Abstract
Background
OnabotulinumtoxinA 20 U reduces glabellar line (GL) severity at maximum frown for approximately 3 to 4 months. Small studies have suggested that >20-U doses may increase the efficacy and duration of response for GLs.
Objectives
The aim of this study was to evaluate safety, pharmacodynamic response, and treatment satisfaction with onabotulinumtoxinA doses ≥20 U for GLs.
Methods
This 48-week, double-blind study compared 40, 60, and 80 U onabotulinumtoxinA vs 20 U and placebo in women with moderate or severe dynamic GLs on the Allergan Facial Wrinkle Scale. The following parameters were evaluated: the percentage of subjects with investigator-assessed ≥1-grade Facial Wrinkle Scale improvement from baseline at maximum frown (responders) at Week 24; the estimated median duration of response; the proportion of mostly/very satisfied responders on the Facial Line Satisfaction Questionnaire follow-up Items 1 to 5; and treatment-emergent adverse events.
Results
The modified intent-to-treat population (N = 226) had a mean age of 48.0 years, with similar baseline GL severity between treatment groups. Week 24 responder rates were 0% for placebo and 16.0%, 32.0%, 30.6%, and 38.5% for onabotulinumtoxinA 20, 40, 60, and 80 U, with significant (P < 0.05) differences for 40 and 80 U vs 20 U. Median duration of response was longer with all higher doses vs 20 U (≥24.0 vs 19.7 weeks; P < 0.05 vs 20 U at Week 24). Facial Line Satisfaction Questionnaire results indicated high subject satisfaction. The incidence and severity of treatment-emergent adverse events did not exhibit a dose-response effect.
Conclusions
GL treatment with onabotulinumtoxinA doses >20 U demonstrated longer duration of response and higher patient-reported satisfaction vs the on-label 20-U dose with no apparent impact on safety variables.
Level of Evidence: 2

See the Commentary on this article here.
The clinical trial program for onabotulinumtoxinA (BOTOX Cosmetic; Allergan Aesthetics, an AbbVie Company, Irvine, CA) for the treatment of glabellar lines (GLs) demonstrated that 20 U reduced the severity of GLs at maximum frown for approximately 3 to 4 months.1-4 The approved regulatory dose of onabotulinumtoxinA for the treatment of GLs is 20 U.1 However, small dose-ranging studies utilizing somewhat different treatment paradigms have suggested that the efficacy and duration of onabotulinumtoxinA response may increase with doses higher than 20 U in adult subjects with moderate to severe GLs.5,6 One study in female subjects—a double-blind, randomized, parallel-group, dose-ranging trial (N = 80) that had an open-label extension phase (N = 74)—showed a significantly higher rate of relapse (percentage of subjects returning to baseline on the Allergan Facial Wrinkle Scale [FWS] at both rest and maximum frown for 2 consecutive visits) at Month 4 with 10 U onabotulinumtoxinA compared with 40, 30, or 20 U (83% vs 28%, 30%, and 33%, respectively).5 Another study in male subjects (N = 80) found that 40, 60, and 80 U onabotulinumtoxinA were associated with consistently more favorable duration, peak response rate, and improvement from baseline in reducing GLs than 20 U.6 Although these studies suggested a longer duration of response with higher doses of onabotulinumtoxinA, sample sizes were insufficient to accurately determine a dose-duration relationship.5,6 The authors advised that in clinical practice, some individuals, particularly men, may need onabotulinumtoxinA doses much greater than 20 U to experience optimal efficacy for treatment of GLs.5,6 Other dose-ranging studies varied with respect to both the dose range and number of injection sites utilized (eg, 20 U divided between 2 injection sites to a range of doses spread across multiple sites), making direct comparisons of dosing regimens impossible.2,7,8
The purpose of this study was to evaluate the safety and pharmacodynamic response, which included the relationship of dose to the drug’s effect at the muscle target corresponding with 40, 60, and 80 U of onabotulinumtoxinA compared with the on-label 20-U dose in female subjects with moderate to severe dynamic GLs. In addition, treatment satisfaction was assessed.
METHODS
Study Design
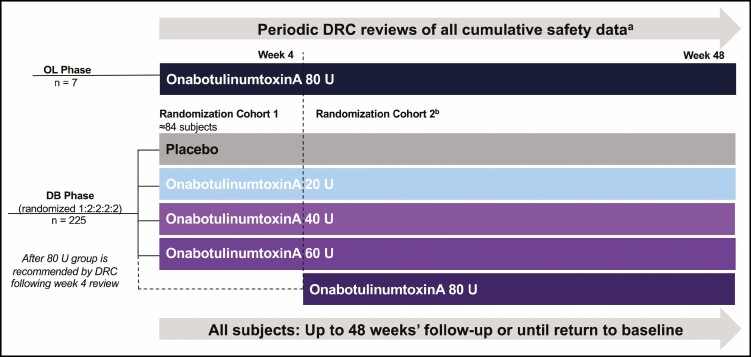
A Phase 1b, 48-week, 9-center US study (Figure 1) compared a single 40-, 60-, and 80-U dose of onabotulinumtoxinA vs a single 20-U dose and placebo. The study had an open-label (OL) phase that allowed for a preliminary safety and tolerability assessment of an 80-U dose, which informed the decision to include the 80-U dose group in the double-blind (DB) randomized phase of the study (Figure 1). This study was approved by a central IRB (Quorum Review Inc., Seattle, WA). The study was conducted between October 2017 and December 2018 and all subjects provided informed written consent.
