Key Points
Question
What is the long-term legacy effect of intensive blood pressure (BP) control on mortality?
Findings
In this secondary analysis of the Systolic Blood Pressure Intervention Trial including 9361 patients, the beneficial effect of intensive BP control on cardiovascular and all-cause mortality was attenuated during 4.5 years of posttrial observational follow-up. During this period, outpatient BP readings indicated that systolic BP increased by an average of 7 mm Hg for participants randomized to intensive treatment.
Meaning
Sustaining BP control to the Systolic Blood Pressure Intervention Trial target of less than 120 mm Hg will be critical for achieving population reductions in cardiovascular mortality.
This secondary analysis of the Systolic Blood Pressure Intervention Trial evaluates the long-term effects of randomization to intensive treatment with the incidence of cardiovascular and all-cause mortality approximately 4.5 years after the trial ended.
Abstract
Importance
The Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive blood pressure control reduced cardiovascular morbidity and mortality. However, the legacy effect of intensive treatment is unknown.
Objective
To evaluate the long-term effects of randomization to intensive treatment with the incidence of cardiovascular and all-cause mortality approximately 4.5 years after the trial ended.
Design, Setting, and Participants
In this secondary analysis of a multicenter randomized clinical trial, randomization began on November 8, 2010, the trial intervention ended on August 20, 2015, and trial close-out visits occurred through July 2016. Patients 50 years and older with hypertension and increased cardiovascular risk but without diabetes or history of stroke were included from 102 clinic sites in the US and Puerto Rico. Analyses were conducted between October 2021 and February 2022.
Interventions
Randomization to systolic blood pressure (SBP) goal of less than 120 mm Hg (intensive treatment group; n = 4678) vs less than 140 mm Hg (standard treatment group; n = 4683).
Main Outcomes and Measures
Extended observational follow-up for mortality via the US National Death Index from 2016 through 2020. In a subset of 2944 trial participants, outpatient SBP from electronic health records during and after the trial were examined.
Results
Among 9361 randomized participants, the mean (SD) age was 67.9 (9.4) years, and 3332 (35.6%) were women. Over a median (IQR) intervention period of 3.3 (2.9-3.9) years, intensive treatment was beneficial for both cardiovascular mortality (hazard ratio [HR], 0.66; 95% CI, 0.49-0.89) and all-cause mortality (HR, 0.83; 95% CI, 0.68-1.01). However, at the median (IQR) total follow-up of 8.8 (8.3-9.3) years, there was no longer evidence of benefit for cardiovascular mortality (HR, 1.02; 95% CI, 0.84-1.24) or all-cause mortality (HR, 1.08; 95% CI, 0.94-1.23). In a subgroup of participants, the estimated mean outpatient SBP among participants randomized to intensive treatment increased from 132.8 mm Hg (95% CI, 132.0-133.7) at 5 years to 140.4 mm Hg (95% CI, 137.8-143.0) at 10 years following randomization.
Conclusions and Relevance
The beneficial effect of intensive treatment on cardiovascular and all-cause mortality did not persist after the trial. Given increasing outpatient SBP levels in participants randomized to intensive treatment following the trial, these results highlight the importance of consistent long-term management of hypertension.
Trial Registration
ClinicalTrials.gov Identifier: NCT01206062
Introduction
Hypertension is a leading modifiable risk factor for cardiovascular disease (CVD).1 Meta-analyses of randomized trials have shown that pharmacological blood pressure (BP) lowering reduces the risk of major cardiovascular events across the spectrum of initial BP.2,3 The Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive treatment, defined by a systolic BP (SBP) target less than 120 mm Hg, reduced the risk of incident CVD and all-cause mortality compared with treatment to an SBP target of less than 140 mm Hg.4 Similar results, in favor of a lower SBP target of 110 mm Hg to less than 130 mm Hg, were also recently observed for a composite cardiovascular outcome in the Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial.5 However, both trials were stopped after a median follow-up of approximately 3.3 years. The longer-term effect of intensive treatment on cardiovascular and all-cause mortality after either trial (ie, the legacy effect)6 has not been evaluated.
The objective of the current study was to evaluate the longer-term legacy effect of intensive treatment in SPRINT on mortality, with passive follow-up using administrative data sources. We linked participants to the National Death Index (NDI) from 2016 through 2020, adding 4.5 years of follow-up after the conclusion of trial visits. A secondary objective was to examine change in attained BP levels following the discontinuation of the trial intervention and study visits. To examine this issue, we extracted longitudinal outpatient measurements of SBP from 2010 to 2020 available in the electronic health record (EHR) for a subset of trial participants.
