Abstract
Introduction
Traumatic brain injury (TBI) is the leading cause of mortality and long-term disability in young adults. Despite the high prevalence of anaemia and red blood cell transfusion in patients with TBI, the optimal haemoglobin (Hb) transfusion threshold is unknown. We undertook a randomised trial to evaluate whether a liberal transfusion strategy improves clinical outcomes compared with a restrictive strategy.
Methods and analysis
HEMOglobin Transfusion Threshold in Traumatic Brain Injury OptimizatiON is an international pragmatic randomised open label blinded-endpoint clinical trial. We will include 742 adult patients admitted to an intensive care unit (ICU) with an acute moderate or severe blunt TBI (Glasgow Coma Scale ≤12) and a Hb level ≤100 g/L. Patients are randomly allocated using a 1:1 ratio, stratified by site, to a liberal (triggered by Hb ≤100 g/L) or a restrictive (triggered by Hb ≤70 g/L) transfusion strategy applied from the time of randomisation to the decision to withdraw life-sustaining therapies, ICU discharge or death. Primary and secondary outcomes are assessed centrally by trained research personnel blinded to the intervention. The primary outcome is the Glasgow Outcome Scale extended at 6 months. Secondary outcomes include overall functional independence measure, overall quality of life (EuroQoL 5-Dimension 5-Level; EQ-5D-5L), TBI-specific quality of life (Quality of Life after Brain Injury; QOLIBRI), depression (Patient Health Questionnaire; PHQ-9) and mortality.
Ethics and dissemination
This trial is approved by the CHU de Québec—Université Laval research ethics board (MP-20-2018-3706) and ethic boards at all participating sites. Our results will be published and shared with relevant organisations and healthcare professionals.
Trial registration number
Keywords: INTENSIVE & CRITICAL CARE, Adult intensive & critical care, Neurological injury, TRAUMA MANAGEMENT
Strengths and limitations of this study.
The multicentre international recruitment and our pragmatic approach will provide generalisable findings.
The blinded outcome assessment will minimise ascertainment bias.
The sample size and sliding dichotomy analysis will increase our ability to detect smaller effect size with similar power for a given population size.
Transfusions administered as part of the initial resuscitation of acute trauma prior to intensive care unit admission will not be protocolised.
Introduction
Traumatic brain injury (TBI) is a significant public health concern and represents the leading cause of mortality and long-term disability in young adults.1 For these patients, the cerebral autoregulation that normally compensates for variations in oxygen delivery is impaired,2 rendering their brain vulnerable to ischaemia and secondary injuries. In the absence of high-quality evidence, several experts have suggested maintaining higher haemoglobin (Hb) levels (>100 g/L) on the assumption that it reduces metabolic distress and improves brain tissue oxygenation.3–5 The adoption of a liberal transfusion strategy has important resource implications since most patients with TBI will develop anemia6 and approximately one-third will be transfused during their hospital stay.7
The evidence to support transfusion strategies in patients with TBI remains scarce. In a systematic review of studies in neurocritical care patients, we found insufficient evidence to support the use of a specific transfusion threshold to improve morbidity and mortality.8 A recent randomised controlled trial showed no effect of red blood cell (RBC) transfusion on neurological outcomes in patients with moderate or severe TBI, although the expected effect size was large and most patients included were not anaemic.9 To date, clinical practice guidelines are based on limited evidence and do not provide clear recommendations regarding RBC transfusion in TBI.10 11 As a result, transfusion practices vary greatly within and between centres12 13; many clinicians extrapolate the evidence supporting the non-inferiority of a restrictive strategy in critically ill patients without TBI14 15 while others advocate for a liberal transfusion strategy pending stronger evidence to support this practice.16
In collaboration with the Canadian Critical Care Trials Group (CCCTG), the Perioperative Anesthesia Clinical Trials group and the Canadian Traumatic Brain Injury Research Consortium (CTRC), we designed the HEMOglobin Transfusion Threshold in Traumatic Brain Injury OptimizatiON (HEMOTION) trial. The primary objective of our international pragmatic randomised open label blinded-endpoint17 trial is to evaluate whether a liberal (higher Hb threshold) versus a restrictive (lower Hb threshold) RBC transfusion strategy improves neurological outcomes in anaemic moderate and severe TBI patients admitted to the intensive care unit (ICU). Secondary objectives will evaluate the effect of transfusion strategies on functional outcome, quality of life, depression and mortality. Tertiary objectives will evaluate the effect of transfusion strategies on the incidence of transfusion-related complications, infections, Hb levels, number of RBC units transfused and ICU and hospital length of stay. Herein, we report the trial protocol according to the SPIRIT statement.18 This trial is registered with ClinicalTrials.gov.
