Abstract
Electrical impedance spectroscopy has clinical relevance in diagnosing malignancy in melanocytic lesions. Sixty-eight lesions with changes during digital follow-up of patients at very high risk of developing melanoma were prospectively included in this study from February to December 2016. Electrical impedance spectroscopy and reflectance confocal microscopy were performed to evaluate their performance in this subset of difficult lesions. Forty-six lesions were considered suspicious on reflectance confocal microscopy and were excised, of these, 19 were diagnosed as melanoma. Fifteen melanomas were detected by electrical impedance spectroscopy, while 4 received a score lower than 4, which suggested no malignancy. The addition of reflectance confocal microscopy improves accuracy while maintaining the same sensitivity. In the case of electrical impedance spectroscopy scores <4, lesions exhibiting changes in follow-up may need short-term monitoring or excision if dermoscopy shows criteria for melanoma. Results of electrical impedance spectroscopy in this subset of very early lesions should be carefully considered due to the risk of false negatives.
Key words: melanoma, dermoscopy, electrical impedance spectroscopy, reflectance confocal microscopy
Early diagnosis of melanoma in very high-risk patients requires specific strategies, such as the 2-step method for digital monitoring, including total-body photography and digital dermoscopy (1, 2). This system has been internationally implemented and is considered cost-efficient (3, 4). The specificity of the surveillance may be improved with the additional use of reflectance confocal microscopy (RCM) (5, 6). This increases the specificity, reducing the number of lesions required to be excised to remove 1 melanoma. Arguments against the implementation of RCM include the cost of the equipment, the time required per lesion and the training required to adequately perform the diagnosis.
SIGNIFICANCE
Electrical impedance spectroscopy is a diagnostic tool developed to support clinicians in detecting early melanoma in the general population. This study examined its use in very high-risk patients with difficult lesions. When electrical impedance spectroscopy detected a lesion as suspicious it was evaluated with reflectance confocal microscopy, which is accurate, and, if lesions were considered suspicious, they were excised. Most of the malignant lesions were detected. In conclusion, electrical impedance spectroscopy can be useful in the detection of melanoma, but showed lower accuracy than reflectance confocal microscopy. Follow-up is recommended if lesions are considered not suspicious, in order to rule out melanoma in these patients.
In this scenario, other diagnostic techniques, such as multispectral imaging and electrical impedance spectroscopy (EIS) (registered as Nevisense®; Scibase, Stockholm, Sweden), which is less expensive and easy to apply for a trained nurse or technician, have been developed, in order to help physicians detect melanoma at early stages (7, 8).
A study by Malvehy et al. (9) in 2014 using EIS to evaluate the accuracy and safety of the instrument concluded that a negative or positive EIS score could be used as guidance to determine whether a lesion should be excised. This was the largest prospective multicentre study performed in the field of melanoma diagnosis, showing that EIS has a sensitivity of 96.6% and specificity 34.4%, in the context of routine dermatological practice. A more recent study by Rocha et al. evaluated the effect of adding EIS measurement to the assessment of lesions undergoing routine short-term digital follow-up, and found it reduced the need for follow-up in almost half of cases (10). The aim of the current study was to analyse the performance of EIS and RCM in examination of changing lesions identified during long-term digital follow-up in patients at very high risk of developing melanoma.
MATERIALS AND METHODS
Patients undergoing total-body photography and digital dermoscopy at our institution at Hospital Clínic Barcelona, who were considered at very high risk of developing melanoma, as previously described (2, 11), were eligible for the current study. Mole Max HDs (Derma Instruments; Vienna, Austria) were used for total-body photography and digital dermoscopy monitoring. High-resolution dermoscopy using Dermlite Foto (3Gen, Dana Farber, San Juan Capistrano, CA, USA) attached to a Canon PowerShot G11 camera was obtained for dermoscopic evaluation of changing lesions detected during follow-up. The study included changing melanocytic lesions detected on digital monitoring prospectively from February to December 2016. Subsequent to written informed consent, every lesion was evaluated with an EIS device (Nevisense, Scibase, ) then sent for RCM to inform management decisions. The clinicians making the decisions to excise the lesions after RCM evaluation (all with more than 5 years of expertise in interpreting RCM) were unaware of the EIS results. Following standard clinical practice, lesions suspicious for malignancy according to RCM were excised, histopathology evaluation performed and managed accordingly. If RCM was not exhibiting criteria of malignancy, lesions were then followed up for 18 months, at intervals of 6 months, to detect additional changes.
