Abstract
Cells of the actinomycete Amycolatopsis methanolica grown on glucose possess only a single, exclusively PPi-dependent phosphofructokinase (PPi-PFK) (A. M. C. R. Alves, G. J. W. Euverink, H. J. Hektor, J. van der Vlag, W. Vrijbloed, D.H.A. Hondmann, J. Visser, and L. Dijkhuizen, J. Bacteriol. 176:6827–6835, 1994). When this methylotrophic bacterium is grown on one-carbon (C1) compounds (e.g., methanol), an ATP-dependent phosphofructokinase (ATP-PFK) activity is specifically induced, completely replacing the PPi-PFK. The two A. methanolica PFK isoenzymes have very distinct functions, namely, in the metabolism of C6 and C1 carbon substrates. This is the first report providing biochemical evidence for the presence and physiological roles of PPi-PFK and ATP-PFK isoenzymes in a bacterium. The novel ATP-PFK enzyme was purified to homogeneity and characterized in detail at the biochemical and molecular levels. The A. methanolica ATP-PFK and PPi-PFK proteins possess a low level of amino acid sequence similarity (24%), clearly showing that the two proteins are not the result of a gene duplication event. PPi-PFK is closely related to other (putative) actinomycete PFK enzymes. Surprisingly, the A. methanolica ATP-PFK is most similar to ATP-PFK from the protozoon Trypanosoma brucei and PPi-PFK proteins from the bacteria Borrelia burgdorferi and Treponema pallidum, both spirochetes, very distinct from actinomycetes. The data thus suggest that A. methanolica obtained the ATP-PFK-encoding gene via a lateral gene transfer event.
In many organisms, the phosphorylation of fructose-6-phosphate (F-6-P) to fructose-1,6-bisphosphate (F-1,6-P2) is catalyzed by an ATP-dependent phosphofructokinase enzyme (ATP-PFK; EC 2.7.1.11) (30, 37). This irreversible ATP-PFK reaction is generally allosterically regulated and is considered to be a major point of regulation in glycolysis (18, 19, 21). However, a second type of PFK enzyme, using pyrophosphate (PPi) as a substrate (but not ATP) (PPi-PFK; EC 2.7.1.90) and catalyzing the reversible phosphorylation of F-6-P to F-1,6-P2, exists in plants and in some bacteria and archaea (31). In bacteria, the PPi-PFK is normally not regulated at the activity level. A third type of PFK enzyme, using ADP as a substrate (ADP-PFK; no EC number available), has been found in the extremophilic archaeon Pyrococcus furiosus and in other hyperthermophilic archaea, e.g., Thermococcus (24, 33).
In Escherichia coli, two ATP-PFK isoenzymes with different kinetic properties and no apparent sequence similarities have been identified (9, 25, 26). PFK1, which accounts for 90% of the total PFK activity (25), is an allosteric enzyme which displays cooperativity with respect to F-6-P and is regulated at the activity level by phosphoenolpyruvate (PEP; an inhibitor) and ADP and GDP (activators). The remaining 10% of PFK activity is accounted for by PFK2. The latter enzyme is not sensitive to PEP and only slightly inhibited by ATP and F-1,6-P2. ADP affects PFK2 activity, with up to 50% inhibition at low F-6-P and ATP concentrations (17). It is unclear whether these two E. coli PFKs have distinct physiological roles (11).
Actinomycetes are best known for their ability to produce secondary metabolites (e.g., antibiotics). Streptomyces and Amycolatopsis species are especially widely used in industrial production processes. Antibiotics are derived from intermediates of primary metabolism, the availability of which may limit overall process productivity. Attempts to construct better-producing strains, therefore, also focus on elimination of possible bottlenecks in central metabolic pathways (e.g., glycolysis) (1) involved in glucose utilization. However, limited information is available about primary metabolism in actinomycetes. Previously, we characterized an ATP-PFK enzyme purified from glucose-grown cells of Streptomyces coelicolor A3(2) (2); this enzyme is allosterically inhibited by PEP. Studies with glucose-grown cells of the actinomycete Amycolatopsis methanolica revealed only a nonallosteric PPi-PFK activity. This enzyme has been purified to homogeneity (a tetramer of 43,000-Da subunit size), and the corresponding gene (pfp) has been characterized (3, 4). PFK activity is also essential for the functioning of the ribulose monophosphate (RuMP) cycle of formaldehyde assimilation (fructose 1,6-bisphosphate [FBP] aldolase cleavage variant; Fig. 1) employed by A. methanolica during growth on one-carbon (C1) compounds, e.g., methanol (13, 15, 16). However, no or only low PPi-PFK activity levels could be detected in cells grown on C1 compounds (references 3 and 13 and this study). Here, we report the biochemical and molecular characterization of a novel ATP-PFK enzyme that is specifically induced during growth of A. methanolica on C1 compounds.
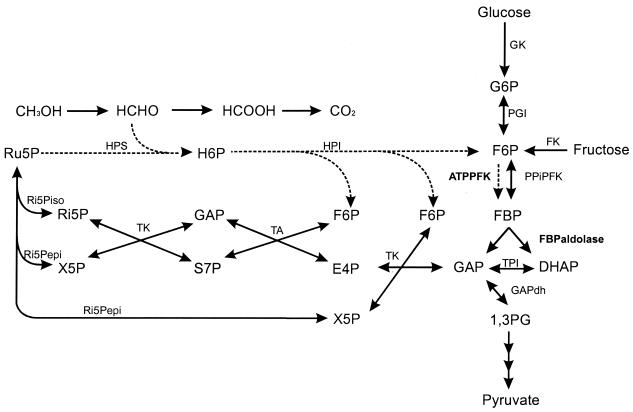
FIG. 1.
Schematic representation of the pathways for glucose and methanol metabolism in A. methanolica. GK, glucose kinase; FK, fructose kinase; PGI, phosphoglucose isomerase; TA, transaldolase; TK, transketolase; Ri5Pepi, ribulose-5-phosphate epimerase; Ri5Piso, ribulose-5-phosphate isomerase; TPI, triose phosphate isomerase, GAPdh, glyceraldehyde-3-phosphate dehydrogenase; S7P, sedoheptulose-7-phosphate; E4P, erythrose-4-phosphate; X5P, xylulose-5-phosphate; Ru5P, ribulose-5-phosphate; H6P, hexulose-6-phosphate; 1,3PG, 1,3-diphosphoglycerate; HPS, hexulose phosphate synthase; HPI, hexulose phosphate isomerase. The RuMP cycle enzymes specifically involved in the FBP aldolase cleavage variant (ATP-PFK and FBP aldolase) are shown by dashed lines.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The plasmids and bacterial strains used are listed in Table 1.The plasmid pMEA300-free A. methanolica WVI strain (42) was grown at 37°C in batch cultures as described previously (13, 14). The medium was supplemented with various carbon sources at different concentrations (see Results). Sugars and organic acids were heat sterilized; amino acids, methanol, and formaldehyde were filter sterilized. Carbon-limited chemostats (working volume, 1 liter) were run at 37°C and pH 7.0 (controlled by automatic adjustment with 2 M NaOH). The medium contained (per liter) KH2PO4, 1.0 g; (NH4)2SO4, 1.5 g; MgCl2, 0.2 g; and 0.2 ml of a trace element solution (39). Formaldehyde-limited continuous cultures were established as described previously (20). The various measurements were performed on samples from cultures in transient state or steady state. A steady-state situation normally became established after five volume displacements.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| A. methanolica WVI | pMEA300-free derivative strain of A. methanolica NCBI 11946 | 42 |
| E. coli DH5α | supE44 ΔlacU169 (φ80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Bethesda Research Laboratories |
| Plasmids | ||
| PDA71 | Rhodococcus-E. coli shuttle vector; cat ecoRI bla | 10 |
| pBluescript KS(+) | Ampr; phagemid derived from pUC18; lacZ | Stratagene |
| PAM101 | 5-kb BamHI DNA fragment containing pfk ligated in BglII-digested pDA71 | This study |
Preparation of extracts, enzyme assays, and measurement of metabolite concentrations.
