Abstract
Background
The use of DTG-containing two-drug regimens is one of the most promising solutions to the need to ease the management of HIV treatment without harming its efficacy and safety. We report long- term results in patients switched, while virologically suppressed, to the combination of dolutegravir (DTG) plus lamivudine (3TC).
Methods
This is a prospective, clinical, uncontrolled cohort enrolling ART-experienced people living with HIV (PLWH) with HIV-RNA < 50 copies/ml for 6 months or longer, negative hepatitis B virus surface antigen, and without known M184V/I mutations. Kaplan-Meiers curves are used to describe persistency of virological suppression on therapy and a Cox regression model to evaluate baseline characteristics and the risk of stopping therapy.
Results
218 individuals switched their regimen since 2015. The mean estimated follow-up was of 64.3 months (95% CI 61.3–67.3) for approximately 1000 patient/years. After 5 years of follow-up, 77.1% were still on the DTG-3TC combination. No virologic failure was detected throughout the whole study period, and only 15 subjects presented single isolated viral blips above 50 copies/ml. Most patients stopped therapy because of reasons unrelated to study drugs (lost to follow-up; patients’ decision; moved to other Centers), but due to the unselected nature of the casuistry; 11 subjects died in the 5 years of follow-up mostly because of pre-existing co-morbidities (6 neoplastic diseases and 2 end-stage liver disease). The median baseline CD4 count was 669 cells/mcl (IQR 483–927). After 5 years it raised to 899 cells/mcl (IQR 646–1160) (P < 0.001) without a significant change of CD8 counts that lowered from 767 cells/mcl (IQR 532–1034) to 683 cells/mcl (IQR 538–988). Consequently, the CD4/CD8 ratio varied from 0.93 (IQR 0.60–1.30) to 1.15 (IQR 0.77–1.45) (P < 0.0001). A non-significant (P = 0.320) increment of mean creatinine, 0.06 mg/dl in magnitude, was observed over the whole follow-up.
Conclusion
These long-term results over 5 years reinforce the durability and good tolerability of DTG-3TC. Our results continue to support the recommended switch use of this 2DR as a well-accepted treatment option for ART-experienced PLWH.
Keywords: Dual ART, Dolutegravir, Lamivudine, Switch, Simplification, Long-term follow-up, Cohort
Introduction
Modern antiretroviral therapy (ART) allows for several treatment options with high efficacy and tolerability, available for both ART-naïve and -experienced people living with HIV (PLWH) [1, 2]. The advent of an integrase inhibitor (INSTI) as dolutegravir (DTG), with high potency and high genetic barrier has allowed to expand strategies for initiating and simplifying ART [3–5]. Moreover, new ART strategies with fewer drugs have been recently introduced in clinical practice and are becoming widely used worldwide [3–8], leading to a scenario where therapies can be increasingly tailored to combine efficacy and tolerability with better ease of intake for PLWH, who consequently may experience an additional benefit in terms of quality of life. Furthermore, the opportunity for single tablet regimens seems to satisfy patient preferences and improve adherence [9–13]. The use of DTG-containing two-drug regimens (2DR), and, in particular, of DTG plus lamivudine (3TC), is one of the most promising solutions to the need to ease the management of HIV treatment without harming its efficacy and safety.
As most of these strategies were introduced in recent years, long-term durability studies are needed to definitively establish the future role of less-drug regimens. We report a prospective, clinical, uncontrolled experience on patients switched, while virologically suppressed, to the combination of dolutegravir plus lamivudine.
Materials and methods
The cohort started in 2015. In 2017, our study group published the short-term results of the cohort 24 weeks after the switch to DTG/3TC combination. The inclusion criteria to build the cohort and the methods applied were described in detail in the previous study [14]. Briefly, we considered for inclusion in this cohort only patients that at the moment of therapeutic switch had a HIV-RNA < 50 copies/ml for 6 months or longer, were negative for hepatitis B virus surface antigen, and were on a stable ART (for > 6 months). Generally, ART was based on a nucleoside backbone plus a third anchor agent, or, in a few cases on other complex regimens. Further, only patients with no previous resistance mutations to either integrase inhibitors or lamivudine were included.
