Abstract
We performed a systematic review to investigate the definition of mild to moderate active ulcerative colitis (UC), and to describe predictors of good response to treatment in clinical trials assessing 5‐ASA and/or budesonide. Thirty‐nine randomized controlled trials were included. The UC Disease Activity Index (UCDAI) was the most frequent score used for defining mild to moderate active UC (16 studies, 41%), followed by Clinical Activity Index in 11 studies (28.2%). Four different cut‐offs were used to define mild to moderate active UC using the UCDAI. The most frequently reported predictors of good response to treatment was a mild and moderate disease activity. There is heterogeneity in the definition of mild to moderate active UC in randomized clinical trials. A standardized definition of mild to moderate active UC used for inclusion of patients in clinical trials is needed.
Keywords: definition, mild, moderate, predictive factor, ulcerative colitis
INTRODUCTION
The development of novel therapeutics and a better understanding of biological pathways of disease had resulted in improved management of patients with ulcerative colitis (UC). 1 In the last decades, the treatment targets have also evolved from symptom control to clinical remission and then to endoscopic remission aiming at achieving more ambitious goals and an ever‐deeper remission. 2 , 3 Although the evaluation of symptom based disease activity is subjective and less reliable than more objective data such as endoscopy, normalization of symptoms is the most desired target by patients as it impacts their quality of life. 4 The severity of UC has historically been dichotomized into arbitrary categories, specifically mild‐to‐moderate and moderate‐to‐severe, depending on number of daily stools, amount of rectal bleeding, vital signs, physician's assessment, and endoscopic evaluation. 5 Several scores have been developed to quantify and standardize the evaluation of clinical disease activity including the Simple Colitis Clinical Activity Index, the Mayo Clinic Score (MCS) (and its modifications), and the Truelove and Witts' criteria for severe disease. 4 , 6 , 7 , 8 , 9 , 10 These classifications allow stratification of disease activity, guide the selection of the optimal therapeutic option for each patient setting and help to monitor the response to treatment. 11 There is no consensus about the definition of mild to moderate disease activity in UC (Table 1). 1 , 12 , 13 The mainstay of therapy for mild to moderate UC is the 5‐aminosalicylic acid (5‐ASA) class of medications. 12 The first line therapy for UC patients with exclusively rectal disease is topical 5‐ASA while oral 5‐ASA alone or in combination with topical therapy is preferred in subjects with left‐side or extensive UC. 1 Patients with inadequate response to optimized 5‐ASA require therapy escalation to budesonide multimatrix system (MMX) or oral prednisone. 12 The lack of a commonly accepted definition of mild to moderate UC makes clinical trial data heterogeneous and non‐replicable, and influences clinical practice by exposing patients to under or over‐treatment. 14
TABLE 1.
Current definitions on mild to moderate active ulcerative colitis (UC) in guidelines
| IBD organization | Year | Definition of mild to moderate ulcerative colitis |
|---|---|---|
| American gastroenterological association | 2019 | Fewer than 4–6 bowel movements per day, mild‐moderate rectal bleeding, absence of constitutional symptoms, low overall inflammatory burden, and absence of features suggestive of high inflammatory activity based upon truelove and Witt's criteria and the Mayo clinic score |
| British society of gastroenterology | 2019 | Mayo score between 3 and 10 |
| European Crohn's and colitis organisation | 2017 | Not applicable |
Abbreviation: IBD, Inflammatory Bowel Disease.
For this reason, we conducted a systematic literature review to investigate the definition of mild to moderate active UC and to describe predictive factors of response to treatment in randomized controlled clinical trials assessing 5‐ASA and/or budesonide.
METHODS
We conducted a systematic review in accordance with the Cochrane Handbook and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension statement for reporting of systematic reviews incorporating network meta‐analysis. 15 , 16
Search strategy
We searched for randomized controlled trials using Pubmed/Embase, and Scopus databases from January 2000 to January 2022. The following Medical Subject Heading terms alone or matched with the Boolean operators “AND” or “OR” were used: “IBD”, “inflammatory bowel disease”, “UC”, “ulcerative colitis”, “5‐ASA”, “5‐aminosalicylic acid”, “mesalazine”, “mesalamine”, “budesonide”, “budesonide MMX”, “budesonide multimatrix”, “mild”, “moderate”. We focused on 5‐ASA and budesonide because these are the only agents established in practice in mild to moderate UC.
Two authors (BC and FD) independently scrutinized titles and abstracts to identify eligible studies. Subsequently, full‐text articles were examined for inclusion. In addition, a hand‐search of the bibliographic lists of selected manuscripts was performed to identify the studies missing from the electronic search. Any disagreements were resolved through discussion between all co‐authors.
Selection criteria
The inclusion criteria were: (a) studies conducted in patients with mild to moderate active UC; (b) randomized controlled trial; (c) study assessing 5‐ASA and/or budesonide; (d) study published in English. Only full text articles were included. All editorials, notes, comments, letters, or review articles were ineligible.
Data extraction
After screening for eligible studies, we extracted data regarding study and patient characteristics, study design, drug exposure, definition of mild to moderate active UC, primary endpoint for evaluation of treatment efficacy, and predictive factors of response to treatment when reported.
Quality of studies
The Jadad score was used to measure the quality of randomized clinical trials. High quality studies were defined as Jadad score of three or greater. 17 Two authors (BC and FD) independently graded the studies, and any disagreements were discussed with another author until resolution.
RESULTS
Study characteristics
A summary of the search and selection process is detailed in Figure 1. A total of 3038 citations (Pubmed/Embase: 930; Scopus: 2108) were identified through the search strategy. After removal of duplicates and careful screening of titles and abstracts, 2950 articles were excluded. Additional 49 studies were excluded after full‐text review of the manuscripts as they did not meet the inclusion criteria, viz. disease in remission (n = 21), not reporting disease activity (n = 7), observational cohort studies (n = 5), systematic reviews or meta‐analysis (n = 5), not evaluating drug efficacy (n = 4), other drug than 5‐ASA or budesonide (n = 4), not reporting definition of mild to moderate active UC or predictive factor of response (n = 2), and other language than English (n = 1). Finally, 39 randomized controlled trials were included. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Table 1 summarizes the main characteristics of the included studies. The study period ranged from 2000 to 2019. A total of 12,046 patients were enrolled. Most studies investigated the efficacy of 5‐ASA (33 studies, 84.6%), 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 53 , 55 , 56 while budesonide was assessed in a quarter of cases (10 studies, 25.6%). 39 , 40 , 42 , 45 , 49 , 50 , 51 , 52 , 54 , 56 Thirty‐five out of 39 studies (89.7%) had a Jadad score of 3 or greater (Table S1).
FIGURE 1.
