Abstract
Introduction
Crizotinib provided meaningful clinical benefit in the initial analysis of a phase 2 study in East Asian patients with advanced ROS1-positive NSCLC (NCT01945021). Nevertheless, overall survival (OS) data were immature. Here, we present the final OS, quality of life (QoL), and safety data after an additional 3 years of follow-up.
Methods
In this phase 2, open-label, single-arm trial, East Asian patients with ROS1-positive advanced NSCLC who had received less than or equal to three systemic therapies previously were treated with crizotinib 250 mg twice daily on a continuous daily dosing schedule in 28-day cycles. The OS (secondary end point) was analyzed for the total population, by country, and by number of previous chemotherapy regimens. QoL and safety were also evaluated.
Results
With a median duration of follow-up of 56.1 months, the median OS was 44.2 months (95% confidence interval: 32.0–not reached) for the total population (N = 127). Differences in median OS were observed among individual countries and with number of previous regimens. The improvement in QoL found in the previous analysis was maintained with the extended follow-up. Treatment-related adverse events led to crizotinib dose reductions or permanent treatment discontinuations in 17.3% and 2.4%, respectively, of the patients.
Conclusions
This is the largest trial of an ALK/ROS1 inhibitor to treat patients with ROS1-positive advanced NSCLC and provides a new benchmark for OS in East Asian patients. The QoL and safety profile with long-term follow-up were consistent with previous reports and support the continued use of crizotinib in the treatment of patients with ROS1-positive advanced NSCLC.
Keywords: Asia, Crizotinib, NSCLC, Phase 2, ROS1
Introduction
In the early 2000s, a number of advances were made in terms of the molecular characterization of NSCLC. Such characterizations identified several potential drug targets, including EGFR, ALK, and ROS1.1, 2, 3 ROS1 is a receptor tyrosine kinase in the same insulin receptor family as ALK and the leukocyte receptor tyrosine kinase.4 ROS1 gene rearrangements are estimated to occur in approximately 1% to 2% of patients with NSCLC, accounting for approximately 17,500 to 35,000 of an estimated 1.8 million new cases of NSCLC worldwide.4, 5, 6 In patients from East Asia with lung adenocarcinoma, the frequency of ROS1-positive NSCLC is believed to be slightly higher at approximately 2% to 3%.7 Although ROS1- and ALK-positive NSCLCs share many clinicopathologic features, ROS1 gene rearrangements do not usually occur in the same tumor as ALK gene rearrangements and, therefore, represent a unique molecular subgroup of patients with NSCLC.8,9 Patients with ROS1-positive advanced NSCLC trend toward being younger than those without ROS1 gene rearrangements, are more frequently never smokers, and generally have a histologic diagnosis of adenocarcinoma.9 ROS1 gene rearrangements are also associated with a high response to chemotherapy, resulting in longer median overall survival (OS) in this group than in patients without ROS1 gene rearrangements, including subgroups with EGFR-mutated and ALK-rearranged NSCLC.10 In addition, tumors with ROS1 gene rearrangements are susceptible to crizotinib, an orally administered, small-molecule tyrosine kinase inhibitor (TKI) of ALK, HGFR (also known as MET), ROS1, and RON.11
In patients with ROS1-positive advanced NSCLC, crizotinib has been found to be highly effective.12,13 In an expansion phase of a multinational phase 1 trial (PROFILE 1001, NCT00585195) in which 53 patients with ROS1-positive NSCLC received crizotinib (250 mg twice daily in continuous 28-day cycles), an objective response rate (ORR) of 72% was observed, with a median progression-free survival (PFS) of 19.5 months.12 Most treatment-related adverse events (TRAEs) were grade 1 or 2 in severity, and the most common (>30%) were vision disorder (87%), nausea (51%), edema (47%), diarrhea (45%), vomiting (38%), elevated transaminases (36%), and constipation (34%).12
We conducted a large phase 2 study (NCT01945021) of crizotinib in 127 patients from East Asia with locally advanced or metastatic ROS1-positive NSCLC with up to three lines of previous therapy. Result of the initial analysis of this study conducted approximately 3 years after the study began revealed a meaningful clinical benefit, including an ORR of 72%, a median PFS of 15.9 months, and a median OS of 32.5 months, with 59.8% of patients still in follow-up at data cutoff.14 Crizotinib was approved for first-line use for patients with ROS1-positive NSCLC in the People’s Republic of China, Japan, South Korea, and Taiwan, on the basis of the results of this study. In addition, there was a trend toward an improvement in patient-reported global quality of life (QoL), with reductions in many lung-specific domains that were considered clinically meaningful.14 Here, we present the final OS data and updated safety and QoL data after more than 3 years of additional follow-up.
