Abstract
Scope:
Blueberries are rich sources of bioactive polyphenols that may provide health benefits when consumed regularly, leading to their increased marketing as dietary supplements. However, the metabolic changes associated with consuming concentrated doses of purified polyphenols, as may be present in dietary supplements, are unknown, especially when considering the colonic metabolites formed. This study aimed to evaluate the pharmacokinetics of high doses of purified blueberry polyphenols.
Methods and results:
Five-month old, ovariectomized Sprague-Dawley rats were acutely dosed with purified blueberry polyphenols (0, 75, 350, and 1000 mg total polyphenols/kg bw) and 45Ca to measure calcium absorption. Blood and urine were collected for 48h after dosing and phenolic metabolites measured via UPLC-MS/MS. The most prominent metabolites were colonically generated cinnamic and hippuric acids. Smaller amounts of other phenolic acids, flavonols, and anthocyanins were also detected. Most metabolites followed a dose-response relationship, though several showed saturated absorption. Maximal metabolite concentrations were reached within 12h for a majority of compounds measured, while some (e.g., hippuric acid) peaked up to 24h post-dosing. Calcium absorption was significantly increased in the highest dose group (p=0.03).
Conclusion:
These results indicate that increased doses of blueberry polyphenols induce changes in intestinal phenolic metabolism and increase calcium absorption.
Keywords: blueberry, polyphenol, colonic metabolism, pharmacokinetics, calcium absorption
1. INTRODUCTION
Fruit and vegetable derived polyphenols may have beneficial health effects by reducing the risk of developing many chronic diseases while also improving cardiovascular and neurocognitive health.[1–4] Blueberries are a particularly rich source of polyphenols, containing high amounts of anthocyanins, flavonols, and chlorogenic acid,[5] and demonstrating many of these same health benefits.[6]
To elucidate the connection between blueberry polyphenols and their health benefits, an understanding of their bioavailability and which forms are most biologically active is necessary. To that end, much recent work has been performed on the absorption, metabolism, and excretion of polyphenols. The metabolism of these compounds, in particular, has been challenging, as polyphenols undergo phase II metabolism (methylation, glucuronidation, and/or sulfation) within small intestinal epithelial cells before being absorbed or effluxed back into the intestinal lumen.[7–9] Small intestine-derived phase II metabolites are poorly absorbed (<2% for many classes of polyphenols),[10, 11] as evidenced by low plasma and urine concentrations (i.e., low Cmax values) and rapid excretion (Tmax 0.5–2h).[12] For years, researchers surmised that because the bioavailability of polyphenols and their in vivo residence time was low, they had a limited ability to exert beneficial health effects.[13]
However, in recent years, the emergence of the gut microbiome as an important and active part of the metabolic transformation of dietary phenolics has offered a new perspective. A large majority of orally ingested polyphenols reach the lower intestine intact, allowing them to interact extensively with the gut microbiota, in a bidirectional manner.[9, 14] Diets high in polyphenols have been reported to shift the composition of the gut microbial communities, often to a putatively healthier state, while also being efficiently metabolized and absorbed.[15] During this process, polyphenols are catabolized to smaller molecular weight phenolic acids that are more extensively absorbed (up to 10-fold higher) than in the small intestine (i.e., higher Cmax and greater AUC) and persist longer in systemic circulation (i.e., later Tmax).[8, 16] This not only increases the overall bioavailability of polyphenols, but raises the possibility that the colonic catabolites may be driving the observed health benefits.[10] Thus, much recent work has focused on understanding these catabolic pathways and fully characterizing the colonic metabolites produced from these interactions.
As knowledge of microbial metabolism of polyphenols continues to expand, there are several areas that remain understudied, including the full pharmacokinetics of colonic catabolites and the dose-response effects of elevated doses as may be found in dietary supplements that may contain up to 100x the amounts typically consumed in the Western diet.[17, 18] This is critical information for designing clinical trials. Most pharmacokinetic studies on polyphenols are completed within 8h of dosing, which may not be long enough to detect a number of colonic catabolites that may be produced as the ingested dose takes ~20h to traverse the full length of the GI tract.[19] Additionally, given the rapid rise – from <2% to 30% of U.S. adults – in popularity of polyphenol-rich herbal and botanical dietary supplements over the past 25 years,[20–23] expanding the range of doses studied is critical to understand the full scope of the metabolism of these compounds.
We evaluated the metabolism and dose-response of blueberry polyphenols. We accomplished this by quantitating urinary and plasma phenolic metabolites for 48h after an acute dose of blueberry polyphenols over a large range of doses. Given that women aged 51–70y are the most frequent consumers of botanical dietary supplements and are also susceptible to menopause-related bone loss,[20] we chose to use the ovariectomized (OVX) rat model for this study because it mimics hormonal changes and bone loss occurring in postmenopausal women.[24] With recent evidence suggesting that blueberry polyphenols may mitigate menopause-associated bone loss and reduce the incidence of osteoporosis in aging females by increasing calcium absorption,[25–27] we also evaluated the influence of blueberry polyphenols on calcium absorption. We hypothesized that the colonic catabolites of blueberry polyphenols would be absorbed much later than and to a much greater extent than small intestinal metabolites and would exhibit a dose-dependent increase in calcium absorption.
2. EXPERIMENTAL SECTION
2.1. Chemicals/Materials and vendors
Commercial standards of cyanidin-3-O-glucoside chloride, delphindin-3-O-glucoside chloride, malvidin-3-O-glucoside chloride, gallic acid, caffeic acid, ferulic acid, ethyl gallate, taxifolin, chlorogenic acid, hippuric acid, 3-hydroxyhippuric acid, 4-hydroxybenzaldehyde, isovanillin, p-anisic acid, 4-hydroxyphenylacetic acid, 3-hydroxyphenylpropionic acid, 3-methoxyphenylacetic acid, isovanillic acid, homovanillic acid, 3-hydroxy-4-methoxyphenylpropionic acid, syringic acid, quercetin, myricetin, chlorogenic acid, quercetin-3-O-glucuronide, protocatechuic acid, p-coumaric acid, catechin, epicatechin, 4-methoxyquercetin, and quercetin-3-O-glucoside as well as sodium carbonate and Folin and Ciocalteu’s reagent (2N) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Caffeic acid glucuronide was supplied by Synthose (Concord, Ontario, Canada). LC-MS grade solvents, including methanol, water, ACN, and formic acid as well as trace metal grade concentrated nitric acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). 45Ca was purchased from PerkinElmer (Waltham, MA, USA). EcoLite (+) scintillation cocktail was purchased from MP Biomedicals (Santa Ana, CA, USA).
2.2. Animal protocols
2.2.1. Study overview.
The study design is illustrated in Figure 1. Upon arrival, animals were maintained on a polyphenol-free diet during a 1-week stabilization. Animals were then randomized to treatment groups (n=8/gp), with all groups (except control) switched to a 5% blueberry diet for the remainder of the study. After 1 week on the blueberry diet, animals were dosed with blueberry phenolics and 45Ca, with blood and urine collected over 48h for pharmacokinetics. Animals were then sacrificed via CO2 asphyxiation, both femurs harvested, and ovariectomy verified by visual inspection.
Figure 1 –
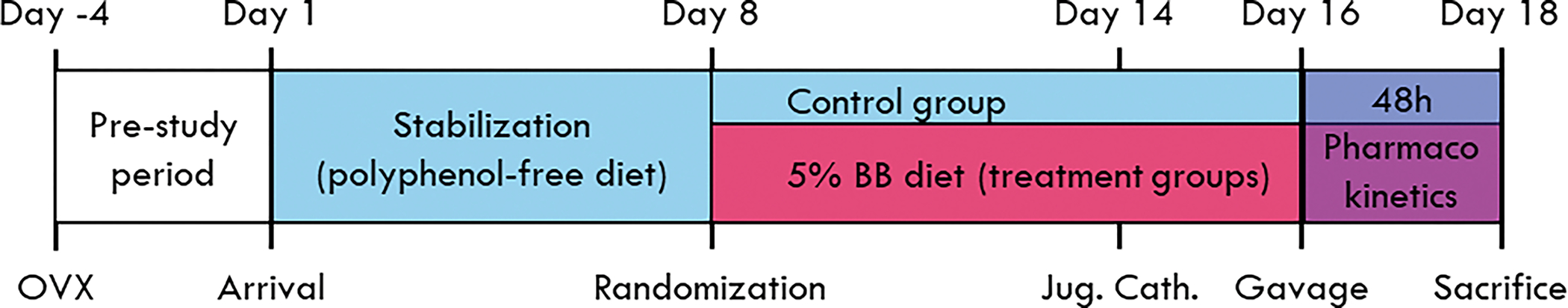
Study design and timeline. All animals were ovariectomized (OVX) by the vendor prior to shipping (day -4). Upon arrival (day 1), animals were stabilized for one week on a polyphenol-free diet. Randomization took place on day 8, with control animals remaining on the polyphenol-free diet and all others placed on a 5% blueberry diet for the remainder of the study. Jugular catheters were placed on day 14, with animals receiving blueberry phenolics and 45Ca via oral gavage two days later. Blood and urine were collected for 48h until sacrifice.
2.2.2. Animal care.
Animal experiments were conducted in adherence to Purdue University Animal Care and Use Committee (PACUC) guidelines, following an approved protocol (1612001508). Thirty-two 5-month old, virgin, ovariectomized, female Sprague Dawley rats were purchased from Envigo (Indianapolis, IN, USA) and individually housed in stainless steel, wire-bottom cages in a temperature and humidity-controlled room with a 12h light/dark cycle and ad libitum access to food and water.
2.2.3. Diets.
Polyphenol-free diets were based on the AIN-93M diet, using corn oil in place of soybean oil to prevent confounding from soy isoflavones. The 5% blueberry chow diet (5% BB) incorporated lyophilized blueberry powder (Wild Blueberry Association of North America, Old Town, ME, USA) into the polyphenol-free base diet. To account for the fiber and sugar content of berries, these components were adjusted in the base diet to maintain isocaloricity with control group. Diets were prepared by Research Diets (New Brunswick, NJ, USA). To minimize phenolic degradation, all diets were stored at −20°C and changed daily for each animal.
