Abstract
Background
Estimates of the relative contribution of different pathogens to all-cause diarrhoea mortality are needed to inform global diarrhoea burden models and prioritize interventions. We aimed to investigate and estimate heterogeneity in the case fatality risk (CFR) of different diarrhoeal pathogens.
Methods
We conducted a systematic review and meta-analysis of studies that reported cases and deaths for 15 enteric pathogens published between 1990 and 2019. The primary outcome was the pathogen-specific CFR stratified by age group, country-specific under-5 mortality rate, setting, study year and rotavirus vaccine introduction status. We developed fixed-effects and multilevel mixed-effects logistic regression models to estimate the pooled CFR overall and for each pathogen, controlling for potential predictors of heterogeneity.
Results
A total of 416 studies met review criteria and were included in the analysis. The overall crude CFR for all pathogens was 0.65%, but there was considerable heterogeneity between and within studies. The overall CFR estimated from a random-effects model was 0.04% (95% CI: 0.026%–0.062%), whereas the pathogen-specific CFR estimates ranged from 0% to 2.7%. When pathogens were included as predictors of the CFR in the overall model, the highest and lowest odds ratios were found for enteropathogenic Escherichia coli (EPEC) [odds ratio (OR) = 3.0, 95% CI: 1.28–7.07] and rotavirus (OR = 0.23, 95% CI: 0.13–0.39), respectively.
Conclusion
We provide comprehensive estimates of the CFR across different diarrhoeal pathogens and highlight pathogens for which more studies are needed. The results motivate the need for diarrhoeal interventions and could help prioritize pathogens for vaccine development.
Keywords: Case fatality ratio, death, mortality, diarrhoea, diarrhoea, heterogeneity
Key Messages.
Reliable estimates of the relative contribution of different diarrhoeal pathogens to all-cause diarrhoea mortality are needed to prioritize interventions and identify important pathogens to target for vaccine development.
Our results provide an updated and comprehensive estimate of the case fatality risk (CFR) across different diarrhoeal pathogens and highlight pathogens for which more studies are needed to estimate their ‘true’ CFR, particularly those with fewer studies and a higher CFR (including enteropathogenic Escherichia coli, enterotoxigenic E. coli and sapovirus).
The estimated overall CFR of 0.65% is substantial, suggesting the need to develop more effective diarrhoeal control strategies.
Introduction
Despite an estimated 80% decline in diarrhoea mortality between 1980 and 2015 among children in low-income and middle-income countries,1 globally in 2015, diarrhoea still caused 1.3 million deaths across the age spectrum, making it the fourth and ninth most common cause of death among children <5 years old and all ages, respectively.2 Thus, there is an urgent need to better understand the factors and pathogens contributing to the diarrhoea mortality burden.
Currently, there are two main models that are used to estimate the global mortality associated with diarrhoeal disease: the Maternal Child Epidemiology Estimation (formerly the Child Health Epidemiology Reference Group) model3–5 and the Institute for Health Metrics and Evaluation model.6,7 Between 2013 and 2019, the two groups reported increasingly divergent mortality estimates among children <5 years old, particularly for Shigella and enterotoxigenic Escherichia coli (ETEC).8,9 Such discrepancies impact decisions around vaccine development, introduction and use. Efforts are needed to fully understand limitations and methodologies used to calculate the estimates for both models.
A potential limitation of both models is that they assume that the distribution of pathogens among inpatients is a suitable proxy for the contribution of each pathogen to overall diarrhoeal deaths. Thus, they implicitly assume the same case fatality risk (CFR) for ‘hospitalizable’ patients across all diarrhoeal pathogens.10 To improve estimates of pathogen-specific diarrhoea mortality and prioritize pathogens for vaccine development, reliable estimates of the overall and pathogen-specific CFR are required. In addition, identifying predictors of heterogeneity in the CFR could inform regions or age strata for targeted interventions in an effort to reduce diarrhoea mortality. We sought to estimate the diarrhoea pathogen-specific CFR and to examine how various factors contribute to the risk of death in order to improve our understanding of overall diarrhoea mortality across the globe.
Methods
Search strategy and selection criteria
We conducted a systematic review to identify studies that could be used to estimate the CFR for 15 enteric diarrhoeal pathogens (identified as causes of diarrhoea in the Global Enteric Multicenter Study11) using three databases (PubMed, EMBASE and Cochrane). The 15 enteric pathogens included seven bacteria [Aeromonas spp., Campylobacter spp., enteropathogenic Escherichia coli (EPEC), ETEC, Salmonella spp., Shigella spp. and Vibrio cholerae], five viruses (adenovirus, astrovirus, norovirus, rotavirus and sapovirus), three parasites (Cryptosporidium spp., Entamoeba histolytica and Giardia lamblia) and ‘other pathogens’ [including Shiga-toxin-producing E. coli, enteroaggregative E. coli and E. coli (type not specified)]; strains included and laboratory detection methods for each pathogen are listed in Supplementary Table S1 (available as Supplementary data at IJE online). The review was restricted to studies that reported cases and deaths published between 1990 and 2019; the search was conducted on 10 July 2019. Inclusion and exclusion criteria are presented in Table 1. The search strategy and the search strings are detailed in the Supplementary Materials, Section 1, whereas the data extraction protocol is presented in the Supplementary Materials, Section 2; the full list of variables extracted is included in Supplementary Table S2 (all available as Supplementary data at IJE online).