Cells were washed in 50 mM Tris-HCl (pH 7.5) buffer containing 5 mM MgCl2 and 5 mM dithiothreitol (buffer A). The cells were disrupted by three passages through a French pressure cell at 140 MPa, followed by centrifugation of the lysate at 40,000 × g for 30 min. The supernatant was used for all enzyme assays (at 37°C) and for purification of the ATP-PFK protein. Triton X-100 (0.01% [wt/vol]) was added to all reaction mixtures containing NADH in order to reduce endogenous NADH consumption. All enzyme assays were done in triplicate; the data presented are averages with a standard deviation of less than 10%. One unit is the amount of enzyme catalyzing the synthesis or the degradation of 1 μmol of product or substrate per min. PFK was assayed in a reaction mixture with 50 mM Tris-HCl buffer (pH 7.5), 5 mM MgCl2, 1 mM KCl, 3 mM NH4Cl, 5 mM dithiothreitol, 0.15 mM NADH, 10 mM F-6-P, 0.9 U of FBP aldolase, 5 U of triose phosphate isomerase, 0.85 U of α-glycerol-3-phosphate dehydrogenase, and limiting amounts of extract. The reaction was started by addition of 5 mM of PPi (for PPi-PFK) or 5 mM ATP (for ATP-PFK). PPi and ATP solutions were adjusted to pH 7.5.
Preparation of cell extracts for NMR analysis.
A. methanolica cells were grown on mineral medium with glucose or methanol and harvested at the end of the exponential growth phase. Cell extracts were prepared by injecting culture samples into liquid nitrogen (using a syringe) followed by addition of perchloric acid to a final concentration of 10% (vol/vol) and vigorously mixing the ingredients on ice for 20 min. The mixture was centrifuged at 20,000 × g for 10 min, and the supernatant was adjusted to neutral pH with 5 M potassium hydroxide. The preparation obtained was freeze-dried for further use. The residue was dissolved in water; EDTA was added to a final concentration of 10 mM, and the pH was adjusted to 8.2. 31P nuclear magnetic resonance (NMR) spectra were acquired at 202.46 MHz with proton broadband decoupling in a Bruker AMX500 spectrometer and a 10-mm-long quadruple-nucleus probe head (31P, 13C, 15N, and 1H). The following acquisition parameters were typically used: spectral width, 12 kHz; pulse width, 18 μs (corresponding to a 70o flip angle); data size, 16 K; repetition delay, 3.8 s. For quantification of phosphorylated metabolites, the signal intensities in fully relaxed spectra (repetition delay, 30.8 s) were compared with the intensity of the resonance due to a known amount of sodium pyrophosphate added to the sample. Identification of resonances was made by adding small amounts of suspected compounds to the sample and observing the increase in the intensity of resonances in the spectrum. The presence of ADP, UDP, ATP, UTP, UMP, UDP-N-acetylglucosamine, and PPi was detected in this way. Polyphosphate was identified from the characteristic chemical shift at approximately −22 ppm. Chemical shifts are referenced with respect to external 85% H3PO4.
Purification of ATP-PFK enzyme.
All chromatographic steps were carried out in a System Prep 10 liquid chromatography system (Pharmacia LKB Biotechnology Inc.) at room temperature. Collected fractions were immediately placed on ice.
(i) Step 1.
Methanol (100 mM)-grown cells were harvested from a batch culture of A. methanolica WVI at the end of the exponential growth phase. Extracts were prepared in buffer A as described before.
(ii) Step 2. Ammonium sulfate precipitation.
Solid (NH4)2SO4 was slowly added to the preparation from step 1 to 30% saturation and stirred for 20 min. The mixture was then centrifuged at 40,000 × g for 15 min. The pellet was resuspended in a minimum volume of buffer A and dialyzed overnight against the same buffer.
(iii) Step 3. Affinity chromatography.
A sample from step 2 was applied to a Levafix Blue G-3A-Sepharose CL-4B (24) column (30 ml) previously equilibrated with buffer A. The column was washed with 3 volumes of buffer A, and ATP-PFK was eluted with buffer A containing 300 mM (NH4)2SO4 (flow rate, 3 ml · min−1; fractions, 4 ml).
(iv) Step 4. Anion-exchange chromatography.
Protein from step 3 was applied to a Resource Q column previously equilibrated with buffer A (flow rate, 2 ml · min−1). Bound proteins were eluted with a linear gradient of 0 to 0.4 M NaCl in buffer A. Fractions containing ATP-PFK activity were pooled, and solid (NH4)2SO4 was slowly added until 30% saturation.
(v) Step 5. Hydrophobic interaction chromatography.
Protein from step 4 was applied to a Phenyl-Superose column (HR5/5) previously equilibrated with buffer A plus 30% (NH4)2SO4 (flow rate, 0.5 ml · min−1). Bound proteins were eluted with a linear decreasing gradient from 30 to 0% (NH4)2SO4 in buffer A. Fractions containing ATP-PFK activity were pooled, and glycerol was added to a final concentration of 40% (vol/vol) before storage at −20°C.
Estimation of molecular mass.
The relative molecular mass of the active enzyme was estimated by gel filtration in buffer A on a Superdex 200 column (XK 16/60) at a flow rate of 1 ml · min−1 with thyroglobulin (molecular mass, 670,000 Da), gamma globulin (158,000 Da), ovalbumin (44,000 Da), myoglobin (17,000 Da), and cobalamine (1,350 Da) as gel filtration standards.
Estimation of subunit size.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (27) was performed with the following prestained marker proteins: phosphorylase B (106,000 Da), bovine serum albumin (80,000 Da), ovalbumin (49,500 Da), carbonic anhydrase (32,500 Da), soybean trypsin inhibitor (27,500 Da), and lysozyme (18,500 Da) (Bio-Rad). The gels were stained by the silver-staining method (43).
Protein determination.
Protein concentrations were determined with the Bio-Rad protein determination kit with bovine serum albumin as a standard (8).
Kinetic studies.
Kinetic parameters were determined at 37°C and pH 7.5 and were calculated with Sigma Plot for Windows 2000 (Jandell Scientific Software) using curve fitting with the Hill or Michaelis-Menten equation. Possible effectors (pH adjusted; at 1 and 4 mM final concentrations) of ATP-PFK were added separately to assays with purified enzyme, at near-Km or K50 (concentration of the substrate at half Vmax in non-Michaelis-Menten kinetics) concentrations of the substrates F-6-P and ATP in the presence of 5 mM MgCl2.
