Abstract
A new series of spirooxindoles based on ethylene derivatives having furan aryl moiety are reported. The new hybrids were achieved via [3 + 2] cycloaddition reaction as an economic one-step efficient approach. The final constructed spirooxindoles have four contiguous asymmetric carbon centers. The structure of 3a is exclusively confirmed using X-ray single crystal diffraction. The supramolecular structure of 3a is controlled by O···H, H···H, and C···C intermolecular contacts. It includes layered molecules interconnected weak C–H···O (2.675 Å), H···H (2.269 Å), and relatively short Cl···Br interhalogen interactions [3.4500(11)Å]. Using Hirshfeld analysis, the percentages of these intermolecular contacts are 10.6, 25.7, 6.4, and 6.2%, respectively. The spirooxindoles along with ethylene derivatives having furan aryl moiety were assessed against breast (MCF7) and liver (HepG2) cancer cell lines. The results indicated that the new chalcone 3b showed excellent activity in both cell lines (MCF7 and HepG2) with IC50 = 4.1 ± 0.10 μM/mL (MCF7) and 3.5 ± 0.07 μM/mL (HepG2) compared to staurosporine with 4.3 and 2.92 folds. Spirooxindoles 6d (IC50 = 4.3 ± 0.18 μM/mL), 6f (IC50 = 10.3 ± 0.40 μM/mL), 6i (IC50 = 10.7 ± 0.38 μM/mL), and 6j (IC50 = 4.7 ± 0.18 μM/mL) exhibited potential activity against breast adenocarcinoma, while compounds 6d (IC50 = 6.9 ± 0.23 μM/mL) and 6f (IC50 = 3.5 ± 0.11 μM/mL) were the most active hybrids against human liver cancer cell line (HepG2) compared to staurosporine [IC50 = 17.8 ± 0.50 μM/mL (MCF7) and 10.3 ± 0.23 μM/mL (HepG2)]. Molecular docking study exhibited the virtual mechanism of binding of compound 3b as a dual inhibitor of EGFR/CDK-2 proteins, and this may highlight the molecular targets for its cytotoxic activity.
1. Introduction
In the last decade, spirooxindole scaffold has been recognized as a potential pharmacophore in drug discovery, particularly for cancer research development.1−3 Spirooxindole has a unique rigid structural architecture with the diversity of pharmaceutical activities which made this nucleus a privileged structure in the new drug discovery. The exploration and discovery of novel drugs for anticancer therapy with lower toxicity and high selectivity is always an area of intensive research. Many examples having spirooxindoles have been reported so far for cancer therapy research and exhibited durable regression for cancer treatment with oral administration in the preclinical advanced stages; as an example MI-888 is a lead compound having spiro-pyrrolidinyl oxindole for p53-MDM2 protein–protein interaction inhibitors.4 MI-773 and MI-219 are other two representative examples having spirooxindole-pyrrolidine derivatives which exhibited cytotoxicity and interfered with the proteasomal degradation of p53.5,6
Several structural pharmacophores have been coadministrated with the spirooxindole privileged structures inspired by research for structural complexities with their diverse bioactivities.7,8 Due to the urgent need to discover a new cancer agent with more targeting to cancer cells and less harmful for the normal tissue, chemists have synthesized many spirooxindoles for this purpose. In particular, spirooxindoles containing furan scaffold, which are called oxa-spirooxindoles, widely exist in many natural and biologically active molecules.8−15 The designed, synthesized, and reported spirooxindoles show anticancer activity and can serve as an anti-tumor agent, CB2 receptor agonist, antagonists of progesterone receptors, antagonists of progesterone receptors, Nav1.7 blocker (XEN907), and selective cyclooxygenase COX-1 with TNF-α and IL-6 Inhibitors.16−19
Spirooxindole core has been continuously attracting the attention of researchers and has become a dynamic area of research due to its outstanding pharmacological properties. Barakat et al. extensively studied this spirooxindole scaffold recently and have reported so many examples so far focusing on the drug discovery research.20−28 In this library, Barakat has reported the synthesis of a new class of new spiro-heterocycles coadministrated with different pharmacophores such thiochromene, benzofuran, benzothiophene, cyclohexanone, pyrrole, and rhodanine scaffolds by the three-component [3 + 2] cycloaddition reaction in a regio- and stereo-selective fashion. All synthesized compounds were subjected to anticancer activity against a variety of cancer cell lines such as PC3, HeLa, MCF-7, MDA-MB231, and so forth, and many of them showed high efficacy against the tested cell lines.
Based on these findings and in continuation of our research program toward the synthesis of multifunctionalized spirooxindole drug skeleton for drug research development, we report here the new spirooxindole system appending the furan structural pharmacophore. The molecular features of the new spirooxindole derivative compound were elucidated based on X-ray diffraction of a single crystal, Hirshfeld analysis, and atoms-in-molecules calculations. Also, the spirooxindoles along with ethylene derivatives having the aryl-furan moiety were assessed against two cancer cell lines including breast adenocarcinoma (MCF7) and liver cancer cell line (HepG2) (Figure 1).
Figure 1.
Some biologically active spirooxindole-based pharmacophores.
2. Results and Discussion
2.1. Chemistry
New spirooxindoles having aryl-furan motif were designed and synthesized according to Scheme 1. The ethylene derivatives 3a–c having the aryl-furan scaffold was mandatory as a dipolarphile for the [3 + 2] cycloaddition reaction approach and were prepared from the acetophenones with the aryl-furan carbaldehydes in basic condition to afford the corresponding chalcones in precipitated form in a high chemical yield. The ethylene derivative 3a was successfully obtained in a single crystalline form by slow diffusion/evaporation in DCM/EtOH, and the crystal was suitable for X-ray diffraction analysis. The required materials for the [3 + 2] cycloaddition reaction were ethylene derivative having aryl-furan motif 3a–c, four amino acids 5a–d, and two substituted isatins 4a,b, which achieved the desired spirooxindoles 6a–j. Ten examples were successfully synthesized in stereoselective and high chemical yield up to 94%. A set of trials were carried out to get any of those final compounds in a crystalline form and only one compound (6b) was provided as crystal; it was not good enough to provide the optimum X-ray data for publication quality but at least gave us the main skeleton of the final compound. Other spectrophotometric tools were employed to prove the chemical structure. The plausible mechanism is depicted in Scheme 2 based on the previous reported literature.29−33
Scheme 1. Synthetic Route for the Ethylene Derivatives 3a–c Engrafted Aryl-Furan Ring.
Scheme 2. A Plausible Mechanism for the 32CA Reaction of Azomethine Ylide to Ethylene Derivative 3a–c to Afford the Spirooxindole Analogues 6a–j.
2.2. Crystal Structure Description of 3a
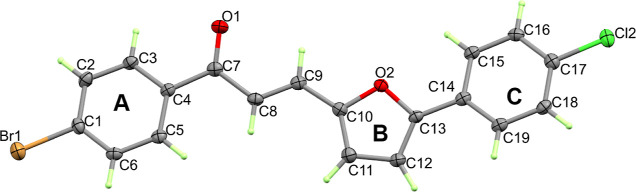
The X-ray structure of 3a is shown in Figure 2 which is found in good agreement with its spectral characterizations. Compound 3a crystallized in the monoclinic crystal system and the P21/c space group with z = 4 and one molecular unit as asymmetric formula (Table 1). The unit cell parameters are a = 19.4870(9) Å, b = 13.8512(6) Å, c = 5.8412(3) Å, β = 92.720(6)°, and V = 1574.87(13) Å3. The list of bond distances and angles is given in Table 2. The structure comprised three aromatic planar ring systems. These rings are abbreviated A, B, and C for simplicity (Figure 2). It is worth to note that the three rings are twisted with one another to different extents. The mean plane of ring A is found twisted with respect to ring B by 12.95°. The corresponding values for ring C is 8.8° with respect to the mean plane of ring B.
