Abstract
Background
Granulocyte–macrophage colony-stimulating factor (GM-CSF) and dysregulated myeloid cell responses are implicated in the pathophysiology and severity of COVID-19.
Methods
In this randomised, sequential, multicentre, placebo-controlled, double-blind study, adults aged 18–79 years (Part 1) or ≥70 years (Part 2) with severe COVID-19, respiratory failure and systemic inflammation (elevated C-reactive protein/ferritin) received a single intravenous infusion of otilimab 90 mg (human anti-GM-CSF monoclonal antibody) plus standard care (NCT04376684). The primary outcome was the proportion of patients alive and free of respiratory failure at Day 28.
Results
In Part 1 (n=806 randomised 1:1 otilimab:placebo), 71% of otilimab-treated patients were alive and free of respiratory failure at Day 28 versus 67% who received placebo; the model-adjusted difference of 5.3% was not statistically significant (95% CI −0.8–11.4%, p=0.09). A nominally significant model-adjusted difference of 19.1% (95% CI 5.2–33.1%, p=0.009) was observed in the predefined 70–79 years subgroup, but this was not confirmed in Part 2 (n=350 randomised) where the model-adjusted difference was 0.9% (95% CI −9.3–11.2%, p=0.86). Compared with placebo, otilimab resulted in lower serum concentrations of key inflammatory markers, including the putative pharmacodynamic biomarker CC chemokine ligand 17, indicative of GM-CSF pathway blockade. Adverse events were comparable between groups and consistent with severe COVID-19.
Conclusions
There was no significant difference in the proportion of patients alive and free of respiratory failure at Day 28. However, despite the lack of clinical benefit, a reduction in inflammatory markers was observed with otilimab, in addition to an acceptable safety profile.
Short abstract
Therapeutic blocking of GM-CSF with otilimab did not significantly improve clinical status in patients with severe COVID-19; however, otilimab demonstrated an acceptable safety profile and reduced markers of inflammation https://bit.ly/3QquyYP
Introduction
Severe COVID-19 is characterised by respiratory and/or multi-organ failure [1]. A subset of patients displays systemic hyperinflammation including dysregulated myeloid cell responses [2–4]. Older age and associated immunosenescence and underlying comorbidities may predispose patients to similar immune abnormalities to those observed in COVID-19 [5, 6], increasing their risk of severe disease and mortality [7–9].
Granulocyte–macrophage colony-stimulating factor (GM-CSF) is implicated in driving hyperinflammation in severe COVID-19 [10–14], with increased circulating concentrations reportedly associated with COVID-19 severity and mortality [12, 15]. This may be due to the putative role of GM-CSF in myeloid cell activation, differentiation, survival and priming to enhance inflammatory cytokine and chemokine production, leading to further myeloid cell recruitment to sites of inflammation. This potentially produces a positive feedback loop driving cytokine and chemokine production, hyperinflammation and tissue damage [10, 11].
Otilimab is a high-affinity, fully human, anti-GM-CSF monoclonal antibody (IgG1λ) that reduces inflammatory activity in rheumatoid arthritis (RA) [16]. GM-CSF inhibition with otilimab was hypothesised to reduce the production of proinflammatory cytokines and chemokines, decrease myeloid cell migration and modulate hyperinflammation, leading to an improved outcome in severe COVID-19 [10]. The otilimab in severe COVID-19-related disease (OSCAR) trial was designed to investigate the efficacy and safety of otilimab in patients with acute respiratory failure and systemic inflammation due to severe COVID-19.
Methods
Study design
OSCAR was a randomised, multicentre, placebo-controlled, double-blind study (GSK study 214094; NCT04376684) conducted at 121 sites across 19 countries (supplementary material). This sequential study was conducted in two parts: Part 1 enrolled patients aged 18–≤79 years between 28 May 2020 and 15 November 2020, with the last patient completing Day 60 on 13 January 2021. Part 1 results indicated a potential benefit of otilimab in a predefined subgroup of patients aged 70–79 years. Therefore, the original protocol was amended to include Part 2, which enrolled only patients aged ≥70 years between 15 February 2021 and 19 June 2021, with the last patient completing Day 60 on 16 August 2021.
Patients were randomised 1:1 in a blinded manner, using interactive response technology (block size of four) to receive otilimab or matched placebo. Patients were monitored daily until Day 28 (or until hospital discharge), with follow-up assessments at Days 42 and 60.
The study was conducted in accordance with the Declaration of Helsinki, Council for International Organisations of Medical Sciences International Ethical Guidelines, International Conference on Harmonisation, Good Clinical Practice and applicable country-specific regulatory requirements. The protocol was approved by relevant institutional review boards. Before enrolment, informed consent was obtained from the patient or their legally authorised representative. An independent data monitoring committee monitored in-stream unblinded safety and efficacy data throughout the study.
Patients
Eligible patients were aged 18–79 years in Part 1 and ≥70 years in Part 2, had a positive SARS-CoV-2 result from any validated test (predominantly reverse transcription PCR) and were hospitalised due to radiographically confirmed pneumonia consistent with COVID-19. All patients had a clinical status of Category 5 or 6 in the modified World Health Organization Ordinal Scale for Clinical Improvement (supplementary methods) [17], defined by recent onset of oxygenation impairment requiring either high-flow oxygen (≥15 L·min−1; Category 5), noninvasive ventilation (Category 5) or invasive mechanical ventilation without additional organ support (Category 6) ≤48 h prior to dosing. Serum concentrations of the inflammatory markers C-reactive protein (CRP) or ferritin were required to be above the upper limit of normal.
Patients were excluded if death was predicted within 48 h; if they had multiple organ failure according to the investigator's opinion and/or a Sequential Organ Failure Assessment [18] score >10; or if they were receiving extracorporeal membrane oxygenation, haemofiltration/dialysis or more than one inotrope or vasopressor of any class. Patients who had received intravenous immunoglobulin, monoclonal antibody or immunosuppressant therapy within the past 3 months or who were currently receiving chronic oral corticosteroids (>10 mg·day−1 prednisone or equivalent) for a non-COVID-19 indication were also excluded. Full eligibility criteria are provided in the supplementary material.
Study treatments
Patients received either a single 1-h intravenous infusion of otilimab 90 mg or placebo on Day 1 and standard of care according to current clinical guidelines and institutional protocols. This otilimab dosing regimen was predicted to result in serum concentrations remaining within the target range for ∼1 week, which was deemed to be sufficient to inhibit the expected levels of GM-CSF in circulation/tissue and induce an anti-inflammatory effect, while allowing a return to normal GM-CSF levels in the recovery phase, during which GM-CSF expression may promote lung repair [10].
End-points and assessments
The primary end-point was the proportion of patients alive and free of respiratory failure (clinical status: Categories 1–4) at Day 28. Key secondary end-points included all-cause mortality at Day 28 (post hoc for Part 1) and Day 60; time to all-cause mortality up to Day 60; participants alive and free of respiratory failure at Days 7, 14, 42 and 60; time to recovery from respiratory failure at Day 28; time to last dependence on supplementary oxygen up to Day 28; time to final intensive care unit discharge up to Day 28; time to first discharge from investigator site up to Day 60 (revised before unblinding in Part 1); time to first hospital discharge to non-hospitalised residence up to Day 60 (revised before unblinding in Part 1); and adverse events (AEs) and serious AEs (SAEs) up to Day 60. Exploratory end-points are provided in the supplementary material.
