Abstract
The development of therapies for SARS-CoV-2 infection, based on virus biology and pathology, and of large- and small-scale randomized controlled trials, have brought forward several antiviral and immunomodulatory drugs targeting the disease severity. Casirivimab/Imdevimab monoclonal antibodies and convalescent plasma to prevent virus entry, Remdesivir, Molnupiravir, and Paxlovid nucleotide analogs to prevent viral replication, a variety of repurposed JAK-STAT signaling pathway inhibitors, corticosteroids, and recombinant agonists/antagonists of cytokine and interferons have been found to provide clinical benefits in terms of mortality and hospitalization. However, current treatment options face multiple clinical needs, and therefore, in this review, we provide an update on the challenges of the existing therapeutics and highlight drug development strategies for COVID-19 therapy, based on ongoing clinical trials, meta-analyses, and clinical case reports.
Keywords: Clinical trials, COVID-19, Cytokine storm, Drugs in clinic, Drugs in development, SARS-CoV-2
Graphical Abstract
1. Introduction
The COVID-19 pandemic led to the mortality of millions of people worldwide and caused a major economic burden across the world. Coronaviruses are single-stranded RNA viruses that can infect a wide range of organisms [1] and have caused three massive outbreaks: (i) severe acute respiratory syndrome (SARS) caused by severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) in 2003; (ii) Middle East respiratory syndrome (MERS) caused by Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012; (iii) COVID-19 caused by SARS-CoV-2 starting from 2019 [2].
SARS-CoV-2 is an enveloped, single-stranded positive-sense RNA virus [3]. The size of the genome of SARS-CoV-2 is around 30 kb, comprising genes encoding spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins, as well as other open reading frames regions encoding nonstructural proteins. SARS-CoV-2, like other coronaviruses, can infect animal cells because their S protein binds to angiotensin-converting enzyme 2 (ACE2) as the cellular receptor, and this virus-receptor interaction is a key factor that determines the range and susceptibility of their hosts [4], [5]. After binding ACE2, SARS-CoV-2 enters the target cells via endocytosis machinery, resulting in the internalization and downregulation of ACE2 [6]. It is evident from different studies the occurrence of a multistep SARS-CoV-2 entry process [7] and that there is a large array of proteins apart from ACE2, which play crucial roles in the SARS-CoV-2 tropism, often in synergistic and complementary ways [8]. The COVID-19 viral tropism depends on the susceptibility and permissiveness of a specific host cell. During the epidemic, patients often presented with respiratory-like illnesses that progressed to severe pneumonia, suggesting that the lung is the primary tropism of SARS-CoV-2 [9].
The main symptoms upon infection with COVID-19 include fever, myalgia, cough, dyspnea, rhinorrhea, loss of smell/taste, sore throat, headaches, nausea/vomiting, diarrhea, and chest pain that may lead to multi-organ failure [10]. There are several potential treatment options for COVID-19 patients, including supportive care, respiratory support, antiviral treatment, therapeutic-specific antibodies, convalescent plasma and immunomodulatory agents including biological treatment. SARS-CoV-2 infection-induced over-activation of the immune system can lead to a cytokine storm in the host [2], [11]. Even with the above treatments, acute respiratory distress syndrome (ARDS) caused by the cytokine storm is the major cause of mortality in critically ill patients [12], [13]. Although a large number of anti-viral and immunomodulatory drugs have been proposed to target the key processes of viral infection and the advanced cytokine storm stage, only a few of them have shown significant, potent therapeutic efficacy and safety and are used in practice. Among the variety of COVID-19 clinical trials, the SOLIDARITY [14] and RECOVERY [15], [16] trials were pivotal in therapy guidelines for the management of COVID-19 patients. The Infectious Disease Society of America (IDSA) and the USA National Institutes of Health (NIH) [17] and World Health Organization (WHO) [18] have produced comprehensive guidelines for the treatment and management of patients with COVID-19. In applying these treatment recommendations to patients with COVID-19 it is crucial to classify the patient’s disease severity, to target patients that will most benefit from the various drugs for COVID-19 and to minimize adverse events.
In this review, we summarize the current clinical and investigational therapeutic options for the management of patients with COVID-19 derived from the IDSA guidelines based on the patient’s current clinical stage of disease, emphasizing agents that have efficacy, demonstrated through well-designed randomized controlled clinical trials, and highlight future directions for developing novel pharmacological therapies as evident from ongoing different clinical trials and case reports. Albeit, vaccines appear to be safe and effective tools to prevent severe COVID-19 hospitalization and death against all virus variants of concern [19], [20], [21], they are not included in the present survey.
2. Clinical presentation of SARS-CoV-2-induced pathology
Infection with SARS-CoV-2 causes severe “flu-like” symptoms, indicative of interferon activation, that can progress to life-threatening systemic inflammation and multi-organ dysfunction such as acute respiratory distress (ARDS), pneumonia, cardiogenic shock, renal failure, and death [22]. The most abundant symptoms are fever, cough, and dyspnea, accounting for 83%, 82%, and 31% of patients with COVID-19 [23]. The incubation period for COVID-19 is short, about 5–6 days. As the pandemic progressed there is growing evidence that COVID-19 encompasses not only rapid respiratory/gastrointestinal illnesses, but can also lead to myocardial inflammation [24] and neurological and neuropsychiatric impairments [25]. Furthermore, severe COVID-19 is not solely restricted to the aged population as initially reported, children and young adults are also at risk [26]. Lymphocytopenia, neutrophilia, elevated inflammation-related indices, and coagulation-related indicators have been consistently reported in older (≥65 years old) relative to young and middle-aged patients with COVID-19 [27]. At the cellular level, a lower capacity of CD4+ and CD8+ T-cells to produce IFN-γ and IL-2, as well as an impairment in T-cell activation from dendritic cells (DCs) in patients with acute COVID-19 (≥55 years old) could compromise an optimal adaptive immune response [28]. High levels of proinflammatory macrophages and neutrophils have been observed in the bronchoalveolar lavage fluid of COVID-19 patients [29], explaining the elevations of proinflammatory cytokines (e.g., IL-6 and IL-8) and inflammatory chemokines (e.g., CCL2), relative to patients with non-severe COVID-19 [29], [30]. This inflammatory cytokine storm is the primary determinant for the severe pathophysiological evolution presented in critically ill COVID-19 patients [31]. The proinflammatory mediators elevated C-reactive protein (CRP) from the liver, signal transducer and activator of transcription 3 (STAT3)-IL-6 signaling [32] eventually contributing to the lung disease. This increase in CRP directly correlates with elevated serum IL-6 levels observed in patients with COVID-19 [29]. The recruitment of activated neutrophils and monocytes may be driven by pulmonary endothelial cell dysfunction through vascular leakage, tissue edema, endothelitis, and disseminated intravascular coagulation (DIC), expressed by elevated serum D-dimer and prolonged prothrombin time. Therefore, it is reasonable to propose that in this pathology, the SARS-CoV-2 direct insult and immune cell recruitment promote vascular leakage, multi-organ failure, and cardiovascular collapse.
3. Current pharmacological therapies against COVID-19
From a pharmacotherapy point of view, it is crucial to choose specific drugs following the COVID-19 patient’s disease severity, considering that subtle differences exist in the known benefits and adverse effects of the various therapies. Fig. 1 presents the rational of initiating antiviral and immunomodulatory drugs for COVID-19, according to timing and immune response phases and the four categories of severity of the disease. Mild COVID-19 is characterized by clinical features suggestive of upper respiratory tract involvement, without another organ involvement. Moderate COVID-19 includes pulmonary involvement without hypoxia. Most patients improve with supportive care at this stage, but patients with risk factors can progress to a more severe or critical disease and even death and hence, may benefit from pharmacotherapies. From a pharmacological mechanistic perspective, early during the infection, when SARS-CoV-2 viral load is high and the patient express mild or moderate symptoms, drug treatments targeting viral replication can be more effective since the adaptive immune system has not revealed yet an adequate response. Antiviral drugs (e.g., Remdesivir, Molnupiravir, Nirmatrelvir/Ritonavir) and neutralizing monoclonal antibody therapies (Table 1, Supplementary Material-Data in Brief), the earlier provided, the more efficacious they likely would be. There are subgroups of patients like immunocompromised patients, with high viral load even later in the disease process, who may still benefit from antiviral treatments, neutralizing monoclonal antibody therapies and convalescent plasma. Patients with severe COVID-19 are those who have pulmonary disease with hypoxia on room air needing treatment with low-flow oxygen. Most existing diagnostic criteria for severe COVID-19, consider an oxygen saturation level less than 93% and tachypnea (respiratory rate >30) (Fig. 1). This severity of critically ill patients requires more ventilator or oxygenation support, with either high-flow oxygen or noninvasive ventilation. High-flow oxygen therapy involves delivery of oxygen via special devices at rates up to 10–15 L/min. These patients are susceptible to a secondary infection phase with increase adaptive immune response. Azithromycin is a macrolide antimicrobial agent which acts against a broad range of gram-positive and gram-negative bacteria and plays an immunomodulatory role and has been widely used with and without ceftriaxone for the treatment of secondary infections in COVID-19 patients in moderate to severe phases, albeit his potential cardiotoxicity [33]. However, recent clinical studies indicate that the combination of Azithromycin and Hydroxychloroquine did not improve survival or length of hospitalization in patients with COVID-19 [34]. Therapeutic anticoagulation often employed in hospitalized patients resulted in a slight reduction in venous thromboembolism, but appears to have no effect on mortality in COVID-19 patients [35].
Fig. 1.
