Abstract
The alphaproteobacterial family Rhizobiaceae is highly diverse, with 168 species with validly published names classified into 17 genera with validly published names. Most named genera in this family are delineated based on genomic relatedness and phylogenetic relationships, but some historically named genera show inconsistent distribution and phylogenetic breadth. The most problematic is Rhizobium , which is notorious for being highly paraphyletic, as most newly described species in the family are assigned to this genus without consideration of their proximity to existing genera, or the need to create novel genera. Moreover, many Rhizobiaceae genera lack synapomorphic traits that would give them biological and ecological significance. We propose a common framework for genus delimitation within the family Rhizobiaceae , wherein genera are defined as monophyletic groups in a core-genome gene phylogeny, that are separated from related species using a pairwise core-proteome average amino acid identity (cpAAI) threshold of approximately 86 %. We further propose that additional genomic or phenotypic evidence can justify division of species into separate genera even if they share greater than 86 % cpAAI. Applying this framework, we propose to reclassify Rhizobium rhizosphaerae and Rhizobium oryzae into Xaviernesmea gen. nov. Data is also provided to support the formation of Peteryoungia aggregata comb. nov., Endobacterium yantingense comb. nov., Neorhizobium petrolearium comb. nov., Pararhizobium arenae comb. nov., Pseudorhizobium tarimense comb. nov. and Mycoplana azooxidifex comb. nov. Lastly, we present arguments that the unification of the genera Ensifer and Sinorhizobium in Opinion 84 of the Judicial Commission is no longer justified by current genomic and phenotypic data. Despite pairwise cpAAI values for all Ensifer species and all Sinorhizobium species being >86 %, additional genomic and phenotypic data suggest that they significantly differ in their biology and ecology. We therefore propose emended descriptions of Ensifer and Sinorhizobium , which we argue should be considered as separate genera.
Keywords: Rhizobiaceae, Xaviernesmea, Ensifer, Sinorhizobium, Rhizobium, genus boundaries
Data Summary
All genome sequences used in this work were previously published, and the assembly accessions are provided in Dataset S1 (available in the online version of this article). Twelve supplementary figures and three supplementary datasets are included in the online version of this article. All raw data (core-proteome average amino acid identity data, whole-proteome average amino acid identity data, average nucleotide identity data, percentage of conserved proteins, and a Newick formatted phylogeny) used to generate the figures presented in this manuscript are available through Figshare at https://doi.org/10.6084/m9.figshare.17076455.v1 [1]. A pipeline to extract the 170 marker proteins used for phylogenetic reconstruction and in calculating core-proteome average amino acid identity values, is available through GitHub at github.com/flass/cpAAI_Rhizobiaceae.
Introduction
The family Rhizobiaceae of the order Alphaproteobacteria was proposed in 1938 and has since undergone numerous, and at times contentious, taxonomic revisions [2, 3]. Currently, this family comprises the genera Agrobacterium , Allorhizobium , Ciceribacter , Endobacterium , Ensifer (syn. Sinorhizobium ), Gellertiella , Georhizobium , Hoeflea , Lentilitoribacter , Liberibacter , Martelella , Mycoplana , ‘ Neopararhizobium ’, Neorhizobium , Pararhizobium , Peteryoungia , Pseudorhizobium , Rhizobium and Shinella (syn. Crabtreella ; https://lpsn.dsmz.de/) [4]. The family Rhizobiaceae contains phenotypically diverse organisms, including N2-fixing legume symbionts (known as rhizobia), plant pathogens, bacterial predators, and other soil bacteria. The agricultural and ecological significance of the family Rhizobiaceae has prompted the isolation and whole genome sequencing of hundreds of strains at a rate outpacing taxonomic refinement of the family. As a result, some species and genera within the family are well known to be paraphyletic [5], while others that are monophyletic likely represent multiple species/genera [6]. In addition, most currently named genera have been delineated based on genomic relatedness – as per current taxonomic guidelines [7] – but lack synapomorphic traits that would give them biological and ecological significance [8].
To aid in the taxonomic classification of this family, here we propose a general framework for defining genera in the family Rhizobiaceae . This framework is based on a set of baseline genomic relatedness measures meant to serve as minimal thresholds for genus demarcation, while allowing for more closely related species to be divided into separate genera when supported by supplemental genomic and/or biological data. By applying this framework, we propose the formation of a new genus – Xaviernesmea – on the basis of the genomic relatedness measures, and provide support for the recently described genus Peteryoungia . In addition, despite genomic relatedness values that would not support genus demarcation based on the proposed baseline thresholds, we argue that current phylogenetic, genomic (e.g. pentanucleotide frequency) and biological (e.g. division by budding) data indicate that the genera Ensifer Casida et al. 1982 [9] and Sinorhizobium Chen 1988 [10] are not synonymous, meaning that the unification of the genera Ensifer and Sinorhizobium in Opinion 84 of the Judicial Commission is no longer justified.
Methods
Dataset
The analysis was performed on a dataset of 94 genomes of Rhizobiaceae strains, among which the majority were type strains of the corresponding species (Dataset S1). As an outgroup, we included the genomes of three Mesorhizobium strains, belonging to the related family Phyllobacteriaceae . Moreover, for calculation of some overall genome relatedness indices (OGRIs) to support additional taxonomic revisions, genomes of two Pararhizobium and two Pseudorhizobium strains were included (Dataset S1). To verify the authenticity of genomes used for taxonomic reclassifications proposed in this paper, we compared the reference 16S rRNA gene sequences (as well as housekeeping gene sequences in ambiguous cases) associated with the original species publication with the sequences retrieved from genome sequences (Dataset S1). Whole genome sequences generated to support new species description in original publications were considered as authentic (Dataset S1).
Core-genome gene phylogeny
The core-genome phylogeny was obtained using the GET_HOMOLOGUES software package version 10032020 [11] and the GET_PHYLOMARKERS software package version 2.2.8_18Nov2018 [12], as described previously [13]. As a result, a set of 170 non-recombining single-copy core marker genes was selected, and a concatenation of their codon-based alignments was used as input for IQ-TREE ModelFinder, with which a search for the best sequence evolution model was conducted. The model ‘GTR+F+ASC+R8’ was selected based on a Bayesian information criterion. The maximum-likelihood (ML) core genome phylogeny was inferred under this model using IQ-TREE [14], with branch supports assessed with approximate Bayes test (-abayes) and ultrafast bootstrap with 1000 replicates (-bb 1000).
Overall genome relatedness indices calculations
Whole-proteome average amino-acid identity (wpAAI; usually simply known as AAI) was computed using the CompareM software (github.com/dparks1134/CompareM) using the aai_wf command with default parameters, i.e., ortholog identification with DIAMOND [15], e-value <1e-3, percent identity >30 %, and alignment length >70 % the length of the protein.
Core-proteome average amino-acid identity (cpAAI) was computed as the proportion of substitutions in pairwise comparisons of sequences from the 170 non-recombining, single-copy core marker genes identified using GET_PHYLOMARKERS [12], using a custom R script that notably relied on the dist.aa() function from the ‘ape’ package [16].
Percentage of conserved proteins (POCP) was determined using publicly available code (github.com/hoelzer/pocp) and the ortholog identification thresholds defined by Qin et al. [17], namely, e-value <1e-5, percent identity >40 %, and alignment length >50 % the length of the protein. This pipeline involved the reannotation of genomes with Prodigal version 2.6.3 [18] and ortholog identification using the blast+ package, version 2.10.1 [19].
The average nucleotide identity (ANIb) comparisons were conducted using PyANI version 0.2.9, with scripts employing the blast+ algorithm to align the input sequences (https://github.com/widdowquinn/pyani). The digital DNA–DNA hybridization (dDDH) computations were performed with the Genome-to-Genome Distance Calculator (GGDC 2.1; https://ggdc.dsmz.de/distcalc2.php) using the recommended blast+ alignment and formula 2 (identities/HSP length) [20].
16S rRNA gene phylogeny
The RNA fasta files for the 157 Sinorhizobium or Ensifer strains analysed in our recent study [21] were downloaded from the National Centre for Biotechnology Information database, and all 16S rRNA gene sequences ≥1000 nt were extracted. The 16S rRNA gene sequences were aligned using mafft version 7.3.10 with the localpair option [22], and trimmed using trimAl version 1.4.rev22 with the automated1 option [23]. A ML phylogeny was prepared using raxmlHPC-HYBRID-AVX2 version 8.2.12 with the GTRCAT model [24]. The final phylogeny is the bootstrap best tree following 756 bootstrap replicates, as determined by the extended majority-rule consensus tree criterion.
Code availability
To facilitate the adoption of our framework for genus demarcation in the family Rhizobiaceae , a custom pipeline was prepared to extract the 170 marker proteins from other genomes. The pipeline, together with the 170 marker proteins from the 97 strains analysed in the current study, is available at github.com/flass/cpAAI_Rhizobiaceae. Briefly, the pipeline will use tblastn [19] to identify genes encoding orthologs of all 170 marker proteins in the input genomes, which will then be extracted and translated. Each set of protein orthologs is then aligned with mafft [22] or Clustal Omega [25] to the pre-computed alignment of the orthologs from the 97 strains analysed here, and a concatenated alignment prepared. The output files can then be used for phylogenetic reconstruction or cpAAI calculations, with sample code for cpAAI calculations also provided.
Results and discussion
Overall genomic relatedness indices measurements in the family Rhizobiaceae
To develop a framework for genus demarcation within the family Rhizobiaceae , we examined a selection of 94 genomes of Rhizobiaceae isolates, most of which are species type strains. Liberibacter , an obligate intra-cellular pathogen with a highly reduced genome, was excluded from our selection of organisms to avoid biassing the analysis by overly reducing the conserved gene set. We reasoned that good practices for genome sequence-based genus delineation should consider both phylogenetic relatedness of species based on a concatenated alignment of core-genome genes (Figs 1 and S1), and one or more OGRI measurement. We initially considered four OGRIs, calculated as described in the Methods: (i) average nucleotide identity (ANIb), (ii) whole-proteome average amino acid identity (wpAAI); (iii) core-proteome average amino acid identity (cpAAI) based on the proportion of substitutions between the concatenated translated sequences of the core marker gene set used for the core-genome phylogeny; and (iv) the percentage of conserved proteins (POCP) as defined by Qin et al. [17]. Digital DNA–DNA hybridization (dDDH; Dataset S3) was also performed for some strains to verify they represented distinct species; however, dDDH was not considered when defining genera.