Figure 1.
Study design. aPeriodic DRC reviews were conducted when ≥7 subjects in the OL onabotulinumtoxinA 80-U group completed Weeks 2 and 4 visits, as well as monthly visits throughout the DB phase. bFollowing review of Week 4 OL data, the DRC recommended whether subjects should be enrolled in the DB randomization cohort 2, which included an onabotulinumtoxinA 80-U group. Subjects were randomized according to a computer-generated randomization scheme obtained from an interactive web response system. DB, double-blind; DRC, data review committee; OL, open-label.
Subjects
The study included female subjects only, aged 18 years and older, with moderate (Grade 2) or severe (Grade 3) dynamic GLs on the FWS as assessed by the investigator (4-point scale of GL severity: 0 = none, 1 = mild, 2 = moderate, and 3 = severe), and excluded subjects previously treated with botulinum toxin of any serotype to the upper face within 1 year of Day 1 of the study.
Treatments
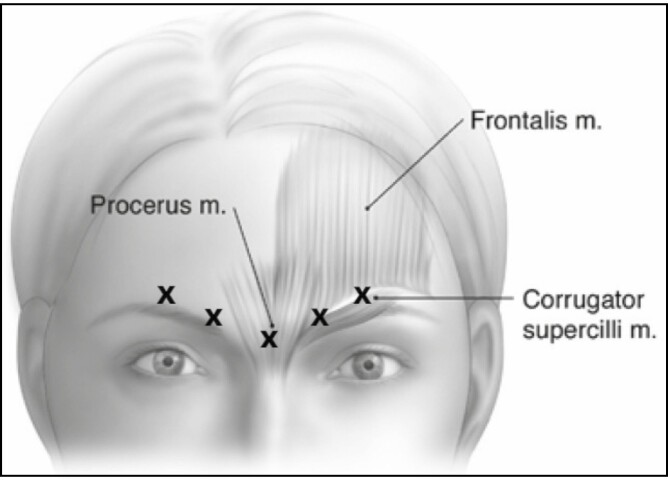
Following the randomization scheme detailed in Figure 1, qualified subjects received on Day 1 a single dose of onabotulinumtoxinA (20, 40, 60, or 80 U) or placebo administered according to the on-label 5-site injection pattern in the glabellar muscle complex. This injection pattern consisted of a single 0.05-mL injection into the procerus muscle and 2 bilateral 0.05-mL injections into each of the corrugator muscles (Figure 2). A lower volume per injection (0.05 mL) than that indicated on the product label (0.1 mL)1 was used to mitigate potential adverse events (AEs) that may be associated with localized diffusion of injected toxin when doses and injection volumes are increased.9,10 Injection volumes were kept consistent across all onabotulinumtoxinA doses and placebo to maintain blinding to treatment.
Figure 2.
Single-dose, 5-point intramuscular injection pattern.
Assessments
Safety
The purpose of the OL phase of the study was to evaluate the safety of the highest onabotulinumtoxinA dose to be tested in the DB phase (80 U), administered via 5 intramuscular injections to the glabellar complex. The Data Review Committee conducted periodic safety reviews of all subjects in the onabotulinumtoxinA 80-U group during the Week 2 and Week 4 OL visits, and monthly during the DB phase. The incidences of treatment-emergent adverse events (TEAEs), including severity and causality, serious AEs leading to discontinuation, and change from baseline in vital signs, were evaluated in both the OL and DB phases. Cumulative safety data from all enrolled subjects were reviewed throughout both the OL and DB phases. The safety population included all subjects who received at least 1 injection of study treatment (OL and DB phases). All summaries determined for the safety population were based on the treatment each subject received.
Pharmacodynamic Response and Treatment Satisfaction
Follow-up visits occurred 1 and 2 weeks after treatment, and every 4 weeks thereafter up to Week 48, or until the subject returned to baseline GL severity at maximum frown (as assessed on the FWS by the investigator), whichever occurred first. The primary efficacy endpoint was the responder rate, defined as the percentage of subjects achieving at least a ≥1-grade improvement from baseline GL severity on the FWS at maximum frown, assessed by the evaluating investigator at Week 24. The ≥1-grade improvement from baseline responder rates, both at maximum frown and at rest, assessed by the investigator and by the subject at each visit, were secondary efficacy endpoints. Additional secondary endpoints included the proportion of responders, defined as subjects who reported “mostly” or “very satisfied” on Items 1 to 5 (pertaining to subject satisfaction) on the proprietary Facial Line Satisfaction Questionnaire (FLSQ) Follow-up Version developed by Allergan,11 and the estimated median duration of response. The median duration of response was defined as the time to return to baseline GL severity for subjects who achieved a ≥1-grade improvement at Week 4 based on the investigator FWS at maximum frown. The FLSQ was completed by subjects in writing at the investigator’s office with assigned subject numbers as identifiers. All efficacy endpoints compared the onabotulinumtoxinA 40-, 60-, and 80-U groups with the onabotulinumtoxinA 20-U group.