Resistance had to be determined by genotypic analysis before the start of ART or afterward in the occasion of viral blips before the current regimen was started. Patients were not included if they had a virological failure following their last genotypic test. Subjects were switched to a dual combination of dolutegravir (50 mg once daily) plus lamivudine (300 mg once daily).
The possibility of the switch to DTG and 3TC was previously discussed individually with each patient, among other available drug regimens and according to available data [2, 15, 16]; in all patients, the decision to switch therapy was taken on clinical grounds as they presented a clinically relevant reason, either because of concomitant diseases, altered laboratory tests, drug adverse events or risk of drug-to-drug interactions.
Since Italian guidelines considered DTG/3TC combination a well-established alternative option in ART management [17], no experimental procedure (e.g. randomization) was applied. The switch was independent from the decision to include the patients in this cohort, thereafter the local EC ruled out the requirement of formal ethics approval; all patients gave their informed consent uniquely for the use of clinical and laboratory data.
Once included, patients were prospectively followed accordingly to current clinical practice as indicated from Italian guidelines with visits after 2, 4 months and thereafter every 3–6 months. Each visit included a physical examination and blood analysis (including HIV-RNA) performed using standard methods.
For the only seek of this report, the primary endpoint was the virological response, defined as the proportion of patients with HIV viral load below 50 copies/ml 5 years after the switch, similarly patients with greater than > 50 copies/ml were asked to perform a new test within a months and in the case of confirmed positivity were considered virological failures.
Several other commonly collected data were used to evaluate secondary endpoints. Safety and tolerability were studied by analyzing questioning of patients at each visit and on the basis of physical examination and laboratory analysis. We evaluated immunological changes in terms of CD4+, CD8+, cell/counts and CD4/CD8 ratio variations and we also collected changes in creatinine and blood lipid content as possible markers of drug toxicity.
Data are presented as medians and interquartile range or percentages. Student’s t-test for paired samples was employed to identify significant changes in immunological, renal and metabolic functions. Kaplan-Meier curves were used to describe persistency on therapy; a Cox regression model was adopted to evaluate baseline characteristics and the risk of stopping therapy. We did all statistical analyses using SPSS version 17.
Results
Two-hundred eighteen individuals who switched their regimen (between January 2015 and July 2016) were enrolled and prospectively followed. Table 1 summarizes baseline characteristics.
Table 1.
Baseline characteristics of the cohort (n = 281)
| Characteristics | Median (IQR) or number (%) |
|---|---|
| Age (years) | 52 (47–59) |
|
Gender Male female |
211 (75.2%) 70 (24.8%) |
|
Origin Italy Other Countries |
264 (94.0%) 17 (6.0%) |
|
Risk factor for HIV Heterosexual contacts MSM IVDU Other |
129 (45.9%) 78 (27.8%) 72 (25.6%) 2 (0.7%) |
|
Co-infection HCV Yes No |
46 (16.5%) 235 (83.5%) |
| Time on ART (years) | 10.2 (4.6–17.2) |
| Previous ART regimens (number) | 3 (2–5) |
| Time with HIV-RNA < 50 copies/ml (months) | 75 (33–122) |
| Baseline CD4 (cells/mcL) | 669 (483–927) |
| Baseline CD8 (cells/mcL) | 767 (532–1034) |
| CD4/CD8 ratio | 0.93 (0.61–1.30) |
Median age of the cohort was 52 (IQR 42–59); male individuals were 75.2% and most of individuals were of Italian origin (94%). Subjects had a long ART history (median 10 years [IQR 4.6–17.2]) and were on average on their 3rd line of therapy. Median time of viral suppression before switch was 75 months (IQR 33–122). Before switch, most of individuals (93.6%) were taking a triple-drug regimen, being the most common backbones tenofovir + emtricitabine (59.2%) or abacavir + lamivudine (27.5%). The most common anchor drugs were efavirenz (18.8%) and rilpivirine (16.5%) among NNRTIs, and either boosted darunavir (14.7%) or boosted atazanavir (14.2%) among PIs. A previous exposure to INSTI was documented in 22.5% of individuals.
