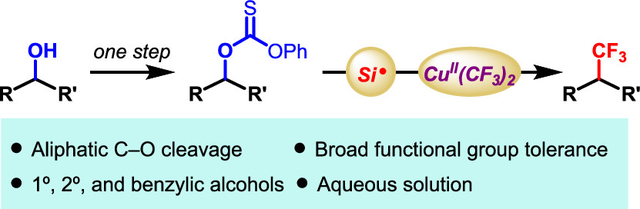
Abstract
The site-selective trifluoromethylation of aliphatic systems remains an important challenge. This work describes a light-driven, copper-mediated trifluoromethylation of O-alkyl thiocarbonates. The reaction provides broad functional group tolerance (e.g., alkyne, alkene, phenol, free alcohol, electron-rich and -deficient arenes), thereby offering orthogonality and practicality for trifluoromethylation. A radical organometallic mechanism is proposed.
Graphical Abstract
The installation of trifluoromethyl groups into organic molecules remains an important goal in synthetic organic chemistry. Trifluoromethyl moieties can impart unique properties to pendant molecules—including high electro-negativity, lipophilicity, and metabolic stability—which has driven their use in the fields of pharmaceuticals, agrosciences, and materials.1 For decades, efforts to develop practical trifluoromethylation methods have led to remarkable progress.2 More recently, several reports describing the formation of the C(sp3)–CF3 bond have emerged using nucleophilic trifluoromethylation,3 electrophilic trifluoromethylation,4 and trifluoromethyl radical addition to olefins.2b,5 However, the use of highly specific electrophiles, nucleophiles, and terminal alkenes has partly limited their practical utility. In contrast, the selective trifluoromethylation of unactivated alkyl groups remains highly underdeveloped when compared to that of activated alkyl counterparts.6 Recent work on the trifluoromethylation of alkyl radical intermediates offers a more direct approach to a wider range of trifluoromethylated compounds.7 Since alkyl radicals can be generated from readily available aliphatic acids,7d,e halides,7a–c or in some cases, C(sp3)–H bonds,7f–j this approach offers great diversity in potential starting materials (Scheme 1a).
Scheme 1.
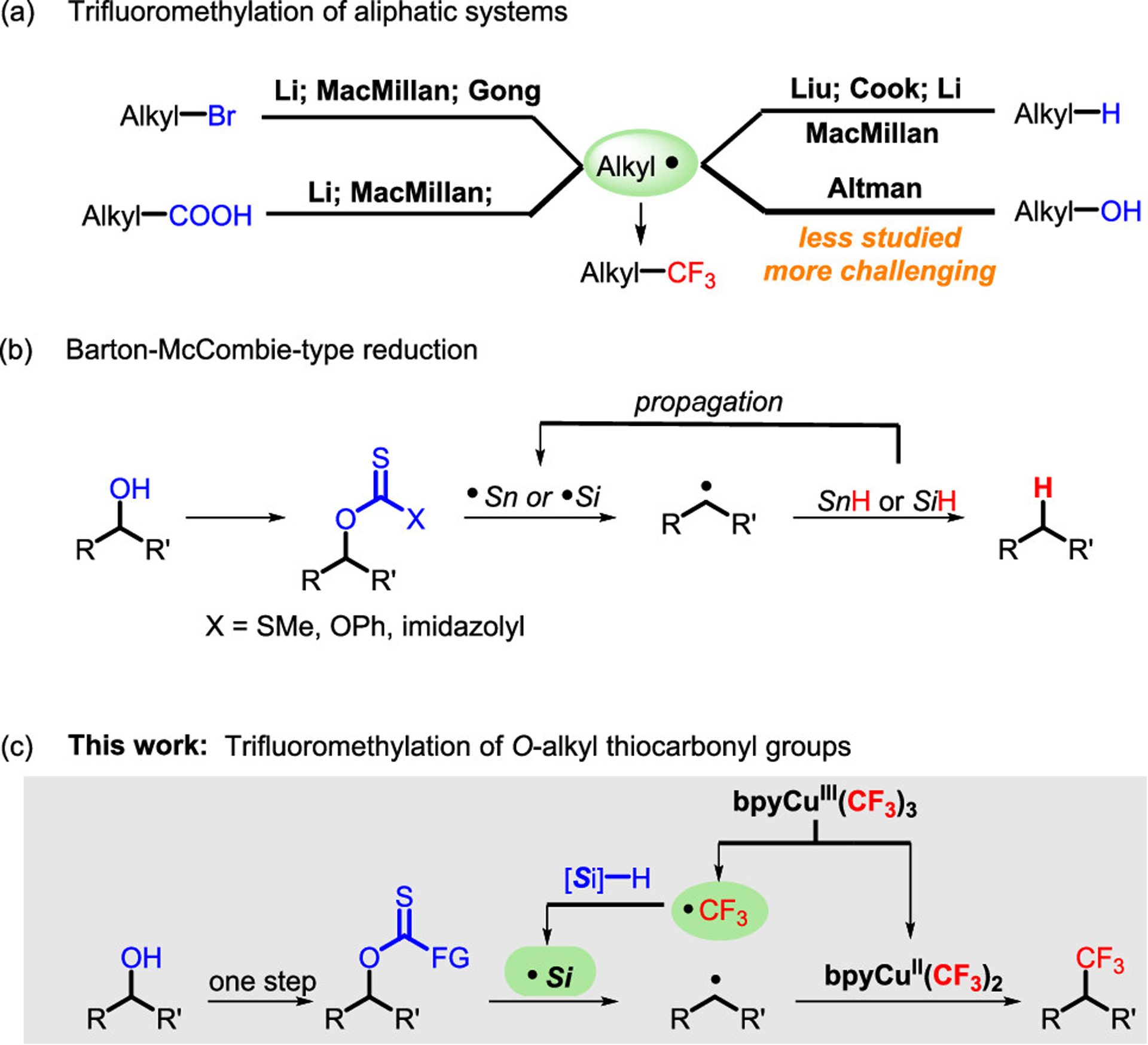
(a) Trifluoromethylation of Aliphatic Systems. (b) Barton–McCombie-Type Reduction. (c) Our Proposed Trifluoromethylation of O-Alkyl Thiocarbonyl Groups
Alcohols represent one of the most abundant functional groups in both natural and unnatural organic molecules. Scission of alcohol-based C–O bonds offers an intriguing but challenging approach to small molecule diversification.8 Interestingly, the formation of alkyl radicals from alcohol derivatives provides a promising opportunity for realizing this approach.9 Work toward this goal has revealed interesting new methods with creative approaches to this problem. For example, Ni- or photoredox-catalyzed radical coupling of oxalates, developed by Gong,10 Overman,11 and MacMillan,12 represent a powerful platform for the transformation of alcohols. Additionally, xanthates, thiocarbonates, and thiocarbamates—bench stable and readily available alcohol derivatives—have a rich history in Barton–McCombie-type reactions to generate carbon-centered radicals (Scheme 1b).9a,13 Recent work by Altman has demonstrated that xanthates can be applied for Cu0-mediated C–CF3 bond formation.14 Molander15 and Rousseaux16 reported the cross coupling of xanthates or thiocarbamates to generate a C(sp3)–C(sp2) bond under Ni or Ni/photoredox dual catalysis. However, these reactions only proceed with benzylic-activated xanthates or thiocarbamates.
