Abstract
Objectives. To estimate whether state Medicaid expansions’ relationships to breast, cervical, and colorectal cancer screening differ by race/ethnicity.
Methods. Analyses conducted in 2021 used 2011–2016 and 2018–2019 Behavioral Risk Factor Surveillance System data on adults aged 40 to 64 years with household incomes below 400% of the federal poverty guideline (FPG; n = 537 250). Triple-difference analyses compared cancer screening in Medicaid expansion versus nonexpansion states, before versus after expansion, among people with incomes above versus below the eligibility cutoff (138% FPG). Race/ethnicity and ethnicity-by-language interaction terms tested for effect modification.
Results. Associations between Medicaid expansions and cancer screening were significant for past-2-year mammograms and past-5-year colorectal screening. Effect modification analyses showed elevated mammography among non-Hispanic Asian women (+9.0 percentage points; 95% confidence interval [CI] = 3.2, 14.8) and Hispanic women (+6.0 percentage points; 95% CI = 2.0, 10.1), and Papanicolaou tests among Hispanic women (+4.2 percentage points; 95% CI = 0.1, 8.2). Findings were not limited to English- or Spanish-speaking respondents and were robust to insurance status controls.
Conclusions. Medicaid expansions yielded statistically significant increases in income-eligible Asian and Hispanic women’s mammography and Hispanic women’s Pap testing relative to non-Hispanic White women. Neither language proficiency nor insurance status explained these findings. (Am J Public Health. 2022;112(11):1630–1639. https://doi.org/10.2105/AJPH.2022.307027)
Cancer screening has reduced cancer incidence and mortality in the United States, with substantial benefits from mammograms, cervical cytology (i.e., Papanicolaou [Pap] tests), and colorectal screening in particular.1 Mammography is correlated with a 19% decrease in breast cancer mortality2; 1 breast cancer death is averted for every 180 women screened triennially between the ages of 50 and 70 years.3 Cervical cancer screening has led to a decrease in mortality from 2000 to 2015, with benefits from screening far outweighing any associated harms.1 For colorectal cancer, the third leading cause of cancer for men and women, consistent screening among adults aged 45 to 75 years could avoid around 24 to 28 deaths per 1000 adults screened.4
Yet, the benefits of cancer screening are not distributed evenly throughout the US population: cancer screening rates vary substantially by race and ethnicity. While the incidence of colorectal cancer is elevated among non-Hispanic Black men (27%) and women (22%), non-Hispanic Black adults are less likely to be screened for colorectal cancer than their non-Hispanic White counterparts. Similarly, Hispanic women exhibit the highest cervical cancer incidence but are less likely to be screened for cervical cancer than non-Hispanic White and non-Hispanic Black women.5
These differences in screening contribute to disparities in cancer outcomes.6 For example, non-Hispanic Black women have a higher prevalence of advanced-stage breast tumors than non-Hispanic White women, and the highest mortality rates from cervical cancer, attributable, in part, to later-stage diagnoses.7,8 Other factors also influence these disparities. For low-income individuals in particular, health care resources and service availability may not match patient needs.9 With certain ethnic groups, screening rates appear lower for foreign-born relative to US-born individuals.10 And, critically, physician behaviors and attitudes may reflect racial and ethnic biases,11 manifesting as differences in patient treatment.12–14
Decreasing differences in cancer screening is a key first step toward reducing disparities in cancer-related outcomes. As access to health care remains a dominant reason for racial and ethnic disparities in patient outcomes, initiatives that increase insurance coverage may help close these screening gaps. Indeed, the Affordable Care Act (ACA) has been associated with higher rates of primary care visits and preventive services among young adults15; reduced racial and ethnic disparities in insurance coverage,16,17 particularly for Hispanic individuals who preferred Spanish over English18; and fewer uninsured visits to primary care physicians among all racial and ethnic groups, but particularly for Hispanic patients with Medicaid. Still, in Medicaid expansion states, non-Hispanic White patients experienced the largest decreases in uninsured visits.19
More than two thirds of US states have implemented ACA Medicaid expansions, largely in 2014, extending program eligibility to most adults with household incomes below 138% of the federal poverty guideline (FPG; according to the US Department of Health and Human Services). As many states’ Medicaid programs already covered low-income parents, ACA-related expansions particularly affected coverage among adults without dependent children (henceforth “childless adults”). Research on Medicaid expansions’ effects on cancer screening is mixed. Some studies show significant impacts on colorectal cancer screening and Pap tests (i.e., cervical cancer screening) but not mammograms,20,21 while others find no consequent increase in rates of mammograms or Pap tests in low-income women.22 Overall screening utilization increased more in traditional cost-sharing programs versus those with enhanced cost-sharing,23 which might affect disparities if beneficiary characteristics differ between such plans. To our knowledge, none of these studies considered whether screening responses differed by racial and ethnic groups.
