Abstract
In China, lung cancer is a primary cancer type with high incidence and mortality. Risk factors for lung cancer include tobacco use, family history, radiation exposure, and the presence of chronic lung diseases. Most early‐stage non‐small cell lung cancer (NSCLC) patients miss the optimal timing for treatment due to the lack of clinical presentations. Population‐based nationwide screening programs are of significant help in increasing the early detection and survival rates of NSCLC in China. The understanding of molecular carcinogenesis and the identification of oncogenic drivers dramatically facilitate the development of targeted therapy for NSCLC, thus prolonging survival in patients with positive drivers. In the exploration of immune escape mechanisms, programmed cell death protein 1 (PD‐1)/programmed death‐ligand 1 (PD‐L1) inhibitor monotherapy and PD‐1/PD‐L1 inhibitor plus chemotherapy have become a standard of care for advanced NSCLC in China. In the Chinese Society of Clinical Oncology's guidelines for NSCLC, maintenance immunotherapy is recommended for locally advanced NSCLC after chemoradiotherapy. Adjuvant immunotherapy and neoadjuvant chemoimmunotherapy will be approved for resectable NSCLC. In this review, we summarized recent advances in NSCLC in China in terms of epidemiology, biology, molecular pathology, pathogenesis, screening, diagnosis, targeted therapy, and immunotherapy.
Keywords: non‐small cell lung cancer, screening, targeted therapy, immunotherapy, epidermal growth factor receptor (EGFR) mutation, programmed cell death protein 1 (PD‐1), programmed death‐ligand 1 (PD‐L1), clinical trials, clinical guidelines
Abbreviations
- ABCP
atezolizumab‐bevacizumab‐carboplatin‐paclitaxel
- ADC
antibody drug conjugate
- ALK
anaplastic lymphoma kinase
- APC
APC regulator of WNT signaling pathway
- ARMS
amplification refractory mutation system
- ATM
ATM serine/threonine kinase
- BCP
bevacizumab‐carboplatin‐paclitaxel
- BLM
BLM RecQ like helicase
- BRCA2
BRCA2 DNA repair associated
- BRAF
BRaf proto‐oncogene, serine/threonine kinase
- BSC
best supportive care
- CanSPUC
Cancer Screening Program in the Urban China
- CCRT
concurrent chemoradiotherapy
- CHEK2
checkpoint kinase 2
- COPD
chronic obstructive pulmonary disease
- CSCO
Chinese Society of Clinical Oncology
- CTCs
circulating tumor cells
- ctDNA
circulating tumor DNA
- DCR
disease control rate
- DDR2
discoidin domain receptor 2
- DFS
disease‐free survival
- DoR
duration of response
- EGFR
epidermal growth factor receptor
- ERBB2
V‐erb‐b2 avian erythroblastic leukemia viral oncogene homolog 2
- FANC
Fanconi anemia complementation group
- FDA
Food and Drug Administration
- FGFR1
fibroblast growth factor receptor 1
- FPR
false positive rate
- GGNs
ground‐glass nodules
- GWAS
genome wide association study
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- ICIs
immune checkpoint inhibitors
- ITT
intention‐to‐treat
- KRAS
kirsten rat sarcoma viral oncogene
- LDCT
low‐dose computed tomography
- LUAD
lung adenocarcinoma
- LUSC
lung squamous cell carcinoma
- mAbs
monoclonal antibodies
- MDT
multidisciplinary team
- MET
MET proto‐oncogene, receptor tyrosine kinase
- MPR
major pathologic response
- MSH6
mutS homolog 6
- NCCN
National Comprehensive Cancer Network
- NCCR
National Central Cancer Registry
- NHC
National Health Commission
- NLST
National Lung Screening Trial
- NMPA
National Medical Products Administration
- NRAS
NRAS proto‐oncogene
- NSCLC
non‐small cell lung cancer
- OR
odds ratio
- ORR
objective response rate
- OS
overall survival
- PAHs
polycyclic aromatic hydrocarbons
- PM
particulate matter
- pCR
pathological complete response
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death‐ligand 1
- PET‐CT
positron emission tomography‐computed tomography
- PFS
progression‐free survival
- PIK3CA, phosphoinositide‐3‐kinase, catalytic
αpolypeptide
- PM2.5
particulate matter with a diameter < 2.5 μm
- PM10
particulate matter with a diameter < 10 μm
- RCT
randomized controlled trial
- RET
transfection proto‐oncogene gene
- ROS1
ROS proto‐oncogene 1,receptor tyrosine kinase
- SDHB
succinate dehydrogenase complex iron sulfur subunit B
- SNPs
single nucleotide polymorphisms
- T‐DM1
ado‐trastuzumab emtansine
- T‐Dxd
trastuzumab deruxtecan
- TKIs
tyrosine‐kinase inhibitors
- TPS
tumor cell proportion score
- TTFields
tumor‐treating fields
- TTF1
transcription termination factor 1
- VATS
video‐assisted thoracoscopic surgery
- WT
wild‐type
- 20ins
exon 20 insertion
- 19del
exon 19 deletion
1. BACKGROUND
Lung cancer is a global health problem, among which non‐small cell lung cancer (NSCLC) accounts for 80%‐85%. According to the global cancer statistics, more than 2 million individuals were estimated to be newly diagnosed with lung cancer annually [1, 2, 3, 4, 5, 6]. Regrettably, about half of all new lung cancer cases were in Asia [3, 4, 5]. Over the past decade, the incidence of lung cancer in the United States dropped by approximately 1%‐3% annually [2]. According to the cancer statistics collected by the American Cancer Society, it was estimated that about 236,740 new cases of lung cancer were diagnosed in the United States in 2022 [2]. The mortality of lung cancer exhibited a descending trend in the United States between 1990 and 2022, with an estimated 130,180 deaths in 2022 [2]. However, in recent years, the number of new cases of lung cancer in China has continued to rise, and lung cancer is still the major reason for cancer‐related deaths worldwide [1, 2, 3, 4, 5, 6].
In this review, we presented an overview of advances in the biology, molecular pathology, pathogenesis, screening, and diagnosis of NSCLC in China. We also summarized and discussed the progress in targeted therapy and immunotherapy with immune checkpoint inhibitors (ICIs) for NSCLC on the basis of current clinical and experimental research data.
2. EPIDEMIOLOGY
Over the past few years, there has been an upward trend of lung cancer incidence and mortality in China. The 2020 global cancer statistics reported by the International Agency for Research on Cancer revealed that an estimated 820,000 new lung cancer diagnoses and 715,000 lung cancer‐related deaths occurred in China in 2020 [3, 4]. According to the national cancer statistics from the National Central Cancer Registry (NCCR) of China in 2015, about 733,300 Chinese patients were newly diagnosed with lung cancer, while 610,200 Chinese patients with lung cancer died (35.92/100,000 for incidence; 28.02/100,000 for mortality) [1]. Remarkably, in China, there were striking differences in the incidence and mortality of lung cancer between different sexes, ages, and regions [1, 6]. According to the 2015 lung cancer statistics from the NCCR of China, the incidence and mortality of lung cancer in males were almost twice as high as those in females (the age‐standardized mortality rate in China: 40.11/100,000 for males vs. 16.54/100,000 for females) [6]. In 2015, of all the newly diagnosed lung cancer cases in China, 520,300 were males and 266,700 were females [6]. The incidence of lung cancer was relatively low in Chinese population under the age of 45 years (28.7 thousands) [1, 6]. However, there were dramatic increases in the incidence and mortality of lung cancer over the age of 45 years. Although the overall incidence of lung cancer was higher in urban areas than in rural areas, the age‐standardized mortality rate was lower among Chinese urban residents (27.82/100,000 vs. 28.25/100,000) [1, 6]. It was estimated that 460,200 urban individuals and 326,800 rural individuals were newly diagnosed with lung cancer in China in 2015 [6].
The high incidence of lung cancer in China might be ascribed to several reasons. Firstly, the initiation, popularization, and application of effective screening tests across the country boost the detection rate of patients with pulmonary nodules and early‐stage lung cancer. Secondly, progress in the accuracy and specificity of diagnostic technology might increase the detection rate of lung cancer. Population aging in China is another important factor since lung cancer is an age‐related disease. Moreover, there are many other risk factors for lung cancer that cannot be ignored, such as smoking history, second‐hand tobacco smoke, and cooking oil fume. During 2000‐2015, the steadily decreasing trend in the mortality of lung cancer in China might be attributed to the promotion of popular science education on lung cancer prevention, high early detection rates, and the development of improved systemic therapies [2, 7–11]. In particular, technological and therapeutic advances have brought remarkable improvements in the outcomes for NSCLC patients.
3. BIOLOGY AND MOLECULAR PATHOLOGY
3.1. Histopathology
Historically, NSCLCs are classified according to histopathological characteristics. The most common histological subtype of NSCLC is adenocarcinoma, while squamous cell carcinoma is the second most common [1, 2, 12]. The proportions of histological subtypes vary according to race [13, 14, 15]. Among all NSCLC subtypes, lung adenocarcinoma (LUAD) accounted for almost 47% of cases in Western patients, while about 55%‐60% of cases in Chinese patients [13, 15]. Among 17,920 Chinese patients who were diagnosed with lung cancer during 1998‐2007, 45.12% (n = 8,085) had LUAD, 28.02% (n = 5,021) had lung squamous cell carcinoma (LUSC), 1.31% (n = 234) had large cell carcinoma, and 0.45% (n = 81) had pulmonary sarcomatoid carcinoma [13]. In 2011 and 2012, the relative frequencies of LUAD, LUSC, and large cell carcinoma in Chinese male patients with lung cancer were 43.36%, 32.23%, and 3.46%, respectively [16]. Among Chinese female patients with lung cancer, the relative frequency of LUAD was the highest in 2011 and 2012 (76.49% had LUAD vs. 5.97% had LUSC vs. 2.83% had large cell carcinoma) [17].
3.2. Genetic alterations
Due to the development of molecular testing and the deepening of tumor‐related pathway research, genetic alterations in Chinese patients with NSCLC were found (Table 1). In a retrospective study recruiting 3,774 Chinese patients with NSCLC, we found that epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma viral oncogene (KRAS) were two of the most common oncogenic alterations (EGFR: 39.0%, 1,470/3,774; KRAS: 8.0%, 80/3,774) [18, 19, 20]. In a Chinese cohort of 884 patients with stage I‐IV lung cancer, the mutation rates of EGFR and KRAS were 57.7% (n = 510) and 10.3% (n = 91), respectively [21]. As shown in Table 1, in the Chinese population with lung cancer, the alteration frequencies of anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), BRaf proto‐oncogene, serine/threonine kinase (BRAF), transfection proto‐oncogene gene (RET), and human epidermal growth factor receptor 2 (HER2) were 2.4%‐5.5%, 7.4%‐10.3%, 0.6%‐1.1%, 0.6%‐1.5%, 1.7%‐4.3%, respectively [18, 19, 20, 21, 22].
TABLE 1.
Frequency of oncogenic alterations in NSCLC patients in China and the United States
| Frequency of oncogenic alterations | ||||
|---|---|---|---|---|
| Driver gene | Zhou et al. [18, 19, 20] | Xing et al. [21] | Chen et al. [22] | The United States [26, 29] |
| EGFR | 39.0% | 57.7% | 50.9% | 17.2% |
| ALK | 5.5% | 2.4% | 4.3% | 3.1% |
| KRAS | 8.0% | 10.3% | 7.4% | 30.8% |
| ROS1 | 2.1% | 0.6% | 0.6% | 1.0% |
| BRAF | 0.6% | 1.1% | 1.1% | 5.0% |
| RET | 1.5% | 0.6% | 1.1% | 1.7% |
| HER2 | 1.7% | 4.3% | 1.9% | 3.0% |
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, BRaf proto‐oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; KRAS, kirsten rat sarcoma viral oncogene; NSCLC, non‐small cell lung cancer; RET, transfection proto‐oncogene gene; ROS1, ROS proto‐oncogene 1,receptor tyrosine kinase.
Data from many NSCLC screening programs indicated histopathological, geographical, and racial specificity in the frequencies of oncogenic driver alterations [18–21, 23–29]. In China, EGFR mutation is the most common genetic alteration in LUAD and non‐/mild‐smokers, while it is less common in LUSC (ranging from 0.2% to 3.9%) [18, 21–26]. In a Chinese LUAD cohort, besides EGFR (63.1%, 855/1,356), other common genetic alterations included ALK (5.2%, 70/1,356), KRAS (8.0%, 108/1,356), ROS1 (0.8%, 11/1,356), BRAF (1.3%, 18/1,356), RET (1.3%, 17/1,356), and HER2 (2.4%, 32/1,356) [22]. In LUSC, an increasing number of driver gene alterations were found, such as fibroblast growth factor receptor 1 (FGFR1), discoidin domain receptor 2 (DDR2), phosphoinositide‐3‐kinase, catalytic, αpolypeptide (PIK3CA), phosphate and tensin homology, and platelet‐derived growth factor receptor [23, 25]. Of 310 Chinese patients with LUSC, two patients harbored FGFR1 fusions (0.6%), and one patient harbored DDR2 alteration (0.2%) [22]. In Chinese patients with LUSC, the mutation rate of PIK3CA was 13.0% (7/54) [21]. Striking ethnic discrepancies were revealed in the frequency of EGFR mutation, ranging from 20% to 76% in Asian patients, and 6% to 36% in European patients [24, 26, 28]. EGFR mutations were more common in Chinese patients than in American patients (Table 1; 39.0%‐57.7% vs. 17.2%) [26]. In 2007, ALK gene rearrangements were firstly identified in NSCLC [30]. Several statistical datasets revealed no obvious ethnic variation in ALK gene rearrangements in NSCLC. The proportion of ALK gene fusion in NSCLC was estimated to range from 2% to 16% in unselected populations, with a slight difference between Asian and Western populations (2.4%‐16.3% vs. 3.0%‐16.4%) [31, 32, 33, 34, 35, 36, 37]. Conversely, the prevalence of KRAS mutation was higher in Caucasian NSCLC patients (20%‐30%) compared with Chinese NSCLC patients [38, 39, 40, 41]. The KRASG12C mutation was the most common type of KRAS mutation. In the Chinese population, KRASG12C mutation prevalence was 3%‐4.6% [42, 43]. KRASG12C showed a 10.5% mutation rate in a European cohort [44].
4. PATHOGENESIS
4.1. Environmental risk factors
Tobacco smoking is considered a leading risk factor for lung cancer (Figure 1) [1, 6, 45]. In China, an age‐period‐cohort analysis based on the Global Burden of Disease study found an increasing trend in smoking‐related deaths from lung cancer between 1990 and 2017 [45]. In 2017, the mortality rate of smoking‐related lung cancer in China was 41.94/100,000 in males and 5.14/100,000 in females [45]. Globally, China accounts for the highest cigarette production and tobacco consumption. According to the 2018 Global Adult Tobacco survey reported by the Chinese Center for Disease Control and Prevention, 308 million Chinese adults were smokers [6]. In the same year, the number of Chinese residents who exposed to second‐hand smoke had reached 732 million [6]. There is growing evidence that approximately 80%‐90% of lung cancer cases were significantly associated with active or passive smoking [2, 46, 47]. According to the cancer statistics reported in 2022, over 80% of lung cancer patients had a history of smoking [2]. Compared with never‐smokers, the risk of lung cancer increased by around 4 to 10 times in smokers, increasing by up to 10 to 25 times in heavy smokers [48]. It was reported that the lower the age a person starts smoking, the higher their risk of lung cancer. Stopping smoking is highly recommended since the risk of lung cancer dropped year by year after cessation [46, 47, 48].
FIGURE 1.
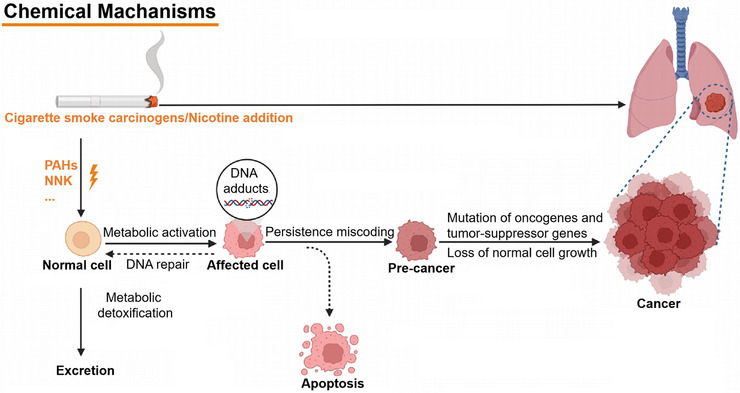
Carcinogenic mechanisms of smoking in lung cancer. Abbreviations: PAHs, polycyclic aromatic hydrocarbons; NNK, nitrosamine 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone.
Air pollution is responsible for about 5% of lung cancer‐related deaths [49, 50, 51, 52]. According to the Global Burden of Disease Study, in China, the mortality of lung cancer attributable to air pollution in 2017 was 9.4/100,000 [53]. In a Chinese cohort, the age‐standardized mortality of lung cancer attributable to ambient air pollution was higher than for indoor air pollution (7.4/100,000 vs. 2.0/100,000) [53]. Common indoor air pollution sources include harmful volatiles from decorating materials and cooking oil fumes [50]. In China, the rate of lung cancer among females who have never smoked might be related closely to the use of unhealthy cooking methods and unventilated kitchens. By means of gas chromatography‐mass spectrometry, Chen et al. [50] found that habitual cooking was an important risk factor for lung cancer among Chinese non‐smoking females, with odds ratios (ORs) of 5.39. In a Chinese cohort of 71,320 never‐smoking females, the researchers demonstrated that inadequate ventilation in kitchens increased the lung cancer risk, with a hazard ratio (HR) of 1.49 [54]. Outdoor air pollution, including emissions from vehicle exhausts and industry, has been blamed as an essential risk factor for lung cancer [51, 52]. In 2017, through comparing air pollution data from 33 Chinese provinces, it was found that the levels of exposure to ambient particulate matter (PM) in Beijing, Hebei, Shandong, Henan, and Xinjiang were higher than those in other regions (63.901‐84.013 μg/m3 vs. 19.020‐63.900 μg/m3) [53]. According to the data extracted from the National Urban Air Quality Real‐time Publishing Platform during 2013‐2015, the daily concentrations of PM with a diameter < 2.5 μm (PM2.5), PM with a diameter < 10 μm (PM10), and ozone in Beijing were higher than those in Chongqing and Guangzhou [55]. The daily levels of PM2.5 and PM10 were significantly related to lung cancer deaths in Chongqing and Guangzhou, while no significance was found in Beijing [55]. Temperature and humidity were significantly associated with the environmental air pollution levels in some cities, municipalities, and special administrative regions in China, including Guangzhou, Beijing, Chongqing, Shanghai, and Hong Kong [55, 56, 57]. When compared with the warm season, higher daily PM2.5, PM10, and sulfur dioxide levels were found in the cold season, indicating that the high incidence and mortality of lung cancer in some cities in China might be related to the composition airborne particulates in the cold season [55, 56]. Moreover, the interaction between temperature and air pollution had a considerable effect on lung cancer incidence and mortality [55]. In China, the harmful influences of environmental pollution caused by rapid urbanization, modernization, and industrialization should be considered. Radiation exposure is a convincing additional risk factor for lung cancer. Radiation promoted the overexpression of oncogenes and the inactivation of tumor suppressor genes, thus leading to the occurrence and progression of lung cancer [58, 59]. Lung cancer‐related occupational exposure included asbestos, methyl chloride, chromium, nickel, and polycyclic aromatic hydrocarbons (PAHs) [60, 61, 62, 63, 64, 65]. In a Chinese cohort involving 2,346 cases, Liu et al. [64] found that exposure to occupational carcinogens was higher in areas with high/middle prevalence of lung cancer than in low‐prevalence areas (58.02%‐65.83% vs. 38.57%, respectively). The production of coke, aluminum, iron, and steel takes place in PAH‐related industries. In 2022, a meta‐analysis of 385 Chinese cases demonstrated that occupational exposure to PAHs dramatically increased the risk of lung cancer (the pooled relative risk: 1.75; 95% confidence interval: 1.33‐2.30) [65]. Together, a combination of various risk factors contributes to the development of lung cancer. Environmental risk factors are largely modifiable. Early detection and active prevention of environmental risk factors are considered as effective ways to reduce the risk of lung cancer.