Direct trifluoromethylation of unactivated thiocarbonyl systems remains an important goal.17 In a search for a general and operationally simple methodology, we envisioned that bpyCu(CF3)3 (i.e., Grushin’s reagent)18 could be used for the trifluoromethylation of O-alkyl xanthates or thiocarbonates. The homolysis of bpyCu(CF3)3 could generate CF3-based radical and a highly reactive CuII(CF3)2 species.7a,g,19 The CF3-based radical could react with a suitable silane to generate an Si-based radical for the subsequent reaction with thiocarbonates. The coupling reaction between the newly formed alkyl radical and CuII(CF3)2 species finally gives the trifluoromethylated product.20 At the outset, it was unclear whether the coupling process would outcompete the direct reduction of the alkyl radical by silane.
With the above design in mind, we began the investigation with the trifluoromethylation of unactivated secondary thiocarbonate 1a. After extensive examination of the reaction conditions, we identified that visible-light irradiation of bpyCu(CF3)3/1a in the presence of super silane (TTMSS) and Na2S2O8 provided the trifluoromethylation product 3a in 86% yield (Table 1, entry 1). Surprisingly, no desired product was detected when nBu3SnH was used, despite its wide application in Barton–McCombie-type reactions (Table 1, entry 2). Triethylsilane and triisopropylsilane were also inferior to super silane (Table 1, entries 3–4). The greater activity of TTMSS may be due more facile reaction with trifluoromethyl radical to produce the active Si-based radical. Switching the copper complex to 2b decreased the yield, but no product was formed when complex 2c or 2d was employed (Table 1, entries 5–7). These results suggest that a Cu(I)CF3 species was not the active CF3 source for the reaction even under the reaction conditions. Further investigation revealed that the Si-based radical activated the thiocarbonate preferentially to the CF3 radical (Table 1, entry 8). Sodium persulfate (Na2S2O8) was found to significantly increase the reaction yield (Table 1, entry 9). The persulfate likely serves as a complementary radical initiator for the generation of silyl radical. Interestingly, water proved beneficial in the reaction (Table 1, entry 10). This could be due to the improved solubility of the persulfate in aqueous solvent, and the potential of lowering the reductive elimination energy barrier by aqua complex.7g The blue light serves to homolyze 2a to give CF3 radical and Cu(II) species (Table 1, entry 11). In addition, the use of 1 equiv thiocarbonate or excess 2a only gave the desired product in moderate yield respectively (Table 1, entries 13 and 14).
Table 1.
Optimization of Reaction Conditionsa
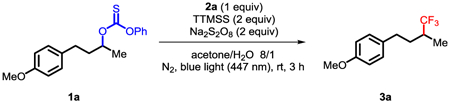
| ||
|---|---|---|
| entry | deviation | yieldb (%) |
| 1 | none | 86 |
| 2 | nBu3SnH instead of TTMSS | 4 |
| 3 | Et3SiH instead of TTMSS | 20 |
| 4 | iPr3SiH instead of TTMSS | 40 |
| 5 | 2b instead of 2a | 74 |
| 6 | 2c instead of 2a | nd |
| 7 | 2d instead of 2a | nd |
| 8 | no TTMSS | nd |
| 9 | no Na2S2O8 | 15 |
| 10 | no H2O | 18 |
| 11 | no hν | nd |
| 12 | under air | 10 |
| 13 | 1 equiv of 1a | 57 |
| 14 | 1 equiv of 1a, 2 equiv of 2a | 40c |

| ||
Performed with 1a (0.1 mmol, 2 equiv or 0.05 mmol, 1 equiv), 2a (0.05 mmol, 1 equiv or 0.1 mmol, 2 equiv) in solvent (0.056 M) for 3 h at room temperature. nd = not detected.
Yields were based on the Cu reagent and reported on the basis of 19F NMR analysis using PhCF3 as an internal standard.
Yield was based on 1a
With the above optimized conditions in hand, we next evaluated a range of unactivated thiocarbonates (Scheme 2a). The electron-rich arenes were well tolerated in this protocol, thus giving the trifluoromethylation products 3a, 3b, and 3f in moderate-to-high yields. For the substrates with different amides and phthalimide, the trifluoromethylation also delivered products 3c–3e in good yields. The reaction was also compatible with molecules bearing alkyne (1g) or conjugated and terminal alkenes (1h, 1i), thereby highlighting the selectivity of the underlying radical reactions. In spite of numerous reports detailing the reaction of unsaturated C–C bonds and arenes with CF3 radical,2,5 such functional groups were well tolerated in the reaction. Under these reaction conditions, the CF3 radical generated from Grushin’s reagent is quenched rapidly by TTMSS to release Si radical, which avoids reaction of the CF3 radical with arenes or other units of unsaturation. In competition experiments with primary and secondary thiocarbonates (e.g., 1j), the secondary thiocarbonate was selectively trifluoromethylated with only a trace amount of the primary trifluoromethylated product. The reaction tolerated tosylated (1k) and silylated (1l) substrates, with selective trifluoromethylation in high yields. Heteroarenes were well tolerated in the reaction, giving the product 3m in 70% yield. Cyclic seven- and six-membered rings were suitable, which delivered the trifluoromethylation products 3n–3q in moderate yields. In addition, trifluoromethylation of thymidine derivative provided CF3 analogue 3r in 39% yield and excellent diastereoselectivity, which clearly shows the potential of facile trifluoromethylation of complex molecules. Primary thiocarbonates 1s and 1t were subjected to the trifluoromethylation, producing the products in 33 and 28% yields, respectively. We attribute the low yields of primary substrates to likely slower initiation and higher energy carbon-based radicals relative to secondary substrates.
Scheme 2.
