Abstract
Context
We recently demonstrated increased cellular proliferation in the pancreatic ductal gland (PDG) compartment of organ donors with type 1 diabetes, suggesting that PDGs may harbor progenitor cells capable of pancreatic regeneration.
Objective
We evaluated the impact of diabetes and pancreatic inflammation on PDG and interlobular duct (ILD) cellular proliferation and profiles.
Methods
Endocrine hormone expression (insulin, glucagon, somatostatin, pancreatic polypeptide) and proliferating Ki67+ cells were localized within the PDG and ILD compartments by multicolor immunohistochemistry in cross-sections from the head, body, and tail regions of pancreata from those with (n = 31) or without type 1 diabetes (n = 43). Whole-slide scanned images were analyzed using digital pathology.
Results
Type 1 diabetes donors with insulitis or histologically identified pancreatitis had increased cellular replication in the ILD and PDG compartments. Interestingly, while cellular proliferation within the pancreatic ductal tree was significantly increased in type 1 diabetes (PDG mean = 3.36%, SEM = 1.06; ILD mean = 2.78%, SEM = 0.97) vs nondiabetes(ND) subjects without pancreatic inflammation (PDG mean = 1.18%, SEM = 0.42; ILD mean = 0.74%, SEM = 0.15, P < 0.05), robust replication was also observed in ND donors with pancreatitis (PDG mean = 3.52%, SEM = 1.33; ILD mean = 2.18%, SEM = 0.54, P < 0.05). Few polyhormonal cells were present in the ILD (type 1 diabetes = 0.04 ± 0.02%; ND = 0.08 ± 0.03%, P = 0.40) or PDG compartment (type 1 diabetes = 0.02 ± 0.01%; ND = 0.08 ± 0.13%, P = 0.63).
Conclusion
These data suggest that increased pancreatic ductal cell replication is associated with sustained pancreatic inflammation; however, as replicating cells were hormone-negative, PDGs do not appear to represent a compelling endogenous source of hormone-positive endocrine cells.
Keywords: cellular replication, inflammation, insulitis, pancreatitis, pancreatic duct glands, interlobular duct, type 1 diabetes
Distinctive pathological hallmarks of the pancreas in type 1 diabetes include loss of insulin producing beta cells, extensive fibrosis, and a decrease in total organ mass [1–4]. Despite this, accumulating evidence suggests that, even in longstanding type 1 diabetes, there may be an endogenous attempt to regenerate beta cells: such evidence includes measurable insulin secretion (ie, C-peptide) [5, 6], as well as the consistent finding of small numbers of insulin-positive cells in the pancreas of subjects with type 1 diabetes, often in close approximation to pancreatic ducts [7, 8]. The pancreatic ductal glands (PDGs) represent a distinct anatomical niche within the pancreas [9, 10], forming blind-ending outpouchings from the main pancreatic duct and its branches. The PDG compartment is purported to serve as a potential progenitor or stem cell niche within the pancreas [11, 12].
We recently reported an increased frequency of epithelial cell replication within the PDG compartment, together with a reduced frequency of insulin-expressing cells within PDG and adjacent interlobular duct (ILD) epithelium of organ donors with type 1 diabetes [13]. While we identified an increase in progenitor-like cells in type 1 diabetes PDGs, most were seemingly fated toward an exocrine lineage [13]; however, that report did not assess for pancreatic endocrine hormones other than insulin, nor the impact of pancreatic inflammation on ILD and PDG replication. In this study, we assessed whether the identity or frequency of hormone-expressing cells within ILDs and PDGs was altered in the setting of exocrine (pancreatitis) or endocrine inflammation (insulitis).
Materials and Methods
Human Subjects and Sample Selection
Human pancreata were recovered from cadaveric organ donors, according to guidelines established by the University of Florida Institutional Review Board and processed by the Network for Pancreatic Organ donors with Diabetes (nPOD) as previously chronicled [14]. Cases (31 type 1 diabetes and 43 nondiabetes control [ND]) were selected based upon nPOD histopathological reports and divided into 3 partially overlapping, age-, sex-, and body mass index (BMI)-matched cohorts: (1) 21 long-duration type 1 diabetes (3-84 years diabetes duration) vs 21 ND without pancreatitis (pancreatitis−); (2) 12 ND with pancreatitis (pancreatitis+) vs 12 ND pancreatitis−; (3) 10 type 1 diabetes without pancreatitis with insulitis (pancreatitis−, insulitis+; 0-8 years diabetes duration) vs 10 ND insulitis−, pancreatitis− donors (Table 1). The presence of pancreatitis was based on histopathological observations and not clinical diagnosis from the medical record. Specifically, pancreatitis score (0 = none, 1 = mild, 2 = moderate, 3 = severe) and/or designation as insulitis+ or insulitis− were obtained from the nPOD Aperio database [14], which provides blinded assessment by 2 independent observers with > 99% concordance in their findings based on established histologic criteria [1, 15–23]. Formalin-fixed paraffin-embedded (FFPE) tissue blocks from the pancreatic head (PH), body (PB), and tail (PT) regions were selected from each donor based on ILD and PDG compartment presence. Due to biological variability in pancreatic ductal presence, a minimum threshold of 2 ILDs (median = 5; range, 2-7) along with their associated PDG (median = 85; range, 4-382) compartments per region from each case was required for case selection.
Table 1.