Biomarker and pharmacokinetic assessments
Blood samples for otilimab and GM-CSF–otilimab complex concentrations were collected on Days 1, 2, 7 and 14. Further details of pharmacokinetic (PK) and exposure-response analyses are provided in the supplementary material.
Free GM-CSF was assessed using an ultrasensitive immunoassay based on single molecule array (Simoa™) technology. Target engagement was estimated from the target-mediated drug disposition model [19] developed using baseline concentrations of free GM-CSF and concentrations of free GM-CSF, otilimab and GM-CSF–otilimab complex over time.
Blood samples were collected at screening and on Days 2 (Part 1 only), 4 and 7 for measurement of serum concentrations of inflammatory markers using electrochemiluminescence-based immunoassays and neutrophil-to-lymphocyte ratios (NLRs) derived from clinical haematology panels.
Statistical analysis
Parts 1 and 2 were analysed separately. Full details are provided in the statistical analysis plan (SAP) (supplementary material). Part 1 used a group sequential design to control for multiplicity, with interim analyses for futility and efficacy. In Part 1 and Part 2, a sample size of 800 and 346 patients provided ∼90% and 80% power to detect a difference of 12% and 15%, respectively, in the proportion of patients alive and free of respiratory failure at a one-sided 2.5% significance level and an assumed placebo response rate of 45%.
The primary end-point was assessed using logistic regression, adjusting for treatment, sex (Part 2 only), age and clinical status at baseline. Missing data in the overall primary analysis were imputed using multiple imputation, assuming data were missing at random and adjusting for analysis covariates. The primary end-point was also analysed in predefined stratification factors based on clinical status, age (post hoc in Part 2), clinical status by age (Part 1 only) and sex (Part 2 only), as described in the SAP (supplementary material).
Given that OSCAR was a single-dose trial, and dosing was anticipated to occur very quickly following randomisation, it was assumed that any patients who were randomised but did not receive treatment were those who withdrew consent or were randomised in error. Because these patients would have no post-baseline data, the population for primary analyses included all patients who were randomised and received study drug (modified intent-to-treat (mITT)). The SAP was finalised before the clinical database was locked. For ease of interpretation, two-sided p-values with 5% significance level are presented.
Results
Baseline population findings
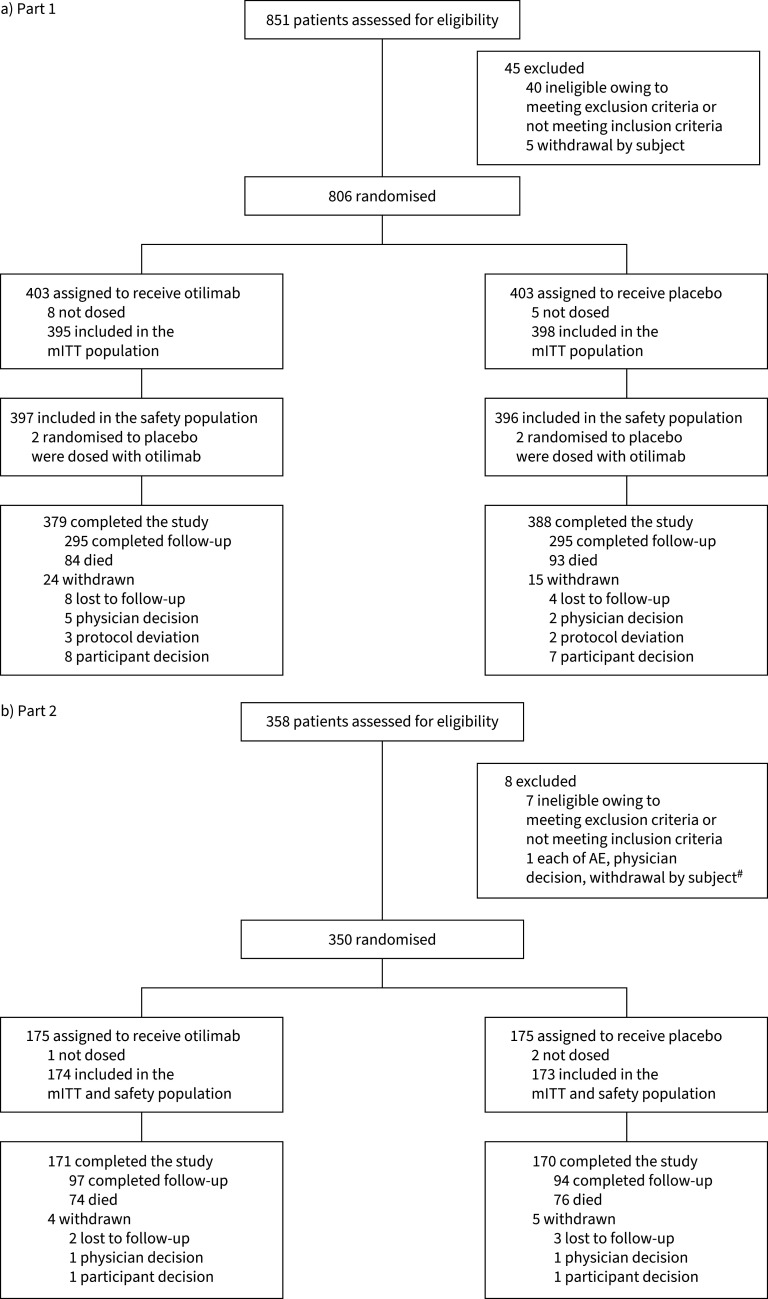
In Part 1, 793 patients were included in the mITT population (otilimab n=395; placebo n=398), with patients aged 70–79 years accounting for 23% of the overall population; in Part 2, 347 patients were included in the mITT population (otilimab n=174; placebo n=173) (figure 1). In both parts, baseline demographics and disease characteristics were generally well balanced between groups and were reflective of severe COVID-19 (table 1). Compared with Part 1, a larger proportion of patients in Part 2 were in Category 5.
FIGURE 1.
CONSORT flow diagram in OSCAR study Part 1 (a) and Part 2 (b). mITT: modified intent-to-treat; AE: adverse event. #: patients may have more than one reason for failure.
TABLE 1.