The main reasons for therapeutic switch were concomitant diseases and abnormality of laboratory tests followed by drug-related adverse events or possible adverse events or a potential drug-drug interaction. Several individuals had a mix of reasons. Each patient presented a median of 2 chronic non AIDS-related co-morbidities. The most frequent co-morbidities were: bone diseases (34.4%), hypertension (30.2%), liver diseases, including end-stage liver disease (24.3%), metabolic abnormalities (21.1%) and diabetes (12.4%), cardiovascular (12.3%), renal (11.0%) and neoplastic diseases (6.4%).
Because of these pathologies, patients took a median of 2 (IQR 1–3) and up to 11 different drugs including, but not limited to, diuretics, beta-blockers, calcium-antagonists, aspirin, statins, benzodiazepines, vitamins, proton pump inhibitors, insulin, metformin.
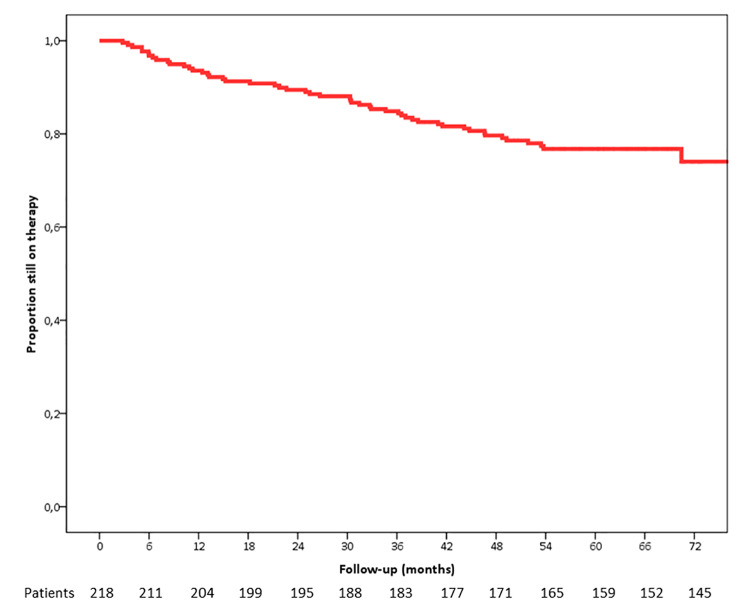
The mean estimated follow-up was of 64.3 months (95% CI 61.3–67.3) for approximately 1000 patient/years. After 5 years of follow-up, 77.1% were still on the DTG/3TC combination (Fig. 1). No virologic failure was detected throughout the whole study period, and only 15 subjects presented single isolated viral blips above 50 copies/ml (not confirmed). Reasons for drug discontinuations are reported in Table 2.
Fig. 1.
Kaplan-Meier curve representing the proportion of subjects still on the DTG-3TC combination
Table 2.
Reasons leading to DTG/3TC discontinuation
| Reason | Number |
|---|---|
|
Death Neoplastic disease End-stage liver disease Myocardial infarction Sepsis Unknown case |
11 6 2 1 1 1 |
|
Adverse events Weight gain Insomnia/neurological symptoms Mialgia Other |
13 3 5 3 2 |
|
Drug unrelated causes Lost to follow-up Patient’s decision Moved to other center Anti-neoplastic therapy |
20 10 6 3 1 |
|
Back to triple drug regimen Poor adherence Not reported |
4 2 2 |
Most patients stopped therapy for reasons unrelated to study drugs (lost to follow-up; patients’ decision; moved to other Centers) but due to the unselected nature of the casuistry; 11 subjects died in the 5 years of follow-up mostly because of pre-existing co-morbidities (6 had neoplastic diseases and 2 had end-stage liver disease). No patient who dropped from the study for any reason had a viral load > 50 copies at the moment of withdrawal.