Characteristics of donors with type 1 diabetes (T1D) and nondiabetes donors (ND), with and without pancreatitis
| nPOD case no. | Age (y) | BMI | Diabetes duration (y) | Sex | Pancreas regions evaluateda | RRID | nPOD case no. | Age (y) | BMI | Sex | Pancreas regions evaluateda | RRID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1D | ND pancreatitis− | |||||||||||
| 6031 | 39 | 24.5 | 35 | M | PH | RRID: SAMN15879088 | 6012b | 68 | 23.7 | F | PH, PT | RRID: SAMN15879069 |
| 6035 | 32 | 27.1 | 28 | M | PH, PB, PT | RRID: SAMN15879092 | 6013b | 65 | 24.2 | M | PH, PT | RRID: SAMN15879070 |
| 6036 | 49 | 25.5 | 34 | F | PH, PB, PT | RRID: SAMN15879093 | 6015b | 39 | 32.2 | F | PH, PT | RRID: SAMN15879072 |
| 6040 | 50 | 31.6 | 20 | F | PH, PB, PT | RRID: SAMN15879097 | 6017 | 59 | 24.8 | F | PH, PT | RRID: SAMN15879074 |
| 6045c,d | 26 | 23.1 | 8 | M | PH, PB, PT | RRID: SAMN15879102 | 6021 | 72 | 24.5 | F | PH, PT | RRID: SAMN15879078 |
| 6050a | 82 | N/A | 58 | M | PH, PB, PT | RRID: SAMN15879107 | 6022 | 75 | 30.6 | M | PH, PT | RRID: SAMN15879079 |
| 6051 | 20 | 22.7 | 13 | M | PH, PB, PT | RRID: SAMN15879108 | 6030 | 30 | 27.1 | M | PH, PB, PT | RRID: SAMN15879087 |
| 6054 | 35 | 30.4 | 30 | F | PH, PB, PT | RRID: SAMN15879111 | 6034 | 32 | 25.2 | F | PH, PB, PT | RRID: SAMN15879091 |
| 6061 | 28 | 22.1 | 23 | M | PH, PB, PT | RRID: SAMN15879118 | 6048 | 30 | 20.6 | M | PH, PB, PT | RRID: SAMN15879105 |
| 6063 | 4 | 23.8 | 3 | M | PH, PB, PT | RRID: SAMN15879120 | 6057 | 22 | 26.0 | M | PH, PB, PT | RRID: SAMN15879114 |
| 6064c,d,e | 20 | 22.6 | 9 | F | PH, PB, PT | RRID: SAMN15879121 | 6060 | 24 | 32.7 | M | PH, PB, PT | RRID: SAMN15879117 |
| 6068a | 72 | N/A | 69 | F | PH, PB, PT | RRID: SAMN15879125 | 6091 | 27 | 35.6 | M | PH, PB, PT | RRID: SAMN15879148 |
| 6070 | 23 | 21.6 | 7 | F | PH, PB, PT | RRID: SAMN15879127 | 6096 | 16 | 18.8 | M | PH, PB, PT | RRID: SAMN15879153 |
| 6076 | 26 | 18.8 | 15 | M | PH, PB, PT | RRID: SAMN15879133 | 6098 | 18 | 22.8 | M | PH, PT | RRID: SAMN15879155 |
| 6085c,d | 89 | 20.3 | 84 | M | PH, PB, PT | RRID: SAMN15879142 | 6102 | 45 | 35.1 | F | PH, PB, PT | RRID: SAMN15879159 |
| 6088 | 31 | 27.0 | 5 | M | PH, PB, PT | RRID: SAMN15879145 | 6104 | 41 | 20.5 | M | PH, PB, PT | RRID: SAMN15879161 |
| 6089c,d | 14 | 26.0 | 8 | M | PH, PB, PT | RRID: SAMN15879146 | 6126 | 25 | 25.1 | M | PH, PB, PT | RRID: SAMN15879183 |
| 6119 | 28 | 19.4 | 14 | M | PH, PB, PT | RRID: SAMN15879176 | 6130 | 5 | 18.5 | M | PH, PB, PT | RRID: SAMN15879187 |
| 6128 | 34 | 22.2 | 31 | F | PH, PB, PT | RRID: SAMN15879185 | 6131 | 24 | 24.8 | M | PH, PB, PT | RRID: SAMN15879188 |
| 6135 | 44 | 28.7 | 21 | M | PH, PB, PT | RRID: SAMN15879192 | 6134 | 27 | 20.1 | M | PH, PB, PT | RRID: SAMN15879191 |
| 6138c,d | 49 | 33.7 | 41 | F | PH, PB, PT | RRID: SAMN15879195 | 6140 | 38 | 21.7 | M | PH, PB, PT | RRID: SAMN15879197 |
| Mean | 36.6 | 24.7 | 25.6 | 38% F | Mean | 37.2 | 25.5 | 28% F | ||||
| SEM | 4.7 | 0.9 | 4.6 | SEM | 4.3 | 1.1 | ||||||
| T1D Insulitis+ Pancreatitis- e | ND Pancreatitis- | |||||||||||
| 6247f | 25 | 24.3 | 0.6 | M | PT | RRID: SAMN15879303 | 6075 | 16 | 14.9 | M | PT | RRID: SAMN15879132 |
| 6265f | 11 | 12.9 | 8 | M | PT | RRID: SAMN15879319 | 6091 | 27 | 35.6 | M | PH | RRID: SAMN15879148 |
| 6268 | 12 | 26.6 | 3 | F | PT | RRID: SAMN15879322 | 6131 | 24 | 24.8 | M | PH | RRID: SAMN15879188 |
| 6323 | 22 | 24.7 | 6 | F | PH | RRID: SAMN15879377 | 6160 | 22 | 23.9 | M | PH | RRID: SAMN15879216 |
| 6324f | 29 | 26.2 | 2 | M | PH | RRID: SAMN15879378 | 6178 | 24 | 27.5 | F | PT | RRID: SAMN15879234 |
| 6325f | 20 | 31.2 | 6 | F | PB | RRID: SAMN15879379 | 6227 | 17 | 26.4 | F | PB | RRID: SAMN15879283 |
| 6371 | 12 | 16.6 | 2 | F | PB | RRID: SAMN15879424 | 6235 | 30 | 25.4 | M | PB | RRID: SAMN15879291 |
| 6399 | 17 | 32.0 | 0 | M | PH | RRID: SAMN15879452 | 6253 | 19 | 34.3 | F | PT | RRID: SAMN15879309 |
| 6405f | 29 | 42.5 | 0.6 | F | PH | RRID: SAMN15879458 | 6377 | 9 | 16.6 | M | PT | RRID: SAMN15879430 |
| 6414 | 23 | 28.4 | 0.4 | M | PT | RRID: SAMN15879467 | 6279 | 19 | 34.0 | M | PB | RRID: SAMN15879333 |
| Mean | 20 | 26.5 | 2.9 | 50% F | Mean | 20.7 | 26.3 | 30% F | ||||
| SEM | 2.2 | 2.6 | 0.9 | SEM | 1.9 | 2.2 | ||||||
| ND Pancreatitis+ c | ND Pancreatitis- | |||||||||||
| 6012b,g | 68 | 23.7 | — | F | PH, PT | RRID: SAMN15879069 | 6009 | 45 | 30.6 | M | PH, PB, PT | RRID: SAMN15879066 |
| 6020 | 60 | 29.8 | — | M | PH, PT | RRID: SAMN15879077 | 6013b | 65 | 24.2 | M | PH, PT | RRID: SAMN15879070 |
| 6047 | 7 | 23.9 | — | M | PH, PB, PT | RRID: SAMN15879104 | 6015b | 39 | 32.2 | F | PH, PT | RRID: SAMN15879072 |
| 6060b,g | 24 | 32.7 | — | M | PH, PB, PT | RRID: SAMN15879117 | 6137 | 8 | 24.2 | F | PH, PB, PT | RRID: SAMN15879194 |
| 6254 | 38 | 30.5 | — | M | PH, PB, PT | RRID: SAMN15879310 | 6168 | 51 | 25.2 | M | PH, PB, PT | RRID: SAMN15879224 |
| 6288 | 55 | 37.7 | — | M | PH, PB, PT | RRID: SAMN15879342 | 6178 | 24 | 27.5 | F | PH, PB, PT | RRID: SAMN15879234 |
| 6290 | 58 | 22.5 | — | M | PH, PB, PT | RRID: SAMN15879344 | 6339 | 23 | 25.0 | M | PH, PB, PT | RRID: SAMN15879393 |
| 6293 | 9 | 18.6 | — | F | PH, PB, PT | RRID: SAMN15879347 | 6368 | 38 | 20.7 | M | PH, PB, PT | RRID: SAMN15879421 |
| 6317g | 15 | 29.8 | — | M | PH, PB, PT | RRID: SAMN15879371 | 6369 | 44 | 18.8 | M | PH, PB, PT | RRID: SAMN15879422 |
| 6331g | 27 | 24.0 | — | F | PH, PB, PT | RRID: SAMN15879385 | 6387 | 15 | 18.1 | M | PH, PB, PT | RRID: SAMN15879440 |
| 6391 | 10 | 19.0 | — | M | PH, PB, PT | RRID: SAMN15879444 | 6406 | 6 | 16.8 | M | PH, PB, PT | RRID: SAMN15879459 |
| 6425g | 38 | 28.3 | — | F | PH, PB, PT | RRID: SAMN15879478 | 6415 | 10 | 14.8 | M | PH, PB, PT | RRID: SAMN15879468 |
| Mean | 34.1 | 26.7 | — | 33% F | Mean | 30.7 | 23.2 | 25% F | ||||
| SEM | 6.3 | 1.7 | — | SEM | 5.5 | 1.6 | ||||||
Abbreviations: BMI, body mass index; N/A, not available; PB, pancreas body; PH, pancreas head; PT, pancreas tail.
