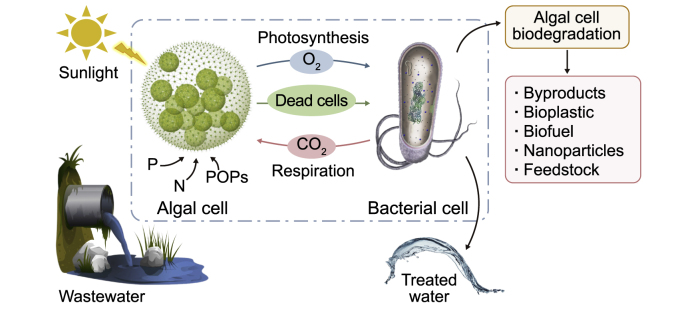
Abstract
The rapid expansion of both the global economy and the human population has led to a shortage of water resources suitable for direct human consumption. As a result, water remediation will inexorably become the primary focus on a global scale. Microalgae can be grown in various types of wastewaters (WW). They have a high potential to remove contaminants from the effluents of industries and urban areas. This review focuses on recent advances on WW remediation through microalgae cultivation. Attention has already been paid to microalgae-based wastewater treatment (WWT) due to its low energy requirements, the strong ability of microalgae to thrive under diverse environmental conditions, and the potential to transform WW nutrients into high-value compounds. It turned out that microalgae-based WWT is an economical and sustainable solution. Moreover, different types of toxins are removed by microalgae through biosorption, bioaccumulation, and biodegradation processes. Examples are toxins from agricultural runoffs and textile and pharmaceutical industrial effluents. Microalgae have the potential to mitigate carbon dioxide and make use of the micronutrients that are present in the effluents. This review paper highlights the application of microalgae in WW remediation and the remediation of diverse types of pollutants commonly present in WW through different mechanisms, simultaneous resource recovery, and efficient microalgae-based co-culturing systems along with bottlenecks and prospects.
Keywords: Bioremediation, Wastewater treatment, Environmental applications, Microalgae, Co-culturing
Graphical abstract
Highlights
-
•
1. Conventional methods for wastewater treatment (WWT) are highly complex and expensive.
-
•
2. Microalgae cultivation is a sustainable and cost-effective approach for WWT.
-
•
Microalgae have a great capacity to eradicate pollutants via several mechanisms.
-
•
Co-culturing of microalgae with other microorganisms enhances WWT.
1. Introduction
Water is an essential resource and a pivotal feedstock in numerous industries, including pharmaceuticals, electronics, food, beverage, petrochemicals, agrochemicals, oil and gas industries, and domestic purposes [1]. The direct disposal of the contaminated water discharged from these applications poses high risks to the environment and is an increasing concern due to its myriad contaminants. Wastewater (WW) contains various compounds, some of them at concentrations that are highly toxic to organisms. In addition, significant amounts of inorganic and organic nutrients are released into the environment, which is reflected by high chemical oxygen demand (COD) and biological oxygen demand (BOD). Eutrophication of aquatic environments caused by excessive loadings of phosphorus (P) and nitrogen (N) results in environmental concerns such as the generation of solid wastes and unpleasant emissions to the air [2,3]. It also promotes the growth of unwanted microbes that threaten other aquatic life and deteriorate the quality of drinking water, which contributes to common health-related issues in regions neighboring the discharge area [4]. Heavy metals (HMs) are the most widespread class of pollutants in WW. Direct inhalation, ingestion, and contact of these toxic substances, even at very low concentrations, can cause significant threats to human health and escalate the risk of developing cancers [5,6]. In recent years, there have been growing concerns about the occurrence of so-called emerging pollutants in waterbodies, their related hazards and impacts on both human health and aquatic life. Emerging pollutants are synthetic organic compounds that, despite having been present in the environment for a long time, have just recently been recognized due to the growing knowledge of their dangers and advances in analytical procedures (i.e., lower detection limits) [[8], [9], [10]]. These emerging pollutants include various categories, including per- and polyfluoroalkyl substances (PFASs), flame retardants, pharmaceutical compounds (PCs), illicit drugs, pesticides, and artificial sweeteners [4,7]. The detrimental effects on human health and the environment are not yet completely understood [[11], [12], [13]].
When water is contaminated and purification becomes indispensable, selecting the best treatment strategy is necessary to achieve the desired purification objectives [14,15]. Conventional methods include different physical and chemical treatments [[16], [17], [18]]. One of the most efficient WW treatments (WWTs) is a chemical treatment aiming to alter the water characteristics, such as decreasing the turbidity (removal of suspended solids), pH adjustment, and eliminating dissolved materials in water, thereby improving water quality. The predominant approaches in chemical treatment include chlorination, coagulation/flocculation, chloramination, ultraviolet light, and ozonation [14,19]. Although these methods are more effective when used in secondary WWT, high costs, dewatering limitations, and the high efforts needed for maintenance are deemed the major shortcomings of these technologies [14,20]. In contrast, biological approaches depend on the metabolic activities of microorganisms to decompose and convert pollutants of WW to biomass and associated gases (CO2, CH4, N2, and SO2), thereby decreasing the values of BOD and COD in the effluents and improving their quality [21]. Biological treatments involve biodegradation bleaching using different microorganisms, including bacteria, fungi, yeast, and microalgae [[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Biological treatments aim to construct a system that facilitates the treatment of disposed WW based on the decomposition yield potentials. Biological WWTs appear simple at first glance as they utilize natural processes, but they are complex and still not completely understood at the intersection of biology and biochemistry [31]. Almost all biological procedures are low-cost treatments under aerobic or anaerobic conditions. However, there are several major restrictions associated with biological methods: (i) the need for a large land area and significant time for adequate treatment; (ii) the imperfect removal of pollutants; (iii) pollutants with complex chemical structures are recalcitrant toward degradation using conventional processes; and (iv) biological WWTs provide only limited flexibility in terms of design and operation long term [32]. Generally, biological treatments are used as a secondary treatment process to mainly eliminate substances remaining after primary treatment [14]. Primary treatment is primarily the sedimentation of solid wastes.
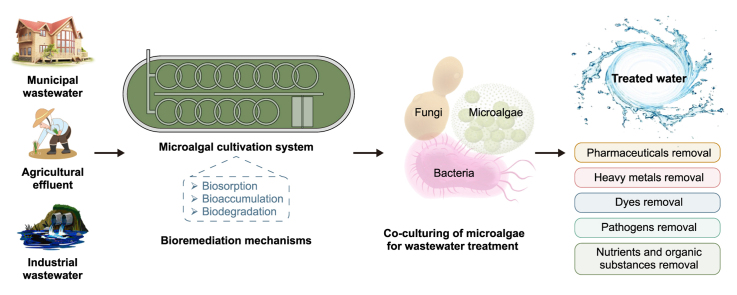
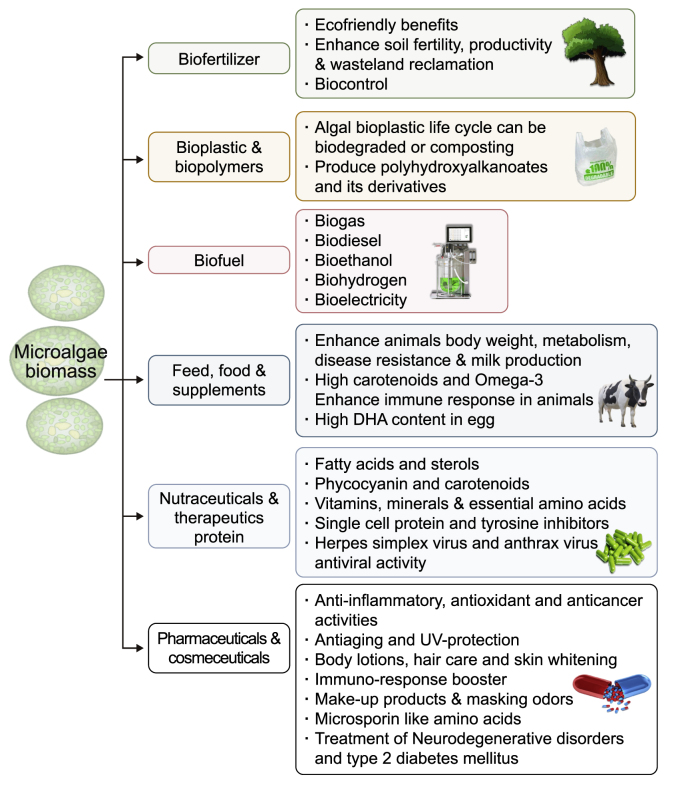
Numerous microalgal species, including Scenedesmus, Chlorella, Botryococcus, Phormidium, Limnospira (formerly Arthrospira, Spirulina), and Chlamydomonas, have been confirmed to have an excellent capability for the bioremediation of nutrients, HMs, emerging pollutants and pathogens from WW [33,34]. Several microalgae species, such as Scenedesmus, Chlorella, Euglena, Oscillatoria, Chlamydomonas, and Ankistrodesmus, have grown efficiently in WW [35]. Moreover, they exhibit great tolerance against WW toxins. Thus, microalgae-based techniques have recently gained significant attention for treating municipal, industrial, agro-industrial, and livestock WW. Microalgae can minimize the risk of eutrophication by removing P and N components [36]. These are considered multifunctional alternatives for biological treatment, transforming undesirable inorganic and organic ingredients into valued biomass [12]. Furthermore, microalgae collected from certain treatment ponds can be used as a source of food and feedstock for multiple products and also play multifarious roles in diverse industries, as shown in Fig. 1. Another promising strategy is co-culturing of microalgae with other microbes to enhance WWT efficiency. In a co-culturing approach, microalgae cooperate symbiotically with heterotrophic microorganisms such as yeast, bacteria, and fungi, resulting in the exchange of nutrients and metabolites, which, in turn, increase algae biomass yield and enhance bioremediation [37,38]. The production of biofuels and biomass from microalgae has been boosted through co-culturing techniques. These techniques involve the utilization of numerous microbial interactions, which are accomplished by cultivating multiple strains of different species, with the intention that one strain will compensate for an enzymatic activity absent in the other strains [39]. Therefore, they develop an artificial conglomerate based on a mutually beneficial relationship to satisfy the prerequisites that must be met.
Fig. 1.
Biorefinery of microalgal biomass after wastewater treatment for biofuel, biofertilizer, and high-value products.
Global water shortages will be one of the greatest social and economic challenges of the 21st century. Microalgae-based WWT could recover nutrients while producing clean water. Microalgae help dispose and treat industrial, agricultural, and municipal WWs. Microalgae strains can withstand the harsh environments of modern industrial and municipal wastes. The shift towards a circular bioeconomy that relies on resource diversification has also prompted the reorganization of traditional WWT processes into a low-carbon, integrated biorefinery model that can accommodate multiple waste streams. The rapid rise in interest in microalgal WW remediation can be explained by the changes in how the treatment is viewed. Therefore, microalgae-based WWT is now a serious competitor to conventional WWT since the major bottlenecks of nutrient assimilation and high microalgae population have been partially mitigated. This review paper aims to collate advances and new knowledge emerged in recent years for microalgae-based WWT.
2. Microalgae and their marvels
Microalgae show exceptional photosynthetic efficiency compared to terrestrial plants, resulting in higher growth rates and biomass productivities, sometimes ten times higher than vascular plants [40]. Microalgae can be farmed without competing for arable land resources, producing around 50% of the atmospheric oxygen. Microalgae are an almost untapped resource, with a species number estimated between 30,000 to more than 1,000,000, but only 15 can be commercially cultivated at a large scale [41]. In recent years, microalgae have been used to detoxify various toxicants with different properties and characteristics released from the agricultural, industrial, and domestic sectors. To date, a wide range of microalgae has been reported to treat domestic WW, such as Scenedesmus abundans, Botryococcus sp., Chlorella variabilis, Chlorella sp., Scenedesmus sp., Scenedesmus obliquus, Chlorella vulgaris, and Chlorella sorokiniana, as depicted in Table 1 [[42], [43], [44], [45], [46], [47], [48]].
Table 1.
Biomass production of microalgae grown in wastewaters.
| Microalgae | Waste source | Biomass production | Reference |
|---|---|---|---|
| Chlorella variabilis | Domestic wastewater | 1.72 g L−1 | [42] |
| Scenedesmus abundans | Domestic wastewater | 3.55 g L−1 | [43] |
| Scenedesmus obliquus | Municipal wastewater | 0.22 g L−1 d−1 | [44] |
| Chlorella sp. | Domestic wastewater | 0.73–1.38 mg L−1 d−1 | [45] |
| Scenedesmus sp. | Municipal wastewater | 1.81 g L−1 | [46] |
| Scenedesmus sp. | Municipal wastewater | 1.1 g L−1 | [47] |
| Chlorella sorokiniana | Municipal wastewater | 1 g L−1 | [48] |
Microalgae-based systems have superior capacities to bioremediate WW, and they have the ability to remove 45–65% of BOD and COD [49,50]. Microalgae-based WWT systems present natural disinfection abilities and are more effective for reducing nutrient contamination than conventional WWT methods, which have shortcomings such as high operational costs and unavoidable secondary pollution from chemical processes [35]. On the contrary, microalgae are the most promising decontaminating agents for numerous pollutants as they have high surface-to-volume ratios, thus, high biosorption capacities. They can remove noxious constituents, and many of them can shift between photoautotrophic, mixotrophic, and heterotrophic growth [51].
Most microalgae are photoautotrophs, so they need less energy for growth. In addition, microalgae guarantee cost-effective remediation of pollutants when integrated with other WWT techniques. In general, microalgae have immense adaptability to grow and thrive in harsh environments, rendering them efficient and multifunctional candidates for enhanced WWT [52]. The phytoremediation process is an environmentally friendly mechanism that also extracts components that can be applied for many purposes. The mechanism involves the removal of nutrients from WW and the sequestration of CO2 for biomass increase and synthesis of bioproducts. Nitrogen in sewage wastes results from metabolic interconversions of additional derived compounds, whereas 50% of P originates from synthetic detergents. However, significant amounts of inorganic N and P after primary and secondary treatment may remain, leading to significant eutrophication, reflected in harmful microalgal blooms and/or massive growth of macrophytes [53].
Microalgae assimilate carbon (C), N, P, and micronutrients (HMs), which are then transformed into organic compounds [54]. The concentrations of essential nutrients, such as N, and other nonrenewable resources, such as P, existing in WW are sufficient to assist in carbon neutrality [55]. As a result, this allows for a reduction in the overall treatment costs and the influence it has on the environment. From an environmental point of view, microalgae constitute a sustainable option as they have an immense capacity for CO2 fixation in the ambient environment through photosynthesis. Primary photosynthetic products are then converted into high-value compounds, which makes this approach also interesting from an economical perspective. Therefore, the utility of microalgae can assist in reducing global warming without causing pollution [55].
Conventional WWTs involve several processes to remove C, N, and P. Microalgae-based WWT technology is a more effective approach because bioremediation is achieved in a single step [55]. To recompense its production cost, harvested microalgal biomass can also be transformed into biobased high-value compounds, including biohydrogen, biohydrocarbons, bioalcohols, and health supplements [56]. Up to 70 tons of biomass and 15,000 L of oil per hectare of land can be produced annually using new revolutionary processes. Microalgae may improve the yield of high-quality biomass for producing biobased fuels that can be commercially used for airplanes and vehicles [57]. Another study evaluated the entire process of producing biofuel from microalgae cultivated anaerobically in digested WW [58]. It was shown that microalgae reduced 57.4% of eutrophication problems, 22.6% of ozone, and 2.7% of global warming [58]. As a result, biofuel production from microalgae coupled with WWT is a promising approach. Furthermore, microalgal biomass can be used for other purposes, for instance, as a feedstock to produce carbohydrates, proteins, vitamins, and lipids [55]. Using microalgae for bioremediation also can recover resources for economic reuse via biorefinery of microalgal/bacterial biomass for a variety of low- and high-value by-products such as microalgal plastics, fertilizer, and fibers, in addition to protein-rich feed [59]. Over the past 30 years, microalgae's potential to decontaminate inorganic, organic, and emerging pollutants has been comprehensively summarized and discussed. Fig. 2 depicts various bioremediation mechanisms of pollutants through microalgal metabolism [[60], [61], [62]].
Fig. 2.
Bioremediation mechanisms of pollutants through microalgal metabolism.