At the enrollment, the median CD4 count was 669 cells/mcL (IQR 483–927); after 5 years it raised to 899 cells/mcL (IQR 646–1160) (P < 0.001), without a significant change of CD8 counts that lowered from 767 cells/mcL (IQR 532–1034) to 683 cells/mcL (IQR 538–988). Consequently, the CD4/CD8 ratio varied from 0.93 (IQR 0.60–1.30) to 1.15 (IQR 0.77–1.45) (P < 0.0001). A non-significant (P = 0.320) increment of mean creatinine, 0.06 mg/dl in magnitude, was observed over the whole follow-up and was more marked in the first two months raising the baseline value 0.94 mg/dl (IQR 0.78–1.11) to 0.97 mg/dl (IQR 0.84–1.13), but thereafter leveled on these values being the median after 5 years 1.01 mg/dl (IQR 0.84–1.20). The lipid profile was not influenced by switching to the dual regimen. Median total cholesterol variation was − 10 mg/dl (from 188 mg/dl [IQR 158–217] to 178 mg/dl [IQR 162–178]); median HDL-cholesterol change was − 1 mg/dl (from 47 mg/dl [IQR 38–56] to 46 mg/dl [IQR 39–60]) and triglycerides variation was − 4 mg/dl (from 109 mg/dl, [IQR 91–138] to 105 mg/dl [IQR 85–162]).
Discussion
With highly-effective antiretroviral drugs, the need of simpler, more manageable and less impactful regimens is becoming one of the priority in more and more patients in our clinics. From a physician’s perspective, the main consideration about whether to switch from a triple combination therapy to an equally effective dual regimen or not is the supposed possibility of reducing long-term side effects, making a pro-active switch. From the patients’ point of view, the success of 2DR in real-practice is driven also by the ease and the handling of these drugs, in particular if used as single-tablet regimens, with the least impact on their daily life [15, 18].
The indication of DTG/3TC regimen in the switching strategy is the result of randomized clinical trials (RCTs) such as the recently updated TANGO study, which supports the effective virologic suppression at 144 weeks of DTG/3TC combination, non-inferior to tenofovir alafenamide (TAF)-based ART [19], in patients without prior virologic failure, NRTI resistance, or HBV co-infection. More recently, the results of the SALSA study reinforced this indication [20].
Besides RCTs, real-life studies have gathered lots of data about the use of DTG/3TC, with interesting aspects and considerations. In particular, these data come from evaluation on patient populations that, for different reasons, would have not be considered eligible for a clinical trial but that would mostly benefit from the switch in terms of less drug-drug interactions, less toxicities, ease of use, better tolerability and, as consequence, better adherence.
A meta-analysis conducted by Patel et al. confirmed the effectiveness and safety of DTG/3TC in clinical practice, combining the data reported in several real-world studies and, therefore, supporting outcome from RCTs; in particular, virologic suppression ranged from 97 to 100% and 92–100% at 48-weeks and 96-weeks of DTG/3TC in the real-life studies considered and available so far [21]. Although virologic failure was a rare event in all these cohort analysis, they do not go beyond a follow-up of 144 weeks, making potentially valid a questioning about long-term outcomes.
Our study reports data for a follow-up over 5 years and a total drug exposure of about 1000 patient/years. In our prospective cohort study, the main reasons for DTG/3TC discontinuation were related to the worsening of pre-existing comorbidities or to drug unrelated events, rather than virologic failures or DTG/3TC-related adverse events.
These results support the hypothesis that DTG in dual-formulations has a well-known high genetic barrier to resistance development together with a high adherence rate, thus limiting the risk of virologic failure and development of resistance inducing mutations. With respect to this, to the best of our knowledge, in only one case, a new NRTI mutation (M41M/L) appeared at failure of the 2DR with DTG/3TC [18], yet with an uncertain role (if any) in the occurrence of failure itself; only recently the emergence of R263K and S230N mutations in the integrase region was detected by genotypic deep sequencing in a patient failing DTG/3TC [22].
Our study has some limitations. First, in clinically unselected uncontrolled cohorts there is always the risk of bias due to unmeasured confounding, such as drug adherence levels or not reporting adverse effects. For this reason, we decided to report only adverse events leading to drug discontinuation or to clinically relevant decision (e.g. change in therapy). Another caveat was that we censored patients who were lost to follow-up. Such censoring could have led to bias in increasing rate of treatment failure. Finally, our study results could not be applied to those health care systems that do not have HIV case management programs and free ART available.