Immunohistochemistry
FFPE pancreas serial cross-sections (4 μm thick) were deparaffinized and rehydrated. Heat-induced epitope retrieval was performed using Borg Decloaker (Biocare Medical, Pacheco, CA) according to the manufacturer's instructions prior to staining with 2 antibody panels: (i) rabbit anti-Ki67 (clone EPR3610, diluted 1:1000, RRID: AB_10562976; Abcam, Waltham, MA, USA), rabbit anti-insulin (clone EPR17359, diluted 1:2000, RRID: AB_2716761; Abcam), mouse anti-glucagon (clone K79bB10, diluted 1:1,000, RRID: AB_297642; Abcam) (Fig. 1A); and (ii) rabbit anti-somatostatin (polyclonal, diluted 1:1,000, RRID: AB_2688022; Agilent Technologies, Inc., Santa Clara, CA, USA), rabbit anti-insulin (clone EPR17359, diluted 1:2,000, RRID: AB_2716761; Abcam), and mouse-pancreatic polypeptide (clone MM0858-31R25, diluted 1:750, RRID:AB_2904511; Abcam) (Fig. 1B). Chromogen-based immunohistochemistry (IHC) staining was detected using a Mach2 Double Stain1/Mach2 Double Stain 2 HRP-AP Polymer Detection Kit according to the manufacturer's instructions (Biocare Medical, Pacheco, CA) with Betazoid DAB (Ki67 or somatostatin), Warp Red (insulin), and Ferangi Blue (glucagon or pancreatic polypeptide; all from Biocare Medical). Slides were counterstained with hematoxylin.
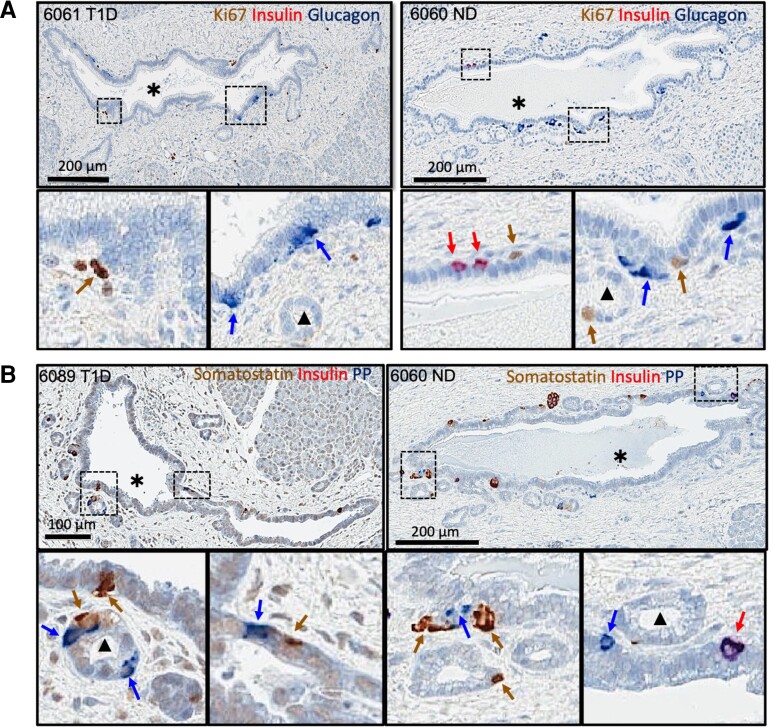
Figure 1.
Representative IHC-stained pancreatic tissue sections from type 1 diabetes (T1D) and nondiabetes (ND) donors. (A) T1D case (left, nPOD 6061, 28-year-old male, 23.1 years T1D duration) and ND case (right, nPOD 6060, 24-year-old male) stained for Ki67 (brown), insulin (red), and glucagon (blue). (B) T1D case (left, nPOD 6089, 14.3-year-old male, 8 years T1D duration) and ND case (right, nPOD 6060) stained for somatostatin (brown), insulin (red), and pancreatic polypeptide (PP, blue). Red arrows show insulin-positive cells. Brown arrows show Ki67-positive (A) or somatostatin-positive cells (B). Blue arrows show glucagon-positive (A) or pancreatic polypeptide-positive cells (B). The black asterisk (*) indicates an ILD. Black triangle (▴) indicates the PDG.
Morphometric Analysis of Pancreatic Ductal Structures
IHC-stained slides were scanned using an Aperio CS2 slide scanner (Leica Biosystems Imaging, Inc.) at 20× magnification and examined with Aperio ImageScope software version 12.1.0.5029. Quantification of Ki67+ and endocrine hormone+ cells was conducted by 2 blinded individuals. ILD and PDG compartments were identified according to the previously published definition [11]. Specifically, ILDs were defined as large ducts lined by cuboidal epithelium embedded in the pancreatic mesenchyme. PDGs were identified as small blind-ending outpouches lined by columnar epithelium with basal nuclei and abundant supranuclear cytoplasm stemming directly from or adjacent to the ILD. ILD and the PDG compartments were outlined using the digital annotation pen tool (Fig. 2). The aggregate number of pancreatic ductal cells for each pancreas region and donor was calculated using the Aperio ImageScope cytonuclear algorithm with parameters adjusted to trace all recognizable nuclei within the pancreatic ductal tree. Manual counting was performed to quantify the total number of Ki67+ cells and endocrine hormone cells (somatostatin, glucagon, pancreatic polypeptide, and insulin) observed in ILD and PDGs. Endocrine hormone+ and Ki67+ cell densities were calculated as the number of positive cells divided by total cells in either ILD or PDG (Supplementary Fig. S1 [24]).
