVID-19?
With the frequent evolution of SARS-CoV-2, SARS-CoV-2 can also pose as an increasing threat to children. Vaccination, even with challenges including serious adverse events and uneven distribution of vaccines, is currently considered one of the most effective ways in mitigating the COVID-19 global pandemic. The need for children to be vaccinated against COVID-19 can be summarized as follows.
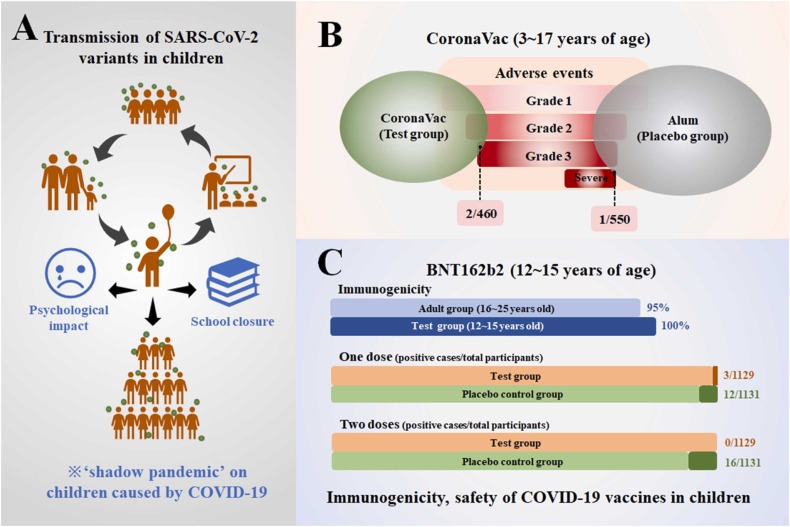
Firstly, the impact of SARS-CoV-2 on children is a key link in a series of chain reactions. Although children with COVID-19 have mild symptoms and low transmissibility, they are the nexus of SARS-CoV-2 transmission between families, schools and societies, with a ‘shadow pandemic’ on the economics and politics in adult society (Fig. 5A) [66], [88], [89], [90], [91], [92], [93], [94]. The ‘shadow pandemic’ has spawned school closures and mandatory home isolation policies around the world. As a result, children are facing more psychological problems from schools and families than in previous years [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105]. Therefore, children's COVID-19 vaccine is related to the progress of the epidemic worldwide, so completion of children’s vaccination requires the participation of the global society.
Fig. 5.
(A) The transmission of SARS-CoV-2 variants in children and the shadow pandemic on children caused by COVID-19; the immunogenicity, safety of (B) CoronaVac and (C) BNT162b2 in clinical trials.
Secondly, fully vaccinated populations have a greater advantage in controlling the incidence of COVID-19 over unvaccinated populations [45], [46], [47], [48], [106], [107]. Children aged 3–17 years vaccinated with inactivated SARS-CoV-2 vaccine (CoronaVac) displayed low severity of post-vaccination adverse events and a high safety profile, supported by data from a phase 1/2 clinical trial [106] (Fig. 5B). Available data indicates that the RNA vaccine (BNT162b2) is 95% immunogenic in 16–25 years old and 100% immunogenic in 12–15 years old, confirming the absolute superiority of RNA vaccines in preventing SARS-CoV-2 infection [48]. Other studies have shown that full vaccination of two or three doses of RNA vaccine can significantly increase the level of neutralizing antibodies in the recipients, preventing the occurrence of a global pandemic amid the frequent emergence of new variants of SARS-CoV-2 both in adults and children [45], [48], [108], [109], [110], [111], [112], [113]. Robust clinical data also confirmed that the COVID-19 vaccine is safe in children with specific diseases, including congenital immunodeficiency, congenital heart disease, pediatric inflammatory bowel disease and allergy [114], [115], [116], [117], [118], [119], [120], [121], [122]. Given that some parents are still hesitant about vaccine safety, reducing the single-dose volume of vaccines for children and extending the vaccination interval has been proven to reduce the occurrence of adverse events, which is expected to become the vaccination strategy for children in the future [111], [123], [124]. Thus, aside from those who are severely allergic to vaccine ingredients, children should be vaccinated against COVID-19 to slow the evolution of SARS-CoV-2 and the progression of the global pandemic.
Lastly, the current COVID-19 pandemic is extremely severe, which requires comprehensive vaccination around the world. Post-vaccination breakthrough infections caused by the SARS-CoV-2 variant, like the Delta and Omicron variant, is a common phenomenon, indicating that the SARS-CoV-2 variants have evolved the capability for immune evasion [10], [108], [125], [126]. In addition, it can be seen that physical barrier alone is not enough to fend off the COVID-19 pandemic for people of different ages, races and genders, so it is necessary to establish an immune barrier for all members through vaccination. In particular, children as a special group face some unique challenges, such as difficulty in strictly implementing physical protection, often being outside the scope of public health strategies, and lack of awareness of self-protection, making children one of the most vulnerable groups to be susceptible to new variants of SARS-CoV-2 [49], [52], [127], [128]. Moreover, the uneven distribution of vaccines in various countries and regions has become an obstacle in delaying the vaccination of children [129], [130], [131], [132]. It is reassuring that this issue is being effectively alleviated through the development of vaccines that are gradually maturing around the world, and it cannot be an obstacle from prioritizing vaccinating children [133], [134], [135], [136], [137]. Consequently, to accelerate the process of fully vaccinating all human beings against COVID-19, more clinical data related to vaccines for children are needed to dispel the concerns of parents regarding vaccine safety.
4. The status of vaccination against COVID-19 and related side effects in children
Due to the widespread vaccination of adults against COVID-19, a strong immune barrier against severe COVID-19 has been established in the population, which cannot be broken even by multiple evolutions of SARS-CoV-2 [10], [14]. Conversely, children are not widely vaccinated against COVID-19, which exposes them to SARS-CoV-2 without the protection of vaccines, increasing the risk of infection [53], [88], [138]. According to available data, as of December 31, 2021, nearly 9.17 billion doses of COVID-19 vaccine have been administered globally, of which 86.4%−90.4% have been administered to adults, whereas only 9.6%−13.6% have been administered to children [21], [139]. WHO approved the application of mRNA vaccines to children aged 16–17 as early as December 2020, and then expanded the authorized age to 12–17 years old in August 2021 (Fig. 1B) [48], [140]. Following, the non-replicating viral vector and inactivated vaccines were successively authorized by WHO for children over 12 years old [106], [141], [142]. Table 1 lists the status and immune efficacy of authorized COVID-19 vaccines for children, including mRNA vaccines (Pfizer-BioNTech, Moderna), non-replicating viral vector (Oxford/AstraZeneca, Ad26COVS1) and inactivated vaccines (Sinopharm, Sinovac). In particular, the Pfizer-BioNTech vaccine was considered one of the most effective vaccines, showing 100% immunogenicity and significant safety, which has been recommended by the Center for Disease Control and Prevention (CDC) as a booster for children vaccinators [143], [144], [145]. Moreover, Sinovac, which has a vaccination count of more than 2.3 billion doses (as of January 29, 2022) in 52 countries, shows 100% neutralizing antibody activity in children under 3 years old in international multicenter phase III clinical trials [146], [147]. However, despite the high safety and immunogenicity of vaccines authorized for children by WHO, this age group represents a low proportion of the total number of vaccinated populations. For example, results of a survey conducted in Spain between January 8, 2021 and January 21, 2022, showed significant differences in the proportion of age groups who were fully vaccinated. More than 90.49% of the population over 50 years old were fully vaccinated, whereas this group accounted for less than 44.41% in children under 14 years old. Moreover, less than 0.05% of children under 9 were fully vaccinated [45], [139]. Therefore, it is evident that the number of vaccinated children is far less than that of adults.
