Abstract
Numerous per- and polyfluoroalkyl substances (PFAS) are of growing concern worldwide, due to their ubiquitous presence, bioaccumulation and adverse effects. Surface waters in the United States have displayed elevated concentrations of PFAS, but so far discrete water sampling has been the commonly applied sampling approach. Here we field-tested a novel integrative passive sampler, a microporous polyethylene (PE) tube, and derived sampling rates (Rs) for 9 PFAS in surface waters. Three sampling campaigns were conducted, deploying PE tube passive samplers in the effluent of two wastewater treatment plant (WWTP) effluent sites plants (WWTPs) and across Narragansett Bay (RI, US) for one month each in 2017/2018. Passive samplers exhibited linear uptake of PFAS in the WWTP effluents over 16–29 days, with in-situ Rs for nine PFASs ranging from 10 mL day−1 (PFPeA) to 29 mL day−1 (PFOS). Similar sampling rates of 19 ± 4.8 mL day−1 were observed in estuarine field deployments. Applying these Rs values in a different WWTP effluent predicted dissolved PFAS concentrations mostly within 50% of their observations in daily composite water samples, except for PFBA (where predictions from passive samplers were 3x greater than measured values), PFNA (1.9), PFDA (1.7) and PFPeS (0.1). These results highlight the potential use of passive samplers as measurement and assessment tools of PFAS in dynamic aquatic environments.
Keywords: PFOS, PFOA, sampling rate, wastewater treatment plan effluent, surface water, Narragansett Bay
Graphical Abstract
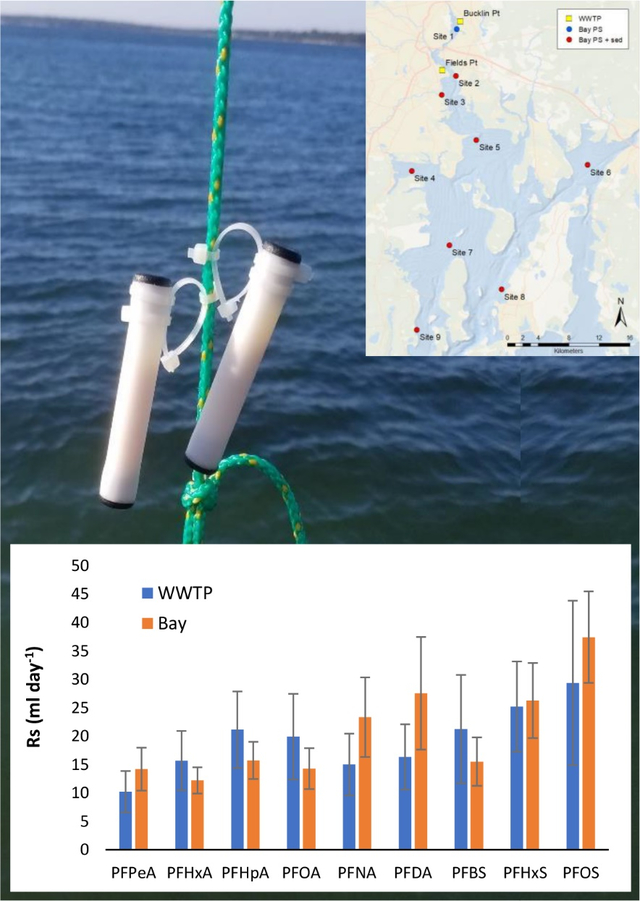
A novel integrative passive sampler was field-tested and sampling rates (Rs) derived for 9 PFAS in two waste water treatment plant effluents and in Narragansett Bay in surface waters.
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are a class of chemicals that have been used in commercial, industrial, and consumer products since the 1950s. Today the class includes over 9,000 chemicals with increasingly diverse chemistries (Lindstrom et al. 2011; Wang et al. 2017; US EPA 2020). These chemicals have been found globally distributed in air, surface water, sediment, biota, and drinking water due to their environmental mobility and remarkable persistence in the environment (Wang et al. 2017).
A variety of sampling methods and extraction protocols have been developed to measure the distribution of PFAS across complex and divergent environmental matrices, including both discrete and limited use of passive sampling (Lai et al. 2019). Discrete sampling provides a snapshot of contaminant levels at a discrete point in time. Passive samplers accumulate organic pollutants via kinetic or equilibrium sampling; kinetic sampling generates a time-weighted mean concentration of freely dissolved compounds in water or air (Vrana et al. 2005). Concentrations derived using passive sampling are therefore more representative of mean contaminant levels compared to discrete sampling techniques. In general, passive samplers also provide other benefits including low cost, small size, ease of use, and reduced complexity of the chemical extraction process (Lohmann et al. 2012). They can be deployed over large spatial and temporal scales, reducing or eliminating the need for frequent environmental sampling campaigns (Ghosh et al. 2014).
Passive samplers like polyethylene (PE) sheets and semi-permeable membrane devices have been widely used and shown to be suitable for sampling hydrophobic organic compounds (Górecki and Namieśnik 2002; Vrana et al. 2005). Recent work incorporating passive sampling materials and design has demonstrated the utility of passive sampling for amphiphilic and hydrophilic compounds. The Polar Organic Chemical Integrative Sampler (POCIS) has been used to sample dissolved polar compounds, including PFAS and pharmaceuticals. It incorporates a metal ring sandwiching a charged powdered adsorbent which binds the polar compound, between two thin (100–200 μm) polyethersulfone membranes (Alvarez et al. 2004). However, the kinetic uptake and sampling rate of PFAS is dependent on the flow rate of the surrounding medium, and the sorbent choice (Kaserzon et al. 2012; Kaserzon et al. 2014; Gobelius et al. 2019; Hale et al. 2021). Additional complications of the standard POCIS sampler include the potential of sorption to the polyethersulfone membrane (Endo et al. 2019). Alternatives to POCIS include diffusive gradients in thin film (DGT) samplers for PFAS (Challis et al. 2016; Guan et al. 2018), though their performance under ambient conditions in the field has only recently been assessed (Fang et al. 2021). Additionally, the use of isotopic performance reference compounds (PRCs) in passive samplers of ionic compounds is difficult due to anisotropy and competition for sorption sites (Vrana et al. 2005).
A novel passive sampler design, consisting of a hollow microporous PE tube filled with charged powdered adsorbent, has been proposed and initial tests in groundwater contaminated with aqueous film forming foam (AFFF) have been performed (Kaserzon et al. 2019). The PE tubes have thick (2 mm) microporous walls that provide a diffusion layer sufficiently thick to dominate the overall mass transfer from the bulk water to the sorbent hence flow/turbulence does not affect sampling kinetics much. Pollutant uptake is presumably limited by passive diffusion through the tube’s porous PE walls. These passive samplers have been previously used for the assessment of polar herbicides (Fauvelle, Montero, et al. 2017; Cárdenas-Soracá et al. 2020), and licit and illicit drugs in surface waters with some success (McKay et al. 2020; Verhagen et al. 2021), but require further validation to evaluate sampling performance under a wider range of environmentally relevant conditions, including dynamic water bodies, such as estuaries.
Estuaries act as an interface between freshwater systems and ocean environments and as such encompass a wide range of physical and chemical conditions based on ever-changing tidal flow, freshwater inputs, and anthropogenic inputs (Nixon and Buckley 2002). PFAS have previously been identified in estuarine surface water, introduced via wastewater treatment plant (WWTP) effluent, riverine flow, septic system leakage, atmospheric deposition, and (near) coastal AFFF use (Moody and Field 2000; Möller et al. 2010; Schaider et al. 2016; Ruyle et al. 2021). Elucidating the distribution and behavior of PFAS in multiple compartments of estuarine environments remains a research priority given the proximity of estuaries to anthropogenic PFAS sources, the highly variable environmental conditions found in estuaries, and the importance of estuarine habitats for key ecosystem services (Sharp et al. 1984; Vasconcelos et al. 2011; Munoz et al. 2017).