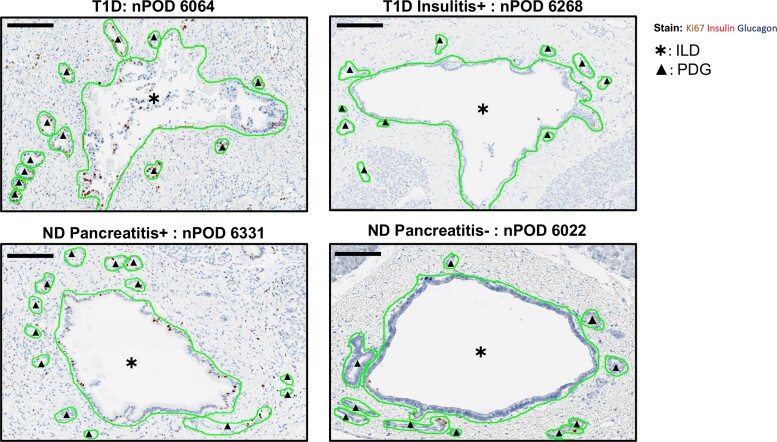
Figure 2.
Representative IHC-stained pancreatic tissue sections showing Ki67+ expression within annotated ILD and PDG from type 1 diabetes (T1D) and nondiabetes (ND) donors. Representative IHC images of FFPE pancreas sections stained for Ki67+ (brown), insulin (red), and glucagon (blue) annotated in Aperio ImageScope software version 12.1.0.5029. Annotations are shown in green for T1D donors with a combination of histologically identified pancreatitis and insulitis (A) (nPOD 6064), (B) with documented insulitis (T1D insulitis+ nPOD 6268), (C) an ND donor with histologically identified pancreatitis (ND pancreatitis+ nPOD 6331), and (D) an ND donor without documented pancreatitis (ND pancreatitis- donor nPOD 6022). Black asterisks (*) indicate ILD compartment. Black triangles (▴) indicate PDG compartment. Scale bars: 800 μm.
Statistical Analysis
All data were normally distributed; hence, parametric tests were used for statistical analyses. Data are displayed as mean ± standard error of the mean (SEM) with analysis by unpaired t test, F-test, Pearson correlation, or one-way ANOVA with Tukey's post hoc test (GraphPad Prism 9, San Diego, CA). Statistical significance was defined as P < 0.05.
Data and Resource Availability
The donors included in the long-duration type 1 diabetes vs ND pancreatitis− cohort (Table 1, upper panel) were previously evaluated for PDG and ILD cellular replication from two-color IHC slides stained for insulin and Ki67 [13]. However, the current report presents our analysis of an entirely new dataset, including 2 three-color IHC panels visualized on newly sectioned slides. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. Resources analyzed during the current study are available to approved investigators upon request from the nPOD repository (https://www.jdrfnpod.org/for-investigators/request-npod-samples/).
Results
Cellular Replication Is Increased in ILD and PDG From Individuals With Long Duration Type 1 Diabetes
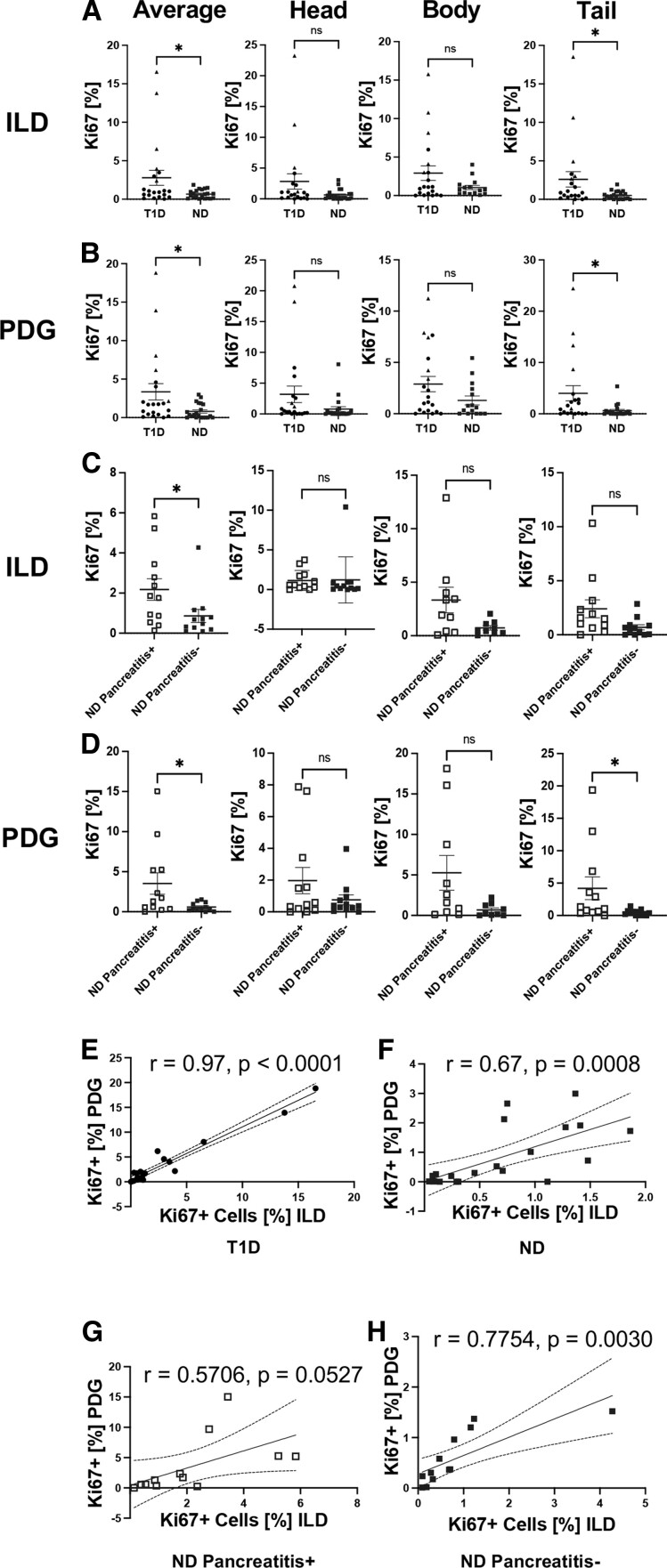
Ki67 nuclear immunoreactivity (Fig. 2) was quantified in the ILD and PDG compartments of PH, PB, and PT sections from established type 1 diabetes vs ND donors (Table 1) to enumerate replicating ductal epithelial cells. Overall, a three-fold increase in the Ki67+ cell frequency in ILDs and PDGs of type 1 diabetes donors was observed in comparison with ND donors (P = 0.03 and P = 0.02, respectively; Fig. 3A and B). With respect to specific pancreatic regional differences, the tail region of the type 1 diabetes pancreas had a significant increase in Ki67+ frequency within the ILDs (P = 0.03) and PDGs (P = 0.02) while the head and body regions demonstrated comparable Ki67+ replication frequencies in the ILD (Fig. 3A) and PDG (Fig. 3B) compartments from these donor groups. However, the type 1 diabetes donors exhibited significant heterogeneity in Ki67+ frequency in all 3 regions as compared with ND donors (F-test, P < 0.0001).
