Abbreviations
- AGEs
advanced glycation endproducts
- BMI
body mass index
- CEL
N‐epsilon‐(1‐carboxyethyl)‐lysine
- CI
confidence intervals
- CML
N‐epsilon‐(carboxymethyl)‐lysine
- DQ
dietary questionnaires
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HR
hazard ratios
- MG‐H1
N‐delta‐[5‐hydro‐5‐methyl‐4‐imidazolon‐2‐yl]‐ornithine
- RAGE
receptor of AGEs
- SD
standard deviation
- sRAGE
soluble receptor of AGEs
- UPLC‐MS/MS
ultra‐performance liquid chromatography‐tandem mass spectrometry
Dear Editor,
In the European region, which shares 22.8% of the global cancer burden for 10% of the global population, there were around 4.4 million new cancer cases and 1.9 million deaths from cancer in 2020 [1]. The reasons for the high cancer incidence rates are complex; however, diet and dietary components are among the main contributors to cancer risk [2]. In modern‐day living, a growing proportion of people include in their diets ultra‐processed foods. Byproducts of food processing and home‐prepared foods are so‐called dietary advanced glycation endproducts (AGEs), which are reactive metabolites emerging during the breakdown of reducing sugar. AGEs production is preponderant in dry high‐heat processes (e.g., baking, roasting); hence foods such as cakes, crisps, crackers, cereal products, meat and meat‐derived products represent a major source of dietary AGEs [3].
AGEs have been associated with chronic inflammatory diseases and may also play a role in carcinogenesis. However, the evidence from prospective human studies of the potential involvement of dietary AGEs in cancer development is limited [4, 5, 6].
This study aimed to examine the association between the intake of three well‐characterized dietary AGEs, N‐epsilon‐carboxymethyl‐lysine (CML), N‐espsilon‐1‐carboxyethyl‐lysine (CEL), and N‐delta‐(5‐hydro‐5‐methyl‐4‐imidazolon‐2‐yl) ornithines (MG‐H1), and the risk of overall and site‐specific cancers in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
After exclusions (Supplementary Figure S1), a sample of 450,111 participants (70% women) was available for analysis (Supplementary Table S1). After a median follow‐up of 14.9 years, 48,702 participants were ascertained with a first primary cancer (Supplementary Materials and Methods). The mean intakes of dietary AGEs (1 standard deviation, SD) were 2.2 mg/day (0.93), 3.1 mg/day (1.3), and 21.7 mg/day (9.7) for CEL, CML, and MG‐H1, respectively (data not shown).
None of the three AGEs (per 1 SD increment) was associated with the risk of overall cancer (Figure 1, Supplementary Figure S2 and S3). Weak positive associations were observed between a higher intake of MG‐H1 and the risk of multiple myeloma (HR1SD: 1.10; 99% CI: 0.97–1.25) and prostate cancer (HR1SD: 1.03; 99% CI: 1.00–1.07), respectively (Figure 1). However, the 99% CI of these associations also included the null. Positive associations with other site‐specific cancers were less pronounced, had wider CIs, or were less robust among never smokers (Figure 1, Supplementary Figure S2 and S3). In contrast, strong inverse associations were observed between higher intakes of MG‐H1, CML, and CEL and the risk of laryngeal cancer with HR1SD of 0.75 (99% CI: 0.62–0.91), 0.74 (99% CI: 0.61–0.90), and 0.76 (99% CI: 0.63−0.92), respectively (Figure 1, Supplementary Figure S2 and S3). MG‐H1 was inversely associated with esophageal cancer risk (HR1SD: 0.90; 99% CI: 0.78–1.04) (Figure 1). A similar inverse association was observed between CML and the risk of adenocarcinoma of the esophagus (Supplementary Figure S2). All other associations of the three AGEs with site‐specific cancers were either less pronounced or null (Figure 1, Supplementary Figure S1 and S2).
FIGURE 1.
