Abstract
The aim of the study was to evaluate the application of global longitudinal strain (GLS) and myocardial work (MW) at rest and during exercise in healthy sedentary or trained participants, to test their ability to improve echocardiographic information and to complement prescribing exercise, cardiac screening, or rehabilitation programs.
Methods:
Thirty healthy males were divided into three groups of 10, sedentary (G1), resistance (G2) and power (G3) athletes, underwent a standard clinical evaluation protocol and exercise stress testing echocardiography.
Results:
During stress, all showed increased left ventricular ejection fraction and mitral annulus tissue Doppler (E'). G1 showed a decrease in left atrial volume (LAVi) as opposed to an increase in G3. E/E 'a decrease in G2, unlike the increase in G3. All groups showed increase of Strain (GLS average AV, Longitudinal LS, Medio-Basal MB Apical AP), global constructive work (GCW), and Global wasted work. G1 showed increase for global work efficiency, G2 and G3 for global work index (GWI). G3 showed a greater variation of E/E', LAVi, GWI and GCW compared to G1 and G2, greater of GLS AV, LS-AP compared to G2. Only G3 showed differences for GLS AV versus LS-AP. The relative regional strain ratio showed a greater value in G3 versus G1 at the end of stress compared to rest.
Conclusions:
The new echocardiographic applications to study the physiological adaptation could open new perspectives for the diagnostic and therapeutic development through the prescription of personalized exercises and screening and follow-up of the early pathological changes of the athlete's heart.
Keywords: Athletes’ heart, echocardiography, exercise echo, myocardial work, strain
INTRODUCTION
The morphological and functional changes observed in athletes' hearts (AH) define a condition called AH. The European Association of Cardiovascular Imaging (EACVI), American College Cardiology (ACC) and American Heart Association (AHA) suggested the correct interpretation of imaging in the evaluation of athletes and new standards for electrocardiography (ECG) interpretation, fitness, and sports equipment.[1,2,3]
In 1975 Morganroth, using M-mode echocardiography, described cardiac adaptations to sports activities according to two “extreme” models: left ventricular hypertrophy “concentric” power athletes (PA), and “eccentric” Endurance athletes (EA).[4] Subsequently, several elaborations showed a spectrum of morphostructural modifications more heterogeneous than the hypothesis of Morganroth in consideration of long-term cardiovascular adaptations induced by various sports and age groups.[3,5]
Currently, the speckle tracking echocardiography (STE) and its derived parameters, such as global longitudinal strain (GLS) and the myocardial work (MW), can further define the characteristics of AH.
The left ventricular GLS (LVGLS) has emerged in the last decade as a reliable tool for the study of myocardial mechanics by adding information on cardiac performance compared with traditional LV systolic function parameters, such as ejection fraction left ventricular ejection fraction (LVEF).[6] Several studies have shown that a reduction in LVGLS is uncommon in AH, it cannot be regarded a physiological adaptation to training and may be helpful to clarify the nature of cardiovascular adaptations in specific circumstances.[7,8]
The MW can be considered an advancement of the GLS, able to study LV performance related to changes in the effort, as it incorporates afterload and provides a measure of myocardial efficiency.[9,10,11,12] LV pressure-strain loop area and derived global MW indices correlate with invasive measurements.[13]
“Exercise stress testing echocardiography” (ESTE) is a widely used method for simultaneous assessment of myocardial function and hemodynamics during physiological stress. The LVGLS evaluation during stress may provide an incremental value and enable further recognition of early myocardial dysfunction,[14,15] but they are still few data on changes in GLS and MW during exercise in healthy.[16]
Our hypothesis is that the new echocardiographic technologies, such as GLS and MW during ESTE, can better assess the AH adaptations and provide more information than standard echocardiography.
MATERIALS AND METHODS
Study population
The study was conducted at the Echo-Lab of our Cardiology Unit. From January 2019 to February 2020, healthy volunteers, students or graduates, practicing competitive or recreational sports, were consecutively enrolled. We evaluated 30 male participants, selected on the basis of optimal echo images during stress, aged between 18 and 35 (26.9 ± 6.3 years), divided into three groups: G1, 10 sedentary or practicing any kind of sport for <1 h/week (<4 h in the last year). G2 and G3 groups were made up of athletes who had passed the medical examination for the release of sports fitness for competitive activity and regularly enrolled in their respective national sports federations.