This study was hence performed to field-test a novel passive sampler for PFAS in two different dynamic surface water environments—first in the effluent of two WWTPs in Providence, Rhode Island (RI), USA, while daily 24 hour composite samples for PFAS were collected; followed by a field campaign in Narragansett Bay (NB), a well-mixed, tidally influenced estuary in RI, USA (Pilson 1985). In summary, the aims were to elucidate i) the integrative uptake of PFAS by PE tube passive samplers in a wastewater treatment plant effluent; ii) the sampling rates of different PFAS relative to daily composite PFAS samples; iii) the validity of the thus derived sampling rates in a parallel wastewater treatment plant effluent; and iv) performance of the PE tube samplers across an estuary.
MATERIALS & METHODS
Chemicals, reagents and materials.
A total of 24 PFAS were evaluated in this study, including C4–C14 perfluorocarboxylates (PFCAs), C4-C10 perfluorosulfonates (PFSAs), three fluorotelomer sulfonates (FTS), and three sulfonamide precursors (Tables S1 and S2). Analytical standards, including mass-labeled surrogates, were purchased from Wellington Laboratories (Guelph, ON, Canada) (see SI). HPLC grade methanol, ammonium hydroxide, and ammonium acetate were purchased from Fisher Scientific (Waltham, MA, USA). Ultrapure water was obtained from a Milli-Q system fit with an HPLC water polisher or via HPLC grade water purchased from Fisher Scientific (Waltham, MA, USA). Weak-anion exchange solid phase extraction cartridges (150 mg/6 cc, Oasis WAX) and bulk hydrophilic-lipophilic-balanced (HLB) sorbent were from Waters Inc. (Milford, MA, USA). Microporous high-density polyethylene Filtroplast tubing (FL10, 2.5 μm filtration grade, 12 mm outer diameter, 2 mm membrane thickness) were purchased from Pall Corp. (Germany) and push-in polyethylene plugs to seal tube samplers were from McMaster-Carr Supply Company (Elmhurst, IL, USA).
Preparation of PE tube samplers.
Microporous PE tubing was cut to a length of 7 cm, filled with 0.6 g of HLB sorbent and capped with push-in polyethylene plugs, creating an exposed surface area of 18.8 cm2 and a surface area to mass sorbent ratio of 31 cm2 g−1. The sorbent-packed sampler was conditioned prior to field deployment via agitation in methanol, 0.1% ammonium hydroxide in methanol, and ultrapure water for 24 hours each, respectively.
Wastewater treatment plant deployments.
The PFAS passive samplers were field-tested in two WWTP final, disinfected, effluents due to their elevated concentrations of PFAS, controlled and consistent conditions (such as flow rate and temperature) and availability of daily composite water samples for PFAS analysis. At Field’s Point, a WWTP servicing 225,000 customers (discharge 160 million L day−1) from Providence, RI, passive samplers were deployed in triplicate for 2, 4, 8, 16, and 29 day periods each. In parallel, triplicate passive samplers were also deployed at a second WWTP, Bucklin Point, serving 160,000 customers (discharge 70 million L day−1, see Figure S1) to test the derived Rs values in a controlled study (as water concentrations were known). Composite water samples (1 L) were collected daily, made up of effluent sub-samples collected every hour for a given 24-hour period and combined. Field blanks were included for both discrete samples and passive samplers.
Estuary field trial deployments.
Previous work suggests a gradient of organic pollutant concentrations across Narragansett Bay (NB), with higher concentrations in the north, near larger population centers (Sacks and Lohmann 2011). At each of the chosen nine NB sites (Figure 1), PE-tube passive samplers were deployed in duplicate anchored to sediment traps, roughly 3 m above the seafloor, from September to October 2017. Surface grab water samples (top 10 cm) were collected at each site during sampler deployment and recovery. Narragansett Bay is partially well mixed, so we typically do not expect major gradients between surface and deeper waters, particularly in the fall.
Figure 1. Map of Narragansett Bay and WWTP Sampling Locations.
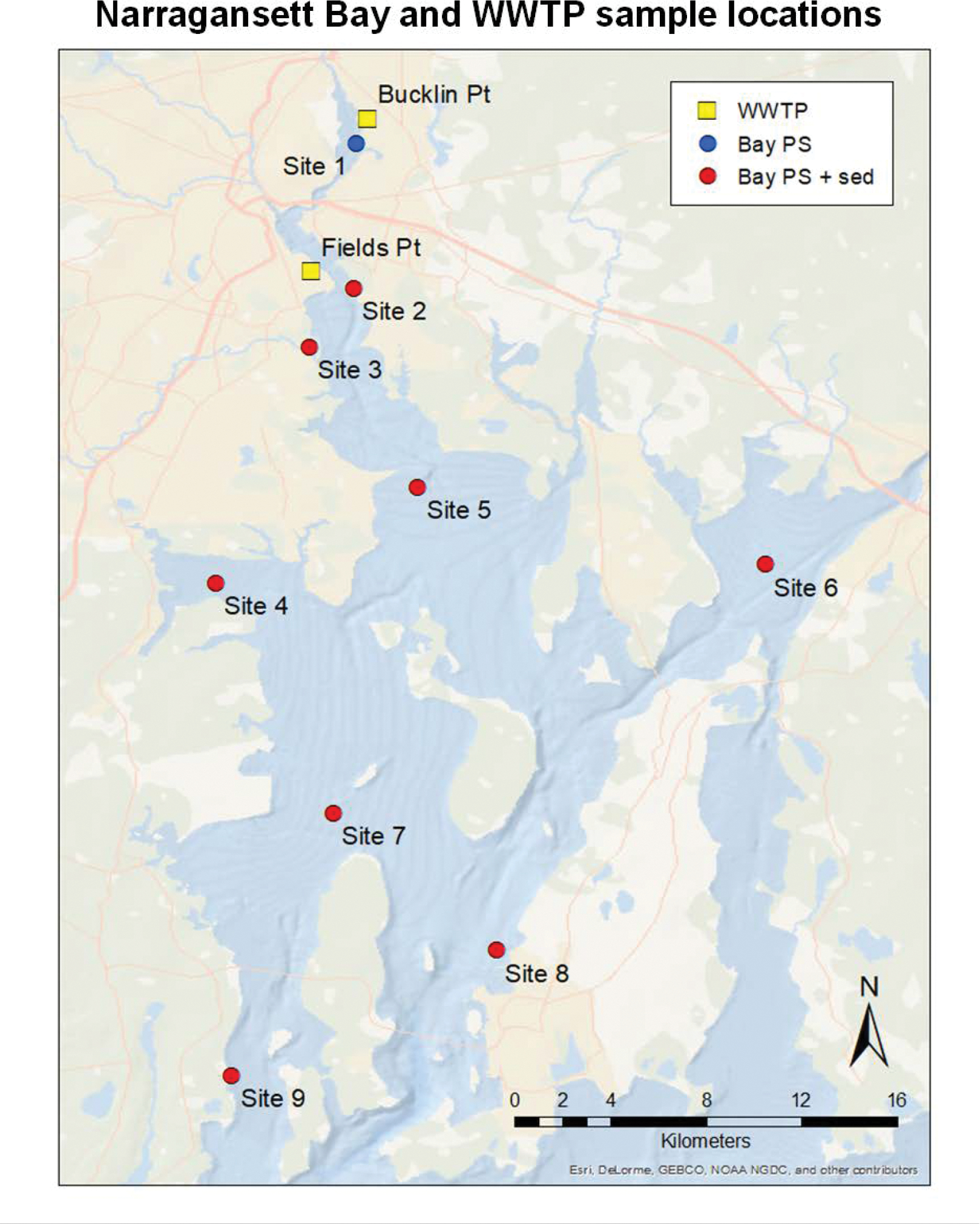
Yellow square: Wastewater treatment plant passive sampler deployments and effluent grabs. Blue Circle: Bay passive sampler deployment and surface water grabs (no sediment trap). Red circle: Bay passive sampler deployment, surface water grabs and sediment trap deployment. Site 1 (Phillipsdale Landing, PDL); Site 2 (Field’s Point Bay, FPB); Site 3 (Pawtuxet River, PR); Site 4 (Greenwich Bay, GWB); Site 5 (Nyatt Point, NP); Site 6 (Mount Hope Bay, MHB); Site 7 (Quonsett Point, QP); Site 8 (Newport, NEW); Site 9 (Bay Campus, BC).