Figure 2.
Thermal ellipsoids at 30% probability level showing atom numbering of 3a.
Table 1. Crystal Data.
| 3a | |
|---|---|
| CCDC | 2165902 |
| empirical formula | C19H12BrClO2 |
| fw | 387.65 |
| temp (K) | 120(2) |
| λ (Å) | 0.71073 |
| cryst syst. | monoclinic |
| space group | P21/c |
| a (Å) | 19.4870(9) |
| b (Å) | 13.8512(6) |
| c (Å) | 5.8412(3) |
| β (deg) | 92.720(6) |
| V (Å3) | 1574.87(13) |
| Z | 4 |
| ρcalc (Mg/m3) | 1.635 |
| μ (Mo Kα) (mm–1) | 2.786 |
| no. reflns. | 14253 |
| unique reflns. | 3887 |
| completeness to θ = 25.242° | 99.8% |
| GOOF (F2) | 1.065 |
| Rint | 0.0558 |
| R1a (I ≥ 2σ) | 0.0547 |
| wR2b (I ≥ 2σ) | 0.1134 |
R1 = Σ||Fo| – |Fc||/Σ|Fo|.
wR2 = {Σ[w(Fo2 – Fc2)2]/Σ[w(Fo2)2]}1/2.
Table 2. Selected Bond Lengths [Å] and Angles [deg] for 3a.
| atoms | distance | atoms | distance |
|---|---|---|---|
| Br(1)–C(1) | 1.889(4) | O(2)–C(13) | 1.368(4) |
| Cl(2)–C(17) | 1.732(4) | O(2)–C(10) | 1.380(4) |
| O(1)–C(7) | 1.234(4) |
| atoms | angle | atoms | angle |
|---|---|---|---|
| C(13)–O(2)–C(10) | 107.3(3) | C(2)–C(1)–Br(1) | 120.2(3) |
| C(6)–C(1)–C(2) | 121.0(4) | C(3)–C(2)–C(1) | 118.3(4) |
| C(6)–C(1)–Br(1) | 118.8(3) |
The supramolecular structure of 3a is controlled by different types of intermolecular contacts (Figure 3). The most important contacts are emphasized by different colors in the packing scheme shown in Figure 4. H···O (magenta), H···H (purple), C···C (orange), and Br···Cl (turquoise) are the main contacts in this crystal structure. The presence of short Br···Cl (3.450 Å) and C···C (C8vC15; 3.323 Å and C9···C13; 3.399 Å) contacts revealed the presence of significant interhalogen and π–π stacking interactions, respectively. The rest of the interactions are O···H and H···H and are depicted in Table 3.
Figure 3.
Most important intermolecular contacts in the crystal structure of 3a.
Figure 4.
Packing of the molecular units via H···O (magenta), H···H (purple), C···C (orange), and Br···Cl (turquoise) in 3a.
Table 3. Different Contacts and Their Distances (Å).
| contact | distance | symm. code |
|---|---|---|
| Br1···Cl2 | 3.4500(11) | –1 + x, y, –1 + z |
| C8···C15 | 3.323(4) | 1 – x, 1 – y, 1 – z |
| C9···C13 | 3.399(4) | 1 – x, 1 – y, 1 – z |
| O1···H16 | 2.675 | 1 – x, 1 – y, 2 – z |
| H3···H16 | 2.269 | 1 – x, 1 – y, 2 – z |
2.3. Hirshfeld Surface Analysis
Crystal structure stability is governed by intermolecular interactions among different fragments in the crystal. Hirshfeld analysis is a simple tool for decomposition of different intermolecular contacts in the crystal. Different Hirshfeld surfaces including dnorm, shape index, and curvedness maps are shown in Figure 5. The dnorm map contains a number of red spots which represent the regions at which the important and short distance contacts occurred. Summary of all contacts and their percentages based on Hirshfeld calculations is presented in Figure 6.
Figure 5.

Hirshfeld surfaces of 3a.
Figure 6.

Intermolecular contacts and their percentages in 3a.
Analysis of these interactions using fingerprint plot and dnorm maps is given in Figure 7. The major contacts are H···H, H···C, and O···H interactions, and their percentages are 25.7, 27.6, and 10.6%, respectively. It is worth noting that the red spots shown in the dnorm map are related to the Br···Cl (6.2%), C···C (6.4%), and H···H contacts. Hence, the hydrogen–hydrogen interactions are not only the most common but also considered strong. The H3···H16 contact (2.06 Å) is the shortest of these interactions. Also, the presence of short Br1···Cl2 (3.45 Å) revealed the presence of interhalogen interactions, whereas the short C13···C9 (3.399 Å) and C16···C9 (3.323 Å) contacts revealed the importance of the π–π stacking interactions. The latter is further confirmed by the presence of red/blue triangles in the shape index map (Figure 5). The O1···H16 contact appeared as a blue region in the dnorm map. The corresponding interaction distance is found to be 2.674 Å based on Hirshfeld analysis. This value is slightly greater than the vdWs radii sum (2.61 Å) of the O and H atoms. Other contacts shown in this figure are considered weak interactions and have less contribution in the molecular packing of 3a.
Figure 7.
Decomposed dnorm maps (A) and fingerprint plots (B) of short contacts in 3a.
2.4. Biological Activity of the Synthesized Compounds
The ethylene derivatives having the aryl-furan moiety and the spirooxindoles were assessed against two cancer cell lines including breast and liver carcinoma by the MTT assay. Interestingly, the new chalcone 3b discovered is the most active member between the synthesized compounds against both cell lines, with IC50 = 4.1 ± 0.10 and 3.5 ± 0.07 μM/mL for MCF7 and HepG2, respectively, and more potent than the standard drug used as staurosporine [IC50 = 17.8 ± 0.50 μM/mL (MCF7) and 10.3 ± 0.23 μM/mL (HepG2)]. The other two chalcones 3a and 3c show moderate activity (Table 4).
Table 4. Cytotoxicity Results of the Ethylene Derivatives Having the Aryl-Furan Moiety and the Spirooxindoles against Breast and Liver Carcinoma.