2.2.4. Dosing Regimen.
VitaBlue Pure American Blueberry Extract, containing 28.8% total phenolics (w/w), was donated by FutureCeuticals (Momence, IL, USA) for use in this study. Oral gavage slurries were prepared with water at the following doses: 0, 75, 350, and 1000 mg phenolics/kg bw. The 75 mg/kg bw dose corresponds to an adult human consuming approximately 1–2 cups of fresh blueberries per day (i.e., a “dietary dose”, calculated using the FDA’s rat to human conversion factor).[28] The 350 and 1000 mg/ kg bw doses group were several-fold higher to mimic higher concentrations as may be present in dietary supplements. Immediately prior to receiving the oral gavage, each animal underwent a one-time, 8h fast.
2.2.5. Jugular catheter surgery, dosing, and blood/urine collection.
Two days prior to beginning pharmacokinetics, jugular catheters were placed in the right jugular vein of all animals as previously described.[29] Animals were anesthetized with isoflurane and given bruprinex (i.p., 0.1 mg/kg bw) to minimize pain. Catheters were flushed with heparinized saline (20 units/mL) every 12h to keep them patent.
Blood was collected via the implanted catheter immediately prior to (baseline) and every 6h after oral gavage until sacrifice. In total, 9 blood draws were collected (0, 6, 12, 18, 24, 30, 36, 42, and 48h). Plasma was separated from whole blood via immediate centrifugation in heparinized microfuge tubes at 4°C and 3500 g for 10 min. Plasma was acidified to a final concentration of 0.1% formic acid, flushed with nitrogen, and frozen at −80°C until analysis.
Urine was collected in 12h increments throughout the study. To capture baseline phenolic metabolites, 2×12h urine collections (0–12 and 12–24h) were obtained from all animals in the 24h leading up to the jugular catheter surgery. Then, after gavage, 4×12h urine collections (0–12, 12–24, 24–36, and 36–48h) were obtained. Collected urine was centrifuged to remove particulates, acidified to a final concentration of 0.1% formic acid, blanketed with nitrogen, and frozen at −80°C until analysis.
2.2.6. 45Ca absorption.
Immediately after gavaging animals with blueberry phenolics, six animals per group were given a second oral gavage containing 20 μCi 45Ca and 100 mg calcium acetate (equivalent to ~25% daily calcium intake to replace calcium missed during fasting) in water. The remaining two animals in each group were also given a 500 μL oral gavage containing 100 mg calcium acetate and water, but were dosed with 10 μCi 45Ca via jugular catheter. At sacrifice, both femurs were harvested, manually cleaned to remove soft tissue, wrapped in saline soaked gauze, and stored at −80°C until analysis.
2.3. Polyphenol analyses
2.3.1. Extraction and purification of phenolics in starting materials.
Lyophilized whole blueberries, VitaBlue Pure blueberry extract, animal diets, and individual gavage doses were extracted in triplicate, as described elsewhere.[30] Extracts were resolubilized with 2% formic acid in water and purified via solid phase extraction (SPE) using Oasis HLB 1cc extraction cartridges (Waters, Milford, MA, USA), as described elsewhere. [30]
2.3.2. Extraction and purification of phenolic metabolites.
Phenolics in plasma and urine samples were extracted via SPE using the strataX, polymeric reversed phase microelution 96 well plate with a capacity of 2 mg/well (Phenomenex, Torrence, CA, USA). Wells were preconditioned with 200 μL 1% formic acid in methanol followed by 200 μL 1% formic acid in water. Samples were then loaded as a mixture of the biological sample (100 μL plasma or 50 μL urine), 200 μL 1% formic acid in water, and 20 μL of 50 μM taxifolin as an extraction efficiency control. Samples were washed with 2 × 200 μL 0.1% formic acid in water, and then dried under nitrogen for 30 minutes. Samples were eluted with 100 μL 0.1% formic acid in methanol into a 96-well plate (350 uL Acquity 96-well plate, Waters, Milford, MA, USA). All steps were aided by gentle, positive pressure nitrogen gas delivered via Waters Positive Pressure-96 Processor. To the eluate was added 20 μL 50 μM ethyl gallate as a volume control. Eluted samples were immediately capped with a pre-slit silicon mat (Cap-mat 96 well 7 mm round plug pre-slit silicone/PTFE, Waters) and frozen at −80°C until analysis.
2.3.3. Quantification of total phenolics in starting materials.
Total phenolics were quantified in crude extracts via the Folin method and corrected for water-soluble interferences (e.g., sugars and ascorbic acid) as described elsewhere.[31, 32]
2.3.4. Quantitation of phenolics via UPLC-MS/MS.
After purification via SPE, individual phenolics were quantified via UPLC-MS/MS using a Waters UPLC Acquity I Class system equipped with a TQD detector. Samples were injected and phenolics separated using an Acquity BEH C18 column (2.1 um, 1.7 mm id x 50 mm) with a flow rate of 0.5 mL/min. Samples were eluted using a biphasic gradient of solvent A (0.1% formic acid in acetonitrile) and solvent B (2.0% formic acid in water (for ESI+ mode) or 0.1% formic acid in water (for ESI-)) as follows: 0 min, 100% B; 0.5 min, 94% B; 2 min, 91% B; 3 min, 87% B; 4.5 min, 65% B; 5.2 min, 100% B; 6 min, 100% B. MS conditions were as follows: capillary voltage, 0.5 kV; probe temp, 150°C; source temp, 600°C; desolvation gas flow, 1000 L/hr; cone gas flow, 50 L/hr.
Identification and quantification of each compound was based on authentic standards, using calibration curves ranging from 0.001–100μM. When standards were not available, compounds (especially phase II metabolites) were confirmed based on retention times and the presence of multiple ion transitions consistent with each compound.[33] A complete list of phenolic compounds and metabolites measured, including corresponding MRMs and standards used for quantitation, is shown in Table SI-1.
2.4. Fractional calcium absorption
Total femoral calcium deposition was used to determine fractional calcium absorption, as described elsewhere.[34, 35] Briefly, each femur was ashed in a muffle furnace for 5d at 600°C, dissolved overnight in concentrated nitric acid, and diluted to 25 mL with ultrapure water. 1 mL of the resulting solution was mixed with 15 mL Ecolite in a scintillation vial and 45Ca quantified by liquid scintillation counting (Tri-Carb 2910 TR Liquid Scintillation Analyzer, PerkinElmer, Waltham, MA, USA). Fractional absorption was calculated as a ratio of oral:i.v. 45Ca in femurs as previously described.[35, 36]
2.5. Statistics
Statistics were completed using SAS (SAS Institute, Raleigh, NC). When data were not normal, appropriate transformations were performed before analysis to ensure normality. Outliers were detected and removed using Tukey’s method. Plasma AUC was calculated using the trapezoidal method and qualitatively observed levels for Cmax and Tmax reported. Comparisons for total excretion and AUC were made via one-way ANOVA, while individual points on pharmacokinetic curves were analyzed via two-way ANOVA (factors: time and dose). Post hoc analyses were carried out with Tukey’s HSD test and significance defined as p<0.05 unless otherwise noted. Guidance in SAS coding was provided by the Statistical Consulting Service at Purdue University.
3. RESULTS AND DISCUSSION
3.1. Phenolic profiles of raw materials, rat diets, and gavage doses.
3.1.1. Phenolic profiles of raw materials.
Two commercially available raw materials were used in this study: lyophilized blueberry powder (FD) and concentrated blueberry polyphenol extract (CE). Both materials were derived from commercially available wild blueberries and contained 3.75% and 28.8% (w/w) total polyphenols, respectively (Table 1). These raw materials were used to create the rat chow diets and gavage doses.