Figure 3.
Increased cellular proliferation in the ILD and PDG compartments of pancreas from donors with type 1 diabetes (T1D) or pancreatitis. Frequencies of Ki67+ cells in the (A) ILD and (B) PDG epithelium from T1D (N = 21; black circles: pancreatitis−, n = 16; black triangles: pancreatitis+, n = 5) vs age-, sex-, and BMI-matched ND pancreatitis− donors (blue filled squares, N = 21). Frequencies of Ki67+ cells in the (C) ILD and (D) PDG epithelium from ND pancreatitis+ (blue open squares, N = 12) vs a separate cohort of age-, sex-, and BMI-matched ND pancreatitis− donors (blue filled squares, N = 12). Each data point represents an average of all sections evaluated per donor (left, see Table 1) or one section evaluated per region per donor from the pancreatic head, body, and tail as labeled on the figure. Data are presented as mean ± SEM with unpaired t test, * P < 0.05. Variances assessed using the F-test, * P < 0.05. (E-H) Positive correlation between Ki67+ cell frequency in the ILD and PDG compartments from (E) T1D, (F) ND, (G) ND pancreatitis+, and (H) ND pancreatitis− cases. Pearson correlation; r values and P values are presented on the figure.
Previous reports demonstrated increased pancreatic ductal cell proliferation in response to pancreatitis during the development of pancreatic cancer [12, 25, 26]; hence, we hypothesized that the presence of pancreatic inflammation might explain the significant variance in ILD and PDG (F-test, P < 0.0001) cell replication seen in individuals with type 1 diabetes (Fig. 3A and 3B). We identified that type 1 diabetes donors with histological evidence of pancreatitis in the nPOD pathology report exhibited the highest Ki67+ cellular frequency in the ILDs and PDGs (Fig. 3A and 3B, triangles). Among the type 1 diabetes group, 3 donors with high ductal cell replication (6045, 6089, 6138) had histologically defined pancreatitis, while 1 donor (6064) displayed a combination of pancreatitis and insulitis. These findings were consistent with previous reports of inflammation associated with increased pancreatic ductal replication and indicate the potential for exocrine-endocrine influence on ductal replication in subjects with type 1 diabetes [13].
Cellular Replication Is Increased in ILD and PDG of ND Subjects With Pancreatitis
To interrogate inflammation as a primary factor affecting pancreatic ductal cell proliferation, we characterized ILD and PDG cellular replication in ND individuals with and without pancreatitis (Fig. 2, Table 1). ND pancreatitis+ subjects had significantly increased proliferation in the ILD (P = 0.04) and PDG (P = 0.04), overall, in comparison with ND pancreatitis− organ donors (Fig. 3C and 3D). We again assessed if there were any interpancreatic regional differences in ductal replication in the context of inflammation. A statistically significant increase in Ki67+ replication was found in the tail region PDGs of ND pancreatitis+ donors compared with ND pancreatitis− donors (Fig. 3D, P = 0.04). However, we did not see this pattern represented in the tail region ILDs, nor the head and body region ILDs or PDGs of the ND pancreatitis+ donors (Fig. 3C and 3D). The PDG and ILD Ki67+ cell frequencies were positively correlated in type 1 diabetes, ND pancreatitis+ and ND pancreatitis− individuals (Fig. 3E-3H), indicating that increased proliferation observed in inflamed pancreata is present throughout the entire pancreatic ductal tree and not exclusively localized to either the PDG or ILD compartment.
Cellular Replication Is Increased in ILD and PDG of Type 1 Diabetes Donors With Insulitis or Pancreatitis
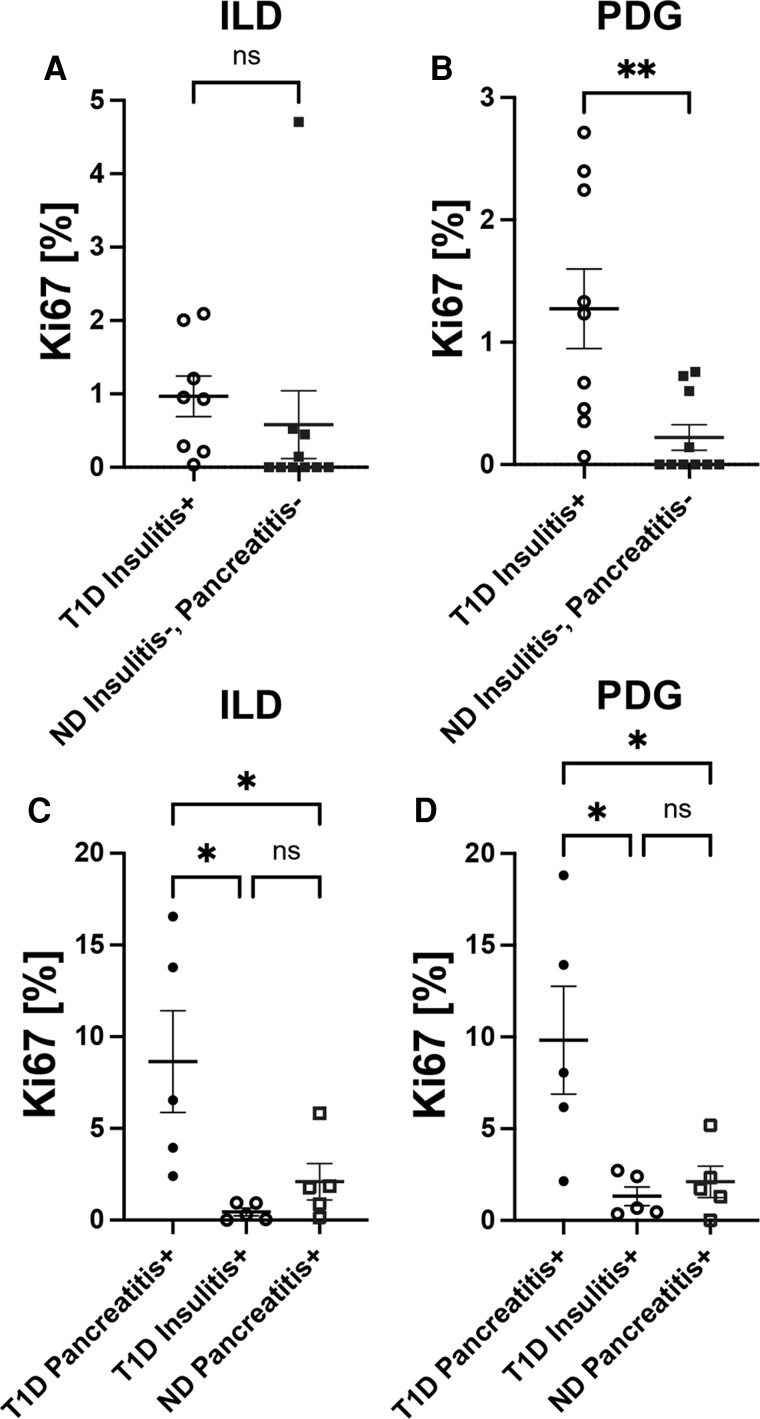
The link between islet inflammation and proliferation of pancreatic ducts has been documented by our group in type 2 diabetes [27] and longstanding type 1 diabetes [13]. Therefore, we explored whether islet inflammation (ie, insulitis) [1, 15] in subjects with shorter duration type 1 diabetes (Table 1) might influence pancreatic ductal cell replication (Fig. 2). We observed that type 1 diabetes insulitis+, pancreatitis− donors had increased replication in the PDG, but not ILD, as compared with ND pancreatitis− individuals (Fig. 4A and 4B). These findings are concordant with current understanding of insulitis and the pancreatic ductal replication axis, and they corroborate previous observations in T2D [27].
