Abstract
In today’s scenario, when cancer cases are increasing rapidly, anticancer herbal compounds become imperative. Studies on the molecular mechanisms of action of polyphenols published in specialized databases such as Web of Science, Pubmed/Medline, Google Scholar, and Science Direct were used as sources of information for this review. Natural polyphenols provide established efficacy against chemically induced tumor growth with fewer side effects. They can sensitize cells to various therapies and increase the effectiveness of biotherapy. Further pharmacological translational research and clinical trials are needed to evaluate theirs in vivo efficacy, possible side effects and toxicity. Polyphenols can be used to design a potential treatment in conjunction with existing cancer drug regimens such as chemotherapy and radiotherapy.
Keywords: cancer, polyphenols, phytochemicals, migration, invasion, pharmacology, anticancer molecular mechanisms
1 Introduction
All happy healthy bodies are alike; every unhealthy human body is unhappy in its way. That is to say, the healthy cells of the human body collaborate on a structural and functional level to maintain the homeostasis and architecture of the body. Alteration of signaling pathways and mechanisms can influence the rate of cell proliferation and the risk of benign or malignant cell proliferation (Ali et al., 2022; Hossain et al., 2022; Javed et al., 2022). Transformed cells acquire new properties that can have a significant impact on local structures or the whole organism. Cells that proliferate acquire new properties (e.g., gain independence from the systems that control the progression of the cell cycle, and develop the ability to invade other tissues) that can alter the body’s homeostasis and predispose to pathological conditions that can have an unfortunate prognosis (Sharifi-Rad et al., 2021a; Jain et al., 2021; Kitic et al., 2022).
Cancer is one of the most prevalent diseases globally, affecting 10 million people (https://www.who.int/news-room/fact-sheets/detail/cancer). According to the WHO, cases will increase from 14 million to 27 million by 2050, with a global fatality rate of 17.5 million (Sharma et al., 2018; Briguglio et al., 2020). Cancer is a complicated phenotype with an infinite replicative capacity unaffected by growth cues (Zlatian et al., 2015; Buga et al., 2019). The capability of cancer cells to avoid cell death activation, persistent angiogenesis, tissue invasion, and metastasis is unrivalled (Docea et al., 2012; Mitrut et al., 2016). Considering this, metastasis is one of the primary causes of cancer-related mortality (Javed et al., 2022). However, a big part of cancer-related fatalities can be mitigated or averted by avoiding risk factors and following evidence-based preventative interventions (Hossain et al., 2022; Kitic et al., 2022). Several medications and therapies such as chemotherapy and radiation have been used to treat cancer (Rizvi et al., 2021b; Quetglas-Llabrés et al., 2022). However, these treatments and drugs have adverse effects, such as developing drug resistance in patients over time and non-specific toxicity to normal cells (Sharifi-Rad et al., 2021a; Dhyani et al., 2022). Nonetheless, these modern treatments can extend patients’ lives by weeks or months (GBD Colorectal Cancer Collaborators, 2022). As a result, developing a treatment that can achieve an objective measure of efficacy is critical; in this regard, plant-based drugs with established efficacy, fewer side effects, and safety are crucial (Sharifi-Rad et al., 2021c; Sharifi-Rad et al., 2021d; Sharifi-Rad al., 2022; Semwal et al., 2022). Plant polyphenols’ ability to protect against chemically induced and spontaneous tumor growth is well documented and deserves additional attention (Hadi et al., 2000; Hadi et al., 2010). The polyphenols, either in individuals or in groups, can be used to design treatment or combined therapy with distinct molecular mechanisms, ultimately resulting in greater efficacy (Azmi et al., 2006; Halliwell, 2007; Gupte and Mumper, 2009). Apart from their anti-cancer properties, polyphenols can also be used in conjunction with chemotherapy and radiotherapy (Asensi et al., 2011; Rizvi et al., 2021a).
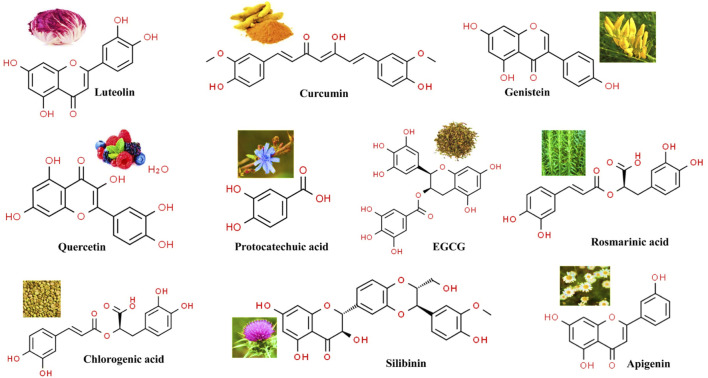
This updated review deals with different anti-cancer potential activities viz., Quercetin, Epigallocatechin, Curcumin, Silibinin, Apigenin, Luteolin, Genistein, Protocatechuic acid, chlorogenic acid and Eupatorine within several realms of existing knowledge on these molecules such as the mechanism of action and different promising scientific studies on their antitumor effect (Figure 1). This review will be a valuable resource for developing secondary metabolite-based anti-cancer therapy.
FIGURE 1.
Chemical structures of most representative polyphenols employed as anticancer agents.
2 Methodology
In the current comprehensive review, the anticancer properties of the polyphenols presented according to their molecular mechanisms of action were analyzed. To compile this study, the papers published in the following specialized databases Web of Science, Pubmed/Medline, Google Scholar, Science Direct were studied. The following MeSH terms were used for the search: “Antineoplastic Agents/pharmacology”, “Antineoplastic Agents/therapeutic use”, “Apoptosis/drug effects”, “Cell Proliferation/drug effects”, “Chemoprevention”, “Cell Line, Tumor”, “Disease Models, Animal”, “Diet”, “Neoplasms/drug therapy”, “Neoplasms/prevention and control”, “Polyphenols/pharmacology”, “Polyphenols/therapeutic use”. In vitro and in vivo experimental pharmacological studies analyzed were processed to select the best data on the antitumor, anticancer, signaling and molecular anticancer mechanisms of polyphenols. The most important obtained data were summarized in tables and figures.
3 Cellular and molecular mechanisms of polyphenols in cancer: Current data from in vitro and in vivo studies
3.1 Anticancer mechanisms of quercetin
Quercetin exhibits multifactorial anti-tumor activity by decreasing cancer risk, growth, as well as the escalation of cancerous cells. Quercetin represses tumor growth of a variety of cancer cell lines, including breast, colorectal, head, lung, ovarian, melanoma, and leukemia, in a dose-dependent manner (Hashemzaei et al., 2017) (Table 1).
TABLE 1.
Anticancer perspectives of polyphenols along with their mechanism of action and signaling pathway.
| Compound | Cancer type | Model | Mechanism of action | Target | References |
|---|---|---|---|---|---|
| Quercetin | Thyroid | SW480 | ↑ apoptosis | Pro-NAG-1/GDF15 | (Hong et al., 2021a; Hong et al., 2021b) |
| HEK293 | |||||
| U2OS | |||||
| BT-20 in vitro | |||||
| Breast | MCF-7 | ↑ autophagy | Akt-mTOR | Jia et al. (2018) | |
| MDA-MB-231 in vitro | |||||
| MCF-7 in vitro | ↑ apoptosis | PI3K/Akt/mTOR | Li et al. (2018) | ||
| MDA-MB-231 in vitro | ↑apoptosis cell cycle arrest | Foxo3a | Nguyen et al. (2017) | ||
| MDA-MB-231 in vitro | cell cycle arrest | Akt/AMPK/mTOR | (Rivera rivera et al., 2016; Lu et al., 2018) | ||
| Oral | HCCLM3 in vitro | ↓invasion | p-Akt1, MMP-2.MMP-9 | Lu et al. (2018) | |
| ↓migration | |||||
| Male Syrian hamsters in vivo | ↑apoptosis | NF-κB | Zhang et al. (2017) | ||
| Liver | PC3 | ↑apoptosis cell cycle arrest | PI3K, AKT, ERK1/2 | Erdogan et al. (2018) | |
| LNCaP in vitro | |||||
| Ovarian | A2780S in vivo | ↑ apoptosis | Caspase-3, -9, MCL-1, Bcl-2, Bax | Gao et al. (2012) | |
| ↓ angiogenesis | |||||
| EGCG | Breast | MCF-7 in vitro | ↓proliferation | P53 and Bcl-2 | Huang et al. (2017) |
| ↓apoptosis | |||||
| T47D in vitro | ↓proliferation | PI3K/AKT | Moradzadeh et al. (2017) | ||
| ↑apoptosis | |||||
| SUM-149 | ↑apoptosis | ER-α36 | Pan et al. (2016) | ||
| SUM-190 | |||||
| MDA-MB-231 in vitro | |||||
| Ovarian | SKOV3 in vitro | ↓proliferation | AQP5, NF-κB, p65, IκBα | Yan et al. (2012) | |
| ↑apoptosis | |||||
| Leukemia | NB4 in vitro | ↓proliferation cell cycle regression | DNMT1, DNMT3a, DAPK1 | Shi et al. (2018) | |
| NB4 in vitro | ↑apoptosis | SHP-1-p38αMAPK-Bax | Gan et al. (2017) | ||
| HL-60 in vitro | ↑apoptosis | Bcl-2, caspase-3 | Han and Kim, (2009) | ||
| Lymphoma | BCBL-1 | ↑apoptosis ↑autophagy |
MAPK | Tsai et al. (2017) | |
| BC-1 in vitro | |||||
| Jeko-1 | ↑apoptosis | Fas, Bax, Bcl-2 | Wang et al. (2015b) | ||
| Raji in vitro | |||||
| Renal | 786-0ACHN in vitro | ↓cell invasion | MMP-2, MMP-9 | Chen et al. (2016) | |
| Caki-1 in vitro | ↓suppression inactivation | Src, JNK | Sato et al. (2013) | ||
| Head and neck | Tu686 in vitro | ↑apoptosis | Bim, p21, p27, Bcl-2 | Haque et al. (2015) | |
| Oral | C3H/HeJ | ↑cell proliferation | HGF/c-Met | Koh et al. (2011) | |
| SCC VII/SF in vitro | ↓migration | ||||
| ↓invasion | |||||
| SCC-9 in vitro | ↓cell growth | MMP-9 | Chen et al. (2011) | ||
| ↓invasion | |||||
| Thyroid carcinoma | TT TPC-1 ARO in vitro |
↓angiogenesis | EGFR/RAS/RAF/MEK/ERK | Wu et al. (2019) | |
| ↑apoptosis | |||||
| 8505C in vitro | ↑suppression | TGF-β1/Smad | Li et al. (2019) | ||
| ↓ invasion | |||||
| ↓migration | |||||
| Osteosarcoma | MG-63 | ↓cell proliferation cell cycle arrest | miR-1/c-MET | Zhu and Wang, (2016) | |
| U-2OS in vitro | |||||
| ↑apoptosis | |||||
| MG63 | ↑apoptosis | miR-126 | Jiang et al. (2014) | ||
| U-2OS in vitro | |||||
| Glioblastoma | GBM02 | ↑apoptosis ↑autophagy |
Grube et al. (2018) | ||
| GBM15 | |||||
| GBM16,17 in vitro | |||||
| Hepatocellular carcinoma | HCC-LM3 | ↑cancer cell death | PFK | Li et al. (2016) | |
| Huh-7 | |||||
| HepG2 | |||||
| SMMC-7721 in vivo, in vitro | |||||
| Curcumin | Pancreatic | Patu8988 | cell cycle arrest ↑apoptosis |
YAP/TAZ | Zhou et al. (2016) |
| Panc-1 in vitro | |||||
| Glioblastoma | A172 in vitro | ↑autophagy | Lee et al. (2019) | ||
| Breast | MCF-7 in vitro | ↑apoptosis | Mcl-1 | Koohpar et al. (2015) | |
| Silibinin | Colorectal | CT26 in vitro | ↓angiogenesis ↑autophagy |
Bax, Caspase-3, COX-2 | Sameri et al. (2021) |
| ↑apoptosis ↓migration | |||||
| Glioblastoma | A172 in vitro | ↑apoptosis | mTOR, YAP | Bai et al. (2018) | |
| ↓autophagy | |||||
| Breast | MCF7 in vitro | ↑cell autophagy | BNIP3 | Jiang et al. (2015) | |
| Ovarian | SKOV-3 in vitro | ↑ apoptosis | P53, P21 | Pashaei-asl et al. (2018) | |
| A2780 SKOV3 in vitro, in vivo |
↓tumor growth | p-ERK, p-Akt | Cho et al. (2013) | ||
| Apigenin | Cervical | HeLa in vitro | ↓cell-renewal capability | CK2α | Liu et al. (2015) |
| Breast | A549 in vitro | ↓proliferation | PI3K/Akt | Zhou et al. (2017) | |
| ↑migration ↓invasiveness | |||||
| MDA-MD-231 in vitro, in vivo) | cell cycle arrest | cyclin A, cyclin B, CDK1, p21WAF1/CIP1 | Tseng et al. (2017) | ||
| MDA-MB-468, 4T1 in vitro | ↑immune response | IFN-γ, PD-L1, STAT1 | Coombs et al. (2016) | ||
| SKBR3 in vitro | ↑apotosis | p-JAK2 and p-STAT3, VEGF | Seo et al. (2015) | ||
| Lung | H1299 H460 in vitro |
↓cell proliferation | GLUT 1 | Lee et al. (2016b) | |
| ↑apoptosis | |||||
| Prostate | PC3 in vitro | cell cycle arrest | p21 and p27; caspases-8,-3, TNF-α | (Shukla et al., 2015; Erdogan et al., 2016) | |
| ↑apoptosis | |||||
| DU145 in vitro | cell cycle arrest | IKK—IκBα | Shukla et al. (2015) | ||
| ↑apoptosis | |||||
| ↓tumorigenesis | |||||
| PC3-M LNCaP C4-2B in vitro |
↓cell proliferation ↓metastases |
Smad2/3, Src/FAK/Akt | Mirzoeva et al. (2014) | ||
| Colorectal | SW480 in vitro | ↓cell proliferation | Wnt/β-catenin | Xu et al. (2016) | |
| ↓migration | |||||
| ↓invasiveness | |||||
| DLD1 | ↓cell proliferation ↓migration | NEDD9 | Dai et al. (2016) | ||
| SW480 in vitro, in vivo | |||||
| ↓invasiveness | |||||
| HCT116 in vitro | ↓proliferation | cyclin B1, Cdc2, Cdc25c | Lee et al. (2014) | ||
| ↑apoptosis | |||||
| ↑autophagy | |||||
| Renal cell | ACHN | cell cycle arrest | p53 | Meng et al. (2017) | |
| 786–0 | |||||
| Caki-1 in vitro, in vivo | |||||
| Head and neck squamous carcinoma | HSC-3 | ↓cancer cell markers | CD44, NANOG, CD105 | Ketkaew et al. (2017) | |
| HN-8 | |||||
| HN- 30 in vitro | |||||
| Luteolin | Prostate | PC3 | ↑apoptosis | miR-301 | Han et al. (2016) |
| LNCaP in vitro | |||||
| Hepatocellular carcinoma | SMMC-7721 in vitro | ↑apoptosis | BL-2, caspase-8 | Cao et al. (2017) | |
| ↑autophagy | |||||
