Abstract
The aim of this randomized controlled trial was to evaluate the wound-healing effect and antimicrobial properties of a novel stabilized hypochlorous acid solution on acute wounds, using a suction blister wound model. One suction blister was raised and de-roofed on each forearm in 20 healthy volunteers. Stabilized hypochlorous acid/control (sterile 0.9% NaCl) solutions were assigned to either wound by randomization. Wounds were irrigated and treated on days 0, 2 and 4. Re-epithelialization was assessed blindly by digital planimetry, and bacterial growth was assessed as the number of colony-forming units cultured from surface swabs. Hypochlorous acid solution increased the degree of re-epithelialization on day 4 by 14% compared with the control solution (95% confidence interval (CI) 6.8–20%, p = 0.00051) and was not inferior (p < 0.0001) to the control solution on day 10 (0.3%, 95% CI –1.3–1.9%). Median bacterial counts were lower with stabilized hypochlorous acid compared with control and were further reduced after irrigation and treatment of both groups on day 4, but remained lower in the stabilized hypochlorous acid group compared with the control group. This study demonstrates immediate and durable antimicrobial action and a bene ficial effect on acute wound healing after irrigation and treatment with a stabilized hypochlorous acid formulation.
Key words: antiseptic, wound management, wound healing, clinical trial
Contamination of acute surgical or non-surgical wounds may lead to infection and poor wound healing (1, 2). Irrigation decontaminates wounds, and its effectiveness depends on the pressure and volume of the solution applied (3). Antimicrobial substances are commonly applied in modern wound management.
A novel wound irrigation solution, composed of 0.016% hypochlorous acid (HOCl) stabilized in 0.25% acetic acid (CH3COOH) buffer at pH 4.2–5.0 (SoftOx Solutions AS, Oslo, Norway) was introduced recently (4). HOCl is indistinguishable from the endogenous substance produced by phagocytic cells. The mode of action of HOCl solution is the physical removal of foreign bodies, debris, and microorganisms through the flushing procedure and by its antimicrobial activity (5). There is a delicate balance between the desired toxicity against microbes without harmful effects on the host cells (6). Cellular studies indicate that stabilized HOCl can increase wound healing at appropriate concentrations (7). In a murine model, stabilized HOCl (0.015%) showed anti-inflammatory effects and beneficial effects on cutaneous wound healing (8). In an exploratory clinical study, irrigation and topical application of HOCl solution to split-thickness skin graft donor sites indicated beneficial effects on epithelialization without raising major safety concerns apart from pain reactions to topical HOCl (4); however, this study was uncontrolled (4).
SIGNIFICANCE
Antimicrobial compounds are often used in wound management. Although most of these show the desired antimicrobial activities, many are cytotoxic toward cells involved in the wound-healing process. Stabilized hypochlorous acid is a simple, but effective, broad-spectrum antimicrobial compound without known resistance issues when dosed at optimum concentration and duration. In standardized human acute wounds, stabilized hypochlorous acid appears to promote re-epithelialization and control the bacterial bioburden. These findings suggest that hypochlorous acid is a promising antimicrobial agent. Further studies are warranted to identify indications in which uncontrolled bacterial growth is a clinical issue.
To follow up on these promising findings, an open-label, evaluator-blinded, non-inferiority, paired study was performed, in which the stabilized HOCl solution was compared with sterile saline (0.9% NaCl; Irri-flex, Fresenius Kabi AG, Bad Homburg, Germany). A suction-blister injury wound model was chosen because it allows the assessment of epidermal regeneration without causing scarring (9–16). The primary objective of the current randomized controlled trial (RCT) was to evaluate wound healing, and the secondary objectives were microbiological control, pain levels and safety with stabilized HOCl vs control treatment. The primary endpoint was the degree of re-epithelialization (%) on day 10. Secondary endpoints included the degree of re-epithelialization (%) on day 4, colony-forming units (CFUs) prior to and after irrigation and treatment on day 4, and the subjective pain level directly after irrigation and treatment on days 0, 2 and 4.
