Abstract
Background and Objectives
Our objective was to investigate cellular and humoral immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in a cohort of people with multiple sclerosis (pwMS) on pulsed B-cell–depleting treatment (BCDT). In particular, we intended to evaluate a possible association between immune responses and the timing of vaccination under BCDT.
Methods
We conducted a cross-sectional study among pwMS on pulsed BCDT or without disease-modifying treatment after completed SARS-CoV-2 vaccination. Samples were collected during routine clinical visits at the Multiple Sclerosis Center Dresden, Germany, between June 2021 and September 2021. Blood was analyzed for SARS-CoV-2 spike protein–specific antibodies and interferon-γ release of CD4 and CD8 T cells on stimulation with spike protein peptide pools. Lymphocyte subpopulations and total immunoglobulin levels in the blood were measured as part of clinical routine.
Results
We included 160 pwMS in our analysis, comprising 133 pwMS on BCDT (n = 132 on ocrelizumab and n = 1 on rituximab) and 27 without disease-modifying treatment. Humoral and cellular anti–SARS-CoV-2 responses were reciprocally regulated by the time between the last BCDT cycle and vaccination. Although antibody responses increased with prolonged intervals between the last BCDT cycle and vaccination, CD4 and CD8 T-cell responses were higher in pwMS vaccinated at early time points after the last BCDT cycle compared with untreated pwMS. T-cellular vaccination responses correlated with total, CD3 CD4, and partly with CD3 CD8 lymphocyte counts. Humoral responses correlated with CD19 lymphocyte counts. Status post coronavirus disease 2019 infection led to significantly increased SARS-CoV-2–specific T-cell and antibody responses.
Discussion
Delaying BCDT is currently discussed as a strategy to optimize humoral responses to SARS-CoV-2 vaccination. However, T cells represent an important line of defense against SARS-CoV-2 infection as well, especially in light of emerging variants of concern. We observed enhanced CD4 and CD8 T-cellular responses in pwMS receiving vaccination at early time points after their last BCDT cycle. These data may influence clinical decision making with respect to vaccination strategies in patients receiving BCDT.
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is recommended for people with multiple sclerosis (pwMS). Unfortunately, immune responses to vaccination can be restrained by certain disease-modifying medications used in the treatment of MS, such as anti-CD20 monoclonal antibodies and sphingosine-1-phosphate receptor modulators. Concerning the treatment with B-cell–depleting agents ocrelizumab and rituximab, most of the published studies report diminished humoral SARS-CoV-2 vaccine responses.1-4 The extent of humoral responses seems to depend on the timing of vaccination with regard to the last cycle of B-cell–depleting treatment (BCDT).2,3,5 Therefore, postponing BCDT to enable an effective antibody response to vaccination is being discussed.
However, the humoral response represents only 1 side of the coin. T-cellular antiviral responses are assumed to confer protection from SARS-CoV-2 infection and severe coronavirus disease 2019 (COVID-19) in patients under BCDT, too.1,6,7 The influence of BCDT on T-cell responses is less well studied because they are more difficult to analyze. Although some studies showed diminished T-cellular responses to SARS-CoV-2 vaccination in patients under BCDT, others provided proof of skewed or even enhanced responses compared with pwMS without immunomodulatory treatment or healthy controls.1-3,8 Hence, the role of T-cellular vaccine responses in patients under BCDT is still unclear, and even less is known about their dependence on the timing of vaccination with regard to the last BCDT cycle.
Our objective was to investigate cellular and humoral immune responses to SARS-CoV-2 vaccination in a cohort of pwMS on BCDT in contrast to pwMS without disease-modifying treatment. In particular, we intended to evaluate a possible association between immune responses and the timing of vaccination under BCDT.