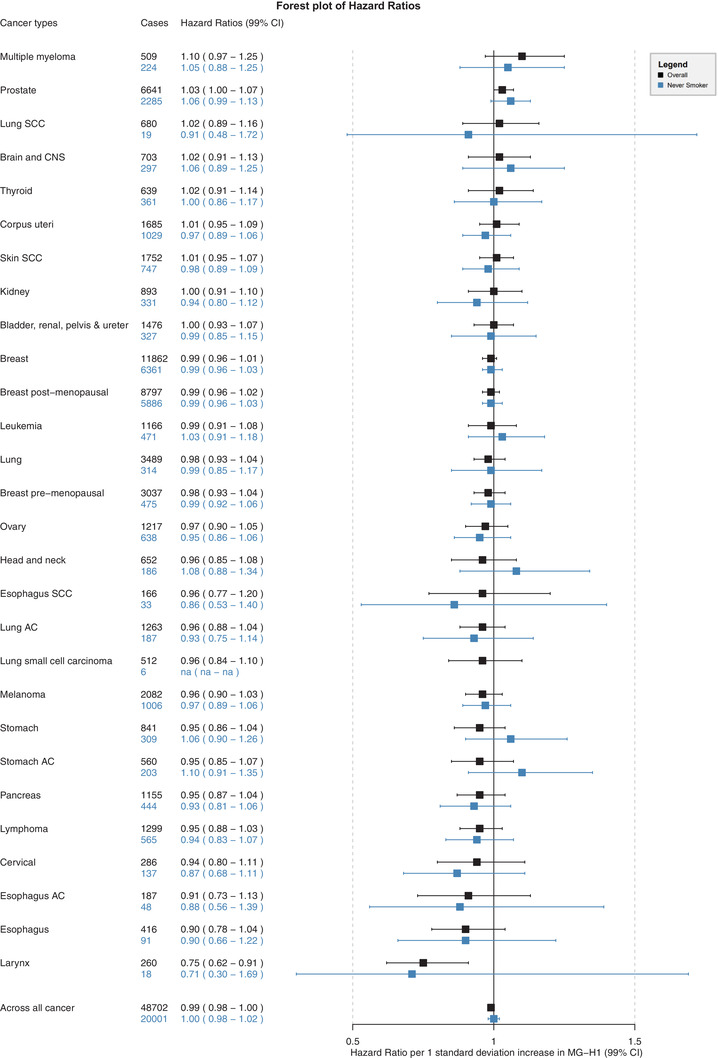
Hazard ratios and 99% confidence intervals (CI) for associations between MG‐H1 and risk of cancer overall and several individual cancer sites in the full cohort and in never smokers. MG‐H1, N‐delta‐(5‐hydro‐5‐methyl‐4‐imidazolon‐2‐yl)‐ornithine; CNS, central nervous system; AC, adenocarcinoma; SCC, squamous cell carcinoma; na, not applicable ‐ sample size for lung small cell carcinoma was too low to estimate associations among never smokers. Number of cases and hazard ratios (99% CI) are shown for full cohort (in black) and among never smokers (in blue). Hazard ratios and 99% CI per 1 standard deviation increase in MG‐H1 derived from Cox proportional hazard models. Each model was adjusted for total energy intake (kcal/day), BMI (kg/m2), baseline alcohol intake (g/day), smoking intensity (never, currently smokes 1‐15 cigarettes/day, currently smokes 16‐25 cigarettes/day, currently smokes 26 + cigarettes/day, former smoker who quit less than 10 years ago, former smoker who quit 11‐20 years ago, former smoker who quit more than 20 years ago, current occasional smoker of pipes or cigars, and missing), the Cambridge physical activity index (inactive, moderately inactive, moderately active, active, and missing), educational level (none, primary completed, technical/professional, longer education incl. university degree, and missing), self‐reported prevalent diabetes (no, yes, missing), and the modified relative Mediterranean Diet Score (mrMDS). Models were stratified by sex, age at recruitment (1‐year categories), and center; however, depending on the anatomical cancer site, additional stratification variables were applied to achieve proportionality of hazard assumption
For five cancer sites, there was evidence for non‐linear associations as modeled with restricted cubic splines (Supplementary Figure S4). These associations were U‐shaped, and the 99% CI included the null over a wide range of AGE intakes, except for prostate cancer. The HR for the risk of prostate cancer comparing the 90th percentile of MG‐H1 intake (33.6 mg/day) to the 10th percentile (11.8 mg/day) was 1.09 (99% CI: 1.01–1.16) (Supplementary Figure S4).