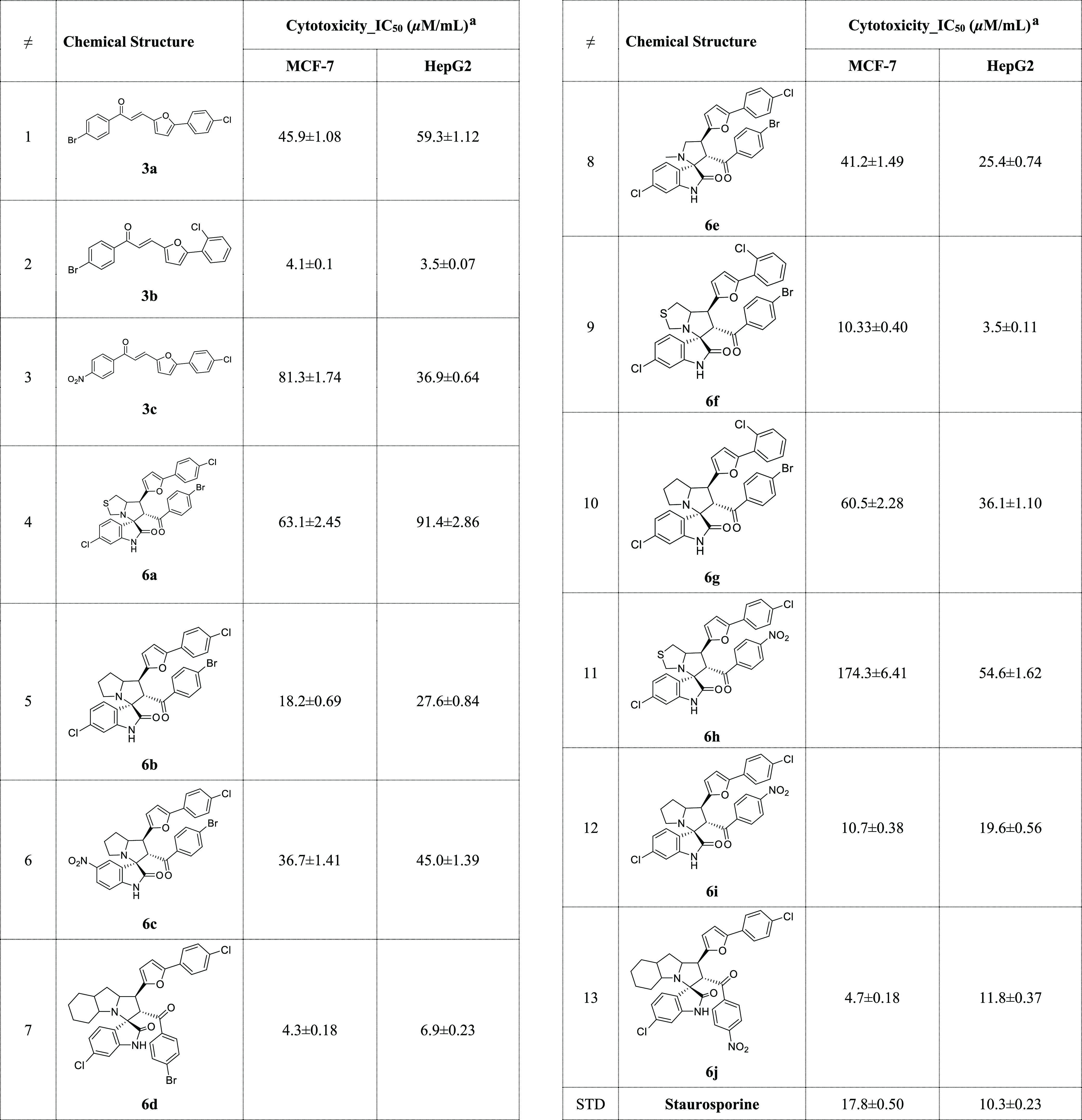
IC50 values are expressed as mean ± SD of three independent trials.
Although compounds 3a and 3b are two isomers with different positions for chlorine substitutions (o/p-substitution), they exhibited different cytotoxicity against the two tested cell lines. This may be due to the relevance of chlorine substitution to interact with the corresponding amino acids in the effective target binding site, which will affect the stability of drug–target complexes and the ability of the complex to have a biological response.
For the synthesized spirooxindoles, for the breast cancer cell line (MCF7), spirooxindole having the bulky fused with the pyrrolidine ring 6d exhibited the most active hybrid between these series with IC50 = 4.3 ± 0.18 μM/mL more potent with 4 folds and the standard staurosporine (IC50 = 17.8 ± 0.50 μM/mL). Next in the reactivity towards cytotoxicity was compound spirooxindole 6j which instead of the Br-atom on the benzoyl ring in compound 6d replaced by more electron withdrawing group, the reactivity slightly decreased which the IC50 equal to 4.7 ± 0.18 μM/mL. Compounds 6f (IC50 = 10.3 ± 0.40 μM/mL) and 6i (IC50 = 10.7 ± 0.38 μM/mL) still provided better cytotoxicity compared to staurosporine. The remaining spirooxindoles provided moderate toxicity against breast adenocarcinoma. Spirooxindoles 6f (IC50 = 3.5 ± 0.11 μM/mL) and 6d (IC50 = 6.9 ± 0.23 μM/mL) were the most active hybrids against human liver cancer cell line (HepG2) compared to staurosporine [IC50 = 10.3 ± 0.23 μM/mL (HepG2)]. The remaining spirooxindoles provided moderate toxicity, and the IC50 ranged from 11.8 ± 0.37 to 91.4 ± 2.86 μM/mL.
2.5. Molecular Docking Study
As cyclic-dependent kinase (CDK-2) and epidermal growth factor receptor (EGFR) are key proteins that mediate and trigger the proliferation of cancer cells, their inhibition is an interesting target for apoptosis induction upon treatment of a chemotherapeutic drug.34−36 A molecular docking study was performed to highlight the virtual mechanism of binding toward EGFR and CDK-2 proteins. As seen in Figure 8, compound 3b was docked inside the EGFR binding site with the binding energy of −19.63 kcal/mol, and it formed one H-bind interaction with Met 769 as the H-bond acceptor. Additionally, compound 3b was docked inside the CDK-2 active site with the binding energy of −18.6 Kcal/mol, and it formed one H-bond with Lys 89. Hence, compound 3b had a good binding affinity toward EGFR and CDK-2 proteins, and these targets may be the effective target for its cytotoxic activity.
Figure 8.
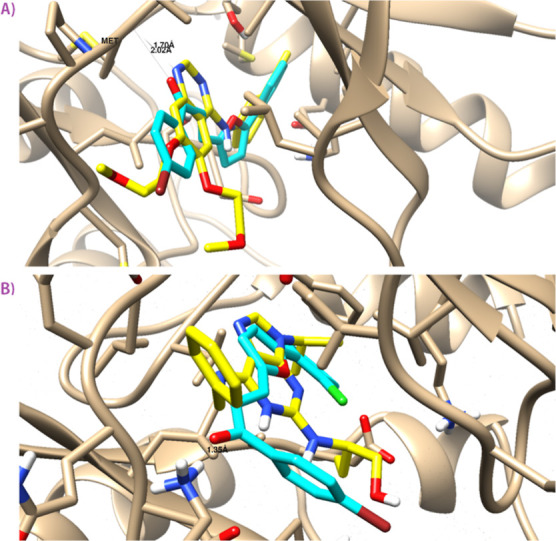
Binding disposition and ligand–receptor interactions of compound 3b inside the EGFR (A) and CDK-2 (B) proteins. Three-dimensional images were generated by Chimera-UCSF. Cocrystallized ligand (yellow-colored) and docked compound (cyan-colored).
3. Conclusions
We concluded that a set of spirooxindoles having aryl-furan moiety have been successfully synthesized and assessed against two cancer cell lines. The results provided promising data for breast adenocarcinoma and could be utilized as a lead compound for further development. Structural analysis of the newly synthesized compound was performed with the aid of X-ray single crystal structure and Hirshfeld calculations. The reported X-ray structure of 3a agreed very well with its spectral characterizations. Its supramolecular structure is controlled by many intermolecular contacts such as O···H, H···H, and C···C intermolecular contacts as well as the Br···Cl interhalogen interactions. All intermolecular contacts occurring in the crystal are quantitatively analyzed based on Hirshfeld calculations. The percentages of these contacts are 10.6, 25.7, 6.4, and 6.2%. The biological activity concluded that the new chalcone 3b showed excellent activity in both MCF7 and HepG2 with IC50 = 4.1 ± 0.1 μM/mL (MCF7) and 3.5 ± 0.07 μM/mL (HepG2) compared to staurosporine with 4.3 and 2.92 folds. The synthesized spirooxindole compounds 6d (IC50 = 4.3 ± 0.18 μM/mL), 6f (IC50 = 10.3 ± 0.40 μM/mL), 6i (IC50 = 10.7 ± 0.38 μM/mL), and 6j (IC50 = 4.7 ± 0.18 μM/mL) show high antiproliferative activity in vitro against breast adenocarcinoma. On the other hand, compounds 6d (IC50 = 6.9 ± 0.23 μM/mL) and 6f (IC50 = 3.5 ± 0.11 μM/mL) were the most active hybrids against human liver cancer cell line (HepG2) compared to staurosporine [IC50 = 17.8 ± 0.50 μM/mL (MCF7) and 10.3 ± 0.23 μM/mL (HepG2)]. These compounds could be useful for further cancer research development.