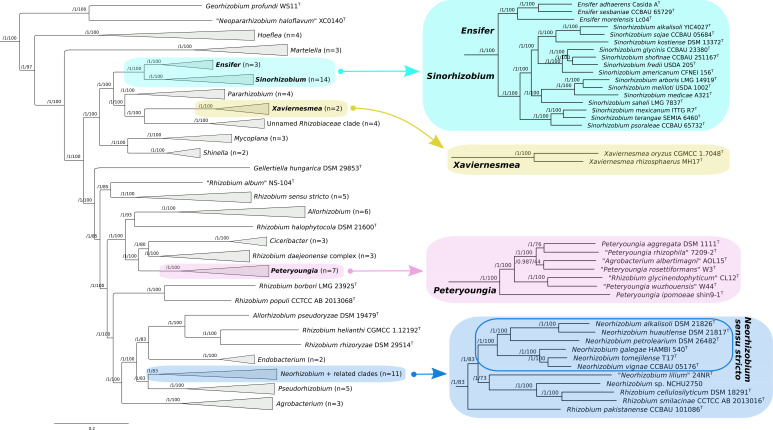
Fig. 1.
Maximum-likelihood core-genome phylogeny of the family Rhizobiaceae . A maximum-likelihood phylogeny 94 Rhizobiaceae strains is shown. The number of strains included in each collapsed clade is indicated. Clades of focus in the current study are expanded along the righthand side of the figure. The phylogeny is built from the concatenated alignments of 170 nonrecombinant loci using IQ-TREE [14]. The numbers on the nodes indicate the approximate Bayesian posterior probabilities support values (first value) and ultra-fast bootstrap values (second value). The tree was rooted using three Mesorhizobium spp. sequences as the outgroup. The scale bar represents the number of expected substitutions per site under the best-fitting GTR+F+ASC+R8 model. An expanded phylogeny is provided as Fig. S1.
On the assumption that genera are not artificial divisions of a continuum of species, but that they instead represent biologically meaningful differentiation of groups of species, we reasoned that an OGRI threshold for delimiting genera should correspond to a drop in the OGRI frequency distribution. We therefore plotted histograms of all pairwise comparisons to identify potential genera boundaries (Figs 2 and S2–S4). It was previously suggested that a 50 % POCP threshold is a good measure of genus boundaries in other families [17]. However, we found that 3885 out of the 4371 (89 %) pairwise comparisons in our Rhizobiaceae dataset gave a POCP value ≥50 %, with no clear breaks in the frequency distribution (Fig. S2). We therefore concluded that POCP is not a useful OGRI measurement for defining genera in the family Rhizobiaceae . Similar observations were also reported for some other taxa, such as members of the roseobacter group [26].
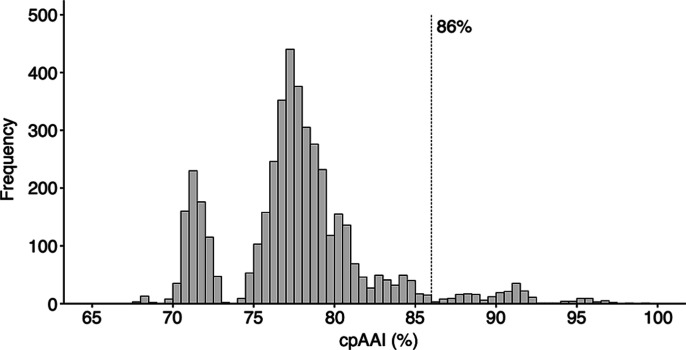
Fig. 2.
Distribution of core-proteome AAI (cpAAI) comparisons of the family Rhizobiaceae . Pairwise cpAAI values were calculated based on 170 nonrecombinant loci from the core-genome of 94 members of the family Rhizobiaceae. Results are summarized as a histogram with a bin width of 0.5 %.
cpAAI data was recently used to delineate genera among other bacterial families [26, 27] and stands as a promising metric for genus demarcation in the family Rhizobiaceae (Fig. 2). We observed a break in the frequency distribution at ~93 % to~94 %, but it was too stringent to use for genus demarcation as it would result in the majority of the 94 strains being classified into their own genera. Likewise, the break at ~73 % to~74 % was too lenient for genus delimitation as all strains would be grouped as a single genus, except for those belonging to the genera Martelella , Hoeflea , ‘ Neopararhizobium ’, and Georhizobium . Instead, the drop in the frequency distribution at 86–86.7 % (inclusive), within which only five of the 4371 pairwise comparisons fell, appeared to be a reasonable threshold to aid with defining genera in the family Rhizobiaceae (Fig. 2). The drop in the frequency distribution at ~86 % was also visible in scatterplots showing the relationships between cpAAI and either wpAAI or ANIb (Fig. S5). Notably, this corresponds nicely with a recent study that used a cpAAI threshold of 86 % in genus demarcation in the roseobacter group of the class α-Proteobacteria [26]. Using a cpAAI threshold of ~86 %, combined with the phylogeny of Fig. 1, we were able to largely preserve the current taxonomy of the family Rhizobiaceae , recovering the genera Agrobacterium , Ciceribacter , Endobacterium , Ensifer (as previously defined), Gellertiella , Georhizobium , Mycoplana , ‘ Neopararhizobium ’, Peteryoungia , Pseudorhizobium , Pararhizobium and Shinella (Figs 3 and S6). Such a threshold would, however, split the genera Allorhizobium , Hoeflea , Martelella , Neorhizobium and Rhizobium sensu stricto into two or more genera.
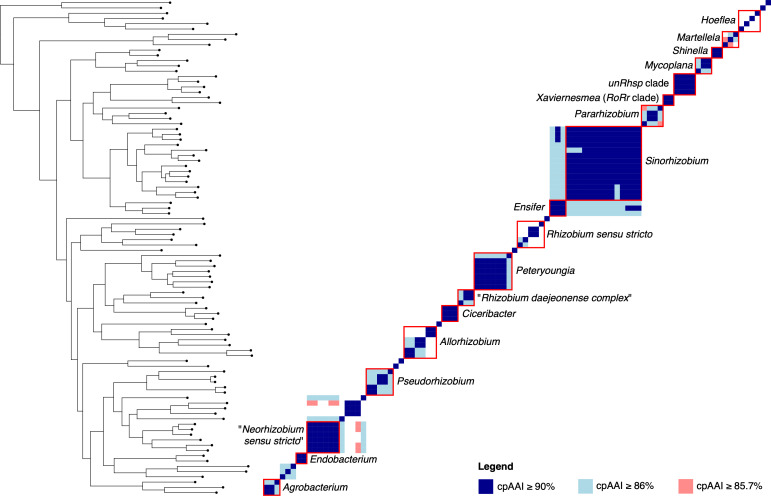
Fig. 3.
Core-proteome AAI (cpAAI) matrix of the family Rhizobiaceae . A matrix showing the pairwise cpAAI values for each pair of 94 members of the family Rhizobiaceae . Values were clustered using the core-genome gene phylogeny of Figs 1 and S1. Several named genera are indicated with red boxes, as indicated. A version of this matrix with a colour scheme representing the full range of cpAAI values is provided as Fig. S6.
Both wpAAI and ANIb were correlated with cpAAI (Fig. S5), although neither relationship was linear. However, there was less support for the presence of genus-level drops in the wpAAI or ANIb frequency distributions (Figs S3 and S4). Nonetheless, the wpAAI frequency distribution density increased sharply below 76.5 %, while there was a sharp increase in the ANIb frequency distribution below 78.5 %. Although noisier, a wpAAI threshold of 76.5 % or a ANIb threshold of 78.5 % returned similar genus demarcations as did a cpAAI threshold of ~86 %, with a few exceptions (Figs S7–S10). In the case of wpAAI, the genus Martelella was recovered as a single genus, Hoeflea was split into fewer genera, and the separation between the genus Ciceribacter and its sister taxon was less clear. When using ANIb, Shinella and Mycoplana were combined as a single genus, Allorhizobium was split into three genera instead of two, the genus Martelella was recovered as a single genus, and the separation between the genus Ciceribacter and its sister taxon was less clear.
Proposal for a framework for genus delineation in the family Rhizobiaceae
Based on the results summarized above, we propose that genera within the family Rhizobiaceae be defined as monophyletic groups (as determined by a phylogenetic reconstruction using a core-genome analysis approach; Fig. 1) separated from related species using a pairwise cpAAI threshold of approximately 86 % calculated as described in the methods. We specify ‘approximately 86%’ to provide some flexibility in the threshold to allow for differences in the evolution of each genus; for example, a cpAAI value of 85.7 % appears better suited for the genus Pararhizobium . We strongly recommend the use of cpAAI over wpAAI or ANIb due to (1) its natural agreement – by construction – with the core-genome gene phylogeny, (2) clearer gaps in its distribution of values among Rhizobiaceae , and (3) the fact that it would not be sensitive to the wide genome size variation within the Rhizobiaceae , notably due to the variation in presence of large mobile genetic elements, including symbiotic and tumor-inducing megaplasmids. We do not, however, propose that cpAAI serve as the sole information source for genus demarcation as nearly all biological rules have exceptions. We therefore propose that genus demarcation using a cpAAI threshold higher than 86 % can be justified by the presence of alternate genomic or phenotypic evidence (as proposed below for splitting of the genus Ensifer ), while a lower cpAAI threshold may be appropriate when considering historical classifications of genera within the family.