MATERIALS AND METHODS
The study was approved by the Danish Medicines Agency and the Committee of Health Research Ethics in the Capital Region of Denmark (H-20035973) and carried out at the Copenhagen Wound Healing Center, Department of Dermatology, University of Copenhagen, Copenhagen, Denmark, in accordance with the Declaration of Helsinki. The trial was submitted to ClinicalTrials. gov as NCT04771819 on 23 November 2020.
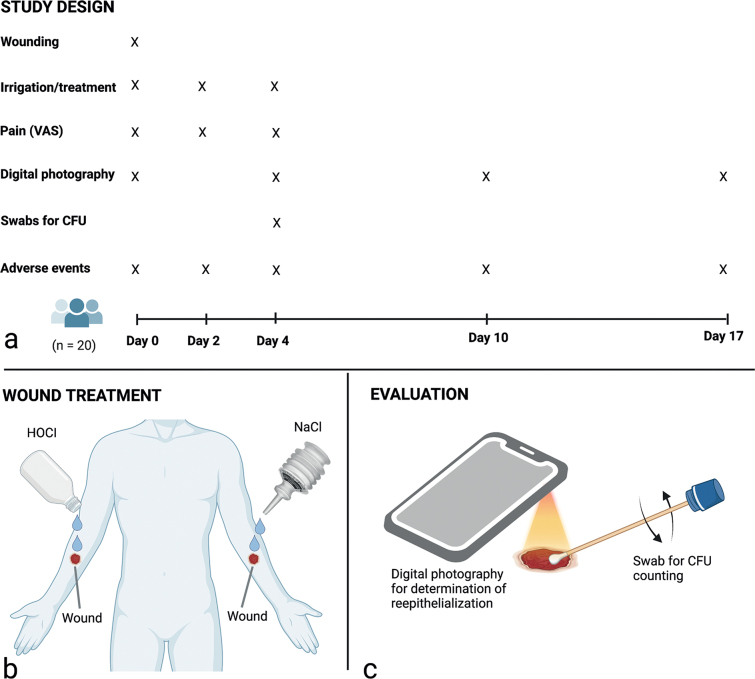
The study design, treatment procedures and assessment of treatment effects are summarized in Fig. 1. Inclusion of participants was performed by 2 investigators (EAB and LS). Treatments and clinical assessments were performed by the study nurses.
Fig. 1.
(a) Study design. (b) Treatments were randomized to the wound on the left or right forearm of the subjects, who served as their own controls. (c) Digital photographs were taken with an iPhone with an attached macro lens (days 0 and 4) or Handyscope (days 10 and 17) for blinded measurements of wound area and determination of re-epithelialization, and a sterile swab was used to quantitate colony-forming units (CFUs). Figure created with BioRender.com (Biorender, Toronto, ON, Canada).
Participants
The inclusion criteria were subjects in the age range 18–60 years, who had healthy forearm skin, and provided informed written consent. The exclusion criteria were diseases that may interfere with wound healing (e.g. diabetes and autoimmune diseases); active skin disease; daily smoking; pregnancy; systemic immunosuppressive treatment; uncontrolled pain that may interfere with the study outcome as judged by the investigator; allergy to hypochlorous acid, acetic acid or any other remedies/material used; participation in other clinical investigations; inability to read or understand Danish; and any other conditions that may make follow-up or investigation inappropriate or the subject unsuitable for study enrolment. Subjects who met all inclusion criteria and none of the exclusion criteria were included.
Induction of epidermal wounds
The NP-4 model manufactured by Electronic Diversities (Finksburg, MD, USA) was used to induce 1 10-mm blister in the middle of the volar aspect of each forearm (15, 17). The instrument was set to a negative pressure of 400 mmHg. The epidermal roof of the formed blisters was excised with a sterile scalpel.