4.2. Genetic susceptibility
Heredity is a unique risk factor in lung cancer. It was found that about 12%‐21% of lung cancer cases could be attributed to genetic alterations [66]. Genetic susceptibility can be divided into two main categories: single nucleotide polymorphisms (SNPs) and germline mutations of key genes. In lung cancer, some genome wide association studies (GWASs) identified several lung cancer susceptibility loci, including rs2228000 [67], rs2228001 [67], 1p31.1(rs71658797) [68], 6q27(rs6920364) [68], 3q29(rs2131877) [69], 5p15(rs2736100) [69], 1q21.1 (rs17160062) [70], 2p23.3 (rs670343) [70], 2p15 (rs9309336) [70], and 17q21.2 (rs9252) [70]. Meanwhile, several susceptibility loci were also identified among the Chinese population, including 10p14 (rs1663689) [71], 5q32 (rs2895680) [71], 20q13.2 (rs4809957) [71], and 13q12.12 (rs753955) [72]. Of note, through a meta‐analysis of existing GWASs with a total of 13,327 cases and 13,328 controls of Chinese descent as well as 13,793 cases and 14,027 controls of European descent, Dai et al. [73] revealed 19 susceptibility loci to be significantly related to NSCLC risk, among which, 6 were completely novel. Furthermore, after rigorous selection and validation, they successfully constructed a polygenic risk score specific to the Chinese population using 19 SNPs, including 2p14 (rs17038564), 3q26.2 (rs2293607), 3q28 (rs11375254), and 14q13.1 (rs1200399), which could be utilized efficiently to identify the subjects at high risk of lung cancer [73].
Germline mutations of EGFR, V‐erb‐b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2), checkpoint kinase 2 (CHEK2), and cyclin‐dependent kinase inhibitor 2A were detected in lung cancer patients [74, 75, 76]. In a large‐scale study of 31,926 Chinese lung cancer patients, 22 types of EGFR germline mutations were identified in 64 lung cancer individuals (0.2%), among which G863D was the most frequent type of mutation (14.1%, 9/64) [75]. In 12,833 Chinese lung cancer cases, 0.12% harbored EGFR germline mutation (n = 14) [76]. In addition, 8 types of EGFR germline mutations (K757R, R831H, D1014N, G724S, V786M, T790M, L792F, and L844V) and ERBB2‐V1128I germline mutation were identified [76]. In a Chinese cohort of 780 lung cancer patients and 1,113 healthy individuals, Li et al. [77] identified 14 LUAD‐related germline mutations and 9 LUSC‐related germline mutations (both P < 0.05). Concretely, germline mutations of APC regulator of WNT signaling pathway (APC) (OR = 13.897), ATM serine/threonine kinase (ATM) (OR = 25.878), BLM RecQ like helicase (BLM) (OR = 9.941 for rs191789336 and 17.883 for rs189925962), BRCA2 DNA repair associated (BRCA2) (OR = 17.883), BRCA1 interacting helicase 1 (OR = 15.949), cadherin 1 (OR = 9.941), Fanconi anemia complementation group (FANC) A (OR = 13.893), mutL homolog 1 (OR = 21.877), mutS homolog 6 (MSH6) (OR = 45.989), RET (OR = 13.897), succinate dehydrogenase complex iron sulfur subunit B (SDHB) (OR = 17.883), serine peptidase inhibitor Kazal type 1 (OR = 5.49) was associated with increased risk for LUAD, while FANCD2 (OR = 0.146) was associated with decreased risk for LUAD [77]. In LUSC, the ORs for germline mutations of APC, ATM, BLM, BRCA2, CHEK2, FANCC, MSH6, RET, and SDHB were 32.503, 32.503, 21.813, 76.587, 10.901, 21.813, 121.545, 32.503, and 76.587, respectively. Further studies of genetic alterations might help screen lung cancer susceptibility genes and provide optimal surveillance strategies for relatives at risk, leading to the development of genetic counseling and clinically precise diagnoses.
4.3. Other risk factors
Other risk factors, such as age, unhealthy diet, alcohol consumption, chronic disease, social psychology, and body mass index, also had influences on lung cancer development [78, 79, 80, 81, 82, 83, 84, 85, 86]. An epidemiological study indicated that increasing age might have a strong correlation with the incidence of lung cancer [79]. Patients with chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), asthma, and diffuse pulmonary fibrosis, were also at an increased risk of lung cancer [80, 81, 82, 83]. In a Chinese case‐control study involving 2,283 lung cancer cases and 2,323 control cases, a history of COPD appeared to increase lung cancer risk (OR = 2.88) [87]. Similar results were found in another study that recruited 1,069 lung cancer patients and 1,132 cancer‐free individuals from Guangdong Province, China (OR = 1.29) [88]. Moreover, the study results suggested that emphysema (OR = 1.55) and chronic bronchitis (OR = 1.22) indicated a high risk of lung cancer in the Chinese population [88]. The low consumption of fruits and vegetables might raise the risk of lung cancer [64, 89]. When compared with areas of high lung cancer incidence, the proportions of Chinese residents who regularly consumed vegetables and fruits (between 3 and 7 days per week) were higher in the areas of low lung cancer incidence (vegetables: 58.01% vs. 78.90%; fruits: 61.91% vs. 63.56%) [64]. Some studies highlighted the relationship between cancer risk and personality traits [84, 85]. In addition, as a novel epidemiological approach, Mendelian randomization also identified several possible risk factors related to lung cancer, including lifetime cannabis use [90], telomere length [91], insomnia [92], adult height [93], elevated platelet count [94], and high vitamin B12 status [95]. However, the link between lung cancer and the above risk factors remains both elusive and controversial (Figure 2). More long‐term studies are required to investigate and confirm the relationships.
FIGURE 2.
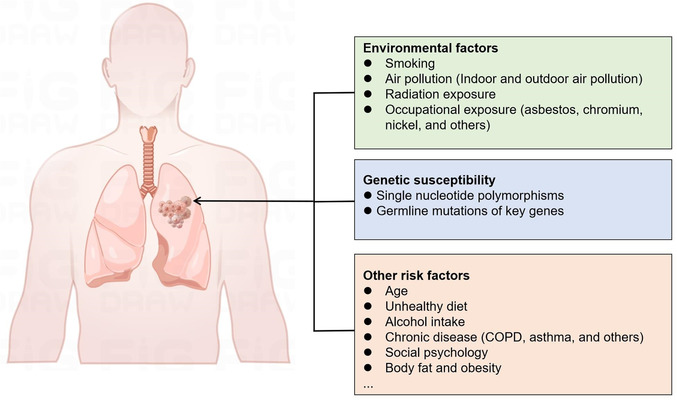
List of environmental factors, genetic susceptibility, and other risk factors for lung cancer. Abbreviations: COPD, chronic obstructive pulmonary disease.
5. SCREENING AND DIAGNOSIS
5.1. Imaging and screening
Imaging examinations have important implications for screening early lung cancer, tracking disease progression, and evaluating therapeutic efficacy [96, 97]. The low‐dose computed tomography (LDCT), as an effective method for improving the prognosis and reducing the mortality of lung cancer, is highly recommended for lung cancer screening in the China Guideline for the Screening and Early Detection of Lung Cancer [98]. This guideline was drafted by a multidisciplinary expert group that was appointed by China's National Health Commission (NHC) and initiated by the National Cancer Center of China. In recent 20 years, with China NHC funding, the Rural People's Republic of China Screening Program and the Cancer Screening Program in the Urban China (CanSPUC) were launched, and more than one million participants have been enrolled [6, 99‐101]. Recent data indicated that hundreds of thousands of individuals underwent LDCT scanning (13,000 rural residents and 163,752 urban residents) [6, 99–101]. The percentage of lung cancer cases in the national screening cohort was nearly 1% [6]. According to preliminary data, the compliance of individuals enrolled in the CanSPUC program raised gradually from 2013 to 2017 [101].
Besides the national screening programs, some lung cancer screening programs with LDCT were also conducted by provinces and hospitals in China. The detection rates for lung cancer were reported as 0.238% (27/11,332) in a Shanghai community study [102], 0.92% (6/650) in a Tianjin study [103], 0.6% (26/4,690) in a research of Cancer Hospital, Chinese Academy of Medical Sciences [104], 0.06% (53/88,596) in a Beijing study [105], and 1.19% (12/1,008) in a Shanxi study [106]. So far, the study of the National Lung Cancer Cohort which aims to collect samples from patients with lung cancer and those at high risk and the China National Cancer Early Screening trial which aims to evaluate the roles of LDCT in cancer mortality are ongoing. The meaningful and positive results from the above‐mentioned national studies are highly anticipated.
The definition of positive screening might affect the false positive rate (FPR) of LDCT in lung cancer. The cutoff values for positive lung nodules in the United States National Lung Screening Trial (NLST) [107] and the International Early Lung Cancer Action Program [108] were 4 mm and 6 mm, respectively. In the NLST study, the FPR of LDCT in lung cancer detection was 96.4% [107]. By applying the NLST definition to Chinese populations, the FPR of LDCT in lung cancer was revealed to be as high as 93.7% [109]. An exploratory study revealed that applying a critical value of 6 mm dramatically decreased the FPR by 35.5% [110]. In a Chinese cohort, the application of 6 mm as the threshold of positive nodules resulted in a 20% reduction in the FPR [111]. In the China Guideline for the Screening and Early Detection of Lung Cancer, the cutoff value for positive lung nodules was 6 mm [98].
To maximize socio‐economic benefits and minimize screening‐associated harms (such as radiation exposure and false positives), the identification of high‐risk populations is of great importance. In China, the guideline drafted by the National Cancer Center assume a screening age of 50‐74 years. When combined with one of the following conditions, those within this range should be regarded as being at high risk: a history of smoking at least 30 packs/year (including former smokers with < 15 years’ cessation), a passive smoking history ≥ 20 years, a history of COPD, a history of occupational exposure ≥ 1 year (to asbestos, radon, beryllium, chromium, cadmium, nickel, silicon, soot, or coal smoke), and first‐degree relatives diagnosed with lung cancer [98].
Generally, lung nodules can be further divided into three types: solid nodules, part‐solid nodules, and pure ground‐glass nodules (GGNs). The flow chart depicts the management and follow‐up recommendations for lung nodules in China (Figure 3) [98]. In the first case, when a pure GGN < 8.0 mm or a solid/part‐solid nodule < 6.0 mm is found, patients should participate in the annual screening next year. When a pure GGN between 8.0‐15.0 mm or the solid component of a solid/part‐solid nodule between 6.0‐15.0 mm is observed, patients are recommended to return for evaluation after 3 months. If the nodule continues to grow, the multidisciplinary team (MDT) assessment is recommended to determine whether clinical intervention is essential. Otherwise, patients ought to join the annual screening program next year. In the last case, if a pure GGN ≥ 15.0 mm or the solid component of a solid/part‐solid nodule ≥ 15.0 mm is found, the two following strategies are recommended. (i) Repeat assessment after 1 month, with or without the use of anti‐inflammatory therapies during this period. If the nodule is completely resolved, then patients are recommended for screening the following year. If the nodule is resolved partially, patients should repeat LDCT after another 3 months. At that time, if the nodule is still enlarging, an MDT evaluation should be performed; otherwise, patients should join the annual screening program the following year. If the nodule is not resolved, then an MDT evaluation is required. (ii) Conduct a biopsy or positron emission tomography‐CT (PET‐CT) examination for the solid/part‐solid nodule. If the result is positive, then an MDT assessment should be taken; otherwise, a repeat evaluation after 3 months is recommended. Depending on the result, patients should undergo an MDT assessment (if the nodule is enlarging or persisting) or participate in the annual screening program next year (if the nodule is resolved) [98].
FIGURE 3.
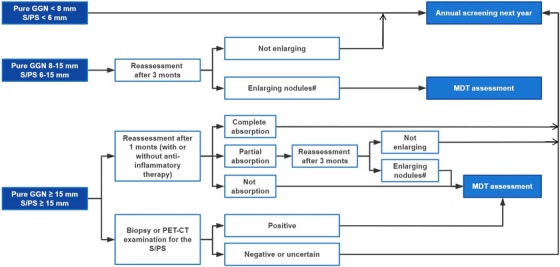
Flow chart for management and follow‐up recommendation of lung nodules in China. Abbreviations: GGN, ground‐glass nodules; MDT, multidisciplinary team; PET‐CT, positron emission tomography‐computed tomography; PS, part‐solid nodules; S, solid nodules; #, increasing diameter ≥ 2 mm.
Regardless of the high sensitivity of the technique, the overdiagnosis of LDCT in lung cancer should not be neglected [112, 113]. Radiomics is a novel technology developed in recent years that might improve the specificity and accuracy of lung cancer diagnosis [114]. Radiomics systems extract and integrate characteristics from CT, magnetic resonance imaging, and PET‐CT images to predict malignancy, histological subtypes, gene mutation status, gene expression levels, and prognosis. In terms of lung cancer diagnosis, several studies highlighted the extraordinary performance of radiomics [115, 116, 117, 118, 119, 120, 121, 122]. In the Chinese population, Ni et al. [118] collected high‐resolution CT images of 1,431 patients with GGNs for the construction of an automatic GGN detector. The accuracy of the automatic network was 85.2%, with 83.7% sensitivity and 86.3% specificity. Progress in radiomics is creating new opportunities for the establishment of accurate imaging diagnoses. Nevertheless, in clinical practice, radiomics is still at the exploratory stage. Plenty of problems lie ahead. For instance, the specificity and accuracy of radiomics diagnostic models need further improvement. The algorithms applied for the construction of lung cancer models require constant refinement as well.
5.2. Histopathology and molecular pathology
Pathology and cytology are still regarded as the gold standard for lung cancer diagnosis. Both cytologic specimens and tissue samples are suitable for pathological diagnosis [123, 124, 125, 126]. In the Chinese Society of Clinical Oncology's (CSCO) Guideline for NSCLC, transcription termination factor 1 (TTF1) and napsin A are specific markers for LUAD, while P40, P63, and cytokeratin5/6 are recommended for identifying LUSC. Therefore, TTF1, P40, P63, and some other factors should be tested using immunohistochemistry to differentiate LUAD and LUSC from other NSCLC subtypes [127].
Molecular testing for gene alterations is recommended as a routine test for patients with newly diagnosed NSCLC. In clinical practice, amplification refractory mutation system (ARMS), immunohistochemistry, polymerase chain reaction‐related technologies, and fluorescence in situ hybridization are widely used. In China, in consideration of detection speed, drug availability, and economic cost, panel‐based testing was more commonly used [128]. The driver gene panel mainly includes EGFR, ALK, KRAS, ROS1, BRAF, RET, HER2, MET proto‐oncogene, receptor tyrosine kinase (MET), neurotrophic tropomyosin receptor tyrosine kinase 3, and NRAS proto‐oncogene (NRAS). A large‐scale, retrospective trial extracted 226,227 lung cancer samples from 49 hospitals to investigate the status of molecular testing in China [129]. By the end of 2019, 11 (22.4%) hospitals provided gene panel testing for lung cancer. It was estimated that the number of molecular testing cases in China grew from 2010 to 2019 (P < 0.001). In 2019, the highest proportion of molecular testing cases was in East China (n = 19,492), while the lowest was in Northwest China (n = 1,051). In the CTONG 1506 study [130], during 2015‐2016, 665 (71.4%) of 932 patients with stage IIIB/IV non‐squamous NSCLC from 12 tertiary hospitals in China had molecular testing. A retrospective, real‐world study involving 2,809 stage III‐IV NSCLC patients from 31 hospitals in China (NCT02620657) revealed that the EGFR screening rate was significantly higher in tier‐1 cities than in tier‐2/3 cities (69.04% vs. 13.95%‐37.30%, P < 0.05) [131]. In a multicenter big‐data research project on Chinese lung cancer pathology, in a cohort with resectable NSCLC, a total of 75,941 lung cancer cases were collected from 23 tertiary hospitals in China [132]. During 2013‐2017, 20,139 (26.5%) patients with stage I‐III NSCLC received EGFR mutation testing, which was more than double the number of patients who underwent KRAS testing (n = 9,441). Moreover, the proportion of hospitals conducting ALK testing increased from 52.2% (12/23) in 2013 to 91.3% (21/23) in 2017.
Low‐throughput and false negative possibilities are two major defects for the panel‐based testing. Meanwhile, many emerging approaches were applied to molecular testing, such as matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry and pyrosequencing [133, 134, 135, 136, 137]. However, more studies are needed to verify and improve the clinical applicability, accuracy, and effectiveness of these novel technologies in NSCLC.
In the recent Chinese Guideline for NSCLC [127], the detection of programmed death‐ligand 1 (PD‐L1) expression by immunohistochemistry is recommended for NSCLC since PD‐L1 is a useful predictive biomarker for ICIs. The Blueprint PD‐L1 Immunohistochemistry Comparability Project compared the performances of five PD‐L1 immunohistochemistry assays (22C3, 28‐8, SP142, SP263, and 73‐10) used in clinical trials [138, 139]. The interchangeability of the 22C3, 28‐8, and SP263 assays was found. Notably, assay 73‐10 exhibited higher sensitivity than other assays. In the EXPRESS trial [140], a real‐word study, the expression levels of PD‐L1 in NSCLC patients were evaluated across 18 countries. Among 2,368 eligible specimens with PD‐L1 expression data, the positive rate of PD‐L1 (tumor cell proportion score [TPS] ≥ 1%) was 52% (n = 1,232). Notably, the prevalence of PD‐L1 TPS ≥ 1% was slightly higher in Asia‐Pacific than in Europe and the Americas (53% vs. 47%‐52%). In a Chinese cohort of 329 NSCLC patients, 46 (14%) had positive PD‐L1 expression [141]. Specifically, in LUSC (n = 108), the prevalence of PD‐L1 positive expression and high expression (TPS ≥ 50%) was 34.3% (n = 37) and 13.9% (n = 15), respectively. In LUAD (n = 221), only one (0.5%) case had PD‐L1 TPS ≥ 50%. However, the prevalence of PD‐L1 expression was dramatically higher in the Caucasian population when compared with the Chinese population. In an unselected Caucasian cohort, the percentages of NSCLC patients with PD‐L1 TPS ≥ 1% and ≥ 50% were 63% (n = 499) and 30% (n = 240), respectively [142].