3. Wastewater treatment using microalgae
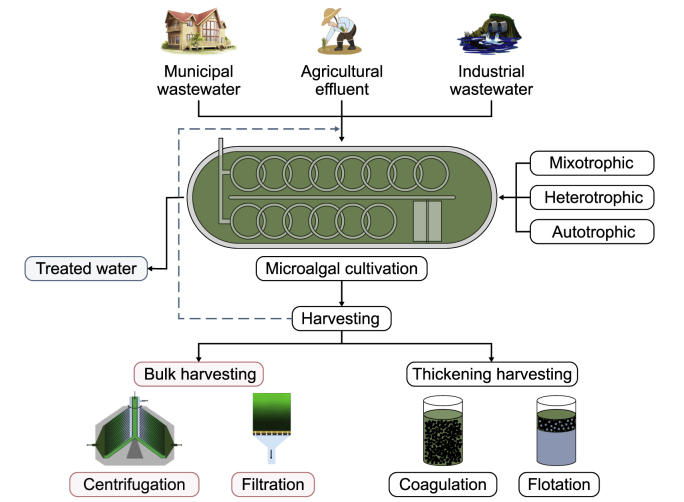
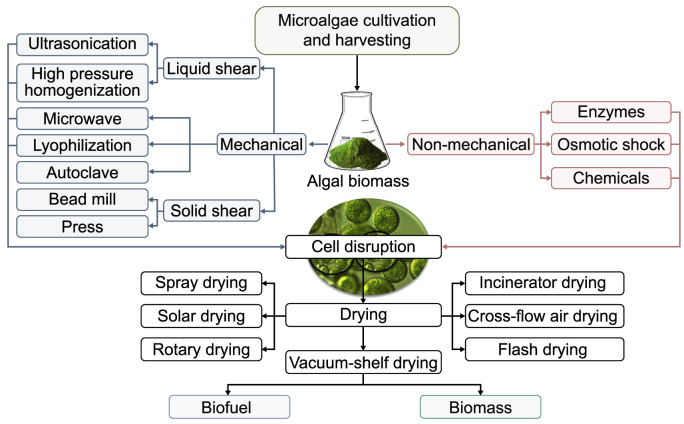
Generally, the traditional practices used for WWT include primary and secondary treatment approaches (Fig. 3, Fig. 4). Primary treatment targets the removal of solid particles, while secondary treatment aims to decompose organic matter via microbial processes. Yet, such treatments yield relatively high operational and maintenance costs [63]. In chemical methods, iron and aluminum salts are commonly applied for precipitation, which results in a significant amount of sludge that needs to be disposed of or requires additional treatment. Biological methods entail large infrastructures and produce a significant amount of activated sludge that should be processed, requiring extra energy input, increasing overall budget, and adding process complexity [64]. Thus, microalgae-based bioremediation is considered an efficient alternative for upgrading the traditional WWT systems as it offers a reliable solution to deal with liquid or solid wastes induced by conventional methods (Fig. 3) and also converts them into value-added products (Fig. 4). An efficient strategy to reduce the microalgae production cost is coupling the microalgal cultivation with other common WWT systems [64]. Due to the high capability of microalgae in nutrient uptake and production of substantial biomass, research to investigate their efficiency on the commercial side has been increasing. Compared to vascular plants, the growth rate of microalgae is much higher, and microalgae cultivation as a step of WWT has no harmful impacts on the environment. Within 13 h of cultivation, microalgae biomass can double its original biomass [65,66]. Microalgae have the capability to survive harsh conditions like WW and only need restricted land area for cultivation, resulting in less land competition for other uses such as agriculture, animal farms, industry, and human residential zone.
Fig. 3.
Wastewater treatment using microalgae.
Fig. 4.
Techniques for biomass and biofuel production during microalgal wastewater treatment.
Methods for microalgae cultivation include autotrophic, heterotrophic, and mixotrophic growth (Fig. 3). Autotrophically grown algae use CO2 as the carbon source and therefore reduce the amount in the atmosphere. Each microalgae biomass transforms about 1.8 pounds of CO2 [55,64]. Some microalgal species are obligatory heterotrophic and utilize organic carbon from industrial waste like ethanol, glucose, acetate, and glycerol, whereas others are mixotrophic, utilizing facultative CO2 in addition to organic carbon [69]. Besides CO2, P and N are also assimilated and converted to proteins, carbohydrates, lipids, and other value-added products [12,67,68]. Considering that WW is usually rich in nutrients, including microalgae in WWT reduces production costs and the overall carbon footprint. Different studies have recently focused on the microalgae-based treatment of different WW streams, such as municipal, agricultural, and industrial effluents (Fig. 3).
Operation conditions of the culture include irradiance, CO2 and nutrients supply, temperature, salinity, and pH affect microalgal proficiency and biomolecule production [66]. For instance, at 60 μmol m−2 s−1 photosynthetically active radiation (PAR), Ankistrodesmus falcatus was able to produce more than 35% of total lipid (containing around 65% neutral lipid), with no remarkable effect of light intensity on the protein productivity [70]. The maximum lipid productivity was recorded in a medium with soil extract in Chlorella vulgaris. The biomass yield was recorded at 6.8 and 2.24 g L−1 in the modified basal medium and modified soil extract medium, respectively [71].
4. Remediation mechanisms and influencing factors
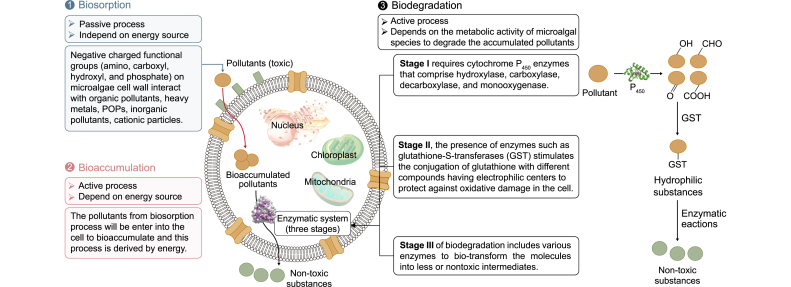
Environmental remediation by using microalgae involves different mechanisms (i.e., biosorption, bioaccumulation, and biodegradation (Fig. 2).
4.1. Biosorption
Biosorption is considered a passive process, where the sorbent is a biological material that can bind and concentrate pollutants from water. Biosorption, in other words, is a mass transfer process in which a material is displaced from the liquid phase and bonded to the surface of a solid. Biosorption includes physical, chemical, and metabolic-independent processes supported by different mechanisms comprising precipitation, ion exchange, surface complexation, electrostatic interaction, absorption, and adsorption [37]. The mechanisms underlying this phenomenon require a biosorbent (solid phase sorbent) and a target sorbate that is dissolved or dispersed in water. The biomaterial engaged could be living or dead microorganisms and even their components. The immense affinity of the biosorbent toward the target sorbate is the driving force for the attraction of sorbate species, and the total capacity is responsible for the number of sorbate molecules [61,72]. The process is continued until an equilibrium is reached between the adsorbed substance by the biosorbent and its remaining concentration in the liquid. In addition, the degree of biosorbent affinity for a specific sorbate is responsible for its distribution among the solid and liquid phases [69].
In the early 1970s, biosorption was first observed in several microalgae when the radionuclides and HMs discharged from a nuclear reactor were concentrated in microalgae. The cell wall of microalgae is directly responsible for biosorption, and its chemical composition plays a vital role during the process and determines the mechanism by which this phenomenon occurs (Fig. 2). In addition, pores exist on microalgal surfaces, and the surface charge assists biosorption. Different chemical groups present in the cell wall of microalgae, such as carboxyl, hydroxyl, and sulfate, perform as binding sites and are also effective ion exchangers that contribute to the complexation of metal ions and adsorption of organic substances from polluted water [73]. Moreover, other molecules such as lipids, proteins, and nucleic acids may be deposited on the cell surface; however, they are predominantly present in the cytoplasm and plasma membrane and are capable of binding to metal cations through their various functional groups (carboxylic, aminic, thiolic, imidazolic, thioesteric, N, and oxygen in peptide links) [74]. In general, the cell wall structure of microalgae includes a fibril matrix that provides high mechanical strength, whereas its flexibility is due to the amorphous fraction, and both these fractions, along with the intercellular spaces present on the cell wall, could enhance the process of biosorption [75]. Furthermore, the porosity and roughness of the microalgae surface can influence HM adsorption [76]. Small cell sizes increase the adsorbent surface area by providing more contact area per biomass unit [77]. The greater the number of biosorbent particles, the greater the number of active sites for biosorption; however, more biomass does not necessarily indicate more biosorption properties, as metal cation captured in an aqueous medium may be reduced due to active site repulsion between microalgae surfaces, which decreases the removal efficiency [78].
HMs, such as manganese (Mn), molybdenum (Mo), cobalt (Co), copper (Cu), zinc (Zn), iron (Fe), and boron (B), are trace elements essential for cell metabolism and enzymatic processes. Some of these are toxic at elevated concentrations; other elements, such as chromium (Cr), cadmium (Cd), mercury (Hg), lead (Pb), and arsenic (As), are generally toxic to microalgae. HMs boost the growth and metabolism of microalgae in case of trace levels of toxic metal ions according to the hormesis phenomenon [79]. In addition, the active binding sites on the cell surface can form complexes with specific pollutants in water, which stimulates flocculation and causes a reduction of total dissolved and suspended solids [14]. HMs ions biosorption by microalgae proceeds through a two-step mechanism. A metabolism-independent process that encounters rapid and reversible binding of adsorbate onto active sites on the surface of microalgae is followed by a slow stage that refers to positive intracellular diffusion and primarily involves the metabolic activity of microalgae (Fig. 2). Besides polymeric substances such as exopolysaccharides with uronic groups and peptides, the cell wall of microalgae may consist of cellulose, alginates, lipids, and proteins, which offer numerous functional groups, including hydroxyl, phosphate (PO43−), amino, sulfonate, carboxyl and thiol (Fig. 2). Microalgae capture both cationic and anionic species of HMs because they have a large number of deprotonated carboxyl and sulfate groups as well as monomeric alcohols that stimulate biosorption [80]. Extracellular polymeric substances (EPS) derived from algae can accelerate metal biosorption, and their efficacy depends on the EPS structure, solution properties, metal species, and operational conditions [[81], [82]]. Mota et al. [81] found that EPS released by Cyanothece sp. can remove HMs from contaminated water due to organic functional groups, namely carboxyl and hydroxyl, rather than ion exchange. At low pH, the EPS of Nostoc linckia exhibited biosorption capability for Co(II) and Cr(IV), which was attributed to interactions between metal ions and negatively charged functional groups of EPS [83]. The interaction of Chlorella vulgaris EPS with cadmium decreased intracellular Cd2+ concentration, improving the capacity of C. vulgaris to remove NH4+-N and PO43−-P [84]. Investigating the chemical structure of a biosorbent provides substantial and precise information to predict its affinity and capacity for specific HMs ions. Nuclear magnetic resonance spectroscopy and Fourier-transform infrared spectroscopy (FTIR) are two valuable analytical techniques that can help scientists define the particular functional groups accountable for complexation with HMs and determine the exact underlying mechanisms [[85], [86], [87]].
The optimal pH for biosorption varies depending on the metal and the microalgae species. In general, the pH varies from 3.0 to 6.5. Values lower than 3.0 cause dissociated H+ ions to compete for active sites on the surface of cells, while metals can precipitate in their hydroxide forms at values over 6.5, rendering biosorbents useless and resulting in the accumulation of highly toxic sludge [78]. Conversely, the adsorption of Cr4+ was achieved by Nostoc linckia [83] and Nannochloris oculate [88] at pH 2.0. Due to the limited number of active sites on the cell surface, metal concentration and competition are two major factors for biomass saturation [78]. Kotrba et al. [89] stated that metal cations of low atomic number compete with target actions for active sites on the surface of cells, thus reducing HMs removal efficiency. The temperature was shown to influence HM absorption in several studies, which might be favorable [90] or negative [91] for the application in WWT. In other studies, however, the temperature had a negligible impact on metal sorption [92].
4.2. Bioaccumulation
Unlike biosorption, bioaccumulation is an active metabolic process described as using various substrates in the cell lumen. Bioaccumulation requires energy and is reasonably slower than biosorption (Fig. 2). Using bioaccumulation, living cells are used to detoxify wastes; they take up substances and then accumulate or metabolize them. This process is considered an essential pathway for removing inorganic and organic pollutants (i.e., sulfates, nitrates, phosphates, HMs, and pesticides) in which these substances can be transferred inside the cells [61]. Although the mechanisms of bioaccumulation and biosorption are substantially different processes, it is difficult to quantify biosorbed and bioaccumulated pollutants because the two mechanisms are dynamically interchanging. Microalgae accumulate different pollutants together with nutrients and microelements present [60]. Microalgae can resist pollutants at low concentrations due to their capabilities for adapting to the environment. Furthermore, microalgae have an immense tolerance toward a wide category of pollutants from domestic, agricultural, and industrial sectors, which enhances their bioremediation ability [93,94].
Bioaccumulation is quantified using a bioconcentration factor, which is the ratio of the concentration of a contaminant adsorbent to the medium [95]. Bioadsorption is the preceding step for bioaccumulation. Nevertheless, not all adsorbed molecules can be bioaccumulated [4]. For instance, Spirogyra can uptake 850,000 times more radio-phosphorus than the surrounding water [96]. Cladophora sp. can accumulate the lipophilic organic pollutants of triclosan and triclocarban, widely used as antimicrobial agents, to as high as 50–400 ng g−1 of microalgae. In contrast, the environmental concentration did not exceed 200 ng kg−1 [97].
Pollutants travel along a concentration gradient from a high (external) to a low (internal) concentration region with no energy required [98]. Because of the hydrophobicity of the cell membrane, low-molecular-weight materials that are nonpolar and lipid-soluble could probably penetrate the cell membrane via passive diffusion. In contrast, polar compounds, high-molecular-weight molecules, and ions cannot diffuse through the cell membrane. The bioaccumulation of sulfamethoxazole, carbamazepine, trimethoprim, and florfenicol has been previously reported, wherein the abovementioned pollutants entered microalgae cells by passive diffusion [99]. Furthermore, changes in cell membrane permeability caused by pollutants or a stressful environment can lead to passive diffusion, which is mediated by membrane depolarization or hyperpolarization. For example, when the cyanobacterium Anabaena CPB4337 was exposed to perfluorinated alkyl acid compounds, like perfluorooctane sulfonate and perfluorooctanoic acid, its sensitivity to herbicides changed, with certain herbicides becoming more harmful and others becoming less toxic [100]. Moreover, bioaccumulation of levofloxacin by Chlorella vulgaris was considerably enhanced from 34 to 101 μg g−1 with adding 1% (w/v) NaCl [101]. Passive-assisted diffusion is the process by which pollutants go across the cell membrane with the assistance of transporter proteins, which support polar molecules entering the cell. The third process is active transport across the cell membrane, which necessitates energy since the molecules diffuse against a concentration gradient [102].
Irrespective of the mechanism, external and internal physicochemical parameters such as pH, contact time, temperature, and the concentration of pollutants significantly impact bioaccumulation [102]. For instance, antibiotic bioaccumulation in the planktonic food web was found to be considerably influenced by temperature [103]. The relationships between temperature and bioaccumulation were biphasic. Bioaccumulation of carbamazepine in Chlamydomonas mexicana and Scenedesmus obliquus improved with increasing cultivation time and carbamazepine concentration [104]. One challenge of bioaccumulation is that some bioaccumulated substances stimulate reactive oxygen species. Free radical (alkoxy radicals, hydroxyl radical (•OH), superoxide radical (•O2−), perhydroxyl radical (•HO2), singlet oxygen (1O2), and nonradicals (hydrogen peroxide (H2O2)) are formed inside the cells, which fundamentally control cellular metabolism and could cause oxidative harm to biomolecules, cellular dysfunction, and ultimately cell death [36,105]. To promote the efficiency of microalgae in bioremediation practice, the physicochemical environment must be optimized because the rate and capacity of microalgae depend on those parameters in the bioaccumulation process. Moreover, screening microalgae species that are tolerant to high pollutant concentrations is an effective strategy to achieve higher capacities and rates of bioaccumulation.