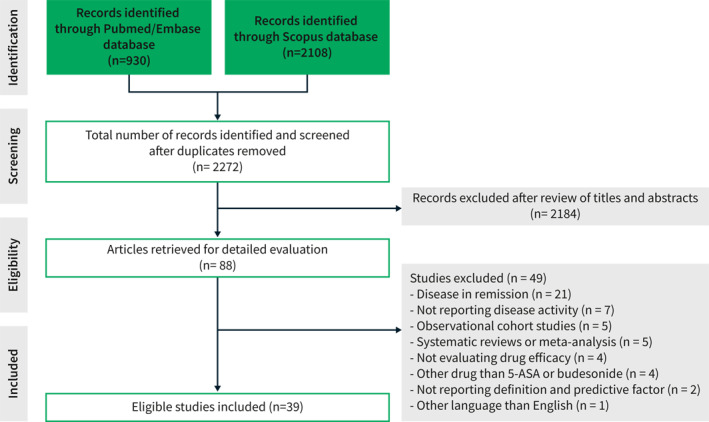
Summary of the literature search and selection process (flow diagram)
Definition of mild to moderate active ulcerative colitis
Thirty‐nine randomized controlled trials reported the definition of mild to moderate active UC (Table 2). In the majority of studies (33 studies, 84.6%), disease activity was assessed by combined clinical and endoscopic scores 18 , 19 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 34 , 35 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 (Table 3). In 6 studies (15.4%), mild to moderate UC was defined as clinical activity alone. 20 , 23 , 30 , 33 , 36 , 39
TABLE 2.
Definition of mild to moderate active ulcerative colitis (UC) in randomized clinical trials
| First author | Year | Number of patients | Study arms | Definition of mild to moderate ulcerative colitis (inclusion criterion) | Primary endpoint |
|---|---|---|---|---|---|
| Pokrotnieks et al. 18 | 2000 | 111 | Mesalazine foam enema or placebo enema | CAI >4 and EI of ≥4 | Clinical remission at week 6 defined as a CAI ≤4, associated with a decrease of least 2 points from baseline |
| Farup et al. 19 | 2001 | 227 | Mesalazine 4 g daily given as prolonged‐release granules in packets of 1 g or prolonged‐release tablets of 0.5 g | UCDAI between 3–5 and 6–8 | Change from baseline in UCDAI |
| Vecchi et al. 20 | 2001 | 67 | Mesalazine 4 g orally plus placebo enema, or mesalazine 2 g orally plus mesalazine 2 g enema | CAI from 4 to 12 | Clinical remission (CAI <4) or clinical remission/improvement (reduction of CAI of 50% from baseline) at week 6 and time to clinical remission/improvement |
| Raedler et al. 21 | 2004 | 362 | Mesalazine micropellets or tablets | CAI for components 1–4 ≥4 and EI ≥4 | Clinical remission at week 8 defined as the sum of CAI components 1–4 < or = 2 |
| Gionchetti et al. 22 | 2005 | 217 | Beclomethasone dipropionate 3 mg enema o.d. or mesalamine enema daily | DAI ≥3 and ≤10 | Decrease in DAI at week 6 |
| Hanauer et al. 23 | 2005 | 386 | Mesalamine 2.4 g/day or 4.8 g/day | PGA of 1 or 2 | Overall improvement at week 6 defined as either complete remission or a clinical response. Complete remission was defined as normal stool frequency, no rectal bleeding, a PFA score of 0, normal endoscopy findings and a PGA score of 0. Clinical response was defined as a decrease in the PGA score of at least one point from baseline, plus improvement in at least one other clinical assessment parameter (stool frequency, rectal bleeding, PFA or endoscopy findings) and no worsening in any of the other clinical assessments. |
| Marakhouski et al. 24 | 2005 | 233 | 1.5 g mesalazine pellets or tablets | CAI of 6–12 and EI ≥4 | Complete response (clinical remission) defined as CAI ≤4 at week 8 |
| Marteau et al. 25 | 2005 | 127 | Mesalazine enema or placebo | UCDAI score ⩾3 and ⩽8 | Remission at week 4 defined as UCDAI <2 |
| D’Haens et al. 26 | 2006 | 38 | MMX mesalazine 1.2 or 2.4 or 4.8 g/day | UCDAI score of 4–10 with a sigmoidoscopy score of ≥1 and a PGA score of ≤2 | Remission defined as UCDAI < or = 1, a score of 0 for rectal bleeding and stool frequency, and > or = 1 ‐point reduction in sigmoidoscopy score at week 8 |
| Miner et al. 27 | 2006 | 159 | Enema of 120 mg alicaforsen or 240 mg alicaforsen or 4 g mesalazine | DAI of 4–10 | Decrease in DAI at week 6 relative to baseline |
| Biancone et al. 28 | 2007 | 92 | Beclomethasone diproprionate enema or foam or mesalazine enema or foam | DAI ranging from 3 to 9, and an endoscopic score ranging from 1 to 2 | Remission at week 4, defined as DAI <3 |
| Eliakim et al. 29 | 2007 | 330 | Low‐volume or high‐volume 5‐aminosalicylic acid foam | CAI >4 and EI ≥4 | Clinical remission defined as CAI < or = 4) at week 6 |
| Hanauer et al. 30 | 2007 | 301 | Oral mesalamine 2.4 g/day or 4.8 g/day | PGA score of 1 or 2 | Overall improvement at week 6 defined as either complete remission or a clinical response. Complete remission was defined as normal stool frequency, no rectal bleeding, a PFA score of 0, normal endoscopy findings and a PGA score of 0. Clinical response was defined as a decrease in the PGA score of at least one point from baseline, plus improvement in at least one other clinical assessment parameter (stool frequency, rectal bleeding, PFA or endoscopy findings) and no worsening in any of the other clinical assessments. |
| Kamm et al. 31 | 2007 | 343 | MMX mesalamine 2.4 g/day or 4.8 g/day, or ASACOL 2.4 g/day or placebo | Modified UCDAI score of 4–10 with a sigmoidoscopy score ≥1 and a PGA score ≤2 | Clinical and endoscopic remission (modified UCDAI of < or = 1 with rectal bleeding and stool frequency scores of 0, no mucosal friability, and a > or = 1‐point reduction in sigmoidoscopy score at week 8 |
| Lichtenstein et al. 32 | 2007 | 280 | MMX mesalamine 2.4 g/day given twice daily or 4.8 g/day given once daily or placebo | Modified UCDAI of 4–10, with a sigmoidoscopy score ≥1 and a PGA score ≤2 | Clinical and endoscopic remission (modified UCDAI score of < or = 1, with a score of 0 for rectal bleeding and stool frequency, and at least a 1‐point reduction in sigmoidoscopy score) at week 8 |
| Cortot et al. 33 | 2008 | 375 | Mesalamine foam or mesalamine enema | CAI for components 1–4 ≥4 | Clinical remission at week 4 defined as CAI 1–4 < or = 2 |
| Lichtenstein et al. 34 | 2008 | 517 | MMX mesalazine 2.4 g/day or 4.8 g/day or placebo | UCDAI score of 4–10 with a sigmoidoscopy score ≥1 and a PGA score ≤2 | Clinical and endoscopic remission at week 8 defined as a total modified UCDAI score of ≤1, calculated as: scores of 0 for rectal bleeding and stool frequency, a combined PGA score and sigmoidoscopy score of ≤1, no mucosal friability and, at least, a one‐point reduction from baseline in the sigmoidoscopy score |
| Kruis et al. 35 | 2009 | 380 | 3 g OD or 1 g TID mesalazine granules | CAI >4 and EI ⩾4 | Clinical remission (CAI < or = 4) at week 8 |
