Abstract
Introduction:
Induction of general anesthesia is often associated with hypotension and is a common scenario faced by anesthesiologists. Intraoperative hypotension can have detrimental effects and cause various adverse effects leading to an extended hospital stay. Patients' preinduction volume status can have an effect on postinduction blood pressure. Ultrasonography is a useful tool for measuring intravascular volume status. We studied the ability of ultrasonographic measurement of subclavian vein (SCV) and inferior vena cava (IVC) diameter, collapsibility index (CI) to predict hypotension after induction of general anesthesia.
Materials and Methods:
We included 120 patients in our study. SCV measurements during spontaneous and deep inspiration and IVC measurements were taken before induction and postinduction blood pressure was monitored. Patients with mean arterial blood pressure <60 mmHg or with a 30% decrease from baseline were considered to be having hypotension.
Results:
The CI of IVC with a cutoff 37% showed sensitivity of 94% and specificity of 84% which was statistically significant. The CI of 36% of SCV during deep breathing was found to have high sensitivity and specificity of 90% and 87%.
Conclusion:
Our study in spontaneously breathing preoperative patients shows that SCV CI in deep breathing and IVC CI is very sensitive and reliable in predicting postinduction hypotension. Bedside ultrasound measurements can be easily done to obtain valuable information to recognize patients who could be at risk from postinduction hypotension.
Keywords: Inferior vena cava collapsibility index, intraoperative hypotension, subclavian vein collapsibility index
INTRODUCTION
Induction of general anesthesia is often associated with hypotension and is a common scenario faced by anesthesiologists. The degree of hypotension can depend on age, comorbidities, and anesthetic drugs used during general anesthesia.[1] Intraoperative hypotension can have detrimental effects and cause adverse events such as myocardial infarction, stroke, and acute kidney injury,[2] leading to extended hospital stay.[3] According to Monk et al.,[4] every minute of hypotension in the operating room can increase 1-year mortality. Myocardial injury can occur with a decrease in blood pressure, as shown in a recent study.[5] Therefore, preventing early intraoperative hypotension and maintaining hemodynamic stability can improve patient outcomes.[6]
Patients' preinduction volume status can have an effect on postinduction hypotension,[7] considering long periods of fasting for elective surgeries. Although great strides have been made in the area of preoperative optimization in elective surgeries, decreased intravascular volume status seems to be the most common cause of hypotension post induction. It enhances other factors such as hypotensive action of anesthetic drugs. Recognizing latent hypovolemia in a hemodynamically stable individual will give the perioperative physician an opportunity to give adequate fluids before induction.[8] Propofol is a commonly used anesthetic induction agent, administration of which causes hypotension via its myocardial depressant effect and smooth muscle relaxation.[9]
There are several methods of assessing intravascular volume status, most of which are invasive. Ultrasonography, as a useful noninvasive tool, is gaining popularity and is widely used by many anesthesiologists in the operating room with its advantages being reliability and portability.[10] It has no complications as with several other invasive monitoring techniques. The role of inferior vena cava (IVC) measurements in predicting hypotension has been studied previously and found to be useful[7,8] in critical care and in perioperative period. Subclavian vein (SCV) collapsibility assessment could be a good alternative to IVC as noted in the intensive care population.[10] It can be used when screening of IVC is difficult.[11] There are not many studies comparing SCV and inferior vena measurements in predicting postinduction hypotension.
We aimed to assess the ability of ultrasonographic measurement of SCV-diameter, collapsibility index (CI) to predict hypotension after induction of general anesthesia, to assess the ability of ultrasonographic measurement of IVC maximum diameter, CI to predict hypotension after induction of general anesthesia, and to compare the predictive power of IVC CI to SCV CI in predicting general anesthesia-induced hypotension. We also compared the time taken to obtain data from IVC and SCV.
MATERIALS AND METHODS
Study design
This was an observational cohort study.
