Abstract
Miscibility in biopolymeric blends is a critical process that requires evaluation of the effect of surfactants or coupling agents under conditions similar to processing. Different mixtures in the molten state of plasticized starch and polylactic acid in the presence of a surfactant (Tween 20) at different concentrations were studied. This allowed knowing the rheological, thermal and surface behavior of the mixtures. The results of the dynamic rheological analysis showed increases in viscosity in the presence of the surfactant, in which strong interactions were produced at high shear rates that reflect possible crosslinking between the polymer chains, in addition to intermolecular interactions that were evidenced in the infrared spectrum. Likewise, the storage and loss modulus showed transitions mainly from viscous to elastic typical for thermoplastics. The thermogravimetric analysis did not show significant changes between the mixtures. However, the calorimetric analysis showed changes in the crystallinity of the mixtures, the tensoactive promotes greater freedom of movement and rearrangements in the microstructure with decrease of interface between polymers, and less compaction of the material induced by the emulsion. Analysis derived from biopolymeric films against contact with water shows significant changes. Interaction with water in short times (in the order of minutes) according to the sessile drop technique, favors hydrophilicity by increasing the concentration of Tween 20. However, interaction with water for prolonged times (in the order of hours), shows that the absorption reaches saturation in samples a stabilization in the absorption is observed. The results demonstrate that the miscibility of PLA in AS was achieved in the presence of the tween, under conventional processing conditions. The stability of the different formulations allows the production of films for packaging and biomedical applications.
Keywords: Biopolymers, Miscibility, Contact angle, Surface wettability, Achira starch
Biopolymers; Miscibility; Contact angle; Surface wettability; Achira starch.
1. Introduction
Synthetic polymers have a history of more than 100 years of innovation, produced from chemical compounds derived from fossil sources, these marked the expansion of the petrochemical industry generating a massive applicability of different uses and fields. In the packaging sector, the results of world production present figures around 368 million tons per year. These materials, because of their favorable characteristics, among which low cost, low density, rapid transformation, acceptable optical, mechanical and barrier properties, as well as different functionalities, stand out in the market, and today they generate dependency in the sector of food, pharmaceutical, agricultural packaging, among others. However, despite their advantages, these materials have caused an undesirable influence on the environment, especially when used in single-use applications. This leads the consumer to the immediate devaluation of the material. Consequently, strategies continue to be developed from the management of recycling and the commitment to the development of new biodegradable and/or compostable polymeric materials that directly point to sustainability [1]. Agricultural products, by-products and co-products are potential raw materials, these include cellulose, starch, chitin, collagen, gluten. Another source for these materials are biotechnological and microbiological treatments such as polylactic acid and polyhydroxyalkanoates, respectively. The search for new sources of biopolymers is of great importance, in the case of starch, unconventional raw materials are used, avoiding competition with the food sector. Starch from the root of Achira (Canna edulis), which is cultivated in South America. This starch is profitable and has favorable physicochemical properties according to the prototypes developed as biodegradable materials [2]. The mixture of plasticized starch and polylactic acid has been presented as an ambitious proposal from biodegradable packaging that seeks to overcome low chemical compatibility. In the mixture, the benefits that relate the thermal, mechanical, and surface properties focused on the barrier are recognized [3, 4].
The first studies of mixtures between starch and polylactic acid were carried out in the presence of methylenediphenyl diisocyanate (MDI), which manages to copolymerize in situ and reduces interfacial tension, showing an adequate interaction with improved mechanical properties: higher tension and elongation below the glass transition temperature [5]. The variation of starch influenced proportionally with the absorption of water, although these showed surface resistance [6]. Subsequently, glycerol-plasticized starch (TPS) mixed with PLA exhibited a certain degree of miscibility evidenced in calorimetric analysis (DSC), and detriment of mechanical properties [7]. According to crystallization kinetic studies from isotherms obtained in TPS/PLA blends, it was possible to demonstrate that TPS induces heterogeneous nucleation, and also improves the crystallization capacity of the PLA matrix, compared to pure PLA [8]. The limitation due to phase separation demonstrated experimentally and theoretically [9] has been the reason in other works that show how different dispersing agents and co-plasticizers influence it, such as: citric acid, acetic acid, lactic acid, oleic acid and maleic anhydride [10, 11, 12, 13, 14, 15]. A significant advance in surface activity of the TPS/PLA blend occurred by previously functionalizing the starch with citric acid in synergy with polyethylene glycol (PEG) and oil polyol (VOP). It is important to mention that PEG acted as a lubricant for both biopolymers, allowing chemical interactions from crosslinking [16]. Likewise, the addition of plasticizers has been a widely discussed topic in its individual components [17] as well as mixture [18] (starch/PLA), these are used alone or in combination. Wang et al. evaluated the addition of glycerol, formamide and water, as starch and PLA plasticizers in a single step, in the optimization favorable results are given for formamide/water in which an adequate dispersion is achieved. However, the mechanical properties showed high stiffness due to poor interaction. Another study of the combination of plasticizers was carried out using glycerol and sorbitol in which the phenomenon of migration from the TPS phase towards PLA could be observed during the mixture in the molten state with coplasticized TPS [19].
In addition to the conventional plasticization of starch with glycerol to produce TPS, the plasticization of PLA with citrate and adipate esters has been studied [20, 21]. Some studies have focused attention on the variables of the extrusion process: residence time and temperature. In parallel, functional groups are modified, increasing reactivity to promote trans-esterifications between them. Indeed, improvement in the mechanical properties of the mixtures has been found [22].
The chemical grafting strategy through ternary mixtures of modified starch with isocyanate groups (HGST), PLA and castor oil increase crystallinity in short times and mechanical properties compared to the ternary mixture where pure starch is used [23]. Likewise, by replacing up to 25% of PLA with modified TPS using epoxydized soybean oil, the fluidity of the system is increased, projecting the use of the material to the injection and blow molding processes [24]. Similar results were obtained when incorporating epoxidized cardoon oil plasticizer, which continue to show weak interactions, leading to a negative deviation from the mixing rule [25]. Later studies used epoxidized sesame oil and this improved barrier properties, in turn thermal stability, although in the mechanical part: it increased deformation sacrificing resistance. However, a phase fading effect was observed in this material [26].
