Abstract
There is increasing global concern of severe acute hepatitis of unknown etiology in young children. In early 2022, our center for liver transplantation in the Netherlands treated five children who presented in short succession with indeterminate acute liver failure. Four children underwent liver transplantation, one spontaneously recovered. Here we delineate the clinical course and comprehensive diagnostic workup of these patients. Three of five patients showed a gradual decline of liver synthetic function and had mild neurological symptoms. Their clinical and histological findings were consistent with hepatitis. These three patients all had a past SARS‐CoV‐2 infection and two of them were positive for adenovirus DNA. The other two patients presented with advanced liver failure and encephalopathy and underwent dialysis as a bridge to transplantation. One of these children spontaneously recovered. We discuss this cluster of patients in the context of the currently elevated incidence of severe acute hepatitis in children.
Keywords: acute liver failure, adenovirus, hepatitis, liver transplantation
Key Summary.
We report a cluster of five cases of indeterminate pediatric acute liver failure (PALF) in The Netherlands
We describe detailed diagnostic and clinical information on the course of disease of affected children
We discuss these cases in light of the current global increase in cases of severe acute hepatitis in young children
INTRODUCTION
Pediatric acute liver failure is a rare clinical syndrome that results from the rapid decline of liver function in a previously healthy child. 1 , 2 Although many different etiologies can lead to PALF, a cause cannot be found in approximately 50% of cases. 3 , 4 The clinical course of PALF is notoriously variable: some patients require intensive care unit (ICU) admission for mechanical ventilation and hemodynamic support within hours, while others appear clinically well and can be maintained on a pediatric ward for several days to weeks. 2 In patients whose liver function spontaneously improves, recovery is typically complete. However, up to 60% of indeterminate PALF cases have progressive disease trajectories with a high risk of mortality. 2 , 5 Liver transplantation is generally curative, but negates the chance of spontaneous recovery and has been associated with increased morbidity and mortality when compared to transplantations for chronic liver disease. Judicious balancing of these risks is challenged further by a paucity of prognostic tools, as well as the unpredictable availability of donor organs in emergency setting. 6
In the spring of 2022, our national referral center for pediatric liver transplantation in the Netherlands treated five children with PALF of unknown etiology. This series represented an unprecedented peak in the incidence of indeterminate PALF, which has averaged approximately 2 cases/year over the last 2 decades (Figure 1 and references 7 , 8 ). Around the same time, pediatric care providers in other parts of Europe and the United Kingdom had become suspicious of a rise in the incidence of severe hepatitis in young children, which led to international initiatives aimed at assessing the incidence of pediatric acute liver disease. While the results of these initial surveys appeared reassuring in terms of severe morbidity and mortality, 9 , 10 ongoing investigations are aimed at characterizing the underlying cause of recent cases of hepatitis of unknown etiology. 