| Sandborn et al. 36 | 2009 | 772 | Mesalamine 4.8 g/day or 2.4 g/day | PGA equal to 2 points, with a score of ≥1 point in both the stool frequency and rectal bleeding clinical assessments and a score of ≥2 points in the sigmoidoscopy assessment with a positive friability assessment | Overall improvement at week 6, defined as improvement in the PGA with no worsening in any individual clinical assessment |
| Scherl et al. 37 | 2009 | 249 | 3.3 g of balsalazide or placebo tablets twice daily | MMDAI score between 6 and 10, inclusive, with an individual subscale score ≥2 for rectal bleeding and mucosal appearance | Clinical improvement (> or = 3 point improvement in MMDAI) and improvement in rectal bleeding (> or = 1 point improvement) at week 8 |
| Andus et al. 38 | 2010 | 354 | Mesalamine 1 g suppository at bedtime or one mesalamine 0.5 g suppository thrice daily | DAI between 3 and 11 | Remission at week 6 defined as DAI <4 |
| D’Haens et al. 39 | 2010 | 36 | Budesonide‐MMX 9 mg tablets or placebo | CAI <14 | Clinical improvement (meaning either remission, defined as a CAI ≤4 or a CAI reduction by at least 50% of the baseline value) at week 4 |
| Hartmann et al. 40 | 2010 | 237 | Budesonide or mesalazine enemas | CAI >4 and EI >2 | Clinical remission at week 4 defined as CAI <4 |
| Ito et al. 41 | 2010 | 225 | Mesalamine 2.4 g/day or 3.6 g/day | UCDAI of 3–8 and a bloody stool score of 1 or greater | Decrease in UCDAI at week 8 |
| Gross et al. 42 | 2011 | 343 | 9 mg budesonide or 3 g mesalazine | CAI ≥6 and EI ≥4 | Clinical remission at week 8 defined as CAI ≤4 with stool frequency and rectal bleeding subscores of “0” |
| Hiwatashi et al. 43 | 2011 | 123 | 4 g/day mesalazine or 2.25 g/day | UCDAI score of 6–8 points | Improvement in all 4 UCDAI variables (stool frequency, rectal bleeding, mucosal appearance, and physician's overall assessment of disease) at week 8 |
| Lamet et al. 44 | 2011 | 99 | Mesalamine 1 g suppository administered QHS or 500 mg suppository administered BID | DAI between 4 and 11 | Decrease of DAI at week 6 |
| Sandborn et al. 45 | 2012 | 509 | Budesonide MMX (9 mg or 6 mg) or mesalamine or placebo | UCDAI score of 4–10 points | Remission at week 8 defined as combined clinical and endoscopic remission with a UCDAI score ≤1 point, with subscores of 0 for both rectal bleeding and stool frequency, no mucosal friability on colonoscopy, and a ≥1‐point reduction from baseline in the endoscopic index score |
| Flourié et al. 46 | 2013 | 206 | Mesalazine (4 g/day) either OD or BD | UCDAI score of 3–8 | Clinical and endoscopic remission at week 8 defined as UCDAI score ≤1 |
| Watanabe et al. 47 | 2013 | 129 | 1 g mesalazine or placebo suppository | UCDAI score between 4 and 8 | Endoscopic remission (mucosal score of 0 or 1) at week 4 |
| Probert et al. 48 | 2014 | 127 | Oral mesalazine 4 g/day, plus 1 g mesalazine enema or placebo enema | UCDAI score ≥3 and ≤8 | Remission at week 4 defined as UCDAI <2 |
| Travis et al. 49 | 2014 | 410 | Budesonide MMX 9 mg or 6 mg, or Entocort EC 9 mg or placebo | UCDAI score ≥4 and ≤10 | Combined clinical and endoscopic remission, defined as UCDAI ≤1 with a score of 0 for rectal bleeding and stool frequency, no mucosal friability on colonoscopy, and a ≥1‐point reduction in endoscopic index score at week 8 |
| Sandborn et al. 50 | 2015 | 546 | Budesonide foam or placebo | MMDAI between 5 and 10, inclusive, with subscale ratings of ≥2 for endoscopic appearance and rectal bleeding | Remission at week 6 defined as an endoscopy subscore ≤1, rectal bleeding subscore of 0, and improvement or no change from baseline in the stool frequency subscore of the Mayo score |
| Sandborn et al. 51 | 2015 | 672 | Budesonide MMX 9 mg or budesonide MMX 6 mg or placebo | UCDAI score between 4 and 10 | Combined clinical and colonoscopic remission at week 8 defined as a UCDAI ≤1, with no rectal bleeding (UCDAI subscore = 0), normal stool frequency (UCDAI subscore = 0), normal mucosa with no evidence of friability at full colonoscopy and an endoscopic index score ≥1 point lower than baseline |
| Bosworth et al. 52 | 2016 | 546 | Budesonide foam or placebo | MMDAI score ≥5 but ≤10, with subscale ratings ≥2 for rectal bleeding and endoscopic appearance | Remission at week 6 defined as MMDAI endoscopy subscale score ≤1, MMDAI rectal bleeding subscale score 0, and improvement or no change from baseline in MMDAI stool frequency subscale score |
| D’Haens et al. 53 | 2017 | 817 | 3.2 g of oral mesalazine, administered as two 1600 mg tablets once, or four 400 mg tablets twice daily | MCS ≥5, a rectal bleeding subscore ≥1, and a MCES score ≥2 | Clinical and endoscopic remission at week 8 defined as stool frequency and rectal bleeding subscores of 0 and MCS ≤2 with no individual subscore >1 |
| Rubin et al. 54 | 2017 | 458 | Budesonide multimatrix 9 mg or placebo | UCDAI ≥4 and ≤10, mucosal appearance subscore ≥1, and physician's rating of disease activity score of 1 or 2 | Combined clinical and endoscopic remission at week 8 defined as total UCDAI score ≤1, with subscale scores of 0 for rectal bleeding, stool frequency, and mucosal appearance |
| Dignass et al. 55 | 2018 | 306 | One 1000 mg mesalazine tablet or two registered 500 mg mesalazine tablets, both taken three times daily | CAI >4 and ≤12 and EI of 4 or greater | Clinical remission at week 8 defined as CAI ≤4, with stool frequency and rectal bleeding subscores of 0 |
| Kruis et al. 56 | 2019 | 337 | Budesonide suppositories (2 mg BUS) or 4 mg BUS or 1 g mesalamine suppositories or the combination of 2 mg BUS and 1 g MES | Modified UCDAI 4–10 with an endoscopic subscore of ≥1 | Time to resolution of clinical symptoms, defined as the first of 3 consecutive days with a score of 0 for rectal bleeding and stool frequency |
Abbreviations: BID, twice a day; CAI, Clinical Activity Index; DAI, Disease Activity Index; EI, Endoscopic Index; MCES, Mayo Clinic Endoscopic Subscale; MCS, Mayo Clinic Score; MES, Mayo endoscopic score; MMDAI, Modified Mayo Disease Activity Index; MMX, multimatrix system; PFA, Patient’s Functional Assessment; PGA, Physician’s Global Assessment; QHS, every bedtime; UCDAI, Ulcerative Colitis Disease Activity Index.