Taxonomic implications of the proposed framework
Following the criteria for genus demarcation outline above would notably lead to the formation of several new genera for species currently assigned to the genus Rhizobium , which is notoriously paraphyletic. They also imply that a few genera ( Neorhizobium , Allorhizobium , Martelella , Hoeflea , and Rhizobium sensu stricto) may be candidates for division. We also note that there is a clear break in the distribution of cpAAI values at ~73 % to ~74 % that may represent an appropriate threshold for delimiting the family Rhizobiaceae . If adopted, this threshold would result in the genera Martelella and Hoeflea being transferred to their own families, while the genera Georhizobium and ‘ Neopararhizobium ’ would form another family. However, a proposal for family-level demarcations in the order Rhizobiales is outside the scope of this work.
Proposal of a new genus encompassing the species R. oryzae and ‘ R. rhizosphaerae ’
In a recent study presenting a phylogeny of 571 Rhizobiaceae and Aurantimonadaceae strains (ML tree based on 155 concatenated core proteins) [28], the type strains of the species R. oryzae ( Allorhizobium oryzae ) [29] and ‘ R. rhizosphaerae ’ formed a well-delineated clade (with 100 % bootstrap support) that was clearly separated from the closest validly published genus type, i.e. Pararhizobium giardinii strain H152T. This pattern was also evident from an ML phylogeny of 797 Rhizobiaceae produced in another study based on the concatenation of 120 near-universal bacterial core genes [6]. The analyses presented in the current study further support the separation of the R. oryzae /‘ R. rhizosphaerae ’ clade (RoRr clade; two species type strains) not only from the Pararhizobium clade (four species type strains), but also from a sister clade consisting solely of rhizobial strains from unnamed species (unRhsp clade; including Rhizobium sp. strains Leaf383, Leaf371, 9140 and NFR03) (Fig. 1); all three clades in the phylogenetic tree are supported by 100 % bootstrap values. All within-clade pairwise cpAAI values were above 85.7, 91 and 94 % for the Pararhizobium clade, RoRr clade, and the unnamed Rhizobium clade, respectively (Fig. 3). In contrast, all pairwise cpAAI values between the RoRr clade and the Pararhizobium or unRhsp clades were less than 81%, while pairwise cpAAI values between the Pararhizobium and unRhsp clades were below 83.5 % (Fig. 3). All three clades thus represent separate genera according to the criteria proposed above, and this remains true when the analysis is repeated with an expanded set of strains (Fig. S11). We therefore propose to define a new genus encompassing the RoRr clade, for which we propose the name Xaviernesmea (see below for formal description). As no strains belonging to the unRhsp clade have been deposited in any international culture collection, we leave the task of describing new species and genera within this clade to others who have access to these strains.
Taxonomy of the ‘ R. aggregatum complex’
The ‘ Rhizobium aggregatum complex’ was initially identified as a sister taxon of the genus Agrobacterium [29], with subsequent work demonstrating that it is instead located on a clade neighbouring the genus Allorhizobium [13]. Moreover, the latter study suggested that ‘ R. aggregatum complex’ includes members of the genus Ciceribacter and that it may represent a novel genus on the basis of phylogenetic and multiple OGRI data, although the authors advised that further investigation was required [13]. It was recently suggested that the ‘ R. aggregatum complex’ be split into two genera [30]. It was proposed that R. daejeonense , R. naphthalenivorans and R. selenitireducens be transferred to the genus Ciceribacter , while R. ipomoeae , Rhizobium rhizophilum , R. rosettiformans and R. wuzhouense be transferred to the novel genus Peteryoungia along with the novel species ‘ Peteryoungia desertarenae ’ [30]; some of these changes have recently been validated [31].
The analyses presented in the current study included 13 strains belonging to the ‘ R. aggregatum complex’ (Fig. 1). The genus demarcation framework proposed here supports the previous studies indicating that the ‘ R. aggregatum complex’ is separate from the genus Allorhizobium . A group of seven species that included all Peteryoungia species present in our analysis ( Rhizobium aggregatum DSM 1111T, ‘ Agrobacterium albertimagni ’ AOL15T, ‘Rhizobium glycinendophyticum’ CL12T, Peteryoungia ipomoeae shin9-1T, ‘ Peteryoungia rhizophila ’ 7209-2T, ‘ Peteryoungia rosettiformans ’ W3T and ‘ Peteryoungia wuzhouensis ’ W44T) formed a monophyletic group with 100 % bootstrap support (Fig. 1). All pairwise cpAAI values within this group were >88 %, while all pairwise cpAAI values against the other six ‘ R. aggregatum complex’ species were <84.7 % (Fig. 3). These results support the formation of the genus Peteryoungia [30], which should also include R. aggregatum , as well as ‘ A. albertimagni ’, and ‘R. glycinendophyticum’. We therefore propose that R. aggregatum be transferred to the genus Peteryoungia (see below for formal description). The species ‘ A. albertimagni ’ and ‘R. glycinendophyticum’ should also be transferred to Peteryoungia once their names are validly published.
The remaining six ‘ R. aggregatum complex’ species formed a monophyletic group that could be further sub-divided into two clades. One clade corresponded to a group of three Ciceribacter species including the genus type strain, while the other clade contained R. daejeonense DSM 17795T, C. naphthalenivorans TSY03bT and C. selenitireducens ATCC BAA-1503T (Fig. 1). All within-group cpAAI values were >86.5 % while all between-group cpAAI values were ≤85.4 %, providing support for these two clades representing separate genera. However, the bootstrap support for the split of these two clades in the phylogeny is only 80 %, and the topology of the tree in this region (Fig. 1) differs from the tree reported by Rahi et al., wherein R. daejeonense , C. naphthalenivorans and C. selenitireducens were not monophyletic (see Fig. 2 of [30]). Overall, the data presented here are not in agreement with R. daejeonense , C. naphthalenivorans and C. selenitireducens belonging to the genus Ciceribacter . Instead, we propose this clade be referred to as the ‘ R. daejeonense complex’ pending further study – enabled by the availability of additional genomes of strains belonging to these clades – to resolve whether these species belong to the genus Ciceribacter or whether they should be transferred to a novel genus.
Proposal for the emendation of the genus Sinorhizobium as a distinct genus from Ensifer
Taxonomy of the genus Ensifer / Sinorhizobium has been the subject of discussion since the early 2000s. The genus Ensifer was proposed in 1982 to describe Ensifer adhaerens , a bacterial predator [9]. Subsequently, the genus Sinorhizobium was proposed in 1988 when Rhizobium fredii was reclassified as Sinorhizobium fredii [10], which was followed by the emendation of this genus by de Lajudie et al. in 1994 [32]. In 2002, as the 16S rRNA gene sequence of E. adhaerens became available, the Subcommittee on the Taxonomy of Agrobacterium and Rhizobium (hereafter ‘the subcommittee’) of the International Committee on Systematics of Prokaryotes (ICSP) noted that this taxon is a part of Sinorhizobium [33]. Although the subcommittee pointed out that the name Ensifer has priority, conservation of the name Sinorhizobium was endorsed in contravention of the rules of the International Code of Nomenclature of Prokaryotes (ICNP). Neighbour-joining trees reconstructed from 16S rRNA gene sequences or partial recA gene sequences, together with phenotypic data, provided further data interpreted as supporting the synonymy and unification of the genera Sinorhizobium and Ensifer , leading Willems et al. to propose the new combination ‘ Sinorhizobium adhaerens ’ [34]. Accordingly, in their Request for an Opinion to the Judicial Commission, Willems et al. officially proposed to conserve the name Sinorhizobium [34]. As the primary argument for conservation of the name Sinorhizobium , the authors indicated that the name Ensifer would cause misunderstanding and confusion in the scientific community. A few months later, in a Request for an Opinion to the Judicial Commission, J. M. Young argued that Ensifer , not Sinorhizobium , was the valid name for the unified genus, as Ensifer had priority [35]. At the same time, J. M. Young emended the description of the genus Ensifer , and transferred previously described Sinorhizobium species to this genus [35]. The Judicial Commission of the ICSP (Judicial Opinion 84) later confirmed that Ensifer had priority over Sinorhizobium , pointed out that the name ‘ Sinorhizobium adhaerens ’ is not validly published, and supported the transfer of members of the genus Sinorhizobium to Ensifer [36]. In this Opinion, it was claimed that the transfer of the members of the genus Sinorhizobium to the genus Ensifer would not cause confusion. The subcommittee, however, disagreed with this justification [37]. J. M. Young criticized these actions of the subcommittee [38], which was also later acknowledged by Tindall [39]. As predicted by Willems et al. [34], adoption of the genus name Ensifer continues to be met with resistance from many rhizobiologists [40].
Earlier phylogenetic studies noted that E. adhaerens was an outgroup of the genus Ensifer [41], providing some support that E. adhaerens represented a distinct genus; however, it was suggested that further evidence would be required prior to redefining genera within this clade [41]. Significant phylogenomic and phenotypic data now exists providing strong evidence that the genera Ensifer and Sinorhizobium as defined Casida 1982 [9] and Chen et al. 1988 [10], respectively, refer to closely related, yet separate, taxa. At least seven studies, including the Genome Taxonomy Database, have presented phylogenetic trees containing two well-defined clades within the genus Ensifer [21, 28, 42–46]. These phylogenies were built on the basis of gene (up to 1652 genes) or protein (up to 155 proteins) sequences using ML or Bayesian inference analysis approaches, indicating that the observed clades are robust to the choice of phylogenetic approach. Notably, our recent study presents an ML phylogeny where the genus Ensifer is split into two clades of 12 and 20 genospecies with 100 % bootstrap support for the split, which we then defined as the ‘nonsymbiotic’ and ‘symbiotic’ clades, respectively [21]. The split is also observed in an ML phylogeny of the 16S rRNA genes of the same strains, with 62 % bootstrap support (Fig. S12). We similarly see a split of the genus Ensifer into two clades of three species type strains (including E. adhaerens Casida AT, the type strain of the type species of the genus Ensifer Casida 1982) and 12 species type strains (including E. fredii USDA 205T, the type strain of the type species of the genus Sinorhizobium Chen et al. 1988) in our core-genome gene phylogeny, with 100 % bootstrap support (Fig. 1), representing the nonsymbiotic and symbiotic clades, respectively. However, all between-clade cpAAI values were above the suggested 86 % threshold as a baseline criterion for genus delimitation. Despite this, and following our proposed framework, we argue that there is sufficient other genomic and phenotypic data supporting the division of this genus (cf. Figs 3–5 of [21]). We describe the distinctive traits and respective synapomorphies of these clades in Table 1 and below.