Methods and analysis
Trial settings and eligibility criteria
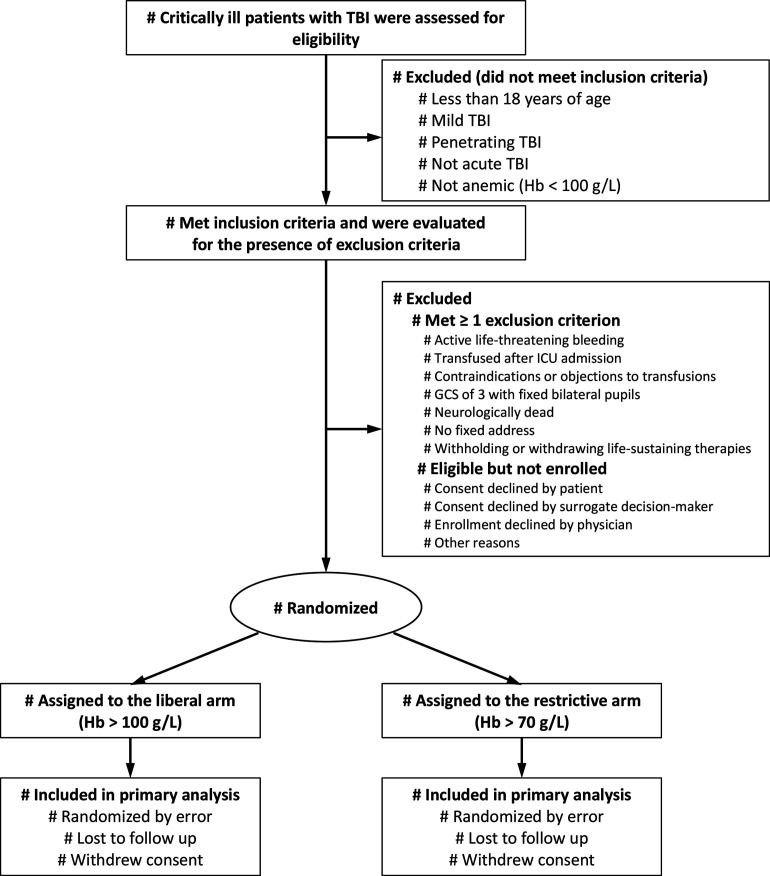
The HEMOTION trial is being conducted in level 1 and level II trauma centres in Canada, the United Kingdom, Brazil and France since September 2017. We are recruiting adult patients (≥18 years old) admitted to the ICU with an acute (hospital admission within 24 hours of injury) moderate or severe (Glasgow Coma Score (GCS) ≤12)19 blunt TBI and a Hb level ≤100 g/L. We exclude patients who receive transfusion after ICU admission, have contraindications or known objection to transfusions or have no fixed address. We also exclude patients who meet the criteria for neurological determination of death, those with a GCS of 3 in combination with bilateral fixed dilated pupils, those with active life-threatening bleeding associated with haemorrhagic shock, and patients for whom a decision to withhold or withdraw life-sustaining therapies has been made at the time of screening. Patients who received transfusion prior to ICU admission (eg, in the emergency room or in the operating room), as part of the initial acute trauma resuscitation, are eligible. Research coordinators at each participating site screens daily all critically ill adult patients with TBI to determine eligibility. Table 1 depicts the schedule of interventions, data collection and outcome assessments. In the final report, we will report excluded patients and reasons for non-enrolment using the Consolidated Standards of Reporting Trials flow diagram20 (figure 1).
Table 1.
Schedule of enrolment, interventions, data collection and outcome assessments
| Trauma | ICU | Hospital | 6 months | |
| Enrolment | ||||
| Eligibility screen | ✔️ | |||
| Informed consent | ✔️ | |||
| Allocation | ✔️ | |||
| Intervention—transfusion strategy | ||||
| Liberal (Hb>100 g/L) or restrictive (Hb>70 g/L) | ✔️ | |||
| Pre-randomisation data collection* | ||||
| Demographics | ✔️ | |||
| Trauma characteristics | ✔️ | |||
| Physical examination | ✔️ | ✔️ | ||
| Laboratory results | ✔️ | ✔️ | ||
| Secondary insults | ✔️ | ✔️ | ||
| Cointerventions | ✔️ | ✔️ | ||
| Neurosurgical and non-neurosurgical interventions | ✔️ | ✔️ | ||
| Blood product transfusions | ✔️ | ✔️ | ||
| Transfusion reactions | ✔️ | ✔️ | ||
| Daily data collection | ||||
| Physical examination | ✔️ | |||
| Laboratory results | ✔️ | |||
| Secondary insults | ✔️ | |||
| Cointerventions | ✔️ | |||
| Neurosurgical and non-neurosurgical interventions | ✔️ | |||
| Blood product transfusions | ✔️ | |||
| Transfusion complications | ✔️ | ✔️ | ||
| Protocol deviation/violation | ✔️ | |||
| Trial outcomes | ||||
| Primary outcome | ||||
| Glasgow Outcome Scale extended | ✔︎ | |||
| Secondary outcomes | ||||
| Mortality | ✔️ | ✔️ | ✔️ | |
| Functional Independence Measure | ✔️ | |||
| EuroQoL 5-Dimension 5-Level | ✔️ | |||
| Quality of Life after Brain Injury (QOLIBRI) | ✔️ | |||
| Patient Health Questionnaire-9 | ✔️ | |||
| Tertiary outcomes | ||||
| Red blood cells transfusion | ✔️ | |||
| Lowest Hb | ✔️ | |||
| Infections | ✔️ | |||
| Length of mechanical ventilation | ✔️ | |||
| Length of stay | ✔️ | ✔️ | ||
| Transfusion complications | ✔️ |
*Performed retrospectively after randomisation.