4.3. Biodegradation
One of the most effective processes to eliminate pollutants from effluents is biodegradation, in which complex compounds degrade into simple and safe chemical building blocks (Fig. 2). In contrast to bioaccumulation and biosorption, which involve microorganisms acting as biological filters to concentrate pollutants and separate them from the surrounding water, bioremediation entails the breakdown of target pollutants either through complete mineralization of parent molecules to CO2 and H2O or through biotransformation, which contains a series of enzymatic reactions to produce different metabolic intermediates [98,101].
The fundamental mechanisms of biodegradation can be divided into two categories: (i) metabolic degradation, in which pollutants serve as the electron donor/acceptor and carbon source for microalgae, and (ii) cometabolism, in which pollutants serve as both an electron donor and a carbon source for non-living matter [106,107]. Microalgae-mediated biodegradation takes place extra- or intracellularly, or in a combination of both locations, with extracellular breakdown followed by intracellular decomposition of the breakdown intermediates [107]. Extracellular degradation is based on EPS excreted mainly by microalgae. It remains restricted to the exterior cell walls, allowing microalgae to mineralize pollutants in their dissolved state and function as an external digestive system. Furthermore, EPS works as an emulsifier in the form of biosurfactants to increase pollutant bioavailability in the environment, allowing for future bioaccumulation by microalgae. The exact mechanisms behind the interaction between pollutants and the EPS emitted by microalgae are yet unknown, calling for further research.
Microalgal biodegradation of organic pollutants encompasses three stages of complex enzymatic processes. Stage I requires cytochrome P450 enzymes that includes hydroxylase, carboxylase, decarboxylase, and monooxygenase. The function of these enzymes is to increase the hydrophilicity of the pollutant through the addition or unmasking of a hydroxyl group through either oxidation-reduction reactions or hydrolysis [36,98] (Fig. 2). In stage II, the presence of enzymes such as glutathione-S-transferases and glucosyltransferases can stimulate the conjugation of glutathione with different compounds having electrophilic centers (CONH2, epoxide ring, and COOH) to protect against oxidative damage in the cell [105] (Fig. 2). Stage III of detoxification includes various enzymes (such as dehydrogenase, glutamyl-tRNA reductase, carboxylase, mono(di)oxygenase, laccases, transferase, hydrolases, pyrophosphatase, and dehydratase) to biotransform the molecules into less or nontoxic intermediates [108] (Fig. 2).
Cometabolism is indicated by the conversion of a non-living element in the mandatory occurrence of organisms that biodegrade other compounds. These organic substrates function as electron donors for the cometabolism of non-living matter and biomass production [102]. For stimulating the microalgae-mediated biodegradation of recalcitrant pollutants, some studies have reported an effective strategy by adding other nutrient substances or organic substrates to form a cometabolic system [109]. For instance, the addition of sodium acetate was found to enhance ciprofloxacin removal efficiency by Ceratozamia mexicana from 13% to 56% [110]. An increase in the removal efficiency of parent molecules, along with regulating the transformation products and pathways, was primarily related to acetate concentration. In particular, the type of nutrient substance and its concentration had the maximum effect among other factors [110]. The effect of carbon sources on the cometabolism of pollutants by microalgae has been systematically investigated [109]. Although the findings demonstrated that the highest EPS, enzyme concentrations and optimal removal efficiency were achieved with sugar-based carbon sources, the removal efficiency of micropollutants might be diminished with the presence of some organic substrates, probably owing to catabolite repression [109]. As a result, it is critical to assess the effects of different carbon sources to select the best appropriate source and to verify that it is suitable for use in the biodegradation of pollutants in WW. Meanwhile, this invention will increase biomass recovery and revenue for various sectors. Generally, it has been reported that the three mechanisms can go hand by hand in microalgal WWT as biodegraded particles can be adsorbed on their surface, followed by the transfer of particles into the cells by active processes. Eventually, enzymatic reactions promote precipitation, biotransformation, and bioaccumulation inside the cells [62].
5. Environmental applications of microalgae
5.1. Bioremediation of industrial effluents
The industrial revolution and technological development are the main causes of water pollution due to the direct or indirect release of toxic pollutants. Several treatment technologies, such as activated sludge, membrane technology, advanced oxidation, and electrochemical treatments, have been developed to eliminate such pollutants. However, most of these approaches have shortcomings, such as incomplete removal of HMs, high maintenance and operational cost, and generation of toxic sludge or other waste by-products. Bioremediation, especially microalgae-based WWT, is one of the most promising, eco-friendly, and sustainable alternatives, which can metabolize hazardous materials into non-toxic substances. It is an affordable process with double benefits on purifying WW and concurrently biomass harvesting due to their efficient ability to uptake inorganic nutrients and HMs as well as the sorption of numerous toxic pollutants from WW (Fig. 3, Fig. 4). This section discusses the application of microalgae for WWT in the bioremediation process of toxic contaminations (HMs, dyes, and pharmaceuticals).
5.1.1. Heavy metals removal
HMs are a class of metals with an atomic density of more than 4000 kg m−3 [111]. Due to their non-biodegradable properties, abundant sources, toxicity, and accumulative behavior, HMs pollution is a critical problem worldwide. HMs are naturally toxic and cause serious diseases to humans and animals, even at low concentrations [112]. Being classified into three categories, HMs may be radionuclides (U, Ra, Am, and Th), precious metals (Au, Pd, Pt, and Ru), and toxic metals (Cu, Cr, As, Zn, Ni, Ag, Sn, Co, and Pb) [113,114]. Besides naturally occurring, HMs enter aquatic systems through industrial discharges and agricultural runoff. Various treatment approaches are available to remove HMs from the aquatic environment with different success. In any case, these conventional treatment methods cause high operation and maintenance costs, producing secondary waste [111]. Therefore, it is critical to developing robust, environment-friendly, and economically viable treatments.
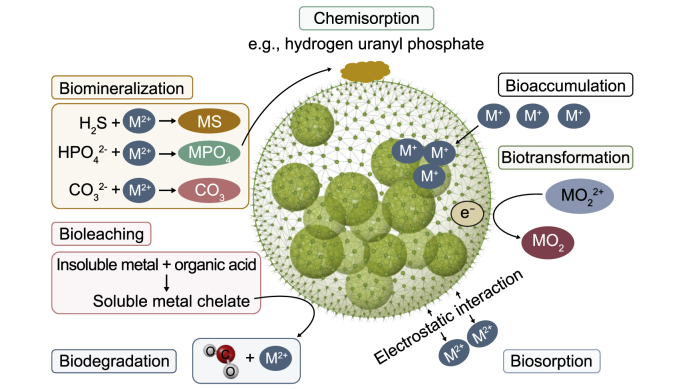
The site characteristics, the extent of HMs contamination, and the regulatory limits for the HMs of concern in that regulatory domain all impact the remediation strategy chosen. Chemical, physical, and biological approaches are the three fundamental categories of remediation techniques for removing HMs from WW using various types of microalgae (Fig. 5) [115,116]. The principal binding sites for the HMs removal from WW are the amino, hydroxyl, carboxyl, and sulfate functional groups found in microalgal cell walls [117]. Because of the presence of these negatively charged groups, which make it possible for ions to be bound from the surrounding environment, the outermost layer of the cell wall is the first participant in removing HMs [7,118]. The removal of HMs by microalgae is primarily influenced by their properties, the type of HMs, and the characteristics of the effluent. Microalgae (in freshwater) are the most common species successfully used to remove various HMs from WW. A few green microalgae, including Chlorella miniata, C. vulgaris, C. reinhardtii, and Sphaeroplea, have been successfully implemented for the toxic HMs removal from the WW [13,119]. Table 2 summarizes the majority of previous HMs remediation by microalgae [[120], [121], [122], [123], [124], [125], [126]].
Fig. 5.
Metal–microbe interactions mechanism during the bioremediation process.
Table 2.
Remediation of heavy metals (HMs) using microalgae.
| HMs | HMs concentration (mg L−1) | Treatment method | Microalgae | Biomass concentration (g) | Biomass | Reference |
|---|---|---|---|---|---|---|
| Cadmium | 50 | Biosorption | Spirulina platensis | 1 | Non-living | [120] |
| Cadmium | 50 | Biosorption | Spirulina platensis | 0.6 | Non-living | [120] |
| Chromium | 50 | Biosorption | Spirulina platensis | 1 | Non-living | [120] |
| Chromium | 50 | Biosorption | Spirulina sp. | 3 | Non-living | [121] |
| Chromium | 50 | Biosorption | Chlorella vulgaris | 2 | Non-living | [122] |
| Cadmium | 50 | Biosorption | Anabaena sphaerica | 0.25 | Non-living | [123] |
| Lead | 50 | Biosorption | Anabaena sphaerica | 0.25 | Non-living | [123] |
| Lead | 100 | Biosorption | Pseudochlorococcum typicum | 4.52 μg chl a ml−1 | living | [124] |
| Mercury | 100 | Biosorption | Pseudochlorococcum typicum | 4.52 μg chl a ml−1 | living | [124] |
| Lead | – | Biosorption | Chlorella vulgaris | 2 | Non-living | [125] |
| Nickel | – | Biosorption | Chlorella vulgaris | 2 | Non-living | [125] |
| Zinc | – | Fixed-bed column | Chlorella vulgaris | 2 | Non-living | [125] |
| Arsenic | 6 | Biosorption | Maugeotia genuflexa | 4 | Non-living | [126] |
5.1.2. Dyes removal
Due to numerous applications in various industries, dyes are becoming more widely used. Dyes discharged into ecosystems have unappealing aesthetic effects and cause serious environmental implications. Phycoremediation technology used to clean dye-contaminated WW has gained prominence in recent years. Algal cell walls contain a variety of functional groups that aid in the dye removal process. Numerous variables are critical for dye removal, including contact time, pH, temperature, initial dye concentration, and adsorbent dosage. As a result, these criteria are considered when determining the efficacy of microalgal dye abatement [127].
Dye-contaminated WWT is commonly accomplished through traditional techniques such as chemical, physicochemical, and biological approaches. Numerous chemical and physical procedures, containing adsorption, advanced oxidation process, flocculation, ozonation, ultrafiltration, photocatalytic oxidation, coagulation, and chemical and electrochemical coagulation, have been used in the past to remove dyes [128]. Numerous limitations have been identified with all of the methods mentioned above, including ineffective dye removal, sludge production, a short O3 half-life (20 min), the generation of byproducts, the discharge of aromatic amines, high electricity costs, ineffective against dispersing and vat dyes, and repetitive or frequent chemical use resulting in secondary pollution problems [127]. As a result, an alternative technique for dye abatement is considered necessary. Bioremediation is regarded as the best alternative to traditional physicochemical treatment owing to its promising advantages of being affordable and non-hazardous.
Phycoremediation is the process by which pollutants such as nutrients, dyes, HMs, and xenobiotics are removed or biotransformed from dye-contaminated WW and CO2 from waste air using microalgae (for environmental cleanup) [129]. Phycoremediation is widely used for various purposes, including nutrient removal from WW, acidic and metal-containing WWT, the degradation and transformation of resistant compounds, and detecting dangerous compounds using microalgal-based biosensors [130]. Microalgal absorption of dyes has several advantages, including being environmentally benign, having a high adsorption efficiency, being a simple process, utilizing readily available materials, and being inexpensive [131]. Microalgae were utilized to abate various types of dyes in many studies, as summarized in Table 3 [[132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149]].
Table 3.
Dyes abatement using microalgae.
| Dyes | Microalgae | The influent concentration of dye (mg L−1) | Removal efficiency (%) | Reference |
|---|---|---|---|---|
| Methylene blue | Chlorella vulgaris | 100 | 83.04 | [132] |
| Reactive Black 5 | Chlorella vulgaris | 200 | 80 | [133] |
| Direct Blue 71 | Chlorella vulgaris | 200 | 78 | [133] |
| Disperse Red 1 | Chlorella vulgaris | 300 | 84 | [133] |
| Malachite green | Oscillatoria sp. | 5 | 93 | [134] |
| Safranin | Oscillatoria sp. | 5 | 52 | [134] |
| Congo red | Chlorella vulgaris | 50 | 100 | [135] |
| Yellow dye | Chlorella vulgaris | 10 | 3.12 | [136] |
| Aniline blue | Chlorella vulgaris | 25 | 58 | [137] |
| Malachite green | Haematococcus sp. | 100 | 67 | [138] |
| Acid Black 210 | Spirulina platensis | 125 | 98.55 | [139] |
| Acid Blue 7 | Spirulina platensis | 125 | 97.05 | [139] |
| Blue dye | Oscillatoria sp. | – | 76.48 | [140] |
| Red dye | Oscillatoria sp. | – | 62.63 | [140] |
| Blue dye | Spirogyra sp. | – | 78.29 | [140] |
| Red dye | Spirogyra sp. | – | 64.21 | [140] |
| Methylene blue | Desmodesmus sp. | 20 | 98.6 | [141] |
| Rhodamine B | Coelastrella sp. | 100 | 80 | [142] |
| Malachite green | Chlorella vulgaris | 6 | 91.61 | [143] |
| Malachite green | Nostoc sp. | 100 | 97.13 | [144] |
| Malachite green | Vaucheria sp. | – | 85.9 | [145] |
| Monoazo and diazo | Scenedesmus bijuga | – | 68 | [146] |
| Malachite green | Chlorella sp. | – | 80.7 | [147] |
| Malachite green | Cosmarium sp. | 10 | 87.1 | [148] |
| Synazol | Spirogyra sp. | – | 85 | [149] |
5.1.3. Pharmaceuticals
Pharmaceutical compounds (PCs) are a broad category of chemical substances widely used worldwide. The agricultural operation, hospital effluents, industrial pollution, and domestic trash all pollute aquatic environments with PC residues. Although most PC concentrations in the environment are in the range of ng L−1 to a few mg L−1, there is growing evidence that they have negative ecological effects on both target and non-target microorganisms, such as inhibiting microbes' growth, changing microbial communities, decreasing soil microbial activity, and affecting denitrification rates [150]. N, N-nitrosodimethylamine (NDMA) is formed when amine-based PCs (e.g., doxylamine, nizatidine, carbinoxamine, and ranitidine) interact with disinfectants used in drinking water disinfection. NDMA poses a significant health risk to humans owing to its carcinogenic properties, and California's Office of Environmental Health Hazard Assessment has established a health objective of no more than 3 ng L−1 of this species in WW [150].
The scientific community is gaining interest in microalgae-mediated PC bioremediation. Its benefits include being solar-powered, having low operational costs, being environmentally friendly, and contributing to carbon fixation and turnover [151,152]. Microalgae have the capacity to overcome several constraints associated with bacteria and fungi, which need a stoichiometric balance of carbon and other nutrients for growth and pollutant breakdown. Due to their adaptability, mixotrophic microalgae may be suitable for removing pollutants from WW [151]. The ability of mixotrophic microalgae to extract and absorb PCs from WW has been reported using a variety of microalgal species (Table 4) [80,[153], [154], [155], [156], [157], [158]]. Microalgae growth in WW decreases the demand for chemical fertilizers/nutrients, which would be needed to prepare artificial growth media, and therefore it decreases the environmental impact. To promote a more sustainable application in the biofuel industry derived from microalgae biomass, a ‘zero-waste’ concept can be performed by using WW as a source for the culture of mixotrophic microalgae (WWT via microalgal remediation), followed by the use of biomass as a viable raw material for sustainable biofuel generation [157]. The mechanisms, such as biosorption, bioaccumulation, and biodegradation, are all involved in removing PCs by microalgae from the environment, as shown in Fig. 6 and Table 5 [101,156,[158], [159], [160], [161]]. It has been reported that the removal mechanisms of various PCs utilizing microalgae vary depending on the physicochemical properties of the targeted PCs [4]. For example, a study conducted by De Godos et al. [162] reported that Chlorella vulgaris could remove more than 50% tetracycline in a high-rate algal pond through adsorption due to the interaction of positively charged molecules on the cell surface. Sulfamethoxazole, carbamazepine, trimethoprim, and florfenicol were bioaccumulated through passive diffusion using Chlorella sp [161]. Zhang et al. [163] demonstrated that Scenedesmus dimorphus can biodegrade about 85% of 17 α-estradiol within seven days.
Table 4.