Table 1.
The inclusion and exclusion criteria used for study selection
| Criteria | Study population | Study design | Publication requirements | Outcomes |
|---|---|---|---|---|
| Inclusion | Persons of any age | Studies with primary data | Articles written in English, French, Spanish, Polish and Portuguese languages | CFR from diarrhoea caused by any of the 15 pathogens of interest |
| Published between 1 January 1990 and 10 July 2019 | Information about the number of deaths was included and/or follow-up data were presented for all participants | |||
| Exclusion |
|
|
|
Selection methods did not allow for CFR calculation |
CFR, case fatality risk.
Risk of bias assessment
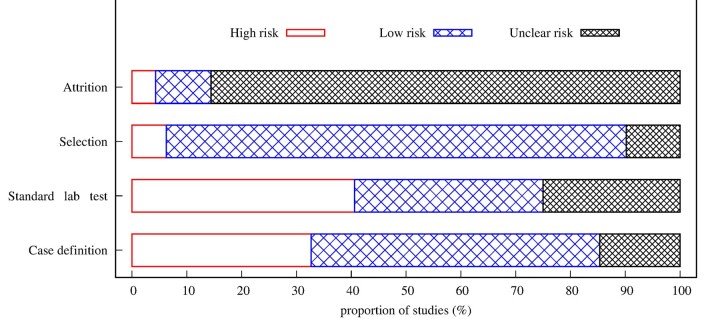
The risk of bias assessment was performed based on the Cochrane risk of bias tool12 using three criteria (Supplementary Table S3, available as Supplementary data at IJE online): measurement bias (case definition and whether a standardized laboratory test was used; see Supplementary Table S1 and Supplementary Materials, Section 2, available as Supplementary data at IJE online), selection bias and attrition bias. For each eligible article, the potential sources of bias were classified as low, unclear and high risk of bias.
Statistical analysis
For each study and for strata within each study, we estimated the crude CFR by dividing the number of deaths by the total number of diarrhoea cases and calculating the corresponding binomial 95% CI. We then combined information across studies using fixed-effects and random-effects binomial-normal generalized linear models to estimate the CFR for each pathogen while controlling for age group (<1 year, <5 years, ≥5 years and other), country-specific under-5 mortality rate (U5MR) (very low, low or high), setting (hospital-based, community-based or other—studies of outbreaks or surveillance databases/disease registries), study year and rotavirus vaccine introduction status (before or after rotavirus vaccine introduction). We compared model-based estimates to the crude CFR. We investigated heterogeneity among studies using the I2 statistic.13 We present estimates from both fixed-effects and random-effects models to represent the population average CFR (assuming studies are representative of the global population mean despite any observed heterogeneity) and the expected CFR if a new study were to be conducted, respectively.14 Potential bias from small-studies effects was assessed using funnel plots and Egger’s regression intercept test.15
To estimate the overall and relative risk of death while controlling for possible predictors as well as between-study and within-study variation, we used multilevel mixed-effects logistic regression models. A detailed description of the models, model code and how each predictor was categorized can be found in Supplementary Materials, Sections 3–4 (available as Supplementary data at IJE online). Data for all included studies are provided in Supplementary Appendix 1 and complete references for all included studies are provided in Supplementary Materials, Section 8 (available as Supplementary data at IJE online). The statistical analyses were conducted using the ‘metafor’ package in R.16
Sensitivity analyses
To examine the robustness of our analysis, we performed three different sensitivity analyses. First, we included data from the Global Enteric Multicenter Study (GEMS). We included case-only data from both the 3-year GEMS and 1-year GEMS-1A follow-up study. Second, we restricted our analysis to only hospital-based studies of children <5 years old. Finally, we included country-specific HIV prevalence estimates as an additional predictor in our model. We categorized the HIV prevalence into three categories (low, medium and high) based on tertile cut-points.