Some of the chemical modifications in starch have been etherification [27], silanization [28], esterification [29] that have been shown to decrease the hydrophilicity and particle size of starch, minimizing the difference in surface energy in TPS/PLA blends. The grafts on starch are more fluid in the process, less fragile in the final solid, as the microdroplets of the TPS (disperse phase) are properly distributed on the PLA [30, 31]. In the case of maleinized linseed oil added to the Starch/PLA mixture, a plasticizing effect is evident and it allows some reactions with hydroxyl groups both in the final PLA chains and in the starch chains, which results in an extension effect. and string matching [32]. In the same way, when analyzing the influence of maleinized hemp oil as a green plasticizer and/or compatibilizer in PLA/TPS blends, an increase in elongation at break and toughness values was achieved [33]. From these results it can be concluded that the maleinized oils mobilize the chains of the macromolecules and increase the free volume, as well as the degree of crystallinity. Another work shows the process of moderate in situ interaction with chemical grafting using glycidyl methacrylate (GMA) on the carboxyl or hydroxyl group of PLA [34]. On the other hand, certain degrees of acetylation in starch restrict intermolecular interactions (formation of hydrogen bonds), and as a consequence increase hydrophobicity and chain mobility. This causes fluidity in the material, optimal for potential use as flexible packaging [35, 36, 37].
Other modification strategies constitute amphoteric surfactants, Fonseca García et al. [38], formulated different concentrations of starch/chitosan with Pluronic by the casting method, explored the physicochemical properties such as the water vapor barrier and resistance to water, the morphological properties, thermal and mechanical. The surfaces of the composite starch/chitosan films obtained were more homogeneous with increasing Pluronic content. The solubility in water and the water vapor barrier capacity (WVP), as well as the mechanical behavior of the films, were improved with the incorporation of poloxamer at concentrations starting at 3% w/v. This polaxamer was tested in TPS/PLA blends by casting that allowed the dispersion of PLA microspheres on the TPS matrix [39]. Since the obtaining of blends by melting, the addition of amphiphilic molecules on TPS/PLA has been shown to facilitate processability of thermoplastic starch by significantly lowering the Tg without thermally destabilizing the system. By incorporating high contents of PLA (70%), the fluidity in the process and the tensile strength were increased. However, the type of amphiphile in contents of less than 50% PLA also managed to influence these variables, in such a way that Zeina was able to depress the fluidity of the mixture and give the material greater rigidity. While Tween 60 and linoleic acid cause ductility [40]. Other results in relation to Tween 80 with TPS/poly(butylene adipate-co-terephthalate) (PBAT) blends, the increase in the surfactant presented a higher water vapor diffusion, which indicates that the surfactant increased the free volume between the adjacent starch chains and causes detriment of mechanical properties [41]. With the above, it was proposed to evaluate the miscibility of TPS/PLA biopolymeric blends in the presence of an amphoteric surfactant Tween 20 obtained in melt mixture, and to know its influence on the rheological, structural, thermal, morphological and surface properties. The results are expected to understand the phenomenon of structural interaction and propose a formulation that can be representative and applied in the packaging sector, specifically single-use packaging that will help minimize environmental impact.
2. Materials and methods
2.1. Materials
Achira starch was supplied by Surtialmidon (Huila, Colombia) with a density of 1.59 g/mL. The plasticizers were obtained from Sigma Aldrich, sorbitol with a density of 1.49 g/mL (purity ≥98%). The lactic acid was supplied Chemí with purity: 85% and density of w 1.21 g/mL. The surfactant Tween 20, was obtained from Sigma Aldrich, with a purity >40% and density of 1.22 g/mL.
2.2. Preparation of polymeric blends TPS/T and TPS-PLA/T
The development of polymer blends is based on the methodology proposed by Caicedo and Calambas, 2021 [10]. The respective amounts of achira starch (AS), sorbitol, lactic acid, Tween 20 (T) and PLA, were mixed for 10 min in the torque rheometer Thermo Scientific equipment HAAKE Rheomix, at a rotor speed of 50 rpm and temperature of 160 °C.
The amount of lactic acid is proportional to the starch content (5% by weight). Additionally, the surfactant content replaces 10% (T10) or 20% (T20) by weight of the total plasticizer content. So, the respective amounts of components to the blends were added according to Table 1.
Table 1.
Proportions of achira starch, sorbitol, lactic acid, Tween 20, and PLA.
| Sample | Achira starch % | Sorbitol % | Tween 20 % | Lactic Acid % | PLA % |
|---|---|---|---|---|---|
| AS100-PLA0-T0 | 60 | 37 | 3 | ||
| AS100-PLA0-T10 | 60 | 33 | 4 | 3 | - |
| AS100-PLA0-T20 | 60 | 29 | 8 | 3 | - |
| AS75-PLA25-T0 | 45 | 27.8 | - | 2.2 | 25 |
| AS75-PLA25-T10 | 45 | 24.8 | 3 | 2.2 | 25 |
| AS75-PLA25-T20 | 45 | 21.8 | 6 | 2.2 | 25 |
| AS50-PLA50-T0 | 30 | 18.5 | - | 1.5 | 50 |
| AS50-PLA50-T10 | 30 | 16.5 | 2 | 1.5 | 50 |
| AS50-PLA50-T20 | 30 | 14.5 | 4 | 1.5 | 50 |
2.3. Fourier transformed infrared spectroscopy (FTIR)
FTIR analysis of blends was carried out in ATR mode with an equipment PerkinElmer espectrum3. The samples were analyzed to 16 scans and a wave number range between 400 cm−1 and 4000 cm−1 and a resolution of 4 cm−1.
2.4. Rheological analysis
The rheological behavior of the materials was studied with a rotational rheometer DHR-2, TA Instruments, USA. This study was done at 220 °C and the parallel plate configuration using the equilibrium flow test with a gap of 1000 μm, a diameter of 25 mm and an angle of 57.53°. The shear rate was in the range of 0.01–1000 rad/s at a deformation controlled of 10%.
2.5. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC)
Thermogravimetric analysis (TGA) was performed with the TGA/DSC 2 STAR System thermogravimetric analyzer equipment, Mettler Toledo, USA. Using a nitrogen purge with a flow rate of 60 mL/min and 10 ± 0.5 mg of the sample at a heating rate of 10 °C/min, from 30 °C to 500 °C.
Differential scanning calorimetry (DSC) analysis was carried out using a TA Q-2000 equipment The samples were subjected to heating–cooling–heating cycles, at a rate of 20 °C/min, a temperature range of 0 °C–200 °C, and with nitrogen purge (50 mL/min). For the evaluation of thermal properties, the results of the second heating run were used because of the elimination of the thermal history of the test specimens. In this analysis 10 mg of the sample.
2.6. Scanning electron microscopy analysis
The cryogenically fractured surfaces of TPS/T and TPS-PLA/T were coated with a thin layer of gold. These were observed using a JEOL, JCM 50000 (Tokyo, Japan) scanning electron microscope (SEM). A voltage of 10 kV and high vacuum was applied, observed at 500 X magnification.
2.7. Contact angle analysis
The films of the biopolymeric blends for this analysis were obtained in a press at a temperature of 110 °C and a pressure of 2 tons. Measurements of the contact angle with water were carried out using a Ramé-Hart Model 250 goniometer at room temperature. A 20 μL drop of distilled water was placed on the surface of the samples. After stabilization (60 s), the image was recorded and the contact angle was measured by ImageJ software.