| Cholangiocarcinoma | n CCA | cell cycle arrest | JAK/STAT3 | Aneknan et al. (2014) | |
| KKU-M156 in vitro | ↑apoptosis | ||||
| ↓migration | |||||
| Pancreatic | BxPC-3 in vitro | ↓cell proliferation | GSK-3β, NF-κB, p65 | Johnson and Mejia, (2013) | |
| Colon | Balb/C mice in vivo | ↑apoptosis | MMP-2, -9, TIMP-2 | Pandurangan et al. (2014) | |
| Oral | CD44 in vitro | ↓invasiveness clonogenicity | IL-6/STAT3 | Tu et al. (2016) | |
| Gastric | MKN45 | ↑mirnas expression | Notch1, PI3K, AKT, mTOR, ERK, STAT3 | Pu et al. (2018) | |
| BGC823 in vitro, in vivo | |||||
| BGC-823 | ↑apoptosis | miR-34a, Bcl-2 | Wu et al. (2015) | ||
| SGC-7901 in vitro | |||||
| Neuroblastoma | SH-SY5Y in vitro | cell cycle arrest | Wang et al. (2015a) | ||
| ↑apoptosis | |||||
| Breast | MDA-MB-231 in vitro, in vivo | apoptosis | STAT3 | Yang et al. (2014) | |
| Glioblastoma | U87MG | ↑apoptosis | miR-7-1-3p | Chakrabarti and Ray, (2016) | |
| T98G in vivo | ↑autophagy | ||||
| Genistein | Breast | MCF-7 | cell cycle arrest | Jiang et al. (2018) | |
| MDA-MB-231 in vitro | ↓proliferation | ||||
| ↑inhibition | |||||
| Hepatocellular | Hepa1-6 in vitro | ↑apoptosis | Sanaei et al. (2018) | ||
| ↓proliferation | |||||
| Cervical | HeLa in vitro | ↑apoptosis | GRP78, CHOP | Yang et al. (2016b) | |
| Liver | HepG2 in vitro | ↑apoptosis cell cycle arrest | Bax, Bcl-2, caspase-3, 9 | Zhang et al. (2019) | |
| Protocatechuic acid | Gastric carcinoma | B16/F10 in vitro | ↓cell migration | Ras/Akt/NF-kB | Lin et al. (2011) |
| ↓invasion | |||||
| Breast | MCF-7 | ↑apoptosis | Caspase-3, 9 | Yin et al. (2009) | |
| A549 | ↓metastasis | ||||
| HepG2 | ↓invasion | ||||
| HeLa | |||||
| LNCaP in vitro | |||||
| Rosmarinic acid | Colorectal | CT26 in vitro | cell cycle arrest | EMT, MMPs, AMPK | Han et al. (2018a) |
| ↑apoptosis | |||||
| Pancreatic | Panc-1 | ↓cell viability | miR-506, MMP2/16 | Han et al. (2019) | |
| SW1990 in | ↓growth | ||||
| vitro | ↓invasion | ||||
| ↓migration | |||||
| Human melanoma | A375 in vitro | ↑apoptosis | ADAM17/EGFR/AKT/GSK3β | Huang et al. (2021) | |
| ↓proliferation | |||||
| ↓migration | |||||
| Breast | Mice | ↓inflammation | NF- κB, p53 caspase-3 | Mahmoud et al. (2021) | |
| in vivo | ↓angiogenesis | ||||
| ↑apoptosis | |||||
| Head and Neck Squamous Carcinoma | UM- SCC-6 | ↓cell viability | MAPK/ERK | Tumur et al. (2015) | |
| UM-SCC- | ↓ migration | ||||
| 10B in vitro | ROS | ||||
| Colon | Wistar rats in vivo | ↓cell proliferation | IL-6, COX-2, p65 | Karthikkumar et al. (2015) | |
| Gastric | MKN45 in vitro | ↓tumorgenesis | miR-155, IL-6/STAT3 | Han et al. (2015) | |
| Chlorogenic Acid | Hepatocellular carcinoma | Hep-G2 | ↑apoptosis | MAPK, NF-κB, TGF-β | Jiang et al. (2021) |
| Huh-7 in vitro | |||||
| Breast | Females in vivo | ↑apoptosis ↓metastasis |
NF-κB/EMT | Zeng et al. (2021) | |
| 4T1 in vitro | ↑apoptosis | Bax and Bcl-2 | Changizi et al. (2020) | ||
| Tumor cells | HepG2 A549 in vitro |
↓migration | mTORC2/F-actin | Tan et al. (2019) | |
| ↓invasiveness | |||||
| ↓tumor growth | |||||
| Glioblastoma | G422 in vitro | ↑apoptosis | LPS/IFNγ | Xue et al. (2017) | |
| Leukemia | U937 | ↑apoptosis | Liu et al. (2013) | ||
| K562 in vitro | ↓proliferation | ||||
| Eupatorin | Breast | 4T1 in vivo | ↓tumor development | MMP-9, NF-κB, NK1.1, CD8+ | Abd Razak et al. (2020) |
| ↓metastasis | |||||
| MCF-7 | Cell cycle arrest, invasion, migration | Phospho-Akt | Razak et al. (2019) | ||
| MDA-MB-231 in vitro | |||||
| MDA-MB-468 in vitro | cell cycle arrest | CYP1 | Androutsopoulos et al. (2008) | ||
| Cervical | HeLa in vitro | cell cycle arrest | p53, p21, Bax | Lee et al. (2016a) | |
| ↑apoptosis | |||||
| Leukemia | HL-60 | ↑apoptosis | c-jun N-terminal kinases | Estevez et al. (2014) | |
| U937 | |||||
| Molt-3 in vitro | |||||
Symbols: ↑increase, ↓decrease. Abbreviations: ADAM17/EGFR/AKT/GSK3β, a disintegrin and metalloproteinase-17/epidermal growth factor receptor/protein kinase B/Glycogen synthase kinase3β; Akt/AMPK/mTOR, protein kinase B/Adenosine monophosphate-activated protein kinase/mammalian target of rapamycin; Akt-mTOR, protein kinase B/mammalian target of rapamycin; AQP5, aquaporin-5; Bax, Bcl2-Associated X Protein; Bcl-2, B-cell leukemia/lymphoma 2 protein; Bim, B cell lymphoma-2-like 11; BL-2, Burkitt’s lymphoma-2; CD8+, cluster of differentiation 8; Cdc2, cell-division cycle 2; CD44, cluster of differentiation 44; CD105, endoglin; CDK1, cyclin-dependent kinase-1; CHOP, cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone; CK2α, catalytic subunit of protein kinase CK2; COX-2, cyclooxygenase-2; CYP1, cytochrome P450 1; DAPK1, death associated protein kinase 1; DNMT1, DNA (cytosine-5)-methyltransferase 1; DNMT3a, DNA methyltransferase 3 alpha; EGFR/RAS/RAF/MEK/ERK, epidermal growth factor receptor/rat sarcoma virus/rapidly accelerated fibrosarcoma/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase; EMT, epithelial-mesenchymal transition; ERK1/2, extracellular signal-regulated kinase 1/2; ER-α36, estrogen receptor-alpha36; Fas, cell surface death receptor; Foxo3a, forkhead box class O 3a; GLUT 1, glucose transporter 1; GRP78, glucose-regulating protein 78; GSK-3β, glycogen synthase kinase-3 beta; HGF/c-Met, hepatocyte growth factor/c-mesenchymal-epithelial transition factor; IFN-γ, interferon-gamma; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IKK–IκBα, IκB kinase-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; LPS/IFNγ, lipopolysaccharide/interferon-gamma; MAPK, mitogen-activated protein kinase; MCL-1, myeloid cell leukemia 1; miR-1/c-MET, microRNA-1/mesenchymal-epithelial transition factor; miR-126, microRNA-126; miR-301, microRNA-301; MMPs, matrix metalloproteinases; mTORC2/F-actin, mTOR complex 1/filamentous actin; NEDD9, neural precursor cell expressed, developmentally downregulated nine; NF-κB, nuclear factor kappa B; NK1.1, natural killer 1.1; p-ERK, phosphorylated-extracellular signal-regulated kinase; PD-L1, programmed death-1; PFK, phosphofructokinase; pro-NAG-1/GDF15, pro-nonsteroidal anti-inflammatory drug-activated gene-1/growth differentiation factor 15; p21WAF1/CIP1, wild-type activating fragment-1/cyclin-dependent kinase inhibitory protein-1; PI3K/Akt/mTOR, phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin; p-Akt1, phosphorylated-serine/threonine kinase 1; p-JAK2, phosphorylated Janus kinase 2; p-STAT3, phosphorylated signal transducer and activator of transcription 3; SHP-1-p38αMAPK-Bax, Src homology 2 domain-containing protein tyrosine phosphatase 1 mitogen-activated protein kinase; Src, Proto-oncogene tyrosine-protein kinase; STAT1, signal transducer and activator of transcription 1; TGF-β1/Smad, transforming growth factor-β1/suppressor of mothers against decapentaplegic; TIMP-2, tissue inhibitor of metalloproteinase-2; TNF-α, tumour necrosis factor-alpha; VEGF, vascular endothelial growth factor receptor; Wnt, wingless-related integration site; YAP/TAZ, yes-associated protein/transcriptional coactivator with PDZ-binding motif.
Many experimental pharmacological studies have revealed that quercetin exerts the anti-tumor effect by changing the cell cycle, metastasis progression, angiogenesis, cell proliferation inhibition, and promotion of apoptosis, thus, affecting autophagy (Tang et al., 2020). Additionally, the anti-tumor activity of quercetin involves the regulation of epigenetics in tumor cells, which further regulate mRNA expression, and DNA methylation. Therefore, it increases the bioavailability of the drug to the tumor cell by the inhibition of BCRP, MRP1, and P-gp (P-glycoprotein) (Kedhari Sundaram et al., 2019; Panthi et al., 2020).
Anti-tumor property of quercetin is well depicted by its ability to interfere in various cellular signal pathways and inhibits enzymes responsible for carcinogens activation (Rauf et al., 2018; Panthi et al., 2020). Quercetin causes arrest at the G2/M or G1 phase in different cell types. Moreover, quercetin-mediated apoptosis results from the induction of stress proteins, microtubule disruption, and cytochrome c release from mitochondria, followed by caspases activation (Panthi et al., 2020). Quercetin triggers arrest of the G2/M phase in human esophageal squamous cell carcinoma at mRNA plus protein levels via up and downregulation of p73, p21waf1, and cyclin B1, respectively. Quercetin reduces the propagation of cancer cells via the inhibition of intracellular signaling, i.e., PI3K, EGFR, and Her2/neu. Quercetin induces apoptosis of cancer cells by modulating the modulation of survival signaling pathways (Akt, NF-kB) or regulatory molecules, which are linked to cell death (Nguyen et al., 2017) (Table 1).
3.1.1 In vitro studies
Various in-vitro and xenograft models have shown the inhibitory role of quercetin in the growth of cancers which are revealed in apoptosis promotion, inhibition of proliferation, angiogenesis, and metastasis, making it a potential antitumor agent (Panthi et al., 2020; Tang et al., 2020) (Table 1). Quercetin possesses its antitumor activity in a dose varied from 3 to 50 µM on numerous cell lines, i.e., 3.5 μM for the B16-BL6 murine cancer cell line, PC-3 and DU-145 human prostate cancers were inhibited at 25 μM and in human breast carcinoma cells confirmed inhibition at 10 μM (Gibellini et al., 2011; Panthi et al., 2020). The ability to trigger apoptosis of quercetin in cancerous cells makes this molecule an appealing preference for various cancer therapies (Figure 2). Quercetin arrests the cell cycle and induces apoptosis in conjunction with p53 phosphorylation, which stabilizes p53 at mRNA and protein levels in HepG2 cells. In HCT116 colon carcinoma cells, apoptosis of the tumor cells is caused by p53 to the quercetin-mediated expression of NAG-1 (Gibellini et al., 2011). However, a recent study has revealed that quercetin induces apoptosis through only pro-NAG-1 expression; however, mature NAG-1 did not induce apoptosis which was mediated by the C/EBP transcription factor in thyroid cancer cell lines (Hong et al., 2021a) (Table 1).
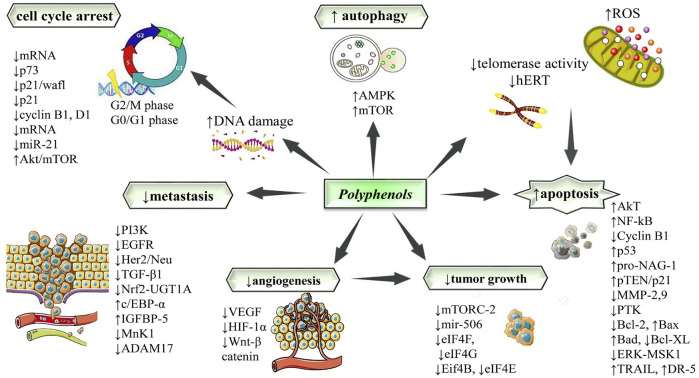
FIGURE 2.
Potential molecular targets and signaling pathways for the antitumor effect of polyphenols. Symbols: ↑increase, ↓decrease. Abbreviations: ADAM17, ADAM metallopeptidase domain 17; Akt/mTOR, protein kinase B/mammalian target of rapamycin; AMPK, adenosine monophosphate-activated protein kinase; BAD, Bcl-2 antagonist of cell death; Bax, Bcl2-Associated X Protein; Bcl-2, B-cell leukemia/lymphoma 2 protein; Bcl-xL, B-cell lymphoma-extra large; c/ebp-α, CCAAT/enhancer-binding protein-alpha; DR-5, death receptor 5; EGFR, epidermal growth factor receptor; ERK/MSK, extracellular signal-regulated kinase/mitogen- and stress-activated kinase 1; Her2/Neu, human epidermal growth factor receptor 2/neutrophills; HIF-1-alpha, hypoxia-inducible factor 1-alpha; IGFBP-5, insulin-like growth factor-binding protein-5; hTERT, human telomerase reverse transcriptase; miR, microRNA; MMP, matrix metalloproteinase; MnK-1, a family of serine/threonine kinases; mRNA, messenger ribonucleic acid; mTOR, mammalian target of rapamycin; mTORC2, mTOR Complex 2; NF-κB, nuclear factor kappa B; Nrf1, nuclear respiratory factor 1; PI3K: phosphatidylinositol 3-kinase; pro-NAG-1, pro-nonsteroidal anti-inflammatory drug-activated gene-1; pTEN, phosphatase and TENsin homolog deleted on chromosome 10; PTK, protein tyrosine kinase; TGF-β1, transforming growth factor-β1; TRAIL, tumor-necrosis factor related apoptosis-inducing ligand; UGT1A, UDP glucuronosyltransferase 1 family; VEGF, vascular endothelial growth factor receptor; Wnt, wingless-related integration site.
H1299 lung carcinoma cell line was discovered to have extra liable to cytotoxicity compared to the A549 lung carcinoma line (Gibellini et al., 2011). Quercetin exerted significant inhibition of cell proliferation and induction of apoptosis in human renal cell adenocarcinoma cells via the survivin mRNA inhibition, protein expression, and caspase 3 activation (Reyes-Farias and Carrasco-Pozo, 2019). The proteasomal degradation of survivin takes place by quercetin, which in flip increases the concentration of cyclin B1 and p53 proteins, thereby enhancing surviving and p21 protein expression and inhibiting apoptosis (Gibellini et al., 2011).