Table 1.
Vaccination status of the current authorized COVID-19 vaccines for children (ages 0–17 years) around the world.
| Platform types | Vaccine | Authorized events |
Vaccine effectiveness in children (95% CI) |
Booster dose | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Approval institution | Date | Number of authorized countries | Ages indication for children (year) |
|||||
| mRNA Vaccines | mRNA-BNT162b2 Other names: Pfizer-BioNTech; COMIRNATY |
*WHO EUL; *CRS EUR |
12/31/2020 | 134 | 12–17 (WHO); 5–17 (CDC) |
100% (immunogenicity) (USA, China, and South Africa) |
FDA: administration of a third primary series dose for children aged over 12 years and immunocompromise individuals aged 5–11 years Intervals: at least 5 months |
[48], [110], [112], [140], [148], [149] |
| mRNA-1273 Other names: Moderna; Spikevax |
*WHO EUL; *CRS EUR |
04/30/2021 | 85 | 12–17 (WHO) | 98.8% (serologic response) (USA) |
Age: 18 years and older Intervals: 5 months |
[46], [149], [150] | |
| Viral vector | ChAdOx1 nCoV-19 Other names: Oxford/AstraZeneca; Vaxzevria; AZD1222 |
*WHO EUL; *CRS EUR *ART Endorsed |
02/15/2021 | 137 | Pause | 76% (one dose) 81.3% (two doses) for adult (ages 18–55 years) 83.5% (two doses) for adult (ages over 65 years) (USA, Chile, and Peru) |
Age: 18–70 years old Intervals: at least 28 days |
|
| [123], [151], [152], [153], [154] | ||||||||
| Ad26. COV 2. SOther names: Janssen (Johnson & Johnson); Ad26COVS1 |
*WHO EUL; *CRS EUR *ART Endorsed |
12/03/2021 | 106 | Pause | 66.9% (prevention of severe COVID-19); 76.7% (severe-critical COVID-19) for adult (USA, Brazil, and South Africa) |
Age: 18 years and older Intervals: 2 months |
[154], [155], [156], [157], [158] | |
| Inactivated vaccine | BBIBP-CorV Other names: Sinopharm (Beijing); Covilo |
*WHO EUL; *CRS EUR *ART Endorsed |
05/07/2021 | 88 | 3–17 (Phase 3 clinical trial) | 100% (neutralization activity) (China) |
Age: 3 years and older Intervals: at least 28 days (two or three doses) |
[113], [142], [159], [160] |
| CoronaVac Other names: Sinovac |
*WHO EUL; *CRS EUR *ART Endorsed |
06/01/2021 | 52 | 6 months and more (Phase 3 clinical trial) |
100% (neutralization antibody activity) 100% (immunogenicity) (China) |
Age: 3 years and older Intervals: 28 days (second dose) 6 months (third dose) |
[106], [141], [161] | |
*WHO EUL: World Health Organization Emergency Use Listing
*CRS EUR: Caribbean Regulatory System Emergency Use Recommendation
*ART: Africa Regulatory Taskforce (ART) Endorsed
Part of the data come from official reports of individual countries or regions and do not represent global average.
The evaluation of efficacy and adverse effects of various vaccines is based on a comprehensive assessment of infection rates, mortality, doses, post-vaccination infections, occurrence and duration of post-vaccination adverse events.
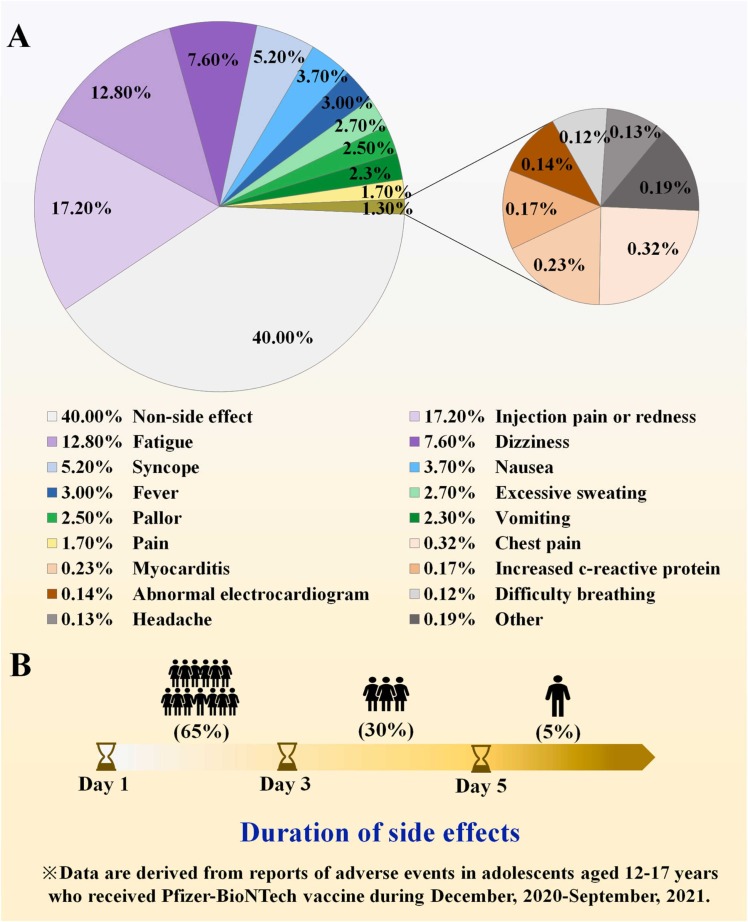
Findings from global surveys on the acceptance of vaccinating children against COVID-19 reveals that parental concerns mostly consist of side effects and doubts about incomplete clinical data describing vaccine efficacy [26], [31], [32], [43], [44], [154], [162], [163], [164]. Yet, the incidence of adverse events after vaccinating children was below 3%, among which the most common side effects were injection site discomfort, fatigue, dizziness, and in rare cases, myocarditis due to MIS-C ( Fig. 3A) [69], [71], [158], [165], [166], [167], [168], [169]. These symptoms were generally mild, 65% of which disappeared within 1–3 days after onset, and only 5% required hospitalization for further treatment (Fig. 3B) [165], [170], [171], [172]. In addition, serious adverse events with COVID-19 vaccines were rare, with fewer than 0.01% of deaths which were related to underlying diseases rather than the vaccine itself [38], [45], [47], [145]. For example, AstraZeneca and J&J adenoviral vector vaccines have been suspended in children for causing vaccine-induced fatal prothrombotic immune thrombocytopenia (VIPIT), although this side effect was found to occur only in women between the ages of 30 and 65 [67], [68], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182]. Side effects were also shown to be related to the type of vaccine. Sinopharm vaccines, for instance, were found to be safe and showed a lower prevalence of adverse events compared with other vaccines[183], [184]. As COVID-19 vaccines related adverse events appear to have negligible impact on vaccinated children, it follows that vaccinating children remains a safe option in preventing the COVID-19 epidemic from further developing.