11 To contribute to our understanding of this recent epidemiological trend, we here delineate the clinical course of the five Dutch patients with indeterminate PALF that were treated in short succession at our center.
FIGURE 1.
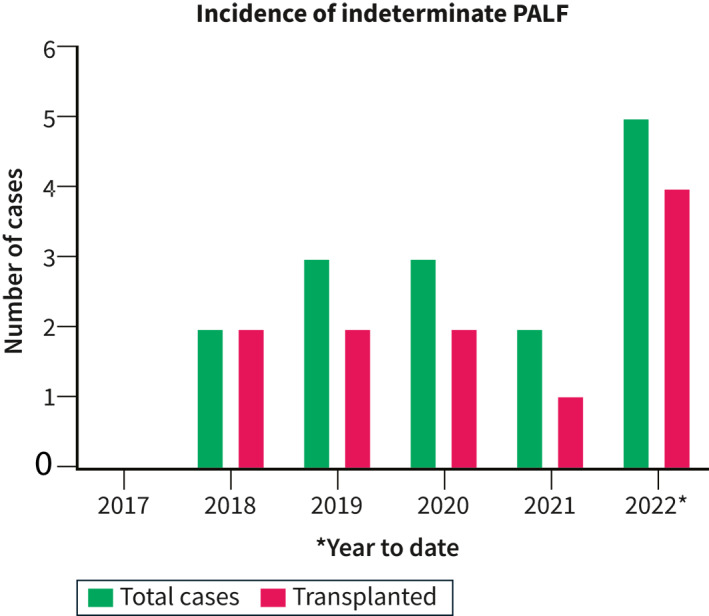
Incidence of indeterminate Pediatric acute liver failure (PALF) in our national transplant center. Blue bars represent the total number of cases, red bars represent the proportion of cases undergoing liver transplantation
Case 1 (Week 11)
An 11‐month‐old male was admitted to a regional hospital with a 4‐day history of vomiting, diarrhea and anorexia. Acetaminophen had been given in age‐appropriate doses over the last 3 days. Within several hours after initiation of oral rehydration therapy, he was noted to have a reduced level of consciousness. Diagnostic work‐up revealed severe hypoglycemia (0.6 mmol/L), elevated transaminases (alanine aminotransferase (ALT) 2547 U/l, aspartate aminotransferase 3475 U/l), low bilirubin (16 μmol/L) and an International Normalized Ratio (INR) of 3.4 (Figure 2). The patient was intubated on site and transported to our transplant center. On admission, he was sedated (E1M1Vtube) and his pupils were reactive to light. His ammonia level was 83 μmol/L and his INR had increased to 5.7. Continuous veno‐venous hemodiafiltration (CVVHDF) was instigated and the patient was listed for liver transplantation with high urgency priority. He was transplanted the next day with a partial graft from a living donor. Etiological work‐up of PALF was significant for multiple viral agents. His nasopharyngeal aspirate tested positive for SARS‐CoV‐2 RNA (cycle threshold (Ct) value 25) and enterovirus and/or rhinovirus (typing unsuccessful, Ct 25). A stool sample on admission was positive for SARS‐CoV‐2 RNA (Ct 27), enterovirus RNA (Ct 31), rotavirus RNA (Ct 12, genotype G3P 8 ) and adenovirus DNA (Ct 28, genotype C2). Low level of adenovirus could also be detected in plasma (Ct 38). The post‐operative course was complicated by respiratory symptoms that required mechanical ventilation for 9 days which were ascribed to his SARS‐CoV‐2 co‐infection, but he could ultimately be discharged in good clinical condition 6 weeks after hospitalization. Trio whole exome sequencing analysis showed no pathogenic mutations in genes associated with liver disease. Histological evaluation of the liver explant demonstrated massive hepatic necrosis with periportal steatotic changes with no signs of inflammatory activity (Figure 3).
FIGURE 2.
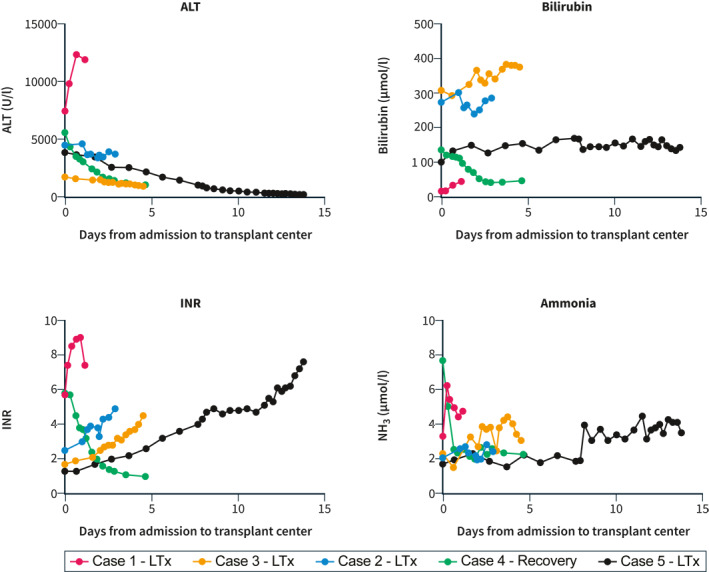
Biochemical parameters. Evolution of alanine aminotransferase (ALT), bilirubin, International Normalized Ratio (INR) and ammonia levels from time of first admission to the transplant center until transplantation (case 1, 2, 3 and 5) or discharge (case 4)
FIGURE 3.