Hb, haemoglobin; ICU, intensive care unit.
Figure 1.
Flow diagram. GCS, Glasgow Coma Scale; Hb, haemoglobin; ICU, intensive care unit; TBI, traumatic brain injury.
Assignment of interventions
On reaching a Hb ≤100 g/L and after a site investigator confirms eligibility, the research coordinator uses a secure, web-based, central, concealed, computerised randomisation portal to allocate patients in a 1:1 ratio to either a liberal (experimental) or a restrictive (control) RBC transfusion strategy. Randomisation is done with variable permuted blocks of 4 and 6, stratified by site. Staff members of the methods centre of the Ottawa Health Research Institute (OHRI) who are not involved in trial implementation generated the randomisation sequence.
Interventions
Once randomised, the trial intervention is initiated within 3 hours in patients meeting the threshold for transfusion in their respective group to avoid prolonged exposure to Hb levels below this threshold.
Experimental intervention: liberal transfusion strategy
Patients in the liberal transfusion strategy group receive an RBC transfusion if their Hb is ≤100 g/L. This threshold, shown to be effective in maintaining adequate cerebral oxygenation,3–5 is considered acceptable by clinicians caring for critical care patients with neurological injuries.16 21
Control intervention: restrictive transfusion strategy
Patients in the restrictive transfusion strategy group receive an RBC transfusion only if their Hb is ≤70 g/L. We have chosen this threshold because it is the most studied restrictive RBC transfusion threshold14 15 and reflects the current standard of care in non-bleeding critically ill patients without neurological or coronary artery diseases.11 It also is a frequently used and accepted threshold for clinicians who care for brain-injured patients.16
Duration of treatment
The allocated transfusion strategy is applied throughout the ICU stay until ICU discharge, death or a decision to withdraw life-sustaining therapy is made, whichever comes first. The study procedures are also implemented in the operating room, provided the patient is still admitted to the ICU. A single unit at a time is transfused when the Hb threshold is reached unless there is an active and uncontrolled bleeding requiring urgent care. Additional RBC transfusions are given if the post-transfusion Hb level remains below the assigned threshold. In both groups, RBCs are transfused within 3 hours after the Hb transfusion threshold is reached.
Compliance
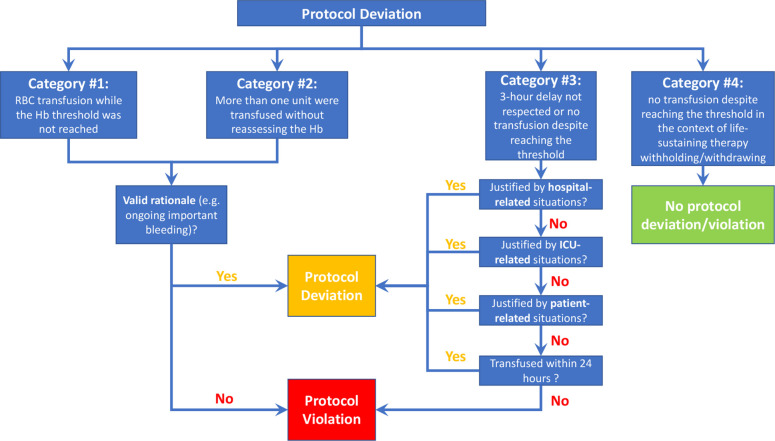
Potential protocol deviations and violations are reported to the Coordinating Centre within 72 hours and further classified into four categories (figure 2), reflecting the following situations wherein: (1) an RBC transfusion occurred while the Hb threshold is not reached, (2) more than one unit is transfused without reassessing the Hb level between transfusions, (3) the delay between reaching the transfusion threshold and transfusion is greater than 3 hours or a transfusion never occurred despite reaching the transfusion threshold and (4) no transfusion occurred in the context of life-sustaining therapy withdrawal. Using a standard operating procedure, an adjudication committee will determine whether each reported event represents a protocol violation, a protocol deviation or neither (see online supplemental appendix 1).
Figure 2.