Statistical Methods
For the primary efficacy endpoint and for the secondary efficacy endpoints involving responder rates at each visit, descriptive statistics (frequency and percentage) of responders were presented by treatment group. The 95% CIs of responder rates and the 95% CIs of the difference in responder rates between subjects who received onabotulinumtoxinA 40, 60, and 80 U compared with 20 U were constructed by the Wilson method. P values for pairwise comparisons were calculated with a Cochran-Mantel-Haenszel test stratified by center/site. Missing data were imputed from the worst postbaseline FWS grade for the onabotulinumtoxinA groups and the best postbaseline FWS grade for the placebo group.
The estimated median duration of response was calculated by the Kaplan-Meier method for Week 4 responders (ie, subjects with at least a ≥1-grade improvement from baseline GL severity at maximum frown); missing data were not imputed. Efficacy analyses were conducted in the modified intent-to-treat (mITT) population, which consisted of all randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment and included only subjects in the DB phase. For the TEAE safety analyses, missing severity data were imputed as severe, and missing relationship to treatment data were imputed as related.
RESULTS
Subjects
The study enrolled 233 female subjects, 7 in the OL phase and 226 in the DB phase. The safety population included all subjects (N = 233) who received at least 1 injection (OL, n = 7; DB, n = 226). All 226 subjects in the DB phase had at least 1 postbaseline efficacy assessment and were included in the mITT population; the OL phase only included safety assessments and did not include efficacy assessments.
Demographic characteristics were similar among treatment groups, and similar numbers of subjects were randomized across onabotulinumtoxinA groups in the DB phase (Table 1). Of the 226 subjects in the mITT population, 88.9% were White, with a mean [standard deviation] age of 48.0 [12.2] years. The placebo and onabotulinumtoxinA groups had comparable baseline GL severity at maximum frown.
Table 1.
Subject Demographics and Baseline Characteristics
| OL phasea | DB phase | Totalc n = 233 |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | 80 U n = 7 |
Placebo n = 25 |
20 U n = 50 |
40 U n = 50 |
60 U n = 49 |
80 U n = 52 |
Totalb n = 226 |
|
| Age (years), mean [SD] | 54.3 [8.4] | 44.8 [14.2] | 49.1 [11.6] | 50.0 [11.3] | 49.5 [11.4] | 45.2 [12.8] | 48.0 [12.2] | 48.2 [12.1] |
| Range | 46–68 | 24–70 | 24–71 | 31–76 | 24–79 | 19–72 | 19–79 | 19–79 |
| Race, n (%) | ||||||||
| White | 7 (100) | 18 (72.0) | 46 (92.0) | 46 (92.0) | 46 (93.9) | 45 (86.5) | 201 (88.9) | 208 (89.3) |
| Black/African American | 0 | 3 (12.0) | 2 (4.0) | 2 (4.0) | 1 (2.0) | 1 (1.9) | 9 (4.0) | 9 (3.9) |
| Asian | 0 | 3 (12.0) | 0 | 1 (2.0) | 0 | 3 (5.8) | 7 (3.1) | 7 (3.0) |
| Other | 0 | 0 | 1 (2.0) | 0 | 2 (4.1) | 2 (3.8) | 5 (2.2) | 5 (2.1) |
| Native Hawaiian/Pacific Islander | 0 | 1 (4.0) | 1 (2.0) | 1 (2.0) | 0 | 1 (1.9) | 4 (1.8) | 4 (1.7) |
| Ethnicity, n (%) | ||||||||
| Not Hispanic or Latino | 5 (71.4) | 22 (88.0) | 42 (84.0) | 43 (86.0) | 40 (81.6) | 44 (84.6) | 191 (84.5) | 196 (84.1) |
| Hispanic or Latino | 2 (28.6) | 3 (12.0) | 8 (16.0) | 7 (14.0) | 9 (18.4) | 8 (15.4) | 35 (15.5) | 37 (15.9) |
| Baseline glabellar line severity at maximum frown | ||||||||
| Investigator-rated FWS | ||||||||
| Moderate | 12 (48.0) | 18 (36.0) | 18 (36.0) | 21 (42.9) | 21 (40.4) | 90 (39.8) | ||
| Severe | 13 (52.0) | 32 (64.0) | 32 (64.0) | 28 (57.1) | 31 (59.6) | 136 (60.2) | ||
| Subject-rated FWS | ||||||||
| Mild | 0 | 0 | 0 | 0 | 1 (1.9) | 1 (0.4) | ||
| Moderate | 10 (40.0) | 9 (18.0) | 14 (28.0) | 11 (22.4) | 13 (25.0) | 57 (25.2) | ||
| Severe | 15 (60.0) | 41 (82.0) | 36 (72.0) | 38 (77.6) | 38 (73.1) | 168 (74.3) | ||
DB, double-blind; FWS, Facial Wrinkle Scale; OL, open-label; SD, standard deviation.
aSubjects in the OL phase were treated as enrolled subjects but not modified intent-to-treat subjects.
bTotal includes all subjects in the DB phase.
cTotal includes all subjects in the OL and DB phases.
Safety
The majority of TEAEs across the OL and DB phases were reported as mild or moderate in severity, and the incidence and severity of TEAEs did not show any onabotulinumtoxinA dose-response effect for any category of adverse events. There were also no clinically meaningful changes from baseline in vital signs during the study. Table 2 summarizes the incidence of TEAEs, including serious AEs and AEs leading to discontinuation. One subject experienced mild eyelid ptosis (80-U group; OL phase) and 1 subject experienced eyebrow ptosis (20-U group; DB phase); both events resolved without sequelae.