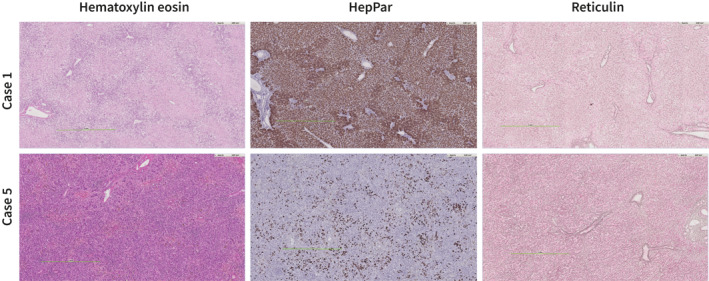
Explant histology of representative cases. Case 1. Hematoxylin eosin (HE) stain shows massive acute necrosis of the hepatocytes with a retained architecture with minimal portal inflammation. HepPar immunohistochemistry stains differentiate viable and necrotic hepatocytes. Reticulin stain shows a preserved architecture without collapse. Case 5. HE stain shows portal and lobular inflammation with venulitis of central veins and perivenular congestion and massive ductular reaction. HepPar shows extensive loss of hepatocytes. In the reticulin collapse of fibers between the ductular reaction. All images were captured via digital pathology
Case 2 (Week 11)
A 3‐year‐old female was admitted to a regional hospital for clinical work‐up of jaundice that had first been noticed 1 day earlier. Her history reported some non‐specific viral symptoms 3–4 weeks prior to presentation and around the same time family members had tested positive for SARS‐CoV‐2. In the 2 days prior to admission, she developed abdominal pain and vomiting. She was clinically well without neurological symptoms. Laboratory diagnostics at admission revealed an ALT of 4000 U//l, a total bilirubin of 165 μmol/L and INR of 1.4 (Figure 2). In the course of 4 days, her INR slowly deteriorated to 2.2 and she was transferred to the clinical ward of our liver transplant center. After 2 days, she had an episode of drowsiness which was scored as hepatic encephalopathy grade II, possibly III. She was admitted to the pediatric intensive care unit (PICU), but due to rapid neurological improvement CVVHDF was not initiated. After 2 days, liver synthesis had deteriorated further with an INR of 4.9 at which time she was decided to undergo living‐related liver transplantation. The post‐operative course was complicated by hepatic artery thrombosis on post‐operative day 10, which was successfully resolved by surgical exploration and revision of the anastomosis. She was discharged home after 5 weeks in good condition. Molecular diagnostics showed adenovirus in plasma (Ct 36) and SARS‐CoV‐2 IgG was positive (indicative of a past infection, Table 1). Histology of her liver explant was consistent with acute liver failure of unknown etiology, with lobular disarray and spotty necrosis of remaining hepatocytes, a moderate portal and lobular lymphocytic infiltrate, collapse and condensation of reticulin within a preserved architecture, and ductular reaction in zones 1 and 2. Trio‐WES revealed a variant of unknown significance in one of the ABCB4 alleles, but loss of ABCB4 has not been associated with acute liver failure in a previously healthy child.
TABLE 1.
Diagnostic work‐up and clinical characteristics of five patients who presented with indeterminate Pediatric acute liver failure (PALF) in the spring of 2022
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age | 11 months | 3 years | 8 years | 17 months | 2 years |
| Sex | Male | Female | Female | Female | Female |
| HISTORY | |||||
| Past medical history | None | None | None | None | Cow's milk allergy |
| Growth and development | Within normal range | Within normal range | Within normal range | Within normal range | Within normal range |
| Medication use prior to episode | None | None | None | None | None |
| Medications prior to referral | Acetaminophen at admission in age‐appropriate dosing for 3 days, stopped upon detection of PALF. Vitamin K. | Vitamin K. | Ursodeoxycholic acid and vitamin K. | Acetaminophen at admission in age‐appropriate dosing for 2 days, stopped upon detection of PALF. Vitamin K. | Vitamin K. |
| Presenting symptoms | Vomiting Diarrhea Anorexia Drowsiness | Vomiting Jaundice Acholic stools | Vomiting Jaundice Itching Acholic stools | Vomiting Fever Jaundice Acholic stools | Jaundice Abdominal pain Fatigue |
| ETIOLOGY | |||||
| Hepatitis A | N/A | Negative | Negative | Negative | Negative |
| Hepatitis B | N/A | Negative | Negative | Negative | Negative |
| Hepatitis C | N/A | Negative | Negative | Negative | Negative |
| Hepatitis E | N/A | Negative | Negative | Negative | Negative |