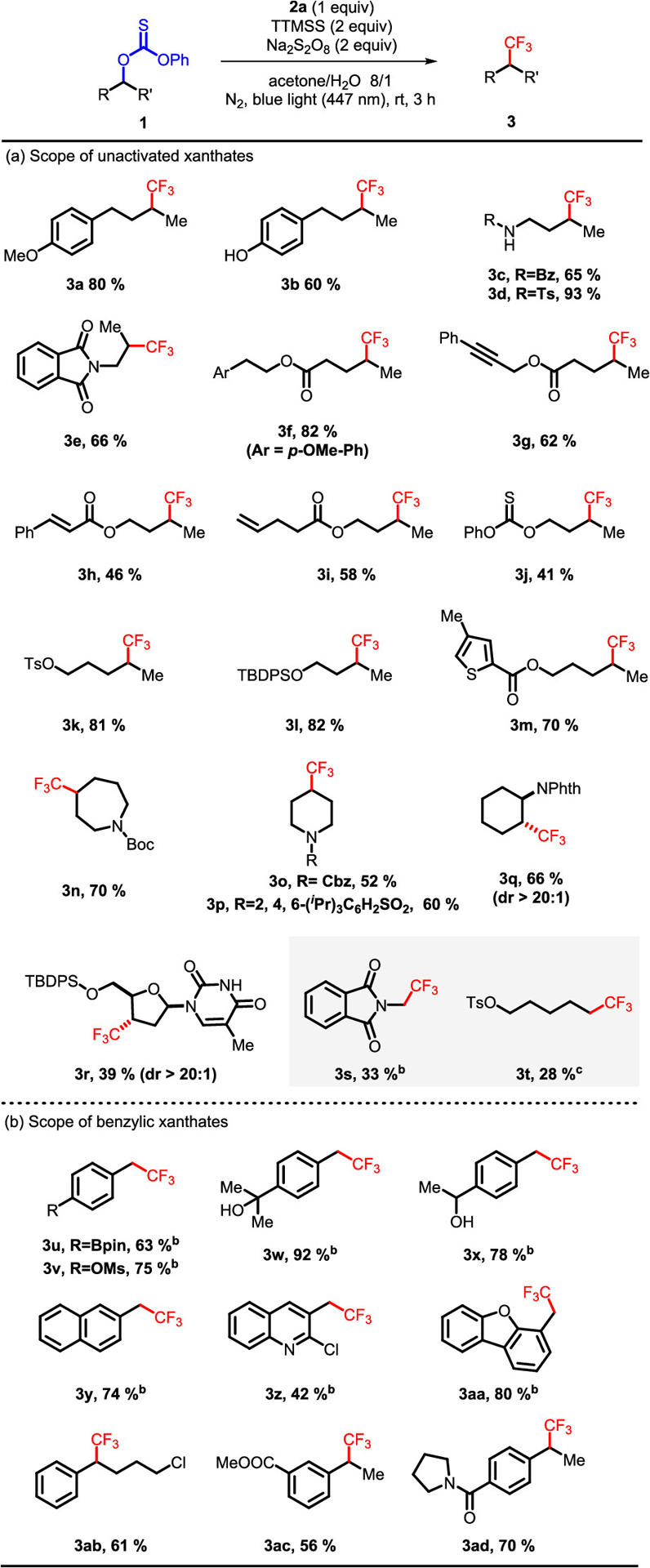
Scope of Thiocarbonatesa
aUnless otherwise specified, all reactions were performed with thiocarbonate 1 (0.4 mmol, 2 equiv), 2a (0.2 mmol, 1 equiv), Na2S2O8 (0.4 mmol, 2 equiv), and TTMSS (0.4 mmol, 2 equiv) in acetone/H2O 8/1 (0.056 M) for 3 h at room temperature. Isolated yields. bThiocarbonate 1 (0.2 mmol, 1 equiv), Na2S2O8 (0.2 mmol, 1 equiv), and TTMSS (0.2 mmol, 1 equiv) were used. cThiocarbonate 1 (0.2 mmol, 1 equiv) was used; 2-butanone was used instead of acetone; 70 °C; reaction time was 6 h.
Next, benzylic thiocarbonates were surveyed in the reaction (Scheme 2b). For substrates derived from primary benzylic alcohols, thiocarbonates with a range of functional groups provided moderate to good yields of the products. The tolerance of Bpin (1u), OMs (1v), alcohols (1w, 1x), electron-deficient (1y, 1z) and -rich arenes (1aa) implies great potential for this methodology in diverse contexts. In particular, the tolerance of secondary and tertiary alcohols makes it more competitive than the trifluoromethylation of alkyl halides, since the halides are usually derived from alcohols and additional steps would be required to protect the free alcohols. For thiocarbonates derived from secondary benzylic alcohols, substrates bearing chloride, ester, and amide afforded the secondary trifluoromethylated products (3ab–3ad) in moderate yields.
To explore the mechanism, we performed a series of straightforward experiments (Scheme 3). First, when we subjected thiocarbonate 4 to the trifluoromethylation reaction, cyclization product 5 was obtained in 53% yield, wherein the linear trifluoromethylation product 5′ was not detected in the reaction (Scheme 3a), thereby supporting a secondary carbon-based radical intermediate. Additionally, significant quantities of fluoroform is produced in the reaction. Treatment of thiocarbonate 1d with deuterium-labeled silane (TMS3SiD) under the standard conditions gave primarily DCF3 (Scheme 3b), suggesting that the majority of CF3 radical is quenched by silane (see the SI). Furthermore, NMR monitoring of the trifluoromethylation of model substrate 1a shows that the production of 3a is directly related to the amount/concentration of Grushin’s reagent 2a (Scheme 3c). During the first 30 min of irradiation, the yield of 3a reached 81% with complete consumption of the Grushin’s reagent. After 30 min, the rate of product formation decreases significantly, with only 5% additional yield achieved after 2.5 h (see the Supporting Information for details).
Scheme 3.