The reproducibility of the passive samplers under different deployment designs was tested in July 2019. Six ‘caged’ and six ‘naked’ passive samplers were deployed near the surface of the Pawtuxet River site at 0.5 m depth for three weeks, and water samples were collected upon deployment and recovery. The naked samplers consisted of bare PE tube zip-tied to the anchored line (the style of samplers deployed at both WWTPs), and the caged samplers were housed in a PE mesh cage to minimize biofouling on the samplers (the style of samplers deployed throughout NB) (See sampler design and photos in Figure S1).
Sample Extraction.
Water samples were stored in 1 L HDPE bottles at −15°C and thawed to room temperature for extraction. Aliquots of 300 to 500 mL were each spiked with 10 ng of mass-labeled surrogates. The water samples were extracted using Oasis WAX solid phase extraction cartridges conditioned and eluted according to previously published methods (Taniyasu et al. 2005; Yamashita et al. 2005).
Passive samplers were scrubbed and rinsed with deionized water to remove algal growth. Intact passive samplers, composed of the PE tube packed with sorbent, were centrifuged three times for three minutes at a relative centrifugal force of 1,300 to remove water. Passive samplers were then transferred to 15 mL polypropylene tubes and 6 mL of methanol was added. The solvent was spiked with mass-labelled surrogates and allowed to equilibrate for 24 hours. The methanol was then decanted to a fresh polypropylene tube; this extraction process was repeated for a total of four solvent extractions per passive sampler, and extracts combined.
Instrumental analysis.
Extracts were evaporated to 250 μL and reconstituted with 750 μL 2 mM ammonium acetate in ultrapure water, yielding a final sample makeup of 3:1 aqueous to organic phases. Reconstituted extracts were centrifuged at 21,3000 × g to remove any remaining particles, and the supernatant transferred to autosampler vials. Samples were analyzed via liquid chromatography- tandem mass spectrometry in negative electrospray ionization (-ESI) mode, as detailed elsewhere (Robuck et al. 2020). Compound identification and quantification was performed using the isotope dilution approach, based on a five-point calibration curve ranging from 0.25–50 ng mL−1.
QA/QC.
Field blanks, process blanks and instrument blanks were included during collection, extraction, and analysis for water and passive samplers. Blank concentrations were at 0–20% of the measured samples; sample concentrations were hence not blank corrected. Method detection limits (MDL) were derived for both water and passive samplers as follows: if no analyte signal was detected in process or field blanks, instrumental detection limits (IDL), representing the analyte concentration with a signal-to-noise ratio of 10, were used as MDL. If an analyte was detected in process and/or field blanks, MDLs were calculated as average value plus 3 times the standard deviation (SD) of the concentrations in all blanks. If the observed levels in field blanks were significantly higher compared to process blanks, only values from field blanks were used for the MDL determination (see SI Table S3).
Thresholds for recoveries were acceptable between 60–130%. Recoveries of mass-labeled internal standard for nine of the most abundant compounds (PFBS, PFHxS, PFOS, PFPeA, PFHxA, PFHpA, PFOA, PFNA, and PFDA) ranged from 60% to 90%. These 9 PFAS were combined to Σ9PFAS. Longer chain and precursor compounds had more variable recoveries (20–300%), probably due to varying sorption artifacts during handling, and were not included in further analysis. Only PFAS with detection frequencies > 50% were considered for the further analysis (Antweiler and Taylor 2008), values < MDL were replaced with ½ MDL (Antweiler 2015; George et al. 2021).
Calculation of sampling rates, Rs.
The general uptake equation for a passive sampler in which compound uptake is kinetically controlled by either the water boundary layer, membrane transport, or a combination of both is:
| (1), |
where Ns is the amount in the passive sampler (ng),
Ksw is the sorbent-water sorption coefficient (L kg−1),
Cw is the average water concentration during sampler deployment (ng L−1),
Rs is the sampling rate (L d−1),
t is time (d), and
ms is the mass of the sorbent used (kg).
Sampling rates (Rs, in L day−1) were calculated using a first-order kinetic model (Kaserzon et al. 2019), assuming samplers were in the linear uptake regime:
| (2). |
Weighted linear regressions were performed in GraphPadPrism® Version 9.3.1.
RESULTS AND DISCUSSION
Reproducibility of PFAS uptake by passive samplers.
Twelve passive samplers were co-deployed at site 9 in NB simultaneously to test the reproducibility of the PE tube samplers during a three week deployment. Passive samplers accumulated Σ9PFAS at an average of 9.1 ± 0.8 ng, consistent across all replicates (Figure S5, Table S16). For seven of the target compounds detected in all passive samplers (PFHxS, PFOS, PFPeA, PFHxA, PFHpA, PFOA, and PFNA), the relative standard deviation between replicates was between 15% and 28% (Figure S6) indicating good reproducibility. A somewhat higher variability was observed for PFBS (48%) and PFDA (36%). For PFDA, this might reflect a combination of lower concentrations and a stronger sorption to surfaces; we are unsure why PFBS varied between replicates. Overall, these passive samplers can be used to reproducibly accumulate dissolved PFAS under ambient conditions.
A comparison was made whether the uptake of PFAS differed between two different sampler deployment configurations–either ‘caged’ or ‘naked’, bare PE tube samplers (Figure S1). The ‘caged’ approach had been used for deployments in NB for additional protection against physical damage and enhanced chance of biofouling. By visual observation, the cage did not reduce biofouling, and there was no significant difference for the accumulated mass by the 2 sampler deployment types for nine dominant PFAS (PFBS, PFHxS, PFOS, PFPeA, PFHxA, PFHpA, PFOA, PFNA, and PFDA, Table S23). The effect of the cage on water-side resistance to uptake was hence negligible, so protective cages can be used in the field if warranted.
PFAS uptake by passive samplers in WWTP effluent.
Concentrations of PFAS.
The Field’s Point WWTP effluent was dominated by PFCAs, primarily PFBA, PFPeA, PFHxA, PFHpA, and PFOA, with those five PFCAs combined at 82 ± 7 ng L−1 (standard error, SE), contributing 68 % to Σ24PFAS in composite water samples. Sulfonates (PFBS, PFPeS, PFHxS, PFHpS, and PFOS) combined to 29 ± 2 ng L−1, 24% of total PFAS (Figure S2, Table S5). Individual neutral precursors were typically < 1 ng L−1; longer chain PFAS (> C9) and 6:2 FTS were each ≤ 3.0 ng L−1. Across the month-long sampling period, effluent PFAS concentrations remained fairly constant, with Σ24PFAS of 120 ± 4 ng L−1 (Table S4).
At the other WWTP, Bucklin Point, PFCAs also contributed most to total PFAS, with the same 5 PFCAs combined at 85 ± 2.0 ng L−1 (Table S8). The 5 PFSA combined to an additional 42 ± 4.9 ng L−1, while the other PFASs contributed little, except for 6:2 FTS at an average of 8.3 ± 1.0 ng L−1.
The effluent concentrations observed here were similar to those reported for other WWTPs in the United States (Schultz et al. 2006; Loganathan et al. 2007) and in Australia (Coggan et al. 2019), with the exception of PFOA. These stable PFAS concentrations (relative standard errors were < 10% for most – ionic- compounds across the 29 days, see Tables S4 and S8) allowed for an ideal calibration of the PFAS uptake by the passive samplers.
The accumulation of PFAS in the PE-tube samplers deployed in the Field’s Point effluent exhibited a linear uptake for the compounds observed (Figure 2, Table S6, Table S19), with passive sampler Σ9PFAS amounts of 7.7, 13, 16, 35, and 66 ng after 2, 4, 8, 16, and 29 day deployments, respectively. Samplers accumulated the largest amount of PFHxA, at 17 ng, with several longer chain compounds and precursors below detection limits (PFUdA, PFDoA, PFTrDa, PFTeDA, PFNS, PFDS, 4:2 FTS, 8:2 FTS). PFCAs comprised the predominant portion of the ∑24PFAS, similar to the concurrently collected effluent grab samples, making up 55 % of the passive sampler load, with sulfonates making up 32 %.