Table 1.
Characteristics differentiating the previously-defined nonsymbiotic and symbiotic clades of the genus Ensifer , corresponding to the emended genera Ensifer and Sinorhizobium , respectively
|
Characteristic |
Nonsymbiotic clade (emended genus Ensifer ) |
Symbiotic clade (emended genus Sinorhizobium ) |
Reference |
|---|---|---|---|
|
GANTC sites per kb |
0.9–1.3 (mean: 1.06) |
1.5 to 1.8 (mean: 1.70) |
[47] |
|
Number of CDS |
5816–7682 (mean: 6876) |
5516 to 8629 (mean: 6550) |
[21] |
|
Ribosomal RNA operons |
5 |
3 |
[21] |
|
Carries nod and nif genes |
No* |
Yes |
[21] |
|
Bacterial predation ability |
Yes |
No |
|
|
Division by budding |
Yes |
No |
[50] |
|
Growth in unmodified LB medium |
Yes |
Poor |
[21] |
|
Starch hydrolysis |
Yes |
No |
[51] |
|
Growth at 37 ˚C |
No (generally) |
Yes (generally) |
[21] |
|
Fatty acids |
More C16 : 0 3OH |
More C18 : 1 ω9c |
[51] |
|
Carbon sources used (Biolog PM1/PM2) |
69–87 (mean: 81) |
50–81 (mean: 65) |
[21] |
|
pH tolerance (Biolog PM9) |
Better low pH tolerance |
Better high pH tolerance |
[21] |
*Except for the species E. sesbaniae, whose nine strains are legume symbionts.
The genome-wide frequency of the pentanucleotide GANTC is higher in all genomes of the symbiotic clade compared to all genomes of the nonsymbiotic clade, with a statistically significant average difference of 60 % (1.70 vs 1.06 GANTC sites per kb, P-value<1×10−10 using a two-sample t-test) [47]. As the GANTC motif is methylated by the highly conserved cell cycle-regulated methyltransferase CcrM [48, 49], this difference may reflect an important difference in the cell cycle biology of these two clades [47]. Indeed, species of the nonsymbiotic clade ( E. adhaerens and E. morelensis ) are capable of division by budding, unlike species of the symbiotic clade [50]. It has also been shown that the ability to hydrolyze starch [51] and robustly grow in LB broth lacking Ca2+ and Mg2+ ion supplementation [21] is specific to the nonsymbiotic clade. Stress tolerance of the two clades also differs (based on an analysis of 10 representative strains), with strains of the nonsymbiotic clade generally being more tolerant to alkaline conditions while strains of the symbiotic clade were generally more acid-tolerant and heat-tolerant [21]. Although many catabolic abilities could be found in at least a subset of each clade, which is unsurprising given both clades have open pangenomes, species of the nonsymbiotic clade are capable of catabolizing an average of 81 (out of 190 tested) carbon sources compared to an average of 65 for the symbiotic clade [21]. These differences in general phenotypic traits, together with the additional genomic and phenotypic differences outlined in Table 1, indicate marked differences in the biology of strains from these two clades. Indeed, at least two genospecies of the nonsymbiotic clade have been described as bacterial predators [9, 52, 53], a lifestyle that has not been attributed to any members of the symbiotic clade. Moreover, these two clades display significant differences in relation to their interactions with plant species, specifically, a biased distribution of the nod and nif genes required for establishment of nitrogen-fixing symbiosis with legumes [21, 45]. A recent study showed that whereas the core nodABC and nifHDK genes were present in strains from all 20 genospecies of the symbiotic clade, they were observed in just one of the 12 genospecies belonging the nonsymbiotic clade ( E. sesbaniae , with all nine reported strains, isolated from three different geographic origins, being symbiotic) [21, 51]. Symbiotic traits are linked to the presence of an accessory megaplasmid in the genome, and thus should not be considered relevant in delineating taxa [7]. However, this almost unique ability of genomes from the symbiotic clade to host symbiotic megaplasmids with respect to their relatives from the nonsymbiotic clade likely reflects differences in their genetic background. These discrepancies in symbiotic potential could thus be interpreted as a further marker of differentiated biology between these two clades.
Taken together, these genomic and phenotypic data suggest that the organisms in these two clades significantly differ in their biology and ecology, reminiscent of the stable ecotype model for bacterial species [54]. Notably, the type species of the genus Ensifer ( E. adhaerens ) is found within the nonsymbiotic clade, while the original type species of the genus Sinorhizobium ( S. fredii ) is found within the symbiotic clade. Given we established the taxonomic position of these type species-containing clades to be well separated, we argue that the proposal of Willems et al. [34] to unify the genera Ensifer Casida 1982 and Sinorhizobium Chen et al. 1988, and the Judicial Opinion 84 enacting the transfer of the members of the genus Sinorhizobium to the genus Ensifer [36], are no longer supported. Instead, we propose that Ensifer Casida 1982 and Sinorhizobium Chen et al. 1988 refer to closely related sister genera, of which Ensifer and Sinorhizobium , respectively, are the legitimate names in accordance with Rules 51a and 23a of the ICNP [55]. We note that the subcommittee has previously indicated support for this proposal [56], while also stating they are not in favour of creating subgenera for these taxa [40]. Formal genus and species emendations and circumscriptions are provided below.
Taxonomy of the genus Neorhizobium
More study is required to resolve the taxonomic relationships between the ‘Neorhizobium sensu stricto’ clade (Fig. 1) – which includes N. vignae , N. alkalisolii, N. hautlense, N. galegae and N. tomejilense , as well as Rhizobium petrolearium – and related taxa. The core-genome gene phylogeny (Fig. 1) and the cpAAI data (Fig. 3) suggest that ‘Neorhizobium lilium’ represents a new genus, as does the clade formed by Neorhizobium sp. NCHU2750, Rhizobium smilacinae , and Rhizobium cellulosilyticum . However, because bootstrap values provided only moderate support for the topology of the tree in the extended Neorhizobium clade and the clades were not well-resolved by the cpAAI data, we defer the proposal of new genera until publication of further genomic evidence.
Additional taxonomic implications of the proposed framework for genus delimitation
R. petrolearium DSM 26482T formed a clade with ‘Neorhizobium sensu stricto’ (Fig. 1). Pairwise cpAAI values were all >90 % when R. petrolearium was compared against ‘Neorhizobium sensu stricto’ species type strains (Fig. 3). We therefore propose that R. petrolearium be transferred to the genus Neorhizobium (see below for formal description).
Rhizobium tarimense CCTC AB 2011022T formed a clade with the genus Pseudorhizobium (Fig. 1). Pairwise cpAAI values were all >88 % when R. tarimense was compared against the four Pseudorhizobium species type strains, but <85 % when compared against all other species (Fig. 3). We therefore propose that R. tarimense be transferred to the genus Pseudorhizobium (see below for formal description).
Rhizobium arenae MIM27T formed a clade with the genus Pararhizobium . Pairwise cpAAI values were 87.4, 87.4 and 85.7 % when R. arenae was compared against the three Pararhizobium species type strains, but <84 % when compared against all other species (Fig. 3). We therefore propose that R. arenae be transferred to the genus Pararhizobium (see below for formal description).
Rhizobium azooxidifex DSM 100211T formed a clade with ‘ Mycoplana subbaraonis ’ JC85T and Mycoplana dimorpha DSM 7138T (Fig. 1). Pairwise cpAAI values between these three species type strains were all >89 %, while cpAAI values against strains outside of this clade were all <85 % (Fig. 3). We therefore propose that R. azooxidifex be transferred to the genus Mycoplana (see below for formal description).
Rhizobium yantingense CCTCC AB 2014007T formed a clade with Endobacterium cereale (Fig. 1). The pairwise cpAAI value between these two species was >95 %, while cpAAI values against strains outside of this clade were all <84 % (Fig. 3). We therefore propose that R. yantingense be transferred to the genus Endobacterium (see below for formal description).
To confirm the distinct taxonomic positions of the above-mentioned species and support their transfer to the respective genera, we compared them to other genus members using ANIb and dDDH indices. These indices are regarded as standard measures of relatedness between prokaryotic species that were widely used for species delimitation [57, 58]. In all cases, the obtained values were clearly below the thresholds for species delimitation (95–96 % for ANI or 70 % for DDH) (Datasets S2 and S3), confirming the authenticity of these species.
In addition, the following species are candidates as type species for new genera: ‘R. album’, R. populii, R. borbori and R. halophytocola . The reclassification of R. album into a new genus was also suggested by Young et al. [6]. Moreover, R. helianthi CGMC 1.12192T, R. rhizoryzae DSM 29514T and Allorhizobium pseudoryzae DSM 19479T formed a monophyletic group as a sister taxon to the genus Endobacterium (Fig. 1). This clade of three species type strains is another candidate for reclassification as a new genus as pairwise cpAAI values within the clade were all >86.5 % while all cpAAI values with species outside of the clade were all <83 % (Fig. 3).
Description of Xaviernesmea gen. nov.
Xaviernesmea (gza.vje.nem’e.a.; N.L. fem. n., in honour of Dr. Xavier Nesme, taxonomist of agrobacteria and rhizobia who pioneered the use of reverse ecology approaches to infer the ecology of Agrobacterium genomic species from comparative genomic analyses [8]).
Cells are Gram-negative, rod-shaped, and aerobic. Oxidase- and catalase-positive. Can utilize adonitol, raffinose and succinic acid. The pH range for growth is pH 5.0–11.0 [59]. The G+C content of the genomic DNA is in the range 62.8–64.7 mol%. The genus Xaviernesmea has been separated from other Rhizobiaceae genera based on a core-genome phylogeny and whole- and core-proteome relatedness indices (wpAAI and cpAAI).
The type species is Xaviernesmea oryzae.