Results
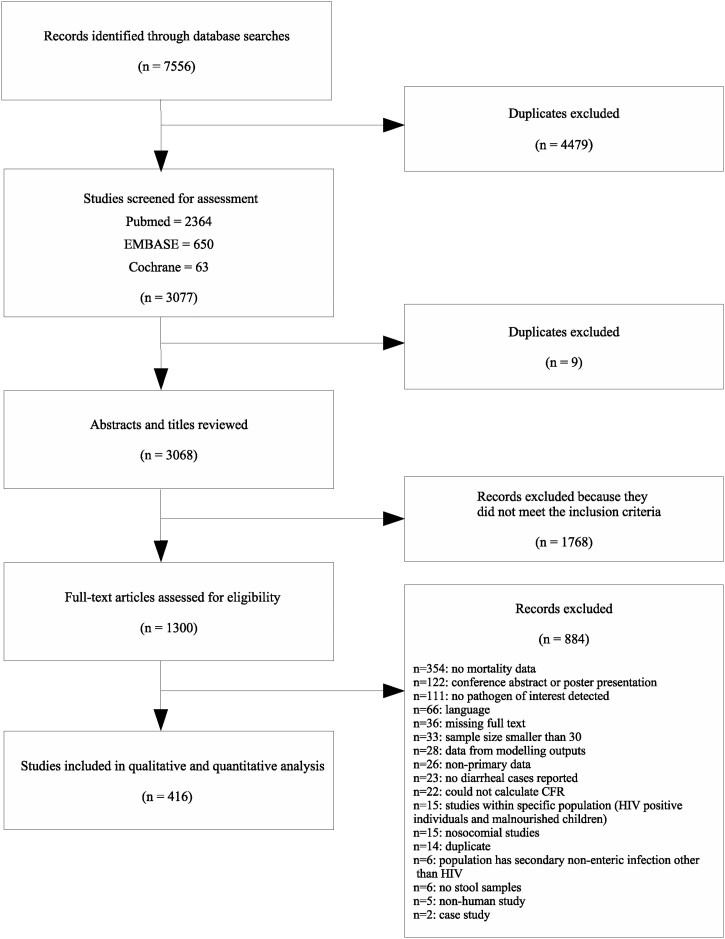
A total of 7556 studies were identified based on the search strategy, of which 416 studies met the criteria and were included in the analysis. Figure 1 shows the flow diagram of the study selection process. The PRISMA checklist is provided in Supplementary Appendix 2 (available as Supplementary data at IJE online). We identified more than twice as many studies for rotavirus (n = 239) compared with all other pathogens (Supplementary Figure S1, available as Supplementary data at IJE online); the fewest number of studies were identified for sapovirus (n = 7) and ‘other’ pathogens (n = 5). There were a considerable number (n = 96) of multi-pathogen studies included in the final analysis. Studies from 108 countries were identified (Supplementary Figure S2, available as Supplementary data at IJE online). There is a clear disparity in the study distribution, with 10% of studies conducted in India, and only seven countries account for more than a third of the studies.
Figure 1.
Flowchart of the study selection process. In total, there were 901 individual observations for the 416 studies; some studies had multiple observations for different pathogens and/or age categories. There was substantial variation in the number of studies for each predictor category (Supplementary Figure S3, available as Supplementary data at IJE online).
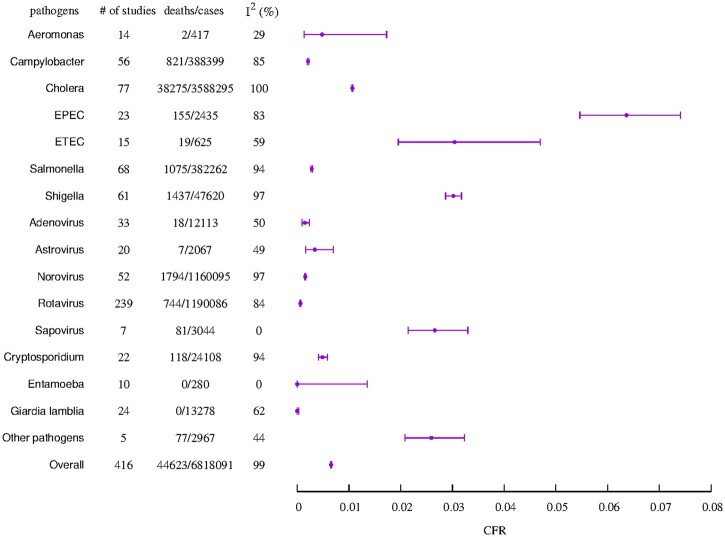
The crude CFR for all diarrhoeal pathogens was 0.65% (95% CI, 0.65–0.66%) with an I2 value of 99% indicating high heterogeneity across studies. The pathogen-specific CFR showed substantial variation, ranging between 0% for G. lamblia (95% CI: 0–0.03%) and E. histolytica (95% CI: 0–1.35%) and 6.4% (95% CI: 5.46–7.41%) for EPEC, and I2 values ranging from 0% to 100% (Figure 2). The pathogen-specific crude CFRs from case-only GEMS data are provided in Supplementary Table S4 (available as Supplementary data at IJE online). When the included studies were restricted to hospital-based studies among children <5 years old, the high heterogeneity remained (I2 = 92%), with a wide variation across pathogens (I2 = 0–99%) (Supplementary Table S5, available as Supplementary data at IJE online). Generally, the estimated CFRs for the bacterial pathogens were higher compared with those for both viral and parasitic pathogens [bacterial: 0.95% (95% CI: 0.94–0.96%); parasitic: 0.33% (95% CI: 0.27–0.39%) and viral: 0.11% (95% CI: 0.11–0.12%)]. Summary estimates of the crude CFR and corresponding binomial 95% CIs are shown in Figure 2, whereas estimates of the CFR from the fixed-effects and random-effects models are presented in Table 2. Most studies reported zero deaths (Supplementary Materials, Section 8, available as Supplementary data at IJE online); hence, the estimated CFR from the random-effects models tended to be lower than those from the fixed-effect models (Table 2). The wide 95% prediction intervals from the random-effects models indicate that the estimated CFR is associated with a high degree of uncertainty, particularly for pathogens with fewer studies (Table 2).