Study period
The study period was from April 2021 to October 2021.
We included the American Society of Anesthesiologists Physical Status Classification (ASA PS) Classes I and II elective surgical patients undergoing surgery under general anesthesia in the age group 18–60 years.
We excluded patients <18 years and more than 60 years, patients with heart failure, autonomic nervous system disorder, portal hypertension, respiratory distress, valvular heart disease, emergency surgery, anticipated difficult airway, major peripheral vascular disease, and systolic blood pressure more than 180 mmHg and <90 mmHg.
The sample size was calculated assuming at least 70% sensitivity for subclavian CI and 0.46[7] incidence of hypotension after GA, with 80% power, and alpha = 0.05 and a minimum sample size of 96 was required.[12] We included 120 patients to maintain adequate power.
IRB Ethical Committee approval (IEC/10/2021-March 06, 2021) was obtained for our study. Written informed consent was obtained from all eligible patients. On the operating table, the patient was laid supine and routine preinduction monitors were attached and baseline values of heart rate, oxygen saturation, and blood pressure were recorded. If an invasive arterial line was indicated for the surgery, it was inserted before induction. M-Turbo USG system manufactured by FUGIFILM SONOSITE, Inc., USA, was used for IVC and SCV examination. A standard curvilinear probe was used for the measurement of IVC diameter measurement. Once good visualization was obtained, IVC was measured in time–motion mode, 1–2 cm caudal to hepatic vein-IVC junction, in subxiphoid transabdominal long-axis view. Measurement over one respiratory cycle was obtained and diameter between two interior walls was noted.[13]

CI was recorded as a percentage. For patients having CI of more than 50%, data were not included in the study due to ethical concerns.
The same anesthesiologist measured the SCV. Right SCV was measured using a linear array probe, placed in the sagittal plane at deltopectoral triangle.[10] CI was calculated during spontaneous and deep breathing.
Patients were induced according to the standard regimen. Preinduction hemodynamic data were recorded. Midazolam 0.05 mg.kg−1, glycopyrrolate 0.005–0.01 mg.kg−1, and 2–3 μg.kg−1 of fentanyl were given as premedication intravenous (i.v.), followed by 1–2.5 mg.kg−1 of propofol for induction i.v. Tracheal intubation was performed after muscle relaxation with vecuronium 0.1 mg.kg−1 i.v. Anesthesia was maintained with isoflurane in oxygen-enriched air. Systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate were recorded every minute for invasive and every 2 min with noninvasive blood pressure monitoring during the 10-min period before skin incision was done.
Patients with mean arterial blood pressure <60 mmHg or with a 30% decrease from baseline were considered to be having hypotension.
Hypotension was treated with rescue vasopressors and fluids.
Data processing and analysis
Data were entered into a Microsoft Excel sheet. Statistical analysis was done using R software (R core team, R foundation for statistical computing, Vienna, Austria). Categorical variables were shown as percentage. Data were expressed as mean ± standard deviation for continuous variables. Receiver operator characteristics (ROC) curve analysis was done for SCV and IVC diameter and CI to predict hypotension. Sensitivity and specificity for the optimal cutoff were calculated. P < 0.05 was considered to be statistically significant.
RESULTS
We included data of 120 eligible patients in our study. Three eligible patients had to be excluded because IVC visualization was poor, 8 patients had IVC-CI was more than 50% and could not be included due to ethical concerns, and 2 patients had to be excluded due to unanticipated difficult intubation. Out of 120 patients, fifty patients developed hypotension. Table 1 shows patient characteristics.
Table 1.