Potential protocol deviations and violations. ICU, intensive care unit.
bmjopen-2022-067117supp001.pdf (98.8KB, pdf)
Cointerventions
No intervention other than the allocated transfusion threshold is protocolised. Standard therapeutic strategies according to the Brain Trauma Foundation guidelines are recommended.10
Outcome measures
Our primary and secondary outcome measures are validated in patients with TBI and aligned with the Common Data Elements developed by the National Institute of Neurological Disorder and Strokes.22 All primary and secondary outcomes are assessed centrally by trained research personnel blinded to the intervention to minimise the risk of bias during data collection. We chose a 6-month assessment as it is the most common time frame used in modern TBI trials and corresponds to the plateau phase of recovery.23 Tertiary outcomes are assessed at participating sites, using standardised definitions (see online supplemental appendix 2).
bmjopen-2022-067117supp002.pdf (59KB, pdf)
Primary outcome
We are using the Glasgow Outcome Scale extended (GOSe) to assess neurological outcome at 6 months.24 The GOSe scale is reliable, sensitive to change25 26 and is the most widely used clinical and patient-oriented outcome in this population.27–31 It comprises eight ranking levels from 1 (death, least favourable outcome) to 8 (upper good recovery, most favourable outcome).
Secondary outcomes
We are assessing ICU, hospital and 6-month mortality. At 6 months, we measure the Functional Independence Measure (FIM).32 The FIM has been used for over three decades in TBI patients to assess their progression during rehabilitation. The scale is sensitive to change and evaluates the amount of assistance required to perform 18 basic daily activities (13 physical and five cognitive components).33 34 Each component is scored on a 7-point scale, with higher scores indicating a greater degree of independence. We also evaluate the quality of life using the EuroQoL 5-Dimension 5-Level (EQ-5D-5L) (generic scale) and the Quality of Life after Brain Injury (QOLIBRI) (TBI-specific scale) questionnaires.35–37 To evaluate depression, we use the self-reported Patient Health Questionnaire (PHQ-9), which includes nine items that assess the frequency of depressive symptoms in the past 2 weeks.38
Tertiary outcomes
We are capturing the number of RBC units transfused in the ICU, lowest daily Hb, infections, duration of mechanical ventilation and ICU and hospital length of stay. We are also assessing complications related to transfusion.
Data collection
At enrolment, the study team collects baseline characteristics, prerandomisation cointerventions and episodes of secondary cerebral injury, which are defined as thresholds at which therapeutic intervention is recommended by practice guidelines10 (see tables 1 and 2). We also collect time from eligibility to randomisation and from randomisation to study intervention implementation. Daily, we collect data on secondary injury episodes and cointerventions. At ICU discharge, we collect the length of stay and the duration of mechanical ventilation. At hospital discharge, we collect non-neurosurgical procedures, infections and transfusion reactions that occurred during the hospital stay as well as the reports of the brain imaging (CT and MRI), length of stay, discharge status and location, documentation of prognostic assessment, justifications provided by clinicians for discontinuing life-sustaining therapies and occurrence of death by neurological criteria.
Table 2.
Secondary cerebral injury definitions
| Definition | |
| Hypoxemia | Oxygen saturation<90% for ≥ 5 min on pulse oxymetry |
| Hypotension | Systolic blood pressure<90 mm Hg for≥5 min |
| Intracranial hypertension | Intracranial pressure>25 mm Hg for≥5 min |
| Brain tissue hypoxia | Brain tissue oxygen tension(PbtO2)< 15 mm Hg for≥5 min or Brain tissue oxygen saturation(SbtO2)> 20% below baseline for≥5 min or SbtO2<60% for ≥ 5 min |
To limit loss to follow-up, we are gathering complete contact information for patients, their family practitioners and caregivers. Local research coordinators send personalised reminders and confirm upcoming interviews with patients. We use flexible schedules for centralised outcome assessment. We obtain survival status of patients lost to follow-up from public registries or by reaching the primary care team. In our previous multicentre, TBI-Prognosis prospective cohort study, we had no losses to follow-up at 6 months using those strategies.39
Data management
The HEMOTION Coordinating Centre, located at the CHU de Québec-Université Laval Research Centre (Québec City, Québec, Canada), oversees the trial coordination. Source documents are kept at each participating site in locked filing cabinets and offices accessible by the site investigators and their authorised personnel. Coded information is entered in a web-based electronic database and stored at the Ottawa Methods Center at OHRI, which meets Health Canada recommendations and Good Clinical Practice for paper-based and electronic document control system. OHRI personnel has secure access to all trial data, but staff from the Coordinating Centre remain blinded to the intervention allocation.