Prior to EIS measurement, the skin was moistened for 30 s with saline solution, after which a reference measurement of healthy skin close to the lesion was performed. The procedure was then repeated on the lesion under study. The system measures the overall electrical resistance and reactance at 35 different frequencies logarithmically distributed between 1.0 kHz and 2.5 MHz at 4 depth settings with a total of 10 permutations. The applied voltage and resulting current is limited to 150 mV and 75 μA, respectively, and is not felt by the patient. Measurements take approximately 8 s, and, within seconds, the system computes both a score (0–10) and a dichotomous output (EIS negative/positive) at a fixed cut-off point. The threshold limit is set at 4, i.e. scores < 4 are EIS-negative and scores of ≥ 4 are EIS-positive.
To capture RCM images, a Vivascope 1500® (MAVIG GmbH, Munich, Germany) was used. Mosaic images were captured horizontally by VivaBlock® (Caliber Imaging and Diagnostics, Rochester, NY, USA).
Excised lesions were formalin fixed and paraffin embedded, total inclusion and step sectioning performed for haematoxylin and eosin staining. HMB45, S-100, SOX10 and double immunohisto-chemistry with MelanA and Ki-67 was performed when needed.
Pearson’s χ2 test and Student’s t-test were preliminarily performed to compare categorical and continuous variables, respectively, and to evaluate potential differences in the distribution of variables among groups.
This study had 2 co-primary aims: to demonstrate the accuracy of both the Nevisense device in this specific difficult scenario, and the accuracy of dermoscopy using a well-established score: the total dermoscopy score (TDS) from the ABCD rule of dermoscopy, where each of the criteria is multiplied by a given factor to yield a total dermoscopy score. TDS values less than 4.75 indicate a benign melanocytic lesion, values between 4.8 and 5.45 indicate a suspicious lesion, and values of 5.45 or greater are highly suggestive of melanoma, as reviewed by Carrera et al. (12), and to compare the outcome with the performance of RCM in clinical practice: (i) a 1-sided exact 95% confidence bound of the sensitivity in detecting cutaneous melanoma of > 90% (sensitivity ≥ 0.90 to detect melanoma); (ii) non-random result at the given sensitivity, i.e. sensitivity + specificity > 1.0. The analysis was conducted on eligible and evaluable lesions.
All analyses were performed using Stata/MP 15.0 Statistical Software (StataCorp, College Station, TX, USA).
RESULTS
A total of 68 lesions were identified to have significant changes (11) and were included in the study. The mean TDS was 4.26 (1.51, range 1–8.8) and mean EIS 4.75 ± 1.92 (range 0–9). Of the 68 lesions included, after the evaluation with RCM, 46 were considered suspicious and consequently excised. Histopathological diagnosis evaluated by 2 different dermatopathologists was melanoma in 19 lesions and naevi in the remaining 27. Of the 19 melanomas detected, 11 were in situ and 8 were invasive (mean Breslow thickness was 0.52 mm, range 0.16–0.94 mm). Two melanomas were detected in one patient with previous personal and familial history of melanoma. The mean ± standard deviation (SD) age of patients included in the study was 48 ± 12.9 years (range 18–79); 49% were women. Sixty percent had a previous personal history of melanoma and 39% had a familiar history of melanoma. The mean age of patients with excised lesions was 46.61 years and for patients with non-excised lesions 49.59 years. The mean age of patients with a diagnosis of melanoma was slightly higher than those with non-melanoma (48.95 ± 18.5 compared with 47.04 ± 10.1; p = 0.002).
The clinical characteristics of 18 patients with a diagnosis of melanoma are shown in Table I and indicate the extreme level of risk of the patients: 12 previously diagnosed with multiple primary melanomas, 10 belonging to melanoma families, and 6 carriers of mutations in CDKN2A.
Table I.