Materials and Methods
The complete methods of this study were previously published and will only be summarized here.14 The study was initiated in September 2013 at 37 sites across East Asia. The original data cutoff was July 30, 2016. The date of database lock was July 1, 2020, for this final analysis. The institutional review board or independent ethics committee at each participating site approved the protocol, which complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice Guidelines, and the Declaration of Helsinki, including local laws. All patients provided informed consent.
Patients
Patients were enrolled if they were more than or equal to 18 years of age with locally advanced or metastatic NSCLC that was positive for ROS1 gene rearrangements and negative for ALK gene rearrangements. Detection of a ROS1 gene rearrangement was carried out by central testing using a validated Amoy real-time polymerase chain reaction assay (AmoyDx; Amoy Diagnostics, Xiamen, People’s Republic of China). In patients with ROS1-positive tumors, negative status for ALK rearrangements was determined using validated tests, including the Amoy real-time polymerase chain reaction, immunohistochemistry (Ventana Medical Systems, Oro Valley, AZ), or the Vysis ALK fluorescence in situ hybridization test (Abbott Laboratories, Chicago, IL). Patients were required to have had three or fewer previous lines of systemic therapies for advanced-stage disease, at least one measurable tumor lesion(s) (as assessed by the Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) that had not been irradiated, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients with brain metastases were eligible if they were asymptomatic or were neurologically stable for at least 2 weeks if treated.
Study Design
Crizotinib was administered at a starting dose of 250 mg twice daily on a continuous daily dosing schedule in 28-day cycles. Treatment was continued until RECIST-defined disease progression, unacceptable toxicity, or withdrawal of consent. Dosing modifications were allowed if patients experienced a crizotinib-associated adverse event (AE). Patients could continue treatment beyond RECIST-defined progression if they had ongoing clinical benefit.
The primary efficacy end point for the initial analysis of this study was ORR by independent radiology review; secondary end points were PFS, OS, duration of response, time to first tumor response, disease control rate, QoL, and safety.14 The final analyses reported herein evaluated the final OS, QoL, and safety end points.
Study Assessments
Tumor assessments were performed at baseline, every 8 weeks until cycle 8, and then every 12 weeks until disease progression or treatment discontinuation. All patients were followed for survival for at least every 2 months after discontinuing study treatment until death or they discontinued the study.
Tumor responses were assessed using RECIST version 1.1. OS was defined as the time, in months, from the date of the first dose of crizotinib to the date of death owing to any cause. OS, the only efficacy end point assessed herein, was analyzed for the total population, by country, and by number of previous chemotherapy regimens. The following tools were used to assess QoL for disease- and treatment-related symptoms, functioning, and global QoL: European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30) and the corresponding Lung Cancer Module (QLQ-LC13).15,16 The EORTC QLQ-C30 consists of 30 questions grouped into five functional domains (physical, role, cognitive, emotional, and social); a global health status/QoL scale; three symptom scales (fatigue, pain, and nausea and vomiting); and six single items that assess additional symptoms (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea); including the perceived financial burden of the treatment. Scores range from 0 to 100. A higher score for a functional-scale item represents healthier functioning, and a high score for global health status correlates with a high QoL; by contrast, a higher score for a symptom-scale item represents a higher level of symptoms or problems.17 QoL results are only presented for the first 60 cycles of crizotinib treatment because smaller percentages (≤20%) of patients were found at later time points. AEs were classified by type, incidence, severity, timing, seriousness, and relatedness to treatment and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistics
The safety analysis population was defined as all enrolled patients who received at least one dose of crizotinib. In tables illustrating data by country, data for South Korea and Taiwan were combined owing to the small number of patients in each country. Kaplan-Meier methodology was applied to estimate OS in the safety analysis population, and two-sided 95% confidence intervals (CIs) are provided. QoL end points were analyzed in the patient-reported outcome-assessable population (all patients in the safety analysis population who completed a baseline and one or more postbaseline patient-reported outcome assessment). Changes in EORTC QLQ-C30 and QLQ-LC13 scores of 10 or more points from baseline were considered clinically meaningful and were considered statistically significant if the 95% CI for the change did not include 0.18
Results
Patients
Between September 2013 and January 2015, a total of 127 patients with ROS1-positive NSCLC from East Asia (People’s Republic of China, Japan, South Korea, and Taiwan) were enrolled and received at least one dose of crizotinib. The greatest percentage of patients were from the People’s Republic of China (n = 74; 58.3%) and Japan (n = 26; 20.5%), with the smallest percentage from Taiwan (n = 15; 11.8%) and South Korea (n = 12; 9.4%) (Table 1). The proportions of patients with 0, 1, 2, or 3 previous chemotherapy regimens were 18.9%, 41.7%, 24.4%, and 15.0%, respectively. The median age of patients was 51.5 years, 97.6% had adenocarcinoma, and 95.3% had metastatic disease (Table 1). There were 49 patients (38.6%) who received subsequent anticancer therapy, including patients who received subsequent commercial crizotinib.