TABLE 3.
Ulcerative colitis (UC) disease activity assessment indices
| Index name | Abbreviation | Range | Variables |
|---|---|---|---|
| Clinical activity index | CAI | 0–29 | Number of stools |
| Blood in stools | |||
| Investigator's global assessment of symptomatic state | |||
| Abdominal pain or cramps | |||
| Temperature due to colitis | |||
| Extraintestinal manifestations | |||
| Laboratory findings (erythrocyte sedimentation rate, hemoglobin) | |||
| Disease activity index | DAI | 0–12 | Stool frequency |
| Rectal bleeding | |||
| Mucosal appearance | |||
| Physician's rating of disease activity | |||
| Endoscopic index | EI | 0–12 | Granulation scattering reflected light |
| Vascular pattern | |||
| Vulnerability of mucosa | |||
| Mucosal damage (mucus, fibrin, exsudates, erosions, ulcer) | |||
| Mayo clinic score | MCS | 0–12 | Stool frequency |
| Rectal bleeding | |||
| Findings of flexible proctosigmoidoscopy | |||
| Physician's global assessment | |||
| Modified Mayo disease activity index | MMDAI | 0–12 | Bowel frequency |
| Rectal bleeding | |||
| Physician's global assessment | |||
| Endoscopy/sigmoidoscopy finding | |||
| Ulcerative colitis disease activity index | UCDAI | 0–12 | Stool frequency |
| Rectal bleeding | |||
| Mucosal appearance | |||
| Physician's rating of disease activity |
The UC Disease Activity Index (DAI) (UCDAI) was the most frequently used score (16 studies, 41%). 19 , 25 , 26 , 31 , 32 , 34 , 41 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 54 , 56 Four different UCDAI cut‐offs were used to define mild to moderate active UC. More than half of included studies reported an UCDAI ≥4 and ≤10 with a sigmoidoscopy score of ≥1 and a Physician's Global Assessment (PGA) score ≤2 (9 studies, 26 , 31 , 32 , 34 , 45 , 49 , 51 , 54 , 56 56,3%), followed by an UCDAI ≥3 and ≤8 (5 studies, 19 , 25 , 41 , 46 , 48 31,3%), between 6 and 8 (1 study, 43 6,2%) and between 4 and 8 (1 study, 47 6,2%).
The CAI was used in 11 studies (28.2%). 18 , 20 , 21 , 24 , 29 , 33 , 35 , 39 , 40 , 42 , 55 Five different CAI cut‐offs were used to define mild to moderate active UC. More than half of included studies reported a CAI >4 (6 studies, 18 , 21 , 29 , 33 , 35 , 40 54.5%), followed by a CAI between 6 and 12 (2 studies, 24 , 55 18.2%), a CAI ≥6 (1 study, 42 9.1%), a CAI <14 (1 study, 39 9.1%), and between 4 and 12 (1 study. 20 9.1%). Clinical Activity Index was associated with the Endoscopic Index (EI) in 8 studies 18 , 21 , 24 , 29 , 35 , 40 , 42 , 55 : EI ≥ 4 (7 studies, 87.5%) and >2 (1 study, 12.5%).
The DAI was used in 5 studies (12.8%). 22 , 27 , 28 , 38 , 44 Five different DAI cut‐offs were used to define mild to moderate active UC. One study reported a DAI between 4 and 11 44 (20%), followed by a DAI between 3 and 9 and an endoscopic score ranging from 1 to 2 (1 study, 28 20%), between 3 and 11 (1 study, 38 20%), between 4 and 10 (1 study, 27 20%) and between 3 and 10 (1 study, 22 20%).
The Modified Mayo Disease Activity Index (MMDAI) was used in 3 studies (7.7%). 37 , 50 , 52 Two different MMDAI cut‐offs were used to defined mild to moderate active UC: between 5 and 10, inclusive, with subscale ratings of ≥2 for endoscopic appearance and rectal bleeding (2 studies, 50 , 52 66.7%), and between 6 and 10, inclusive, with subscale ratings of ≥2 for endoscopic appearance and rectal bleeding (1 study, 37 33.3%).
The PGA alone was used in 3 studies (7.7%). 23 , 30 , 36 Two different PGA cut‐offs ware used to define mild to moderate active UC: PGA score of 1 or 2 (2 studies, 23 , 30 66.7%), and a PGA equal to 2 points (1 study, 36 33.3%).
The MCS was used in one study (2.6%). 53 Mild to moderate active UC was defined as MCS ≥5, a rectal bleeding subscore ≥1, and a Mayo Clinic Endoscopic Subscale score ≥2.
Predictive factors of response to treatment
Eleven randomized controlled trials (28.2%) reported predictive factors of response to treatment (Table 4). 22 , 23 , 24 , 29 , 30 , 35 , 36 , 38 , 41 , 49 , 56 Most studies investigated the efficacy of 5‐ASA (10 studies, 90.9%), 22 , 23 , 24 , 29 , 30 , 35 , 36 , 38 , 41 , 56 while budesonide was assessed in one study (9.1%). 49 The most frequently reported predictor was a mild disease activity (4 studies, 36.4%). 24 , 35 , 38 , 56 Other predictive factors were moderate disease activity (3 studies, 27.3%), 22 , 30 , 41 previous treatment with steroids (3 studies, 27.3%), 23 , 30 , 36 no extraintestinal manifestation (2 studies, 18.2%), 24 , 38 shorter disease duration (1 study, 9.1%), 29 proctitis (1 study, 9.1%), 41 distal disease (1 study, 9.1%), 35 female (1 study, 38 9.1%), male (1 study, 9.1%), 49 Eastern European patients (1 study, 9.1%), 49 younger patients (1 study, 9.1%), 49 and previous treatment with oral mesalamine or rectal therapies (1 study, 9.1%). 36
TABLE 4.