Table 2.
AE Summary (Safety Populationa)
| OL phasea | DB phase | Total n = 208 |
|||||
|---|---|---|---|---|---|---|---|
| Category, n (%) | 80 U n = 7 |
Placebo n = 25 |
20 U n = 50 |
40 U n = 50 |
60 U n = 49 |
80 U n = 52 |
|
| Subjects with ≥1 TEAE | 1 (14.3) | 2 (8.0) | 12 (24.0) | 14 (28.0) | 13 (26.5) | 12 (23.1) | 52 (25.0) |
| Headache | 0 | 0 | 2 | 3 | 7 | 1 | 13 (6.3) |
| Seasonal allergy | 0 | 1 | 0 | 3 | 2 | 1 | 6 (2.9) |
| Influenza | 0 | 1 | 2 | 2 | 1 | 0 | 5 (2.4) |
| URTI | 0 | 0 | 3 | 0 | 0 | 1 | 4 (1.9) |
| Bronchitis | 0 | 0 | 1 | 2 | 0 | 0 | 3 (1.4) |
| Any treatment-related AE | 1 (14.3) | 0 | 1 (2.0) | 2 (4.0) | 3 (6.1) | 3 (5.8) | 10 (4.8) |
| Headache | 0 | 0 | 0 | 2 | 3 | 1 | 6 (2.9) |
| Eyelid ptosis | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.5) |
| Contusion | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.5) |
| Brow ptosis | 0 | 0 | 1 | 0 | 0 | 0 | 1 (0.5) |
| Madarosis | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.5) |
| Serious AE | 0 | 0 | 0 | 0 | 1 (2.0) | 0 | 1 (0.5) |
| Cervical cancer | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.5) |
| AEs leading to study discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AE, adverse event; DB, double-blind; OL, open-label; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection.
aAll subjects (N = 233) who received at least 1 injection (OL phase, n = 7; DB phase, n = 226).
Pharmacodynamic Response and Treatment Satisfaction
Primary Endpoint
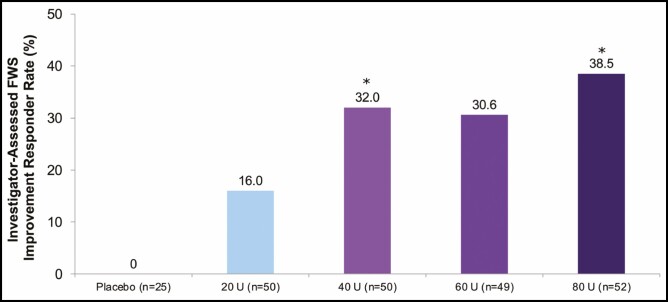
The primary endpoint, ie, the proportion of subjects with at least a ≥1-grade improvement from baseline in investigator-assessed FWS ratings of GL severity at maximum frown at Week 24, was achieved (Figure 3) and was statistically significant, favoring 40 and 80 U (P < 0.05), but not 60 U (P = 0.09), compared with the 20-U group.
Figure 3.
Proportion of responders (subjects with a ≥1-grade FWS improvement from baseline at maximum frown) at Week 24 (primary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center (P value for 60 U vs 20 U not significant). DB, double-blind; FWS, Facial Wrinkle Scale; mITT, modified intent-to-treat.
Secondary Endpoints
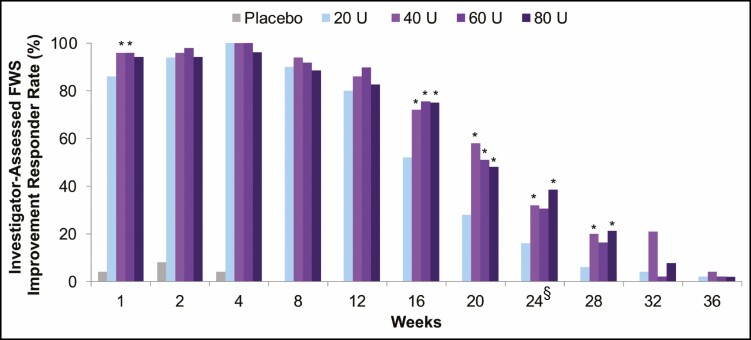
For responder rates at each visit based on investigator-assessed GL severity at maximum frown with the FWS (Figure 4), between-group differences for onabotulinumtoxinA 40, 60, and 80 U compared with 20 U were statistically significant, favoring onabotulinumtoxinA 40 U at Weeks 1, 16, 20, 24 and 28, onabotulinumtoxinA 60 U at Weeks 1, 16, and 20, and onabotulinumtoxinA 80 U at Weeks 16, 20, 24, and 28 (P < 0.05).
Figure 4.
Proportion of responders at each visit (subjects with a ≥1-grade FWS improvement from baseline at maximum frown), assessed by investigator (secondary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center. §Primary time point. DB, double-blind; FWS, Facial Wrinkle Scale; mITT, modified intent-to-treat.