Fig. 3.
(A) The types and proportions of adverse events reported from children who vaccinated with COVID-19 vaccines. (B) The duration of post-vaccination adverse events of COVID-19 vaccine in children.
5. The COVID-19 vaccine mechanism in children
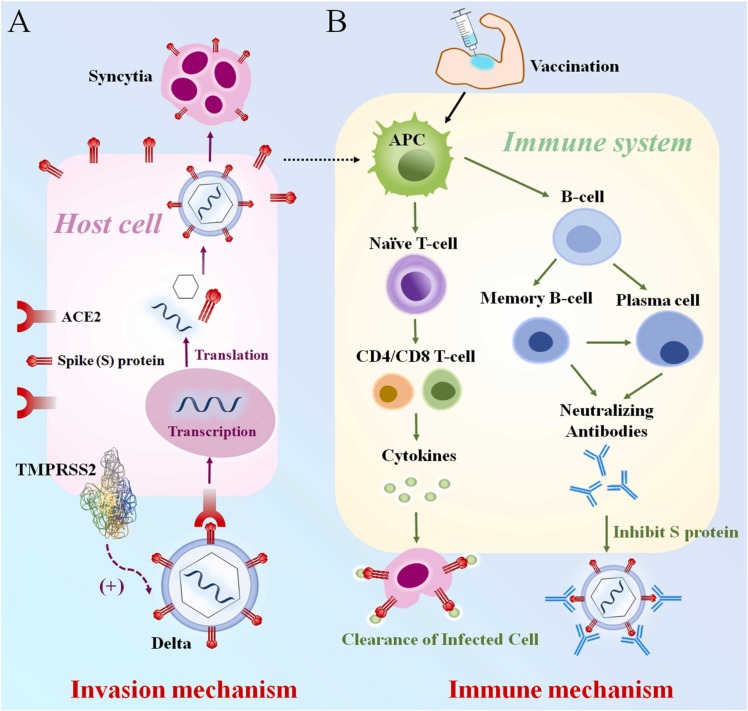
COVID-19 vaccines have strong immune efficacy and safety in stopping the global COVID-19 pandemic [110], [111]. Therefore, understanding the mechanism of SARS-CoV-2 infection and the COVID-19 vaccine immunization process in children is a preferential method in designing the appropriate vaccine formulation. Design of the vaccine starts from understanding the basic structure of the Spike (S) protein, which is the characteristic structure for SARS-CoV-2 to enter host cells. The S protein is contained by a receptor binding domain (RBD) and an N-terminal domain (NTD). The mutations on RBD and NTD determine the type of SARS-CoV-2 variant and the severity of COVID-19 [185], [186], [187], [188], [189]. SARS-CoV-2 can bind to the ACE2 receptor under the mediation of transmembrane serine protease 2 (TMPRSS2), and sends RNA into the host cell for intracellular transcription and translation [185], [190], [191]. More ACE2 receptors subside in the lung, heart and kidney, which tends to promote membrane fusion, leading to more syncytia than other organs ( Fig. 4A) [189], [192], [193], [194], [195], [196], [197], [198], [199]. The vaccines developed according to the above process can activate humoral and cellular immunity by inducing the antigen presenting cells (APC) of children, wherein humoral immunity produces neutralizing antibodies that can bind to the ACE2 receptor, preventing the invasion of SARS-CoV-2 [200], [201], [202], [203], [204]. On the other hand, cytokines produced by the cellular immune system, such as IL-6, can rapidly label and eliminate infected host cells [205], [206], [207]. These two immune pathways are the strongest barriers to ensuring that children are not infected with SARS-CoV-2 (Fig. 4B). The above processes in children are not fundamentally different from those in adults. Due to the special immune characteristics of children, however, children with COVID-19 have milder symptoms and lower viral loads. Furthermore, children can induce similar or higher levels of neutralizing antibodies compared to adults [28], [51], [208]. In essence, children have stronger innate immunity compared to adults, which leads to early control of infection at the site of entry and a lower chance of transmission between children, but it also makes children more susceptible to MIS-C [50], [54], [200], [209]. In addition, it is theorized that the low expression of ACE2 in children, the weak affinity of SARS-CoV-2 with ACE2 in children, and the strong regeneration ability of the pediatric lung epithelium are all protective factors for children suffering from COVID-19 [210], [211], [212], [213]. Even so, the infection of SARS-CoV-2 is a common phenomenon in children. Therefore, the decisive role of vaccines in fighting SARS-CoV-2 infection in children cannot be ignored, and it is imperative to widely vaccinate children. Fig. 5.
Fig. 4.
(A) The mechanism of SARS-CoV-2 invasion in children and (B) immune mechanism of children vaccinated against COVID-19.
6. Conclusion
The COVID-19 pandemic has ravaged the world, which has affected children both physically and mentally. It is one of the most threatening diseases that children are facing this century. Since children play an essential role in the evolution and transmission chain of SARS-CoV-2, vaccinating children under strict physical protection is necessary. Currently, widely authorized vaccines have demonstrated about 100% superiority in both efficacy and safety against SARS-CoV-2 infections between the age of 12–17 years. Although a few worrisome adverse events have caused parental hesitation, it is worth mentioning that those post-vaccination side effects were almost exclusively mild, such as injection site discomfort, fatigue and dizziness, which relieved or resolve within 5 days in 95% of cases. Upon examining clinical trials and the evolution of the virus, we note that children are key factors in ending the pandemic and the vaccination of children can effectively help reduce societal harm and prevent the intensification of the pandemic. More clinical data is required for the various types of vaccines for children to provide a safer and more effective solution in ending COVID-19.
Conflict of Interest
The authors declared that they have no conflicts of interest to this work.