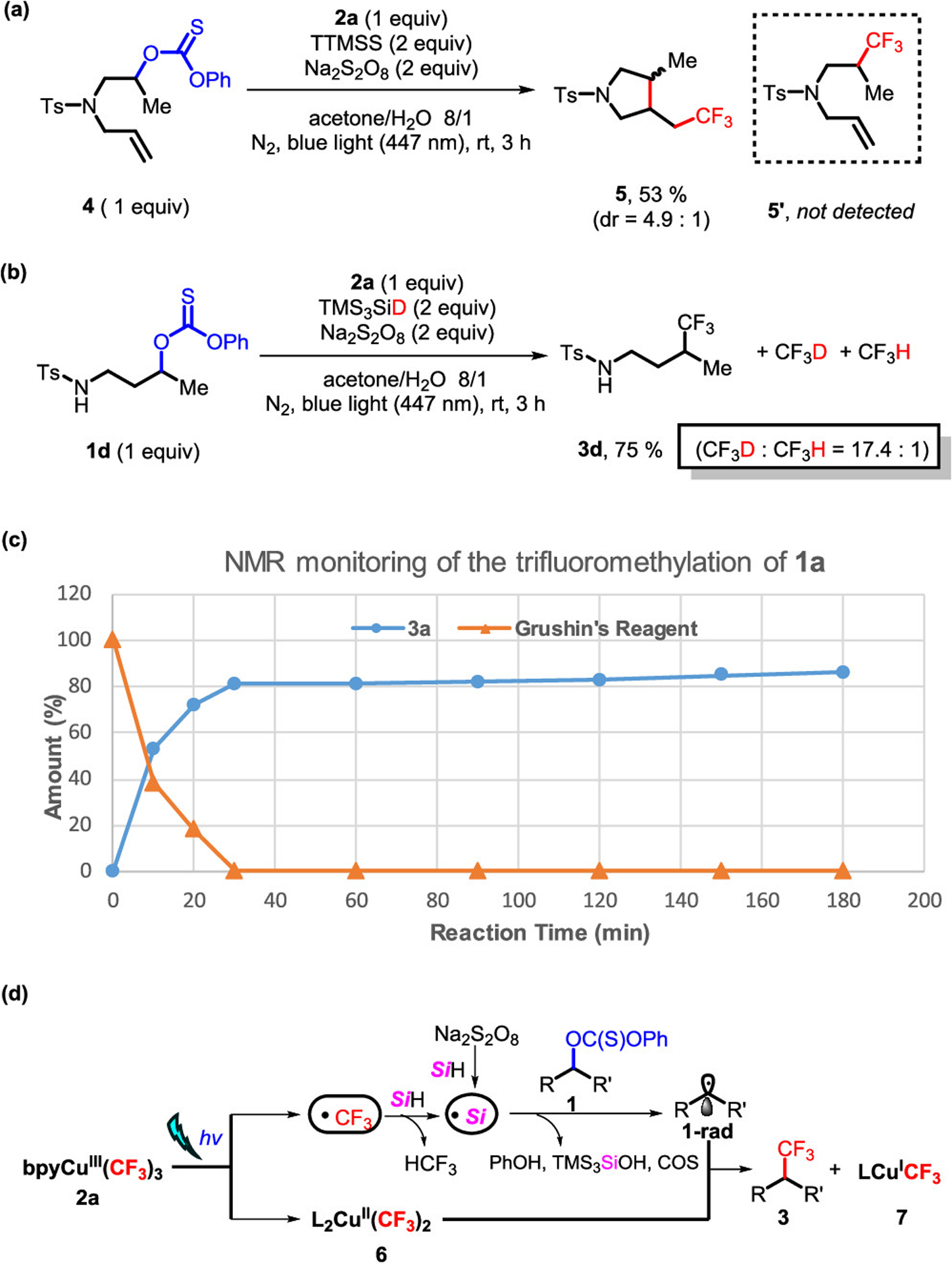
Mechanistic Studies
Taken together, these studies support the basic mechanistic outline in Scheme 3d. Blue-light irradiation serves to facilitate the homolysis of 2a and enables the rapid generation of CF3 radical and Cu(II) species 6.7g,19 Then, the reaction between CF3 radical and silane produces the Si-based radical7a,g that goes on to react with the thiocarbonate. Another possible pathway to generate the Si-based radical is the reaction of silane with persulfate, which is likely a slower process. Meanwhile, the persulfate may also facilitate the coupling process through the transition-metal counterion effect of sulfate as suggested previously.19 The Si-based radical subsequently reacts with the thiocarbonate to release alkyl radical 1-rad, which is the same process as Barton–McCombie-type reactions.13 The representative byproducts (e.g., phenol, TMS3SiOH) were also observed in the reaction.21 Finally, the alkyl radical 1-rad undergoes a coupling reaction with Cu(II) species 6 to provide the trifluoromethylation product along with the Cu(I) species 7.7a,d,g,19,20
In the reaction, the CF3 radical is quenched with silane (TTMSS/BDE ~84 kcal/mol) to form fluoroform (BDE ~106 kcal/mol),22 which inhibits radical attack of arenes or unsaturated C–C bonds, thereby leading to the broad functional group tolerance observed in this chemistry. Experimentally, small quantities of Barton–McCombie reduction product were observed (<10%) along with the remaining silane. This is likely due to the rapid H atom abstraction from silane by the CF3 radical to suppress Barton–McCombie product formation. Moreover, the coupling between 1-rad and 6 appears faster than the reductive hydrogenation process, thus preferentially producing the desired products.
In summary, we have disclosed a mild, Cu-mediated deoxygenative trifluoromethylation of unactivated and benzylic thiocarbonates. It represents a practical method for the radical trifluoromethylation of unactivated alcohol-derived substrates. The protocol tolerates a wide range of functional groups and electron-rich and -deficient arenes and can be used in the trifluoromethylation of complex molecules. As such, this methodology should find general use in a range of chemical applications.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funds from Indiana University in partial support of this work. We also gratefully acknowledge the NIH (GM121840) for partial support of this work. Eli Lilly & Co. and Amgen supported this work through the Lilly Grantee Award and the Amgen Young Investigator Award. We thank IU mass spectrometry for the HRMS (NSF CHE1726633).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.0c04039
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c04039.
Experimental details, compound characterization, and NMR data (PDF)
The authors declare no competing financial interest.
Contributor Information
Zhi-Yun Liu, Department of Chemistry, Indiana University, Bloomington, Indiana 47405-7102, United States.
Silas P. Cook, Department of Chemistry, Indiana University, Bloomington, Indiana 47405-7102, United States.