5.3. Liquid biopsy
Considering the difficulty of obtaining tissue samples, liquid biopsy has developed rapidly. Liquid biopsy has the advantages of safety, non‐invasiveness, repeatability, easy performance, and high patient compliance. Circulating tumor DNA (ctDNA) could be used for the molecular diagnosis of lung cancer and the detection of tumor mutation burden [143, 144, 145, 146]. Notably, ctDNA and cell‐free DNA extracted from peripheral blood samples have been approved by the US Food and Drug Administration (FDA) only for EGFR mutation. In particular, it was reported that the gene mutation results of plasma samples were highly consistent with those of tissue samples [147]. The recently published International Association for the Study of Lung Cancer consensus declared that liquid biopsy is a complementary to tissue‐based analysis among patients with oncogene‐addicted NSCLC [148]. In China, the Super‐ARMS® EGFR Mutation Detection Kit is the only companion diagnostic product approved by the China National Medical Products Administration (NMPA) for the detection of EGFR mutations in ctDNA derived from plasma. Li et al. [149] demonstrated its superior performance through comparing blood samples and matched tumor tissue samples from patients with advanced LUAD, with 82.0% sensitivity and 100% specificity. For the clinical detection of folate receptor‐positive circulating tumor cells (CTCs) in peripheral blood, the CytoploRare Detection Kit (Genosaber Biotech, Shanghai, China) was approved by the China NMPA and the China FDA. In a study of 756 Chinese participants (473 NSCLC patients and 283 cancer‐free cases), folate receptor‐positive CTCs in peripheral blood exhibited superior sensitivity (72.46% in the training cohort and 76.37% in the validation cohort) and specificity (88.65% in the training cohort and 82.39% in the validation cohort) in the diagnosis of NSCLC [150]. In another study of 197 lung cancer patients and 171 benign/healthy individuals, baseline folate receptor‐positive CTCs was an effective biomarker for lung cancer diagnosis, with high sensitivity (77.7%) and specificity (89.5%) [151]. In recent years, the promising prospect and reliable performance of folate receptor‐positive CTCs in lung cancer diagnosis among Chinese population were also elucidated in other studies [152, 153, 154].
6. TREATMENT
Currently, there is an increasing number of therapeutic options for NSCLC, including surgery, radiotherapy, chemotherapy, traditional Chinese medicine, targeted therapy, immunotherapy, antibody‐drug conjugates (ADCs), and bispecific antibodies. Detailed discussions of surgery and radiotherapy are out of the scope of this article. In the review, we mainly focus on the advances in targeted therapy and immunotherapy for NSCLC.
6.1. Resectable NSCLC
6.1.1. Surgery
For resectable stage I‐III NSCLC patients, anatomical resection with regional lymph node dissection is the standard of care in China. The mode of anatomical resection includes lobectomy, pulmonary wedge resection, and pneumonectomy. It is recommended that regional lymph node dissection at least includes three hilar lymph node stations and three mediastinal lymph node stations [155]. In China, video‐assisted thoracoscopic surgery (VATS), as a rational alternative to open thoracotomy, was increasingly used [156, 157, 158]. A retrospective study of 7,726 Chinese NSCLC patients found that VATS lobectomy was related to better perioperative outcomes in comparison with open lobectomy [159]. According to the lung cancer statistics from the National Cancer Center of China, between 2005 and 2014, the proportion of patients with lung cancer who received surgery was 47.4% [160]. Over the past decade (2005‐2014), an increasing trend in the clinical application of VATS was found (< 5% in 2005 vs. 34.4% in 2014) [160]. During 2016‐2019, it was estimated that the VATS adoption rate was up to 80% in Chinese patients with lung cancer [6, 161]. In European population, during 2007‐2012, the application rate of VATS increased from 10.7% to 18.8% [162]. The number of the VATS procedures also increased annually in the United States (10% in 2002 vs. 29% in 2007) [163].
6.1.2. Adjuvant targeted therapy
The clinical application of neoadjuvant and adjuvant targeted therapies is still in its infancy. In NSCLC, osimertinib is the first adjuvant drug approved by the China NMPA (Table 2). The multicenter ADAURA study (NCT02511106) enrolled 682 EGFR‐mutated NSCLC patients who received surgery worldwide, among which the majority of participants were from Asia (64%). In the overall study population, adjuvant osimertinib improved the 2‐year disease‐free survival (DFS) rate (89% vs. 52%) as well as decreased the risk of both relapse and death (HR = 0.20, P < 0.001) in stage IB‐IIIA EGFR‐mutated patients compared with the placebo [164, 165]. In the 2022 European Lung Cancer Congress, the ADAURA trial reported the subgroup analysis results [166]. Among Chinese stage IB‐IIIA EGFR‐mutated patients (n = 159), adjuvant osimertinib also exhibited DFS advantage in comparison with the placebo (median DFS: not reached vs. 24.9 months; HR = 0.18, P < 0.001) [166]. The EVAN study [167] and the SELECT study [168] highlighted the clinical value of adjuvant erlotinib therapy in NSCLC. In the Chinese population, the phase II EVAN study indicated that the median DFS was significantly longer in the adjuvant erlotinib group than in the chemotherapy group (42.41 months vs. 20.96 months, P < 0.001) [167]. In 2021, the CTONG1104 study declared that the DFS advantage (median DFS: 30.8 months vs. 19.8 months) of adjuvant gefitinib over chemotherapy failed to translate to overall survival (OS) benefit (median OS: 75.5 months vs. 62.8 months, P > 0.05) in Chinese patients with stage II‐IIIA EGFR‐mutated NSCLC [169]. The EVIDENCE study (NCT02448797) illustrated that adjuvant icotinib exhibited better DFS compared with chemotherapy among Chinese stage IB‐IIIA EGFR‐mutated patients, achieving an impressive median DFS of 46.95 months and a 3‐year DFS rate of 63.88% [170]. Currently, the Chinese guideline for adjuvant therapy recommends osimertinib for all stage IB‐IIIA EGFR‐mutated patients. Gefitinib and icotinib can be utilized for stage IIA‐IIIB EGFR‐mutated patients, while erlotinib is only recommended for stage IIIA‐IIIB EGFR‐mutated patients.
TABLE 2.
Targeted drugs that approved by the China NMPA, US FDA, and EMA
| Drug | The China NMPA‐approved indications | The US FDA‐approved indications | The EMA‐approved indications |
|---|---|---|---|
| Osimertinib | (1) EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC; (2) EGFR T790M‐mutant, previously EGFR‐TKI treated, advanced NSCLC; (3) EGFR‐positive (19del and L858R), metastatic, resected NSCLC. | (1) EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC; (2) EGFR T790M‐mutant, previously EGFR‐TKI treated, advanced NSCLC; (3) EGFR‐positive (19del and L858R), metastatic, resected NSCLC. | (1) EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC; (2) EGFR T790M‐mutant, locally advanced/metastatic NSCLC; (3) EGFR‐positive (19del and L858R), metastatic, resected NSCLC. |
| Dacomitinib | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC. | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC. |
| Afatinib | (1) EGFR‐positive, treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously platinum‐based chemotherapy treated, locally advanced/metastatic LUSC. | (1) Treatment‐naïve, metastatic NSCLC with EGFR sensitive mutations; (2) Previously platinum‐based chemotherapy treated LUSC; (3) EGFR‐positive (19del and L858R), metastatic NSCLC. | (1) Treatment‐naïve, locally advanced/metastatic NSCLC with EGFR sensitive mutations; (2) Previously platinum‐based chemotherapy treated, locally advanced/metastatic LUSC. |
| Gefitinib | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC. | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC. | EGFR‐positive (19del and L858R), treatment‐naïve, metastatic NSCLC. |
| #Almonertinib | (1) EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC; (2) EGFR T790M‐mutant, previously first/second‐generation EGFR‐TKI treated, NSCLC. | / | / |
| #Furmonertinib | EGFR T790M‐mutant, previously first/second‐generation EGFR‐TKI treated, advanced NSCLC. | / | / |
| #Icotinib | (1) EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously platinum‐based chemotherapy treated, locally advanced/metastatic NSCLC; (3) EGFR‐positive (19del and L858R), stage II‐IIIA, resected NSCLC. | / | / |
| Erlotinib | (1) EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously platinum‐based chemotherapy treated, EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC; (3) Maintenance therapy for EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC after 4 cycles platinum‐based chemotherapy. | (1) EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously platinum‐based chemotherapy treated, EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC; (3) Maintenance therapy for EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC after 4 cycles platinum‐based chemotherapy; (4) Plus ramucirumab for EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC. | (1) EGFR‐positive (19del and L858R), treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously platinum‐based chemotherapy treated, EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC; (3) Maintenance therapy for EGFR‐positive (19del and L858R), locally advanced/metastatic NSCLC after 4 cycles platinum‐based chemotherapy. |
| Amivantamab | / | Second‐line therapy for EGFR exon 20 insertion‐positive patients. | / |
| Crizotinib | ALK/ROS1‐positive, treatment‐naïve, advanced NSCLC. | ALK/ROS1‐positive, treatment‐naïve, advanced NSCLC. | ALK/ROS1‐positive, treatment‐naïve, advanced NSCLC. |
| Alectinib | ALK‐positive, treatment‐naïve, locally advanced/metastatic NSCLC. | (1) ALK‐positive, treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously crizotinib treated, ALK‐positive, advanced NSCLC. | (1) ALK‐positive, treatment‐naïve, locally advanced/metastatic NSCLC; (2) Previously crizotinib treated, ALK‐positive, advanced NSCLC. |
| Ceritinib | (1) ALK‐positive, treatment‐naïve, advanced NSCLC; (2) Previously crizotinib treated, ALK‐positive, advanced NSCLC. | (1) ALK‐positive, treatment‐naïve, advanced NSCLC; (2) Previously crizotinib treated, ALK‐positive, advanced NSCLC. | (1) ALK‐positive, treatment‐naïve, advanced NSCLC; (2) Previously crizotinib treated, ALK‐positive, advanced NSCLC. |
| #Ensatinib | Previously crizotinib treated, ALK‐positive, advanced NSCLC. | / | / |
| Brigatinib | / | Previously crizotinib treated, ALK‐positive, advanced NSCLC. | Previously crizotinib treated, ALK‐positive, advanced NSCLC. |
| Lorlatinib | / | (1) ALK‐positive, advanced NSCLC; (2) Previously ALK inhibitor treated (crizotinib or alectinib or ceritinib), ALK‐positive, metastatic NSCLC. | (1) ALK‐positive, treatment‐naïve, advanced NSCLC; (2) Previously ALK inhibitor treated (crizotinib or alectinib or ceritinib), ALK‐positive, metastatic NSCLC. |
| Pralsetinib | Previously treated, RET fusion‐positive NSCLC. | RET fusion‐positive NSCLC. | / |
| #Savolitinib | Previously platinum‐based chemotherapy treated, MET exon 14‐altered, locally advanced/metastatic NSCLC. | / | / |
| Capmatinib | / | MET exon 14‐altered, metastatic NSCLC. | / |
| Tepotinib | / | MET exon 14‐altered, metastatic NSCLC. | / |
| Dabrafenib plus trametinib | / | BRAF V600E‐positive, treatment‐naïve, metastatic NSCLC. | / |
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, BRaf proto‐oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; FDA, Food and Drug Administration; LUSC, lung squamous cell carcinoma; MET, MET proto‐oncogene, receptor tyrosine kinase; NMPA, National Medical Products Administration of China; NSCLC, non‐small cell lung cancer; RET, transfection proto‐oncogene gene; ROS1, ROS proto‐oncogene 1,receptor tyrosine kinase; 19del, exon 19 deletion; #, domestic drug.
6.1.3. Neoadjuvant and adjuvant immunotherapy
The clinical value of neoadjuvant immunotherapy‐based therapy was assessed in some clinical trials [171]. The multicenter, open‐label, phase III CheckMate‐816 study (NCT02998528) compared the efficacy of neoadjuvant immunotherapy with neoadjuvant platinum‐based chemotherapy [172, 173]. All 358 patients enrolled in the CheckMate‐816 trial had resectable IB‐IIIA NSCLC without known EGFR/ALK sensitive mutations. When compared with neoadjuvant chemotherapy, neoadjuvant nivolumab plus platinum‐doublet chemotherapy had higher pathological complete response (pCR) rate, major pathological response (MPR) rate, and objective response rate (ORR) in the intention‐to‐treat (ITT) populations. The multicenter, single‐arm, phase II LCMC3 trial (NCT02927301) evaluated the efficacy of neoadjuvant atezolizumab monotherapy [174, 175]. The MPR rate was 21% with neoadjuvant atezolizumab therapy. For NSCLC patients with PD‐L1 TPS ≥ 50%, the MPR rate was increased to 33%. Chinese experts also evaluated the neoadjuvant application of sintilimab among patients with resectable IA‐IIIB NSCLC in a phase I study, obtaining a MPR rate of 40.5%, an ORR of 20% and a pCR rate in primary tumors of 16.2% [176]. Currently, in terms of neoadjuvant immunotherapy for NSCLC, the Chinese expert consensus has been published [177], and the main consensus are summarized as follows. (i) Neoadjuvant ICI monotherapy or ICI plus chemotherapy is a promising regimen for patients with resectable stage IB‐IIIA NSCLC. (ii) Considering the limited predictive performance of biomarkers in neoadjuvant immunotherapy, it is unnecessary to apply biomarker detection for patient selection in clinic. However, for EGFR/ALK‐positive patients, neoadjuvant ICI monotherapy should be used judiciously. (iii) It is recommended to perform 2‐4 cycles of neoadjuvant immunotherapy and conduct reviews every 2 cycles to assess treatment efficacy. (iv) PET‐CT plus serum tumor markers and/or ctDNA load are recommended for the assessment of efficacy of neoadjuvant immunotherapy. (v) The appropriate operative opportunity is 4‐6 weeks after the end of neoadjuvant immunotherapy. (vi) The negative influences of neoadjuvant immunotherapy on surgery remain ambiguous. (vii) For neoadjuvant immunotherapy, the main outcome indicators should include MPR and pCR. (viii) For patients with resectable NSCLC who are sensitive to neoadjuvant immunotherapy, 1‐year maintenance immunotherapy is suitable. (ix) Immunotherapy or induction chemotherapy may provide surgical chance for borderline resectable locally advanced NSCLC [177].
The phase III Impower010 (NCT02486718) study established the essential position of adjuvant atezolizumab in NSCLC [178, 179]. In that study, 1,005 patients who had undergone resection for stage IB‐IIIA NSCLC and had received adjuvant chemotherapy were randomly assigned to the adjuvant atezolizumab group and the best supportive care (BSC) group. In the stage II‐IIIA NSCLC subgroup, the median DFS in those who received atezolizumab was significantly higher than in patients who received BSC (42.3 months vs. 35.3 months, P = 0.02). Although median DFS was not available for patients with PD‐L1 TPS ≥ 1%, among patients with stage II‐IIIA NSCLC and all ITT patients who received adjuvant immunotherapy, the results of a survival analysis suggested better DFS in the adjuvant atezolizumab group. In 2021, based on the above data, atezolizumab was recommended for the post‐surgical treatment of NSCLC patients by the US FDA. The combination of neoadjuvant immunotherapy plus chemotherapy and adjuvant immunotherapy is a new attempt in NSCLC. The NADIM trial (NCT03081689), a multicenter, single‐arm, phase II clinical trial, demonstrated promising survival benefits with neoadjuvant nivolumab plus chemotherapy combined with adjuvant nivolumab [180]. After a long‐term follow‐up, the median DFS was 21.4 months. Moreover, the 3‐year OS rate was over 80% in the ITT cohort.
6.1.4. Neoadjuvant and adjuvant chemotherapy
For NSCLC patients with resectable disease, several clinical trials indicated the potential benefits of adjuvant chemotherapy [181, 182, 183, 184, 185, 186, 187, 188]. A meta‐analysis of 4,584 eligible cases found that adjuvant platinum‐based chemotherapy significantly prolonged OS in patients with stage IB‐III NSCLC [189]. No significant difference was found among different platinum‐based regimens [189]. The efficacy of neoadjuvant chemotherapy on NSCLC was controversial [190, 191, 192, 193, 194, 195]. In comparison with surgery, a meta‐analysis involving 15 randomized controlled trials (RCTs) and 2,385 patients showed that the 5‐year absolute improvement in OS, relapse‐free survival, and time to distant recurrence for NSCLC patients using neoadjuvant chemotherapy were 5%, 6%, and 10%, respectively [195]. Importantly, neoadjuvant chemotherapy might increase the chances of surgery for some patients with NSCLC [196]. Among 624 resectable NSCLC patients, the NATCH study found that the preoperative chemotherapy arm was not superior to the adjuvant chemotherapy arm in terms of DFS [192]. After 7.5 years of follow‐up, the IALT study of 1867 resectable NSCLC patients found that the survival benefits of chemotherapy decreased over time [181, 182].
6.2. Locally advanced NSCLC
6.2.1. Chemoradiotherapy
Concurrent chemoradiotherapy (CCRT) and sequential chemoradiotherapy are standard treatments for locally advanced NSCLC [197, 198, 199, 200, 201]. In China, CCRT is used widely. The phase III RTOG 9410 trial suggested better survival in the CCRT group than in the sequential chemoradiotherapy group [198]. A meta‐analysis of 1,205 NSCLC patients also highlighted longer OS in patients who received CCRT than in those who received sequential chemoradiotherapy [197]. In the Chinese population, a phase III trial found that OS did not differ significantly between stage III NSCLC patients who were assigned to the etoposide and cisplatin plus concurrent radiotherapy arm or the carboplatin and paclitaxel plus concurrent radiotherapy arm [200].
6.2.2. Immunotherapy plus chemoradiotherapy
In 2021, the multicenter PACIFIC study (NCT02125461) of stage III NSCLC reported the 5‐year follow‐up data [202]. After CCRT, the median OS and progression‐free survival (PFS) for patients in the durvalumab group were 47.5 months and 16.9 months, respectively (Figure 4). It is worth noting that more than 40% of patients who received immunotherapy survived 5 years [202, 203, 204].
FIGURE 4.
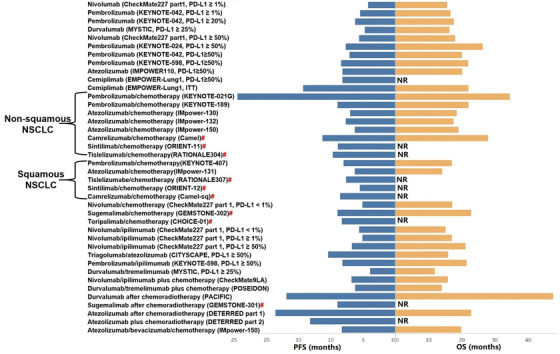
OS and PFS of patients with unresectable NSCLC after first‐line immunotherapy in phase 3 randomized controlled trials. Abbreviations: ITT, intention‐to‐treat; NR, not reach; NSCLC, non‐small cell lung cancer; OS, overall survival; PD‐L1, programmed death‐ligand 1; PFS, progression‐free survival; #, phase III clinical trials on innovative immune checkpoint inhibitors from China.
In Chinese patients with stage III NSCLC, the phase III GEMSTONE‐301 trial (NCT03728556) revealed that after CCRT or sequential chemoradiotherapy, sugemalimab significantly improved patient outcomes (Figure 4) [205, 206]. Among all enrolled cases, the median PFS assessed by the blinded independent central review was 9.0 months with sugemalimab and 5.8 months with placebo (P < 0.05).
In locally advanced NSCLC, the efficacy and safety of atezolizumab after chemoradiotherapy were explored in the phase II DETERRED‐part 1 study. Two phase II clinical trials, the Keynote‐799 study (NCT03631784) [207] and the DETERRED‐part 2 study [208], were conducted to explore the use of first‐line single ICI plus CCRT in locally unresectable advanced NSCLC. The recent follow‐up data of these trials presented promising ORRs and well tolerability with immunotherapy plus chemoradiotherapy.
6.3. Advanced NSCLC with positive driver alterations
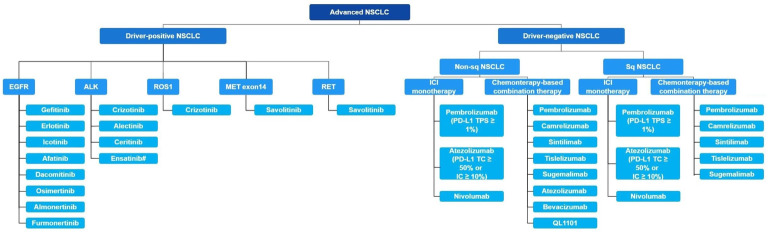
For advanced NSCLC, we summarized treatment regimens approved by the China NMPA in Figure 5.
FIGURE 5.