Methods
Patients
We conducted a cross-sectional study among pwMS attending routine clinical visits at the MS Center Dresden, Germany, between June 14 and September 22, 2021. Inclusion criteria were MS diagnosis, age >18 years, BCDT or no disease-modifying treatment, and completed SARS-CoV-2 vaccination. At the beginning of our trial, it was clinical practice to vaccinate patients on ocrelizumab or rituximab at least 12 weeks after the last infusion based on the available data on vaccine responses under pulsed BCDT from the VELOCE study.9 Experts recommended to keep a distance of at least 4–6 weeks before and after infusions.10-12 As we aimed to analyze the effect of vaccination timing during BCDT on the efficacy of immunization, patients on BCDT were classified according to the time between the last treatment cycle and the first SARS-CoV-2 vaccination. Groups were defined as 31–90 days between the last BCDT infusion and vaccination, 91–180 days, or more than 180 days. The first group had hence been vaccinated less than 12 weeks after the last infusion, the second group had been vaccinated more than 12 weeks after the last infusion in line with the timing of vaccination in the VELOCE study, and for the third group, the usual treatment regimen comprising BCDT infusions every 6 months had been interrupted or delayed leading to an interval of more than 180 days between the last BCDT cycle and vaccination. No patient was vaccinated less than 31 days after the last BCDT cycle according to expert consensus.10-12 For all patients on BCDT, at least 4 weeks passed between the last SARS-CoV-2 vaccination and the next BCDT cycle.
Analysis of SARS-CoV-2–Specific T-Cell Responses
Lithium heparin blood and serum sampling for the analysis of vaccination responses took place during routine clinical visits. CD4 and CD8 interferon-γ (IFN-γ) secretion after stimulation with SARS-CoV-2 spike protein peptide pool antigens 1 and 2 was measured through the SARS-CoV-2 QuantiFERON test (Qiagen, Hilden, Germany).2 Blood samples were incubated for 16–24 hours with SARS-CoV-2 peptide pools or with mitogen as a positive control. IFN-γ release to the negative control of each sample was subtracted from responses to antigens 1 and 2. The positivity cutoff was defined as 0.15 IU/mL, as previously described.2 Response levels to antigen 1 represent IFN-γ release by CD4 T cells, and IFN-γ release to antigen 2 represents the activity of both CD4 and CD8 T cells.
Detection of SARS-CoV-2–Specific Antibodies
Immunoglobulin (Ig) G antibodies against the SARS-CoV-2 spike protein receptor-binding domain (RBD) in serum samples were quantified through electrochemiluminescence immunoassay on a COBAS e801 module (Roche, Basel, Switzerland). The seropositivity cutoff was defined as 0.8 U/mL.13 The lower detection limit was 0.43 U/mL, and values under 0.43 U/mL were set to half the detection limit, that is, 0.215 U/mL. The upper detection limit was 25,000 U/mL, which was only exceeded by 1 patient whose value was set to 25,000 U/mL. The assigned unit (U/mL) corresponds to the World Health Organization international standard binding antibody units per milliliter.13
Counts of Lymphocyte Subpopulations and Total Immunoglobulin Quantities
Counts of lymphocyte subpopulations and total IgG, IgM, and IgA quantities in the blood were analyzed as part of clinical routine by the Institute of Clinical Chemistry and Laboratory Medicine at the University Hospital Carl Gustav Carus Dresden, Germany.
Statistical Analysis
Data were analyzed descriptively calculating means and SDs for the total study sample and relevant subgroups. For the classification of the distribution of outcomes, Q-Q plots were created. Correlations between outcomes were calculated as Spearman's Rho. Differences between subgroups were tested with χ2 tests and Mann-Whitney U tests. IFN-γ release and antibody titers were analyzed through generalized linear models with Gamma-log link function and reported as model estimates (mean and 95% CI). Type of MS, age, sex, time since last BCDT, disability (through the Expanded Disability Status Scale [EDSS]), previous COVID-19, type of vaccination, and time between vaccination and sampling served as fixed factors. Bonferroni correction for pairwise testing was applied.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of the University Hospital Dresden (EK348092014 and BOEK35012021). Written informed consent was obtained from all study participants.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