Table 1.
Baseline Patient and Disease Characteristics (Safety Analysis Population)
| Characteristics | Crizotinib |
|||||||
|---|---|---|---|---|---|---|---|---|
| By Country |
By Number of Previous Regimens |
|||||||
| Total (N = 127) | People’s Republic of China (n = 74) | Japan (n = 26) | South Korea or Taiwan (n = 27) | 0 (n = 24) | 1 (n = 53) | 2 (n = 31) | 3 (n = 19) | |
| Median age, y (min, max) | 51.5 (22.8, 79.7) | 49.5 (22.8, 79.7) | 56.3 (30.2, 79.1) | 52.7 (33.8, 73.8) | 57.6 (26.7, 79.7) | 49.1 (22.8, 79.1) | 57.7 (33.8, 76.3) | 50.6 (38.4, 75.4) |
| Sex, n (%) | ||||||||
| Male | 54 (42.5) | 34 (45.9) | 10 (38.5) | 10 (37.0) | 12 (50.0) | 20 (37.7) | 15 (48.4) | 7 (36.8) |
| Female | 73 (57.5) | 40 (54.1) | 16 (61.5) | 17 (63.0) | 12 (50.0) | 33 (62.3) | 16 (51.6) | 12 (63.2) |
| History of smoking, n (%) | 36 (28.3) | 19 (25.7) | 10 (38.5) | 7 (25.9) | 6 (25.0) | 18 (34.0) | 9 (29.0) | 3 (15.8) |
| ECOG PS, n (%) | ||||||||
| 0 | 34 (26.8) | 9 (12.2) | 10 (38.5) | 15 (55.6) | 4 (16.7) | 19 (35.8) | 8 (25.8) | 3 (15.8) |
| 1 | 93 (73.2) | 65 (87.8) | 16 (61.5) | 12 (44.4) | 20 (83.3) | 34 (64.2) | 23 (74.2) | 16 (84.2) |
| Histologic classification, n (%) | ||||||||
| Adenocarcinoma | 124 (97.6) | 71 (95.9) | 26 (100.0) | 27 (100.0) | NA | NA | NA | NA |
| Squamous cell carcinoma | 1 (0.8) | 1 (1.4) | 0 | 0 | ||||
| Other | 2 (1.6) | 2 (2.7) | 0 | 0 | ||||
| Extent of disease, n (%) | ||||||||
| Locally advanced only | 6 (4.7) | 4 (5.4) | 0 | 2 (7.4) | NA | NA | NA | NA |
| Metastatic | 121 (95.3) | 70 (94.6) | 26 (100) | 25 (92.6) | ||||
| Number of previous regimens, n (%) | ||||||||
| 0 | 24 (18.9) | 18 (24.3) | 2 (7.7) | 4 (14.8) | NA | NA | NA | NA |
| 1 | 53 (41.7) | 27 (36.5) | 14 (53.8) | 12 (44.4) | ||||
| 2 | 31 (24.4) | 17 (23.0) | 6 (23.1) | 8 (29.6) | ||||
| 3 | 19 (15.0) | 12 (16.2) | 4 (15.4) | 3 (11.1) | ||||
ECOG PS, Eastern Cooperative Oncology Group performance status; max, maximum; min, minimum; NA, not available.
Overall Survival
With a median follow-up of 56.1 months (95% CI: 52.1–59.4), the median OS for the total population was 44.2 months (95% CI: 32.0–not reached [NR]19) (Fig. 1). The probabilities of survival at 24, 36, and 48 months were 66.4% (95% CI: 57.2–74.0), 54.1% (95% CI: 44.7–62.6), and 46.7% (95% CI: 37.3–55.4), respectively. When stratified by country, median OS for patients from the People’s Republic of China and South Korea/Taiwan was similar to that of the overall population (48.5 mo and 43.7 mo versus 44.2 mo, respectively) but was numerically lower for those patients from Japan (31.2 mo) (Fig. 1). Median OS was numerically greatest for those with no previous chemotherapy regimens (51.5 mo) and lowest for those with one previous chemotherapy regimen (33.5 mo; Table 2). Median OS for those with two and three previous regimens was 44.4 months and 48.0 months, respectively.