References
- 1.SeyedAlinaghi S., Mirzapour P., Dadras O., et al. Characterization of SARS-CoV-2 different variants and related morbidity and mortality: a systematic review. Eur J Med Res. 2021;26:51. doi: 10.1186/s40001-021-00524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awadasseid A., Wu Y., Tanaka Y., Zhang W. SARS-CoV-2 variants evolved during the early stage of the pandemic and effects of mutations on adaptation in Wuhan populations. Int J Biol Sci. 2021;17:97–106. doi: 10.7150/ijbs.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey W.T., Carabelli A.M., Jackson B., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh D.D., Parveen A., Yadav D.K. SARS-CoV-2: emergence of new variants and effectiveness of vaccines. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.777212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistry P., Barmania F., Mellet J., et al. SARS-CoV-2 variants, vaccines, and host immunity. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirabara S.M., Serdan T.D.A., Gorjao R., et al. SARS-COV-2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdul A., Abdul B.A.S., Amy K.S. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19), 2022. [PubMed]
- 9.Thakur V., Ratho R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. 2021 doi: 10.1002/jmv.27541. [DOI] [PubMed] [Google Scholar]
- 10.Mahase E. Covid-19: Omicron and the need for boosters. BMJ. 2021;375:n3079. doi: 10.1136/bmj.n3079. [DOI] [PubMed] [Google Scholar]
- 11.Safari I., Elahi E. Evolution of the SARS-CoV-2 genome and emergence of variants of concern. Arch Virol., 2021. [DOI] [PMC free article] [PubMed]
- 12.Salleh M.Z., Derrick J.P., Deris Z.Z. Structural Evaluation of the Spike Glycoprotein Variants on SARS-CoV-2 Transmission and Immune Evasion. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22147425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson C.B., Zhang L., Farzan M., Choe H. Functional importance of the D614G mutation in the SARS-CoV-2 spike protein. Biochem Biophys Res Commun. 2021;538:108–115. doi: 10.1016/j.bbrc.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araf Y., Akter F., Tang Y.D., et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022 doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X., Hong W., Pan X., Lu G., Wei X. SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm, 2020: 2021. [DOI] [PMC free article] [PubMed]
- 16.Kannan S., Shaik Syed Ali P., Sheeza A. Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur Rev Med Pharm Sci. 2021;25:8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- 17.Spencer A.J., Morris S., Ulaszewska M., et al. The ChAdOx1 vectored vaccine, AZD2816, induces strong immunogenicity against SARS-CoV-2 B.1.351 and other variants of concern in preclinical studies. 2021: 10.1101/2021.06.08.447308. [DOI] [PMC free article] [PubMed]
- 18.Abdulla Z.A., Al-Bashir S.M., Al-Salih N.S., Aldamen A.A., Abdulazeez M.Z. A Summary of the SARS-CoV-2 Vaccines and Technologies Available or under Development. Pathogens. 2021:10. doi: 10.3390/pathogens10070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitiello A., Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021;29:645–649. doi: 10.1007/s10787-021-00811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.We are tracking the progress of COVID-19 vaccine candidates to monitor the latest developments, 2022.
- 21.Our World in Data. Coronavirus (COVID-19) Vaccinations, 2022.
- 22.Fiolet T., Kherabi Y., MacDonald C.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zinatizadeh M.R., Zarandi P.K., Zinatizadeh M., Yousefi M.H., Amani J., Rezaei N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharm. 2022;146 doi: 10.1016/j.biopha.2021.112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada T., Zhou H., Samanovic M.I., et al. Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants. bioRxiv. 2021 doi: 10.1101/2021.07.19.452771. [DOI] [Google Scholar]
- 25.Haveri A., Ekström N., Solastie A., et al. Persistence neutralizing antibodies a year SARS-CoV-2 Infect. 2021 doi: 10.1101/2021.07.13.21260426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan F., Zhao H., Nicholas S., Maitland E., Liu R., Hou Q. Parents' Decisions to Vaccinate Children against COVID-19: A Scoping Review. Vaccin (Basel) 2021:9. doi: 10.3390/vaccines9121476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson L.C., Soveri A., Lewandowsky S., et al. Fearing the disease or the vaccine: The case of COVID-19. Pers Individ Dif. 2021;172 doi: 10.1016/j.paid.2020.110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 29.Bhalala U.S., Gist K.M., Tripathi S., et al. Characterization and Outcomes of Hospitalized Children With Coronavirus Disease 2019: A Report From a Multicenter, Viral Infection and Respiratory Illness Universal Study (Coronavirus Disease 2019) Registry. Crit Care Med. 2022;50:e40–e51. doi: 10.1097/CCM.0000000000005232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olusanya O.A., Bednarczyk R.A., Davis R.L., Shaban-Nejad A. Addressing Parental Vaccine Hesitancy and Other Barriers to Childhood/Adolescent Vaccination Uptake During the Coronavirus (COVID-19) Pandemic. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.663074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skjefte M., Ngirbabul M., Akeju O., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teherani M., Banskota S., Camacho-Gonzalez A., et al. Intent to Vaccinate SARS-CoV-2 Infected Children in US Households: A Survey. Vaccines. 2021:9. doi: 10.3390/vaccines9091049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afifi T.O., Salmon S., Taillieu T., Stewart-Tufescu A., Fortier J., Driedger S.M. Older adolescents and young adults willingness to receive the COVID-19 vaccine: Implications for informing public health strategies. Vaccine. 2021;39:3473–3479. doi: 10.1016/j.vaccine.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman R.D., Krupik D., Ali S., et al. Caregiver Willingness to Vaccinate Their Children against COVID-19 after Adult Vaccine Approval. Int J Environ Res Public Health. 2021:18. doi: 10.3390/ijerph181910224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh M., Bharti B., Bharti S., Gupta S. Needle Fear among Children during Mass Measles Rubella (MR) Injectable Vaccination Campaign in North India: an Observational Study. J Child Adolesc Trauma. 2021 doi: 10.1007/s40653-021-00352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandstetter S., Boehmer M.M., Pawellek M., et al. Parents' intention to get vaccinated and to have their child vaccinated against COVID-19: cross-sectional analyses using data from the KUNO-Kids health study. Eur J Pediatr. 2021;180:3405–3410. doi: 10.1007/s00431-021-04094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.She J., Liu L., Liu W. Providing children with COVID-19 vaccinations is challenging due to lack of data and wide-ranging parental acceptance. Acta Paediatr. 2022;111:35–44. doi: 10.1111/apa.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zakeri M., Li J., Sadeghi S.D., Essien E.J., Sansgiry S.S. Strategies to decrease COVID-19 vaccine hesitancy for children. J Pharm Health Serv Res. 2021;12:539–544. [Google Scholar]