Figure 4.
Increased cellular proliferation in the ductal epithelium of type 1 diabetes (T1D) donors with insulitis or pancreatitis. Frequency of Ki67+ cells in (A) ILD and (B) PDG epithelium is higher in T1D insulitis+ subjects (black open circles, n = 10) compared with ND insulitis−, pancreatitis− subjects (blue filled squares, n = 10). Frequency of Ki67+ cells in the (C) ILD and (D) PDG epithelium of T1D pancreatitis+, insulitis− (black closed circles, n = 5) vs T1D, insulitis+, pancreatitis− subjects (black open circles, n = 5) vs ND pancreatitis+ subjects (blue open squares, n = 5). Each data point in (A) and (B) represents a specific insulitis+ section and matched control section evaluated per donor; see Table 1. Each data point in (C) and (D) represents an average of 1 to 3 sections evaluated per donor from the pancreatic head, body, and tail regions for T1D pancreatitis+ and ND pancreatitis+ donors and a specific insulitis+ section for T1D insulitis+ donors; see Table 1. (A-B) Data are presented as mean ± SEM with unpaired t test, P > 0.05. (C-D) Data are presented as mean ± SEM with one-way ANOVA with Tukey's post hoc test, * P < 0.05.
We further identified that type 1 diabetes individuals with histopathologically defined pancreatitis [16–23] had a significantly increased Ki67+ cell frequencies in the ILD (Fig. 4C) and PDG (Fig. 4D) compartments as compared with ND pancreatitis+ and type 1 diabetes insulitis+, pancreatitis− donor groups, and in these latter groups, cellular replication was comparable. These data demonstrate that while endocrine (insulitis) and exocrine (pancreatitis) inflammation can independently influence pancreatic ductal cell proliferation, the effect is more pronounced in individuals with type 1 diabetes and pancreatitis, highlighting the potential impact of the endocrine-exocrine axis on pancreatic histopathology [28]. Interestingly, the pancreatitis score, obtained from the nPOD Aperio database, correlated significantly with replication in both the ILD (Pearson's r = 0.58, P = 0.03) and PDG (r = 0.55, P = 0.04) of type 1 diabetes cases. In contrast, among ND pancreatitis+ cases, a significant correlation between pancreatitis severity and ductal replication was observed only within the PDG (r = 0.76, P = 0.004), but not the ILD compartment (r = 0.48, P = 0.11).
No Effect of Age or Diabetes Duration on Pancreatic Ductal Replication
Clinical features such as age, gender, or BMI were not significantly different across the 3 matched donor cohorts (Table 1). However, because pancreatic growth, cellular replication potential, and function have been reported to decrease with age [29], we performed Pearson correlation analyses to assess for associations between ILD and PDG Ki67+ cell frequency and donor age or type 1 diabetes duration. No significant correlation was observed between age and Ki67+ cells present within either ductal compartment of donors with type 1 diabetes (ILD [r = −0.10, P = NS] or PDG [r = 0.03, P = NS]) and their matched ND pancreatitis- controls (ILD [r = −0.33, P = NS] or PDG [r = −0.41, P = NS]). In addition, type 1 diabetes duration did not correlate with ductal Ki67+ cell frequency (ILD, r = −0.95, P = NS; PDG, r = 0.03, P = NS). However, Ki67+ cell frequency negatively correlated with age in ND pancreatitis+ donors (ILD [r = −0.64, P = 0.02] and PDG [r = −0.74, P = 0.01]), in accord with previous reports detailing pancreatic organogenesis [30].
No Evidence of Islet Regeneration From ILDs or PDGs in Type 1 Diabetes or Pancreatitis
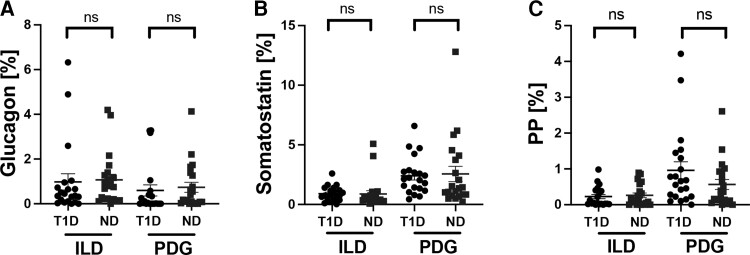
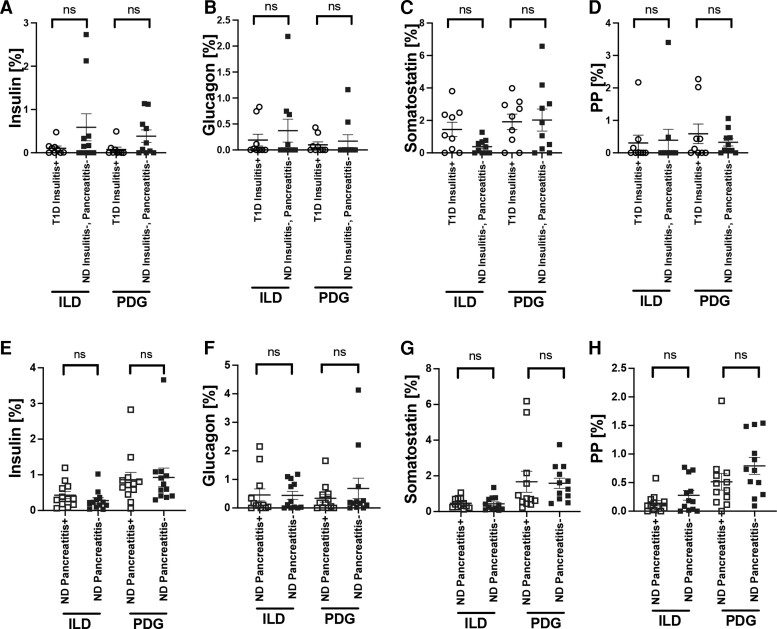
Finally, we investigated whether perturbations in endocrine hormone-expressing cells might be present within the pancreatic ductal epithelium from donors with increased ILD and/or PDG cellular proliferation. Insulin-expressing cells, although infrequent, were identified in the ILD and PDG compartments of type 1 diabetes donors however, as expected, were significantly decreased in insulitis- type 1 diabetes vs ND subjects (P < 0.0001, Supplementary Fig. S2 [24]). No differences were observed in the frequency of somatostatin, glucagon, or pancreatic polypeptide cells in either the ILD or PDG compartment from type 1 diabetes vs ND donors (Fig. 5). Moreover, the frequencies of endocrine hormone-positive cells within ILD and PDG compartments were not significantly altered in the presence of insulitis or pancreatitis (Fig. 6). Small numbers of polyhormonal cells were present in the ILD (type 1 diabetes: 0.04 ± 0.02%; ND: 0.08 ± 0.03%, P = NS) and PDG (type 1 diabetes: 0.02 ± 0.01%; ND: 0.08 ± 0.13%, P = NS) compartments. All Ki67+ cells identified in the pancreatic ductal tree were hormone-negative. We did not observe a subpopulation of hormone-positive replicating cells. Thus, the pancreatic ductal compartment in humans does not appear to increase production of endocrine cell subtypes in individuals with type 1 diabetes or pancreatitis, despite increased ductal replication, suggesting that the pancreatic ductal compartment in humans does not initiate a compensatory response.