Athletes received sport-specific training protocols, were always doping-test negative, and did not use anabolic steroids. The participants recruited from groups G2 and G3 practiced competitive activity for more than 24 months and in the past 12 months. G2 included 10 competitive EA, mainly engaged in aerobic training (all cyclists) for at least 4 h a week (≥16 h/month in the last year); G3 included 10 competitive PA practicing anaerobic sports (weightlifting and throwing) for at least 4 h/week (≥16 h/month in the past year). All participants have undergone normal physical examination, blood test, standard ECG, and echocardiogram.
The study was conducted in accordance with the Helsinki Declaration. All participants provided written informed consent before entering the study.
Exercise stress testing echocardiography
Exercise stress test
The “Maximal Exercise-Stress Test” was performed on the “e-Bike EL Ergometer,” using the Case system. The participants, in a semi-supine inclined left position, started the exercise test with 2 min warm-up, followed by a 60W load increased by 30W every 2 min to target heart rate (HR). The pedaling speed was maintained at 60 rpm until muscle exhaustion, with a subsequent 7 min recovery to 30W. The contraindicating symptoms included chest discomfort, severe exhaustion, pain in lower limbs, pathological abnormalities in the electrocardiographic trace, or arterial blood pressure (ABP) increase ≥250/115 mmHg. The ABP measurements were performed by a cuff connected to the ECG monitoring system during all phases. During the test any cardiological signs and symptoms, increased ABP, ECG changes of ischemia or arrhythmias, metabolic equivalents (METS), double product (DP = SBP × HR), and increased workload (Watt), were detected. At the end of the test, all patients were asked to wait 30 min before being discharged.
Echocardiography
A complete echocardiographic exam was performed by Vivid E95 (GE Horten, Norway) equipped with a probe M5S, according to the standards of our laboratory and the EACVI/ASE recommendations.[17] The image acquisitions were performed at rest and at 85% of the expected maximum HR, using the dedicated software (EchoPAC AFI-Stress). Before starting the test, we explained to the participants how to collaborate with a controlled breathing during the acquisition phases of the Echo images.
Standard echo
LV quantitative analysis M-2D-Doppler was performed according to the current recommendations.[17,18] For this study were considered: LV diastolic diameter (LVIDd), mass (LVMi) indexed for body surface area (BSA) using M-Mode; Left atrial volume (LAVi) indexed for BSA and LVEF by the 2D-biplane method; peak velocity of the mitral flow, early (E), atrial (A) and their ratio, by Pulsed Wave Doppler (PW); the average of the early diastolic (E') and systolic (S') velocities by tissue Doppler imaging at septal and lateral mitral annulus, and the ratio E/E'.
Advanced echo
Images for the GLS calculation were acquired in the three standard apical views, 60–70 fpm, and at least 7 cardiac cycles for each view to minimize unusable images to maximum ESTE.[19]
The ESTE analyses were performed offline using the dedicated software (Echopac v. 2.02). We calculated the GLS in a 17-segment bull's-eye model and strain of the single segments (LS) to evaluate the regional mechanical [Figure 1a]. We considered the average systolic peak value of the 12 middle-basal segments (LS-MB), the 5 apical (LS-AP), and the regional deformation ratio between apex and base (LS-AP/LS-MB).[20,21]
Figure 1.
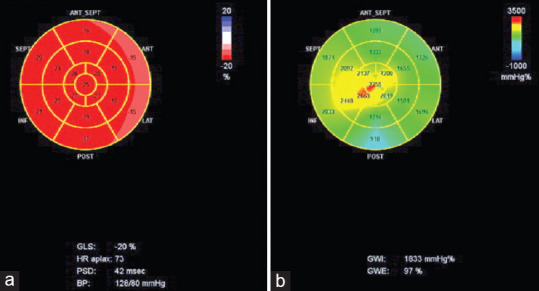
A bull's eye with the Global Longitudinal Strain (1a-red map) and Global Work Index (1b-green map). GLS = Global Longitudinal Strain Average of all segments. PSD = peak strain dispersion. BP = Blood pressure. GWI: Global Work Index; GWE: Global Work Efficiency. HR APLAX = HR at apical long axis view
The quantification of MW was performed using the same software package. It was measured by areas Pressure strain loop (PSL), obtained from noninvasive pressure curves LV with acquired deformation with STE, as proposed by Russell et al.[10] The software has derived the noninvasive-PSL after taking the GLS and integrated with the BP values and the time of valvular events. GLS and SBP were synchronized by aligning the timing of the valve events, which have been set from PW at mitral and aortic valve and then confirmed by 2DE evaluation of the long axis apical view. MW was evaluated from mitral valve closure to mitral valve opening. A bull's eye with the segmental and global work index (GWI) (the area within the curve total work from mitral valve closure to mitral valve opening) values was also provided [Figure 1b]. We also achieved global work (GW) global constructive work (GCW) work performed during shortening in systole adding negative work during lengthening in isovolumetric relaxation), global wasted work (GWW) negative work performed during lengthening in systole adding work performed during shortening in isovolumetric relaxation; and efficiency (GWE) constructive work divided by the sum of constructive and wasted work).[21] All parameters were calculated at rest (resting) and at peak exercise (stress).