2.8. Water absorption
To study the water absorption behavior of the films, they were cut to a size of 2 × 2 cm and dried for 4 h at 50 °C, later; films were weighed and soaked in distilled water for 6 h at room temperature, subsequently, the weight of the drained films was taken. The water absorption (%W) was calculated with Eq. (1):
| (1) |
Where Wt is the weight of films after the exposed time interval and W0 is the initial dry weight of the sample.
2.8.1. Statistical analysis
Analysis of variance (ANOVA) was performed by Tukey’s test (0.05 level of significance) to compare the mean differences of the biopolymer film formulations. All statistical analyzes were performed with IBM SPSS Statistics for Windows version 25 (New York, United States).
3. Results and discussion
3.1. Infrared spectra
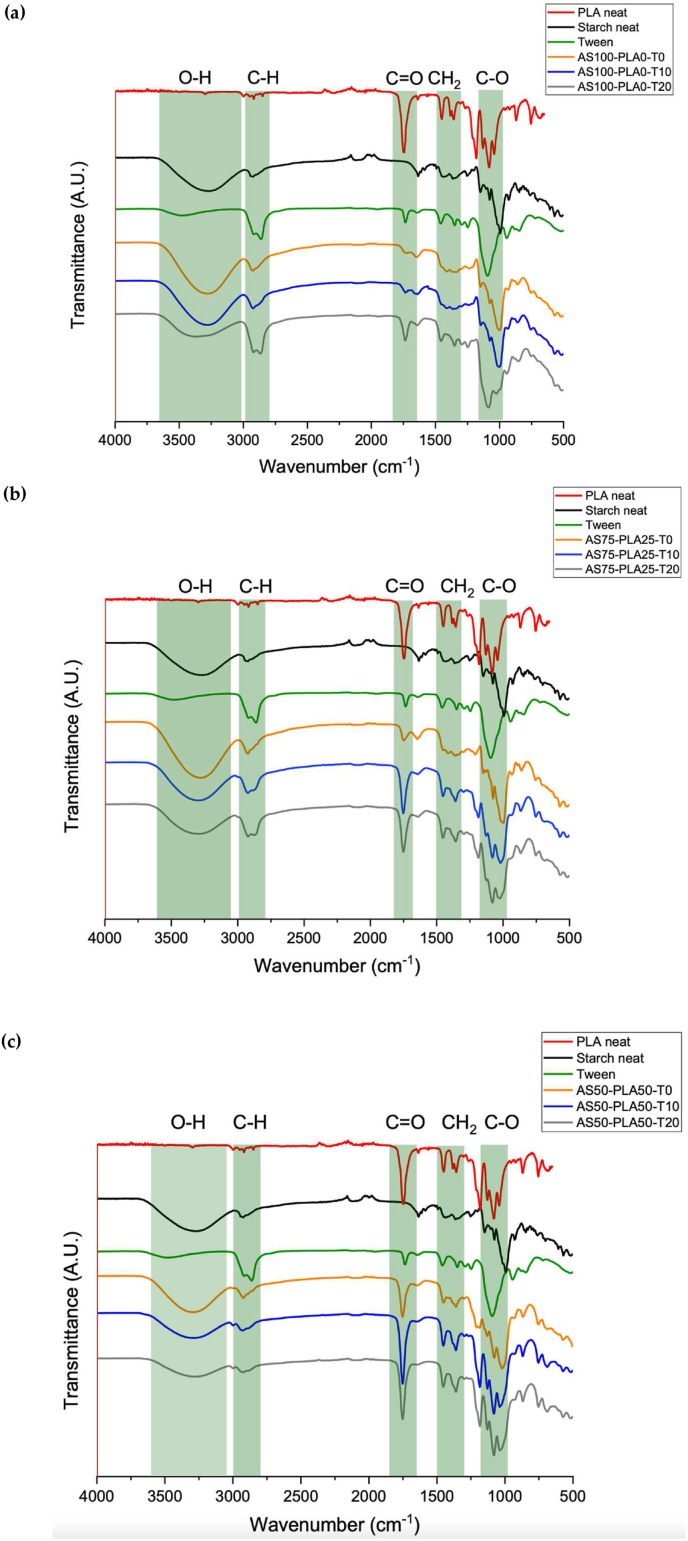
The FTIR spectrum (Figure 1) allowed us to observe the characteristic bands of the functional groups related to the polymers (TPS and PLA) and the non-ionic surfactant (Tween 20), as well as mixtures of TPS/PLA with different proportions of Tween 20. Figure 1 allows observe the curves of the spectra obtained by each group of mixtures. Between 2900 cm−1 and 3500 cm−1 there is a broad band corresponding to the hydroxyl groups (-OH) of the polysaccharides of starch, water and sorbitol, from the same functional group another band appears related to bending at 1642 cm−1 of the absorbed water molecules. In addition, the spectrum shows two signals around 2900 cm−1, and another one at 1345 cm−1, corresponding to the asymmetric and symmetric stretching of the methylene group (-CH2) and C–H, respectively. A band that allows recognizing structural changes is due to the bending of the C–O–H group that appears at 1012 cm−1 [42]. Finally, the 953 cm−1 and 924 cm−1 bands are characteristic of oscillatory vibrations of the CH3 group in the helical structure.
Figure 1.
IR spectra of the TPS/PLA with different Tween 20 contents. (a) AS100-PLA0, (b) AS75-PLA25 and (c) AS50-PLA50.