Methods
Trial Design
The design and methods of the trial have been published previously,4,7 and the trial protocol can be found in Supplement 1. Briefly, it was a multicenter randomized clinical trial that compared 2 strategies for managing SBP in older adults with hypertension who were at increased risk of CVD. Participants were 50 years or older and had an SBP between 130 and 180 mm Hg at the screening visit, depending on the use and number of antihypertensive agents prescribed. Participants were considered to have an increased cardiovascular risk if they had clinical or subclinical CVD, chronic kidney disease (CKD), or a Framingham Risk Score of 15% or greater or if they were 75 years or older. Individuals residing in a nursing home, with a diagnosis of dementia (based on medical record review), and treated with medications prescribed for dementia were excluded, as were persons with prevalent diabetes, history of stroke, proteinuria level greater than 1 g per day, or polycystic kidney disease. Individuals at 102 sites in the US and Puerto Rico were randomized (1:1) by the data coordinating center to an SBP goal of less than 120 mm Hg (intensive treatment group; n = 4678) or a goal of less than 140 mm Hg (standard treatment group; n = 4683), using random permuted blocks with the randomization stratified by clinic site. Randomization began on November 8, 2010, and ended in March 2013. On August 20, 2015, the Director of the National Heart, Lung, and Blood Institute accepted the data and safety monitoring board’s recommendation to inform the investigators and participants of the primary outcome results and decided to stop the trial early for benefit. In addition to the trial’s intervention phase, which spanned November 8, 2010, through August 20, 2015, the current study includes as part of the trial phase an additional period of follow-up through July 1, 2016, where the study still provided antihypertensive medications prior to each participants’ final closeout visit (eFigure 1 in Supplement 2). Observational follow-up continued through December 2020. The study was approved by the institutional review boards at each participating site, and each participant provided written informed consent. The study is registered at ClinicalTrials.gov (NCT01206062).
Baseline Study Measurements
Sociodemographic data were collected at baseline, with race and ethnicity collected via self-report using fixed categories to satisfy the National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. In the current study, the estimated glomerular filtration rate (eGFR) was calculated by the race-free 2021 Chronic Kidney Disease–Epidemiology Collaboration creatinine equation.8 Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA).9 Lower cognitive function was defined as scoring at or below the estimated age-specific and education-specific normative 10th percentile from the Irish Longitudinal Study of Ageing,10 after adding 3 points to the scores of non-White participants.11 We defined frailty status at baseline using a 36-item Frailty Index (FI) based on the model of deficit accumulation.12 The FI is calculated as the sum of the score for each deficit divided by the total number of nonmissing items. We categorized frailty status as fit (FI score of 0.10 or less), less fit (FI between 0.10 and 0.21), or frail (FI score greater than 0.21).
NDI Linkage
Outcomes of interest included all-cause and cardiovascular mortality. Methods of ascertainment and adjudication through the course of trial follow-up have been previously described.4 In the final report of trial results, mortality was ascertained through a US NDI search completed in December 2016.4 For the current analysis, we completed an NDI search including deaths through December 2020. Possible matches were identified according to NDI guidelines.13 Deaths were treated as confirmed if they were a Class 1 match, or a Class 2, 3, or 4 match with a probabilistic score above cutoffs recommended by the NDI.13 Deaths ascertained in 2020 were based on the NDI preliminary data release. CVD mortality for NDI-based follow-up used the NDI Plus System, which automatically identifies underlying causes of death from death certificates, including conversion to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. We defined CVD mortality as any death containing the ICD-10 codes of I00 to I99.14
BP Measurement in the Trial
Sites were provided with the Professional Digital Blood Pressure Monitor (Omron Healthcare), model 907XL, for BP measurement in the trial.15 Training on BP measurement technique emphasized proper positioning of participants, measurement of arm circumference and use of proper cuff size, and the importance of a 5-minute rest period before obtaining the 3 seated BP values. The trial Manual of Procedures recommended that the staff leave the room during the rest period but return to take the BPs at the end of the 5-minute rest but did not require staff attendance or absence during the BP measurement.
EHR Ancillary Study
Methods for the linkage of participants to their medical record number and the extraction of vital sign data have been previously described.16 We identified 3074 participants with 3 or more EHR reports of outpatient BP measurements during the trial. After excluding 130 participants without EHR data following July 2016 (ie, conclusion of the trial phase), a total of 2944 patients were included for the ancillary BP analysis. Because encounter-type information was inconsistently available (eg, outpatient, inpatient, observation), we defined a BP measurement as outpatient if there was not a BP measurement on the preceding or following day and if there were 2 or less BP measurements on a particular day. We averaged outpatient EHR BP readings when there were 2 on the same day.
Statistical Analysis
Given the a priori expectation that treatment group differences may not be constant as a function of follow-up time (ie, the proportional hazards assumption was likely to be invalid), we modeled treatment group differences as a function of time using 2 approaches. The first approach split each participant’s follow-up time into nonoverlapping trial and observational phases and estimated regression coefficients for intensive treatment separately during each phase.17 The second approach estimated a regression coefficient for intensive treatment as a continuous function of time since randomization.18,19 All analyses accounted for correlation within study sites,20 and analyses of cardiovascular mortality accounted for the competing risk of noncardiovascular mortality.21
We examined the trajectory of SBP following the conclusion of the trial using outpatient SBPs extracted from the EHR. Mean between-group differences in outpatient SBP were estimated using linear mixed models. Models included random effects for participant and clinic site and an interaction between treatment group and time since randomization, which was modeled using B-splines. All analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.1.2 (The R Foundation) with assistance from multiple R packages.22,23,24,25,26 All hypothesis tests were 2-sided, and P values less than .05 were considered statistically significant. No adjustments for multiple comparisons were made.