Figure 1.
OS by country (safety analysis population). 95% CIs based on the Brookmeyer and Crowley method. CI, confidence interval; NR, not reached; OS, overall survival.
Table 2.
Median OS by Number of Previous Chemotherapy Regimens (Safety Analysis Population)
| Number of Previous Regimens | Median OS, mo (95% CI) |
|---|---|
| 0 (n = 24) | 51.5 (23.3–NR) |
| 1 (n = 53) | 33.5 (20.6–NR) |
| 2 (n = 31) | 44.4 (21.7–NR) |
| 3 (n = 19) | 48.0 (19.5–NR) |
95% CIs based on the Brookmeyer and Crowley method.
CI, confidence interval; NR, not reached; OS, overall survival.
Quality of Life
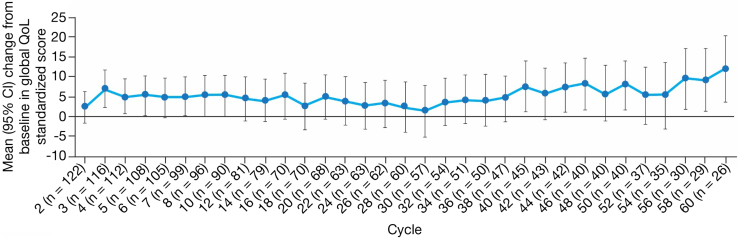
Of those on treatment, completion rates of the EORTC QLQ-LC13 were high at 96.7% to 100%, and 100.0% of the patients on treatment completed the EORTC QLQ-C30. Results from the EORTC QLQ-C30 revealed that an improvement from baseline was observed for global QoL as early as cycle 2, with statistically significant improvements found at cycles 3 to 5, 7, 40, 44, 46, 50, and 56 to 60 (Fig. 2). For most cycles to cycle 60, more than half of the patients on treatment were noted to have improved (≥10-point increase from baseline) or stable scores in the functioning domains: physical (improved: 16.7%–32.3%; stable: 50.9%–73.3%), role (improved: 13.8%–33.3%; stable: 45.5%–66.7%), emotional (improved: 21.6%–28.6%; stable: 51.4%–62.1%), cognitive (improved: 14.0%–29.5%; stable: 39.3%–56.6%), and social (improved: 21.6%–36.1%; stable: 41.8%–67.5%).
Figure 2.
Change from baseline in global QoL standardized score (PRO-assessable population). EORTC QLQ-C30 was used for the global QoL assessment. CI, confidence interval; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; EORTC QLQ-LC13, European Organization for Research and Treatment of Cancer Lung Cancer Module; PRO, patient-reported outcome; QoL, quality of life.
Symptoms from EORTC QLQ-C30 with the highest proportion of patients with improvement (≥10-point decrease from baseline) from cycle 2 to cycle 60 were appetite loss (19.0%–33.3%), fatigue (23.0%–50.0%), dyspnea (24.8%–41.3%), insomnia (24.6%–37.2%), and pain (33.6%–51.9%). Symptoms from EORTC QLQ-LC13 with the highest proportion of patients with improvement from cycle 2 to cycle 60 were coughing (42.6%–50.5%), pain in the chest (25.9%–35.3%), dyspnea (22.1%–35.3%), and pain in the arm or shoulder (24.3%–35.3%).
Safety
Median duration of crizotinib treatment was 101.7 weeks (range: 0.6–291.9 wk) for the entire study population, 114.2 weeks (range: 3.9–280.3 wk) for the People’s Republic of China, 44.4 weeks (range: 0.6–268.0 wk) for Japan, and 111.0 weeks (range: 1.4–291.9 wk) for South Korea/Taiwan. Treatment-emergent AEs occurred in 100.0% of the patients, of which 97.6% (n = 124) were considered treatment related. Serious TRAEs were found in 8.7% of the patients. Overall, 65 patients (51.2%) died during the study; disease progression was the most common cause of death (in 53 of 65 patients). One death due to an AE (respiratory failure) was considered treatment related because the investigator reported the relationship as unknown. TRAEs leading to permanent treatment discontinuation, dose reduction, or dosage interruption occurred in 2.4%, 17.3%, and 30.7% of the patients, respectively. Most TRAEs were grade 1 or 2 in severity, with 32.3% of the patients reporting a TRAE of grade 3 or 4 in severity. The most common TRAEs of any grade were elevated transaminases (66.9%), vision disorder (48.0%), diarrhea (41.7%), and nausea (41.7%) (Table 3). The most common grade 3 or 4 TRAEs were neutropenia (11.8%) and elevated transaminases (7.1%).