4. Materials and Methods
All technical instruments and chemicals used in this study are provided in the Supporting Information.
4.1. General Procedure for the Synthesis of the Chalcones (Ethylene Derivatives 3a–c)
The synthesis of ethylene derivatives 3a–c was performed according to the reported procedure by mixing equimolar of the acetophenone derivatives 1a,b [1-(4-bromophenyl)ethan-1-one 1a; 1-(4-nitrophenyl)ethan-1-one 1b] with the corresponding aldehydes 2a,b [5-(4-chlorophenyl)furan-2-carbaldehyde 2a; 5-(2-chlorophenyl)furan-2-carbaldehyde 2b] in the presence of basic condition (NaOH, 2 equiv) in ethanolic solution to give the corresponding ethylene derivatives in a precipitated form which were used for the next step without any further purification.
4.1.1. (E)-1-(4-Bromophenyl)-3-(5-(4-chlorophenyl)furan-2-yl)prop-2-en-1-one 3a
1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 8.5 Hz, 2H), 7.71–7.53 (m, 5H), 7.42–7.35 (m, 3H), 6.83–6.72 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 188.59, 155.58, 155.54, 151.39, 151.35, 151.29, 137.04, 134.59, 133.25, 132.10, 132.05, 131.60, 130.89, 130.76, 130.61, 130.56, 129.50, 128.80, 128.33, 128.28, 128.23, 128.04, 127.96, 127.88, 127.04, 125.99, 125.95, 125.64, 124.57, 120.73, 119.83, 118.91, 117.95, 117.04, 110.08, 109.20; chemical formula: C19H12BrClO2; elemental analysis: C, 58.87; H, 3.12; Found: C, 58.89; H, 3.14.
4.1.2. (E)-1-(4-Bromophenyl)-3-(5-(2-chlorophenyl)furan-2-yl)prop-2-en-1-one 3b
1H NMR (400 MHz, CDCl3): δ 7.96 (t, J = 7.6 Hz, 1H), 7.91 (t, J = 7.8 Hz, 2H), 7.69–7.56 (m, 3H), 7.52–7.40 (m, 2H), 7.37 (t, J = 7.6 Hz, 1H), 7.33–7.22 (m, 2H), 6.86 (dd, J = 7.3, 3.8 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 188.68, 152.83, 150.89, 137.03, 132.11, 132.06, 131.95, 131.07, 131.01, 130.87, 130.57, 129.58, 129.03, 128.85, 128.36, 127.97, 127.91, 127.56, 126.76, 119.43, 118.62, 114.57, 113.49; chemical formula: C19H12BrClO2; elemental analysis: C, 58.87; H, 3.12; Found: C, 58.90; H, 3.15.
4.1.3. (E)-3-(5-(4-Chlorophenyl)furan-2-yl)-1-(4-nitrophenyl)prop-2-en-1-one 3c
1H NMR (400 MHz, chloroform-D): δ 8.34 (d, J = 8.4 Hz, 2H), 8.16 (d, J = 8.3 Hz, 2H), 7.70 (d, J = 8.3 Hz, 2H), 7.63 (d, J = 15.0 Hz, 1H), 7.42 (t, J = 10.2 Hz, 3H), 6.88 (s, 1H), 6.80 (s, 1H). 13C NMR (101 MHz, chloroform-D): δ 188.18, 156.13, 151.06, 150.14, 143.22, 134.90, 131.88, 129.41, 129.33, 128.12, 125.93, 123.96, 109.03; chemical formula: C19H12ClNO4; elemental analysis: C, 64.51; H, 3.42; N, 3.96; Found: C, 64.50; H, 3.41; N, 4.00.
4.2. General Method for the Synthesis of the Spirooxindoles Scaffold 6a–j
An equimolar (0.5 mmol) of the ethylene derivatives 3a–c with isatin derivatives 4a,b and amino acids 5a–d in methanol was heated under reflux for 5 h to provide the final products 6a–j as solid materials in almost quantitative yield upon slow evaporation overnight. In the case of compound 6b, the crystal quality was not good enough to solve the X-ray structure in a suitable form enough for publication quality but at least provided a reasonable structure for the spiro-compound.
4.2.1. (3R,6′S,7′S)-6′-(4-Bromobenzoyl)-6-chloro-7′-(5-(4-chlorophenyl)furan-2-yl)-1′,6′,7′,7a′-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one 6a
1H NMR (400 MHz, CDCl3): δ 8.43 (s, 1H), 7.51 (t, J = 9.1 Hz, 3H), 7.45–7.22 (m, 7H), 7.02 (d, J = 8.1 Hz, 1H), 6.67 (s, 1H), 6.52 (d, J = 3.5 Hz, 1H), 6.32 (d, J = 3.5 Hz, 1H), 4.81 (d, J = 11.7 Hz, 1H), 4.52 (dt, J = 8.9, 4.1 Hz, 1H), 4.15–4.03 (m, 1H), 3.88 (d, J = 11.0 Hz, 1H), 3.46 (d, J = 11.0 Hz, 1H), 3.20 (d, J = 3.9 Hz, 2H). 13C NMR (101 MHz, CDCl3): δ 194.82, 179.72, 152.73, 151.47, 141.76, 136.28, 135.26, 133.13, 132.03, 131.90, 131.75, 130.15, 129.89, 129.71, 129.57, 129.45, 129.09, 129.01, 128.89, 125.07, 124.84, 122.89, 122.65, 120.91, 111.06, 110.78, 106.60, 106.26, 74.31, 72.00, 58.69, 56.40, 45.33, 37.09; IR (KBr, cm–1): 3425, 3310, 2935, 2840, 1720, 1610, 1500; chemical formula: C30H21BrCl2N2O3S; elemental analysis: C, 56.27; H, 3.31; N, 4.37; S, 5.01; Found: C, 56.17; H, 3.35; N, 4.45; S, 5.09.
4.2.2. (1′S,2′S,3R)-2′-(4-Bromobenzoyl)-6-chloro-1′-(5-(4-chlorophenyl)furan-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 6b
1H NMR (400 MHz, CDCl3): δ 8.58 (s, 1H), 7.47 (d, J = 8.6 Hz, 2H), 7.41 (s, 4H), 7.32–7.23 (m, 2H), 7.12 (d, J = 8.1 Hz, 1H), 6.99 (dd, J = 8.0, 2.1 Hz, 1H), 6.77–6.72 (m, 1H), 6.51 (d, J = 3.5 Hz, 1H), 6.26 (d, J = 3.2 Hz, 1H), 4.95 (d, J = 11.6 Hz, 1H), 4.46 (s, 1H), 4.08 (t, J = 10.6 Hz, 1H), 2.73 (d, J = 12.4 Hz, 2H), 2.23 (dt, J = 13.1, 6.7 Hz, 1H), 1.98 (h, J = 6.6, 6.0 Hz, 2H), 1.90 (dd, J = 12.9, 6.6 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 195.27, 170.74, 152.47, 142.24, 136.10, 135.26, 132.95, 131.67, 129.34, 129.19, 129.09, 128.48, 128.07, 124.63, 123.36, 122.02, 121.86, 114.28, 109.15, 108.99, 108.06, 106.10, 73.42, 67.22, 46.85, 19.53; IR (KBr, cm–1): 3422, 3318, 2930, 2850, 1725, 1610, 1500; chemical formula: C31H23BrCl2N2O3; elemental analysis: C, 59.83; H, 3.73; N, 4.50; Found: C, 59.79; H, 3.75; N, 4.61.