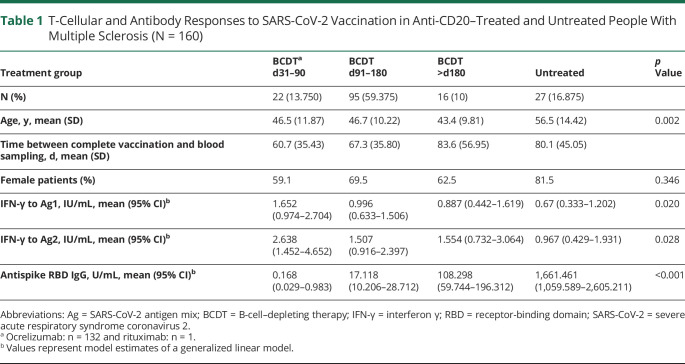
We included 160 pwMS (80.6% relapsing remitting, 13.1% primary progressive, and 6.3% secondary progressive), with an age of 48 ± 11.79 years (mean ± SD) in our analysis. The study population comprised 133 (83.1%) patients on BCDT and 27 (16.9%) patients without disease-modifying treatment. In the BCDT group, 132 pwMS received ocrelizumab and 1 received rituximab. Ocrelizumab infusions were administered at a dose of 600 mg every 6 months and rituximab at a dose of 500 mg every 6 months. Ocrelizumab infusions were delayed for vaccination in 17 patients; the time between the last ocrelizumab infusion and first vaccination in this group was between 130 and 183 days. One patient had paused ocrelizumab treatment since August 2019 because of pregnancy. Another patient had ended ocrelizumab treatment in November 2019 because of its lacking effect on secondary progression. The total duration of BCDT until analysis was 938.2 ± 545.17 days (mean ± SD). Total treatment duration did not significantly differ between the 3 BCDT groups (p > 0.05). Of the untreated pwMS, 12 were treatment naive, 13 had received disease-modifying therapies more than 12 months before the first SARS-CoV-2 vaccination, and 3 had received disease-modifying treatment within 12 months before the first vaccination but had ended it at least 85 days before the first vaccination. Previous treatments included IFN-β, glatiramer acetate, teriflunomide, dimethyl fumarate, laquinimod, mitoxantrone, and azathioprine.
Patients in the untreated group were significantly older than patients in the other 3 groups (p < 0.05; Table 1). Sex, type of MS, EDSS, status post SARS-CoV-2 infection, type of vaccination, and time between vaccination and blood sampling did not differ significantly between the 4 groups.
Table 1.
T-Cellular and Antibody Responses to SARS-CoV-2 Vaccination in Anti-CD20–Treated and Untreated People With Multiple Sclerosis (N = 160)
Previous SARS-CoV-2 infection was reported for 16 patients (BCDT: n = 11 and untreated: n = 5). Most patients (90%) were vaccinated with either 2 doses of messenger RNA (mRNA) vaccine (n = 139) or 1 dose each of a vector and mRNA vaccine (n = 5). Complete viral vector vaccination was performed in 9 patients (5.6%), whereas 7 patients (4.4%) received 1 mRNA vaccine dose after SARS-CoV-2 infection. Blood samples for the evaluation of T-cellular and humoral responses to vaccination were drawn 70.2 ± 40.16 days (mean ± SD) after completed vaccination.
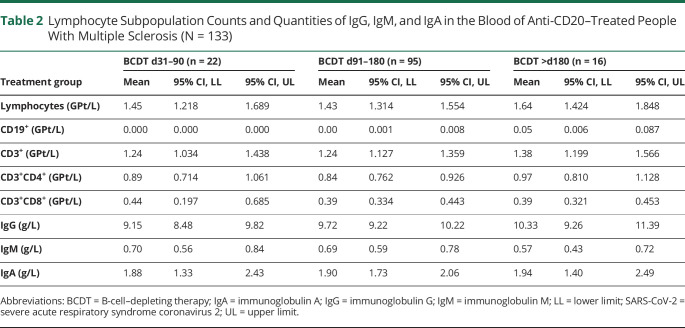
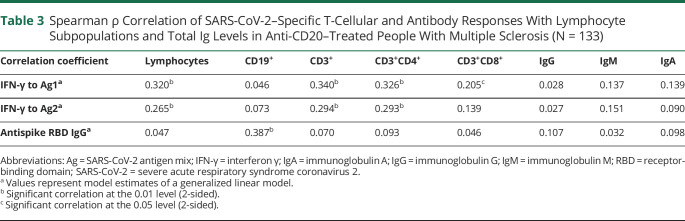
After vaccination, a positive T-cellular response to SARS-CoV-2 spike protein was seen in 77.4% (n = 103) and a positive anti-RBD antibody response in 28.6% (n = 38) of patients on BCDT compared with 70.4% (n = 19) and 100% (n = 27) of untreated patients, respectively. Patients vaccinated early after their last BCDT cycle (i.e., day 31–90) presented higher CD4 and CD8 T-cellular responses than patients without immunomodulation (Figure, A and B, Table 1). There was no difference in T-cellular responses between untreated pwMS and BCDT patients vaccinated later than day 90. Although patients on BCDT demonstrated lower antibody levels compared with untreated patients, anti-RBD antibody titers markedly increased with prolonged intervals between vaccination and the last BCDT cycle (Figure, C, Table 1). SARS-CoV-2–specific T-cellular responses in pwMS on BCDT correlated significantly with total, CD3, and CD3 CD4 lymphocyte counts in the blood (Tables 2 and 3). T-cell response to SARS-CoV-2 peptide pool 1 also correlated with CD3 CD8 lymphocyte counts. Antibody responses to SARS-CoV-2 in patients on BCDT showed a significant correlation with CD19 lymphocyte counts. There was no significant correlation between vaccination responses and levels of total IgG, IgM, or IgA in the blood.