Statistical analysis
Statistical analysis was performed using SPSS v. 26.0.(IBM SPSS v 26.0 New York, USA) Continuous variables, reported as mean ± standard deviation, were compared by t-test. The linear relationships between the deformation parameters and other continuous variables were evaluated with the Pearson's correlation method. All significant variables on univariate analysis were included in multivariate regression step by step, after excluding those that showed collinearity (Pearson r > 0.6). A P < 0.05 indicates statistical significance and goodness of adapted model expressed with the statistical R-squared.
The echocardiogram was always performed by the IM operator. The offline analysis of the captured images is always by the DB operator. The image quality was optimal, and no LV segment was excluded from the analysis. The reproducibility (intra/inter-observer variability) of LV deformation of our laboratory was previously reported.[22]
RESULTS
We found a good intraclass correlation (r = 0.790, P < 0.0001) for strain component (intra-observer 0.978; inter-observer 0.957).
Study population
All participants were male, matched by age (G1: 27 ± 6; G2:28 ± 5; G3: 27 ± 5 y). G3 showed higher BSA than G2 (2 ± 0.1 vs. 19 ± 0.1 sqm P < 0.030) and DBP values at rest than G1 (72 ± 9 vs. 62 ± 6 mmHg P < 0.013). At the end of the stress, G2 reached higher levels of METS than G1 and G3 (12 ± 2.4 vs. 9.4 ± 1.4 P < 0.009 and vs. 9.9 ± 1.2 P < 0.026 ml/kg/min) and Watts (225 ± 49 vs. 182 ± 22 P < 0.022 and vs. 195 ± 32 W). G3 showed a greater increase in SBP than the other groups (74 ± 19 vs. 63 ± 28 and vs. 57 ± 13 Δ %, P < 0.036 G3 vs. G2).
Echo findings
Standard echo
Resting
G2 and G3 showed only LVEF greater than G1 [Table 1].
Table 1.
Echo findings at rest
| G1 | G2 | G3 | P between groups | |
|---|---|---|---|---|
| Standard | ||||
| LVEF (%) | 60±3.5 | 64.1±3.2 | 63.3±4.7 | 0.0138* 0.0368# |
| SVi (ml/mq) | 32.97±9.5 | 34.24±9.4 | 32.22±6.1 | |
| E/E’ (m/s) | 7.32±1.04 | 7.19±2.02 | 6.69±1.52 | |
| E’ (m/s) | 0.11±0.02 | 0.13±0.04 | 0.11±0.003 | |
| S’ (m/s) | 0.08±0.01 | 0.08±0.02 | 0.08±0.01 | |
| RWT (cm) | 0.34±0.09 0.09 | 0.39±0.06 | 0.38±0.06 | |
| LVMi (ml/mq) | 71.1±15.3 15.3 | 84.2±20.7 | 76.7±9.1 | |
| LVIDd (cm) | 4.91±0.4 0.43 | 4.85±0.5 | 4.81±0.4 | |
| LAVi (ml/mq) | 16.5±3.8 | 17.8±5.3 | 17.8±2.6 | |
| Advanced | ||||
| GLS-AV (%) | −19.5±1.3 | −19.8±1.5 | −18.6±1.17 | 0.020# |
| LS-MB (%) | −18.1±1.1 | −17.9±1.9 | −16.9±0.8 | |
| LS-AP (%) | −22.0±3.2 | −24.0±2.1 | −22.7±2.9 | |
| GWI (mmHg %) | 1633±235 | 1846±332 | 1720±159 | |
| GCW (mmHg %) | 1970±269 268.71 | 2201±370 | 1983±217 | |
| GWW (mmHg %) | 69±41 | 77±41 | 87±40 | |
| GWE (mmHg %) | 96±2 | 96±2 | 95±2 |
*G2 versus G1, # G3 versus G1, § G3 versus G2. LV=Left ventricular, LVEF=LV ejection fraction, LVMi=LV mass index by body surface area, LVIDd=LV internal diastolic diameter, SVi=Stroke Volume index by body surface area, LAVi=Left Atrial Volume indexed by body surface area, RWT=Relative Wall Thickness, S’=Peak velocity of systolic mitral annular motion as determined by pulsed wave Doppler, E/E’=Ratio between early flow velocity at mitral valve (E) and tissue velocity wave (E’) at mitral annulus, GLS-AV=Global longitudinal strain-average, LS=Longitudinal strain, LS-AP=LS apical segments, LSMB=LS strain medio-basal segments, MD=Mechanical dispersion, GCW=Global constructive work, GW=Global work, GWE=GW efficiency, GWI=GW index, GWW=Global wasted work, G1=Sedentary, G2=Resistance, G3=Power
Stress
Compared to rest, G1 showed an increase in LVEF and E', a decrease in LAVi; G2 an increase in LVEF and E', a decrease in E/E'; G3 showed an increase in LVEF, E/E', E' and LAVi. The increase in LVEF and LAVi in G2 and G3 was greater than G1. The increase in the E/E' and LAVi in G3 was greater than G2 [Table 2].