4.2.3. (1′S,2′S,3R)-2′-(4-Bromobenzoyl)-1′-(5-(4-chlorophenyl)furan-2-yl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 6c
1H NMR (400 MHz, CDCl3): δ 8.96 (s, 1H), 8.23–8.05 (m, 2H), 7.57–7.34 (m, 7H), 7.27 (d, J = 8.1 Hz, 3H), 6.83 (d, J = 8.7 Hz, 1H), 6.51 (d, J = 3.5 Hz, 1H), 6.27 (d, J = 3.5 Hz, 1H), 5.01 (d, J = 11.6 Hz, 1H), 4.39 (dt, J = 12.2, 6.4 Hz, 1H), 4.12 (dd, J = 14.2, 8.3 Hz, 2H), 2.66 (p, J = 6.9, 5.9 Hz, 2H), 2.21 (dt, J = 12.4, 6.5 Hz, 1H), 2.08–1.81 (m, 5H), 1.24 (t, J = 7.0 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 194.94, 180.62, 171.49, 152.46, 151.98, 146.37, 143.36, 135.20, 132.99, 132.07, 129.61, 129.24, 129.16, 128.94, 126.83, 125.77, 124.85, 123.40, 110.44, 109.24, 106.40, 73.16, 69.08, 61.38, 60.60, 48.25, 46.67, 30.82, 27.62, 14.28.; IR (KBr, cm–1): 3434, 3325, 2922, 2850, 1715, 1608, 1512; chemical formula: C31H23BrClN3O5; elemental analysis: C, 58.83; H, 3.66; N, 6.64; Found: C, 58.85; H, 3.69; N, 6.71.
4.2.4. (1′S,2′S,3R)-2′-(4-Bromobenzoyl)-6-chloro-1′-(5-(4-chlorophenyl)furan-2-yl)-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one 6d
1H NMR (400 MHz, CDCl3): δ 8.09 (s, 1H), 7.51–7.35 (m, 6H), 7.31–7.23 (m, 2H), 7.14 (d, J = 8.0 Hz, 1H), 7.01 (dd, J = 8.0, 1.7 Hz, 1H), 6.63 (s, 1H), 6.49 (d, J = 3.6 Hz, 1H), 6.20 (d, J = 3.6 Hz, 1H), 5.01 (d, J = 11.8 Hz, 1H), 4.48 (d, J = 8.6 Hz, 1H), 4.00 (t, J = 11.0 Hz, 1H), 3.10 (d, J = 4.3 Hz, 1H), 2.23–2.09 (m, 1H), 2.16 (s, 1H), 1.94 (dd, J = 12.4, 6.4 Hz, 1H), 1.81 (ddd, J = 11.9, 8.5, 6.1 Hz, 1H), 1.60–1.43 (m, 3H), 1.38 (t, J = 12.5 Hz, 1H), 1.17–0.82 (m, 5H); 13C NMR (101 MHz, CDCl3): δ 195.28, 179.74, 178.80, 171.53, 152.56, 152.16, 141.51, 135.49, 132.80, 132.14, 131.58, 129.35, 129.30, 129.26, 128.82, 128.44, 124.62, 122.78, 121.78, 110.95, 108.21, 106.11, 106.07, 71.84, 47.83, 40.45, 34.69, 29.40, 28.77; IR (KBr, cm–1): 3420, 3317, 2919, 2823, 1715, 1600, 1515; chemical formula: C35H29BrCl2N2O3; elemental analysis: C, 62.15; H, 4.32; N, 4.14; Found: C, 62.19; H, 4.27; N, 4.06.
4.2.5. (3R,3′S,4′S)-3′-(4-Bromobenzoyl)-6-chloro-4′-(5-(4-chlorophenyl)furan-2-yl)-1′-methylspiro[indoline-3,2′-pyrrolidin]-2-one 6e
1H NMR (400 MHz, CDCl3): δ 8.42 (s, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.36 (s, 4H), 7.34 (d, J = 5.4 Hz, 1H), 7.26 (d, J = 7.6 Hz, 3H), 7.04 (d, J = 8.0 Hz, 1H), 6.92 (dd, J = 8.1, 2.1 Hz, 1H), 6.58 (d, J = 2.0 Hz, 1H), 6.51 (d, J = 3.4 Hz, 1H), 6.25 (d, J = 3.4 Hz, 1H), 4.59 (d, J = 6.4 Hz, 2H), 3.74–3.64 (m, 1H), 3.51–3.43 (m, 1H); 13C NMR (101 MHz, CDCl3): δ 195.96, 178.40, 170.35, 152.11, 141.68, 135.67, 135.31, 132.79, 131.83, 131.40, 129.30, 128.91, 128.62, 124.81, 110.18, 106.10, 72.91, 59.96, 57.51, 43.06; IR (KBr, cm–1): 3434, 3320, 2930, 2834, 1725, 1620, 1520; chemical formula: C29H21BrCl2N2O3; elemental analysis: C, 58.41; H, 3.55; N, 4.70; Found: C, 58.47; H, 3.61; N, 4.79.
4.2.6. (3R,6′S,7′S)-6′-(4-Bromobenzoyl)-6-chloro-7′-(5-(2-chlorophenyl)furan-2-yl)-1′,6′,7′,7a′-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one 6f
1H NMR (400 MHz, CDCl3): δ 8.17 (s, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.42–7.23 (m, 7H), 7.17 (d, J = 7.5 Hz, 1H), 7.06–6.97 (m, 2H), 6.65 (s, 1H), 6.37 (d, J = 3.2 Hz, 1H), 4.84 (d, J = 11.7 Hz, 1H), 4.51 (td, J = 6.8, 5.4, 3.5 Hz, 1H), 4.14–4.04 (m, 1H), 3.88 (d, J = 10.9 Hz, 1H), 3.50–3.41 (m, 1H), 3.21 (d, J = 5.4 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 194.84, 179.81, 151.35, 150.04, 141.60, 136.19, 135.31, 131.87, 130.85, 130.09, 129.60, 128.96, 128.20, 127.84, 126.97, 122.77, 121.06, 111.86, 110.78, 109.73, 74.28, 71.82, 59.16, 55.27, 45.29, 37.19, 31.69, 22.75, 14.24; IR (KBr, cm–1): 3428, 3318, 2925, 2840, 1722, 1618, 1508; chemical formula: C30H21BrCl2N2O3S; elemental analysis: C, 56.27; H, 3.31; N, 4.37; S, 5.01; Found: C, 56.35; H, 3.29; N, 4.41; S, 5.00.
4.2.7. (1′S,2′S,3R)-2′-(4-Bromobenzoyl)-6-chloro-1′-(5-(2-chlorophenyl)furan-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 6g
1H NMR (400 MHz, CDCl3): δ 8.69 (s, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.37 (d, J = 7.7 Hz, 2H), 7.28–7.19 (m, 1H), 7.13 (dd, J = 8.0, 5.2 Hz, 2H), 7.03–6.97 (m, 3H), 6.72 (s, 1H), 6.30 (d, J = 3.1 Hz, 1H), 4.97 (d, J = 11.2 Hz, 1H), 4.36 (dt, J = 9.8, 6.0 Hz, 1H), 4.06 (t, J = 10.6 Hz, 1H), 2.63 (t, J = 6.4 Hz, 2H), 2.18 (dd, J = 12.1, 6.2 Hz, 1H), 1.99–1.80 (m, 4H).; 13C NMR (101 MHz, CDCl3): δ 195.50, 180.56, 152.39, 149.62, 141.93, 135.69, 135.40, 131.88, 130.77, 129.89, 129.66, 129.07, 128.89, 128.59, 127.94, 127.71, 126.92, 123.05, 122.59, 111.87, 111.24, 108.86, 73.20, 68.98, 61.33, 48.25, 46.51, 31.68, 30.94, 27.37, 22.75, 14.23; IR (KBr, cm–1): 3430, 3309, 2918, 2827, 1716, 1608, 1508; chemical formula: C31H23BrCl2N2O3; elemental analysis: C, 59.83; H, 3.73; N, 4.50; Found: C, 59.80; H, 3.75; N, 4.55.