Sample size
Our sample size was calculated based on the proportion of patients who will experience an unfavourable outcome (GOSe ≤4).24 27 28 Assuming a 40% risk of unfavourable outcome in the control group,27 28 a sample size of 712 patients will allow us to detect an absolute risk reduction of 10% with a power of 80% and a type 1 error of 5%. Our sample size is conservative as it was based on the simple dichotomous cut-off and most used definition of an unfavourable outcome in TBI using the GOSe. Based on simulated data, a sliding dichotomy approach will increase our ability to observe the planned effect size with 95% power. To account for an estimated 2% dropout rate (consent withdrawals and losses to follow-up) based on observed aggregate rates at the interim analysis, the final sample size was increased to 742.40
Statistical methods
All analyses will be performed according to the intention-to-treat principle by biostatisticians blinded to the intervention and reported using 95% CIs. Patient characteristics will be presented with means, medians or proportions, as appropriate. The primary outcome will be presented as quantile-specific ORs using a sliding dichotomy approach to account for the whole ordinal scale. With the sliding dichotomy approach, the point of dichotomy of the GOSe for an unfavourable outcome varies according to the baseline prognostic risk. This approach has been advocated by several trialists41 and used in recent TBI trials to increase the ability to detect smaller effect size with similar power.27 28 We will assess the baseline prognosis risk with the externally validated International Mission for Prognosis and Analysis of Clinical Trials in TBI prognostic model, which includes admission characteristics (hypoxemia, hypotension and CT scan and laboratory results).42 Patients will be split into a minimum of three quantiles according to their baseline prognostic risk. Patients categorised in the worst predicted prognosis quantile will be considered to have an unfavourable outcome if the 6-month GOSe is ≤3 (ie, death, vegetative state or lower severe disability). We will use multiple imputation to simulate missing data values using imputation models for independent variables in respective analysis models with the number of imputations corresponding to the fraction of missing data, in line with recommendations.43
We will perform the following secondary analyses for the primary outcome: per protocol analysis, best case-worst-case scenarios for patients with missing primary outcome, proportional odds analysis (provided the distribution of the GOSe meets the proportional odds assumption,44 and analysis of the GOSe as a binary variable (GOSe≤4 vs >4)) using a χ2 test and multivariable logistic regression. In sensitivity analyses, we will compare results generated using multiple imputation to complete-case results.
Duration of mechanical ventilation and length of stay will be compared using Cox shared frailty regression to account for the competing risk of mortality.45 Other secondary outcomes, including the number of RBC units transfused and the lowest daily Hb, will be compared between groups using generalised linear models with appropriate link functions and conditional distributions.
Subgroup analyses
We will perform subgroup analyses for our primary outcome according to age, sex, TBI severity (moderate vs severe), country, presence of heart disease, occurrence of decompressive craniectomy or surgical drainage prior to randomisation and occurrence of transfusion prior to ICU admission. We will use the Instrument to assess the Credibility of Effect Modification ANalyses to judge the credibility of apparent effect modification among subgroups.46
Data safety and monitoring
We adopted the Data Safety and Monitoring Committee (DSMC) charter template from the DAMOCLES Study Group (see online supplemental appendix 3).47 The DSMC includes an international expert in transfusion medicine, a senior biostatistician and epidemiologist and a neurologist with expertise in neurocritical care. Periodically, the DSMC will independently review reports received directly from the Ottawa Methods Centre, including blinded serious adverse events (SAE) reports, protocol adherence, indicators of trial management (eg, enrollment, consent). The DSMC will also blindly evaluate the primary outcome at the interim analysis of 50% enrollment using the Haybittle-Peto criterion (p<0.001).48 49
bmjopen-2022-067117supp003.pdf (271.6KB, pdf)
Serious adverse events
Our rationale for reporting SAE is in agreement with a statement on academic trials in critically ill patients.50 Several potential SAEs are already reported as outcomes, defined a priori, while other events are commonly expected ICU events. Potential SAEs not reported as study outcomes or that are not common ICU events will be defined as any postrandomisation adverse occurrence or event that is determined to be directly attributable to the study intervention, that requires inpatient hospitalisation after discharge or prolongation of existing hospitalisation; that results in persistent or significant disability/incapacity; or that results in a congenital anomaly/birth defect; that is life threatening; that results in death. Any event that ICU physicians or site investigators label as unexpected will be described fully. These will be collated and submitted to the DSMC.
Data monitoring
The HEMOTION Coordinating Centre team verifies data entered for completeness and accuracy (eg, range checks for data value), generate queries and communicate with the sites as required. The frequency of the verifications depends on the site enrolment rates, with high enrolling sites having more than one monitoring visit. We are conducting remote continuous monitoring activities, including monitoring visits (remotely or on-site if required), and will perform a final closeout virtual visit for each site.
Patient and public involvement
Representatives from Brain Injury Canada, a non-governmental organisation whose vision is to promote a better quality of life for people affected by acquired brain injury,51 were involved in the trial design and are involved in its conduction. Patient and caregiver engagement ensures that our study objectives are tailored to their needs.