Baseline characteristics
| Part 1 | Part 2 | |||||
| Overall population | Age 70–79 years# | Overall population | ||||
| Characteristic | Otilimab (N=403) | Placebo (N=403) | Otilimab (n=88) | Placebo (n=92) | Otilimab (N=175) | Placebo (N=175) |
| Male sex | 302 (75) | 275 (68) | 65 (74) | 57 (62) | 102 (58) | 100 (57) |
| Age | 59.8±11.7 | 59.4±11.9 | 74.0±2.8 | 74.0±2.8 | 75.3±4.7 | 75.0±4.7 |
| Age group | ||||||
| Part 1: | ||||||
| <60 years | 178 (44) | 185 (46) | 0 | 0 | – | – |
| 60–69 years | 135 (33) | 127 (32) | 0 | 0 | – | – |
| 70–79 years | 90 (22) | 91 (23) | 88 (100) | 92 (100) | – | – |
| Part 2: | ||||||
| <70 years¶ | – | – | – | – | 9 (5) | 5 (3) |
| 70–79 years | – | – | – | – | 126 (72) | 136 (78) |
| ≥80 years | – | – | – | – | 40 (23) | 34 (19) |
| Weight (kg) | 88.0±20.9 | 88.2±20.9 | 84.6±20.2 | 80.0±14.2 | 83.9±16.2 | 81.9±16.5 |
| Race or ethnic group | ||||||
| American Indian or Alaska Native | 30 (8) | 24 (6) | 3 (3) | 4 (4) | 8 (5) | 3 (2) |
| Asian | 57 (14) | 73 (19) | 12 (14) | 18 (20) | 5 (3) | 15 (9) |
| Black or African American | 26 (7) | 25 (6) | 5 (6) | 3 (3) | 6 (3) | 6 (3) |
| White | 272 (69) | 262 (67) | 67 (77) | 64 (71) | 155 (89) | 150 (86) |
| Hispanic or Latino | 125 (31) | 116 (29) | 13 (15) | 18 (20) | 58 (33) | 37 (21) |
| Clinical status | ||||||
| Category 5: Hospitalised, high-flow oxygen, noninvasive ventilation | 311 (77) | 311 (77) | 63 (72) | 68 (74) | 150 (86) | 148 (85) |
| Category 6: Hospitalised, mechanical ventilation | 89 (22) | 89 (22) | 24 (27) | 23 (25) | 25 (14) | 27 (15) |
| ICU status | ||||||
| Not in ICU and not on mechanical ventilation | 97 (24) | 98 (24) | 13 (15) | 17 (18) | 79 (45) | 83 (47) |
| In ICU and not on mechanical ventilation | 209 (52) | 211 (52) | 49 (56) | 52 (57) | 69 (39) | 62 (35) |
| In ICU and on mechanical ventilation | 97 (24) | 94 (23) | 26 (30) | 23 (25) | 27 (15) | 30 (17) |
| Biomarkers + | ||||||
| CRP (mg·L−1) | 111.8±86.0 | 116.3±84.5 | 109.7±79 | 128.8±82.2 | 96.1±79.4 | 93.5±77.7 |
| Ferritin (μg·L−1) | 1247.7±1242.9 | 1147.4±1041.6 | 1493.1±1916 | 1248.4±1201.3 | 1482.3±1697.3 | 1177.4±1060.7 |
| GM-CSF (ng·L−1) | 0.71±0.84 | 0.72±0.76 | 0.82±1.19 | 0.73±0.71 | 0.82±1.44 | 0.80±0.95 |
| Residence prior to hospital admission | ||||||
| Independent or community dwelling | 392 (98) | 391 (97) | NA | NA | 173 (99) | 169 (97) |
| Long-term care facility | 7 (2) | 10 (2) | NA | NA | 2 (1) | 6 (3) |
| Current comorbidity § | ||||||
| Hypertension | 192 (48) | 209 (52) | 59 (67) | 61 (66) | 113 (65) | 129 (74) |
| Diabetes | 147 (36) | 149 (37) | 31 (35) | 39 (42) | 57 (33) | 63 (36) |
| Hyperlipidaemia | 97 (24) | 96 (24) | 35 (40) | 41 (45) | 45 (26) | 53 (30) |
| Heart disorder | 51 (13) | 45 (11) | 21 (24) | 21 (23) | 35 (20) | 47 (27) |
| Pretreatment medications § ,ƒ | ||||||
| Corticosteroids (including dexamethasone) | 332 (84) | 330 (83) | 72 (82) | 74 (80) | 150 (86) | 148 (86) |
| Dexamethasone | 281 (71) | 267 (67) | 64 (73) | 66 (72) | 137 (79) | 125 (72) |
| Remdesivir | 127 (32) | 142 (36) | 28 (32) | 32 (35) | 12 (7) | 22 (13) |
| Convalescent plasma therapy | 20 (5) | 24 (6) | 5 (6) | 4 (4) | NA | NA |
| Immunosuppressants | 0 | 0 | 0 | 0 | 1 (<1) | 0 |
| Anti-IL-6 therapies | 0 | 0 | 0 | 0 | 1 (<1)## | 0 |
| Antiviral | 136 (34) | 155 (39) | 29 (33) | 38 (41) | 29 (17) | 44 (25) |
| COVID-19 vaccine | NA | NA | NA | NA | 2 (4) | 1 (2) |
| Geographic region § | ||||||
| USA | 98 (24) | 90 (22) | 20 (23) | 23 (25) | 1 (<1) | 6 (3) |
| Europe¶¶ | 142 (35) | 160 (40) | 41 (47) | 38 (41) | 69 (39) | 78 (45) |
| Latin America++ | 68 (17) | 53 (13) | 8 (9) | 8 (9) | 53 (30) | 31 (18) |
| Rest of world§§ | 95 (24) | 100 (25) | 19 (22) | 23 (25) | 44 (25) | 49 (28) |
Data are presented as n (%) or mean±sd. ICU: intensive care unit; CRP: C-reactive protein; GM-CSF: granulocyte–macrophage colony-stimulating factor; IL: interleukin; NA: not available. #: baseline characteristics in the Part 1 age 70–79 years subgroup are presented in the modified intent-to-treat population; ¶: patient age was derived from the date of screening visit, year of birth (provided at screening) and an assumed birth date of June 30, and so some patients were recorded as <70 years; +: biomarkers summarised by actual treatment received; §: data in the Part 1 age 70–79 years group are from Day 4; ƒ: a dose or infusion of medication used prior to Day 1 (day of dosing of study drug), irrespective of whether medication continued after dosing; ##: one patient who had received anti-IL-6 therapy was included in error; ¶¶: Belgium, France, Italy, Netherlands, Poland, Spain, UK; ++: Argentina, Brazil, Chile, Colombia, Mexico, Peru; §§: Canada, India, Japan, Russian Federation, South Africa.
Primary end-point: patients alive and free of respiratory failure
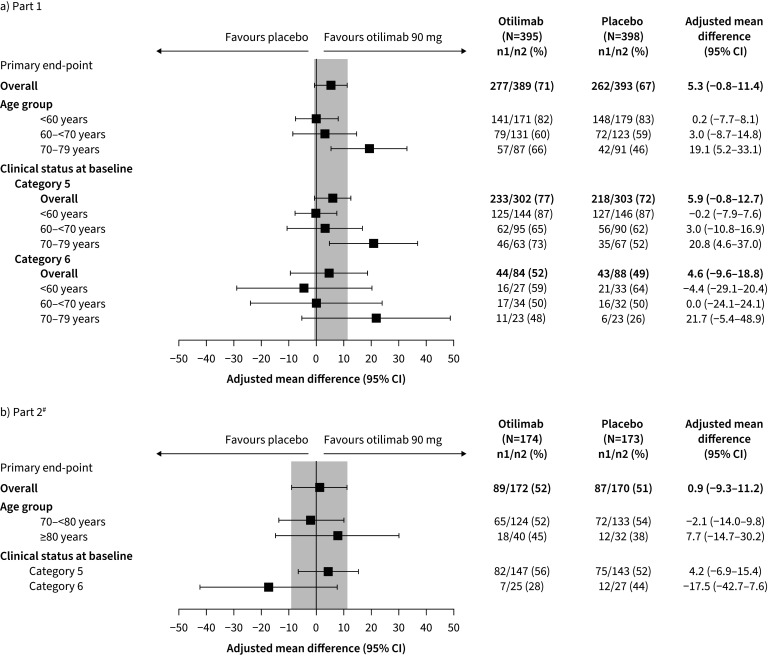
In Part 1, 71% of patients in the otilimab group were alive and free of respiratory failure at Day 28 versus 67% who received placebo; the model-adjusted difference of 5.3% was not statistically significant (95% CI −0.8–11.4%, p=0.09) (figure 2a). Model-adjusted differences for patients in Categories 5 and 6 were 5.9% (95% CI −0.8–12.7%) and 4.6% (95% CI −9.6–18.8%), respectively (figure 2a). In the predefined subgroup of patients aged 70–79 years, the model-adjusted difference was 19.1% (95% CI 5.2–33.1%, nominal p=0.009); this response was consistent regardless of clinical status (figure 2a).