Results
Study Participants
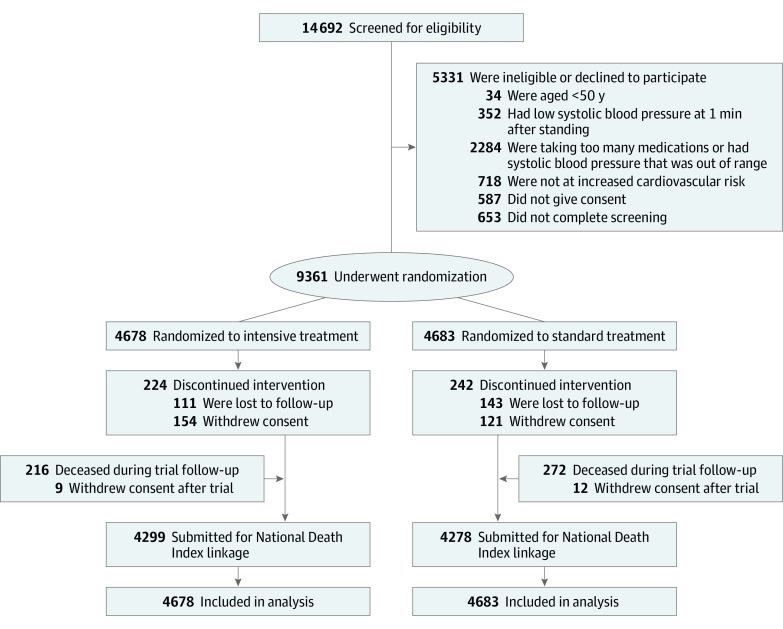
A total of 9361 participants were randomized between November 2010 and March 2013 (Figure 1). The mean (SD) age was 67.9 (9.4) years, with 2636 participants (28.2%) 75 years or older (eTable 1 in Supplement 2). A total of 3332 participants (35.6%) were female, and 2947 (31.5%) were Black. The mean (SD) SBP at baseline was 139.7 (15.6) mm Hg, and 2510 participants (27.0%) had MoCA scores below an age-specific and education-specific normative 10th percentile. Compared with participants not included in the ancillary EHR study, participants included were more likely to be male and older, with lower SBP and higher scores on the MoCA and a higher prevalence of CKD.
Figure 1. Participant Flow in the Systolic Blood Pressure Intervention Trial.
All-Cause Mortality
In both treatment groups, the total median (IQR) follow-up time was 8.8 (8.3-9.3) years. A total of 818 and 826 deaths occurred among participants randomized to intensive and standard treatment, respectively (Table). The hazard ratio (HR) for all-cause mortality comparing intensive with standard treatment was 0.83 (95% CI, 0.68-1.01) during the trial phase, and 1.08 (95% CI, 0.94-1.23) during the observational phase. The continuous time-dependent effect of intensive vs standard treatment indicated a benefit for all-cause mortality from 1.03 to 2.8 years following randomization and was attenuated throughout the remainder of the observational phase (eFigure 2 in Supplement 2). In subgroups based on age, sex, race, CKD, cognitive function, and frailty status, there was no evidence that intensive treatment during the trial phase was associated with benefit for all-cause mortality during the observational phase of follow-up (eFigure 3 in Supplement 2).
Table. All-Cause Mortality by Treatment Group, Phase of Follow-up, and Subgroup.
| Subgroup | Trial phase | Observational phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of CVD deaths/No. at risk | Incidence (95% CI)a | HR (95% CI)b | No. of CVD deaths/No. at risk | Incidence (95% CI)a | HR (95% CI)b | |||||
| Intensive | Standard | Intensive | Standard | Intensive | Standard | Intensive | Standard | |||