Automated amino acid analysis.
For determination of the N-terminal amino acid sequence, about 100 pmol of purified protein was applied to a Pro-spin cartridge (Applied Biosystems, Warrington, United Kingdom) containing a polyvinylidene difluoride membrane according to the manufacturer's protocol. Automated sequencing was performed at Eurosequence BV (Groningen, The Netherlands) on an automatic pulse liquid sequenator (model 477 A; Applied Biosystems) equipped on-line with a reverse-phase high-performance liquid chromatography unit (model 120 A; Applied Biosystems).
Peptide digest and amino acid sequence.
The purified ATP-PFK was digested with trypsin. The resultant peptides were separated by reverse-phase high-performance liquid chromatography using a Nucleosil 10C18 column equilibrated with 0.1% trifluoroacetic acid and eluted with a gradient of 0 to 100% 0.1% trifluoroacetic acid plus 100% acetonitrile over 120 min at a flow rate of 1 ml · min−1. The amino acid sequence of the peptide eluting in fraction 45 (at 48 min) was analyzed as described above.
DNA manipulations.
Chromosomal DNA (40) and plasmid DNA (7) were isolated as described previously. DNA-modifying enzymes were obtained from Boehringer (Mannheim, Germany) and were used according to the manufacturer's instructions.
Southern hybridizations.
Chromosomal DNA from A. methanolica, digested with the appropriate enzymes, was subjected to electrophoresis on a 0.8% agarose gel and transferred to a nylon-Plus membrane (Qiagen, Basel, Switzerland) after alkaline denaturation (32). The membrane was probed at 62°C with an oligonucleotide probe (100 pmol) labeled with the DIG oligonucleotide tailing kit from Boehringer. The membrane was subsequently washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% [wt/vol] SDS for 5 min and twice with 0.1× SSC–0.1% SDS for 5 min.
Construction of a partial genomic library and cloning of the pfk gene.
Chromosomal DNA from A. methanolica was digested overnight with the restriction enzyme BamHI and subjected to sucrose fractionation. DNA fragments varying in size (0.5 to 20 kb) were collected by centrifugation (Sorvall SW-28 rotor) for 24 h at 122,000 × g. Each fraction (500 μl) was dialyzed overnight against water to remove the sucrose. Subsequently, 5 μl of each fraction was subjected to electrophoresis and transferred to a nylon-Plus membrane. Southern hybridizations were performed at 62°C with 2 digoxigenin-labeled degenerated oligonucleotide probes (oligo 1, GAA/G CCG/C GCG/C GAG/A TTC GGC/G GCG/C GTG/C GCG/C GCG/C CGC/G, derived from the peptide QPAEFGAVAAR; and oligo 2, AAC GAA/G GTG/C GAC CCG/C GAC GGG/C GAC CTG/C TGG ATG, derived from the peptide NQVDPDGDLWM), both corresponding to the internal peptide sequence obtained by trypsin digestion of the purified ATP-PFK protein. Both oligonucleotides hybridized with the same 4.4-kb band, confirming that both probes specifically hybridized to the pfk gene. Subsequently, DNA fragments ranging from 4 to 5 kb in size were ligated into the BglII site of plasmid pDA71 and transformed to E. coli DH5α. A positive clone (pAM101) was identified via hybridization and further characterized by restriction analysis and nucleotide sequencing.
Nucleotide sequencing.
Double-stranded DNA was sequenced with T7 polymerase, with unlabeled primers and fluorescein-labeled ATP (41). The nucleotide sequence data was compiled and analyzed using the programs supplied in the PC/GENE software package (Intelligenetics, Mountain View, Calif.).
Sequence comparison and phylogenetic-tree construction.
The PFK alignment was made with Clustal W (36); positions with fully conserved amino acid residues, positions with fully conserved strong groups (STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, and FYW), and positions with fully conserved weaker groups (CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, and HFY) are indicated by the program. The groups are according to the Pam 250 residue matrix. The programs supplied in Treecon (38) were used to determine phylogenetic relationships among the PFK proteins. Bootstrap values (100 replicates) were performed to evaluate the reliability of the tree.
Nucleotide sequence accession number.
The nucleotide sequence presented in this paper was entered into GenBank under accession number AF298119.
RESULTS
PPi-PFK and ATP-PFK activities in cells grown on different carbon substrates.
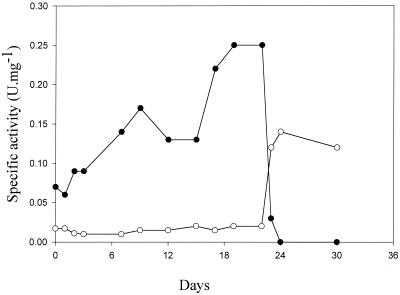
As shown previously, only PPi-PFK activity is present in A. methanolica cells grown on glucose (3, 4) (Table 2). We observed that cells grown on methanol possess a significant ATP-PFK activity. When we screened several growth substrates, it appeared that ATP-PFK activity became specifically induced when the organism was grown on C1 substrates or on betaine (Table 2). Degradation of betaine results in formaldehyde release that is assimilated via the RuMP cycle. Similar observations were made when A. methanolica was grown in carbon-limited chemostat cultures (Fig. 2). ATP-PFK activity was present in cells grown under methanol or formaldehyde limitation only. During growth on glucose, ATP-PFK activity completely disappeared and only PPi-PFK activity was detected. A similar regulatory pattern of induction (by C1 compounds) and repression (by glucose) was observed for the RuMP cycle enzymes hexulose-6-phosphate synthase (HPS) and hexulose-6-phosphate isomerase (HPI) involved in assimilation of C1 compounds (references 3 and 13 and this study). The various observations indicate that PPi-PFK and ATP-PFK have different physiological roles. ATP-PFK is specifically involved in the RuMP cycle of formaldehyde assimilation during growth on C1 compounds; PPi-PFK functions in the glycolytic pathway (Fig. 1).
TABLE 2.
Enzyme activities in A. methanolica WVI cells grown in batch culture on different substrates
| Substrate | Specific activity
(mU · mg−1)
|
|
|---|---|---|
| PPi-PFK | ATP-PFK | |
| Glucose (10 mM) | 180 | 0 |
| Fructose (10 mM) | 120 | 0 |
| Glycerol (20 mM) | 100 | 0 |
| Acetate (20 mM) | 20 | 0 |
| Gluconate (10 mM) | 30 | 0 |
| Succinate (10 mM) | 20 | 0 |
| Phenylalanine (10 mM) | 30 | 0 |
| Methanol (60 mM) | 20 | 120 |
| Formaldehydea (50 mM) | 10 | 220 |
| Betaine (20 mM) | 10 | 180 |
Data from formaldehyde-limited continuous cultures in steady-state at a growth rate of D (dilution rate) = 0.05 h−1.
FIG. 2.