China National Medical Products Administration approved treatment regimens in advanced NSCLC. Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; IC, immune cell; ICI, immune checkpoint inhibitor; MET, MET proto‐oncogene, receptor tyrosine kinase; NSCLC, non‐small cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; RET, transfection proto‐oncogene gene; ROS1, ROS proto‐oncogene 1,receptor tyrosine kinase; sq, squamous; TC, tumor cell.
6.3.1. EGFR
There are more than 40 subdivisions of EGFR mutations, among which, EGFR exon 19 deletion (19del) and exon 21 L858R mutation are the two major sensitive mutations. In an unselected Chinese population with EGFR‐mutated NSCLC, the percentages of exon 21 L858R mutation, 19del, exon 18 G719X, and exon 20 T790M were 54.5% (n = 278), 36.1% (n = 184), 5.1% (n = 26), and 2.7% (n = 14), respectively [21]. Similarly, among 855 LUAD cases with EGFR mutations, L858R comprised the largest proportion (47.1%, n = 402), followed by 19del (42.2%, n = 361), and other mutation types (10.8%, n = 92) [22]. EGFR‐targeted therapy mainly includes EGFR‐tyrosine kinase inhibitors (EGFR‐TKIs) which could block the intracellular domain of the EGFR receptor, and anti‐EGFR monoclonal antibodies (mAbs) which could deprive specific signaling pathways by binding to the extracellular domain of the EGFR receptor.
6.3.1.1. EGFR‐TKI monotherapy
For the first‐line therapy, NSCLC patients with sensitive EGFR mutations could greatly benefit from first‐, second‐, and third‐generation EGFR‐TKIs, with ORRs of 50%‐80% and median PFS of 8.3‐19.3 months [209, 210, 211, 212, 213, 214]. In the phase III FLAURA study (NCT02296125), osimertinib significantly prolonged both PFS and OS in EGFR‐mutated NSCLC patients compared with comparator EGFR‐TKI (gefitinib or erlotinib) [209, 210]. In a Chinese cohort, osimertinib also showed advantages over gefitinib in median PFS (17.8 months vs 9.8 months, P < 0.05) [214]. In 2021, the AENEAS study, a multicenter RCT with 429 Chinese patients, updated the clinical data of aumolertinib, a third‐generation EGFR‐TKI, in untreated advanced NSCLC with EGFR‐19del or L858R mutations [213]. The median PFS of the aumolertinib group was significantly higher than that of the gefitinib group (19.3 months vs 9.9 months, P < 0.05). According to a subgroup analysis, aumolertinib showed promising antitumor effects in brain metastasis NSCLC patients, with a HR of 0.38. In China, domestic aumolertinib has been covered by health insurance areas and was added to the 2021 CSCO Guideline for NSCLC [127].
However, advanced untreated NSCLC patients with EGFR exon 20 insertion (20ins) were resistant to gefitinib, erlotinib, and afatinib, with ORRs of 3%‐8% [211, 215]. The median PFS was just 1.2‐2 months in EGFR 20ins patients who received common EGFR‐TKIs. For pretreated NSCLC patients with EGFR 20ins, three clinical trials exhibited the clinical availability of amivantamab [216], mobocertinib [217], and DZD9008 [218]. The ORRs of second‐line amivantamab, mobocertinib, and DZD9008 in EGFR 20ins‐positive patients were 40%, 28%, and 37.5%, respectively. Based on meaningful data from the CHRYSALIS study (NCT02609776; n = 81) [216] and the phase I/II study (NCT02716116; n = 114) [217], amivantamab and mobocertinib were approved by the US FDA for the treatment of EGFR20ins NSCLC patients. In China, amivantamab was included in the Breakthrough Therapy Program by the China NMPA and written into the 2021 CSCO Guideline for NSCLC. For EGFR20ins NSCLC patients, the marketing authorization applications for mobocertinib and DZD9008 have been accepted by the China NMPA and received the priority review.
Clinically, resistance to EGFR‐TKIs is inevitable. T790M mutation is one of resistance mechanisms to EGFR‐TKIs in NSCLC [219, 220]. For EGFR T790M mutation, two clinical studies, the phase III AURA3 study (n = 419) [221] and the phase II AURA study (n = 201) [222], confirmed the encouraging efficacy and safety of osimertinib in NSCLC patients who had disease progression after the first‐line EGFR‐TKI therapy. Results from these two studies showed that ORR and median PFS were significantly higher in the osimertinib group than in the chemotherapy group. Recently, almonertinib was also used for pretreated NSCLC patients with EGFR T790M mutations [223]. The ORR and median PFS of the almonertinib group were 52% and 11 months, respectively. In 2021, almonertinib, a domestic drug, was approved by the China NMPA for the second‐line treatment of EGFRT790M NSCLC (Table 2). Furmonertinib is another targeted drug that granted by the China NMPA for Chinese EGFRT790M patients (Table 2). A phase IIb trial (NCT03452592) reported an ORR of 94% when using furmonertinib to treat EGFRT790M patients [224]. Additionally, NSCLC patients with brain metastases also benefited from almonertinib and furmonertinib.
6.3.1.2. EGFR‐TKI plus chemotherapy
The addition of chemotherapy to anti‐EGFR targeted therapy effectively prevented drug resistance, thus improving the survival of patients with advanced EGFR‐mutated NSCLC. The encouraging efficacy of the first‐line gefitinib plus chemotherapy in advanced NSCLC was shown in some clinical trials [225, 226, 227, 228]. In 2017, a phase II RCT (NCT02148380) involving 121 Chinese LUAD patients with sensitive EGFR mutations found that the first‐line gefitinib plus chemotherapy significantly pronged both PFS and OS in comparison with the chemotherapy group or the gefitinib group (median PFS: 17.5 months vs. 5.7 months vs. 11.9 months; median OS: 32.6 months vs. 24.3 months vs. 25.8 months; both P < 0.05) [225]. Another phase II trial, the JMIT study (NCT01469000) of 191 advanced EGFR‐mutated patients, also demonstrated higher median PFS with gefitinib plus chemotherapy compared with gefitinib alone (15.8 months vs. 10.9 months, P < 0.05) [228]. In the JMIT study, a total of 52 patients (27.2%) were enrolled from China. In terms of untreated EGFR‐mutated NSCLC, two phase III studies, the Japan NEJ009 study [226] and the India NORONHA study [227], further illustrated better prognosis in gefitinib plus chemotherapy groups than in gefitinib monotherapy groups.
According to a prospective RCT (NCT02031601) of 179 Chinese patients with EGFR‐mutated NSCLC, the first‐line icotinib combined with chemotherapy significantly improved PFS, ORR, and disease control rate (DCR) compared with the icotinib group (PFS: 16.0 months vs. 10.0 months; ORR: 77.8% vs. 64.0%; DCR 91.1% vs. 79.8%; both P < 0.05) [229]. The ENSURE clinical trial highlighted another promising combination strategy (erlotinib plus chemotherapy) in the Chinese population [230]. Regardless of the sequential order, erlotinib plus chemotherapy raised the survival benefits of Chinese patients with advanced NSCLC compared with monotherapy (median OS: 51.6 months vs. 23.0 months, P < 0.001).
The roles of chemotherapy in combination with EGFR‐mAbs in the first‐line therapy were also investigated in some clinical studies. In non‐squamous NSCLC, the open‐label, phase III INSPIRE study (NCT00982111) found no significant difference in survival between the first‐line chemotherapy plus necitumumab and chemotherapy alone [231]. On the contrary, in stage IV LUSC, the phase III SQUIRE trial (NCT00981058) revealed a remarkable survival improvement in the chemotherapy plus necitumumab group versus the chemotherapy cohort [232]. However, the efficacy and safety of the above EGFR‐mAbs in the Chinese population remain unclear. It is expected that more clinical trials will be conducted in China.
6.3.2. ALK
Over the past decade (2010‐2019), there was an escalating trend in the ALK screening rate in Chinese patients with lung cancer (6.4% in 2010 vs. 80.9% in 2019) [129]. Table 2 summarizes the ALK inhibitors that have been approved by the China NMPA, US FDA, and the European Medicines Agency for lung cancer treatment. Crizotinib, an oral drug, is the first ALK‐TKI for ALK‐positive patients [233, 234]. The second‐/third‐generation of ALK‐TKIs, including ceritinib, alectinib, brigatinib, lorlatinib, and ensartinib, displayed higher central nervous system permeability [235, 236, 237, 238]. In the 2021 CSCO Guideline for NSCLC, crizotinib, alectinib, and ceritinib were recommended for the first‐line therapy of stage IV ALK‐positive NSCLC (grade I recommendation), while ensartinib was recommended for previously crizotinib treated, ALK‐positive, advanced NSCLC (grade II recommendation).
For the first‐line therapy of ALK‐positive NSCLC, the phase III PROFILE1014 trial indicated better performance of crizotinib than chemotherapy [233]. The phase III PROFILE1029 trial enrolled 207 untreated patients with ALK‐positive NSCLC, most of whom were Chinese (n = 183). The PROFILE1029 trial reached the primary end point, suggesting that crizotinib significantly prolonged PFS compared with chemotherapy (11.1 months vs. 6.8 months, P < 0.05) [234]. In Asian patients with ALK‐positive NSCLC, two phase III studies, the ALEX study and the ALESIA study, compared the efficacy of the first‐line alectinib and crizotinib, indicating that better survival and intracranial ORRs in the alectinib group [239, 240]. Ceritinib, brigatinib, and lorlatinib had better clinical outcomes when compared with crizotinib [236, 237, 238]. In 2021, the eXalt3 study, a phase III RCT, highlighted the encouraging effect of ensartinib in Asian patients with NSCLC [241]. The median PFS of ensartinib was superior to that of crizotinib (25.8 months vs. 12.7months, P < 0.01). Based on the extraordinary performance of ensartinib in phase II and III clinical trials [241, 242], the China NMPA approved the clinical use of ensartinib in Chinese patients with ALK‐positive NSCLC (Table 2). The approval of ceritinib in Chinese patients was also received from the China NMPA this year. The open label, phase III, ASCEND‐4 RCT (NCT01828099) suggested that the prognosis of ALK‐positive patients was prolonged in the ceritinib group compared with the chemotherapy group [237]. The median PFS was 16.6 months in the ceritinib group and 8.1 months in the chemotherapy group. As novel ALK‐TKIs, ensartinib and ceritinib might provide a new option for ALK‐positive NSCLC patients.
For crizotinib‐resistant NSCLC, some studies have demonstrated the considerable effects of ceritinib, alectinib, brigatinib, lorlatinib, and ensartinib [243, 244, 245, 246]. However, the resistance mechanisms of ALK‐TKIs are not well understood, and more investigations are needed. Moreover, only a limited number of clinical trials have been conducted on drugs for NSCLC patients with progression after second‐ and third‐generation ALK‐TKIs therapy.
6.3.3. KRAS
In a Chinese cohort, the median PFS and median OS of KRAS‐mutated NSCLC patients who received first‐line pemetrexed‐based chemotherapy were 6.4 months and 25.4 months, respectively [247]. Emerging KRAS G12C inhibitors made a breakthrough in targeted therapy, thus bringing survival benefits for NSCLC with KRAS mutations. In 2021, two KRAS inhibitors, sotorasib and adagrasib, were written into the clinical guidelines and approved by US FDA for use in KRAS‐positive NSCLC (Table 2). The phase II CodeBreaK100 study (NCT03600883) found that the median PFS was 6.8 months and the median OS was 12.5 months in KRAS G12C‐mutated NSCLC patients who received sotorasib. In addition, the DCR of sotorasib was 80.6% in KRAS G12C‐positive NSCLC patients [248]. The recent CSCO Guideline recommends the clinical use of sotorasib in KRAS G12C‐positive NSCLC.
The impressive DCR and ORR of adagrasib were found in the KRYSTAL‐1 study (NCT03785249) [249]. About 96% of patients with KRAS p.G12C‐mutant NSCLC experienced disease control. An objective response was found in more than 40% KRAS p.G12C‐mutant NSCLC patients. Notably, the median PFS of patients receiving adagrasib was 11.1 months. In NSCLC, targeted therapy for KRAS mutations is both challenging and meaningful. Many KRAS inhibitors are currently undergoing preclinical experiments and clinical trials.
6.3.4. ROS1
In a Chinese cohort with 226,227 NSCLC patients, the ROS1 testing rate was lower than 10% between 2010 and 2015 [129]. Remarkably, in 2019, 57.2% of NSCLC patients underwent ROS1 tests [129]. To date, crizotinib has been approved by the China NMPA for ROS1‐positive NSCLC patients (Table 2). In NSCLC, targeted therapy for ROS1 has been explored for many years, but its progress is limited. With high level homology in kinase domains between ALK and ROS1, ROS1‐positive NSCLC patients were also shown to be sensitive to crizotinib, ceritinib, and lorlatinib [250, 251, 252, 253]. Among 127 East Asian patients with ROS1‐positive NSCLC, a phase II, open‐label, single‐arm trial (NCT01945021) reported the median PFS with the first‐line crizotinib to be 15.9 months, and the ORR was 71.7% in the whole cohort and 71.6% for Chinese patients [253].
In 2021, our team reported the latest clinical data of the TRUST study (NCT04395677), which was a multicenter phase II clinical trial in China [254]. Taletrectinib, a novel ROS1 inhibitor, exhibited encouraging efficacy in treatment‐naïve patients with crizotinib‐resistant and brain‐metastatic patients with ROS1 mutation. The first‐line group had the highest ORR (90.5%), followed by the brain metastatic group (83.3%) and the crizotinib‐resistant group (43.8%). According to the side effects analysis, taletrectinib was well tolerated in NSCLC.
6.3.5. BRAF
The most common type of BRAF mutation is BRAF‐V600E, with a frequency of around 50% [255]. BRAF‐G469A/V mutation accounts for 35% of all BRAF mutation types, while BRAF‐D594G makes up only 7%. BRAF‐V600 mutation occurs more frequently in never‐smoking females [256]. The US FDA approved the use of dabrafenib plus trametinib in NSCLC patients with BRAF‐V600E‐mutation (Table 2). According to the phase II study data, 23 of 36 advanced BRAF‐V600E‐mutant NSCLC patients achieved complete or partial response with the first‐line dabrafenib in combination with trametinib (ORR, 64%) [257]. The median PFS of the dabrafenib plus trametinib group was 10.9 months. Future clinical trials with a larger sample size are expected. In the 2021 CSCO Guideline for NSCLC, the recommendation grade of dabrafenib plus trametinib in BRAF‐V600E‐mutant NSCLC patients was raised from grade III to grade II.
6.3.6. RET
In recent years, two RET inhibitors, selpercatinib and pralsetinib, have broken the stalemate in the therapy of RET‐mutant NSCLC. Two phase I/II studies, the LIBR ETTO‐001 study (NCT03157128) and the ARROW trial (NCT03037385), displayed the curative advantages and promising antitumor activities of selpercatinib and pralsetinib in RET‐driven NSCLC [258, 259]. In 2021, the efficacy and safety of selpercatinib and pralsetinib was explored in Chinese NSCLC patients with RET fusion. At the World Conference on Lung Cancer, the phase II LIBRETTO‐321 study (NCT04280081) presented data on the use of selpercatinib in Chinese populations [260]. The ORR of selpercatinib assessed by an independent review committee was 69.2%. Notably, over 90% of enrolled patients remained responsive after a median follow‐up of 9.7 months. In terms of pralsetinib efficacy, another clinical trial indicated that the confirmed ORR of pralsetinib in previously untreated patients with RET‐positive NSCLC was 66.7%, with 80% for pretreated patients [261]. Both selpercatinib and pralsetinib demonstrated good safety and tolerability in Chinese NSCLC cohorts. It is noteworthy that no selpercatinib or pralsetinib‐related deaths were recorded. In the LIBRETTO‐321 study, 45.5% of selpercatinib‐related adverse effects occurred at grade1 and 2. The most common selpercatinib‐related adverse effect was alanine aminotransferase increase (62.3%), while aspartate aminotransferase increase (80.9%) was the most frequent pralsetinib‐related adverse effect. These two US FDA‐approved RET inhibitors have dramatically changed the treatment landscape for RET‐fusion NSCLC. In 2021, pralsetinib was approved by the China NMPA for the treatment of RET‐positive NSCLC patients (Table 2).
6.3.7. HER2
In China, pyrotinib is the first targeted drug recommended for HER2‐positive NSCLC patients. In a multicenter, open‐label, single‐arm, phase II clinical trial (NCT02834936), our team found satisfactory clinical efficacy of pyrotinib in HER2‐positive NSCLC [262]. The median OS with second‐line pyrotinib therapy was 14.4 months. The median PFS was 6.9 months. Additionally, our study found tolerable and manageable adverse events of pyrotinib. Treatment‐related adverse effects ≥ grade 3 occurred in 28.3% of patients, and no treatment‐related deaths were recorded. Currently, increasing numbers of HER2‐targeted drugs for NSCLC have been developed and have entered clinical trials.
Ado‐trastuzumab emtansine (T‐DM1) and trastuzumab deruxtecan (T‐Dxd) were written into the National Comprehensive Cancer Network (NCCN) Guideline for advanced NSCLC patients with HER2 mutations. The encouraging antitumor effect of T‐DM1 in NSCLC was observed in a phase II basket study (NCT02675829) [263]. In 2021, a multicenter, phase II DESTINY‐Lung01 trial (NCT03505710) presented the preliminary results of T‐Dxd, a novel HER2‐targeted ADC, in HER2‐positive NSCLC [264]. In HER2‐positive NSCLC cohort, the confirmed ORR of T‐Dxd was 54.9%, and the median PFS was 8.2 months.
6.3.8. MET
For NSCLC with MET exon 14 alterations, the satisfactory antitumor activities of crizotinib, capmatinib, tepotinib, and savolitinib were presented in some clinical trials. Among 65 response‐evaluated patients with MET exon 14‐altered NSCLC, the ORR of crizotinib was 32% [265]. In the crizotinib cohort, the median duration of response (DoR) and PFS were 9.1 months and 7.3 months, respectively. The multiple‐cohort, phase II GEOMETRY mono‐1 trial (NCT02414139) indicated the antitumor activity and safety of capmatinib in MET exon14‐mutant and MET‐amplified NSCLC. For the first‐line capmatinib therapy, the ORR was 68% in patients with MET exon 14 skipping mutations and 40% in those with MET amplification. For the second‐/third‐line capmatinib treatment, the ORR was 41% in the MET exon 14‐mutant group and 29% in the MET amplification group [266]. Results from the open‐label phase II VISION study (NCT02864992) revealed that tepotinib significantly improved the clinical outcomes of MET exon14‐mutant NSCLC patients, with an ORR of 46%‐71% [267]. According to the above results, the recent NCCN Guideline recommends the use of crizotinib, capmatinib, and tepotinib in MET‐positive NSCLC. However, capmatinib and tepotinib are yet to be approved by the China NMPA. In China, savolitinib, a domestic drug independently developed by Chinese pharmaceutical enterprises, was approved by the China NMPA in 2021 (Table 2). The 2021 CSCO Guideline for NSCLC also added savolitinib. The encouraging antitumor activity of savolitinib was verified in a phase II study (NCT02897479) of pulmonary sarcomatoid carcinoma and other subtypes of NSCLC [268]. In the savolitinib group, the DoR was 8.3 months, with a PFS of 6.8 months. The DCR with savolitinib was up to 90%. Nowadays, Chinese MET‐mutant patients can benefit from second‐line therapy of savolitinib.
6.4. Wild‐type (WT) advanced NSCLC
6.4.1. Immunotherapy
With increasing awareness of immune escape mechanisms, immunotherapy has become an effective treatment approach for NSCLC patients (Figure 6). Notably, the impairment of antigen presentation, neoantigen loss/silencing, immune suppression‐related genes or pathways, oncogenic alterations, and immune cells/cytokines in tumor microenvironments are associated with immune escape. In the last decade, immunotherapy has been a major innovation in the field of lung cancer therapy. Currently, ICIs used for the treatment of NSCLC include nivolumab, pembrolizumab, durvalumab, atezolizumab, tislelizumab, camrelizumab, and sintilimab (Figure 5).