Table 2.
Echo findings at stress
| G1 | G2 | G3 | Between groups | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Stress versus rest | Stress versus rest | Stress versus rest | |||||
| Standard | |||||||
| LVEF (%) | 71±5.7 | 0.001 | 78±4.4 | 0.0001 | 76±3.1 | 0.0001 | 0.014* |
| SVi (ml/mq) | 30.33±3.1 | 37.38±11.8 | 34.5±6.2 | 0.037# | |||
| E/E’ (m/s) | 6.35±2.91 | 5.88±1.92 | 0.043 | 7.91±2.08 | 0.055 | 0.037§ | |
| E’ (m/s) | 0.20±0.05 | <0.001 | 0.16±0.05 | 0.036 | 0.17±0.05 | 0.007 | 0.05 |
| LAVi (ml/mq) | 14.1±2.3 | 17.5±5.1 | 24.0±6.1 | 0.006 | 0.044* 0.001# 0.025§ |
||
| Advanced | |||||||
| GLS-AV (%) | −23.4±1.3 | 0.0001 | −23.6±1 | 0.0001 | −23.7±1.2 | 0.0001 | |
| LS-MB (%) | −20.9±2.1 | 0.002 | −21±1.3 | 0.0009 | −20.2±1.1 | 0.0001 | |
| LS-AP (%) | −28.8±3.3 | 0.0017 | −30.1±4.6 | 0.0004 | −32.1±3.4 | 0.0001 | 0.050# |
| GWI (mmHg %) | 1907±429 | 2378±668 | 0.0185 | 2475±454 | 0.0002 | 0.038* 0.010# |
|
| GCW (mmHg %) | 3318±542 | 0.0001 | 3508±1081 | 0.0009 | 4037±474 | 0.0001 | 0.005# |
| GWW (mmHg %) | 184±114 | 0.0046 | 157±70 | 0.0028 | 239±123 | 0.0072 | 0.040§ |
| GWE (mmHg %) | 94±4 | 0.0413 | 96±2 | 93±3 | 0.035§ | ||
*G2 versus G1, # G3 versus G1, § G3 versus G2. LV=Left ventricular, LVEF=LV ejection fraction, SVi=Stroke volume index by body surface area, LAVi=Left atrial volume indexed by body surface area, E/E’=Ratio between early flow velocity at mitral valve (E) and tissue velocity wave (E’) at mitral annulus, GLS-AV=Global longitudinal strain-average, LS=Longitudinal strain, LS-AP=LS apical segments, LS-MB=LS medio-basal segments, GCW=Global constructive work, GW=Global work, GWE=GW efficiency, GW=GW index, GWW=Global wasted work
Advanced echo
Resting
Only LS-MB in G3 was greater than G1.
Stress
Compared to rest, GLS-AV, LS-MB, LS-AP, GCW, GWW had increased in all groups. In G1, GWE also increased, and in G2 and G3, GWI. In G2 the increase in GWI was greater than in G1. In G3 the increase in LS AP, GWI and GCW was greater than in G1, and that of E/E', LAVi and GWW greater than in G2; GWE in G3 was smaller than in G2.