Description of Xaviernesmea oryzae comb. nov.
Xaviernesmea oryzae (o.ry’zae. L. gen. fem. n. oryzae, of rice, referring to the host of isolation of the type strain).
Basonym: Rhizobium oryzae Peng et al. 2008 [60].
Homotypic synonym: Allorhizobium oryzae (Peng et al., 2008) Mousavi et al. 2015.
The description is as provided by Peng et al. 2008 [60] and Mousavi et al. 2015 [29]. X. oryzae can be differentiated from other species of the genus Xaviernesmea based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.8 mol%. Its approximate genome size is 5.39 Mbp.
The type strain is Alt 505T (=LMG 24253T=CGMCC 1.7048T), isolated from Oryza alta growing in the Wild Rice Core Collection Nursery of South China Agricultural University. The NCBI RefSeq Assembly accession number for the genome sequence is GCF_900109605.1.
Description of Xaviernesmea rhizosphaerae sp. nov.
Xaviernesmea rhizosphaerae (rhi.zo.sphae’rae. N.L. gen. fem. n. rhizosphaerae, of the rhizosphere, referring to host plant compartment of isolation of the type strain).
The description is as provided by Zhao et al. [59]. X. rhizosphaerae can be differentiated from other species of the genus Xaviernesmea based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 64.7 mol%. Its approximate genome size is 5.18 Mbp.
The type strain is MH17T (=ACCC 19963T=KCTC 52414T), which was isolated from the roots of rice collected from Beijing, PR China. The NCBI RefSeq assembly accession number for the genome sequence is GCF_001938945.1. We note that the name ‘ Rhizobium rhizosphaerae ’ that was proposed in the original publication [59] has yet to be validly published.
Emended description of the genus Ensifer Casida 1982
The description is as given by Casida 1982 [9] with the following emendations. The optimal growth temperature is 27–28 °C. Some species can grow at 37 °C. Capable of growth in unmodified lysogeny broth (LB). Capable of hydrolysing starch. Resistant to multiple antibiotics including ampicillin and erythromycin. The genomic G+C content is ~61–63 mol%. The genomic GANTC pentanucleotide frequency is ~0.9–1.3 sites per kb. Most strains carry five rRNA operons. The genus can be differentiated from other genera based on core-genome gene phylogenies.
The type species is Ensifer adhaerens .
The emended genus contains the species E. adhaerens , E. morelensis and E. sesbaniae .
The species E. alkalisoli , E. americanum , E. arboris , E. fredii , E. garamanticum, E. glycinis , E. kostiensis , E. kummerowiae , E. medicae , E. meliloti , E. mexicanum, E. numidicus , E. psoraleae , E. saheli , E. sofinae, E. sojae and E. terangae are transferred to the genus Sinorhizobium .
Emended description of the genus Sinorhizobium Chen et al. 1988 emend. de Lajudie et al. 1994
The description is as given by de Lajudie et al. [32] with the following emendations, drawing also from Young [35]. The optimal growth temperature is 25–33 °C, but some strains can grow at 12 °C and others can grow at 44 °C. Optimum pH is 6–7, but some strains can grow at pH 5.0 and others at pH 10.0. Starch is not utilized. Ammonium salts, nitrate, nitrite, and many amino acids can serve as nitrogen sources for most strains. Most strains produce cytochrome oxidase and catalase. The genomic G+C content is ~61–64 mol%. The genomic GANTC pentanucleotide frequency is ~1.5–1.8 sites per kb. Most strains carry three rRNA operons. The genus can be differentiated from other genera based on core-genome gene phylogenies.
The type species is Sinorhizobium fredii .
The emended genus contains the species S. alkalisoli, S. americanum , S. arboris , S. fredii , S. garamanticum, S. glycinis, S. kostiense , S. kummerowiae , S. medicae , S. meliloti , S. mexicanum, S. numidicum, S. psoraleae, S. saheli , S. sofinae, S. sojae and S. terangae .
Description of Sinorhizobium alkalisoli comb. nov.
Sinorhizobium alkalisoli (al.ka.li.so’li. N.L. neut. n. alkali, alkali (from Arabic al-qaliy); L. neut. n. solum, soil; N.L. gen. neut. n. alkalisoli, of alkaline soil, referring to the saline-alkali soil where the bacterium was isolated).
Basonym: Ensifer alkalisoli Li et al. 2016.
The description is as provided by Li et al. 2016 [61]. S. alkalisoli can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.2 mol%. Its approximate genome size is 6.13 Mbp.
The type strain is YIC4027T (=HAMBI 3655T=LMG 29286T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_001723275.1.
Emended description of Sinorhizobium americanum Toledo et al. 2004
Sinorhizobium americanum (a.me.ri.ca’num. N.L. neut. adj. americanum, American, referring to the isolation of the type strain from the Colorado Plateau).
Homotypic synonym: Ensifer americanus (Toledo et al. 2004) Wang et al. 2015 emend. Hördt et al. 2020.
The description is as provided by Hördt et al. 2020 [5]. S. americanum can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.3 mol%. Its approximate genome size is 6.75 Mbp.
The type strain is CFNEI 156T (=ATCC BAA-532T=CIP 108390T=DSM 15007T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_001651855.1.
Emended description of Sinorhizobium arboris Nick et al. 1999
Sinorhizobium arboris (ar’bo.ris. L. fem. n. arbour, tree; L. gen. fem. n. arboris, of a tree).
Homotypic synonym: Ensifer arboris (Nick et al. 1999) Young 2003 emend. Hördt et al. 2020.
The description is as provided by Hördt et al. 2020 [5]. S. arboris can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.0 mol%. Its approximate genome size is 6.85 Mbp.
The type strain is HAMBI 1552T (=ATCC BAA-226T=DSM 13375T=LMG 14919T=NBRC 100383T=TTR 38T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_000427465.1.
Emended description of Sinorhizobium fredii (Scholla and Elkan 1984) Chen et al. 1988
Sinorhizobium fredii (fred'i.i. N.L. gen. neut. n. fredii, of Fred, named after of E.B. Fred).
Homotypic synonym: Ensifer fredii (Scholla and Elkan 1984) Young 2003 emend. Hördt et al. 2020.
Heterotypic synonym: Ensifer xinjiangensis (Chen et al. 1988) Young 2003 [43]
The description is as provided by Hördt et al. 2020 [5]. S. fredii can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.2 mol%. Its approximate genome size is 7.15 Mbp.
The type strain is USDA 205T (=ATCC 35423T=CCUG 27877T=DSM 5851T=HAMBI 2075T=ICMP 11139T=IFO 14780T=JCM 20967T=LMG 6217T=NBRC 14780T=NRRL B-14241T=NRRL B-14594T=PRC 205T). The NCBI RefSeq Assembly accession number for the genome sequence is GCF_001461695.1.
Description of Sinorhizobium garamanticum comb. nov.
Sinorhizobium garamanticum (ga.ra.man’ti.cum. N.L. neut. adj. garamanticum, pertaining to Garamante, Garamantian, the country of Garamantes, from which the strains were isolated).
Basonym: Ensifer garamanticus Merabet et al. 2010.
The description is as provided by Merabet et al. 2010 [62]. S. garamanticum can be differentiated from other species of the genus Sinorhizobium by phylogenetic analysis based on several housekeeping (recA, glnA, gltA, thrC and atpD) genes and 16S rRNA gene sequencing. The genomic G+C content of the type strain is approximately 62.4 mol% (HPLC).
The type strain is ORS 1400T (=CIP 109916T=LMG 24692T).
Description of Sinorhizobium glycinis comb. nov.
Sinorhizobium glycinis (gly.ci’nis. N.L. gen. n. glycinis, of the botanical genus Glycine, the soybean, named for its nodulation characteristics and symbiotic genes).
Basonym: Ensifer glycinis Yan et al. 2016.
The description is as provided by Yan et al. 2016 [63]. S. glycinis can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.4 mol%. Its approximate genome size is 6.04 Mbp.
The type strain is CCBAU 23380T (=HAMBI 3645T=LMG 29231T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_001651865.1.
Emended description of Sinorhizobium kostiense Nick et al. 1999
Sinorhizobium kostiense (kos.ti.en’se. N.L. neut. adj. kostiense, pertaining to Kosti, the region in Sudan where most of these organisms have been isolated).
Homotypic synonym: Ensifer kostiensis (Nick et al. 1999) Young 2003.
The description is as provided by Nick et al. 1999 [64]. S. kostiense can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.7 mol%. Its approximate genome size is 6.33 Mbp.
The type strain is DSM 13372T (=ATCC BAA-227T=HAMBI 1489T=LMG 15613T=LMG 19227T=NBRC 100382T=TTR 15T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_017874595.1.
Emended description of Sinorhizobium kummerowiae Wei et al. 2002
Sinorhizobium kummerowiae (kum.me.ro’wi.ae. N.L. gen. fem. n. kummerowiae, of Kummerowia, a genus of leguminous plants, referring to the host from which the bacterium was isolated).
Homotypic synonym: Ensifer kummerowiae (Wei et al. 2002) Young 2003.
The description is as provided by Wei et al. 2002 [65]. S. kummerowiae can be differentiated from other species of the genus Sinorhizobium by phylogenetic analysis based on 16S rRNA gene sequences. The genomic G+C content of the type strain is approximately 61.6 mol% (Tm).
The type strain is CCBAU 71714T (=CGMCC 1.3046T=CIP 108026T=NBRC 100799T).
Emended description of Sinorhizobium medicae Rome et al. 1996
Sinorhizobium medicae (me’di.cae. L. gen. fem. n. medicae, of/from lucerne (plant belonging to the genus Medicago).
Homotypic synonym: Ensifer medicae (Rome et al. 1996) Young 2003.
The description is as provided by Rome et al. 1996 [66]. S. medicae can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.2 mol%. Its approximate genome size is 6.53 Mbp.
The type strain is A 321T (=11-3 21 aT=HAMBI 2306T=ICMP 13798T=LMG 19920T=NBRC 100384T=USDA 1037T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_009599935.1.