Clinical dermoscopic features of 19 melanomas included
| N | Age, years/Sex | Background | Location | EIS score | DP pattern | TDS | Breslow |
|---|---|---|---|---|---|---|---|
| 1 | F/18 | Familiar MM, CDKN2 mutation | Leg | 1 | Multicomponent | 4.7 | Melanoma in situ arising on naevus |
| 2 | F/44 | DNS | Trunk | 2 | Globular | 5.1 | SSM 0.42 |
| 3 | F/78 | Familiar MM, Multiple MM | Trunk | 3 | Reticular | 4.3 | Melanoma in situ |
| 4 | F/78 | Familiar MM, Multiple MM | Leg | 3 | Reticulo globular | 3.2 | Melanoma in situ |
| 5 | F/41 | DNS, Familiar MM | Trunk | 4 | Reticular | 4.7 | Melanoma in situ arising on naevus |
| 6 | M/46 | Multiple MM | Leg | 4 | Reticular | 4.1 | SSM 0.78 |
| 7 | M/46 | Multiple MM | Trunk | 9 | Reticular | 4.6 | Melanoma in situ arising on congenital naevus |
| 8 | M/48 | Multiple MM | Trunk | 4 | Reticular-inverted network | 5.0 | Melanoma in situ arising on compound naevus |
| 9 | M/40 | Multiple MM | Trunk | 5 | Reticular | 5.4 | Melanoma in situ on naevus |
| 10 | M/68 | Multiple MM | Trunk | 5 | Reticular-inverted network | 4.1 | SSM 0.32 |
| 11 | M/43 | Familiar MM, CDKN2 mutation, Multiple MM | Arm | 6 | Reticular | 5.3 | SSM 0.16 |
| 12 | F/37 | DNS, Familiar MM, Multiple MM | Trunk | 6 | Reticular | 4.9 | Melanoma in situ arising on congenital naevus |
| 13 | M/31 | DNS, Familiar MM | Trunk | 6 | Reticular | 3.8 | SSM 0.6 arising on congenital naevus |
| 14 | M/44 | DNS, Familiar MM | Trunk | 7 | Reticular | 3.3 | Melanoma in situ |
| 15 | F/77 | Actinic Damage | Arm | 7 | Reticular | 6.0 | Melanoma in situ |
| 16 | M/79 | Multiple MM, CDKN2A mutation | Trunk | 7 | Reticular | 5.6 | Melanoma in situ |
| 17 | M/36 | Familiar MM, Multiple MM, CDKN2 mutation | Trunk | 9 | Multicomponent | 7.7 | SSM 0.43 |
| 18 | M/36 | Familiar MM, Multiple MM CDKN2 mutation | Trunk | 8 | Multicomponent | 8.8 | SSM 0.94 arising on congenital naevus |
| 19 | M/36 | Familiar MM, Multiple MM CDKN2 mutation | Leg | 7 | Multicomponent | 7.2 | SSM 0.54 |
EIS: electrical impedance spectroscopy; DP: dermoscopy; TDS: total dermoscopy score; MM: malignant melanoma; DNS: dysplastic naevus syndrome; SSM: superficial spreading melanoma.
Total dermoscopy score
Mean TDS was 4.2 ± 1.5, with no differences between melanoma and naevi (5.1 ± 0.3 vs 3.9 ± 0.2; p = 0.38) nor between mean TDS for excised lesions and those not excised (4.68 ± 1.49 vs 3.38 ± 1.17; p = 0.43). Mean TDS for invasive melanomas was 5.76 ± 1.79 higher compared with melanoma in situ 4.7 ± 1.9, p = 0.006.
Of the 19 melanomas, TDS scored as malignant (TDS > 5.45) in only 5 lesions, and 5 additional lesions scored as suspicious (TDS > 4.8 and < 5.45). TDS for suspicion of malignancy (TDS > 4.8) had a sensitivity of 52.66% and specificity of 61.22%. For in situ melanoma, TDS sensitivity was 45.45% and specificity 57.89% (positive predictive value (PPV) 17.24 and negative predictive value (NPV) 84.62). Regarding invasive melanoma, TDS sensitivity was 62.5% and specificity 60%, with a PPV of 21.43% and a NPV of 90.74%.