Table 1 –
Phenolic content of raw materials, rat diets, and gavage doses.a
| Polyphenol | Raw Materials | Rat Diets | Gavage Doses | |||||
|---|---|---|---|---|---|---|---|---|
| FD | CE | PPF | 5% BB | Control | Low | Medium | High | |
|
| ||||||||
| Anthocyanins | ||||||||
| Cyanidins | ||||||||
| Arabinoside | 48.5 ± 7.19 | 227 ± 14.2 | nd | 1.85 ± 0.47 | nd | 0.63 ± 0.14 | 3.59 ± 1.03 | 6.98 ± 1.18 |
| Galactoside | 102 ± 16.5 | 581 ± 52.8 | nd | 3.92 ± 1.11 | nd | 1.54 ± 0.35 | 8.60 ± 2.70 | 16.0 ± 3.14 |
| Glucoside | 102 ± 16.8 | 271 ± 24.4 | nd | 3.97 ± 0.98 | nd | 0.74 ± 0.16 | 4.20 ± 1.18 | 8.15 ± 1.32 |
| Delphinidins | ||||||||
| Arabinoside | 31.1 ± 4.51 | 484 ± 28.1 | nd | 1.07 ± 0.24 | nd | 1.26 ± 0.28 | 8.03 ± 2.01 | 15.6 ± 2.62 |
| Galactoside + Glucoside | 58.6 ± 7.72 | 662 ± 35.3 | nd | 2.43 ± 0.45 | nd | 1.78 ± 0.26 | 11.8 ± 2.72 | 23.4 ± 3.98 |
| Malvidins | ||||||||
| Arabinoside | 144 ± 22.5 | 2313 ± 95.3 | nd | 5.44 ± 1.19 | nd | 6.33 ± 1.12 | 38.4 ± 8.72 | 79.2 ± 11.7 |
| Galactoside | 237 ± 24.3 | 3976 ± 153 | nd | 11.6 ± 1.22 | nd | 10.7 ± 0.78 | 76.2 ± 9.22 | 169 ± 13.2 |
| Glucoside | 319 ± 40.7 | 3248 ± 136 | nd | 15.6 ± 1.52 | nd | 8.75 ± 0.54 | 63.7 ± 6.73 | 142 ± 12.1 |
| Peonidins | ||||||||
| Arabinoside | 42.0 ± 6.64 | 202 ± 13.7 | nd | 1.61 ± 0.42 | nd | 0.58 ± 0.13 | 3.19 ± 0.91 | 6.24 ± 1.06 |
| Galactoside | 24.5 ± 3.9 | 180 ± 18.3 | nd | 0.95 ± 0.27 | nd | 0.49 ± 0.11 | 2.58 ± 0.81 | 4.84 ± 0.94 |
| Glucoside | 42.2 ± 6.29 | 164 ± 10.8 | nd | 1.72 ± 0.36 | nd | 0.45 ± 0.08 | 2.65 ± 0.62 | 5.27 ± 0.82 |
| Petunidins | ||||||||
| Arabinoside | 48.0 ± 7.47 | 847 ± 65.6 | nd | 1.84 ± 0.48 | nd | 2.34 ± 0.57 | 13.6 ± 3.75 | 26.4 ± 4.38 |
| Galactoside | 78.9 ± 12.1 | 1814 ± 170 | nd | 3.07 ± 0.88 | nd | 4.75 ± 1.13 | 27.4 ± 7.70 | 51.8 ± 9.02 |
| Glucoside | 132 ± 20.0 | 946 ± 105 | nd | 5.12 ± 1.23 | nd | 2.59 ± 0.61 | 14.6 ± 4.25 | 27.8 ± 4.99 |
| Phenolic Acids | ||||||||
| Benzoic Acids | ||||||||
| Gallic acid | 0.04 ± 0.07 | 9.8 ± 2.43 | nd | trace | nd | 0.083 ± 0.018 | 0.44 ± 0.05 | 0.86 ± 0.25 |
| Protocatechuic acid | 0.85 ± 0.17 | 13 ± 3.05 | nd | 0.07 ± 0.01 | nd | 0.047 ± 0.007 | 0.26 ± 0.06 | 0.51 ± 0.19 |
| Cinnamic Acids | ||||||||
| Caffeic acid | 0.51 ± 0.2 | 32.5 ± 7.4 | nd | 0.27 ± 0.07 | nd | 0.085 ± 0.022 | 0.44 ± 0.13 | 0.73 ± 0.23 |
| Chlorogenic acid | 593 ± 65.9 | 1916 ± 165 | nd | 21.9 ± 3.61 | nd | 5.21 ± 0.72 | 26.8 ± 5.07 | 52.1 ± 9.08 |
| Ferulic acid | 4.48 ± 1 | 97.2 ± 8.18 | nd | 0.58 ± 0.12 | nd | 0.27 ± 0.05 | 1.39 ± 0.26 | 2.67 ± 0.61 |
| Feruloylquinic acid | 16.2 ± 2.3 | 60.3 ± 5.75 | nd | 0.6 ± 0.14 | nd | 0.18 ± 0.03 | 0.86 ± 0.15 | 1.64 ± 0.27 |
| Flavan-3-ols | ||||||||
| Catechin | 8.91 ± 1.44 | 43.8 ± 5.68 | nd | 0.39 ± 0.06 | nd | 0.083 ± 0.023 | 0.45 ± 0.05 | 0.79 ± 0.19 |
| Epicatechin | 5.91 ± 0.74 | 13.6 ± 3.1 | nd | 0.22 ± 0.04 | nd | 0.025 ± 0.009 | 0.12 ± 0.02 | 0.20 ± 0.08 |
| Gallocatechin | 2.93 ± 0.51 | 3.2 ± 0.58 | nd | 0.1 ± 0.02 | nd | 0.0065 ± 0.002 | 0.034 ± 0.004 | 0.059 ± 0.024 |
| Epigallocatechin | nd | 30.1 ± 7.58 | nd | nd | nd | 0.091 ± 0.031 | 0.36 ± 0.09 | 0.63 ± 0.18 |
| Flavonols | ||||||||
| Myricetin | 2.68 ± 0.45 | 38.8 ± 5.19 | nd | 0.1 ± 0.03 | nd | 0.11 ± 0.04 | 0.49 ± 0.16 | 0.86 ± 0.21 |
| Kaemperol | trace | trace | nd | trace | nd | trace | trace | trace |
| Galactoside + Glucoside | 53.9 ± 8.2 | 194 ± 34 | nd | 1.78 ± 0.58 | nd | 0.46 ± 0.13 | 2.24 ± 0.89 | 3.48 ± 1.01 |
| Quercetin | 11.7 ± 2.23 | 951 ± 93.1 | nd | 1.12 ± 0.29 | nd | 2.24 ± 0.71 | 11.7 ± 3.47 | 21.4 ± 4.62 |
| Galactoside + Glucoside | 181 ± 24.9 | 1095 ± 108 | nd | 8.35 ± 1.58 | nd | 3.01 ± 0.56 | 14.9 ± 3.94 | 28.2 ± 5.53 |
| Rutin | 82.7 ± 13.5 | 245 ± 29 | nd | 3.63 ± 0.81 | nd | 0.71 ± 0.11 | 3.67 ± 0.77 | 7.64 ± 1.10 |
| Total Phenolics (via Folin assay) | 3747 ± 68.1 | 28824 ± 1608 | nd | 141 ± 11.6 | nd | 75.3 ± 3.30 | 342 ± 16.1 | 1011 ± 11.7 |
FD = freeze dried whole blueberries; CE = concentrated blueberry phenolic extract; PPF = polyphenol-free diet; nd = not detected; trace = compound detected, but below LOQ.
Raw materials and rat diets shown in mg phenolic/100 g dw; gavage doses shown in mg phenolic/kg bw. Data are comprised of three analytical replicates and presented as mean ± SD.
To further characterize these materials, a total of 30 individual phenolics were quantified. As shown in Table 1, anthocyanins were the most prevalent class of phenolics present, comprising nearly half of the total phenolics. In both FD and CE, malvidin glycosides were the most prevalent anthocyanins, followed by petunidin glycosides. Other classes of phenolics, including phenolic acids, flavan-3-ols, and flavonols, were quantified. The most prevalent of these were chlorogenic acid and quercetin species, which collectively accounted for ~20% of total phenolics assayed by LC-MS. Comparatively lower levels of benzoic acids and flavan-3-ols were observed.
3.1.2. Phenolic profiles of rat diets.
The rat chow diets were created by incorporating either 0% or 5% of the FD berries into the AIN-93M diet (denoted PPF and 5% BB diet, respectively). During the manufacturing of these diets, thermal and oxidative degradation occur, resulting in phenolic losses. In preliminary experiments with several vendors, we have observed significant differences in phenolic content and potential losses (data not shown). For this study, we chose the manufacturer that demonstrated minimal losses, though we note ~25% of total phenolics were lost in the creation of our 5% BB diet (Table 1). When comparing the relative amounts of individual phenolics in the 5% BB diet with those in the FD berries, the losses appear to occur evenly across all compounds measured, indicating that the phenolic composition of the 5% BB diet is similar to the FD berries.
No significant difference in food consumption or food efficiency ratio was found between groups (data not shown). Based on total diet consumption while on the 5% BB diet, rats consumed 50–60 mg total polyphenols/kg bw/d from their diets (data not shown). This is nearly as much as the low dose received via oral gavage.
3.1.3. Phenolic profiles of gavage doses.
Gavage doses were created using the CE, with target doses of 75, 350, and 1000 mg total polyphenol/kg bw. As shown in Table 1, our actual doses were quite close to these targets. And, as expected, the relative amounts of individual phenolics in the gavage doses mirrored the amounts in the CE.
3.2. Urinary excretion of phenolic metabolites.
3.2.1. Summary of urinary phenolic excretion.
A total of 43 phenolic metabolites were detected in the urine, including 17 anthocyanins, 19 phenolic acids, 2 hippuric acids, and 5 flavonols. A majority of the metabolites demonstrated dose-dependent excretion and were maximally detected within 12h of dosing (Table 2). Blueberry polyphenols were extensively metabolized, with <5% of total urinary metabolites being detected in their unmetabolized forms. The most prominent metabolites were trans-cinnamic acids, followed by hippuric acids, with smaller amounts of other phenolic acids, flavonols, and anthocyanins also detected (Figure 2). These metabolites exhibited a dose-dependent shift in excretion, with higher doses showing increased proportions of cinnamic and phenylpropionoic acids and a concomitant decrease in the proportion of hippuric acids.