To address this gap in the literature, we tested whether state Medicaid expansions’ associations with cervical, colorectal, and breast cancer screening differed by race and ethnicity.
METHODS
We analyzed nationally and state-representative annual data on noninstitutionalized adults from the Behavioral Risk Factor Surveillance System (BRFSS), a repeated cross-sectional survey focused on health-related behaviors and outcomes. We considered the 2011–2016 and 2018–2019 waves, dropping 2017 as it omitted cancer-screening questions. Changes in the sampling structure and weighting methodology precluded comparing pre-2011 data to later waves.
Analytic Samples
To assess Medicaid expansion effects, we restricted our analytic samples to adults residing in US states with no minors in their household (“childless adults”), who were not age-eligible for insurance through dependent coverage provisions (older than 25 years) or Medicare (younger than 65 years). We omitted those reporting a household income above 400% of the FPG, because wealthier respondents provide less plausible counterfactuals for the behavior of Medicaid-eligible individuals. We did not consider states that expanded Medicaid or Medicaid-equivalent coverage to adults with household incomes up to 138% of FPG statewide before 2014—that is, Massachusetts (through its 2006 health care reform), Vermont (via the Vermont Health Access Plan), and the District of Columbia (Medicaid expansion; see “Medicaid Expansions” under Appendix section I, available as a supplement to the online version of this article at https://ajph.org). As previous work categorizes New York and Delaware alongside the other 3 as having substantial early expansions,24 sensitivity tests also omit those states to ensure that estimates reflect the more homogenous set of ACA Medicaid expansions implemented in 2014 and 2015.
Analytic samples are further winnowed by age and gender to consider only those within date-concordant US Preventive Services Task Force (USPSTF) screening recommendations—specifically, adults aged 50 to 64 years for colonoscopies or sigmoidoscopies (2008), women aged 50 to 64 years for mammograms (2009), and women aged 40 to 64 years for Pap tests (2003 and 2012).25 While USPSTF recommendations suggest beginning cervical cancer screening at age 21, we limited that outcome’s analytic sample to women aged 40 years and older because of a concern that pregnancies might affect screening. (The vast majority of US mothers give birth before the age of 40 years.)
Outcomes of interest were binary indicators for 3 cancer screening variables: whether a respondent had been screened for breast cancer using mammograms in the past 2 years, cervical cancer using Pap testing in the past 3 years, or colon cancer using sigmoidoscopies or colonoscopies in the past 5 years.
Exposures
The exposure of interest was an interaction term between indicators for whether the respondent’s state of residence had expanded Medicaid to 138% of FPG by their survey date and whether they would have been income-eligible for Medicaid if their state expanded (i.e., household income < 138% FPG). While most state Medicaid expansions went into effect on January 1, 2014, several were delayed as states pursued waivers (e.g., to pursue a private option where state funds subsidize eligible adults’ insurance payments). To capture lasting responses, our exposure variable indicates expansions that went into effect before 2016 only, with specification checks dropping later adopters to clarify if or how their inclusion affects estimates.
To clarify whether responses to the expansion differed by race/ethnicity, the exposure was further interacted with race/ethnicity indicators. First, we used a race/ethnicity variable dividing respondents into mutually exclusive categories: non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, other non-Hispanic race, and missing race/ethnicity. Subsequent analyses considered ethnicity-by-language—distinguishing non-Hispanic respondents, Hispanic respondents who completed the BRFSS survey in English, and Hispanic respondents who completed it in Spanish—to clarify whether findings might reflect reduced language barriers in health care (e.g., related to the Affordable Care Act’s requirement that patients be notified of and provided with language services).
Covariates
Analyses adjusted for several sociodemographic covariates: indicators for 10-year age groups, sex (in colorectal screening analyses), household income below 138% of FPG, education (did not graduate high school, graduated high school, attended college or technical school, graduated from college or technical school), the categorical race/ethnicity variables described previously, and an indicator for whether the survey was administered by cell phone (as compared with landline). Other covariates included binary indicators for survey year, state of residence, and, for some robustness checks, having health insurance.
Statistical Analysis
We conducted analyses with Stata version 15.0 (StataCorp LP, College Station, TX). First, a table of summary statistics compared cancer screening rates and insurance coverage in states that did versus did not expand Medicaid, before versus after the Medicaid expansion. Second, multivariable regressions used a triple-difference specification to estimate the relationship between Medicaid expansion and each screening outcome, effectively comparing people below versus at or above 138% of the FPG, in states that did versus did not expand Medicaid, before versus after that expansion.