Figure. T-Cellular and Antibody Responses to SARS-CoV-2 Vaccination in pwMS on BCDT or Without Disease-Modifying Treatment (N = 160).
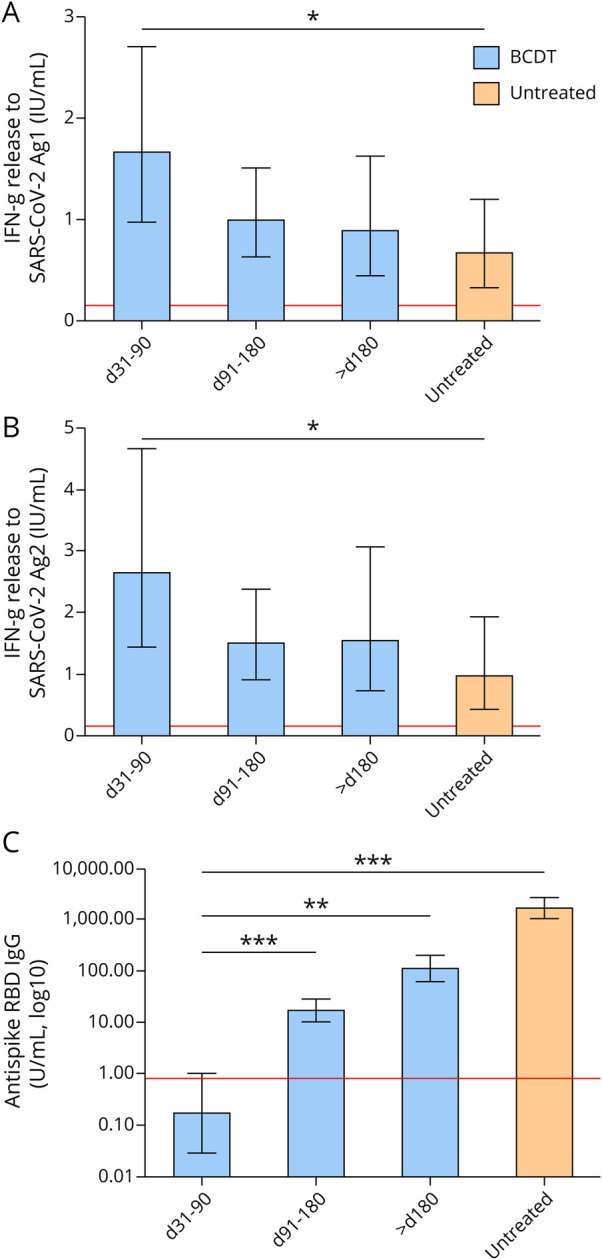
Immune responses to SARS-CoV-2 vaccination in pwMS on BCDT (blue) and untreated pwMS (orange) are presented. Patients on BCDT were further categorized according to the time between the first SARS-CoV-2 vaccination and the last BCDT cycle (day [d] 31–90, d91–180, and >d180). Means with 95% CIs are presented. Data were analyzed through generalized linear models with Gamma-log link function and Bonferroni correction for multiple pairwise comparisons. Asterisks indicate the level of statistical significance (*p < 0.05, **p < 0.01, and ***p < 0.001). (A, B) T-cellular responses to SARS-CoV-2 were evaluated using a combination of spike protein peptides stimulating CD4 and CD8 T cells. IFN-γ secretion was measured using ELISA in duplicates each. The red line indicates the cutoff level of positivity (0.15 IU/mL). A, Pairwise comparison d31–90 vs untreated: p = 0.029. B, Pairwise comparison d31–90 vs untreated: p = 0.043. (C) IgG antibody responses to the spike protein RBD are shown (the cutoff level of seropositivity 0.8 U/mL). The assigned unit (U/mL) corresponds to the World Health Organization international standard binding antibody units per milliliter. Pairwise comparisons between all 4 treatment groups were significant (p = 0.002 for d31–90 vs >d180; p = 0.002 for d91–180 vs >d180; and p < 0.001 for all other pairwise comparisons). BCDT = B-cell–depleting treatment; IFN-γ = interferon γ; pwMS = people with multiple sclerosis; RBD = receptor-binding domain; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 2.