Table 3.
Most Frequent (≥10% of Total) Treatment-Related AEs by Grade (Safety Analysis Population)
| Patients With AE, n (%) | Crizotinib (N = 127) |
||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4a | Total | |
| Total | 34 (26.8) | 49 (38.6) | 35 (27.6) | 5 (3.9) | 124 (97.6) |
| Elevated transaminasesb | 61 (48.0) | 15 (11.8) | 7 (5.5) | 2 (1.6) | 85 (66.9) |
| Vision disorderb | 59 (46.5) | 1 (0.8) | 0 | 0 | 61 (48.0) |
| Diarrhea | 41 (32.3) | 11 (8.7) | 1 (0.8) | 0 | 53 (41.7) |
| Nausea | 45 (35.4) | 6 (4.7) | 2 (1.6) | 0 | 53 (41.7) |
| Neutropeniab | 8 (6.3) | 20 (15.7) | 12 (9.4) | 3 (2.4) | 43 (33.9) |
| Vomiting | 39 (30.7) | 4 (3.1) | 0 | 0 | 43 (33.9) |
| Constipation | 34 (26.8) | 7 (5.5) | 0 | 0 | 41 (32.3) |
| Edemab | 30 (23.6) | 3 (2.4) | 1 (0.8) | 0 | 34 (26.8) |
| Leukopeniab | 10 (7.9) | 20 (15.7) | 3 (2.4) | 0 | 33 (26.0) |
| Blood creatinine increasedb | 19 (15.0) | 6 (4.7) | 0 | 0 | 25 (19.7) |
| Decreased appetite | 16 (12.6) | 5 (3.9) | 1 (0.8) | 0 | 22 (17.3) |
| Dysgeusia | 16 (12.6) | 1 (0.8) | 0 | 0 | 17 (13.4) |
| Fatigue | 9 (7.1) | 4 (3.1) | 2 (1.6) | 0 | 15 (11.8) |
| Bradycardiab | 11 (8.7) | 1 (0.8) | 2 (1.6) | 0 | 14 (11.0) |
| Blood alkaline phosphatase increased | 11 (8.7) | 2 (1.6) | 0 | 0 | 13 (10.2) |
AE, adverse event.
One patient had grade 5 AE (respiratory failure), which occurred after the primary analysis date and was considered treatment related because the investigator relationship was reported as unknown.
This item comprised a cluster of AEs that represent similar clinical symptoms or syndromes.
Discussion
Here, we present the final data on OS, QoL, and safety from the largest phase 2 study to date of East Asian patients with ROS1-positive advanced NSCLC. After a median follow-up of 56.1 months (95% CI: 52.1–59.4), the median OS for the total population was 44.2 months (95% CI: 32.0–NR). There were some numerical differences in OS between countries and by number of previous chemotherapy regimens, but the CIs were overlapping, perhaps owing to the low number of patients in some groups. In addition, OS was lowest for Japan, but only two patients (7.7%) had received crizotinib as first-line therapy for advanced disease, whereas 18 patients (24.3%) from the People’s Republic of China had received crizotinib as first-line therapy for advanced disease, so it is possible that the patients from Japan had more advanced disease at the time of study enrollment. Nevertheless, these results are consistent with the OS found in patients with ROS1-positive NSCLC treated with crizotinib from other geographic locations.12,19 In the PROFILE 1001 study based in the United States, Australia, Japan, and South Korea (NCT00585195; N = 53, including 21 Asian patients), after a median follow-up of 62.6 months, the OS was 51.4 months (95% CI: 29.3–NR).12 A phase 2 European study (NCT02183870; N = 30) of crizotinib in ROS1-positive patients with NSCLC reported a median OS that was NR after 44.9 months of follow-up, and the 24-month OS probability was 66%, which is similar to what was observed in this study.19 Studies of other ROS1 inhibitors in the setting of ROS1-positive advanced NSCLC are limited and do not focus on East Asian patients. Results of an integrated analysis of three ongoing phase 1 or 2 trials of the multitargeting TKI entrectinib, in ROS1-positive NSCLC (n = 53) with a median follow-up of 15.5 months, revealed median OS that was NR (95% CI: 15.1–NR) and 85% of patients were alive at 12 months.20 In a small, open-label phase 2 study of ceritinib, a TKI most active against ALK but also IGF-1 receptor and ROS1,21 in patients with ROS1-positive NSCLC (n = 28), median OS was 24 months (95% CI: 5–43), with a 12-month OS rate of 56% after a median follow-up of 14 months.22 A phase 1/2 study of the ALK/ROS1 TKI lorlatinib did not report OS.23 A retrospective study of eight assessable patients with ROS1-positive NSCLC investigated the clinical activity of the ALK/ROS1 inhibitor brigatinib, but OS data were not reported.24 Last, one previously published simulated treatment comparison analyzed four phase 1/2 studies to compare the efficacy of crizotinib and entrectinib in ROS1-positive NSCLC.25 This study found a higher but not significant ORR favoring crizotinib (risk ratio [RR] = 1.04, 95% CI: 0.85–1.28). In the adjusted comparison, the 12-month landmark OS difference for crizotinib estimated a not significant 1% increased risk of death (RR = 1.01, 95% CI: 0.90–1.12). The study concluded that crizotinib and entrectinib have comparable efficacy in ROS1-positive NSCLC. Although OS data with other ROS1 TKIs are still emerging, and baseline differences in the study populations and relatively small trial populations complicate direct crosstrial comparisons, our findings reveal that treatment with crizotinib provided meaningful clinical benefit in East Asian patients with ROS1-positive advanced NSCLC. Data on postprogression therapy were not available for this study; this lack of available data represents one limitation of the data interpretation.