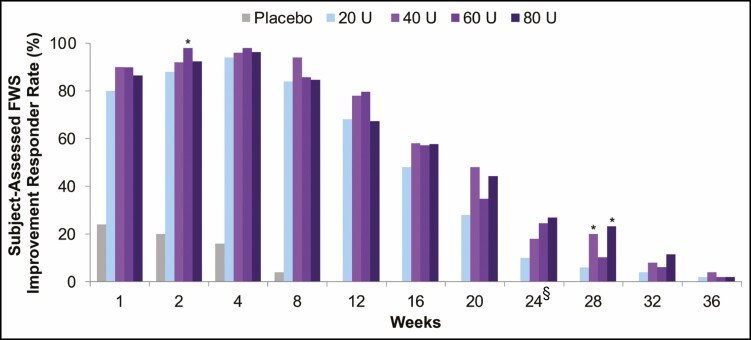
For responder rates at each visit based on subject-assessed GL severity at maximum frown according to the FWS (Figure 5), there were higher proportions of responders in the onabotulinumtoxinA 40-, 60-, and 80-U groups compared with the onabotulinumtoxinA 20-U group at almost all time points. The responder rates were statistically superior at Week 2 for onabotulinumtoxinA 60 U and at week 28 for onabotulinumtoxinA 40 and 80 U.
Figure 5.
Proportion of responders at each visit (subjects with a ≥1-grade FWS improvement from baseline at maximum frown), assessed by subject (secondary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center. §Primary time point. DB, double-blind; FWS, Facial Wrinkle Scale; mITT, modified intent-to-treat.
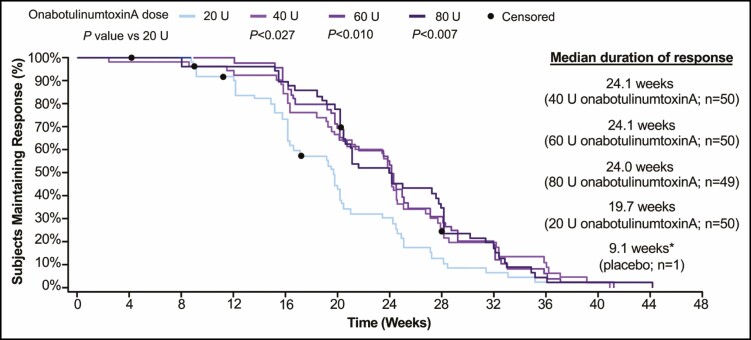
Median duration of response (ie, time to return to baseline GL severity at maximum frown) for Week 4 responders (subjects achieving a ≥1-grade improvement at maximum frown) as assessed by the investigator was longer in the 40-, 60-, and 80-U groups (24.1, 24.1, and 24.0 weeks, respectively) compared with the 20-U group (19.7 weeks) (Figure 6). Statistically significant (P < 0.05) between-group differences in the median duration of response favored all higher onabotulinumtoxinA doses over 20 U at Week 24.
Figure 6.
Time to return to baseline FWS grade at maximum frown for Week 4 respondersa (secondary endpoint, mITT populationb). aResponders were defined as subjects with a ≥1-grade improvement in GL severity at Week 4 on the investigator FWS at maximum frown. Percentages were calculated based on number of Week 4 responders within each treatment group. Censored subjects (•) did not return to baseline investigator FWS at the last visit where FWS at maximum frown was assessed. P values were based on log-rank test. bAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *Median duration of response for placebo group was based on data from 1 responder; therefore, a Kaplan-Meier curve of these data is uninterpretable with respect to duration. DB, double-blind; FWS, Facial Wrinkle Scale; GL, glabellar line; mITT, modified intent-to-treat.
Treatment Satisfaction
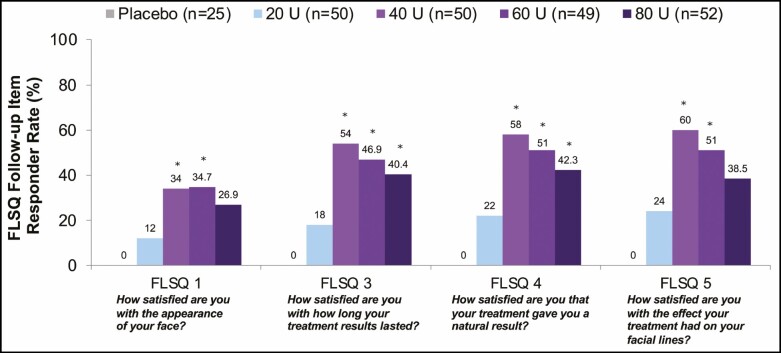
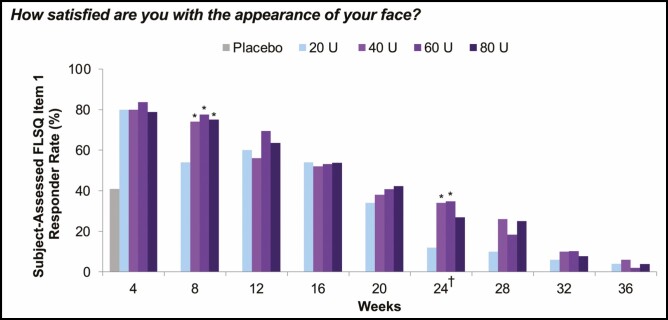
At almost every time point up to Week 36 for each FLSQ satisfaction item, there were greater proportions of responders (defined as mostly or very satisfied) in the 40-, 60-, and 80-U groups compared with the 20-U group. At Week 24, greater proportions of responders in the 40-, 60-, and 80-U groups compared with the 20-U group indicated high subject satisfaction across FLSQ Items 1 and 3 through 5, with statistically significant (P < 0.05) between-group differences favoring the higher onabotulinumtoxinA doses compared with 20 U (Figure 7). For Item 1 (How satisfied are you with the appearance of your face?), the proportion of responders in the 40-, 60-, and 80-U groups was ≥26.9% compared with 12.0% in the 20-U group at Week 24, and between-group differences were statistically significant (P < 0.05) compared with 20 U for the 40-U (Weeks 8 and 24), 60-U (Weeks 8 and 24), and 80-U groups (Week 8) (Figure 8). In response to FLSQ Item 2 (How satisfied are you with how long it took your treatment to work?), all treatment groups had similarly high responder rates at Week 4, with no significant differences in satisfaction with the onset of onabotulinumtoxinA effect.