Profiles of ATP- and PPi-dependent PFK activities in cell extracts of A. methanolica grown in carbon- and energy source-limited continuous culture on the substrates glucose, formaldehyde, and methanol at a growth rate of D (dilution rate) = 0.05 h−1. Days 0 to 6, 50 mM methanol; days 6 to 10, 50 mM methanol plus 5 mM formaldehyde; days 10 to 14, 50 mM methanol plus 12 mM formaldehyde; days 14 to 22, 50 mM formaldehyde; day 22 to end, 5 mM glucose. ●, ATP-PFK; ○, PPi-PFK.
Purification and characterization of ATP-PFK.
The ATP-PFK enzyme was purified to homogeneity (1,230-fold, with a final yield of 23%) from methanol-grown cells. The ATP-PFK binds to a Levafix blue G-3A-Sepharose Cl-4B column (22), resulting in a 294-fold purification in a single step (Table 3). Subsequent anion-exchange (Resource Q) and hydrophobic-interaction (Phenyl-Superose) column chromatography yielded a homogeneous preparation of ATP-PFK, as judged by SDS-polyacrylamide gel electrophoresis and silver staining.
TABLE 3.
Purification of ATP-PFK from methanol-grown cells of A. methanolica WVI
| Step | Procedure | Protein (mg) | Total activity (U) | Sp act (U · mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | Crude extract | 160 | 20.8 | 0.13 | 1 | 100 |
| 2 | (NH4)2SO4 (30%) | 80 | 13.6 | 0.17 | 1.3 | 65 |
| 3 | Levafix blue | 0.2 | 10 | 50 | 385 | 48 |
| 4 | Resource Q | 0.08 | 7.2 | 90 | 692 | 35 |
| 5 | Phenyl-Superose | 0.03 | 4.8 | 160 | 1,230 | 23 |
Characterization of the pure ATP-PFK enzyme revealed an Mr of 110,000 for the holoenzyme and a subunit size of 50,000, suggesting a dimeric structure. The enzyme stored in buffer with 40% glycerol at −20°C retained its full activity over a period of 6 months.
The enzyme showed an absolute specificity for its substrate, F-6-P, which could not be replaced by fructose-1-phosphate or glucose-6-phosphate. The phosphate donor ATP could be replaced by GTP or UTP with a remaining activity of 100 and 40%, respectively. Also, Mg2+ ions were essential for ATP-PFK activity, with an apparent Km for Mg2+ of about 0.025 mM (data not shown). Similar observations with respect to substrate specificity have been made for ATP-PFK enzymes from other bacterial sources (18).
Several metabolites (at 1 and 4 mM final concentrations) were tested for effects on ATP-PFK activity, with 5 mM F-6-P and Mg2+ and 1 mM ATP present. PPi caused 20 and 50% inhibition, and AMP caused 20 and 40% inhibition (at 1 and 4 mM), respectively. ADP, citrate, PEP, FBP, sedoheptulose-7-P, erythrose-4-P, pyruvate, xylulose-5-P, ribulose-5-P, and sedoheptulose-7-phosphate (all at 1 and 4 mM concentrations except for fructose 2,6-bisphosphate at 0.1 mM) had no effect on ATP-PFK enzyme activity.
Kinetics of the ATP-PFK enzyme.
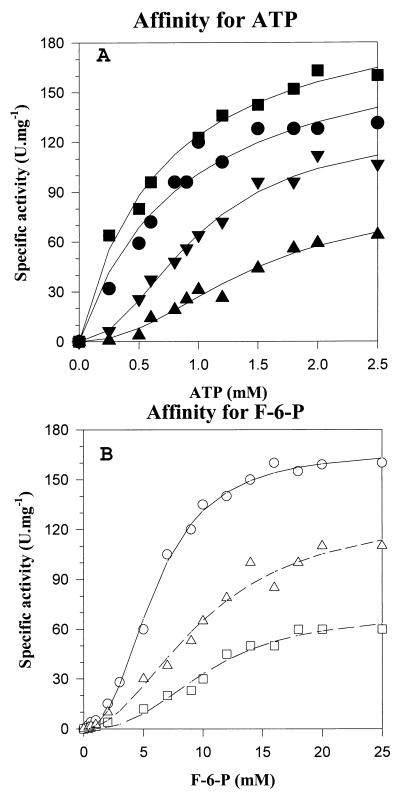
The kinetic parameters of the purified ATP-PFK enzyme were determined in the presence of 5 mM Mg2+ (Fig. 3 and Table 4). Michaelis-Menten kinetics was observed with respect to ATP (Fig. 3A). The apparent Km value for ATP was estimated as 0.6 ± 0.1 mM. PPi was an inhibitor of ATP-PFK, decreasing the Vmax and affinity of the enzyme for ATP. With 2 and 4 mM PPi (Fig. 3A and Table 4), the data points could be better fitted with the Hill equation, giving a K50 value for ATP of 1.0 to 1.5 ± 0.1 mM with a Hill coefficient (n) of 2. With respect to the affinity of the enzyme for the substrate F-6-P, all data points could be better fitted with the Hill equation. PPi also decreased the Vmax and affinity of the enzyme for the substrate F-6-P. The affinity for F-6-P was rather low and varied between K50 values of 6 and 10 mM (Fig. 3B and Table 4) with a Hill coefficient of 2.
FIG. 3.
Kinetics of ATP-PFK from A. methanolica and effect of PPi on ATP-PFK activity. (A) ATP-PFK activity versus ATP concentration at a fixed F-6-P concentration (10 mM). ▪, 0 mM PPi; ●, 1 mM PPi; ▾, 2 mM PPi; ▴, 4 mM PPi. (B) ATP-PFK activity versus F-6-P concentration at fixed ATP concentrations. ○, 0 mM PPi; ▵, 2 mM PPi; □, 4 mM PPi. Kinetic parameters are given in Table 4.
TABLE 4.
Kinetics of the ATP-PFK enzyme from A. methanolica WVI and effects of PPi on affinities for substrates F-6-P and ATP
| Substrate | Vmaxapp (U · mg−1) | Kapp (mM) | K50 (mM) | n | PPi (mM) |
|---|---|---|---|---|---|
| ATPa | 180 ± 4.0 | 0.6 ± 0.1 | Absent | ||
| 130 ± 5.0 | 0.9 ± 0.07 | 1 | |||
| 110 ± 5.0 | 1.0 ± 0.1 | 2 | 2 | ||
| 60 ± 4.0 | 1.5 ± 0.1 | 2 | 4 | ||
| F-6-Pb | 167 ± 3.0 | 6.0 ± 0.17 | 2 | Absent | |
| 100 ± 1.2 | 10 ± 1.0 | 2 | 2 | ||
| 80 ± 5 | 10 ± 0.8 | 2 | 4 |
At [F-6-P] = 10 mM.
At [ATP] = 1 mM.
N-terminal amino acid sequence.
The first 26 amino acids of the ATP-PFK protein were determined (in two separate experiments) to be TLHLDDLRVRLLGERRYDSPFXEVRHT. Surprisingly, no homology was observed with PFK enzyme sequences present in databases or with any other class of proteins. When we compared the initial yield for the automated protein sequencing (ca. 40 and 45 pmol) with the amount of protein applied to the membrane used (ca. 50 pmol), we concluded that the sequence obtained was from the purified ATP-PFK protein and not from a possible contaminant.