Figure 5.
No compensatory increase in hormone-expressing endocrine cells in the ILD or PDG compartments of type 1 diabetes (T1D) subjects. Frequency of glucagon+ (A), somatostatin+ (B) and pancreatic polypeptide (PP)+ cells (C) in ILD and PDG compartments. Data are presented as mean ± SEM for T1D (black circles, n = 21) and nondiabetes (ND) donors (blue filled squares, n = 21). Each data point represents an average of 1 to 3 sections per donor (from the pancreatic head, body, and/or tail regions; see Table 1). Unpaired t test, P > 0.05 all.
Figure 6.
No significant difference in hormone-expressing endocrine cells in the ILD or PDG compartments of nondiabetes (ND) subjects with or without pancreatitis, or type 1 diabetes (T1D) subjects with insulitis as observed from 2 age-matched cohorts. Frequency of insulin+ (A), glucagon+ (B), somatostatin+ (C), and pancreatic polypeptide (PP)+ cells (D) in ILD and PDG. Data are presented as mean ± SEM for age-matched T1D insulitis+ donors (black open circles, n = 10) and ND insulitis−, pancreatitis− donors (blue filled squares, n = 10). Frequency of insulin+ (E), glucagon+ (F), somatostatin+ (G) and pancreatic polypeptide (PP)+ cells (H) in ILD and PDG. Data are presented as mean ± SEM for age-matched ND pancreatitis+ donors (blue open squares, n = 12) and ND pancreatitis− donors (blue filled squares, n = 12). Each data point represents an average of 1 to 3 sections per donor (from the pancreatic head, body, and/or tail regions; see Table 1). Unpaired t test, P > 0.05 all.
Discussion
Using an integrated image-based, in situ approach, changes in pancreatic ductal cellular morphology and frequency were characterized, demonstrating an association between inflammation in type 1 diabetes and pancreatic ductal cell replication. Specifically, type 1 diabetes subjects have a significant increase in ILD- and PDG-associated Ki67+ cells compared with ND controls, and this observation was driven by increased replication in the pancreas tail region from type 1 diabetes donors. Variable interregional ductal replication could potentially be due to the differences in islet distribution and composition in the pancreas, as well as the heterogeneous impact of type 1 diabetes pathogenesis on the pancreatic cellular architecture [31]. Thus, our results indicate that the PDG compartment in humans does not respond to the loss of beta cells by increasing the production of insulin, glucagon, somatostatin, or pancreatic polypeptide-expressing endocrine cell subtypes. Moreover, we did not observe an increase in polyhormonal cells in PDG or ILD epithelium, suggesting that, at least in this location, reprogramming of other endocrine cells through transdifferentiation is likely not occurring [32].
Both histologically defined pancreatitis and islet-specific insulitis were associated with an increase in PDG replication. Specifically, increased Ki67+ frequency was observed in ND pancreatitis+ and type 1 diabetes insulitis+ donors. However, the most robust increase in replication was observed in type 1 diabetes donors with a combination of exocrine and endocrine inflammation. Pancreatic ducts, with their unique PDG niche, have long been recognized as a potential source of new cells [33]. Recently, attention has been drawn to pancreatic ducts because of their ability to proliferate in response to severe injury [34]. Moreover, PDGs have been reported to express early pancreatic developmental genes and might serve as a cellular niche for newly forming beta cells [11, 12]. While we previously demonstrated the sensitivity of pancreatic ducts to inflammation [13], the findings reported herein suggest that both exocrine and endocrine inflammation can initiate replication within the ducts in type 1 diabetes and that the increase in pancreatic ductal cell proliferation is not accompanied by alterations in endocrine cell frequency or identity. While our study has not yet characterized the mechanisms driving inflammation-associated increased pancreatic ductal cell replication in pancreata from type 1 diabetes subjects, recent studies have shown that proinflammatory cytokine and chemokine signaling could promote an endocrine transcriptional profile in exocrine ductal cells [35]. Thus, we propose that the activation of cellular proliferation in the pancreatic ducts via inflammatory signaling might be considered a potential mechanism underlying these findings.
In terms of limitations, studies from organ donor tissues are inherently cross-sectional, and fixed-tissue samples preclude the clear identification of a causal relationship between inflammation and pancreatic ductal proliferation. Additional dynamic testing, using platforms such as pancreas tissue slice culture [36], are needed to delineate the underlying mechanisms linking insulitis, pancreatitis, and ductal plasticity. In the future, we also expect that emerging high-content imaging technologies will allow for simultaneous assessment of ILD-specific, PDG-specific, and inflammation-specific markers to further elaborate on the in situ association between increased ductal cell proliferation and inflammation. This said, through this study, we have extensively characterized previously unknown pancreatic ductal cell changes in human type 1 diabetes, thus revealing the potential for inflammation-driven pancreatic ductal cell plasticity to be harnessed for future translational diabetes therapeutic approaches.
Acknowledgments
None.