Predictors of good response to treatment in mild to moderate active ulcerative colitis (UC)
| First author | Year | Number of patients | Study arms | Definition of mild to moderate UC (inclusion criterion) | Predictive factors of response |
|---|---|---|---|---|---|
| Pokrotnieks et al. 18 | 2000 | 111 | Mesalazine foam enema or placebo enema | CAI >4 and EI of ≥4 | ND |
| Farup et al. 19 | 2001 | 227 | Mesalazine 4g daily given as prolonged‐release granules in packets of 1g or prolonged‐release tablets of 0.5 g | UCDAI between 3–5 and 6–8 | ND |
| Vecchi et al. 20 | 2001 | 67 | Mesalazine 4g orally plus placebo enema, or mesalazine 2g orally plus mesalazine 2g enema | CAI from 4 to 12 | ND |
| Raedler et al. 21 | 2004 | 362 | Mesalazine micropellets or tablets | CAI for components 1–4 ≥4 and EI ≥4 | ND |
| Gionchetti et al. 22 | 2005 | 217 | Beclomethasone dipropionate 3 mg enema o.d. or mesalamine enema daily | DAI ≥3 and ≤10 | Moderate disease |
| Hanauer et al. 23 | 2005 | 386 | Mesalamine 2.4 g/day or 4.8 g/day | PGA of 1 or 2 | Previous treatment with steroids |
| Marakhouski et al. 24 | 2005 | 233 | 1.5 g mesalazine pellets or tablets | CAI of 6–12 and EI ≥4 | Mild disease activity |
| No extraintestinal manifestation | |||||
| Marteau et al. 25 | 2005 | 127 | Mesalazine enema or placebo | UCDAI score ⩾3 and ⩽8 | ND |
| D’Haens et al. 26 | 2006 | 38 | MMX mesalazine 1.2 or 2.4 or 4.8 g/day | UCDAI score of 4–10 with a sigmoidoscopy score of ≥1 and a PGA score of ≤2 | ND |
| Miner et al. 27 | 2006 | 159 | Enema of 120 mg alicaforsen or 240 mg alicaforsen or 4g mesalazine | DAI of 4–10 | ND |
| Biancone et al. 28 | 2007 | 92 | Beclomethasone diproprionate enema or foam or mesalazine enema or foam | DAI ranging from 3 to 9, and an endoscopic score ranging from 1 to 2 | ND |
| Eliakim et al. 29 | 2007 | 330 | Low‐volume or high‐volume 5‐aminosalicylic acid foam | CAI >4 and EI ≥4 | Shorter disease duration (<5 years) |
| Hanauer et al. 30 | 2007 | 301 | Oral mesalamine 2.4 g/day or 4.8 g/day | PGA score of 1 or 2 | Moderate disease |
| Previous treatment with steroids | |||||
| Kamm et al. 31 | 2007 | 343 | MMX mesalamine 2.4 g/day or 4.8 g/day, or ASACOL 2.4 g/day or placebo | Modified UCDAI score of 4–10 with a sigmoidoscopy score ≥1 and a PGA score ≤2 | ND |
| Lichtenstein et al. 32 | 2007 | 280 | MMX mesalamine 2.4 g/day given twice daily or 4.8 g/day given once daily or placebo | Modified UCDAI of 4–10, with a sigmoidoscopy score ≥1 and a PGA score ≤2 | ND |
| Cortot et al. 33 | 2008 | 375 | Mesalamine foam or mesalamine enema | CAI for components 1–4 ≥4 | ND |
| Lichtenstein et al. 34 | 2008 | 517 | MMX mesalazine 2.4 g/day or 4.8 g/day or placebo | UCDAI score of 4–10 with a sigmoidoscopy score ≥1 and a PGA score ≤2 | ND |
| Kruis et al. 35 | 2009 | 380 | 3g OD or 1g TID mesalazine granules | CAI >4 and EI ⩾4 | Mild disease |
| Distal disease | |||||
| Sandborn et al. 36 | 2009 | 772 | Mesalamine 4.8 g/day or 2.4 g/day | PGA equal to 2 points, with a score of ≥1 point in both the stool frequency and rectal bleeding clinical assessments and a score of ≥2 points in the sigmoidoscopy assessment with a positive friability assessment | Previous treatment with steroids, oral mesalamine, rectal therapies |
| Scherl et al. 37 | 2009 | 249 | 3.3 g of balsalazide or placebo tablets twice daily | MMDAI score between 6 and 10, inclusive, with an individual subscale score ≥2 for rectal bleeding and mucosal appearance | ND |
| Andus et al. 38 | 2010 | 354 | Mesalamine 1g suppository at bedtime or one mesalamine 0.5 g suppository thrice daily | DAI between 3 and 11 | Female |
| Mild disease | |||||
| No extraintestinal manifestation | |||||
| D’Haens et al. 39 | 2010 | 36 | Budesonide‐MMX 9 mg tablets or placebo | CAI <14 | ND |
| Hartmann et al. 40 | 2010 | 237 | Budesonide or mesalazine enemas | CAI >4 and EI >2 | ND |
| Ito et al. 41 | 2010 | 225 | Mesalamine 2.4 g/day or 3.6 g/day | UCDAI of 3–8 and a bloody stool score of 1 or greater | Proctitis |
| Moderate disease | |||||
| Gross et al. 42 | 2011 | 343 | 9mg budesonide or 3g mesalazine | CAI ≥6 and EI ≥4 | ND |
| Hiwatashi et al. 43 | 2011 | 123 | 4g/day mesalazine or 2.25 g/day | UCDAI score of 6–8 points | ND |
| Lamet et al. 44 | 2011 | 99 | Mesalamine 1g suppository administered QHS or 500 mg suppository administered BID | DAI between 4 and 11 | ND |
| Sandborn et al. 45 | 2012 | 509 | Budesonide MMX (9 mg or 6 mg) or mesalamine or placebo | UCDAI score of 4–10 points | ND |
| Flourié et al. 46 | 2013 | 206 | Mesalazine (4 g/day) either OD or BD | UCDAI score of 3–8 | ND |
| Watanabe et al. 47 | 2013 | 129 | 1g mesalazine or placebo suppository | UCDAI score between 4 and 8 | ND |
| Probert et al. 48 | 2014 | 127 | Oral mesalazine 4 g/day, plus 1g mesalazine enema or placebo enema | UCDAI score ≥3 and ≤8 | ND |
| Travis et al. 49 | 2014 | 410 | Budesonide MMX 9 mg or 6 mg, or Entocort EC 9 mg or placebo | UCDAI score ≥4 and ≤10 | Younger patients (aged ≤43.5 years) |
| Men | |||||
| Eastern European patients | |||||
| Sandborn et al. 50 | 2015 | 546 | Budesonide foam or placebo | MMDAI between 5 and 10, inclusive, with subscale ratings of ≥2 for endoscopic appearance and rectal bleeding | ND |
| Sandborn et al. 51 | 2015 | 672 | Budesonide MMX 9 mg or budesonide MMX 6 mg or placebo | UCDAI score between 4 and 10 | ND |
| Bosworth et al. 52 | 2016 | 546 | Budesonide foam or placebo | MMDAI score ≥5 but ≤10, with subscale ratings ≥2 for rectal bleeding and endoscopic appearance | ND |
| D’Haens et al. 53 | 2017 | 817 | 3.2 g of oral mesalazine, administered as two 1600 mg tablets once, or four 400 mg tablets twice daily | MCS ≥5, a rectal bleeding subscore ≥1, and a MCES score ≥2 | Treatment failure: |
| Younger age | |||||
| Higher endoscopic disease activity | |||||
| Higher histopathologic disease activity | |||||
| Higher leucocyte concentration | |||||
| Rubin et al. 54 | 2017 | 458 | Budesonide multimatrix 9 mg or placebo | UCDAI ≥4 and ≤10, mucosal appearance subscore ≥1, and physician's rating of disease activity score of 1 or 2 | ND |
| Dignass et al. 55 | 2018 | 306 | One 1000 mg mesalazine tablet or two registered 500 mg mesalazine tablets, both taken three times daily | CAI >4 and ≤12 and EI of 4 or greater | ND |
| Kruis et al. 56 | 2019 | 337 | Budesonide suppositories (2 mg BUS) or 4 mg BUS or 1g mesalamine suppositories or the combination of 2 mg BUS and 1 g MES | Modified UCDAI 4–10 with an endoscopic subscore of ≥1 | Mild disease |
Abbreviations: BID, twice a day; CAI, Clinical Activity Index; DAI, Disease Activity Index; EI, Endoscopic Index; MCES, Mayo Clinic Endoscopic Subscale; MCS, Mayo Clinic Score; MES, Mayo endoscopic score; MMDAI, Modified Mayo Disease Activity Index; MMX, multimatrix system; ND, non disponible; PGA, Physician’s Global Assessment; QHS, every bedtime; TID, three times a day; UCDAI, Ulcerative Colitis Disease Activity Index.