FIGURE 2.
Proportion of patients alive and free of respiratory failure at Day 28 in a) Part 1 and b) Part 2 (primary end-point). #: analysis of the primary end-point in patients by clinical status at baseline stratified by age group was not conducted in Part 2 owing to the low number of patients aged ≥80 years.
In Part 2, 52% of patients who received otilimab were alive and free of respiratory failure at Day 28 versus 51% who received placebo (model-adjusted difference: 0.9%, 95% CI −9.3–11.2%, p=0.86) (figure 2b). For patients in Categories 5 and 6, the model-adjusted difference was 4.2% (95% CI −6.9–15.4%) and −17.5% (95% CI −42.7–7.6%), respectively (figure 2b). Model-adjusted differences were −2.1% (95% CI −14.0–9.8%, p=0.73) in patients aged 70–<80 years and 7.7% (95% CI −14.7–30.2%, p=0.51) in patients aged ≥80 years (figure 2b). Post hoc analyses of the primary end-point by baseline characteristic are presented in supplementary figure S1.
Secondary end-point: all-cause mortality
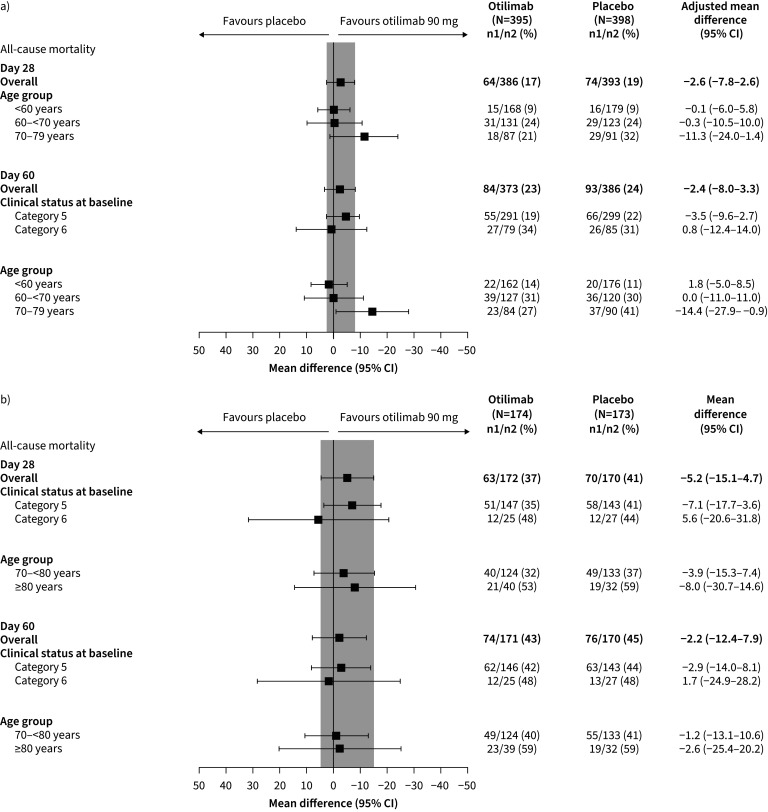
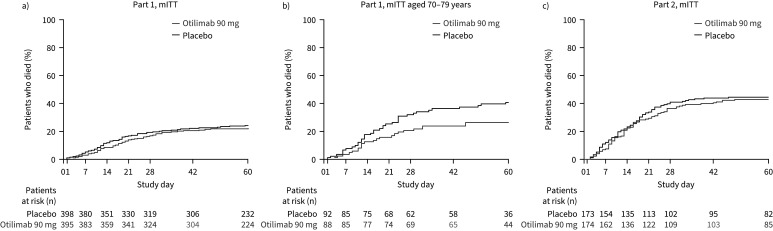
In Part 1, all-cause mortality at Day 60 was 23% in the otilimab group compared with 24% in the placebo group (model-adjusted difference −2.4%, 95% CI −8.0–3.3%, p=0.41) (figure 3a). In the 70–79 years subgroup, there was lower mortality at Day 60 with otilimab (27%) versus placebo (41%) (model-adjusted difference −14.4%, 95% CI −27.9– −0.9%, nominal p=0.04).
FIGURE 3.
All-cause mortality in a) Part 1 at Day 28 (post hoc#) and Day 60 (pre-specified), and in b) Part 2 at Day 28 and Day 60 (pre-specified). #: Day 28 analysis in Part 1 was conducted post hoc, thus data are not available by clinical status at baseline.
In Part 2, all-cause mortality at Day 28 was 37% in the otilimab group compared with 41% in the placebo group (model-adjusted difference −5.2%, 95% CI −15.1–4.7%, p=0.31) (figure 3b). Mortality at Day 60 was 43% in the otilimab group and 45% in the placebo group, with a model-adjusted difference of −2.2% (95% CI −12.4–7.9%, p=0.67). No significant differences in mortality at Days 28 or 60 were observed in the predefined subgroups of either part.
Additional secondary and exploratory efficacy end-points
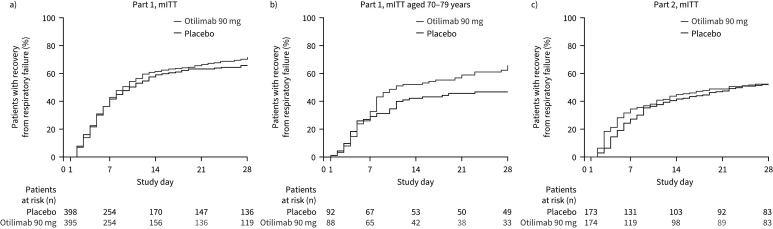
Generally, there were no significant differences in time-to-event analyses in the Part 1 mITT population between treatment groups (figures 4a and 5a, supplementary figure S2a–g). However, improvements with otilimab versus placebo were observed in the 70–79 years subgroup (figures 4b and 5b, supplementary figure S2a–d), with treatment effects apparent 7–10 days post-infusion.
FIGURE 4.
Kaplan–Meier time to recovery from respiratory failure up to Day 28 in the a) modified intent-to-treat (mITT) population and b) post hoc 70–79 years group of Part 1, and c) in the mITT population of Part 2 (secondary end-point).
There was a short-term numerical benefit of otilimab versus placebo in most time-to-event analyses in Part 2, including time to recovery from respiratory failure, as well as an early delay in time to invasive mechanical ventilation; separation between groups was observed from around Day 3 and they converged around Day 10 (figure 4c, supplementary figure S2a, b, e, g). There was no difference between otilimab and placebo in time to all-cause mortality up to Day 60 (figure 5c).
FIGURE 5.
Kaplan–Meier time to all-cause mortality up to Day 60 in the a) modified intent-to-treat (mITT) population and b) post hoc 70–79 years group of Part 1, and c) in the mITT population of Part 2 (secondary end-point).