Antiviral and immunotherapies of COVID-19 are targeting the immune response and disease severity. ACE2, angiotensin-converting enzyme 2; ATR, angiotensin-receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor; JNK, c-Jun N-terminal kinases; IL, interleukin; PAMP, pathogen-associated molecular pattern inflammatory signal molecule; DAMP, damage-associated molecular pattern inflammatory signal molecule; PCR, polymerase chain reaction; PaO2/FiO2, the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2 expressed as a fraction), known as the Horowitz index; ARDS/MIS A/C, acute respiratory distress syndrome/ multisystem inflammatory syndrome type A (fever, elevated inflammatory markers, and multiple organ system involvement) and type C (pediatric inflammatory multisystem syndrome (PIMS)(; ECMO, extracorporeal membrane oxygenation.
Glucocorticoids are potent anti-inflammatory drugs that mitigate the risk of ARDS in COVID-19 and other viral pneumonia, by inhibition of the development of cytokine storm and multi-organ damage. Glucocorticoids are recommended for use in the severe and critically ill patients in the hyper-inflammatory systemic phase, because they have shown the highest 28-day mortality benefit when used in these subpopulations. Dexamethasone 6 mg daily for 10 days is preferred, but doses up to 20 mg daily can also be applied [15]. Hydrocortisone 50 mg administered intravenously every 6 h is an alternative regimen [36]. Dexamethasone is known to suppress IL-1 signaling pathways, specifically c-Jun N-terminal kinase (JNK)-p38 kinase, leading to suppression of macrophage release of downstream cytokines, such as IL-6, IL-8, and TNF [108]. Similarly, Hydrocortisone use in patients with severe sepsis has been shown to significantly decrease IL-1β, interferon-γ (IFN-γ), TNF-α, and IL-6 levels [109]. Methylprednisolone has been reported to have some benefit in acute lung injury [110]. In addition, due to their immunosuppressive activity, glucocorticoids could also delay virus clearance in COVID-19 patients as they did in SASR and MERS patients [111]. In addition to glucocorticoids, IL-6 inhibitors (Tocilizumab, preferred over Sarilumab) or JAK inhibitors (Baricitinib, preferred over Tofacitinib) are recommended for those patients who present with elevated levels of inflammatory markers like C-reactive protein (CRP, Table 2 Supplementary Material-Data in Brief, Fig. 2). However, the clinical trials done so far, have not identified specific subpopulations of critically ill patients already being treated with corticosteroids that would benefit with additional treatment with IL-6 or JAK inhibitors. The IDSA guidelines recommend Dexamethasone, in critically ill patients with COVID-19 requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (Fig. 1). Interestingly, the most recent analysis of the pharmacological therapies in 737 unique clinical trials indicated that the most commonly used interventions for COVID- 19 patients were drugs such as antiparasitics (62% of the trials), antivirals (57%), antibiotics (31%), oxygen (17%), antithrombotics/anticoagulants (14%), vitamins (13%), immunomodulatory agents (13%), glucocorticoids (12%), analgesics/antipyretics (12%). Moreover, various combinations of those interventions were used, with up to seven different types of combinations [37].
Fig. 2.
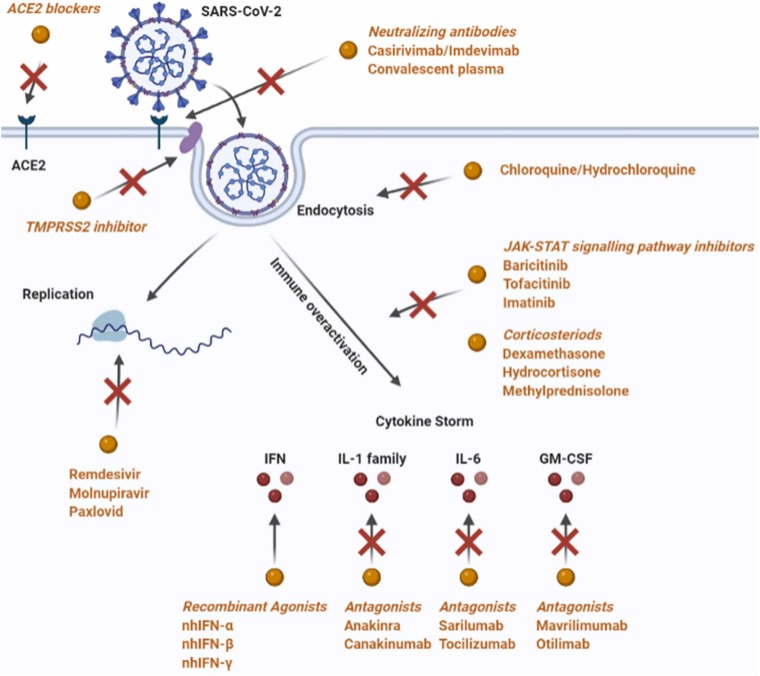
Therapeutic targets in SARS-CoV-2 infection. Antiviral drugs target the essential steps for viral infection. ACE2 blockers, TMPRSS2 inhibitor, and neutralizing antibodies disrupt the binding of SARS-CoV-2 to ACE2 and other protein acceptors. Chloroquine and Hydroxychloroquine suppress the endocytosis process. Remdesivir, Molnupiravir and Paxlovid inhibit viral replication. JAK-STAT signaling pathway inhibitors, corticosteroids, and recombinant agonists and antagonists of cytokines and interferons serve as immunosuppressants to modulate the cytokine storm induced by the hyper-activation of the immune system.
4. Antiviral selected drugs for COVID 19
SARS-CoV-2 infection is mediated by the binding of viral spike proteins (S-protein) to human cells through a two-step process, which involves angiotensin-converting enzyme-2 (ACE2) and angiotensin receptors (ATR) and hijacking the androgen-receptor regulated, transmembrane serine protease (TMPRSS)− 2 for viral entry due to its proteolytic cleavage of S-protein. The renin-angiotensin system (RAS) plays an important role in the development of ARDS. ACE2 is one of the enzymes involved in the RAS cascade. Virus S-protein binds to ACE2 to form a complex suitable for cellular endocytosis (Fig. 2). The downregulation of ACE2 results in the excessive accumulation of angiotensin II, and it has been demonstrated that the stimulation of the angiotensin II type 1a receptor (AT1R) increases pulmonary vascular permeability, explaining the increased lung pathology when the activity of ACE2 is decreased. Upon endocytosis of the virus in the host cell, it replicates increasing the viral load. Antiviral drugs directed for the early infection and inflammatory phases of the COVID-19 disease (Fig. 1), target these essential steps of viral infection, including viral binding and attachment, endocytosis, and replication in the host cell [38]. As of June 2022, about 688 investigational anti-viral clinical trials have been registered to Clinicaltrial.gov and some selected main representatives according to the mechanism of action are presented in Table 1 (Supplementary Material-Data in Brief) and Fig. 2 and discussed below.
4.1. Pipeline and approved drugs targeting the SARS-CoV-2 virus spike protein and host cell recognition receptors
The monoclonal neutralizing antibodies, anti-spike protein S1 subunit-receptor binding domain, such as Casirivimab/Imdevimab, Bamlanivimab/Etesevimabd, Sotrovimab and Bebtelovimab that block viral tropism, received FDA emergency use authorization for COVID-19 therapy (Table 1, Supplementary Material-Data in Brief). Among the various therapeutic and prophylactic strategies developed to contain the COVID-19 epidemic, immune-based SARS-CoV-2 elimination by passive immunization with COVID-19 polyclonal convalescent plasma transfusion has been also proven effective when administered in the early phase of the disease (within 72 h from symptom onset) and with a high titer ( >1:160) of anti-SARS-CoV-2 neutralizing antibodies. This passive immunoglobulin therapy is one of the potential adjunctive therapies now being used for COVID-19 patients [39], [40]. The rationale for such passive immunization is that plasma collected from patients after they recovered from COVID-19 might contain antibodies (IgG, IgA, IgM, IgE, and IgD) to SARS-CoV-2 and can be used to reduce the viremia and disease mortality in patients with life-threatening SARS-CoV-2 infections. However, larger clinical trials are required in order to further consolidate this therapy [41]. High-dose intravenous immunoglobulin (IVIG), based on clinical practical experience in autoimmune, inflammatory, and other infectious diseases, has been also been investigated, but the current evidence from the literature does not support the use of IVIG in COVID-19 patients [42].
Another preclinical therapeutic approach is based on strategies aimed at blocking ACE2 with monoclonal antibodies (Table 1, Supplementary Material-Data in Brief). Blockade of the ACE2 receptors could be also achieved through rationally designed small molecules [43], peptides [44], and exogenous administration of ACE2 [44]. The administration of a large amount of soluble form of ACE2 could represent an intriguing opportunity, since excessive ACE2 may competitively bind SARS-CoV-2 to neutralize or delay viral entry in the host cell and/or rescue ACE2 activity, which negatively regulates RAS and therefore, may confer a protective effect in lung injury. The safety and efficiency of recombinant human ACE2 were tested in a small cohort of healthy volunteers in phase 1 and in patients with ARDS in a phase 2 clinical study of COVID-19 patients [45]. However, the usage of ACE2 inhibitors to block the SARS-CoV-2 viral entry requires additional studies as there are conflicting findings and severe health complications reported for these inhibitors in COVID-19 patients. In preclinical research, in silico screening identified several novel compounds that can bind ACE2 or S-protein of SARS-CoV-2 and interfere with the interaction between this virus and its receptors [46]. In another study, compounds that can potentially inhibit the spike-ACE2 interaction were screened from 2701 FDA-approved drugs by using drug screening and in silico modeling. The findings indicated that the most potent effect of disrupting the S protein-ACE2 interaction was Thiostrepton, Oxytocin, Nilotinib, and Hydroxycamptothecin [47], calling for repurposing them for COVID-19 by characterization of their efficacy in clinical trials. Besides the in silico studies, the efficacy of potential drugs that can block the interactions between the S protein and ACE2 was also supported by in vitro and animal studies. For example, Ceftazidime and Dalbavancin effectively prevented SARS-CoV-2 infection in Vero E6 cells [48], [49], and Dalbavancin was active in mouse and rhesus macaque models [49]. However, although a large number of drugs have been identified by computational studies, clinical studies are required to confirm their safety and efficacy and ACE2-blocking monoclonal antibodies and inhibitors are not yet in clinical use.