Removal of pharmaceutical pollutants from the environment by microalgae.
| Wastewater (WW) category | Pollutants | Microalgae | Removal efficiency (%) | Reference |
|---|---|---|---|---|
| Lake Mead water | Ibuprofen | Nannochloris sp. | 40 | [153] |
| Lake Mead water | Trimethoprim | Nannochloris sp. | 10 | [153] |
| Lake Mead water | Ciprofloxaci | Nannochloris sp. | 100 | [153] |
| Lake Mead water | Carbamazepine | Nannochloris sp. | 20 | [153] |
| Lake Mead water | Triclosan | Nannochloris sp. | 100 | [153] |
| Urine, anaerobically treated black water, and synthetic urine | Diclofenac | Chlorella sorokiniana | 40–60 | [154] |
| Urine, anaerobically treated black water, and synthetic urine | Ibuprofen | Chlorella sorokiniana | 100 | [154] |
| Urine, anaerobically treated black water, and synthetic urine | Paracetamol | Chlorella sorokiniana | 100 | [154] |
| Urine, anaerobically treated black water, and synthetic urine | Metoprolol | Chlorella sorokiniana | 100 | [154] |
| Urine, anaerobically treated black water, and synthetic urine | Carbamazepine | Chlorella sorokiniana | 30 | [154] |
| Urine, anaerobically treated black water, and synthetic urine | Trimethoprim | Chlorella sorokiniana | 40 | [154] |
| Urban or synthetic WW | Carbamazepine | Microalgae consortia in high-rate algal ponds dominated by Chlorella sp. and Scenedesmus sp. | 20 | [155] |
| WW digestate and growth medium | Estradiol | Selenastrum capricornutum | 88–100 | [156] |
| Urban WW | Acetaminophen | Microalgae consortia in high-rate algal ponds | 99 | [80] |
| Urban WW | Ibuprofen | Microalgae consortia in high-rate algal ponds | 99 | [80] |
| Urban WW | Carbamazepine | Microalgae consortia in high-rate algal ponds | 62 | [80] |
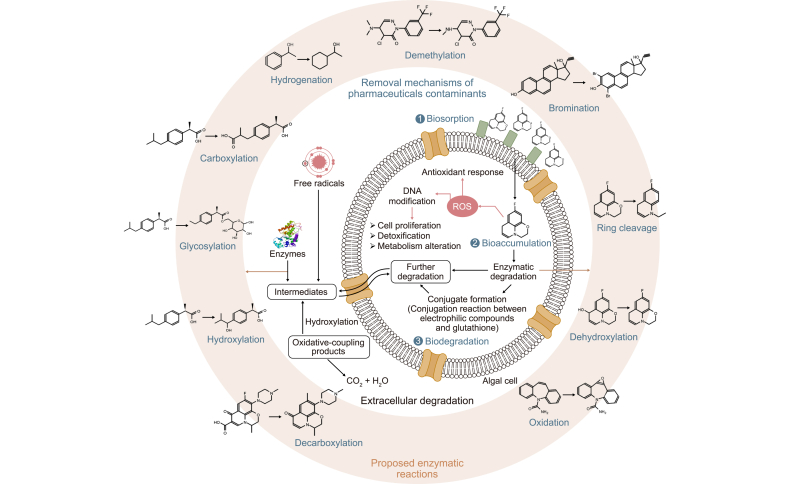
Fig. 6.
Mechanisms involved in the removal of pharmaceutical compounds by microalgae.
Table 5.
Mechanisms of removal for pharmaceutical compounds (PCs) by microalgae.
| Microalgae | PCs | Mechanism | Removal (%) | Reference |
|---|---|---|---|---|
| Desmodesmus subspicatus | 17α-ethinylestradiol | Adsorption & biodegradation | 68 | [158] |
| Chlorella pyrenoidosa | Norgestrel | Biodegradation | 60 | [159] |
| Chlamydomonas reinhardtii | β-estradiol | Adsorption & biodegradation | 100 | [156] |
| Chlorella vulgaris | Metronidazole | Adsorption | 100 | [160] |
| Chlorella sp. | Florfenicol | Bioaccumulation, biodegradation & adsorption | 97 | [161] |
| Scenedesmus obliquus | Enrofloxacin | Bioaccumulation, bioadsorption, and/or biodegradation | 23 | [101] |
| Chlamydomonas mexicana | Enrofloxacin | Bioaccumulation, bioadsorption, and/or biodegradation | 25 | [101] |
Extracellular enzymes produced by microalgae break down certain antibiotics into less toxic or even non-toxic intermediates [164]. These intermediates are then bioaccumulated and biodegraded by enzymes in cells. In addition, the degree of complexity of the structures determines the degree of biodegradability [4]. Both ketoprofen and ibuprofen, classified as nonsteroidal anti-inflammatory drugs, are eliminated from WW in a manner that is distinct from one another. Using vascular plants and microalgae-mediated treatment, ibuprofen was eliminated with higher efficiency than ketoprofen [156]. Paracetamol and ibuprofen were completely destroyed by Chlorella sorokiniana [154]. The EPS matrix facilitates the adsorption, accumulation, and eventually biodegradation of various substances [156]. The presence of carboxyl, hydroxyl, and phosphoryl functional groups are responsible that microalgae being predominately negatively charged, which turned out to be favorable for capturing positively charged PC molecules [160]. The rapid uptake of PCs onto the cell wall using EPS has been demonstrated by many recent surveys [156]. In general, the studies suggest that PCs are eliminated by several different mechanisms, with bioadsorption as the most prevalent one. The contribution of bioadsorption is variable depending on the microalgae used and the micropollutant being absorbed [154].
Some PCs that are adsorbed on the surface of microalgae can enter the cell and bioaccumulate [93]. Bioaccumulation, as an active process, requires energy. First-line defenses consist of EPS and the cell wall. Quantifying bioaccumulation and bioadsorption is a difficult task because both processes are ongoing. After 14 days of cultivation in the dark, Bai and Acharya [153] found that the green microalgae Nannochloris sp. still had a low uptake of trimethoprim (11%) and carbamazepine (13%), but 27% of the triclosan was already taken up within the first seven days. Certain bioaccumulated PCs contribute to producing reactive oxygen species, which harm cell metabolism [105].
5.2. Bioremediation of domestic effluents
5.2.1. Nutrients and organic substance removal
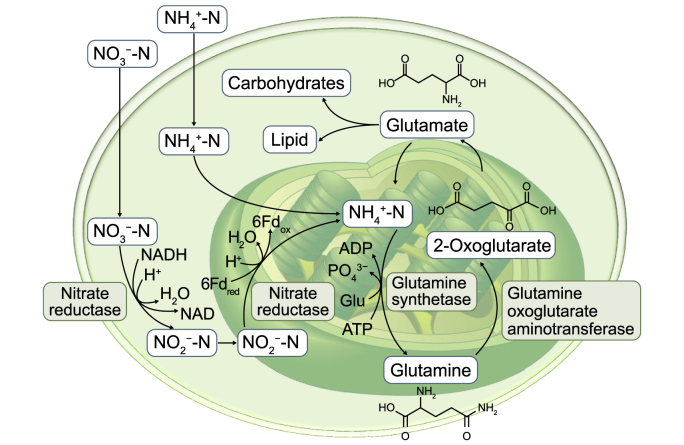
Microalgae-based WW bioremediation offers superior elimination of nutrients, meeting the growingly strict discharge and reuse standards as microalgae depend mainly on P and N for proteins synthesis, as shown in Fig. 7. Furthermore, the indirect role of microalgae in organic carbon and nutrients degradation is indicated in Fig. 8 as microalgae utilize CO2 through photosynthesis generating oxygen which is required for degradation of organic forms of carbon, N and P and convert them to CO2 and inorganic N and P by heterotrophic bacteria. Microalgae could utilize these inorganics in further photosynthesis for biomass synthesis [55].
Fig. 7.
Nitrogen removal mechanisms by microalgal cells in wastewater.
Fig. 8.
Synergic interaction between aerobic bacteria and microalgae [55].
Implementing microalgae to treat WW still faces several challenges, including harvesting the biomass to recover molecules synthesized by microalgae and the potential presence of toxic compounds when microalgae are cultured in livestock WW, such as antibiotics [165,166]. On the other hand, recent attention has been focused on microalgae-based WWT due to its low energy requirements, the robust ability of microalgae to grow in various environmental conditions, and the ability to recover and transform WW nutrients into high-value bioactive compounds. However, microalgae biomass recovery efficiency is one of the most significant challenges faced by microalgae-based WWT due to its inherent characteristics, such as its small size, negatively charged surface, and similar density to water [167,168]. The production of EPS gives an organism the ability to form multicellular structures, which in turn increases the efficiency with which it can harvest due to the formation of cell aggregates in the medium, which leads to self-flocculation [169]. Moreover, meeting the optimal light requirement is one of the challenges of microalgae-based WWT since livestock WW contains large amounts of suspended solids and turbidity, which both reduce light penetration into the medium and inhibit microalgae growth. This reduction in light penetration has a detrimental effect on the photosynthetic rate, especially for microalgae that cannot float on the surface of the water [33]. Immobilizing matrices facilitate biomass recovery, one of the major challenges for the large-scale implementation of microalgae-based WWT. Despite this, the positive and negative interactions between microalgae, the immobilizing matrix, and the livestock WW medium remain unknown. To make use of the livestock WW-treated microalgal biomass, more research must be conducted on the subjects of lowering the cost of harvesting the biomass and developing technologies that ensure its safety [33].
A wide range of microalgal strains was well grown in municipal WWT, but a limited range can be incorporated in agricultural and industrial WW. Generally, different types of WW have various nutrients and pollutants content, and each microalgal strain has its ideal growth condition. So, nutrients should be formulated to provide the optimal condition for microalgal growth [170]. Table 6 summarizes some microalgal species utilized in WWT for nutrients and organics removal [[171], [172], [173], [174], [175], [176], [177], [178], [179]]. Microalgae were involved through constructed wetland-microbial fuel cells integrated with a sand filter to eradicate the cost of external mechanical aeration systems and to eliminate pollutants [180]. COD removal (17.59%) was ascribed mainly to the symbiotic role between microalgae and aerobic bacteria as microalgae supplied aerobic bacteria with oxygen required to oxidize organic matter.
Table 6.
Microalgae-based wastewater treatment (WWT) for nutrients and organics removal.
| Microalgae | Wastewater type | Removal efficiency | Reference |
|---|---|---|---|
| Scenedesmus obliquus | Piggery WW | 91.43% (BOD), 83.11% (COD), 83.74% (TN), and 54.69% (TP) | [171] |
| Chlorella vulgaris | Brewery WW | 91.43% (BOD), 83.11% (COD), 83.74% (TN), and 54.69% (TP) | [172] |
| Scenedesmus spp. | Anaerobic membrane bioreactor effluent (domestic WW) | N and P removal ranged from 90 to 99% | [173] |
| Halochlorella rubescens | Secondary municipal WW | 83.2% (NO3−-N) and 73.2% (PO43−) | [174] |
| Chlorella sorokiniana and Scenedesmus obliquus | Industrial and municipal WW + stormwater | 63.6% (COD), 4.2% (TN), and 82.7% (TP) | [175] |
| Scenedesmus obliquus | Stimulated municipal WW | 99.2% (NH4+), 91.2% (PO4), and 83.6% (TOC) | [176] |
| Chlorella sorokiniana-activated sludge consortium | Synthetic municipal WW | 98% (NH4+), 96% (PO43−), and 88% (COD) | [177] |
| Chlorella vulgaris with activated sludge | Synthetic municipal WW | 81% (NH3), 39% (PO43−), and 98% (COD) | [178] |
| Galdieria sulphuraria | Primary-treated WW | 6.26 mg L−1 d−1 (NH3), 1.41 mg L−1 d−1 (PO43−), and 16.4 mg L−1 d−1 (COD) | [179] |
BOD, Biological oxygen demand; COD, Chemical oxygen demand; NH3, Ammonia; NH4+, Ammonium; PO43−, Phosphate; TN, Total nitrogen; TOC, Total organic carbon; TP, Total phosphorus.
The effect of light in a hybrid microalgal-bacterial consortia on NH4+-based competition and microalgal growth was evaluated by González-Camejo et al. [173]. In case of NH4+-N removal, light intensity boosted NH4+-N assimilation by microalgae over NH4+-N oxidation by nitrifying bacteria due to microalgal proliferation. The upward pattern was observed for biomass productivity with the increase of light intensity reaching up to 94.3 mg VSS L−1 d−1 at a light intensity of 125 μE m−1 s−1. The rise of luminous intensity exhibited utmost COD removal from dairy WW [181]. Arcila and Buitrón [182] demonstrated that the utmost values of biomass productivity, dissolved oxygen (DO), PO43−-P removal (92%), and pH were attained under high irradiance conditions with a temperature of 26 °C, and these conditions impeded the nitrification process. The enhanced performance of P removal was attributed to the P precipitation mechanism under higher pH values. Conversely, with low irradiance levels, higher removal efficiencies for TN (60%) and COD (89%) were observed with moderate PO43−-P removal (28%).
The effect of the light/dark regime and initial sludge/microalgae ratio for treating domestic WW using a hybrid consortium of Chlorella sp. and activated sludge were investigated [177]. The hybrid culture exhibited superior nutrients and COD removal, better settleability, as well as less oxygen consumption, compared to activated sludge only under the condition of 12 h light/12 h dark with five days of cultivation with an initial sludge/microalgae ratio of 1:2, showing the economic and practical feasibility of Chlorella sp. and activated sludge cooperation as a less-dependent light system for treating domestic WW.
Temperature and pH are important factors that need to be optimized for microalgae-based treatment systems. González-Camejo et al. [173] proved that the microalgal-bacterial treatment system was efficient in N and P removal at around 22 °C. The increase in temperature led to the proliferation of nitrifying bacteria resulting in the domination of NH4+-N removal by these bacteria over microalgae assimilation. Valizadeh and Davarpanah [181] mentioned that the optimum pH for COD removal from dairy WW was 8.0, and a further increase in pH values would diminish the microalgal proliferation rate. In addition, a higher pH value can impede microalgae metabolism and nutrient uptake through assimilation [183,184].
Nutrient concentrations and C/N and N/P ratios could affect microalgal growth and hence the treatment performance. The nutrient concentrations remarkedly oscillated according to the withdrawal points of the municipal WWT plant. Wang et al. [185] concluded that microalgae cultivation in nutrient-rich sewage sludge was more convenient from the viewpoint of microalgal growth and the removal of COD, PO43−-P, NO3−-N, and NH4+-N compared to primary- and secondary-treated effluents. Zheng et al. [186] mixed piggery WW with brewery WW to determine the optimal C/N ratio for nutrient removal and microalgal growth. At an optimum C/N ratio of 7.9, NH4+-N, TN, TP, and COD removal efficiencies reached 100%, 96%, 90%, and 93%, respectively. The cultivation of Scenedesmus sp. in molasses WW with a C/N ratio of 15 achieved better treatment with removal efficiencies of 87.2%, 90.5%, and 88.6% for COD, TN, and TP, respectively, at 10 °C [187]. AlMomani and Örmeci [188] studied the feasibility of utilizing C. vulgaris, Neochloris oleoabundans, and mixed indigenous microalgae in terms of microalgal growth and removal of organic carbon, N, and P from primary effluent, secondary effluent, and centrate. These results indicated that the suitable culture for the growth rate varied for different WW sampling points, and none of the WW types was the most favored for cultivating all microalgal species. The highest growth rate for C. vulgaris and N. oleoabundans was observed in primary WW with a C:N:P ratio of 24:5:1 and for mixed microalgae culture in secondary sludge with a C:N:P ratio of 4.4:1:1.5. Therefore, nutrient structure and its ratios in different municipal effluents in microalgae treatment systems should be completely evaluated. You et al. [189] stated that nutrient concentrations and ratios could be balanced by mixing different WW types to meet the standards of WW discharges. The impact of emerging pollutants on the treatment performance should also be studied.
5.2.2. Removal of pathogens
The existence of pathogens in WW causes severe threats to public health associated with the potential outbreak of many diseases [18]. Microalgae exhibit a promising approach for pathogenic microorganisms’ removal and inactivation through different mechanisms, including nutrient competition, pH elevation and oxygenation, attachment and sedimentation, and microalgal toxins [190]. Microalgae devour nutrients and carbon from water which are the core energy components for bacterial growth. The depletion of these components by microalgal assimilation may be conducive to bacteria starvation leading finally to the death of bacteria [190].