Internal peptide sequence.
The amino acid sequence of one peptide in the ATP-PFK tryptic digest (NQVDPDGDLWMSVLETTXQPAEFGAVAAR) was further analyzed, resulting in identification of a further 28 amino acids. Database searches using this sequence as the query again revealed no similarity with known PFK proteins or with any other class of proteins.
Cloning, sequencing, and analysis of the A. methanolica pfk gene.
The strategy used for isolation of the pfk gene from A. methanolica was to design two oligonucleotide probes (see Materials and Methods) based on the internal peptide amino acid sequence. Initial hybridizations on digested genomic DNA with both probes yielded a unique 4.4-kb hybridization signal. We therefore concluded that both probes specifically hybridized to the pfk gene. Screening of a partial genomic library (1,500 transformants) yielded pAM101. Nucleotide sequencing of the 5-kb pAM101 insert revealed an open reading frame (ORF) of 1,379 bp coding for a polypeptide of 459 amino acids with a calculated molecular mass of 48,500 Da. The G+C content of this ORF (70%) is similar to the high G+C content of other A. methanolica and S. coelicolor genes and of other actinomycete genomes (1, 2, 4, 44). A total of 23 out of the 26 amino acids identified in the ATP-PFK N-terminal amino acid sequence were found in the deduced amino acid sequence; the internal peptide sequence of 28 amino acids could be perfectly identified in this sequence. Database searches using the deduced amino acid sequence as the query revealed high similarity scores with other PFK proteins (see below). We conclude that this ORF encodes the A. methanolica ATP-PFK protein.
Sequence comparison of PFK proteins.
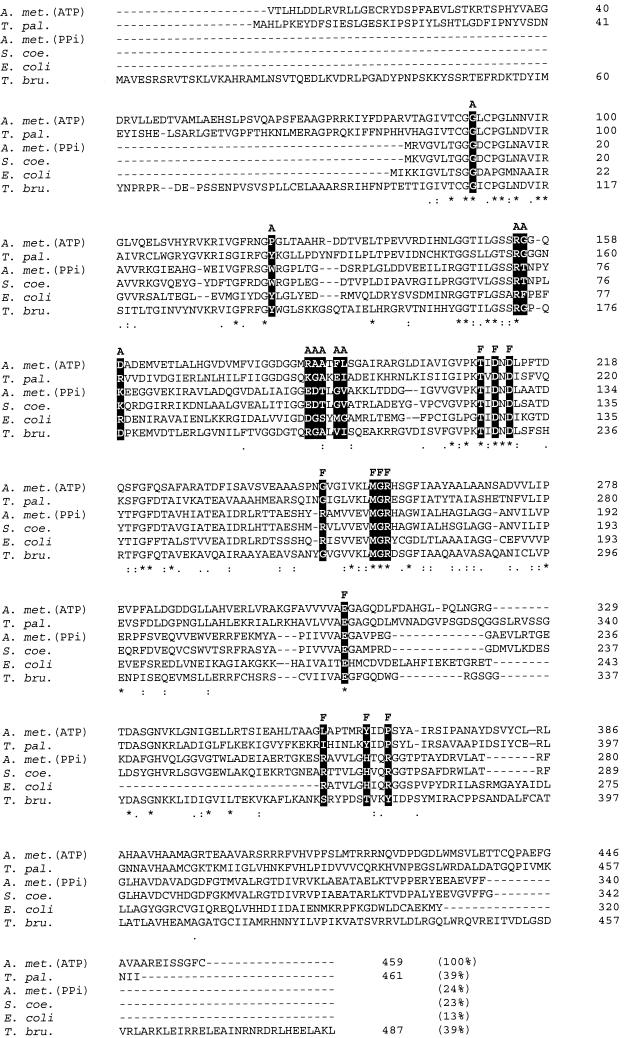
The A. methanolica PFK amino acid sequences were aligned with those of other ATP-PFK and PPi-PFK proteins from various sources. A subset of the full alignment (available on request) is shown in Fig. 4. In particular, the comparison between the A. methanolica ATP-PFK and PPi-PFK enzymes and the S. coelicolor A3(2) ATP-PFK enzyme yielded surprising and interesting results. Only 24% similarity was observed between the two A. methanolica PFK proteins, while 70% identity was observed between the S. coelicolor A3(2) ATP-PFK and the A. methanolica PPi-PFK (2, 4). Similarity between the ATP-PFKs from A. methanolica and S. coelicolor A3(2) was also low (23%).
FIG. 4.
Clustal W (36) multiple alignment of the deduced amino acid sequence of the ATP-PFK from A. methanolica with the sequences of the PFKs from S. coelicolor A3(2) (National Center for Biotechnology Information [NCBI] accession number 008333), E. coli (NCBI accession number KIECFA), T. pallidum (NCBI accession number A71366), and T. brucei (NCBI accession number AAC47836) and the PPi-PFK from A. methanolica (NCBI accession number Q59126). The percent similarity between the ATP-PFK from A. methanolica and each of the other PFKs is indicated in parentheses. A and F indicate residues (solid boxes) involved in binding of ATP and F-6-P, respectively, in the E. coli ATP-PFK enzyme (34). *, position with a fully conserved amino acid residue; :, position with a fully conserved strong group (STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, and FYW); ., position with a fully conserved weaker group (CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, and HFY).
Based on the three-dimensional crystal structure of the E. coli ATP-PFK enzyme (34), a total of 11 amino acid residues involved in binding of the F-6-P substrate have been identified. Identical residues are present in S. coelicolor A3(2) ATP-PFK and in A. methanolica PPi-PFK (Fig. 4); 7 of the 11 residues could be identified in A. methanolica ATP-PFK (Fig. 4). Interestingly, the same four residues varied in ATP-PFK from the protozoon Trypanosoma brucei and in PPi-PFK from the bacterium Treponema pallidum (Fig. 4). The mutations Arg 162 Ser and Arg 243 Ser in the E. coli ATP-PFK drastically decreased the affinity of the enzyme for F-6-P (6), in line with the low affinity of the A. methanolica ATP-PFK for F-6-P.
With respect to the amino acid residues assigned for the binding of ATP in E. coli PFK, the situation in the A. methanolica ATP-PFK enzyme differs more drastically: 8 out of 10 residues assigned for ATP binding in the E. coli PFK enzyme are not conserved in the A. methanolica ATP-PFK enzyme (Fig. 4). As previously noted (2), not all the residues involved in ATP binding in E. coli ATP-PFK are conserved in the ATP-PFK of S. coelicolor A3(2). Remarkably, the same differences were observed in the PPi-PFK of A. methanolica. We observed that the ATP-PFK and PPi-PFK enzymes of A. methanolica are quite distinct with respect to the identities of these residues. In fact, in this respect the A. methanolica ATP-PFK more closely resembles the T. brucei ATP-PFK and the T. pallidum PPi-PFK (Fig. 4).
Phylogenetic relationships with other PFKs.