Currently, available angiotensin receptor (ATR) antagonists such as Telmisartan, Eprosartan, Losartan and Valsartan are used as antihypertensive drugs in the clinic, by blocking the excessive angiotensin-mediated AT1R activation, and upregulation of ACE2 were considered for COVID-19 therapy (Table 1, Supplementary Material-Data in Brief). Albeit the controversy over the efficacy of ATR antagonists in the therapy of COVID-19 patients [50], they were found in clinical trials and case studies to reduce morbidity and mortality in hospitalized patients with pre-existing hypertension, by anti-inflammatory effects [51], but they are not yet in clinical practice.
Targeting of serine protease TMPRSS2 (Transmembrane Serine Protease-2) by either androgen blockade or direct inhibition is another approach in preclinical research and clinical trials for therapy of early SARS-CoV-2 infection. N-0385, a ketobenzothiazole-based peptidomimetic provided a high level of protection and a therapeutic benefit after either single or multiple administrations in K18-hACE2 mice preclinical model of COVID-19 disease (Table 1, Supplementary Material-Data in Brief). Camostat mesylate is a TMPRSS2 inhibitor that has been shown to inhibit SARS-CoV-2 viral entry and hence viral replication [52]. Both Camostat and Nafamostat, approved drugs for the treatment of pancreatitis, are under clinical trials as potential repurposing drug candidates for COVID-19 therapy [53].
4.2. Chloroquine (CQ) and Hydroxychloroquine (HCQ): Drugs targeting virus endocytosis
The cell entry of SARS-CoV-2 is mediated by endocytosis, which is also a therapeutic target for the treatment of COVID-19. The most extensively studied drugs that target the virus endocytic pathway are chloroquine (CQ) and hydroxychloroquine (HCQ) [54] (Table 1, Supplementary Material-Data in Brief, Fig. 2). FDA gave emergency approval to CQ and HCQ in March 2020. It was reported that HCQ treatment effectively reduced the mortality of critical COVID-19 patients [55]. Although HCQ is considered to be safer than CQ, both drugs can cause severe or even lethal adverse effects [56]. Due to these adverse effects, on June 2020, these drugs were withdrawn by FDA for clinical applications.
4.3. Remdesivir and Molnupiravir: Targeting the RNA-dependent RNA polymerase (RdRp)
Remdesivir (GS-5734) is an adenosine analog that disrupts viral replication by inhibiting the RNA-dependent RNA polymerase (RdRp) [57] and acts as a terminator of RNA elongation. The clinical study showed that Remdesivir effectively shortened the recovery time and prevented the progression of respiratory disease in COVID-19 patients (Table 1, Supplementary Material-Data in Brief). Remdesivir became the first FDA-approved drug for treating COVID-19 [58]. The FDA issued an emergency-use authorization on May 2020, considering the benefits of the drug outweigh its risks in patients. Remdesivir is not recommended unless the potential benefit outweighs the potential risk in adults and pediatric patients (>28 days old) with an estimated glomerular filtration rate (eGFR less than 30 mL per minute) or in full-term neonates (≥7 days and ≤28 days old) with serum creatinine ≥ 1 mg/dL. Possible side effects of Remdesivir include elevated liver function tests, an indication of inflammation or damage to the liver, and infusion-related reactions like low blood pressure, nausea, vomiting, sweating, and shivering. It is important to stress that besides its inhibitory effect on RdRp, recent evidence indicate that Remdesivir is blocking different steps of viral tropism. Remdesivir has a high affinity to the SARS-CoV-2 spike, ACE2, and TMPRSS2, proposing that it may also inhibit viral entry [59]. Remdesivir has a high affinity to the main proteases of SARS-CoV-2, suggesting that it may disrupt viral endocytosis and/or replication [60], [61]. The effect was found to be more potent than that of Lopinavir (ABT-378), another widely used viral protease inhibitor [59]. In addition, Remdesivir may also affect viral assembly as it showed a high affinity to SARS-CoV-2 M protein and 3–5′ exoribonuclease (nsp14) [59]. Remdesivir is not recommended for treating mild or moderate COVID-19 patients as it failed to show a significant therapeutic effect [62]. The final results of the WHO Solidarity randomized trial and updated meta-analyses indicate that Remdesivir has no significant effect on patients with COVID-19 who are already being ventilated. However, among other hospitalized patients, it presented a small positive effect against death and/or progression to ventilation [63]. Nevertheless, its efficacy in reducing mortality has not been clearly demonstrated. Molnupiravir, the first oral antiviral for COVID-19 is another safe RdRp inhibitor effective at reducing the risk of hospitalization and death in people with COVID-19, who are at increased risk of developing the severe disease (Table 1, Supplementary Material-Data in Brief). Molnupiravir has been authorized for use in people who have mild to moderate COVID-19 and at least one risk factor for developing severe illness. Such risk factors include obesity, older age (>60 years), diabetes mellitus, or heart disease. Molnupiravir is effective mainly when used within 5-days of the onset of symptoms. A 5-days course seems to be safe without any obvious short-term side effects [64]. Additional drugs such as Favipiravir and Ribavirin target RdRp and Galidesivir targets the RNA polymerase, inhibiting viral RNA synthesis and evaluated in different clinical trials for COVID-19 [65].
4.4. Nirmatrelvir plus Ritonavir: targeting SARS-CoV-2 main protease in combination with a Cytochrome P450 inhibitor
Nirmatrelvir plus Ritonavir (Paxlovid™; Pfizer) is a co-package combination of Nirmatrelvir and Ritonavir tablets, intended for co-administration and developed for the treatment and post-exposure prophylaxis of COVID-19. Nirmatrelvir, is a potent peptidomimetic selective and reversible inhibitor of SARS-CoV-2 Mpro main protease (3 C-like or nsp5 protease), while Ritonavir is a human immunodeficiency virus type 1 (HIV-1) protease inhibitor and Cytochrome P450, family 3, subfamily A (CYP3A) inhibitor. As a result of this inhibition viral replication is prevented. Ritonavir acts as a pharmacokinetic enhancer, increasing the systemic exposure of Nirmatrelvir and decreasing its metabolism, and prolonging its half-life when the drugs are administered together. Nirmatrelvir plus Ritonavir received its first conditional authorization in December 2021 in the United Kingdom, for the treatment of COVID-19 in adults who do not require supplemental oxygen, and who are at increased risk for progression to severe COVID-19 [66]. January 2022, Nirmatrelvir plus Ritonavir (Paxlovid) received authorization in the EU for use in the same indication and is authorized by FDA for emergency use in the USA for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (≥ 12 years of age and weighing ≥ 40 kg) at increased risk for progression to severe COVID-19. Treatment of symptomatic COVID-19 with Paxlovid resulted in a reduced risk of progression to severe COVID − 19 by 89% lower than the risk with a placebo, without evidence of severe safety concerns (Table 1, Supplementary Material-Data in Brief). Additional drug combinations such as Darunavir (protease inhibitor) and Cobicistat (pharmacokinetic enhancer) or Danoprevir (protease inhibitor) is registered in clinical trials for COVID-19.
Other anti-viral drugs such as Clevudine, a synthetic pyrimidine analog that targets DNA polymerase and reverse-transcriptase enzyme, Oseltamivir, a neuraminidase inhibitor, and Umifenovir, a fusion inhibitor blocking the viral entry and replication, are being evaluated in investigational clinical trials for COVID-19. All these different approaches of anti-viral pharmacological therapy for COVID-19, early eliminate and inactivate the SARS-CoV-2 virus to prevent or limit the immune dysregulation of the host and therefore the severity of the disease.
5. Immunotherapy selected drugs for COVID-19
From the very beginning of the COVID-19 pandemic, it became clear that dysregulation of immune responses against SARS-CoV-2 is one of the main features of disease pathogenesis, especially in patients with severe disease, and studies aimed at rebalancing the immune response by different modulators initiated early on. SARS-CoV-2 infection-induced hyper-inflammation (cytokine storm) is a major cause of death [2], [67]. In addition to the anti-viral therapies, immunotherapies that broadly or specifically target the immune response have been approved or are considered in different investigational clinical trials for COVID-19 [68]. Table 2 (Supplementary Material-Data in Brief) and Fig. 2 present approved and selected investigational clinical trials on immunotherapy based on glucocorticoids, pan-immune suppressors of inflammation, protein tyrosine-kinase inhibitors of the JAK-STAT signaling pathway, anti-IL-1, 6 and GM-CSF cytokines and interferons, stimulators of anti-viral defense.
5.1. Glucocorticoids
A variety of glucocorticoids, including Dexamethasone, Hydrocortisone, Methylprednisolone, etc., have been evaluated in clinical trials (Table 1, Supplementary Material-Data in Brief). The largest study, conducted by the RECOVERY collaborative group, resulted in the regulatory approval of Dexamethasone as the standard of care for patients with SARS-CoV-2 requiring oxygen therapy. Dexamethasone and Methylprednisolone are effective in reducing the severity of COVID-19 and associated comorbidities such as chronic obstructive pulmonary diseases [69]. Although acute glucocorticoid treatment is still considered to be the best therapy for severe COVID-19, chronic glucocorticoid treatment could cause the occurrence of a variety of adverse diseases such as arteriosclerosis, hypertension, diabetes, etc. Albeit glucocorticoid treatment is essential for critically ill patients experiencing ARDS [70], glucocorticoid drugs represent a double-edged sword in COVID-19 therapy [71], [72]. Attention should be given to their profound adverse effects, especially for those with comorbid conditions [73].