REFERENCES
- (1).(a) Muller K; Faeh C; Diederich F Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [DOI] [PubMed] [Google Scholar]; (b) Hagmann WK The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem 2008, 51, 4359–4369. [DOI] [PubMed] [Google Scholar]; (c) Ilardi EA; Vitaku E; Njardarson JT Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery. J. Med. Chem 2014, 57, 2832–2842. [DOI] [PubMed] [Google Scholar]; (d) Fujiwara T; O’Hagan D Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem 2014, 167, 16–29. [Google Scholar]; (e) Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Applications of fluorine in medicinal chemistry. J. Med. Chem 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]; (f) Zhou Y; Wang J; Gu Z; Wang S; Zhu W; Aceña JL; Soloshonok VA; Izawa K; Liu H Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev 2016, 116, 422–518. [DOI] [PubMed] [Google Scholar]; (g) Meanwell NA Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem 2018, 61, 5822–5880. [DOI] [PubMed] [Google Scholar]
- (2).(a) Furuya T; Kamlet AS; Ritter T Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Merino E; Nevado C Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev 2014, 43, 6598–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Besset T; Schneider C; Cahard D Tamed Arene and Heteroarene Trifluoromethylation. Angew. Chem., Int. Ed 2012, 51, 5048–5050. [DOI] [PubMed] [Google Scholar]; (d) Barata-Vallejo S; Lantaño B; Postigo A Recent Advances in Trifluoromethylation Reactions with Electrophilic Trifluoromethylating Reagents. Chem. - Eur. J 2014, 20, 16806–16829. [DOI] [PubMed] [Google Scholar]; (e) Alonso C; Martínez de Marigorta E; Rubiales G; Palacios F Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev 2015, 115, 1847–1935. [DOI] [PubMed] [Google Scholar]; (f) Xu XH; Matsuzaki K; Shibata N Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev 2015, 115, 731–764. [DOI] [PubMed] [Google Scholar]; (g) Egami H; Sodeoka M Trifluoromethylation of Alkenes with Concomitant Introduction of Additional Functional Groups. Angew. Chem., Int. Ed 2014, 53, 8294–8308. [DOI] [PubMed] [Google Scholar]; (h) Chu L; Qing F-L Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)-trimethylsilane as a Nucleophilic CF3 Source. Acc. Chem. Res 2014, 47, 1513–1522. [DOI] [PubMed] [Google Scholar]; (i) Tomashenko OA; Grushin VV Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev 2011, 111, 4475–4521. [DOI] [PubMed] [Google Scholar]; (j) Shibata N; Mizuta S; Kawai H Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron: Asymmetry 2008, 19, 2633–2644. [Google Scholar]; (k) Ma J-A; Cahard D Update 1 of: Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev 2008, 108, PR1–PR43. [DOI] [PubMed] [Google Scholar]; (l) Ma J-A; Cahard D Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev 2004, 104, 6119–6146. [DOI] [PubMed] [Google Scholar]; (m) Kumadaki I; Ando A; Sato K; Tarui A; Omote M Trifluoromethylation of Organic Compounds and Related Reactions. Synthesis 2010, 1865–1882. [Google Scholar]
- (3).(a) Langlois BR; Billard T; Roussel S Nucleophilic trifluoromethylation: Some recent reagents and their stereoselective aspects. J. Fluorine Chem 2005, 126, 173–179. [Google Scholar]; (b) Liu X; Xu C; Wang M; Liu Q Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev 2015, 115, 683–730. [DOI] [PubMed] [Google Scholar]; (c) Ruppert I; Schlich K; Volbach W Die ersten CF3-substituierten organyl(chlor)silane. Tetrahedron Lett. 1984, 25, 2195–2198. [Google Scholar]; (d) Prakash GKS; Krishnamurti R; Olah GA Fluoride-Induced Trifluoromethylation of Carbonyl Compounds with Trifluoromethyltrimethylsilane (TMS-CF3). A Trifluoromethide Equivalent. J. Am. Chem. Soc 1989, 111, 393–395. [Google Scholar]; (e) Chen Q-Y; Wu S-W Methyl Fluorosulphonyldifluoroacetate; a New Trifluoromethylating Agent. J. Chem. Soc., Chem. Commun 1989, 11, 705–706. [Google Scholar]
- (4).(a) Umemoto T Electrophilic Perfluoroalkylating Agents. Chem. Rev 1996, 96, 1757–1778. [DOI] [PubMed] [Google Scholar]; (b) Shibata N; Matsnev A; Cahard D Shelf-stable electrophilic trifluoromethylating reagents: A brief historical perspective. Beilstein J. Org. Chem 2010, 6, 65 DOI: 10.3762/bjoc.6.65. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Barata-Vallejo S; Lantaño B; Postigo A Recent Advances in Trifluoromethylation Reactions with Electrophilic Trifluoromethylating Reagents. Chem. - Eur. J 2014, 20, 16806–16829. [DOI] [PubMed] [Google Scholar]; (d) Prieto A; Baudoin O; Bouyssi D; Monteiro N Electrophilic trifluoromethylation of carbonyl compounds and their nitrogen derivatives under copper catalysis. Chem. Commun 2016, 52, 869–881. [DOI] [PubMed] [Google Scholar]; (e) Umemoto T; Ishihara S Power-Variable Electrophilic Trifluoromethylating Agents. S-, Se-, and re-(Trifluoromethyl)-dibenzothio-, -seleno-, and tellurophenium Salt System. J. Am. Chem. Soc 1993, 115, 2156–2164. [Google Scholar]; (f) Eisenberger P; Gischig S; Togni A Novel 10-I-3 Hypervalent Iodine-Based Compounds for Electrophilic Trifluoromethylation. Chem. - Eur. J 2006, 12, 2579–2586. [DOI] [PubMed] [Google Scholar]; (g) Noritake S; Shibata N; Nakamura S; Toru T; Shiro M Fluorinated Johnson Reagent for Transfer-Trifluoromethylation to Carbon Nucleophiles. Eur. J. Org. Chem 2008, 20, 3465–3468. [Google Scholar]