Figure 7.
Proportion of responders reporting mostly or very satisfied on FLSQ follow-up items at Week 24 (secondary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center. DB, double-blind; FLSQ, Facial Line Satisfaction Questionnaire; mITT, modified intent-to-treat.
Figure 8.
Proportion of responders reporting mostly or very satisfied on FLSQ follow-up Item 1 (secondary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center. †Primary time point. DB, double-blind; FLSQ, Facial Line Satisfaction Questionnaire; mITT, modified intent-to-treat.
DISCUSSION
This Phase 1b study demonstrated that the on-label onabotulinumtoxinA dose (20 U) as well as higher onabotulinumtoxinA doses (40, 60, and 80 U) are safe, well tolerated, and effective for the treatment of moderate to severe GLs. TEAEs were consistent with the known safety profile of onabotulinumtoxinA treatment of GLs1,12 and there was no apparent impact of dose on safety variables. In the primary endpoint analysis, proportions of responders in the onabotulinumtoxinA 40-, 60-, and 80-U groups were greater than in the 20-U group, and doses higher than 20 U resulted in increased duration of treatment response and patient-reported satisfaction. Although the 60-U dose failed to reach significance vs the 20-U dose for the primary endpoint, upon closer examination, this difference was caused by only 2 subjects not achieving a ≥1-grade improvement in GLs from baseline. A limited additional pharmacodynamic response was observed at doses higher than onabotulinumtoxinA 40 U, suggesting that a “ceiling effect,” or maximal attainable response, may have been reached, typical of dose-response kinetics.13
Interestingly, subjects tended to grade their GLs at maximum frown more severely than the investigators. Although the statistical significance of these differences was not determined in this study, it is a trend consistent with other aesthetic studies14,15 and may be reflective of a more intense bias of subjects toward the severity of the facial areas they consider to be treatment concerns. However, investigator and subject improvement ratings were more comparable with one another by Week 24.
The current study was designed to evaluate the pharmacodynamic response of onabotulinumtoxinA doses higher than that approved globally for the treatment of moderate to severe GLs (20 U). Considering the range of doses studied spanned a 4-fold increase over the approved dose, reconstitution was adapted to administer each dose in a lower volume than previous onabotulinumtoxinA studies for GL treatment (0.05 mL/injection vs 0.1 mL/injection per label). Administering a higher dose in a more concentrated solution may have mitigated known local spread of toxin effects and contributed to the favorable safety profile of the higher doses in this study. For example, the summary of TEAEs listed on the onabotulinumtoxinA product label indicates that the incidence of eyelid ptosis was 3% for subjects treated with 20 U; 1 in contrast, the incidence of eyelid ptosis in the current study was 0.5%, occurring only in subjects treated in the OL phase with onabotulinumtoxinA 80 U. Further studies may provide insights into whether the clinical performance profile differentiates across injection volumes, particularly because head-to-head studies of 0.1- and 0.05-mL injection volumes have not been published.
The current study had more subjects at baseline with severe rather than moderate GLs at maximum frown; however, the distribution of GL severity was comparable across all treatment groups, and subject FLSQ self-assessments (follow-up Items 1-5) consistently showed greater subject satisfaction with higher onabotulinumtoxinA doses compared with onabotulinumtoxinA 20 U. Notably, higher onabotulinumtoxinA doses had no detrimental effect on subjects’ satisfaction with the natural look of their treatment; rather, the opposite occurred: with doses greater than 20 U at Week 24, significantly higher proportions of subjects reported “mostly” or “very satisfied” on FLSQ follow-up Item 4 (How satisfied are you that your treatment gave you a natural result?). This study demonstrated that higher doses of onabotulinumtoxinA were more effective than 20 U and subjects were generally satisfied that their treatments resulted in natural outcomes. At all time points up to Week 36, greater proportions of subjects also reported that they were mostly or very satisfied with how long it took the treatment to work in the 40-, 60-, and 80-U groups compared with the 20-U group (FLSQ follow-up Item 3). However, a slight decrease in subject satisfaction from 40 to 60 U and/or to 80 U was also observed in this study after Week 24. The greater proportion of investigator-assessed responders after Week 24 in the 40-U group relative to the 60-U group (Weeks 28, 32, and 36) and the 80-U group (Weeks 32 and 36) may have been a factor in this observation. It should be noted that this study was not powered to detect the statistical significance between the higher doses of onabotulinumtoxinA.