| Adenovirus | Positive (feces Ct 28, plasma Ct 38) | Positive (plasma Ct 38) Negative (NPA, feces) | Negative (plasma) | Positive (NPA Ct 31) Negative (plasma) Negative (feces) | Negative (plasma, feces) positive (feces Ct38 5 days after admission |
| Influenza A | Negative | Negative | Negative | Negative | Negative |
| Enterovirus (EV)/rhinovirus | Positive (NPA Ct 25, EV feces Ct 31)Negative (plasma) | Negative (NPA and feces) | Negative | Negative | Negative (feces and plasma) |
| EBV | IgG EBNA and VCA negative, DNA negative in follow up | Negative (IgG and IgM negative) | IgM negative, IgG EBNA and VCA positive (prior in other hospital EBNA borderline) | Positive (IgM and IgG VCA positive, IgG EBV NA negative, plasma 3.21 log IU/ml) | Negative (IgG and IgM negative) |
| CMV | Negative (IgG and IgM negative) | Negative (IgG and IgM negative) | Negative (IgG and IgM negative) | Negative (IgG and IgM negative) | Negative (IgG and IgM negative) |
| HSV 1/2 | Negative | Negative | Negative | Negative | Negative |
| VZV | Negative | Negative | Negative | Negative | Positive (plasma Ct 28, IgG antibodies negative) |
| HHV‐6 | Negative | Negative | Negative | Negative | Negative |
| Parechovirus | Negative | Negative (NPA, plasma, feces) | Negative (plasma) | Negative | Negative |
| Parvo B19 | Negative | Negative | Negative | Negative | Negative |
| SARS‐COV 2 | Positive (NPA Ct 25, feces Ct 27) | Negative in NPAIgG nucleocapsid positive (prior SARS‐CoV‐2 infection) | Negative in NPAIgG nucleocapsid positive (prior SARS‐CoV‐2 infection) | Negative in NPAIgG spike and nucleocapsid negative | IgG nucleocapsid positive (prior SARS‐CoV‐2 infection) |
| Toxicological screen (incl acetaminophen) | Acetaminophenl in therapeutic range, N/A for other compounds | Paracetamol negative, N/A for other compounds | N/A | Paracetamol in therapeutic range, N/A for other compounds | N/A |
| Total IgG (g/L) | N/A | 16.7 | 9.3 | 11.6 | 12.2 |
| Anti‐LKM antibodies | N/A | Negative | Negative | Negative | Negative |
| Anti‐SMA antibodies | N/A | Negative | Negative | Negative | Negative |
| ANA screen | N/A | Negative | Negative | Negative | Negative |
| Wilson disease | N/A | Negative | Negative | Negative | Negative |
| Alpha‐1 antitrypsin def | N/A | Negative | Negative | Negative | Negative |
| HLH | N/A | Negative | Negative | Negative | Negative |
| Vascular liver disease | Negative | Negative | Negative | Negative | Negative |
| Metabolic disease | No diagnosis from metabolic screen | No diagnosis from metabolic screen | No diagnosis from metabolic screen | No diagnosis from metabolic screen | No diagnosis from metabolic screen |
| Genetic | Trio‐WES negative | Trio‐WES negative | Trio‐WES negative | N/A | Trio‐WES negative |
| CLINICAL CONDITION | |||||
| Ascites | Trace amount | None | Trace amount | Trace amount | Trace amount |
| Splenomegaly | No | No | No | Mild (8.5 cm + 3 SD) | No |
| Mechanical ventilation | Yes | No | No | Yes | No |
| Inotropy | Yes | No | No | Yes | No |
| Renal replacement therapy | Yes | No | No | Yes | No |
| KING’S COLLEGE CRITERIA | |||||
| Hepatic encephalopathy | Grade II‐III | Briefly grade II, then spontaneously improved | Peak grade I, spontaneously resolved | Grade II‐III before intubation and sedation | Peak grade I |
| INR > 6.5 | Yes | No | No | Yes | Yes |
| INR > 3.5 | Yes | Yes | Yes | Yes | Yes |
| Age < 10 years | Yes | Yes | Yes | Yes | Yes |
| Jaundice >7 days before HE | No | Yes | Yes | No | Yes |
| Bilirubin >290 μmol/L | No | Yes | Yes | No | No |
| Unfavorable etiology (including indeterminate) | Yes | Yes | Yes | Yes | Yes |
| EXPLANT HISTOLOGY | |||||
| Weight (g) | 324 | 294 | 495 | N/A | 278 |
| Pre‐existing fibrosis/cirrhosis | No | No | No | N/A | No |
| Inflammation (P = portal, L = lobular, PL = portolobular) | Minimal p | PL | p > L | N/A | PL |
| Central venulitis | No | Yes | Yes | N/A | Yes |
| Necrosis | Massive | Focally in remaining hepatocytes | Focally in remaining hepatocytes | N/A | Focally, extensive loss |
| Ductular reaction, severity, zones | Minimal, zone 1 | Moderate, zones 2 and 3 | Moderate, zone 1 | N/A | Extensive, all zones |