- 39.Bagateli L.E., Saeki E.Y., Fadda M., Agostoni C., Marchisio P., Milani G.P. COVID-19 Vaccine Hesitancy among Parents of Children and Adolescents Living in Brazil. Vaccines. 2021:9. doi: 10.3390/vaccines9101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldakhil H., Albedah N., Alturaiki N., Alajlan R., Abusalih H. Vaccine hesitancy towards childhood immunizations as a predictor of mothers' intention to vaccinate their children against COVID-19 in Saudi Arabia. J Infect Public Health. 2021;14:1497–1504. doi: 10.1016/j.jiph.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musa S., Dergaa I., Abdulmalik M.A., Ammar A., Chamari K., Ben Saad H. BNT162b2 COVID-19 Vaccine Hesitancy among Parents of 4023 Young Adolescents (12-15 Years) in Qatar. Vaccines. 2021:9. doi: 10.3390/vaccines9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladhani S.N. Crossing the Rubicon: A fine line between waiting and vaccinating adolescents against COVID-19. J Infect. 2021;83:294–297. doi: 10.1016/j.jinf.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thunstrom L., Ashworth M., Finnoff D., Newbold S.C. Hesitancy Toward a COVID-19 Vaccine. Ecohealth. 2021;18:44–60. doi: 10.1007/s10393-021-01524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szilagyi P.G., Shah M.D., Delgado J.R., et al. Parents' Intentions and Perceptions About COVID-19 Vaccination for TheirChildren: Results From a National Survey. Pediatrics. 2021:148. doi: 10.1542/peds.2021-052335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastorino R., Pezzullo A.M., Villani L., et al. Change in age distribution of COVID-19 deaths with the introduction of COVID-19 vaccination. Environ Res. 2022;204 doi: 10.1016/j.envres.2021.112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali K., Berman G., Zhou H., et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu F., Jin P., Zhu T., et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomised, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis., 2021. [DOI] [PMC free article] [PubMed]
- 48.Frenck R.W., Klein N.P., Kitchin N., et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonker L.M., Neilan A.M., Bartsch Y., et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J Pedia. 2020;227 doi: 10.1016/j.jpeds.2020.08.037. 45-52 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S., Siddique R., Hao X., et al. The COVID-19 infection in children and its association with the immune system, prenatal stress, and neurological complications. Int J Biol Sci. 2022;18:707–716. doi: 10.7150/ijbs.66906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta S., Smith L., Diakiw A. Avoidance of COVID-19 for Children and Adolescents and Isolation Precautions. Pediatr Clin North Am. 2021;68:1103–1118. doi: 10.1016/j.pcl.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viner R., Waddington C., Mytton O., et al. Transmission of SARS-CoV-2 by children and young people in households and schools: A meta-analysis of population-based and contact-tracing studies. J Infect. 2021 doi: 10.1016/j.jinf.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau C.M., Adams N.M., Geary C.D., et al. Epigenetic control of innate and adaptive immune memory. Nat Immunol. 2018;19:963–972. doi: 10.1038/s41590-018-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borrelli M., Corcione A., Castellano F., Fiori Nastro F., Santamaria F. Coronavirus Disease 2019 in Children. Front Pedia. 2021;9 doi: 10.3389/fped.2021.668484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu X., Zhang L., Du H., et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhochak N., Singhal T., Kabra S.K., Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pedia. 2020;87:537–546. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta N.S., Mytton O.T., Mullins E.W.S., et al. SARS-CoV-2 (COVID-19): What Do We Know About Children? A Systematic Review. Clin Infect Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapustova L., Petrovicova O., Banovcin P., et al. COVID-19 and the differences in physiological background between children and adults and their clinical consequences. Physiol Res. 2021;70:S209–S225. doi: 10.33549/physiolres.934759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kloc M., Ghobrial R.M., Kuchar E., Lewicki S., Kubiak J.Z. Development of child immunity in the context of COVID-19 pandemic. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molteni E., Sudre C.H., Canas L.S., et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–718. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dionne A., Son M.B.F., Randolph A.G. An update on multisystem inflammatory syndrome in children related to SARS-CoV-2. Pedia Infect Dis J. 2022;41:e6–e9. doi: 10.1097/INF.0000000000003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schleiss M.R., John C.C., Permar S.R. Children are the key to the Endgame: A case for routine pediatric COVID vaccination. Vaccine. 2021;39:5333–5336. doi: 10.1016/j.vaccine.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang J., Lee S.B., Lee S.W., et al. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J Autoimmun. 2021;122 doi: 10.1016/j.jaut.2021.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz N.H., Sorvoll I.H., Michelsen A.E., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jain S.S., Steele J.M., Fonseca B., et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021:148. doi: 10.1542/peds.2021-053427. [DOI] [PubMed] [Google Scholar]
- 70.Das B.B., Kohli U., Ramachandran P., et al. Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18Years of Age. J Pediatr. 2021;238:26–32. doi: 10.1016/j.jpeds.2021.07.044. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snapiri O., Danziger C.R., Shirman N., et al. Transient Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19 Vaccine. Pediatr Infect Dis J. 2021;40:E360–E363. doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kabeerdoss J., Pilania R.K., Karkhele R., Kumar T.S., Danda D., Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheuma Int. 2021;41:19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellino S., Punzo O., Rota M.C., et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020:146. doi: 10.1542/peds.2020-009399. [DOI] [PubMed] [Google Scholar]
- 74.Mania A., Faltin K., Mazur-Melewska K., et al. Clinical Picture and Risk Factors of Severe Respiratory Symptoms in COVID-19 in Children. Viruses. 2021:13. doi: 10.3390/v13122366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard-Jones A.R., Burgner D.P., Crawford N.W., et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58:46–53. doi: 10.1111/jpc.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metbulut A.P., Ozkaya Parlakay A., Bayhan G.I., et al. Evaluation of cutaneous symptoms in children infected with COVID-19. Pedia Allergy Immunol. 2021;32:1120–1125. doi: 10.1111/pai.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayor S. Covid-19: UK studies find gastrointestinal symptoms are common in children. BMJ. 2020;370:m3484. doi: 10.1136/bmj.m3484. [DOI] [PubMed] [Google Scholar]
- 78.Govil-Dalela T., Sivaswamy L. Neurological Effects of COVID-19 in Children. Pedia Clin North Am. 2021;68:1081–1091. doi: 10.1016/j.pcl.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K.G. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology. 2020;159:1571–1574. doi: 10.1053/j.gastro.2020.05.079. e1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J.E., Asfour A., Sewell T.B., et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743 doi: 10.1016/j.neulet.2020.135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soleimani A., Soleimani Z. Presentation and outcome of congenital heart disease during Covid-19 pandemic: a review. Curr Probl Cardiol. 2022;47 doi: 10.1016/j.cpcardiol.2021.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arizaga-Ballesteros V., Gutierrez-Mendoza M.A., Villanueva-Sugishima K.R., Santos-Guzman J. Pediatric inflammatory multisystem syndrome or multisystem inflammatory syndrome in children: a new thread in pandemic era. Glob Pedia Health. 2021;8 doi: 10.1177/2333794X211050311. 2333794×211050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giacomet V., Barcellini L., Stracuzzi M., et al. Gastrointestinal Symptoms in Severe COVID-19 Children. Pedia Infect Dis J. 2020;39:e317–e320. doi: 10.1097/INF.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 84.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poisson K.E., Zygmunt A., Leino D., et al. Lethal Pediatric Cerebral Vasculitis Triggered by Severe Acute Respiratory Syndrome Coronavirus 2. Pedia Neurol. 2022;127:1–5. doi: 10.1016/j.pediatrneurol.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akcay N., Ogur M., Emin Menentoglu M., et al. Acute Cerebellitis in MIS-C: A Case Report. Pedia Infect Dis J. 2022;41:e16–e18. doi: 10.1097/INF.0000000000003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu P.Y., Gragnani C.M., Timmerman J., et al. Pediatric Household Transmission of Severe Acute Respiratory Coronavirus-2 Infection-Los Angeles County, December 2020 to February 2021. Pediatr Infect Dis J. 2021;40:E379–E381. doi: 10.1097/INF.0000000000003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkinson D., Finlay I., Pollard A.J., Forsberg L., Skelton A. Should we delay covid-19 vaccination in children? BMJ. 2021;374:n1687. doi: 10.1136/bmj.n1687. [DOI] [PubMed] [Google Scholar]