- (5).(a) Studer AA “Renaissance” in Radical Trifluoromethylation. Angew. Chem., Int. Ed 2012, 51, 8950–8958. [DOI] [PubMed] [Google Scholar]; (b) Koike T; Akita M Trifluoromethylation by visible-light-driven photoredox catalysis. Top. Catal 2014, 57, 967–974. [Google Scholar]; (c) Koike T; Akita M Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon–Carbon Multiple Bonds. Acc. Chem. Res 2016, 49, 1937–1945. [DOI] [PubMed] [Google Scholar]
- (6).For selected examples of trifluoromethylation of allyl groups and benzyl groups, see:; (a) Ambler BR; Altman RA Copper-Catalyzed Decarboxylative Trifluoromethylation of Allylic Bromodifluoroacetates. Org. Lett 2013, 15, 5578–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parsons AT; Buchwald SL Copper-Catalyzed Trifluoromethylation of Unactivated Olefins. Angew. Chem., Int. Ed 2011, 50, 9120–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang X; Ye Y; Zhang S; Feng J; Xu Y; Zhang Y; Wang J Copper-Catalyzed C(sp3)–C(sp3) Bond Formation Using a Hypervalent Iodine Reagent: An Efficient Allylic Trifluoromethylation. J. Am. Chem. Soc 2011, 133, 16410–16413. [DOI] [PubMed] [Google Scholar]; (d) He Z; Tan P; Hu J Copper-Catalyzed Trifluoromethylation of Polysubstituted Alkenes Assisted by Decarboxylation. Org. Lett 2016, 18, 72–75. [DOI] [PubMed] [Google Scholar]; (e) Jiang X; Qing F-L Cu-Mediated trifluoromethylation of benzyl, allyl and propargyl methanesulfonates with TMSCF3. Beilstein J. Org. Chem 2013, 9, 2862–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Dubinina GG; Furutachi H; Vicic DA Active Trifluoromethylating Agents from Well-Defined Copper(I)–CF3 Complexes. J. Am. Chem. Soc 2008, 130, 8600–8601. [DOI] [PubMed] [Google Scholar]; (g) Kawai H; Furukawa T; Nomura Y; Tokunaga E; Shibata N Cu-Mediated Chemoselective Trifluoromethylation of Benzyl Bromides Using Shelf-Stable Electrophilic Trifluoromethylating Reagents. Org. Lett 2011, 13, 3596–3599. [DOI] [PubMed] [Google Scholar]; (h) Ambler BR; Zhu L; Altman RA Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates. J. Org. Chem 2015, 80, 8449–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Shen H; Liu Z; Zhang P; Tan X; Zhang Z; Li C Trifluoromethylation of Alkyl Radicals in Aqueous Solution. J. Am. Chem. Soc 2017, 139, 9843–9846. [DOI] [PubMed] [Google Scholar]; (b) Chen Y; Ma G; Gong H Copper-Catalyzed Reductive Trifluoromethylation of Alkyl Iodides with Togni’s Reagent. Org. Lett 2018, 20, 4677–4680. [DOI] [PubMed] [Google Scholar]; (c) Kornfilt DJP; MacMillan DWC Copper-Catalyzed Trifluoromethylation of Alkyl Bromides. J. Am. Chem. Soc 2019, 141, 6853–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tan X; Liu Z; Shen H; Zhang P; Zhang Z; Li C Silver-Catalyzed Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc 2017, 139, 12430–12433. [DOI] [PubMed] [Google Scholar]; (e) Kautzky JA; Wang T; Evans RW; MacMillan DWC Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc 2018, 140, 6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Paeth M; Carson W; Luo J-H; Tierney D; Cao Z; Cheng M-J; Liu W Copper-Mediated Trifluoromethylation of Benzylic Csp3–H Bonds. Chem. - Eur. J 2018, 24, 11559–11563. [DOI] [PubMed] [Google Scholar]; (g) Guo S; AbuSalim DI; Cook SP Aqueous Benzylic C–H Trifluoromethylation for Late-Stage Functionalization. J. Am. Chem. Soc 2018, 140, 12378–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Xiao H; Liu Z; Shen H; Zhang B; Zhu L; Li C Copper-Catalyzed Late-Stage Benzylic C(sp3)–H Trifluoromethylation. Chem. 2019, 5, 940–949. [DOI] [PubMed] [Google Scholar]; (i) Liu Z; Xiao H; Zhang B; Shen H; Zhu L; Li C Copper-Catalyzed Remote C(sp3)–H Trifluoromethylation of Carboxamides and Sulfonamides. Angew. Chem., Int. Ed 2019, 58, 2510–2513. [DOI] [PubMed] [Google Scholar]; (j) Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam Y; Sherer EC; MacMillan DWC The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem 2020, 12, 459–467. [DOI] [PubMed] [Google Scholar]; (k) Jiang C; Wang L; Zhang H; Chen P; Guo Y-L; Liu G Enantioselective Copper-Catalyzed Trifluoromethylation of Benzylic Radicals via Ring Opening of Cyclopropanols. Chem. 2020, 6, 2407–2419. [Google Scholar]
- (8).(a) Oyeyemi VB; Keith JA; Carter EA Trends in Bond Dissociation Energies of Alcohols and Aldehydes Computed with Multireference Averaged Coupled-Pair Functional Theory. J. Phys. Chem. A 2014, 118, 3039–3050. [DOI] [PubMed] [Google Scholar]; (b) Bisz E; Szostak M Iron-Catalyzed C–O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. [DOI] [PubMed] [Google Scholar]; (c) Cornella J; Zarate C; Martin R Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity. Chem. Soc. Rev 2014, 43, 8081–8097. [DOI] [PubMed] [Google Scholar]; (d) Su B; Cao Z-C; Shi Z-J Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res 2015, 48, 886–896. [DOI] [PubMed] [Google Scholar]; (e) Tollefson EJ; Hanna LE; Jarvo ER Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers and Esters. Acc. Chem. Res 2015, 47, 2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]; Select examples:; (f) Kuwano R; Kondo Y Palladium-Catalyzed Benzylation of Active Methine Compounds without Additional Base: Remarkable Effect of 1,5-Cyclooctadiene. Org. Lett 2004, 6, 3545–3547. [DOI] [PubMed] [Google Scholar]; (g) Guan B-T; Xiang S-K; Wang B-Q; Sun Z-P; Wang Y; Zhao K-Q; Shi Z-J Direct Benzylic Alkylation via Ni-Catalyzed Selective Benzylic sp3 C–O Activation. J. Am. Chem. Soc 2008, 130, 3268–3269. [DOI] [PubMed] [Google Scholar]; (h) Yu D-G; Wang X; Zhu R-Y; Luo S; Zhang X-B; Wang B-Q; Wang L; Shi Z-J Direct Arylation/Alkylation/Magnesiation of Benzyl Alcohols in the Presence of Grignard Reagents via Ni-, Fe-, or Co-Catalyzed sp3 C–O Bond Activation. J. Am. Chem. Soc 