Changes (Δ%) of echo findings in stress to resting
G3 showed a higher Δ% E/E', LAVi, GWI and GCW than G1 and G2, and a higher Δ%GLS-AV, LS-AP compared to G2. In the analysis of relative regional strain between groups, changes of Δ% of the regional LS [Figure 2a] showed differences only in G3: GLS-AV vs. Δ%LS-AP (P < 0.007), with Δ%GLS-AV < Δ%LS-AP; Δ%GLS-AV vs. Δ%LS-MB (P < 0.004) with Δ%GLS-AV > Δ%LS-MB; Δ%LS-AP vs. Δ%LS-MB (P < 0.002) with Δ% LS-AP > %ΔLS-MB [Table 3]. In the analysis between groups of the relative regional strain ratio, G3 showed a higher value than G1 [Figure 2b]; while, in the analysis within groups, from rest to stress of the relative regional strain ratio, only G3 group showed an increase.
Figure 2.
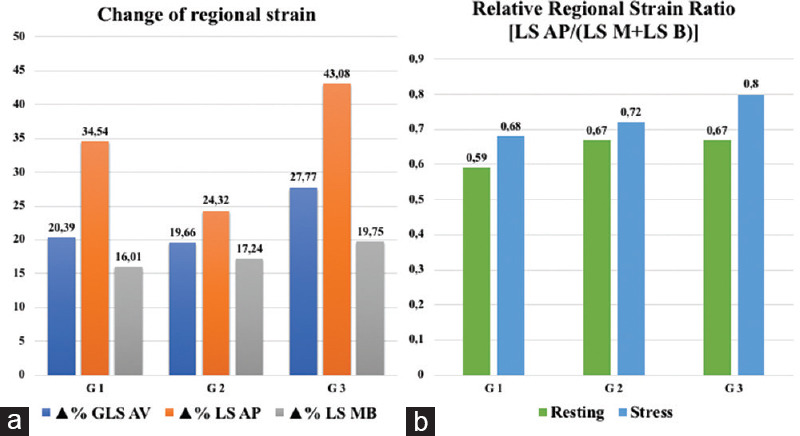
Changes (Δ%) of Echo findings in Stress to Resting. Analysis of relative regional strain within each group (2a) and analysis between groups of the relative regional strain ratio (2b). GLSAV: Global Longitudinal Strain Average; LSMB: Longitudinal Strain Medio-Basal; LSAP: Longitudinal Strain Apical
Table 3.
Changes (Δ%) stress to resting
| G1 | G2 | G3 | P | |
|---|---|---|---|---|
| Standard | ||||
| SVi (ml/mq) | 0.14±32.45 | 9.36±18.28 | 11.63±36.39 | |
| E/E’ (m/s) | −12.96±39.37 | −16.92±18.56 | 20.43±28.89 | 0.044#
0.003§ |
| E’ (m/s) | 83.40±59.78 | 41.87±51.44 | 52.57±49 | |
| LVEF (%) | 19.62±14.15 | 21.32±8.92 | 20.68±10.54 | |
| LAVi (ml/mq) | −11.19±19.67 | 5.32±35.03 | 35.31±29.88 | <0.001#
0.054§ |
| Advanced | ||||
| GLS AV (%) | 20.39±9.14 | 19.66±8.2 | 27.77±8.72 | 0.046§ |
| LS MB (%) | 16.01±12.52 | 17.24±12.69 | 19.75±7.39 | |
| LS AP (%) | 22.36±15.32 | 18.46±11.05 | 28.93±9.46 | 0.029§ |
| GWI (mmHg %) | 17.88±26.4 | 29.8±34.38 | 43.91±21.56 | 0.026# |
| GCW (mmHg %) | 71.58±39.55 | 57.73±36.33 | 104.81±24.94 | 0.037#
0.003§ |
| GWW (mmHg %) | 184.09±151.15 | 136.2±95.46 | 253.59±286.21 | |
| GWE (mmHg %) | −2.4±3.16 | −0.5±1.71 | −1.74±3.73 |
# G3 versus G1, § G3 versus G2. SVi=Stroke volume index by body surface area, LV=Left ventricular, LVEF=LV ejection fraction, E/E’=Ratio between early flow velocity at mitral valve (E) and tissue velocity wave (E’) at mitral annulus, E’=Peak velocity of early diastolic mitral annular motion as determined by pulsed wave doppler, LAVi=Left atrial volume indexed by body surface area, GLS-AV=Global longitudinal strain-average, LS=Longitudinal strain, LS MB=LS medio-basal segments, LS AP=LS apical segments, GW=Global work, GWI=GW index, GWE=GW efficiency, GCW=Global constructive work, GWW=Global wasted work
Pearson's correlations
Resting: G1 showed a positive correlation of GWI and GCW with SBP, negative of GWI with GLS-AV and LS-AP, and of GWE with LS-AP. G2 showed a positive correlation of GWI and GCW with SBP, of GCW with LAVi, negative of both with GLS-AV, of GWI with LS-MB.