In the exploratory end-point of change from baseline in inspiratory oxygen fraction (FiO2), a greater reduction was observed in patients receiving otilimab versus placebo in the Part 1 mITT population, Part 1 70–79 years subgroup and Part 2 mITT population up to Day 14 (supplementary figure S2h).
Safety end-points
In both parts, no safety signals related to otilimab were identified. Overall safety findings, including the scope of AEs and SAEs, were reflective of the severe COVID-19 population, and no clinically meaningful differences in AEs, including the rates of secondary infections, were observed (table 2).
TABLE 2.
Adverse events
| Part 1 | Part 2 | |||||
| AE | Safety population | Age 70–79 years | Safety population | |||
| Otilimab (n=397) | Placebo (n=396) | Otilimab (n=89) | Placebo (n=91) | Otilimab (n=174) | Placebo (n=173) | |
| Any AE | ||||||
| Patients with ≥1 event | 274 (69) | 265 (67) | 73 (82) | 68 (75) | 140 (80) | 133 (77) |
| Any SAE | ||||||
| Patients with ≥1 event | 124 (31) | 147 (37) | 33 (37) | 49 (54) | 90 (52) | 90 (52) |
| Most common AEs ≥5% in any group | ||||||
| Constipation | 39 (10) | 35 (9) | 16 (18) | 14 (15) | 16 (9) | 15 (9) |
| Pneumonia | 43 (11) | 29 (7) | 13 (15) | 11 (12) | 12 (7) | 17 (10) |
| Acute kidney injury | 23 (6) | 25 (6) | 8 (9) | 11 (12) | 14 (8) | 12 (7) |
| Anaemia | 18 (5) | 22 (6) | 5 (6) | 8 (9) | 11 (6) | 10 (6) |
| Respiratory failure | 19 (5) | 21 (5) | 6 (7) | 9 (10) | 7 (4) | 8 (5) |
| Hypotension | 14 (4) | 16 (4) | 1 (1) | 6 (7) | 10 (6) | 13 (8) |
| Atrial fibrillation | 12 (3) | 18 (5) | 5 (6) | 9 (10) | 9 (5) | 12 (7) |
| Septic shock | 18 (5) | 16 (4) | 4 (4) | 2 (2) | 10 (6) | 6 (3) |
| Pulmonary embolism | 13 (3) | 25 (6) | 2 (2) | 9 (10) | 3 (2) | 7 (4) |
| Hypoxaemia | 10 (3) | 13 (3) | 1 (1) | 8 (9) | 10 (6) | 12 (7) |
| MODS | 12 (3) | 16 (4) | 3 (3) | 5 (5) | 6 (3) | 11 (6) |
| Hypokalaemia | 15 (4) | 16 (4) | 7 (8) | 6 (7) | 8 (5) | 4 (2) |
| Diarrhoea | 15 (4) | 18 (5) | 4 (4) | 6 (7) | 4 (2) | 5 (3) |
| UTI | 13 (3) | 14 (4) | 3 (3) | 5 (5) | 5 (3) | 10 (6) |
| Pneumothorax | 17 (4) | 15 (4) | 3 (3) | 6 (7) | 6 (3) | 3 (2) |
| Pyrexia | 20 (5) | 15 (4) | 3 (3) | 6 (7) | 1 (<1) | 4 (2) |
| Hyperglycaemia | 12 (3) | 14 (4) | 4 (4) | 3 (3) | 10 (6) | 4 (2) |
| Delirium | 17 (4) | 17 (4) | 4 (4) | 5 (5) | 3 (2) | 2 (1) |
| Hyperkalaemia | 17 (4) | 13 (3) | 5 (6) | 7 (8) | 4 (2) | 4 (2) |
| Hypertension | 17 (4) | 10 (3) | 6 (7) | 3 (3) | 6 (3) | 5 (3) |
| Acute respiratory failure | 10 (3) | 11 (3) | 5 (6) | 3 (3) | 6 (3) | 9 (5) |
| Hepatocellular injury | 6 (2) | 5 (1) | 5 (6) | 1 (1) | 14 (9) | 10 (6) |
| Hypernatraemia | 20 (5) | 10 (3) | 2 (2) | 6 (7) | 3 (2) | 1 (<1) |
| Insomnia | 12 (3) | 5 (1) | 3 (3) | 2 (2) | 8 (5) | 7 (4) |
| Sepsis | 7 (2) | 12 (3) | 1 (1) | 6 (7) | 6 (3) | 3 (2) |
| Decubitus ulcer | 16 (4) | 9 (2) | 8 (9) | 3 (3) | 0 | 2 (1) |
| Fluid overload | 1 (<1) | 2 (<1) | 0 | 1 (1) | 9 (5) | 5 (3) |
| Most common SAEs ≥5% any group | ||||||
| Respiratory failure | 17 (4) | 18 (5) | 6 (7) | 8 (9) | 6 (3) | 8 (5) |
| MODS | 12 (3) | 15 (4) | 3 (3) | 5 (5) | 6 (3) | 8 (5) |
| Septic shock | 14 (4) | 13 (3) | 4 (4) | 2 (2) | 8 (5) | 5 (3) |
| Acute respiratory failure | 9 (2) | 10 (3) | 5 (6) | 3 (3) | 6 (3) | 9 (5) |
| Pneumonia | 7 (2) | 9 (2) | 1 (1) | 5 (5) | 6 (3) | 5 (3) |
| COVID-19# | 3 (<1) | 5 (1) | 1 (1) | 1 (1) | 6 (3) | 9 (5) |
| Pulmonary embolism | 6 (2) | 11 (3) | 2 (2) | 5 (5) | 1 (<1) | 3 (2) |
| Patients with AEs of special interest | ||||||
| Serious infections | 50 (13) | 58 (15) | 12 (13) | 17 (19) | 37 (21) | 29 (17) |
| Cytokine release syndrome | 0 | 2 (<1) | 0 | 1 (1) | 3 (2) | 1 (<1) |
| Serious hypersensitivity reactions | 1 (<1) | 1 (<1) | 1 (1) | 0 | 0 | 0 |
| Infusion site reactions | 1 (<1) | 1 (<1) | 1 (1) | 0 | 0 | 0 |
| Neutropaenia | 1 (<1) | 0 | 0 | 0 | 0 | 0 |
Data are presented as n (%). AE: adverse event; SAE: serious adverse event; MODS: multiple organ dysfunction syndrome; UTI: urinary tract infection. #: COVID-19, as per protocol, was only to be reported as an AE if the signs and symptoms of COVID-19 were more severe than expected.
Biomarkers
Similar free GM-CSF concentrations were observed in both parts at baseline and Day 1 (table 1 and supplementary table S1). In Part 1, free GM-CSF levels in the otilimab arm at Day 2, proximal to maximum concentration (Cmax), were reduced by at least 95% to a mean of 0.037 ng·L−1, with 255 of 381 samples (67%) falling below the assay lower limit of quantification (0.036 ng·L−1); levels in the placebo arm remained unchanged. Day 2 data were not collected in Part 2, and post-Day 2 data are not available.