| Collapse and condensation of reticulin | No | Yes | Yes | N/A | Yes |
| Hepatocyte steatosis/vacuolization | Yes, diffuse in remaining hepatocytes | Sporadically | Sporadically | N/A | No |
| OUTCOME | |||||
| Liver transplantation | Yes | Yes | Yes | No | Yes |
| Type of transplant | Living donor | Living donor | Living donor | Living donor | |
| Survival | Yes | Yes | Yes | Yes | Yes |
| OTHER COMMENTS | Rotavirus positive (feces Ct 12) | VUS in ABCB4 Edema of gallbladder on ultrasound, suspect for hepatitis | Edema of gallbladder on ultrasound, suspect for hepatitis | Sapovirus positive Edema of gallbladder on ultrasound, suspect for hepatitis |
Abbreviations: ANA = Anti Nuclear Antibodies; CMV = Cytomegalo Virus; Ct = cycle threshold; EBV = Epstein‐Barr Virus; HHV = Human Herpes Virus; HLH = Hemophagocytic Lymphohistiocytosis; HSV = Herpes Simplex Virus; INR = International Normalized Ratio; LKM = Liver Kidney Microsomes; N/A = Not available; NPA = Nasopharyngeal Aspirate; SD = Standard Deviation; SMA = Smooth Muscle Antigen; VUS = Variant of Unknown Significance; VZV = Varicella Zoster Virus; SARS‐COV = Severe Acute Respiratory Syndrome Coronovirus; VCA = Viral capsid antigen; EBNA = Epstein Barr Nuclear Antigen
Case 3 (Week 13)
An 8‐year‐old female was referred to our center after a 2‐week episode of jaundice that was preceded by 2 days of vomiting and abdominal pain. One week after onset of this episode she had an ALT level of 2200 U/l, a total bilirubin of 214 μmol/L and an INR of 1.5, at which time she was managed as an outpatient in another hospital. When her INR had increased to 2.0, she was transferred to our clinical ward (Figure 1). After 2 days she was admitted to our PICU with a clinical picture consistent with grade I‐II encephalopathy and she was listed for high urgency liver transplantation. In the next days, her clinical condition remained stable and her neurology improved, but liver synthesis continued to decline. At an INR of 4.5 she underwent living‐related liver transplantation. Her recovery was uneventful and she could be discharged home in good condition after 4 weeks. Explant histology showed signs of hepatitis, predominantly portal rather than lobular with extensive hepatocyte loss in zones 2 and 3, ductular reaction and collapse and condensation of reticulin within a preserved architecture. No viral agents were identified despite sampling of nasopharyngeal aspirate, feces, serum and plasma samples. The patient had a previous SARS‐CoV‐2 infection as evidenced by circulating IgG antibodies. A trio‐WES analysis did not identify mutations in any of the genes associated with (acute) liver disease.
Case 4 (Week 15)
A 1.5‐year‐old female presented to a local emergency department with fever, vomiting and jaundice for 1 day. Her parents reported an intercurrent illness approximately 4 weeks earlier from which she had fully recovered within a few days. She appeared weak and cried inconsolably. She had transaminases above 2500 U/l, a total bilirubin of 156 μmol/L, hypoglycemia (1.2 mmol/L), an INR of 6.7 and an ammonia level >500 μmol/L. She was intubated by the regional PICU team and transported to our center. Upon arrival she was scored as E1M4Vtube (with sedation), with light reactive pupils and bilateral Babinski reflexes. She was immediately started on CVVHDF and listed for emergency liver transplantation. In the first 24 h after PICU admission, her liver synthesis started to improve and, while undergoing CVVHDF, her ammonia level decreased from 200 μmol/L at admission to 64 μmol/L the next day. At day 3, liver function had improved sufficiently to withhold CVVHDF, and the patient continued to make a complete recovery. Etiologic workup showed a primary Epstein‐Barr virus (EBV) infection (IgM positive, IgG VCA positive, IgG EBNA negative, and EBV DNA plasma viral load of 3.2 log IU/ml). A nasopharyngeal swab came back positive for adenovirus DNA (Ct 31, genotype C2), but adenovirus was not detected in samples from stool or plasma. She had no IgG or IgM antibodies against SARS‐CoV‐2. On day 4 after admission, she was transferred back to her regional hospital and has made a complete recovery.