The median duration of response (time to return to baseline GL severity), which was approximately 6 months in the onabotulinumtoxinA 40-, 60-, and 80-U groups compared with approximately 5 months in the onabotulinumtoxinA 20-U group, favored the higher onabotulinumtoxinA doses over 20 U at Week 24 (P < 0.05, all comparisons). Phase 3 clinical trial data for daxibotulinumtoxinA in the treatment of moderate to severe GLs at a dose of 40 U showed a duration of response (median time until none or mild GL severity was lost) of 24 weeks.16 In the Phase 2 BELMONT clinical trial, there were no statistical differences in clinical efficacy measures (ie, 1- and 2-point improvements on the FWS scales) between the 20-U doses of daxibotulinumtoxinA and onabotulinumtoxinA at any time point (Weeks 4, 16, or 24) as assessed by investigators as well as by subjects; significant differences over onabotulinumtoxinA 20 U were only achieved for the higher daxibotulinumtoxinA doses (ie, 40 or 60 U).17 The median duration of response reported in the BELMONT study for daxibotulinumtoxinA was 20.0 weeks for 20 U, 23.6 weeks for 40 U, and 20.9 weeks for 60 U. These findings, coupled with the current data, suggest that longer duration is largely a factor of higher dose, as the median duration of response in our study was also longer for the 40-U and higher onabotulinumtoxinA doses when compared with onabotulinumtoxinA 20 U (≥24.0 weeks for 40, 60, and 80 U vs 19.7 weeks for the approved 20 U for GLs). Other studies conducted with onabotulinumtoxinA, as well as studies conducted with other botulinum toxins for treatment of GL severity (abobotulinumtoxinA and incobotulinumtoxinA), also support extended duration of responses with escalating doses.18-20 However, units of onabotulinumtoxinA are not interchangeable with those of any other botulinum toxin preparation and cannot be compared to or converted into units of any other product.21 In addition, the facial wrinkle photonumeric scales used across the various referenced botulinum toxin A studies are proprietary to each manufacturer and also render direct comparisons of duration not possible.
A potential limitation of our study is that it may not fully represent median duration of response. Only investigators’ assessments of subjects’ return to baseline GL severity were used to determine whether subjects exited the study, with no subject-reported assessments of return to baseline GL severity or duration of response nor jointly agreed-upon investigator-subject FWS ratings considered in study exit determination. Thus, based on the investigator ratings alone, most subjects exited by Week 36. One study on onabotulinumtoxinA treatment of forehead lines suggested that duration of response reported on the FWS by subjects may exceed those reported by investigators by almost 7 days for the 40-U dose.22
CONCLUSIONS
This pharmacodynamic dose-response study demonstrated greater patient-reported satisfaction and response duration with onabotulinumtoxinA doses greater than 20 U for moderate to severe GLs, with no apparent impact on the safety profile with an injection volume (0.05 mL) lower than that specified on the onabotulinumtoxinA product label (0.1 mL).
Acknowledgments
Writing and editorial assistance was provided to the authors by Regina Kelly, MA, of Peloton Advantage (Parsippany, NJ), an OPEN Health company. The authors would also like to acknowledge the study management contributions of Miles McLennan, who was associate vice president for Global Medical Trial Management, AbbVie (Irvine, CA).
Contributor Information
John H Joseph, Clinical Testing of Beverly Hills, Beverly Hills, CA, USA.
Dee Anna Glaser, Department of Dermatology, Saint Louis University School of Medicine, St. Louis, MO, USA.
Sara Sangha, Research and Development, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA.
John Maltman, Research and Development, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA.
Xiaofang Lei, Research and Development, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA.
Mitchell F Brin, Research and Development, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA.
Disclosures
Dr Joseph is an investigator and advisory board member for Allergan Aesthetics, an AbbVie company (Irvine, CA); clinical investigator for Galderma (Lausanne, Switzerland), Merz (Raleigh, NC), Evolus (Newport Beach, CA), Revance (Newark, CA), and Croma (Leobendorf, Austria); speaker or consultant for Galderma, Merz, and Evolus; advisor for Revance and Croma; and shareholder for Evolus and Revance. Dr Maas is an investigator for Allergan Aesthetics, an AbbVie company. Dr Palm is an advisory board member, paid consultant, speaker, and clinical investigator for Allergan Aesthetics, an AbbVie company, and for Galderma; clinical investigator and speaker for Merz; clinical investigator for Revance; and speaker for Evolus. Dr Lain is an advisory board member, paid consultant, and clinical investigator for Allergan Aesthetics, an AbbVie company; and advisory board member, paid consultant, and clinical investigator for Galderma. Dr Glaser is an uncompensated investigator for Allergan Aesthetics, an AbbVie company, Galderma, Revance, Dermira (Menlo Park, CA), ATACAMA (Wellesley, MA), and Brickell (Miami, FL); and speaker or consultant for AbbVie, Galderma, Dermira, and OrthoDerm (Bridgewater, NJ). Dr Bruce is an advisory board member, paid consultant, and clinical investigator for Allergan Aesthetics, an AbbVie company. Dr Yoelin is a paid consultant, researcher (paid with grants), and speaker for Allergan Aesthetics, an AbbVie company; paid consultant for Galderma, Revance, and Evolus; and researcher (paid with grants) for Merz. Dr Cox is a principal investigator, advisory board member, and consultant to AbbVie, Croma, Evolus, and Galderma; advisory board member and consultant to Merz; principal investigator, advisory board member, consultant, and paid speaker for Revance; and stockholder in Revance and Candesant (San Francisco, CA). Dr Fagien is an advisory board member, investigator, and paid consultant for Allergan Aesthetics, an AbbVie company; clinical investigator for Galderma and Merz; and advisory board member, investigator, and paid consultant for Evolus. Drs Sangha, Maltman, Lei, and Brin are employees of AbbVie, and may own AbbVie stock.