| Overall | 241/4678 | 290/4683 | 12.5 | 15.1 | 0.83 (0.68-1.01) | 577/4274 | 536/4260 | 31.9 | 29.6 | 1.08 (0.94-1.23) |
| Age group, y | ||||||||||
| <75 | 115/3361 | 137/3364 | 8.2 | 9.7 | 0.84 (0.63-1.11) | 246/3123 | 257/3129 | 18.1 | 18.9 | 0.96 (0.78-1.17) |
| ≥75 | 126/1317 | 153/1319 | 24.3 | 29.8 | 0.82 (0.62-1.07) | 331/1151 | 279/1131 | 73.3 | 61.6 | 1.21 (1.00-1.46) |
| Sex | ||||||||||
| Male | 170/2994 | 212/3035 | 13.8 | 17.2 | 0.80 (0.64-1.01) | 400/2745 | 353/2743 | 34.6 | 30.3 | 1.14 (0.97-1.34) |
| Female | 71/1684 | 78/1648 | 10.2 | 11.4 | 0.89 (0.61-1.28) | 177/1529 | 183/1517 | 27.0 | 28.2 | 0.94 (0.74-1.20) |
| Racec | ||||||||||
| Black | 68/1454 | 81/1493 | 11.5 | 13.2 | 0.85 (0.59-1.24) | 151/1321 | 150/1363 | 26.7 | 25.7 | 1.02 (0.78-1.32) |
| Non-Black | 173/3224 | 209/3190 | 13.0 | 16.0 | 0.80 (0.64-1.01) | 426/2953 | 386/2897 | 34.2 | 31.4 | 1.10 (0.94-1.28) |
| Chronic kidney diseased | ||||||||||
| No | 126/3485 | 163/3491 | 8.7 | 11.3 | 0.77 (0.59-1.01) | 349/3250 | 316/3232 | 25.0 | 22.7 | 1.08 (0.91-1.29) |
| Yes | 112/1170 | 125/1161 | 23.6 | 26.5 | 0.89 (0.66-1.19) | 227/1012 | 220/1007 | 55.5 | 53.8 | 1.02 (0.82-1.26) |
| Cognitive function, percentilee | ||||||||||
| >10th | 152/3389 | 212/3397 | 10.8 | 15.1 | 0.70 (0.55-0.89) | 408/3125 | 357/3101 | 30.7 | 26.9 | 1.13 (0.96-1.33) |
| ≤10th | 83/1257 | 77/1253 | 16.5 | 15.3 | 1.13 (0.79-1.62) | 165/1131 | 176/1135 | 34.7 | 36.8 | 0.98 (0.76-1.26) |
| Frailty statusf | ||||||||||
| Fit (Frailty Index ≤0.10) | 18/751 | 21/729 | 5.7 | 6.9 | 0.90 (0.43-1.87) | 34/685 | 42/715 | 13.4 | 11.3 | 1.26 (0.72-2.2) |
| Prefrail (0.10 < Frailty Index ≤ 0.21) | 93/2359 | 107/2406 | 9.5 | 10.7 | 0.90 (0.66-1.24) | 251/2244 | 261/2193 | 27.8 | 26.1 | 1.07 (0.87-1.30) |
| Frail (Frailty Index >0.21) | 127/1538 | 161/1523 | 20.4 | 26.4 | 0.75 (0.57-0.98) | 251/1317 | 274/1349 | 49.9 | 46.2 | 1.07 (0.87-1.30) |
Abbreviations: CVD, cardiovascular disease; HR, hazard ratio.
Incidence represents the rate of events per 1000 person-years of follow-up.
Hazard ratios for intensive treatment vs standard treatment based on a Cox proportional hazards regression model with a time-varying effect across the trial phase.