Emended description of Sinorhizobium meliloti (Dangeard 1926) de Lajudie et al. 1994
Sinorhizobium meliloti (me.li.lo’ti. N.L. masc. n. Melilotus, generic name of sweet clover; N.L. gen. masc. n. meliloti, of Melilotus).
Homotypic synonym: Ensifer meliloti (Dangeard 1926) Young 2003.
The description is as provided by de Lajudie et al. 1994 [32]. S. meliloti can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.0 mol%. Its approximate genome size is 7.34 Mbp.
The type strain is USDA 1002T (=ATCC 9930T=CCUG 27879T=CFBP 5561T=DSM 30135T=HAMBI 2148T=ICMP 12623T=IFO 14782T=JCM 20682T=LMG 6133T=NBRC 14782T=NCAIM B.01520T=NRRL L-45T=NZP 4027T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_009601385.1.
Description of Sinorhizobium mexicanum comb. nov.
Sinorhizobium mexicanum (me.xi.ca’num. N.L. neut. adj. mexicanum, of or belonging to Mexico, where the strains were isolated).
Basonym: Ensifer mexicanus Lloret et al. 2011.
The description is as provided by Lloret et al. 2011 [67]. S. mexicanum can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.4 mol%. Its approximate genome size is 7.14 Mbp.
The type strain is ITTG R7T (=ATCC BAA-1312T=CFN ER1001T=CIP 109033T=DSM 18446T=HAMBI 2910T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_013488225.1.
Description of Sinorhizobium numidicum comb. nov.
Sinorhizobium numidicum (nu.mi’di.cum. N.L. neut. adj. numidicum, pertaining to the country of Numidia, Numidian, the Roman denomination of the region in North Africa from which the majority of the organisms were isolated).
Basonym: Ensifer numidicus Merabet et al. 2010.
The description is as provided by Merabet et al. 2010 [62]. S. numidicus can be differentiated from other species of the genus Sinorhizobium by phylogenetic analysis based on several housekeeping (recA, glnA, gltA, thrC and atpD) genes and 16S rRNA gene sequencing. The genomic G+C content of the type strain is approximately 62.8 mol% (HPLC).
The type strain is ORS 1407T (=CIP 109850T=LMG 27395T).
Description of Sinorhizobium psoraleae comb. nov.
Sinorhizobium psoraleae (pso.ra.le’ae N.L. gen. fem. n. psoraleae, of Psoralea, referring to the main host of the species).
Basonym: Ensifer psoraleae Wang et al. 2013.
The description is as provided by Wang et al. 2013 [51]. S. psoraleae can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.3 mol%. Its approximate genome size is 7.43 Mbp.
The type strain is CCBAU 65732T (=HAMBI 3286T=LMG 26835T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_013283645.1.
Emended description of Sinorhizobium saheli de Lajudie et al. 1994
Sinorhizobium saheli (sa’hel.i. N.L. gen. neut. n. saheli, of the Sahel, the region in Africa from which they were isolated).
Homotypic synonym: Ensifer saheli (de Lajudie et al. 1994) Young 2003 emend. Hördt et al. 2020.
The description is as provided by Hördt et al. 2020 [5]. S. saheli can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 63.6 mol%. Its approximate genome size is 5.99 Mbp.
The type strain is LMG 7837T (=ATCC 51690T=DSM 11273T=HAMBI 215T=ICMP 13648T=NBRC 100386T=ORS 609T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_001651875.1.
Description of Sinorhizobium shofinae comb. nov.
Sinorhizobium shofinae (sho.fi’nae. N.L. fem. gen. n. shofinae from Shofine, a company name, referring to the fact that the type strain of this species was isolated from root nodule of soybean grown in the farm of the company, Shandong Shofine Seed Technology Company Ltd., located in Jiaxiang County, Shandong Province of China).
Basonym: Ensifer shofinae Chen et al. 2017 emend. Hördt et al. 2020.
The description is as provided by Hördt et al. 2020 [5]. S. shofinae can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 59.9 mol%. Its approximate genome size is 6.21 Mbp.
The type strain is CCBAU 251167T (=HAMBI 3507T=LMG 29645T=ACCC 19939T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_001704765.1.
Description of Sinorhizobium sojae comb. nov.
Sinorhizobium sojae (so'ja.e. N.L. gen. n. sojae, of soja, of soybean, referring to the source of the first isolates).
Basonym: Ensifer sojae Li et al. 2011 emend. Hördt et al. 2020.
The description is as provided by Hördt et al. 2020 [5]. S. sojae can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 60.9 mol%. Its approximate genome size is 6.09 Mbp.
The type strain is CCBAU 5684T (=DSM 26426T=HAMBI 3098T=LMG 25493T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_000261485.1.
Emended description of Sinorhizobium terangae de Lajudie et al. 1994
Sinorhizobium terangae [te’ran.gae. N.L. n. terengae, hospitality (from West African Wolof n. terenga, hospitality); N.L. gen. n. terangae, of hospitality, intended to mean that this species is isolated from different host plants].
Homotypic synonym: Ensifer terangae (de Lajudie et al. 1994) Young 2003.
The description is as provided by de Lajudie et al. 1994 [32]. S. terangae can be differentiated from other species of the genus Sinorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.4 mol%. Its approximate genome size is 6.79 Mbp.
The type strain is SEMIA 6460T (=ATCC 51692T=DSM 11282T=HAMBI 220T=ICMP 13649T=LMG 7834T=NBRC 100385T=ORS 1009T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_014197705.1.
Description of Endobacterium yantingense comb. nov.
Endobacterium yantingense (yan. ting. en’se. N.L. neut. adj. yantingense referring to Yanting district, Sichuan Province, PR China, where the organism was isolated).
Basonym: Rhizobium yantingense Chen et al. 2015.
The description is as provided by Chen et al. 2015 [68]. E. yantingense can be differentiated from another species of the genus Endobacterium ( Endobacterium cereale corrig. Menéndez et al. 2021) based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 59.5 mol%. Its approximate genome size is 5.82 Mbp.
The type strain is H66T (=CCTCC AB 2014007T=LMG 28229T), which was isolated from the surfaces of weathered rock (purple siltstone) in Yanting (Sichuan, PR China). The JGI IMG accession number for the genome sequence is Ga0196656.
Description of Neorhizobium petrolearium comb. nov.
Neorhizobium petrolearium (pe.tro.le.a’ri.um. L. fem. n. petra, rock; L. neut. olearium related to oil, of or belonging to oil; N.L. neut. adj. petrolearium related to mineral oil).
Basonym: Rhizobium petrolearium Zhang et al. 2012.
The description is as provided by Zhang et al. 2012 [69]. N. petrolearium can be differentiated from another species of the genus Neorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 60.5 mol%. Its approximate genome size is 6.97 Mbp.
The type strain, SL-1T (=ACCC 11238T=KCTC 23288T), was isolated from petroleum-contaminated sludge samples in Shengli oilfield, Shandong Province, China. The JGI IMG accession number for the genome sequence is Ga0196653.
Description of Pararhizobium arenae comb. nov.
Pararhizobium arenae (a.re’nae. L. fem. gen. n. arenae of sand, the isolation source of the type strain).
Basonym: Rhizobium arenae Zhang et al. 2017.
The description is as provided by Zhang et al. 2017 [70]. P. arenae can be differentiated from another species of the genus Pararhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 59.8 mol%. Its approximate genome size is 4.94 Mbp.
The type strain is MIM27T (=KCTC 52299T=MCCC 1K03215T), isolated from sand of the Mu Us Desert, PR China. The NCBI RefSeq Assembly accession number for the genome sequence is GCF_001931685.1.
Description of Peteryoungia aggregata comb. nov.
Peteryoungia aggregata (ag.gre.ga’ta. L. fem. part. adj. aggregata, joined together, referring to the frequent formation of rosettes).
Basonym: Rhizobium aggregatum Kaur et al. 2011 [71].
Homotypic synonym: Blastobacter aggregatus Hirsch and Muller 1986 [72].
The description is as provided by Kaur et al. 2011 [71]. P. aggregata can be differentiated from another species of the genus Peteryoungia based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 62.7 mol%. Its approximate genome size is 4.81 Mbp.
The type strain is IFAM 1003T (= DSM 1111T=ATCC 43293T). The JGI IMG accession number for the genome sequence is Ga0196658.
Description of Pseudorhizobium tarimense comb. nov.
Pseudorhizobium tarimense (ta.rim.en’se. N.L. neut. adj. tarimense, pertaining to Tarim basin in Xinjiang Uyghur autonomous region of China, where the type strain was isolated).
Basonym: Rhizobium tarimense Turdahon et al. 2013.
The description is as provided by Turdahon et al. 2013 [73]. P. tarimense can be differentiated from other species of the genus Pseudorhizobium based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 61.2 mol%. Its approximate genome size is 4.83 Mbp.
The type strain is CCTCC AB 2011011T (=NRRL B-59556T=PL-41T). The JGI IMG accession number for the genome sequence is Ga0196649.
Description of Mycoplana azooxidifex comb. nov.
Mycoplana azooxidifex [a.zo.o.xi’di.fex. N.L. neut. n. azooxidum, dinitrogenmonoxide; L. masc. suff. -fex, the maker; N.L. masc. n. azooxidifex, the dinitrogenmonoxide maker (nominative in apposition)].
Basonym: Rhizobium azooxidifex Behrendt et al. 2016.
The description is as provided by Behrendt et al. 2016 [74]. M. azooxidifex can be differentiated from other species of the genus Mycoplana based on OGRI calculations (ANI and dDDH). The genomic G+C content of the type strain is 64.3 mol%. Its approximate genome size is 5.89 Mbp.
The type strain is DSM 100211T (=Po 20/26T=LMG 28788T). The NCBI RefSeq assembly accession number for the genome sequence is GCF_014196765.1.