Figure 2.
Summary of crude case fatality risk estimate and associated binomial confidence intervals for each pathogen and overall. The total number of studies, cases and deaths for each pathogen is indicated, along with the pathogen-specific and overall I2 measure of heterogeneity. The ‘other pathogens’ include Shiga toxin-producing Escherichia coli, enteroaggregative E. coli and E. coli (type not specified).
Table 2.
Estimated case fatality risk (CFR) (deaths per 1000 cases) from fixed-effects and random-effects binomial-normal models for each pathogen and overall. For the random-effect models, we included a random effect for each study rather than each observation. Effect estimates from the sensitivity analysis including Global Enteric Multicenter Study (GEMS) data are also presented
| Pathogen | Fixed-effects model estimated CFR (deaths per 1000 cases) (95% CI) | Random-effects model estimated CFR (deaths per 1000 cases) (95% CI) | Random-effects model estimated 95% prediction interval (deaths per 1000 cases) |
|---|---|---|---|
| Aeromonas | 4.8 (1.05, 21.56) | 0 (0, 1000) | (0, 1000) |
| Campylobacter | 2.11 (1.97, 2.27) | 0.01 (0, 1.96) | (0, 427.11) |
| Cholera | 10.67 (10.56, 0.77) | 11.41 (8.47, 15.35) | (1.12, 106.27) |
| EPEC | 63.66 (54.21, 74.61) | 0.347 (0, 67.99) | (0, 940.32) |
| ETEC | 30.4 (18.77, 48.87) | 3.51 (0.14, 80.82) | (0, 815.14) |
| Salmonella | 2.81 (2.65, 2.99) | 0.4 (0.07, 2.23) | (0, 202.66) |
| Shigella | 30.18 (28.66, 31.77) | 2.03 (0.69, 5.92) | (0, 467.87) |
| Adenovirus | 1.49 (0.92, 2.34) | 0.54 (0.03, 8.99) | (0, 68.49) |
| Astrovirus | 3.39 (1.54, 7.41) | 0 (0, 1000) | (0, 1000) |
| Norovirus | 1.55 (1.48, 1.62) | 0.25 (0.03, 1.95) | (0, 108.66) |
| Rotavirus | 0.63 (0.58, 0.67) | 0.1 (0.043, 0.22) | (0, 35.06) |
| Sapovirus | 26.61 (20.33, 34.76) | 26.61 (20.33, 34.76) | (20.33, 34.76) |
| Cryptosporidium | 4.89 (4.05, 5.91) | 1 (1.02, 1.029) | (0, 750.19) |
| Entamoeba | 0 (0, 1000) | – | – |
| Giardia lamblia | 0 (0, 1000) | – | – |
| Other pathogens | 26 (19, 35) | – | – |
| Overall (all diarrheal pathogens) | 6.54 (6.48, 6.61) | 0.4 (0.26, 0.62) | (0, 175.37) |
| Overall (all diarrheal pathogens) including GEMS data | 6.59 (6.53, 6.65) | 0.74 (0.52, 1.05) | (0, 186.15) |
EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli.
The study-specific funnel plots were asymmetrical for some pathogens and overall (Supplementary Materials, Section 7, available as Supplementary data at IJE online), indicating potential small-studies effects. The regression intercepts and P-values obtained from Egger's tests are provided in Supplementary Table S6 (available as Supplementary data at IJE online). When we excluded studies with zero deaths, only rotavirus indicated a potential for small-studies effects.
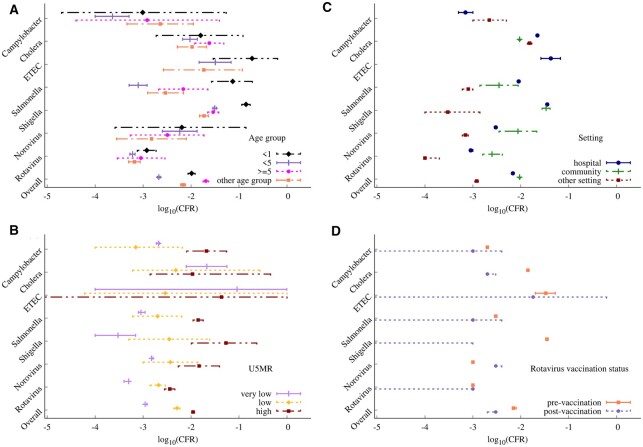
Pathogen-specific fixed-effects models showed a high variability in the CFR across the different predictors for some of the important pathogens (Figure 3); variability in the CFR across the predictors was qualitatively similar for the random-effects models (Supplementary Figure S4, available as Supplementary data at IJE online). A higher CFR was estimated among infants <1 year of age for Salmonella, Shigella and ETEC, whereas a slightly higher CFR for those aged ≥5 years was estimated for cholera and Campylobacter (Figure 3A). The CFR was lowest in the very low U5MR strata and highest in the high U5MR strata for all pathogens except cholera and ETEC (Figure 3B). There was no clear pattern in the estimated CFR for different study settings across pathogens (Figure 3C), although a slightly higher CFR was estimated for community-based studies for the viral pathogens (rotavirus and norovirus) and for hospital-based studies for the bacterial pathogens (cholera, Salmonella and ETEC). The estimated CFR was lower after rotavirus vaccine introduction for all pathogens except norovirus (Figure 3D), although this mostly reflected a decline in the CFR over time rather than the specific impact of rotavirus vaccine introduction (Supplementary Figure S5, available as Supplementary data at IJE online). Results for pathogen-specific multilevel mixed-effects models (across the six pathogens with >50 studies) showed a similar influence for the various predictors (Supplementary Table S7, available as Supplementary data at IJE online).