In prostate cancer cells, quercetin complements the TNF-associated apoptosis-inducing ligand (TRAIL), which in turn causes apoptosis through induction of the expression of the death receptor (DR)-5. The upregulation of DR-5 and downregulation of β-FLIP make contributions to the recuperation of TRAIL sensitivity which further inhibits hepatocellular carcinoma cells by the use of quercetin. The superior TRAIL-caused apoptosis using quercetin takes vicinity via inhibiting the expression of the ERK-MSK1 pathway (Gibellini et al., 2011).
In a recent study, the expansion of ovarian cancer cells was inhibited by quercetin by a mechanism related to TGF-β1 (Shafabakhsh and Asemi, 2019). TGF-β1 possesses potent hematopoietic regulatory properties, depending on their stage progenitor differentiators; it either stimulates or inhibits the noncancerous myeloid progenitor’s growth. Quercetin inhibits the cell cycle and promotes apoptosis in breast cancer and leukemia cell xenograft models via Akt/mTOR pathway in a dose-dependent manner (Rivera rivera et al., 2016) (Table 1).
3.1.2 In vivo studies
The effect of quercetin on tumor growth was analyzed in-vivo by angiogenesis and metastasis. Prostate tumor inhibition by quercetin in vivo in a mouse model is upregulated by the expression of Thrombospondin-1, which inhibits tumor propagation (Yang F. et al., 2016). (Zhao et al., 2016) reported that quercetin inhibited angiogenesis by mediating the calcineurin/NFAT pathway in the BALB/c mice breast cancer model. Quercetin’s inhibitory effect on metastasis is depicted by in-vivo experiments. Quercetin suppresses EMT by interfering with EGFR’s signaling pathway, thereby reducing VEGF expression (Jia et al., 2018).
3.2 Anticancer mechanisms of epigallocatechin gallate
The antitumor activity of EGCG is attributed to its capacity to mediate signaling pathways and regulate cells’ undesired survival and proliferation. The cell death by EGCG is initiated by the intrinsic pathways in various cancers (Table 1).
EGCG suppresses ERK1/2, NF-κB, and Akt-mediated signaling and activates p53 and PTEN/p21 for cell apoptosis along with the alteration of the Bcl-2 protein ratio (Almatroodi et al., 2020). Induction of apoptosis takes place through some pathways which incorporate intrinsic and extrinsic pathways, regulatory proteins, the strain on the endoplasmic reticulum through the activation of caspase-mediated pathways, death receptors, downregulation of several anti-apoptotic proteins, upregulation of Bad, and Bax pro-apoptotic proteins in human adrenal cancers cells (Wu et al., 2019) (Figure 2). The cell cycle arrest is triggered by EGCG at G0/G1 phase and occurs via the regulation of several cyclins in pancreatic cancer cells.
In cyclins, EGCG stops cyclin D1 and turns on p21, which in addition consists of ERK, IKK, and PI3K signaling pathways in which colorectal cancer cells are being inhibited from proliferating (Rady et al., 2018) (Figure 2). In cervical cancer, EGCG prevented the spread, invasion, and migration of HeLa cells via down-and upregulation of MMP-9 and TIMP-1 genes, respectively (Sharma et al., 2012). EGCG inhibited tumor growth by activating VEGF/VEGFR axis, interrupting the HIF-1a expression and other foremost growth factors, inactivation of PAR2-AP, ERK1/2, and NF-κB pathways were blocked (Zhou et al., 2012). In esophageal tumor cells, EGCG suppresses cellular viability through the reduction of p-ERK1/2, c-Jun, and COX-2, which in addition reasons the activation of caspase-3 in conjunction with the suppression of the COX-2 expression (Ye et al., 2012). EGCG significantly suppresses the glycolytic enzymes’ mRNA levels in breast cancer cells (Wei et al., 2018). In colorectal-cancer-cell, EGCG activates caspase-3 for apoptosis, PARP, downregulation of STAT3 and phosphorylated STAT3 (p-STAT3), decreased Bcl-2 MCL-1, vimentin, along with the increase in E-cadherin (Luo et al., 2021).
EGCG set up its important function for inhibition of glioma and showed Mitogen-activated protein kinase pathway (MAPK) involvement in apoptosis and proliferation (Figure 2). EGCG treatment with TRAIL induces rapid apoptosis which could be a striking approach for treating several gliomas (Li et al., 2014).
3.2.1 In vitro studies
EGCG possesses an anti-tumor effect on various cancer cells in-vitro, including adrenal and breast. Cervical, colorectal, gastric, liver, lung, ovarian, prostate, and skin cancer cells (Gan et al., 2018). (Huang et al., 2017) stated that EGCG suppressed breast cancer cell proliferation in a concentration-structured manner. The comparison of expression of P53 in the EGCG-combined with the si-P53 group revealed the enhanced expression of the former in the combination of EGCG than that of the si-P53 group. (Luo et al., 2014) reported a dramatic decrease in cell growth in breast cancer cells on treatment with a range of quantities of EGCG in comparison with control cells. Moreover, expression of HIF-1α protein in addition to vascular endothelial growth factor declined in cancer cells in a dose-dependent approach on pre-treatment with progressing concentrations of EGCG. In ovarian cancer cell growth, EGCG was reported to show inhibition of growth in all cell lines in a dose-dependent manner; thereby, apoptosis and cell cycle arrest was triggered (Kim et al., 2004). (Wu et al., 2019) reported that EGCG inhibited thyroid carcinoma cells’ viability and proliferation as well as cell cycle progression.
Another study suggested that the pro-EGCG remedy affected tumor angiogenesis in endometrial cancers. Pro-EGCG contributes to the tumor angiogenesis inhibition in xenograft animal models which takes place through downregulation of vascular endothelial factor A, and HIF-1 α. Pro-EGCG remedy reduced vascular endothelial factor A secretion from endometrial cancer cells. In EGCG-treated endometrial adenocarcinoma, the expressions of the sex hormones estrogen and progesterone receptor were decreased along with the decrease in MAPK signals and phospho-Akt. Further over, EGCG arrested cells in the G0/G1 cell cycle phase (Park et al., 2012). An interesting study to assess the characteristic of EGCG effects on the metabolism of pancreatic adenocarcinoma cells was undertaken. Results found that the adenocarcinoma cells treated with catechin enormously suppressed lactate production and anaerobic glycolysis in addition to glucose consumption. A pioneering examination confirmed the blended better antitumor reaction of curcumin plus EGCG on prostate cancer cells, which in any other case had been immune to chemotherapy, and apoptosis inducers. The co-remedy of EGCG and curcumin enhanced the protein expressions of p21. However, their expression was unchanged on treatment with each compound alone (Eom et al., 2015).
An in-vitro look on bladder cancer cells showed that EGCG prompted morphological modifications and inhibition in a dose- and time-established fashion. In addition, EGCG-treated with bladder tumor cells found sub-G1 populations similarly to activation of caspase-3 and -9. Bladder cancer cells’ proliferation was decreased and controlled by EGCG treatment in vitro migration, followed by the downregulation of N-cadherin and Akt signaling inactivation (Jankun et al., 2014).
3.2.2 In vivo studies
In-vivo findings tested that EGCG brought about cell arrest in the G1 phase, and apoptosis was induced via ROS generation (Lu et al., 2015). The intraperitoneal injection of EGCG inhibited the gastric cancer cells’ growth and declined the expression of mRNA of endothelial growth factors in tumor cells in a dose-dependent fashion (Cheng et al., 2020). (Sur et al., 2016) evaluated the chemopreventive and therapeutic efficacy of EGCG in combination with theaflavin on hedgehog pathways during CCl4/N-nitosodi-ethylamine-induced mouse liver carcinogenesis. Findings revealed that both the compounds in combination limited the hepatocellular carcinoma development at the 30th week after the carcinogen had been given, depicting their potential chemopreventive action in the continuous treated group than in the post-treated group. EGCG/theaflavin remedies precipitated reduction in apoptosis and proliferation, respectively. EGCG has a defensive effect on the growth, liver, and pulmonary metastases of colon cancers in nude mice by the activation of the Nrf2-UGT1A signal pathway (Almatroodi et al., 2020).
A phase I study on the safety and effectiveness of EGCG mouthwash was performed, which was given in addition to radiation in head and neck cancer episodes. The assigned dose of EGCG mouthwash was directed in a standard 3 plus 3 dose-escalation design. EGCG administration decreased oral mucosal injury in patients (Zhu et al., 2020).
3.3 Anticancer mechanisms of curcumin
Curcumin activates the formation of ROS, enhances intracellular calcium levels, and changes cell membrane potential, which further activates apoptotic pathways in tumor cells (Liu and Ho, 2018). Curcumin disrupts the maintained stability of the mitochondrial membrane, inflicting the elevated suppression of the Bcl-2 and Bcl-xL apoptotic protein expression (Tomeh et al., 2019). Curcumin’s anticancer mechanism involves a decrease in glucose absorption and lactate generation (Warburg effect) in cancer cells which takes place through downregulation of pyruvate kinase-M2 (PKM2), executed via suppression of the rapamycin-hypoxia-inducible issue 1α (Tomeh et al., 2019). Overall, the anti-tumor activity of curcumin in the cell causes an increase in growth suppressor factors, decreases associated proliferative pathways, and Wnt-βcatenin, acts on angiogenesis (VEGF), and enhances apoptosis (Allegra et al., 2017) (Table 1).
3.3.1 In vitro studies
Curcumin has anti-cancer properties against breast cancer on Her2-positive cell lines (SKBR3 and BT474), having a lower inhibitory concentration (IC50) for curcumin compared to triple-negative cell lines. The decrease in IC50 is directly proportional to the expression of ER rather than Her2. ER-negative cell lines, i.e., SKBR3 and MBA-MB-231, have a lower IC50 for curcumin than ER-positive cell lines like BT474 and MCF7 (Liu and Ho, 2018). When treated with curcumin, the malignant colorectal cells reduce M(1)G levels without any significant change in COX-2 protein levels. Treatment of HCT 116 colorectal cancer cells with curcumin pronounced the arrest of the cell cycle in the G2/M phase via the miR-21 gene together with the inhibition of the proliferation of tumor cells.
Treatment of curcumin on human mammary epithelial and MCF-7 breast cancer cells reported a substantial decline in telomerase activity in a concentration-dependent manner associated with the downregulation of hTERT (Tomeh et al., 2019). The growth of oral mucosal epithelial cell lines and squamous cell carcinoma was inhibited by curcumin with the least consequence on normal oral epithelial cells. Curcumin reduced the efficiency of the eIF4F translational complex of mucosal cells via suppressing phosphorylation of eIF4G and eIF4B, combined with a decrease in total levels of eIF4E and Mnk1. Antitumour effects of curcumin were found to be effective in another investigation in oral cancer cells, which triggered the promoter activity of insulin-like growth factor binding protein-5 (IGFBP-5) and CCAAT/enhancer-binding protein alpha (C/EBP-α).
Curcumin triggers p53-established cell demise in basal cell carcinoma and breast cancer. However, in neuroblastoma, mammary epithelial carcinoma, and colon cancer, curcumin remedy unexpectedly via upregulated p53 expression, triggered nuclear translocation of p53, accompanied by p21 and Bax expression induction (Allegra et al., 2017; Giordano and Tommonaro, 2019). Curcumin in tumor tissue enhances the p53 molecule expression, modulates the pathway responsible for apoptosis in ovarian cancer as well as induces the p53 expression in nasopharyngeal carcinoma by mediating serine phosphorylation of p53 (Sultana et al., 2021).
3.3.2 In vivo studies
The inhibition of IGFBP-5 and C/EBP-alpha by curcumin was mediated through p38 activation and caused a decline of tumorigenesis in a mouse xenograft model in vivo (Wilken et al., 2011). (Kocaadam and Şanlier, 2017) examined the antitumor property of curcumin in various head and neck squamous carcinoma cell lines, CCL23 (laryngeal), CAL27, UM-SCC14A, and UMSCC1 (oral). Curcumin decreased the expression of NF-κB and controlled the expression of gene products, phospho-IκB-α, as well as inhibition of nuclear localization (Kocaadam and Şanlier, 2017). In-vivo studies on mice tormented by colorectal cancer showed a better reaction to radiation remedy in aggregation with curcumin owing to its assets to target nuclear factor (NF-κB) (Tomeh et al., 2019).
3.4 Anticancer mechanisms of silibinin
Silibinin inhibits the cell’s metabolic activity and induces apoptosis via caspase 3 and PARP-1 regulation in a concentration- and time-dependent fashion. Silibinin induces autophagy upregulation of protein which was associated with microtubule formation (Bai et al., 2018). Silibinin reduced tumor growth by downregulating extracellular signal-regulated kinase and Akt in human ovarian cells (Cho et al., 2013). Silibinin triggered autophagic cell death in breast cancer cells; the usage of mitochondrial potential became a result of ROS formation and ATP depletion, causing stimulation of expression of Bcl-2 adenovirus E1B 19-kDa-interacting protein 3 (Jiang et al., 2015). Together, apoptosis and autophagy were strongly induced by silibinin through the interference of various pathways, up- and downregulation of the expression of caspase-3, Atg5, Atg7, Bcl-2, COX-2, HIF-1α, VEGF, MMP-2, 9, respectively (Sameri et al., 2021) (Figure 2) (Table 1).
3.4.1 In vitro studies
In human hepatocellular carcinoma (HCC), silibinin powerfully repressed the growth of HepG2 and Hep3B cell lines and showed relatively more potent cytotoxicity in Hep3B cells, causing apoptosis induction. In HepG2, silibinin caused G1and G2-M phase arrest in Hep3B cells.
A study conducted by (Mateen et al., 2010) demonstrated the effect of silibinin on non-small cell lung cancer (NSCLC) subtypes and bronchoalveolar carcinoma cells. The cell growth was affected with silibinin treatment, and the cell cycle was reported to arrest at the G1 phase in a dose and time-dependent manner. Silibinin decreased kinase expression in all of the cell lines; however, no impact was found on CDK4 activity in H460 cells, with the concomitant reduction in retinoblastoma protein phosphorylation. Silibinin strongly caused a decline in cell viability and death in MCF-7 cells related to expanded p53 expression (Noh et al., 2011). (Kaur et al., 2010) studied the effect of treatment of silibinin on the expansion of tumorous human CRC cells in culture as well as in xenograft model. Results concluded that silibinin treatment in SW480 inhibited cell growth, prompted cell death, and reduced nuclear-cytoplasmic β-catenin; however, no longer in wild type, depicting its effect on the β-catenin pathway along with related biological responses. Anticancer properties of silibinin investigated on CT26 mouse colon cell lines showed a reduction in proliferation, cell survival, angiogenesis, and migration. Silibinin significantly reduced CT26 cells’ survival along with up-and downregulation of various signal pathways (Sameri et al., 2021).
In-vitro studies have demonstrated that silibinin mediates Kip1/p27 declines cyclin D1, D3, cyclin-dependent kinase (CDK)-2, and CDK-4 expressions in both the cell lines. In Hep3B cells, silibinin reduces the protein concentrations of G2-M regulators with the inhibition of CDK-2, CDK-4, and CDC2 activity in HCC cells (Varghese et al., 2005).