Randomization, treatment, and subject-reported pain on days 0, 2 and 4
After induction of the 2 wounds, the wound on the left arm was randomized to irrigation and treatment with either stabilized HOCl or saline control, and the wound on the right arm received the opposite treatment to the wound on the left arm. Randomization codes were obtained from a separate list. The allocation sequence was computer-generated (SAS Statistical Software, SAS Institute, Cary, NC, USA). Distribution of the 2 treatments between the left and right arms was equal in the enrolled subjects. The solutions (i.e. 100 ml stabilized HOCl or 120 ml sterile saline) were poured onto the wound for 5 s from the containers held 10 cm above the wound, as shown in Fig. 1b. Then, the wound was covered for 15 min with sterile non-woven swabs (OneMed, Helsinki, Finland) soaked in the solutions. The subject-reported visual analogue scale (VAS) pain intensity was then assessed on a scale ranging from 0 to 10 cm, where 0 cm was equal to no pain, and 10 cm was the worst imaginable pain for the subject. Finally, the wound was covered with an island dressing composed of a non-adherent absorbent pad in the centre of a vapour-permeable, but water- and bacterial-proof, transparent adhesive film (Leukomed® T plus, BSN Medical GmbH, Hamburg, Germany).
Digital photography and image analysis for determination of re-epithelialization
On days 0, 4, 10, and 17, wounds were photographed before application (day 0) and after removal of the island dressing. Overview images of the wounds and a millimetre scale bar with the subject number, left or right forearm, date, and an arrow pointing toward the hand and parallel to the arm applied adjacent to the wound were acquired by a digital camera (iPhone 5/5S, model: A1753), as shown in Fig. 1c. On days 0 and 4, close-up images of the wounds were taken with a macro lens (21×; Olloclip, Foothill Ranch, CA, USA) attached to an iPhone (18). On days 10 and 17, a Handyscope (FotoFinder Systems GmbH, Bad Birnbach, Germany) was connected to the iPhone (18). The device captures digital images (polarized light) at 20× magnification. A 5-mm calibration scale was included in each image. The wound on the left arm was photographed first.
The digital photographs were imported into ImageJ software (ImageJ 1.52a, NIH, Bethesda, MD, USA) and were analysed twice by a blinded investigator (MSÅ). The wound areas on day 0 (ADay 0), day 4 (ADay 4), day 10 (ADay 10) and day 17 (ADay 17) were used to calculate the degree of re-epithelialization as a percentage (%) using the following formula (15):
Bacterial swabs and determination of colony-forming units
Sterile swabs (Σ-Transwab®, MWE, Corsham, UK) were used for sampling from the wounds on day 4 before and after irrigation/treatment with the solutions on both arms, as shown in Fig. 1c. Swabs were rotated at an angle of 45° in the centre of the wound 3 times clockwise and then 3 times anticlockwise (15). The swab stick was then immersed in 1 ml of the Σ-Transwab transportation system medium.
The swabs were processed for CFU determination within 24 h by the Biofilm Test Facility, Department of Immunology and Microbiology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark. Swabs were vortexed and sonicated for 5 min, and 10-fold dilutions were performed in sterile saline. One hundred microlitres of the samples were applied to 5% horse blood agar plates (Statens Serum Institut, Copenhagen, Denmark) and to blue agar plates selective for Gram-negative bacteria using a modified Conradi-Drigalski diagnostic substrate (4, 15). The plates were incubated for 24 h at 37°C under normoxic conditions. CFUs were counted visually per plate, and the results are expressed as CFUs/swab. The analyses were performed without knowledge of the origin of the swabs.
Sample size calculation
The sample size calculation was based on the primary non-inferiority endpoint that normal wound healing (re-epithelialization in %) after HOCl treatment was not inferior to the control treatment. The Δ (absolute difference in the degree of re-epithelialization) between the HOCl and control was calculated for each subject, where a positive value means more re-epithelialization with HOCl than the control and a negative value indicates the opposite. A Δ value > –10% was considered non-inferior. A standard deviation (SD) of 15% was assumed (10, 14, 15), and with a power of 80% (1–β) and a significance level of 5% (α), 16 subjects were needed. Twenty subjects were included to account for a dropout rate of 20%.
Data management and statistical analyses
Data were entered into an eCRF (Clindox, Sevenoaks, UK) specifically designed for this study.