Figure 3.
Fixed-effect model estimates of case fatality risk stratified by the predictors of interest overall and for seven select pathogens. The predictors include (a) age group, (b) country-specific under-5 mortality rate (U5MR), (c) study setting and (d) country rotavirus vaccine introduction status. The different line types represent the strata within each predictor. Estimates are plotted on the log10 scale for visualization purposes. Random-effect estimates are provided in Supplementary Figure S4 (available as Supplementary data at IJE online).
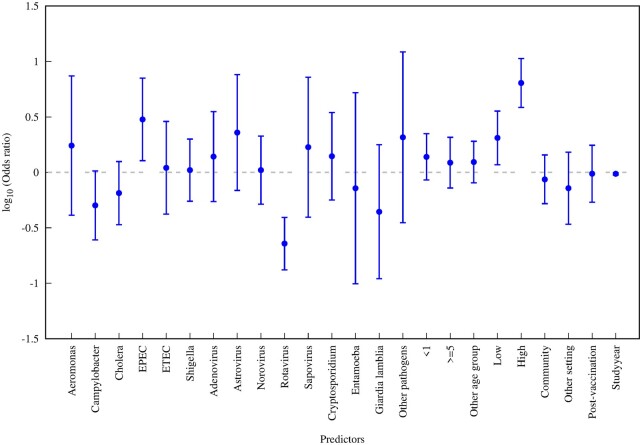
The results from the overall multilevel mixed-effects model are shown in Figure 4. Compared with Salmonella (reference pathogen), the CFR was estimated to be substantially higher for EPEC and substantially lower for rotavirus. The estimated CFR was slightly higher among infants <1 year of age and slightly lower among children and adults ≥5 years old. Our model revealed a strong gradient across U5MR strata, with the estimated CFR for the low and high categories about 2-fold and 6-fold higher, respectively, compared with the very low (reference) category. The estimated CFR from community-based and other studies was slightly lower than hospital-based studies. The estimated CFR for studies carried out after rotavirus vaccine introduction was slightly lower than pre-vaccination, but most of the decline in the CFR was associated with study year. By study decade relative to 1978–1989 (reference), the OR was 0.40 (95% CI: 0.17–0.94) for 1990–1999, 0.15 (95% CI: 0.07–0.33) for 2000–2009 and 0.26 (95% CI: 0.11–0.60) for 2010–2019.
Figure 4.
Odds ratios for overall multilevel mixed-effects logistic regression model. The reference explanatory categorical variable was Salmonella (pathogen), under-5 (age group); very low (U5MR strata), hospital (setting) and pre-vaccination (rotavirus vaccine introduction status). U5MR refers to country-specific under-5 mortality rate.
Compared with the main analysis, the sensitivity analysis including the GEMS data revealed a comparable overall effect size (Table 2) and similar trends in the associations across the predictors (Supplementary Figure 6, available as Supplementary data at IJE online). Also, similar associations were seen in the subgroup analysis limited to hospital-based studies of children <5 years old (Supplementary Figure 7, available as Supplementary data at IJE online). When HIV prevalence was included as an additional predictor in the model, the associations were again similar, but the CFR was estimated to be ∼4-fold higher in high HIV prevalence settings (Supplementary Figure 8, available as Supplementary data at IJE online).
Overall, 45.2% of studies were classified as having a low risk of bias for all the criteria assessed, whereas 21.0% of studies were high risk and 33.8% of studies had an unclear risk. The major source of risk was measurement bias (case definition: 32.7% and whether standardized laboratory test was used: 40.6%) (Figure 5). Most of the studies (85.6%) were classified as unclear risk of attrition bias since the maximum follow-up time was not specified (Figure 5). The risk of bias for individual studies is provided in Supplementary Appendix 3 (available as Supplementary data at IJE online).
Figure 5.
Summary of the risk of bias assessment for all included studies. Unfilled: high risk, lightly shaded: low risk and heavily shaded: unclear. Standardized lab test refers to whether studies employed standard laboratory tests.
Discussion
Our analyses highlight the extent to which estimated diarrhoea-specific CFRs vary across pathogens. Overall, the CFR was <1%, but there were considerable differences in the estimated CFR across pathogens and, in addition, considerable heterogeneity between studies that could not be fully explained by any of the covariates we examined, including age group, country U5MR, study setting (hospital-based or community-based), rotavirus vaccine introduction status and study year. The pathogen-specific and overall models showed differences in the strength and direction of associations between CFRs and the explanatory variables used in the regression analyses. By comprehensively examining associations across all (major) diarrhoeal pathogens, we are able to compare and contrast predictors of the CFR for different pathogens, unlike studies that only focus on a single pathogen.