Nevertheless, these long-term results over 5 years reinforce the durable efficacy, high barrier to treatment-emergent resistance, and good tolerability of DTG/3TC. Our results continue to support the recommended switch use of this 2DR as a well-accepted treatment option for virologically suppressed ART-experienced PLWH.
Acknowledgements
Not applicable.
Abbreviations
- ART
Antiretroviral Therapy.
- HCV
Hepatitis C Virus.
- HIV
Human Immunodeficiency Virus.
- IVDU
Intravenous Drug Users.
- MSM
Men who have Sex with Men
Authors’ contributions
FM conceived and coordinated the study, managed patients, performed the statistical analysis and helped to draft the manuscript. RG conceived the study, managed patients, collected data and helped to draft the manuscript. LP managed patients, collected data. MD managed patients, collected data. DV collected data. AnC managed patients, collected data, helped to draft the manuscript. AdC helped to draft the manuscript. CM conceived the study, managed patients, helped to draft the manuscript. All Authors read and approved the final manuscript.
Funding
No funding.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available since their containing information that could compromise the privacy of patients but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
FM has served as a consultant on advisory boards for Abbvie, Bristol-Myers Squibb, Gilead, Janssen and ViiV; he has received lecture fees from Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck Sharp and Dome, and has received research and educational grants from Abbvie, Bristol-Myers Squibb, Gilead, GlaxoSmithKline and Janssen-Cilag.
RG has served as consultant on advisory boards for Abbvie, Bristol-Myers Squibb, ViiV Healthcare and received lecture fees from Bristol-Myers Squibb, Janssen-Cilag, Roche, ViiV Healthcare, Merck Sharp and Dome.
CM has served as a consultant on advisory boards for Abbvie, Bristol-Myers Squibb, Gilead, ViiV, Janssen, she has received lecture fees from Bristol-Myers Squibb, Gilead,ViiV, Merck Sharp and Dome, and has received research and educational grants from Gilead and ViiV. Other authors: no conflict.
Ethics approval and consent to participate
No experimental procedure (e.g. randomization) was applied, and drugs were used according to a considered alternative option in Italian Guidelines. In all patients, the decision to switch therapy was taken on clinical grounds as they presented a clinically relevant reason, either because of concomitant diseases, altered laboratory tests, drug adverse events or risk of drug-to-drug interactions. The drug combination was presented as a possible alternative and discussed individually according to clinical needs. The possibility to use the dual combination was discussed according to available data and to results obtained with similar dual therapies. Alternatives were presented according to the specific clinical situation. The choice of the dual regimen was therefore a part of the patient/doctor relationship during normal clinical practice. Although patients were followed prospectively, none of them switched therapy after the decision to perform this analysis, consequently the local EC ruled out that no formal ethics approval was required and patients gave their informed consent solely for the use of clinical and laboratory data.
Consent for publication
Not Applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents., A Working Group of the Office of AIDS Research, Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
- 2.EACS Guidelines [Internet]. EACSociety. [cited 2022 Jan 22]. Available from: https://www.eacsociety.org/guidelines/eacs-guidelines/ EACS European Aids Clinical Society. Guidelines. Version 11.0. October 2021. Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf.