Continuous variables were analysed using a paired t-test. CFU values were not normally distributed and were analysed using the Wilcoxon signed-rank test. The correlation between CFUs before treatment and the degree of re-epithelialization on day 4 was assessed by the Spearman rank-order test. Two-sided statistical analyses were performed using SPSS Statistics 26.0 software (IBM, Armonk, NY, USA). Data are presented as the mean ± SD or Δ with 95% confidence intervals (95% CI) unless otherwise stated. The statistical significance was set to p < 0.05.
RESULTS
This RCT compared the effects of stabilized HOCl on wound healing and antimicrobial activity with control in a human wound-healing model.
Participant flow
The recruitment of participants started on 4 November 2020. Twenty remunerated healthy volunteers (age range 18–59 years, 33 ± 12 years), 11 males and 9 females, were included consecutively from 23 November 2020 to 22 March 2021, and the last participant was completed on 8 April 2021. The participants received the allocated treatments except 1 individual who did not attend the day 4 clinical visit due to COVID-19 quarantine, but provided digital images of the 2 wounds. Another participant withdrew from the study after day 10 due to personal reasons and was lost to the day 17 follow-up, but was included in the intention-to-treat population.
Clinical observations
On day 0, confluent blisters had formed in approximately 1.5 h, and the blister roofs were excised. The wound size did not differ (p = 0.14) between the HOCl (55 ± 16 mm2) and control groups (60 ± 15 mm2).
Stinging, burning or pain reactions were reported by 10 subjects, strongest on day 0 for 9 subjects and strongest on day 4 for 1 subject, immediately after irrigation with stabilized HOCl. These sharp reactions were transient and usually disappeared within seconds. The subjective VAS levels were assessed directly after the 15-min treatment period and were low, with a mean value on day 0 of 0.24 ± 0.52 cm for HOCl and 0.05 ± 0.22 cm for control, values on day 2 of 0.12 ± 0.31 cm for HOCl and 0 cm for control, and values on day 4 of 0.08 ± 0.25 cm for HOCl and 0.03 ± 0.11 cm for control; none of these differences were statistically significant.
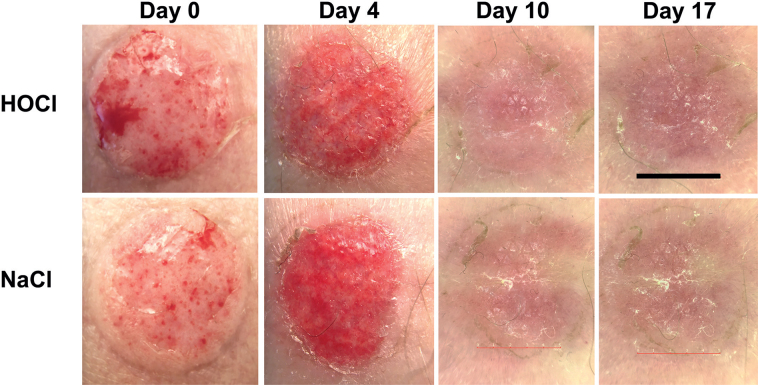
All wounds were moist on removal of the protective dressing until day 4. The general impression of the principal investigator (EAB) was that the HOCl-treated wounds were less red than the control wounds. Wound complications were rare, although mild abnormal inflammatory signs were observed on day 2 in 1 wound treated with HOCl and in 2 wounds in the control group. Representative courses of treatment with HOCl and control saline are shown in Fig. 2.
Fig. 2.
Course of treatment with stabilized hypochlorous acid (HOCl) or saline control (NaCl) on days 0 (before irrigation and application of HOCl/NaCl), 4, 10, and 17. Scale bar: 6 mm.
Wound-healing measurements
The HOCl solution increased the degree of re-epithelialization compared with that of the control on day 4, with an estimated absolute difference (Δ) in the degree of re-epithelialization of 14% (95% CI 6.8–20%, p = 0.00051). The individual re-epithelialization results from day 4 are depicted in Fig. 3. On day 10, the estimated Δ was 0.3% (95% CI –1.3–1.9%). The accompanying non-inferiority p-value was < 0.0001 (for primary data see Table SI).
Fig. 3.