Our results should be interpreted with caution when attempting to rank diarrhoeal pathogens by the estimated CFR due to disparities in the number of studies for each pathogen (ranging from 5 to 239) and the potential for small-studies effects. For example, although the highest CFR was estimated for EPEC, there was considerable heterogeneity (I2 = 83%) among the 23 studies that contributed to this estimate. Most studies with non-zero deaths were conducted in higher mortality settings (e.g. for EPEC, three out of the five studies with non-zero deaths were conducted in high U5MR settings) or conducted only among children <5 years of age (e.g. for EPEC, four out of the five studies with non-zero deaths were conducted among children <5 years of age). Studies conducted in these settings are usually associated with higher CFRs.6,17,18 Second, pathogens represented by a smaller number of studies may be at higher risk of small-studies bias (i.e. studies reporting a higher number of deaths may be more likely to be published), such that the high overall estimated CFR may be due to a few isolated studies with high CFR (e.g. EPEC had five studies with non-zero deaths out of the 23 studies). Nevertheless, for multi-pathogen studies involving EPEC and other diarrhoeal pathogens with a non-zero CFR, the estimated CFR for EPEC was either first or second highest.19–21 Pathogens with low estimated CFRs also had fewer studies, e.g. G. lamblia (24) and E. histolytica (10). However, most of these studies were conducted in very low U5MR countries (G. lamblia: 75% and E. histolytica: 50%) and were not limited to children <5 years old (e.g. 88% of G. lamblia and 67% of E. histolytica observations were for the ‘other’ age category, which typically included individuals of all ages or only adults). More studies are required for pathogens such as EPEC, ETEC and sapovirus, for which a limited number of studies suggest a higher CFR, to better quantify their relative contribution to overall diarrhoea deaths.
The estimated CFR for rotavirus was substantially lower than that of other diarrhoeal pathogens [odds ratio (OR) = 0.23, P < 0.0001 compared with Salmonella]. This may in part be driven by the large number of studies for rotavirus, including many from countries with a very low U5MR (46%), and 82% of observations with zero deaths. It may also be because rotavirus diarrhoea can be effectively treated with oral rehydration solution (ORS),22,23 as indicated by the lower CFR among hospital-based studies compared with community-based studies for rotavirus. Substantial declines in diarrhoeal mortality have been observed following the introduction of rotavirus vaccines in numerous countries, which suggests that rotavirus is a substantial contributor to diarrhoeal deaths.24–26 Many of these deaths may occur among infants and children with poor access to healthcare facilities, which could lead to underestimation of the CFR for rotavirus in typical study populations.
The widely available predictors we examined could explain some but not all of the variability in the estimated CFR across pathogens. The CFR was consistently higher in studies conducted in high U5MR countries, which could indicate poorer access to healthcare in these settings. The CFR was slightly higher for hospital-based studies relative to community-based studies overall. However, the CFR was higher in community-based studies for viral pathogens (rotavirus and norovirus), which may reflect their acute presentation, such that most deaths occur among individuals whose access to healthcare is limited or delayed. The overall CFR also tended to be slightly higher among infants <1 year old. This is consistent with a previous systematic review27 and may result from infants having a less fully developed immune systems or being more prone to dehydration. In contrast, the results for cholera showed a slightly higher CFR among those aged ≥5 years. Most of these studies were conducted in cholera-endemic settings,28–30 indicating that cholera mortality occurs among older age groups in endemic regions. However, this could be due to differences in cholera case definitions by age.31 A similar high CFR has been found among adults >60 years old for Shigella6 and all-cause diarrhoea.32
Our results indicate that the CFR from diarrhoea has decreased over time. This may be partly due to improvements in water, sanitation and hygiene (WASH) infrastructure and socio-economic development (leading to reductions in childhood malnutrition) leading to a reduction in the severity of diarrhoeal episodes,33–35 as well as the increased use of ORS and zinc for treatment of diarrhoea.7,36,37 In addition, improvements in healthcare quality and access over time could have contributed to reductions in the CFR.38 Further reductions in the CFR of diarrhoea overall and for non-rotavirus pathogens also occurred following the introduction of rotavirus vaccination, suggesting that preventing rotavirus gastroenteritis may have added benefits of decreasing vulnerability to severe disease caused by other diarrhoeal pathogens, although most reductions were modest. However, our estimates of the impact of rotavirus vaccine introduction may be biased due to misclassification. We assumed all studies carried out after rotavirus vaccine introduction in the country were post rotavirus vaccine introduction, but some of these studies may have been carried out within unvaccinated populations (e.g. older age groups).
When we included GEMS data in a sensitivity analysis, the crude CFR was similar (systematic review data: 0.65%; with GEMS data: 0.66%) and the overall trends remained similar across the different predictors, suggesting that adding more data may not substantially change the strength of the associations. The crude estimated CFR is within the range of the crude CFR estimates from both GEMS and GEMS1A case-only data.39 When we limited the analysis to hospital-based studies of children <5 years old, the results were also similar: the highest and lowest odds ratios were found for EPEC and rotavirus, respectively. However, the estimated CFRs were slightly higher in the hospital-based subgroup compared with the overall model, which likely reflects the greater severity of hospitalized cases and higher risk of diarrhoea mortality among children.