The inverse associations between higher dietary intakes of the three AGEs and the risk of cancer of the larynx were robust in sensitivity analyses. The point estimate of associations was similar among never smokers, although the estimates were more imprecise because of the reduced sample size (Figure 1, Supplementary Figure S2 and S3). The P‐values for heterogeneity across levels of smoking status were equal to 0.65, 0.94, and 0.74 for MG‐H1, CML, and CEL, respectively (Supplementary Table S2). Similarly, there was no evidence for effect modification across subgroups of sex, age, geographical region, prevalent diabetes, or BMI categories (Supplementary Table S2). Furthermore, there was no evidence for residual confounding by alcohol consumption for the associations with laryngeal cancer (Supplementary Table S3). In sensitivity analysis for other alcohol‐related cancers, replacing the alcohol adjustment of baseline alcohol intake with lifetime alcohol drinking patterns led to a stronger inverse association between MG‐H1 intake and the risk of esophageal cancer (HR1SD: 0.85; 99% CI: 0.75–0.98) (Supplementary Table S3). Additional adjustment for main food sources of AGEs (Supplementary Table S4), i.e., red and processed meat, fish, cereals and cereal products, cakes and biscuits, dairy, and ultra‐processed foods, did not alter associations with the risk of laryngeal cancer nor across all cancer (Supplementary Table S5).
Previous clinical and experimental studies suggested that AGEs are proinflammatory, increase oxidative stress and activate pro‐carcinogenic transcription factors such as NFκB and STAT3 as well as cell signaling pathways like mitogen‐activated protein kinase (MAPK) by binding on the receptor for AGEs (RAGE) [7]. These are characteristics potentially relevant to many different cancers. However, besides RAGE, AGEs can bind to other cell surface receptors like AGER1, which has a highly AGE‐specific binding capacity. Like RAGE, AGER1 is present in most cells and tissues, including macrophages, mesangial cells, and mononuclear cells and expedites the absorption and clearance of AGEs. Importantly, AGER1 inhibits AGE‐RAGE activation and RAGE signaling pathways as well as the generation of ROS and proinflammatory cytokines [7]. Another potential explanation of the observed null associations in our study involves the soluble RAGE (sRAGE) receptor, which acts as a possible defense system that supports the body against the adverse effects of AGE/RAGE interaction [8]. We speculate that the mechanism of the observed inverse association with laryngeal cancer could also be indirect through reduced laryngopharyngeal reflux rather than systemic. Ingestion of fatty and/or spicy foods relax the lower esophageal sphincter, delay stomach emptying, and lead to acid reflux, causing cancer‐promoting chronic damage. In contrast, foods rich in AGEs, such as cereals, cakes, biscuits, or stewed meats, pass more quickly through the stomach and may be less implicated in acid reflux.
Conflicting with the concept of RAGE acting as a potent trigger of tumor growth and malignant conversion are reports that RAGE might also have tumor‐suppressive functions in certain cell types [9, 10]. For example, RAGE is highly expressed in lung tissue, and its expression is significantly reduced in lung carcinomas [10]. This may suggest that lung cancer progressions are intensified by the loss of RAGE function [9]. Indeed, re‐expression of RAGE in lung tumor cell lines reduced their proliferation and diminished tumor growth in athymic mice [10].
The results of our study should also be interpreted with consideration of the following limitations. First, collecting diet and other lifestyle information only at baseline did not allow to account for potential changes in diet or lifestyle during follow‐up. Cooking and/or processing methods and conditions, at home and commercially, can increase AGE content in foods. Although we accounted for these preparation methods as much as possible when we assigned AGE values to consumed foods, we cannot completely rule out measurement error bias in assessing dietary AGEs exposure. Last, there always remains the possibility of unmeasured confounding.