Sociodemographic data were collected at baseline, with race and ethnicity collected via self-report using fixed categories to satisfy the National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. In the Systolic Blood Pressure Intervention Trial, the subgroups of Black and non-Black were prespecified.
Chronic kidney disease defined as an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 based on the 2021 Chronic Kidney Disease–Epidemiology Collaboration creatinine equation.
Age-specific and education-specific normative 10th percentile from the Irish Longitudinal Study of Ageing, after adding 3 points to the scores of non-White participants.
Scores range from 0 to 1, with higher values indicating greater frailty.
Cardiovascular Mortality
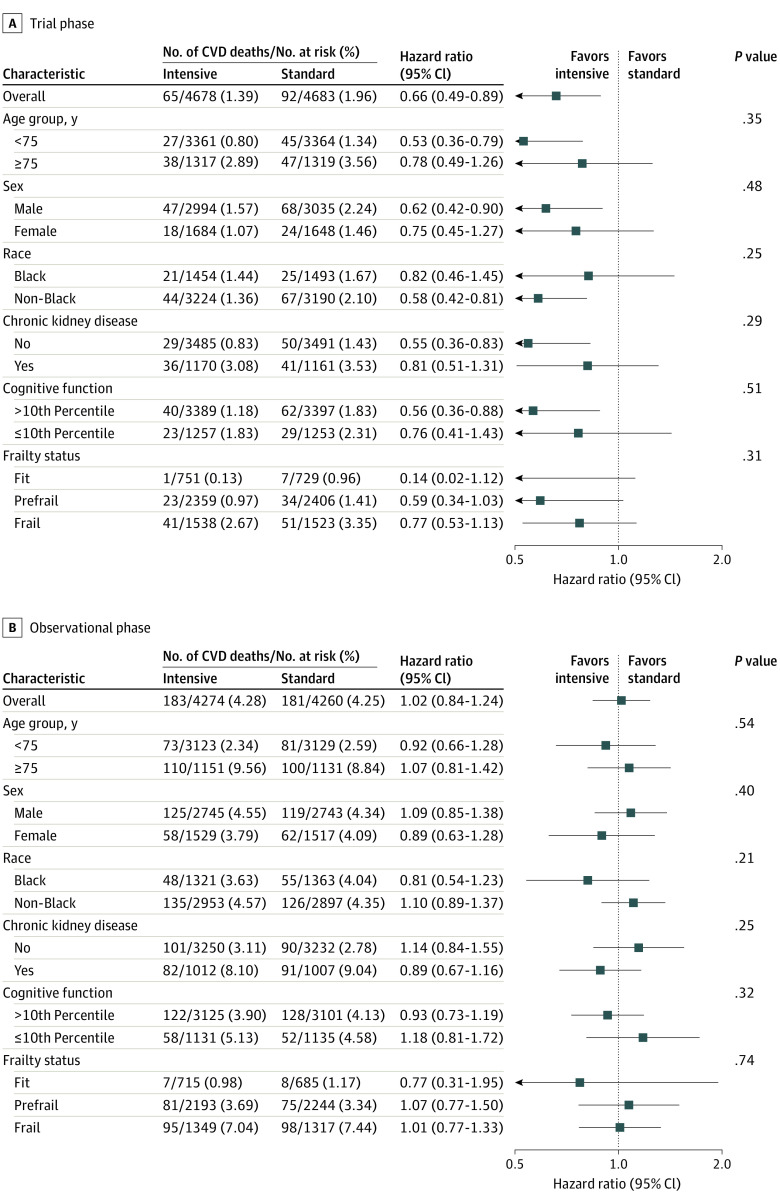
During the 8.8 years of follow-up, a total of 248 and 273 CVD deaths occurred among participants randomized to intensive and standard treatment, respectively (eTable 2 in Supplement 2). The HR for CVD mortality among participants randomized to intensive vs standard treatment was 0.66 (95% CI, 0.49-0.89) during the trial phase and 1.02 (95% CI, 0.84-1.24) during the observational phase. The time-dependent effect of intensive vs standard treatment indicated a benefit for CVD mortality from 2.3 to 5.6 years from randomization and was attenuated throughout the remainder of the observational phase (Figure 2). The same pattern of effect was observed when we examined subgroup-specific treatment effects (Figure 3). There was no evidence of heterogeneity for any subgroup during either phase of trial follow-up.
Figure 2. Cardiovascular Disease (CVD) vs Non-CVD Mortality by Treatment Group.
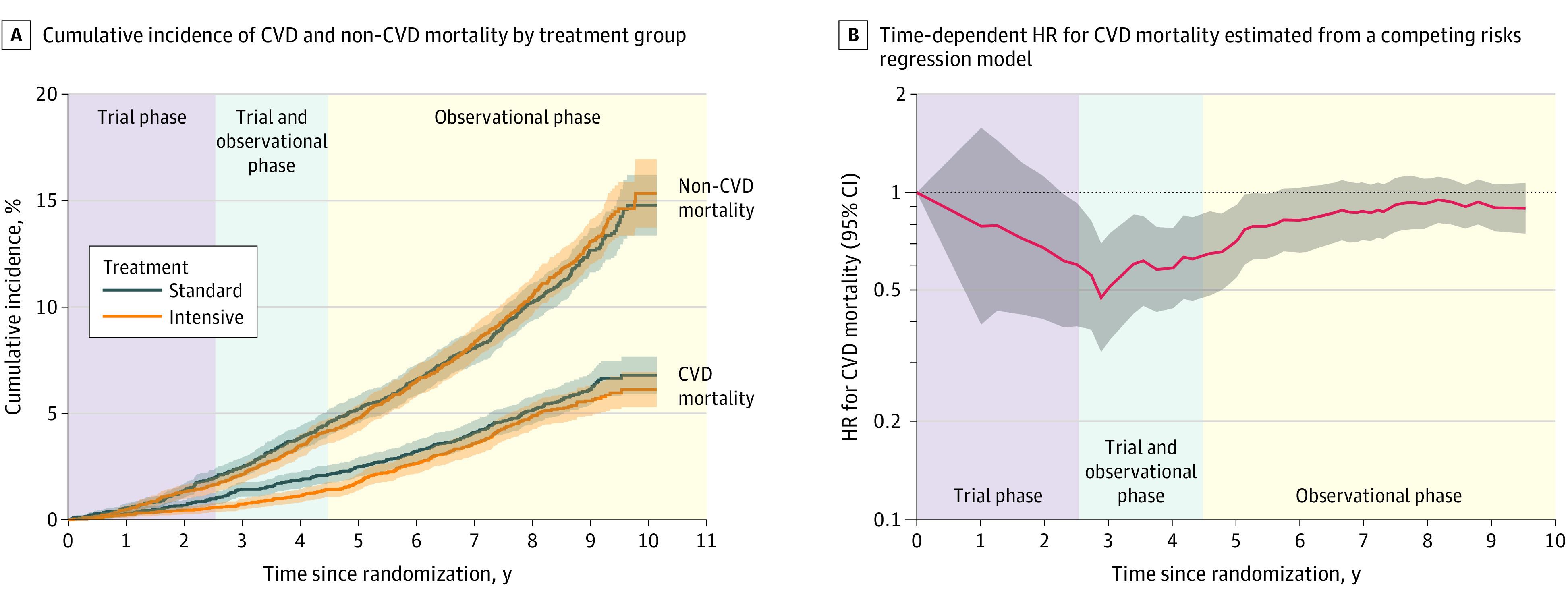
A, Cumulative incidence of CVD and non-CVD mortality by treatment group. B, Time-dependent hazard ratio (HR) for cardiovascular mortality estimated from a competing-risks regression model comparing intensive treatment with standard treatment. The trial phase and observational phase do not overlap for individual participants, but due to participants being randomized over time, there is an overlap in the trial and observational phase for the trial population when time is measured relative to the date of randomization. Trial phase encompasses follow-up through the end of study closeout visits (eFigure 1 in Supplement 2).