Table 2 –
Total urinary excretion of phenolic metabolites for 48h after gavage.a
| Total Urinary Excretion (nmol) | Qualitative Tmax b | Cmax (nmol excreted) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low | Medium | High | Control | Low | Medium | High | Control | Low | Medium | High | |
|
| ||||||||||||
| Anthocyanins | ||||||||||||
| Cyanidin | ||||||||||||
| Galactoside | nd | 0.19 ± 0.04 w | 0.41 ± 0.08 x | 0.68 ± 0.08 y | - | np | 0–12h | 0–12h | - | np | 0.19 ± 0.04 | 0.46 ± 0.09 |
| Glucoside | nd | 0.17 ± 0.03 w | 0.31 ± 0.08 x | 0.41 ± 0.04 y | - | np | 0–24h | 0–12h | - | np | 0.11 ± 0.05 | 0.22 ± 0.04 |
| Glucuronide | nd | nd | 0.05 ± 0.02 w | 0.13 ± 0.05 x | - | - | 0–12h | 0–12h | - | - | 0.02 ± 0.01 | 0.09 ± 0.04 |
| Total Cyanidins | - | 0.36 | 0.77 | 1.22 | ||||||||
| Delphinidin | ||||||||||||
| Galactoside | nd | 0.76 ± 0.06 w | 2.08 ± 0.21 x | 2.81 ± 0.23 y | - | np | 0–12h | 0–12h | - | np | 0.98 ± 0.20 | 1.74 ± 0.22 |
| Glucoside | nd | 0.79 ± 0.07 w | 1.94 ± 0.19 x | 2.00 ± 0.29 x | - | np | 0–24h | 0–24h | - | np | 0.81 ± 0.14 | 0.94 ± 0.22 |
| Glucuronide | nd | 4.21 ± 0.92 w | 12.7 ± 2.18 x | 31.4 ± 1.33 y | - | np | 0–12h | 0–12h | - | np | 6.35 ± 1.60 | 20.8 ± 5.1 |
| Sulfate | nd | 31.8 ± 5.83 w | 46.9 ± 6.01 x | 54.4 ± 4.23 x | - | np | 0–24h | 0–24h | - | np | 18.2 ± 2.4 | 20.2 ± 2.4 |
| Total Delphinidins | - | 37.5 | 63.6 | 90.6 | ||||||||
| Malvidin | ||||||||||||
| Galactoside | nd | 4.75 ± 0.44 w | 13.5 ± 2.51 x | 22.7 ± 1.30 y | - | np | 0–12h | 0–12h | - | np | 7.36 ± 2.00 | 14.2 ± 1.3 |
| Glucoside | nd | 6.06 ± 0.66 w | 14.3 ± 0.88 x | 19.7 ± 1.02 y | - | np | 0–12h | 0–12h | - | np | 6.57 ± 0.57 | 10.7 ± 0.9 |
| Total Malvidins | - | 10.8 | 27.8 | 42.4 | ||||||||
| Peonidin | ||||||||||||
| Galactoside | nd | 0.03 ± 0.01 w | 0.08 ± 0.01 x | 0.14 ± 0.02 y | - | np | 0–12h | 0–12h | - | np | 0.04 ± 0.01 | 0.09 ± 0.02 |
| Glucoside | nd | 0.05 ± 0.01 w | 0.08 ± 0.02 x | 0.14 ± 0.02 y | - | np | 0–12h | 0–12h | - | np | 0.03 ± 0.01 | 0.09 ± 0.02 |
| Glucuronide | nd | 2.12 ± 0.83 w | 3.01 ± 1.01 w,x | 3.38 ± 1.27 x | - | np | np | np | - | np | np | np |
| Sulfate | nd | 0.59 ± 0.17 | 0.64 ± 0.13 | 0.58 ± 0.08 | - | np | np | np | - | np | np | np |
| Total Peonidins | - | 2.79 | 3.83 | 4.24 | ||||||||
| Petunidin | ||||||||||||
| Galactoside | nd | 0.44 ± 0.09 w | 1.05 ± 0.30 x | 2.49 ± 0.32 y | - | np | 0–12h | 0–12h | - | np | 0.49 ± 0.23 | 1.73 ± 0.38 |
| Glucoside | nd | 0.44 ± 0.08 w | 0.90 ± 0.25 x | 1.47 ± 0.17 y | - | np | 0–12h | 0–12h | - | np | 0.37 ± 0.14 | 0.94 ± 0.19 |
| Glucuronide | nd | 0.69 ± 0.25 w | 1.81 ± 0.47 x | 5.63 ± 0.56 y | - | np | 0–12h | 0–12h | - | np | 0.92 ± 0.24 | 3.49 ± 0.46 |
| Sulfate | nd | 0.32 ± 0.09 w | 0.43 ± 0.08 x | 0.44 ± 0.09 w,x | - | np | 12–24h | 12–24h | - | np | 0.16 ± 0.03 | 0.15 ± 0.02 |
| Total Petunidins | - | 1.89 | 4.19 | 10.0 | ||||||||
| Total Anthocyanins | - | 53.3 | 100.2 | 148.5 | ||||||||
| Phenolic Acids | ||||||||||||
| Benzaldehydes (BALD) | ||||||||||||
| 4-OH-BALD | nd | nd | trace | trace | - | - | - | - | - | - | - | - |
| 3-OH-4-OMe-BALD | nd | nd | trace | trace | - | - | - | - | - | - | - | - |
| Total BALD | - | - | - | - | ||||||||
| Benzoic Acids (BzA) | ||||||||||||
| Gallic acid | nd | nd | trace | trace | - | - | - | - | - | - | - | - |
| 3-OH-4-OMe-BzA | nd | nd | trace | trace | - | - | - | - | - | - | - | - |
| Syringic acid | nd | 1.90 ± 0.32 w | 17.0 ± 2.89 x | 48.2 ± 4.49 y | - | 0–12h | 0–12h | 0–12h | - | 1.03 ± 0.30 | 12.2 ± 3.0 | 35.1 ± 6.0 |
| BzA sulfate | 11.2 ± 1.72 w,x | 7.96 ± 1.20 y | 12.6 ± 1.75 x | 9.62 ± 1.40 w,y | np | np | np | np | np | np | np | np |
| BzA glucuronide | 1.45 ± 0.30 w | 1.14 ± 0.16 x | 2.25 ± 0.21 y | 1.95 ± 0.31 y | np | np | 12–24h | 12–24h | np | np | 0.85 ± 0.17 | 0.76 ± 0.17 |
| Total BzA | 12.7 | 11.0 | 31.9 | 59.8 | ||||||||
| Phenyl Acetic Acids (PAA) | ||||||||||||
| 4-OH-PAA | 3.08 ± 0.81 w | 0.95 ± 0.20 x | 1.87 ± 0.52 y | 1.22 ± 0.23 x | np | 0–24h | 0–24h | 0–24h | np | 0.30 ± 0.12 | 0.84 ± 0.38 | 0.51 ± 0.16 |
| 3-OMe-PAA | nd | nd | nd | trace | - | - | - | - | - | - | - | - |
| 3-OH-4-OMe-PAA | nd | 33.3 ± 8.61 w | 34.4 ± 6.10 w | 28.6 ± 3.82 w | - | np | np | np | - | np | np | np |
| Total PAA | 3.08 | 34.3 | 36.3 | 29.8 | ||||||||
| Phenyl Propionic Acids (PPA) | ||||||||||||
| 3-OH-PPA | nd | 57.2 ± 11.4 x | 328 ± 49.6 y | 579 ± 67.1 z | - | 0–12h | 0–12h | 12–24h | np | 25.4 ± 6.9 | 177 ± 24 | 275 ± 34 |
| 3-OH-4-OMe-PPA | nd | 2.00 ± 1.01 w | 4.77 ± 1.99 x | 6.76 ± 5.20 x | - | 0–12h | 12–24h | 12–24h | - | 0.82 ± 0.58 | 2.69 ± 1.83 | 2.48 ± 2.69 |
| Total PPA | nd | 59.2 | 333 | 586 | ||||||||
| trans-Cinnamic acids | ||||||||||||
| Caffeic acid | nd | 4.37 ± 0.99 w | 8.78 ± 3.77 x | 8.52 ± 1.73 x | np | np | 0–12h | 0–12h | np | np | 3.18 ± 1.64 | 3.97 ± 1.38 |
| Caffeic acid sulfate | 1.30 ± 0.03 w | 9.55 ± 4.01 x | 15.4 ± 6.56 x | 18.1 ± 2.69 x | np | np | 0–12h | 0–12h | np | np | 6.43 ± 4.57 | 9.74 ± 0.78 |
| Caffeic acid glucuronide | 443 ± 28.4 w | 402 ± 61.8 w | 677 ± 97.7 x | 897 ± 163 y | - | np | 0–12h | 0–12h | - | np | 261 ± 50 | 379 ± 134 |
| Ferulic acid | nd | 5.76 ± 1.26 w | 22.6 ± 4.34 x | 38.8 ± 0.81 y | np | np | 0–24h | 0–24h | np | np | 11.7 ± 3.0 | 29.5 ± 5.9 |
| Ferulic acid sulfate | 4.01 ± 1.03 w | 5.17 ± 1.70 w | 15.2 ± 3.47 x | 37.8 ± 13.7 y | np | np | 0–12h | 0–12h | np | np | 7.41 ± 3.52 | 18.7 ± 14.9 |
| Ferulic acid glucuronide | 469 ± 82.8 w | 936 ± 147 x | 3611 ± 450 y | 4772 ± 674 y | - | np | 0–12h | 0–12h | - | np | 2069 ± 354 | 2943 ± 302 |
| Chlorogenic acid | nd | 40.1 ± 10.4 w | 256 ± 13.5 x | 360 ± 61.1 y | - | np | 0–24h | 0–12h | - | np | 66.1 ± 23.4 | 238 ± 67 |
| Total Cinnamic Acids | 917 | 1403 | 4606 | 6122 | ||||||||
| Total Phenolic Acids | 935 | 1507 | 5007 | 6798 | ||||||||
| Hippuric Acids | ||||||||||||
| Hippuric acid | 304 ± 70.2 w | 706 ± 66.8 x | 1611 ± 154 y | 1528 ± 193 y | np | np | 12–24h | 12–24h | np | np | 700 ± 78 | 598 ± 146 |
| 3-OH-hippuric acid | 3.94 ± 1.01 w | 18.4 ± 3.14 x | 64.2 ± 13.4 y | 42.6 ± 9.1 z | np | np | 12–24h | 12–24h | np | np | 31.8 ± 8.1 | 26.3 ± 7.6 |
| Total Hippuric Acids | 308 | 724 | 1675 | 1571 | ||||||||
| Flavonols | ||||||||||||
| Myricetin | nd | trace | trace | trace | - | - | - | - | - | - | - | - |
| Quercetin | nd | 11.5 ± 4.47 w | 19.7 ± 7.26 x | 26.9 ± 2.24 x | - | 0–12h | 0–12h | 0–12h | - | 3.88 ± 1.46 | 10.1 ± 7.4 | 11.5 ± 4.0 |
| Quercetin glucuronide | nd | 0.49 ± 0.07 w | 1.48 ± 0.18 x | 2.70 ± 0.19 y | - | np | 0–12h | 0–12h | - | np | 0.72 ± 0.14 | 1.83 ± 0.28 |
| 4-OMe-quercetin | nd | 0.60 ± 0.25 w | 1.39 ± 0.32 x | 2.49 ± 0.61 y | - | 0–12h | 0–12h | 12–24h | - | 0.19 ± 0.08 | 0.75 ± 0.33 | 1.29 ± 1.02 |
| Me-quercetin glucuronide | nd | 0.82 ± 0.16 w | 2.93 ± 0.58 x | 7.17 ± 1.31 y | - | np | 0–12h | 0–12h | - | np | 1.61 ± 0.43 | 4.16 ± 0.58 |
| Total Flavonols | nd | 13.4 | 25.5 | 39.3 | ||||||||
| Total Phenolics Excreted | 1243 | 2298 | 6808 | 8557 | ||||||||
nd = not detected; trace = compound detected, but below LOQ; np = no peak observed.
Note: only phenolics detected via chromatography presented here. Data are comprised of biological replicates (n=6–8) and presented as mean ± SD. Superscript letters indicate significant differences between doses (p<0.05 with Tukey’s HSD test).