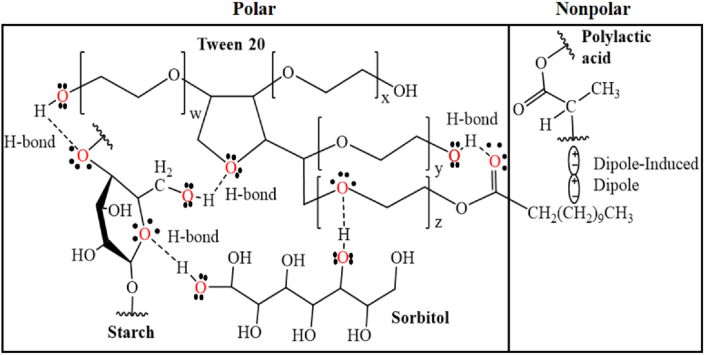
In the same order, the bands for the PLA spectrum are presented, the first one is observed between 2995 cm−1 and 2950 cm−1. This same group bands are exhibited at 1376 cm−1 and 1356 cm−1 attributed to symmetric and asymmetric deformations, respectively. At 1453 cm−1 a band corresponding to the bending vibrations of the CH3 group is observed. Another typical and intense band is shown centered at 1749 cm−1, this one corresponding to stretching of the carbonyl group (C=O) of the polyester. In addition, an absorption sequence for the asymmetric stretching of the C–O bond at 1183 cm−1, 1127 cm−1, 1080 cm−1 and 1039 cm−1 is shown. Finally, in the fingerprint region, two bands at 868 cm−1 and 755 cm−1 were related to the C–C stretching vibration that corresponded to the amorphous and crystalline phase of PLA, respectively [10]. In the case of the signals evidenced in Tween 20, the vibration of the hydroxyl (O–H) is observed through a broad band centered at 3489 cm−1. C–H stretching is observable at 2925 cm−1 and 2840 cm−1, as well as for methylene –CH2 at 1460 cm−1. The ester group of the structure shows vibrations between carbon and oxygen in different ways, with the carbonyl C=O exhibiting a band at 1735 cm−1. While the C–O vibration is observable at 1361 cm−1, 1285 cm−1, 1238 cm−1 and 1095 cm−1. The monosubstituted C–H strains appear at 942 cm−1. Regarding the increase in the concentration of the surfactant in the thermoplastic starch matrix (AS100-P0), increases proportional to the concentration of Tween 20 are observed in the band corresponding to C=O and C–O at 1735 cm−1 and 1095 cm−1. The Tween 20 leads to a decrease in the intensity of the band associated with the hydroxyl that appears at 3360 cm−1, which indicates restriction in the vibrations of this functional group due to intermolecular interactions that give rise to hydrogen bonds between the surfactant and the starch. van der Waals interactions are evidenced by a decrease in the intensity of bands at 2925 cm−1 and 2840 cm−1 (asymmetric stretch and symmetric stretch, respectively), these results are consistent with those reported in similar works (starch/PLA blends) [38]. The spectra were normalized based on the reference band at 1012 cm−1 (C–O indicated in Figure 1 a, b, c) [43]. According to what was previously argued, a proposal of the interactions that are generated between the biopolymers and the Tween is presented, the mixture is presented in two polar and non-polar phases. The most representative intermolecular interactions are hydrogen bonds in the polar phase and van der Waals interactions in the nonpolar phase, as shown in Figure 2.
Figure 2.
Proposed structure of the possible intermolecular interactions between the components of the (AS-Sor)-PLA-Tween blend.
3.2. Rheological analysis
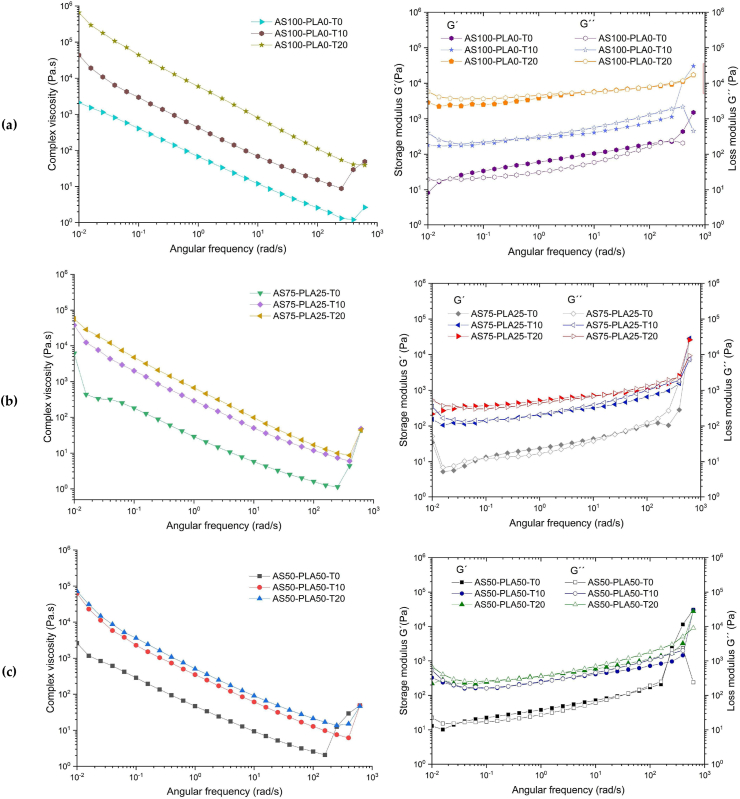
According to the complex viscosity (η∗) results obtained from the dynamic analysis (Figures 3a, 3b and 3c), it is observed in the TPS control sample (AS100-PLA0) that the surfactant decreases the free volume, achieving a greater integration of the components in the mixture, this leads to an increase in the viscosity proportionally with the concentration of the surfactant. This miscibility phenomenon also occurs in TPS/PLA blends (AS75-PLA25 and AS50-PLA50). In general, it is observed that as shearing increases, there is a thinning in the viscosity of the polymer, however, around an angular frequency of 100 rad/s, an increase in viscosity begins that can be caused by reactions of crossover This behavior has been evidenced in previous works and discussed by other authors [10, 44]. Likewise, the result of the analysis of the storage (G') and loss (G'') modulus (Figures 3a, 3b and 3c) show a gradual increase as the shear increases with a moderate viscous to elastic transition, once the 100 rad/s are exceeded, an overshoot occurs in the resistance of the fluid, taking solid consistency. Due to the predominance of elastic behavior, this is preferably in mixtures with a lower content of surfactant. Hence, the surfactant does not promote plasticization in the mixtures, on the contrary, it achieves interactions that limit the mobility of the polymer chains, promoting ordered microstructures [45].
Figure 3.
Rheological behavior of the TPS/PLA/Tween, 1) ɳ∗ vs angular frequency and 2) G'' and G' vs angular frequency. (a) AS100-PLA0, (b) AS75-PLA25 and (c) AS50-PLA50.