Figure 3. Cardiovascular Disease (CVD) Mortality by Treatment Group According to Subgroups.
Sociodemographic data were collected at baseline, with race and ethnicity collected via self-report using fixed categories to satisfy the National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. In the Systolic Blood Pressure Intervention Trial, the subgroups of Black and non-Black were prespecified. Chronic kidney disease was defined as an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 based on the 2021 Chronic Kidney Disease–Epidemiology Collaboration creatinine equation. Cognitive function groups based on age-specific and education-specific normative 10th percentile from the Irish Longitudinal Study of Ageing, after adding 3 points to the scores of non-White participants. Frailty status based on a 36-item Frailty Index, for which scores range from 0 to 1, with higher values indicating greater frailty. Trial phase encompasses follow-up through the end of study closeout visits (eFigure 1 in Supplement 2).
BP
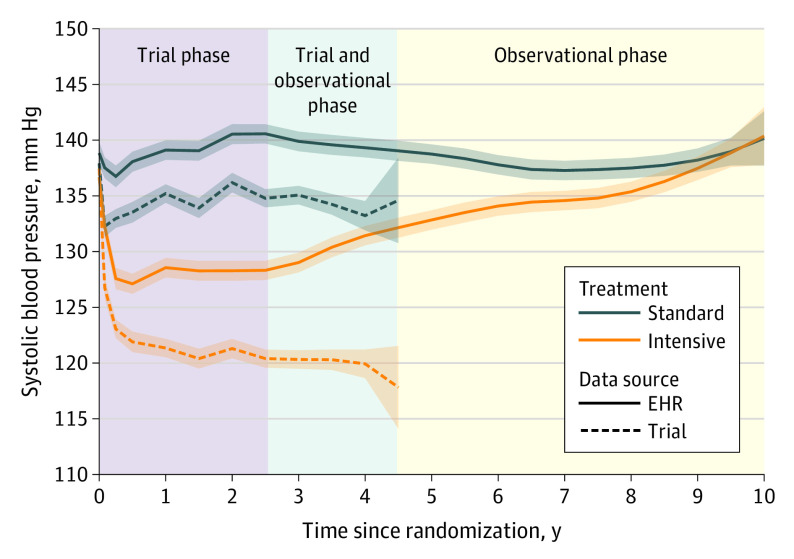
Among a subset of 2944 trial participants, the median (IQR) number of outpatient BP measurements extracted from the EHR during the observational phase of follow-up was 20 (10-34). The estimated mean SBP among participants randomized to intensive treatment was 132.8 mm Hg (95% CI, 132.0-133.7) at 5 years following randomization and 140.4 mm Hg (95% CI, 137.8-143.0) at 10 years following randomization (Figure 4). For participants randomized to standard treatment, mean SBP was estimated to be 138.8 mm Hg (95% CI, 137.9-139.6) at 5 years following randomization and 140.2 mm Hg (95% CI, 137.7-142.6) at 10 years following randomization. The between-group difference in mean SBP levels (intensive treatment group minus standard treatment group) was 5.9 mm Hg (95% CI, 5.2-6.7) at 5 years following randomization and was reduced to −0.21 mm Hg (95% CI, −3.6 to 3.2) at 10 years following randomization (eFigure 4 in Supplement 2).
Figure 4. Mean Systolic Blood Pressures by Treatment Group Comparing Trial Measurements With Outpatient Readings Extracted From the Electronic Health Record (EHR).
Estimates based on a linear mixed model with random intercepts for participant and clinic site, with years since randomization modeled using B-splines. Shaded areas denote pointwise 95% CIs. The trial phase and observational phase do not overlap for individual participants, but due to participants being randomized over time, there is an overlap in the trial and observational phase for the trial population when time is measured relative to the date of randomization. Trial phase encompasses follow-up through the end of study closeout visits (eFigure 1 in Supplement 2).
Discussion
The current study analyzed all-cause and CVD mortality among trial participants up to 10 years following randomization, finding that the mortality benefits associated with intensive treatment quickly attenuated after the trial intervention was discontinued. Time-varying estimates of the benefit of intensive treatment for all-cause mortality were attenuated at 2.8 years following randomization, while the benefit for CVD mortality was attenuated at 5.6 years following randomization. Findings from our ancillary study of outpatient SBP measured in routine clinical practice indicated that the difference in SBP between treatment groups diminished steadily over time, with no detectable difference in SBP approximately 9 years after randomization. These results, in combination with the primary findings of the trial, indicate that the beneficial effect of intensive treatment among adults with hypertension appears to diminish quickly if intensive BP control is not sustained.