Trial oversight
The HEMOTION Steering Committee is comprised of coinvestigators with expertise in TBI and neurocritical care, neurosurgery, haematology, transfusion research, trauma, critical care and large-scale multicentre trials. Knowledge users from various organisations and their representatives are also part of the Steering Committee. These organisations are the Institut national d’excellence en santé et service sociaux, Canadian Anesthesiologists Society, Canadian Blood Services and Brain Injury Canada. We have established an Executive Committee to address day-to-day clinical and methodological issues. The Executive Committee is composed of the three principal investigators and is supported by the project manager and trial coordinator. The HEMOTION trial is being conducted under the auspices of the CCCTG, an inclusive group of healthcare professionals that promotes and assists in the implementation of investigator-initiated, patient-oriented, multicentre research in critically ill patients. The trial is also conducted in collaboration with the Canadian Perioperative Anesthesia Clinical Trials Group and the CTRC that was created to enhance collaborations among Canadian scientists working in anesthesiology and perioperative medicine, and on different aspects of the continuum of care of patients with TBI, respectively.
Ethics and dissemination
Research ethics approval and consent process
We obtained approval from the research ethics board prior to the initiation of the trial at each participating centre (see online supplemental appendix 4). Since all patients with TBI are temporarily unable to provide an informed consent, initial consent is sought from a surrogate decision-maker (see Informed Consent Form in online supplemental appendix 5). If a surrogate decision-maker is not available, a deferred informed consent approach is used where authorised by the local research ethics board as the research risk to patients is minimal, and the studied transfusion strategies are part of usual care in many centres12 13 and considered acceptable by clinicians caring for these patients.16 21 A deferred consent approached has been previously used in RBC transfusion strategy trials with no safety issues.52 53 Should the patient regain capacity to consent, the consent to continue participation is sought. If the study intervention is suspended for any reason, we pursue data collection unless consent is denied.
bmjopen-2022-067117supp004.pdf (86.2KB, pdf)
bmjopen-2022-067117supp005.pdf (198.9KB, pdf)
Protocol amendments
All past and future changes to the protocol are approved by research ethics committees prior to implementation. Shortly after the ethics approval was obtained and recruitment began, we amended the protocol to detail one exclusion criteria, modify the size of the permuted blocks used for randomisation, specify the number of interim analyses and shorten the time frame to report protocol violation to the Coordinating Centre (online supplemental appendix 6). In the spring of 2022, we implemented additional amendments and increased the sample size to compensate for postrandomisation exclusions, consent withdrawals and losses-to-follow-up observed at the interim analysis. We detailed the adjudication process for protocol deviations and violations, corrected some administrative details (number of participating sites and countries, updated references) and modified the prognostic model to be used in the sliding dichotomy analysis.
bmjopen-2022-067117supp006.pdf (91KB, pdf)
Confidentiality
Confidentiality is maintained by coded identification, password-protected files and websites, locked filing cabinets and offices. Direct identifiers are removed and replaced with a code. Site investigators can re-identify specific patients, if required by authorised persons. The code list is kept in secured cabinets and offices at each participating site, only accessible by the site investigators and their authorised personnel. Electronic data are physically and virtually secured in the data centre physically located at OHRI.
Dissemination
The findings from this trial will be shared with relevant brain injury organisations and healthcare professionals, through the publication of manuscripts, conference presentations and seminars. Based on the findings, this trial will engage knowledge translation specialists to build an implementation strategy to reach as many stakeholders and members of the medical community as possible, to help reduce transfusion-related practice variation and thereby promote better outcomes for patients with TBI.
Current trial status
Recruitment began in September 2017 at the CHU de Québec—Université Laval and is currently ongoing at 34 recruiting sites in Canada, the United Kingdom, Brazil and France. The recruitment was initially planned to end in spring 2021. As of March 2022, 75% of the target sample size was achieved. Due to the COVID-19 pandemic and the increase of the sample size, the recruitment is expected to be completed in winter 2023.
Supplementary Material
Acknowledgments
We want to thank the HEMOTION Trial Team members, the Canadian Critical Care Trials Group, the Canadian Perioperative Anesthesia Clinical Trials Group and the Canadian Traumatic Brain Injury Research Consortium for the ongoing support and collaboration. More importantly, we want to thank patients and their caregivers for their important contribution in participating in the HEMOTION trial.