2012, 134, 14638–14641. [DOI] [PubMed] [Google Scholar]; (i) Martin-Montero R; Krolikowski T; Zarate C; Manzano R; Martin R Stereospecific Nickel-Catalyzed Borylation of Secondary Benzyl Pivalates. Synlett 2017, 28, 2604–2608. [Google Scholar]; (j) Taylor BLH; Swift EC; Waetzig JD; Jarvo ER Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Alkyl Ethers: Enantioselective Synthesis of Diarylethanes. J. Am. Chem. Soc 2011, 133, 389–391. [DOI] [PubMed] [Google Scholar]; (k) Oelke AJ; Sun J; Fu GC Nickel-Catalyzed Enantioselective Cross-Couplings of Racemic Secondary Electrophiles That Bear an Oxygen Leaving Group. J. Am. Chem. Soc 2012, 134, 2966–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Harris MR; Hanna LE; Greene MA; Moore CE; Jarvo ER Retention or Inversion in Stereospecific Nickel-Catalyzed Cross-Coupling of Benzylic Carbamates with Arylboronic Esters: Control of Absolute Stereochemistry with an Achiral Catalyst. J. Am. Chem. Soc 2013, 135, 3303–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]; Select examples for free alcohol:; (m) Wu H-B; Ma X-T; Tian S-K Palladium-catalyzed stereospecific cross-coupling of enantioenriched allylic alcohols with boronic acids. Chem. Commun 2014, 50, 219–221. [DOI] [PubMed] [Google Scholar]; (n) Cao Z-C; Yu D-G; Zhu R-Y; Wei J-B; Shi Z-J Direct cross-coupling of benzyl alcohols to construct diarylmethanes via palladium catalysis. Chem. Commun 2015, 51, 2683–2686. [DOI] [PubMed] [Google Scholar]; (o) Suga T; Ukaji Y Nickel-Catalyzed Cross-Electrophile Coupling between Benzyl Alcohols and Aryl Halides Assisted by Titanium Co-reductant. Org. Lett 2018, 20, 7846–7850. [DOI] [PubMed] [Google Scholar]; (p) Akkarasamiyo S; Margalef J; Samec JSM Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction of Naphthyl and Quinolyl Alcohols with Boronic Acids. Org. Lett 2019, 21, 4782–4787. [DOI] [PubMed] [Google Scholar]; (q) Jefferies LR; Cook SP Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols. Org. Lett 2014, 16, 2026–2029. [DOI] [PubMed] [Google Scholar]; (r) Marcyk PT; Jefferies LR; AbuSalim DI; Pink M; Baik M-H; Cook SP Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides. Angew. Chem., Int. Ed 2019, 58, 1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Marcyk PT; Cook SP Synthesis of Tetrahydroisoquino-lines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination. Org. Lett 2019, 21, 6741–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Crich D; Quintero L Radical chemistry associated with the thiocarbonyl group. Chem. Rev 1989, 89, 1413–1432. [Google Scholar]; (b) Dolan SC; MacMillan J A New Method for the Deoxygenation of Tertiary and Secondary Alcohols. J. Chem. Soc., Chem. Commun 1985, 22, 1588–1589. [Google Scholar]; (c) Coppa F; Fontana F; Lazzarini E; Minisci F; Pianese G; Zhao L A Novel, Simple and Cheap Source of Alkyl Radicals from Alcohols, Useful for Heteroaromatic Substitution. Chem. Lett 1992, 21, 1295–1298. [Google Scholar]; (d) Chenneberg L; Baralle A; Daniel M; Fensterbank L; Goddard J-P; Ollivier C Visible Light Photocatalytic Reduction of O-Thiocarbamates: Development of a Tin-Free Barton–McCombie Deoxygenation Reaction. Adv. Synth. Catal 2014, 356, 2756–2762. [Google Scholar]; (e) Stache EE; Ertel AB; Rovis T; Doyle AG Generation of Phosphoranyl Radicals via Photoredox Catalysis Enables Voltage–Independent Activation of Strong C–O Bonds. ACS Catal. 2018, 8, 11134–11139. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Han J-B; Guo A; Tang X-Y Alkylation of Allyl/Alkenyl Sulfones by Deoxygenation of Alkoxyl Radicals. Chem. - Eur. J 2019, 25, 2989–2994. [DOI] [PubMed] [Google Scholar]; (g) Gao Y; Wu Z; Yu L; Wang Y; Pan Y Alkyl Carbazates for Electrochemical Deoxygenative Functionalization of Heteroarenes. Angew. Chem., Int. Ed 2020, 59, 10859–10863. [DOI] [PubMed] [Google Scholar]; (h) Wu J; Bär RM; Guo L; Noble A; Aggarwal VK Photoinduced Deoxygenative Borylations of Aliphatic Alcohols. Angew. Chem., Int. Ed 2019, 58, 18830–18834. [DOI] [PubMed] [Google Scholar]; (i) Friese FW; Studer A Deoxygenative Borylation of Secondary and Tertiary Alcohols. Angew. Chem., Int. Ed 2019, 58, 9561–9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Ye Y; Chen H; Sessler JL; Gong H Zn-Mediated Fragmentation of Tertiary Alkyl Oxalates Enabling Formation of Alkylated and Arylated Quaternary Carbon Centers. J. Am. Chem. Soc 2019, 141 (2), 820–824. [DOI] [PubMed] [Google Scholar]; (b) Gao M; Sun D; Gong H Ni-Catalyzed Reductive C–O Bond Arylation of Oxalates Derived from α-Hydroxy Esters with Aryl Halides. Org. Lett 2019, 21, 1645–1648. [DOI] [PubMed] [Google Scholar]; For iron-catalyzed coupling reaction of oxalates, see:; (c) Chen H; Ye Y; Tong W; Fang J; Gong H Formation of allylated quaternary carbon centers via C–O/C–O bond fragmentation of oxalates and allyl carbonates. Chem. Commun 2020, 56, 454–457. [DOI] [PubMed] [Google Scholar]; (d) Ye Y; Chen H; Yao K; Gong H Iron-Catalyzed Reductive Vinylation of Tertiary Alkyl Oxalates with Activated Vinyl Halides. Org. Lett 2020, 22, 2070–2075. [DOI] [PubMed] [Google Scholar]