- 90.Hevia F.J., Vergara-Lope S., Velasquez-Duran A., Calderon D. Estimation of the fundamental learning loss and learning poverty related to COVID-19 pandemic in Mexico. Int J Educ Dev. 2022;88 doi: 10.1016/j.ijedudev.2021.102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serrano-Díaz N., Aragón-Mendizábal E., Mérida-Serrano R. Families’ perception of children’s academic performance during the COVID-19 lockdown. Comunicar. 2022;30:59–68. [Google Scholar]
- 92.Howard-Jones A.R., Bowen A.C., Danchin M., et al. COVID-19 in children: I. Epidemiology, prevention and indirect impacts. J Paediatr Child Health. 2022;58:39–45. doi: 10.1111/jpc.15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alyasin S., Kanannejad Z., Esmaeilzadeh H., Nabavizadeh H., Ghatee M.A., Amin R. Relationship between Bacillus Calmette Guerin Vaccination Policy and Coronavirus Disease-2019 (COVID-19) Incidence. Iran J Allergy Asthma Immunol. 2021;20:106–113. doi: 10.18502/ijaai.v20i1.5417. [DOI] [PubMed] [Google Scholar]
- 94.Chen A.T., Stacey H.D., Marzok A., et al. Effect of inactivated influenza vaccination on human coronavirus infection: Secondary analysis of a randomized trial in Hutterite colonies. Vaccine. 2021;39:7058–7065. doi: 10.1016/j.vaccine.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massad E., Amaku M., Covas D.T., Lopez L.F., Bezerra Coutinho F.A. Estimating the effects of reopening of schools on the course of the epidemic of COVID-19. Epidemiol Infect. 2021:149. doi: 10.1017/S0950268821000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J.T., Mei S., Luo S., et al. A global assessment of the impact of school closure in reducing COVID-19 spread. Philos Trans A Math Phys Eng Sci. 2022;380 doi: 10.1098/rsta.2021.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burrows A.G., Ellis A.K. Psychological impacts of coronavirus disease 2019 on people with asthma, allergic rhinitis, and food allergy. Ann Allergy Asthma Immunol. 2021 doi: 10.1016/j.anai.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ravichandran S., Tang J., Grubbs G., et al. SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19. Nat Immunol. 2021;22:1452–1464. doi: 10.1038/s41590-021-01051-8. [DOI] [PubMed] [Google Scholar]
- 99.Piroth L., Cottenet J., Mariet A.-S., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feinberg M.E., J A.M., Lee J.K., et al. Impact of the COVID-19 Pandemic on Parent, Child, and Family Functioning. Fam Process. 2021 doi: 10.1111/famp.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams S.H., Schaub J.P., Nagata J.M., Park M.J., Brindis C.D., Irwin CE Jr. Young Adult Perspectives on COVID-19 Vaccinations. J Adolesc Health. 2021;69:511–514. doi: 10.1016/j.jadohealth.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agarwal V., L G B.K.S. Impact of COVID-19 on the mental health among children in China with specific reference to emotional and behavioral disorders. Int J Hum Rights Healthc. 2020;14:182–188. [Google Scholar]
- 103.Manchia M., Gathier A.W., Yapici-Eser H., et al. The impact of the prolonged COVID-19 pandemic on stress resilience and mental health: A critical review across waves. Eur Neuropsychopharmacol. 2022;55:22–83. doi: 10.1016/j.euroneuro.2021.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun J., Singletary B., Jiang H., Justice L.M., Lin T.J., Purtell K.M. Child behavior problems during COVID-19: Associations with parent distress and child social-emotional skills. J Appl Dev Psychol. 2022;78 doi: 10.1016/j.appdev.2021.101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abrams E.M., Greenhawt M., Shaker M., Pinto A.D., Sinha I., Singer A. The COVID-19 pandemic: Adverse effects on the social determinants of health in children and families. Ann Allergy Asthma Immunol. 2022;128:19–25. doi: 10.1016/j.anai.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han B., Song Y., Li C., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson E.J., Campbell J.D., Creech C.B., et al. Warp Speed for Coronavirus Disease 2019 (COVID-19) Vaccines: Why Are Children Stuck in Neutral? Clin Infect Dis. 2021;73:336–340. doi: 10.1093/cid/ciaa1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoffmann M., Kruger N., Schulz S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng Y.J., Wang X.C., Feng L.Z., et al. Expert consensus on COVID-19 vaccination in children. World J Pedia. 2021;17:449–457. doi: 10.1007/s12519-021-00465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Falsey A.R., Frenck R.W., Jr., Walsh E.E., et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gerber J.S., Offit P.A. COVID-19 vaccines for children. Science. 2021;374:913. doi: 10.1126/science.abn2566. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Beltran W.F., St, Denis K.J., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021 doi: 10.1101/2021.12.14.21267755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ai J., Zhang H., Zhang Y., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alexander J.L., Moran G.W., Gaya D.R., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6:218–224. doi: 10.1016/S2468-1253(21)00024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Connelly J.A., Chong H., Esbenshade A.J., et al. Impact of COVID-19 on Pediatric Immunocompromised Patients. Pediatr Clin North Am. 2021;68:1029–1054. doi: 10.1016/j.pcl.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Unsal H., Sekerel B.E., Sahiner U.M. Allergic reactions against Covid-19 vaccines. Turk J Med Sci. 2021;51:2233–2242. doi: 10.3906/sag-2104-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liotti L., Bianchi A., Bottau P., et al. COVID-19 Vaccines in Children with Cow's Milk and Food Allergies. Nutrients. 2021:13. doi: 10.3390/nu13082637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ellen Kuenzig M., Windsor J.W., Barrett L., et al. Crohn's and Colitis Canada's 2021 Impact of COVID-19 and Inflammatory Bowel Disease in Canada: Executive Summary. J Can Assoc Gastroenterol. 2021;4:S1–S9. doi: 10.1093/jcag/gwab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Emami A., Javanmardi F., Akbari A., Asadi-Pooya A.A. COVID-19 in patients with Down syndrome. Neurol Sci. 2021;42:1649–1652. doi: 10.1007/s10072-021-05091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rouger-Gaudichon J., Bertrand Y., Boissel N., et al. COVID19 and acute lymphoblastic leukemias of children and adolescents: Updated recommendations (Version 2) of the Leukemia Committee of the French Society for the fight against Cancers and leukemias in children and adolescents (SFCE) Bull Du Cancer. 2021;108:490–500. doi: 10.1016/j.bulcan.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruan W., Nguyen H., Wyatt A., et al. High Seroconversion Rate Against Severe Acute Respiratory Syndrome Coronavirus 2 in Symptomatic Pediatric Inflammatory Bowel Disease Patients. J Pediatr Gastroenterol Nutr. 2021;73:363–366. doi: 10.1097/MPG.0000000000003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luxi N., Giovanazzi A., Capuano A., et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021;44:1247–1269. doi: 10.1007/s40264-021-01131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cohen R., Ashman M., Taha M.-K., et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51:418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma P., Mishra S., Basu S., Tanwar N., Kumar R. Breakthrough infection with SARS-CoV-2 and its predictors among healthcare workers in a medical college and hospital complex in Delhi, India, 2021: 10.1101/2021.06.07.21258447.