The baseline and disease characteristics of the population were generally similar to other reports of East Asian patients with ROS1-positive advanced NSCLC and patients from Europe and the United States.12,26, 27, 28 Studies of patients with ROS1-rearranged advanced NSCLC have found an association with non- or light-smoking history and younger age compared with patients with NSCLC without ROS1 gene rearrangements.9,29 Our population is consistent with these findings as it comprised patients of whom only 28.3% had a history of smoking and with a median age of 51.5 years. Although in the total population there were slightly more females than males, in the Japanese and South Korean or Taiwanese populations there was a greater percentage of females compared with the total population. One study from Europe has found a greater percentage of ROS1 fusions in females (10.2%) than males (0.4%; p = 1.2E-10).29
The trend toward improvement in patient-reported global QoL found in the initial analysis was maintained with the extended follow-up described here.14 Because maintaining QoL is an important goal of cancer therapy30 and poorer QoL is associated with shorter survival of patients with cancer,31 these results are clinically relevant and further support the use of crizotinib in this patient population. Results are presented for the first 60 cycles of treatment because smaller percentages of patients (≤20%) at later time points limited the interpretation of the results.
The safety profile with the long-term follow-up revealed here was consistent with the known safety profile of crizotinib and with the initial report.11,14 As expected, the longer duration of treatment led to an increase in the number of AEs in the course of the study, but as in the previous report, most were grade 1 or 2 in severity. Small increases were found in the numbers of patients with serious TRAEs, grade 3 or 4 TRAEs, TRAEs leading to permanent discontinuation of crizotinib, and TRAEs leading to dosage interruptions and dose reductions. This study represents one of the longest durations of crizotinib treatment in a clinical trial (approximately 24 mo). The consistency of these safety findings in an Asian population compared with other studies should give reassurance to physicians and their patients when considering the long-term use of crizotinib in ROS1-positive NSCLC.
In conclusion, these results represent the largest phase 2 trial of an ALK/ROS1 inhibitor to treat patients with ROS1-positive advanced NSCLC and the first trial of its kind conducted in East Asia. With approximately 5 years of follow-up and a median OS of 44.2 months, these data provide a new benchmark for OS in this population. Taken together, the long OS, maintenance of QoL, and favorable AE profile through long-term use support the continued use of crizotinib in the treatment of patients with ROS1-positive advanced NSCLC in East Asia.
CRediT Authorship Contribution Statement
Yi-Long Wu: Conceptualization, Investigation, Writing—original draft, Writing—review and editing.
Shun Lu, Jianying Zhou, Takashi Seto, Myung-Ju Ahn, Koichi Goto: Writing—review and editing.
James Chih-Hsin Yang: Methodology, Writing—review and editing.
Wu-Chou Su: Data curation, Writing—review and editing.
Noboru Yamamoto: Investigation, Writing—review and editing.
Dong-Wan Kim: Conceptualization, Investigation, Writing—review and editing.
Jolanda Paolini: Data curation, Formal analysis, Methodology, Supervision, Writing—review and editing.
Tiziana Usari, Laura Iadeluca: Data curation, Formal analysis, Methodology, Writing—review and editing.
Keith D. Wilner: Data curation, Formal analysis, Methodology, Supervision, Writing—review and editing.