Funding
This study was funded by Allergan Aesthetics, an AbbVie company (Irvine, CA). Neither honoraria nor other form of payment was made for authorship.
REFERENCES
- 1. Botox Cosmetic [package insert]. Allergan plc; 2020. [Google Scholar]
- 2. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-9. [DOI] [PubMed] [Google Scholar]
- 3. Carruthers JD, Lowe NJ, Menter MA, Gibson J, Eadie N, Fagien S. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin A for patients with glabellar lines. Plast Reconstr Surg. 2003;112(5 suppl):21S-32S. [DOI] [PubMed] [Google Scholar]
- 4. Glogau R, Kane M, Beddingfield F, et al. OnabotulinumtoxinA: a meta-analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg. 2012;38(11):1794-803. [DOI] [PubMed] [Google Scholar]
- 5. Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatol Surg. 2005;31(4):414-22; discussion 422. [DOI] [PubMed] [Google Scholar]
- 6. Carruthers A, Carruthers J. Prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytids. Dermatol Surg. 2005;31(10):1297-303. [DOI] [PubMed] [Google Scholar]
- 7. Pribitkin EA, Greco TM, Goode RL, Keane WM. Patient selection in the treatment of glabellar wrinkles with botulinum toxin type A injection. Arch Otolaryngol Head Neck Surg. 1997;123(3):321-6. [DOI] [PubMed] [Google Scholar]
- 8. Lowe NJ, Lask G, Yamauchi P, Moore D. Bilateral, double-blind, randomized comparison of 3 doses of botulinum toxin type A and placebo in patients with crow’s feet. J Am Acad Dermatol. 2002;47(6):834-40. [DOI] [PubMed] [Google Scholar]
- 9. Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994;9(1):31-9. [DOI] [PubMed] [Google Scholar]
- 10. Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004;140(11):1351-4. [DOI] [PubMed] [Google Scholar]
- 11. Pompilus F, Burgess S, Hudgens S, Banderas B, Daniels S. Development and validation of a novel patient-reported treatment satisfaction measure for hyperfunctional facial lines: Facial Line Satisfaction Questionnaire. J Cosmet Dermatol. 2015;14(4):274-85. [DOI] [PubMed] [Google Scholar]
- 12. Cavallini M, Cirillo P, Fundaro SP, et al. Safety of botulinum toxin A in aesthetic treatments: a systematic review of clinical studies. Dermatol Surg. 2014;40(5):525-36. [DOI] [PubMed] [Google Scholar]
- 13. Banham S, Taylor D. Pharmacodynamics and pharmacokinetics chapter 4. In: Haddad PM, Nutt DJ, eds. Seminars in Clinical Psychopharmacology, 3rd ed. Cambridge University Press; 2020:124-150. [Google Scholar]
- 14. Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA for injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg. 2020;145(1):45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type A for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901 [DOI] [PubMed] [Google Scholar]
- 16. Bertucci V, Solish N, Kaufman-Janette J, et al. DaxibotulinumtoxinA for injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, Phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol. 2020;82(4):838-45. [DOI] [PubMed] [Google Scholar]
- 17. Carruthers J, Solish N, Humphrey S, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a Phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg. 2017;43(11):1321-1331. [DOI] [PubMed] [Google Scholar]
- 18. Nicholson GS, Canty D, Brideau-Andersen A, Broide RS. BoNT/A exhibits a linear correlation between dose, peak efficacy and duration of effect in functional assays [poster]. Presented at: Biennial TOXINS, Copenhagen, Denmark, January 16-19, 2019.
- 19. Joseph JH, Eaton LL, Robinson J, Pontius A, WilliamsEF, III.. Does increasing the dose of abobotulinumtoxinA impact the duration of effectiveness for the treatment of moderate to severe glabellar lines? J Drugs Dermatol. 2016;15(12):1544-1549. [PubMed] [Google Scholar]
- 20. Polacco MA, Singleton AE, Barnes CH, Maas C, Maas CS. A double-blind, randomized clinical trial to determine effects of increasing doses and dose-response relationship of incobotulinumtoxinA in the treatment of glabellar rhytids. Aesthet Surg J. 2021;41(6):NP500-NP511. [DOI] [PubMed] [Google Scholar]
- 21. Botox [package insert]. Allergan USA; 2021. [Google Scholar]
- 22. Solish N, Rivers JK, Humphrey S, et al. Efficacy and safety of onabotulinumtoxinA treatment of forehead lines: a multicenter, randomized, dose-ranging controlled trial. Dermatol Surg. 2016;42(3):410-9. [DOI] [PubMed] [Google Scholar]