4.2.8. (3R,6′S,7′S)-6-Chloro-7′-(5-(4-chlorophenyl)furan-2-yl)-6′-(4-nitrobenzoyl)-1′,6′,7′,7a′-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one 6h
1H NMR (400 MHz, CDCl3): δ 8.38 (s, 1H), 8.08 (d, J = 8.6 Hz, 2H), 7.54 (dd, J = 17.7, 8.6 Hz, 5H), 7.30 (d, J = 8.2 Hz, 2H), 7.04 (dd, J = 8.1, 2.1 Hz, 1H), 6.65 (d, J = 1.8 Hz, 1H), 6.54 (d, J = 3.1 Hz, 1H), 6.38 (d, J = 3.6 Hz, 1H), 4.85 (d, J = 11.7 Hz, 1H), 4.53 (dt, J = 9.7, 3.9 Hz, 1H), 4.15–4.00 (m, 1H), 3.88 (d, J = 11.1 Hz, 1H), 3.46 (d, J = 11.1 Hz, 1H), 3.20 (d, J = 3.9 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 194.92, 179.53, 152.88, 151.11, 150.38, 141.79, 141.08, 136.60, 133.25, 131.08, 129.93, 129.18, 129.07, 129.04, 129.01, 128.94, 124.96, 123.69, 123.57, 122.96, 120.70, 110.18, 106.47, 74.11, 71.71, 60.61, 59.94, 55.31, 45.22, 37.09, 14.28; IR (KBr, cm–1): 3422, 3320, 2920, 2850, 1730, 1620, 1511; chemical formula: C30H21Cl2N3O5S; elemental analysis: C, 59.41; H, 3.49; N, 6.93; S, 5.29; Found: C, 59.44; H, 3.51; N, 7.02; S, 5.36.
4.2.9. (1′S,2′S,3R)-6-Chloro-1′-(5-(4-chlorophenyl)furan-2-yl)-2′-(4-nitrobenzoyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 6i
1H NMR (400 MHz, CDCl3): δ 8.92 (s, 1H), 8.36 (d, J = 8.3 Hz, 1H), 8.23 (d, J = 8.7 Hz, 1H), 8.05 (d, J = 8.6 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 7.46 (s, 0H), 7.26 (d, J = 8.2 Hz, 2H), 7.12 (d, J = 11.9 Hz, 1H), 7.02 (d, J = 8.2 Hz, 1H), 6.68 (s, 1H), 6.51 (d, J = 3.0 Hz, 1H), 6.28 (d, J = 3.5 Hz, 1H), 4.98 (d, J = 11.6 Hz, 1H), 4.32 (dt, J = 9.9, 6.3 Hz, 1H), 4.15–3.96 (m, 1H), 2.57 (dt, J = 6.6, 4.2 Hz, 2H), 2.15 (dt, J = 12.8, 6.3 Hz, 1H), 1.90 (p, J = 6.4 Hz, 2H), 1.82 (dt, J = 13.5, 6.3 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 195.56, 180.70, 171.46, 152.40, 150.76, 150.34, 142.00, 141.20, 140.87, 135.93, 132.95, 129.73, 129.18, 129.08, 129.02, 128.95, 128.89, 128.85, 128.41, 127.43, 124.83, 124.61, 124.34, 124.05, 123.69, 123.34, 122.96, 122.76, 111.36, 109.52, 109.12, 108.76, 106.40, 106.17, 73.18, 69.02, 64.69, 62.38, 60.58, 48.14, 46.41, 31.02, 27.42, 14.27; IR (KBr, cm–1): 3423, 3317, 2935, 2845, 1723, 1606, 1516; chemical formula: C31H23Cl2N3O5; elemental analysis: C, 63.28; H, 3.94; N, 7.14; Found: C, 63.34; H, 3.99; N, 7.24.
4.2.10. (1′S,2′S,3R)-6-chloro-1′-(5-(4-chlorophenyl)furan-2-yl)-2′-(4-nitrobenzoyl)-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one 6j
1H NMR (400 MHz, CDCl3): δ 8.12 (d, J = 8.7 Hz, 3H), 7.59 (d, J = 8.7 Hz, 2H), 7.49 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.1 Hz, 2H), 7.13 (d, J = 8.0 Hz, 1H), 7.04 (d, J = 8.5 Hz, 1H), 6.61 (s, 1H), 6.51 (d, J = 3.6 Hz, 1H), 6.26 (d, J = 3.6 Hz, 1H), 5.05 (d, J = 11.7 Hz, 1H), 4.48 (q, J = 8.3, 7.5 Hz, 1H), 3.98 (t, J = 10.9 Hz, 1H), 3.12–3.05 (m, 1H), 2.17 (dq, J = 9.9, 4.8 Hz, 1H), 1.97–1.89 (m, 1H), 1.81 (dt, J = 13.7, 6.3 Hz, 1H), 1.52 (q, J = 14.4 Hz, 3H), 1.36 (q, J = 12.6 Hz, 1H), 1.02 (dddd, J = 48.7, 25.8, 9.8, 3.6 Hz, 3H), 0.84 (d, J = 14.7 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 195.39, 181.09, 174.14, 152.31, 152.24, 150.37, 141.50, 141.35, 135.69, 132.93, 129.21, 129.15, 128.93, 128.85, 124.86, 123.68, 122.49, 122.16, 111.04, 109.01, 106.38, 71.61, 68.01, 63.26, 60.57, 57.93, 53.55, 47.20, 41.75, 37.62, 28.32, 27.59, 24.52, 19.92, 14.27; IR (KBr, cm–1): 3429, 3314, 2927, 2837, 1720, 1610, 1505; chemical formula: C35H29Cl2N3O5; elemental analysis: C, 65.43; H, 4.55; N, 6.54; Found: C, 65.45; H, 4.54; N, 6.64.
4.3. X-ray Structure Determinations
The crystal of 3a was immersed in cryo-oil, mounted in a loop, and measured at a temperature of 120 K. The X-ray diffraction data were collected on a Rigaku Oxford Diffraction Supernova diffractometer using Mo Kα radiation. The CrysAlisPro37 software package was used for cell refinement and data reduction. An analytical absorption correction (CrysAlisPro37) was applied to the intensities before structure solution. The structure was solved by intrinsic phasing (SHELXT38) method. Structural refinement was carried out using SHELXL39 software with the SHELXLE40 graphical user interface. Hydrogen atoms were positioned geometrically and constrained to ride on their parent atoms, with C–H = 0.95 Å and Uiso = 1.2Ueq (parent atom).
4.4. Hirshfeld Surface Analysis
Crystal Explorer 17.5 program41 was used to perform the Hirshfeld topology analysis.
4.5. Molecular Docking Study
The investigated compounds were docked against the protein structures of EGFR (PDB = 1M17) and CDK-2 (PDB = 2A4L) using AutoDock Vina software following routine work.42−45 Vina was used to improve protein and ligand structures and favor them energetically. Proteins and compound structures were prepared and optimized using Maestro. Then, the binding sites inside proteins were determined using grid-box dimensions around the cocrystallized ligands. Binding activities interpreted molecular docking results in terms of binding energy and ligand–receptor interactions. The visualization was then done with Chimera.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0040).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03790.