Lymphocyte Subpopulation Counts and Quantities of IgG, IgM, and IgA in the Blood of Anti-CD20–Treated People With Multiple Sclerosis (N = 133)
Table 3.
Spearman ρ Correlation of SARS-CoV-2–Specific T-Cellular and Antibody Responses With Lymphocyte Subpopulations and Total Ig Levels in Anti-CD20–Treated People With Multiple Sclerosis (N = 133)
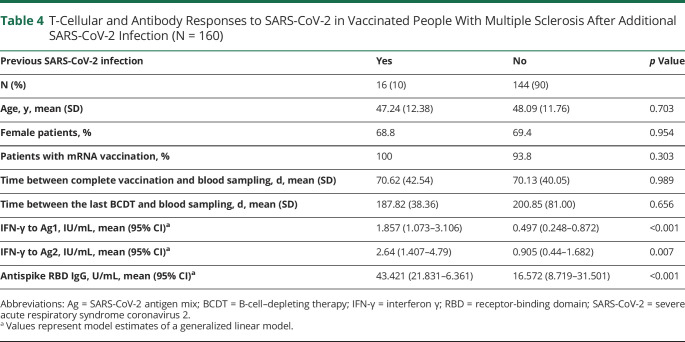
Anti-RBD antibody titers were lower in viral vector–vaccinated patients compared with those immunized with mRNA vaccine (4.00 U/mL [1.37–11.65] vs 38.16 U/mL [21.56–67.53]; mean [95% CI]; p = 0.002). Previous SARS-CoV-2 infection was a dominant factor for the extent of T-cellular and humoral responses after vaccination (Table 4). Notably, all observed effects remained stable after exclusion of the 16 pwMS with previous SARS-CoV-2 infection in a sensitivity analysis underlining the robustness of the reported results.
Table 4.
T-Cellular and Antibody Responses to SARS-CoV-2 in Vaccinated People With Multiple Sclerosis After Additional SARS-CoV-2 Infection (N = 160)
Discussion
Our study analyzed the dependence of humoral and T-cellular responses to SARS-CoV-2 on the timing of vaccination during BCDT in a real-world cohort of pwMS. As for humoral responses, our results could confirm published studies showing an increase of antiviral antibodies with prolonged intervals between BCDT and vaccination. By itself, this observation would support the currently discussed suggestion to delay BCDT as a strategy to optimize vaccination responses.4,5,14
However, recent studies indicate that T cells are able to induce protection from SARS-CoV-2 infection and severe COVID-19 in patients on BCDT as well, which is especially relevant in the face of emerging virus variants.1,6,7 Unfortunately, the extent of T-cellular responses to SARS-CoV-2 vaccination under BCDT is not well known. One study showed decreased T-cell activity in anti-CD20–treated patients,2 whereas other reports described similar or even enhanced T-cell responses under BCDT.1,3,8 In 2 studies, patients on BCDT presented increased CD8 T-cell responses to SARS-CoV-2 vaccination compared with controls.1,8 As for CD4 T-cell responses, one study indicated that some subsets of activated type 1 helper T cells are maintained under BCDT, whereas circulating follicular helper T cells vanish prematurely after initial induction.1 A different study implicated that SARS-CoV-2–specific CD4 T cells develop equivalently in B-cell–depleted and immunocompetent patients, but that the cells express different levels of activation markers.8 However, the aforementioned studies did not delineate the association of antiviral T-cellular responses with the timing of vaccination during BCDT. In our study, the magnitude not only of CD8 but also of CD4 T-cellular responses was higher in B-cell–depleted compared with untreated pwMS when vaccination took place within 31–90 days after the last BCDT cycle. When vaccination was performed later than 90 days after the last BCDT cycle, no difference in CD4 or CD8 T-cell responses between BCDT and untreated groups was observed. Our data hence support the hypothesis of a compensatory increase in T-cell–mediated responses to SARS-CoV-2 in the absence of B cells and antibodies. Moreover, they challenge the suggestion to postpone BCDT for the improvement of vaccine responses and may influence clinical decision making regarding vaccination strategies. The often suggested strategy to delay anti-CD20 treatment to increase humoral vaccination responses is not suitable for some patients because of clinical or radiologically detectable disease activity so that alternate approaches are needed. As it is unclear whether virus-specific antibodies, T cells, or a combination of the 2 is necessary for protection from SARS-CoV-2 infection and severe COVID-19 disease