Qualitative Tmax represents the urine collection during which excretion of the metabolite was greatest; np indicates no peak excretion (i.e., consistent excretion at all time points), 0–12h indicates peak excretion within 12h after dosing, 12–24h indicates peak excretion 12–24h after dosing, and 0–24h indicates peak excretion was similar at 0–12h and 12–24h.
Figure 2 –
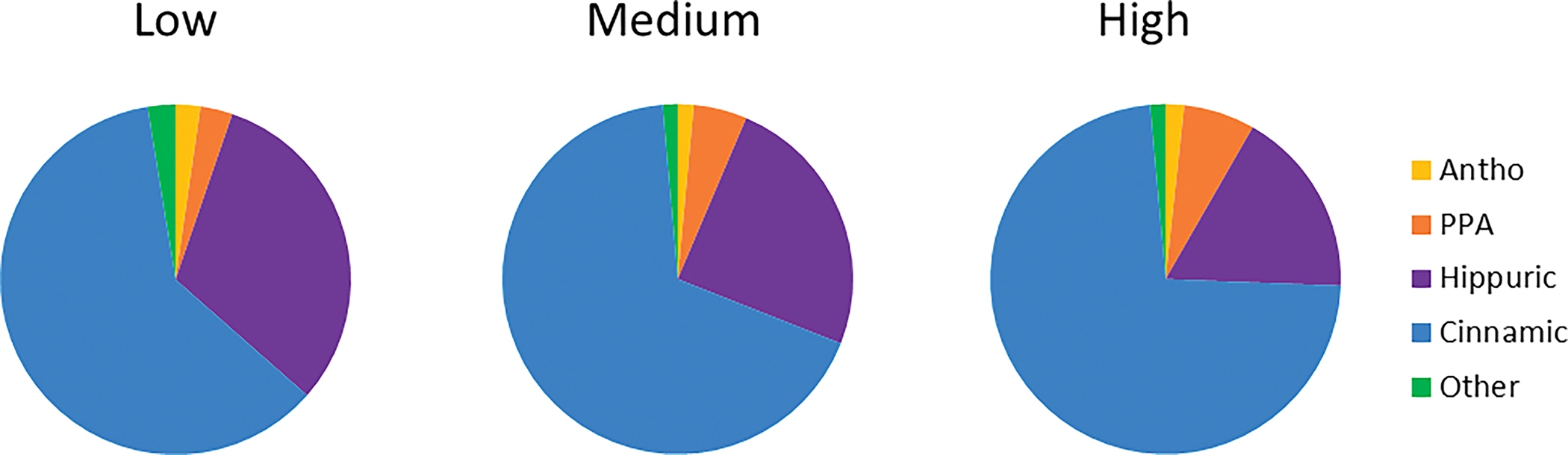
Dose dependent excretion of phenolics over 48h. For all doses, cinnamic acids were the predominant metabolite found in urine, followed by hippuric acids. As dose increased, hippuric acids decreased as a percentage of total metabolites excreted, while cinnamic acids and phenylpropionic acids increased. (Antho = sum of all anthocyanins excreted; PPA = sum of phenylpropionic acids and phase-II metabolites excreted; Hippuric = sum of hippuric acids excreted; Cinnamic = sum of cinnamic acids and phase-II metabolites excreted; Other = sum of benzoic acids, phenylacetic acids, and flavonols excreted.)
3.2.2. Urinary excretion of phenolic acids.
Of the phenolic acids quantitated in the urine, 7 were maximally excreted within 12h after dosing, with pharmacokinetic curves similar to the one shown in Figure 3a. For the other phenolic acids, 4 exhibited similar excretion in both the 0–12h and 12–24h urine collections (exemplified in Figure 3c), while the 3 remaining metabolites did not have a clear peak excretion time. (Cmax and Tmax values can be found in Table 2; Pharmacokinetic curves for all phenolic acids can be found in the Supporting Information, Figures SI-1–14.)
Figure 3 –
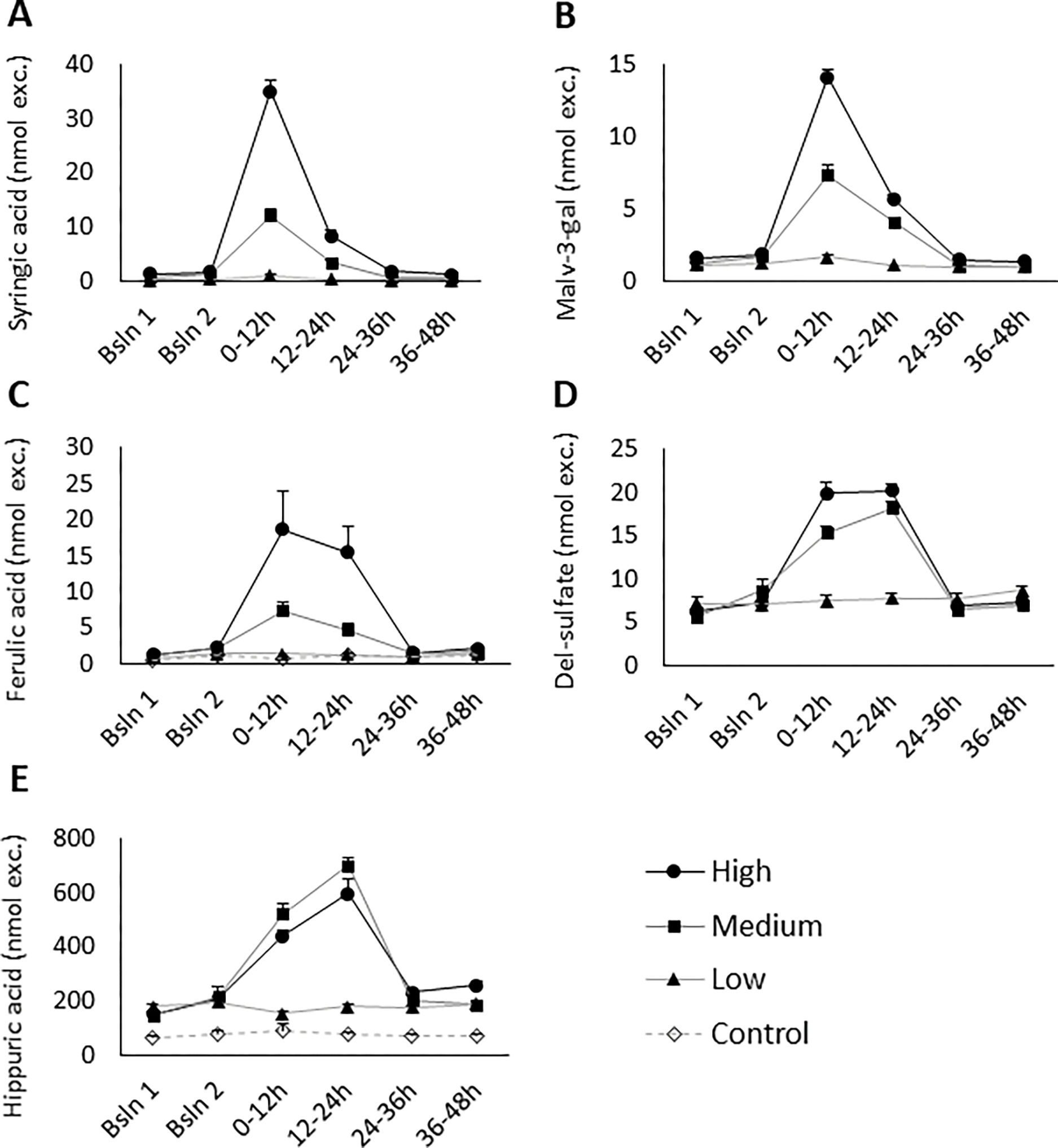
Urinary excretion pharmacokinetics of selected metabolites. Most phenolic acid, flavonol, and anthocyanin metabolites demonstrated a dose-response relationship and were maximally excreted within 12h of dosing (exemplified by syringic acid (A) and malvidin-3-O-galactoside (B)), while others had similar levels of excretion at both the 0–12h and 12–24h urine collections (e.g., ferulic acid (C) and delphinidin sulfate (D)). Hippuric acid (E), one of the last metabolites formed in colonic metabolism, exhibited maximal excretion during the 12–24h urine collection and appeared to have saturated absorption at the medium dose. Data shown as mean ± SEM. Bsln 1 = Baseline 0–12h; Bsln 2 = Baseline 12–24h.
Cinnamic acid derivatives accounted for more than half of all urinary metabolites observed. These metabolites can come from a variety of parent compounds, though their main precursors are thought to be anthocyanins [9] and chlorogenic acids.[37, 38] Additionally, cinnamic acid derivatives have been observed in other studies using polyphenol-rich berries. In a recent clinical study of cranberries, for example, a total of 24 cinnamic acid derivatives were observed, including ferulic and caffeic acid glucuronides and sulfates. The authors note that the cinnamic acids are most likely derived from both chlorogenic acid and anthocyanins present in the cranberries.[39] Similar results were observed after blueberry intake in healthy adults, as cinnamic acid derivatives (especially glucuronidated and sulfated forms) were prominent metabolites.[40–42] However, in contrast to our results, in which cinnamic acid derivatives accounted for up to 75% of total urinary metabolites, cinnamic acid derivatives in these studies accounted for <20% of total urinary metabolites. This may be due to a number of factors, though the most likely are the large amounts of hippuric acids observed in these studies (see below) or the lack of authentic standards for all cinnamic acid derivatives, which can significantly alter the quantitation of these metabolites.[8]
Other phenolic acids, including benzoic acids, phenylacetic acids (PAA), and phenylpropionic acids (PPA), are commonly reported as colonic metabolites of polyphenols, though their total contribution to phenolic metabolism varies widely. In previous studies of blueberries, phenolic acid metabolites varied considerably, with different studies reporting benzoic acids,[40, 43] PAA,[41, 42] or benzaldehydes and PAA [44] as the most prominent phenolic acid metabolites. In contrast to these studies, we found higher amounts of PPA in urine than PAA or benzoic acids.
3.2.3. Urinary excretion of flavonoids.
Flavonol metabolites observed in urine followed similar excretion patterns observed for phenolic acids, with 3 maximally excreted within 12h of dosing and 1 having similar excretion in the 0–12h and 12–24h urine collections (Figure SI-15–18).