Covariates adjusted for the aforementioned respondent sociodemographics, year fixed effects (to absorb general time trends), state fixed effects (to adjust for time-invariant state characteristics), an indicator for Medicaid expansion (absorbing effects of unobserved factors correlated with expansion that affected respondents above and below the eligibility cutoff), and 2 sets of interaction terms absorbing screening differences specific to those below 138% of FPG, for 2014 on versus earlier (< 138% FPG * year ≥ 2014) as well as time-invariant differences between expansion versus nonexpansion states (< 138% FPG * expansion state). Thus, the exposure variable’s coefficient will not be biased by nationwide changes in the lower income group’s screening rates concurrent with the ACA, nor by average (time-invariant) differences in expansion versus nonexpansion states’ screening rates (see Appendix section I for further details).
To clarify whether the relationship between Medicaid expansions and cancer screening differed by race/ethnicity, we repeated these analyses with an additional term interacting the primary exposure’s effect with respondent race/ethnicity indicators (< 138% FPG * expanded Medicaid * race/ethnicity). Finally, we replicated that specification with ethnicity-by-language indicators in the exposure interaction terms in place of race/ethnicity. Because of concerns about attenuation bias in nonlinear models with large numbers of fixed effects, regression analyses used sample-weighted linear probability models instead of logistic regressions, with standard errors clustered by state (the level of the policy intervention).26,27
Robustness checks added insurance status as a covariate to confirm whether the expansion response was explained by being insured per se as opposed to other factors (e.g., changes in cost-sharing for preventive care, language access requirements). Further sensitivity checks dropped odd survey years (when more than half of US states omitted the BRFSS cancer screening module), excluded states that expanded Medicaid after 2015 to clarify if or how their inclusion affects estimates, dropped respondents in New York and Delaware to consider whether implications vary when omitting the full set of states others categorize as having substantive early expansions,24 and sequentially dropped states accounting for the largest populations of 3 Hispanic subgroups in the 50 US states (i.e., Florida [Cuban], New York [Puerto Rican], and California [Mexican])—to clarify whether a specific subgroup was responsible for our findings.
This study’s protocol was not preregistered.
RESULTS
For each analytic sample, summary statistics show higher rates of cancer screening and insurance coverage among respondents with household incomes at 138% to 400% of FPG relative to below 138% of FPG (Table 1). Within income groups, insurance rates were consistently higher for adults in expansion states compared with nonexpansion states even without differentiating pre- versus postexpansion periods, particularly for those below 138% of FPG. Corresponding differences in screening rates were not statistically significant.
TABLE 1—
Summary Statistics: United States, Behavioral Risk Factor Surveillance System, 2011–2016 and 2018–2019
| Non‒Expansion State | Expansion State | Δ (Expansion ‒ Non-Expansion) | ||||
| < 138% FPG, % (95% CI) | 138%–400% FPG, % (95% CI) | < 138% FPG, % (95% CI) | 138%–400% FPG, % (95% CI) | < 138% FPG, pp (95% CI) | 138%–400% FPG, pp (95% CI) | |
| Pap samplea | ||||||
| Past 3-y Pap | 60.4 (58.0, 62.7) | 68.2 (66.2, 70.2) | 64.1 (60.5, 67.8) | 68.0 (65.6, 70.4) | 3.8 (−0.6, 8.1) | −0.2 (−3.3, 3.0) |
| Insured | 65.6 (60.1, 71.0) | 79.5 (75.8, 83.1) | 79.8 (77.6, 82.1) | 85.2 (83.6, 86.7) | 14.3** (8.3, 20.2) | 5.7** (1.8, 9.6) |
| Mammogram sampleb | ||||||
| Past 2-y mammogram | 67.0 (64.2, 69.8) | 72.3 (70.6, 73.9) | 68.8 (65.2, 72.4) | 72.5 (70.1, 74.9) | 1.8 (−2.8, 6.4) | 0.2 (−2.7, 3.1) |
| Insured | 68.7 (63.3, 74.2) | 81.8 (78.6, 85.0) | 81.2 (78.8, 83.6) | 86.7 (85.3, 88.0) | 12.5** (6.5, 18.4) | 4.9** (1.4, 8.4) |
| Colorectal screening samplec | ||||||
| Past 5-y colorectal screening | 38.7 (35.8, 41.6) | 44.7 (41.9, 47.6) | 38.7 (36.4, 41.0) | 43.5 (40.9, 46.1) | −0.02 (−3.7, 3.7) | −1.2 (−5.1, 2.7) |
| Insured | 67.9 (61.4, 74.5) | 80.4 (77.0, 83.9) | 78.6 (76.3, 80.8) | 84.8 (83.6, 86.0) | 10.6** (3.7, 17.5) | 4.4* (0.8, 8.0) |
Note. CI = confidence interval; Pap = Papanicolaou. pp = percentage points. Sample-weighted averages give rates of each outcome variable for adults without dependent children in the corresponding analytic sample, based on data from the 2011–2016 and 2018–2019 Behavioral Risk Factor Surveillance System on US states other than Massachusetts and Vermont (i.e., the District of Columbia and US territories are also omitted).
Women aged 40–64 y (n = 114 523).
Women aged 50–64 y (n = 97 277).