The STEP trial enrolled 8511 Chinese patients aged 60 to 80 years with hypertension and randomized patients to an SBP target of 110 to less than 130 mm Hg (intensive treatment group) or a target of 130 to less than 150 mm Hg (standard treatment group).6 The STEP trial found an HR of 0.72 (95% CI, 0.39-1.32) with intensive vs standard treatment for incident CVD after a median follow-up of 3.34 years but did not find evidence of a benefit for all-cause mortality. In the current trial, the protective effect of intensive treatment for all-cause mortality was attenuated several years before attenuation of the protective effect for CVD mortality. These results in combination with findings from the STEP trial suggest weaker evidence for reduced all-cause vs CVD mortality risk with intensive BP control, consistent with the suggestion that the non-CVD mortality benefit in SPRINT was driven by an increased visit frequency among participants randomized to intensive treatment.27
Previous studies have found rising BP levels among US adults during the period of the current study. General population studies of adults living in the US with hypertension found that the prevalence of uncontrolled BP (SBP of 140 mm Hg or greater or diastolic BP of 90 mm Hg or greater) increased from 2013 to 2017.28 In addition, an analysis of data from 464 585 adults enrolled in a Quest Diagnostics wellness program found that SBP was between 1 mm Hg and 3 mm Hg greater, depending on age group and sex, in April through December of 2020 vs their corresponding values throughout 2019.29 The current study shows that even for adults who have maintained more intensive BP control for 3 years or more, relaxation of BP control quickly erodes the beneficial effect on CVD mortality. Combined with previous findings on rising BP levels among US adults, data from the current study emphasize the need for implementation of sustainable population-level and community-level strategies to improve BP control in the US.
A lingering question from this study is what were the primary drivers of the increase in BP in the intensive treatment group following the trial? There are several likely contributors, ranging from medication adherence (antihypertensive medications were no longer being provided for free), ascertainment bias (some participants likely received primary care from a different health system vs their trial site), differences in hypertension management strategies and therapeutic inertia as participants transitioned hypertension care back to their primary care physician, or physiologic differences in achieving more intensive BP control, given that the cohort was 4.5 years older. Unfortunately, as part of the EHR ancillary study, we only have access to medication data in the Veterans Affairs system and do not have information about who participants had encounters with, and so our ability to inform this question is quite limited. We believe this will be an important topic for future research in trials such as the STEP trial and other ongoing trials investigating more intensive BP control.
In October 2020, the US Surgeon General published a call to action to control hypertension.30 Evidenced-based strategies to improve BP control addressed in the call to action include implementing treatment protocols, using integrated care teams, providing clinicians feedback on their performance, and promoting shared patient-clinician management with self-measured BP monitoring. During the trial, participants received team-based care consistent with strategies outlined in the 2020 call to action, and clinicians received real-time feedback on individual and aggregate participant BP control. After the trial phase, when these protocols were no longer followed by the primary care clinicians, the incidence of all-cause mortality approximately doubled in both treatment groups. These data emphasize the benefit that can be realized by implementing the goals and strategies of the 2020 US Surgeon General’s call to action. Future research should continue to evaluate strategies for obtaining consistent BP control in clinical settings to reduce the burden of CVD, which remains the leading cause of death for US adults.
Limitations
This study has several additional limitations. First, while we restricted analyses to high-quality NDI matches, misclassification in linking participants to the NDI is possible. Second, while some studies have shown reasonable performance of using NDI diagnosis codes for defining CVD mortality,14,31 it is certainly subject to misclassification, and it is not as robust as the adjudication process used in the primary follow-up for the trial. Third, data on the incidence of nonfatal cardiovascular events were not available during the observational phase of follow-up. Fourth, information about SBP control after the trial was limited to routine outpatient SBP values in a subgroup of the trial participants and was extracted from the EHR, which is known to have poor concordance with the standardized BP measurement process used during the trial.16 While this prohibits definite conclusions about the absolute level of BP in both treatment groups, as well as pinpointing when the between-group SBP difference may or may not have completely attenuated, the observation of steadily increasing SBP for participants in the intensive treatment and relatively stable, or slightly decreased, SBP in the standard treatment group following the trial is likely still valid.
Conclusions
In conclusion, while intensive treatment produced beneficial effects on mortality during the trial, there was no evidence that this produced sustained benefits on cardiovascular and all-cause mortality subsequent to discontinuing the intervention protocol. Given steadily increasing mean SBP levels in participants randomized to intensive treatment after the trial, these results suggest that maintaining more intensive BP targets throughout adulthood will likely be essential for long-term CVD risk management.