Photosynthesis required for microalgal growth results in pH elevation and the richness of oxygen concentration in the WW. These changes in pH and DO levels can deactivate pathogen growth in WW. Mezzari et al. [191] reported that the inhibition of antibiotic multi-resistant Salmonella typhimurium within 48 h was allied with amendable pH and DO levels resulting from the microalgal photosynthetic activity. In a recent study reported by Liu et al. [52], it was shown that two microalgal species, namely Mougeotia sp. and Hydrodicty sp., had the capability of increasing pH and DO levels in WW stabilization ponds affecting the removal of E. coli, total coliforms, Enterococci and Clostridium perfringens. A maximum inactivation rate was attained at pH 10.5 for all pathogens except Enterococci at pH 4.0. The reduction of E. coli and total coliforms was observed at high (20 mg L−1) and low (1 mg L−1) DO concentrations, while these pathogens can survive at medium (8.6 mg L−1) DO concentrations. Enterococci showed a high inactivation resistance at low DO levels, while it was deactivated at high DO. Conversely, C. perfringens was slightly decreased with the increase of DO. Alsenani et al. [192] stated that extracts of three microalgae species exhibited strong antimicrobial activities against gram-positive bacteria, while no inhibition was observed with gram-negative bacteria. Additionally, he revealed the reason for the antibacterial activity difference was due to the different structures of the cell wall, with the outer membrane of gram-negative species acting as a barrier.
The effect of microalgal biomass concentrations on fecal coliform (FC) destruction in different WW (weak/medium strength and a mixture of treated and raw WW) under light and dark mode was assessed [193]. Under light conditions, the FC decay rate had an upward trend with the increase in chlorophyll-a concentration as light promotes the production of toxic forms of oxygen species within the abundance of DO damaging the cytoplasmic membrane of bacteria. Light-mediated damage is also stimulated by elevated pH resulting from microalgae. Higher decay rates were obtained in medium strength WW relative to low strength WW at a chlorophyll-a concentration of 13.9 mg L−1. However, adding raw WW to the treated WW lowered the decay rate because of the pH drop and DO depletion. On the other hand, Ansa et al. [193] attributed the FC decay in darkness to producing a substance by microalgae which may impede FC growth. It has also been reported that some microalgal species produce a toxin named microcystin-LR, which has detrimental effects on pathogens and fecal bacteria [194]. On the other hand, microalgae can cooperate with fungi for nutrients and HMs removal from WW [195].
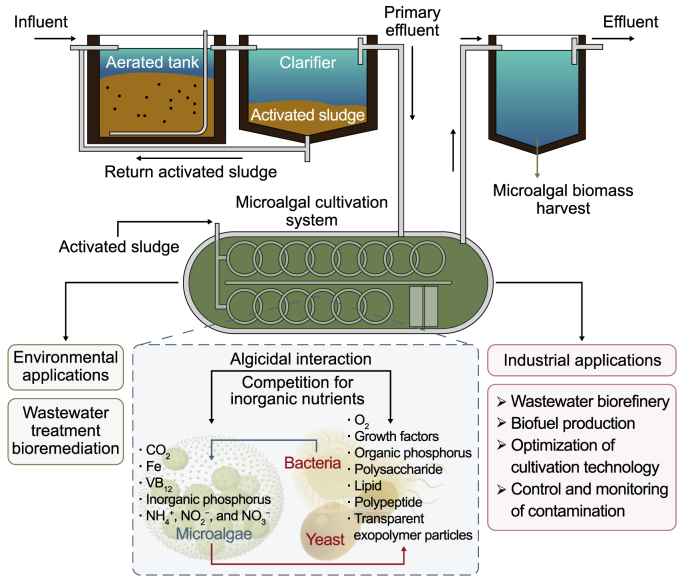
6. Co-culturing of microalgae for wastewater treatment
Recently, the integration of microalgae with activated sludge, bacteria, fungi, and nanoparticles has been widely adopted to benefit from a co-culture system during bioremediation. These possible co-culture systems will be introduced as follows:
6.1. Microalgae-bacteria
Carbon fixation by microalgae through photosynthesis can release organic carbon and oxygen, and then heterotrophic bacteria degrade a carbon source in an abundance of oxygen into CO2, which is needed as a reagent in photosynthesis. This synergistic relationship (Fig. 9) can be exploited to enrich biomass production and nutrient removal from WW [196]. In a sequencing batch bioreactor, microalgae were incorporated to maximize nutrient uptake. The results indicated that microalgal-bacterial symbiosis could promote TN removal from 38.5% to 65.8% and TP removal from 31.9% to 89.3% [197]. Compared with a microalgae-only system, the co-culture of Chlorella with WW-borne bacteria (Bacillus firmus and Beijerinckia fluminensis) can increase the removal efficiencies of COD, TN, and TP from vinegar production WW by 22.1%, 20.0%, and 18.1%, respectively [198]. The co-culture comprising microalgae C. sorokiniana with bacteria Pusillimonas cultivated on striped food waste permeates from an anaerobic digester effluent can decrease TN, NH4+, and COD by 34–67%, 65–97%, and 14–60%, respectively [199].
Fig. 9.
Potential microalgal hydride cultivation systems applications in industrial and environmental applications.
6.2. Microalgae-activated sludge
The superiority of microalgae-activated sludge association in WWT (Fig. 9) emanated from microalgal assimilation of nutrients and COD removal enhancement via activated sludge, surpassing the conventional mono-systems [200]. The sludge/microalgae ratio depends on WW characteristics and composition. In municipal WW, at a low sludge/microalgae ratio (0.5), the growth of microalgae doubled, and more nutrient removal was achieved (66% removal of N and 100% of P in one day) relative to pure microalgal culture [201]. In another study, Mujtaba et al. [202] used the co-culture of suspended activated sludge and immobilized C. vulgaris in a single reactor, and the enhancement in nutrients removal was observed with the decrease of inoculum ratio of activated sludge/C. vulgaris reaching an utmost value of 0.5% with N and P removals of 99.8% and 100%, respectively, within two days. Conversely, the differences in carbon matter degradation at different inoculum ratios were negligible in the range of 90–95% COD removal. In a mixed culture of the photobioreactor, the microalgae/activated sludge ratio of 3:1 was selected to attain efficient organics and nutrient removal (86% TN, 70% TP, and 99% COD) [200].
6.3. Microalgae-fungi
Many studies have also investigated co-culturing microalgae with fungi and their symbiotic role in WW bioremediation. The synergic interaction between microalgae and fungi/yeast is abbreviated as generating O2 by microalgae for biomass synthesis. Then yeast utilizes O2 for cellular respiration resulting in carbon substance disintegration releasing CO2 required for microalgal photosynthesis [203]. Walls et al. [204] co-cultured microalgae with yeast supplemented with glucose to achieve high biomass production and nutrient removal rates. The heterotrophic cultivation with 10 and 20 g L−1 glucose yielded 1.85 and 2.74 g L−1 biomass concentration and removed 91% and 94% orthophosphate, 93% and 97% nitrate, and 93% and 95% total NH4+-N in three days, respectively [204]. A hybrid culture consisting of C. vulgaris and Aspergillus sp. in molasses WW can remove 70.7% COD, 67.1% TN, 94.7% NH4+-N, and 88.4% TP versus 26%, 44.4%, 79%, and 33% for mono-microalgae and 59%, 18.2%, 19%, and 40% for mono-fungi [205]. Co-culturing Chlorella sp. with Penicillium sp. by treating the byproduct of the hydrothermal carbonization of wet biomass can achieve 46.13% COD, 13% TN, 6% NH4+-N, and 88.4% TP [2]. Consequently, the co-cultivation system performs better relative to a mono-system of microalgae in removing nutrients in WW.
6.4. Microalgae-nanoparticles
With numerous advantages of high surface-to-volume ratio, porosity, and biocompatibility, qualified nanofibers are considered ideal supporting carriers over other conventional supporting materials for the immobilization and encapsulation of microorganisms, including microalgae, to produce bio-integrated hybrid materials with higher efficiency of pollutants removal, ease of application, and reusability [18]. Eroglu et al. [206] utilized a cross-linked chitosan nanofiber mat in immobilizing microalga C. vulgaris as a nontoxic support surface exhibiting diverse advantages associated with NO3− removal from water and combined with microalgal harvesting, dewatering, and processing steps into a single stage. The microalgal-immobilized chitosan nanofiber featured simplicity, robustness with six months of contact time with water, and a higher elimination rate of nitrate (87 ± 4%) compared with 32 ± 3% for non-immobilized nanofiber. The biocomposite nanofiber exhibited superior decolorization capacity achieving a 72.97% and 30.25% reduction for RB5 and RB221, respectively, versus 12.36% and 5.51%, respectively, for a pristine polysulfone nanofiber [207]. Vasistha et al. [208] incorporated microalgae with ZnO nanoparticles, and the combination was evaluated in terms of nutrient reductions from primary and secondary treated sewage WW. Co-cultured bionanofiber achieved removal efficiencies of 97.5%, 87.20%, 82.21% and 95.3%, 85.2%, 81.5% for TOC, TN, and TP from primary and secondary treated sewage WW, respectively. Luo et al. [209] used nano-TiO2 in a microalgae-TiO2 coupling system to improve the utilization of humic acid by microalgae from piggery biogas slurry, eventually removing about 50% of the humic acid.
7. Recommendations and future perspectives
Microalgal WWT technologies in biorefinery and valorization applications still face challenges, such as difficult harvesting requirements, high demand for specific nutrients, contamination by other microorganisms, immature downstream processing, and complicated operation and monitoring of the whole system.
-
(1)
Most of the referenced studies and research do not go beyond pilot scales, and bringing them to an industrial scale will expose microalgae to harsher environmental and seasonal conditions and variations in pollutant concentrations, which can reduce the treatment efficiency and biomass yield. As a result, innovative and resilient microalgal consortia with greater adaptation to challenging environmental conditions while maintaining the ability to degrade pollutants should be investigated.
-
(2)
Further genetic/metabolic engineering studies should be conducted to ensure the generation of genome-modified microalgal cells with improved qualities related to unfavorable circumstances adaption and higher performance for pollutant removal and bioproduct yields.
-
(3)
Separating microalgal biomass from treated WW after bioremediation is a major challenge, especially in suspended cultivation. To deal with this, attaching microalgae cultivation to a media/supporter might be applied, making the separation process more accessible and reducing the hydraulic retention time.
-
(4)
Like the attached growth process for biological WWT, the attached cultivation has several problems, including fouling and the need for mechanical biomass collecting systems, which should be considered and solved.
-
(5)
Economic feasibility studies of microalgae-based WWT and comparisons to traditional methods must be examined, as only a few studies have been accomplished. On the other hand, the lack of basic design and operation guidelines for microalgae-based WWT motivates researchers to work harder to provide basic guidance and recommendations to increase the resilience and flexibility of microalgal strains to deal with various types of WW.
-
(6)
Researchers used microalgae to reduce typical pollutants such as nutrients and HMs in the abovementioned studies. As a result, it is necessary to conduct microalgae to further remediate dyes, other HMs, and emerging pollutants such as personal care products and antibiotics. Furthermore, several studies have focused on using microalgae to remove extra nutrients from secondary treated wastewater. As a result, additional research on introducing microalgae into the biological treatment process and a thorough understanding of the interactions between microalgae and existing bacteria in WW are needed.
-
(7)
One of the main limitations of large-scale wastewater treatment plants (WWTPs) is inhibiting microalgal growth due to the WW's dark color and hazardous pollutants. To overcome this challenge, it is suggested to control the influents with proper input and/or to pretreat before introducing microalgae. Also, the vast diversity of nutrients and their strength in WW require several pretreatments to achieve an ideal nutrient balance for the microalgae. In such cases, researchers should utilize optimized mixtures of various sources of WW as a single, well-balanced nutrient media for microalgae.
-
(8)
Estimating industrial WW and microalgae cultivation modes might differ according to geographic zones due to sunlight availability and temperature variations. Therefore, colder zones should focus on photobioreactor-based microalgae cultivation with the aim of high-value-added product generation from microalgae grown on WW.
-
(9)
Another main challenge that faces the operation of the large-scale WWTPs, especially the microalgae-based WWT processes, is the complicated operation and monitoring of the whole process (e.g., pH, temperature, microalgae cells conditions, BOD, and DO), which requires developing new technologies such as online monitoring and remote control.
8. Conclusions
Since the first step towards utilizing microalgae in WWT, a considerable number of research efforts have been directed towards developing this promising application. Microalgae can serve as a remarkable role-player while used in WWT, being an affordable process with multiple benefits. Microalgae have a great capacity to eradicate diverse classes of pollutants and hazardous materials generated from domestic agricultural runoffs, effluents, textile, printing, pharmaceutical, and electroplating industries via several mechanisms. The bioremediation in microalgae does not produce secondary pollution. Moreover, the disposed microalgal biomass can be used as raw materials to produce medicines, biofuel, fertilizers, and nutritional food with significant economic benefits. Despite prior studies showing that microalgae-based WWT outperforms conventional WWT technologies in terms of nutrient and HMs removal, carbon capture, and biomaterial generation, their widespread application still faces some challenges. Therefore, WWT using microalgae and biorefinery applications calls for further studies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31772529), the National Key R&D Program of China (2018YFE0107100), and the Priority of Academic Program Development of Jiangsu Higher Education Institutions (PAPD 4013000011).
Contributor Information
Sameh Samir Ali, Email: samh@ujs.edu.cn, samh_samir@science.tanta.edu.eg.
Shih-Hsin Ho, Email: stephen6949@hit.edu.cn.
Jianzhong Sun, Email: jzsun1002@ujs.edu.cn.