Case 5 (Week 17)
A 2‐year‐old female presented to her pediatrician with a 1‐day history of jaundice and abdominal pain, preceded by several weeks of fatigue and short episodes of vomiting for the last 4 months. She had recovered from a SARS‐CoV‐2 infection approximately 10 weeks before presentation at the hospital. Her physical examination was significant for numerous varicella lesions. Her ALT level was 3600 IU/L and bilirubin 83 μmol/L, with normal INR and ammonia level. The following day she was admitted to our hospital. She was managed on our clinical ward and received intravenous acyclovir. There was complete healing of vesicular lesions. Liver synthesis showed a gradual decline (Figure 2) and she was listed for liver transplantation on the fourth day of admission. At day 7 her INR was above 4.0 and she was transferred to our ICU. Hepatic encephalopathy was briefly assessed at grade I‐II, but normalized to 0‐I during the remainder of her ICU stay. At day 9, living‐related liver transplantation was scheduled but deferred at the last minute because of an apparent biochemical stabilization. However, the following days INR continued to increase to >7.0 and factor V levels dropped below 20%. Transplantation was ultimately carried out at day 13 of admission. In addition to the primary varicella zoster infection (DNA titer lowest Ct value 28 in plasma and IgM antibodies positive), she tested positive for sapovirus in her stool sample at admission (Ct 33). Repeat stool sample 5 days later showed a very low adenovirus DNA load (Ct 38), which had been negative at admission in fecal and blood sample. Trio‐WES was negative. Histological evaluation of the explant showed a portal and lobular inflammatory infiltrate with condensation and collapse of reticulin with a retained architecture, some lobular disarray and a profound ductular reaction (Figure 3). The patient is still recovering in our hospital but is overall doing well.
DISCUSSION
Here we have summarized the clinical course of five children who have been treated for acute liver failure (INR ≥2.0, transaminases elevated >1000 U/ml and some degree of hepatic encephalopathy) in our national pediatric liver transplantation center between mid‐March and early May 2022. Despite a comprehensive diagnostic workup (Table), a conclusive etiological diagnosis for PALF could not be made in any of the five patients. All children met the King's College criteria used to justify high urgency liver transplantation 12 , and four out of five patients indeed received a liver transplant. The patients progressed along two disparate disease trajectories: Cases 1 and 4 presented with fulminant disease with already significantly elevated INR at first analysis, developed a high degree of encephalopathy and received renal replacement therapy as a bridge to transplantation. Case 1 underwent liver transplantation within 36 h of admission, but Case 4 spontaneously recovered despite severity of disease. Cases 2, 3 and 5 were jaundiced at time of first hospital presentation but still had normal neurological exams and an INR <2.0. These three children demonstrated a more insidious course of disease, with gradually deteriorating INR and self‐limiting episodes of mild hepatic encephalopathy and only modestly elevated levels of ammonia. Upon histological examination of explanted livers, these three children all showed signs of hepatitis with no fibrosis or cirrhosis that would indicate chronic liver disease (Table). Both these disease trajectories are common to indeterminate PALF. 5 Patients who spontaneously recover often do so in the first few days after presentation, whereas gradual progression of disease during the first week is characterized by a poor prognosis. 5
The cases described here were treated during a period of increased (inter)national incidence of severe hepatitis in children. Indeed, the experience at our center was one of several signals that motivated a survey amongst participating centers of the European Reference Network for Rare Liver Diseases. 9 Similar cues appeared in Scotland, where 13 cases of acute hepatitis of unknown origin were observed between Jan 1 and April 12th of 2022 13 , and the rest of the UK, where as of May 20th nearly two hundred cases have been identified of severe hepatitis in young children. 11 of these children (6%) required liver transplantation. 11 Cumulative data from 14 European countries at that time (May 22nd) amassed to 125 cases of acute hepatitis in children, of which six (5%) have received a liver transplant. 14 The case definition for the UK and European registrations hinges on an ALT level >500 U/l in patients ≤10 years of age in whom hepatitis A‐E, metabolic, genetic, congenital or mechanical causes have been excluded. Consequently, all five patients that we describe here (in fact: all patients with indeterminate PALF) meet this definition. It is therefore tempting to speculate that the peak in incidence of indeterminate PALF is the direct corollary of the currently increased burden of acute liver disease within the pediatric population.