Electrical impedance spectroscopy
EIS identified 53 lesions as suspicious (a score of more than 3, as previously described). Of these, 15 were melanomas and 38 were naevi. Four melanomas had an EIS score < 4 (Fig. 1). Sensitivity and specificity of EIS was 78% and 22%, respectively. PPV was 28% and NPV 73%. Mean EIS in excised lesions was 4.98 ± 1.90 vs 4.27 ± 1.93 in non-excised lesions (p = 0.9). Mean EIS in melanoma was 5.42 ± 2.26 and in naevi 4.49 ± 1.73 (p = 0.11). In the case of in situ melanoma EIS had 72.73% sensitivity and 21.05% specificity (PPV 15.09%, NPV 80%). For invasive melanoma EIS had 87.5% sensitivity and 76.67 specificity with PPV 17% and NPV of 92%.
Fig. 1.
Dermoscopy of 4 melanomas with an electrical impedance spectroscopy score below 4. (A) Case 1. Asymmetrical melanocytic lesion with multicomponent pattern, an irregular light-brown network on the periphery of the lesion, dotted vessels in the centre of the lesion and an area with inverted network (total dermoscopy score (TDS) 4.7). (B) Case 2. Asymmetrical melanocytic lesion, with an irregular globular pattern: globules of different size and colour at the periphery and a papillomatous component in the centre of the lesion (TDS 5.1). (C) Case 3. Asymmetrical melanocytic lesion with an atypical hyperpigmented network (TDS 4.3). (D) Case 4. Asymmetrical melanocytic lesion with different shades of brown and a few irregular globules on the periphery (TDS 3.2).
Reflectance confocal microscopy
In this study, the sensitivity and specificity of RCM was 100% and 44%, respectively. PPV was 41% and NPV 100%. For in situ melanoma, RCM was 100% sensitive and 40.35% specific (PPV 24.44% and NPP 100%). Regarding invasive melanoma, RCM had 100% sensitivity and 38.33% specificity, with PPV 17.78% and NPV 100%. Clinical and dermoscopic features of the 19 melanomas are shown in Table I.
DISCUSSION
RCM following dermoscopic selection is as sensitive as, but more specific than, any other imaging technique for the diagnosis of melanoma (13). Limitations to its full implementation in the dermatological field include the cost of the equipment and the need for specific training for both the imaging and interpretation of images for diagnosis. On the other hand, EIS is a less expensive technique that does not require training for interpretation and has been developed as a tool to support clinicians in detecting early melanoma in the general population (9, 14). The current study focused on very high-risk patients (> 50 naevi and a personal or familial history of melanoma) (2, 15). In these types of patients, the clinical diagnosis of a melanocytic lesion should be interpreted according to each patient’s personal history (phenotype, genotype, family history, age, etc.), since in such cases a very low TDS could still be associated with a malignant lesion (16).
In the current study, EIS was not able to detect 4 melanomas that had a relatively high TDS, which reduced the sensitivity compared with a previous study in the general population (9). Dermoscopic images of 4 lesions can be seen in Fig. 1. Case 1 showed the dermoscopic features of early melanomas in low phototype, including dotted vessels and shiny white streaks on a light-brown atypical pigment network pattern. Digital follow-up showed increased size, with pigmentation at the periphery and new melanoma-associated structures, such as white shiny streaks. Dermoscopic digital follow-up images and histological sections of this difficult lesion are shown in Fig. 2. Putting all the information together, this lesion should be excised (17). Considering case 2, the presence of dark globules in an asymmetrical distribution in dermoscopy was indicative of excision, because of the suspicion of a Spitzoid lesion (17) or melanoma. The other 2 lesions were small and with fewer suspicious features in dermoscopy, on a patient with multiple previous melanomas and familial history of melanoma. In particular, case 3 was an asymmetrical lesion in terms of colour, with only reticular structure, while case 4 showed the presence of reticular and globular patterns together in the context of a very small diameter lesion. Both lesions were melanoma in situ, and, in both cases, digital follow-up was able to detect significant changes compared with the previous images.
Fig. 2.