3.4.2 In-vivo studies
(Cho et al., 2013) reported that the silibinin in vitro and in vivo reduces tumor growth via downregulation of the extracellular signal-regulated kinase (ERK) and Akt in human ovarian cancer cells. Silibinin, when given orally to A2780 cells, shrinks the tumor volume, decreases Ki-67-positive cells, increases transferase-mediated dUTP nick end labelling -positive cells, caspase-3 activation, p-ERK, and p-Akt inhibition.
3.5 Anticancer mechanisms of apigenin
Apigenin triggers cell cycle arrest at various proliferation laps, regulates intrinsic apoptotic pathways, and promotes different anti-inflammatory pathways (Iizumi et al., 2013; Lapchak and Boitano, 2014). Apigenin enhances the expression of anti-oxidant enzymes plus induces the inhibition of metastasis and angiogenesis (Rezai-Zadeh et al., 2008) (Table 1).
Various signaling pathways are modulated by apigenin in tumor cells which include phosphoinositide 3-kinase and Wnt/β-catenin pathways (Yan et al., 2017). Apigenin upregulates the Bad and Bax, which are pro-apoptotic proteins, and/or downregulates pro-survival associates, i.e., Bcl-xL and Bcl-2, thus activating the apoptotic process within the cell. Cancer cell proliferation is inhibited using apigenin through modulation of the cell cycle and blockage at checkpoints, i.e., G2/M or G0/G1, that is related to the suppression of cyclin B1 and its activating partners, together with the increase of expression of cell cycle inhibitors, ROS accumulation and hence, DNA damage (Lee et al., 2014) (Table 1). In cervical cancer, apigenin changes Bax/Bcl-2 ratio and triggers apoptosis, followed by inhibition of the Raf/MEK/ERK signaling pathway (Zhang et al., 2019). Autophagy is induced through AMPK activation, which is triggered by apigenin, and the mTOR signaling pathway is also inhibited (Sung et al., 2016).
3.5.1 In vitro studies
(Zhang et al., 2019) examined the anticancer property of apigenin on human cervical cancer cells. Apigenin at IC50 of 15 µM suppressed the colony formation of HeLa cells, altered the Bax/Bcl-2 ratio, triggered apoptosis, and altered the Raf/MEK/ERK signaling pathway. (Zhang et al., 2008) reported that apigenin triggered blockage of the cell cycle in addition to apoptosis and inhibited the expression of cyclin B1 in KYSE-510 and OE33 cells. In KYSE-510 cells, apigenin showed induction of expression of p21/waf1 and promoted G2/M phase cell cycle arrest in KYSE-510 and OE33cells. Higher PIG3 mRNA and protein expression due to apigenin processing indicated the involvement of PIG3 in apigenin-induced apoptosis in both KYSE-510 and OE33 cells. The increase in activity of caspase-3 and -9 fragments recommended that PIG3 induction was mediated through apigenin which further caused esophageal cancer cell apoptosis via interfering with the mitochondrial pathway.
(Masuelli et al., 2011) revealed that apigenin triggered tumor cell death in tongue, pharyngeal cancers, and cancer in FaDu cells by destabilization of the EGFR/ErbB2 signaling pathway. In head and neck squamous carcinoma cells, apigenin downregulates the phosphorylation of EGFR and ErbB2 at precise sites and is regarded to be associated with the stimulation of Ras/Raf/Erk-1/-2 and Akt pathways. Apigenin treatment causes arrest of cell growth and induces apoptosis in many tumors by the modulation of diverse signaling pathways. The authors also analyzed the interactions between apigenin and TRAIL in NSCLC cells. Results demonstrated apigenin and TRAIL produced a synergistic effect for the induction of apoptosis of NSCLC cells by upregulating death receptors-4, and -5 in a p53-dose-dependent fashion. Additionally, the pro-apoptotic proteins were upregulated, while the anti-apoptotic proteins, viz. Bcl-xl and Bcl-2 were reported to be downregulated. Apigenin inhibited the activation of NF-κB, AKT, and ERK.
3.5.2 In vivo studies
In the mouse xenograft model, the combined treatment of apigenin and TRAIL entirely inhibited tumor proliferation as compared to the treatment of apigenin or TRAIL alone. In total, apigenin enhanced antitumor activity in NSCLC cells which was induced by TRAIL through the inhibition of several pro-survival regulators.
3.6 Anticancer mechanisms of luteolin
Luteolin inhibits the progression of cancerous cells, protects from carcinogenic stimuli, activates cell division blocks, and helps in the initiation of cell death via activating numerous signaling pathways. Luteolin also reverses the epithelial-mesenchymal transition (EMT) by altering a mechanism that involves cytoskeleton contraction resulting in a change in the expression of epithelial biomarker E-cadherin in addition to a decrease in expression of the mesenchymal biomarkers. In addition, luteolin elevates the intracellular concentration of ROS via the stimulating ER stress, which is lethal as well as mitochondrial dysfunction in glioblastoma cells (Imran et al., 2019). Modulation of ROS levels, topoisomerase I and II, P13K, NF-kappaB and AP-I activity reduction, and p53 stabilization are mechanisms contributing to the anti-tumor activity of luteolin (Lopez-Lazaro, 2009). Luteolin inhibits specific critical cancer, which is followed by the activation of various signaling pathways like mTOR and MAPK. Programmed cell death is mediated through the cell cycle arrest. In colon cancer, cells treated with luteolin block the cycle of the cell at the G2/M phase. Proteins involved in G2/M transition, specifically cyclin B and CDC2, were downregulated, whilst upregulated proteins were CDK2 and cyclin A. Luteolin arrests the G2 phase in non-small-cell lung cancer cells by the inhibition of the cyclin A expression plus CDC2 phosphorylation (Ganai et al., 2021).
3.6.1 In vitro studies
In vitro studies on luteolin have revealed that luteolin inhibits breast cancer cell proliferation, is stimulated by IGF-1, arrests cell cycle development, plus induces cell death in a time-and dose-dependent manner (Table 1). Luteolin declined IGF-1-stressful Erk1/2 phosphorylation depicting its inhibitory effect. In breast cancer cells, luteolin increases or decreases the expression of numerous estrogen stimulating genes and cell cycle pathway genes and modifications histone H4 acetylation that’s positioned on the PLK-1 promoter (Yang et al., 2014). In colon cancer, luteolin exhibits a withdrawing effect on nitric oxide synthase (iNOS) and COX-2 expressions and a suppressing one on MMP-2 and -9 expressions. Recent research has discovered that treatment with dimethylhydrazine induced renal bleeding in addition to colon polyps in rats with the enhancement in COX-2 and oxidative stress. Luteolin treatment declined the concentrations of iNOS and COX-2 (Abdel hadi et al., 2015).
Luteolin treatment (15 μM, 24 h) to human pancreatic cancer cells significantly declined nuclear GSK-3β and NF-κB p65 expression (Bothe et al., 2010). The chemopreventive plus chemotherapeutic actions of luteolin against prostate cancer were demonstrated in the vastly persistent Du145-III prostate cancer cells. The study reported that luteolin decreased the growth of these cells and suppressed cancer cell intrusion. (Johnson et al., 2015) reported that luteolin hampered PCa cell growth that was androgen-sensitive and independent, caused the downregulation of miR 301, and triggered cell death in LNCaP PC3 cells.
In prostate fibroblastoma, luteolin caused inhibition of myofibroblast phenotypes and extracellular matrix contraction, which was induced by TGF-β; suppression of TGF-β signaling involving activation of AKT and ERK; suppressed activation of RhoA (Johnson and Mejia, 2013) (Table 1).
A recent study by Chakrabarti and Ray (2016) investigated the consequence of taking two different luteolin concentrations, viz. 25 and 50 μM, on the neuroblastoma cells in a dose-dependent manner. Luteolin aroused apoptosis, triggered G0/G1 cell cycle arrest, plus lowered the mitochondrial membrane potential. Luteolin in oral cancers affected the phosphorylation of ataxia-telangiectasia in conjunction with pathways of DNA repair. Luteolin reduced the growth of the SCC-4 cells and multiplied the death of tumor cells by interfering with the expression of some cyclins, cyclin-established kinase (CDKs) (Wang F. et al., 2015). In head and neck squamous cell carcinoma, luteolin inhibited the tumor expansion plus histone acetylation, promoted arrest of the cell cycle, declined the movement of cells as well as altered gene expression, and upregulation of p53 induced plus miRNA systems (Tu et al., 2016).
Luteolin played an influential part in human NSCLC cell line A549 against tumor cell propagation by inducing cell death along with suppression of movement of cells. Apoptosis induction involves numerous steps in cells, viz. Caspases activation, alteration of MEK phosphorylation, Bcl-2 family proteins expression and downstream of kinase ERK, along with Akt phosphorylation (Figure 2). The luteolin’s anticancer properties were well explained on NCIH460 cells via induction of cell-death that was mediated by Sirt1 (Imran et al., 2019).
In-vitro studies on esophageal carcinoma cells demonstrated that antitumor activity of luteolin on cancer cells occurred through the activation or suppression of several mechanisms which included the activation of apoptotic cell death plus caspase-3, and induction of arrest of the cell cycle at the G2/M phase (Ganai et al., 2020; Ganai et al., 2021). Luteolin also lowered the mitochondrial membrane potential and enhanced the regulatory protein of the cell cycle in addition to the increase in the concentrations of apoptosis-related proteins.
3.6.2 In vivo studies
In a recent study, azoxymethane (AOM) intraperitoneal administration triggered colon carcinogenesis in Balb/C mice via an increase in the levels of tumor markers. Oral administration of luteolin appreciably declined the concentrations of tumor markers as well as expressions of MMP-2 and -9 (Naso et al., 2016). In pancreatic most cancers cells, in vivo apoptosis was brought about by luteolin through the suppression of the K-ras/GSK-3β/NF-κB pathway with the release of cytochrome C, caspase-3activation, and decline in Bcl-2/Bax ratio (Pandurangan et al., 2014). In a xenograft mouse model, at a specific dose, luteolin reduced the pro-inflammatory cytokines and TNF-α stages in PC3 cells similarly to decreased weight and extent of tumors, affected the cell capability, triggered cell death, and downregulated the ERK, AKT, mTOR, MMPs expressions (Han et al., 2016) (Figure 2).
In another study, in a xenograft model in mice with NSCLC cells, luteolin and Infra-Red radiation co-treatment activated apoptotic cell death, and declined the expression of B-cell lymphoma 2 (Bcl-2) along with caspase-complex activation. Also, luteolin inhibited the progression of xenograft mouse models of esophageal squamous cell carcinoma (Wu et al., 2015).
3.7 Anticancer mechanisms of genistein
Genistein possesses antitumor activities by the modulation of multiple signaling pathways, i.e., extracellular signal-regulated kinase 1/2 (ERK1/2), protein-tyrosine kinase (PTK), nuclear transcription factor-kB (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3 kinase/Akt (PI3K/Akt), matrix metalloproteinases (MMPs) and, Bcl-2-related X protein (Bax) (Lee et al., 2012; Spagnuolo et al., 2015; Bhat et al., 2021) (Figure 2). Genistein at higher concentration results in apoptosis mediated by inhibition of various proteins associated with primary tumor growth, i.e., cell cycle regulators (cyclin class) and the Akt family of proteins. However, when genistein is given at a lower concentration via diet inhibits the transforming growth factor (TGF) pathway, which affects pro-metastatic actions such as cancer cell detachment and invasion (Pavese et al., 2010). Genistein induces endoplasmic reticulum (ER) stress by upregulation of glucose-regulated protein 78 (GRP78) and CHOP expression. ER stress inhibitor also enhances genistein-induced cell death (Yang F. et al., 2016) (Table 1).
3.7.1 In vitro studies
In vitro studies of genistein revealed that in ovarian cancer cells, it triggers cell cycle arrest in the G2/M phase, and induces caspase-3 activity hence, enhances the apoptotic population. As the genistein concentration increases concurrently after 48 h of exposure, an increase in cell proportion in the G2/M phase was also noticed (Choi et al., 2007). Genistein in HO-8910 cells changed the protein levels, which are related to the checkpoint pathway, resulting in the inhibition of cancer cell propagation (Ouyang et al., 2009). Pretreatment with genistein by Solomon et al. (2008) in platinum-sensitive and -resistant ovarian cancer cells inhibited cell growth by NF-κB inactivation.
A recent study (Yang F. et al., 2016) reported that in cervical cancer cells, cell death triggered by genistein is mediated by endoplasmic reticulum (ER) stress and suppresses the HeLa cells’ viability in a dose-dependent fashion. Additionally, genistein also played an important part in triggering ER stress in HeLa cells mediated through the upregulation of glucose-regulated protein 78 and CHOP expression. Genistein helps in the initiation of cell death in several hepatocellular carcinoma cells (HCCs). Genistein exhibits potential anti-invasive and anti-metastatic activities against 12-O-tetra decanoyl phorbol-13-acetate-mediated metastasis which proceeds through downregulation of MMP-9 and NF-kB, and activator protein 1 transcription factors occurring via inhibition of MAPK (Spagnuolo et al., 2015).
Genistein triggered apoptosis in the low-invasive MCF-7 and the high-attack in breast cancer cell lines (10–100 mM) in a dose-dependent approach (Spagnuolo et al., 2015). Genistein declined the cell viability as well as the colony-forming potential of HepG2 liver cancer cells in a dose-and concentration-dependent manner (IC50 = 25 μM). Genistein triggered the ROS generation, thus, favoring cell death. Apoptosis induction by genistein is linked with the upregulation of cytosolic cytochrome c, with the downregulation of Bcl-2 in HepG2 cells (Zhang et al., 2019). Genistein significantly inhibits the propagation of hepatocellular carcinoma and induces death of the cell in a concentration and time-dependent manner. During genistein treatment, the reported enhanced percentage of cell death at a concentration (20 μM) with the progression of time 24, 48, and 72 h were 35, 42, and 65%, respectively (Sanaei et al., 2018).
3.7.2 In vivo studies
Genistein administration in ovariectomized rats increased uterine weight. An antagonistic effect of genistein on the estradiol increases uterine epithelial height (Diel et al., 2006). A protective effect of genistein against the progression of endometrial cancer along with atypical endometrial hyperplasia in mice was observed by the modulation of the expression of genes known to be linked with estrogen plus cytokines (Lee et al., 2012). Genistein significantly suppressed the attack of Bel 7,402 cells and altered the cell cycle, cell death, and angiogenesis in the renal parenchyma of nude mice with a xenograft transplant. In treating mice with genistein, tumor growth was significantly slowed down in male BALB/C nu/nu mice that were injected with Bel 7,402 cells (Gu et al., 2005).