Figure 2. Kinetic uptake over time (days) of PFHxA, PFHpA, PFOA, PFBS, PFHxS, and PFOS* (ng) in Field’s Point WWTP passive samplers.
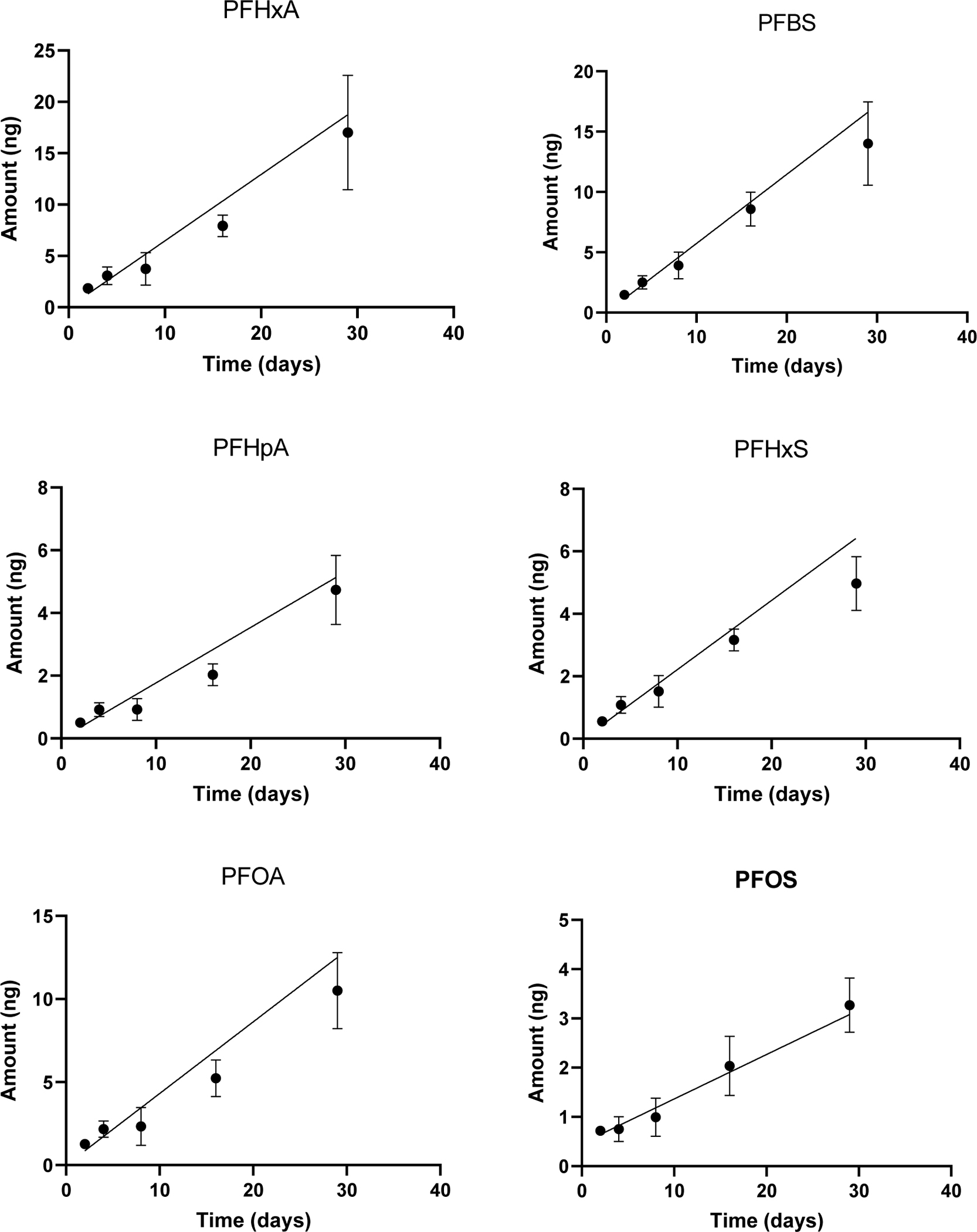
* Displayed are weighted linear fits with forced intercept, except for PFOS where a regression without forced intercept was used. Regression details are given in Table S19.
At Bucklin Point, the accumulation of PFAS in the effluent increased up to 16 days, with little additional accumulation observed beyond 16 days. Accumulation profiles were dominated by PFPeA, PFHxA, PFOA, and PFBS (Table S10).
Sampling Rates.
Sampling rates were derived for passive samplers relative to averaged water concentrations at Field’s Point after 2, 4, 8, 16 and 29 days. For the 29-day results, sampling rates ranged from 10 mL day−1 (PFPeA) to 34 mL day−1 (6:2 FTS), with a mean of 19 ± 7.4 mL day−1 (standard error), see Table 1.
Table 1.
Comparison of 4-week-derived sampling rates (Rs, in mL day−1) derived from the Field’s Point and Buckling Point waste water treatment plants (WWTPs), and across Narragansett Bay.
| Fields Point WWTP | Bucklin Point WWTP | Bay | ||||
|---|---|---|---|---|---|---|
| Rs (mL day−1) | ± SE | Rs (mL day−1) | ± SE | Rs (mL day−1) | ± SE | |
| PFBA | 3.4 | 1.3 | 10 | 7.8 | 13 | 4.2 |
| PFPeA | 10 | 3.6 | 11 | 1.9 | 14 | 4.2 |
| PFHxA | 16 | 5.2 | 14 | 3.1 | 12 | 2.3 |
| PFHpA | 21 | 6.7 | 17 | 4.1 | 16 | 3.3 |
| PFOA | 20 | 7.6 | 20 | 3.2 | 14 | 3.6 |
| PFNA | 15 | 5.4 | 28 | 4.0 | 23 | 7.0 |
| PFDA | 16 | 5.7 | 28 | 4.7 | 28 | 9.9 |
| PFBS | 21 | 9.5 | 16 | 5.2 | 16 | 4.3 |
| PFPeS | 33 | 12.4 | 2.9 | 3.9 | N/A | N/A |
| PFHxS | 25 | 7.9 | 15 | 10 | 26 | 6.6 |
| PFHpS | 17 | 4.2 | 17 | 5.5 | N/A | N/A |
| PFOS | 29 | 14.5 | 17 | 3.6 | 37 | 8.1 |
| 6:2-FTS | 34 | 15.9 | 27 | 15.4 | N/A | N/A |
| N-Me FOSAA | 10 | 8.5 | N/A | N/A | 34 | 8.3 |
| EtFOSAA | 13 | 4.7 | N/A | N/A | 29 | 13 |
| Average | 20 | 8.0 | 18 | 5.4 | 23 | 6.4 |
SE – standard error; N/A – not available
Rs were derived from eq. (2).
WWTP sampling rates were derived relative to daily composite water samples, while Narragansett Bay sampling rates were derived relative to average of two (deployment and retrieval) grab samples.
There was potentially an increase of Rs with the molar mass of the compound (Figure S9), but this was inconclusive. Sampling rates were overall within a factor of 3.5 from smallest to greatest, and, given the measurement uncertainties, no major differences in Rs emerged from these field trials. A general increase of Rs with molecular weight or size was reported earlier (Kaserzon et al. 2019).
Linear uptake.
The duration of linear uptake is an important consideration for integrative passive samplers, such as this PE tube, and helps determine the optimal deployment period. Sampling rates decreased with increasing length of deployments, (see Figure 3) with faster initial uptake. This might be due to some contribution from adsorption and residual PFAS in the passive samplers early on, but stabilized for the 8, 16 and 29-day sampling rates, in particular for the PFCAs, at about 15 mL day−1 (range 3.4–21 mL day−1). It could also indicate some degree of sorbent-controlled kinetics (Booij 2021). Overall, though, the Field’s Point WWTP time series displayed linear uptake for the nine compounds examined by the PE tube samplers over 29 days (Figure 2, Table S19).