Anthocyanins and anthocyanin metabolites (i.e., glucuronidated and sulfated anthocyanins) were also detected in the urine. Of those quantitated, 12 were maximally excreted within 12h of dosing (exemplified in Figure 3b), 3 had similar excretions during the 0–12h and 12–24h time points (exemplified in Figure 3d), and 2 did not exhibit a discernable peak excretion. (See Table 2 for Cmax and Tmax as well as Figures SI-19–35 for pharmacokinetic urinary excretion.) The relative excretion of unmetabolized 3-O-glucoside and 3-O-galactoside anthocyanins vs. the phase II glucuronidated and sulfated forms varied based on the aglycone (Figure SI-36). Cyanidins and malvidins were observed almost exclusively as unmetabolized 3-O-glucoside and 3-O-galactoside anthocyanins, while delphinidins and peonidins were extensively metabolized to the glucuronidated and sulfated forms; petunidins were a mixture of both unmetabolized and metabolized forms.
Although observed in small amounts, anthocyanin and flavonol derivatives were detected in the urine. This is noted in other studies,[40, 44] though it is rare for others to report the presence of unmetabolized anthocyanin glucosides and galactosides in the urine, especially in the dose-dependent manner observed here. However, despite the lack of reporting on these metabolites in the literature, it is known that small amounts of unmetabolized anthocyanins are present in systemic circulation, though the mechanism for their absorption is currently unknown.[45]
3.2.4. Urinary excretion of hippuric acids.
The two hippuric acid metabolites did not follow the excretion patterns exhibited by other metabolites. These metabolites were maximally excreted during the 12–24h urine collection (Figures 3e and SI-37–38). There was no difference in the amount of hippuric acid excreted after dosing with either the medium or high dose.
Hippuric acids were the second most prominent urinary metabolite observed in the current study. Hippuric acid and its derivatives are frequently noted as a major metabolite in studies of polyphenol metabolism, and they can be derived in large quantities from flavanols,[46] anthocyanins,[9, 47] and chlorogenic acids.[37, 38] In studies on polyphenol-rich berries, hippuric acids are often observed as the most prominent metabolite, accounting for up to half of total metabolites quantitated.[41, 48–51] We found hippuric acid to be a major metabolite, but as shown in Figures 3e and SI-37–38, there appears to be a saturation effect, whereby urinary excretion of hippuric acid is no different between the medium and high dose groups. As a result, hippuric acid accounts for a smaller portion of total polyphenol metabolites as the dose increases (Figure 2). This may indicate that hippuric acid production is reaching a point of saturation by the medium dose. It could also be the result of hippuric acid being one of the end stage metabolites formed in the microbial metabolism of polyphenols, meaning that, at higher doses, the total polyphenol content may be too high for the colonic microbes to fully metabolize prior to fecal excretion (see section 3.2.5 for details).
3.2.5. Metabolic sinks in colonic metabolism of blueberry polyphenols.
A summary of colonic metabolism of polyphenols is shown in Figure 4. Although not commonly discussed in relation to polyphenol metabolism, the ideas of rate limiting steps in metabolism, competition for metabolites, and “metabolic sinks” where various metabolites may accumulate are common to many metabolic pathways. Given the large quantities of cinnamic acid sulfates and glucuronides, it appears that the phase II metabolism of cinnamic acids occurs more readily than the conversion to PPA. However, once converted to PPA, the metabolites appear to be rapidly metabolized through the rest of the chain, being converted to different phenolic acid metabolites and accumulating as hippuric acids. As the dose increases, the decreasing proportion of hippuric acids (accompanied by the relative increases in PPA and cinnamic acids) suggests that this conversion is the rate limiting step. This may also slow the production at earlier steps of the process, which would cause an accumulation of other forms (in this case, PPA). Additionally, this notion of competition and diversion of metabolites at the cinnamic acid step is supported by the timing of peak production of metabolites. As shown in Table 2, cinnamic acids and the phase II metabolites are observed most prominently within the first 12h after dosing, while other phenolic acids (PPA, PAA, and benzoic acids) were generally observed somewhat later, with hippuric acids not peaking until 12–24h after dosing. This indicates that cinnamic acids were formed in large amounts and were largely diverted to phase II metabolism rather than being further metabolized into phenolic and hippuric acids. However, of those that were shuttled through the phenolic acid pathway, it appears that most went all the way to the terminal hippuric acid step until the saturation point was reached.
Figure 4 –
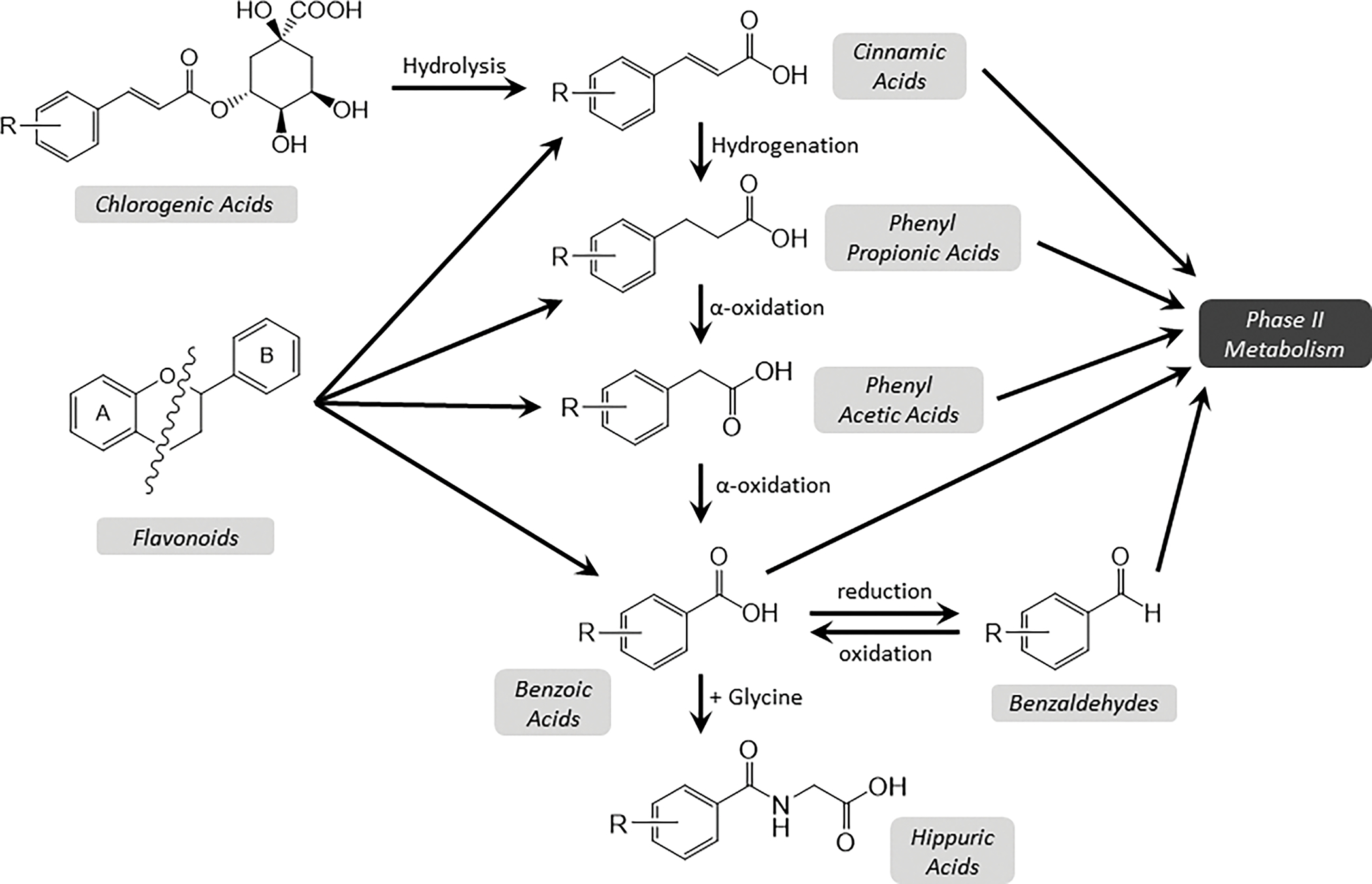
Summary of colonic catabolism of major blueberry polyphenols. Chlorogenic acids are hydrolyzed to cinnamic acids, while the heterocyclic ring of flavonoids (anthocyanins and flavonols in blueberries) is cleaved, producing two, smaller molecular weight phenolic acids from the A and B rings. These smaller phenolic acids can then be further metabolized via glucuronidation or sulfation (phase II metabolism) or to other phenolic acids. Hippuric acids are formed by conjugation with glycine, and are the terminal step in the metabolic chain. Various metabolites within each family are formed by different substitutions on the benzene ring (represented by –R).
3.3. Plasma pharmacokinetics.
A total of 16 metabolites (6 anthocyanins, 6 phenolic acids, 3 flavonols, and hippuric acid) were detected in the plasma (Table 3). Anthocyanin responses were low and inconsistent, which is not surprising given their rapid metabolism and short half-lives in vivo. Of the remaining 10 metabolites, 7 were quantitated, with most demonstrating a dose-dependent response and exhibiting peak plasma concentrations 6h after dosing (Table 3 and Figures SI-39–45). Most plasma metabolites had Tmax values consistent with urinary excretion. Metabolites that had early Tmax values in the urine were detected early in the plasma, and later appearing metabolites tended to appear later in both urine and plasma.