Trial Protocol
eTable 1. Baseline Characteristics of Randomized Participants, Overall and by Inclusion in Electronic Health Record Ancillary Study
eTable 2. Competing Risks of CVD and Non-CVD Mortality by Treatment Group, Phase of Follow-up, and According to Subgroups
eFigure 1. Timeline for Follow-up of Mortality in SPRINT
eFigure 2. All-Cause Mortality by Treatment Group
eFigure 3. All-Cause Mortality by Treatment Group, Phase of Follow-up, and According to Subgroups
eFigure 4. Mean Difference in Systolic Blood Pressure During Follow-up
Data Sharing Statement
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimi K, Bidel Z, Nazarzadeh M, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration . Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398(10305):1053-1064. doi: 10.1016/S0140-6736(21)01921-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775-781. doi: 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis CE, Fine LJ, Beddhu S, et al. ; SPRINT Research Group . Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. 2021;384(20):1921-1930. doi: 10.1056/NEJMoa1901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wander GS, Bansal M. Legacy effect in medicine—the expanding horizon! Indian Heart J. 2018;70(6):769-771. doi: 10.1016/j.ihj.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Zhang S, Deng Y, et al. ; STEP Study Group . Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268-1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 7.Ambrosius WT, Sink KM, Foy CG, et al. ; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532-546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Eneanya ND, Coresh J, et al. ; Chronic Kidney Disease Epidemiology Collaboration . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 10.Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. J Am Geriatr Soc. 2013;61(suppl 2):S279-S290. doi: 10.1111/jgs.12195 [DOI] [PubMed] [Google Scholar]
- 11.Sachs BC, Chelune GJ, Rapp SR, et al. Robust demographically-adjusted normative data for the Montreal Cognitive Assessment (MoCA): results from the systolic blood pressure intervention trial. Clin Neuropsychol. Published online September 1, 2021. doi: 10.1080/13854046.2021.1967450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pajewski NM, Williamson JD, Applegate WB, et al. ; SPRINT Study Research Group . Characterizing frailty status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71(5):649-655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics . National Death Index: user’s guide. Accessed December 1, 2021. https://www.cdc.gov/nchs/data/ndi/ndi_users_guide.pdf
- 14.Olubowale OT, Safford MM, Brown TM, et al. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the National Death Index: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2017;6(5):e004966. doi: 10.1161/JAHA.116.004966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KC, Whelton PK, Cushman WC, et al. ; SPRINT Research Group . Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71(5):848-857. doi: 10.1161/HYPERTENSIONAHA.117.10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drawz PE, Agarwal A, Dwyer JP, et al. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern Med. 2020;180(12):1655-1663. doi: 10.1001/jamainternmed.2020.5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. doi: 10.21037/atm.2018.02.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinussen T, Scheike TH. Dynamic Regression Models for Survival Data. Springer Science & Business Media; 2007. [Google Scholar]
- 19.Therneau T, Crowson C, Atkinson E. Using time dependent covariates and time dependent coefficients in the Cox model. Accessed DATE. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf
- 20.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074-1078. doi: 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 21.Scheike TH, Zhang MJ. Flexible competing risks regression modeling and goodness-of-fit. Lifetime Data Anal. 2008;14(4):464-483. doi: 10.1007/s10985-008-9094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeger B. Table.glue: make and apply customized rounding specifications for tables. Accessed February 15, 2022. https://github.com/bcjaeger/table.glue
- 23.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 24.Therneau TM. A package for survival analysis in R. Accessed February 15, 2022. https://CRAN.R-project.org/package=survival
- 25.Scheike TH, Zhang MJ. Analyzing competing risk data using the R timereg package. J Stat Softw. 2011;38(2):1-15. doi: 10.18637/jss.v038.i02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau WM. The targets R package: a dynamic make-like function-oriented pipeline toolkit for reproducibility and high-performance computing. J Open Source Softw. 2021;6(57):2959. doi: 10.21105/joss.02959 [DOI] [Google Scholar]
- 27.Kreutz R, Brunström M, Thomopoulos C, Carlberg B, Mancia G. Do recent meta-analyses truly prove that treatment with blood pressure-lowering drugs is beneficial at any blood pressure value, no matter how low? a critical review. J Hypertens. 2022;40(5):839-846. doi: 10.1097/HJH.0000000000003056 [DOI] [PubMed] [Google Scholar]
- 28.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffin LJ, Kaufman HW, Chen Z, et al. Rise in blood pressure observed among US adults during the COVID-19 pandemic. Circulation. 2022;145(3):235-237. doi: 10.1161/CIRCULATIONAHA.121.057075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Substance Abuse and Mental Health Services Administration (US); Office of the Surgeon General (US) . The Surgeon General’s Call to Action to Control Hypertension. US Department of Health and Human Services; 2020. [Google Scholar]
- 31.Quin JA, Hattler B, Shroyer ALW, et al. ; Department of Veteran Affairs (CSP#517-FS) ROOBY Follow-up Study’s Endpoints Committee . Concordance between administrative data and clinical review for mortality in the randomized on/off bypass follow-up study (ROOBY-FS). J Card Surg. 2017;32(12):751-756. doi: 10.1111/jocs.13379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics of Randomized Participants, Overall and by Inclusion in Electronic Health Record Ancillary Study
eTable 2. Competing Risks of CVD and Non-CVD Mortality by Treatment Group, Phase of Follow-up, and According to Subgroups
eFigure 1. Timeline for Follow-up of Mortality in SPRINT
eFigure 2. All-Cause Mortality by Treatment Group
eFigure 3. All-Cause Mortality by Treatment Group, Phase of Follow-up, and According to Subgroups
eFigure 4. Mean Difference in Systolic Blood Pressure During Follow-up
Data Sharing Statement