Patient characteristics
| Variables | Value |
|---|---|
| Age (years) | 41.35±9.68 |
| BMI | 26.32±3.02 |
| NPO (h) | 10.56±1.41 |
| Baseline HR (bpm) | 78±7.56 |
| Baseline MAP (mmHg) | 96±8.82 |
BMI=Body mass index, NPO=Nill per oral, HR=Heart rate, MAP=Mean arterial pressure, BPM=Beats per minute
The surgical procedures included gynecology (n = 21), ENT (n = 10), urology (n = 21), spine (n = 24), general surgery (n = 43), and neurosurgery (n = 1). Out of these, 23% of patients had hypertension, 22.5% had diabetes mellitus, 4.1% thyroid disorders, and 0.8% had history of epilepsy. About 44.17% of patients were male and 55.83% were female. Table 2 shows patient characteristics, inferior vena cava, and superior vena cava ultrasound measurements in patients with and without postinduction hypotension.
Table 2.
Patient characteristics, inferior vena cava, and superior vena cava ultrasound measurements in patients with and without postinduction hypotension
| Variables | Hypotension | P | |
|---|---|---|---|
|
| |||
| Yes | No | ||
| Age (years) | 41.2±9.65 | 41.46±9.77 | 0.88 |
| BMI | 26.62±3.18 | 26.10±2.90 | 0.35 |
| NPO (h) | 10.96±1.38 | 10.27±1.36 | 0.007 |
| Baseline HR (bpm) | 78.82±7.28 | 77.41±7.76 | 0.317 |
| Baseline MAP (mmHg) | 96.74±9.53 | 95.47±8.30 | 0.44 |
| SCV spontaneous minimum (cm) | 0.549±0.15 | 0.686±0.16 | 0.0001 |
| SCV spontaneous maximum (cm) | 0.72±0.17 | 0.85±0.18 | 0.001 |
| SCV spontaneous CI (%) | 24.27±5.28 | 19.54±6.47 | 0.001 |
| SCV deep minimum (cm) | 0.45±0.12 | 0.65±0.15 | 0.0001 |
| SCV deep maximum (cm) | 0.77±0.18 | 0.89±0.19 | 0.0005 |
| SCV deep CI (%) | 42.21±6.31 | 26.30±7.80 | 0.0001 |
| IVC minimum (cm) | 1.11±0.17 | 1.48±0.35 | 0.0001 |
| IVC maximum (cm) | 1.88±0.26 | 1.93±0.39 | 0.419 |
| IVC CI (%) | 40.44±3.58 | 23.32±9.42 | 0.0001 |
| Propofol (mg.kg−1) | 1.69±0.18 | 1.71±0.19 | 0.64 |
Values are mean±SD. BMI=Body mass index, NPO=Nil per oral, HR=Heart rate, BPM=Beats per minute, MAP=Mean arterial pressure, SCV=Subclavian vein, IVC=Inferior vena cava, CI: Collapsibility index, SD=Standard deviation
Maximum diameters – Receiver operator characteristics curve analysis
The maximum diameter of SCV during spontaneous and deep inspiration breathing had an optimal cutoff of 0.69 cm (P ≤ 0.001) and 0.7 cm (P = 0.0002), respectively, in ROC curve. The sensitivity was 88% in both cases and similar specificity of 50% and 44% for spontaneous and deep breathing, respectively.
The maximum diameter of IVC had an optimal ROC cutoff of 1.97 cm, with a sensitivity of 44% and specificityof 74% (P = 0.164).
Collapsibility index – Receiver operator characteristics curve analysis
The relationship between postinduction hypotension and SCV-CI during spontaneous breathing (cutoff-23.44%) showed a low sensitivity of only 64% and specificity of 77% [Figure 1], while during deep breathing, CI of 36% [Figure 2] showed a sensitivity of 90% and specificity of 87% and an area under the curve of 0.944 (P < 0.0001). Calculation of IVC-CI and association with significant hypotension with a cutoff of 37% [Figure 3] on ROC curve analysis showed sensitivity of 94% and specificity of 84%, area under the curve was 0.9281 (P < 0.0001).
Figure 1.