The risk of bias assessment revealed an overall low risk of bias for nearly half of all included studies. However, a potential limitation in our analysis is that most of the studies did not indicate follow-up time, and there was a wide disparity in the follow-up time (between 1 day and 15 months) for studies that did, leading to possible attrition bias. Studies with shorter follow-up time may miss a significant number of deaths, leading to underestimation of the CFR. Nearly half of deaths in the GEMS study occurred >14 days after enrolment.39 Furthermore, studies may be likely to miss deaths that occur outside of health facilities (e.g. in the home). Variation in pathogen detection methods and improvements in the sensitivity over time can also present additional biases in the analysis. This underscores the value of studies such as the Child Health and Mortality Prevention Surveillance (CHAMPS) network, which seek to characterize the multiple causes of deaths occurring in communities through minimally invasive tissue samples, advanced diagnostics and verbal autopsies.40
Model-based estimates of mortality caused by different diarrhoeal pathogens assume that deaths caused by each pathogen are proportional to the distribution of pathogens among severe and/or hospitalized cases.3–7 Thus, the models implicitly assume that the CFR is the same for all diarrhoeal pathogens after accounting for severity leading to hospitalization. Our analysis suggests that there may be additional variability in the CFR for different diarrhoeal pathogens. Global burden models could account for differences in the risk of death among severe cases by multiplying pathogen prevalence among hospitalized or severe cases by estimates of the OR for the risk of death from each pathogen based on this study, which may help to improve estimates of global mortality caused by different pathogens.
To our knowledge, this is the first study to estimate the CFR across multiple diarrhoeal pathogens. Our estimated overall CFR of 0.65% is substantial, suggesting the need to scale up and sustain ongoing control strategies. In addition, our results highlight the existence of marked heterogeneity in the estimated CFR both within and between studies and across pathogens. Our results not only provide an updated and comprehensive estimate of the CFR across different diarrhoeal pathogens, but also highlight pathogens for which more studies are needed, particularly those with fewer studies and a higher CFR (including EPEC, ETEC and sapovirus). Our results could also help prioritize vaccine development and pathogen-specific interventions. Developing vaccines for important diarrhoeal pathogens, as well as WASH and nutritional interventions, will help to reduce the overall diarrhoea burden and may have benefits that extend beyond reductions in mortality.
Ethics approval
Not applicable for this paper.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
V.E.P., B.A.L., J.L., M.H. and B.G. conceived of and supervised the study. J.L., D.H., J.S., B.M., H.A., A.B., A.B.W. and M.D. developed the protocol and performed the systematic review. E.O.A. and V.E.P. performed the statistical analyses. B.A.L., M.H. and B.G. provided feedback on the analyses. E.O.A. wrote the manuscript. All authors edited the manuscript. All authors read, reviewed and approved the final manuscript. The opinions expressed herein do not necessarily reflect the decisions of the World Health Organization.
Funding
This work was supported by funding from the World Health Organization (Contract 2020/993515–0 to V.E.P.) and the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01AI112970 to V.E.P.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of Andre Peralta-Santos and Catherine Troja in developing the approach used in the systematic review.
Conflict of interest
B.A.L. reports personal fees and grant funding from Takeda Pharmaceuticals outside of the submitted work. V.E.P. reports reimbursement from Merck and Pfizer for travel to Scientific Input Engagements unrelated to the current work and V.E.P. is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee (IVIR-AC).
Contributor Information
Ernest O Asare, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Yale University, New Haven, CT, USA.
Dianna Hergott, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Jessica Seiler, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Brooks Morgan, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Helena Archer, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Alison B Wiyeh, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Boya Guo, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Matt Driver, Department of Epidemiology, University of Washington, Seattle, WA, USA.
Birgitte Giersing, Vaccine Product Delivery Research, Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Mateusz Hasso-Agopsowicz, Vaccine Product Delivery Research, Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Jairam Lingappa, Departments of Global Health, Medicine, and Pediatrics, University of Washington, Seattle, WA, USA.
Benjamin A Lopman, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Virginia E Pitzer, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Yale University, New Haven, CT, USA.