Stress: G2 showed a positive correlation of GCW with SBP and LAVi. G3 showed GWW positively with LS-AP and negatively with LAVi; GWE positively with LAVi and negatively with LS-AP.
DISCUSSION
New advanced echocardiographic applications expand the observation on adaptations during peak exercise phases. EA perform isotonic exercise, characterized by normal or reduced peripheral vascular resistance and increased cardiac output, reflecting the high aerobic involvement of large muscle groups.[22,23] The power sports are characterized by isometric exercise, increased peripheral vascular endurance during training, and greater global afterload imposed on the ventricle, which results in concentric rather than eccentric chambers' remodeling.
Our data agree with this pathophysiological background. Our athletes showed LVMi average values greater than the sedentary, but LVIDd slightly less well in G3. The conventional parameters of cardiac function remain normal and did not differ between the groups, while the cardiac deformation characteristics differ between trained athletes aerobic and anaerobic. The wide variations in blood pressure during the workout could alter the vascular structure with deterioration of the elastic fibers of the vessels.[23] The resulting increase in vascular stiffness could therefore have a negative impact on the LV deformational properties and ventricular-arterial coupling. Furthermore, since the use/abuse of anabolic drugs affects a significant impairment of LVGLS as a result of the pro-fibrotic load,[24,25,26] special attention should be paid to the hidden use of illicit drugs.
Our study showed that, at peak of stress, various forms of training lead to specific cardiac adaptation patterns: PA (G3) show changes in SBP and %SBP more than the EA (G2).[27,28] According to Rowland,[28] we have no significant differences between G1 and G2 groups for E' and E/E' at rest and stress. E/E' during stress and its variation Δ% compared to rest, reveal an opposite trend between G1 and G2, these tend to have lower values (improvement) than G3, which tends to increase (worsen), although none showed pathological values.
Furthermore, we found LAVi values during stress lower than the rest phase in G2 and higher in G3. With increased LV stiffness or noncompliance, the left atrium (LA) pressure rises to maintain adequate LV filling, and the higher atrial wall tension leads to chamber dilation and the stretch of the atrial myocardium.[28] In pathological hypertrophy, the compensatory LA contraction is impaired, as a result of both increased workloads imposed on the LA myocardium and of intrinsic LA dysfunction. Since LA function reflects and influences LV diastolic filling, its reduction may contribute to a decrease in LV preload and stroke volume, according to the Frank–Starling mechanism.[29] Sengupta in 24 recreational athletes noted LA dilation immediately after half marathon persisting 72 h after completion, and has suggested a preservation of LVEF but subclinical LV diastolic dysfunction.[30]
The global and regional strain values found at rest are similar to those observed by other studies.[19] In G1 and G2, the contribution provided by the MB and AP regions during Stress is no different and LS-AP is always greater in both the groups. In G2 there is a homogeneous contribution from all myocardial regions, while in G1 AP is greater (in both cases no statistical relevance). In G3, we obtained significant differences between myocardial regions. This data is also confirmed by the analysis of the regional deformation ratio that only in G3 shows differences between the various ventricular regions with a greater apical component than the MB region. Only G3 showed a different trend compared to G2 and a significant increase in LS-AP from rest to stress, probably due to the different modes of cardiac adaptation to stress.
For all groups the same maximum effort was considered for the acquisition of stress images (85% theoretical HR for age). The HR is also similar for all groups. However, the SBP values reached at the peak are different, higher in G3 than G1 and G2, as a result of adaptations to the increase in peripheral resistance. These higher values would be supported by the greater contribution of the ventricular apex during systole. Conversely, the lower contribution of the middle and basal region and the increase of E/E' and LAVi could express an early diastolic dysfunction, similar to what evidenced by Sengupta in young runners immediately after the race.[30]
The resting MW parameters observed in our study are comparable with those obtained in other studies.[21] In the stress phase, G2 and G3 show GWI values greater than G1. This may be related to the fact that it represents the overall work within the PSL.[10,11] GWE is reduced in all groups, but higher in G2 than in G3. This parameter is expresses the contractile efficiency of the myocardium as a percentage of constructive work compared to all the work done by the myocardium, and is not influenced by the BSP values. As GWW and GCW are parameters dependent on GLS and SBP, the basic information is provided to us by GWE, it follows that, although in G3 GCW and GWW are higher, in terms of efficiency G2 shows higher values. This behavior could be linked to the same observations on diastolic function: a higher value of GWW could be due to adaptation to pressure levels, with a consequent increase in the GCW required to support high working values produced not useful for the systole (GWW).