- 127.Jehn M., McCullough J.M., Dale A.P., et al. Association Between K–12 School Mask Policies and School-Associated COVID-19 Outbreaks, 2021;70:1372–1373. [DOI] [PMC free article] [PubMed]
- 128.Munro A.P.S., Faust S.N. COVID-19 in children: current evidence and key questions. Curr Opin Infect Dis. 2020;33:540–547. doi: 10.1097/QCO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 129.The Lancet Infectious D. COVID-19 vaccine equity and booster doses. The Lancet Infectious Diseases, 2021;21. [DOI] [PMC free article] [PubMed]
- 130.Regional Health-Americas TL. COVID-19 vaccine equity in the Americas. Lancet Reg Health Am, 2022;5:100189. [DOI] [PMC free article] [PubMed]
- 131.Hyder A.A., Hyder M.A., Nasir K., Ndebele P. Inequitable COVID-19 vaccine distribution and its effects. Bull World Health Organ. 2021;99 doi: 10.2471/BLT.21.285616. 406-406A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foy B.H., Wahl B., Mehta K., Shet A., Menon G.I., Britto C. Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study. Int J Infect Dis. 2021;103:431–438. doi: 10.1016/j.ijid.2020.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zimmermann P., Pittet L.F., Finn A., Pollard A.J., Curtis N. Should children be vaccinated against COVID-19? Arch Dis Child. 2022;107 doi: 10.1136/archdischild-2021-323040. [DOI] [PubMed] [Google Scholar]
- 134.Nichol A.A., Mermin-Bunnell K.M. The ethics of COVID-19 vaccine distribution. J Public Health Policy. 2021;42:514–517. doi: 10.1057/s41271-021-00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Emanuel E.J., Persad G., Kern A., et al. An ethical framework for global vaccine allocation. Science. 2020;369:1309–1312. doi: 10.1126/science.abe2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malone B., Kim E., Jennings R., Pacheco R.A., Kieu A. COVID-19 Vaccine Distribution in a Community With Large Numbers of Immigrants and Refugees. Am J Public Health. 2022;112:393–396. doi: 10.2105/AJPH.2021.306608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang H.S., Costa V., Racine-Brzostek S.E., et al. Association of Age With SARS-CoV-2 Antibody Response. Jama Netw Open. 2021:4. doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu Y., Bloxham C.J., Hulme K.D., et al. A Meta-analysis on the Role of Children in Severe Acute Respiratory Syndrome Coronavirus 2 in Household Transmission Clusters. Clin Infect Dis. 2021;72:e1146–e1153. doi: 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Share of people who completed the initial COVID-19 vaccination protocol by age, Spain, 2022.
- 140.Thomas S.J., Moreira E.D., Jr., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fernandes E.G., Lopez-Lopes G.I.S., Silva V.O., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in inadvertently vaccinated healthy children. Rev Inst Med Trop Sao Paulo. 2021;63 doi: 10.1590/S1678-9946202163083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia S., Zhang Y., Wang Y., et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. The Lancet. Infectious diseases. 2021. [DOI] [PMC free article] [PubMed]
- 143.Pfizer-BioNTech COVID-19 Vaccine (also known as COMIRNATY) Overview and Safety, 2022. Available from: 〈https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html〉.
- 144.Moderna COVID-19 Vaccine (also known as Spikevax) Overview and Safety, 2022. Available from: 〈https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html〉.
- 145.Garcia-Grimshaw M., Ceballos-Liceaga S.E., Hernandez-Vanegas L.E., et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin Immunol. 2021;229 doi: 10.1016/j.clim.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.SINOVAC is officially used for emergency use by Chinese teenagers, 2021. Available from: 〈http://www.sinovac.com/news/shownews.php?id=1271〉.
- 147.Phase III clinical study in South Africa suggests that SINOVAC is safe in people as young as 6 months old, 2021. Available from: 〈http://www.sinovac.com/news/shownews.php?id=1363〉.
- 148.WHO recommendation BioNtech Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – COMIRNATY®, 2022. Available from: 〈https://extranet.who.int/pqweb/vaccines/who-recommendation-covid-19-mrna-vaccine-nucleoside-modified-comirnaty〉.