FIGURE 6.
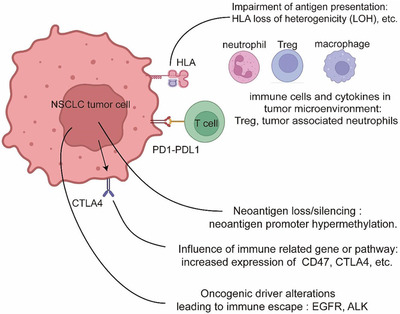
Mechanisms of immune escape in NSCLC. Abbreviations: ALK, anaplastic lymphoma kinase; CTLA4, cytotoxic T‐lymphocyte‐associated protein 4; EGFR, epidermal growth factor receptor; HLA, human leukocyte antigen; NSCLC, non‐small cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; Treg, regulatory T cell.
6.4.1.1. Immunotherapy monotherapy
Several phase III clinical trials emphasized the clinical importance of the first‐line ICI monotherapy in NSCLC (Figure 4). In the KEYNOTE 024 trial (NCT02142738), pembrolizumab significantly prolonged both PFS and OS in NSCLC patients with high PD‐L1 expression (PD‐L1 TPS ≥ 50%) [269, 270]. After a median follow‐up of 46.9 months, the KEYNOTE‐042 study (NCT02220894) reported considerable OS improvement in the first‐line pembrolizumab group regardless of the PD‐L1 TPS (median OS: PD‐L1 TPS ≥ 1%, 16.7 months; PD‐L1 TPS ≥ 50%, 20.0 months) [271]. In advanced NSCLC with PD‐L1 TPS ≥ 50%, the phase III IMPOWER110 study (NCT02409342) showed longer OS and PFS in the atezolizumab monotherapy group compared with the chemotherapy group (median OS: 20.2 months vs. 13.1 months; median PFS: 8.2 months vs. 5.0 months) [272]. The China NMPA approved atezolizumab for treatment‐naïve NSCLC patients with high PD‐L1 expression or tumor‐infiltrating immune cells (≥ 10%) in 2021 (Table 3). In the 2021 CSCO Guideline for NSCLC, for the first‐line therapy of lung cancer patients with high PD‐L1 expression or tumor‐infiltrating immune cells ≥ 10%, atezolizumab was added as a grade‐1 recommendation [127].
TABLE 3.
Immune checkpoint inhibitors that approved by the China NMPA, US FDA, and EMA
| Drug | The China NMPA‐approved indication | The US FDA‐approved indication | EMA‐approved indication |
|---|---|---|---|
| Pembrolizumab | (1) PD‐L1 TPS ≥ 1%, treatment‐naïve, locally advanced/metastatic NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel or nab‐paclitaxel for treatment‐naïve, metastatic LUSC; (3) Plus pemetrexed and platinum‐based chemotherapy for treatment‐naïve, metastatic, non‐squamous NSCLC. | (1) PD‐L1 TPS ≥ 1%, treatment‐naïve, locally advanced/metastatic NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel or nab‐paclitaxel for treatment‐naïve, metastatic LUSC; (3) Plus pemetrexed and platinum‐based chemotherapy for treatment‐naïve, metastatic, non‐squamous NSCLC; (4) Previously platinum‐based chemotherapy treated, advanced/metastatic NSCLC with PD‐L1 positive expression. | (1) PD‐L1 TPS ≥ 50%, treatment‐naïve, locally advanced/metastatic NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel or nab‐paclitaxel for treatment‐naïve, metastatic LUSC; (3) Plus pemetrexed and platinum‐based chemotherapy for treatment‐naïve, metastatic, non‐squamous NSCLC; (4) Previously chemotherapy treated, locally advanced/metastatic NSCLC with PD‐L1 TPS ≥ 1%. |
| Nivolumab | Previously platinum‐based chemotherapy treated, locally advanced/metastatic NSCLC without EGFR/ALK aberrations. | (1) Previously platinum‐based chemotherapy treated, metastatic NSCLC; (2) Plus ipilimumab and chemotherapy for treatment‐naïve, advanced/relapsed NSCLC; (3) Plus ipilimumab for treatment‐naïve, metastatic NSCLC with PD‐L1 TPS ≥ 1% and without EGFR/ALK aberrations. | (1) Previously chemotherapy treated, locally advanced/metastatic NSCLC; (2) Plus ipilimumab and chemotherapy for treatment‐naïve, metastatic NSCLC. |
| Atezolizumab | (1) PD‐L1 TC ≥ 50% or IC ≥ 10%, treatment‐naïve, metastatic NSCLC without EGFR/ALK aberrations; (2) Plus pemetrexed and platinum‐based chemotherapy for treatment‐naïve, metastatic, non‐squamous NSCLC without EGFR/ALK aberrations. | (1) PD‐L1 TC ≥ 50% or IC ≥ 10%, treatment‐naïve, metastatic NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and nab‐paclitaxel for treatment‐naïve, metastatic, non‐squamous NSCLC without EGFR/ALK aberrations; (3) Plus bevacizumab and carboplatin and paclitaxel for treatment‐naïve, advanced NSCLC without EGFR/ALK aberrations; (4) Previously platinum‐based chemotherapy treated, metastatic NSCLC; (5) Previously platinum‐based chemotherapy treated, stage II‐IIIA, resected NSCLC with PD‐L1 TC ≥ 1%. | (1) Plus carboplatin and nab‐paclitaxel for treatment‐naïve, metastatic, non‐squamous NSCLC without EGFR/ALK aberrations; (2) Plus bevacizumab and carboplatin and paclitaxel for treatment‐naïve, advanced, non‐squamous NSCLC; (3) Previously chemotherapy treated, locally advanced/metastatic NSCLC. |
| Durvalumab | Stage III, unresectable NSCLC after concurrent chemoradiotherapy. | Stage III, unresectable NSCLC after concurrent chemoradiotherapy. | PD‐L1 TPS ≥ 1%, unresectable NSCLC after concurrent chemoradiotherapy. |
| #Camrelizumab | (1) Plus pemetrexed and carboplatin for treatment‐naïve, advanced, non‐squamous NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel for treatment‐naïve, advanced LUSC without EGFR/ALK aberrations. | / | / |
| #Sintilimab | (1) Plus pemetrexed and carboplatin for treatment‐naïve, advanced, non‐squamous NSCLC without EGFR/ALK aberrations; (2) Plus gemcitabine and platinum‐based chemotherapy for treatment‐naïve, locally advanced/metastatic LUSC without oncogenic aberrations. | / | / |
| #Tislelizumab | (1) Plus pemetrexed and platinum‐based chemotherapy for treatment‐naïve, locally advanced/metastatic, non‐squamous NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel or nab‐paclitaxel for treatment‐naïve, advanced LUSC; (3) Previously treated, non‐squamous and squamous NSCLC. | / | / |
| #Sugemalimab | (1) Plus pemetrexed and carboplatin for treatment‐naïve, metastatic, non‐squamous NSCLC without EGFR/ALK aberrations; (2) Plus carboplatin and paclitaxel for treatment‐naïve, metastatic LUSC. | / | / |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; FDA, Food and Drug Administration; IC, immune cells; LUSC, lung squamous cell carcinoma; NMPA, National Medical Products Administration; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death‐ligand 1; TC, tumor cells; TPS, tumor cell proportion score; #, domestic drug.
6.4.1.2. Immunotherapy plus chemotherapy
Some clinical trials investigated the efficacy of single ICI plus chemotherapy as the first‐line treatment for advanced NSCLC (Figure 4). Based on the results, the 2021 CSCO Guideline for NSCLC was updated. In non‐squamous NSCLC without driver gene alterations, three domestic drugs, including camrelizumab, sintilimab, and tislelizumab, were approved by the China NMPA (Table 3). We conducted the open‐label, multicenter, phase III CameL study (NCT03134872) to compare the first‐line efficacy and safety of camrelizumab plus chemotherapy with chemotherapy alone for patients with non‐squamous NSCLC [273]. In the Chinese population, higher PFS (median: 11.3 months vs. 8.3 months, P = 0.001) and ORR (60.5% vs. 38.6%) were found in the camrelizumab plus chemotherapy group compared with the chemotherapy alone group. In addition, the camrelizumab in combination with chemotherapy regimen was well tolerated, with a grade ≥ 3 treatment‐related adverse effects proportion of 38%. In the camrelizumab plus chemotherapy group, the incidence of reactive cutaneous capillary endothelial proliferation was the highest (78%). The ORIENT‐11 study (NCT03607539), a phase III RCT, indicated sintilimab plus chemotherapy slower disease progression in Chinese NSCLC patients versus chemotherapy alone (median PFS: 8.9 months vs. 5.0 months, P < 0.001) [274]. The ORR was higher in the sintilimab plus chemotherapy group than in the chemotherapy group (51.9% vs. 29.8%). According to the RATIONALE‐304 study (NCT03594747), Chinese NSCLC patients who received tislelizumab plus chemotherapy demonstrated better prognosis than those who received only chemotherapy (median PFS: 9.7 months vs. 7.6 months, P < 0.05) [275]. Significantly higher ORR was observed in the tislelizumab plus chemotherapy group compared with the chemotherapy group (57.4% vs. 36.9%).
In LUSC, the use of camrelizumab, sintilimab, and tislelizumab were validated in some clinical trials (Figure 4). In the phase III CameL‐sq study (NCT03668496), our team found excellent efficacy and manageable toxicity with camrelizumab plus chemotherapy in LUSC [276]. The addition of camrelizumab to chemotherapy significantly prolonged both PFS (median: 8.5 months vs. 4.9 months, P < 0.001) and OS (median: not reached vs. 14.5 months, P < 0.001) in Chinese populations with LUSC. The antitumor effect of sintilimab plus gemcitabine and platinum was validated in the phase III ORIENT‐12 study (NCT03629925) involving 543 Chinese patients with advanced or metastatic LUSC [277]. The disease progression risk was significantly declined in the sintilimab plus chemotherapy group compared with the chemotherapy group (HR = 0.536, P < 0.001). For the tislelizumab plus chemotherapy regimen, positive results were found in the phase III RATIONALE‐307 study (NCT03594747) [278]. In Chinese patients with LUSC (n = 355), in comparison with chemotherapy, the tislelizumab plus chemotherapy regimen was associated with better PFS (median: 7.6 months vs. 5.5 months, P < 0.001), DoR (median: 8.2‐8.6 months vs. 4.2 months), and ORR (72.5%‐74.8% vs. 49.6%).
Two emerging therapeutics, suglizumab and toripalimab, were applied for the first‐line treatment of WT NSCLC patients (Figure 4). Recently, we updated the follow‐up data of the GEMSTONE‐302 trial (NCT03789604), a phase III RCT [279]. The median PFS was significantly higher in Chinese patients treated with the first‐line sugemalimab plus chemotherapy compared with placebo plus chemotherapy (median PFS: 9.0 months vs 4.9 months, P < 0.05). There was a trend of higher 2‐year OS rate in the combination immunotherapy group compared with the control group (47.1% vs. 38.1%). The safety analysis suggested that 23% of individuals in the sugemalimab plus chemotherapy group and 20% of participants in the control group experienced treatment‐related serious adverse events. In December 2021, sugemalimab was approved by the China NMPA for the first‐line treatment of metastatic NSCLC patients (Table 3). The phase III CHOICE‐01 trial indicated longer PFS in the toripalimab plus chemotherapy group compared with the placebo plus chemotherapy group (median PFS: 8.3 months vs 5.6 months, P < 0.05) [280]. Better ORR (63.4% vs. 41.7%, P < 0.05) and DoR (median: 8.3 months vs. 4.2 months) were also found in the toripalimab plus chemotherapy group than the chemotherapy group. In the CHOICE‐01 trial, the addition of toripalimab to chemotherapy did not increase the adverse effects rate at grade 3 or worse compared with chemotherapy (76.3% vs. 80.1%). The toxicity of the above two novel drugs was manageable, further suggesting their considerable potential for clinical treatment.
6.4.2. Anti‐angiogenesis therapy plus chemotherapy
Since the proposal of tumor angiogenesis in 1971 [281], anti‐angiogenic drugs have been gradually designed and used in NSCLC patients. Angiogenesis inhibitors could effectively inhibit tumor proliferation and metastasis. Some clinical studies explored the efficacy of anti‐angiogenesis therapy plus chemotherapy in NSCLC. Regretfully, most of these results were unsatisfactory [282, 283]. It was not until 2015 that positive results from the phase III BEYOND trial (NCT01364012) brought new hope for the use of chemotherapy combined with anti‐angiogenesis therapy in NSCLC [284]. In Chinese non‐squamous NSCLC, the clinical prognosis was improved significantly in the bevacizumab plus carboplatin and paclitaxel arm compared with the placebo plus carboplatin and paclitaxel arm (median PFS, 9.2 months vs. 6.5 months, P < 0.05; median OS, 24.3 months vs. 17.7 months, P = 0.0154). A phase III RCT (NCT03169335) enrolled 535 Chinese patients with advanced non‐squamous NSCLC to compare the efficacy and safety of QL1101 and bevacizumab [285]. QL1101 is a domestic bevacizumab analog. In the overall population, the ORR (52.8% vs. 56.8%), 18‐month OS rate (28.3% vs. 33.5%), and safety profile (any treatment‐related adverse effects: 99.26% vs. 99.62%) of first‐line therapy with QL1101 plus chemotherapy were similar to that of bevacizumab plus chemotherapy, respectively. Bevacizumab and QL1101 were approved by the China NMPA for lung cancer treatment (Table 4). In the CSCO Guideline for NSCLC, bevacizumab or QL1101 plus chemotherapy was recommended for the first‐line therapy of stage IV, WT, non‐squamous NSCLC. In advanced NSCLC, the encouraging therapeutic effect of anlotinib was verified in a phase III, multicenter, ALTER 0303 RCT (NCT02388919) [286]. In comparison with the placebo cohort, the anlotinib cohort of 296 Chinese NSCLC patients demonstrated better median PFS (5.4 months vs. 1.4 months) and median OS (9.6 months vs. 6.3 months). For the third‐line therapy of NSCLC, anlotinib has been approved by the China NMPA (Table 4). In 2021, a phase Ib, single‐arm trial (NCT03628521) firstly presented considerable efficacy and manageable toxicity of anlotinib plus sintilimab in treatment‐naïve patients with advanced NSCLC [287]. The median PFS was 15 months, and the ORR was 72.7%.
TABLE 4.
Anti‐angiogenic drugs that approved by the China NMPA, US FDA, and EMA
| Drug | The China NMPA‐approved indication | The US FDA‐approved indication | The EMA‐approved indication |
|---|---|---|---|
| Bevacizumab | Plus platinum‐based chemotherapy for treatment‐naïve, unresectable, metastatic/relapsed, non‐squamous NSCLC. | Plus platinum‐based chemotherapy for treatment‐naïve, unresectable, metastatic/relapsed, non‐squamous NSCLC. | Plus platinum‐based chemotherapy for treatment‐naïve, unresectable, metastatic/relapsed, non‐squamous NSCLC. |
| #QL1101 | Plus platinum‐based chemotherapy for treatment‐naïve, unresectable, metastatic/relapsed, non‐squamous NSCLC. | / | / |
| #Anlotinib | Third‐line therapy for advanced NSCLC. | / | / |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; NMPA, National Medical Products Administration; NSCLC, non‐small cell lung cancer; #, domestic drug.
6.4.3. Immunotherapy plus angiogenesis inhibitor and chemotherapy
The IMpower150 study (NCT02366143), an open‐label phase III RCT, explored the efficacy of the first‐line treatment with chemotherapy plus angiogenesis inhibitor and ICI in advanced, non‐squamous NSCLC [288, 289, 290, 291, 292]. Compared with the bevacizumab‐carboplatin‐paclitaxel (BCP) group (median PFS = 6.8 months, median OS = 14.7 months) and the atezolizumab‐carboplatin‐paclitaxel group (median PFS = 6.3 months, median OS = 19.0 months), the PFS and OS in the atezolizumab‐BCP (ABCP) group (median PFS = 8.4 months, median OS = 19.5 months) were the highest. In addition, a subgroup analysis suggested that metastatic patients, EGFR‐mutated patients, ALK‐positive patients, KRAS‐mutant patients could benefit from ABCP therapy. Another phase III RCT, the IMpower 151 (NCT04194203) study, is aimed at evaluating the efficacy and safety of ABCP and atezolizumab‐bevacizumab‐carboplatin‐pemetrexed in untreated, stage IV, non‐squamous NSCLC. The clinical data from the IMpower 151 study are highly anticipated.
6.4.4. Chemotherapy
In NSCLC, several chemotherapy drugs are used frequently, including cisplatin, carboplatin, nedaplatin, gemcitabine, paclitaxel, pemetrexed, docetaxel, and vinorelbine. For the first‐line therapy, several large population‐based studies(e.g., ECOG 1594, SWOG9509, TAX326, EORTC 08975, ILCP, JMDB, and WJOG5208L) suggested the vital roles of doublet chemotherapy in advanced or relapsed NSCLC patients without driver gene alterations [293, 294, 295, 296, 297, 298, 299, 300, 301, 302, 303]. Paclitaxel liposome (Lipusu®), a domestic drug developed by a Chinese pharmaceutical company, is the world's first commercial liposomal formulation of paclitaxel. In 2022, the LIPUSU study (NCT02996214), a multicenter open‐label phase III RCT, highlighted the significantly lower toxicity of Lipusu combined with cisplatin than gemcitabine combined with cisplatin (treatment interruption rate: 10.9% vs. 26.4%; treatment termination rate: 14.3% vs. 23.1%; both P < 0.05) in the first‐line therapy of LUSC [304]. Among all enrolled Chinese patients (n = 490), PFS (median: 5.2 months vs. 5.5 months), OS (median: 14.6 months vs. 12.5 months), ORR (41.8% vs. 45.9%), and DCR (90.3% vs. 88.1%) did not differ significantly between the two arms. Some efficacy‐related cytokines were also found in the LIPUSU trial, such as tumor necrosis factor‐alpha, interferon‐gamma, interleukin‐6, and interleukin‐8. Lipusu has been approved by the China NMPA for the first‐line treatment of advanced NSCLC.
In Chinese patients with advanced NSCLC, nanoparticle polymeric micellar paclitaxel showed promising efficacy and manageable toxicity in a phase III, open‐label, multicenter RCT (NCT02667743) [305]. The median PFS was significantly longer in the polymeric micellar paclitaxel plus cisplatin group than the solvent‐based paclitaxel plus cisplatin group (6.4 months vs. 5.3 months, P < 0.05). Notably, the ORR was 50% with polymeric micellar paclitaxel plus cisplatin versus 26% with solvent‐based paclitaxel plus cisplatin. In 2021, polymeric micellar paclitaxel was approved by the China NMPA for the treatment of NSCLC.
6.4.5. Emerging therapies
Several emerging treatments have generated considerable research attention in recent years, such as ADCs and tumor‐treating fields (TTFields). Much progress has been made with ADCs in HER2‐mutant NSCLC. The investigations into other targets, including HER3, TROP2, CEACAM5 and MET in NSCLC are still ongoing [306]. TTFields, a low intensity, intermediate frequency, alternating electric fields, is one of physical therapy in cancer. TTFields could promote cancer cell death and inhibit tumor growth via interfering with the correct alignment of chromosomes and tubulin [307, 308]. In this therapy, TTFields mainly attack dividing cells. A phase I/II trial verified that as a second‐line therapy for NSCLC, the combination of TTFields and pemetrexed was safe and potentially more effective than pemetrexed alone [309]. Among 42 enrolled patients with stage IIIB‐IV NSCLC, 26 (61.9%) experienced partial response (n = 6) or stable disease (n = 20). The median OS was 13.8 months. Currently, the prospective phase III LUNAR RCT is underway, and patients are being recruited (NCT02973789).