References
- 1. Black R, Fontaine O, Lamberti L et al. Drivers of the reduction in childhood diarrhea mortality 1980-2015 and interventions to eliminate preventable diarrhea deaths by 2030. J Glob Health 2019;9:020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang H, Naghavi M, Allen C et al. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ 2008;86:710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker CF, Black RE. Diarrhoea morbidity and mortality in older children, adolescents, and adults. Epidemiol Infect 2010;138:1215–26. [DOI] [PubMed] [Google Scholar]
- 5. Walker CLF, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PLoS One 2012;7:e29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Troeger C, Blacker BF, Khalil IA et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018;18:1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reiner RC, Wiens KE, Deshpande A et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000-17: analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:1779–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanata CF, Fischer-Walker CL, Olascoaga AC et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013;8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalil IA, Troeger C, Blacker BF et al. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect Dis 2018;18:1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prudden HJ, Hasso-Agopsowicz M, Black RE et al. Meeting Report: WHO Workshop on modelling global mortality and aetiology estimates of enteric pathogens in children under five. Cape Town, 28-29th November 2018. Vaccine 2020;38:4792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotloff KL, Nataro JP, Blackwelder WC et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013;382:209–22. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Altman DG, Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. New York: John Wiley & Sons, 2008. [Google Scholar]
- 13. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. J R Stat Soc A 2018;181:205–27. [Google Scholar]
- 15. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 17. Miller AC, Ramananjato RH, Garchitorena A et al. Baseline population health conditions ahead of a health system strengthening program in rural Madagascar. Glob Health Action 2017;10:1329961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garchitorena A, Miller AC, Cordier LF et al. Early changes in intervention coverage and mortality rates following the implementation of an integrated health system intervention in Madagascar. BMJ Glob Health 2018;3:e000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dutta P, Mitra U, Rasaily R et al. Assessing the cause of in-patients pediatric diarrheal deaths: an analysis of hospital records. Indian Pediatr 1995;32:313–21. [PubMed] [Google Scholar]
- 20. Fagundes-Neto U, Andrade JAB. Acute diarrhea and malnutrition: lethality risk in hospitalized infants. J Am Coll Nutr 1999;18:303–308. [DOI] [PubMed] [Google Scholar]
- 21. O'Reilly CE, Jaron P, Ochieng B et al. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005-2007: a cohort study. PLoS Med 2012;9:e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nappert G, Barrios JM, Zello GA, Naylor JM. Oral rehydration solution therapy in the management of children with rotavirus diarrhea. Nutr Rev 2000;58:80–87. [DOI] [PubMed] [Google Scholar]
- 23. Kauna R, Sobi K, Pameh W, Vince JD, Duke T. Oral rehydration in children with acute diarrhoea and moderate dehydration-effectiveness of an ORS tolerance test. J Trop Pediatr 2019;65:583–91. [DOI] [PubMed] [Google Scholar]
- 24. Bar-Zeev N, King C, Phiri T, VacSurv Consortium et al. Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study. Lancet Glob Health 2018;6:e1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richardson V, Hernandez-Pichardo J, Quintanar-Solares M et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010;362:299–305. [DOI] [PubMed] [Google Scholar]
- 26. Costa I, Linhares AC, Cunha MH et al. Sustained decrease in gastroenteritis-related deaths and hospitalizations in children less than 5 years of age after the introduction of rotavirus vaccination: a time-trend analysis in Brazil (2001). Pediatr Infect Dis J 2016;35:e180–90. [DOI] [PubMed] [Google Scholar]
- 27. Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Abbassi A, Ahmed S, Al-Hadithi T. Cholera epidemic in Baghdad during 1999: clinical and bacteriological profile of hospitalized cases. East Mediterr Health J 2005;11:613. [PubMed] [Google Scholar]
- 29. Lenglet A, Khamphaphongphane B, Thebvongsa P et al. A cholera epidemic in Sekong Province, Lao People's Democratic Republic, December 2007-January 2008. Jpn J Infect Dis 2010;63:204–07. [PubMed] [Google Scholar]
- 30. Tripurari K, Deepak B, Aakash S, Prakash NJ. Vibrio cholerae outbreak in Batala Town, Punjab, India 2012. J Commun Dis 2017;49:35–40. [Google Scholar]
- 31. World Health Organization (WHO) Global Task Force on Cholera Control. Cholera Outbreak: Assessing the Outbreak Response and Improving Preparedness. Geneva: World Health Organization, 2004. [Google Scholar]
- 32. Lew JF, Glass RI, Gangarosa RE, Cohen IP, Bern C, Moe CL. Diarrheal deaths in the United States, 1979 through 1987: a special problem for the elderly. JAMA 1991;265:3280–84. [PubMed] [Google Scholar]
- 33. Ferdous F, Das SK, Ahmed S et al. Severity of diarrhea and malnutrition among under five-year-old children in rural Bangladesh. Am J Trop Med Hyg 2013;89:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rice AL, Sacco L, Hyder A, Black RE. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ 2000;78:1207–21. [PMC free article] [PubMed] [Google Scholar]
- 35. Jahan Y, Moriyama M, Hossain S et al. Relation of childhood diarrheal morbidity with the type of tube well used and associated factors of Shigella sonnei diarrhea in rural Bangladesh site of the Global Enteric Multicenter Study. Trop Med Health 2019;47:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Victora CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ 2000;78:1246–55. [PMC free article] [PubMed] [Google Scholar]
- 37. Schroder K, Battu A, Wentworth L et al. Increasing coverage of pediatric diarrhea treatment in high-burden countries. J Glob Health 2019;9:0010503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvajal-Vélez L, Amouzou A, Perin J et al. Diarrhea management in children under five in sub-Saharan Africa: does the source of care matter? A countdown analysis. BMC Public Health 2006;16:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levine MM, Nasrin D, Acácio S et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Global Health 2020;8:e204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Breiman RF, Blau DM, Mutevedzi P et al. ; the CHAMPS Consortium. Postmortem investigations and identification of multiple causes of child deaths: an analysis of findings from the Child Health and Mortality Prevention Surveillance (CHAMPS) network. PLoS Med 2021;18:e1003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.