3.8 Anticancer mechanisms of protocatechuic acid
Protocatechuic acid (PCA) exerts pro-apoptotic and anti-proliferative properties in different tissues. Anti-tumor property of PCA involves the stimulation of c-Jun N-terminal kinase, p38 subgroups of the mitogen, which are further activated by the protein kinase (MAPK) family (Khan et al., 2015). PCA blocks the retinone-induced apoptotic death of cells. PCA influences the activity of cyclooxygenase (COX) inducible isoenzyme as well as nitric oxide synthase along with regulating proteins of the cell cycle, or inflammatory cytokines, comprising the part of oncogenesis (Kakkar and Bais, 2014). Another mechanism of action for the anti-cancer activity of PCA follows the downregulation of the Ras/Akt/NF-kB pathway by targeting activation of RhoB, further leading to a drop in MMP-2-mediated cellular actions in cancer cells (Lin et al., 2011). In five cancer cells, concentration-dependent PCA lowered cell viability, increased lactate dehydrogenase leakage due to increased DNA fragmentation, lowered mitochondrial membrane potential, and declined the Na+-K+-ATPase activity; thus, elevated caspase-3 and caspase-8 activities, therefore, promoted cell death in cancer cells (Yin et al., 2009) (Table 1).
3.8.1 In vitro studies
The apoptotic effects of PCA on different types of cancers, namely, lung cancer, human breast cancer, and prostate cancer cell, in a dose-dependent approach (1, 2, 4, 8 μmol/L) were studied. The authors concluded that PCA declined cell feasibility, increased leakage of lactate dehydrogenase, DNA fragmentation, suppressed mitochondrial membrane potential, and decreased Na+-K+-ATPase activity. Although PCA elevated caspase-3 activity in 5 cancer cells still, at the concentration of 2–8 μmol/L, PCA considerably enhanced caspase-8 activity, lowered intercellular adhesion molecule expression in cancer cells, and declined vascular endothelial growth factor production. Additionally, PCA reduced interleukins (IL)-6 and IL-8 in cancer cell lines (Yin et al., 2009).
3.8.2 In vivo studies
Metastasis mouse models in vivo were analyzed to study the effect of PCA on cancer cell attacks. The study concluded that PCA at non-cytotoxic concentrations inhibited cell migration and invasion. PCA treatment caused decreased expression of MMP-2 subsequently, a rise in MMP tissue inhibitor. PCA-inhibition of MMP-2 activity expression was consorted by NF-kB inactivation, which was further mediated via RhoB/protein kinase C and Ras/Akt cascade pathways. Taken together, PCA reduced the progression of B16/F10 melanoma cells in mice (Lin et al., 2011).
3.9 Anticancer mechanisms of rosmarinic acid
RA’s antitumor mechanism of action involves significant suppression of cell viability, progression, invasiveness, and migration thus, induction of cell death. Anti-tumor effect of RA occurs via regulation of the miR-506/MMP2/16 axis in pancreatic tumor cells. The treatment of RA suppresses the tumor expansion of pancreatic cells and enhances and suppresses the expression of miR-506 and MMP2/16/Ki-67, respectively (Han Z. et al., 2018). RA possesses an inhibitory effect on melanoma cells’ proliferation, movement, and invasion via inhibiting expression of the ADAM17/EGFR/AKT/GSK3β axis (Huang et al., 2021). In colorectal cancer, the anticancer effect of RA causes the inhibition of COX-2 activation by repression of binding of activator protein-1 (AP-1) and c-Fos inducing agents (Hossan et al., 2014). The anti-metastatic effect of RA occurs via the AMPK phosphorylation, and colorectal cancer proliferation is slowed down, which further proceeds by the initiation of cell cycle arrest and apoptosis, followed by inhibition of expression of MMP-2 and MMP-9. In conclusion, RA effects on EMT and MMPs expressions result in AMPK activation (Han Z. et al., 2018). In leukemia, RA suppresses the activation of NF-kB-dependent anti-apoptotic proteins via phosphorylation inhibition, I kappa-B-alpha degradation, and p50 and p65 nuclear translocation (Moon et al., 2010) (Figure 2).
3.9.1 In vitro studies
The effects of RA on pancreatic tumor progression and its primary molecular mechanisms were explored by Han et al. (2019) (Table 1). Authors concluded that RA considerably suppressed vital cellular functions in pancreatic tumor cells, finally inducing cell death in pancreatic cells. Further, RA significantly up-regulated and knocked down the expression of miR-506 in pancreatic cancer cells, accompanied by the suppressive effects of RA on cell growth and EMT, and prohibited increased impact of RA on cell apoptosis in pancreatic tumor cells. Additionally, RA treatment concealed the MMP2/16 expression in pancreatic cancer cells.
In another study, anticancer effects of RA in A375 cells melanoma cells via downregulation of ADAM17 and expression of ADAM17/EGFR/AKT/GSK3β were also evaluated. Results revealed that A375 cells treated with RA had decreased cell viability, propagation, invasive abilities, melanin content, and decreased expression of MMP-2 and MMP-9 proteins as compared to normal cells. RA increased the expression of pro-apoptotic proteins and suppressed Bcl-2 expression (Huang et al., 2021). (Han Z. et al., 2018) reported that RA inhibited the metastatic properties of CRC cells through the phosphorylation of AMPK. RA suppressed the CRC cells propagation, brought about cell cycle arrest, and hence, initiated apoptosis. RA mediated EMT through the growing the expression of an epithelial marker, E-cadherin, and lowering the expression of the mesenchymal markers and N-cadherin (Figure 2). RA treatment caused CRC cells’ invasion and migration suppression and declined the expressions of MMP-2 and MMP-9.
In human leukemia U937 cells, RA was reported to trigger apoptosis which was in turn induced by TNF-α and generation of ROS. RA inhibited NF-kB activation via phosphorylation inhibition, followed by the degradation of IκBαand nuclear translocation of p50 and p65, which is associated with NF-kB-dependent anti-apoptotic proteins suppression (Hossan et al., 2014). RA inhibited the movement of human bone-homing breast cancer cells suggesting that RA affected the bone metastasis in breast carcinoma via activation of the NF-kB ligand (RANKL)/RANK/osteoprotegerin (OPG) pathway with the simultaneous repression of the IL-8 expression (Xu et al., 2010).
3.9.2 In vivo studies
In vivo studies have exposed that RA inhibited the tumor growth dose-dependently of pancreatic cancer cells and enhanced the expression of miR-506, although lowered the MMP2/16 and Ki-67 expression in xenograft nude mice. Mice treated with oral RA suppressed tumor induction NF-κB, TNF-α, VEGF serum, and VEGF receptors. RA induced apoptosis by restoring the expressions of Bcl-2, Bax, and caspase-3. In Ehrlich solid tumor mice, RA, in combination with paclitaxel, significantly decreased the growth of the tumor with enhancement in levels of apoptotic markers (Mahmoud et al., 2021).
In the swiss albino xenograft model, RA administration suppressed tumor formation, positively altered the antioxidant level, and stabilized the 7,12-dimethyl-benz(a)anthracene (DMBA)-induced alterations in the apoptotic markers (Sharmila and Manoharan, 2012).
3.10 Anticancer mechanisms of chlorogenic acid
Mechanism of action of CA involves induction of apoptosis upregulation of p21 leading to G0/G1 cell cycle arrest, reducing the potential of the mitochondrial membrane of the cell resulting in mitochondrial dysfunction, and escalating the activation of the caspase-3 pathway, ultimately causing cell death (Dai et al., 2012; Huang et al., 2019). The apoptosis effect of CA occurs via the increase in p53 and Bax protein expression with a decrease in Bcl-2 expression followed by activation of the caspase-3 pathway (Changizi et al., 2020) (Figure 2). The BH3-protein Bcl-2 binding component 3 (BBC3) is upregulated by CA, which causes mitochondrial death (Jiang et al., 2021). CA inhibits the migration and invasive capacity of tumor cells, modulates mTOR2-related pathways, and reduces the phosphorylation of p-protein kinase C alpha (PKCα), Akt, along with declined expression of Rictor and F-actin (factors responsible for cell growth and organization of actin cytoskeleton). Directly involves the mTOR2/F-actin pathway for tumor suppression (Tan et al., 2019). CA inhibits the NF-B/EMT signaling pathway, concluding its anticancer and antimetastatic properties (Zeng et al., 2021).
3.10.1 In vitro studies
In human hepatoma cells (HCC), CA dose-dependently inhibits the activity of cell lines, i.e., Hep-G2 and Huh-7. CA alters the NF-κB signaling pathway and turns on the mitochondrial cell death of HCC through the upregulation of (BBC3) (Jiang et al., 2021). The inhibitory effect of CA in-vitro (A549 and HepG2 cell lines) and in vivo (female BALB/c nude mice) on tumor cells and its associated possible mechanisms engaged in filamentous actin (F-actin) organization was also investigated. Results demonstrated that CA effectively inhibited the increase of tumor cells.
A study conducted by Changizi et al. (2020) analyzed the effect of CA on 4T1 breast cancer cells. Highest CA concentration up-and down-regulated Bax and Bcl-2, respectively, in 4T1 treated cells. Further, outcomes showed growth in the expression of P53 and caspase-3 for the duration of remedy in CA-handled 4T1 cells. CA inhibits proliferation and stimulates preprophase apoptosis, arrests the cell cycle at the G0/G1 segment in human acute promyelocytic leukemia HL-60 cells on the greatest dose of 10 µM (Liu et al., 2013).
The anti-cancer effect of CA was evaluated in another study by Huang et al. (2019). Treatment of tumour cells with CA caused elevation in SUMO1 expression via acting on 3′UTR and stabilization of mRNA. Elevated levels of SUMO1 resulted in c-Mycsumoylation, downregulation of the miR-17 family, and p21 upregulation causing the G0/G1 cell cycle seize.
3.10.2 In vivo studies
In vivo, CA administration to mice bearing tumor cell-implanted xenografts inhibited the growth of the tumor. Alteration in various signal pathways includes modulation of mTORC2 associated signaling pathways, decreased phosphorylation of p-protein kinase C alpha (PKCα), Akt, declined expression of Rictor and F-actin known for the activation of cell growth and organization of the actin cytoskeleton indicating the involvement of mTORC2/F-actin pathway in tumor suppression induced by CA (Tan et al., 2019).
CA antitumor effect was evaluated in a recent study (Zeng et al., 2021) in a subcutaneous tumor mouse model possessing 4T1 cells. Authors discovered that CA noticeably slowed down the tumor growth with the prolongation of the survival rate of tumor-bearing mice. CA exerted antitumor and anti-metastatic consequences using a modification of the NF-κB/EMT signaling pathway.
3.11 Anticancer mechanisms of action of eupatorin
Eupatorin showed an antitumor effect on human breast cancer cells and mediated CYP1- metabolism. The cytotoxic effect of eupatorin was investigated on the human breast carcinoma cell line as well as another cell line that was extracted from normal mammary tissue, MCF-10A. Eupatorin blocks the cell cycle in the G2/M phase in CYP1-expressing cell line MDA-MB-468; however, the authors did not report any effect on MCF-10A cells; hence, no expression of CYP1 enzymes was observed. Together, the presence of CYP1 family metabolism resulted in the flavone eupatorin selective activation in breast cancer cells but not in normal breast cells (Androutsopoulos et al., 2008) (Table 1).
Razak et al. (2019) operating on human breast carcinoma strains concluded that eupatorin triggered apoptosis, suppressed invasion, migration, and angiogenesis of cell strains, i.e., MDA-MB-231 and MCF-7 cells through inhibition of the Phospho-Akt pathway, and cell cycle obstruction. Cytotoxic consequences of eupatorin have been located on both cells; however, they remained non-poisonous to the ordinary cells of MCF-10a in a time- and dose-structured approach. However, eupatorin was reported to be mild cytotoxic on both cells after 24 h of treatment. A lower dose of eupatorin (5 μg/ml) prompted apoptosis via the intrinsic pathway and caspase-9 in assessment to caspase-8. The eupatorin exerted arrest of the cell cycle in MCF-7 and MDA-MB-231 cells at Gθ/G1 in a time-hooked-up fashion. In vivo studies have revealed that eupatorin up-regulated pro-apoptotic genes; however, SMAC/Diablo inhibited the Phospho-Akt pathway. In another study (Pavlopoulou et al., 2015), reported that eupatorin suppressed cell proliferation in human tumor cells. Eupatorin blocks cells at the G2-M phase and triggers cell death, activating a couple of caspases cytochrome-c release and breakdown of poly (ADP-ribose) polymerase in human leukaemia cells. Eupatorin initiates the mitogen-activated protein kinases phosphorylation and cell apoptosis through c-jun N-terminal kinases/stress-activated kinases inhibition. Cell death via eupatorin is mediated via every apoptotic pathway and ROS mechanism.
3.11.1 In vitro studies
In HeLa cells (HCT116 cells) human cervical carcinoma cells, cell cycle arrest started after 12 h of eupatorin treatment at the G2/M segment, followed by induction of apoptosis. Eupatorin down- and up-regulated cyclin D1 and B1 after 3 and 12 h of eupatorin treatment, respectively. Eupatorin lowered p53, p21, and Bax protein concentrations via initiation of the breakdown of caspase-3, -7, and poly (ADP-ribose) polymerase. Eupatorin-triggered p21 and Bax expressions which were p53 and Egr-1-pathways dependent, respectively. The authors concluded that eupatorin induced cytotoxicity in HeLa human cervical carcinoma cells, which was dysregulated via G2/M cell cycle blockage and triggered apoptosis via the activation of the p53-pathway (Lee K. et al., 2016) (Table 1).
4 Therapeutic perspectives on polyphenols as potential anticancer agents
Most polyphenols are present in foods in the form of esters, glycosides and polymers that cannot be absorbed in their native form in the human gastrointestinal tract (Sharifi-Rad et al., 2022; Semwal et al., 2022). Thus, before absorption of the molecules must be hydrolyzed by saliva, intestinal enzymes or need to be metabolized by the colonic microflora. Subsequently, to be absorbed, polyphenols are subjected to conjugation in intestinal cells and then methylation, sulfation and/or hepatic glucuronidation (Sharifi-Rad et al., 2021b). Thus, the structural integrity and proper functioning of the digestive tract are essential for the optimal absorption of polyphenols. The variability of the biological activity of polyphenols beyond the particularities of the human body also depends on the biological properties of the classes and subclasses of polyphenols (Rudrapal et al., 2022). For the anticancer effect, polyphenols can also be consumed in the form of natural supplements. In the case of approved natural supplements, they must be administered according to the recommendations made by the manufacturer, which can be found on the product packaging (Tsoukalas et al., 2019a; Tsoukalas et al., 2021b) Although obtained from 100% natural ingredients, these supplements can have unwanted effects if not administered correctly (Tsoukalas et al., 2019b; Tsoukalas et al., 2021a). Therefore, future translational studies are needed to determine the effective therapeutic dose in humans and the route of administration.
A therapeutic limitation is represented by the possibility of polyphenols interfering with the absorption of nutrients or drugs. For example, they can reduce the body’s ability to absorb iron, thiamine or folic acid (Cory et al., 2018). More research needs to be done to better understand these potential interactions. To increase therapeutic efficacy, nanotechnologies have been tried to increase the absorption and reduced bioavailability of some polyphenols, and numerous studies have confirmed this benefit. Food supplements with polyphenols do not endanger health, but there are uncertainties about the side effects of polyphenol supplements (Rana et al., 2022). Therefore, new studies are needed in the future to overcome the biopharmaceutical problems of polyphenols in cancer. Also, future research are needed to evaluate the isolated and particular effects of certain classes/subclasses of polyphenolic compounds on various types of cancer, respectively extrapolating the possible anticancer benefits to the general population or particular high-risk groups.