3.3. Thermal properties
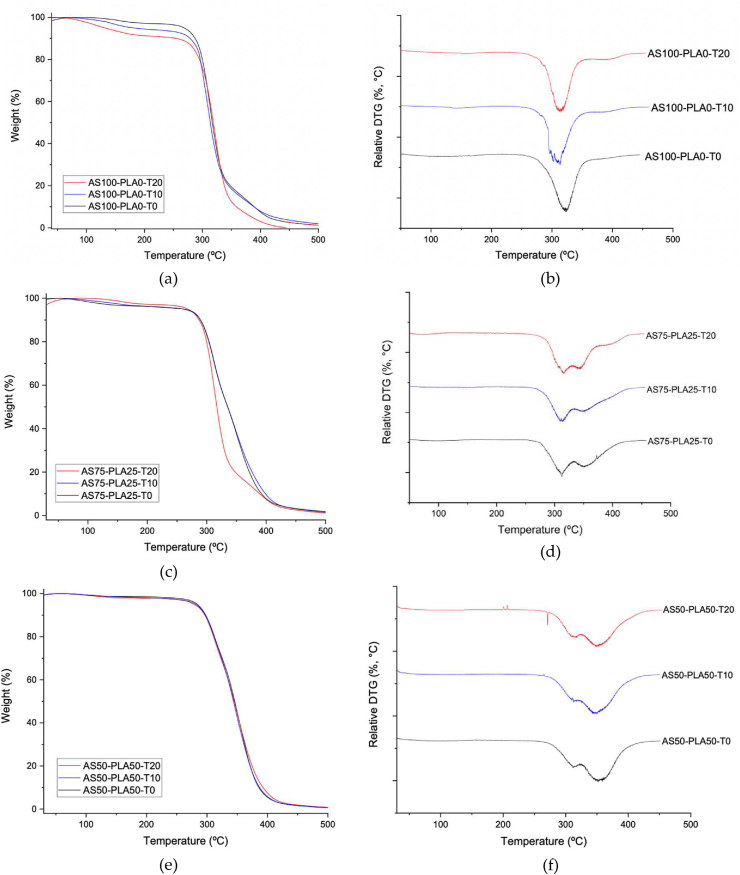
The thermal decomposition of the TPS/surfactant and TPS-PLA/surfactant samples are presented in Figure 4 (a), (c), (e), where the mass losses as a function of temperature are observed. They have differentiated in two important areas: 1) a low mass loss between 30 °C and 180 °C, due initially to the loosening of weakly bound water and later to strongly bound water [10]. 2) The greatest loss in mass corresponds to the degradation of the components of the mixture, on the one hand, the TPS between 311 °C and 320 °C and on the other, the PLA between 344 °C and 352 °C.
Figure 4.
Thermogravimetric analysis (TGA) (a), (c), (e) and derivative thermogravimetry (DTG) (b), (d), (f) curves for TPS-PLA blends.
Figure 4 (b), (d), (f) shows the first derivative of the thermogravimetry (DTG) of the TPS-PLA samples. Two differentiated weight losses are observed, which confirm the separation of phases present in the system, this behavior has also been observed by other authors [10, 24].
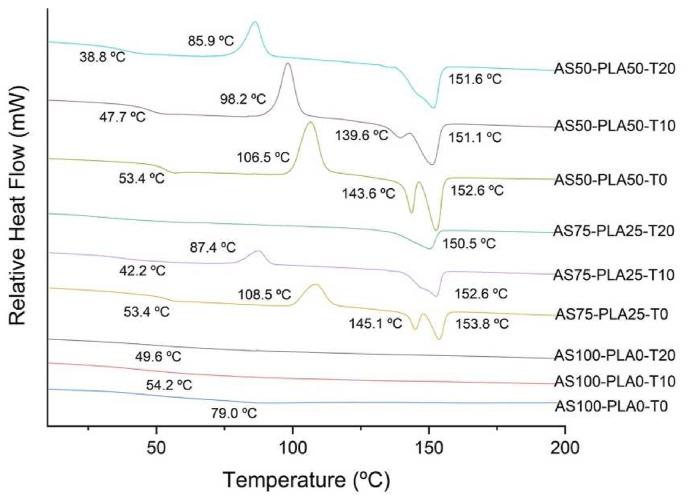
It was observed that by increasing the TPS content in the mixtures, the T10 as Tonset decreases (see Table 2), evidencing the hydrophilic character of the starch, and lower thermal stability compared to the samples with higher PLA content. This deterioration in thermal properties is due to the greater presence of amorphous phase of TPS after processing [33]. On the other hand, in the TPS-PLA mixtures, it is evident that the addition of Tween 20 generates an increase of 2 °C–3 °C in the starch degradation temperature (Td1), while the PLA degradation temperature (Td2) decreases around 4 °C. This favors an approach between the two degradation temperatures, which demonstrates the ability of Tween 20 to promote the miscibility of TPS and PLA.
Table 2.
Thermal analysis parameter summary for TPS-PLA blends (first loss at 10% mass temperature (T10), initial degradation temperature (Tonset), maximum degradation temperature (Td), first and second order thermal transitions (Tm, Tc and Tg).
| Sample | T10 |
Tonset |
Td1 |
Td2 |
Tg |
Tc |
Tm |
ΔHc |
ΔHm |
XC |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | J/g | J/g | % | ||||||||||
| AS100-PLA0-T0 | 287.1 | 258.9 | 319.4 | - | 79.0 | - | - | - | - | - | - | - | - |
| AS100-PLA0-T10 | 278.7 | 258.7 | 313.7 | - | 54.2 | - | - | - | - | - | - | - | - |
| AS100-PLA0-T20 | 254.7 | 257.8 | 312.9 | - | 49.6 | - | - | - | - | - | - | - | - |
| AS75-PLA25-T0 | 288.8 | 261.5 | 311.4 | 351.1 | 53.4 | 108.6 | 145 | 153.8 | 12.9 | 2.9 | 6.8 | 0.68 | 0.85 |
| AS75-PLA25-T10 | 289.2 | 261.0 | 313.2 | 348.6 | 42.2 | 87.6 | - | 152.7 | 6.7 | - | 13.9 | - | 0.89 |
| AS75-PLA25-T20 | 287.1 | 261.9 | 316.0 | 344.4 | - | - | - | 150.5 | - | - | 11.2 | - | 0.48 |
| AS50-PLA50-T0 | 297.6 | 266. 7 | 311.2 | 352.6 | 53.4 | 106.5 | 143.7 | 152.6 | 23.7 | 6.2 | 14.7 | 0.64 | 0.83 |
| AS50-PLA50-T10 | 297.0 | 268.6 | 312.5 | 347.7 | 47.7 | 98.3 | 139.3 | 151.1 | 21.8 | 8.4 | 18.3 | 0.65 | 0.86 |
| AS50-PLA50-T20 | 296.3 | 268.7 | 314.4 | 348.0 | 38.8 | 86.3 | - | 151.7 | 13.8 | - | 19.8 | - | 0.72 |
Figure 5 presents the curve of the second heating of the TPS-PLA mixtures with and without surfactant, the first and second-order transition temperatures are presented in Table 2. The samples that contain 100% starch, only one transition is observed of second-order around 79 °C for AS100-PLA0-T0, while a decrease in the transition temperature of 25 °C and 30 °C is evident when increasing the content of surfactant between 10% and 20%, respectively. This effect is probably due to the molecular disorganization caused by Tween 20, which generates greater mobility of the PLA chains [46]. The same effect is observed on the glass transition temperature (Tg) of the TPS and PLA blends, with the increase in the content of surfactant. This result was also observed by Fonseca-García et al., in mixtures of TPS and PLA, with Pluronic® F127, an amphiphilic triblock copolymer that acts as a surfactant. These interactions are generated between the starch and the PLA chains that cause a nucleation effect. The interactions allow the structure greater freedom of movement [37]. Additionally, other authors have observed that the Tg is reduced in PLA/TPS blends with respect to PLA, due to a plasticizing effect exerted by TPS on the PLA matrix [25].