Acknowledgments
This study was sponsored by Pfizer Inc. Pfizer participated in the study design; collection, analysis, and interpretation of data; and review and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication and had final responsibility for the decision to submit the article for publication. The authors thank the patients, their families, teams of investigators, research nurses, study coordinators, operations staff, and the Chinese Thoracic Oncology Group who made this work possible. Editorial and medical writing support was provided by Meredith Rogers, MS, CMPP, of CMC AFFINITY, McCann Health Medical Communications, and Alana Dorfstatter, PharmD, of ClinicalThinking, Inc., which were funded by Pfizer.
Data Sharing Statement
On request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Footnotes
Disclosure: Dr. Wu reports receiving speaker fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Pfizer, Roche, and Sanofi; research funding (institution) from AstraZeneca, Bristol Myers Squibb, Pfizer, and Roche. Dr. Lu reports receiving consulting/advisory fees from AstraZeneca, Boehringer Ingelheim, GenomiCare, Hutchison MediPharma, Roche, Simcere Pharmaceutical, and ZaiLab; speakers’ fees from AstraZeneca, Hansoh, and Roche; and research funding from AstraZeneca, Bristol Myers Squibb, Heng Rui, Hutchison MediPharma, and Roche. Dr. Yang reports receiving grants and personal and institution consulting/advisory fees from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck KGaA, Merck Sharp & Dohme, Novartis, Roche/Genentech, Takeda Oncology, and Yuhan Pharmaceuticals; institution consulting/advisory fees from Eli Lilly, Johnson & Johnson, Glaxo, and Puma Technology; and personal consulting/advisory fees from Ono Pharmaceuticals and Pfizer. Dr. Seto reports receiving grants from Pfizer Japan during the conduct of the study; receiving honoraria from AstraZeneca KK, Bristol Myers Squibb, Covidien Japan, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Kyowa Hakko Kirin, Mochida Pharmaceutical, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Novartis Pharma, Ono Pharmaceutical, Pfizer Japan, Taiho Pharmaceutical, Takeda Pharmaceutical, and Thermo Fisher Scientific; receiving research funding (institution) from AbbVie, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Kissei Pharmaceutical, Loxo Oncology, Merck Biopharma, Merck Sharp & Dohme, Novartis Pharma, Pfizer Japan, and Takeda Pharmaceutical; and being an employee of Precision Medicine Asia. Dr. Yamamoto reports receiving honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Ono Pharmaceutical, and Sysmex; consulting/advisory fees from Boehringer Ingelheim, Cimic, Eisai, Otsuka, and Takeda Pharmaceuticals; and research funding (institution) from Astellas Pharma, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Chiome Bioscience, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly & Co, Genmab, GlaxoSmithKline, Janssen Pharma, Kyowa Hakko Kirin, Merck Sharp & Dohme, Merck, Novartis, Ono Pharmaceutical, Otsuka, Pfizer, Quintiles, Sumitomo Dainippon, Taiho Pharmaceutical, and Takeda Pharmaceuticals. Dr. Kim reports receiving grants and nonfinancial support from Pfizer during the conduct of the study; research funding to his institution from Alpha Biopharma, Amgen, AstraZeneca/MedImmune, Boehringer Ingelheim, Chong Keun Dang, Daiichi Sankyo, Hanmi, Janssen, Merus, Mirati Therapeutics, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, and Yuhan; and travel support for advisory board meetings from Amgen and Daiichi Sankyo. Goto reports receiving grants and personal fees from Pfizer Japan, Inc., during the conduct of the study; honoraria (personal fees) from Amgen, Amgen KK, Astellas BioPharma KK, Amoy Diagnostics, AstraZeneca KK, Boehringer Ingelheim Japan, Bristol Myers Squibb KK, Bayer Yakuhin, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, Guardant Health, Janssen Pharmaceutical KK, Kyowa Hakko Kirin, Life Technologies Japan, Merck Sharp & Dohme KK, Novartis Pharma KK, Ono Pharmaceutical, Otsuka Pharmaceutical, Taiho Pharmaceutical, and Takeda Pharmaceutical; and grants (institution) from Amgen, Amgen KK, Astellas BioPharma KK, AstraZeneca KK, Boehringer Ingelheim Japan, Bristol Myers Squibb KK, Bayer Yakuhin, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan KK, Haihe Biopharma, Ignyta, Janssen Pharmaceutical KK, Kissei Pharmaceutical, Kyowa Hakko Kirin, Loxo Oncology, Medical & Biological Laboratories, Merck BioPharma, Merus N.V., Merck Sharp & Dohme KK, NEC Corporation, Novartis Pharma KK, Ono Pharmaceutical, Spectrum Pharmaceuticals, Sumitomo Dainippon Pharma, Sysmex Corporation, Taiho Pharmaceutical, Takeda Pharmaceuticals, and Turning Point Therapeutics. Mrs. Paolini, Mrs. Usari, Dr. Iadeluca, and Dr. Wilner are employees of Pfizer and hold Pfizer stock/stock options. Mrs. Usari holds stock in Viatris. The remaining authors declare no conflict of interest.