One study (2.6%) reported predictive factors of treatment failure 53 : younger age, higher endoscopic disease activity, higher histopathologic disease activity, and higher leucocyte concentration.
DISCUSSION
During the last 2 decades, various scores have been used to evaluate disease activity in mild to moderate UC. In this systematic review we summarized evidence from 39 randomized controlled trials assessing 5‐ASA and/or budesonide, reporting the definition of mild to moderate active UC. Six different scores were used to define mild to moderate active UC, highlighting relevant heterogeneity in the literature. The UCDAI was the most adopted score with a value between 4 and 10 as a threshold to indicate mild to moderate disease. This composite index includes a rectal bleeding score (RBS), a stool frequency subscore (SFS), endoscopic appearance, and physician global assessment. In contrast, mild to moderate active UC was defined using the MCS in only one study in our review.
Recently, a similar systematic review by Sedano and colleagues investigated the definition of mild to moderate UC using data from ClinicalTrials.gov. 57 A total of 61 ongoing studies were included (38 (62%) placebo‐controlled studies, 11 (18%) active comparator controlled, and 11 (18%) uncontrolled). Of note, the MCS was the most frequently used score (50/61, 82%) while the UCDAI was detected only in a small percentage of cases (8/61, 13.1%). The authors proposed a definition of mild to moderate active UC based on the MCS: MCS of 4‐9 using Mayo endoscopic score (MES) ≥2 in combination with RBS ≥1, and SFS ≥1 or MES ≥1 and Geboes score >2B.0 or Robarts Histopathology Index ≥10 and/or feacal calprotectin >250 μg/g. 57 Probably these contrasting data are associated to the considered study period. We considered studies conducted in the last 20 years, while Sedano et al. considered ongoing studies. Furthermore, the MCS is easy to use and is commonly used in clinical practice.
Regulatory authorities have highlighted the limitations of the MCS and UCDAI. 58 One of the major limitations is the PGA subscore. 58 This single general item cannot adequately capture whether benefit is achieved in the important signs and symptoms, and it not derived directly from the patient. 58 Accordingly, the PGA subscore should not be used to assess disease activity. 58
In our systematic review, we also investigated predictive factors of response to treatment with 5‐ASA or budesonide in mild to moderate active UC. Only one third of the included studies reported predictive factors of response to treatment. Mild and moderate disease activity, previous treatment with steroids, no extraintestinal manifestation, shorter disease duration, and distal disease were reported as predictive factors of response to treatment.
To the best of our knowledge, this is the first systematic review of randomized controlled trials describing the definition of mild to moderate UC and predictive factors of response to treatment, focusing only on 5‐ASA and budesonide. In addition, other strength of our article is the high quality of the included studies as demonstrated by the rate of studies with a Jadad score greater than 3. However, some limitations need to be pointed out. In fact, the included studies are very heterogeneous. This is partly explained by the considerable time span between studies and the lack of commonly accepted guidelines on this topic. Almost all recent randomized controlled trials focus on use of biologics of small molecules in patients with moderately to severely active UC, for which regulatory guidance has recommended appropriate efficacy assessments, endpoint definitions, and timing to measure these outcomes. 57 , 58 However there is no consensus definition for mild to moderate disease activity resulting in heterogeneous study populations. 57 , 59 The heterogeneity of available studies in the selection of patients suggest that the development of an international consensus on the definition of mild to moderate active UC used for inclusion of patients in clinical trials would be useful to allow harmonization. Other symptoms, such as faecal urgency for example, might be interesting to include in the definition of mild to moderate active UC.
In conclusion, this review found substantial heterogeneity in the scoring indexes used and their definitions of mild to moderate active UC. Similar definition of mild to moderate active UC should be considered to minimize heterogeneity and ensure validity of future randomized controlled trials. The definition of mild to moderate active UC should combine clinic and endoscopic evaluation, in line with the current trend of making evaluations more objective and standardized.
AUTHOR CONTRIBUTIONS
Silvio Danese and Laurent Peyrin‐Biroulet conceived the study. Bénédicte Caron wrote the article and created tables and figures. Vipul Jairath, Ferdinando D’Amico, Kristine Paridaens, Fernando Magro, Silvio Danese, and Laurent Peyrin‐Biroulet critically reviewed the content of the paper and supervised the project. The manuscript was approved by all authors.
CONFLICT OF INTEREST
B Caron reports lecture and/or consulting fees from Abbvie, Amgen, Celltrion, Janssen, Ferring, Takeda. V Jairath reports consulting/advisory board fees from AbbVie, Alimentiv Inc (formerly Robarts Clinical Trials), Arena pharmaceuticals, Asahi Kasei Pharma, Asieris, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genentech, Gilead, Janssen, Merck, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Reistone Biopharma, Roche, Sandoz, Second Genome, Takeda, Teva, Topivert, Vividion; speaker's fees from, Abbvie, Ferring, Galapagos, Janssen Pfizer Shire, Takeda, Fresenius Kabi. F D’Amico declares no conflict of interest. K Paridaens is an employee of Ferring Pharmaceuticals. F Magro received a fee for presenting from: AbbVie, Ferring, Falk, Hospira, PharmaKern, MSD, Schering, Laboratórios Vitoria, Vifor Pharma, OM Pharma. S Danese has served as a speaker, consultant, and advisory board member for Schering‐Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma and Vifor. L Peyrin‐Biroulet reports personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; stock options: CTMA.
Supporting information
Supplementary Material 1
ACKNOWLEDGEMENT
This work was supported by Ferring Pharmaceuticals.