Figure 5.
DSC thermograms of TPS-PLA blends.
As for the TPS-PLA blends with and without surfactant, there is a significant decrease in the temperature (TC) and the enthalpy (ΔHC) of crystallization, with the increase in the surfactant. This effect is more notable for mixtures with 75% starch, where, by adding 10% of surfactant, the TC presents a shift of approximately 20 °C, towards lower temperatures and its enthalpy is reduced by 50%. From the above, it is concluded that, with a higher content of water and sorbitol in starch and the addition of surfactant [47], the speed of crystallization and molecular disorganization are favored. Due to the fact that there is greater freedom of movement of the chains as an effect of the interactions. That occur with the amphiphilic macromolecule of the surfactant [38], this becomes more evident by increasing the content of Tween to 20%.
The melting peaks of the TPS-PLA mixtures are related to the melting temperatures of the α and β conversion crystals of PLA [29], in the temperature range of 150 °C–153 °C and 139 °C–145 °C, respectively. Where, the melting peak at lower temperature corresponds to crystals with higher reorganization, while the melting peak at higher temperature corresponds to a more organized crystal structure [44]. As already mentioned, the addition of the surfactant favors molecular disorder. Therefore, a lower presence of β-type PLA crystals is evident, which causes the disappearance of this peak in AS75-PLA25-T20 blend, and β to α reconversion in AS50-PLA50-T20 blend.
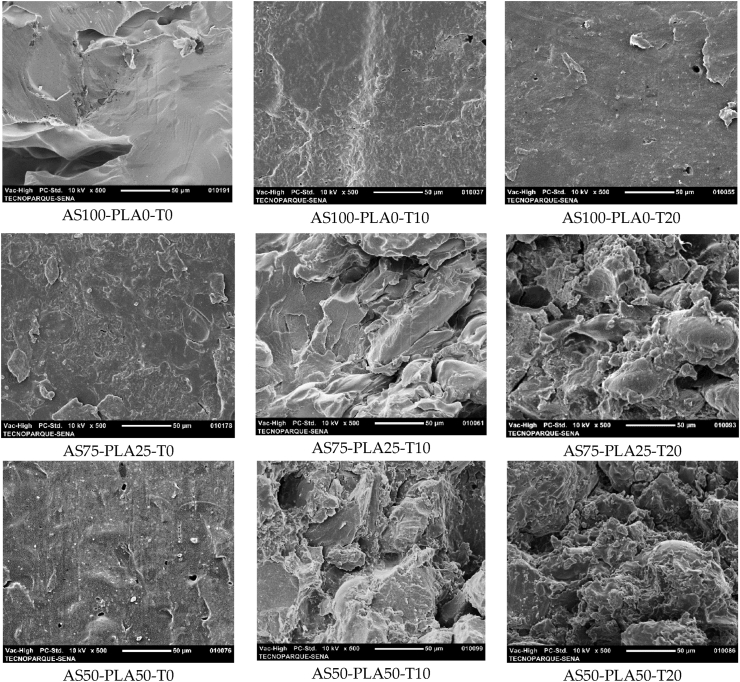
3.4. Morphology
Figure 6 shows the micrographs of the rupture surface of the biopolymeric samples based on starch and their respective mixtures with PLA in the presence of Tween as a surfactant. In general, the samples without surfactant present compact structures, in the case of the sample AS100-PLA0-T0 (control) a smooth surface is also exhibited. However, incorporating the surfactant promotes roughness and some defects on the surface as a consequence of the emulsion. This is due to the rearrangements between the polymeric chains and the smaller organic molecules (plasticizer and surfactant) that interact allowing miscibility. The formation of hydrogen bonds between the surfactant and the starch was evidenced in the FTIR analysis and has been discussed in similar systems in previous works.
Figure 6.
Micrographs by SEM ofTPS/PLA blend: Transversal section of film.
The micrograph of the AS75-PLA25-T0 mixture allows us to appreciate the interface between the TPS and the PLA. The higher content of TPS in the mixtures shows a greater amount of defects, such as pores and agglomerates. This effect was also observed by Brandelero et al. [41], in TPS and PBAT films that present discontinuous and porous forms with the addition of surfactant (Tween 80).
When adding 10% of surfactant to the TPS-PLA blends, a discontinuous and irregular morphology is presented, where the phases present are not easily recognized, evidencing greater miscibility of TPS and PLA. By increasing the content of surfactant, the matrix of TPS and PLA is observed, surrounding some unplasticized starch granules, as a consequence of the hydrogen bonds formed between the surfactant and the starch. These unplasticized granules compromise the plasticization of the TPS-PLA blend, as was also observed from the rheological results of the mixtures. This effect is more evident in the AS75-PLA25-T20 sample, due to the greater presence of starch that can be encapsulated by the matrix, without plasticizing. Brandelero et al., observed this same effect in mixtures of TPS–PBAT and 0.5%–1% of Tween 80 (T), where the formation of a greater number of TPS agglomerates is observed when the T/TPS ratio increases [48].
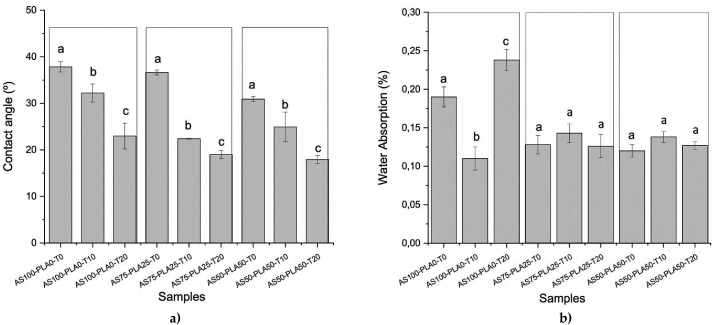
3.5. Contact angle and water absorption
The results of contact angle and water absorption are presented in Figure 7a, it was observed that the contact angle decreases with the increase of the surfactant for all the mixtures. These results suggest that the hydrogen bonds that occur between the starch and the surfactant oriented the polar groups of the Tween 20 molecule towards the surface, generating an increase in the hydrophilicity of the material. The same effect was observed by Ortega-Toro et al., who determined that the polar epoxide groups of epoxidized sesame oil in TPS-PLA mixtures are oriented toward the surface of the film [25].
Figure 7.
a) Average contact angle values and b) water absorption for TPS-PLA. a–c Different letters in the same file of each property: contact angle and water absorption, indicate significative differences (p < 0.05). The comparison is made between homologous blends (indicated with the boxes in the figure). ∗ Mean of three replications ±standard deviation.