Supplementary Data
Funding information
The work of N.K. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 429 677 233. Research in the G.C.D laboratory is supported by a NSERC Discovery Grant, and funding from Queen’s University. A.M. and C.F. are supported by MICRO4Legumes grant (Italian Ministry of Agriculture) and ALL-IN project (H2020 ERA-NETs SUSFOOD2 and CORE Organic Cofund, under the Joint SUSFOOD2/CORE Organic Call 2019). This research was funded in part by the Wellcome Trust Grant [206194]. For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Acknowledgements
The authors gratefully acknowledge Prof. George M. Garrity (Michigan State University, East Lansing, MI, USA) for valuable help interpreting the ICNP, and Prof. Peter Young for helpful feedback on the manuscript. This research was enabled, in part, through computational resources provided by Compute Ontario (computeontario.ca), Compute Canada (computecanada.ca), and BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI) (031A537B, 031A533A, 031A538A, 031A533B, 031A535A, 031A537C, 031A534A, 031A532B).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AAI, average amino-acid identity; ANI, average nucleotide identity; ANIb, average nucleotide identity based on blast; cpAAI, core-proteome average amino-acid identity; dDDH, digital DNA-DNA hybridization; GGDC, genome-to-genome distance calculator; HPLC, high performance liquid chromatography; ICNP, International Code of Nomenclature of Prokaryotes; ICSP, International Committee on Systematics of Prokaryotes; IMG, integrated microbial genomes; JGI, Joint Genome Institute; ML, maximum-likelihood; NCBI, National Center for Biotechnology; OGRI, overall genomic relatdness index; POCP, percentage of conserved proteins; RoRr, R.oryzae/'R. rhizosphaerae'; Tm, melting temperature; unRhsp, unnamed rhizobium species; wpAAI, whole-proteome average amino-acid identity.
Three supplementary datasets and twelve supplementary figures are available with the online version of this article.
References
- 1.Kuzmanovic N, Fagorzi C, Mengoni A, Lassalle F, diCenzo G. Taxonomy of rhizobiaceae revisited: proposal of a new framework for genus delimitation. Figshare. 2022 doi: 10.6084/m9.figshare.17076455.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn HJ. Taxonomic relationships of certain non-sporeforming rods in soil. J Bacteriol. 1938;36:320–321. [Google Scholar]
- 3.Alves LMC, de SJ, Varani A de M, Lemos E de M. The family Rhizobiaceae. The Prokaryotes. 2014:419–437. [Google Scholar]
- 4.Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70:5607–5612. doi: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hördt A, López MG, Meier-Kolthoff JP, Schleuning M, Weinhold L-M, et al. Analysis of 1,000+ type-strain genomes substantially improves taxonomic classification of alphaproteobacteria. Front Microbiol. 2020;11:468. doi: 10.3389/fmicb.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young JPW, Moeskjær S, Afonin A, Rahi P, Maluk M, et al. Defining the Rhizobium leguminosarum species complex. Genes. 2021;12:111. doi: 10.3390/genes12010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lajudie PM, Andrews M, Ardley J, Eardly B, Jumas-Bilak E, et al. Minimal standards for the description of new genera and species of rhizobia and agrobacteria. Int J Syst Evol Microbiol. 2019;69:1852–1863. doi: 10.1099/ijsem.0.003426. [DOI] [PubMed] [Google Scholar]
- 8.Lassalle F, Muller D, Nesme X. Ecological speciation in bacteria: reverse ecology approaches reveal the adaptive part of bacterial cladogenesis. Res Microbiol. 2015;166:729–741. doi: 10.1016/j.resmic.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Casida LE. Ensifer adhaerens gen. nov., sp. nov.: a bacterial predator of bacteria in soil. Int J Syst Bacteriol. 1982;32:339–345. doi: 10.1099/00207713-32-3-339. [DOI] [Google Scholar]
- 10.Chen WX, Yan GH, Li JL. Numerical taxonomic study of fast-growing soybean rhizobia and a proposal that Rhizobium fredii be assigned to Sinorhizobium gen. nov. Int J Syst Bacteriol. 1988;38:392–397. doi: 10.1099/00207713-38-4-392. [DOI] [Google Scholar]
- 11.Contreras-Moreira B, Vinuesa P. GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl Environ Microbiol. 2013;79:7696–7701. doi: 10.1128/AEM.02411-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinuesa P, Ochoa-Sánchez LE, Contreras-Moreira B. GET_PHYLOMARKERS, a software package to select optimal orthologous clusters for phylogenomics and inferring pan-genome phylogenies, used for a critical geno-taxonomic revision of the genus Stenotrophomonas . Front Microbiol. 2018;9:771. doi: 10.3389/fmicb.2018.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzmanović N, Biondi E, Overmann J, Puławska J, Verbarg S, et al. Revisiting the taxonomy of Allorhizobium vitis (i.e. agrobacterium vitis) using genomics - emended description of All. vitis sensu stricto and description of Allorhizobium ampelinum sp. nov. bioRxiv. 2020 doi: 10.1101/2020.12.19.423612. [DOI] [Google Scholar]
- 14.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 16.Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 17.Qin Q-L, Xie B-B, Zhang X-Y, Chen X-L, Zhou B-C, et al. A proposed genus boundary for the prokaryotes based on genomic insights. J Bacteriol. 2014;196:2210–2215. doi: 10.1128/JB.01688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagorzi C, Ilie A, Decorosi F, Cangioli L, Viti C, et al. Symbiotic and nonsymbiotic members of the genus Ensifer (syn. Sinorhizobium) are separated into two clades based on comparative genomics and high-throughput phenotyping. Genome Biol Evol. 2020;12:2521–2534. doi: 10.1093/gbe/evaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth JS, Whitman WB. Phylogenomic analyses of a clade within the Roseobacter group suggest taxonomic reassignments of species of the genera Aestuariivita, Citreicella, Loktanella, Nautella, Pelagibaca, Ruegeria, Thalassobius, Thiobacimonas and Tropicibacter, and the proposal of six novel genera. Int J Syst Evol Microbiol. 2018;68:2393–2411. doi: 10.1099/ijsem.0.002833. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 28.Lassalle F, Dastgheib SMM, Zhao F-J, Zhang J, Verbarg S, et al. Phylogenomics reveals the basis of adaptation of Pseudorhizobium species to extreme environments and supports a taxonomic revision of the genus. Syst Appl Microbiol. 2021;44:126165. doi: 10.1016/j.syapm.2020.126165. [DOI] [PubMed] [Google Scholar]
- 29.Mousavi SA, Willems A, Nesme X, de Lajudie P, Lindström K. Revised phylogeny of Rhizobiaceae: proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst Appl Microbiol. 2015;38:84–90. doi: 10.1016/j.syapm.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Rahi P, Khairnar M, Hagir A, Narayan A, Jain KR, et al. Peteryoungia gen. nov. with four new species combinations and description of Peteryoungia desertarenae sp. nov., and taxonomic revision of the genus Ciceribacter based on phylogenomics of Rhizobiaceae . Arch Microbiol. 2021;203:3591–3604. doi: 10.1007/s00203-021-02349-9. [DOI] [PubMed] [Google Scholar]
- 31.Oren A, Garrity GM. Valid publication of new names and new combinations effectively published outside the ijsem. Int J Syst Evol Microbiol. 2021;71:005096. doi: 10.1099/ijsem.0.005096. [DOI] [PubMed] [Google Scholar]
- 32.De Lajudie P, Willems A, Pot B, Dewettinck D, Maestrojuan G, et al. Polyphasic taxonomy of rhizobia: emendation of the genus Sinorhizobium and description of Sinorhizobium meliloti comb. nov., Sinorhizobium saheli sp. nov., and Sinorhizobium teranga sp. nov. Int J Syst Bacteriol. 1994;44:715–733. doi: 10.1099/00207713-44-4-715. [DOI] [Google Scholar]
- 33.Lindstrom K, Martinez-Romero ME. International Committee on Systematics of Prokaryotes: Subcommittee on the taxonomy of Agrobacterium and Rhizobium . Int J Syst Evol Microbiol. 2002;52:2337. doi: 10.1099/ijs.0.02524-0. [DOI] [PubMed] [Google Scholar]
- 34.Willems A, Fernández-López M, Muñoz-Adelantado E, Goris J, De Vos P, et al. Description of new Ensifer strains from nodules and proposal to transfer Ensifer adhaerens Casida 1982 to Sinorhizobium as Sinorhizobium adhaerens comb. nov. Request for an opinion. Int J Syst Evol Microbiol. 2003;53:1207–1217. doi: 10.1099/ijs.0.02264-0. [DOI] [PubMed] [Google Scholar]
- 35.Young JM. The genus name Ensifer Casida 1982 takes priority over Sinorhizobium Chen et al. 1988, and Sinorhizobium morelense Wang et al. 2002 is a later synonym of Ensifer adhaerens Casida 1982. Is the combination “Sinorhizobium adhaerens” (Casida 1982) Willems et al. 2003 legitimate? Request for an Opinion. Int J Syst Evol Microbiol. 2003;53:2107–2110. doi: 10.1099/ijs.0.02665-0. [DOI] [PubMed] [Google Scholar]
- 36.Judicial Commission of the International Committee on Systematics of Prokaryotes The genus name Sinorhizobium Chen et al. 1988 is a later synonym of Ensifer Casida 1982 and is not conserved over the latter genus name, and the species name “Sinorhizobium adhaerens” is not validly published. Opinion 84. Int J Syst Evol Microbiol. 2008;58:1973. doi: 10.1099/ijs.0.2008/005991-0. [DOI] [PubMed] [Google Scholar]