Several hypotheses are currently under investigation that may explain the sudden rise of hepatitis in children. 11 Since the earliest reports, it has been clear that adenovirus DNA could be detected in a majority of cases (68% in the most recently published version of the dataset 11 ). Infection with adenovirus has hence been prominently featured as a potential explanation, either by itself or in combination with an environmental modifier or viral co‐infection. 11 Amongst these co‐factors, past or current infection with SARS‐CoV‐2 has been suggested as a plausible candidate, because SARS‐CoV‐2 represents a novel immunological stimulus that only recently has become endemic. 11 While these studies are underway, the relationship between acute severe hepatitis in children and our cluster of indeterminate PALF patients remains speculative. Comprehensive screening for viral infections is part of our routine work‐up of PALF patients (Table) and, in line with results from the UK series, adenovirus was detected in four out of five cases, albeit at an overall low abundance and in different biomaterials. In clinical practice, however, it is often impossible to unequivocally link the detection of a viral agent to the ensuing liver pathology. 15 Non‐hepatitis viruses (e.g. enterovirus, adenovirus, varicella zoster or EBV) infect nearly all young children but almost never cause overt liver disease, let alone PALF. This suggests that when an association with a non‐hepatitis virus is suspected, PALF may depend more on (unknown) modifying factors in the host than on the viral agent itself. It is therefore possible that temporal clustering of PALF cases may be a reflection of such an idiosyncratic, (post)infectious epiphenomenon that affects the susceptible children in a population that recently experienced a sudden change from an abnormally low to an abnormally high burden of infectious diseases after rapid alleviation of COVID‐19 restrictions in our country during the first quarter of 2022. Whether a previous SARS‐CoV‐2 infection itself modifies this susceptibility is a topic of ongoing investigation in the UK series. 11 , 16 It is noteworthy that the three patients in our series (Case 2, 3 and 5) who had clinical and histological signs of hepatitis all had a past SARS‐CoV‐2 infection (IgG antibodies positive) whereas the two patients with rapidly progressive PALF and severe neurological symptoms either had an active, primary SARS‐CoV‐2 infection at time of PALF (Case 1) or no evidence of a previous or current infection (Case 4). Similar to the UK and other parts of Europe, the Omicron variant was the predominant SARS‐CoV‐2 strain circulating in the beginning of 2022 in the Netherlands.
A striking discrepancy with the UK series is the transplant rate. In the UK registry, only 5%–6% of cases underwent liver transplantation. 11 , 14 Therefore, if our cluster of PALF patients would be accounted for in full by the international rise in severe hepatitis cases, the Netherlands should have had approximately 100 cases in order to arrive at a similar transplant rate of 5%. Despite initiatives from the Dutch government and Pediatric Society as well as widespread attention across professional and public channels, only 14 cases of severe hepatitis in children have been formally registered thus far. However, in contrast to the UK, where the National Health System has been able to centrally link ICD‐10 codes with laboratory results, the Dutch registration is dependent in full on the active participation of local providers because a centralized system to couple these data is lacking. It is therefore likely that registration of Dutch cases is incomplete and hence, is skewed towards the severe cases that were registered at our national pediatric liver transplant center. An alternative explanation for our high transplant rate could be that we transplanted children who would have spontaneously recovered otherwise. We believe this is unlikely, as all five patients met Kings College criteria, which are thought to predict a high risk of death without transplantation and have been applied to guide decisions on emergency organ allocation for decades. 17 However, these criteria were derived primarily from adult data and before the advent of liver transplantation (and thus modern supportive care). 12 Data from the PALF study group have previously shown that children with indeterminate PALF who meet Kings College criteria are still more likely to recover (67%) than to die (33%) in case a donor organ does not become available. 6 Our first three patients presented before international awareness was raised for increased severe hepatitis and were transplanted after expedited work‐up of a living donor. In the fifth patient, we allowed more time for spontaneous recovery to occur under meticulous monitoring of biochemistry and neurological condition, but ultimately, she also was transplanted when the INR had increased to >7.0.
Clinical details or biochemical parameters of the 11 patients who have been transplanted in the UK have so far not been made publicly available. Our previous European survey 9 identified too few patients who required a transplantation to allow comparison with our series. It is also unclear from available data which percentage of pediatric patients who meet the case definition for severe acute hepatitis progress to PALF (i.e. International Normalized Ratio ≥2.0 or INR ≥1.5 with any degree of encephalopathy) but nevertheless recover with their native liver. Going forward in the current epidemic of severe hepatitis in children, we believe it will be of critical importance to focus not only on case numbers and possible etiologies, but also to keep a detailed track of the clinical trajectories these patients follow. When the natural history of patients with acute severe hepatitis becomes better known, this knowledge will be instrumental in guiding therapeutic decisions, including the need for transplantation, in those patients at the most severe side of the spectrum.
NOTE
The Medical Ethical Committee of the University Medical Center Groningen approved the ongoing investigations into the etiology of severe hepatitis cases in our center (M22.296493). Informed consent for publication of these clinical data was obtained from the parents of all five patients described (by RdK).
The University Medical Center Groningen is a member of the European Reference Network for hepatological diseases (ERN RARE‐LIVER).
CONFLICT OF INTEREST
The Authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
We thank the members of the working group on Acute Liver Failure from the European Reference Network for hepatological diseases (ERN RARE‐LIVER) for helpful discussions on the emerging incidence of acute severe hepatitis in children.