References
- 1.Morseletto P., Mooren C.E., Munaretto S. Circular economy of water: definition, strategies and challenges. Circ. Econ. Sustain. 2022:1–5. [Google Scholar]
- 2.Chen M., Chang L., Zhang J., Guo F., Vymazal J., He Q., Chen Y. Global nitrogen input on wetland ecosystem: the driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020;4 doi: 10.1016/j.ese.2020.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Luo D., Zhang X., Huang R., Cao Y., Liu G., Zhang Y., Wang H. Biochar-based slow-release of fertilizers for sustainable agriculture: a mini review. Environ. Sci. Ecotechnol. 2022;10 doi: 10.1016/j.ese.2022.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hena S., Gutierrez L., Croué J.P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: a review. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.124041. [DOI] [PubMed] [Google Scholar]
- 5.Mo Z., Tai D., Zhang H., Shahab A. A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water. Chem. Eng. J. 2022;443 [Google Scholar]
- 6.Abidli A., Huang Y., Rejeb Z.B., Zaoui A., Park C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: recent progress, challenges, and future perspectives. Chemosphere. 2022;292 doi: 10.1016/j.chemosphere.2021.133102. [DOI] [PubMed] [Google Scholar]
- 7.Singh D.V., Bhat R.A., Upadhyay A.K., Singh R., Singh D.P. Microalgae in aquatic environs: a sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innovat. 2021;21 [Google Scholar]
- 8.Munyaneza J., Jia Q., Qaraah F.A., Hossain M.F., Wu C., Zhen H., Xiu G. A review of atmospheric microplastics pollution: in-depth sighting of sources, analytical methods, physiognomies, transport and risks. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153339. [DOI] [PubMed] [Google Scholar]
- 9.Ali S.S., Elsamahy T., Al-Tohamy R., Zhu D., Mahmoud Y.A., Koutra E., Metwally M.A., Kornaros M., Sun J. Plastic wastes biodegradation: mechanisms, challenges and future prospects. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146590. [DOI] [PubMed] [Google Scholar]
- 10.Ali S.S., Elsamahy T., Koutra E., Kornaros M., El-Sheekh M., Abdelkarim E.A., Zhu D., Sun J. Degradation of conventional plastic wastes in the environment: a review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021;771 doi: 10.1016/j.scitotenv.2020.144719. [DOI] [PubMed] [Google Scholar]
- 11.Mastropetros S.G., Koutra E., Amouri M., Aziza M., Ali S.S., Kornaros M. Comparative assessment of nitrogen concentration effect on microalgal growth and biochemical characteristics of two chlorella strains cultivated in digestate. Mar. Drugs. 2022;20:415. doi: 10.3390/md20070415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastropetros S.G., Pispas K., Zagklis D., Ali S.S., Kornaros M. Biopolymers production from microalgae and cyanobacteria cultivated in wastewater: recent advances. Biotechnol. Adv. 2022;60 doi: 10.1016/j.biotechadv.2022.107999. [DOI] [PubMed] [Google Scholar]
- 13.Koutra E., Mastropetros S.G., Ali S.S., Tsigkou K., Kornaros M. Assessing the potential of Chlorella vulgaris for valorization of liquid digestates from agro-industrial and municipal organic wastes in a biorefinery approach. J. Clean. Prod. 2021;280 [Google Scholar]
- 14.Al-Tohamy R., Ali S.S., Li F., Okasha K.M., Mahmoud Y.A., Elsamahy T., Jiao H., Fu Y., Sun J. A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022;231 doi: 10.1016/j.ecoenv.2021.113160. [DOI] [PubMed] [Google Scholar]
- 15.Basu S., Dutta A., Mukherjee S.K., Hossain S.T. New Trends in Removal of Heavy Metals from Industrial Wastewater. Elsevier; 2021. Exploration of green technology for arsenic removal from groundwater by oxidation and adsorption using arsenic-oxidizing bacteria and metal nanoparticles; pp. 177–211. [Google Scholar]
- 16.El-Aswar E.I., Zahran M.M., El-Kemary M. Optical and electrochemical studies of silver nanoparticles biosynthesized by Haplophyllum tuberculatum extract and their antibacterial activity in wastewater treatment. Mater. Res. Express. 2019;6 [Google Scholar]
- 17.El-Aswara E.I., Gaber S.E., Zahran M.M. Characterization of biosynthesized silver nanoparticles by Haplophyllum tuberculatum plant extract under microwave irradiation and detecting their antibacterial activity against some wastewater microbes. Desalination Water Treat. 2020;195:275–285. [Google Scholar]
- 18.El-Aswar E.I., Ramadan H., Elkik H., Taha A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022;301 doi: 10.1016/j.jenvman.2021.113908. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf A., Sodiq A., Giwa A., Eke J., Pikuda O., De Luca G., Di Salvo J.L., Chakraborty S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020;266 [Google Scholar]
- 20.Daud N.M., Abdullah S.R., Hasan H.A., Dhokikah Y. Integrated physical-biological treatment system for batik industry wastewater: a review on process selection. Sci. Total Environ. 2022;819 doi: 10.1016/j.scitotenv.2022.152931. [DOI] [PubMed] [Google Scholar]
- 21.Dalvi V., Naaz F., Nigam H., Jain R., Samuchiwal S., Kalia S., Kumar R., Mathur M., Bano F., Malik A., Singh A. Removal of pollutants from wastewater via biological methods and shifts in microbial community profile during treatment process. Wastewater Treatment Reactors. 2021:19–38. [Google Scholar]
- 22.Al-Tohamy R., Kenawy E.R., Sun J., Ali S.S. Performance of a newly isolated salt-tolerant yeast strain Sterigmatomyces halophilus SSA-1575 for azo dye decolorization and detoxification. Front. Microbiol. 2020;11:1163. doi: 10.3389/fmicb.2020.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Tohamy R., Sun J., Fareed M.F., Kenawy E.R., Ali S.S. Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmatomyces halophilus SSA1575, valued for textile azo dye wastewater processing and detoxification. Sci. Rep. 2020;10:1–6. doi: 10.1038/s41598-020-69304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S.S., Shaaban M.T., Abomohra A.E., El-Safity K. Macroalgal activity against multiple drug resistant Aeromonas hydrophila: a novel treatment study towards enhancement of fish growth performance. Microb. Pathog. 2016;101:89–95. doi: 10.1016/j.micpath.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Ali S.S., Al-Tohamy R., Sun J., Wu J., Huizi L. Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel. 2019;236:1128–1145. [Google Scholar]
- 26.Ali S.S., Al-Tohamy R., Xie R., El-Sheekh M.M., Sun J. Construction of a new lipase-and xylanase-producing oleaginous yeast consortium capable of reactive azo dye degradation and detoxification. Bioresour. Technol. 2020;313 doi: 10.1016/j.biortech.2020.123631. [DOI] [PubMed] [Google Scholar]
- 27.Ali S.S., Al-Tohamy R., Koutra E., El-Naggar A.H., Kornaros M., Sun J. Valorizing lignin-like dyes and textile dyeing wastewater by a newly constructed lipid-producing and lignin modifying oleaginous yeast consortium valued for biodiesel and bioremediation. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123575. [DOI] [PubMed] [Google Scholar]
- 28.Ali S.S., Sun J., Koutra E., El-Zawawy N., Elsamahy T., El-Shetehy M. Construction of a novel cold-adapted oleaginous yeast consortium valued for textile azo dye wastewater processing and biorefinery. Fuel. 2021;285 [Google Scholar]
- 29.Darwesh O.M., Ali S.S., Matter I.A., Elsamahy T. Nanosensors and Nanodevices for Smart Multifunctional Textiles. Elsevier; 2021. Nanotextiles waste management: controlling of release and remediation of wastes; pp. 267–286. [Google Scholar]
- 30.Danso B., Ali S.S., Xie R., Sun J. Valorisation of wheat straw and bioethanol production by a novel xylanase-and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel. 2022;310 [Google Scholar]
- 31.Pereliaeva E.V., Dmitrieva M.E., Morgunova M.M., Belyshenko A.Y., Imidoeva N.A., Ostyak A.S., Axenov-Gribanov D.V. The use of Baikal psychrophilic actinobacteria for synthesis of biologically active natural products from sawdust waste. Fermentation. 2022;8:213. [Google Scholar]
- 32.Roy M., Saha R. Dyes and their removal technologies from wastewater: a critical review. Intell. Environ. Data Monit. Pollut. Manag. 2021:127–160. [Google Scholar]
- 33.López-Sánchez A., Silva-Gálvez A.L., Aguilar-Juárez Ó., Senés-Guerrero C., Orozco-Nunnelly D.A., Carrillo-Nieves D., Gradilla-Hernández M.S. Microalgae-based livestock wastewater treatment (MbWT) as a circular bioeconomy approach: enhancement of biomass productivity, pollutant removal and high-value compound production. J. Environ. Manag. 2022;308 doi: 10.1016/j.jenvman.2022.114612. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad I., Abdullah N., Koji I., Yuzir A., Mohamad S.E. Potential of microalgae in bioremediation of wastewater. Bull. Chem. React. Eng. Catal. 2021;16:413–429. [Google Scholar]
- 35.El-Sheekh M., Abdel-Daim M.M., Okba M., Gharib S., Soliman A., El-Kassas H. Green technology for bioremediation of the eutrophication phenomenon in aquatic ecosystems: a review. Afr. J. Aquat. Sci. 2021;46:274–292. [Google Scholar]
- 36.Priyadharshini S.D., Babu P.S., Manikandan S., Subbaiya R., Govarthanan M., Karmegam N. Phycoremediation of wastewater for pollutant removal: a green approach to environmental protection and long-term remediation. Environ. Pollut. 2021;290 doi: 10.1016/j.envpol.2021.117989. [DOI] [PubMed] [Google Scholar]
- 37.Chia W.Y., Tang D.Y., Khoo K.S., Lup A.N., Chew K.W. Nature's fight against plastic pollution: algae for plastic biodegradation and bioplastics production. Environ. Sci. Ecotechnol. 2020;4 doi: 10.1016/j.ese.2020.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Show P.L., Ngo H.H., Ho S.H. Algae-mediated antibiotic wastewater treatment: a critical review. Environ. Sci. Ecotechnol. 2022;9 doi: 10.1016/j.ese.2022.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray A., Nayak M., Ghosh A. A review on co-culturing of microalgae: a greener strategy towards sustainable biofuels production. Sci. Total Environ. 2022;802 doi: 10.1016/j.scitotenv.2021.149765. [DOI] [PubMed] [Google Scholar]
- 40.Santos F.M., Gonçalves A.L., Pires J.C. Academic Press; 2019. Negative Emission Technologies. InBioenergy with Carbon Capture and Storage; pp. 1–13. [Google Scholar]
- 41.Martinez-Porchas M., Martinez-Cordova L.R., Lopez-Elias J.A., Porchas-Cornejo M.A. Microbial Biodegradation and Bioremediation. Elsevier; 2014. Bioremediation of aquaculture effluents; pp. 539–553. [Google Scholar]
- 42.Tran D.T., Van Do T.C., Nguyen Q.T., Le T.G. Simultaneous removal of pollutants and high value biomaterials production by Chlorella variabilis TH03 from domestic wastewater. Clean Technol. Environ. Policy. 2021;23:3–17. [Google Scholar]
- 43.SundarRajan P., Gopinath K.P., Arun J., GracePavithra K., Pavendan K., AdithyaJoseph A. An insight into carbon balance of product streams from hydrothermal liquefaction of Scenedesmus abundans biomass. Renew. Energy. 2020;151:79–87. [Google Scholar]
- 44.Ling Y., Sun L.P., Wang S.Y., Lin C.S., Sun Z., Zhou Z.G. Cultivation of oleaginous microalga Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochem. Eng. J. 2019;148:162–169. [Google Scholar]
- 45.El Asli A., El Mesbahi N., Oubakalla R., El Fels L., Hafidi M., Liang Y. Domestic wastewater treatment and lipid accumulation for biodiesel production by an isolated heterotrophic microalgae from an arid climate zone. Asia J. Appl. Microbiol. 2019;6:1–9. [Google Scholar]
- 46.Tripathi R., Gupta A., Thakur I.S. An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae. Scenedesmus sp. ISTGA1. Renewable Energy. 2019;135:617–625. [Google Scholar]
- 47.Arias D.M., Solé-Bundó M., Garfi M., Ferrer I., García J., Uggetti E. Integrating microalgae tertiary treatment into activated sludge systems for energy and nutrients recovery from wastewater. Bioresour. Technol. 2018;247:513–519. doi: 10.1016/j.biortech.2017.09.123. [DOI] [PubMed] [Google Scholar]
- 48.de Souza Leite L., Hoffmann M.T., Daniel L.A. Microalgae cultivation for municipal and piggery wastewater treatment in Brazil. J. Water Proc. Eng. 2019;31 [Google Scholar]
- 49.Al-Jabri H., Das P., Khan S., Thaher M., AbdulQuadir M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—a review. Water. 2020;13:27. [Google Scholar]
- 50.Geremia E., Ripa M., Catone C.M., Ulgiati S. A review about microalgae wastewater treatment for bioremediation and biomass production—a new challenge for Europe. Environments. 2021;8:136. [Google Scholar]
- 51.Laraib N., Hussain A., Javid A., Hafeez-ur-Rehman M., Bukhari S.M., Rashid M., Ali W. Microorganisms for Sustainable Environment and Health. Elsevier; 2020. Recent advancements in microalgal-induced remediation of wastewaters; pp. 205–217. [Google Scholar]
- 52.Liu L., Hall G., Champagne P. The role of algae in the removal and inactivation of pathogenic indicator organisms in wastewater stabilization pond systems. Algal Res. 2020;46 [Google Scholar]
- 53.Sawayama S., Rao K.K., Hall D.O. Nitrate and phosphate ion removal from water by Phormidium laminosum immobilized on hollow fibres in a photobioreactor. Appl. Microbiol. Biotechnol. 1998;49:463–468. [Google Scholar]
- 54.Chen X., Li Z., He N., Zheng Y., Li H., Wang H., Wang Y., Lu Y., Li Q., Peng Y. Nitrogen and phosphorus removal from anaerobically digested wastewater by microalgae cultured in a novel membrane photobioreactor. Biotechnol. Biofuels. 2018;11 doi: 10.1186/s13068-018-1190-0. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chai W.S., Tan W.G., Munawaroh H.S., Gupta V.K., Ho S.H., Show P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: a review. Environ. Pollut. 2021;269 doi: 10.1016/j.envpol.2020.116236. [DOI] [PubMed] [Google Scholar]
- 56.Hashemian M., Ahmadzadeh H., Hosseini M., Lyon S., Pourianfar H.R. Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts. Woodhead Publishing; 2019. Production of microalgae-derived high-protein biomass to enhance food for animal feedstock and human consumption; pp. 393–405. [Google Scholar]
- 57.Jackrel S.L., Narwani A., Bentlage B., Levine R.B., Hietala D.C., Savage P.E., Oakley T.H., Denef V.J., Cardinale B.J. Ecological engineering helps maximize function in algal oil production. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00953-18. e00953-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li G., Lu Z., Zhang J., Li H., Zhou Y., Zayan A.M., Huang Z. Life cycle assessment of biofuel production from microalgae cultivated in anaerobic digested wastewater. Int. J. Agric. Biol. Eng. 2020;13:241–246. [Google Scholar]
- 59.Sutherland D.L., Heubeck S., Park J., Turnbull M.H., Craggs R.J. Seasonal performance of a full-scale wastewater treatment enhanced pond system. Water Res. 2018;136:150–159. doi: 10.1016/j.watres.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Rempel A., Gutkoski J.P., Nazari M.T., Biolchi G.N., Cavanhi V.A., Treichel H., Colla L.M. Current advances in microalgae-based bioremediation and other technologies for emerging contaminants treatment. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2020.144918. [DOI] [PubMed] [Google Scholar]
- 61.Mustafa S., Bhatti H.N., Maqbool M., Iqbal M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: prospects, challenges and opportunities. J. Water Proc. Eng. 2021;41 [Google Scholar]
- 62.Chan S.S., Khoo K.S., Chew K.W., Ling T.C., Show P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: microalgae-bacteria consortium-A review. Bioresour. Technol. 2022;344 doi: 10.1016/j.biortech.2021.126159. [DOI] [PubMed] [Google Scholar]
- 63.Crini G., Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019;17:145–155. [Google Scholar]
- 64.Kalra R., Gaur S., Goel M. Microalgae bioremediation: a perspective towards wastewater treatment along with industrial carotenoids production. J. Water Proc. Eng. 2021;40 [Google Scholar]
- 65.Mohsenpour S.F., Hennige S., Willoughby N., Adeloye A., Gutierrez T. Integrating micro-algae into wastewater treatment: a review. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.142168. [DOI] [PubMed] [Google Scholar]
- 66.Aron N.S., Khoo K.S., Chew K.W., Veeramuthu A., Chang J.S., Show P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Proc. Eng. 2021;39 [Google Scholar]