Respondents aged 50–64 y (n = 174 701).
P < .05; **P < .01.
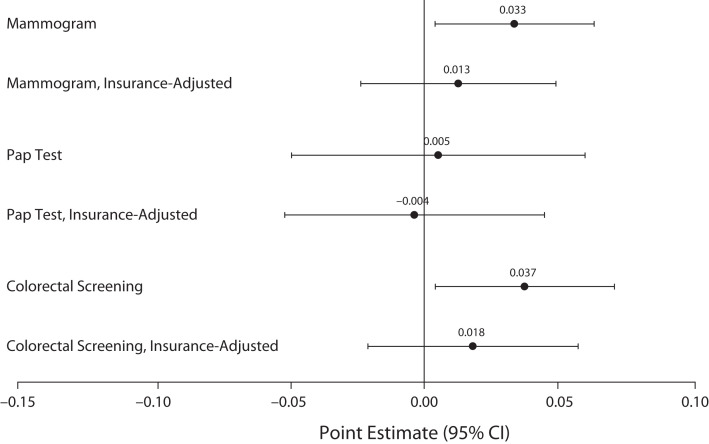
Comparing pre- versus postexpansion trends, triple-difference analyses linked Medicaid expansions to significant 3.3-percentage-point increases in past-2-year mammograms (95% confidence interval [CI] = 0.4, 6.3) among women aged 50 to 64 years under 138% of the FPG, and 3.7-percentage-point increases in past-5-year colorectal screening among adults aged 50 to 64 years under 138% of the FPG (95% CI = 0.4, 7.0), relative to those between 138% and 400% of FPG (Figure 1). Associations with Pap tests among women aged 40 to 64 years were also positive but statistically nonsignificant, at 0.5 percentage points (95% CI = −4.9, 5.9).
FIGURE 1—
Screening Responses to Medicaid Expansions: United States, Behavioral Risk Factor Surveillance System, 2011–2016 and 2018–2019
Note. CI = confidence interval; Pap = Papanicolaou. Sample weighted linear probability models estimated triple-difference specifications to approximate the relationship between state Medicaid expansions and cancer screening indicators among adults without dependent children who are not age-eligible for Medicare—specifically, comparing respondents in states that did vs did not expand Medicaid, before vs after expansions, with incomes below the expanded-access cutoff (138% of the federal poverty guideline [FPG; according to the US Department of Health and Human Services]) vs at or above it but below 400% of FPG. Point estimates and 95% CIs estimated screening responses to Medicaid expansions, with the analytic sample limited to those younger than 65 years for whom the screening was recommended—that is, women aged 50–64 years for biennial mammograms, women aged 40 to 64 years for Pap tests every 3 years, and respondents aged 45–64 years for colorectal screenings (colonoscopies or sigmoidoscopies) every 5 years. See Table A (available as a supplement to the online version of this article at https://ajph.org) for output in tabular form with P values.
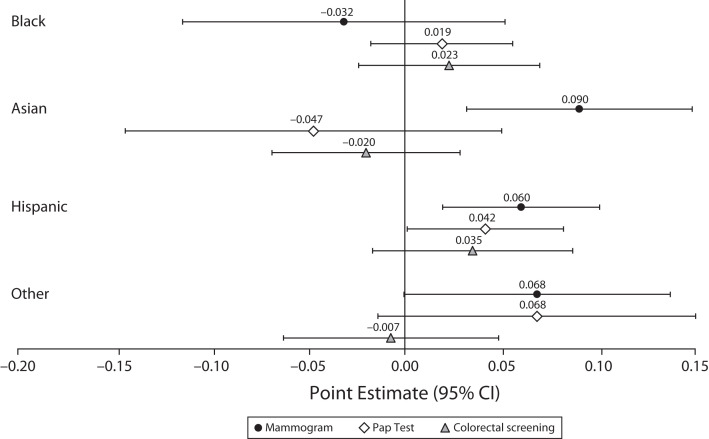
To clarify whether screening responses to state Medicaid expansions differed by race/ethnicity, Figure 2 presents coefficients and 95% CIs estimating how each subgroup’s cancer screening response differed from that of non-Hispanic White adults in the same analytic sample. Findings suggest greater responsiveness among Hispanic women for both mammograms (6.0 percentage points; 95% CI = 2.0, 10.1) and Pap tests (4.2 percentage points; 95% CI = 0.1, 8.2). Responses among other racial groups did not show a statistically significant difference from those of non-Hispanic White respondents, except for mammography among non-Hispanic Asian women (9.0 percentage points; 95% CI = 3.2, 14.8). Notably, the non-Hispanic White reference group’s screening responses were small and statistically nonsignificant in all cases (results not shown).