5.2. JAK-STAT signaling pathway inhibitors
The JAK-signal transducer and activator of transcription (JAK-STAT) signaling pathway mediates extracellular interleukins and interferons signal by membrane receptor activation and is a key inflammatory pathway that regulates the production of pro-inflammatory cytokines [74], [75]. Over-activation of the JAK-STAT pathway could lead to a cytokine storm in critically ill patients [76]. JAK inhibitors have been clinically used for the treatment of several inflammatory and autoimmune diseases, [77], [78]. For this reason, JAK inhibitors have been repurposed as pharmacological therapies for COVID-19 (Table 2, Supplementary Material-Data in Brief). The STOP-COVID clinical trial investigated the efficacy of the JAK inhibitor Tofacitinib, in 289 hospitalized patients with mild to moderate disease. Patients were randomized to receive oral Tofacitinib 10 mg twice a day, for 14 days, versus a placebo. At day 28, the composite outcome of death or respiratory failure was lower in the Tofacitinib group compared to the placebo. Beginning of 2020, it was predicted by studies of artificial intelligence that the JAK inhibitor Baricitinib could potentially inhibit cell entry and of SARS-CoV-2 [79]. This hypothesis was subsequently further supported by mechanistic studies [80]. In a meta-analysis study of eleven reports on the safety and efficacy of JAK-inhibitors Ruxolitinib and Baricitinib in COVID-19 patients, it was found that Baricitinib therapy caused a lower risk of death [81]. In COV-BARRIER, ACTT-2, and other clinical trials, systemic Baricitinib treatment revealed quite confidently that it decreased all-cause mortality in hospitalized individuals with moderate to severe COVID-19. Moderate-certainty evidence indicated that systemic JAK inhibitors probably decreased the risk of worsening clinical status and make little or no difference in the rate of adverse events of any grade, whilst they probably decrease the occurrence of serious adverse events (Table 2, Supplementary Material-Data in Brief). Because of its potent anti-inflammatory properties, Baricitinib was recommended by WHO for the treatment of patients with severe or critical COVID-19 on January 2022 [82]. Imatinib, another JAK inhibitor was tested in COUNTER-COVID and INVENT-COVID clinical trials and conferred clinical benefits in hospitalized patients with COVID-19, but further studies are required to validate these findings. Given the key role of the JAK-STAT pathway in immune responses, the use of JAK inhibitors may result in broad adverse effects. For example, the use of Baricitinib may delay viral clearance and increase the risk of secondary infections such as the herpes virus, due to its immunosuppressive activity and carries the risk of increased thromboembolic events [83]. Many lines of evidence suggest that SARS-CoV-2 infection may result in the down-regulation of JAK signaling in certain cell lines, and inhibition of JAK may promote viral infection [84]. In addition, a recent study showed that patients who have previously received treatment of JAK inhibitors Baricitinib, Tofacitinib, and Upadacitinib may show diminished responsiveness to COVID-19 vaccination [85]. Among the studied cases with different ages and different drug pre-treatments, older patients or patients treated with Upadacitinib tend to have higher non-response rates [85]. Although this aspect still requires further investigation, it raises an important concern that the use of immunosuppressive drugs may weaken the protection by vaccination. In summary, oral treatment with 4 mg/day up to 14 days of JAK inhibitors is effective in preventing SARS-CoV-2 progression and has the potential to decrease mortality in patients requiring low-flow oxygen but not in those with severe symptoms. JAK inhibitors are in general used in hospitals concomitantly with systemic delivered glucocorticoids.
5.3. Anti-cytokine therapy
To find safe and effective drugs to mitigate the cytokine storm, repurpose of the interleukin (IL) − 1 blockade agents is one of the safest ways to stop this innate immune response and may offer an important therapeutic option in the hyper-inflammatory phase of COVID-19 [86]. Therefore, drugs that block the IL-1 receptor, such as Anakinra, or drugs that block IL-1 signaling, such as Canakinumab, can potentially interrupt the vicious hyper-inflammatory loop. These drugs have been investigated in clinical trials as potential therapies for COVID-19 (Table 2, Supplementary Material-Data in Brief). Anakinra is a recombinant human IL-1 receptor antagonist. It is approved by the FDA to treat rheumatoid arthritis and neonatal-onset multisystem inflammatory disease. It is used “off-label” to treat severe chimeric antigen receptor T cell-mediated cytokine release syndrome and macrophage activation syndrome (MAS)/secondary hemophagocytic lymphohistiocytosis. Canakinumab is a human monoclonal antibody that targets the β-subunit of IL-1 and is approved by the FDA for the treatment of systemic juvenile idiopathic arthritis and Still’s diseases. In the SAVE and SAVE-MORE trials, hospitalized patients who had moderate or severe COVID-19 pneumonia and plasma-soluble urokinase plasminogen activator receptor (suPAR) levels ≥ 6 ng/mL were randomized to receive either Anakinra or placebo. The studies found that patients who received Anakinra had a lower risk of clinical progression of COVID-19, compared to those who received a placebo. CORIMUNO-ANA-1, a randomized controlled trial that compared the use of Anakinra to usual care in 116 hospitalized patients who were hypoxemic but did not require high-flow oxygen or ventilation, did not improve outcomes in patients with mild-to-moderate COVID-19 pneumonia [87]. Administration of Anakinra may be beneficial for treating severe COVID-19 patients with secondary hemophagocytic lymphohistocytosis (sHLH), but larger clinical studies are required to validate this concept [88]. After reviewing the results of the clinical trials and taking into consideration the fact that suPAR assays are not widely available to guide the use of Anakinra, FDA concluded that there is insufficient evidence to recommend either for or against the use of Anakinra for the treatment of COVID-19 in hospitalized patients.
CAN-COVID, a randomized controlled trial that evaluated Canakinumab in hospitalized patients with COVID-19 who were hypoxemic but did not require ventilator support, reported that the use of Canakinumab did not improve the likelihood of survival without invasive mechanical ventilation. Because of these results, FDA does not recommend Canakinumab for the treatment of COVID-19, except in a clinical trial (BIIa) (Table 2, Supplementary Material-Data in Brief).
IL-6 is a key cytokine in the pathogenesis of severe to critical SARS-CoV-2 infection and excessive IL-6 levels may lead to an imbalance in innate and adaptive immunity [89]. Therefore, IL-6 antagonism has an important role in the management of severe and critical phases of COVID-19. Sarilumab and Tocilizumab are human monoclonal antibodies binding and antagonizing soluble as well as membrane-bound interleukin-6 receptors, hindering IL-6 from exerting its pro-inflammatory effects. Multiple studies have examined the therapeutic efficacy of a single dose of 400 mg of Tocilizumab (8 mg/kg), or Sarilumab at 400 mg, delivered intravenously, in hospitalized patients with SARS-CoV-2, resulting in variable findings due to the administration of the drug at different stages of the disease process and variable severity of the disease. In CORIMUNO, REMAP-CAP, RECOVERY, and other clinical trials, Tocilizumab improved survival and other clinical outcomes in hospitalized COVID-19 patients with hypoxia and systemic inflammation. These benefits were seen regardless of the amount of respiratory support and were added to the benefits of systemic corticosteroids. Tocilizumab reduced all-cause mortality in all studies and reduced mechanical ventilation and length of stay in randomized controlled trials in hospitalized COVID-19 patients. Based on these findings it received approval from EUA FDA on June 2021 for therapy of COVID-19 (Table 2, Supplementary Material-Data in Brief). In REMAP-CAP, SARICOR, PROSPERO, Sarilumab improved outcomes, including survival in critically ill patients with COVID-19 receiving organ support in intensive care units. Meta-analysis of clinical trials of patients hospitalized for COVID-19 indicated that administration of IL-6 antagonists, compared with usual care or a placebo, was associated with lower 28-day all-cause mortality. COVID-19 guidelines recommend its use intravenously as an alternative to Tocilizumab. In conclusion, early use of IL-6 antagonists is beneficial for hypoxemic patients with COVID-19 requiring oxygen. The use of IL-6 receptor inhibitor therapy in patients on mechanical ventilation is most probably too late and unlikely to improve outcomes. IL-6 antagonists are used in hospitals in conjunction with corticosteroids.
Besides IL-1 and IL-6, other proinflammatory cytokines are involved in COVID-19-mediated cytokine storms and inflammation. An interesting investigational approach was to inhibit neutrophil recruitment to the lung by blocking the granulocyte- macrophage colony-stimulating factor (GM-CSF). Otilimab is an IgG1 monoclonal antibody that binds to GM-CSF and hinders its interaction with its receptor GM-CSFRα. Mavrilimumab is an IgG4 human monoclonal antibody targeting GM-CSF receptor-α and antagonizing its interaction with GM-CSF. In the double-blind randomized clinical trial OSCAR, the benefit-risk of a single infusion of Otilimab was investigated in the treatment of hospitalized participants with severe COVID-19 related pulmonary disease with new onset hypoxia requiring significant oxygen support or early invasive mechanical ventilation. A significant rate of survival without progression to respiratory failure at day 28 was recorded, in particular in patients aged 70 years or older compared to placebo (Table 2, Supplementary Material-Data in Brief). In several other clinical trials, Mavrilimumab treatment was associated with improved clinical outcomes compared with standard care in non-mechanically ventilated patients with severe COVID-19 pneumonia and systemic hyper-inflammation. However, the benefits for those patients remained uncertain, given the insufficient controlled studies and the small sample size. These studies call for additional clinical trials since the GM-CSF blockade strategy may have broad immunomodulatory effects given that it could affect the secretion of multiple pro-inflammatory cytokines and chemokines by myeloid cells. The GM-CSF-based therapies are worthwhile to be further pursued but in the design of the clinical trials care should be taken concerning dose, delivery route, timing of administration, and disease severity for each therapeutic approach [90].