- 67.Koutra E., Economou C.N., Tsafrakidou P., Kornaros M. Bio-based products from microalgae cultivated in digestates. Trends Biotechnol. 2018;36:819–833. doi: 10.1016/j.tibtech.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Koutra E., Grammatikopoulos G., Kornaros M. Selection of microalgae intended for valorization of digestate from agro-waste mixtures. Waste Manag. 2018;73:123–129. doi: 10.1016/j.wasman.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 69.Mantzorou A., Navakoudis E., Paschalidis K., Ververidis F. Microalgae: a potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018;15:1815–1830. [Google Scholar]
- 70.George B., Pancha I., Desai C., Chokshi K., Paliwal C., Ghosh T., Mishra S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus–A potential strain for bio-fuel production. Bioresour. Technol. 2014;171:367–374. doi: 10.1016/j.biortech.2014.08.086. [DOI] [PubMed] [Google Scholar]
- 71.Ortiz Montoya E.Y., Casazza A.A., Aliakbarian B., Perego P., Converti A., de Carvalho J.C. Production of Chlorella vulgaris as a source of essential fatty acids in a tubular photobioreactor continuously fed with air enriched with CO2 at different concentrations. Biotechnol. Prog. 2014;30:916–922. doi: 10.1002/btpr.1885. [DOI] [PubMed] [Google Scholar]
- 72.El-Sheekh M.M., Metwally M.A., Allam N., Hemdan H.E. Simulation treatment of industrial wastewater using microbiological cell immobilization technique. Iran. J. Sci. Technol. Trans. A-Science. 2020;44:595–604. [Google Scholar]
- 73.Soto-Ramírez R., Lobos M.G., Córdova O., Poirrier P., Chamy R. Effect of growth conditions on cell wall composition and cadmium adsorption in Chlorella vulgaris: a new approach to biosorption research. J. Hazard Mater. 2021;411 doi: 10.1016/j.jhazmat.2021.125059. [DOI] [PubMed] [Google Scholar]
- 74.Pennesi C., Rindi F., Totti C., et al. In: Marine Macrophytes: Biosorbents BT - Springer Handbook of Marine Biotechnology. Kim S.-K., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2015. pp. 597–610. [Google Scholar]
- 75.Kumar V., Jaiswal K.K., Verma M., Vlaskin M.S., Nanda M., Chauhan P.K., Singh A., Kim H. Algae-based sustainable approach for simultaneous removal of micropollutants, and bacteria from urban wastewater and its real-time reuse for aquaculture. Sci. Total Environ. 2021;774 [Google Scholar]
- 76.Wang J., Chen C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Mathew B.B., Jaishankar M., Biju V.G., Beeregowda K.N. Role of bioadsorbents in reducing toxic metals. J. Toxicol. 2016;2016 doi: 10.1155/2016/4369604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nateras-Ramírez O., Martinez-Macias M.R., Sánchez-Machado D.I., López-Cervantes J., Aguilar-Ruiz R.J. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresour. Technol. Rep. 2021;17 [Google Scholar]
- 79.Erofeeva E.A. Environmental hormesis: from cell to ecosystem. Curr. Opin. Environ. Sci. Health. 2022 doi: 10.1016/j.coesh.2022.100378. [DOI] [Google Scholar]
- 80.Matamoros V., Gutiérrez R., Ferrer I., García J., Bayona J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J. Hazard Mater. 2015;288:34–42. doi: 10.1016/j.jhazmat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Xiao R., Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016;34:1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Mota R., Rossi F., Andrenelli L., Pereira S.B., De Philippis R., Tamagnini P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016;100:7765–7775. doi: 10.1007/s00253-016-7602-9. [DOI] [PubMed] [Google Scholar]
- 83.Mona S., Kaushik A. Chromium and cobalt sequestration using exopolysaccharides produced by freshwater cyanobacterium Nostoc linckia. Ecol. Eng. 2015;82:121–125. [Google Scholar]
- 84.Chen B., Li F., Liu N., Ge F., Xiao H., Yang Y. Role of extracellular polymeric substances from Chlorella vulgaris in the removal of ammonium and orthophosphate under the stress of cadmium. Bioresour. Technol. 2015;190:299–306. doi: 10.1016/j.biortech.2015.04.080. [DOI] [PubMed] [Google Scholar]
- 85.Liu R., Li S., Tu Y., Hao X. Capabilities and mechanisms of microalgae on removing micropollutants from wastewater: a review. J. Environ. Manag. 2021;285 doi: 10.1016/j.jenvman.2021.112149. [DOI] [PubMed] [Google Scholar]
- 86.Ahmad A., Bhat A.H., Buang A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: kinetic and equilibrium modeling. J. Clean. Prod. 2018;171:1361–1375. [Google Scholar]
- 87.Adhiya J., Cai X., Sayre R.T., Traina S.J. Binding of aqueous cadmium by the lyophilized biomass of Chlamydomonas reinhardtii. Colloids Surf. A Physicochem. Eng. Asp. 2002;210 1-1. [Google Scholar]
- 88.Kim E.J., Park S., Hong H.J., Choi Y.E., Yang J.W. Biosorption of chromium (Cr (III)/Cr (VI)) on the residual microalga Nannochloris oculata after lipid extraction for biodiesel production. Bioresour. Technol. 2011;102:11155–11160. doi: 10.1016/j.biortech.2011.09.107. [DOI] [PubMed] [Google Scholar]
- 89.Andrès Y., Gérente C. Microbial Biosorption of Metals. Springer; Dordrecht: 2011. Removal of rare earth elements and precious metal species by biosorption; pp. 179–196. [Google Scholar]
- 90.Furuhashi Y., Honda R., Noguchi M., Hara-Yamamura H., Kobayashi S., Higashimine K., Hasegawa H. Optimum conditions of pH, temperature and preculture for biosorption of europium by microalgae Acutodesmus acuminatus. Biochem. Eng. J. 2019;143:58–64. [Google Scholar]
- 91.Lee H., Shim E., Yun H.S., Park Y.T., Kim D., Ji M.K., Kim C.K., Shin W.S., Choi J. Biosorption of Cu (II) by immobilized microalgae using silica: kinetic, equilibrium, and thermodynamic study. Environ. Sci. Pollut. Control Ser. 2016;23:1025–1034. doi: 10.1007/s11356-015-4609-1. [DOI] [PubMed] [Google Scholar]
- 92.Das N., Das D. Recovery of rare earth metals through biosorption: an overview. J. Rare Earths. 2013;31:933–943. [Google Scholar]
- 93.Wu Y., Li T., Yang L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: a review. Bioresour. Technol. 2012;107:10–18. doi: 10.1016/j.biortech.2011.12.088. [DOI] [PubMed] [Google Scholar]
- 94.Mojiri A., Baharlooeian M., Kazeroon R.A., Farraji H., Lou Z. Removal of pharmaceutical micropollutants with integrated biochar and marine microalgae. Microorganisms. 2020;9:4. doi: 10.3390/microorganisms9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gobas F.A., Burkhard L.P., Doucette W.J., Sappington K.G., Verbruggen E.M., Hope B.K., Bonnell M.A., Arnot J.A., Tarazona J.V. Review of existing terrestrial bioaccumulation models and terrestrial bioaccumulation modeling needs for organic chemicals. Integrated Environ. Assess. Manag. 2016;12:123–134. doi: 10.1002/ieam.1690. [DOI] [PubMed] [Google Scholar]
- 96.Sen B., Alp M.T., Sonmez F., Kocer M.A., Canpolat O. Relationship of algae to water pollution and waste water treatment. Water Treat. 2013:335–354. [Google Scholar]
- 97.Coogan M.A., Edziyie R.E., La Point T.W., Venables B.J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 98.Sutherland D.L., Ralph P.J. Microalgal bioremediation of emerging contaminants-Opportunities and challenges. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114921. [DOI] [PubMed] [Google Scholar]
- 99.Song C., Wei Y., Qiu Y., Qi Y., Li Y., Kitamura Y. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: experimental study. Bioresour. Technol. 2019;272:529–534. doi: 10.1016/j.biortech.2018.10.080. [DOI] [PubMed] [Google Scholar]
- 100.Rodea-Palomares I., Makowski M., Gonzalo S., González-Pleiter M., Leganés F., Fernández-Piñas F. Effect of PFOA/PFOS pre-exposure on the toxicity of the herbicides 2, 4-D, Atrazine, Diuron and Paraquat to a model aquatic photosynthetic microorganism. Chemosphere. 2015;139:65–72. doi: 10.1016/j.chemosphere.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 101.Xiong J.Q., Kurade M.B., Jeon B.H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2017;313:1251–1257. [Google Scholar]
- 102.Xiong Q., Liu Y.S., Hu L.X., Shi Z.Q., Cai W.W., He L.Y., Ying G.G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020;175 doi: 10.1016/j.watres.2020.115656. [DOI] [PubMed] [Google Scholar]
- 103.Tang J., Wang S., Tai Y., Tam N.F., Su L., Shi Y., Luo B., Tao R., Yang Y., Zhang X. Evaluation of factors influencing annual occurrence, bioaccumulation, and biomagnification of antibiotics in planktonic food webs of a large subtropical river in South China. Water Res. 2020;170 doi: 10.1016/j.watres.2019.115302. [DOI] [PubMed] [Google Scholar]
- 104.Xiong J.Q., Kurade M.B., Abou-Shanab R.A., Ji M.K., Choi J., Kim J.O., Jeon B.H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016;205:183–190. doi: 10.1016/j.biortech.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 105.Xiong J.Q., Kim S.J., Kurade M.B., Govindwar S., Abou-Shanab R.A., Kim J.R., Roh H.S., Khan M.A., Jeon B.H. Combined effects of sulfamethazine and sulfamethoxazole on a freshwater microalga, Scenedesmus obliquus: toxicity, biodegradation, and metabolic fate. J. Hazard Mater. 2019;370:138–146. doi: 10.1016/j.jhazmat.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 106.Leng L., Wei L., Xiong Q., Xu S., Li W., Lv S., Lu Q., Wan L., Wen Z., Zhou W. Use of microalgae based technology for the removal of antibiotics from wastewater: a review. Chemosphere. 2020;238 doi: 10.1016/j.chemosphere.2019.124680. [DOI] [PubMed] [Google Scholar]
- 107.Tiwari B., Sellamuthu B., Ouarda Y., Drogui P., Tyagi R.D., Buelna G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017;224:1–2. doi: 10.1016/j.biortech.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 108.Ding T., Yang M., Zhang J., Yang B., Lin K., Li J., Gan J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard Mater. 2017;330:127–134. doi: 10.1016/j.jhazmat.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Vo H.N., Ngo H.H., Guo W., Nguyen K.H., Chang S.W., Nguyen D.D., Liu Y., Liu Y., Ding A., Bui X.T. Micropollutants cometabolism of microalgae for wastewater remediation: effect of carbon sources to cometabolism and degradation products. Water Res. 2020;183 doi: 10.1016/j.watres.2020.115974. [DOI] [PubMed] [Google Scholar]
- 110.Xiong Q., Hu L.X., Liu Y.S., Zhao J.L., He L.Y., Ying G.G. Microalgae-based technology for antibiotics removal: from mechanisms to application of innovational hybrid systems. Environ. Int. 2021;155 doi: 10.1016/j.envint.2021.106594. [DOI] [PubMed] [Google Scholar]
- 111.Vardhan K.H., Kumar P.S., Panda R.C. A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J. Mol. Liq. 2019;290 [Google Scholar]
- 112.Edelstein M., Ben-Hur M. Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018;234:431–444. [Google Scholar]
- 113.Pavithra K.G., Kumar P.S., Jaikumar V., Vardhan K.H., SundarRajan P. Microalgae for biofuel production and removal of heavy metals: a review. Environ. Chem. Lett. 2020;18:1905–1923. [Google Scholar]
- 114.Kafil M., Berninger F., Koutra E., Kornaros M. Utilization of the microalga Scenedesmus quadricauda for hexavalent chromium bioremediation and biodiesel production. Bioresour. Technol. 2022;346 doi: 10.1016/j.biortech.2021.126665. [DOI] [PubMed] [Google Scholar]
- 115.Abd Ellatif S., El-Sheekh M.M., Senousy H.H. Role of microalgal ligninolytic enzymes in industrial dye decolorization. Int. J. Phytoremediation. 2021;23:41–52. doi: 10.1080/15226514.2020.1789842. [DOI] [PubMed] [Google Scholar]
- 116.Sinaei M., Loghmani M., Bolouki M. Application of biomarkers in brown algae (Cystoseria indica) to assess heavy metals (Cd, Cu, Zn, Pb, Hg, Ni, Cr) pollution in the northern coasts of the Gulf of Oman. Ecotoxicol. Environ. Saf. 2018;164:675–680. doi: 10.1016/j.ecoenv.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 117.Srinivasa Rao P., Kalyani S., Suresh Reddy K.V., Krishnaiah A. Comparison of biosorption of nickel (II) and copper (II) ions from aqueous solution by sphaeroplea algae and acid treated sphaeroplea algae. Separ. Sci. Technol. 2005;40:3149–3165. [Google Scholar]
- 118.Danouche M., El Ghachtouli N., El Arroussi H. Phycoremediation mechanisms of heavy metals using living green microalgae: physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mazur L.P., Cechinel M.A., de Souza S.M., Boaventura R.A., Vilar V.J. Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: a review. J. Environ. Manag. 2018;223:215–253. doi: 10.1016/j.jenvman.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 120.Gunasundari E. Adsorption isotherm, kinetics and thermodynamic analysis of Cu (II) ions onto the dried algal biomass (Spirulina platensis) J. Ind. Eng. Chem. 2017;56:129–144. [Google Scholar]
- 121.Rezaei H. Biosorption of chromium by using Spirulina sp. Arab. J. Chem. 2016 Nov 1;9(6):846–853. [Google Scholar]
- 122.Sibi G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ. 2016;1:172–177. [Google Scholar]
- 123.Abdel-Aty A.M., Ammar N.S., Ghafar H.H., Ali R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013;4:367–374. doi: 10.1016/j.jare.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shanab S., Essa A., Shalaby E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates) Plant Signal. Behav. 2012;7:392–399. doi: 10.4161/psb.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferreira L.S., Rodrigues M.S., De Carvalho J.C., Lodi A., Finocchio E., Perego P., Converti A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011;173:326–333. [Google Scholar]
- 126.Sarı A., Uluozlü Ö.D., Tüzen M. Equilibrium, thermodynamic and kinetic investigations on biosorption of arsenic from aqueous solution by algae (Maugeotia genuflexa) biomass. Chem. Eng. J. 2011;167:155–161. [Google Scholar]
- 127.Ayele A., Getachew D., Kamaraj M., Suresh A. Phycoremediation of synthetic dyes: an effective and eco-friendly algal technology for the dye abatement. J. Chem. 2021:2021. [Google Scholar]
- 128.Tahir U., Yasmin A., Khan U.H. Phytoremediation: potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci. 2016;28:119–130. [Google Scholar]
- 129.Sharif A., Nasreen Z., Bashir R., Kalsoom S. 8. Microbial degradation of textile industry effluents: a review. Pure Appl. Biol. (PAB) 2020;9:2361–2382. [Google Scholar]
- 130.Preeti K., Veena S. Bioremediation of textile dyes by using blue green algae. Int. J. Sci. Res. Dev. 2017;5:1322–1324. [Google Scholar]
- 131.Tsai W.T., Chen H.R. Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. J. Hazard Mater. 2010;175:844–849. doi: 10.1016/j.jhazmat.2009.10.087. [DOI] [PubMed] [Google Scholar]
- 132.Chin J.Y., Chng L.M., Leong S.S., Yeap S.P., Yasin N.H., Toh P.Y. Removal of synthetic Dye by Chlorella vulgaris microalgae as natural adsorbent. Arabian J. Sci. Eng. 2020;45:7385–7395. [Google Scholar]
- 133.Ishchi T., Sibi G. Azo dye degradation by Chlorella vulgaris: optimization and kinetics. Int. J. Biol. Chem. 2020;14:1–7. [Google Scholar]
- 134.Gelebo G.G., Tessema L.H., Kehshin K.T., Gebremariam H.H., Gebremikal E.T., Motuma M.T., Ayele A., Getachew D., Benor S., Suresh A. Phycoremediation of synthetic dyes in an aqueous solution using an indigenous Oscillatoria sp., from Ethiopia. Ethiopian J. Sci. Sustain. Dev. 2020;7:14–20. [Google Scholar]
- 135.Naji N.S., Salman J.M. Effect of temperature variation on the efficacy of Chlorella vulgaris in decolorization of Congo red from aqueous solutions. Biochem. Cell. Arch. 2019;19:4169–4174. [Google Scholar]
- 136.Raymond E.S., Omo K.M. Biodecolourization of yellow dye using chlorella vulgaris and sphaerocystics schroeteri. Am. J. Biol. Environ. Stat. 2019;5:1–6. [Google Scholar]
- 137.Arteaga L.C., Zavaleta M.P., Eustaquio W.M., Bobadilla J.M. Removal of aniline blue dye using live microalgae Chlorella vulgaris. J. Energy Environ. Sci. 2018;2:6–12. [Google Scholar]
- 138.Liu J.H., Zhang L., Zha D.C., Chen L.Q., Chen X.X., Qi Z.M. Biosorption of malachite green onto Haematococcus pluvialis observed through synchrotron Fourier-transform infrared microspectroscopy. Lett. Appl. Microbiol. 2018;67:348–353. doi: 10.1111/lam.13043. [DOI] [PubMed] [Google Scholar]
- 139.AL HAMADİ A., Güven U.R., KATIRCIOĞLU H., OSMANAĞAOĞLU Ö. Adsorption of azo dyes from textile wastewater by Spirulina Platensis. Eurasian J. Environ. Res. 2017;1:19–27. [Google Scholar]
- 140.Brahmbhatt N.H., Jasrai R.T. The role of algae in bioremediation of textile effluent. Methods. 2016;21:28. [Google Scholar]