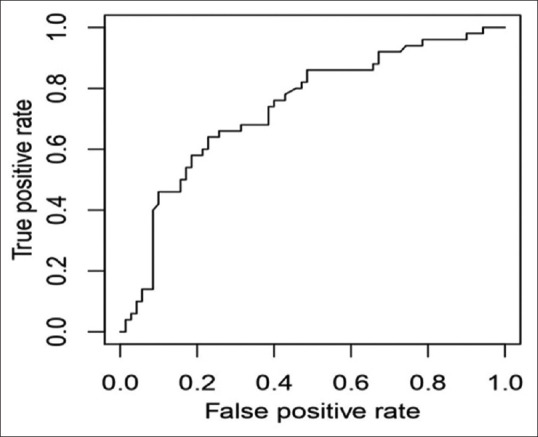
Receiver operator characteristics curve of subclavian vein collapsibility index (spontaneous breathing)
Figure 2.
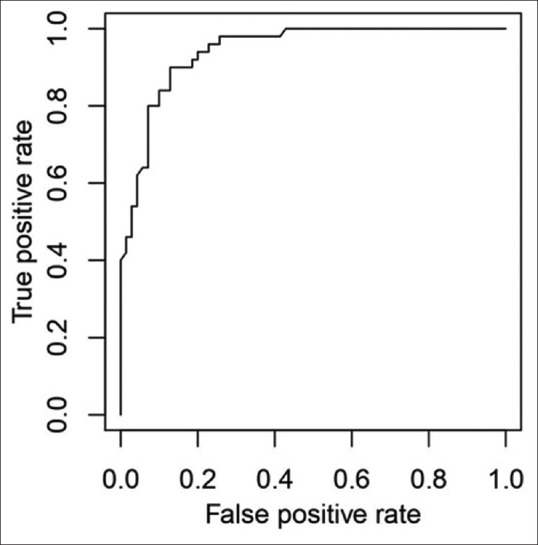
Receiver operator characteristics curve of subclavian vein collapsibility index (deep breathing)
Figure 3.
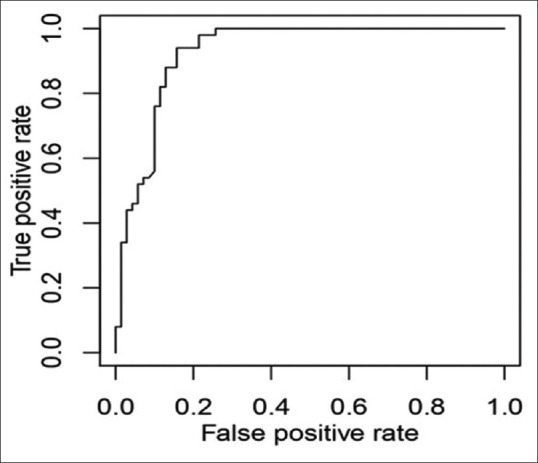
Receiver operator characteristics curve of inferior vena cava collapsibility index
On doing regression analysis between SCV-CI during spontaneous breathing and IVC-CI, a weak linear relationship was noted (R2 = 0.160), while SCV-CI during deep breathing and IVC-CI showed a better linear relationship (R2 = 0.55).
Time taken to obtain data from the two veins: subclavian vein had a mean time of 40.37 s and for IVC, the mean time taken was 48.44 s (P < 0.0001).
DISCUSSION
Low arterial blood pressure can have deleterious effects during noncardiac surgery, however, short it may be.[14] The definition of intraoperative hypotension including early intraoperative hypotension varies with some using systolic blood pressure and others using mean arterial blood pressure.[15,16] Any degree of intraoperative drop in arterial blood pressure can have adverse outcomes. This can be prevented if causative factors are identified. We have considered MAP as an indicator of hypotension with >30% decrease from baseline or a fall in MAP to <60 mmHg. Hypotension after general anesthesia before any surgical intervention is attributed either to patient factors and/or anesthetic agents.[15] Our measurements were taken before any surgical intervention, and the lowest value in the first 10 min after the administration of induction agent was taken rather than the average of values.