Caron B, Jairath V, D’Amico F, Paridaens K, Magro F, Danese S, et al. Definition of mild to moderate ulcerative colitis in clinical trials: a systematic literature review. United European Gastroenterol J. 2022;10(8):854–67. 10.1002/ueg2.12283
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. 10.1093/ecco-jcc/jjab178 [DOI] [PubMed] [Google Scholar]
- 2. Peyrin‐Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat‐to‐target. Am J Gastroenterol. 2015;110(9):1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 3. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 4. Walsh AJ, Bryant RV, Travis SPL. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13(10):567–79. 10.1038/nrgastro.2016.128 [DOI] [PubMed] [Google Scholar]
- 5. Danese S, Fiorino G, Peyrin‐Biroulet L. Positioning therapies in ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(6):1280–90. 10.1016/j.cgh.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 6. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. 10.1136/gut.43.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9. 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Sands BE, Wolf DC, Valentine JF, Safdi M, Katz S, et al. Repifermin (keratinocyte growth factor‐2) for the treatment of active ulcerative colitis: a randomized, double‐blind, placebo‐controlled, dose‐escalation trial. Aliment Pharmacol Therapeut. 2003;17(11):1355–64. 10.1046/j.1365-2036.2003.01589.x [DOI] [PubMed] [Google Scholar]
- 9. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041–8. 10.1136/bmj.2.4947.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132(2):763–86. 10.1053/j.gastro.2006.12.038 [DOI] [PubMed] [Google Scholar]
- 11. Peyrin‐Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14(3):348–54.e17. 10.1016/j.cgh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 12. Ko CW, Singh S, Feuerstein JD, Falck‐Ytter C, Falck‐Ytter Y, Cross RK , et al. AGA clinical practice guidelines on the management of mild‐to‐moderate ulcerative colitis. Gastroenterology. 2019;156(3):748–64. 10.1053/j.gastro.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solitano V, D’Amico F, Fiorino G, Paridaens K, Peyrin‐Biroulet L, Danese S. Key strategies to optimize outcomes in mild‐to‐moderate ulcerative colitis. J Clin Med. 2020;9:E2905. 10.3390/jcm9092905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for systematic reviews of interventions. 2nd ed. Chicexter UK: John Wiley and Sons; 2019. [Google Scholar]
- 16. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C , et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 18. Pokrotnieks J, Marlicz K, Paradowski L, Margus, Zaborowski, Greinwald. Efficacy and tolerability of mesalazine foam enema (Salofalk foam) for distal ulcerative colitis: a double‐blind, randomized, placebo‐controlled study. Aliment Pharmacol Ther. 2000;14(9):1191–8. 10.1046/j.1365-2036.2000.00784.x [DOI] [PubMed] [Google Scholar]
- 19. Farup PG, Hinterleitner TA, Lukás M, Hebuterne X, Rachmilewitz D, Campieri M, et al. Mesalazine 4 g daily given as prolonged‐release granules twice daily and four times daily is at least as effective as prolonged‐release tablets four times daily in patients with ulcerative colitis. Inflamm Bowel Dis. 2001;7(3):237–42. 10.1097/00054725-200108000-00009 [DOI] [PubMed] [Google Scholar]
- 20. Vecchi M, Meucci G, Gionchetti P, Beltrami M, Di Maurizio P, Beretta L, et al. Oral versus combination mesalazine therapy in active ulcerative colitis: a double‐blind, double‐dummy, randomized multicentre study. Aliment Pharmacol Ther. 2001;15(2):251–6. 10.1046/j.1365-2036.2001.00913.x [DOI] [PubMed] [Google Scholar]
- 21. Raedler A, Behrens C, Bias P. Mesalazine (5‐aminosalicylic acid) micropellets show similar efficacy and tolerability to mesalazine tablets in patients with ulcerative colitis‐‐results from a randomized‐controlled trial. Aliment Pharmacol Ther. 2004;20(11‐12):1353–63. 10.1111/j.1365-2036.2004.02282.x [DOI] [PubMed] [Google Scholar]
- 22. Gionchetti P, D’Arienzo A, Rizzello F, Manguso F, Maieron R, Lecis PE, et al. Topical treatment of distal active ulcerative colitis with beclomethasone dipropionate or mesalamine: a single‐blind randomized controlled trial. J Clin Gastroenterol. 2005;39(4):291–7. 10.1097/01.mcg.0000155124.74548.61 [DOI] [PubMed] [Google Scholar]
- 23. Hanauer SB, Sandborn WJ, Kornbluth A, Katz S, Safdi M, Woogen S, et al. Delayed‐release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. 2005;100(11):2478–85. 10.1111/j.1572-0241.2005.00248.x [DOI] [PubMed] [Google Scholar]
- 24. Marakhouski Y, Fixa B, Holomán J, Hulek P, Lukas M, Batovsky M, et al. A double‐blind dose‐escalating trial comparing novel mesalazine pellets with mesalazine tablets in active ulcerative colitis. Aliment Pharmacol Ther. 2005;21(2):133–40. 10.1111/j.1365-2036.2005.02312.x [DOI] [PubMed] [Google Scholar]
- 25. Marteau P, Probert CS, Lindgren S, Gassul M, Tan TG, Dignass A, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54(7):960–5. 10.1136/gut.2004.060103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Haens G, Hommes D, Engels L, Baert F, Van Der Waaij L, Connor P , et al. Once daily MMX mesalazine for the treatment of mild‐to‐moderate ulcerative colitis: a phase II, dose‐ranging study. Aliment Pharmacol Ther. 2006;24(7):1087–97. 10.1111/j.1365-2036.2006.03082.x [DOI] [PubMed] [Google Scholar]
- 27. Miner PBJ, Wedel MK, Xia S, Baker BF. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left‐sided ulcerative colitis: a randomized, double‐blind, active‐controlled trial. Aliment Pharmacol Ther. 2006;23(10):1403–13. 10.1111/j.1365-2036.2006.02837.x [DOI] [PubMed] [Google Scholar]
- 28. Biancone L, Gionchetti P, Blanco GDV, Orlando A, Annese V, Papi C, et al. Beclomethasone dipropionate versus mesalazine in distal ulcerative colitis: a multicenter, randomized, double‐blind study. Dig Liver Dis. 2007;39(4):329–37. 10.1016/j.dld.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 29. Eliakim R, Tulassay Z, Kupcinskas L, Adamonis K, Pokrotnieks J, Bar‐Meir S, et al. Clinical trial: randomized‐controlled clinical study comparing the efficacy and safety of a low‐volume vs. a high‐volume mesalazine foam in active distal ulcerative colitis. Aliment Pharmacol Ther. 2007;26(9):1237–49. 10.1111/j.1365-2036.2007.03468.x [DOI] [PubMed] [Google Scholar]
- 30. Hanauer SB, Sandborn WJ, Dallaire C, Archambault A, Yacyshyn B, Yeh C, et al. Delayed‐release oral mesalamine 4.8 g/day (800 mg tablets) compared with 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: the ASCENDI trial. Can J Gastroenterol. 2007;21(12):827–34. 10.1155/2007/862917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T , et al. Once‐daily, high‐concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132(1):66–75. 10.1053/j.gastro.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 32. Lichtenstein GR, Kamm MA, Boddu P, Gubergrits N, Lyne A, Butler T , et al. Effect of once‐ or twice‐daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(1):95–102. 10.1016/j.cgh.2006.10.025 [DOI] [PubMed] [Google Scholar]
- 33. Cortot A, Maetz D, Degoutte E, Delette O, Meunier P, Tan G, et al. Mesalamine foam enema versus mesalamine liquid enema in active left‐sided ulcerative colitis. Am J Gastroenterol. 2008;103(12):3106–14. 10.1111/j.1572-0241.2008.02152.x [DOI] [PubMed] [Google Scholar]