FIGURE 2—
Differential Screening Responses to Medicaid Expansions by Race/Ethnicity: United States, Behavioral Risk Factor Surveillance System, 2011–2016 and 2018–2019
Note. CI = confidence interval; Pap = Papanicolaou. Sample weighted linear probability models estimated triple-difference specifications to approximate the relationship between state Medicaid expansions and cancer screening indicators among adults without dependent children who are not age-eligible for Medicare—specifically, comparing respondents in states that did vs did not expand Medicaid, before vs after expansions, with incomes below the expanded-access cutoff (138% of the federal poverty guideline [FPG; according to the US Department of Health and Human Services]) vs at or above it but below 400% of FPG. Point estimates and 95% CIs plotted here estimated whether these screening responses differed by race/ethnicity, relative to the response among non-Hispanic White respondents. For each outcome, the analytic sample was limited to those younger than 65 years for whom the screening was recommended—that is, women aged 50–64 years for biennial mammograms, women aged 40–64 years for Pap tests every 3 years, and respondents aged 45–64 years for colorectal screenings (colonoscopies or sigmoidoscopies) every 5 years. See Table B (available as a supplement to the online version of this article at https://ajph.org) for output in tabular form with P values.
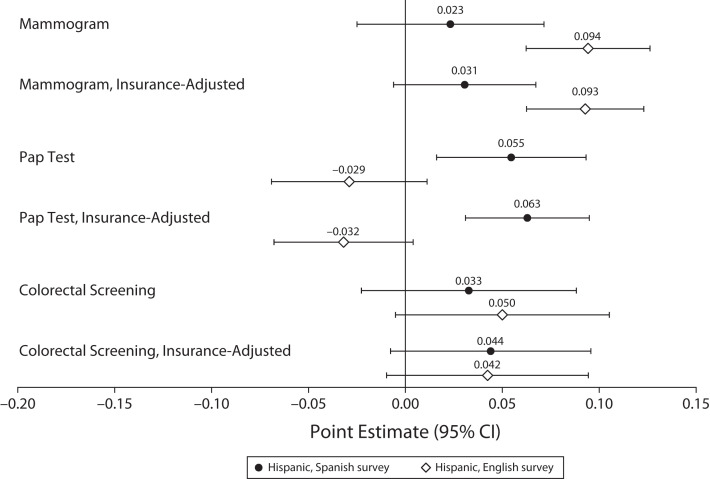
To better understand what might drive higher screening responses among Hispanic women, we repeated analyses interacting the Medicaid expansion variable with ethnicity-by-language indicators in place of race/ethnicity indicators (Figure 3), with and without a covariate for observed insurance status, to clarify whether the responses are explained by insurance alone (as opposed to concurrent changes in cost sharing, language accessibility, or cancer prevention outreach). Relative to non-Hispanic women, English-speaking Hispanic women showed a 9.4-percentage-point increase in rates of mammography (95% CI = 6.2, 12.6) and Spanish-speaking Hispanic women showed a 5.5-percentage-point increase in rates of Pap tests (95% CI = 1.6, 9.3) in response to Medicaid expansions. Moreover, for both of these screenings, the coefficient estimates for Spanish- versus English-speaking Hispanic women were statistically different, with P values of .01 for mammography and .001 for Pap tests.
FIGURE 3—
Differential Screening Responses to Medicaid Expansions by Ethnicity and Survey Language: United States, Behavioral Risk Factor Surveillance System, 2011–2016 and 2018–2019
Note. CI = confidence interval; Pap = Papanicolaou. Sample weighted linear probability models estimated triple difference specifications to approximate the relationship between state Medicaid expansions and cancer screening indicators among adults without dependent children who are not age-eligible for Medicare—specifically, comparing respondents in states that did vs did not expand Medicaid, before vs after expansions, with incomes below the expanded-access cutoff (138% of the federal poverty guideline [FPG]) vs at or above it but below 400% of FPG. Point estimates and 95% CIs plotted here estimate whether these screening responses differed between Hispanic respondents who completed the survey in English, Hispanic respondents who completed the survey in Spanish, and the non-Hispanic reference group. For each outcome, the analytic sample was limited to those younger than 65 years for whom the screening was recommended—that is, women aged 50–64 years for biennial mammograms, women aged 40–64 years for Pap tests every 3 years, and respondents aged 45–64 years for colorectal screenings (colonoscopies or sigmoidoscopies) every 5 years. See Table C (available as a supplement to the online version of this article at http://ajph.org) for output in tabular form with P values.
These findings held even when the specification explicitly controlled for reported insurance status, suggesting that the drivers extended beyond changes in insurance status alone. Results were similar when we limited consideration to survey waves when cancer screening questions were fielded nationwide (Figure A, available as a supplement to the online version of this article at https://ajph.org) and when we omitted states that expanded Medicaid after 2015 and before 2020 (Figure B, available as a supplement to the online version of this article at https://ajph.org). Omitting states with substantial early Medicaid expansions yielded comparable results for mammography and Pap tests, as well as significant positive associations between Medicaid expansion and colorectal screening among both English- and Spanish-speaking Hispanic adults: 6.1 percentage points (95% CI = 1.0, 11.2) and 5.2 percentage points (95% CI = 1.0, 9.5), respectively (Figure C, available as a supplement to the online version of this article at https://ajph.org).