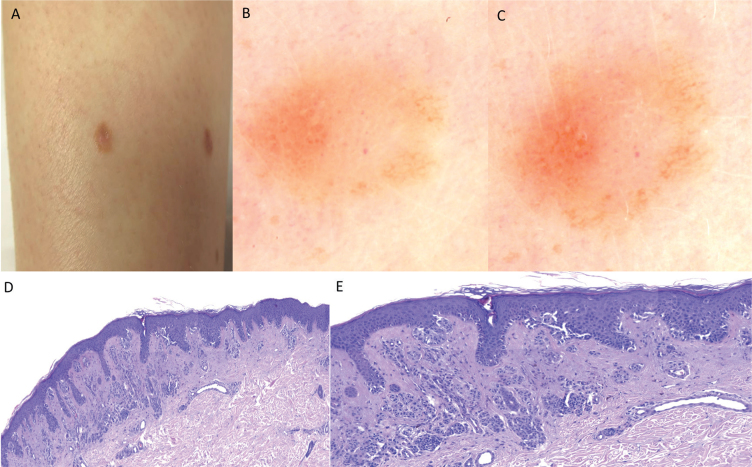
(A) Melanoma in situ (electrical impedance spectroscopy (EIS) <4) on the lower leg of a woman with atypical mole syndrome and personal and familial history of melanoma (5 mm diameter). (B and C) One-year digital dermoscopy follow-up of case 1. Asymmetrical melanocytic lesion with multicomponent pattern, irregular growth of the brown network at the periphery of the lesion can be appreciated after 1 year, persistent dotted vessels in the centre of the lesion and area with subtle inverted network. (D) Histopathological section of the lesion, melanoma arising on a naevus, single/isolated atypical melanocytic proliferation, continuous on suprapapillary areas and confluence of different sized nests. At dermal level, deep melanocytic proliferation (magnification × 10). (E) Detail of D. At greater magnification, isolated melanocytic proliferation with confluence in suprapapillary areas and pagetoid cells (magnification × 20).
The 4 lesions all showed an atypical epidermal pattern at RCM with pagetoid cells, giving reason to suspect a malignant lesion (Fig. 3). Moreover, case 2 also showed some dermal malignant features (different refractivity of dermal nests), reflecting the existence of dermal invasive structures.
Fig. 3.
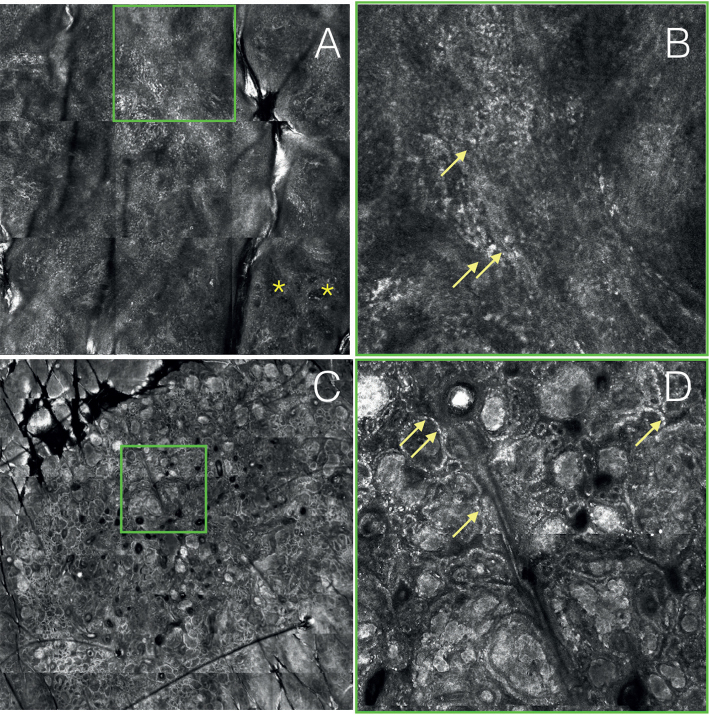
Reflectance confocal microscopy (RCM) in 2 of the melanomas from Fig. 1. (A) RCM at basal cell layer (case 3): atypical cobblestone and roundish and dendritic pagetoid infiltration of the epidermis. In the lower right part, presence of non-edged papillae (*). (B) RCM of a particular of A: disarranged honeycomb and dendritic pagetoid cells. (C) RCM VivaBlock® at the junctional layer (case 2): presence of edged and non-edged papillae. Dense and dense/sparse nests with different refractivity. (D) RCM of a particular of C: cellular polymorphism inside the nests. Atypical hyper-refractile nucleated cells are visible (arrows).