5 Conclusion
In the attention to experimental evidence stated and mentioned withinside the review, it is miles glaring that polyphenols display more capability than other compounds by blocking more than one target in pathways and mechanisms associated with cancer progression. Various in-vitro studies suggest that the polyphenols modulate Nrf 2 and NF-kB activation in cells and also influence the MAPK and PI3K function in the cells, thereby establishing a prominent role in cancer cell progression. In connection, the possibility of combining conventional drugs with polyphenols (in individual or combination) offers valuable advantages for developing more anti-cancer therapies with greater efficacy. Although, there is no doubt about the role of oxidation in the occurrence of mutations in DNA and the increased risk of cancer, the isolated action of a particular polyphenol or polyphenolic compounds, in general, is unlikely to have a distant effect on the gastrointestinal tract - for example, in the mammary gland or lungs. While data on the beneficial effects of polyphenolic compounds as anticancer agents, it has been observed that all these benefits depend not only on the food content of polyphenols, but especially on their bioavailability in the gastrointestinal tract, and their ability to be absorbed into the circulatory system to reach the cells where they manifest their protective effect. Nevertheless, the usage of dietary polyphenols as whole foods can be a promising aspect of cancer prevention.
Author contributions
Conceptualization and design were performed by JS-R, AS, DC, and WC; validation, investigation, data curation, and writing were performed by ES, DA, JS-R, PS, CH, and PD; review and editing were performed by PD, AS, WC, DC, and JS-R.; supervision DC, JS-R, CH, and WC. All the authors contributed equally, read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this study was funded by Région Centre-Val de Loire (grant number INNO-COSM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Bax, Bcl2-Associated X Protein; Bcl-2, B-cell lymphoma 2; BCRP, Breast Cancer Resistance Protein; Cyt-c, cytochrome-c; CDH1, cadherin-1; IGFR, insulin-like growth factor receptor; MAPK, mitogen-activated protein kinase; MRP1, multidrug resistance protein1; mTOR, mammalian target of rapamycin; P13K/Akt, phosphatidylinositol 3-kinase/protein kinase B; p53, tumor protein p53; RTKs, receptor tyrosine kinases; RAS: rat sarcoma virus; TNF-α, tumour necrosis factor-alpha; VEGFR2, vascular endothelial growth factor receptor 2,.
References
- Abd razak N., Yeap S. K., Alitheen N. B., Ho W. Y., Yong C. Y., Tan S. W., et al. (2020). Eupatorin suppressed tumor progression and enhanced immunity in a 4T1 murine breast cancer model. Integr. Cancer Ther. 19, 1534735420935625. 10.1177/1534735420935625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel hadi L., DI Vito C., Marfia G., Ferraretto A., Tringali C., Viani P., et al. (2015). Sphingosine kinase 2 and ceramide transport as key targets of the natural flavonoid luteolin to induce apoptosis in colon cancer cells. PLOS ONE 10, e0143384. 10.1371/journal.pone.0143384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali E. S., Akter S., Ramproshad S., Mondal B., Riaz T. A., Islam M. T., et al. (2022). Targeting ras-ERK cascade by bioactive natural products for potential treatment of cancer: An updated overview. Cancer Cell Int. 22, 246. 10.1186/s12935-022-02666-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra A., Innao V., Russo S., Gerace D., Alonci A., Musolino C. (2017). Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Invest. 35, 1–22. 10.1080/07357907.2016.1247166 [DOI] [PubMed] [Google Scholar]
- Almatroodi S. A., Almatroudi A., Khan A. A., Alhumaydhi F. A., Alsahli M. A., Rahmani A. H. (2020). Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 25, E3146. 10.3390/molecules25143146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsopoulos V., Arroo R. R. J., Hall J. F., Surichan S., Potter G. A. (2008). Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res. 10, R39. 10.1186/bcr2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneknan P., Kukongviriyapan V., Prawan A., Kongpetch S., Sripa B., Senggunprai L. (2014). Luteolin arrests cell cycling, induces apoptosis and inhibits the JAK/STAT3 pathway in human cholangiocarcinoma cells. Asian pac. J. Cancer Prev. 15, 5071–5076. 10.7314/apjcp.2014.15.12.5071 [DOI] [PubMed] [Google Scholar]
- Asensi M., Ortega A., Mena S., Feddi F., Estrela J. M. (2011). Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 48, 197–216. 10.3109/10408363.2011.631268 [DOI] [PubMed] [Google Scholar]
- Azmi A. S., Bhat S. H., Hanif S., Hadi S. M. (2006). Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 580, 533–538. 10.1016/j.febslet.2005.12.059 [DOI] [PubMed] [Google Scholar]
- Bai Z.-L., Tay V., Guo S.-Z., Ren J., Shu M.-G. (2018). Silibinin induced human glioblastoma cell apoptosis concomitant with autophagy through simultaneous inhibition of mTOR and YAP. Biomed. Res. Int. 2018, 6165192. 10.1155/2018/6165192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S. S., Prasad S. K., Shivamallu C., Prasad K. S., Syed A., Reddy P., et al. (2021). Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 43, 1502–1517. 10.3390/cimb43030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H., Götz C., Stobbe-Maicherski N., Fritsche E., Abel J., Haarmann-Stemmann T. (2010). Luteolin enhances the bioavailability of benzo(a)pyrene in human colon carcinoma cells. Arch. Biochem. Biophys. 498, 111–118. 10.1016/j.abb.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Briguglio G., Costa C., Pollicino M., Giambò F., Catania S., Fenga C. (2020). Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 1, 9. 10.3892/ijfn.2020.9 [DOI] [Google Scholar]
- Buga A. M., Docea A. O., Albu C., Malin R. D., Branisteanu D. E., Ianosi G., et al. (2019). Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol. Lett. 17, 4170–4175. 10.3892/ol.2019.9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Zhang H., Cai X., Fang W., Chai D., Wen Y., et al. (2017). Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell. Physiol. biochem. 43, 1803–1812. 10.1159/000484066 [DOI] [PubMed] [Google Scholar]
- Chakrabarti M., Ray S. K. (2016). Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo . Apoptosis 21, 312–328. 10.1007/s10495-015-1198-x [DOI] [PubMed] [Google Scholar]
- Changizi Z., Moslehi A., Rohani A., Eidi A. (2020). Chlorogenic acid inhibits growth of 4T1 breast cancer cells through involvement in Bax/Bcl2 pathway. J. Cancer Res. Ther. 16, 1435–1442. 10.4103/jcrt.JCRT_245_19 [DOI] [PubMed] [Google Scholar]
- Chen P.-N., Chu S.-C., Kuo W.-H., Chou M.-Y., Lin J.-K., Hsieh Y.-S. (2011). Epigallocatechin-3 gallate inhibits invasion, epithelial− mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 59, 3836–3844. 10.1021/jf1049408 [DOI] [PubMed] [Google Scholar]
- Chen S. J., Yao X. D., Peng B., Xu Y. F., Wang G. C., Huang J., et al. (2016). Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp. Ther. Med. 11, 1243–1248. 10.3892/etm.2016.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Lu X., Feng B. (2020). A review of research progress of antitumor drugs based on tubulin targets. Transl. Cancer Res. 9, 4020–4027. 10.21037/tcr-20-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Suh D. S., Moon S. H., Song Y. J., Yoon M. S., Park D. Y., et al. (2013). Silibinin inhibits tumor growth through downregulation of extracellular signal-regulated kinase and Akt in vitro and in vivo in human ovarian cancer cells. J. Agric. Food Chem. 61, 4089–4096. 10.1021/jf400192v [DOI] [PubMed] [Google Scholar]
- Choi E. J., Kim T., Lee M.-S. (2007). Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 80, 1403–1408. 10.1016/j.lfs.2006.12.031 [DOI] [PubMed] [Google Scholar]
- Coombs M. R. P., Harrison M. E., Hoskin D. W. (2016). Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 380, 424–433. 10.1016/j.canlet.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. (2018). The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 5, 87. 10.3389/fnut.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., VAN Wie P. G., Fai L. Y., Kim D., Wang L., Poyil P., et al. (2016). Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicol. Appl. Pharmacol. 311, 106–112. 10.1016/j.taap.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X. Y., Zeng X. X., Peng F., Han Y. Y., Lin H. J., Xu Y. Z., et al. (2012). A novel anticancer agent, SKLB70359, inhibits human hepatic carcinoma cells proliferation via G0/G1 cell cycle arrest and apoptosis induction. Cell. Physiol. biochem. 29, 281–290. 10.1159/000337609 [DOI] [PubMed] [Google Scholar]
- Dhyani P., Quispe C., Sharma E., Bahukhandi A., Sati P., Attri D. C., et al. (2022). Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 22, 206. 10.1186/s12935-022-02624-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P., Hertrampf T., Seibel J., Laudenbach-Leschowsky U., Kolba S., Vollmer G. (2006). Combinatorial effects of the phytoestrogen genistein and of estradiol in uterus and liver of female Wistar rats. J. Steroid Biochem. Mol. Biol. 102, 60–70. 10.1016/j.jsbmb.2006.09.022 [DOI] [PubMed] [Google Scholar]
- Docea A. O., Mitrut P., Grigore D., Pirici D., Calina D. C., Gofita E. (2012). Immunohistochemical expression of TGF beta (TGF-beta), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal variant of gastric adenocarcinomas. Romanian J. Morphol. Embryology 53, 683–692. [PubMed] [Google Scholar]
- Eom D.-W., Lee J. H., Kim Y.-J., Hwang G. S., Kim S.-N., Kwak J. H., et al. (2015). Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 48, 461–466. 10.5483/bmbrep.2015.48.8.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan S., Doganlar O., Doganlar Z. B., Serttas R., Turkekul K., Dibirdik I., et al. (2016). The flavonoid apigenin reduces prostate cancer CD44+ stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 162, 77–86. 10.1016/j.lfs.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Erdogan S., Turkekul K., Dibirdik I., Doganlar O., Doganlar Z. B., Bilir A., et al. (2018). Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 107, 793–805. 10.1016/j.biopha.2018.08.061 [DOI] [PubMed] [Google Scholar]
- Estevez S., Marrero M. T., Quintana J., Estevez F. (2014). Eupatorin-induced cell death in human leukemia cells is dependent on caspases and activates the mitogen-activated protein kinase pathway. PLoS One 9, e112536. 10.1371/journal.pone.0112536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Zhong L., Shan Z., Xiao C., Xu T., Song H., et al. (2017). Epigallocatechin-3-gallate induces apoptosis in acute promyelocytic leukemia cells via a SHP-1-p38α MAPK-Bax cascade. Oncol. Lett. 14, 6314–6320. 10.3892/ol.2017.6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan R.-Y., Li H.-B., Sui Z.-Q., Corke H. (2018). Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 58, 924–941. 10.1080/10408398.2016.1231168 [DOI] [PubMed] [Google Scholar]
- Ganai S. A.-O., Sheikh F. A., Baba Z. A., Mir M. A., Mantoo M. A., Yatoo M. A. (2020). Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. [DOI] [PubMed] [Google Scholar]
- Ganai S. A., Sheikh F. A., Baba Z. A., Mir M. A., Mantoo M. A., Yatoo M. A. (2021). Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 35, 3509–3532. 10.1002/ptr.7044 [DOI] [PubMed] [Google Scholar]
- Gao X., Wang B., Wei X., Men K., Zheng F., Zhou Y., et al. (2012). Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 4, 7021–7030. 10.1039/c2nr32181e [DOI] [PubMed] [Google Scholar]
- GBD Colorectal Cancer Collaborators (2022). Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol. Hepatol. 7. 10.1016/S2468-1253(22)00044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L., Pinti M., Nasi M., Montagna J. P., DE Biasi S., Roat E., et al. (2011). Quercetin and cancer chemoprevention. Evid. Based. Complement. Altern. Med. 2011, 591356. 10.1093/ecam/neq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., Tommonaro G. (2019). Curcumin and cancer. Nutrients 11, E2376. 10.3390/nu11102376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube S., Ewald C., Kögler C., Lawson Mclean A., Kalff R., Walter J. (2018). Achievable central nervous system concentrations of the green tea catechin EGCG induce stress in glioblastoma cells in vitro . Nutr. Cancer 70, 1145–1158. 10.1080/01635581.2018.1495239 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhu C.-F., Iwamoto H., Chen J.-S. (2005). Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J. Gastroenterol. 11, 6512–6517. 10.3748/wjg.v11.i41.6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A., Mumper R. J. (2009). Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 35, 32–46. 10.1016/j.ctrv.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Asad S. F., Singh S., Ahmad A. (2000). Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 50, 167–171. 10.1080/152165400300001471 [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Ullah M. F., Azmi A. S., Ahmad A., Shamim U., Zubair H., et al. (2010). Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for chemoprevention of cancer. Pharm. Res. 27, 979–988. 10.1007/s11095-010-0055-4 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2007). Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 73, 341–347. 10.1016/j.cardiores.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Han D. H., Kim J. H. (2009). Difference in growth suppression and apoptosis induction of EGCG and EGC on human promyelocytic leukemia HL-60 cells. Arch. Pharm. Res. 32, 543–547. 10.1007/s12272-009-1410-z [DOI] [PubMed] [Google Scholar]
- Han K., Meng W., Zhang J.-J., Zhou Y., Wang Y.-L., Su Y., et al. (2016). Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. Onco. Targets. Ther. 9, 3085–3094. 10.2147/OTT.S102862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Yang S., Cai Z., Pan D., Li Z., Huang Z., et al. (2015). Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des. devel. Ther. 9, 2695–2703. 10.2147/DDDT.S82342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.-H., Kee J.-Y., Hong S.-H. (2018a). Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front. Pharmacol. 9, 68. 10.3389/fphar.2018.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Ma L., Zhao L., Feng W., Zheng X. (2019). Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 115, 108878. 10.1016/j.biopha.2019.108878 [DOI] [PubMed] [Google Scholar]
- Han Z., Ma X., Wei M., Zhao T., Zhan R., Chen W. (2018b). SSR marker development and intraspecific genetic divergence exploration of Chrysanthemum indicum based on transcriptome analysis. BMC genomics 19, 291. 10.1186/s12864-018-4702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A., Rahman M. A., Chen Z. G., Saba N. F., Khuri F. R., Shin D. M., et al. (2015). Combination of erlotinib and EGCG induces apoptosis of head and neck cancers through posttranscriptional regulation of Bim and Bcl-2. Apoptosis 20, 986–995. 10.1007/s10495-015-1126-0 [DOI] [PubMed] [Google Scholar]
- Hashemzaei M., Delarami Far A., Yari A., Heravi R. E., Tabrizian K., Taghdisi S. M., et al. (2017). Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo . Oncol. Rep. 38, 819–828. 10.3892/or.2017.5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Lee J., Moon H., Ryu C. H., Seok J., Jung Y.-S., et al. (2021a). Quercetin induces anticancer activity by upregulating pro-NAG-1/GDF15 in differentiated thyroid cancer cells. Cancers 13, 3022. 10.3390/cancers13123022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Lee J., Moon H., Ryu C. H., Seok J., Jung Y.-S., et al. (2021b). Quercetin induces anticancer activity by upregulating pro-NAG-1/GDF15 in differentiated thyroid cancer cells. Cancers 13, 3022. 10.3390/cancers13123022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain R., Ray P., Sarkar C., Islam M. S., Khan R. A., Khalipha A. B. R., et al. (2022). Natural compounds or their derivatives against breast cancer: A computational study. Biomed. Res. Int. 2022, 5886269. 10.1155/2022/5886269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossan M. S., Rahman S., Bashar A. B. M., Jahan R., Nahian A., Rahmatullah M. (2014). Rosmarinic acid: A review of its anticancer action. WORLD J. Pharm. Pharm. Sci. 3, 57–70. [Google Scholar]