Tumor necrosis factor α (TNFα), one of the pro-inflammatory cytokines commonly upregulated in acute lung injury, triggers cytokine storm and facilitates SARS-CoV-2 interaction with ACE2. TNF-α inhibitors, therefore, may serve as an effective therapeutic strategy for attenuating disease progression in severe SARS-CoV-2 infection [91]. These TNFα blocking agents have long been used in the clinical setting to treat inflammatory and autoimmune diseases and therefore, TNFα blockade can be achieved by monoclonal antibodies (such as Infliximab, Adalimumab, etc.), fusion proteins (Etanercept) and dominant negative proteins (INB03). Indeed several clinical trials are ongoing in patients with rheumatoid arthritis and intestinal bowel disorders with pulmonary complications due to COVID-19 infection. From the preliminary results, it appears that therapy with TNF-α inhibitors is associated with a lower probability of hospitalization and severe COVID-19 when compared to other treatments for an underlying inflammatory disease [92]. Allocetra-OTS is a cell-based therapeutic composed of donor early apoptotic cells, comprising allogeneic mononuclear enriched cell suspension with at least 40% early apoptotic cells that markedly improved the cytokine and chemokine storm and restored the impaired mitochondrial and glycolytic function in white blood cells leading to increased survival [93]. Definitely, cytokine-targeted therapy for COVID-19 represents an investigational attractive pharmacological approach that may further succeed by enrolling stratified patients based on the establishment of defined biomarkers for different phases of the disease.
5.4. Stimulation of antiviral defense by Interferons
Interferons are divided into type I (IFN-α and IFN-β), type II (IFN-γ), and type III (IFN-λ) and have a fundamental role in the innate immune system, being part of the first line of defense against viral infections. Differential induction of type I and III interferon genes in the upper respiratory tract of patients with COVID-19 were reported [94]. Therapy in clinical trials with recombinant super-compound interferon was associated with a shorter time of clinical improvement than IFN-α in the treatment of moderate-to-severe COVID-19 when combined with baseline antiviral agents. Treatment with IFN-α2b significantly reduced the duration of detectable virus in the upper respiratory tract and parallel reduced the duration of elevated blood levels for the inflammatory markers, and the average days of hospitalization were found lower (Table 2, Supplementary Material-Data in Brief). Early intervention with IFN-α, either within five days from the onset of symptoms or at hospital admission, confers better clinical outcomes [95]. Emerging data suggest that IFN-I-mediated boosting of patients’ immunity, achieved directly through the exogenous administration of IFN-β early post- viral infection, might play a therapeutic role against SARS-CoV-2 [96]. In SG016, UW-20–074, and other clinical trials, IFN-β does not appear to provide an increased survival benefit in hospitalized patients with COVID-19 but may help reduce the risk of intensive care admission. The addition of interferon-β to the standard of care resulted in a significant reduction in time to clinical improvement but no significant benefit in terms of reduction in mortality and length of hospital stay in moderate-to-severe cases of COVID-19. In several clinical case studies, a positive effect of IFNγ was noted on the rate of clinical stabilization and recovery of patients with community-acquired pneumonia and viral infections. IFNγ may be considered potential booster-adjuvant immunotherapy in a subset of immunocompromised COVID-19 patients, and concomitant IFNγ therapy may support antibiotic strategies. Presently, according to the COVID-19 treatment guidelines, based on the results from the present clinical trials, and concerning the lack of thorough evidence on the occurrence of adverse effects, the FDA recommends against the administration of interferons (α, β, and λ) in hospitalized and non-hospitalized patients, unless in the setting of a clinical trial.
6. Resistance to current pharmacological therapies in new SARS-CoV-2 variants
Since the beginning of the SARS-CoV-2 outbreak, the virus has been continuously adapting to its hosts and efforts have been made to continuously map its genome and to identify possible mutations that may offer the virus a selective advantage. The newly emerged strains can rapidly become the dominant variant all over the world due to their higher transmissibility and enhanced replication in human airway epithelial cells [97]. For example, the 501Y.V1 (B.1.1.7), 501Y.V2 (B.1.351) and the P.1 variants carry an N501Y mutation in the RBD of the spike protein that increases their binding affinity to ACE2 receptor, potentiating the virus transmission [98], [99]. The 501Y.V2 and P.1 variants have two additional mutations in the spike protein (E484K and K417N/P) that may not only further enhance binding affinity but also help the virus escape recognition affecting their neutralization by antibodies [99], [100]. The nearest emerged variant, Omicron, has 37 mutations on the spike gene, endowing it with much higher infectivity [101]. These changes indicate their ability to evade immunity generated in response to a previous infection or vaccination. Moreover, mutations on viral proteins that serve as key drug targets may lead to the loss of potency of current anti-viral therapies. It was reported that both monoclonal antibodies and convalescent sera showed low or no neutralizing activity against the Omicron variant. [102]. A Pfizer booster dose could successfully elicit neutralization activity against the omicron variant, though the neutralization efficiency is much lower than that against the D614G and Delta variants [102]. A recent study tested the efficiency of several monoclonal antibodies and antiviral drugs on the omicron variant as well as a number of other earlier variants. Among the tested monoclonal antibodies, Casirivimab, Tixagevimab, Cilgavimab, and Sotrovimab showed remarkably reduced neutralizing activity against the Omicron variant, characterized by much higher FRNT50 values [103]. Strikingly, the Omicron variant was almost completely resistant to neutralization by Etesevimab, Bamlanivimab, and Imdevimab. Cochrane COVID-19 study register reviewed the randomized controlled trials that evaluated SARS-CoV-2-neutralizing monoclonal antibodies, including fragments, alone or combined, versus an active comparator, placebo, or no intervention, for pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) of COVID-19 [104]. For PrEP, there is a decrease in the development of clinical COVID-19 symptoms (high certainty), infection with SARS-CoV-2 (moderate certainty), and admission to the hospital (low certainty) with Tixagevimab/Cilgavimab. There is low certainty of a decrease in infection with SARS-CoV-2, and development of clinical COVID-19 symptoms, and a higher rate for all-grade adverse effects with Casirivimab/Imdevimab. The Omicron variant also carries mutations on RdRp and the main protease of SARS-CoV-2, indicating that it may escape from current antiviral treatments [105]. To test this possibility, Takashita et al. tested Remdesivir, Molnupiravir, and PF-07304814 on the Omicron variant and found that in vitro the inhibitory concentration of 50% values of these three compounds against the Omicron variant were similar to those against the early strains, indicating that they may still be effective [103]. However, the effectiveness has not yet been verified by clinical studies. Also, the current anti-viral therapies are still at risk of losing potency as the viruses are continuously mutating.
7. Prospective of drug development against COVID-19
COVID-19 has already resulted in millions of deaths worldwide, and it is essential to develop efficient therapies as quickly as possible. Rapid progress has been made to understand the precise machinery of viral infection and immune responses. The SARS-CoV-2 infection leads to dysregulation of immune pathways. Therapies focusing on suppressing cytokine activity demonstrate some success in clinical trials and case reports. Current evidence supports the use of Dexamethasone in hospitalized patients requiring oxygen. Early use of IL-6 inhibitors is beneficial in hypoxemic patients and CRP level can serve to identify patients who may benefit from IL-6 inhibitors. JAK inhibition in combination with glucocorticoids is emerging as a potential therapeutic option for patients with moderate to severe symptoms. However, antiviral therapy is not effective in hospitalized patients with severe symptoms. The clinical data on Anakinra, hyperimmune immunoglobulin/convalescent plasma are yet limited and inconclusive. Although more mechanistic studies are still needed to identify potential therapeutic targets, developing new drugs based on existing knowledge should be a promising solution at the current stage. Conventional methods include structure-based drug design, which is a highly validated method that has been previously used in developing anti-viral drugs against HIV-1 and MERS-CoV [106], [107]. Artificial intelligence-based drug design is a newly emerged approach that has been recently used to discover novel drugs based on machine and deep and reinforcement learning algorithms [108], [109]. Combining these drug design approaches with virtual drug screening and high-throughput screening approaches could greatly aid in the process of developing novel lead compounds for COVID-19 therapy [110]. Nevertheless, developing a new drug could take years, and quicker approaches must be taken to combat this devastating pandemic. Repurposing pre-existing drugs is a quick solution to this developing demand, as their dosage, safety, and mechanism of action are already well established. Drug repurposing against COVID-19 has been successful by using cell-based or computational screening. For example, Remdesivir is a repurposed anti-viral drug and has become an FDA-approved drug for treating COVID-19 [111]. However, most of the drug repurposing is still at the computational level, lacking experimental validation and requiring investigational clinical trials [112]. As the lethal cytokine storm may occur in advanced-stage COVID-19 patients, early detection of viral infection, the establishment of valid clinical biomarkers, and treatment with anti-viral drugs are essential. However, anti-viral therapies are at risk of losing potency because the targeted viral proteins are continuously mutating. A combination of anti-viral therapies with a different mechanism of action is a solution to the possible occurrence of strains resistant to the certain anti-viral drugs. In addition, developing broad-spectrum antiviral drugs is crucial for not only controlling the ongoing COVID-19 pandemic but also the possible outbreak of new viruses in the future. Immunotherapy in COVID-19 needs to be further progressed by clinical trials to establish solid knowledge and clinical experience and to determine the optimal patient-directed strategy based on validated biomarkers of the different phases of disease severity. The development of immunotherapies approaches must take into consideration the immune heterogeneity of clinically-similar COVID-19 patients and their incorporation in the clinic will help both in therapy decisions as well for application in other severe viral infections.