- 141.Al-Fawwaz A.T., Abdullah M. Decolorization of methylene blue and malachite green by immobilized Desmodesmus sp. isolated from North Jordan. Int. J. Environ. Sustain Dev. 2016;7:95. [Google Scholar]
- 142.Baldev E., MubarakAli D., Ilavarasi A., Pandiaraj D., Ishack K.S., Thajuddin N. Degradation of synthetic dye, Rhodamine B to environmentally non-toxic products using microalgae. Colloids Surf. B Biointerfaces. 2013;105:207–214. doi: 10.1016/j.colsurfb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 143.Kousha M., Farhadian O., Dorafshan S., Soofiani N.M., Bhatnagar A. Optimization of malachite green biosorption by green microalgae—Scenedesmus quadricauda and Chlorella vulgaris: application of response surface methodology. J. Taiwan Inst. Chem. Eng. 2013;44:291–294. [Google Scholar]
- 144.Gajare S.M., Menghani S. Biosorption of malachite green by naturally grown algal biomass from Girna river, Jalgaon District, Maharashtra. J. Algal. Biomass. Utln. 2012;3:60–65. [Google Scholar]
- 145.Khataee A.R., Dehghan G., Ebadi E., Pourhassan M. Central composite design optimization of biological dye removal in the presence of macroalgae Chara sp. CLEAN–Soil, Air, Water. 2010;38:750–757. [Google Scholar]
- 146.Omar H.H. Algal decolorization and degradation of monoazo and diazo dyes. Pakistan J. Biol. Sci. 2008;11:1310–1316. doi: 10.3923/pjbs.2008.1310.1316. [DOI] [PubMed] [Google Scholar]
- 147.Daneshvar N., Ayazloo M., Khataee A.R., Pourhassan M. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresour. Technol. 2007;98:1176–1182. doi: 10.1016/j.biortech.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 148.Daneshvar N., Ayazloo M., Khataee A.R., Pourhassan M. Biodegradation of the textile dye malachite green by Microalgae Cosmarium sp. Int. J. Sci. High Technol. Environ. Sci. 2005 [Google Scholar]
- 149.Khalaf M.A. Biosorption of reactive dye from textile wastewater by non-viable biomass of Aspergillus Niger and Spirogyra sp. Bioresour. Technol. 2008;99:6631–6634. doi: 10.1016/j.biortech.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 150.Kelly B.C., Ikonomou M.G., Blair J.D., Morin A.E., Gobas F.A. Food web specific biomagnification of persistent organic pollutants. Science. 2007;317:236–239. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- 151.Hassaan M.A., El Nemr A., Hassaan A. Health and environmental impacts of dyes: mini review. Am. J. Environ. Sci. Eng. 2017;1:64–67. [Google Scholar]
- 152.Daneshvar E., Antikainen L., Koutra E., Kornaros M., Bhatnagar A. Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: treatment of wastewater and lipid extraction. Bioresour. Technol. 2018;255:104–110. doi: 10.1016/j.biortech.2018.01.101. [DOI] [PubMed] [Google Scholar]
- 153.Bai X., Acharya K. Algae-mediated removal of selected pharmaceutical and personal care products (PPCPs) from Lake Mead water. Sci. Total Environ. 2017;581:734–740. doi: 10.1016/j.scitotenv.2016.12.192. [DOI] [PubMed] [Google Scholar]
- 154.de Wilt A., Butkovskyi A., Tuantet K., Leal L.H., Fernandes T.V., Langenhoff A., Zeeman G. Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J. Hazard Mater. 2016;304:84–92. doi: 10.1016/j.jhazmat.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 155.Matamoros V., Uggetti E., García J., Bayona J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: a laboratory scale study. J. Hazard Mater. 2016;301:197–205. doi: 10.1016/j.jhazmat.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 156.Hom-Diaz A., Llorca M., Rodríguez-Mozaz S., Vicent T., Barceló D., Blánquez P. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 2015;155:106–113. doi: 10.1016/j.jenvman.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 157.Xiong J.Q., Kurade M.B., Jeon B.H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018;36:30–44. doi: 10.1016/j.tibtech.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 158.Maes H.M., Maletz S.X., Ratte H.T., Hollender J., Schaeffer A. Uptake, elimination, and biotransformation of 17α-ethinylestradiol by the freshwater alga Desmodesmus subspicatus. Environ. Sci. Technol. 2014;48(20):12354–12361. doi: 10.1021/es503574z. [DOI] [PubMed] [Google Scholar]
- 159.Peng F.Q., Ying G.G., Yang B., Liu S., Lai H.J., Liu Y.S., Chen Z.F., Zhou G.J. Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): transformation kinetics and products identification. Chemosphere. 2014;95:581–588. doi: 10.1016/j.chemosphere.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 160.Hena S., Gutierrez L., Croué J.P. Removal of metronidazole from aqueous media by C. vulgaris. J. Hazard Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121400. [DOI] [PubMed] [Google Scholar]
- 161.Song C., Wei Y., Qiu Y., Qi Y., Li Y., Kitamura Y. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: experimental study. Bioresour. Technol. 2019;272:529–534. doi: 10.1016/j.biortech.2018.10.080. [DOI] [PubMed] [Google Scholar]
- 162.de Godos I., Muñoz R., Guieysse B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard Mater. 2012;229:446–449. doi: 10.1016/j.jhazmat.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y., Habteselassie M.Y., Resurreccion E.P., Mantripragada V., Peng S., Bauer S., Colosi L.M. Evaluating removal of steroid estrogens by a model alga as a possible sustainability benefit of hypothetical integrated algae cultivation and wastewater treatment systems. ACS Sustain. Chem. Eng. 2014;2:2544–2553. [Google Scholar]
- 164.Naghdi M., Taheran M., Brar S.K., Kermanshahi-Pour A., Verma M., Surampalli R.Y. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018;234:190–213. doi: 10.1016/j.envpol.2017.11.060. [DOI] [PubMed] [Google Scholar]
- 165.Ahmed S.F., Mofijur M., Parisa T.A., Islam N., Kusumo F., Inayat A., Badruddin I.A., Khan T.Y., Ong H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere. 2022;286 doi: 10.1016/j.chemosphere.2021.131656. [DOI] [PubMed] [Google Scholar]
- 166.Maryjoseph S., Ketheesan B. Microalgae based wastewater treatment for the removal of emerging contaminants: a review of challenges and opportunities. Case Stud. Chem. Environ. Eng. 2020;2 [Google Scholar]
- 167.Cheng P., Chen D., Liu W., Cobb K., Zhou N., Liu Y., Liu H., Wang Q., Chen P., Zhou C., Ruan R. Auto-flocculation microalgae species Tribonema sp. and Synechocystis sp. with T-IPL pretreatment to improve swine wastewater nutrient removal. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138263. [DOI] [PubMed] [Google Scholar]
- 168.Cheng P., Chu R., Zhang X., Song L., Chen D., Zhou C., Yan X., Cheng J.J., Ruan R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020;318 doi: 10.1016/j.biortech.2020.124054. [DOI] [PubMed] [Google Scholar]
- 169.Bernaerts T.M., Gheysen L., Kyomugasho C., Kermani Z.J., Vandionant S., Foubert I., Hendrickx M.E., Van Loey A.M. Comparison of microalgal biomasses as functional food ingredients: focus on the composition of cell wall related polysaccharides. Algal Res. 2018;32:150–161. [Google Scholar]
- 170.Li K., Liu Q., Fang F., Luo R., Lu Q., Zhou W., Huo S., Cheng P., Liu J., Addy M., Chen P. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour. Technol. 2019;291 doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- 171.Prandini J.M., Da Silva M.L., Mezzari M.P., Pirolli M., Michelon W., Soares H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016;202:67–75. doi: 10.1016/j.biortech.2015.11.082. [DOI] [PubMed] [Google Scholar]
- 172.Choi H.J. Parametric study of brewery wastewater effluent treatment using Chlorella vulgaris microalgae. Environ. Eng. Res. 2016;21:401–408. [Google Scholar]
- 173.Gonzalez-Camejo J., Barat R., Pachés M., Murgui M., Seco A., Ferrer J. Wastewater nutrient removal in a mixed microalgae–bacteria culture: effect of light and temperature on the microalgae–bacteria competition. Environ. Technol. 2018;39:503–515. doi: 10.1080/09593330.2017.1305001. [DOI] [PubMed] [Google Scholar]
- 174.Shi J., Podola B., Melkonian M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014;154:260–266. doi: 10.1016/j.biortech.2013.11.100. [DOI] [PubMed] [Google Scholar]
- 175.De Francisci D., Su Y., Iital A., Angelidaki I. Evaluation of microalgae production coupled with wastewater treatment. Environ. Technol. 2018;39:581–592. doi: 10.1080/09593330.2017.1308441. [DOI] [PubMed] [Google Scholar]
- 176.Qu Z., Duan P., Cao X., Liu M., Lin L., Li M. Comparison of monoculture and mixed culture (Scenedesmus obliquus and wild algae) for C, N, and P removal and lipid production. Environ. Sci. Pollut. Control Ser. 2019;26:20961–20968. doi: 10.1007/s11356-019-05339-z. [DOI] [PubMed] [Google Scholar]
- 177.Fan J., Chen Y., Zhang T.C., Ji B., Cao L. Performance of Chlorella sorokiniana-activated sludge consortium treating wastewater under light-limited heterotrophic condition. Chem. Eng. J. 2020;382 [Google Scholar]
- 178.Corpuz M.V., Borea L., Senatore V., Castrogiovanni F., Buonerba A., Oliva G., Ballesteros F., Jr., Zarra T., Belgiorno V., Choo K.H., Hasan S.W. Wastewater treatment and fouling control in an electro algae-activated sludge membrane bioreactor. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147475. [DOI] [PubMed] [Google Scholar]
- 179.Tchinda D., Henkanatte-Gedera S.M., Abeysiriwardana-Arachchige I.S., Delanka-Pedige H.M., Munasinghe-Arachchige S.P., Zhang Y., Nirmalakhandan N. Single-step treatment of primary effluent by Galdieria sulphuraria: removal of biochemical oxygen demand, nutrients, and pathogens. Algal Res. 2019;42 [Google Scholar]
- 180.Gupta S., Nayak A., Roy C., Yadav A.K. An algal assisted constructed wetland-microbial fuel cell integrated with sand filter for efficient wastewater treatment and electricity production. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128132. [DOI] [PubMed] [Google Scholar]
- 181.Valizadeh K., Davarpanah A. Design and construction of a micro-photo bioreactor in order to dairy wastewater treatment by micro-algae: parametric study. Energy Sources, Part A Recovery, Util. Environ. Eff. 2020;42:611–624. [Google Scholar]
- 182.Arcila J.S., Buitrón G. Influence of solar irradiance levels on the formation of microalgae-bacteria aggregates for municipal wastewater treatment. Algal Res. 2017;27:190–197. [Google Scholar]
- 183.Wang J.H., Zhang T.Y., Dao G.H., Xu X.Q., Wang X.X., Hu H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017;101:2659–2675. doi: 10.1007/s00253-017-8184-x. [DOI] [PubMed] [Google Scholar]
- 184.Koutra E., Kopsahelis A., Maltezou M., Grammatikopoulos G., Kornaros M. Effect of organic carbon and nutrient supplementation on the digestate-grown microalga. Parachlorella kessleri. Bioresource technology. 2019;294 doi: 10.1016/j.biortech.2019.122232. [DOI] [PubMed] [Google Scholar]
- 185.Wang L., Min M., Li Y., Chen P., Chen Y., Liu Y., Wang Y., Ruan R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010;162:1174–1186. doi: 10.1007/s12010-009-8866-7. [DOI] [PubMed] [Google Scholar]
- 186.Zheng H., Liu M., Lu Q., Wu X., Ma Y., Cheng Y., Addy M., Liu Y., Ruan R. Balancing carbon/nitrogen ratio to improve nutrients removal and algal biomass production in piggery and brewery wastewaters. Bioresour. Technol. 2018;249:479–486. doi: 10.1016/j.biortech.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 187.Ma C., Wen H., Xing D., Pei X., Zhu J., Ren N., Liu B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels. 2017;10:1–3. doi: 10.1186/s13068-017-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.AlMomani F.A., Örmeci B. Performance of Chlorella vulgaris, Neochloris oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate. Ecol. Eng. 2016;95:280–289. [Google Scholar]
- 189.You X., Yang L., Zhou X., Zhang Y. Sustainability and carbon neutrality trends for microalgae-based wastewater treatment: a review. Environ. Res. 2022 Jun 1;209 doi: 10.1016/j.envres.2022.112860. [DOI] [PubMed] [Google Scholar]
- 190.Gupta S., Bux F. Application of microalgae in wastewater treatment. Appl. Microalgae Wastewater Treat. 2019;1 [Google Scholar]
- 191.Mezzari M.P., Prandini J.M., Kich J.D., da Silva M.B. Elimination of antibiotic multi-resistant salmonella typhimurium from swine waste water by microalgae-induced antibacterial mechanisms. Embrapa Suínos e Aves-Artigo em periódico indexado (ALICE) 2017 [Google Scholar]
- 192.Alsenani F., Tupally K.R., Chua E.T., Eltanahy E., Alsufyani H., Parekh H.S., Schenk P.M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharmaceut. J. 2020;28:1834–1841. doi: 10.1016/j.jsps.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ansa E.D., Lubberding H.J., Gijzen H.J. The effect of algal biomass on the removal of faecal coliform from domestic wastewater. Appl. Water Sci. 2012;2:87–94. [Google Scholar]
- 194.Mohamed Z.A. Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology. 2008;17:504–516. doi: 10.1007/s10646-008-0204-2. [DOI] [PubMed] [Google Scholar]
- 195.Lin W., Chen L., Tan Z., Deng Z., Liu H. Application of filamentous fungi in microalgae-based wastewater remediation for biomass harvesting and utilization: from mechanisms to practical application. Algal Res. 2022;62 [Google Scholar]
- 196.Gonçalves A.L., Pires J.C., Simões M. Wastewater polishing by consortia of Chlorella vulgaris and activated sludge native bacteria. J. Clean. Prod. 2016;133:348–357. [Google Scholar]
- 197.Tang C.C., Tian Y., Liang H., Zuo W., Wang Z.W., Zhang J., He Z.W. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae-assisted sequencing batch biofilm reactor. Bioresour. Technol. 2018;250:185–190. doi: 10.1016/j.biortech.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 198.Huo S., Kong M., Zhu F., Qian J., Huang D., Chen P., Ruan R. Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: enhancement of nutrients removal and influence of algal biomass generation. Algal Res. 2020;45 [Google Scholar]
- 199.Paddock M.B., Fernández-Bayo J.D., VanderGheynst J.S. The effect of the microalgae-bacteria microbiome on wastewater treatment and biomass production. Appl. Microbiol. Biotechnol. 2020;104:893–905. doi: 10.1007/s00253-019-10246-x. [DOI] [PubMed] [Google Scholar]
- 200.Nguyen T.T., Binh Q.A., Bui X.T., Ngo H.H., Vo H.N., Lin K.Y., Guo W., Lin C., Breider F. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: exploring their role under different inoculation ratios. Bioresour. Technol. 2020;314 doi: 10.1016/j.biortech.2020.123754. [DOI] [PubMed] [Google Scholar]
- 201.Mujtaba G., Lee K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017;120:174–184. doi: 10.1016/j.watres.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 202.Mujtaba G., Rizwan M., Kim G., Lee K. Removal of nutrients and COD through co-culturing activated sludge and immobilized Chlorella vulgaris. Chem. Eng. J. 2018;343:155–162. [Google Scholar]
- 203.Leng L., Li W., Chen J., Leng S., Chen J., Wei L., Peng H., Li J., Zhou W., Huang H. Co-culture of fungi-microalgae consortium for wastewater treatment: a review. Bioresour. Technol. 2021;330 doi: 10.1016/j.biortech.2021.125008. [DOI] [PubMed] [Google Scholar]
- 204.Walls L.E., Velasquez-Orta S.B., Romero-Frasca E., Leary P., Noguez I.Y., Ledesma M.T. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019;151 [Google Scholar]
- 205.Yang L., Li H., Wang Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019;275:35–43. doi: 10.1016/j.biortech.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 206.Eroglu E., Agarwal V., Bradshaw M., Chen X., Smith S.M., Raston C.L., Iyer K.S. Nitrate removal from liquid effluents using microalgae immobilized on chitosan nanofiber mats. Green Chem. 2012;14:2682–2685. [Google Scholar]
- 207.San Keskin N.O., Celebioglu A., Uyar T., Tekinay T. Microalgae immobilized by nanofibrous web for removal of reactive dyes from wastewater. Ind. Eng. Chem. Res. 2015;54:5802–5809. [Google Scholar]
- 208.Vasistha S., Khanra A., Rai M.P. Influence of microalgae-ZnO nanoparticle association on sewage wastewater towards efficient nutrient removal and improved biodiesel application: an integrated approach. J. Water Proc. Eng. 2021;39 [Google Scholar]
- 209.Luo L., Luo S., Wang H., Hu K., Lin X., Liu L., Yan B. Effect of nano-TiO2 on humic acid utilization from piggery biogas slurry by microalgae. Bioresour. Technol. 2021;337 doi: 10.1016/j.biortech.2021.125414. [DOI] [PubMed] [Google Scholar]