Table 3 –
Phenolics detected in plasma.a
| Plasma AUC (uM*h) | Qualitative Tmax b | Cmax (uM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low | Medium | High | Control | Low | Medium | High | Control | Low | Medium | High | |
|
| ||||||||||||
| Phenolic Acids | ||||||||||||
| Syringic acid | nd | nd | nd | trace | - | - | - | - | - | - | - | - |
| 3-OH-PPA | nd | nd | 2.46 ± 0.95 w | 8.00 ± 2.22 x | - | - | 12h | 12h | - | - | 0.161 ± 0.092 | 0.507 ± 0.107 |
| Caffeic gcnd | nd | 0.36 ± 0.06 w | 0.55 ± 0.11 x | 0.92 ± 0.20 y | - | np | 6h | 6h | - | np | 0.021 ± 0.008 | 0.084 ± 0.020 |
| Ferulic acid | nd | nd | nd | trace | - | - | - | - | - | - | - | - |
| Ferulic sulf | 1.88 ± 0.26 w | 6.16 ± 1.50 x | 6.33 ± 3.04 x | 9.12 ± 2.89 x | np | np | 6h | 6h | np | np | 0.262 ± 0.169 | 0.825 ± 0.317 |
| Ferulic gcnd | nd | 5.78 ± 0.75 w | 7.17 ± 1.05 x | 11.3 ± 0.72 y | - | np | 6h | 6h | - | np | 0.217 ± 0.073 | 0.797 ± 0.141 |
| Hippuric acid | 3.19 ± 0.36 w | 25.8 ± 3.08 x | 47.7 ± 5.13 y | 74.3 ± 7.54 z | np | np | 12h | 24–36h | np | np | 1.535 ± 0.510 | 2.065 ± 0.806 |
| Flavonols | ||||||||||||
| Quer gcnd | nd | 0.32 ± 0.01 w | 0.34 ± 0.05 w | 0.57 ± 0.14 x | - | np | 6h | 6h | - | np | 0.008 ± 0.002 | 0.023 ± 0.005 |
| 4-OMe-quer | nd | nd | nd | trace | - | - | - | - | - | - | - | - |
| Me-quer gcnd | nd | 3.27 ± 0.29 w | 3.56 ± 0.73 w | 4.31 ± 0.20 x | - | np | np | 6–18h | - | np | np | 0.104 ± 0.007 |
nd = not detected; trace = compound detected, but below LOQ; np = no peak observed; gcnd = glucuronide; sulf = sulfate; quer = quercetin; OH = hydroxy; PPA = phenyl propionic acid; OMe = methoxy.
Several anthocyanins (delphinidin glucuronide, delphinidin sulfate, peonidin glucuronide, petunidin-3-O-galactoside, petunidin-3-O-glucoside, and petunidin glucuronide) were detected at trace levels in medium and high doses, though responses were inconsistent and below LOQ. Data are comprised of biological replicates (n=6–8) and presented as mean ± SD. Superscript letters indicate significant differences between doses (p<0.05 with Tukey’s HSD test).
Qualitative Tmax represents the blood collection during which plasma levels of the metabolite was greatest; np indicates no peak observed; time ranges demonstrate similar amounts quantitated at multiple time points.
3.4. Calcium absorption.
To link phenolic absorption to physiological effects, fractional calcium absorption was measured using 45Ca, an isotopic tracer. When comparing all groups to control (using Dunnett’s test), fractional calcium absorption was significantly higher in the highest dose group (p = 0.034, Figure 5). This relationship was demonstrated for both the right and left femurs when analyzed separately and together. Additionally, when comparing all groups to each other (using Tukey’s test), fractional calcium absorption was nearly significantly higher for the high dose compared to control group (p = 0.058) and high dose compared to medium dose (p = 0.056).
Figure 5 –
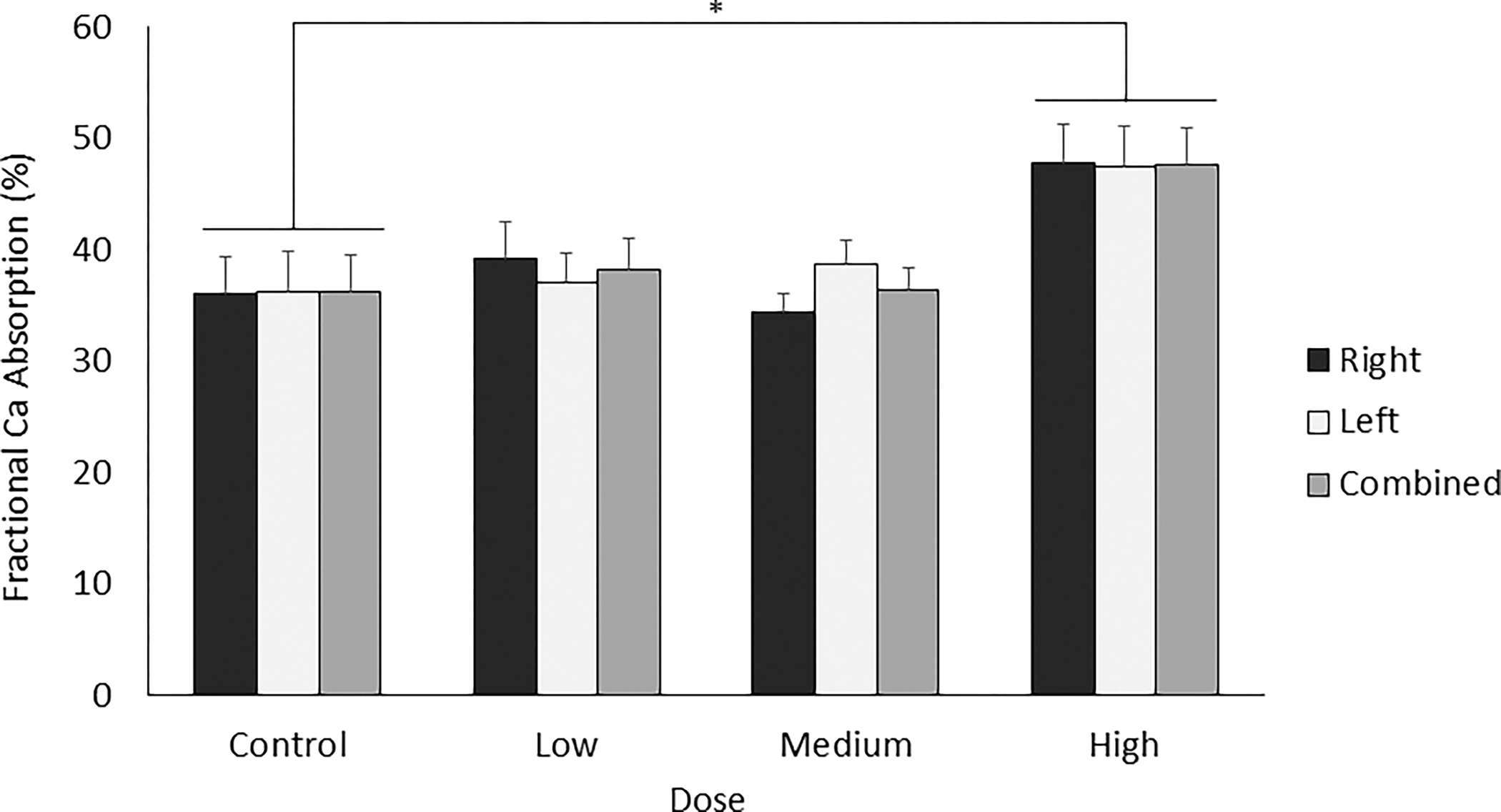
Fractional 45Ca absorption. Fractional calcium absorption was significantly higher in the high dose group for both the right and left femurs separately and when analyzed together. *p<0.05 significantly different from respective control, using Dunnett’s test. Data shown as mean ± SEM.
Maintaining bone health throughout the lifespan is key to preventing osteoporosis, and this can be accomplished through either increasing calcium absorption, increasing bone formation, or inhibiting bone resorption.[52] Our results indicate that calcium absorption is only increased with the highest dose of blueberry polyphenols; the control, low, and medium dose groups exhibit 45Ca absorption levels that are typically observed for this animal model (~35%).[35, 53, 54] Our results agree with a previous study of grape polyphenols in rats, where, at low doses of polyphenols, calcium absorption was not acutely altered by administration of a grape-enriched diet.[55] These studies indicate that polyphenols consumed at dietary doses do not acutely alter calcium absorption.
Although grape and blueberry polyphenols did not to alter calcium absorption at low doses, a recent study found that repeated administration of low doses of blueberry polyphenols increased bone calcium retention.[56] Low doses of polyphenols may improve bone quality through pathways other than increased calcium absorption. This should direct future mechanistic studies aimed at understanding the effects of polyphenols on bone health.
4. CONCLUDING REMARKS
The strength and innovation of this study is the unique perspective it provides on the dose-dependent shift in the production of colonic metabolites after an acute dose of purified blueberry polyphenols as may be present in dietary supplements. Given the growing popularity and use of botanical dietary supplements, our approach of using a concentrated phenolic extract at elevated doses is directly relevant to dietary supplement consumption. By focusing on the pharmacokinetics and colonic metabolism of elevated doses, this study provides a novel glimpse into metabolic shifts that may occur when consuming botanical dietary supplements. Additionally, by not only measuring dose-dependent phenolic metabolism but also measuring the influence on calcium absorption, we have demonstrated that shifts in phenolic metabolism can have physiological effects.
However, there were also several limitations to the current study. First, the lack of authentic standards for some of the metabolites may have caused inaccurate estimates of the actual amounts present, as certain metabolites (e.g., sulfates) ionize more and may easily be overestimated.[8] This is a challenge that many studies of phenolic metabolism face, as commercially available standards are rarely found for specific metabolites and are challenging to synthesize. Second, the time points chosen to sample and measure plasma metabolites likely missed peak plasma concentrations for many metabolites, as many phenolics are absorbed and excreted quickly (Tmax <2h).[12] Our original hypothesis was that many of the colonic metabolites would be observed at later times in the plasma, leading us to select later times for blood collection. Our hypothesis appears to hold true for the urinary appearance of metabolites, but we were unable to detect many of these metabolites in appreciable amounts in the plasma. Finally, using metabolic cages to collect urine samples from animals presents two important challenges in measuring phenolic metabolites: oxidation and contamination. Because urine was collected continuously in 12h increments, samples were exposed to oxygen for several hours and small food particles and debris were observed. We judged this to be the best method for collecting urine from the animals and, because none of the major phenolic metabolites observed are present in the chow diet, this had a minimal impact on the results and their interpretation.