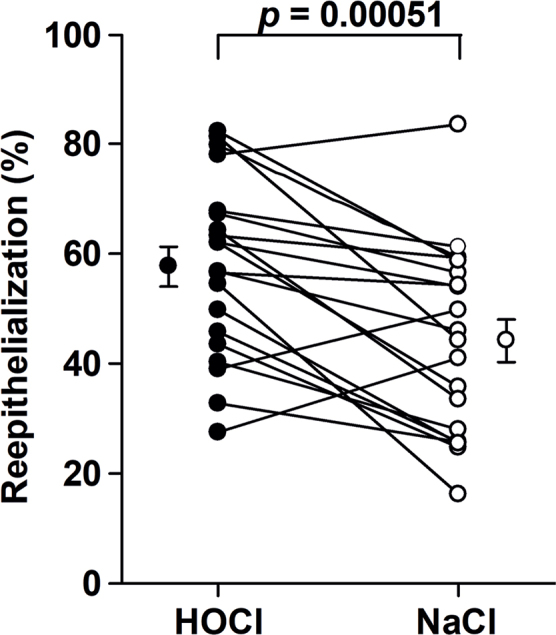
Re-epithelialization paired data from the 20 volunteers on day 4. Mean ± standard error of mean (stabilized hypochlorous acid (HOCl), 58 ± 3.6%; NaCl, 44 ± 3.8%).
Effect of sex on re-epithelialization day 4
The estimated Δ between males and females was 5.1% (95% CI –10–20%, p = 0.50) for HOCl-treated wounds and 1.3% (95% CI –15–18%, p = 0.87) for control-treated wounds.
Bacteriology day 4
Surface swabs were taken from day 4 wounds for normoxic culturing before and after treatment. In the HOCl group, bacterial growth was detected in 12 wounds before treatment and in 7 wounds after HOCl treatment. The corresponding figures for the control group were 16 and 15 wounds. Median bacterial counts were significantly lower with HOCl than the control and were reduced after irrigation and treatment in both groups, but remained lower in the HOCl group, as shown in Table I (for primary data see Table SII).
Table I.
Bacterial counts (colony-forming units (CFUs)/swab) on day 4 before and after irrigation and treatment with stabilized hypochlorous acid (HOCl) and the control (sterile saline (0.9% NaCl))
| HOCI (n = 19) | NaCI (n = 19) | p-value | |
|---|---|---|---|
| Before treatment, median (IQR) | 70 (0–3,300) | 11,500 (20–52,000) | 0.015 |
| After treatment, median (IQR) | 0(0–390) | 1,100 (10–30,000) | 0.0023 |
| p-value | 0.023 | 0.0044 |
IQR: interquartile range; CFUs: colony-forming units (derived from 5% blood agar plates. No swarming from Gram-negative species such as Proteus sp. was detected; thus, counting the blue plates was unnecessary).
Correlation between re-epithelialization and colony-forming units on day 4
There were no significant correlations between CFU and re-epithelialization on day 4 in the HOCl group (r = –0.073, p = 0.77, n = 19) or the control group (r = 0.21, p = 0.39, n = 19).
Adverse events
During the study, 8 adverse events were noted and judged unrelated to the HOCl solution; these manifested as mild skin irritation reactions beneath the adhesive film dressing in 4 patients. The other 4 adverse events were erysipelas around the ear in 1 patient, another patient had inguinal skin infection, and the third patient was febrile and had hypertension. No serious adverse events occurred.
DISCUSSION
This RCT demonstrated that wound healing (re-epithelialization) after combined irrigation and treatment with stabilized HOCl was not inferior to that of the saline control on day 10. In contrast, re-epithelialization was accelerated at an earlier stage with the stabilized HOCl formulation. This finding was unforeseen, and it is possible that the early beneficial effect on re-epithelialization resulted in a shortened time to complete wound closure. Daily monitoring between day 4 and day 10 would have been required to detect an effect on the time to complete wound closure.
It should be emphasized that the accuracy of the method (digital planimetry) used to determine re-epithelialization has been validated against histological assessment of 60 suction blister wounds on day 4 (15). Evaluation of re-epithelialization on day 4 provides a high degree of sensitivity to detect differences between treatments (maximum sensitivity corresponds to 50% re-epithelialization) (15).