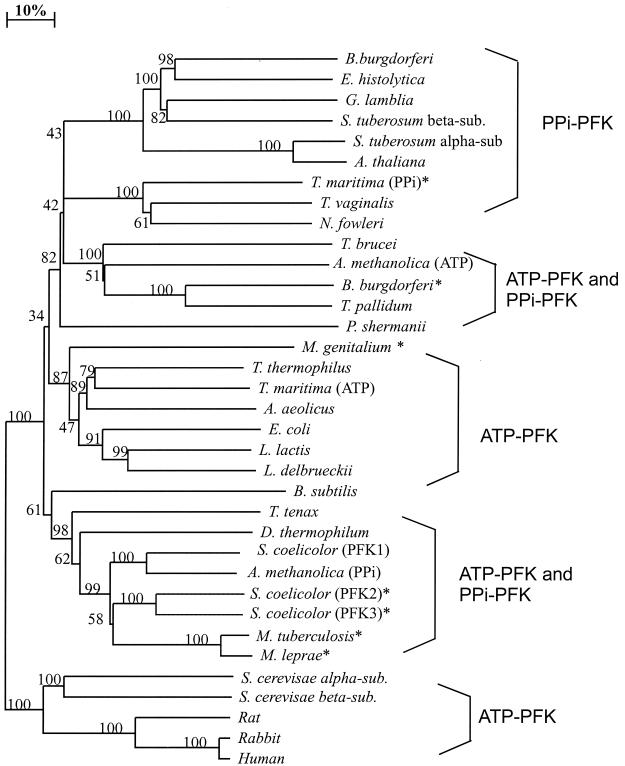
A phylogenetic tree of PFK enzymes, based on alignment of full-length protein sequences, clearly showed that the previously characterized S. coelicolor A3(2) ATP-PFK (PFK1) (2) and A. methanolica PPi-PFK (4) are closely related (Fig. 5). Additional putative actinomycete PFKs have been identified in the genome sequences of Mycobacterium tuberculosis (http://www.tigr.org /tdb), Mycobacterium leprae (http://www.sanger.ac.uk/projects /M-leprae), and S. coelicolor A3(2) (PFK2 and PFK3) (http: //www.sanger.ac.uk/projects/S-coelicolor). Most actinomycete PFK proteins cluster closely together phylogenetically (Fig. 5). A clear exception is the novel ATP-PFK of A. methanolica, which shares little sequence similarity with the other six actinomycete PFKs. In fact, the ATP-PFK from A. methanolica is clearly more closely related to the ATP-PFK of the protozoon T. brucei and the PPi-PFKs of the spirochetes Borrelia burgdorferi and T. pallidum (Fig. 5). These data thus suggest that A. methanolica has obtained the ATP-PFK enzyme via lateral gene transfer rather than via gene duplication events.
FIG. 5.
Unrooted phylogenetic tree of PFK proteins. The tree is based on distance analysis (neighbor-joining method) of selected available sequences of ATP-PFK and PPi-PFK proteins from the following sources in the National Center for Biotechnology Information database (accession numbers are given in parentheses): B. burgdorferi (D70102), Entamoeba histolytica (S68243), Giardia lamblia (S52081), Solanum tuberosum beta subunit (P21343), S. tuberosum alpha subunit (A36094), Arabidopsis thaliana (AC015450.3), Thermotoga maritima (PPi-PFK) (G72396), Trichomonas vaginalis (AAD13344), Naegleria fowleri (S54978), T. brucei (AAC47836), A. methanolica (ATP-PFK) (AF298119), B. burgdorferi (putative PFK) (F70190), T. pallidum (A71366), Propionibacterium shermanii (A41169), Mycoplasma genitalium (G64223), Thermus thermophilus (P21777), T. maritima (ATP-PFK) (C72406), Aquifex aeolicus (O67605), E. coli (KIECFA), Lactococcus lactis (JN0614), Lactobacillus delbrueckii (A48663), Bacillus subtilis (A69675), Thermoproteus tenax (CAA74985), Dictyoglomus thermophilum (AF268276.1), S. coelicolor A3(2) (PFK1) (OO8333), A. methanolica (PPi-PFK) (Q59126), S. coelicolor A3(2) (putative PFK) PFK2 (AL138978.1), S. coelicolor A3(2) (putative PFK) PFK3 (AL391017.1), M. tuberculosis (O53257), M. leprae (O33106), Saccharomyces cerevisae alpha subunit (NP011756.1), S. cerevisae beta subunit (NP013932), rat (A53047), rabbit (P00125128), and human muscle (227448). Bootstrap values, based on 100 replicates, are indicated at the branch points. The asterisks indicate putative PFKs.
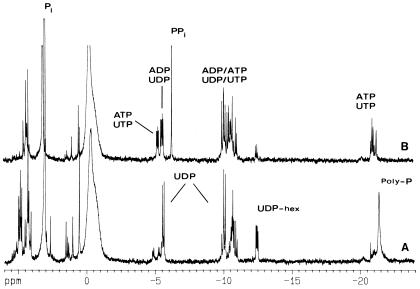
31P-NMR analysis of A. methanolica cell extracts of glucose- and methanol-grown cultures.
Aqueous suspensions of lyophilized A. methanolica cells grown on mineral medium with methanol (Fig. 6A) or glucose (Fig. 6B) as a carbon substrate were subjected to 31P-NMR for identification of phosphocompounds. Striking differences were observed between the two growth conditions. (i) Interestingly, PPi appeared only in glucose-grown cells (5 nmol · mg [dry weight]−1). Much larger amounts of longer-chain inorganic polyphosphates were detected in methanol-grown cells. (ii) In methanol-grown cells, using the RuMP cycle (Fig. 1), a greater variety of phosphomonoesters, e.g., sugar phosphates (including F-6-P) and UMP, appeared. (iii) In glucose-grown cells, ADP, ATP, UDP, and UTP are present at similar levels ([ATP] = [UTP] = 2.4 nmol · mg [dry weight]−1, [UDP] = 3.8 nmol · mg [dry weight]−1, and [ADP] = 4.4 nmol · mg [dry weight]−1). (iv) UDP was by far the major nucleotide in methanol-grown cells (4.5 nmol · mg [dry weight]−1); other nucleotides were detected, but only in small amounts ([ADP] = 1 nmol · mg [dry weight]−1 and [UDP-N-acetylglucosamine] = 2 nmol · mg [dry weight]−1). The level of nucleotide triphosphates in these cells was also very low compared with that in glucose cells. (v) UDP-hexoses also accumulated at higher levels in methanol-grown cells. Two major UDP-hexose compounds were present. One of these was identified as UDP-N-acetylglucosamine. The other compound had resonances that did not coincide with UDP-glucose, UDP-glucuronic acid, or UDP-glucosamine, and it remained unidentified.
FIG. 6.
31P-NMR spectra of perchloric acid extracts of A. methanolica cells grown on mineral medium containing 60 mM methanol (spectrum A) or 10 mM glucose (spectrum B). Resonance assignments are indicated above each peak: ADP, ATP, UDP, UTP, UDP-hex (uridine diphosphohexoses, such as UDP-N-acetylglucosamine), PPi, Pi (inorganic phosphate), and poly-P (polyphosphate).