Otilimab also induced rapid reductions in other key inflammatory markers compared with placebo in the 7 days after infusion (supplementary figure S3). Data from the 70–79 years subgroup of Part 1 were similar to the total Part 1 population. In both parts, greater reductions in interleukin (IL)-6 and IL-10 were observed with otilimab versus placebo at Day 2 and/or 4, converging by Day 7. CRP concentrations decreased from baseline in both groups, although Part 2 showed greater reductions with otilimab by Day 7. CC chemokine ligand 17 (CCL17) concentrations increased in the placebo group, but not in the otilimab group in both parts, and a greater reduction from baseline in NLR was observed with otilimab at Days 4 and 7 in Part 2; however, the effect with placebo varied between study parts, as did the patterns observed for macrophage chemotactic protein-1 (MCP-1) and IL-8.
PK
Similar serum concentrations of otilimab (supplementary figure S4 and table S1) and GM-CSF–otilimab complex concentrations (supplementary figure S5 and table S1) were observed in both parts. The target engagement model predicted 91%, 74% and 23% target engagement at Days 2, 4 and 7, respectively.
Across all patients in both parts, the PK model-derived mean otilimab exposure parameters Cmax and area under the concentration-time curve (AUC), following a single dose of 90 mg, were 18.9 μg·mL−1 and 50.7 μg·day·mL−1, respectively. The population clearance rate of otilimab was 1.67 L·day−1, and effective half-life was 3.65 days.
Clinical response (patients alive and free of respiratory failure on Day 28, all-cause mortality at Day 60 and improvements in clinical status over time) when stratified by placebo and quartile of otilimab exposure (AUC or Cmax) suggested that a higher otilimab exposure was associated with better response (supplementary figure S6); however, patients in the lowest quartile group had a worse response than those in the placebo group. Day 7 and 14 data for the proportion of patients alive and free of respiratory failure were similar to Day 28 data. There was no clear relationship between exposure and serious infection or change in CRP, IL-6, CCL17 or MCP-1.
Discussion
In this large study of hospitalised adults with COVID-19 aged 18–79 years (Part 1) and ≥70 years (Part 2), administration of otilimab was not associated with a significant difference in the proportion of patients alive and free of respiratory failure at Day 28.
In Part 1, otilimab was associated with a nonsignificant increase in the proportion of patients alive and free of respiratory failure at Day 28. However, significantly more patients in a predefined subgroup aged 70–79 years receiving otilimab met this end-point compared with those receiving placebo. There was also a corresponding decrease in all-cause mortality at Day 60. Immunosenescence and “inflammaging”, associated with normal ageing of the immune system, may predispose older patients with COVID-19 to inappropriate, myeloid cell-driven hyperinflammation [5, 6]. Further evidence emerged at the time of the Part 1 analysis that supported the potential role of GM-CSF and myeloid cells in COVID-19 pathogenesis [10–13].
Based on Part 1 findings and the high mortality rate observed in older patients with severe COVID-19 [9], Part 2 specifically evaluated the potential clinical benefit in patients aged ≥70 years. This extension of the study did not, however, confirm the significant difference between otilimab and placebo for the primary end-point observed in Part 1. Despite a credible hypothesis, it is likely that observations in a single subgroup in Part 1 were due to chance. Other confounding factors may have also contributed to the differences in results, including slight variations in patient demographics, risk profiles and clinical status between parts, in addition to variability in mortality rates across geographies [20], improvements in standard of care and patient management, and the changing prevalence and virulence of viral variants [21] at the different stages of the pandemic. Additional study limitations include the use of an estimated birth date (with only the year of birth recorded) to determine patient age and low patient numbers in certain subgroups, which made it difficult to perform some sub-analyses.
Low systemic target engagement levels after Day 4 may have impacted efficacy. However, patients with the lowest otilimab exposure generally had a worse clinical response than placebo-treated patients. This suggests a potential bidirectional interaction between PK and response, whereby patients with more severe disease have increased otilimab clearance, causing an apparent exposure-response relationship. Thus, exposure-response data cannot indicate whether a higher dose of otilimab would provide any additional benefit. Furthermore, while a potential early benefit in respiratory status was observed within the first ∼10 days of dosing in Part 2, the apparent benefit in the ≥70 years subgroup in Part 1 was only observed after Day 10, despite a decrease in otilimab concentration over Days 1–7, suggesting a delay in treatment effect. Therefore, multiple doses may not have been more effective. However, given that the findings of an overall benefit in most of the time-to-event analyses through to Day 28 in the ≥70 years subgroup of Part 1 were not replicated in Part 2 (except for decreased FiO2 requirement), despite a similar population, the observed differences between parts during the early stages of the studies are unlikely to be real.
In both parts of OSCAR, otilimab treatment resulted in lower concentrations of the putative pharmacodynamic biomarker for otilimab activity, CCL17 [22], in the 7 days post-infusion with no convergence with placebo, indicating successful target engagement and inhibition of pathways downstream of GM-CSF. Inflammatory markers IL-6 and IL-10 are generally increased in hospitalised patients with COVID-19 and are associated with disease severity [23]. In the RECOVERY study, inhibition of IL-6 reduced mortality and improved clinical outcomes [24]. The reduction in these cytokines observed with otilimab may be associated with the delay in clinical deterioration observed in the first week in Part 2. However, the otilimab group converged with placebo by Day 7, coinciding with the decrease in target engagement from 95% at the end of infusion to 23% by Day 7. This could be due to the shorter than previously observed effective half-life of otilimab in patients with COVID-19.
Elevated NLR is a predictor for critical disease [25], and neutrophils have been proposed to have an important role in COVID-19 pneumonia [2, 4, 26]. Otilimab was associated with decreased NLR from baseline up to Day 7 in Part 2, which suggests an early reduction in circulating neutrophil numbers and/or repopulation of lymphocytes and potential dampening of the hyperinflammatory response following GM-CSF inhibition [26, 27]. Because all observed biomarker changes were systemic, it is unclear whether these changes were reflected in the lungs, where multiple mechanisms may lead to lung injury.
The lack of a clinically meaningful benefit of otilimab in this severe COVID-19 population may be due to the highly complex and only partially characterised multiplicity of cytokines, chemokines and cellular components involved in COVID-19 pathophysiology. With new evidence continually emerging, combination therapies, targeting multiple pathways [28, 29], have been adopted into treatment regimens and guidelines [1]. Furthermore, the timing of intervention may be key. OSCAR included patients with already profound respiratory failure and systemic hyperinflammation. However, a window of opportunity may exist in the early stage of hyperinflammation, before progression to significant respiratory failure [11]. This is suggested by the results of the LIVE-AIR study in which anti-GM-CSF lenzilumab was less effective in patients with higher CRP concentrations [30]. Both parts of OSCAR demonstrated the ability of otilimab to decrease FiO2 more rapidly in all age groups to Day 12–14. This apparent improvement in gaseous exchange in the lungs was not, however, associated with improved clinical outcomes.