Author Contributions
Conceptualization, A.B.; methodology, M.S.A. and A.A.A.; software, S.M.S. and M.H.; validation, M.S.A., N.H.A.-S., and A.A.A.; formal analysis, M.S.A., N.H.A.-S., M.H., and A.A.A.; investigation, M.S.A.; resources, M.S.A. and A.B.; data curation, A.B. and S.M.S.; writing—original draft preparation, A.B. and S.M.S.; writing—review and editing, A.B. and S.M.S.; visualization, A.B., M.S.A., and N.H.A.-S.; supervision, A.B. and M.S.A.; project administration, A.A.A.; funding acquisition, M.S.A. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Panda S. S.; Jones R. A.; Bachawala P.; Mohapatra P. P. Spirooxindoles as Potential Pharmacophores. Mini-Rev. Med. Chem. 2017, 17, 1515–1536. 10.2174/1389557516666160624125108. [DOI] [PubMed] [Google Scholar]
- Zhou L. M.; Qu R. Y.; Yang G. F. An Overview of Spirooxindole as a Promising Scaffold for Novel drug Discovery. Expert Opin. Drug Discovery 2020, 15, 603–625. 10.1080/17460441.2020.1733526. [DOI] [PubMed] [Google Scholar]
- Yu B.; Yu D. Q.; Liu H. M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Yu S.; Sun W.; Liu L.; Lu J.; McEachern D.; Shargary S.; Bernard D.; Li X.; Zhao T.; Zou P.; Sun D.; Wang S. A Potent Small-Molecule Inhibitor of the MDM2-p53 Interaction (MI-888) Achieved Complete and Durable Tumor Regression in Mice. J. Med. Chem. 2013, 56, 5553–5561. 10.1021/jm4005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Guan S.; Zhao Y.; Yu Y.; Wang Y.; Shi Y.; Mao X.; Yang K. L.; Sun W.; Xu X.; Yi J. S.; Yang T.; Yang J.; Nuchtern J. G. Novel MDM2 Inhibitor SAR405838 (MI-773) Induces p53-Mediated Apoptosis in Neuroblastoma. Oncotarget 2016, 7, 82757. 10.18632/oncotarget.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M.; Yang J.; Xu X.; Sebolt J. T.; Wang S.; Sun Y. Efficacy of MDM2 Inhibitor MI-219 Against Lung Cancer Cells Alone or in Combination with MDM2 Knockdown, a XIAP Inhibitor or Etoposide. Anticancer Res. 2010, 30, 3321. [PubMed] [Google Scholar]
- Zhao B. L.; Du D. M. Organocatalytic Cascade Michael/Michael Reaction for the Asymmetric Synthesis of Spirooxindoles Containing Five Contiguous Stereocenters. Chem. Commun. 2016, 52, 6162–6165. 10.1039/c6cc00705h. [DOI] [PubMed] [Google Scholar]
- Galliford C. V.; Scheidt K. A. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]
- Liu J.; Peng H.; Yang Y.; Jiang H.; Yin B. Regioselective and Stereoselective Pd-Catalyzed Intramolecular Arylation of Furans: Access to Spirooxindoles and 5H-Furo[2,3-c]quinolin-4-ones. J. Org. Chem. 2016, 81, 9695–9706. 10.1021/acs.joc.6b01774. [DOI] [PubMed] [Google Scholar]
- Pan L. N.; Sun J.; Shi R. G.; Yan C. G. Diastereoselective synthesis of dispiro[indoline-3,3′-furan-2′,3″-pyrrolidine] via [3 + 2]cycloaddition reaction of MBH maleimides of isatins and 1,3-dicarbonyl compounds. Org. Chem. Front. 2020, 7, 3202–3208. 10.1039/d0qo00845a. [DOI] [Google Scholar]
- Yin B.-L.; Lai J. Q.; Zhang Z. R.; Jiang H. F. A Novel Entry to Spirofurooxindoles Involving Tandem Dearomatization of Furan Ring and Intramolecular Friedel–Crafts Reaction. Adv. Synth. Catal. 2011, 353, 1961–1965. 10.1002/adsc.201100034. [DOI] [Google Scholar]
- Gupta N.; Bhojani G.; Tak R.; Jakhar A.; Khan N. U. H.; Chatterjee S.; Kureshy R. I. Highly Diastereoselective Syntheses of Spiro-Oxindole Dihydrofuran Derivatives in Aqueous Media and Their Antibacterial Activity. ChemistrySelect 2017, 2, 10902–10907. 10.1002/slct.201702314. [DOI] [Google Scholar]
- Liu Z.; Fang J.; Yan C. Efficient Synthesis of Spiro[furan-3,3′-indoline] Derivatives via Reactions of Pyridinium Salts with Isatinylidene Acetoacetates. Chin. J. Chem. 2013, 31, 1054–1058. 10.1002/cjoc.201300407. [DOI] [Google Scholar]
- Ball-Jones N. R.; Badillo J. J.; Franz A. K. Strategies for the Enantioselective Synthesis of Spirooxindoles. Org. Biomol. Chem. 2012, 10, 5165–5181. 10.1039/c2ob25184a. [DOI] [PubMed] [Google Scholar]
- Kumarswamyreddy N.; Kesavan V. Stereoselective and Regioselective Assembly of Spirooxindole [2,1-b]furan Motifs through a Tandem Friedel-Crafts Alkylation/5-exo-dig-Cyclization. Eur. J. Org. Chem. 2016, 5301–5308. 10.1002/ejoc.201601030. [DOI] [Google Scholar]
- Kumarswamyreddy N.; Kesavan V. Enantioselective Synthesis of Dihydrospiro[indoline-3,4′-pyrano[2,3-c]pyrazole] Derivatives via Michael/Hemiketalization Reaction. Org. Lett. 2016, 18, 1354–1357. 10.1021/acs.orglett.6b00287. [DOI] [PubMed] [Google Scholar]
- Xie X.; Peng C.; He G.; Leng H. J.; Wang B.; Huang W.; Han B. Asymmetric Synthesis of a Structurally and Stereochemically Complex Spirooxindole Pyran Scaffold Through an Organocatalytic Multicomponent Cascade Reaction. Chem. Commun. 2012, 48, 10487–10489. 10.1039/c2cc36011j. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.; Chafeev M.; Liu S.; Sun J.; Raina V.; Chui R.; Young W.; Kwan R.; Fu J.; Cadieux J. A. Discovery of XEN907, a spirooxindole blocker of NaV1.7 for the treatment of pain. Bioorg. Med. Chem. Lett. 2011, 21, 3676–3681. 10.1016/j.bmcl.2011.04.088. [DOI] [PubMed] [Google Scholar]
- Altowyan M. S.; Barakat A.; Al-Majid A. M.; Al-Ghulikah H. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208–1216. 10.1016/j.sjbs.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfy G.; Abdel Aziz Y. M. A.; Said M. M.; El Ashry E. S. H.; El Tamany E. S. H.; Abu-Serie M. M.; Teleb M.; Dömling A.; Barakat A. Molecular Hybridization Design and Synthesis of Novel Spirooxindole-Based MDM2 Inhibitors Endowed with BCL2 Signaling Attenuation; A Step Towards the Next Generation p53 Activators. Bioorg. Chem. 2021, 117, 105427. 10.1016/j.bioorg.2021.105427. [DOI] [PubMed] [Google Scholar]
- Barakat A.; Islam M. S.; Ghawas H. M.; Al-Majid A. M.; El-Senduny F.; Badria F. A.; Elshaier Y.; Ghabbour H. A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2-p53 interaction. Bioorg. Chem. 2019, 86, 598–608. 10.1016/j.bioorg.2019.01.053. [DOI] [PubMed] [Google Scholar]