Propofol by itself can cause hypotension due to its pharmacodynamic effects, but we used the same agent for all the study participants at the prescribed induction dose of 1–2.5 mg.kg−1. The induction dose of propofol varied depending on the response of the patient. The propofol dose among patients with postinduction hypotension was 1.69 ± 0.18 mg.kg−1 and without postinduction hypotension was 1.71 ± 0.19 mg.kg−1.
All our patients were fasting for more than 8 h. As per the ASA fasting guidelines for elective surgery, the recommended fasting time for solids is 8 h and clear liquids is 2 h,[17] but our patients were fasting overnight for liquids also. Fluid responsiveness implies an increase in stroke volume after volume expansion[18] and its relation to collapsibility index of IVC varies, as the index cutoff varies in different studies.[19] There is a paucity of studies for subclavian vein, and hence, there is no known value of collapsibility index which could predict fluid responsiveness or postinduction hypotension. The collapsibility index of 36% of subclavian vein during deep breathing was found to have high sensitivity and specificity of 90% and 87% in our study. We could not find other studies in literature to compare our findings. The value was close to the IVC collapsibility index and also showed a positive linear relationship. The collapsibility index of IVC with a cutoff 37% showed sensitivity of 94% and specificity of 84% which was statistically significant. Zhang and Lester have shown a cutoff value of more than 43% for predicting postinduction hypotension after general anesthesia.[7] A collapsibility index of more than 40% was found to be indicative of fluid responsiveness in a previous study by Muller et al.[20,21] In a study on spontaneously breathing sepsis patients, an IVC-CI value of more than 32% was found to be predictive of volume responsiveness.[22] A collapsibility index of more than 42% was found to have high specificity in intensive care patients by Airapetian et al.[23] IVC-CI value of more than 25% was found to be indicative of fluid responsiveness and it was found that the performance of the value was better when measurements were done by experts rather than beginner sonologists.[24]
The maximum diameter of IVC did not have significant predictive power for postinduction hypotension in our study. The maximum diameter of IVC has shown to be predictive of fluid responsiveness in some studies, while contradictory results have also been seen.[22,23] The IVC diameter size shows wide variations in healthy individuals.[25] The maximum diameter of subclavian vein with a cutoff 0.69 cm during spontaneous breathing and 0.70 cm during deep breathing was found to have a significant association with postinduction hypotension in our study.
All of our patients were of south Indian ethnicity and the BMI had a mean of 26.3 ± 3.02, with none of the patients in Classes II or III obesity. The failure rate of IVC visualization was lower as compared to the western population.[25] Only three patients had to be excluded because IVC visualization was poor. In obese patients, IVC visualization can prove to be technically difficult.
Variations may or may not arise due to the method of BP monitoring,[26] but our study had only two patients who required intra-arterial blood pressure monitoring due to the nature of surgery.
The time taken to obtain data from subclavian vein varied from 19 to 82 s with a mean of 40.371 ± 3.16 s (mean ± standard deviation) and from IVC varied from 14 to 107 s with a mean of 48.44 ± 19.40 s. The subclavian collapsibility indices were easier to obtain than the IVC-CI. SCV could be useful and easier in patients in whom IVC visualization would be difficult as in abdominal guarding, rigidity, distension, abdominal dressings, and morbidly obese patients.[10] Subclavian vein collapsibility indices could have lower chances to be influenced by external compression as compared to femoral vein or internal jugular vein which are more superficially placed.[10] Subclavian vein could have even more relevance in emergent situations where the patient may already be hypovolemic or in a state of fluid overload.