Funding
This research was supported by National Natural Science Foundation of China (32070969), The Science and Technology Development Fund, Macau SAR (File No. 0127/2019/A3, 0113/2018/A3 and 0038/2020/AMJ), The Guangdong Provincial Funding Committee for Basic and Applied Fundamental Research (2022-Natural Science Foundation), the University of Macau (File No. MYRG2018-00134-FHS and MYRG2020-00158-FHS). PL holds the Jacob Gitlin Chair in Physiology and is affiliated and supported by David R. Bloom Center for Pharmacy, Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics, and the Grass Center for Drug Design and Synthesis of Novel Therapeutics at the Hebrew University of Jerusalem, Israel.
CRediT authorship contribution statement
L.R. and Y.J. wrote the manuscript. Z.Z., R.L., S.H., P.L., H.Z., Q. S. and W.Z. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Yizhou Jiang obtained his bachelor's degree from Southern University of Science and Technology (SUSTech) in 2017. He is currently a SUSTech-University of Macau joint Ph. D. student. His major research interests are aging and neurobiology.

Limor Rubin obtained her M.D. degree in 2015 from the Hebrew University of Jerusalem. Thereafter, she completed her residency in Internal Medicine at Hadassah-Hebrew University and from 2020, she is performing a fellowship in Allergy and Clinical Immunology. Present position: Senior Physician, Internal Medicine Department B, Hadassah-Hebrew University Medical Center, Jerusalem, Israel. Main research fields: internal medicine, respiratory diseases, allergy, asthma, common variable immunodeficiency, COVID − 19 immune responses, diagnosis and therapy.

Dr. Qiaozhu Su is an Associate Professor in the School of Biological Sciences at Queen’s University Belfast in the United Kingdom. She leads a research team to decipher the molecular mechanisms linking redox signalling (e.g., CREBH/ERK) with metabolic syndrome and the parasympathetic nervous system in lipid metabolism and insulin resistance. Dr. Su’s research program has been supported by funding from NIH and USDA in the USA, BHF and NICHS in the UK.

Lazarovici Philip. Obtained his Ph.D. degree in pharmacology and toxicology 1980 from The Hebrew University, post graduated 1984 on neurobiology at the Weizmann Institute of Science, Israel and was a visiting scientist from 1984 to 1988 in the Section of Growth Factors at the National Institutes of Child Health and Human Development, NIH, Bethesda, USA. He was a Fogorty visiting scientist at NIH (1990–1998), Drexel University (2004–2011) and Temple University (2012–2015), Philadelphia, USA. Present position: Professor Emeritus, Jacob Gitlin Chair in Physiology, School of Pharmacy, The Hebrew University of Jerusalem, Israel. Main research fields: neuronal and non-neuronal pharmacological activities of nerve growth factor, trkA, p75NTR and α9β1-integrin signaling and role in neuroprotection, angiogenesis and tumor development; toxins, lead compounds, drug discovery and development, cell therapy, stem cells, neurological disorders.

Wenhua Zheng is a professor at University of Macau, Faculty of Health Sciences. Main research fields: neuropharmacology; aging, neuronal degenerative disorders including Alzheimer’s disease and degenerative retinal diseases; molecular mechanisms of neuronal cell death; drug development.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.cytogfr.2022.10.003.
Appendix A. Supplementary material
Supplementary material
References
- 1.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y., Rubin L., Peng T., Liu L., Xing X., Lazarovici P., Zheng W. Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. Int J. Biol. Sci. 2022;18(2):459–472. doi: 10.7150/ijbs.59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel Y., Mizrahi O., Nachshon A., Weingarten-Gabbay S., Morgenstern D., Yahalom-Ronen Y., Tamir H., Achdout H., Stein D., Israeli O., Beth-Din A., Melamed S., Weiss S., Israely T., Paran N., Schwartz M., Stern-Ginossar N. The coding capacity of SARS-CoV-2. Nature. 2021;589(7840):125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- 4.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102(22):7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portales A.E., Mustafa E.R., McCarthy C.I., Cornejo M.P., Couto P.M., Gironacci M.M., Caramelo J.J., Perello M., Raingo J. ACE2 internalization induced by a SARS-CoV-2 recombinant protein is modulated by angiotensin II type 1 and bradykinin 2 receptors. Life Sci. 2022;293 doi: 10.1016/j.lfs.2021.120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katopodis P., Randeva H.S., Spandidos D.A., Saravi S., Kyrou I., Karteris E. Host cell entry mediators implicated in the cellular tropism of SARSCoV2, the pathophysiology of COVID19 and the identification of microRNAs that can modulate the expression of these mediators (Review) Int. J. Mol. Med. 2022;49(2) doi: 10.3892/ijmm.2021.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., Group H.U.S.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodriguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh U.K. Across speciality collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consortium W.H.O.S.T., Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernandez Garcia C., Kieny M.P., Malekzadeh R., Murthy S., Reddy K.S., Roses Periago M., Abi Hanna P., Ader F., Al-Bader A.M., Alhasawi A., Allum E., Alotaibi A., Alvarez-Moreno C.A., Appadoo S., Asiri A., Aukrust P., Barratt-Due A., Bellani S., Branca M., Cappel-Porter H.B.C., Cerrato N., Chow T.S., Como N., Eustace J., Garcia P.J., Godbole S., Gotuzzo E., Griskevicius L., Hamra R., Hassan M., Hassany M., Hutton D., Irmansyah I., Jancoriene L., Kirwan J., Kumar S., Lennon P., Lopardo G., Lydon P., Magrini N., Maguire T., Manevska S., Manuel O., McGinty S., Medina M.T., Mesa Rubio M.L., Miranda-Montoya M.C., Nel J., Nunes E.P., Perola M., Portoles A., Rasmin M.R., Raza A., Rees H., Reges P.P.S., Rogers C.A., Salami K., Salvadori M.I., Sinani N., Sterne J.A.C., Stevanovikj M., Tacconelli E., Tikkinen K.A.O., Trelle S., Zaid H., Rottingen J.A., Swaminathan S. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group R.C., Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., Whitehouse T., Felton T., Williams J., Faccenda J., Underwood J., Baillie J.K., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Lim W.S., Montgomery A., Rowan K., Tarning J., Watson J.A., White N.J., Juszczak E., Haynes R., Landray M.J. Effect of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH, COVID-19 Treatment Guidelines. 〈https://www.covid19treatmentguidelines.nih.gov/〉, 2022 (Accessed 22. 07.09).
- 18.WHO, Therapeutics and COVID-19: living guideline. 〈https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4〉, 2022 (Accessed 22. 09.13).
- 19.Alexandridi M., Mazej J., Palermo E., Hiscott J. The Coronavirus pandemic - 2022: viruses, variants & vaccines. Cytokine Growth Factor Rev. 2022;63:1–9. doi: 10.1016/j.cytogfr.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int. J. Infect. Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhar B.K., Nehira J., Malhotra A., Kotchoni S.O. The race for COVID-19 vaccines: the various types and their strengths and weaknesses. J. Pharm. Pract. 2022 doi: 10.1177/08971900221097248. 8971900221097248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh D., Singh E. An overview of the neurological aspects in COVID-19 infection. J. Chem. Neuroanat. 2022;122 doi: 10.1016/j.jchemneu.2022.102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J., Ushay H.M., Cabana M.D., Medar S.S. Clinical characteristics and outcomes of hospitalized and critically Ill Children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J. Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020;80(6):e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., Yeung P., Chan W.M., Wu A.K., Lung K.C., Tsang O.T., Leung W.S., Hung I.F., Yuen K.Y., Chen Z. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–877. doi: 10.1016/j.immuni.2020.07.026. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 30.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R., Lan Z., Ye J., Pang L., Liu Y., Wu W., Qin X., Guo Y., Zhang P. Cytokine storm: the primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marnell L., Mold C., Du Clos T.W. C-reactive protein: ligands, receptors and role in inflammation. Clin. Immunol. 2005;117(2):104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Farmakis I.T., Minopoulou I., Giannakoulas G., Boutou A. Cardiotoxicity of azithromycin in COVID-19: an overall proportion meta-analysis. Adv. Respir. Med. 2022 doi: 10.5603/ARM.a2022.0022. [DOI] [PubMed] [Google Scholar]
- 34.Sivapalan P., Ulrik C.S., Lapperre T.S., Bojesen R.D., Eklof J., Browatzki A., Wilcke J.T., Gottlieb V., Hakansson K.E.J., Tidemandsen C., Tupper O., Meteran H., Bergsoe C., Brondum E., Bodtger U., Bech Rasmussen D., Graff Jensen S., Pedersen L., Jordan A., Prieme H., Soborg C., Steffensen I.E., Hogsberg D., Klausen T.W., Frydland M.S., Lange P., Sverrild A., Ghanizada M., Knop F.K., Biering-Sorensen T., Lundgren J.D., Jensen J.S., Pro P.A.C.CwgobotP.-C.S.G. Azithromycin and hydroxychloroquine in hospitalised patients with confirmed COVID-19: a randomised double-blinded placebo-controlled trial. Eur. Respir. J. 2022;59(1) doi: 10.1183/13993003.00752-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batista D.R., Floriano I., Silvinato A., Bacha H.A., Barbosa A.N., Tanni S.E., Bernardo W.M. Use of anticoagulants in patients with COVID-19: a living systematic review and meta-analysis. J. Bras. Pneumol. 2022;48(4) doi: 10.36416/1806-3756/e20220041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munch M.W., Meyhoff T.S., Helleberg M., Kjaer M.N., Granholm A., Hjortso C.J.S., Jensen T.S., Moller M.H., Hjortrup P.B., Wetterslev M., Vesterlund G.K., Russell L., Jorgensen V.L., Kristiansen K.T., Benfield T., Ulrik C.S., Andreasen A.S., Bestle M.H., Poulsen L.M., Hildebrandt T., Knudsen L.S., Moller A., Solling C.G., Brochner A.C., Rasmussen B.S., Nielsen H., Christensen S., Strom T., Cronhjort M., Wahlin R.R., Jakob S.M., Cioccari L., Venkatesh B., Hammond N., Jha V., Myatra S.N., Jensen M.Q., Leistner J.W., Mikkelsen V.S., Svenningsen J.S., Laursen S.B., Hatley E.V., Kristensen C.M., Al-Alak A., Clapp E., Jonassen T.B., Bjerregaard C.L., Osterby N.C.H., Jespersen M.M., Abou-Kassem D., Lassen M.L., Zaabalawi R., Daoud M.M., Abdi S., Meier N., la Cour K., Derby C.B., Damlund B.R., Laigaard J., Andersen L.L., Mikkelsen J., Jensen J.L.S., Rasmussen A.H., Arnerlov E., Lykke M., Holst-Hansen M.Z.B., Tostesen B.W., Schwab J., Madsen E.K., Gluud C., Lange T., Perner A. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol. Scand. 2021;65(10):1421–1430. doi: 10.1111/aas.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidahic M., Nujic D., Civljak M., Runjic R., Markotic F., Vidak M., Puljak L. Standard of care for COVID-19 in randomized clinical trials registered in trial registries and published in preprint servers and scholarly journals: a cross-sectional study. BMC Med. Res. Methodol. 2022;22(1):173. doi: 10.1186/s12874-022-01646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J.D., Meng W., Wang X.J., Wang H.C. Broad-spectrum antiviral agents. Front Microbiol. 2015;6:517. doi: 10.3389/fmicb.2015.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenig A., Ishay Y., Kharouf F., Rubin L. Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasma-based products. Clin. Immunol. 2021;227 doi: 10.1016/j.clim.2021.108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piechotta V., Iannizzi C., Chai K.L., Valk S.J., Kimber C., Dorando E., Monsef I., Wood E.M., Lamikanra A.A., Roberts D.J., McQuilten Z., So-Osman C., Estcourt L.J., Skoetz N. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst. Rev. 2021;5 doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein F.C., Levin H.S. Epidemiology of pediatric closed head injury: incidence, clinical characteristics, and risk factors. J. Learn. Disabil. 1987;20(9):518–525. doi: 10.1177/002221948702000903. [DOI] [PubMed] [Google Scholar]