- 34. Lichtenstein GR, Kamm MA, Sandborn WJ, Lyne A, Joseph RE. MMX mesalazine for the induction of remission of mild‐to‐moderately active ulcerative colitis: efficacy and tolerability in specific patient subpopulations. Aliment Pharmacol Ther. 2008;27(11):1094–102. 10.1111/j.1365-2036.2008.03688.x [DOI] [PubMed] [Google Scholar]
- 35. Kruis W, Kiudelis G, Rácz I, Gorelov IA, Pokrotnieks J, Horynski M, et al. Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double‐blind, double‐dummy, randomised, non‐inferiority trial. Gut. 2009;58(2):233–40. 10.1136/gut.2008.154302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandborn WJ, Regula J, Feagan BG, Belousova E, Jojic N, Lukas M, et al. Delayed‐Release oral mesalamine 4.8 g/day (800‐mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009;137(6):1934–43.e3. 10.1053/j.gastro.2009.08.069 [DOI] [PubMed] [Google Scholar]
- 37. Scherl EJ, Pruitt R, Gordon GL, Lamet M, Shaw A, Huang S, et al. Safety and efficacy of a new 3.3g b.i.d. tablet formulation in patients with mild‐to‐moderately‐active ulcerative colitis: a multicenter, randomized, double‐blind, placebo‐controlled study. Am J Gastroenterol. 2009;104(6):1452–9. 10.1038/ajg.2009.83 [DOI] [PubMed] [Google Scholar]
- 38. Andus T, Kocjan A, Müser M, Baranovsky A, Mikhailova TL, Zvyagintseva TD, et al. Clinical trial: a novel high‐dose 1 g mesalamine suppository (Salofalk) once daily is as efficacious as a 500‐mg suppository thrice daily in active ulcerative proctitis. Inflamm Bowel Dis. 2010;16(11):1947–56. 10.1002/ibd.21258 [DOI] [PubMed] [Google Scholar]
- 39. D’Haens GR, Kovács A, Vergauwe P, Nagy F, Molnar T, Bouhnik Y, et al. Clinical trial: preliminary efficacy and safety study of a new Budesonide‐MMX® 9 mg extended‐release tablets in patients with active left‐sided ulcerative colitis. J Crohns Colitis. 2010;4(2):153–60. 10.1016/j.crohns.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 40. Hartmann F, Stein J. BudMesa‐Study Group . Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left‐sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32(3):368–76. 10.1111/j.1365-2036.2010.04354.x [DOI] [PubMed] [Google Scholar]
- 41. Ito H, Iida M, Matsumoto T, Suzuki Y, Sasaki H, Yoshida T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double‐blind, randomized study. Inflamm Bowel Dis. 2010;16(9):1567–74. 10.1002/ibd.21193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gross V, Bunganic I, Belousova EA, Mikhailova TL, Kupcinskas L, Kiudelis G, et al. 3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: a double‐blind, double‐dummy, randomised trial. J Crohns Colitis. 2011;5(2):129–38. 10.1016/j.crohns.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 43. Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel‐dosing study. J Gastroenterol. 2011;46(1):46–56. 10.1007/s00535-010-0308-3 [DOI] [PubMed] [Google Scholar]
- 44. Lamet M. A multicenter, randomized study to evaluate the efficacy and safety of mesalamine suppositories 1 g at bedtime and 500 mg Twice daily in patients with active mild‐to‐moderate ulcerative proctitis. Dig Dis Sci. 2011;56(2):513–22. 10.1007/s10620-010-1334-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al. Once‐daily budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143(5):1218–26.e2. 10.1053/j.gastro.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 46. Flourié B, Hagège H, Tucat G, Maetz D, Hebuterne X, Kuyvenhoven JP, et al. Randomised clinical trial: once‐vs. twice‐daily prolonged‐release mesalazine for active ulcerative colitis. Aliment Pharmacol Ther. 2013;37(8):767–75. 10.1111/apt.12266 [DOI] [PubMed] [Google Scholar]
- 47. Watanabe M, Nishino H, Sameshima Y, Ota A, Nakamura S, Hibi T. Randomised clinical trial: evaluation of the efficacy of mesalazine (mesalamine) suppositories in patients with ulcerative colitis and active rectal inflammation ‐‐ a placebo‐controlled study. Aliment Pharmacol Ther. 2013;38(3):264–73. 10.1111/apt.12362 [DOI] [PubMed] [Google Scholar]
- 48. Probert CSJ, Dignass AU, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild‐to‐moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8(3):200–7. 10.1016/j.crohns.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 49. Travis SPL, Danese S, Kupcinskas L, Alexeeva O, D'Haens G, Gibson PR, et al. Once‐daily budesonide MMX in active, mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014;63(3):433–41. 10.1136/gutjnl-2012-304258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandborn WJ, Bosworth B, Zakko S, Gordon GL, Clemmons DR, Golden PL, et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology. 2015;148(4):740–50. 10.1053/j.gastro.2015.01.037 [DOI] [PubMed] [Google Scholar]
- 51. Sandborn WJ, Danese S, D’Haens G, Moro L, Jones R, Bagin R, et al. Induction of clinical and colonoscopic remission of mild‐to‐moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Aliment Pharmacol Therapeut. 2015;41(5):409–18. 10.1111/apt.13076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bosworth BP, Sandborn WJ, Rubin DT, Harper JR. Baseline oral 5‐ASA use and efficacy and safety of budesonide foam in patients with ulcerative proctitis and ulcerative proctosigmoiditis: analysis of 2 phase 3 studies. Inflamm Bowel Dis. 2016;22(8):1881–6. 10.1097/MIB.0000000000000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D’Haens GR, Sandborn WJ, Zou G, Stitt LW, Rutgeerts PJ, Gilgen D, et al. Randomised non‐inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild‐to‐moderate ulcerative colitis. Aliment Pharmacol Ther. 2017;46(3):292–302. 10.1111/apt.14164 [DOI] [PubMed] [Google Scholar]
- 54. Rubin DT, Cohen RD, Sandborn WJ, Lichtenstein GR, Axler J, Riddell RH, et al. Budesonide multimatrix is efficacious for mesalamine‐refractory, mild to moderate ulcerative colitis: a randomised, placebo‐controlled trial. J Crohns Colitis. 2017;11(7):785–91. 10.1093/ecco-jcc/jjx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dignass A, Schnabel R, Romatowski J, Pavlenko V, Dorofeyev A, Derova J, et al. Efficacy and safety of a novel high‐dose mesalazine tablet in mild to moderate active ulcerative colitis: a double‐blind, multicentre, randomised trial. United European Gastroenterol J. 2018;6(1):138–47. 10.1177/2050640617703842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kruis W, Neshta V, Pesegova M, Alekseeva O, Andreev P, Datsenko O, et al. Budesonide suppositories are effective and safe for treating acute ulcerative proctitis. Clin Gastroenterol Hepatol. 2019;17(1):98–106.e4. 10.1016/j.cgh.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 57. Sedano R, Jairath V, Ma C, Sedano R, Hanzel J, Shackelton LM, et al. Design of clinical trials for mild to moderate ulcerative colitis. Gastroenterology. 2022. Jan 6;162(22):S0016–5085. 10.1053/j.gastro.2021.12.284 [DOI] [PubMed] [Google Scholar]
- 58. Colitis U. Clinical trial endpoints guidance for industry. U.S. Food and Drug Administration. Published June 5, 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/ulcerative‐colitis‐clinical‐trial‐endpoints‐guidance‐industry. Accessed December 3, 2020. [Google Scholar]
- 59. Singh S, Chowdhry M, Umar S, Bilal M, Clarke K. Variations in the medical treatment of inflammatory bowel disease among gastroenterologists. Gastroenterol Rep (Oxf). 2018;6(1):61–4. 10.1093/gastro/gox005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