Regarding the absorption of water in the TPS-PLA mixtures (Figure 7b), no significant effect is observed with the use of the surfactant (probably water saturation is reached in the internal structure and the excesses on the surface dissolve). However, for sample AS100-PLA0-T20, the excess of Tween 20 is evident, as there is a significant increase in water absorption.
4. Conclusions
Starch and PLA blends were developed in the molten state using a torque rheometer, later, films were obtained by thermocompression, in the physicochemical analyzes, it was found that the presence of the surfactant Tween 20 promotes miscibility between TPS and PLA, which is evidenced through the shifting of the degradation temperatures and the reduction of the presence of PLA crystals as a consequence of the molecular disorder and the increase in the crystallization rate due to the interactions that occur between the amphiphilic macromolecule of the surfactant and the starch.
On the other hand, the obtaining of a continuous phase in the mixtures AS75-PLA25-T10 and AS50-PLA50-T10 also shows the miscibility of TPS–PLA caused by the surfactant. Although in these mixtures the hydrophilicity of the mixtures is increased due to the orientation toward the surface of the polar groups of the Tween 20 molecule, this study constitutes an important precedent to achieve the scaling of this type of material in the biodegradable packaging sector required today.
Declarations
Author contribution statement
Heidi Lorena Calambas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Carolina Caicedo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Edwin Florez Lopez: Performed the experiments; Wrote the paper.
Funding statement
This work was supported by ASTIN-SENA, Unidad Central del Valle del Cauca and Dirección General de Investigaciones (DGI) of Universidad Santiago de Cali under call no. 01–2022.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Nil.
References
- 1.Varona Beltran G.A. Structural stability of a flexible film obtained from thermoplastic starch with stearic Acid. Rev. Fac. Nac. Agron. 2014;67(2):502–504. [Google Scholar]
- 2.Gómez-Aldapa C.A., Castro-Rosas J., Rangel-Vargas E., Navarro-Cortez R.O., Cabrera-Canales Z.E., Díaz-Batalla L.…Falfan-Cortes R.N. A modified Achira (Canna indica L.) starch as a wall material for the encapsulation of Hibiscus sabdariffa extract using spray drying. Food Res. Int. 2019;119:547–553. doi: 10.1016/j.foodres.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Prieto A.M., Garcia R.C., González M.C. 2018. “Plastics Technology Mexico,” Introducción sobre propiedades de barrera del PET, parte 1.https://www.pt-mexico.com/articulos/introducción-sobre-propiedades-de-barrera-parte-1- [Google Scholar]
- 4.Tyuftin A.A., Kerry J.P. Gelatin films: study review of barrier properties and implications for future studies employing biopolymer films. Food Packag. Shelf Life. 2021;29 [Google Scholar]
- 5.Wang H., Sun X., Seib P. Strengthening blends of poly (lactic acid) and starch with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2001;82(7):1761–1767. [Google Scholar]
- 6.Wang H., Sun X., Seib P. Mechanical properties of poly (lactic acid) and wheat starch blends with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2002;84(6):1257–1262. [Google Scholar]
- 7.Martin O., Avérous L. Poly (lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer. 2001;42(14):6209–6219. [Google Scholar]
- 8.Cai J., Liu M., Wang L., Yao K., Li S., Xiong H. Isothermal crystallization kinetics of thermoplastic starch/poly (lactic acid) composites. Carbohydr. Polym. 2011;86(2):941–947. [Google Scholar]
- 9.Müller P., Bere J., Fekete E., Móczó J., Nagy B., Kállay M.…Pukánszky B. Interactions, structure and properties in PLA/plasticized starch blends. Polymer. 2016;103:9–18. [Google Scholar]
- 10.Wang N., Yu J., Chang P.R., Ma X. Influence of citric acid on the properties of glycerol-plasticized dry starch (DTPS) and DTPS/poly (lactic acid) blends. Starch Staerke. 2007;59(9):409–417. [Google Scholar]
- 11.Caicedo C., Pulgarin H.L.C. Study of the physical and mechanical properties of thermoplastic starch/poly (lactic acid) blends modified with acid agents. Processes. 2021;9(4):578. [Google Scholar]
- 12.Caicedo C., Aguirre Loredo R.Y., Fonseca García A., Ossa O.H., Vázquez Arce A., Calambás Pulgarin H.L., Ávila Torres Y. Rheological, thermal, superficial, and morphological properties of thermoplastic achira starch modified with lactic acid and oleic acid. Molecules. 2019;24(24):4433. doi: 10.3390/molecules24244433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabrat E., Abdillahi H., Rouilly A., Rigal L. Influence of citric acid and water on thermoplastic wheat flour/poly (lactic acid) blends. I: thermal, mechanical and morphological properties. Ind. Crop. Prod. 2012;37(1):238–246. [Google Scholar]
- 14.Abdillahi H., Chabrat E., Rouilly A., Rigal L. Influence of citric acid on thermoplastic wheat flour/poly (lactic acid) blends. II. Barrier properties and water vapor sorption isotherms. Ind. Crop. Prod. 2013;50:104–111. [Google Scholar]
- 15.Orozco V.H., Brostow W., Chonkaew W., Lopez B.L. Preparation and characterization of poly (Lactic acid)-g-maleic anhydride+ starch blends. Macromol. Symp. 2009, February;277(No. 1):69–80. Weinheim: WILEY-VCH Verlag. [Google Scholar]
- 16.Hu H., Xu A., Zhang D., Zhou W., Peng S., Zhao X. High-toughness poly (lactic acid)/starch blends prepared through reactive blending plasticization and compatibilization. Molecules. 2020;25(24):5951. doi: 10.3390/molecules25245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sothornvit R., Krochta J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001;50(3):149–155. [Google Scholar]
- 18.Wang N., Yu J., Chang P.R., Ma X. Influence of formamide and water on the properties of thermoplastic starch/poly (lactic acid) blends. Carbohydr. Polym. 2008;71(1):109–118. [Google Scholar]
- 19.Esmaeili M., Pircheraghi G., Bagheri R., Altstädt V. Poly (lactic acid)/coplasticized thermoplastic starch blend: effect of plasticizer migration on rheological and mechanical properties. Polym. Adv. Technol. 2019;30(4):839–851. [Google Scholar]
- 20.Shirai M.A., Grossmann M.V.E., Mali S., Yamashita F., Garcia P.S., Müller C.M.O. Development of biodegradable flexible films of starch and poly (lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013;92(1):19–22. doi: 10.1016/j.carbpol.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Shirai M.A., Olivato J.B., Demiate I.M., Müller C.M.O., Grossmann M.V.E., Yamashita F. Poly (lactic acid)/thermoplastic starch sheets: effect of adipate esters on the morphological, mechanical and barrier properties. Polímeros. 2016;26:66–73. [Google Scholar]