To clarify whether findings were related to a specific subgroup of the Hispanic population, we repeated the analyses excluding residents of states with the largest Puerto Rican, Cuban, and Mexican populations—that is, New York, Florida, and California, respectively. Findings were similar to the main specification for mammography and Pap tests. Dropping New York led to significant coefficients for colorectal screening as well, consistent with the no-early-expanders specification that also omitted that state (Figures D‒F, available as supplements to the online version of this article at https://ajph.org).
DISCUSSION
To date, most Medicaid expansion studies have observed modest to negligible impacts on cancer screening behaviors despite increases in health care access and reductions in out-of-pocket costs.28 When we estimated average treatment effects, our study’s estimates were consistent with that literature. However, we also expanded upon that work by testing for heterogeneity in these responses. Specifically, Medicaid expansions were associated with greater increases in reported mammography among Asian and Hispanic women, and Pap testing among Hispanic women. Among Hispanic respondents, elevated mammography rates were driven more by English-speaking Hispanic women while greater rates of Pap testing stemmed more from Spanish-speaking Hispanic women. These findings are unique to this study and worth additional exploration.
Critically, differential responses were evident even when we controlled for respondent insurance status, suggesting that factors beyond increased rates of insurance coverage per se may have been important. Considering the US health care landscape between 2011 and 2019 suggests several potential mechanisms. First, ACA provisions requiring coverage of preventive care without cost sharing, including USPSTF-recommended cancer screening, may have increased screening behavior. Second, increased access to mammography services (e.g., via mobile mammography) may have contributed to increased mammography among Asian and Hispanic women.21 Third, between 2008 and 2019, several states increased language access services in Medicaid programs,29 and effective Spanish-language cervical cancer prevention and screening campaigns have been introduced.30 Similarly, observed increases in colorectal cancer screening coincide with national efforts to increase colorectal cancer screening to 80% among eligible individuals by 2020.31 By increasing the proportion of particular racial or ethnic groups with access to care, Medicaid expansions might have amplified screening campaigns’ impacts on those subgroups.16,17
Differential responses, however, help narrow the set of potential explanations. For example, evidence of increased screening among both English- and Spanish-speaking Hispanic women suggests that general changes in language accessibility are unlikely to fully explain these results. Indeed, limited English proficiency might dampen screening responses because language barriers can have a negative impact on health care access and quality,32,33 but this would not explain increased Pap testing among Spanish-speaking Hispanic women. Spanish language preference could also reflect a lack of US citizenship, a predictor of reduced health care access16 that may also be correlated with greater prioritization of cervical cancer screening because of Latin America’s elevated disease burden.34 This could help explain postexpansion increases in cervical cancer screening among Spanish- but not English-speaking Hispanic women. Of course, differential changes in Spanish- versus English-language cancer screening campaigns might also affect these results.
Limitations
This study had several limitations. First, reliance on self-reported data may introduce recall and social desirability biases. Reassuringly, this would only prejudice our differential-response estimates if state Medicaid expansions affected consequent misreporting and those biases were both stronger for a particular racial/ethnic subgroup and differentially so for those below 138% of FPG.
Second, while 12% of the BRFSS data on individuals aged 26 to 64 years lack income information, we did not use multiple imputation to address missing income data for 2 reasons: income observations are unlikely to be missing at random, and each round of imputation could alter the analytic sample (because incomes below 400% FPG are an inclusion criterion), creating further issues in comparing estimates across imputations.
Third, use of an indicator for completing the survey in Spanish is an imperfect proxy for language proficiency and may be correlated with citizenship status, which was not asked about in the BRFSS. If Hispanic respondents who completed the BRFSS survey in Spanish were more likely to come from mixed-immigration-status households and, thus, less likely to enroll in Medicaid,35 we would expect reduced health care utilization in that subgroup relative to those who completed the survey in English. Thus, we might expect our estimates of screening responses among Spanish-speaking Hispanic respondents to be higher if we could limit the sample to citizens and documented residents to ensure that immigration statuses did not restrict respondents’ Medicaid eligibility.
Finally, while triple-difference analyses offer a rigorous, quasi-experimental approach to identifying a policy change’s effects, they are not randomized controlled trials. The plausibility of a causal interpretation here is bolstered by our data: as BRFSS is administered via random-digit dialing, the timing of a person’s survey date relative to their state’s Medicaid expansion is effectively random. To bias the overall policy effect estimates presented here, a confounder would need to be correlated with the Medicaid expansions’ locations and timing, and apply only to respondents under 138% of the FPG. As unmeasured early expansions might meet this criterion, it is reassuring that findings hold when we omitted states with substantive pre-2014 expansions.