- 149.Klein N.P., Lewis N., Goddard K., et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Forchette L., Sebastian W., Liu T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr Med Sci., 2021. [DOI] [PMC free article] [PubMed]
- 151.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Voysey M., Costa Clemens S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rubin E.J., Baden L.R., Morrissey S. Audio interview: vaccinating children. N Engl J Med. 2021;384 doi: 10.1056/NEJMe2109334. [DOI] [PubMed] [Google Scholar]
- 155.Sadoff J., Gray G., Vandebosch A., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sablerolles R.S.G., Rietdijk W.J.R., Goorhuis A., et al. Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming. N Engl J Med. 2022 doi: 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mercado N.B., Zahn R., Wegmann F., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bova C., Vigna E., Gentile M. Multisystem Inflammatory Syndrome after Ad26.COV2.S vaccination. IDCases. 2022;27 doi: 10.1016/j.idcr.2022.e01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Petrovic V., Vukovic V., Patic A., Markovic M., Ristic M. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS-CoV-2 infection-A comparative study from Novi Sad, Serbia. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ai J., Zhang Y., Zhang H., et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: interim results from a prospective open-label study. Emerg Microbes Infect. 2022:1–36. doi: 10.1080/22221751.2022.2025746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han B., Song Y., Li C., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teasdale C.A., Borrell L.N., Shen Y., et al. Parental plans to vaccinate children for COVID-19 in New York city. Vaccine. 2021;39:5082–5086. doi: 10.1016/j.vaccine.2021.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Z., She R., Chen X., et al. Parental acceptability of COVID-19 vaccination for children under the age of 18 years among Chinese doctors and nurses: a cross-sectional online survey. Hum Vaccin Immunother. 2021;17:3322–3332. doi: 10.1080/21645515.2021.1917232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee E.J., Cines D.B., Gernsheimer T., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alamer E., Alhazmi A., Qasir N.A., et al. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12-18 Years in Saudi Arabia. Vaccin (Basel) 2021:9. doi: 10.3390/vaccines9111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lazaros G., Klein A.L., Hatziantoniou S., Tsioufis C., Tsakris A., Anastassopoulou C. The Novel Platform of mRNA COVID-19 Vaccines and Myocarditis: Clues into the Potential Mechanism. Vaccine. 2021;39:4925–4927. doi: 10.1016/j.vaccine.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marshall M., Ferguson I.D., Lewis P., et al. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021:148. doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 168.Saeed B.Q., Al-Shahrabi R., Alhaj S.S., Alkokhardi Z.M., Adrees A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.You J., Shaik N., Chen H. Data Mining on COVID-19 Vaccines: Side Effects. Proc Assoc Inf Sci Technol. 2021;58:869–871. doi: 10.1002/pra2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaur R.J., Dutta S., Bhardwaj P., et al. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J Clin Biochem. 2021;36:427–439. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ledford H. COVID vaccines and blood clots: what researchers know so far. Nature. 2021;596:479–481. doi: 10.1038/d41586-021-02291-2. [DOI] [PubMed] [Google Scholar]
- 174.Ciccone A. SARS-CoV-2 vaccine-induced cerebral venous thrombosis. Eur J Intern Med. 2021;89:19–21. doi: 10.1016/j.ejim.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scully M., Singh D., Lown R., et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roman G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222) Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsieh Y.L., Rak S., SteelFisher G.K., Bauhoff S. Effect of the suspension of the J&J COVID-19 vaccine on vaccine hesitancy in the United States. Vaccine. 2022;40:424–427. doi: 10.1016/j.vaccine.2021.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data. J Autoimmun. 2021;122 doi: 10.1016/j.jaut.2021.102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Satyanarayana M. AstraZeneca’s COVID-19 vaccine related woes grow: C&EN; 2021. Available from: 〈https://pubs.acs.org/doi/full/10.1021/cen-09913-buscon4〉.
- 180.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Elrashdy F., Tambuwala M.M., Hassan S.S., et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ledford H. How could a COVID vaccine cause blood clots? Scientists race to investigate. Nature. 2021;592:334–335. doi: 10.1038/d41586-021-00940-0. [DOI] [PubMed] [Google Scholar]
- 183.Omeish H., Najadat A., Al-Azzam S., et al. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum Vaccin Immunother. 2021:1–8. doi: 10.1080/21645515.2021.1981086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Al Khames Aga Q.A., Alkhaffaf W.H., Hatem T.H., et al. Safety of COVID-19 vaccines. J Med Virol. 2021;93:6588–6594. doi: 10.1002/jmv.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaudhari A.M., Kumar D., Joshi M., Patel A., Joshi C. E156G and Arg158, Phe-157/del mutation in NTD of spike protein in B.1.617.2 lineage. 2021: 10.1101/2021.06.07.447321.
- 187.Xia X. Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses. 2021:13. doi: 10.3390/v13010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rumain B., Schneiderman M., Geliebter A. COVID-19 Due to Wild-Type SARS-CoV-2 More Prevalent in Adolescents and Youth than in Older Adults Based on 19 US States. Fall 2020 Vaccin Availab. 2021 doi: 10.1101/2021.07.06.21260112. [DOI] [Google Scholar]
- 189.Baral P., Bhattarai N., Hossen M.L., et al. Mutation-induced changes in the receptor-binding interface of the SARS-CoV-2 Delta variant B.1.617.2 and implications for immune evasion. Biochem Biophys Res Commun. 2021;574:14–19. doi: 10.1016/j.bbrc.2021.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bonilauri P., Rugna G. Animal Coronaviruses and SARS-COV-2 in Animals, What Do We Actually Know? Life (Basel) 2021:11. doi: 10.3390/life11020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Seyran M., Takayama K., Uversky V.N., et al. The structural basis of accelerated host cell entry by SARS-CoV-2dagger. FEBS J. 2021;288:5010–5020. doi: 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Arora P., Sidarovich A., Krüger N., et al. Increased lung cell entry of B.1.617.2 and evasion of antibodies induced by 2021, 2021: 10.1101/2021.06.23.449568.
- 193.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gomez J., Albaiceta G.M., Garcia-Clemente M., et al. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene. 2020;762 doi: 10.1016/j.gene.2020.145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.AlGhatrif M., Tanaka T., Moore A.Z., Bandinelli S., Lakatta E.G., Ferrucci L. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: results from the InCHIANTI study. Geroscience. 2021;43:619–627. doi: 10.1007/s11357-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Poonam B., Gill P.K. Coronavirus: History. Genome Struct Pathog Corona. 2021;2:325–338. [Google Scholar]
- 197.Rajah M.M., Hubert M., Bishop E., et al. SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation. EMBO J. 2021;40 doi: 10.15252/embj.2021108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Neerukonda S.N., Vassell R., Lusvarghi S., et al. SARS-CoV-2 Delta Variant Displays Moderate Resistance to Neutralizing Antibodies and Spike Protein Properties of Higher Soluble ACE2 Sensitivity, Enhanced Cleavage and Fusogenic Activity. Viruses. 2021:13. doi: 10.3390/v13122485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ou J., Zhang Y., Wang Y., et al. ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants. Signal Transduct Target Ther. 2022;7:43. doi: 10.1038/s41392-022-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Netea M.G., Dominguez-Andres J., Barreiro L.B., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Strengert M., Becker M., Ramos G.M., et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on hemodialysis, 2021. [DOI] [PMC free article] [PubMed]
- 202.Azkur A.K., Akdis M., Azkur D., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Baumjohann D., Fazilleau N. Antigen-dependent multistep differentiation of T follicular helper cells and its role in SARS-CoV-2 infection and vaccination. Eur J Immunol. 2021;51:1325–1333. doi: 10.1002/eji.202049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mahil S.K., Bechman K., Raharja A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roltgen K., Boyd S.D. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;29:1063–1075. doi: 10.1016/j.chom.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng M., Karki R., Williams E.P., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Candia P., Prattichizzo F., Garavelli S., Matarese G. T Cells: Warriors of SARS-CoV-2 Infection. Trends Immunol. 2021;42:18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang M., Liang Y., Yu D., et al. A systematic review of Vaccine Breakthrough Infections by SARS-CoV-2 Delta Variant. Int J Biol Sci. 2022;18:889–900. doi: 10.7150/ijbs.68973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pan P., Shen M., Yu Z., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hou Y., Zhao J., Martin W., et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18:216. doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liguoro I., Pilotto C., Bonanni M., et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pedia. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]