Recent in vitro studies suggest that binding of the SARS-CoV-2 spike protein to circulating mononuclear cells directly induces GM-CSF secretion, providing further evidence of a role for GM-CSF in the immune response to the virus [31]. However, clinical anti-GM-CSF therapy has generated mixed results in various COVID-19 trials. The anti-GM-CSF receptor α subunit mavrilimumab demonstrated efficacy in a phase 2 trial [32]; however, the phase 3 trial did not meet the primary end-point, leading to its discontinuation in COVID-19 [33]. Anti-GM-CSF namilumab demonstrated a reduction in CRP in the CATALYST trial and trends towards clinical improvement, but the study was not powered for these outcomes [34]. Finally, while LIVE-AIR demonstrated that early intervention with lenzilumab decreases CRP and improves the likelihood of survival without ventilation [30, 35], this was not supported by the ACTIV-5/BET-B trial of lenzilumab plus remdesivir, which failed to meet the same primary end-point of survival without ventilation [36]. Furthermore, lenzilumab did not significantly improve mortality rates in the overall population of either trial [30, 36]. This inconclusive evidence for the benefit of anti-GM-CSF monotherapy in COVID-19 may be linked to the varying disease severity of the patient populations and the different end-points used in the different studies. Nevertheless, inflammatory biomarker findings in OSCAR continue to support the ongoing evaluation of otilimab in other immune-inflammatory conditions. Indeed, following two phase 2 studies in RA [16, 22], a large global phase 3 RA programme is ongoing [37–39].
The AE rate for OSCAR was as expected for a population with severe COVID-19 pneumonia, with the most common SAE being respiratory failure. No clinically meaningful difference was observed between all AEs, including, importantly, the rates of COVID secondary infections, and no safety signals related to otilimab treatment were identified.
Treatment with a single dose of otilimab did not improve the proportion of patients alive and free of respiratory failure at Day 28. Target engagement and a reduction in inflammatory markers were observed, in addition to an acceptable safety profile in a severely ill patient population.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01870-2021.SUPPLEMENT (1.1MB, pdf)
Shareable PDF
Acknowledgements
We thank all the patients for their participation in this important research. We thank Gift Nyamundanda and Johannes Freudenberg for their work on the biomarker analyses; the global clinical operations delivery managers, Kai Hove and Jack Siderfin; and the global study data managers, Sam Birleson, Laurence Harvey and Oyiza Momoh. Medical writing support, in the form of editorial checks, figure development and assistance with submission, was provided by Leigh O'Connor-Jones and Clare Cunningham of Fishawack Indicia Ltd UK, part of Fishawack Health (funded by GSK).
Footnotes
This clinical trial has been prospectively registered as NCT04376684. GSK makes available anonymised individual participant data and associated documents from interventional clinical studies which evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access original data for studies that have been re-analysed, other types of GSK sponsored research, study documents without patient-level data and clinical studies not listed, please submit an enquiry via the website.
This article has an editorial commentary: https://doi.org/10.1183/13993003.02091-2022
Author contributions: GSK authors contributed to the conception, design and analysis of data. Study investigators contributed to the acquisition of data. All authors contributed to data interpretation or drafting or critically revising the article. All authors provided final approval and agreement of accountability.
Conflict of interest: J. Patel, D. Bass, A. Cahn, K. Davy, S. Fernandes, A. Gupta, K. Hanrott, D. Inman, E. Jarvis, D. Lakshminarayanan, S. Mukherjee, C. O'Shea, L. Schifano, J.E. Smith, R. Williamson and M. Layton are shareholders and/or employees of GSK. A. Beishuizen, X. Bocca Ruiz, H. Boughanmi, H. Colombo, G.J. Criner, J. de-Miguel-Díez, P.A. Doreski, B. François, T. Hatlen, J.D. Isaacs, N. Kostina, T. Kropotina, J-C. Lacherade, P. Martinez-Ayala, C. McEvoy, F. Meziani, M. Monchi, R. Muñoz-Bermúdez, G. Plantefeve, L.E. Schwab, Z. Shahid, M. Shirano, E. Sprinz, C. Summers, N. Terzi and Y. Trefilova were investigators in the OSCAR trial, which was funded by GSK. X. Bocca Ruiz has served as a clinical trial investigator for AstraZeneca and Zambon. B. François reports consultancy fees with GSK, Enlivex, Inotrem, Takeda, Aridis, Transgene, AM-Pharma, Asahi-Kasai and Biomérieux within the past 36 months. R. Muñoz-Bermúdez has participated on an advisory board for GSK. J.D. Isaacs has received research funding from GSK, Janssen and Pfizer, and personal fees from AbbVie, BMS, Gilead, Roche and UCB, all outside the submitted work, as well as support for event attendance from Eli Lilly and Gilead. A. Beishuizen has received consultancy fees from GSK. C. McEvoy has received research funding from the National Institutes of Health, US Department of Defense, Patient-Centered Outcomes Research Institute, GSK and AstraZeneca. C. Summers’ institution has received research funding from GSK, AstraZeneca, the Wellcome Trust, The Medical Research Council and National Institute for Health Research to support her work outside the area of the submitted manuscript. C. Summers has received personal fees from AbbVie, Roche and GSK. G.J. Criner has received research grants from ALung Technologies Inc., American College of Radiology, American Lung Associations, AstraZeneca, BioScale Inc., Boehringer Ingelheim, BREATH Therapeutics Inc., COPD Foundation, Coridea/ZIDAN, Corvus, Dr Karen Burns of St Michael's Hospital, Fisher & Paykel Healthcare Ltd, Galapagos NV, GSK, Kinevent, Lungpacer Medical Inc., National Heart Lung and Blood Institute, Nurvaira Inc., Patient-Centered Outcomes Research Institute, Pulmonary Fibrosis Foundation, PulmonX, Respironics Inc., Respivant Sciences, Spiration Inc., Steward St Elizabeth's Medical Center of Boston Inc. and Veracyte Inc.; and received personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Broncus Medical, CSA Medical, EOLO Medical, Gala Therapeutics, GSK, Helios Medical, Ion, Merck, Medtronic, Mereo BioPharma, NGM Biopharmaceuticals, Novartis, Olympus, PulmonX, Respironics Inc., Respivant Sciences, The Implementation Group and Verona Pharma. J. Neisen is an employee and shareholder of AstraZeneca, and a shareholder and former employee of GSK. L.E. Schwab reports holding shares in Johnson & Johnson and BMS, and participated on the Holy Cross Health Institutional Review Board. Z. Shahid has received research funding from Karyopharm. M. Shirano is an investigator in separate trials funded by Roche and AstraZeneca, and has received payment for lectures from Gilead Sciences. E. Sprinz participates on a data safety monitoring board/advisory board for, and has received consulting fees and honoraria from, GSK, Janssen and Gilead. M.A. Tidswell received a fee for serving on the independent data monitoring committee for this study, as well as for serving on a data safety monitoring board/advisory board for Spectral Diagnostics Inc., ReAlta Life Sciences Inc., Celltrion Inc., AstraZeneca and Molecular Partners AG. Additionally, M.A. Tidswell has held a research contract with Edesa Biotech Research Inc., RevImmune SAS, Spectral Diagnostics, Beyond Air Inc., National Institutes of Health and National Heart, Lung, and Blood Institute. D. Wyncoll received a fee for serving on the independent data monitoring committee for this study, has served as a study adjudicator for AstraZeneca, and reports consulting fees and/or honoraria from Gilead and Shionogi. J. de-Miguel-Díez, H. Boughanmi, J-C. Lacherade, P. Martinez-Ayala, T. Hatlen, G. Plantefeve, N. Terzi, M. Shirano, N. Kostina, T. Kropotina, Y. Trefilova and M. Monchi have no other conflicts of interest to declare.