In our study, the workload on the bicycle ergometer was the means to bring the heart to high HR, in order to observe the adaptations of different type of athlete's hearts during effort. The greater workloads sustained by the G2 (cyclists) compared to the G3 (body builder) can be explained by the type of training supported by G2; in fact, specific training for a specific athletic gesture (pedaling) leads to improve athletic performance despite a lower muscle mass, as strength does not depend only on muscle mass. The force is also a nervous phenomenon as specific training improves athletic performance thanks to the ability of the nervous system to recruit specific muscle units more efficiently and equipped with a specific enzymatic pool for that specific type of work.
These results highlight the GLS and MW analysis utility, compared to conventional echocardiographic parameters, to identify functional adaptations. The GLS and MW analysis during ESTE is a novelty in the field of AH study. Our data show a different diastolic function behavior of the power group compared to resistance and control group, and a correlation with the deformation of LV.
Limitations
We need to recognize the limitations of this study.
This is a unicentric study with a small sample and needs a broader application to be able to draw extensive conclusions. Nevertheless, it satisfies our goal of verifying the applicability of the method during stress.
The accentuation of breathing during exercise is a limit to the correct acquisition of images. However, an adequate preparation of the patient before the execution of the test can favor its success.
CONCLUSIONS
The heart adaptations observed in athletes, depending on the type of sport, can be detected early with the new techniques. The use of GLS and MW could open new perspectives for the development of personalized diagnostic-therapeutic protocols for the different “types of AH,” for the early screening of pathological alterations, as well as for personalized rehabilitation programs in heart diseases. Despite the interesting results observed, further multicenter studies with larger samples are needed to confirm and take advantage of our findings.
Ethical clearance
The study was conducted in accordance with the Helsinki Declaration. All participants provided written informed consent before entering the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete's heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 2.Pelliccia A, Caselli S, Sharma S, Basso C, Bax JJ, Corrado D, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. Eur Heart J. 2018;39:1949–69. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Levine BD, Washington RL, Washinghton RL, Baggisc AL, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 3: Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: A scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2356–61. doi: 10.1016/j.jacc.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–4. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- 5.Italian Sports Medicine Federation. Cardiological protocols for the judgment of suitability for competitive sport 2017. Rew of Italian Federation of Sports Medicine. 2018;71:1–5. [Google Scholar]
- 6.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: How useful is it in clinical decision making? Eur Heart J. 2016;37:1196–207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marwick TH. Measurement of strain and strain rate by echocardiography: Ready for prime time? J Am Coll Cardiol. 2006;47:1313–27. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Boe E, Russell K, Eek C, Eriksen M, Remme EW, Smiseth OA, et al. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2015;16:1247–55. doi: 10.1093/ehjci/jev078. [DOI] [PubMed] [Google Scholar]
- 9.Galli E, Leclercq C, Hubert A, Bernard A, Smiseth OA, Mabo P, et al. Role of myocardial constructive work in the identification of responders to CRT. Eur Heart J Cardiovasc Imaging. 2018;19:1010–8. doi: 10.1093/ehjci/jex191. [DOI] [PubMed] [Google Scholar]
- 10.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Gjesdal O, et al. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013;305:H996–1003. doi: 10.1152/ajpheart.00191.2013. [DOI] [PubMed] [Google Scholar]
- 11.Schnell F, Matelot D, Daudin M, Kervio G, Mabo P, Carré F, et al. Mechanical dispersion by strain echocardiography: A novel tool to diagnose hypertrophic cardiomyopathy in athletes. J Am Soc Echocardiogr. 2017;30:251–61. doi: 10.1016/j.echo.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Smiseth OA, Donal E, Penicka M, Sletten OJ. How to measure left ventricular myocardial work by pressure-strain loops. Eur Heart J Cardiovasc Imaging. 2021;22:259–61. doi: 10.1093/ehjci/jeaa301. [DOI] [PubMed] [Google Scholar]