Bacteria have been cultured from this wound type previously and CFUs are higher in wounds than adjacent skin on day 4 (15, 19, 20). The CFUs were significantly reduced in wounds treated with stabilized HOCl than with the control solution, which documents the durable bacterial control with HOCl intervention. In addition, CFU measurements directly after treatment showed a further reduction in the bacterial bioburden. The species recovered from the wounds was not determined. In other studies, the growth of coagulase-negative staphylococci dominated in these wounds (15, 19, 20).
The HOCl formulation contains acetic acid (0.25%) as a pH stabilizing excipient. Acetic acid possesses antimicrobial activity and the minimal inhibitory concentrations of acetic acid against different strains of Staphylococcus aureus were determined in the range 0.16–0.31% (21). Thus, the acetic acid component of the HOCl solution may have contributed to the reduced CFUs.
Robson et al. (22) concluded that the increased wound healing with topical HOCl (0.01%) treatment for 30 min of non-infected and infected full-thickness wounds in rats was accompanied by reduced bacterial bioburden compared with that with saline. These beneficial effects were not observed when wounds were exposed to HOCl for 24 h or at higher strengths (22). The current study results for human wounds are strikingly similar to those in rodents. In the same animal model, silver sulfadiazine showed superior antimicrobial activity, but inferior wound-healing capacity, compared with stabilized HOCl (22).
Wound irrigation with saline is an effective method of wound cleansing and prevention of surgical site infections. Reduced bacterial load was documented after saline irrigation, as has been shown in animal wounds (23).
The effect of the microbiota and wound healing per se is debated (24, 25). The current study did not demonstrate a correlation between bacterial colonization and re-epithelialization. Re-epithelialization depends on keratinocyte proliferation and migration (26). In vitro tests showed that stabilized HOCl increased the migration of keratinocytes (7). The total activity of collagenase matrix metalloproteinase (MMP)-1 increases more than 100-fold in these wounds (27) and appears to be obligatory for keratinocyte migration (9, 27–29). Interestingly, HOCl can convert non-catalytic latent MMP-1 into catalytic MMP-1 (30) and may thus facilitate the movement of keratinocytes during the re-epithelialization process.
Another possible mode of action of HOCl on wound healing may be attributed to its anti-inflammatory properties (8, 31, 32), which could advantageously be investigated by sampling cytokines secreted into chambers placed over the wounds and filled with saline (33). Interestingly, HOCl rescued the wound healing ability of keratinocytes derived from the skin of patients with Hailey-Hailey disease by reducing the levels of several pro-inflammatory cytokines (34).
This study has some limitations and strengths. Although the solutions were not masked, the wound areas used for the re-epithelialization calculations were determined by a blinded investigator, and the CFUs were determined in a blinded fashion. Another advantage is that the model allows comparison in the same individual, which eliminates inter-individual differences in, for example, basal cytokine and hormonal levels (35). However, further studies are needed to assess the effect and safety of stabilized HOCl treatment of wounds involving the dermis.
No adverse events related to the use of topical HOCl were observed. The suction blister wound model has been applied to assess pain perception (36). The investigators did not classify the brief immediate and transient sensory reactions after topical HOCl application as adverse events. The high hydrogen ion concentration may have caused these pain reactions via acid-sensing ion channels of nerve endings (37, 38). Hyperalgesia may also be due to the hypotonicity of the HOCl solution. Regardless of causes, the pain scores would probably have been higher if assessed immediately after irrigation of the wounds. HOCl is unlikely to induce antimicrobial resistance.
In conclusion, stabilized HOCl is a promising topical agent for the control of bacterial bioburden and the promotion of wound healing. More studies are needed to elucidate the mechanisms responsible for the observed positive effects of stabilized HOCl on wound healing.
ACKNOWLEDGEMENTS
The authors are grateful to all the participants, the excellent study nurses Stine Ingvertsen and Susan Bermark, and Lasse Andersson Kvich at Costerton Biofilm Center for the microbiological analyses. KKM is medical advisor and GG is employee of SoftOx Solutions AS. Grants were given by SoftOx Solutions AS to the institution for the conduct of the study.