In conclusion, we have shown that increasing doses of blueberry polyphenols result in dose-dependent shifts in phenolic metabolite profiles and increased calcium absorption. Taken together, these results indicate that high doses of blueberry polyphenols may alter gut function. Future studies examining the consequences of these metabolic changes with repeated dosing will help determine if these changes have systemic effects. Repeated dosing studies with high doses of purified polyphenols mimics typical dietary supplement consumption and will help elucidate the safety and potential health consequences – both positive and negative – of this consumption modality.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by the National Institutes of Health (R01AT008754) and internal funds. The authors are thankful for the assistance of Dr. Berdine Martin, Dr. Colby Vorland, Andrea Lobene, and Gretchen Weise in collecting and processing samples collected during this study.
List of Abbreviations:
- 5% BB
5% blueberry chow diet
- CE
concentrated blueberry polyphenol extract
- FD
lyophilized blueberry powder
- MRM
multiple reaction monitoring
- OVX
ovariectomized
- PAA
phenylacetic acid
- PPA
phenylpropionic acid
- PPF
polyphenol-free chow diet
- SPE
solid phase extraction
Footnotes
CONFLICT OF INTEREST
The authors have no competing financial interests to declare.
5. REFERENCES
- [1].Spencer JPE, Brit. J. Nutr. 2010, 104, S40. [DOI] [PubMed] [Google Scholar]
- [2].Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Muller MJ, Oberritter H, Schulze M, Stehle P, Watzl B, Eur. J. Nutr. 2012, 51, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oyebode O, Gordon-Dseagu V, Walker A, Mindell JS, J. Epidemiol. Commun. Health 2014, 68, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A, Antioxid. & Redox Signal. 2013, 18, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yousef GG, Brown AF, Funakoshi Y, Mbeunkui F, Grace MH, Ballington JR, Loraine A, Lila MA, J. Ag. Food Chem. 2013, 61, 4806. [DOI] [PubMed] [Google Scholar]
- [6].Ma LY, Sun ZH, Zeng YW, Luo MC, Yang JZ, Int. J. Molec. Sci. 2018, 19 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marin L, Miguelez EM, Villar CJ, Lombo F, Biomed. Res. Int. 2015, 905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A, Arch. Toxicol. 2014, 88, 1803. [DOI] [PubMed] [Google Scholar]
- [9].Kay CD, Pereira-Caro G, Ludwig IA, Clifford MN, Crozier A, Ann. Rev. Food Sci. Technol. 2017, 8, 155. [DOI] [PubMed] [Google Scholar]
- [10].Williamson G, Clifford MN, Brit. J. Nutr. 2010, 104, S48. [DOI] [PubMed] [Google Scholar]
- [11].Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA, J. Nutr 2001, 131, 1731. [DOI] [PubMed] [Google Scholar]
- [12].Maiz M, Cladis DP, Lachcik PJ, Janle E, Lila MA, Ferruzzi MG, Weaver CM, FASEB J. 2016, 30, 690S. [Google Scholar]
- [13].Williamson G, Kay CD, Crozier A, Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054. [DOI] [PubMed] [Google Scholar]
- [14].Borges G, Lean MEJ, Roberts SA, Crozier A, Food Funct. 2013, 4, 754. [DOI] [PubMed] [Google Scholar]
- [15].Faria A, Fernandes I, Norberto S, Mateus N, Calhau C, J. Ag. Food Chem. 2014, 62, 6898. [DOI] [PubMed] [Google Scholar]
- [16].Williamson G, Clifford MN, Biochem. Pharmacol. 2017, 139, 24. [DOI] [PubMed] [Google Scholar]
- [17].Martin KR, Appel CL, Nutr. Diet. Suppl. 2010, 2, 1. [Google Scholar]
- [18].Chandra P, Rathore AS, Kay KL, Everhart JL, Curtis P, Burton-Freeman B, Cassidy A, Kay CD, Molecules 2019, 24, 4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bove GM, J. Pharmacol. Toxicol. Method. 2015, 74, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF, J. Nutr 2011, 141, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Frances Picciano M, McDowell M, Sempos C, C. NCHS data brief, no 61. 2011, Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- [22].Council for Responsible Nutrition. CRN 2017 Annual Survey on Dietary Supplements. https://www.crnusa.org/resources/crn-2017-annual-survey-dietary-supplements (accessed 25 October 2017).
- [23].Dickinson A, Blatman J, El-Dash N, Franco JC, J. Am. Coll. Nutr. 2014, 33, 176. [DOI] [PubMed] [Google Scholar]
- [24].Thompson DD, Simmons HA, Pirie CM, Ke HZ, Bone 1995, 17, S125–S133. [DOI] [PubMed] [Google Scholar]
- [25].Tucker KL, Hannan MT, Kiel DP, Eur. J. Nutr. 2001, 40, 231. [DOI] [PubMed] [Google Scholar]
- [26].Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, Goltzman D, Morin S, Poliquin S, Kreiger N, CaMos Res G, Am. J. Clin. Nutr. 2011, 93, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maiz M, Henry C, Lachcik PJ, Ferruzzi MG, McCabe G, Lila MA, Weaver CM, FASEB J. 2017, 31 (1), 793.20.27871063 [Google Scholar]
- [28].US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD, 2005; 30p. [Google Scholar]
- [29].Thrivikraman KV, Huot RL, Plotsky PM, Brain Res. Protoc. 2002, 10, 84. [DOI] [PubMed] [Google Scholar]
- [30].Furrer A, Cladis DP, Kurilich A, Manoharan R, Ferruzzi MG, Food Chem. 2017, 218, 47. [DOI] [PubMed] [Google Scholar]
- [31].Moser S, Aragon I, Furrer A, Van Klinken JW, Kaczmarczyk M, Lee BH, George J, Hamaker BR, Mattes R, Ferruzzi MG, Nutr. Res. 2018, 52: 57. [DOI] [PubMed] [Google Scholar]
- [32].George S, Brat P, Alter P, Amiot MJ, J. Ag. Food Chem. 2005, 53, 1370–1373. [DOI] [PubMed] [Google Scholar]
- [33].de Ferrars RM, Cassidy A, Curtis P, Kay CD, Molec. Nutr. Food Res. 2014, 58, 490. [DOI] [PubMed] [Google Scholar]
- [34].Koo J, Weaver CM, Neylan MJ, Miller GD, J. Nutr. Biochem. 1993, 4, 72. [Google Scholar]
- [35].Weaver CM, Martin BR, Costa NMB, Saleeb FZ, Huth PJ, J. Ag. Food Chem. 2002, 50, 4974. [DOI] [PubMed] [Google Scholar]
- [36].Ariefdjohan MW, Martin BR, Lachcik PJ, Weaver CM, J. Ag. Food Chem. 2008, 56, 2649. [DOI] [PubMed] [Google Scholar]
- [37].Mosele JI, Macia A, Motilva MJ, Molecules 2015, 20, 17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stalmach A, Mullen W, Barron D, Uchida K, Yokota T, Cavin C, Steiling H, Williamson G, Crozier A, Drug Metab. Dispos. 2009, 37, 1749. [DOI] [PubMed] [Google Scholar]
- [39].Feliciano RP, Boeres A, Massacessi L, Istas G, Ventura MR, dos Santos CN, Heiss C, Rodriguez-Mateos A, Arch. Biochem. Biophys. 2016, 599, 31. [DOI] [PubMed] [Google Scholar]
- [40].Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A, Molecules 2016, 21, 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rodriguez-Mateos A, Feliciano RP, Cifuentes-Gomez T, Spencer JPE, J. Berry Res 2016, 6, 137. [Google Scholar]
- [42].Istas G, Wood E, Le Sayec M, Rawlings C, Yoon J, Dandavate V, Cera D, Rampelli S, Costabile A, Fromentin E, Rodriguez-Mateos A, Am. J. Clin. Nutr. 2019, 110, 316–329. [DOI] [PubMed] [Google Scholar]
- [43].Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, Spencer JPE, Am. J. Clin. Nutr. 2013, 98, 1179. [DOI] [PubMed] [Google Scholar]
- [44].Zhong SQ, Sandhu A, Edirisinghe I, Burton-Freeman B, Molec. Nutr. Food Res. 2017, 61, 1700405. [DOI] [PubMed] [Google Scholar]
- [45].Kamiloglu S, Capanoglu E, Grootaert C, Van Camp J, Int. J. Molec. Sci. 2015, 16, 21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mullen W, Rouanet JM, Auger C, Teissedre PL, Caldwell ST, Hartley RC, Lean MEJ, Edwards CA, Crozier A, J. Ag. Food Chem. 2008, 56, 12127. [DOI] [PubMed] [Google Scholar]
- [47].Czank C, Cassidy A, Zhang QZ, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD, Am. J. Clin. Nutr. 2013, 97, 995. [DOI] [PubMed] [Google Scholar]
- [48].Gonzalez-Barrio R, Edwards CA, Crozier A, Drug Metab. Dispos. 2011, 39, 1680. [DOI] [PubMed] [Google Scholar]
- [49].Ludwig IA, Mena P, Calani L, Borges G, Pereira-Caro G, Bresciani L, Del Rio D, Lean MEJ, Crozier A, Free Radical Bio. Med. 2015, 89, 758. [DOI] [PubMed] [Google Scholar]
- [50].Xie LY, Lee SG, Vance TM, Wang Y, Kim B, Lee JY, Chun OK, Bolling BW, Food Chem. 2016, 211, 860. [DOI] [PubMed] [Google Scholar]
- [51].Gomes A, Oudot C, Macia A, Foito A, Carregosa D, Stewart D, Van de Wiele T, Berry D, Motilva MJ, Brenner C, dos Santos CN, Nutritents, 2019, 11, 2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hodges JK, Cao SS, Cladis DP, Weaver CM, Nutrients, 2019, 11, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhao YD, Martin BR, Wastney ME, Schollum L, Weaver CM, Exp. Biol. Med. 2005, 230, 536. [DOI] [PubMed] [Google Scholar]
- [54].Marsh CL, Leblanc AD, Johnson PC, Pool SL, Am. J. Physiol. 1983, 245, G438. [DOI] [PubMed] [Google Scholar]
- [55].Hohman EE, Weaver CM, J. Nutr. 2015, 145, 253. [DOI] [PubMed] [Google Scholar]
- [56].Maiz M, PhD Thesis, Purdue University, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