Footnotes
Twitter: @AlexisTurgeon_ @HEMOTION_trial @CCNT_ULAVAL, @RZarychasnki, @shane_w_english, @abdocherty79, @KarenBurnsk, @jgordonboyd, @Ball, @LamontagneFran5, @MaudeStOnge, @Moore, @NSTrauma, @drfoxrob, @LauzierFrancoi1
Contributors: AFT, DAF and FLau originally designed the trial and drafted the manuscript. LC, M-PP, RZ, SE, AD, TW, DG, AHK, DS, KEAB, JGB, JCM, DJK, IB, PCH, FLam, OC, MS-O, PLB, LM, XN, AR, KK, RSG, VL and AF-R contributed to the protocol and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Funding: The HEMOTION trial is funded by peer-reviewed grants from the Canadian Institutes of Health Research (CIHR) (PJT #148934 and FDN #148443). The CIHR have no role in the design of the trial, its conduction, the interpretation of data and the dissemination of the trial results. AFT is the Chairholder of the Canada Research Chair in Critical Care Neurology and Trauma, Flau, LM, MS-O and Flam are recipients of salary support Awards from the Fonds de la Recherche du Québec-Santé (FRQS). RZ is the Chairholder of the Lyonel G Israels Research Chair in Hematology.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1. Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22:341–53. 10.3233/NRE-2007-22502 [DOI] [PubMed] [Google Scholar]
- 2. Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2011;2:492–516. 10.1007/s12975-011-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med 2005;33:1104–8. 10.1097/01.CCM.0000162685.60609.49 [DOI] [PubMed] [Google Scholar]
- 4. Zygun DA, Nortje J, Hutchinson PJ, et al. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med 2009;37:1074–8. 10.1097/CCM.0b013e318194ad22 [DOI] [PubMed] [Google Scholar]
- 5. Leal-Noval SR, Rincón-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med 2006;32:1733–40. 10.1007/s00134-006-0376-2 [DOI] [PubMed] [Google Scholar]
- 6. Utter GH, Shahlaie K, Zwienenberg-Lee M, et al. Anemia in the setting of traumatic brain injury: the arguments for and against liberal transfusion. J Neurotrauma 2011;28:155–65. 10.1089/neu.2010.1451 [DOI] [PubMed] [Google Scholar]
- 7. Boutin A, Chassé M, Shemilt M, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review and meta-analysis. Transfus Med Rev 2016;30:15–24. 10.1016/j.tmrv.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 8. Desjardins P, Turgeon AF, Tremblay M-H, et al. Hemoglobin levels and transfusions in neurocritically ill patients: a systematic review of comparative studies. Crit Care 2012;16:R54. 10.1186/cc11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson CS, Hannay HJ, Yamal J-M, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014;312:36–47. 10.1001/jama.2014.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6–15. 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 11. Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European society of intensive care medicine. Intensive Care Med 2020;46:673–96. 10.1007/s00134-019-05884-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boutin A, Moore L, Green RS, et al. Hemoglobin thresholds and red blood cell transfusion in adult patients with moderate or severe traumatic brain injuries: a retrospective cohort study. J Crit Care 2018;45:133–9. 10.1016/j.jcrc.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 13. Boutin A, Moore L, Lauzier F, et al. Transfusion of red blood cells in patients with traumatic brain injuries admitted to Canadian trauma health centres: a multicentre cohort study. BMJ Open 2017;7:e014472. 10.1136/bmjopen-2016-014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. transfusion requirements in critical care Investigators, Canadian critical care trials group. N Engl J Med 1999;340:409–17. 10.1056/NEJM199902113400601 [DOI] [PubMed] [Google Scholar]
- 15. Carson JL, Stanworth SJ, Dennis JA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev 2021;12:CD002042. 10.1002/14651858.CD002042.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lessard Bonaventure P, Lauzier F, Zarychanski R, et al. Red blood cell transfusion in critically ill patients with traumatic brain injury: an international survey of physicians' attitudes. Can J Anaesth 2019;66:1038–48. 10.1007/s12630-019-01369-w [DOI] [PubMed] [Google Scholar]
- 17. Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (probe) study. A novel design for intervention trials. Blood Press 1992;1:113–9. 10.3109/08037059209077502 [DOI] [PubMed] [Google Scholar]
- 18. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81–4. 10.1016/s0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kramer AH, Diringer MN, Suarez JI, et al. Red blood cell transfusion in patients with subarachnoid hemorrhage: a multidisciplinary North American survey. Crit Care 2011;15:R30. 10.1186/cc9977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maas AIR, Harrison-Felix CL, Menon D, et al. Standardizing data collection in traumatic brain injury. J Neurotrauma 2011;28:177–87. 10.1089/neu.2010.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asselin M, Lachance Y, Lalonde G, et al. Long-term functional outcome in adults with severe TBI: a meta-analysis. Crit Care 2013;17:S127. 10.1186/cc12272 [DOI] [Google Scholar]