- Barakat A.; Haukka M.; Soliman S. M.; Ali M.; Al-Majid A. M.; El-Faham A.; Domingo L. R. Straightforward Regio- and Diastereoselective Synthesis, Molecular Structure, Intermolecular Interactions and Mechanistic Study of Spirooxindole-Engrafted Rhodanine Analogs. Molecules 2021, 26, 7276. 10.3390/molecules26237276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz Y. M. A.; Lotfy G.; Said M. M.; El Ashry E. S. H.; El Tamany E. S. H.; Soliman S. M.; Abu-Serie M. M.; Teleb M.; Yousuf S.; Dömling A.; et al. Design, Synthesis, Chemical and Biochemical Insights into Novel Hybrid Spirooxindole-Based p53-MDM2 Inhibitors with Potential Bcl2 Signaling Attenuation. Front. Chem. 2021, 9, 735236. 10.3389/fchem.2021.735236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majid A. M.; Ali M.; Islam M. S.; Alshahrani S.; Alamary A. S.; Yousuf S.; Choudhary M. I.; Barakat A. Stereoselective Synthesis of the Di-Spirooxindole Analogs Based Oxindole and Cyclohexanone Moieties as Potential Anticancer Agents. Molecules 2021, 26, 6305. 10.3390/molecules26206305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A.; Islam M. S.; Ali M.; Al-Majid A. M.; Alshahrani S.; Alamary A. S.; Yousuf S.; Choudhary M. I. Regio- and Stereoselective Synthesis of a New Series of Spirooxindole Pyrrolidine Grafted Thiochromene Scaffolds as Potential Anticancer Agents. Symmetry 2021, 13, 1426. 10.3390/sym13081426. [DOI] [Google Scholar]
- Islam M. S.; Ghawas H. M.; El-Senduny F. F.; Al-Majid A. M.; Elshaier Y. A.; Badria F. A.; Barakat A. Synthesis of new thiazolo-pyrrolidine-(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. 10.1016/j.bioorg.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Lotfy G.; Said M. M.; El Ashry H.; El Tamany H.; Al-Dhfyan A.; Abdel Aziz Y. M. A.; Barakat A. Synthesis of New Spirooxindole-Pyrrolothiazole Derivatives: Anti-Cancer Activity and Molecular Docking. Bioorg. Med. Chem. 2017, 25, 1514–1523. 10.1016/j.bmc.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Barakat A.; Islam M. S.; Ghawas H. M.; Al-Majid A. M.; El-Senduny F. F.; Badria F. A.; Elshaier Y. A. M.; Ghabbour H. A. Substituted Spirooxindole Derivatives as Potent antiCancer Agents Through Inhibition of Phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. 10.1039/c8ra02358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Gutiérrez M.; Domingo L. R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 267–282. 10.1002/ejoc.201800916. [DOI] [Google Scholar]
- Domingo L. R.; Chamorro E.; Perez P. Understanding the High Reactivity of the Azomethine Ylides in [3 + 2] Cycloaddition Reactions. Lett. Org. Chem. 2010, 7, 432–439. 10.2174/157017810791824900. [DOI] [Google Scholar]
- Domingo L. R.; Kula K.; Ríos-Gutiérrez M. Unveiling the Reactivity of Cyclic Azomethine Ylides in [3+2] Cycloaddition Reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 5938–5948. 10.1002/ejoc.202000745. [DOI] [Google Scholar]
- Domingo L. R.; Ríos-Gutiérrez M.; Pérez P. A Molecular Electron Density Theory Study of the Role of the Copper Metalation of Azomethine Ylides in [3 + 2] Cycloaddition Reactions. J. Org. Chem. 2018, 83, 10959–10973. 10.1021/acs.joc.8b01605. [DOI] [PubMed] [Google Scholar]
- Ríos-Gutiérrez M.; Barakat A.; Domingo L. R. A Molecular Electron Density Theory Study of the [3+2] Cycloaddition Reaction of Pseudo(mono)radical Azomethine Ylides with Phenyl Vinyl Sulphone. Organics 2022, 3, 122–136. 10.3390/org3020010. [DOI] [Google Scholar]
- Faber A. C.; Chiles T. C. Inhibition of Cyclin-Dependent Kinase-2 Induces Apoptosis in Human Diffuse Large B-Cell Lymphomas. Cell Cycle 2007, 6, 2982–2989. 10.4161/cc.6.23.4994. [DOI] [PubMed] [Google Scholar]
- ElZahabi H. S. A.; Nafie M. S.; Osman D.; Elghazawy N. H.; Soliman D. H.; EL-Helby A. A. H.; Arafa R. K. Design, Synthesis and Evaluation of New Quinazolin-4-one Derivatives as Apoptotic Enhancers and Autophagy Inhibitors with Potent Antitumor Activity. Eur. J. Med. Chem. 2021, 222, 113609. 10.1016/j.ejmech.2021.113609. [DOI] [PubMed] [Google Scholar]
- Nafie M. S.; Kishk S. M.; Mahgoub S.; Amer A. M. -Quinoline-based thiazolidinone derivatives as potent cytotoxic and apoptosis-inducing agents through EGFR inhibition. Chem. Biol. Drug Des. 2022, 99, 547–560. 10.1111/cbdd.13997. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M.SADABS-Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, Wisconsin, USA, 2012.
- Sheldrick G. M. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement withSHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübschle C. B.; Sheldrick G. M.; Dittrich B. ShelXle: a Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. 10.1107/s0021889811043202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. J.; McKinnon J. J.; Wolff S. K.; Grimwood D. J.; Spackman P. R.; Jayatilaka D.; Spackman M. A.. Crystal Explorer17; University of Western Australia, 2017. http://hirshfeldsurface.net.
- Nafie M. S.; Tantawy M. A.; Elmgeed G. A. Screening of Different Drug Design Tools to Predict the Mode of Action of Steroidal Derivatives as Anti-cancer Agents. Steroids 2019, 152, 108485. 10.1016/j.steroids.2019.108485. [DOI] [PubMed] [Google Scholar]
- Nafie M. S.; Amer A. M.; Mohamed A. K.; Tantawy E. S. Discovery of Novel Pyrazolo[3,4-b]pyridine Scaffold-Based Derivatives as Potential PIM-1 Kinase Inhibitors in Breast Cancer MCF-7 Cells. Bioorg. Med. Chem. 2020, 28, 115828. 10.1016/j.bmc.2020.115828. [DOI] [PubMed] [Google Scholar]
- Nafie M. S.; Arafa K.; Sedky N. K.; Alakhdar A. A.; Arafa R. K. Triaryl dicationic DNA Minor-Groove Binders with Antioxidant Activity Display Cytotoxicity and Induce Apoptosis in Breast Cancer. Chem.-Biol. Interact. 2020, 324, 109087. 10.1016/j.cbi.2020.109087. [DOI] [PubMed] [Google Scholar]
- Khalifa M. M.; Al-Karmalawy A. A.; Elkaeed E. B.; Nafie M. S.; Tantawy M. A.; Eissa I. H.; Mahdy H. A. Topo II Inhibition and DNA Intercalation by New Phthalazine-Based Derivatives as Potent Anticancer Agents: Design, Synthesis, Anti-Proliferative, Docking, and in vivo Studies. J. Enzyme Inhib. Med. Chem. 2022, 37, 299–314. 10.1080/14756366.2021.2007905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.