Footnotes
The authors have no other conflicts of interest to declare.
REFERENCES
- 1.Angerås MH, Brandberg A, Falk A, Seeman T. Comparison between sterile saline and tap water for the cleaning of acute traumatic soft tissue wounds. Eur J Surg 1992; 158: 347–350. [PubMed] [Google Scholar]
- 2.Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg 1973; 107: 206–210. [DOI] [PubMed] [Google Scholar]
- 3.Nicks BA, Ayello EA, Woo K, Nitzki-George D, Sibbald RG. Acute wound management: revisiting the approach to assessment, irrigation, and closure considerations. Int J Emerg Med 2010; 3: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burian EA, Sabah L, Kirketerp-Møller K, Ibstedt E, Fazli MM, Gundersen G. The safety and antimicrobial properties of stabilized hypochlorous acid in acetic acid buffer for the treatment of acute wounds – a human pilot study and in vitro data. Int J Low Extrem Wounds 2021: 15347346211015656. [DOI] [PubMed] [Google Scholar]
- 5.Ono T, Yamashita K, Murayama T, Sato T. Microbicidal effect of weak acid hypochlorous solution on various microorganisms. Biocontrol Sci 2012; 17: 129–133. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, et al. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds 2007; 6: e5. [PMC free article] [PubMed] [Google Scholar]
- 7.Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. Hypochlorous acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 2014; 26: 342–350. [PubMed] [Google Scholar]
- 8.da Costa MC, Ferreira BA, de Moura FBR, de Lima LG, Araujo FA, Mota FCD. Evaluation of 4% stabilized sodium hypochlo-rite activity in the repair of cutaneous excisional wounds in mice. Injury 2021; 52: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 9.Ågren MS, Chafranska L, Eriksen JO, Forman JL, Bjerrum MJ, Schjerling P, et al. Spatial expression of metallothionein, matrix metalloproteinase-1 and Ki-67 in human epidermal wounds treated with zinc and determined by quantitative immunohistochemistry: a randomised double-blind trial. Eur J Cell Biol 2021; 100: 151147. [DOI] [PubMed] [Google Scholar]
- 10.Ahlström MG, Gjerdrum LMR, Larsen HF, Fuchs C, Sørensen AL, Forman JL, et al. Suction blister lesions and epithelialization monitored by optical coherence tomography. Skin Res Technol 2018; 24: 65–72. [DOI] [PubMed] [Google Scholar]
- 11.Kiistala U. Dermal-epidermal separation. I. The influence of age, sex and body region on suction blister formation in human skin. Ann Clin Res 1972; 4: 10–22. [PubMed] [Google Scholar]
- 12.Kiistala U. Dermal-epidermal separation. II. External factors in suction blister formation with special reference to the effect of temperature. Ann Clin Res 1972; 4: 236–246. [PubMed] [Google Scholar]
- 13.Kiistala U, Mustakallio KK, Rorsman H. Suction blisters in the study of cellular dynamics of inflammation. Acta Derm Venereol 1967; 47: 150–153. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer M, Frederiksen AKS, Nissen NI, Willumsen N, van Hall G, Jorgensen LN, et al. Multinutrient supplementation increases collagen synthesis during early wound repair in a randomized controlled trial in patients with inguinal hernia. J Nutr 2020; 150: 792–799. [DOI] [PubMed] [Google Scholar]
- 15.Larsen HF, Ahlström MG, Gjerdrum LMR, Mogensen M, Ghathian K, Calum H, et al. Noninvasive measurement of reepithelialization and microvascularity of suction-blister wounds with benchmarking to histology. Wound Repair Regen 2017; 25: 984–993. [DOI] [PubMed] [Google Scholar]
- 16.Mirastschijski U, Schwab I, Coger V, Zier U, Rianna C, He W, et al. Lung surfactant accelerates skin wound healing: a translational study with a randomized clinical phase I study. Sci Rep 2020; 10: 2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatje LK, Richter C, Blume-Peytavi U, Kottner J. Blistering time as a parameter for the strength of dermoepidermal adhesion: a systematic review and meta-analysis. Br J Dermatol 2015; 172: 323–330. [DOI] [PubMed] [Google Scholar]