course, individual patient profiles need to be taken into account. In cases in which disease-modifying treatment cannot be delayed and infection incidences are high, it may make sense to vaccinate patients early after their last BCDT pulse in order not to lose time before a possible infection. Our observation that SARS-CoV-2–specific T-cellular responses are enhanced in pwMS vaccinated early after the last BCDT cycle may support this option, especially when methods for the measurement of humoral and T-cellular responses are available. In this case, vaccination responses can be monitored, and vaccination regimen can be adapted individually. For example, if early vaccination does not elicit T-cellular responses in a patient on BCDT, vaccination can be repeated at a later time point after BCDT to elicit a humoral vaccination response. These proposals evidently need confirmation from future studies with regard to clinical efficacy of the different vaccination strategies.
Although standardized anti–SARS-CoV-2 antibody assays are widely accessible, methods to measure T-cell responses are often more complex because of the necessity to process fresh cells. We introduced a simple assay for the measurement of SARS-CoV-2–specific T cells that can easily be implemented in clinical practice. A comprehensive control of vaccination responses is therewith facilitated, enabling clinicians to adapt their vaccination recommendations to each individual patient.
B cells being plasma cell precursors, the decrease in humoral vaccine responses under BCDT is not surprising from an immunologic perspective. Accordingly, the antibody response to SARS-CoV-2 vaccination correlated significantly with counts of CD19 lymphocytes in the BCDT cohort in our study. However, anti-CD20 treatment does not only deplete B cells but has an effect on the T-cell compartment as well. First, B cells are potent antigen presenters for T cells. Therefore, one might expect that BCDT also impairs T-cellular responses to stimuli administered during phases when B cells are absent. Nevertheless, we did not observe decreased T-cellular responses to SARS-CoV-2 vaccination in BCDT compared with immunocompetent pwMS. Other antigen-presenting cells hence seem to be sufficient for the activation of T cells in the context of vaccination in patients on BCDT. Second, anti-CD20 treatment also depletes the compartment of CD20 T cells. Recent evidence suggests that the latter may play an important role in the pathogenesis of MS and that the depletion of CD20-expressing T cells may account for part of the efficacy of anti-CD20 treatment.15 It has further been shown that CD20 T cells repopulate at an earlier time point after pulsed BCDT than CD20 B cells.16 Three months after rituximab infusion, counts of CD20 T cells were higher than the ones of CD20 B cells.16 In our study, we did not only see a similar but an increased T-cellular vaccination response in pwMS vaccinated 1–3 months after the last anti-CD20 treatment cycle compared with untreated pwMS. In other words, the enhanced T-cell response was observed in the group of patients on BCDT who were not only B cell depleted but also assumed to have a higher degree of depletion of CD20 T cells compared with the other analyzed BCDT groups. Possibly, this may suggest a suppressive function of CD20 T cells with regard to T-cellular vaccination responses. It is essential to note that values of actual CD20 T-cell counts are not available for our cohort. Obviously, other mechanisms underlying the observed timing effect are conceivable as well, like a decreased stimulation of regulatory T cells through non–B-cell antigen-presenting cells, a lack of T-cell inhibition due to depletion of regulatory B cells, or increased availability of antigen for T-cell stimulation in the absence of antigen-specific antibodies.