Our study has several strengths. We included a heterogeneous surgical population undergoing various noncardiac surgeries. The same two anesthesiologists did the ultrasound measurements thereby interobserver variations were largely avoided. Experience of the operator doing the scan[24] can influence ultrasonographic measurements, the operators in our study had more than 5 years of experience in the use of ultrasonography in a critical care setting as well as in the perioperative period. Measurements of IVC were not taken at its junction with the right atrium, as it is advisable not to do so for collapsibility index measurements.[27] The site of measurement of scanning may change the measured values. Measuring IVC close to the right atrium reduces the incidence of motion artifacts but could be affected by diaphragmatic contractions.[13] We consistently tried to take measurements from similar sites for both IVC and subclavian vein.
Older populations are more prone to have hemodynamic fluctuations.[1,28] Our patients were in the age group of 18–60 years and patients above 50 years in our study were 17.5%. We included only ASA PS Classification Classes I and II patients to eliminate hemodynamically unstable patients. Patients with ASA PS Classes more than II are more susceptible to have hypotension post induction together with other adverse effects.[29]
Our study has a few limitations. We did not include data of patients with IVC-CI ≥50% in our study due to ethical concerns. We did not include ASA PS Class III and above patients. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were discontinued on the day of surgery according to the institutional practice. Twenty-three percent of patients were hypertensive in our study. There is a conflicting opinion regarding the continuation or discontinuation of these drugs before surgery.[30] We did not study the association between these drugs and hypotension and no subgroup analysis was done.
Studies with larger sample sizes and diverse population including pediatric population are required to validate the findings of our study and to assess the correlation between subclavian vein, IVC indices, and other modalities of intravascular volume assessment.
CONCLUSION
Our study in spontaneously breathing preoperative patients shows that subclavian vein collapsibility index in deep breathing and IVC collapsibility index could be very sensitive and reliable in predicting postinduction hypotension in patients undergoing general anesthesia. SCV CI is comparable to IVC CI in deep breathing. Bedside ultrasound measurements can be easily done to obtain valuable information to recognize patients who could develop postinduction hypotension and thereby help the anesthesiologist to take the necessary precautionary steps which will help a long way in improving outcome and reducing morbidity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jor O, Maca J, Koutna J, Gemrotova M, Vymazal T, Litschmannova M, et al. Hypotension after induction of general anesthesia: Occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth. 2018;32:673–80. doi: 10.1007/s00540-018-2532-6. [DOI] [PubMed] [Google Scholar]
- 2.Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: An observational study. Eur J Anaesthesiol. 2018;35:273–9. doi: 10.1097/EJA.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 4.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 5.Hallqvist L, Mårtensson J, Granath F, Sahlén A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: An observational study. Eur J Anaesthesiol. 2016;33:450–6. doi: 10.1097/EJA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 6.Temesgen N, Fenta E, Eshetie C, Gelaw M. Early intraoperative hypotension and its associated factors among surgical patients undergoing surgery under general anesthesia: An observational study. Ann Med Surg (Lond) 2021;71:102835. doi: 10.1016/j.amsu.2021.102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Critchley LA. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124:580–9. doi: 10.1097/ALN.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 8.Szabó M, Bozó A, Darvas K, Horváth A, Iványi ZD. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anesthesia: An observational study. BMC Anesthesiol. 2019;19:139. doi: 10.1186/s12871-019-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider AC. Propofol compared to etomidate inductions and attenuating propofol induced hypotension. J Anaesth Crit Care. 2017:8. doi: 10.15406/jaccoa. 2017.08.00296. [Google Scholar]