7. CONCLUSIONS
Lung cancer remains a devastating disease that is responsible for substantial incidence and cancer‐related deaths in China. In NSCLC, prognostic improvements are closely associated with multidisciplinary advances in risk factor prevention, screening, targeted therapy, and immunotherapy. In recent decades, understanding of the molecular biology and the clinical characteristics of Chinese NSCLC patients has increased. However, there are both ongoing challenges and considerable scope for further progression in NSCLC in China. The future directions of NSCLC might lie in precise therapy and individualized therapy. To maximize the benefits for Chinese patients with lung cancer, particular efforts should be made in the following areas: effective tumor prevention strategies, rapid identification of oncogenic drivers, broad consensus on screening programs, increased clinical application of molecular testing, and additional prospective clinical trials of novel combination regimens and emerging therapeutics.
DECLARATIONS
AUTHOR CONTRIBUTIONS
CCZ and PXC conceived the review and edited the manuscript. CCZ and PXC contributed to the data collection. PXC, YHL, and YKW analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENTS
Not applicable.
Chen P, Liu Y, Wen Y, Zhou C. Non‐small cell lung cancer in China. Cancer Commun. 2022;42:937–970. 10.1002/cac2.12359
Peixin Chen, Yunhuan Liu and Yaokai Wen contributed equally
REFERENCES
- 1. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer incidence and mortality in China, 2015. Journal of the National Cancer Center. 2021;1(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 4. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo YH, Chiu CH, Scott Kuo CH, Chou TY, Yeh YC, Hsu HS, et al. Lung cancer in Republic of China. J Thorac Oncol. 2021;16(4):519–27. [DOI] [PubMed] [Google Scholar]
- 6. Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in People's Republic of China. J Thorac Oncol. 2020;15(10):1567–76. [DOI] [PubMed] [Google Scholar]
- 7. Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900‐80. J Natl Cancer Inst. 1983;71(3):473–9. [PubMed] [Google Scholar]
- 8. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–907. [DOI] [PubMed] [Google Scholar]
- 9. Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross‐sectional household surveys. Lancet. 2012;380(9842):668–79. [DOI] [PubMed] [Google Scholar]
- 10. Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer‐Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980‐2012. JAMA. 2014;311(2):183–92. [DOI] [PubMed] [Google Scholar]
- 11. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung‐cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. [DOI] [PubMed] [Google Scholar]
- 13. Wang N, Chen WQ, Zhu WX, Xing XM, Lu AP, Yang L. [Incidence trends and pathological characteristics of lung cancer in urban Beijing during period of 1998 ‐ 2007]. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(3):249–54. [PubMed] [Google Scholar]
- 14. Bender E. Epidemiology: The dominant malignancy. Nature. 2014;513(7517):S2–3. [DOI] [PubMed] [Google Scholar]
- 15. Zhang LM, Lin H, Mei H. An analysis of epidemic trends of lung cancer from 1991 to 2005 in Dalian city, liaoning province. Bulletin of Chinese Cancer. 2008(02):84–7. [Google Scholar]
- 16. Zou XN, Lin DM, Wan X, Chao A, Feng QF, Dai Z, et al. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci. 2014;27(1):3–9. [DOI] [PubMed] [Google Scholar]
- 17. Zou XN, Lin D, Chao A, Wan X, Feng Q, Li J, et al. Histological subtypes of lung cancer in Chinese women from 2000 to 2012. Thorac Cancer. 2014;5(5):447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, et al. Clinical features and therapeutic options in non‐small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019;8(6):2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai W, Su C, Li X, Fan L, Zheng L, Fei K, et al. KIF5B‐RET fusions in Chinese patients with non‐small cell lung cancer. Cancer. 2013;119(8):1486–94. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Zhang S, Wu F, Zhao J, Li X, Zhao C, et al. Outcomes of Pemetrexed‐based chemotherapies in HER2‐mutant lung cancers. BMC Cancer. 2018;18(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li D, Ding L, Ran W, Huang Y, Li G, Wang C, et al. Status of 10 targeted genes of non‐small cell lung cancer in eastern China: A study of 884 patients based on NGS in a single institution. Thorac Cancer. 2020;11(9):2580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang R, Zhang Y, Pan Y, Li Y, Hu H, Cai D, et al. Comprehensive investigation of oncogenic driver mutations in Chinese non‐small cell lung cancer patients. Oncotarget. 2015;6(33):34300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pao W, Girard N. New driver mutations in non‐small‐cell lung cancer. Lancet Oncol. 2011;12(2):175–80. [DOI] [PubMed] [Google Scholar]
- 24. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirsch FR, Suda K, Wiens J, Bunn PA, Jr . New and emerging targeted treatments in advanced non‐small‐cell lung cancer. Lancet. 2016;388(10048):1012–24. [DOI] [PubMed] [Google Scholar]
- 26. Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non‐Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA. 2019;321(14):1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, et al. Beyond ALK‐RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han B, Tjulandin S, Hagiwara K, Normanno N, Wulandari L, Laktionov K, et al. EGFR mutation prevalence in Asia‐Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non‐adenocarcinoma histology: The IGNITE study. Lung Cancer. 2017;113:37–44. [DOI] [PubMed] [Google Scholar]
- 29. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba, II , et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448(7153):561–6. [DOI] [PubMed] [Google Scholar]
- 31. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–203. [DOI] [PubMed] [Google Scholar]
- 32. Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4‐ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw AT, Yeap BY, Mino‐Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27(26):4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi T, Sonobe M, Kobayashi M, Yoshizawa A, Menju T, Nakayama E, et al. Clinicopathologic features of non‐small‐cell lung cancer with EML4‐ALK fusion gene. Ann Surg Oncol. 2010;17(3):889–97. [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tufman AL, Edelmann M, Gamarra F, Reu S, Borgmeier A, Schrödl K, et al. Preselection based on clinical characteristics in German non‐small‐cell lung cancer patients screened for EML4‐ALK translocation. J Thorac Oncol. 2014;9(1):109–13. [DOI] [PubMed] [Google Scholar]
- 37. Zheng D, Wang R, Zhang Y, Pan Y, Cheng X, Cheng C, et al. Prevalence and clinicopathological characteristics of ALK fusion subtypes in lung adenocarcinomas from Chinese populations. J Cancer Res Clin Oncol. 2016;142(4):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K‐Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS‐mutant lung adenocarcinomas: The lung cancer mutation consortium experience. J Thorac Oncol. 2019;14(5):876–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheffler M, Ihle MA, Hein R, Merkelbach‐Bruse S, Scheel AH, Siemanowski J, et al. K‐ras mutation subtypes in NSCLC and associated Co‐occuring mutations in other oncogenic pathways. J Thorac Oncol. 2019;14(4):606–16. [DOI] [PubMed] [Google Scholar]
- 41. Liu SY, Sun H, Zhou JY, Jie GL, Xie Z, Shao Y, et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non‐small cell lung cancer patients. Biomark Res. 2020;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Li H, Zhu J, Zhang Y, Liu X, Li R, et al. The Prevalence and Concurrent Pathogenic Mutations of KRAS (G12C) in Northeast Chinese Non‐small‐cell Lung Cancer Patients. Cancer Manag Res. 2021;13:2447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loong HH, Du N, Cheng C, Lin H, Guo J, Lin G, et al. KRAS G12C mutations in Asia: a landscape analysis of 11,951 Chinese tumor samples. Transl Lung Cancer Res. 2020;9(5):1759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finn SP, Addeo A, Dafni U, Thunnissen E, Bubendorf L, Madsen LB, et al. Prognostic Impact of KRAS G12C Mutation in Patients With NSCLC: Results From the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2021;16(6):990–1002. [DOI] [PubMed] [Google Scholar]
- 45. Liu X, Yu Y, Wang M, Mubarik S, Wang F, Wang Y, et al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990‐2017. Cancer Commun (Lond). 2020;40(11):611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fillon M. Pairing smoking cessation with lung cancer screening may save lives. CA Cancer J Clin. 2021;71(4):283–4. [DOI] [PubMed] [Google Scholar]
- 47. Pelosi G, Pasini F. Over‐time risk of lung cancer is largely owing to continuing smoking exposition: A good reason to quit. J Thorac Oncol. 2021;16(8):e57–e9. [DOI] [PubMed] [Google Scholar]
- 48. DeRouen MC, Canchola AJ, Thompson CA, Jin A, Nie S, Wong C, et al. Incidence of lung cancer among never‐smoking Asian American, Native Hawaiian, and Pacific Islander Females. J Natl Cancer Inst. 2022;114(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raaschou‐Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, et al. Particulate matter air pollution components and risk for lung cancer. Environ Int. 2016;87:66–73. [DOI] [PubMed] [Google Scholar]
- 50. Chen KC, Tsai SW, Shie RH, Zeng C, Yang HY. Indoor air pollution increases the risk of lung cancer. Int J Environ Res Public Health. 2022;19(3):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones B. Epidemiology: Time to ESCAPE the city? Air pollution linked to lung cancer. Nat Rev Clin Oncol. 2013;10(9):486. [DOI] [PubMed] [Google Scholar]
- 52. Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope CA III, et al. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin. 2020;70(6):460–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yin P, Brauer M, Cohen AJ, Wang H, Li J, Burnett RT, et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990‐2017: an analysis for the Global Burden of Disease Study 2017. Lancet Planet Health. 2020;4(9):e386–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim C, Gao YT, Xiang YB, Barone‐Adesi F, Zhang Y, Hosgood HD, et al. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int J Cancer. 2015;136(3):632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang N, Mengersen K, Tong S, Kimlin M, Zhou M, Wang L, et al. Short‐term association between ambient air pollution and lung cancer mortality. Environ Res. 2019;179(Pt A):108748. [DOI] [PubMed] [Google Scholar]
- 56. Cheng Y, Kan H. Effect of the interaction between outdoor air pollution and extreme temperature on daily mortality in Shanghai, China. J Epidemiol. 2012;22(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lam HC, Li AM, Chan EY, Goggins WB, 3rd . The short‐term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: a time‐series study. Thorax. 2016;71(12):1097–109. [DOI] [PubMed] [Google Scholar]
- 58. Deas SD, Huprikar N, Skabelund A. Radiation exposure and lung disease in today's nuclear world. Curr Opin Pulm Med. 2017;23(2):167–72. [DOI] [PubMed] [Google Scholar]
- 59. Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102(21):1628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brims FJH, Kong K, Harris EJA, Sodhi‐Berry N, Reid A, Murray CP, et al. Pleural Plaques and the Risk of Lung Cancer in Asbestos‐exposed Subjects. Am J Respir Crit Care Med. 2020;201(1):57–62. [DOI] [PubMed] [Google Scholar]
- 61. Hyland RA, Chrzanowska A, Hannaford‐Turner K, Davis A, Ke H, Bradbury L, et al. Asbestos‐related lung cancer: Clinical characteristics and survival outcomes in an Australian cohort seeking workers compensation. Asia Pac J Clin Oncol. 2022. [DOI] [PubMed] [Google Scholar]
- 62. Baszuk P, Janasik B, Pietrzak S, Marciniak W, Reszka E, Białkowska K, et al. Lung cancer occurrence‐correlation with serum chromium levels and genotypes. Biol Trace Elem Res. 2021;199(4):1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pesch B, Kendzia B, Pohlabeln H, Ahrens W, Wichmann HE, Siemiatycki J, et al. Exposure to welding fumes, hexavalent chromium, or nickel and risk of lung cancer. Am J Epidemiol. 2019;188(11):1984–93. [DOI] [PubMed] [Google Scholar]
- 64. Liu L, Wan X, Chen G, Ma X, Ning B, Yang G. [Risk Factors of Lung Cancer in Xuanwei, Yunnan Province, China]. Zhongguo Fei Ai Za Zhi. 2017;20(8):528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yuan H, Wang Y, Duan H. Risk of lung cancer and occupational exposure to polycyclic aromatic hydrocarbons among workers cohorts ‐ worldwide, 1969‐2022. China CDC Wkly. 2022;4(17):364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dai J, Shen W, Wen W, Chang J, Wang T, Chen H, et al. Estimation of heritability for nine common cancers using data from genome‐wide association studies in Chinese population. Int J Cancer. 2017;140(2):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Köberle B, Koch B, Fischer BM, Hartwig A. Single nucleotide polymorphisms in DNA repair genes and putative cancer risk. Arch Toxicol. 2016;90(10):2369–88. [DOI] [PubMed] [Google Scholar]
- 68. McKay JD, Hung RJ, Han Y, Zong X, Carreras‐Torres R, Christiani DC, et al. Large‐scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017;49(7):1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bossé Y, Amos CI. A decade of GWAS results in lung cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(4):363–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ji P, Ding D, Qin N, Wang C, Zhu M, Li Y, et al. Systematic analyses of genetic variants in chromatin interaction regions identified four novel lung cancer susceptibility loci. J Cancer. 2020;11(5):1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dong J, Hu Z, Wu C, Guo H, Zhou B, Lv J, et al. Association analyses identify multiple new lung cancer susceptibility loci and their interactions with smoking in the Chinese population. Nat Genet. 2012;44(8):895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, et al. A genome‐wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011;43(8):792–6. [DOI] [PubMed] [Google Scholar]
- 73. Dai J, Lv J, Zhu M, Wang Y, Qin N, Ma H, et al. Identification of risk loci and a polygenic risk score for lung cancer: a large‐scale prospective cohort study in Chinese populations. Lancet Respir Med. 2019;7(10):881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gazdar A, Robinson L, Oliver D, Xing C, Travis WD, Soh J, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol. 2014;9(4):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin X, Peng M, Chen Q, Yuan M, Chen R, Deng H, et al. Identification of the unique clinical and genetic features of chinese lung cancer patients With EGFR germline mutations in a large‐scale retrospective study. Front Oncol. 2021;11:774156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu S, Yu Y, Li Z, Yu R, Wu X, Bao H, et al. EGFR and ERBB2 germline mutations in chinese lung cancer patients and their roles in genetic susceptibility to cancer. J Thorac Oncol. 2019;14(4):732–6. [DOI] [PubMed] [Google Scholar]
- 77. Li X, Liu J, Wang K, Zhou J, Zhang H, Zhang M, et al. Polymorphisms and rare variants identified by next‐generation sequencing confer risk for lung cancer in han Chinese population. Pathol Res Pract. 2020;216(4):152873. [DOI] [PubMed] [Google Scholar]
- 78. Katzke VA, Kaaks R, Kühn T. Lifestyle and cancer risk. Cancer J. 2015;21(2):104–10. [DOI] [PubMed] [Google Scholar]
- 79. Bates JHT, Hamlington KL, Garrison G, Kinsey CM. Prediction of lung cancer risk based on age and smoking history. Comput Methods Programs Biomed. 2022;216:106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jiang L, Sun YQ, Langhammer A, Brumpton BM, Chen Y, Nilsen TI, et al. Asthma and asthma symptom control in relation to incidence of lung cancer in the HUNT study. Sci Rep. 2021;11(1):4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mouronte‐Roibás C, Leiro‐Fernández V, Ruano‐Raviña A, Ramos‐Hernández C, Abal‐Arca J, Parente‐Lamelas I, et al. Chronic obstructive pulmonary disease in lung cancer patients: Prevalence, underdiagnosis, and clinical characterization. Respiration. 2018;95(6):414–21. [DOI] [PubMed] [Google Scholar]
- 82. Sutton SS, Magagnoli J, Cummings TH, Hardin JW. Leukotriene inhibition and the risk of lung cancer among U.S. veterans with asthma. Pulm Pharmacol Ther. 2021;71:102084. [DOI] [PubMed] [Google Scholar]
- 83. Jang HJ, Park MS, Kim YS, Chang J, Lee JH, Lee CT, et al. The relationship between the severity of pulmonary fibrosis and the lung cancer stage. J Cancer. 2021;12(10):2807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McGee R, Williams S, Elwood M. Depression and the development of cancer: a meta‐analysis. Soc Sci Med. 1994;38(1):187–92. [DOI] [PubMed] [Google Scholar]
- 85. Lund R, Christensen U, Nilsson CJ, Kriegbaum M, Hulvej Rod N. Stressful social relations and mortality: a prospective cohort study. J Epidemiol Community Health. 2014;68(8):720–7. [DOI] [PubMed] [Google Scholar]
- 86. Gepner Y, Lev‐Ari S, Goldbourt U. Excess body weight and long‐term incidence of lung and colon cancer in men; follow‐up study of 43 years. Int J Environ Res Public Health. 2021;18(19):10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang W, Xie M, Dou S, Cui L, Zheng C, Xiao W. The link between chronic obstructive pulmonary disease phenotypes and histological subtypes of lung cancer: a case‐control study. Int J Chron Obstruct Pulmon Dis. 2018;13:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang H, Yang L, Zou L, Huang D, Guo Y, Pan M, et al. Association between chronic obstructive pulmonary disease and lung cancer: a case‐control study in Southern Chinese and a meta‐analysis. PLoS One. 2012;7(9):e46144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro‐Rosenblatt D, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta‐analysis. Ann Oncol. 2016;27(1):81–96. [DOI] [PubMed] [Google Scholar]