There are several plausible explanations for the relatively low sensitivity of EIS observed in the present study compared with previous studies. First, those lesions detected during digital monitoring were incipient (3 out of 4 were in situ) and small in diameter. The semiology of those lesions was quite scarce in terms of malignant dermoscopic features. The suspicion of melanoma, and thus the indication of excision, was based mainly on observed changes seen through digital dermoscopy and the melanoma-specific criteria identified in RCM. In previous studies the benefits of the addition of RCM in a subset of lesions under digital follow-up has been reported (5, 6, 18, 19). RCM increases accuracy in the detection of melanoma in these very difficult patients. The current results are similar to those reported by our group (5) and Stanganelli et al. (6) in previous studies. Even if our sensitivity was 100%, the results from Stanganelli et al. (6) were inferior in accuracy using RCM and the authors concluded that these negative lesions should be considered for further follow-up to rule out a melanoma even if RCM is negative.
In a previous large prospective study, Nevisense exhibited a high sensitivity for melanoma detection (9). However, Nevisense (EIS) seems to be less sensitive in the detection of incipient malignant lesions. In the multicentre prospective study, sensitivity of EIS for melanoma in situ was significantly lower (93.8%) compared with sensitivity of 100% for lesions with a Breslow thickness of more than 1 mm, or thinner but ulcerated. In a different study by Rocha et al. (10), they used Nevisense (EIS) in lesions that were suspicious at the first visit, to decide whether short-term monitoring was needed. In that study, Nevisense showed good performance, detecting lesions that should be excised without missing melanomas. In contrast to the study by Rocha, the current study focuses on very high-risk patients during long-term follow-up. In this specific context, in our practice we recommend the excision of lesions if they exhibit significant dermoscopic changes, as described previously (2). In order to improve specificity and decrease the number of benign lesions excised, examination with RCM was the most accurate method to approach such patients. However, in circumstances in which RCM would not be feasible because of the long learning curve or because of cost, EIS could be used combined with dermoscopy and digital follow-up. Based on the results of the current study, it could be considered that if the EIS is > 3 or the lesion shows specific criteria of melanoma under dermoscopy (as in cases 1 and 2 in our series) lesions should be excised and for those lesions with non-suspicious dermoscopy and EIS < 4 additional short-term follow-up would be recommended to rule out malignancy.
This study has some limitations. First, the number of lesions included was low and the results may not be representative in a larger cohort of patients. However, the number of melanomas in this selected very high-risk population was significant and, in our opinion, the results are conclusive. Secondly, this is not a multicentre study and, in different populations with different genetic background for melanoma risk, the results could be different. Finally, the time of follow-up of slow-growing melanoma could be longer than the time defined in the current study of 18 months. Not all of the included lesions were excised for histopathology since, when RCM indicated benignity, the lesions were submitted to additional follow-up of 18 months, at intervals of 6 months, to detect additional changes. After 18 months no changes were detected. The risk of misdiagnosing a melanoma in stable lesions after 18 months of follow-up seems to be very low.
In conclusion, in a very high-risk population of patients with atypical moles, early detection of melanoma is challenging, and the addition of RCM to digital FU improves accuracy. EIS can be useful in the detection of melanoma, but showed lower accuracy than RCM. Excision of lesions exhibiting malignant dermoscopic features is suggested. Follow-up is recommended where lesions are considered not suspicious after RCM evaluation, EIS and dermoscopy, so as to rule out melanoma in these patients.
ACKNOWLEDGEMENTS
The study in the Melanoma Unit, Hospital Clínic, Barcelona was supported in part by grants from Fondo de Investigaciones Sanitarias P.I. 12/00840, PI15/00956 and PI15/00716 Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co-funded by “Fondo Europeo de Desarrollo Regional (FEDER). Unión Europea. Una manera de hacer Europa”; by the AGAUR 2014_SGR_603 and 2017_SGR_1134 of the Catalan Government, Spain; by a grant from “Fundació La Marató de TV3, 201331-30”, Catalonia, Spain; by the European Commission under the 6th Framework Programme, Contract nº: LSHC-CT-2006-018702 (GenoMEL); by CERCA Programme / Generalitat de Catalunya and by a Research Grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain.