DISCUSSION
This is the first report providing biochemical evidence for the presence and physiological roles of ATP-PFK and PPi-PFK isoenzymes in a bacterium, the actinomycete A. methanolica. ATP-PFK and PPi-PFK isoenzymes had previously been reported only in higher plants (5). The two PFK isoenzymes differ in subunit composition and native molecular mass: the ATP-PFK is a dimer with 50-kDa subunits, whereas the PPi-PFK is a tetramer with 43-kDa subunits.
The A. methanolica ATP-PFK and PPi-PFK enzymes are clearly very different biochemically (see above), at the amino acid sequence level (24% similarity), phylogenetically (Fig. 5), with respect to the regulation of their synthesis (presence in cells grown on C1 substrates and on sugars, respectively [Table 2]), and with respect to regulation of their activities (Table 4 and Fig. 3). On the basis of these observations, we conclude that earlier in the evolution of the A. methanolica genome the ATP-PFK-encoding gene was acquired via a lateral gene transfer phenomenon.
PFK activity in A. methanolica is required not only for sugar metabolism via the glycolytic pathway (serving both in energy generation and the synthesis of precursors for the synthesis of cell material) but also for the functioning of the RuMP cycle of formaldehyde assimilation (FBP aldolase cleavage variant; serving primarily in carbon assimilation) during growth on C1 compounds (Fig. 1) (13, 15, 16). Similar to the typical RuMP cycle enzymes HPS and HPI, ATP-PFK (but not PPi-PFK) is clearly induced by C1 compounds and repressed by glucose. This ATP-PFK enzyme thus has a specific physiological role in the RuMP cycle during growth on C1 compounds, whereas PPi-PFK makes its most important contribution during growth on sugars (C6 compounds) in the glycolytic pathway.
The A. methanolica ATP-PFK has a poor affinity for F-6-P (between 6 and 10 mM, depending on the ATP concentration). By comparison, the PPi-PFK enzyme has a relatively high affinity for F-6-P (0.4 mM) (3). Most PFK enzymes have an affinity for F-6-P on the order of less than 1 mM (18). The ATP-PFK enzyme assayed in cell extracts displayed an affinity for F-6-P in the same order of magnitude (data not shown), ruling out the possible existence of an activator for this enzyme in vivo. PFK enzymes specifically functioning in the RuMP cycle have not been characterized before. This property of low F-6-P affinity thus may reflect the specific role of ATP-PFK in the RuMP cycle. Assimilation of three formaldehyde molecules via the RuMP cycle, with RuMP as an acceptor molecule, results in the net formation of three F-6-P molecules (Fig. 1). One molecule of F-6-P is converted via PFK and FBP aldolase, providing the cells two molecules of GAP/DHAP, the precursor for biosynthesis of cell material from pyruvate onwards. The two remaining F-6-P molecules, together with the second GAP/DHAP molecule, are used by transaldolase and transketolase enzymes in regeneration of three molecules of RuMP, involving a number of reversible enzyme steps. To ensure a sufficient regeneration of RuMP for formaldehyde assimilation, ATP-PFK action is required only when intracellular F-6-P levels have become rather high.
Glucose metabolism results in relatively high intracellular PPi levels (Fig. 6), which may inhibit activity of the RuMP cycle ATP-PFK (Table 4). This, and the repression of the synthesis of RuMP cycle enzymes by glucose (13) (Table 2 and Fig. 2), will have strong negative effects on the RuMP cycle during growth on mixtures of glucose plus methanol in batch cultures. This explains previous observations that, during growth on such mixtures, methanol is used only as an additional energy source (oxidation to carbon dioxide) and not for carbon assimilation via the RuMP cycle (Fig. 1) (14). The data show that the ATP-PFK regulatory and kinetic properties of A. methanolica are finely tuned to its specific physiological role as a RuMP cycle enzyme.
Little is known at the moment about (poly)phosphate metabolism in actinomycetes. Several interesting observations were made with A. methanolica. During growth on glucose, A. methanolica employs a PPi-PFK (3) and a glucose kinase that uses not only ATP and GTP (3) as phosphogroup donors but also polyphosphates (P5–P75) (this study and data not shown). Similar glucose kinase activities have been studied previously in Actinomyces naeslundii (35) and M. tuberculosis (23). PPi accumulation was readily detectable in glucose-grown cells, whereas no PPi was found in methanol-grown cells (Fig. 6). Similar PPi levels were also reported for other organisms possessing PPi-linked enzyme activities, e.g., Propionibacterium freudenreichii (28, 29) and A. naeslundii (35). PPi is produced in nucleic acid and protein biosynthesis and by metabolic cycling between glycogen and glucose-1-phosphate through the action of UDP-glucose synthase, glycogen synthase, and glycogen phosphorylase (12). Large differences in glycogen levels, however, were not observed between glucose- and methanol-grown cells of A. methanolica (data not shown). It is also possible that glucose uptake in A. methanolica is associated with PPi release, but this remains to be studied experimentally. The source of PPi during growth of A. methanolica thus has yet to be identified.
ACKNOWLEDGMENTS
A.M.C.R.A. was supported by JNICT (Portugal Grants BD808-IF-90; Praxis XXI-BD-2798-94).
We thank H. J. Hektor for assistance in the continuous culture experiments.