Abbreviations
- BMI
body mass index
- FFPE
formalin-fixed, paraffin-embedded
- IHC
immunohistochemistry
- ILD
interlobular duct
- ND
nondiabetes
- nPOD
Network for Pancreatic Organ donors with Diabetes
- PB
pancreatic body
- PDG
pancreas duct gland
- PH
pancreas head
- PT
pancreas tail
- SEM
standard error of the mean
Contributor Information
Shweta Kulkarni, Department of Pathology, Immunology, and Laboratory Medicine, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA.
Amanda L Posgai, Department of Pathology, Immunology, and Laboratory Medicine, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA.
Irina Kusmartseva, Department of Pathology, Immunology, and Laboratory Medicine, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA.
Clive H Wasserfall, Department of Pathology, Immunology, and Laboratory Medicine, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA.
Mark A Atkinson, Department of Pathology, Immunology, and Laboratory Medicine, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA; Department of Pediatrics, Diabetes Institute, College of Medicine, University of Florida, Gainesville, FL 32611, USA.
Alexandra E Butler, Email: aeb91011@gmail.com, Department of Research, Royal College of Surgeons in Ireland-Bahrain, 15503 Adliya, Bahrain.
Funding
These studies were supported by grants from the National Institutes of Health (NIH; R01 DK123292 and P01 AI042288 to MAA). This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project supported by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant#2018PG-TYPE 1 DIABETES053, G-2108-04793). The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/.
Author Contributions
S.K. designed the study, performed the experiments, interpreted the data, and wrote the manuscript. A.L.P. contributed to discussion and revised the manuscript. I.K. performed the experiments and revised the manuscript. C.W., M.A.A., and A.E.B. contributed to the study design and data interpretation and revised the manuscript.
Disclosure
The authors declare that no relevant conflicts of interest exist.
Guarantor Statement: A.E.B. is the guarantor of this work and as such, had full access to all of the data and takes full responsibility for the integrity of the data and accuracy of the data analysis.
Data Availability
Data will be made available upon reasonable request to the corresponding author.
References
- 1. Campbell-Thompson M, Fu A, Kaddis JS, et al. . Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atkinson MA, Campbell-Thompson M, Kusmartseva I, Kaestner KH. Organisation of the human pancreas in health and in diabetes. Diabetologia. 2020;63(10):1966–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. . The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell-Thompson ML, Filipp SL, Grajo JR. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care. 2019;42(2):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis AK, DuBose SN, Haller MJ, et al. . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38(3):476–481. [DOI] [PubMed] [Google Scholar]
- 6. Keenan HA, Sun JK, Levine J, et al. . Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist study. Diabetes. 2010;59(11):2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. [DOI] [PubMed] [Google Scholar]
- 8. Wasserfall C, Nick HS, Campbell-Thompson M, et al. . Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab. 2017;26(3):568–575.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taguchi M, Yamaguchi T, Otsuki M. Induction of PDX-1-positive cells in the main duct during regeneration after acute necrotizing pancreatitis in rats. J Pathol. 2002;197(5):638–646. [DOI] [PubMed] [Google Scholar]
- 10. Taguchi M, Otsuki M. Co-localization of nestin and PDX-1 in small evaginations of the main pancreatic duct in adult rats. J Mol Histol. 2004;35(8-9):785–789. [DOI] [PubMed] [Google Scholar]
- 11. Strobel O, Rosow DE, Rakhlin EY, et al. . Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate shh-induced metaplasia. Gastroenterology. 2010;138(3):1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaguchi J, Liss AS, Sontheimer A, et al. . Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem Cell Res. 2015;15(1):190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moin AS, Butler PC, Butler AE. Increased proliferation of the pancreatic duct gland compartment in type 1 diabetes. J Clin Endocrinol Metab. 2017;102(1):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell-Thompson M, Wasserfall C, Kaddis J, et al. . Network for pancreatic organ donors with diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28(7):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campbell-Thompson ML, Atkinson MA, Butler AE, et al. . The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56(11):2541–2543. [DOI] [PubMed] [Google Scholar]
- 16. Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clain JE, Pearson RK. Diagnosis of chronic pancreatitis. Is a gold standard necessary? Surg Clin North Am. 1999;79(4):829–845. [DOI] [PubMed] [Google Scholar]
- 19. Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332(22):1482–1490. [DOI] [PubMed] [Google Scholar]
- 20. Mergener K, Baillie J. Chronic pancreatitis. Lancet. 1997;350(9088):1379–1385. [DOI] [PubMed] [Google Scholar]
- 21. Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25(7):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyake H, Harada H, Kunichika K, Ochi K, Kimura I. Clinical course and prognosis of chronic pancreatitis. Pancreas. 1987;2(4):378–385. [DOI] [PubMed] [Google Scholar]
- 23. Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86(5 Pt 1):820–828. [PubMed] [Google Scholar]
- 24. Kulkarni SPA, Kusmartseva I, Wasserfall C, Atkinson MA, Butler AE. Data from: Presence of Exocrine and Endocrine Inflammation Increases Cellular Replication in the Pancreatic Duct Compartment in Type 1 Diabetes. Figshare. 2022. https://figshare.com/articles/figure/Supplemental_figures/20236065. [DOI] [PMC free article] [PubMed]
- 25. Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the kras(G12D) mouse model. Diabetes. 2012;61(5):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parsa I, Longnecker DS, Scarpelli DG, Pour P, Reddy JK, Lefkowitz M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985;45(3):1285–1290. [PubMed] [Google Scholar]
- 27. Schludi B, Moin ASM, Montemurro C, et al. . Islet inflammation and ductal proliferation may be linked to increased pancreatitis risk in type 2 diabetes. JCI Insight. 2017;2(13):e92282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep. 2015;15(10):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Löhr JM, Panic N, Vujasinovic M, Verbeke CS. The ageing pancreas: a systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283(5):446–460. [DOI] [PubMed] [Google Scholar]
- 30. Shih HP, Wang A, Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol. 2013;29:81–105. [DOI] [PubMed] [Google Scholar]
- 31. Wang X, Misawa R, Zielinski MC, et al. . Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8(6):e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moin ASM, Butler AE. Alterations in beta cell identity in type 1 and type 2 diabetes. Curr Diab Rep. 2019;19(9):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonner-Weir S, Toschi E, Inada A, et al. . The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5Suppl 2:16–22. [DOI] [PubMed] [Google Scholar]
- 34. Valdez IA, Teo AK, Kulkarni RN. Cellular stress drives pancreatic plasticity. Sci Transl Med. 2015;7(273):273ps2. [DOI] [PubMed] [Google Scholar]
- 35. Valdez IA, Dirice E, Gupta MK, Shirakawa J, Teo AKK, Kulkarni RN. Proinflammatory cytokines induce endocrine differentiation in pancreatic ductal cells via STAT3-dependent NGN3 activation. Cell Rep. 2016;15(3):460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Panzer JK, Hiller H, Cohrs CM, et al. . Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight. 2020;5(8):e134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.