Figure 3.
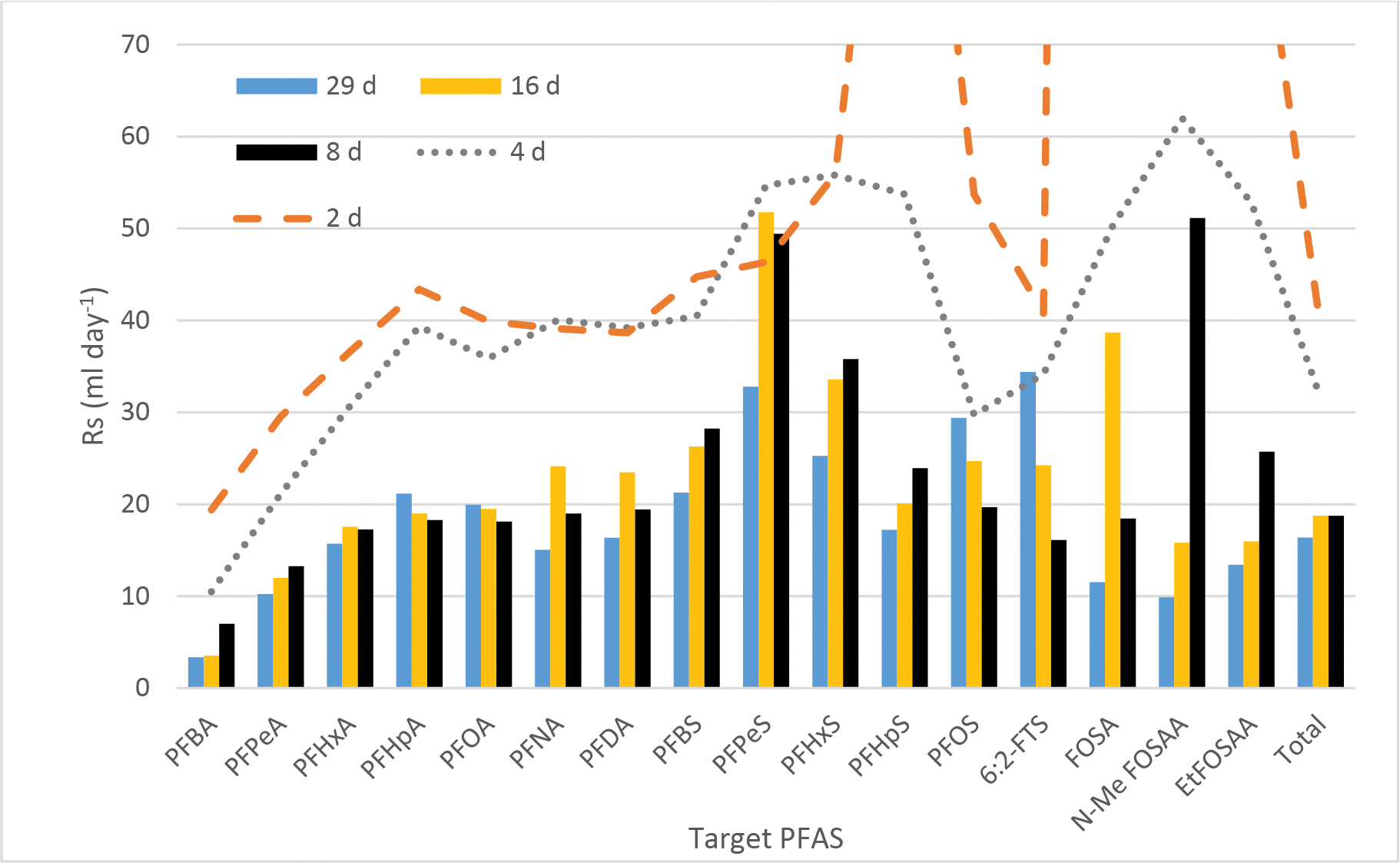
Sampling rates (Rs) from the Field’s Point WWTP after 2, 4, 8, 16 and 29 days (ml d−1).
The time for PFAS to reach 10% of time to equilibrium (t10) in the passive sampler can be derived to determine the maximum deployment period while staying in the linear uptake regime (Lohmann et al. 2012) (equation 3):
| (3), |
where the term equals (1– 0.1) for 10% equilibrium and Ksw were taken from (Urík and Vrana 2019) (Table 2).
Table 2.
Derived times to reach 10% of equilibrium for passive PE tube samplers (filled with 0.6 g HLB)
| Ksw a (mL g−1) | Rs b (mL day−1) | t10 (days) | |
|---|---|---|---|
| PFPA | 5,400 | 10 | 33 |
| PFHxA | 43,000 | 16 | 170 |
| PFHpA | 98,000 | 21 | 290 |
| PFOA | 120000 | 20 | 380 |
| PFNA | 210,000 | 15 | 880 |
| PFDA | 410,000 | 16 | 1,600 |
| PFBS | 16,000 | 21 | 48 |
| PFHxS | 460,000 | 25 | 1,200 |
| PFOS | 460,000 | 29 | 990 |
From (Urík and Vrana 2019)
29-day sampling rates from WWTP effluent (Table 1)
The time to reach equilibrium were derived using equation (3), and ranged from several weeks for PFPeA and PFBS to 4.4 years for PFDA (Table 2). Somewhat similar time scales were estimated for this PE tube sampler in groundwater, with half-times to equilibrium between 120 and 490 days, albeit for a different sorbent (WAX) (Kaserzon et al. 2019).
These calculations indicate that our thirty-day deployment was well within the linear uptake phase for most compounds, with the possible exception of PFBA. However, this is based on the assumption of a well-characterized and well-mixed sorbent without concentration gradients, which might not happen during field deployments. The optimal deployment period is typically constrained by the desired temporal resolution, the need to over-come instrumental detection limits, and an incentive to minimize biofouling. Finding a compromise between these factors would suggest that deployment periods for up to several months could be explored in future sampling campaigns if typical surface water concentrations on seasonal scales were the desired outcome.
Field-validation of Rs.
The Rs values derived for 2, 4, 8, 16 and 29 day deployments at Field’s Point were field-validated in the concurrent Bucklin Point WWTP deployment, where passive PE samplers were also retrieved after 2, 4, 8, 16 and 29 days, while 24 hour composite water samples were collected daily during sampler deployment (Figure 4). The use of the Rs values derived from Field’s Point resulted in an overestimation of the actual dissolved PFAS concentrations at Bucklin Point for the shorter deployment periods, typically by a factor of 2. After 29 days, the overall agreement was very good, with almost all concentrations ratios of passive divided by active sampling within 50% of unity, except for PFBA (ratio of 3), PFNA (1.9), PFDA (1.7) and PFPeS (0.1).
Figure 4: Comparison of passive sampler-derived versus actively measured dissolved PFAS concentrations from 24-hour composite grab sample at Bucklin Point after 29 days.