REFERENCES
- 1.Alves A M C R. Regulation of glucose metabolism in the actinomycetes Amycolatopsis methanolica and Streptomyces coelicolor A3(2). Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1997. [Google Scholar]
- 2.Alves A M C R, Euverink G J W, Dijkhuizen L. Identification of ATP-dependent phosphofructokinase as a regulatory step in the glycolytic pathway of the actinomycete Streptomyces coelicolorA3(2) Appl Environ Microbiol. 1997;63:956–961. doi: 10.1128/aem.63.3.956-961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves A M C R, Euverink G J W, Hektor H J, Hessels G I, van der Vlag J, Vrijbloed J W, Hondmann D H A, Visser J, Dijkhuizen L. Enzymes of glucose and methanol metabolism in the actinomycete Amycolatopsis methanolica. J Bacteriol. 1994;176:6827–6835. doi: 10.1128/jb.176.22.6827-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves A M C R, Meijer W G, Vrijbloed J W, Dijkhuizen L. Characterization and phylogeny of the pfp gene of Amycolatopsis methanolica encoding PPi-dependent phosphofructokinase. J Bacteriol. 1996;178:149–155. doi: 10.1128/jb.178.1.149-155.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balogh A, Wong J H, Wotzel C, Soll J, Cseke C, Buchanan B B. Metabolite-mediated catalyst conversion of PFK and PFP: a mechanism of enzyme regulation in green plants. FEBS Lett. 1984;169:287–292. [Google Scholar]
- 6.Berger S A, Evans P R. Active-site mutants altering the cooperativity of E. coliphosphofructokinase. Nature. 1990;343:575–576. doi: 10.1038/343575a0. [DOI] [PubMed] [Google Scholar]
- 7.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Buschmeir B. Purification and properties of 1-PFK from Escherichia coli. FEMS Microbiol Lett. 1985;29:231–235. [Google Scholar]
- 10.Dabbs E R, Gowan B, Quan S, Andersen S J. Development of improved Rhodococcusplasmid vectors and their use in cloning genes of potential commercial and medical importance. Biotechnologia. 1995;7–8:129–135. [Google Scholar]
- 11.Daldal F, Babul J, Fraenkel D G. An alteration in phosphofructokinase 2 of Escherichia coliwhich impairs gluconeogenic growth and improves growth on sugars. Eur J Biochem. 1982;126:373–379. doi: 10.1111/j.1432-1033.1982.tb06790.x. [DOI] [PubMed] [Google Scholar]
- 12.Dawes E A, Senior P E. The role and regulation of energy reserve polymers in microorganisms. Adv Microb Physiol. 1973;10:135–166. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 13.de Boer L, Euverink G J W, van der Vlag J, Dijkhuizen L. Regulation of methanol metabolism in the facultative methylotroph Nocardiasp. 239 during growth on mixed substrates in batch- and continuous cultures. Arch Microbiol. 1990;153:337–343. [Google Scholar]
- 14.de Boer L, van Rijssel M, Euverink G J W, Dijkhuizen L. Purification, characterization and regulation of a monomeric L-phenylalanine dehydrogenase from the facultative methylotroph Nocardiasp. 239. Arch Microbiol. 1989;153:12–18. [Google Scholar]
- 15.Dijkhuizen L, Levering P R, de Vries G E. The physiology and biochemistry of aerobic methanol-utilizing Gram-negative and Gram-positive bacteria. In: Atkinson T, Sherwood R F, editors. Methane and methanol utilizers. New York, N.Y: Plenum Press; 1992. pp. 149–181. [Google Scholar]
- 16.Dijkhuizen L, Sokolov I G. Regulation of oxidation and assimilation of one-carbon compounds in methylotrophic bacteria. In: Goldberg I, Rokem J S, editors. Biology of methylotrophs. London, United Kingdom: Butterworths-Heinemann; 1984. pp. 127–148. [DOI] [PubMed] [Google Scholar]
- 17.Doelle H W. ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coliK-12. Eur J Biochem. 1975;50:335–342. doi: 10.1111/j.1432-1033.1975.tb09808.x. [DOI] [PubMed] [Google Scholar]
- 18.Fothergill-Gilmore L A, Michels P A M. Evolution of glycolysis. Prog Biophys Mol Biol. 1993;59:105–235. doi: 10.1016/0079-6107(93)90001-z. [DOI] [PubMed] [Google Scholar]
- 19.Fraenkel D G. Glycolysis, pentose phosphate pathway, and Entner-Doudoroff pathway. In: Neidhardt F C, Ingraham J L, Magasanik B, Low K B, Schaechter M, editors. Escherichia coli and Salmonella typhimurium. Cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 142–151. [Google Scholar]
- 20.Hektor H J, Dijkhuizen L. Mutational analysis of primary alcohol metabolism in the methylotrophic actinomycete Amycolatopsis methanolica. FEMS Microbiol Lett. 1996;144:73–81. [Google Scholar]
- 21.Hofmann E. The significance of phosphofructokinase to the regulation of carbohydrate metabolism. Rev Physiol Biochem Pharmacol. 1976;75:1–67. doi: 10.1007/BFb0030484. [DOI] [PubMed] [Google Scholar]
- 22.Hondmann D H A, Visser J. Screening method for large numbers of dye-adsorbents for enzyme purification. J Chromatogr. 1990;510:155–164. doi: 10.1016/s0021-9673(01)93749-5. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh P C, Kowalczyk T H, Philips N F B. Kinetic mechanisms of polyphosphate glucokinase from Mycobacterium tuberculosis. Biochemistry. 1996;35:9772–9781. doi: 10.1021/bi9528659. [DOI] [PubMed] [Google Scholar]
- 24.Kengen S W M, Tuininga J E, Debok F A M, Stams A J M, de Vos W M. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1995;270:30453–30457. doi: 10.1074/jbc.270.51.30453. [DOI] [PubMed] [Google Scholar]
- 25.Kotzlar D, Buc H. Two Escherichia colifructose-6-phosphate kinases. Preparative purification, oligomeric structure and immunological studies. Biochim Biomed Acta. 1977;484:35–48. doi: 10.1016/0005-2744(77)90111-5. [DOI] [PubMed] [Google Scholar]
- 26.Kotzlar D, Buc H. Regulatory properties of phosphofructokinase 2 from Escherichia coli. Eur J Biochem. 1981;117:569–574. doi: 10.1111/j.1432-1033.1981.tb06375.x. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K, Favre K. Maturation of the head of bacteriophage T4. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 28.Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285:1–5. doi: 10.1016/0014-5793(91)80711-b. [DOI] [PubMed] [Google Scholar]
- 29.Mertens E. ATP versus pyrophosphate: glycolysis revisited in parasitic protists. Parasitol Today. 1993;9:122–126. doi: 10.1016/0169-4758(93)90169-g. [DOI] [PubMed] [Google Scholar]
- 30.Mertens E, Müller M. Glucokinase and fructokinase of Trichomonas vaginalis and Tritrichomonas foetus. J Protozool. 1990;37:384–388. doi: 10.1111/j.1550-7408.1990.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 31.Reeves R E, South D J, Blytt H J, Warren L G. Pyrophosphate: D-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function 6-phosphate 1-phosphotransferase. J Biol Chem. 1974;249:7737–7741. [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Selig M, Xavier K B, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- 34.Shirakihara Y, Evans P R. Crystal structure of the complex of phosphofructokinase from Escherichia coliwith its reaction products. J Mol Biol. 1988;204:973–994. doi: 10.1016/0022-2836(88)90056-3. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi N, Kalfas S, Yamada T. Phosphorylating enzymes involved in glucose fermentation of Actinomyces naeslundii. J Bacteriol. 1995;177:5806–5811. doi: 10.1128/jb.177.20.5806-5811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uyeda K. Phosphofructokinase. Adv Enzymol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- 38.van de Peer Y, de Wachter R. Construction of evolutionary distance trees with Treecon for Windows: accounting for variation in nucleotide substitution rate among sites. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/13.3.227. [DOI] [PubMed] [Google Scholar]
- 39.Vishniac W, Santer W. The thiobacilli. Bacteriol Rev. 1957;21:195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogt-Singer M E, Finnerty W R. Construction of an Escherichia coli-Rhodococcus shuttle vector and plasmid transformation in Rhodococcusspp. J Bacteriol. 1988;170:638–645. doi: 10.1128/jb.170.2.638-645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voss H, Schwager C, Wirkner V, Zimmermann J, Erfle H, Hewitt N, Rupp T, Stegemann J, Ansorge W. New procedure for automated DNA sequencing with multiple internal labelling by fluorescent dUTP. Methods Mol Cell Biol. 1992;3:30–34. [Google Scholar]
- 42.Vrijbloed J W, Madon J, Dijkhuizen L. Transformation of the methylotrophic actinomycete Amycolatopis methanolicawith plasmid DNA: stimulatory effect of a pMEA300-encoded gene. Plasmid. 1995;34:96–104. doi: 10.1006/plas.1995.9997. [DOI] [PubMed] [Google Scholar]
- 43.Wray W, Boulikas T, Wray V P, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 44.Wright F, Bibb M J. Codon usage in the G+C rich Streptomycesgenome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]