- 24. Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the glasgow outcome scale. J Neurol Neurosurg Psychiatry 1981;44:285–93. 10.1136/jnnp.44.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pettigrew LEL, Wilson JTL, Teasdale GM. Reliability of ratings on the glasgow outcome scales from in-person and telephone structured interviews. J Head Trauma Rehabil 2003;18:252–8. 10.1097/00001199-200305000-00003 [DOI] [PubMed] [Google Scholar]
- 26. Levin HS, Boake C, Song J, et al. Validity and sensitivity to change of the extended glasgow outcome scale in mild to moderate traumatic brain injury. J Neurotrauma 2001;18:575–84. 10.1089/089771501750291819 [DOI] [PubMed] [Google Scholar]
- 27. Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 2014;371:2457–66. 10.1056/NEJMoa1404304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nichol A, French C, Little L, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet 2015;386:2499–506. 10.1016/S0140-6736(15)00386-4 [DOI] [PubMed] [Google Scholar]
- 29. Roquilly A, Moyer JD, Huet O, et al. Effect of continuous infusion of hypertonic saline vs standard care on 6-month neurological outcomes in patients with traumatic brain injury: the cobI randomized clinical trial. JAMA 2021;325:2056–66. 10.1001/jama.2021.5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrews PJD, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med 2015;373:2403–12. 10.1056/NEJMoa1507581 [DOI] [PubMed] [Google Scholar]
- 31. Cooper DJ, Nichol AD, Bailey M, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the polar randomized clinical trial. JAMA 2018;320:2211–20. 10.1001/jama.2018.17075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keith RA, Granger CV, Hamilton BB, et al. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1987;1:6–18. [PubMed] [Google Scholar]
- 33. Livingston DH, Lavery RF, Mosenthal AC, et al. Recovery at one year following isolated traumatic brain injury: a Western trauma association prospective multicenter trial. J Trauma 2005;59:1298–304. 10.1097/01.ta.0000196002.03681.18 [DOI] [PubMed] [Google Scholar]
- 34. Zhu XL, Poon WS, Chan CCH, et al. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj 2007;21:681–90. 10.1080/02699050701468941 [DOI] [PubMed] [Google Scholar]
- 35. EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 36. Salbach NM, Mayo NE, Hanley JA, et al. Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Arch Phys Med Rehabil 2006;87:1597–604. 10.1016/j.apmr.2006.08.336 [DOI] [PubMed] [Google Scholar]
- 37. von Steinbüchel N, Wilson L, Gibbons H, et al. Quality of life after brain injury (QOLIBRI): scale validity and correlates of quality of life. J Neurotrauma 2010;27:1157–65. 10.1089/neu.2009.1077 [DOI] [PubMed] [Google Scholar]
- 38. Fann JR, Bombardier CH, Dikmen S, et al. Validity of the patient health questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil 2005;20:501–11. 10.1097/00001199-200511000-00003 [DOI] [PubMed] [Google Scholar]
- 39. Turgeon AF, Lauzier F, Zarychanski R, et al. Prognostication in critically ill patients with severe traumatic brain injury: the TBI-prognosis multicentre feasibility study. BMJ Open 2017;7:e013779. 10.1136/bmjopen-2016-013779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials 1981;2:93–113. 10.1016/0197-2456(81)90001-5 [DOI] [PubMed] [Google Scholar]
- 41. Murray GD, Barer D, Choi S, et al. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma 2005;22:511–7. 10.1089/neu.2005.22.511 [DOI] [PubMed] [Google Scholar]
- 42. Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5:e165. 10.1371/journal.pmed.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Little RJ, Rubin DB. Statistical analysis with missing data. John Wiley & Sons, 2019. [Google Scholar]
- 44. Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics 1990;46:1171–8. 10.2307/2532457 [DOI] [PubMed] [Google Scholar]
- 45. Ha D, Jeong J-H, Lee Y. Statistical modelling of survival data with random effects. Springer, 2017. [Google Scholar]
- 46. Schandelmaier S, Briel M, Varadhan R, et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020;192:E901–6. 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. DAMOCLES Study Group, NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711–22. 10.1016/S0140-6736(05)17965-3 [DOI] [PubMed] [Google Scholar]
- 48. Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793–7. 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 49. Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585–612. 10.1038/bjc.1976.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cook D, Lauzier F, Rocha MG, et al. Serious adverse events in academic critical care research. CMAJ 2008;178:1181–4. 10.1503/cmaj.071366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brain injury Canada. Available: https://braininjurycanada.ca/en/about-us [Accessed 16 June 2022].
- 52. Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–8. 10.1056/NEJMoa1500704 [DOI] [PubMed] [Google Scholar]
- 53. English SW, Fergusson D, Chassé M, et al. Aneurysmal subarachnoid hemorrhage-red blood cell transfusion and outcome (Sahara): a pilot randomised controlled trial protocol. BMJ Open 2016;6:e012623. 10.1136/bmjopen-2016-012623 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067117supp001.pdf (98.8KB, pdf)
bmjopen-2022-067117supp002.pdf (59KB, pdf)
bmjopen-2022-067117supp003.pdf (271.6KB, pdf)
bmjopen-2022-067117supp004.pdf (86.2KB, pdf)
bmjopen-2022-067117supp005.pdf (198.9KB, pdf)
bmjopen-2022-067117supp006.pdf (91KB, pdf)