- 42.Focosi D., Franchini M., Tuccori M., Cruciani M. Efficacy of high-dose polyclonal intravenous immunoglobulin in COVID-19: a systematic review. Vaccines. 2022;10(1) doi: 10.3390/vaccines10010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross L.Z.F., Sacerdoti M., Piiper A., Zeuzem S., Leroux A.E., Biondi R.M. ACE2, the receptor that enables infection by SARS-CoV-2: biochemistry, structure, allostery and evaluation of the potential development of ACE2 modulators. Chemmedchem. 2020;15(18):1682–1690. doi: 10.1002/cmdc.202000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saponaro F., Rutigliano G., Sestito S., Bandini L., Storti B., Bizzarri R., Zucchi R. ACE2 in the era of SARS-CoV-2: controversies and novel perspectives. Front Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.588618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C., Neuhold S., Haider D., Stiasny K., Bergthaler A., Puchhammer-Stoeckl E., Mirazimi A., Montserrat N., Zhang H., Slutsky A.S., Penninger J.M. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8(11):1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choudhary S., Malik Y.S., Tomar S. Identification of SARS-CoV-2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach. Front Immunol. 2020;11:1664. doi: 10.3389/fimmu.2020.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsegay K.B., Adeyemi C.M., Gniffke E.P., Sather D.N., Walker J.K., Smith S.E.P., Repurposed A. Drug screen identifies compounds that inhibit the binding of the COVID-19 spike protein to ACE2. Front Pharm. 2021;12 doi: 10.3389/fphar.2021.685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin C., Li Y., Zhang Y., Liu Z., Mu X., Gu C., Liu J., Li Y., Li G., Chen J. Ceftazidime is a potential drug to inhibit SARS-CoV-2 infection in vitro by blocking spike protein-ACE2 interaction. Signal Transduct. Target Ther. 2021;6(1):198. doi: 10.1038/s41392-021-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G., Yang M.L., Duan Z.L., Liu F.L., Jin L., Long C.B., Zhang M., Tang X.P., Xu L., Li Y.C., Kamau P.M., Yang L., Liu H.Q., Xu J.W., Chen J.K., Zheng Y.T., Peng X.Z., Lai R. Dalbavancin binds ACE2 to block its interaction with SARS-CoV-2 spike protein and is effective in inhibiting SARS-CoV-2 infection in animal models. Cell Res. 2021;31(1):17–24. doi: 10.1038/s41422-020-00450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin J., Wang C., Song X., Li X., Miao M. Effects of renin-angiotensin system inhibitors on mortality and disease severity of COVID-19 patients: a meta-analysis of randomized controlled trials. Am. J. Hypertens. 2022;35(5):462–469. doi: 10.1093/ajh/hpac001. [DOI] [PubMed] [Google Scholar]
- 51.Sato K., White N., Fanning J.P., Obonyo N., Yamashita M.H., Appadurai V., Ciullo A., May M., Worku E.T., Helms L., Ohshimo S., Juzar D.A., Suen J.Y., Bassi G.L., Fraser J.F., Arora R.C. C.-C.C.C. Investigators, Impact of renin-angiotensin-aldosterone system inhibition on mortality in critically ill COVID-19 patients with pre-existing hypertension: a prospective cohort study. BMC Cardiovasc. Disord. 2022;22(1):123. doi: 10.1186/s12872-022-02565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breining P., Frolund A.L., Hojen J.F., Gunst J.D., Staerke N.B., Saedder E., Cases-Thomas M., Little P., Nielsen L.P., Sogaard O.S., Kjolby M. Camostat mesylate against SARS-CoV-2 and COVID-19-Rationale, dosing and safety. Basic Clin. Pharmacol. Toxicol. 2021;128(2):204–212. doi: 10.1111/bcpt.13533. [DOI] [PubMed] [Google Scholar]
- 53.Mantzourani C., Vasilakaki S., Gerogianni V.E., Kokotos G. The discovery and development of transmembrane serine protease 2 (TMPRSS2) inhibitors as candidate drugs for the treatment of COVID-19. Expert Opin. Drug Disco. 2022;17(3):231–246. doi: 10.1080/17460441.2022.2029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrezenmeier E., Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 55.Yu B., Li C., Chen P., Zhou N., Wang L., Li J., Jiang H., Wang D.W. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci. China Life Sci. 2020;63(10):1515–1521. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y., Li M.X., Lu G.D., Shen H.M., Zhou J. Hydroxychloroquine/chloroquine as therapeutics for COVID-19: truth under the mystery. Int J. Biol. Sci. 2021;17(6):1538–1546. doi: 10.7150/ijbs.59547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Hobartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12(1):279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FDA, Know Your Treatment Options for COVID-19. 〈https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19〉, 2022 (Accessed 22. 05.19).
- 59.Eweas A.F., Alhossary A.A., Abdel-Moneim A.S. Molecular docking reveals ivermectin and remdesivir as potential repurposed drugs against SARS-CoV-2. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.592908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall D.C., Jr., Ji H.F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deshpande R.R., Tiwari A.P., Nyayanit N., Modak M. In silico molecular docking analysis for repurposing therapeutics against multiple proteins from SARS-CoV-2. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young B., Tan T.T., Leo Y.S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 2021;21(1):20–21. doi: 10.1016/S1473-3099(20)30911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Consortium W.H.O.S.T. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941–1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh A.K., Singh A., Singh R., Misra A. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes Metab. Syndr. 2022;16(2) doi: 10.1016/j.dsx.2022.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumari P., Rawat K., Saha L. Pipeline pharmacological therapies in clinical trial for COVID-19 Pandemic: a recent update. Curr. Pharm. Rep. 2020;6(5):228–240. doi: 10.1007/s40495-020-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertha B.G., Folts J.D. Phasic mitral blood flow and regional left ventricular dimensions: possible mechanism of active assist to ventricular filling. Circulation. 1986;74(4):901–911. doi: 10.1161/01.cir.74.4.901. [DOI] [PubMed] [Google Scholar]
- 67.Coperchini F., Chiovato L., Ricci G., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021;58:82–91. doi: 10.1016/j.cytogfr.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van de Veerdonk F.L., Giamarellos-Bourboulis E., Pickkers P., Derde L., Leavis H., van Crevel R., Engel J.J., Wiersinga W.J., Vlaar A.P.J., Shankar-Hari M., van der Poll T., Bonten M., Angus D.C., van der Meer J.W.M., Netea M.G. A guide to immunotherapy for COVID-19. Nat. Med. 2022;28(1):39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 69.El-Saber Batiha G., Al-Gareeb A.I., Saad H.M., Al-Kuraishy H.M. COVID-19 and corticosteroids: a narrative review. Inflammopharmacology. 2022 doi: 10.1007/s10787-022-00987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore J.B. C.H. June, Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 71.Yang R., Yu Y. Glucocorticoids are double-edged sword in the treatment of COVID-19 and cancers. Int J. Biol. Sci. 2021;17(6):1530–1537. doi: 10.7150/ijbs.58695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solinas C., Perra L., Aiello M., Migliori E., Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 2020;54:8–23. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karn M., Yonghang S., Ghimire S. Corticosteroids in COVID-19: we should be mindful of their acute toxicities. J. Clin. Pharmacol. 2021;61(10):1301–1302. doi: 10.1002/jcph.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grant A.H., Estrada A., 3rd, Ayala-Marin Y.M., Alvidrez-Camacho A.Y., Rodriguez G., Robles-Escajeda E., Cadena-Medina D.A., Rodriguez A.C., Kirken R.A. The Many Faces of JAKs and STATs Within the COVID-19 Storm. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.690477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasselbalch H.C., Skov V., Kjaer L., Ellervik C., Poulsen A., Poulsen T.D., Nielsen C.H. COVID-19 as a mediator of interferon deficiency and hyperinflammation: Rationale for the use of JAK1/2 inhibitors in combination with interferon. Cytokine Growth Factor Rev. 2021;60:28–45. doi: 10.1016/j.cytogfr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2021;133(5):489–507. doi: 10.1080/00325481.2020.1855921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciechanowicz P., Rakowska A., Sikora M., Rudnicka L. JAK-inhibitors in dermatology: current evidence and future applications. J. Dermatol. Treat. 2019;30(7):648–658. doi: 10.1080/09546634.2018.1546043. [DOI] [PubMed] [Google Scholar]
- 78.Jamilloux Y., El Jammal T., Vuitton L., Gerfaud-Valentin M., Kerever S., Seve P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102390. [DOI] [PubMed] [Google Scholar]
- 79.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J., Monteil V., Lauschke V.M., Mirazimi A., Youhanna S., Tan Y.J., Baldanti F., Sarasini A., Terres J.A.R., Nickoloff B.J., Higgs R.E., Rocha G., Byers N.L., Schlichting D.E., Nirula A., Cardoso A., Corbellino M., Sacco Baricitinib Study G. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020;12(8) doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen C.X., Wang J.J., Li H., Yuan L.T., Gale R.P., Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35(9):2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO, WHO recommends two new drugs to treat COVID-19. 〈https://www.who.int/news/item/14–01-2022-who-recommends-two-new-drugs-to-treat-covid-19〉, 2022 (Accessed 22. 05.19).