The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; nor in the preparation, review, approval of the manuscript, or in the decision to submit the manuscript for publication.
This study has been approved by the institutional review board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. Registration 2010/5605.
Footnotes
Conflicts of interest. JM has been scientific consultant for Nevisense.
REFERENCES
- 1.Malvehy J, Puig S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: a two-step method. Clin Dermatol 2002; 20: 297–304. [DOI] [PubMed] [Google Scholar]
- 2.Salerni G, Carrera C, Lovatto L, Puig-Butille J A, Badenas C, Plana E, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol 2012; 67: e17–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moloney FJ, Guitera P, Coates E,Haass N, Ho K, Khoury R, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol 2014; 150: 819–827. [DOI] [PubMed] [Google Scholar]
- 4.Tromme I, Devleesschauwer B, Beutels P, Richez P, Praet N, Sacré L, et al. Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi. PLoS ONE 2014;9: e109339–e109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovatto L, Carrera C, Salerni G, Alós L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatology Venereol 2015; 29: 1918–1925. [DOI] [PubMed] [Google Scholar]
- 6.Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol 2015; 172: 365–371. [DOI] [PubMed] [Google Scholar]
- 7.Aberg P, Nicander I, Hansson J, Geladi P, Holmgren U, Ollmar S. Skin cancer identification using multifrequency electrical impedance – a potential screening tool. IEEE Trans Biomed Eng 2004; 51: 2097–2102. [DOI] [PubMed] [Google Scholar]
- 8.Mohr P, Birgersson U, Berking C, Henderson C, Trefzer U, Lajos K, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Ski Res Technol 2013; 19: 75–83. [DOI] [PubMed] [Google Scholar]
- 9.Malvehy J, Hauschild A, Curiel-Lewandrowski C, Mohr P, Hofmann-Wellenhof R, Motley R, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol 2014; 171: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha L, Menzies SW, Lo S, Avramidis M, Khoury R, Jackett L, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol 2017; 177: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 11.Salerni G, Carrera C, Lovatto L, Martí-Laborda RM, Isern G, Palou J, et al. Characterization of 1152 lesions excised over 10 years using total-body photography and digital dermatoscopy in the surveillance of patients at high risk for melanoma. J Am Acad Dermatol 2012; 67: 836–845. [DOI] [PubMed] [Google Scholar]
- 12.Carrera C, Marchetti MA, Dusza SW, Argenziano G, Braun RP, Halpern AC, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma a web-based international dermoscopy society study. JAMA Dermatol 2016; 10022: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards SJ, Osei-Assibey G, Patalay R, Wakefield V, Karner C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: a systematic review. Clin Exp Dermatol 2017; 42: 266–275. [DOI] [PubMed] [Google Scholar]
- 14.Svoboda RM, Prado G, Mirsky RS, Rigel DS. Assessment of clinician accuracy for diagnosing melanoma on basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol 2019; 80: 285–287. [DOI] [PubMed] [Google Scholar]
- 15.Haenssle HA, Korpas B, Hansen-Hagge C, Buhl T, Kaune KM, Johnsen S, et al. Selection of patients for long-term surveillance with digital dermoscopy by assessment of melanoma risk factors. Arch Dermatol 2010; 146: 257–264. [DOI] [PubMed] [Google Scholar]
- 16.Carrera C, Palou J, Malvehy J, Segura S, Aguilera P, Salerni G, et al. Early stages of melanoma on the limbs of high-risk patients: clinical, dermoscopic, reflectance confocal microscopy and histopathological characterization for improved recognition. Acta Derm Venereol 2011; 91: 137–146. [DOI] [PubMed] [Google Scholar]
- 17.Zalaudek I, Nowotny T, Kittler H, Hofmann-Wellenhof R, Massone C. The brown and black rule: a simple clue to differentiate common naevi from spitzoid neoplasms with a dermoscopic uniform globular (clod) pattern. J Eur Acad Dermatology Venereol 2016; 30: 875–876. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014; 170: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol 2014; 171: 1044–1051. [DOI] [PubMed] [Google Scholar]