Lexmond WS, de Meijer VE, Scheenstra R, Bontemps STH, Duiker EW, Schölvinck EH, et al. Indeterminate pediatric acute liver failure: Clinical characteristics of a temporal cluster of five children in the Netherlands in the spring of 2022. United European Gastroenterol J. 2022;10(8):795–804. 10.1002/ueg2.12269
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Cochran JB, Losek JD. Acute liver failure in children. Pediatr Emerg Care. 2007;23:129–35. 10.1097/pec.0b013e3180308f4b [DOI] [PubMed] [Google Scholar]
- 2. Squires RH, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–8. 10.1016/j.jpeds.2005.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Squires JE, Alonso EM, Ibrahim SH, Kasper V, Kehar M, Martinez M, et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the diagnosis and management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2022;74:138–58. 10.1097/mpg.0000000000003268 [DOI] [PubMed] [Google Scholar]
- 4. Narkewicz MR, Dell Olio D, Karpen SJ, Murray KF, Schwarz K, Yazigi N, et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: an opportunity for quality improvement. J Pediatr. 2009;155:801–6. 10.1016/j.jpeds.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li R, Belle SH, Horslen S, Chen L, Zhang S, Squires RH, et al. Clinical course among cases of acute liver failure of indeterminate diagnosis. J Pediatr. 2016;171:163–70. 10.1016/j.jpeds.2015.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sundaram V, Shneider BL, Dhawan A, Ng VL, Im K, Belle S, et al. King’s College hospital criteria for non‐acetaminophen induced acute liver failure in an international cohort of children. J Pediatr. 2013;162:319–23. 10.1016/j.jpeds.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lexmond WS, Van Dael CML, Scheenstra R, Goorhuis JF, Sieders E, Verkade HJ, et al. Experience with molecular adsorbent recirculating system treatment in 20 children listed for high‐urgency liver transplantation. Liver Transplant. 2015;21:369–80. 10.1002/lt.24037 [DOI] [PubMed] [Google Scholar]
- 8. Sturm E, Lexmond WS, Verkade HJ. Pediatric acute liver failure: variations in referral timing are associated with disease subtypes. Eur J Pediatr. 2015;174:169–75. 10.1007/s00431-014-2363-x [DOI] [PubMed] [Google Scholar]
- 9. Kleine RHde, Lexmond WS, Buescher G, Sturm E, Kelly D, Lohse AW, et al. Severe acute hepatitis and acute liver failure of unknown origin in children: a questionnaire‐based study within 34 paediatric liver centres in 22 European countries and Israel. Euro Surveill. 2022;27. 10.2807/1560-7917.ES.2022.27.19.2200369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beek J van, Fraaij PL, Giaquinto C, Shingadia D, Horby P, Indolfi G, et al. Case numbers of acute hepatitis of unknown aetiology among children in 24 countries up to 18 April 2022 compared to the previous 5 years. Euro Surveill. 2022;27. 10.2807/1560-7917.es.2022.27.19.2200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United Kingdom Health Security Agency (UKHSA). Investigation into acute hepatitis of unknown aetiology in children in England. Technical briefing 3. (May 19 ‐ 2022). Available at https://www.gov.uk/government/publications/acute‐hepatitis‐technical‐briefing
- 12. O’Grady JG, Alexander GJM, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–45. 10.1016/0016-5085(89)90081-4 [DOI] [PubMed] [Google Scholar]
- 13. Marsh K, Tayler R, Pollock L, Roy K, Lakha F, Ho A, et al. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Euro Surveill. 2022;27. 10.2807/1560-7917.es.2022.27.15.2200318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control (ECDC) . Increase in severe acute hepatitis cases of unknown aetiology in children. ISBN 2022; 2022. https://www.ecdc.europa.eu/en/increase‐severe‐acute‐hepatitis‐cases‐unknown‐aetiology‐children. Accessed on 22 5.
- 15. Schwarz KB, Olio DD, Lobritto SJ, Lopez MJ, Rodriguez‐Baez N, Yazigi NA, et al. Analysis of viral testing in nonacetaminophen pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2014;59:616–23. 10.1097/mpg.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brodin P, Arditi M. Severe acute hepatitis in children: investigate SARS‐CoV‐2 superantigens. Lancet Gastroenterology Hepatology. 2022(7):594–5. 10.1016/s2468-1253(22)00166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eurotransplant, ET Liver allocation system (ELAS). 2022. Available at https://www.eurotransplant.org/wp‐content/uploads/2022/03/H5‐ELAS‐MELD‐March‐2022.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