- (11).(a) Lackner GL; Quasdorf KW; Pratsch G; Overman LE Fragment Coupling and the Construction of Quaternary Carbons Using Tertiary Radicals Generated From tert-Alkyl N-Phthalimidoyl Oxalates By Visible-Light Photocatalysis. J. Org. Chem 2015, 80, 6012–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nawrat CC; Jamison CR; Slutskyy Y; MacMillan DWC; Overman LE Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling. J. Am. Chem. Soc 2015, 137, 11270–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jamison CR; Slutskyy Y; Overman LE Fragment Coupling and Formation of Quaternary Carbons by Visible-Light Photoredox Catalyzed Reaction of tert-Alkyl Hemioxalate Salts and Michael Acceptors. Org. Synth 2018, 94, 167–183. [Google Scholar]; (d) Abbas SY; Zhao P; Overman LE 1,6-Addition of Tertiary Carbon Radicals Generated From Alcohols or Carboxylic Acids by Visible-Light Photoredox Catalysis. Org. Lett 2018, 20, 868–871. [DOI] [PubMed] [Google Scholar]; (e) Pitre SP; Muuronen M; Fishman DA; Overman LE Tertiary Alcohols as Radical Precursors for the Introduction of Tertiary Substituents into Heteroarenes. ACS Catal. 2019, 9, 3413–3418. [Google Scholar]; (f) Weires NA; Slutskyy Y; Overman LE Facile Preparation of Spirolactones by an Alkoxycarbonyl Radical Cyclization-Cross-Coupling Cascade. Angew. Chem., Int. Ed 2019, 58, 8561–8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Zhang X; MacMillan DWC Alcohols as Latent Coupling Fragments for Metallaphotoredox Catalysis: sp3–sp2 Cross-Coupling of Oxalates with Aryl Halides. J. Am. Chem. Soc 2016, 138, 13862–13865. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) González-Esguevillas M; Miró J; Jeffrey JL; MacMillan DWC Photoredox-catalyzed deoxyfluorination of activated alcohols with Selectfluor. Tetrahedron 2019, 75, 4222–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Barton DHR; McCombie SW A new method for the deoxygenation of secondary alcohols. J. Chem. Soc., Perkin Trans 1 1975, 0, 1574–1585. [Google Scholar]; (b) Ramaiah M Radical reactions in organic synthesis. Tetrahedron 1987, 43, 3541–3676. [Google Scholar]
- (14).Zhu L; Liu S; Douglas JT; Altman RA Copper-Mediated Deoxygenative Trifluoromethylation of Benzylic Xanthates: Generation of a C-CF3 Bond from an O-Based Electrophile. Chem. - Eur. J 2013, 19, 12800–12805. [DOI] [PubMed] [Google Scholar]
- (15).Vara BA; Patel NR; Molander GA O-Benzyl Xanthate Esters under Ni/Photoredox Dual Catalysis: Selective Radical Generation and Csp3–Csp2 Cross-Coupling. ACS Catal. 2017, 7, 3955–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mills LR; Monteith JJ; Gomes G. dos P.; Aspuru-Guzik A; Rousseaux SAL The Cyclopropane Ring as a Reporter of Radical Leaving-Group Reactivity for Ni-Catalyzed C(sp3)–O Arylation. J. Am. Chem. Soc 2020, 142, 13246–13254. [DOI] [PubMed] [Google Scholar]
- (17).For trifluoromethylation of activated alcohol-based substrates, see:; (a) Clark JH; McClinton MA; Blade RJ The reactions of copper-dibromodifluoromethane-amide systems with alcohols. J. Fluorine Chem 1992, 59, 257–267. [Google Scholar]; (b) Duan J-X; Chen Q-Y Novel synthesis of 2,2,2-trifluoroethyl compounds from homoallylic alcohols: a copper(I) iodide-initiated trifluoromethyl–dehydroxylation process. J. Chem. Soc., Perkin Trans 1 1994, 6, 725–730. [Google Scholar]; (c) Takechi N; Ait-Mohand S; Medebielle M; Dolbier WR Novel Nucleophilic Trifluoromethylation of Vicinal Diol Cyclic Sulfates. Org. Lett 2002, 4, 4671–4672. [DOI] [PubMed] [Google Scholar]; (d) Ambler BR; Zhu L; Altman RA Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates. J. Org. Chem 2015, 80, 8449–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gao X; Xiao Y-L; Wan X; Zhang X Copper-Catalyzed Highly Stereoselective Trifluoromethylation and Difluoroalkylation of Secondary Propargyl Sulfonates. Angew. Chem., Int. Ed 2018, 57, 3187–3191. [DOI] [PubMed] [Google Scholar]; (f) Zhang W; Lin J-H; Wu W; Cao Y-C; Xiao J-C Dehydroxylative Trifluoromethylthiolation, Trifluoromethylation, and Difluoromethylation of Alcohols. Chin. J. Chem 2020, 38, 169–172. [Google Scholar]
- (18).(a) Tomashenko OA; Escudero-Adan EC; Belmonte MM; Grushin VV Simple, Stable, and Easily Accessible Well-Defined CuCF3 Aromatic Trifluoromethylating Agents. Angew. Chem., Int. Ed 2011, 50, 7655–7659. [DOI] [PubMed] [Google Scholar]; (b) Romine AM; Nebra N; Konovalov AI; Martin E; Benet-Buchholz J; Grushin VV Easy Access to the Copper(III) Anion [Cu(CF3)4]−. Angew. Chem., Int. Ed 2015, 54, 2745–2749. [DOI] [PubMed] [Google Scholar]
- (19).Guo S; AbuSalim DI; Cook SP 1,2-(Bis)-trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group. Angew. Chem., Int. Ed 2019, 58, 11704–11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Liu S; Liu H; Liu S; Lu Z; Lu C; Leng X; Lan Y; Shen Q C(sp3)-CF3 Reductive Elimination from a Five-Coordinate Neutral Copper(III) Complex. J. Am. Chem. Soc 2020, 142, 9785–9791. [DOI] [PubMed] [Google Scholar]; (b) Paeth M; Tyndall SB; Chen L-Y.; Hong J-C; Carson WP; Liu X; Sun X; Liu J; Yang K; Hale EM; Tierney DL; Liu B; Cao Z; Cheng M-J; Goddard WA III; Liu W Csp3–Csp3 Bond-Forming Reductive Elimination from Well-Defined Copper(III) Complexes. J. Am. Chem. Soc 2019, 141, 3153–3159. [DOI] [PubMed] [Google Scholar]
- (21).Barton DHR; Doo OJ; Jaszberenyi JC Cs. An improved radical chain procedure for the deoxygenation of secondary and primary alcohols using diphenylsilane as hydrogen atom donor and triethylborane-air as initiator. Tetrahedron Lett. 1990, 31, 4681–4684. [Google Scholar]; These products were confirmed with GCMS and 1H NMR.
- (22).(a) Chatgilialoglu C; Ferreri C; Landais Y; Timokhin VI Thirty Years of (TMS)3SiH: A Milestone in Radical-Based Synthetic Chemistry. Chem. Rev 2018, 118, 6516–6572. [DOI] [PubMed] [Google Scholar]; (b) Amphlett JC; Coomber JW; Whittle E The C-H Bond Dissociation Energy in Fluoroform. J. Phys. Chem 1966, 70, 593–594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