- 22.Wootthikanokkhan J., Wongta N., Sombatsompop N., Kositchaiyong A., Wong-On J., Isarankura na Ayutthaya S., Kaabbuathong N. Effect of blending conditions on mechanical, thermal, and rheological properties of plasticized poly (lactic acid)/maleated thermoplastic starch blends. J. Appl. Polym. Sci. 2012;124(2):1012–1019. [Google Scholar]
- 23.Xiong Z., Zhang L., Ma S., Yang Y., Zhang C., Tang Z., Zhu J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013;94(1):235–243. doi: 10.1016/j.carbpol.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Przybytek A., Sienkiewicz M., Kucińska-Lipka J., Janik H. Preparation and characterization of biodegradable and compostable PLA/TPS/ESO compositions. Ind. Crop. Prod. 2018;122:375–383. [Google Scholar]
- 25.Turco R., Ortega-Toro R., Tesser R., Mallardo S., Collazo-Bigliardi S., Chiralt Boix A.…Santagata G. Poly (Lactic acid)/Thermoplastic starch films: effect of cardoon seed epoxidized oil on their chemicophysical, mechanical, and barrier properties. Coatings. 2019;9(9):574. [Google Scholar]
- 26.Ortega-Toro R., López-Córdoba A., Avalos-Belmontes F. Epoxidised sesame oil as a biobased coupling agent and plasticiser in polylactic acid/thermoplastic yam starch blends. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wokadala O.C., Emmambux N.M., Ray S.S. Inducing PLA/starch compatibility through butyl-etherification of waxy and high amylose starch. Carbohydr. Polym. 2014;112:216–224. doi: 10.1016/j.carbpol.2014.05.095. [DOI] [PubMed] [Google Scholar]
- 28.Jariyasakoolroj P., Chirachanchai S. Silane modified starch for compatible reactive blend with poly (lactic acid) Carbohydr. Polym. 2014;106:255–263. doi: 10.1016/j.carbpol.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Tang Z., Xiong Z., Zhu J. Preparation and characterization of thermoplastic starches and their blends with poly (lactic acid) Int. J. Biol. Macromol. 2015;77:273–279. doi: 10.1016/j.ijbiomac.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Akrami M., Ghasemi I., Azizi H., Karrabi M., Seyedabadi M. A new approach in compatibilization of the poly (lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016;144:254–262. doi: 10.1016/j.carbpol.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Noivoil N., Yoksan R. Oligo (lactic acid)-grafted starch: a compatibilizer for poly (lactic acid)/thermoplastic starch blend. Int. J. Biol. Macromol. 2020;160:506–517. doi: 10.1016/j.ijbiomac.2020.05.178. [DOI] [PubMed] [Google Scholar]
- 32.Ferri J.M., Garcia-Garcia D., Sánchez-Nacher L., Fenollar O., Balart R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly (lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr. Polym. 2016;147:60–68. doi: 10.1016/j.carbpol.2016.03.082. [DOI] [PubMed] [Google Scholar]
- 33.Lerma-Canto A., Gomez-Caturla J., Herrero-Herrero M., Garcia-Garcia D., Fombuena V. Development of polylactic acid thermoplastic starch formulations using maleinized hemp oil as biobased plasticizer. Polymers. 2021;13(9):1392. doi: 10.3390/polym13091392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palai B., Biswal M., Mohanty S., Nayak S.K. In situ reactive compatibilization of polylactic acid (PLA) and thermoplastic starch (TPS) blends; synthesis and evaluation of extrusion blown films thereof. Ind. Crop. Prod. 2019;141 [Google Scholar]
- 35.Jiménez-Regalado E.J., Caicedo C., Fonseca-García A., Rivera-Vallejo C.C., Aguirre-Loredo R.Y. Preparation and physicochemical properties of modified corn starch–chitosan biodegradable films. Polymers. 2021;13(24):4431. doi: 10.3390/polym13244431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri J.M., Garcia-Garcia D., Carbonell-Verdu A., Fenollar O., Balart R. Poly (lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J. Appl. Polym. Sci. 2018;135(4) [Google Scholar]
- 37.Noivoil N., Yoksan R. Compatibility improvement of poly (lactic acid)/thermoplastic starch blown films using acetylated starch. J. Appl. Polym. Sci. 2021;138(2) [Google Scholar]
- 38.Fonseca-García A., Jiménez-Regalado E.J., Aguirre-Loredo R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2022;251 doi: 10.1016/j.carbpol.2020.117009. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca-García A., Osorio B.H., Aguirre-Loredo R.Y., Calambas H.L., Caicedo C. Carbohydrate Polymers; 2022. Miscibility Study of Thermoplastic Starch/polylactic Acid Blends: Thermal and Superficial Properties. 119744. [DOI] [PubMed] [Google Scholar]
- 40.Yokesahachart C., Yoksan R. Effect of amphiphilic molecules on characteristics and tensile properties of thermoplastic starch and its blends with poly (lactic acid) Carbohydr. Polym. 2011;83(1):22–31. [Google Scholar]
- 41.Brandelero R.P.H., Yamashita F., Grossmann M.V.E. The effect of surfactant Tween 80 on the hydrophilicity, water vapor permeation, and the mechanical properties of cassava starch and poly (butylene adipate-co-terephthalate)(PBAT) blend films. Carbohydr. Polym. 2010;82(4):1102–1109. [Google Scholar]
- 42.Warren F.J., Gidley M.J., Flanagan B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—a joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016;139:35–42. doi: 10.1016/j.carbpol.2015.11.066. [DOI] [PubMed] [Google Scholar]
- 43.Hong T., Yin J.Y., Nie S.P., Xie M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: progress, challenge and perspective. Food Chem. X. 2021;12 doi: 10.1016/j.fochx.2021.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagano T., Tamaki E., Funami T. Influence of guar gum on granule morphologies and rheological properties of maize starch. Carbohydr. Polym. 2008;72(1):95–101. [Google Scholar]
- 45.Rodriguez-Gonzalez F.J., Ramsay B.A., Favis B.D. Rheological and thermal properties of thermoplastic starch with high glycerol content. Carbohydr. Polym. 2004;58(2):139–147. [Google Scholar]
- 46.Jullanun P., Yoksan R. Morphological characteristics and properties of TPS/PLA/cassava pulp biocomposites. Polym. Test. 2020;88 [Google Scholar]
- 47.Hu H., Xu A., Zhang D., Zhou W., Peng S., Zhao X. High-toughness poly (lactic acid)/starch blends prepared through Reactive Blending Plasticization and Compatibilization. Molecules. 2020;25(24):5951. doi: 10.3390/molecules25245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandelero R.P.H., Grossmann M.V., Yamashita F. Films of starch and poly(butylene adipate co-terephthalate) added of soybean oil (SO) and Tween 80. Carbohydr. Polym. 2012;90(4):1452–1460. doi: 10.1016/j.carbpol.2012.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.