Even if the estimated policy effect were causal, regression specifications allowing it to differ by race/ethnicity would not confirm whether Medicaid expansion effects on cancer screening were modified by race/ethnicity per se, versus a correlate (e.g., trust in the health care system). While the specific mechanism does not change Medicaid expansions’ overall implications for racial/ethnic disparities in cancer screening, it is an important avenue for future work: if such correlates are susceptible to intervention, that may provide an alternative approach to reducing racial/ethnic disparities in cancer screening and consequent mortality.
Our findings reinforce the importance of continued examination of heterogeneity in policy effects, not only by sex and race/ethnicity, as is more common, but also in terms of less commonly measured factors like preferred language, language proficiency, and citizenship status. As racial and ethnic groups are heterogeneous, research like the efforts of Alcalá et al. to differentiate ACA impacts among Latino subpopulations is also warranted.16 Identifying different benefits from and avenues for intervention to increase preventive health care in underserved groups offers a means to improve overall population health while reducing disparities and, thus, should be a key priority for future work.
Public Health Implications
In this study of nationally and state-representative BRFSS data, state Medicaid expansions were associated with varying changes in cancer screening depending on respondent ethnicity and race. Specifically, relative to non-Hispanic White women, expansions were linked to greater increases in mammography among Asian and Hispanic women, and increased Pap testing among Hispanic women. Moreover, these relationships differed between Hispanic women who completed their survey in English versus Spanish. Critically, insurance status per se did not explain these results, suggesting that there may be other consequential avenues for intervention to reduce disparities in cancer screening. Future research and cancer screening surveillance should consider the role of respondent and family citizenship status, as well as how regulations and interventions affecting language accessibility and access to care might affect cancer screening in subgroups experiencing disproportionate morbidity and mortality.
ACKNOWLEDGMENTS
S. Thomas’s work on this research was supported by an Herb Scarf Summer Research Opportunity and a Tobin Undergraduate Research Assistantship. Both A. S. Friedman and S. C. Suttiratana have other research funded through awards from the National Institutes of Health and US Food and Drug Administration’s Center for Tobacco Products.
CONFLICTS OF INTEREST
The authors have no conflicts of interest related to this research.
HUMAN PARTICIPANT PROTECTION
This research used only publicly available de-identified data and, thus, was deemed exempt from human participant review (institutional review board protocol ID 2000032422).
REFERENCES
- 1.Curry SJ, Byers T, Hewitt ME. Fulfilling the Potential of Cancer Prevention and Early Detection. Washington, DC: National Academies Press; 2003. National Cancer Policy Board. Potential of screening to reduce the burden of cancer. [PubMed] [Google Scholar]
- 2.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 3.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force. Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 5.Gray TF, Cudjoe J, Murphy J, Thorpe RJ, Wenzel J, Han HR. Disparities in cancer screening practices among minority and underrepresented populations. Semin Oncol Nurs. 2017;33(2):184–198. doi: 10.1016/j.soncn.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Carethers JM, Sengupta R, Blakey R, Ribas A, D’Souza G. Disparities in cancer prevention in the COVID-19 era. Cancer Prev Res (Phila). 2020;13(11):893–896. doi: 10.1158/1940-6207.CAPR-20-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 8.Benard VB, Watson M, Saraiya M, et al. Cervical cancer survival in the United States by race and stage (2001‒2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5119–5137. doi: 10.1002/cncr.30906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiscella K, Humiston S, Hendren S, et al. Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take? J Health Care Poor Underserved. 2011;22(1):83–100. doi: 10.1353/hpu.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel MS, Wee CC, McCarthy EP, Davis RB, Ngo-Metzger Q, Phillips RS. Racial and ethnic disparities in cancer screening: the importance of foreign birth as a barrier to care. J Gen Intern Med. 2003;18(12):1028–1035. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha S, Arbelaez JJ, Cooper LA. Patient–physician relationships and racial disparities in the quality of health care. Am J Public Health. 2003;93(10):1713–1719. doi: 10.2105/AJPH.93.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Alba I, Sweningson JM. English proficiency and physicians’ recommendation of Pap smears among Hispanics. Cancer Detect Prev. 2006;30(3):292–296. doi: 10.1016/j.cdp.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Kwon HT, Ma GX, Gold RS, Atkinson NL, Wang MQ. Primary care physicians’ cancer screening recommendation practices and perceptions of cancer risk of Asian Americans. Asian Pac J Cancer Prev. 2013;14(3):1999–2004. doi: 10.7314/APJCP.2013.14.3.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinh QD, Li H, Meyer CP, et al. Determinants of cancer screening in Asian-Americans. Cancer Causes Control. 2016;27(8):989–998. doi: 10.1007/s10552-016-0776-8. [DOI] [PubMed] [Google Scholar]