Cite this article as: Wu YL, Lu S, Yang JCH, et al. Final overall survival, safety, and quality of life results from a phase 2 study of crizotinib in East Asian patients with ROS1-positive advanced NSCLC. JTO Clin Res Rep. 2022;3:100406.
References
- 1.Lynch T.J., Bell D.W., Sordella R., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Soda M., Choi Y.L., Enomoto M., et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Rikova K., Guo A., Zeng Q., et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Davies K.D., Doebele R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19:4040–4045. doi: 10.1158/1078-0432.CCR-12-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization, International Agency for Research on Cancer. Globocan 2020: lung cancer. http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf. Accessed June 14 2021.
- 6.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 7.Kohno T., Nakaoku T., Tsuta K., et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–164. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor J.F., Shaw A.T. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergethon K., Shaw A.T., Ou S.H., et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheffler M., Schultheis A., Teixido C., et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577–10585. doi: 10.18632/oncotarget.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfizer Inc. XALKORI® (crizotinib) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202570s030lbl.pdf. Accessed 11 June 2021; 2021.
- 12.Shaw A.T., Riely G.J., Bang Y.J., et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw A.T., Ou S.H., Bang Y.J., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y.L., Yang J.C., Kim D.W., et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.Bergman B., Aaronson N.K., Ahmedzai S., Kaasa S., Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Aaronson NK, Bjordal K, et al. EORTC Quality of Life Group. EORTC QLQ-C30 scoring manual. 3rd edition. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. Accessed July 22 2022.
- 18.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Michels S.Y.F., Franklin J., Massuti B., et al. Crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial – updated report on progression-free and overall survival. J Clin Oncol. 2019;37 doi: 10.1016/j.jtho.2019.03.020. 9066–9066. [DOI] [PubMed] [Google Scholar]
- 20.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novartis Pharmaceuticals CorpSoration. ZYKADIA® (ceritinib) Prescribing Information. https://www.novartis.us/sites/www.novartis.us/files/zykadia.pdf. Accessed October 11 2021; 2021.
- 22.Lim S.M., Kim H.R., Lee J.S., et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35:2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 23.Shaw A.T., Solomon B.J., Chiari R., et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 24.Dudnik E., Agbarya A., Grinberg R., et al. Clinical activity of brigatinib in ROS1-rearranged non-small cell lung cancer. Clin Transl Oncol. 2020;22:2303–2311. doi: 10.1007/s12094-020-02376-w. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay G., Groff M., Iadeluca L., et al. Effectiveness of crizotinib versus entrectinib in ROS1-positive non-small-cell lung cancer using clinical and real-world data. Future Oncol. 2022;18:2063–2074. doi: 10.2217/fon-2021-1102. [DOI] [PubMed] [Google Scholar]
- 26.Park S., Ahn B.C., Lim S.W., et al. Characteristics and outcome of ROS1-positive non-small cell lung cancer patients in routine clinical practice. J Thorac Oncol. 2018;13:1373–1382. doi: 10.1016/j.jtho.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Yu H., Chang J., et al. Crizotinib in Chinese patients with ROS1-rearranged advanced non‒small-cell lung cancer in routine clinical practice. Target Oncol. 2019;14:315–323. doi: 10.1007/s11523-019-00636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazières J., Zalcman G., Crinò L., et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti A., Barberis M., Di Lorito A., et al. ROS1 gene fusion in advanced lung cancer in women: a systematic analysis, review of the literature, and diagnostic algorithm. JCO Precis Oncol. 2017;1:1–9. doi: 10.1200/PO.16.00010. [DOI] [PubMed] [Google Scholar]
- 30.Yang P. Maximizing quality of life remains an ultimate goal in the era of precision medicine: exemplified by lung cancer. Precis Clin Med. 2019;2:8–12. doi: 10.1093/pcmedi/pbz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan J.A., Zhao X., Novotny P.J., et al. Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. J Clin Oncol. 2012;30:1498–1504. doi: 10.1200/JCO.2010.33.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]