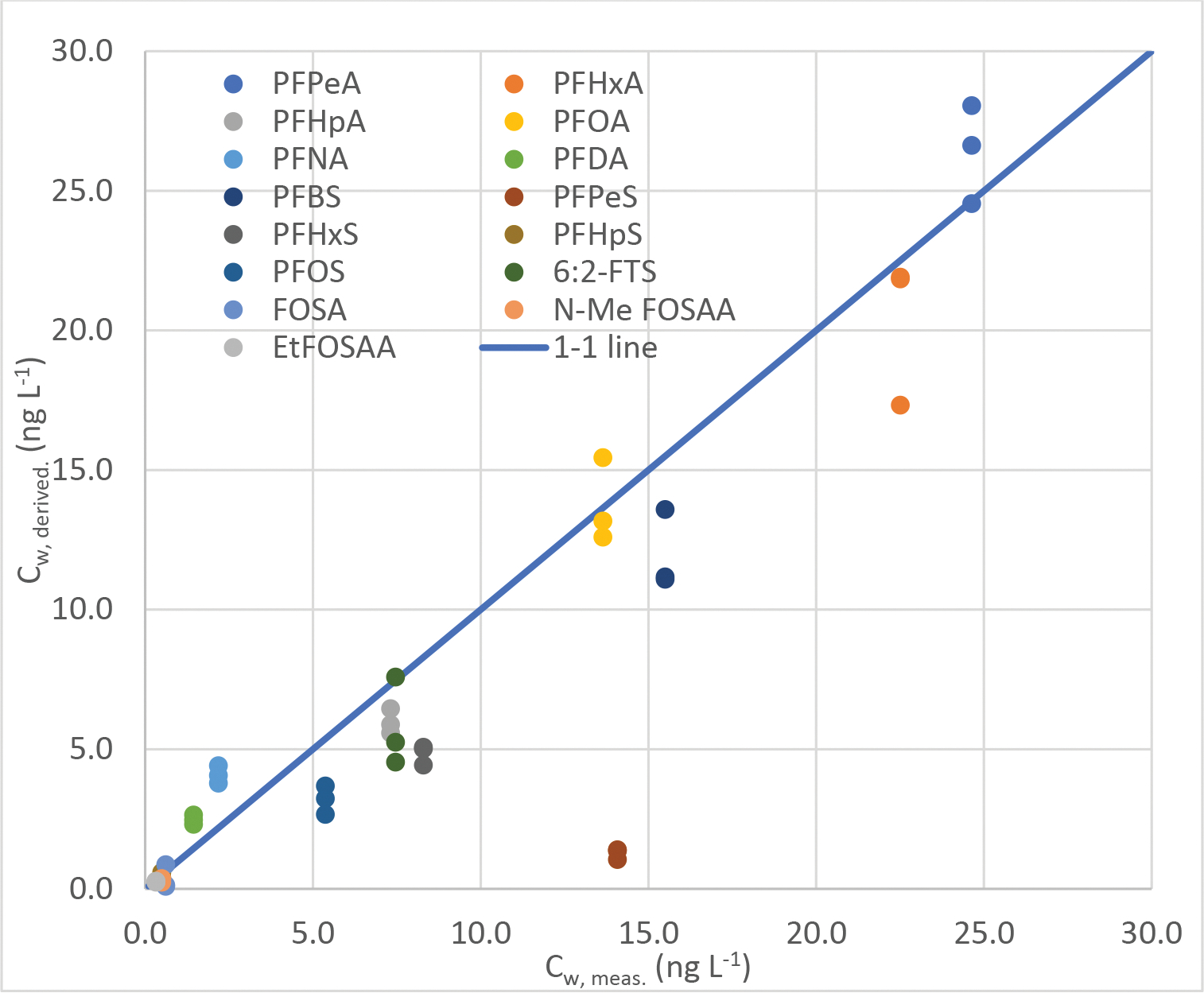
Dissolved concentrations (Cderived) at Bucklin Point were derived from the 3 passive samplers deployed for 29 days using Rs derived from Field’s Point WWTP. Across three replicates at 29 days: Cw,derived = 1.0 Cw,meas; r2 = 0.53; root mean standard error 0.82 (n=48).
A weighted linear regression between observation and prediction was derived (r2=0.53, slope = 1.0 with forced origin, n=48, root mean square error 0.82). Passive sampler-derived concentrations somewhat underestimating observed grab sample results, by an average of 14 %. The two WWTPs serve a considerably different user base (residential vs. industrial) and differ in size and in treatment and disinfection styles, likely resulting in different matrix effects for PFAS in both effluents. Given these differences, we consider this agreement as satisfactory and as validation that passive PE-tube style samplers can be used to derive time-weighted average PFAS concentrations in surface waters.
Narragansett Bay field deployment of PFAS passive samplers.
Surface water concentrations.
Narragansett Bay PFAS surface water concentrations from individual grab water samples ranged from <DL to 102 ng L−1 for Σ9PFAS Figure S3, Table S12). Throughout the Bay, PFCAs dominated over PFSAs, similar to our results for the Field’s Point WWTP effluent (see above). The highest PFAS concentrations were observed in the northern part of NB, near the largest cities (e.g., Providence) and industrial sites; lower concentrations were observed towards the (southern) mouth of the Bay, with a lower human population density and more tidal mixing with the Atlantic Ocean. Average Σ9PFAS in the upper watershed (sites 1–3) were 44 ± 12 ng L−1, compared to 6.4 ± 2.0 ng L−1 for the lower estuary (sites 4–9). This pattern is consistent with those in other urban estuaries, such as San Francisco Bay (Sedlak et al. 2018). The broad range of PFAS concentrations and field conditions created a good opportunity to test how well the PE samplers worked across a range of environmental settings.
Field-derived sampling rates in Narragansett Bay.
The sampling rates for the nine compounds were calculated using the linear uptake model (equation 1), and Rs ranged from 12 to 37 mL day−1, with a mean of 23 ± 6.4 mL day−1 (Table 1). There was good agreement with the Rs values derived after 29-day deployments at both waste water treatment plants, with values within a factor of 2 for all compounds, except for PFBA, N-Me-FOSAA and Et-FOSAA, and no significant differences observed.
PFAS in an estuarine surface water.
Surface water concentrations of PFAS were calculated using the average sampling rate derived from each of the WWTPs for the nine compounds and compared to the grab sample concentration during sampler recovery (Figure S10). There was a general good agreement between the two concentrations in either case (within ± a factor of 3), however some variability remained, given that we observed large fluctuations between the two grab samples at a couple of sites. In such a dynamic environment, with tidal flushing, storm run-off, and variable point sources, these observations further the notion that PFAS concentrations in estuaries fluctuate, and that a long-term passive sampling could be beneficial for obtaining more representative data. Long-term time series PFAS data for NB surface waters show significant fluctuations in concentration and distribution of PFAS (Katz et al. 2022).
Comparison to other PFAS Passive Samplers.
Potential effect of water flow velocity.
The Rs values derived in our field studies (Field’s Point WWTP effluent and NB) were compared with those from Kaserzon et al. (2019) in ground water (Figure S11). Water flow velocities were estimated based on average expected conditions for the three environments and compared to the calculated Rs values. The sampling rate for ground water (3.2 ± 0.6 mL day−1 for a 4 cm tube, or 5.6 ± 1.1 mL day−1 for a 7 cm tube) was lower than the identical sampling rates for the WWTPs (20 ± 8.0 mL day−1 for Fields Point, and 18 ± 5.4 mL day−1 for Bucklin Point) and the Bay (23 ± 6.4 mL day−1). These results might indicate that water flow velocity, and hence boundary layer diffusion, is different for the PE tube sampler in stagnant (groundwater) versus flowing (surface) water. We interpret this that (higher flow) surface waters facilitate a higher sampling rate of the PE tube samplers by reducing the effect of the water boundary layer between the sampler and medium (Fauvelle, Kaserzon, et al. 2017). Once transfer of PFAS through the walls of the PE tubes becomes the rate-limiting step, the uptake rates probably level off. This should be confirmed in future studies.
Comparison to other passive samplers.
Several previous studies have used passive samplers to monitor PFAS in aquatic environments using the POCIS-style sampler while at least one deployed a DGT-style sampler. In a drinking water treatment plant, an average sampling rate of 45 mL day−1 was reported for the classic POCIS-style sampler (Gobelius et al. 2019); correcting for the surface area (46 cm2), the uptake rate was 0.98 mL day−1 cm-2. Hale et al. (2021) relied on a nylon mesh for the standard surface area (46 cm2)), and combined WAX with fluoroflash sorbent to obtain much greater uptake of 20–60 mL day−1 cm-2. A modified POCIS sampler set-up (smaller surface area, greater sorbent amount, and larger pore size in the polyethersulfone membrane than a traditional POCIS in order to maximize sampling rate) was used in an Australian study, and an uptake rate of 17 mL day−1 cm−2 was derived (270 mL day−1 over 16 cm2) (Kaserzon et al. 2012). For the DGT samplers (3.1 cm2), sampling rates of 11–13 mL day−1 were derived, or roughly 4 mL day−1 cm−2 (Wang et al. 2021). Comparatively, the uptake rate produced by the PE tube sampler in NB was 1.0 mL day−1 cm−2 (19 mL day−1 over 19 cm−2). Different sorbents were used in prior studies (WAX vs HLB), and this might affect uptake (Kaserzon et al. 2012). Results from the PE tube sampler were comparable to the classical POCIS configuration, while the PE tube sampler has the benefits of being easily scalable. Side by side deployments of these different passive samplers for PFAS are needed—as was previously performed for herbicides (Hageman et al. 2019)—to determine how the sampling rates and overall performances correlate between these different PFAS passive sampling approaches.