- Huang C.-Y., Han Z., Li X., Xie H.-H., Zhu S.-S. (2017). Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 14, 3623–3627. 10.3892/ol.2017.6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Chen J., Quan J., Xiang D. (2021). Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 12, 3065–3076. 10.1080/21655979.2021.1941699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Wang L.-L., Xue N.-N., Li C., Guo H.-H., Ren T.-K., et al. (2019). Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 9, 6745–6763. 10.7150/thno.34674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizumi Y., Oishi M., Taniguchi T., Goi W., Sowa Y. (2013). The Flavonoid Apigenin Downregulates CDK1 by Directly Targeting Ribosomal Protein S9, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M. A., Khan I. A., et al. (2019). Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 112, 108612. 10.1016/j.biopha.2019.108612 [DOI] [PubMed] [Google Scholar]
- Jain D., Chaudhary P., Varshney N., Bin Razzak K. S., Verma D., Zahra T. R. K., et al. (2021). Tobacco smoking and liver cancer risk: Potential avenues for carcinogenesis. J. Oncol., 5905357. 10.1155/2021/5905357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankun J., Keck R. W., Selman S. H. (2014). Epigallocatechin-3-gallate prevents tumor cell implantation/growth in an experimental rat bladder tumor model. Int. J. Oncol. 44, 147–152. 10.3892/ijo.2013.2174 [DOI] [PubMed] [Google Scholar]
- Javed Z., Khan K., Herrera-Bravo J., Naeem S., Iqbal M. J., Raza Q., et al. (2022). Myricetin: Targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 22, 239. 10.1186/s12935-022-02663-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Huang S., Yin X., Zan Y., Guo Y., Han L. (2018). Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 208, 123–130. 10.1016/j.lfs.2018.07.027 [DOI] [PubMed] [Google Scholar]
- Jiang H., Fan J., Cheng L., Hu P., Liu R. (2018). The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. Onco. Targets. Ther. 11, 8153–8163. 10.2147/OTT.S182239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Wang W., Jin X., Wang Z., Ji Z., Meng G. (2015). Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol. Rep. 33, 2711–2718. 10.3892/or.2015.3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Tao C., He A., He X. (2014). Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 12, 383–387. 10.1186/1477-7819-12-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Nan H., Shi N., Hao W., Dong J., Chen H. (2021). Chlorogenic acid inhibits proliferation in human hepatoma cells by suppressing noncanonical NF-κB signaling pathway and triggering mitochondrial apoptosis. Mol. Biol. Rep. 48, 2351–2364. 10.1007/s11033-021-06267-3 [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Dia V. P., Wallig M., Gonzalez DE Mejia E. (2015). Luteolin and gemcitabine protect against pancreatic cancer in an orthotopic mouse model. Pancreas 44, 144–151. 10.1097/MPA.0000000000000215 [DOI] [PubMed] [Google Scholar]
- Johnson J., Mejia E. (2013). Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro . Food Chem. Toxicol., 83–91. 10.1016/j.fct.2013.07.036 [DOI] [PubMed] [Google Scholar]
- Kakkar S., Bais S. (2014). A review on protocatechuic acid and its pharmacological potential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikkumar V., Sivagami G., Viswanathan P., Nalini N. (2015). Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J. Basic Clin. Physiol. Pharmacol. 26, 185–200. 10.1515/jbcpp-2014-0044 [DOI] [PubMed] [Google Scholar]
- Kaur M., Velmurugan B., Tyagi A., Agarwal C., Singh R. P., Agarwal R. (2010). Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of β-catenin-dependent signaling. Neoplasia 12, 415–424. 10.1593/neo.10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedhari sundaram M., Raina R., Afroze N., Bajbouj K., Hamad M., Haque S., et al. (2019). Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 39, BSR20190720. 10.1042/BSR20190720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketkaew Y., Osathanon T., Pavasant P., Sooampon S. (2017). Apigenin inhibited hypoxia induced stem cell marker expression in a head and neck squamous cell carcinoma cell line. Arch. Oral Biol. 74, 69–74. 10.1016/j.archoralbio.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Khan K., Pal S., Yadav M., Maurya R., Trivedi A. K., Sanyal S., et al. (2015). Prunetin signals via G-protein-coupled receptor, GPR30(GPER1): Stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. J. Nutr. Biochem. 26, 1491–1501. 10.1016/j.jnutbio.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Kim Y.-A., Choi B. T., Lee Y. T., Park D. I., Rhee S.-H., Park K.-Y., et al. (2004). Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 11, 441–446. 10.3892/or.11.2.441 [DOI] [PubMed] [Google Scholar]
- Kitic D., Miladinovic B., Randjelovic M., Szopa A., Sharifi-Rad J., Calina D., et al. (2022). Anticancer potential and other pharmacological properties of prunus armeniaca L.: An updated overview. Plants 11. 10.3390/plants11141885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaadam B., Şanlier N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895. 10.1080/10408398.2015.1077195 [DOI] [PubMed] [Google Scholar]
- Koh Y. W., Choi E. C., Kang S. U., Hwang H. S., Lee M. H., Pyun J., et al. (2011). Green tea (−)-epigallocatechin-3-gallate inhibits HGF-induced progression in oral cavity cancer through suppression of HGF/c-Met. J. Nutr. Biochem. 22, 1074–1083. 10.1016/j.jnutbio.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Koohpar Z. K., Entezari M., Movafagh A., Hashemi M. (2015). Anticancer activity of curcumin on human breast adenocarcinoma: Role of mcl-1 gene. Iran. J. Cancer Prev. 8, e2331. 10.17795/ijcp2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak P. A., Boitano P. D. (2014). Effect of the pleiotropic drug CNB-001 on tissue plasminogen activator (tPA) protease activity in vitro: Support for combination therapy to treat acute ischemic stroke. J. Neurol. Neurophysiol. 5, 214. [PMC free article] [PubMed] [Google Scholar]
- Lee J.-E., Yoon S. S., Moon E.-Y. (2019). Curcumin-induced autophagy augments its antitumor effect against A172 human glioblastoma cells. Biomol. Ther. 27, 484–491. 10.4062/biomolther.2019.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-Y., Kim H. S., Song Y.-S. (2012). Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complement. Med. 2, 96–104. 10.1016/s2225-4110(16)30082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Hyun Lee D., Jung Y. J., Shin S. Y., Lee Y. H. (2016a). The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl. Biol. Chem. 59, 193–199. 10.1007/s13765-016-0160-0 [DOI] [Google Scholar]
- Lee Y.-M., Lee G., Oh T.-I., Kim B. M., Shim D.-W., Lee K.-H., et al. (2016b). Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 48, 399–408. 10.3892/ijo.2015.3243 [DOI] [PubMed] [Google Scholar]
- Lee Y., Sung B., Kang Y. J., Kim D. H., Jang J.-Y., Hwang S. Y., et al. (2014). Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 44, 1599–1606. 10.3892/ijo.2014.2339 [DOI] [PubMed] [Google Scholar]
- Li H., Li Z., Xu Y.-M., Wu Y., Yu K.-K., Zhang C., et al. (2014). Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci. Bull. 30, 67–73. 10.1007/s12264-013-1394-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wu L., Feng J., Li J., Liu T., Zhang R., et al. (2016). In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Sci. Rep. 6, 28479–28518. 10.1038/srep28479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhao N., Lu J., Zhu Q., Liu X., Hao F., et al. (2019). Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 10, 282–291. 10.1080/21655979.2019.1632669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhou N., Wang J., Liu Z., Wang X., Zhang Q., et al. (2018). Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 196, 56–62. 10.1016/j.lfs.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Lin H.-H., Chen J.-H., Chou F.-P., Wang C.-J. (2011). Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 162, 237–254. 10.1111/j.1476-5381.2010.01022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-T., Ho Y.-S. (2018). Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 7, 134–137. 10.1016/j.fshw.2018.06.001 [DOI] [Google Scholar]
- Liu J., Cao X. C., Xiao Q., Quan M. F. (2015). Apigenin inhibits HeLa sphere-forming cells through inactivation of casein kinase 2α. Mol. Med. Rep. 11, 665–669. 10.3892/mmr.2014.2720 [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Zhou C. Y., Qiu C. H., Lu X. M., Wang Y. T. (2013). Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 8, 1106–1110. 10.3892/mmr.2013.1652 [DOI] [PubMed] [Google Scholar]
- Lopez-lazaro M. (2009). Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 9, 31–59. 10.2174/138955709787001712 [DOI] [PubMed] [Google Scholar]
- Lu J., Wang Z., Li S., Xin Q., Yuan M., Li H., et al. (2018). Quercetin inhibits the migration and invasion of HCCLM3 cells by suppressing the expression of p-Akt1, matrix metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. 24, 2583–2589. 10.12659/MSM.906172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q.-Y., Zhang L., Yee J. K., Go V.-L. W., Lee W.-N. (2015). Metabolic consequences of LDHA inhibition by epigallocatechin gallate and oxamate in MIA PaCa-2 pancreatic cancer cells. Metabolomics 11, 71–80. 10.1007/s11306-014-0672-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H.-Q., Xu M., Zhong W.-T., Cui Z.-Y., Liu F.-M., Zhou K.-Y., et al. (2014). EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J. B.U.ON, official J. Balkan Union Oncol. 19, 435–439. [PubMed] [Google Scholar]
- Luo K.-W., Xia J., Cheng B.-H., Gao H.-C., Fu L.-W., Luo X.-L. (2021). Tea polyphenol EGCG inhibited colorectal-cancer-cell proliferation and migration via downregulation of STAT3. Gastroenterol. Rep. 9, 59–70. 10.1093/gastro/goaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M. A., Okda T. M., Omran G. A., Abd-Alhaseeb M. M. (2021). Rosmarinic acid suppresses inflammation, angiogenesis, and improves paclitaxel induced apoptosis in a breast cancer model via NF3 κB-p53-caspase-3 pathways modulation. J. Appl. Biomed. 19, 202–209. 10.32725/jab.2021.024 [DOI] [PubMed] [Google Scholar]
- Masuelli L., Marzocchella L., Quaranta A., Palumbo C., Pompa G., Izzi V., et al. (2011). Apigenin induces apoptosis and impairs head and neck carcinomas EGFR/ErbB2 signaling. Front. Biosci. 16, 1060–1068. 10.2741/3735 [DOI] [PubMed] [Google Scholar]
- Mateen S., Tyagi A., Agarwal C., Singh R. P., Agarwal R. (2010). Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol. Carcinog. 49, 247–258. 10.1002/mc.20595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S., Zhu Y., Li J.-F., Wang X., Liang Z., Li S.-Q., et al. (2017). Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 8, 19834–19842. 10.18632/oncotarget.15771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoeva S., Franzen C. A., Pelling J. C. (2014). Apigenin inhibits TGF‐β‐induced VEGF expression in human prostate carcinoma cells via a Smad2/3‐and Src‐dependent mechanism. Mol. Carcinog. 53, 598–609. 10.1002/mc.22005 [DOI] [PubMed] [Google Scholar]
- Mitrut P., Docea A. O., Kamal A. M., Mitrut R., Calina D., Gofita E., et al. (2016). Colorectal cancer and inflammatory bowel disease. [Google Scholar]
- Moon D.-O., Kim M.-O., Lee J.-D., Choi Y. H., Kim G.-Y. (2010). Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 288, 183–191. 10.1016/j.canlet.2009.06.033 [DOI] [PubMed] [Google Scholar]
- Moradzadeh M., Hosseini A., Erfanian S., Rezaei H. (2017). Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 69, 924–928. 10.1016/j.pharep.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Naso L. G., Lezama L., Valcarcel M., Salado C., Villacé P., Kortazar D., et al. (2016). Bovine serum albumin binding, antioxidant and anticancer properties of an oxidovanadium(IV) complex with luteolin. J. Inorg. Biochem. 157, 80–93. 10.1016/j.jinorgbio.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Nguyen L. T., Lee Y.-H., Sharma A. R., Park J.-B., Jagga S., Sharma G., et al. (2017). Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 21, 205–213. 10.4196/kjpp.2017.21.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh E.-M., Yi M. S., Youn H. J., Lee B. K., Lee Y.-R., Han J.-H., et al. (2011). Silibinin enhances ultraviolet B-induced apoptosis in MCF-7 human breast cancer cells. J. Breast Cancer 14, 8–13. 10.4048/jbc.2011.14.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang G., Yao L., Ruan K., Song G., Mao Y., Bao S. (2009). Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol. Int. 33, 1237–1244. 10.1016/j.cellbi.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Pan X., Zhao B., Song Z., Han S., Wang M. (2016). Estrogen receptor-α36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 130, 85–93. 10.1016/j.jphs.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Pandurangan A. K., Dharmalingam P., Sadagopan S. K. A., Ganapasam S. (2014). Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum. Exp. Toxicol. 33, 1176–1185. 10.1177/0960327114522502 [DOI] [PubMed] [Google Scholar]
- Panthi V. K., Kaushal S., Basnet N., Chaudhary D., Parajuli R. R., Pokhrel P. (2020). International journal of innovative research and reviews A review of quercetin: Anticancer activity, 1–7. [Google Scholar]
- Park S. J., Kim Y. T., Jeon Y. J. (2012). Antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolinhomologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells. Mol. Cells, 363–369. 10.1007/s10059-012-2285-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashaei-asl F., Pashaei-Asl R., Khodadadi K., Akbarzadeh A., Ebrahimie E., Pashaiasl M. (2018). Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 46, 1483–1487. 10.1080/21691401.2017.1374281 [DOI] [PubMed] [Google Scholar]
- Pavese J. M., Farmer R. L., Bergan R. C. (2010). Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 29, 465–482. 10.1007/s10555-010-9238-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlopoulou A., Spandidos D. A., Michalopoulos I. (2015). Human cancer databases (review). Oncol. Rep. 33, 3–18. 10.3892/or.2014.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y., Zhang T., Wang J., Mao Z., Duan B., Long Y., et al. (2018). Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 9, 3669–3675. 10.7150/jca.27183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas-llabrés M. M., Quispe C., Herrera-Bravo J., Catarino M. D., Pereira O. R., Cardoso S. M., et al. (2022). Pharmacological properties of bergapten: Mechanistic and therapeutic aspects. Oxid. Med. Cell. Longev. 2022, 8615242. 10.1155/2022/8615242 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rady I., Mohamed H., Rady M., Siddiqui I. A., Mukhtar H. (2018). Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 5, 1–23. 10.1016/j.ejbas.2017.12.001 [DOI] [Google Scholar]
- Rana A., Samtiya M., Dhewa T., Mishra V., Aluko R. E. (2022). Health benefits of polyphenols: A concise review. J. Food Biochem., e14264. 10.1111/jfbc.14264 [DOI] [PubMed] [Google Scholar]