In conclusion, in this large multinational prospective cohort study across 20 anatomical cancer sites, a higher intake of dietary AGEs was not associated with an increased risk of overall cancer and most cancer types studied. A non‐linear weak positive association was observed between higher intakes of MG‐H1 and the risk of prostate cancer. In contrast, a strong inverse association was observed with the risk of laryngeal cancer. These findings do not support the hypothesis that dietary AGEs contribute to a higher cancer incidence.
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceived and designed the study: HF, MJ. Analyzed the data: RC, HF. Supported data analysis: ALM, EKA, VK, RC, CS. Wrote the manuscript: RC, HF. Has primary responsibility for the final content of the manuscript: HF. Critically reviewed the manuscript for important intellectual content and approved the final version: RC, ALM, VK, EKA, CS, KW, KO, AT, CK, VAK, CLC, MBS, AB, DP, SG, FP, AC, TMS, ITG, GS, MC, EM, PA, SMC, EA, ID, JM, IJ, AE, AP, EW, MJ, HF.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval for the EPIC study was obtained from the International Agency for Research on Cancer and the ethical review boards of the participating institutes. All participants provided written informed consent.
FUNDING
Reynalda Córdova is a recipient of a DOC Fellowship of the Austrian Academy of Sciences. This study was funded by the Fondation de France (FDF, grant no. 00081166, HF and RC, and FDF grant no. 00089811, ALM). MJ and EA acknowledge funding by the Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund (WCRF) International grant programme (WCRF 2015‐1391, PI Dr. Mazda Jenab, International Agency for Research on Cancer).
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Supporting information
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the EPIC study participants and staff for their valuable contribution to this research. The authors would also like to especially thank Mr. Bertrand Hemon and Ms. Corinne Casagrande for preparing the EPIC databases. We acknowledge the use of data from the EPIC‐France cohort, PI Gianluca Severi; the EPIC‐Ragusa cohort, PI Rosario Tumino; the Prospect‐EPIC Utrecht cohort, PI Roel Vermeulen; the EPIC‐Asturias cohort, PI J. Ramón Quirós; the EPIC‐Norfolk cohort, PI Nick Wareham; and the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, for their contribution and ongoing support to the EPIC Study.
DATA AVAILABILITY STATEMENT
Data described in the manuscript, code book, and analytic code will be made available upon request. For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐49. [DOI] [PubMed] [Google Scholar]
- 2. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol: Mech Dis. 2016;11:421‐49. [DOI] [PubMed] [Google Scholar]
- 3. Cordova R, Knaze V, Viallon V, Rust P, Schalkwijk C, Weiderpass E, et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur J Nutr. 2020;59(7):2893‐904. [DOI] [PubMed] [Google Scholar]
- 4. Mayén AL, Aglago EK, Knaze V, Cordova R, Schalkwijk CG, Wagner KH, et al. Dietary intake of advanced glycation endproducts and risk of hepatobiliary cancers: a multinational cohort study. Int J Cancer. 2021;149(4):854‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aglago EK, Mayén AL, Knaze V, Freisling H, Fedirko V, Hughes DJ, et al. Dietary advanced glycation end‐products and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) study. Nutrients. 2021;13(9):3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiao L, Stolzenberg‐Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH‐AARP Diet and Health Study. Am J Clin Nutr. 2015;101(1):126‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Q, Wang Y, Fu L. Dietary advanced glycation end‐products: Perspectives linking food processing with health implications. Compr Rev Food Sci. 2020;19(5):2559‐87. [DOI] [PubMed] [Google Scholar]
- 8. Prasad K, Mishra M. AGE–RAGE stress, stressors, and antistressors in health and disease. Int J Angiol. 2018;27(01):001‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riehl A, Németh J, Angel P, Hess J. The receptor RAGE: bridging inflammation and cancer. Cell Commun Signal. 2009;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartling B, Demling N, Silber R‐E, Simm A. Proliferative stimulus of lung fibroblasts on lung cancer cells is impaired by the receptor for advanced glycation end‐products. Am J Respir Cell Mol Biol. 2006;34(1):83‐91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request. For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php