An important limitation of our study is the inability to draw conclusions on the clinical efficacy of SARS-CoV-2 vaccination. Furthermore, T-cellular responses were defined solely through the release of IFN-γ as the most common marker of T-cell activity. However, other cytokines and activation markers are also of interest for a better understanding of vaccine responses. Longitudinal studies of vaccine responses including the evaluation of clinical efficacy for different vaccination schemes are under way.
In conclusion, our study confirmed the increase of humoral responses to SARS-CoV-2 vaccination with a prolonged distance between the last BCDT cycle and vaccination. However, we were able to show that T-cellular responses to vaccination are increased in anti-CD20–treated as compared to immunocompetent pwMS when vaccination takes place within 31–90 days after the last BCDT pulse. This observation may support early vaccine administration in patients for whom BCDT cannot be delayed because of MS disease activity. We used a simple assay for the measurement of SARS-CoV-2–specific T-cell responses, which may facilitate a more comprehensive monitoring of vaccination responses in clinical routine compared with the sole measurement of humoral responses.
Acknowledgment
The authors thank Michaela Marggraf and Tristan Bensch (Center of Clinical Neuroscience, Department of Neurology, University Hospital Carl Gustav Carus Dresden, Technical University of Dresden, Dresden, Germany) for excellent technical support.
Glossary
- BCDT
B-cell–depleting treatment
- COVID-19
coronavirus disease 2019
- EDSS
Expanded Disability Status Scale
- IFN-γ
interferon γ
- Ig
immunoglobulin
- mRNA
messenger RNA
- MS
multiple sclerosis
- pwMS
people with MS
- RBD
receptor-binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix. Authors
Contributor Information
Christina Woopen, Email: christina.woopen@uniklinikum-dresden.de.
Marie Dunsche, Email: marie.dunsche@uniklinikum-dresden.de.
Rocco Haase, Email: rocco.haase@ukdd.de.
Catarina Raposo, Email: catarina.raposo@roche.com.
Rosetta Pedotti, Email: rosetta.pedotti@roche.com.
Katja Akgün, Email: katja.akguen@ukdd.de.
Study Funding
This study was supported partly by an unrestricted grant from Roche.
Disclosure
C. Woopen, M. Dunsche, and R. Haase report no disclosures relevant to the manuscript. C. Raposo and R. Pedotti are employees and shareholders of F. Hoffmann-La Roche. K. Akgün received personal compensation for consulting services from Roche. T. Ziemssen received personal compensation for consulting services from Roche, is a shareholder of Roche, and is a principal investigator of the AMA-VACC and KYRIOS studies evaluating SARS-CoV-2 vaccination responses in pwMS. Go to Neurology.org/NN for full disclosures.
References
- 1.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990-2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3(11):e789-e797. doi: 10.1016/s2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73:103636. doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:1-8. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021;78(12):1529-1531. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280-1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol. 2020;20(10):581-582. doi: 10.1038/s41577-020-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madelon N, Lauper K, Breville G, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. 2022;75(1):e1037-e1045. doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. doi: 10.1212/wnl.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centonze D, Rocca MA, Gasperini C, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021;268(11):3961-3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woopen C, Schleußner K, Akgün K, Ziemssen T. Approach to SARS-CoV-2 vaccination in patients with multiple sclerosis. Front Immunol. 2021;12:701752. doi: 10.3389/fimmu.2021.701752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhise V, Dhib-Jalbut S. Potential risks and benefits of multiple sclerosis immune therapies in the COVID-19 era: clinical and immunological perspectives. Neurotherapeutics. 2021;18(1):244-251. doi: 10.1007/s13311-021-01008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jochum S, Kirste I, Hortsch S, et al. Clinical utility of Elecsys anti-SARS-CoV-2 S assay in COVID-19 vaccination: an exploratory analysis of the mRNA-1273 phase 1 trial. Front Immunol. 2022;12:798117. doi: 10.3389/fimmu.2021.798117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91(1):89-100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochs J, Nissimov N, Torke S, et al. Proinflammatory CD20+ T cells contribute to CNS-directed autoimmunity. Sci Transl Med. 2022;14(638):eabi4632. doi: 10.1126/scitranslmed.abi4632. [DOI] [PubMed] [Google Scholar]
- 16.Schuh E, Berer K, Mulazzani M, et al. Features of human CD3+CD20+ T cells. J Immunol. 2016;197(4):1111-1117. doi: 10.4049/jimmunol.1600089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.