- Rauf A., Imran M., Khan I. A., Ur-Rehman M., Gilani S. A., Mehmood Z., et al. (2018). Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 32, 2109–2130. 10.1002/ptr.6155 [DOI] [PubMed] [Google Scholar]
- Razak N. A., Abu N., Ho W. Y., Zamberi N. R., Tan S. W., Alitheen N. B., et al. (2019). Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 9, 1514. 10.1038/s41598-018-37796-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-farias M., Carrasco-pozo C. (2019). The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 20, 3177. 10.3390/ijms20133177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-zadeh K., Ehrhart J., Bai Y., Sanberg P. R., Bickford P., Tan J., et al. (2008). Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflammation 5, 41. 10.1186/1742-2094-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera rivera A., Castillo-Pichardo L., Gerena Y., Dharmawardhane S. (2016). Anti-breast cancer potential of quercetin via the Akt/AMPK/mammalian target of rapamycin (mTOR) signaling cascade. PLOS ONE 11, e0157251. 10.1371/journal.pone.0157251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A., Farhan M., Nabi F., Khan R. H., Adil M., Ahmad A. (2021a). Transcriptional control of the oxidative stress response and implications of using plant derived molecules for therapeutic interventions in cancer. Curr. Med. Chem. 28, 8480–8495. 10.2174/0929867328666210218110550 [DOI] [PubMed] [Google Scholar]
- Rizvi A., Merlin M. A., Shah G. M. (2021b). Poly (ADP-ribose) polymerase (PARP) inhibition in cancer: Potential impact in cancer stem cells and therapeutic implications. Eur. J. Pharmacol. 911, 174546. 10.1016/j.ejphar.2021.174546 [DOI] [PubMed] [Google Scholar]
- Rudrapal M., Khairnar S. J., Khan J., Dukhyil A. B., Ansari M. A., Alomary M. N., et al. (2022). Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 13, 806470. 10.3389/fphar.2022.806470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameri S., Mohammadi C., Mehrabani M., Najafi R. (2021). Targeting the hallmarks of cancer: The effects of silibinin on proliferation, cell death, angiogenesis, and migration in colorectal cancer. BMC Complement. Med. Ther. 21, 160. 10.1186/s12906-021-03330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M., Kavoosi F., Valiani A., Ghobadifar M. A. (2018). Effect of genistein on apoptosis and proliferation of hepatocellular carcinoma hepa1-6 cell line. Int. J. Prev. Med. 9, 12. 10.4103/ijpvm.IJPVM_249_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Sekine M., Kobayashi M., Virgona N., Ota M., Yano T. (2013). Induction of the connexin 32 gene by epigallocatechin-3-gallate potentiates vinblastine-induced cytotoxicity in human renal carcinoma cells. Chemotherapy 59, 192–199. 10.1159/000354715 [DOI] [PubMed] [Google Scholar]
- Semwal P., Painuli S., Abu-Izneid T., Rauf A., Sharma A., Daştan S. D., et al. (2022). Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxid. Med. Cell. Longev. 2022, 1035441. 10.1155/2022/1035441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. S., Ku J. M., Choi H. S., Woo J. K., Jang B. H., Go H., et al. (2015). Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med. Rep. 12, 2977–2984. 10.3892/mmr.2015.3698 [DOI] [PubMed] [Google Scholar]
- Shafabakhsh R., Asemi Z. (2019). Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 12, 55. 10.1186/s13048-019-0530-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Bahukhandi A., Dhyani P., Sati P., Capanoglu E., Docea A. O., et al. (2021a). Therapeutic potential of neoechinulins and their derivatives: An overview of the molecular mechanisms behind pharmacological activities. Front. Nutr. 8, 664197. 10.3389/fnut.2021.664197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Dey A., Koirala N., Shaheen S., EL Omari N., Salehi B., et al. (2021b). Cinnamomum species: Bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front. Pharmacol. 12, 600139. 10.3389/fphar.2021.600139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Quispe C., Bouyahya A., EL Menyiy N., EL Omari N., Shahinozzaman M., et al. (2022). Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus Bulbophyllum . Evidence-Based Complementary Altern. Med. 2022. 10.1155/2022/6727609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Quispe C., Butnariu M., Rotariu L. S., Sytar O., Sestito S., et al. (2021c). Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 21, 318. 10.1186/s12935-021-02025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Quispe C., Patra J. K., Singh Y. D., Panda M. K., Das G., et al. (2021d). Paclitaxel: Application in modern oncology and nanomedicine-based cancer therapy. Oxid. Med. Cell. Longev. 2021, 3687700. 10.1155/2021/3687700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Kaur M., Katnoria J. K., Nagpal A. K. (2018). Polyphenols in food: Cancer prevention and apoptosis induction. Curr. Med. Chem. 25, 4740–4757. 10.2174/0929867324666171006144208 [DOI] [PubMed] [Google Scholar]
- Sharma C., Nusri Q. E.-A., Begum S., Javed E., Rizvi T. A., Hussain A. (2012). (-)-Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian pac. J. Cancer Prev. 13, 4815–4822. 10.7314/apjcp.2012.13.9.4815 [DOI] [PubMed] [Google Scholar]
- Sharmila R., Manoharan S. (2012). Anti-tumor activity of rosmarinic acid in 7, 12-dimethylbenz(a)anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Indian J. Exp. Biol. 50, 187–194. [PubMed] [Google Scholar]
- Shi X., Gao H.-Y., Yan W., He X.-W., Yang W. (2018). Effects of EGCG on proliferation, cell cycle and DAPK1 gene methylation of acute promyelocytic leukemia NB4 cell line. Zhongguo shi yan xue ye xue za zhi 26, 1288–1293. 10.7534/j.issn.1009-2137.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Shukla S., Shankar E., Fu P., Maclennan G. T., Gupta S. (2015). Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS One 10, e0138710. 10.1371/journal.pone.0138710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L. A., Ali S., Banerjee S., Munkarah A. R., Morris R. T., Sarkar F. H. (2008). Sensitization of ovarian cancer cells to cisplatin by genistein: The role of NF-kappaB. J. Ovarian Res. 1, 9. 10.1186/1757-2215-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo C., Russo G. L., Orhan I. E., Habtemariam S., Daglia M., Sureda A., et al. (2015). Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 6, 408–419. 10.3945/an.114.008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S., Munir N., Mahmood Z., Riaz M., Akram M., Rebezov M., et al. (2021). Molecular targets for the management of cancer using curcuma longa linn. Phytoconstituents: A review. Biomed. Pharmacother. 135, 111078. 10.1016/j.biopha.2020.111078 [DOI] [PubMed] [Google Scholar]
- Sung B., Chung H. Y., Kim N. D. (2016). Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J. Cancer Prev. 21, 216–226. 10.15430/JCP.2016.21.4.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S., Pal D., Mandal S., Roy A., Panda C. K. (2016). Tea polyphenols epigallocatechin gallete and theaflavin restrict mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. J. Nutr. Biochem. 27, 32–42. 10.1016/j.jnutbio.2015.08.016 [DOI] [PubMed] [Google Scholar]
- Tan S., Dong X., Liu D., Hao S.-M., He F. (2019). Anti-tumor activity of chlorogenic acid by regulating the mTORC 2 signaling pathway and disrupting F-actin organization. [Google Scholar]
- Tang S.-M., Deng X.-T., Zhou J., Li Q.-P., Ge X.-X., Miao L. (2020). Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 121, 109604. 10.1016/j.biopha.2019.109604 [DOI] [PubMed] [Google Scholar]
- Tomeh M. A., Hadianamrei R., Zhao X. (2019). A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 20, E1033. 10.3390/ijms20051033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.-Y., Chen C.-Y., Chiou Y.-H., Shyu H.-W., Lin K.-H., Chou M.-C., et al. (2017). Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. Int. J. Mol. Sci. 19, 16. 10.3390/ijms19010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T. H., Chien M. H., Lin W. L., Wen Y. C., Chow J. M., Chen C. K., et al. (2017). Inhibition of MDA‐MB‐231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation‐mediated p21WAF1/CIP1 expression. Environ. Toxicol. 32, 434–444. 10.1002/tox.22247 [DOI] [PubMed] [Google Scholar]
- Tsoukalas D., Buga A. M., Docea A. O., Sarandi E., Mitrut R., Renieri E., et al. (2021a). Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int. J. Mol. Med. 48, 199. 10.3892/ijmm.2021.5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukalas D., Fragkiadaki P., Docea A. O., Alegakis A. K., Sarandi E., Thanasoula M., et al. (2019a). Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 20, 3701–3708. 10.3892/mmr.2019.10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukalas D., Fragkiadaki P., Docea A. O., Alegakis A. K., Sarandi E., Vakonaki E., et al. (2019b). Association of nutraceutical supplements with longer telomere length. Int. J. Mol. Med. 44, 218–226. 10.3892/ijmm.2019.4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukalas D., Zlatian O., Mitroi M., Renieri E., Tsatsakis A., Izotov B. N., et al. (2021b). A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J. Clin. Med. 10, 624. 10.3390/jcm10040624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu D.-G., Lin W.-T., Yu C.-C., Lee S.-S., Peng C.-Y., Lin T., et al. (2016). Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos. Med. Assoc. 115, 1032–1038. 10.1016/j.jfma.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Tumur Z., Guerra C., Yanni P., Eltejaye A., Waer C., Alkam T., et al. (2015). Rosmarinic acid inhibits cell growth and migration in head and neck squamous cell carcinoma cell lines by attenuating epidermal growth factor receptor signaling. J. Cancer Sci. Ther. 7, 367–374. 10.4172/1948-5956.1000376 [DOI] [Google Scholar]
- Varghese L., Agarwal C., Tyagi A., Singh R. P., Agarwal R. (2005). Silibinin efficacy against human hepatocellular carcinoma. Clin. Cancer Res. 11, 8441–8448. 10.1158/1078-0432.CCR-05-1646 [DOI] [PubMed] [Google Scholar]
- Wang F., Gao F., Pan S., Zhao S., Xue Y. (2015a). Luteolin induces apoptosis, G0/G1 cell cycle growth arrest and mitochondrial membrane potential loss in neuroblastoma brain tumor cells. Drug Res. 65, 91–95. 10.1055/s-0034-1372648 [DOI] [PubMed] [Google Scholar]
- Wang J., Xie Y. A., Feng Y., Zhang L., Huang X., Shen X., et al. (2015b). (-)-Epigallocatechingallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int. J. Oncol. 46, 1507–1515. 10.3892/ijo.2015.2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Mao L., Xu P., Zheng X., Hackman R. M., Mackenzie G. G., et al. (2018). Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 9, 5682–5696. 10.1039/c8fo01397g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken R., Veena M. S., Wang M. B., Srivatsan E. S. (2011). Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 10, 12. 10.1186/1476-4598-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Liu Z., Li J., Zhang Q., Zhong P., Teng T., et al. (2019). Epigallocatechin-3-gallate inhibits the growth and increases the apoptosis of human thyroid carcinoma cells through suppression of EGFR/RAS/RAF/MEK/ERK signaling pathway. Cancer Cell Int. 19, 43. 10.1186/s12935-019-0762-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Huang M., Liu Y., Shu Y., Liu P. (2015). Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 14, 747–755. 10.7785/tcrt.2012.500434 [DOI] [PubMed] [Google Scholar]
- Xu M., Wang S., Song Y., Yao J., Huang K., Zhu X. (2016). Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 11, 3075–3080. 10.3892/ol.2016.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jiang Z., Ji G., Liu J. (2010). Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 76, 956–962. 10.1055/s-0029-1240893 [DOI] [PubMed] [Google Scholar]
- Xue N., Zhou Q., Ji M., Jin J., Lai F., Chen J., et al. (2017). Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 7, 39011–11. 10.1038/srep39011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Yang J., Shen L., Chen X. (2012). Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 285, 459–467. 10.1007/s00404-011-1942-6 [DOI] [PubMed] [Google Scholar]
- Yan X., Qi M., Li P., Zhan Y., Shao H. (2017). Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 7, 50. 10.1186/s13578-017-0179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Jiang X., Song L., Wang H., Mei Z., Xu Z., et al. (2016a). Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo . Oncol. Rep. 35, 1602–1610. 10.3892/or.2015.4481 [DOI] [PubMed] [Google Scholar]
- Yang M.-Y., Wang C.-J., Chen N.-F., Ho W.-H., Lu F.-J., Tseng T.-H. (2014). Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem. Biol. Interact. 213, 60–68. 10.1016/j.cbi.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Yang Y., Yang Y., Dai W., Li X., Ma J., Tang L. (2016b). Genistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 20, 3292–3296. [PubMed] [Google Scholar]
- Ye F., Zhang G.-H., Guan B.-X., Xu X.-C. (2012). Suppression of esophageal cancer cell growth using curcumin, (-)-epigallocatechin-3-gallate and lovastatin. World J. Gastroenterol. 18, 126–135. 10.3748/wjg.v18.i2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M.-C., Lin C.-C., Wu H.-C., Tsao S.-M., Hsu C.-K. (2009). Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 57, 6468–6473. 10.1021/jf9004466 [DOI] [PubMed] [Google Scholar]
- Zeng A., Liang X., Zhu S., Liu C., Wang S., Zhang Q., et al. (2021). Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 45, 717–727. 10.3892/or.2020.7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bao J., Yang J. (2019). Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migration. Arch. Med. Sci. 15, 1001–1009. 10.5114/aoms.2018.78742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhao X.-H., Wang Z.-J. (2008). Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 46, 2042–2053. 10.1016/j.fct.2008.01.049 [DOI] [PubMed] [Google Scholar]
- Zhang W., Yin G., Dai J., Sun Y., Hoffman R. M., Yang Z., et al. (2017). Chemoprevention by quercetin of oral squamous cell carcinoma by suppression of the NF-κB signaling pathway in DMBA-treated hamsters. Anticancer Res. 37, 4041–4049. 10.21873/anticanres.11789 [DOI] [PubMed] [Google Scholar]
- Zhao X., Wang Q., Yang S., Chen C., Li X., Liu J., et al. (2016). Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur. J. Pharmacol. 781, 60–68. 10.1016/j.ejphar.2016.03.063 [DOI] [PubMed] [Google Scholar]
- Zhou F., Zhou H., Wang T., Mu Y., Wu B., Guo D.-L., et al. (2012). Epigallocatechin-3-gallate inhibits proliferation and migration of human colon cancer SW620 cells in vitro . Acta Pharmacol. Sin. 33, 120–126. 10.1038/aps.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Su J., Feng S., Wang L., Yin X., Yan J., et al. (2016). Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget 7, 79076–79088. 10.18632/oncotarget.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Tang M., Liu Y., Zhang Z., Lu R., Lu J. (2017). Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anticancer. Drugs 28, 446–456. 10.1097/CAD.0000000000000479 [DOI] [PubMed] [Google Scholar]
- Zhu K., Wang W. (2016). Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 37, 4373–4382. 10.1007/s13277-015-4187-3 [DOI] [PubMed] [Google Scholar]
- Zhu W., Mei H., Jia L., Zhao H., Li X., Meng X., et al. (2020). Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: A prospective, non-randomised, phase 1 trial. Invest. New Drugs 38, 1129–1136. 10.1007/s10637-019-00871-8 [DOI] [PubMed] [Google Scholar]
- Zlatian O. M., Comanescu M. V., Rosu A. F., Rosu L., Cruce M., Gaman A. E., et al. (2015). Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Romanian J. Morphol. Embryology 56, 175–181. [PubMed] [Google Scholar]