- 18.Ågren MS, Phothong N, Burian EA, Mogensen M, Haedersdal M, Jorgensen LN. Topical zinc oxide assessed in two human wound-healing models. Acta Derm Venereol 2021; 101: adv00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bay L, Kragh KN, Eickhardt SR, Poulsen SS, Gjerdrum LMR, Ghathian K, et al. Bacterial aggregates establish at the edges of acute epidermal wounds. Adv Wound Care (New Rochelle) 2018; 7: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daeschlein G, Alborova J, Patzelt A, Kramer A, Lademann J. Kinetics of physiological skin flora in a suction blister wound model on healthy subjects after treatment with water-filtered infrared-A radiation. Skin Pharmacol Physiol 2012; 25: 73–77. [DOI] [PubMed] [Google Scholar]
- 21.Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, et al. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS One 2015; 10: e0136190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robson MC, Payne WG, Ko F, Mentis M, Donati G, Shafii SM, et al. Hypochlorous acid as a potential wound care agent: part II. Stabilized hypochlorous acid: its role in decreasing tissue bacterial bioburden and overcoming the inhibition of infection on wound healing. J Burns Wounds 2007; 6: e6. [PMC free article] [PubMed] [Google Scholar]
- 23.Badia JM, Torres JM, Tur C, Sitges-Serra A. Saline wound irrigation reduces the postoperative infection rate in guinea pigs. J Surg Res 1996; 63: 457–459. [DOI] [PubMed] [Google Scholar]
- 24.Hansson C, Hoborn J, Moller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbio-logical technique. Acta Derm Venereol 1995; 75: 24–30. [DOI] [PubMed] [Google Scholar]
- 25.Tomic-Canic M, Burgess JL, O’Neill KE, Strbo N, Pastar I. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol 2020; 21: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomic-Canic M, Wong LL, Smola H. The epithelialisation phase in wound healing: options to enhance wound closure. J Wound Care 2018; 27: 646–658. [DOI] [PubMed] [Google Scholar]
- 27.Ågren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol 2001; 10: 337–348. [DOI] [PubMed] [Google Scholar]
- 28.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997; 137: 1445–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michopoulou A, Rousselle P. How do epidermal matrix metal-loproteinases support re-epithelialization during skin healing? Eur J Dermatol 2015; 25: 33–42. [DOI] [PubMed] [Google Scholar]
- 30.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci U S A 1990; 87: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draelos ZD. Antipruritic hydrogel for the treatment of atopic dermatitis: an open-label pilot study. Cutis 2012; 90: 97–102. [PubMed] [Google Scholar]
- 32.Fukuyama T, Martel BC, Linder KE, Ehling S, Ganchingco JR, Baumer W. Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin Exp Allergy 2018; 48: 78–88. [DOI] [PubMed] [Google Scholar]
- 33.Portugal-Cohen M, Kohen R. Non-invasive evaluation of skin cytokines secretion: an innovative complementary method for monitoring skin disorders. Methods 2013; 61: 63–68. [DOI] [PubMed] [Google Scholar]
- 34.Cialfi S, Calabro S, Franchitto M, Zonfrilli A, Screpanti I, Talora C. Hypotonic, acidic oxidizing solution containing hypochlorous acid (HClO) as a potential treatment of Hailey-Hailey disease. Molecules 2019; 24: 4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry 1999; 56: 450–456. [DOI] [PubMed] [Google Scholar]
- 36.Pfeifer AC, Schroeder-Pfeifer P, Schneider E, Schick M, Heinrichs M, Bodenmann G, et al. Oxytocin and positive couple interaction affect the perception of wound pain in everyday life. Mol Pain 2020; 16: 1744806920918692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng YR, Jiang BY, Chen CC. Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing. J Biomed Sci 2018; 25: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Luo Y, Asaki T, Graven-Nielsen T, Cairns BE, Arendt-Nielsen T, et al. Acid-induced experimental muscle pain and hyperalgesia with single and repeated infusion in human forearm. Scand J Pain 2017; 17: 260–266. [DOI] [PubMed] [Google Scholar]