Support statement: This study (GSK ID: 214094; NCT04376684) was funded by GSK. In 2013, GSK assumed exclusive worldwide responsibility of otilimab (previously MOR103) from MorphoSys AG for all development and commercialisation activities in all therapeutic fields. The study was supported in the UK by the National Institute for Health Research (NIHR) Comprehensive Research Network under the Urgent Public Health clinical trial scheme and the NIHR Newcastle Biomedical Research Centre in Ageing and Long-term Conditions. The views expressed are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Rochwerg B, Agarwal A, Siemieniuk RA, et al. . A living WHO guideline on drugs for COVID-19. BMJ 2020; 370: m3379. [DOI] [PubMed] [Google Scholar]
- 2.Schulte-Schrepping J, Reusch N, Paclik D, et al. . Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020; 182: 1419–1440. doi: 10.1016/j.cell.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao M, Liu Y, Yuan J, et al. . Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26: 842–844. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 4.Schurink B, Roos E, Radonic T, et al. . Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 2020; 1: e290–e299. doi: 10.1016/S2666-5247(20)30144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazeldine J, Lord JM. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front Immunol 2020; 11: 573662. doi: 10.3389/fimmu.2020.573662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrobon AJ, Teixeira FME, Sato MN. Immunosenescence and inflammaging: risk factors of severe COVID-19 in older people. Front Immunol 2020; 11: 579220. doi: 10.3389/fimmu.2020.579220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salje H, Tran Kiem C, Lefrancq N, et al. . Estimating the burden of SARS-CoV-2 in France. Science 2020; 369: 208–211. doi: 10.1126/science.abc3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. . The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc 2020; 21: 915–918. doi: 10.1016/j.jamda.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . Risk for COVID-19 Infection, Hospitalization, and Death By Age Group. www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html Date last accessed: 26 November 2021.
- 10.Lang FM, Lee KMC, Teijaro JR, et al. . GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol 2020; 20: 507–514. doi: 10.1038/s41577-020-0357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P, Porter JC, Manson JJ, et al. . Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med 2020; 8: 822–830. doi: 10.1016/S2213-2600(20)30267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. . Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol 2021; 6: eabg9873. doi: 10.1126/sciimmunol.abg9873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Fu B, Zheng X, et al. . Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Nat Sci Rev 2020; 7: nwaa041. doi: 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Kilian C, Turner JE, et al. . Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol 2021; 6: eabf6692. doi: 10.1126/sciimmunol.abf6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hue S, Beldi-Ferchiou A, Bendib I, et al. . Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 202: 1509–1519. doi: 10.1164/rccm.202005-1885OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley CD, Simón-Campos JA, Zhdan V, et al. . Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: a randomised, phase 2b, dose-ranging study. Lancet Rheumatol 2020; 2: e677–e688. doi: 10.1016/S2665-9913(20)30229-0 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . R&D Blueprint: Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. Date last accessed: 9 August 2022.
- 18.Vincent JL, Moreno R, Takala J, et al. . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 19.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 2001; 28: 507–532. doi: 10.1023/A:1014414520282 [DOI] [PubMed] [Google Scholar]
- 20.Asch DA, Sheils NE, Islam MN, et al. . Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med 2021; 181: 471–478. doi: 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies NG, Jarvis CI, van Zandvoort K, et al. . Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021; 593: 270–274. doi: 10.1038/s41586-021-03426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese MC, Berkowitz M, Conaghan PG, et al. . MRI of the joint and evaluation of the granulocyte–macrophage colony-stimulating factor–CCL17 axis in patients with rheumatoid arthritis receiving otilimab: a phase 2a randomised mechanistic study. Lancet Rheumatol 2020; 2: e666–e676. doi: 10.1016/S2665-9913(20)30224-1 [DOI] [PubMed] [Google Scholar]
- 23.Williamson EJ, Walker AJ, Bhaskaran K, et al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recovery Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Liu Y, Xiang P, et al. . Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 2020; 18: 206. doi: 10.1186/s12967-020-02374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazeldine J, Lord JM. Neutrophils and COVID-19: active participants and rational therapeutic targets. Front Immunol 2021; 12: 680134. doi: 10.3389/fimmu.2021.680134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wicks IP, Roberts AW. Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol 2016; 12: 37–48. doi: 10.1038/nrrheum.2015.161 [DOI] [PubMed] [Google Scholar]
- 28.Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell 2021; 184: 1671–1692. doi: 10.1016/j.cell.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temesgen Z, Burger CD, Baker J, et al. . Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med 2021; 10: 237–246. doi: 10.1016/S2213-2600(21)00494-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiang X, Zhu S, Li J, et al. . Monoclonal antibodies capable of binding SARS-CoV-2 spike protein receptor-binding motif specifically prevent GM-CSF induction. J Leukoc Biol 2022; 111: 261–267. doi: 10.1002/JLB.3COVCRA0920-628RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pupim L, Wang TS, Hudock K, et al. . LB0001 mavrilimumab improves outcomes in phase 2 trial in non-mechanically-ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation. Ann Rheum Dis 2021; 80: 198–199. doi: 10.1136/annrheumdis-2021-eular.5012 [DOI] [Google Scholar]
- 33.Kiniksa Pharmaceuticals . Kiniksa Announces Results from Phase 3 Trial of Mavrilimumab in COVID-19-related ARDS. 2021. https://investors.kiniksa.com/news-releases/news-release-details/kiniksaannounces-results-phase-3-trial-mavrilimumab-covid-19 Date last accessed: August 2022.
- 34.Fisher BA, Veenith T, Slade D, et al. . Namilumab or infliximab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multi-arm, multistage, open-label, adaptive, phase 2, proof-of-concept trial. Lancet Respir Med 2021; 10: 255–266. doi: 10.1016/S2213-2600(21)00460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temesgen Z, Burger C, Baker J, et al. . C-reactive protein as a biomarker for improved efficacy of lenzilumab in patients with COVID-19: results from the LIVE-AIR trial. Chest 2021; 160: A2522–A2524. doi: 10.1016/j.chest.2021.08.029 [DOI] [Google Scholar]
- 36.Humanigen Inc. Humanigen Receives Preliminary Topline Data From NIH/NIAID Study of Lenzilumab in ACTIV-5/BET-B. 2022. https://ir.humanigen.com/English/news/news-details/2022/Humanigen-Receives-Preliminary-Topline-Data-From-NIHNIAID-Study-of-Lenzilumab-in-ACTIV-5BET-B/default.aspx Date last accessed: August 2022.
- 37.Clinicaltrials.gov . Efficacy and Safety of GSK3196165 (Otilimab) Versus Placebo and Sarilumab in Participants with Moderately to Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Biological Disease-modifying Antirheumatic Drug (DMARDs) and/or Janus Kinase (JAK) Inhibitors (contRAst 3). https://clinicaltrials.gov/ct2/show/NCT04134728 Date last accessed: 16 February 2022.
- 38.Clinicaltrials.gov . Efficacy and Safety of GSK3196165 Versus Placebo and Tofacitinib in Participants with Moderately to Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Methotrexate (contRAst 1) (NCT03980483). www.clinicaltrials.gov/ct2/show/NCT03980483 Date last accessed: 16 February 2022.
- 39.Clinicaltrials.gov . Efficacy and Safety of GSK3196165 Versus Placebo and Tofacitinib in Participants with Moderately to Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Conventional Synthetic (cs)/Biologic (b) Disease Modifying Anti-rheumatic Drugs (DMARDs) (contRAst 2) (NCT03970837). https://clinicaltrials.gov/ct2/show/NCT03970837 Date last accessed: 16 February 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01870-2021.SUPPLEMENT (1.1MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01870-2021.Shareable (496.4KB, pdf)