- 13.Hubert A, Le Rolle V, Leclercq C, Galli E, Samset E, Casset C, et al. Estimation of myocardial work from pressure-strain loops analysis: An experimental evaluation. Eur Heart J Cardiovasc Imaging. 2018;19:1372–9. doi: 10.1093/ehjci/jey024. [DOI] [PubMed] [Google Scholar]
- 14.Yang LT, Kado Y, Nagata Y, Otani K, Otsuji Y, Takeuchi M. Strain imaging with a bull's-eye map for detecting significant coronary stenosis during dobutamine stress echocardiography. J Am Soc Echocardiogr. 2017;30:159–67. doi: 10.1016/j.echo.2016.10.011. e1. [DOI] [PubMed] [Google Scholar]
- 15.Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: A novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J. 2009;30:330–7. doi: 10.1093/eurheartj/ehn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Andrea A, Radmilovic J, Carbone A, Mandoli GE, Santoro C, Evola V, et al. Speckle tracking evaluation in endurance athletes: The “optimal” myocardial work. Int J Cardiovasc Imaging. 2020;36:1679–88. doi: 10.1007/s10554-020-01871-z. [DOI] [PubMed] [Google Scholar]
- 17.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–10. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 18.Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:101–38. doi: 10.1016/j.echo.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Galderisi M, Lomoriello VS, Santoro A, Esposito R, Olibet M, Raia R, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: A speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23:1190–8. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Esposito R, Galderisi M, Santoro C, Imbriaco M, Riccio E, Maria Pellegrino A, et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naïve Anderson-Fabry disease patients. Eur Heart J Cardiovasc Imaging. 2019;20:438–45. doi: 10.1093/ehjci/jey108. [DOI] [PubMed] [Google Scholar]
- 21.Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;20:582–90. doi: 10.1093/ehjci/jey188. [DOI] [PubMed] [Google Scholar]
- 22.Monte IP, Mangiafico S, Buccheri S, Bottari VE, Lavanco V, Arcidiacono AA, et al. Myocardial deformational adaptations to different forms of training: A real-time three-dimensional speckle tracking echocardiographic study. Heart Vessels. 2015;30:386–95. doi: 10.1007/s00380-014-0520-9. [DOI] [PubMed] [Google Scholar]
- 23.D’Andrea A, Limongelli G, Caso P, Sarubbi B, Della Pietra A, Brancaccio P, et al. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete's heart. Int J Cardiol. 2002;86:177–84. doi: 10.1016/s0167-5273(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 24.Hassan NA, Salem MF, Sayed MA. Doping and effects of anabolic androgenic steroids on the heart: Histological, ultrastructural, and echocardiographic assessment in strength athletes. Hum Exp Toxicol. 2009;28:273–83. doi: 10.1177/0960327109104821. [DOI] [PubMed] [Google Scholar]
- 25.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Picard MH, Hutter AM, Jr, et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail. 2010;3:472–6. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Andrea A, Radmilovic J, Caselli S, Carbone A, Scarafile R, Sperlongano S, et al. Left atrial myocardial dysfunction after chronic abuse of anabolic androgenic steroids: A speckle tracking echocardiography analysis. Int J Cardiovasc Imaging. 2018;34:1549–59. doi: 10.1007/s10554-018-1370-9. [DOI] [PubMed] [Google Scholar]
- 27.Durmić T, Ðjelić M, Gavrilović T, Antić M, Jeremić R, Vujović A, et al. Usefulness of heart rate recovery parameters to monitor cardiovascular adaptation in elite athletes: The impact of the type of sport. Physiol Int. 2019;106:81–94. doi: 10.1556/2060.106.2019.03. [DOI] [PubMed] [Google Scholar]
- 28.Rowland TW, Garrard M, Marwood S, Guerra ME, Roche D, Unnithan VB. Myocardial performance during progressive exercise in athletic adolescent males. Med Sci Sports Exerc. 2009;41:1721–8. doi: 10.1249/MSS.0b013e3181a06cb5. [DOI] [PubMed] [Google Scholar]
- 29.Gabrielli L, Bijnens BH, Brambila C, Duchateau N, Marin J, Sitges-Serra I, et al. Differential atrial performance at rest and exercise in athletes: Potential trigger for developing atrial dysfunction? Scand J Med Sci Sports. 2016;26:1444–54. doi: 10.1111/sms.12610. [DOI] [PubMed] [Google Scholar]
- 30.Sengupta S, Jain R, Burkule N, Olet S, Khandheria BK. Myocardial Work Index: A Novel Method for Assessment of Myocardial Function in South Asian Recreational Athletes. J Patient Cent Res Rev. 2020;7:147–56. doi: 10.17294/2330-0698.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]