- 90. Baumeister SE, Baurecht H, Nolde M, Alayash Z, Gläser S, Johansson M, et al. Cannabis use, pulmonary function, and lung cancer susceptibility: A mendelian randomization study. J Thorac Oncol. 2021;16(7):1127–35. [DOI] [PubMed] [Google Scholar]
- 91. Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, et al. Association between telomere length and risk of cancer and non‐neoplastic diseases: A mendelian randomization study. JAMA Oncol. 2017;3(5):636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huo Z, Ge F, Li C, Cheng H, Lu Y, Wang R, et al. Genetically predicted insomnia and lung cancer risk: a Mendelian randomization study. Sleep Med. 2021;87:183–90. [DOI] [PubMed] [Google Scholar]
- 93. Khankari NK, Shu XO, Wen W, Kraft P, Lindström S, Peters U, et al. Association between adult height and risk of colorectal, lung, and prostate cancer: Results from meta‐analyses of prospective studies and mendelian randomization analyses. PLoS Med. 2016;13(9):e1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhu Y, Wei Y, Zhang R, Dong X, Shen S, Zhao Y, et al. Elevated platelet count appears to be causally associated with increased risk of lung cancer: A mendelian randomization analysis. Cancer Epidemiol Biomarkers Prev. 2019;28(5):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fanidi A, Carreras‐Torres R, Larose TL, Yuan JM, Stevens VL, Weinstein SJ, et al. Is high vitamin B12 status a cause of lung cancer? Int J Cancer. 2019;145(6):1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for lung cancer: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):69s–77s. [DOI] [PubMed] [Google Scholar]
- 97. Jemal A, Fedewa SA. Lung cancer screening with low‐dose computed tomography in the United States‐2010 to 2015. JAMA Oncol. 2017;3(9):1278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. He J, Li N, Chen WQ, Wu N, Shen HB, Jiang Y, et al. [China guideline for the screening and early detection of lung cancer(2021, Beijing)]. Zhonghua Zhong Liu Za Zhi. 2021;43(3):243–68. [DOI] [PubMed] [Google Scholar]
- 99. Zhou Q. MS16.02 NELCIN B3 screening program in China. J Thorac Oncol. 2018;13(10):S272–S3. [Google Scholar]
- 100. Chen WQ, Ni LI, Shi JF, Ren JS, Chen HD, Jiang LI, et al. Progress of cancer screening program in Urban China. China Cancer. 2019;28(01):23–5. [Google Scholar]
- 101. Chen WQ, Ni LI, Cao MM, Ren JS, Shi JF, Chen HD, et al. Preliminary analysis of cancer screening program in Urban China from 2013 to 2017. China Cancer. 2020;29(01):1–6. [Google Scholar]
- 102. Xiaoyang L, Quan L, Shengping W, Yuan L, Lei S, Guodong L, et al. Shanghai community‐based practice of early lung cancer screening with low‐dose spiral computed tomography. Chin Oncol. 2016;26(12):996–1003. [Google Scholar]
- 103. Gao Z, Ye Z, Zhang P, Cui X, Xie Y, Han L. Low‐dose computed tomography screening for lung cancer in Tianjin: a preliminary clinical analysis of baseline screening and follow‐up results. Chinese Journal of Clinical Oncology. 2017;44(20):1034–9. [Google Scholar]
- 104. Tang W, Wu N, Huang Y, Wang J, Zhao S, Xu Z, et al. [Results of low‐dose computed tomography (LDCT) screening for early lung cancer: prevalence in 4 690 asymptomatic participants]. Zhonghua Zhong Liu Za Zhi. 2014;36(7):549–54. [PubMed] [Google Scholar]
- 105. Chun‐Fang Z, Qiang Z, Wei‐min W, Dan F , Yuan H. Detection rates and cost of lung cancer screening with low‐dose helical computed tomography among physical examination people. Chinese Journal of Cancer Prevention and Treatment. 2015;22:247–51. [Google Scholar]
- 106. WANG W, S‐w NIE, S‐q LI, C‐t XU. Diagnosis value of low‐dose spiral CT for early lung cancer. Medical Recapitulate. 2012. [Google Scholar]
- 107. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Henschke CI, Yip R, Shaham D, Zulueta JJ, Aguayo SM, Reeves AP, et al. The regimen of computed tomography screening for lung cancer: Lessons learned over 25 years from the international early lung cancer action program. J Thorac Imaging. 2021;36(1):6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yang W, Qian F, Teng J, Wang H, Manegold C, Pilz LR, et al. Community‐based lung cancer screening with low‐dose CT in China: Results of the baseline screening. Lung Cancer. 2018;117:20–6. [DOI] [PubMed] [Google Scholar]
- 110. Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst. 2014;106(11):dju284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G, Huang Y, et al. China National Lung Cancer screening guideline with low‐dose computed tomography (2018 version). Zhongguo fei ai za zhi = Chinese Journal of Lung Cancer. 2018;21(2):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low‐dose computed tomography screening: A secondary analysis of the Danish lung cancer screening trial. JAMA Intern Med. 2018;178(10):1420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Patz EF, Jr. , Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low‐dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Choi W, Oh JH, Riyahi S, Liu CJ, Jiang F, Chen W, et al. Radiomics analysis of pulmonary nodules in low‐dose CT for early detection of lung cancer. Med Phys. 2018;45(4):1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kamiya A, Murayama S, Kamiya H, Yamashiro T, Oshiro Y, Tanaka N. Kurtosis and skewness assessments of solid lung nodule density histograms: differentiating malignant from benign nodules on CT. Jpn J Radiol. 2014;32(1):14–21. [DOI] [PubMed] [Google Scholar]
- 117. Lyu J, Bi X, Ling SH. Multi‐Level Cross Residual Network for Lung Nodule Classification. Sensors (Basel). 2020;20(10):2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ni Y, Yang Y, Zheng D, Xie Z, Huang H, Wang W. The invasiveness classification of ground‐glass nodules using 3D attention network and HRCT. J Digit Imaging. 2020;33(5):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ren Y, Tsai MY, Chen L, Wang J, Li S, Liu Y, et al. A manifold learning regularization approach to enhance 3D CT image‐based lung nodule classification. Int J Comput Assist Radiol Surg. 2020;15(2):287–95. [DOI] [PubMed] [Google Scholar]
- 120. Shen Y, Xu F, Zhu W, Hu H, Chen T, Li Q. Multiclassifier fusion based on radiomics features for the prediction of benign and malignant primary pulmonary solid nodules. Ann Transl Med. 2020;8(5):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Song Q, Zhao L, Luo X, Dou X. Using deep learning for classification of lung nodules on computed tomography images. J Healthc Eng. 2017;2017:8314740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Trebeschi S, Bodalal Z, Boellaard TN, Tareco Bucho TM, Drago SG, Kurilova I, et al. Prognostic value of deep learning‐mediated treatment monitoring in lung cancer patients receiving immunotherapy. Front Oncol. 2021;11:609054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Butnor KJ, Beasley MB, Cagle PT, Grunberg SM, Kong FM, Marchevsky A, et al. Protocol for the examination of specimens from patients with primary non‐small cell carcinoma, small cell carcinoma, or carcinoid tumor of the lung. Arch Pathol Lab Med. 2009;133(10):1552–9. [DOI] [PubMed] [Google Scholar]
- 124. Kimbrell HZ, Gustafson KS, Huang M, Ehya H. Subclassification of non‐small cell lung cancer by cytologic sampling: a logical approach with selective use of immunocytochemistry. Acta Cytol. 2012;56(4):419–24. [DOI] [PubMed] [Google Scholar]
- 125. Kapila K, Al‐Ayadhy B, Francis IM, George SS, Al‐Jassar A. Subclassification of pulmonary non‐small cell lung carcinoma in fine needle aspirates using a limited immunohistochemistry panel. J Cytol. 2013;30(4):223–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Betz BL, Dixon CA, Weigelin HC, Knoepp SM, Roh MH. The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma. Cancer Cytopathol. 2013;121(9):489–99. [DOI] [PubMed] [Google Scholar]
- 127. Jia Z. Interpretation of updated points of 2021 CSCO guideline for non‐small‐cell lung cancer. Journal of Practical Oncology. 2022;37(1):8–15. [Google Scholar]
- 128. Jiang T, Ren S, Li X, Su C, Zhou C, O'Brien M. The changing diagnostic pathway for lung cancer patients in Shanghai, China. Eur J Cancer. 2017;84:168–72. [DOI] [PubMed] [Google Scholar]
- 129. Li W, Lyu Y, Wang S, Zhou X, Ma J, Xu C, et al. Trends in molecular testing of lung cancer in mainland People's Republic of China over the decade 2010 to 2019. JTO Clin Res Rep. 2021;2(4):100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhou Q, Song Y, Zhang X, Chen GY, Zhong DS, Yu Z, et al. A multicenter survey of first‐line treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous non‐small cell lung cancer in China (CTONG 1506). BMC Cancer. 2017;17(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cheng Y, Wang Y, Zhao J, Liu Y, Gao H, Ma K, et al. Real‐world EGFR testing in patients with stage IIIB/IV non‐small‐cell lung cancer in North China: A multicenter, non‐interventional study. Thorac Cancer. 2018;9(11):1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Current status of postoperative pathological diagnosis of lung cancer in China: a multicenter big data study. Zhonghua bing li xue za zhi = Chinese journal of pathology. 2021;50(8):882–90. [DOI] [PubMed] [Google Scholar]
- 133. Bonaparte E, Pesenti C, Fontana L, Falcone R, Paganini L, Marzorati A, et al. Molecular profiling of lung cancer specimens and liquid biopsies using MALDI‐TOF mass spectrometry. Diagn Pathol. 2018;13(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Giannini R, Lupi C, Sensi E, Alì G, Proietti A, Boldrini L, et al. EGFR and KRAS mutational analysis in a large series of Italian non‐small cell lung cancer patients: 2,387 cases from a single center. Oncol Rep. 2016;36(2):1166–72. [DOI] [PubMed] [Google Scholar]
- 135. Min KW, Kim WS, Jang SJ, Choi YD, Chang S, Jung SH, et al. MassARRAY, pyrosequencing, and PNA clamping for EGFR mutation detection in lung cancer tissue and cytological samples: a multicenter study. J Cancer Res Clin Oncol. 2016;142(10):2209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sakai K, Okamoto I, Takezawa K, Hirashima T, Kaneda H, Takeda M, et al. A novel mass spectrometry‐based assay for diagnosis of EML4‐ALK‐positive non‐small cell lung cancer. J Thorac Oncol. 2012;7(5):913–8. [DOI] [PubMed] [Google Scholar]
- 137. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non‐small‐cell lung cancer. J Clin Oncol. 2005;23(28):6829–37. [DOI] [PubMed] [Google Scholar]
- 138. Hirsch FR, McElhinny A, Stanforth D, Ranger‐Moore J, Jansson M, Kulangara K, et al. PD‐L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208–22. [DOI] [PubMed] [Google Scholar]
- 139. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD‐L1 immunohistochemistry comparability study in real‐life clinical samples: Results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real‐world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non‐small‐cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–9. [DOI] [PubMed] [Google Scholar]
- 141. Pan Y, Zheng D, Li Y, Cai X, Zheng Z, Jin Y, et al. Unique distribution of programmed death ligand 1 (PD‐L1) expression in East Asian non‐small cell lung cancer. J Thorac Dis. 2017;9(8):2579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Skov BG, Rørvig SB, Jensen THL, Skov T. The prevalence of programmed death ligand‐1 (PD‐L1) expression in non‐small cell lung cancer in an unselected, consecutive population. Mod Pathol. 2020;33(1):109–17. [DOI] [PubMed] [Google Scholar]
- 143. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early‐ and late‐stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Diaz LA, Jr. , Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Santarpia M, Liguori A, D'Aveni A, Karachaliou N, Gonzalez‐Cao M, Daffinà MG, et al. Liquid biopsy for lung cancer early detection. J Thorac Dis. 2018;10(Suppl 7):S882–s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sozzi G, Roz L, Conte D, Mariani L, Andriani F, Lo Vullo S, et al. Plasma DNA quantification in lung cancer computed tomography screening: five‐year results of a prospective study. Am J Respir Crit Care Med. 2009;179(1):69–74. [DOI] [PubMed] [Google Scholar]
- 147. Xie F, Zhang Y, Mao X, Zheng X, Han‐Zhang H, Ye J, et al. Comparison of genetic profiles among primary lung tumor, metastatic lymph nodes and circulating tumor DNA in treatment‐naïve advanced non‐squamous non‐small cell lung cancer patients. Lung Cancer. 2018;121:54–60. [DOI] [PubMed] [Google Scholar]
- 148. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. Liquid biopsy for advanced NSCLC: A consensus statement from the international association for the study of lung cancer. J Thorac Oncol. 2021;16(10):1647–62. [DOI] [PubMed] [Google Scholar]
- 149. Li Y, Xu H, Su S, Ye J, Chen J, Jin X, et al. Clinical validation of a highly sensitive assay to detect EGFR mutations in plasma cell‐free DNA from patients with advanced lung adenocarcinoma. PLoS One. 2017;12(8):e0183331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, et al. Folate receptor‐positive circulating tumor cell detected by LT‐PCR‐based method as a diagnostic biomarker for non‐small‐cell lung cancer. J Thorac Oncol. 2015;10(8):1163–71. [DOI] [PubMed] [Google Scholar]
- 151. Wang L, Wu C, Qiao L, Yu W, Guo Q, Zhao M, et al. Clinical significance of folate receptor‐positive circulating tumor cells detected by ligand‐targeted polymerase chain reaction in lung cancer. J Cancer. 2017;8(1):104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu J, Han M, Huang H. Validation of the diagnostic efficiency of folate receptor‐positive circulating tumor cells in lung cancers: a prospective observational study. Transl Cancer Res. 2019;8(4):1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C, et al. Folate receptor‐positive circulating tumor cells as a novel diagnostic biomarker in non‐small cell lung cancer. Transl Oncol. 2013;6(6):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lou J, Ben S, Yang G, Liang X, Wang X, Ni S, et al. Quantification of rare circulating tumor cells in non‐small cell lung cancer by ligand‐targeted PCR. PLoS One. 2013;8(12):e80458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol. 2009;4(5):652–7. [DOI] [PubMed] [Google Scholar]
- 156. Shao W, Liu J, Liang W, Chen H, Li S, Yin W, et al. Safety and feasibility of video‐assisted thoracoscopic surgery for stage IIIA lung cancer. Chin J Cancer Res. 2014;26(4):418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang Z, Zhang Y, Feng H, Yao Z, Teng J, Wei D, et al. Is video‐assisted thoracic surgery lobectomy better than thoracotomy for early‐stage non‐small‐cell lung cancer? A systematic review and meta‐analysis. Eur J Cardiothorac Surg. 2013;44(3):407–14. [DOI] [PubMed] [Google Scholar]
- 158. Cai J, Zhou J, Yang F, Wang J. Adoption Rate of Video‐Assisted Thoracic Surgery for Lung Cancer Varies Widely in China. Chest. 2018;153(4):1073–5. [DOI] [PubMed] [Google Scholar]
- 159. Xu J, Ni H, Wu Y, Cao J, Han X, Liu L, et al. Perioperative comparison of video‐assisted thoracic surgery and open lobectomy for pT1‐stage non‐small cell lung cancer patients in China: a multi‐center propensity score‐matched analysis. Transl Lung Cancer Res. 2021;10(1):402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zang R, Shi JF, Lerut TE, Wang L, Liu CC, Brunelli A, et al. Ten‐year trends of clinicopathologic features and surgical treatment of lung cancer in china. Ann Thorac Surg. 2020;109(2):389–95. [DOI] [PubMed] [Google Scholar]
- 161. Sihoe ADL, Han B, Yang TY, Pan C, Jiang G, Fang VWT. The advent of ultra‐high volume thoracic surgical centers in Shanghai. World J Surg. 2017;41(11):2758–68. [DOI] [PubMed] [Google Scholar]
- 162. Begum S, Hansen HJ, Papagiannopoulos K. VATS anatomic lung resections‐the European experience. J Thorac Dis. 2014;6(Suppl 2):S203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity‐matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–78. [DOI] [PubMed] [Google Scholar]
- 164. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2020;383(18):1711–23. [DOI] [PubMed] [Google Scholar]
- 165. Wu YL, John T, Grohe C, Majem M, Goldman JW, Kim SW, et al. Postoperative chemotherapy use and outcomes from ADAURA: Osimertinib as adjuvant therapy for resected EGFR‐mutated NSCLC. J Thorac Oncol. 2021;17(3):423–33. [DOI] [PubMed] [Google Scholar]
- 166. Jie W, Wu YL, Lu S, Wang Q, Li S, Zhong W, et al. 85P Adjuvant osimertinib in patients (pts) with stage IB–IIIA EGFR mutation‐positive (EGFRm) NSCLC after complete tumour resection: ADAURA China subgroup analysis. Ann Oncol. 2022;33:S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yue D, Xu S, Wang Q, Li X, Shen Y, Zhao H, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation‐positive non‐small‐cell lung cancer (EVAN): a randomised, open‐label, phase 2 trial. Lancet Respir Med. 2018;6(11):863–73. [DOI] [PubMed] [Google Scholar]
- 168. Pennell NA, Neal JW, Chaft JE, Azzoli CG, Jänne PA, Govindan R, et al. SELECT: A phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor‐mutant non‐small‐cell lung cancer. J Clin Oncol. 2019;37(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II‐IIIA (N1‐N2) EGFR‐mutant NSCLC: Final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39(7):713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. He J, Su C, Liang W, Xu S, Wu L, Fu X, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II‐IIIA EGFR‐mutant non‐small‐cell lung cancer (EVIDENCE): a randomised, open‐label, phase 3 trial. Lancet Respir Med. 2021;9(9):1021–9. [DOI] [PubMed] [Google Scholar]
- 171. Kang J, Zhang C, Zhong WZ. Neoadjuvant immunotherapy for non‐small cell lung cancer: State of the art. Cancer Commun (Lond). 2021;41(4):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. PM F, J S, S L. Abstract CT003: Nivolumab + platinum‐doublet chemotherapy vs chemotherapy as neoadjuvant treatment for resectable (Ib‐IIIa) non‐small cell lung cancer in the phase III CheckMate 816 trial. . Cancer Res. 2021;81(13_suppl):CT003. [Google Scholar]
- 173. J S, CL W, F T. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum‐doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non‐small cell lung cancer (NSCLC) [EB/OL]. ASCO 2021 8503. 2021.