- 10.Kent A, Bahner DP, Boulger CT, Eiferman DS, Adkins EJ, Evans DC, et al. Sonographic evaluation of intravascular volume status in the surgical intensive care unit: A prospective comparison of subclavian vein and inferior vena cava collapsibility index. J Surg Res. 2013;184:561–6. doi: 10.1016/j.jss.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Choi MH, Chae JS, Lee HJ, Woo JH. Pre-anaesthesia ultrasonography of the subclavian/infraclavicular axillary vein for predicting hypotension after inducing general anaesthesia: A prospective observational study. Eur J Anaesthesiol. 2020;37:474–81. doi: 10.1097/EJA.0000000000001192. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Fine J. On sample size for sensitivity and specificity in prospective diagnostic accuracy studies. Stat Med. 2004;23:2537–50. doi: 10.1002/sim.1836. [DOI] [PubMed] [Google Scholar]
- 13.De Backer D, Fagnoul D. Intensive care ultrasound: VI. Fluid responsiveness and shock assessment. Ann Am Thorac Soc. 2014;11:129–36. doi: 10.1513/AnnalsATS.201309-320OT. [DOI] [PubMed] [Google Scholar]
- 14.Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Kouz K, Hoppe P, Briesenick L, Saugel B. Intraoperative hypotension: Pathophysiology, clinical relevance, and therapeutic approaches. Indian J Anaesth. 2020;64:90–6. doi: 10.4103/ija.IJA_939_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vos JJ, Scheeren TW. Intraoperative hypotension and its prediction. Indian J Anaesth. 2019;63:877–85. doi: 10.4103/ija.IJA_624_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–93. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 18.Preau S, Bortolotti P, Colling D, Dewavrin F, Colas V, Voisin B, et al. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit Care Med. 2017;45:e290–7. doi: 10.1097/CCM.0000000000002090. [DOI] [PubMed] [Google Scholar]
- 19.Long E, Oakley E, Duke T, Babl FE. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: A systematic review and meta-analysis. Shock. 2017;47:550–9. doi: 10.1097/SHK.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 20.Kalshetty K, Jahan N, Setlur R, Jaiswal A, Dwivedi D. Inferior vena cava collapsibility index for the assessment of fluid responsiveness among spontaneously breathing preoperative fasting patients – An observational study. J Marine Med Soc. 2020;22:151–5. [Google Scholar]
- 21.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care. 2012;16:R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagi AI, Shafik AM, Abdel Fatah AM, Selima WZ, Fathy A. Inferior vena cava collapsibility index as a predictor of fluid responsiveness in sepsis-related acute circulatory failure. Ain Shams J Anesthesiol. 2021;13:75. [Google Scholar]
- 23.Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400. doi: 10.1186/s13054-015-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corl KA, Azab N, Nayeemuddin M, Schick A, Lopardo T, Zeba F, et al. Performance of a 25% inferior vena cava collapsibility in detecting fluid responsiveness when assessed by novice versus expert physician sonologists. J Intensive Care Med. 2020;35:1520–8. doi: 10.1177/0885066619881123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhao L. Volume assessment by inferior vena cava examination: Bedside ultrasound techniques and practical difficulties. Curr Anesthesiol Rep. 2017;7:416–20. [Google Scholar]
- 26.Liu B, Li Q, Qiu P. Comparison between invasive and non-invasive blood pressure in young, middle and old age. Blood Press. 2016;25:155–61. doi: 10.3109/08037051.2015.1110935. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DJ, Allison M, Stone MB. Inferior vena cava percentage collapse during respiration is affected by the sampling location: An ultrasound study in healthy volunteers. Acad Emerg Med. 2010;17:96–9. doi: 10.1111/j.1553-2712.2009.00627.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki S, Kiyohara C, Tokunaga S, Hoka S. Prediction of hemodynamic fluctuations after induction of general anesthesia using propofol in non-cardiac surgery: A retrospective cohort study. BMC Anesthesiol. 2018;18:167. doi: 10.1186/s12871-018-0633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 30.Roshanov PS, Rochwerg B, Patel A, Salehian O, Duceppe E, Belley-Côté EP, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An analysis of the vascular events in noncardiac surgery patIents cOhort evaluatioN prospective cohort. Anesthesiology. 2017;126:16–27. doi: 10.1097/ALN.0000000000001404. [DOI] [PubMed] [Google Scholar]