- 83.Jorgensen S.C.J., Tse C.L.Y., Burry L., Dresser L.D. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ravid J.D., Leiva O., Chitalia V.C. Janus kinase signaling pathway and its role in COVID-19 inflammatory, vascular, and thrombotic manifestations. Cells. 2022;11(2) doi: 10.3390/cells11020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seror R., Camus M., Salmon J.H., Roux C., Dernis E., Basch A., Germain V., Leske C., Brousseau S., Truchetet M.E., Ramon A., Gottenberg J.E., Felten R., Coury F., Colombey A., Prati C., Mariette X., Avouac J. Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry. Lancet Rheuma. 2022;4(1):e8–e11. doi: 10.1016/S2665-9913(21)00314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geng J., Wang F., Huang Z., Chen X., Wang Y. Perspectives on anti-IL-1 inhibitors as potential therapeutic interventions for severe COVID-19. Cytokine. 2021;143 doi: 10.1016/j.cyto.2021.155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.C.-C. group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir. Med. 2021;9(3):295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M.G., Spyridopoulos T., Verheggen R.J., Hoogerwerf J., Lachana A., van de Veerdonk F.L., Giamarellos-Bourboulis E.J. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host Microbe. 2020;28(1):117–123. doi: 10.1016/j.chom.2020.05.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carsetti R., Zaffina S., Piano Mortari E., Terreri S., Corrente F., Capponi C., Palomba P., Mirabella M., Cascioli S., Palange P., Cuccaro I., Milito C., Zumla A., Maeurer M., Camisa V., Vinci M.R., Santoro A., Cimini E., Marchioni L., Nicastri E., Palmieri F., Agrati C., Ippolito G., Porzio O., Concato C., Onetti Muda A., Raponi M., Quintarelli C., Quinti I., Locatelli F. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lang F.M., Lee K.M., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20(8):507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo Y., Hu K., Li Y., Lu C., Ling K., Cai C., Wang W., Ye D. Targeting TNF-alpha for COVID-19: recent advanced and controversies. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.833967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kokkotis G., Kitsou K., Xynogalas I., Spoulou V., Magiorkinis G., Trontzas I., Trontzas P., Poulakou G., Syrigos K., Bamias G. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment. Pharmacol. Ther. 2022;55(2):154–167. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]
- 93.Karbian N., Abutbul A., El-Amore R., Eliaz R., Beeri R., Reicher B., Mevorach D. Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis. 2020;11(7):535. doi: 10.1038/s41419-020-02748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scagnolari C., Pierangeli A., Frasca F., Bitossi C., Viscido A., Oliveto G., Scordio M., Mazzuti L., Di Carlo D., Gentile M., Solimini A., Ceccarelli G., Pugliese F., Mastroianni C.M., d'Ettorre G., Turriziani O., Antonelli G. Virology, C.-S.G.o.S.U. Infectious Diseases, Differential induction of type I and III interferon genes in the upper respiratory tract of patients with coronavirus disease 2019 (COVID-19) Virus Res. 2021;295 doi: 10.1016/j.virusres.2020.198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu L.Y., Feng P.H., Yu M.S., Chen M.C., Lin A.J., Chen J.L., Yu L.H. Current utilization of interferon alpha for the treatment of coronavirus disease 2019: A comprehensive review. Cytokine Growth Factor Rev. 2022;63:34–43. doi: 10.1016/j.cytogfr.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arico E., Bracci L., Castiello L., Urbani F., Casanova J.L., Belardelli F. Exploiting natural antiviral immunity for the control of pandemics: Lessons from Covid-19. Cytokine Growth Factor Rev. 2022;63:23–33. doi: 10.1016/j.cytogfr.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Sheffield C.-G.G., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking CHanges in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Group C.C.-W., Consortium C.-G.U., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tragni V., Preziusi F., Laera L., Onofrio A., Mercurio I., Todisco S., Volpicella M., De Grassi A., Pierri C.L. Modeling SARS-CoV-2 spike/ACE2 protein-protein interactions for predicting the binding affinity of new spike variants for ACE2, and novel ACE2 structurally related human protein targets, for COVID-19 handling in the 3PM context. EPMA J. 2022:1–27. doi: 10.1007/s13167-021-00267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.H., Michailidis E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Luchsinger L., Hillyer C.D., Caskey M., Robbiani D.F., Rice C.M., Nussenzweig M.C., Hatziioannou T., Bieniasz P.D. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D. Mannar, J.W. Saville, X. Zhu, S.S. Srivastava, A.M. Berezuk, K.S. Tuttle, C. Marquez, I. Sekirov, S. Subramaniam, SARS-CoV-2 Omicron Variant: ACE2 Binding, Cryo-EM Structure of Spike Protein-ACE2 Complex and Antibody Evasion, bioRxiv (2021) 2021.12.19.473380. [DOI] [PMC free article] [PubMed]
- 102.Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.H., Porrot F., Staropoli I., Lemoine F., Pere H., Veyer D., Puech J., Rodary J., Baele G., Dellicour S., Raymenants J., Gorissen S., Geenen C., Vanmechelen B., Wawina-Bokalanga T., Marti-Carreras J., Cuypers L., Seve A., Hocqueloux L., Prazuck T., Rey F.A., Simon-Loriere E., Bruel T., Mouquet H., Andre E., Schwartz O. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021 doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 103.Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., Iwatsuki-Horimoto K., Chiba S., Halfmann P., Nagai H., Saito M., Adachi E., Sullivan D., Pekosz A., Watanabe S., Maeda K., Imai M., Yotsuyanagi H., Mitsuya H., Ohmagari N., Takeda M., Hasegawa H., Kawaoka Y. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022 doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirsch C., Park Y.S., Piechotta V., Chai K.L., Estcourt L.J., Monsef I., Salomon S., Wood E.M., So-Osman C., McQuilten Z., Spinner C.D., Malin J.J., Stegemann M., Skoetz N., Kreuzberger N. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Syst. Rev. 2022;6 doi: 10.1002/14651858.CD014945.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.K. Bansal, S. Kumar, Mutational cascade of SARS-CoV-2 leading to evolution and emergence of omicron variant, bioRxiv (2021) 2021.12.06.471389. [DOI] [PMC free article] [PubMed]
- 106.Ghosh A.K., Sridhar P.R., Leshchenko S., Hussain A.K., Li J., Kovalevsky A.Y., Walters D.E., Wedekind J.E., Grum-Tokars V., Das D., Koh Y., Maeda K., Gatanaga H., Weber I.T., Mitsuya H. Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance. J. Med. Chem. 2006;49(17):5252–5261. doi: 10.1021/jm060561m. [DOI] [PubMed] [Google Scholar]
- 107.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J., Zhang Y., Nie W., Luo Y., Deng L. Computational anti-COVID-19 drug design: progress and challenges. Brief. Bioinform. 2022;23(1) doi: 10.1093/bib/bbab484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouchlis V.D., Afantitis A., Serra A., Fratello M., Papadiamantis A.G., Aidinis V., Lynch I., Greco D., Melagraki G. Advances in de Novo drug design: from conventional to machine learning methods. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bajad N.G., Rayala S., Gutti G., Sharma A., Singh M., Kumar A., Singh S.K. Systematic review on role of structure based drug design (SBDD) in the identification of anti-viral leads against SARS-Cov-2. Curr. Res Pharm. Drug Disco. 2021;2 doi: 10.1016/j.crphar.2021.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simonis A., Theobald S.J., Fatkenheuer G., Rybniker J., Malin J.J. A comparative analysis of remdesivir and other repurposed antivirals against SARS-CoV-2. EMBO Mol. Med. 2021;13(1) doi: 10.15252/emmm.202013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cusinato J., Cau Y., Calvani A.M., Mori M. Repurposing drugs for the management of COVID-19. Expert Opin. Ther. Pat. 2021;31(4):295–307. doi: 10.1080/13543776.2021.1861248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material