CONCLUSIONS
Overall, these results demonstrate that PE tube samplers can be used to derive PFAS concentrations in WWTP effluents, and more dynamic surface waters such as estuaries, and provide a suitable long-term monitoring tool of these compounds. The PE tube samplers were shown to be reproducible within 20–30%. Typical sampling rates were on the order of 20 mL day−1 for most PFCAs based on calibration within a WWTP effluent. Very similar sampling rates were derived for a 29-day estuarine field deployment, implying that these Rs values are reasonable approximations for field deployments. Applying these Rs values in a different WWTP effluent resulted in very good predictions, mostly within 50% of their measured dissolved concentrations in the effluent. The field deployments highlighted the dynamic nature of PFAS concentrations in an estuary; adopting passive samplers, such as the PE tubes, would enable ready assessment of typical ambient concentrations and identify the importance of various known and unknown sources of PFAS in urban estuaries. Moving forward, controlled laboratory experiments with consistent PFAS concentrations and varying environmental conditions would be beneficial to calibrate the sampling rates of the samplers across a wide range of environmental conditions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Narragansett Bay Commission and URI’s Marine Ecosystems Research Lab for logistical field support. We acknowledge funding from the NIEHS Superfund Research Program (P42ES027706), SERDP (ER12–1280) and the RI STAC program. The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the authors and do not necessarily represent the views or the policies of the U.S. Environmental Protection Agency.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION
The Supporting Information is available free of charge at https://setac.onlinelibrary.wiley.com/ doi-XXX. The SI contains details on further standard and QA/QC measures, tables of all measured concentrations and percent recoveries, and additional figures.
Data Availability Statement
Data pertaining to this manuscript will be deposited in the Open Science Framework. All relevant data will also be available in the supplemental data.
REFERENCES
- Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE. 2004. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem. 23(7):1640–1648. [DOI] [PubMed] [Google Scholar]
- Antweiler RC. 2015. Evaluation of Statistical Treatments of Left-Censored Environmental Data Using Coincident Uncensored Data Sets. II. Group Comparisons. Env Sci Technol. 49:13439–13446. doi: 10.1021/acs.est.5b02385. [DOI] [PubMed] [Google Scholar]
- Antweiler RC, Taylor HE. 2008. Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environ Sci Technol. 42(10):3732–3738. doi: 10.1021/es071301c. [DOI] [PubMed] [Google Scholar]
- Booij K 2021. Passive Sampler Exchange Kinetics in Large and Small Water Volumes Under Mixed Rate Control by Sorbent and Water Boundary Layer. Environ Toxicol Chem. 40(5):1241–1254. doi: 10.1002/etc.4989. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Soracá DM, Arra-Ríos RO, Mueller JF, Hawker DW, Kaserzon SL. 2020. In-situ calibration of a microporous polyethylene passive sampling device with polar organic micropollutants in the Chillan River, central Chile. Environ Res. 188:109738. doi:doi: 10.1016/j.envres.2020.109738. [DOI] [PubMed] [Google Scholar]
- Challis JK, Hanson ML, Wong CS. 2016. Development and Calibration of an Organic-Diffusive Gradients in Thin Films Aquatic Passive Sampler for a Diverse Suite of Polar Organic Contaminants. Anal Chem. 88(21):10583–10591. doi: 10.1021/acs.analchem.6b02749. [DOI] [PubMed] [Google Scholar]
- Coggan TL, Moodie D, Kolobaric A, Szabo D, Shimeta J, Crosbie ND, Lee E, Fernandes M, Clarke BO. 2019. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen Australian wastewater treatment plants (WWTPs). Heliyon. 5(8):e02316. doi: 10.1016/j.heliyon.2019.e02316. https://www.sciencedirect.com/science/article/pii/S2405844019359766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S, Matsuura Y, Vermeirssen ELM. 2019. Mechanistic Model Describing the Uptake of Chemicals by Aquatic Integrative Samplers: Comparison to Data and Implications for Improved Sampler Configurations. Environ Sci Technol. 53(3):1482–1489. doi: 10.1021/acs.est.8b06225. [DOI] [PubMed] [Google Scholar]
- Fang Z, Li Yuan, Li Yanying, Yang D, Zhang H, Jones KC, Gu C, Luo J. 2021. Development and Applications of Novel DGT Passive Samplers for Measuring 12 Per- and Polyfluoroalkyl Substances in Natural Waters and Wastewaters. Env Sci Technol.: 10.1021/acs.est.0c08092. doi: 10.1021/acs.est.0c08092. [DOI] [PubMed] [Google Scholar]
- Fauvelle V, Kaserzon SL, Montero N, Lissalde S, Allan IJ, Mills G, Mazzella N, Mueller JF, Booij K. 2017. Dealing with Flow Effects on the Uptake of Polar Compounds by Passive Samplers. Environ Sci Technol. 51(5):2536–2537. doi: 10.1021/acs.est.7b00558. [DOI] [PubMed] [Google Scholar]
- Fauvelle V, Montero N, Mueller JF, Banks A, Mazzella N, Kaserzon SL. 2017. Glyphosate and AMPA passive sampling in freshwater using a microporous polyethylene diffusion sampler. Chemosphere. 188:241–248. doi: 10.1016/J.CHEMOSPHERE.2017.08.013. [DOI] [PubMed] [Google Scholar]
- George BJ, Gains-germain L, Broms K, Black K, Furman M, Hays MD, Thomas KW, Simmons JE. 2021. Censoring Trace-Level Environmental Data: Statistical Analysis Considerations to Limit Bias. Env Sci Technol. 55:3786–3795. doi: 10.1021/acs.est.0c02256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh U, Kane Driscoll S, Burgess RM, Jonker MTO, Reible D, Gobas F, Choi Y, Apitz SE, Maruya KA, Gala WR, et al. 2014. Passive sampling methods for contaminated sediments: Practical guidance for selection, calibration, and implementation. Integr Environ Assess Manag. 10(2):210–223. doi: 10.1002/ieam.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobelius L, Persson C, Wiberg K, Ahrens L. 2019. Calibration and application of passive sampling for per- and polyfluoroalkyl substances in a drinking water treatment plant. J Hazard Mater. 362:230–237. doi: 10.1016/J.JHAZMAT.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Górecki T, Namieśnik J. 2002. Passive sampling. TrAC Trends Anal Chem. 21(4):276–291. doi: 10.1016/S0165-9936(02)00407-7. [DOI] [Google Scholar]
- Guan DX, Li YQ, Yu NY, Yu GH, Wei S, Zhang H, Davison W, Cui XY, Ma LQ, Luo J. 2018. In situ measurement of perfluoroalkyl substances in aquatic systems using diffusive gradients in thin-films technique. Water Res. 144(October 2015):162–171. doi: 10.1016/j.watres.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Hageman KJ, Aebig CHF, Luong KH, Kaserzon SL, Wong CS, Reeks T, Greenwood M, Macaulay S, Matthaei CD. 2019. Current-use pesticides in New Zealand streams: Comparing results from grab samples and three types of passive samplers. Environ Pollut. 254:112973. doi: 10.1016/j.envpol.2019.112973. https://www.sciencedirect.com/science/article/pii/S0269749118358925. [DOI] [PubMed] [Google Scholar]
- Hale SE, Canivet B, Rundberget T, Langberg HA. 2021. Using Passive Samplers to Track Per- and Polyfluoroalkyl Substances (PFAS) Emissions From the Paper Industry : Laboratory Calibration and Field Verification. Front Environ Sci. 9(December):1–11. doi: 10.3389/fenvs.2021.796026. [DOI] [Google Scholar]