- 3.Cahn P, Madero JS, Arribas JR, et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment–Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. J Acquir Immune Defic Syndr. 2020;83(3):310–8. doi: 10.1097/QAI.0000000000002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019 Sep;6(9):e576–87. Available at: https://linkinghub.elsevier.com/retrieve/pii/S2352301819301493. Accessed 4 January 2022. [DOI] [PubMed]
- 5.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs Continuing a Tenofovir Alafenamide–Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study. Clin Infect Dis. 2020;71(8):1920–9. doi: 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–72. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 7.Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Virologically Suppressed Adults With Human Immunodeficiency Virus. Clin Infect Dis. 2021;73:e485–93. doi: 10.1093/cid/ciaa988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Antoni ML, Andreatta K, Acosta RK, Chang S, Martin R, White KL. Bictegravir/Emtricitabine/Tenofovir Alafenamide (B/F/TAF) Efficacy in Participants with Pre-Existing Primary Integrase Inhibitor Resistance Through 48 Weeks of Phase 3 Clinical Trials. Open Forum Infectious Diseases 2020; 7:S376–S377. Available at https://academic.oup.com/cid/article/71/8/1920/5697294. Accessed March 2022. [DOI] [PMC free article] [PubMed]
- 9.Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 2010;4:115. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton SS, Hardin JW, Bramley TJ, D’Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care. 2016;22(4):242–8. [PubMed] [Google Scholar]
- 11.Palella FJ, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS. 2014;28:335–44. doi: 10.1097/QAD.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 12.Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. JAIDS. 2014;65:587–96. doi: 10.1097/QAI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton SS, Magagnoli J, Cummings H, Hardin T JW. Adherence after treatment switch from a multiple tablet antiretroviral regimen to a single tablet antiretroviral regimen. Therapie. 2021 Dec;76(6):567–76. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0040595721000639. Accessed March 2022. [DOI] [PubMed]
- 14.Maggiolo F, Gulminetti R, Pagnucco L, et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17(1):215. doi: 10.1186/s12879-017-2311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghetti A, Lombardi F, Gagliardini R, et al. Efficacy and tolerability of lamivudine plus dolutegravir compared with lamivudine plus boosted PIs in HIV-1 positive individuals with virologic suppression: a retrospective study from the clinical practice. BMC Infect Dis. 2019;19. DOI:10.1186/s12879-018-3666-8. [DOI] [PMC free article] [PubMed]
- 16.Baldin G, Ciccullo A, Borghetti A, Di Giambenedetto S. Virological efficacy of dual therapy with lamivudine and dolutegravir in HIV-1-infected virologically suppressed patients: long-term data from clinical practice. J Antimicrob Chemother. 2019;74(5):1461–3. doi: 10.1093/jac/dkz009. [DOI] [PubMed] [Google Scholar]
- 17.Linee Guida Italiane sull’utilizzo dei. farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1 - anno 2017 [Internet]. [cited 2022 Jun 6]. Available from: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2696.
- 18.Galizzi N, Poli A, Galli L, et al. Retrospective study on the outcome of two-drug regimens based on dolutegravir plus one reverse transcriptase inhibitor in virologically-suppressed HIV-infected patients. Int J Antimicrob Agents. 2020;55(3):105893. doi: 10.1016/j.ijantimicag.2020.105893. [DOI] [PubMed] [Google Scholar]
- 19.Osiyemi O, De Wit S, Ajana F, et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine (DTG/3TC) Versus Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With HIV-1: Results Through Week 144 From the Phase 3, Non-inferiority TANGO Randomized Trial. Clin Infect Dis. 2022 Jan; ciac036. [DOI] [PMC free article] [PubMed]
- 20.Llibre JM, Brites C, Cheng C-Y, Osiyemi O, Galera C, Hocqueloux L, et al. Efficacy and Safety of Switching to the 2-Drug Regimen Dolutegravir/Lamivudine Versus Continuing a 3- or 4-Drug Regimen for Maintaining Virologic Suppression in Adults Living With HIV-1: Week 48 Results From the Phase 3, Non-inferiority SALSA Randomized Trial. Clin Infect Dis. 2022 Mar;ciac130. [DOI] [PMC free article] [PubMed]
- 21.Patel R, Evitt L, Mariolis I, et al. HIV Treatment with the Two-Drug Regimen Dolutegravir Plus Lamivudine in Real-world Clinical Practice: A Systematic Literature Review. Infect Dis Ther. 2021;10(4):2051–70. doi: 10.1007/s40121-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revollo B, Viñuela L, de la Mora L, García F, Noguera-Julián M, Parera M, et al. Integrase resistance emergence with dolutegravir/lamivudine with prior HIV-1 suppression. J Antimicrob Chemother. 2022;77(6):1738–40. doi: 10.1093/jac/dkac082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available since their containing information that could compromise the privacy of patients but are available from the corresponding author on reasonable request.