- 15.Lau JS, Adams SH, Park MJ, Boscardin WJ, Irwin CE. Improvement in preventive care of young adults after the Affordable Care Act: the Affordable Care Act is helping. JAMA Pediatr. 2014;168(12):1101–1106. doi: 10.1001/jamapediatrics.2014.1691. [DOI] [PubMed] [Google Scholar]
- 16.Alcalá HE, Chen J, Langellier BA, Roby DH, Ortega AN. Impact of the Affordable Care Act on health care access and utilization among Latinos. J Am Board Fam Med. 2017;30(1):52–62. doi: 10.3122/jabfm.2017.01.160208. [DOI] [PubMed] [Google Scholar]
- 17.Buchmueller TC, Levy HG. The ACA’s impact on racial and ethnic disparities in health insurance coverage and access to care: an examination of how the insurance coverage expansions of the Affordable Care Act have affected disparities related to race and ethnicity. Health Aff (Millwood). 2020;39(3):395–402. doi: 10.1377/hlthaff.2019.01394. [DOI] [PubMed] [Google Scholar]
- 18.Heintzman J, Bailey SR, DeVoe J, et al. In low-income Latino patients, post-Affordable Care Act insurance disparities may be reduced even more than broader national estimates: evidence from Oregon. J Racial Ethn Health Disparities. 2017;4(3):329–336. doi: 10.1007/s40615-016-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angier H, Hoopes M, Marino M, et al. Uninsured primary care visit disparities under the Affordable Care Act. Ann Fam Med. 2017;15(5):434–442. doi: 10.1370/afm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendryx M, Luo J. Increased cancer screening for low-income adults under the Affordable Care Act Medicaid expansion. Med Care. 2018;56(11):944–949. doi: 10.1097/MLR.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 21.Vang S, Margolies LR, Jandorf L. Mobile mammography participation among medically underserved women: a systematic review. Prev Chronic Dis. 2018;15:E140. doi: 10.5888/pcd15.180291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alharbi AG, Khan MM, Horner R, Brandt H, Chapman C. Impact of Medicaid coverage expansion under the Affordable Care Act on mammography and Pap tests utilization among low-income women. PLoS One. 2019;14(4):e0214886. doi: 10.1371/journal.pone.0214886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy GP., Jr The effects of cost sharing on access to care among childless adults. Health Serv Res. 2010;45(6 pt 1):):1720–1739. doi: 10.1111/j.1475-6773.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon K, Soni A, Cawley J. The impact of health insurance on preventive care and health behaviors: evidence from the first two years of the ACA Medicaid expansions. J Policy Anal Manage. 2017;36(2):390–417. doi: 10.1002/pam.21972. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality. 2022. https://www.ahrq.gov/prevention/guidelines/guide/index.html [DOI] [PubMed]
- 26.Abadie A, Athey S, Imbens GW, Wooldridge J. When should you adjust standard errors for clustering? NBER working paper no. 24003. Cambridge, MA: National Bureau of Economic Research; 2017. [Google Scholar]
- 27.Greene W. The behaviour of the maximum likelihood estimator of limited dependent variable models in the presence of fixed effects. Econom J. 2004;7(1):98–119. doi: 10.1111/j.1368-423X.2004.00123.x. [DOI] [Google Scholar]
- 28.Sabik LM, Adunlin G. The ACA and cancer screening and diagnosis. Cancer J. 2017;23(3):151–162. doi: 10.1097/PPO.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youdelman M.2019. https://healthlaw.org/resource/summary-of-state-law-requirements-addressing-language-needs-in-health-care-2
- 30.Baezconde-Garbanati L, Ochoa CY, Murphy ST, et al. 2019. https://www.ncbi.nlm.nih.gov/books/NBK573240/#_NBK573240_pubdet_
- 31.Whitaker DE, Snyder FR, San Miguel-Majors SL, Bailey LO, Springfield SA.2020. [DOI]
- 32.Al Shamsi H, Almutairi AG, Al Mashrafi S, Al Kalbani T. Implications of language barriers for healthcare: a systematic review. Oman Med J. 2020;35(2):e122. doi: 10.5001/omj.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himmelstein J, Himmelstein DU, Woolhandler S, et al. Health care spending and use among Hispanic adults with and without limited English proficiency, 1999‒2018. Health Aff (Millwood). 2021;40(7):1126–1134. doi: 10.1377/hlthaff.2020.02510. [DOI] [PubMed] [Google Scholar]
- 34.Lopez MS, Baker ES, Maza M, et al. Cervical cancer prevention and treatment in Latin America. J Surg Oncol. 2017;115(5):615–618. doi: 10.1002/jso.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen MS, Schpero WL. Household immigration status had differential impact on Medicaid enrollment in expansion and nonexpansion states. Health Aff (Millwood). 2018;37(3):394–402. doi: 10.1377/hlthaff.2017.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]