- 174. Kwiatkowski D, Rusch V, Chaft J, Johnson B, Nicholas A, Wistuba I, et al. Neoadjuvant atezolizumab in resectable non‐small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol. 2019;37:8503‐. [Google Scholar]
- 175. Lee J, Chaft J, Nicholas A, Patterson A, Waqar S, Toloza E, et al. PS01.05 surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. Journal of Thoracic Oncology. 2021;16:S59–S61. [Google Scholar]
- 176. Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD‐1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–26. [DOI] [PubMed] [Google Scholar]
- 177. Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non‐small cell lung cancer. Transl Lung Cancer Res. 2020;9(6):2696–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB‐IIIA non‐small‐cell lung cancer (IMpower010): a randomised, multicentre, open‐label, phase 3 trial. Lancet. 2021;398(10308):1344–57. [DOI] [PubMed] [Google Scholar]
- 179. E F, E V, C Z. LBA9 IMpower010: Sites of relapse and subsequent therapy from a phase III study of atezolizumab vs best supportive care after adjuvant chemotherapy in stage IB‐IIIA NSCLC. Ann Oncol. 2021;32:S1319. [Google Scholar]
- 180. Provencio M, Nadal E, Insa A, García‐Campelo MR, Casal‐Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non‐small‐cell lung cancer (NADIM): an open‐label, multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–22. [DOI] [PubMed] [Google Scholar]
- 181. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small‐cell lung cancer. N Engl J Med. 2004;350(4):351–60. [DOI] [PubMed] [Google Scholar]
- 182. Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin‐based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. [DOI] [PubMed] [Google Scholar]
- 183. Butts CA, Ding K, Seymour L, Twumasi‐Ankrah P, Graham B, Gandara D, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non‐small‐cell lung cancer: updated survival analysis of JBR‐10. J Clin Oncol. 2010;28(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles‐Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB‐IIIA non‐small‐cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27. [DOI] [PubMed] [Google Scholar]
- 185. Kreuter M, Vansteenkiste J, Fischer JR, Eberhardt W, Zabeck H, Kollmeier J, et al. Randomized phase 2 trial on refinement of early‐stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol. 2013;24(4):986–92. [DOI] [PubMed] [Google Scholar]
- 186. Kreuter M, Vansteenkiste J, Fischer JR, Eberhardt WE, Zabeck H, Kollmeier J, et al. Three‐Year Follow‐Up of a Randomized Phase II Trial on Refinement of Early‐Stage NSCLC Adjuvant Chemotherapy with Cisplatin and Pemetrexed versus Cisplatin and Vinorelbine (the TREAT Study). J Thorac Oncol. 2016;11(1):85–93. [DOI] [PubMed] [Google Scholar]
- 187. Strauss GM, Herndon JE, 2nd , Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non‐small‐cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non‐small‐cell lung cancer. N Engl J Med. 2005;352(25):2589–97. [DOI] [PubMed] [Google Scholar]
- 189. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9. [DOI] [PubMed] [Google Scholar]
- 190. Rosell R, Gómez‐Codina J, Camps C, Maestre J, Padille J, Cantó A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non‐small‐cell lung cancer. N Engl J Med. 1994;330(3):153–8. [DOI] [PubMed] [Google Scholar]
- 191. Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB, Jr. , Lee JS, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non‐small‐cell lung cancer. J Natl Cancer Inst. 1994;86(9):673–80. [DOI] [PubMed] [Google Scholar]
- 192. Felip E, Rosell R, Maestre JA, Rodríguez‐Paniagua JM, Morán T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early‐stage non‐small‐cell lung cancer. J Clin Oncol. 2010;28(19):3138–45. [DOI] [PubMed] [Google Scholar]
- 193. Pisters KM, Vallières E, Crowley JJ, Franklin WA, Bunn PA, Jr. , Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early‐stage non‐small‐cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28(11):1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Martins RG, D'Amico TA, Loo BW, Jr. , Pinder‐Schenck M, Borghaei H, Chaft JE, et al. The management of patients with stage IIIA non‐small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw. 2012;10(5):599–613. [DOI] [PubMed] [Google Scholar]
- 195. Group NM‐aC. Preoperative chemotherapy for non‐small‐cell lung cancer: a systematic review and meta‐analysis of individual participant data. Lancet. 2014;383(9928):1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non‐small‐cell lung carcinomas: long‐term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol. 2007;25(3):313–8. [DOI] [PubMed] [Google Scholar]
- 197. Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. [DOI] [PubMed] [Google Scholar]
- 198. Curran WJ, Jr. , Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non‐small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non‐Small‐Cell Lung Cancer: KCSG‐LU05‐04. J Clin Oncol. 2015;33(24):2660–6. [DOI] [PubMed] [Google Scholar]
- 200. Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non‐small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28(4):777–83. [DOI] [PubMed] [Google Scholar]
- 201. Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed‐Cisplatin or Etoposide‐Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non‐Small‐Cell Lung Cancer. J Clin Oncol. 2016;34(9):953–62. [DOI] [PubMed] [Google Scholar]
- 202. DR S, C F‐F, JE G. Five‐year survival outcomes with durvalumab after chemoradiotherapy inunresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol. 2021;39(15_suppl):8511. [Google Scholar]
- 203. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 204. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 205. Zhou Q, Chen M, Wu G, Chang JH, Jiang O, Cui JW, et al. GEMSTONE‐301: a phase III clinical trial of CS1001 as consolidation therapy in patients with locally advanced/unresectable (stage III) non‐small cell lung cancer (NSCLC) who did not have disease progression after prior concurrent/sequential chemoradiotherapy. Transl Lung Cancer Res. 2020;9(5):2008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non‐small‐cell lung cancer in China (GEMSTONE‐301): interim results of a randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(2):209–19. [DOI] [PubMed] [Google Scholar]
- 207. Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non‐small cell lung cancer: The phase 2 KEYNOTE‐799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Augustyn A, Adams DL, He J, Qiao Y, Verma V, Liao Z, et al. Giant circulating cancer‐associated macrophage‐like cells are associated with disease recurrence and survival in non‐small‐cell lung cancer treated with chemoradiation and atezolizumab. Clin Lung Cancer. 2021;22(3):e451–e65. [DOI] [PubMed] [Google Scholar]
- 209. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 210. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. [DOI] [PubMed] [Google Scholar]
- 211. Sutiman N, Tan SW, Tan EH, Lim WT, Kanesvaran R, Ng QS, et al. EGFR mutation subtypes influence survival outcomes following first‐line gefitinib therapy in advanced Asian NSCLC Patients. J Thorac Oncol. 2017;12(3):529–38. [DOI] [PubMed] [Google Scholar]
- 212. Díaz‐Serrano A, Gella P, Jiménez E, Zugazagoitia J, Paz‐Ares Rodríguez L. Targeting EGFR in lung cancer: Current standards and developments. Drugs. 2018;78(9):893–911. [DOI] [PubMed] [Google Scholar]
- 213. Lu S, Wang Q, Zhang G, Dong X, Yang CT, Song Y, et al. Efficacy of aumolertinib (HS‐10296) in patients with advanced EGFR T790M+ NSCLC: Updated post‐national medical products administration approval results from the APOLLO registrational trial. J Thorac Oncol. 2021;17(3):411–22. [DOI] [PubMed] [Google Scholar]
- 214. Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B, et al. Osimertinib versus comparator EGFR TKI as first‐line treatment for EGFR‐mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol. 2021;16(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20‐selective kinase inhibitor in non‐small cell lung cancer. Nat Med. 2018;24(5):638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Park K, Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, et al. Amivantamab in EGFR Exon 20 Insertion‐Mutated Non‐Small‐Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Zhou C, Ramalingam SS, Kim TM, Kim SW, Yang JC, Riely GJ, et al. Treatment outcomes and safety of mobocertinib in platinum‐pretreated patients with EGFR exon 20 insertion‐positive metastatic non‐small cell lung cancer: A Phase 1/2 open‐label nonrandomized clinical trial. JAMA Oncol. 2021;7(12):e214761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yang JC‐H, Wang M, Mitchell P, Fang J, Nian W, Chiu C‐H, et al. Preliminary safety and efficacy results from phase 1 studies of DZD9008 in NSCLC patients with EGFR Exon20 insertion mutations. J Clin Oncol. 2021;39:9008. [Google Scholar]
- 219. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–81. [DOI] [PubMed] [Google Scholar]
- 220. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR‐mutated non‐small cell lung cancer. Br J Cancer. 2019;121(9):725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376(7):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288–96. [DOI] [PubMed] [Google Scholar]
- 223. Yang JC, Camidge DR, Yang CT, Zhou J, Guo R, Chiu CH, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS‐10296) in Pretreated Patients With EGFR‐Mutated Advanced NSCLC: A Multicenter, Open‐label, Phase 1 Trial. J Thorac Oncol. 2020;15(12):1907–18. [DOI] [PubMed] [Google Scholar]
- 224. Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non‐small‐cell lung cancer: a phase 2b, multicentre, single‐arm, open‐label study. Lancet Respir Med. 2021;9(8):829–39. [DOI] [PubMed] [Google Scholar]
- 225. Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, et al. Combination of chemotherapy and gefitinib as first‐line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer. 2017;141(6):1249–56. [DOI] [PubMed] [Google Scholar]
- 226. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib alone versus gefitinib plus chemotherapy for non‐small‐cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115–23. [DOI] [PubMed] [Google Scholar]
- 227. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR‐mutated lung cancer. J Clin Oncol. 2020;38(2):124–36. [DOI] [PubMed] [Google Scholar]
- 228. Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First‐Line Therapy in Patients With Advanced Nonsquamous Non‐Small‐Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol. 2016;34(27):3258–66. [DOI] [PubMed] [Google Scholar]
- 229. Xu L, Qi Q, Zhang Y, Cui J, Liu R, Li Y. Combination of icotinib and chemotherapy as first‐line treatment for advanced lung adenocarcinoma in patients with sensitive EGFR mutations: A randomized controlled study. Lung Cancer. 2019;133:23–31. [DOI] [PubMed] [Google Scholar]
- 230. Wu YL, Zhou C, Lu S, Qin S, Pan H, Wu G, et al. Erlotinib versus gemcitabine/cisplatin in Chinese patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: Crossover extension and post‐hoc analysis of the ENSURE study. Lung Cancer. 2019;130:18–24. [DOI] [PubMed] [Google Scholar]
- 231. Paz‐Ares L, Mezger J, Ciuleanu TE, Fischer JR, von Pawel J, Provencio M, et al. Necitumumab plus pemetrexed and cisplatin as first‐line therapy in patients with stage IV non‐squamous non‐small‐cell lung cancer (INSPIRE): an open‐label, randomised, controlled phase 3 study. Lancet Oncol. 2015;16(3):328–37. [DOI] [PubMed] [Google Scholar]
- 232. Ciuleanu T, Socinski MA, Obasaju C, Luft AV, Szczesna A, Szafrański W, et al. Efficacy and Safety of Necitumumab Continuation Therapy in the Phase III SQUIRE Study of Patients With Stage IV Squamous Non‐Small‐Cell Lung Cancer. Clin Lung Cancer. 2018;19(2):130–8.e2. [DOI] [PubMed] [Google Scholar]
- 233. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167–77. [DOI] [PubMed] [Google Scholar]
- 234. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a Phase III Comparison of First‐Line Crizotinib versus Chemotherapy in East Asian Patients with ALK‐Positive Advanced Non‐Small Cell Lung Cancer. J Thorac Oncol. 2018;13(10):1539–48. [DOI] [PubMed] [Google Scholar]
- 235. Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al. Alectinib versus crizotinib in treatment‐naive anaplastic lymphoma kinase‐positive (ALK+) non‐small‐cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29(11):2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor‐Naive ALK‐Positive Non‐Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA‐1L Trial. J Clin Oncol. 2020;38(31):3592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Soria JC, Tan DSW, Chiari R, Wu YL, Paz‐Ares L, Wolf J, et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): a randomised, open‐label, phase 3 study. Lancet. 2017;389(10072):917–29. [DOI] [PubMed] [Google Scholar]
- 238. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First‐Line Lorlatinib or Crizotinib in Advanced ALK‐Positive Lung Cancer. N Engl J Med. 2020;383(21):2018–29. [DOI] [PubMed] [Google Scholar]
- 239. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated Efficacy and Safety Data and Impact of the EML4‐ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK‐Positive Advanced Non‐Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol. 2019;14(7):1233–43. [DOI] [PubMed] [Google Scholar]
- 240. Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase‐positive non‐small‐cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–46. [DOI] [PubMed] [Google Scholar]
- 241. Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase‐Positive Non‐Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021;7(11):1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Yang Y, Zhou J, Zhou J, Feng J, Zhuang W, Chen J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib‐resistant, ALK‐positive non‐small‐cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. 2020;8(1):45–53. [DOI] [PubMed] [Google Scholar]
- 243. Deeks ED. Ceritinib: a Review in ALK‐Positive Advanced NSCLC. Target Oncol. 2016;11(5):693–700. [DOI] [PubMed] [Google Scholar]
- 244. Markham A. Brigatinib: First Global Approval. Drugs. 2017;77(10):1131–5. [DOI] [PubMed] [Google Scholar]
- 245. Morcos PN, Nueesch E, Jaminion F, Guerini E, Hsu JC, Bordogna W, et al. Exposure‐response analysis of alectinib in crizotinib‐resistant ALK‐positive non‐small cell lung cancer. Cancer Chemother Pharmacol. 2018;82(1):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, Helland Å, et al. Alectinib versus chemotherapy in crizotinib‐pretreated anaplastic lymphoma kinase (ALK)‐positive non‐small‐cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Zheng Y, Lai Q, Zhao H, Li X, Sun X, Xing L. Clinical characteristics and outcomes of Chinese patients with KRAS‐mutant non‐small cell lung cancer after chemotherapy. Cancer Commun (Lond). 2021;41(11):1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021;384(25):2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ou SI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA, et al. First‐in‐Human Phase I/IB Dose‐Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRAS(G12C) Solid Tumors (KRYSTAL‐1). J Clin Oncol. 2022;40(23):2530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lim SM, Kim HR, Lee JS, Lee KH, Lee YG, Min YJ, et al. Open‐Label, Multicenter, Phase II Study of Ceritinib in Patients With Non‐Small‐Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol. 2017;35(23):2613–8. [DOI] [PubMed] [Google Scholar]
- 251. Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel‐Vinay S, et al. Lorlatinib in non‐small‐cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open‐label, single‐arm first‐in‐man phase 1 trial. Lancet Oncol. 2017;18(12):1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1‐Positive Advanced Non‐Small‐Cell Lung Cancer. J Clin Oncol. 2018;36(14):1405–11. [DOI] [PubMed] [Google Scholar]
- 254. Zhou C, Fan H, Wang Y, Wu H, Yang N, Li K, et al. Taletrectinib (AB‐106; DS‐6051b) in metastatic non‐small cell lung cancer (NSCLC) patients with ROS1 fusion: Preliminary results of TRUST. J Clin Oncol. 2021;39:9066. [Google Scholar]
- 255. Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer. 2016;91:23–8. [DOI] [PubMed] [Google Scholar]
- 256. Caparica R, de Castro G, Jr. , Gil‐Bazo I, Caglevic C, Calogero R, Giallombardo M, et al. BRAF mutations in non‐small cell lung cancer: has finally Janus opened the door? Crit Rev Oncol Hematol. 2016;101:32–9. [DOI] [PubMed] [Google Scholar]
- 257. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)‐mutant metastatic non‐small‐cell lung cancer: an open‐label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–16. [DOI] [PubMed] [Google Scholar]
- 258. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of Selpercatinib in RET Fusion‐Positive Non‐Small‐Cell Lung Cancer. N Engl J Med. 2020;383(9):813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Gainor J, Curigliano G, Kim D‐S, Lee DH, Besse B, Baik C, et al. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU‐667) in patients (pts) with advanced RET fusion+ non‐small cell lung cancer (NSCLC). J Clin Oncol. 2020;38:9515. [Google Scholar]
- 260. Lu S, Cheng Y, Huang D, Sun Y, Wu L, Zhou J, et al. MA02.01 Efficacy and Safety of Selpercatinib in Chinese Patients With RET Fusion‐Positive Non‐Small Cell Lung Cancer: A Phase 2 Trial. J Thorac Oncol. 2021;16:S888–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zhou Q, Wu Y, Chang J, Wang H, Fan Y, Zhao J, et al. MA02.02 Efficacy and Safety of Pralsetinib in Chinese Patients with Advanced RET Fusion+ Non‐Small Cell Lung Cancer. J Thorac Oncol. 2021;16:S889–S90. [Google Scholar]
- 262. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2‐Mutant Advanced Lung Adenocarcinoma After Platinum‐Based Chemotherapy: A Multicenter, Open‐Label, Single‐Arm, Phase II Study. J Clin Oncol. 2020;38(24):2753–61. [DOI] [PubMed] [Google Scholar]
- 263. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado‐Trastuzumab Emtansine for Patients With HER2‐Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol. 2018;36(24):2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2‐mutant non‐small‐cell lung cancer. N Engl J Med. 2022;386(3):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Drilon A, Clark JW, Weiss J, Ou SI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14‐mutated or MET‐amplified non‐small‐cell lung Cancer. N Engl J Med. 2020;383(10):944–57. [DOI] [PubMed] [Google Scholar]
- 267. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non‐small‐cell lung cancer with MET Exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once‐daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non‐small‐cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single‐arm, open‐label, phase 2 study. Lancet Respir Med. 2021;9(10):1154–64. [DOI] [PubMed] [Google Scholar]
- 269. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis of KEYNOTE‐024: Pembrolizumab Versus Platinum‐Based Chemotherapy for Advanced Non‐Small‐Cell Lung Cancer With PD‐L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37(7):537–46. [DOI] [PubMed] [Google Scholar]
- 270. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five‐year outcomes with pembrolizumab versus chemotherapy for metastatic non‐small‐cell lung cancer with PD‐L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 272. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First‐Line Treatment of PD‐L1‐Selected Patients with NSCLC. N Engl J Med. 2020;383(14):1328–39. [DOI] [PubMed] [Google Scholar]
- 273. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy‐naive patients with advanced non‐squamous non‐small‐cell lung cancer (CameL): a randomised, open‐label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–14. [DOI] [PubMed] [Google Scholar]
- 274. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First‐Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double‐Blind, Phase 3 Study (Oncology pRogram by InnovENT anti‐PD‐1‐11). J Thorac Oncol. 2020;15(10):1636–46. [DOI] [PubMed] [Google Scholar]
- 275. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first‐line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–22. [DOI] [PubMed] [Google Scholar]
- 276. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first‐line treatment for advanced squamous NSCLC (CameL‐Sq): A phase 3 trial. J Thorac Oncol. 2021;17(4):544–57. [DOI] [PubMed] [Google Scholar]
- 277. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first‐line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double‐blind, phase 3 trial (ORIENT‐12). J Thorac Oncol. 2021;16(9):1501–11. [DOI] [PubMed] [Google Scholar]
- 278. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first‐line treatment for advanced squamous non‐small‐cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum‐based chemotherapy, as first‐line treatment of metastatic non‐small‐cell lung cancer (GEMSTONE‐302): interim and final analyses of a double‐blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23(2):220–33. [DOI] [PubMed] [Google Scholar]
- 280. Wang J, Wang Z, Wu L, Li B, Cheng Y, Li X, et al. MA13.08 CHOICE‐01: A phase 3 study of toripalimab versus placebo in combination with first‐line chemotherapy for advanced NSCLC. J Thorac Oncol. 2021;16(10, Supplement):S927–S8. [Google Scholar]
- 281. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. [DOI] [PubMed] [Google Scholar]
- 282. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. [DOI] [PubMed] [Google Scholar]
- 283. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med. 2006;355(24):2542–50. [DOI] [PubMed] [Google Scholar]
- 284. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: A Randomized, Double‐Blind, Placebo‐Controlled, Multicenter, Phase III Study of First‐Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non‐Small‐Cell Lung Cancer. J Clin Oncol. 2015;33(19):2197–204. [DOI] [PubMed] [Google Scholar]
- 285. Chu T, Lu J, Bi M, Zhang H, Zhuang W, Yu Y, et al. Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non‐squamous non‐small cell lung cancer patients: a phase 3 randomized, double‐blind clinical trial. Cancer Biol Med. 2021;18(3):816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third‐line or further treatment on overall survival of patients with advanced non‐small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, et al. Phase 1b study of sintilimab plus anlotinib as first‐line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–52. [DOI] [PubMed] [Google Scholar]
- 288. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 289. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. [DOI] [PubMed] [Google Scholar]
- 290. West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS‐mutated non‐small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer. 2022;10(2):e003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17(2):309–23. [DOI] [PubMed] [Google Scholar]
- 292. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first‐line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909–24. [DOI] [PubMed] [Google Scholar]
- 293. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med. 2002;346(2):92–8. [DOI] [PubMed] [Google Scholar]
- 294. Kelly K, Crowley J, Bunn PA, Jr. , Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small‐cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–8. [DOI] [PubMed] [Google Scholar]
- 295. Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non‐small‐cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21(16):3016–24. [DOI] [PubMed] [Google Scholar]
- 296. Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, et al. Three‐arm randomized study of two cisplatin‐based regimens and paclitaxel plus gemcitabine in advanced non‐small‐cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group–EORTC 08975. J Clin Oncol. 2003;21(21):3909–17. [DOI] [PubMed] [Google Scholar]
- 297. Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum‐based doublets in advanced non‐small‐cell lung cancer. J Clin Oncol. 2002;20(21):4285–91. [DOI] [PubMed] [Google Scholar]
- 298. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. [DOI] [PubMed] [Google Scholar]
- 299. Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non‐small‐cell lung cancer: Four‐Arm Cooperative Study in Japan. Ann Oncol. 2007;18(2):317–23. [DOI] [PubMed] [Google Scholar]
- 300. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62. [DOI] [PubMed] [Google Scholar]
- 301. Santana‐Davila R, Szabo A, Arce‐Lara C, Williams CD, Kelley MJ, Whittle J. Cisplatin versus carboplatin‐based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J Thorac Oncol. 2014;9(5):702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Pujol JL, Breton JL, Gervais R, Rebattu P, Depierre A, Morère JF, et al. Gemcitabine‐docetaxel versus cisplatin‐vinorelbine in advanced or metastatic non‐small‐cell lung cancer: a phase III study addressing the case for cisplatin. Ann Oncol. 2005;16(4):602–10. [DOI] [PubMed] [Google Scholar]
- 303.!!! INVALID CITATION !!!
- 304. Zhang J, Pan Y, Shi Q, Zhang G, Jiang L, Dong X, et al. Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first‐line treatment of locally advanced or metastatic lung squamous cell carcinoma: A multicenter, randomized, open‐label, parallel controlled clinical study. Cancer Commun (Lond). 2022;42(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Shi M, Gu A, Tu H, Huang C, Wang H, Yu Z, et al. Comparing nanoparticle polymeric micellar paclitaxel and solvent‐based paclitaxel as first‐line treatment of advanced non‐small‐cell lung cancer: an open‐label, randomized, multicenter, phase III trial. Ann Oncol. 2021;32(1):85–96. [DOI] [PubMed] [Google Scholar]
- 306. Desai A, Abdayem P, Adjei AA, Planchard D. Antibody‐drug conjugates: A promising novel therapeutic approach in lung cancer. Lung Cancer. 2022;163:96–106. [DOI] [PubMed] [Google Scholar]
- 307. Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–95. [DOI] [PubMed] [Google Scholar]
- 308. Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104(24):10152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Pless M, Droege C, von Moos R, Salzberg M, Betticher D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non‐small cell lung cancer. Lung Cancer. 2013;81(3):445–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.