- Kaserzon SL, Hawker DW, Booij K, Brien DSO, Kennedy K, Vermeirssen ELM, Mueller JF. 2014. Passive sampling of perfluorinated chemicals in water : In-situ calibration. Environ Pollut. 186:98–103. doi: 10.1016/j.envpol.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Kaserzon SL, Kennedy K, Hawker DW, Thompson J, Carter S, Roach AC, Booij K, Mueller JF. 2012. Development and Calibration of a Passive Sampler for Perfluorinated Alkyl Carboxylates and Sulfonates in Water. Environ Sci Technol Sci Technol. 46(9):4985–4993. doi: 10.1021/es300593a. [DOI] [PubMed] [Google Scholar]
- Kaserzon SL, Vijayasarathy S, Bräunig J, Mueller L, Hawker DW, Thomas KV., Mueller JF. 2019. Calibration and validation of a novel passive sampling device for the time integrative monitoring of per- and polyfluoroalkyl substances (PFASs) and precursors in contaminated groundwater. J Hazard Mater. 366(December 2018):423–431. doi: 10.1016/j.jhazmat.2018.12.010. 10.1016/j.jhazmat.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Katz DR, Sullivan JC, Rosa K, Gardner CL, Robuck AR, Lohmann R, Kincaid C, Cantwell MG. 2022. Transport and fate of aqueous film forming foam in an urban estuary. Environ Pollut.:118963. doi: 10.1016/j.envpol.2022.118963. https://www.sciencedirect.com/science/article/pii/S0269749122001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai FY, Rauert C, Gobelius L, Ahrens L. 2019. A critical review on passive sampling in air and water for per- and polyfluoroalkyl substances (PFASs). TrAC - Trends Anal Chem. 121:115311. doi: 10.1016/j.trac.2018.11.009. [DOI] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated Compounds : Past, Present, and Future. Env Sci Technol. 45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Loganathan BG, Sajwan KS, Sinclair E, Senthil Kumar K, Kannan K. 2007. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 41(20):4611–4620. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Booij K, Smedes F, Vrana B. 2012. Use of passive sampling devices for monitoring and compliance checking of POP concentrations in water. Environ Sci Pollut Res. 19(6):1885–1895. doi: 10.1007/s11356-012-0748-9. [DOI] [PubMed] [Google Scholar]
- McKay S, Tscharke B, Hawker D, Thompson K, O’Brien J, Mueller JF, Kaserzon S. 2020. Calibration and validation of a microporous polyethylene passive sampler for quantitative estimation of illicit drug and pharmaceutical and personal care product (PPCP) concentrations in wastewater influent. Sci Total Environ. 704:135891. doi: 10.1016/j.scitotenv.2019.135891. https://www.sciencedirect.com/science/article/pii/S0048969719358863. [DOI] [PubMed] [Google Scholar]
- Möller A, Ahrens L, Surm R, Westerveld J, Van Der Wielen F, Ebinghaus R, De Voogt P. 2010. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ Pollut. 158(10):3243–3250. doi: 10.1016/j.envpol.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Moody CA, Field JA. 2000. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ Sci Technol. 34(18):3864–3870. doi: 10.1021/es991359u. [DOI] [Google Scholar]
- Munoz G, Budzinski H, Labadie P. 2017. Influence of Environmental Factors on the Fate of Legacy and Emerging Per- and Polyfluoroalkyl Substances along the Salinity/Turbidity Gradient of a Macrotidal Estuary. Environ Sci Technol. 51(21):12347–12357. doi: 10.1021/acs.est.7b03626. 10.1021/acs.est.7b03626. [DOI] [PubMed] [Google Scholar]
- Nixon SW, Buckley BA. 2002. “A strikingly rich zone”-nutrient enrichment and secondary production in coastal marine ecosystems. Estuaries. 25(4):782–796. doi: 10.1007/BF02804905. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0036701197&doi=10.1007%2FBF02804905&partnerID=40&md5=5440ba4cf8d4109f81f57d26292f4f56. [DOI] [Google Scholar]
- Pilson MEQ. 1985. On the residence time of water in Narragansett Bay. Estuaries. 8(1):2–14. doi: 10.2307/1352116. [DOI] [Google Scholar]
- Robuck A, Cantwell M, McCord J, Addison L, Pfohl M, Strynar M, McKinney R, Katz D, Wiley D, Lohmann R. 2020. Legacy and Novel Per- and Polyfluoroalkyl Substances (PFAS) in Juvenile Seabirds from the US Atlantic Coast. Env Sci Technol. 54:12938–12948. doi:DOI: 10.1021/acs.est.0c01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle B, Pickard H, LeBlanc D, Tokranov A, Thackray C, Hu XC, Vecitis CD, Sunderland EM. 2021. Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine. Env Sci Technol.:accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks VP, Lohmann R. 2011. Development and use of polyethylene passive samplers to detect triclosans and alkylphenols in an Urban estuary. Environ Sci Technol. 45(6). doi: 10.1021/es1040865. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Ackerman JM, Rudel RA. 2016. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci Total Environ. 547:470–481. doi: 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field JA. 2006. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ Sci Technol. 40(23):7350–7357. doi: 10.1021/es061025m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak M, Sutton R, Wong A, Lin D. 2018. Per and Polyfluoroalkyl Substances (PFASs) in San Francisco Bay: Synthesis and Strategy. RMP Contribution No. 867. Richmond CA. https://www.sfei.org/sites/default/files/biblio_files/PFAS Synthesis and Strategy.pdf.
- Sharp JH, Pennock JR, Church TM, Tramontano JM, Cifuentes LA. 1984. THE ESTUARINE INTERACTION OF NUTRIENTS, ORGANICS, AND METALS: A CASE STUDY IN THE DELAWARE ESTUARY. In: The Estuary As a Filter. [Google Scholar]
- Taniyasu S, Kannan K, Man KS, Gulkowska A, Sinclair E, Okazawa T, Yamashita N. 2005. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A. 1093(1–2):89–97. doi: 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Urík J, Vrana B. 2019. An improved design of a passive sampler for polar organic compounds based on diffusion in agarose hydrogel. Environ Sci Pollut Res.:15273–15284. doi: 10.1007/s11356-019-04843-6. [DOI] [PubMed] [Google Scholar]
- US EPA. 2020. PFAS Master List of PFAS Substances (Version 2). [accessed 2021 Jan 24]. https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster.
- Vasconcelos RP, Reis-Santos P, Costa MJ, Cabral HN. 2011. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecol Indic. 11(5):1123–1133. doi: 10.1016/j.ecolind.2010.12.012. [DOI] [Google Scholar]
- Verhagen R, Tscharke BJ, Clokey J, Gerber C, Ghetia M, Kaserzon SL, Thomas KV, Mueller JF. 2021. Multisite Calibration of a Microporous Polyethylene Tube Passive Sampler for Quantifying Drugs in Wastewater. Environ Sci Technol. 55(19):12922–12929. doi: 10.1021/acs.est.1c02900. 10.1021/acs.est.1c02900. [DOI] [PubMed] [Google Scholar]
- Vrana B, Allan IJ, Greenwood R, Mills GA, Dominiak E, Svensson K, Knutsson J, Morrison G. 2005. Passive sampling techniques for monitoring pollutants in water. TrAC Trends Anal Chem. 24(10):845–868. [Google Scholar]
- Wang P, Challis JK, Luong KH, Vera TC, Wong CS. 2021. Calibration of organic-diffusive gradients in thin films (o-DGT) passive samplers for perfluorinated alkyl acids in water. Chemosphere. 263:128325. doi: 10.1016/j.chemosphere.2020.128325. https://www.sciencedirect.com/science/article/pii/S0045653520325200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dewitt JC, Higgins CP, Cousins IT. 2017. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Env Sci Technol. 51:2508–2518. doi: 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T. 2005. A global survey of perfluorinated acids in oceans. Mar Pollut Bull. 51(8–12):658–668. doi: 10.1016/j.marpolbul.2005.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data pertaining to this manuscript will be deposited in the Open Science Framework. All relevant data will also be available in the supplemental data.