- 37.Lindström K, Young JPW. International Committee on Systematics of Prokaryotes; Subcommittee on the Taxonomy of Agrobacterium and Rhizobium: minutes of the meetings, 31 August 2008, Gent, Belgium. Int J Syst Evol Microbiol. 2009;59:921–922. doi: 10.1099/ijs.0.012583-0. [DOI] [PubMed] [Google Scholar]
- 38.Young JM. Sinorhizobium versus Ensifer: may a taxonomy subcommittee of the ICSP contradict the Judicial Commission? Int J Syst Evol Microbiol. 2010;60:1711–1713. doi: 10.1099/ijs.0.025163-0. [DOI] [PubMed] [Google Scholar]
- 39.Tindall BJ. The correct name of the taxon that contains the type strain of Rhodococcus equi . Int J Syst Evol Microbiol. 2014;64:302–308. doi: 10.1099/ijs.0.059584-0. [DOI] [PubMed] [Google Scholar]
- 40.de Lajudie P, Young JPW. International Committee on Systematics of Prokaryotes Subcommittee on the Taxonomy of Rhizobia and Agrobacteria Minutes of the meeting by video conference, 11 July 2018. Int J Syst Evol Microbiol. 2019;69:1835–1840. doi: 10.1099/ijsem.0.003335. [DOI] [PubMed] [Google Scholar]
- 41.Martens M, Delaere M, Coopman R, De Vos P, Gillis M, et al. Multilocus sequence analysis of Ensifer and related taxa. Int J Syst Evol Microbiol. 2007;57:489–503. doi: 10.1099/ijs.0.64344-0. [DOI] [PubMed] [Google Scholar]
- 42.Kumar HKS, Gan HM, Tan MH, Eng WWH, Barton HA, et al. Genomic characterization of eight Ensifer strains isolated from pristine caves and a whole genome phylogeny of Ensifer (Sinorhizobium). J Genomics 2017;5:12–15. 10.7150/jgen.17863. [DOI] [PMC free article] [PubMed]
- 43.Martens M, Dawyndt P, Coopman R, Gillis M, De Vos P, et al. Advantages of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium) Int J Syst Evol Microbiol. 2008;58:200–214. doi: 10.1099/ijs.0.65392-0. [DOI] [PubMed] [Google Scholar]
- 44.diCenzo GC, Debiec K, Krzysztoforski J, Uhrynowski W, Mengoni A, et al. Genomic and biotechnological characterization of the heavy-metal resistant, arsenic-oxidizing bacterium Ensifer sp. M14. Genes. 2018;9:E379. doi: 10.3390/genes9080379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrido-Oter R, Nakano RT, Dombrowski N, Ma K-W, AgBiome Team. et al. Modular traits of the rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe. 2018;24:155–167. doi: 10.1016/j.chom.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 47.diCenzo GC, Cangioli L, Nicoud Q, Cheng JHT, Blow MJ, et al. DNA methylation in Ensifer species during free-living growth and during nitrogen-fixing symbiosis with Medicago spp. mSystems. 2022;7:e01092–21. doi: 10.1128/mSystems.01092-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zweiger G, Marczynski G, Shapiro L. A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J Mol Biol. 1994;235:472–485. doi: 10.1006/jmbi.1994.1007. [DOI] [PubMed] [Google Scholar]
- 49.Wright R, Stephens C, Shapiro L. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus . J Bacteriol. 1997;179:5869–5877. doi: 10.1128/jb.179.18.5869-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang ET, Tan ZY, Willems A, Fernández-López M, Reinhold-Hurek B, et al. Sinorhizobium morelense sp. nov., a Leucaena leucocephala-associated bacterium that is highly resistant to multiple antibiotics. Int J Syst Evol Microbiol. 2002;52:1687–1693. doi: 10.1099/00207713-52-5-1687. [DOI] [PubMed] [Google Scholar]
- 51.Wang YC, Wang F, Hou BC, Wang ET, Chen WF, et al. Proposal of Ensifer psoraleae sp. nov., Ensifer sesbaniae sp. nov., Ensifer morelense comb. nov. and Ensifer americanum comb. nov. Syst Appl Microbiol. 2013;36:467–473. doi: 10.1016/j.syapm.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Wilks M. Predation Mediated Carbon Turnover in Nutrient-Limited Cave Environments. MSc Thesis. University of Akron; 2013. [Google Scholar]
- 53.Martin MO. Predatory prokaryotes: an emerging research opportunity. J Mol Microbiol Biotechnol. 2002;4:467–477. [PubMed] [Google Scholar]
- 54.Cohan FM. Towards a conceptual and operational union of bacterial systematics, ecology, and evolution. Philos Trans R Soc Lond B Biol Sci. 2006;361:1985–1996. doi: 10.1098/rstb.2006.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker CT, Tindall BJ, Garrity GM. International Code of Nomenclature of Prokaryotes. Int J Syst Evol Microbiol. 2015;65:4284–4287. doi: 10.1099/ijsem.0.000664. [DOI] [PubMed] [Google Scholar]
- 56.de Lajudie P, Mousavi SA, Young JPW. International Committee on Systematics of Prokaryotes Subcommittee on the Taxonomy of Rhizobia and Agrobacteria Minutes of the closed meeting by videoconference, 6 July 2020. Int J Syst Evol Microbiol. 2021;71:004784. doi: 10.1099/ijsem.0.004784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meier-Kolthoff JP, Klenk H-P, Göker M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int J Syst Evol Microbiol. 2014;64:352–356. doi: 10.1099/ijs.0.056994-0. [DOI] [PubMed] [Google Scholar]
- 59.Zhao J-J, Zhang J, Zhang R-J, Zhang C-W, Yin H-Q, et al. Rhizobium rhizosphaerae sp. nov., a novel species isolated from rice rhizosphere. Antonie van Leeuwenhoek. 2017;110:651–656. doi: 10.1007/s10482-017-0831-9. [DOI] [PubMed] [Google Scholar]
- 60.Peng G, Yuan Q, Li H, Zhang W, Tan Z. Rhizobium oryzae sp. nov., isolated from the wild rice Oryza alta . Int J Syst Evol Microbiol. 2008;58:2158–2163. doi: 10.1099/ijs.0.65632-0. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Yan J, Yu B, Wang ET, Li X, et al. Ensifer alkalisoli sp. nov. isolated from root nodules of Sesbania cannabina grown in saline-alkaline soils. Int J Syst Evol Microbiol. 2016;66:5294–5300. doi: 10.1099/ijsem.0.001510. [DOI] [PubMed] [Google Scholar]
- 62.Merabet C, Martens M, Mahdhi M, Zakhia F, Sy A, et al. Multilocus sequence analysis of root nodule isolates from Lotus arabicus (Senegal), Lotus creticus, Argyrolobium uniflorum and Medicago sativa (Tunisia) and description of Ensifer numidicus sp. nov. and Ensifer garamanticus sp. nov. Int J Syst Evol Microbiol. 2010;60:664–674. doi: 10.1099/ijs.0.012088-0. [DOI] [PubMed] [Google Scholar]
- 63.Yan H, Yan J, Sui XH, Wang ET, Chen WX, et al. Ensifer glycinis sp. nov., a rhizobial species associated with species of the genus Glycine . Int J Syst Evol Microbiol. 2016;66:2910–2916. doi: 10.1099/ijsem.0.001120. [DOI] [PubMed] [Google Scholar]
- 64.Nick G, de Lajudie P, Eardly BD, Suomalainen S, Paulin L, et al. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int J Syst Bacteriol. 1999;49 Pt 4:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- 65.Wei GH, Wang ET, Tan ZY, Zhu ME, Chen WX. Rhizobium indigoferae sp. nov. and Sinorhizobium kummerowiae sp. nov., respectively isolated from Indigofera spp. and Kummerowia stipulacea . Int J Syst Evol Microbiol. 2002;52:2231–2239. doi: 10.1099/00207713-52-6-2231. [DOI] [PubMed] [Google Scholar]
- 66.Rome S, Fernandez MP, Brunel B, Normand P, Cleyet-Marel JC. Sinorhizobium medicae sp. nov., isolated from annual Medicago spp. Int J Syst Bacteriol. 1996;46:972–980. doi: 10.1099/00207713-46-4-972. [DOI] [PubMed] [Google Scholar]
- 67.Lloret L, Ormeño-Orrillo E, Rincón R, Martínez-Romero J, Rogel-Hernández MA, et al. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst Appl Microbiol. 2007;30:280–290. doi: 10.1016/j.syapm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Chen W, Sheng X-F, He L-Y, Huang Z. Rhizobium yantingense sp. nov., a mineral-weathering bacterium. Int J Syst Evol Microbiol. 2015;65:412–417. doi: 10.1099/ijs.0.064428-0. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Li B, Wang H, Sui X, Ma X, et al. Rhizobium petrolearium sp. nov., isolated from oil-contaminated soil. Int J Syst Evol Microbiol. 2012;62:1871–1876. doi: 10.1099/ijs.0.026880-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhang S, Yang S, Chen W, Chen Y, Zhang M, et al. Rhizobium arenae sp. nov., isolated from the sand of Desert Mu Us, China. Int J Syst Evol Microbiol. 2017;67:2098–2103. doi: 10.1099/ijsem.0.001810. [DOI] [PubMed] [Google Scholar]
- 71.Kaur J, Verma M, Lal R. Rhizobium rosettiformans sp. nov., isolated from a hexachlorocyclohexane dump site, and reclassification of Blastobacter aggregatus Hirsch and Muller 1986 as Rhizobium aggregatum comb. nov. Int J Syst Evol Microbiol. 2011;61:1218–1225. doi: 10.1099/ijs.0.017491-0. [DOI] [PubMed] [Google Scholar]
- 72.Hirsch P, Müller M. Blastobacter aggregatus sp. nov., Blastobacter capsulatus sp. nov., and Blastobacter denitrificans sp. nov., new budding bacteria from freshwater habitats. Syst Appl Microbiol. 1985;6:281–286. doi: 10.1016/S0723-2020(85)80032-1. [DOI] [Google Scholar]
- 73.Turdahon M, Osman G, Hamdun M, Yusuf K, Abdurehim Z, et al. Rhizobium tarimense sp. nov., isolated from soil in the ancient Khiyik River. Int J Syst Evol Microbiol. 2013;63:2424–2429. doi: 10.1099/ijs.0.042176-0. [DOI] [PubMed] [Google Scholar]
- 74.Behrendt U, Kämpfer P, Glaeser SP, Augustin J, Ulrich A. Characterization of the N2O-producing soil bacterium Rhizobium azooxidifex sp. nov. Int J Syst Evol Microbiol. 2016;66:2354–2361. doi: 10.1099/ijsem.0.001036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.