Abstract
Cryotherapy is one of the most common treatments for warts; however, pain during treatment and relatively high recurrence rates limit its use. Local hyperthermia has also been used successfully in the treatment of plantar warts. The aim of this study was to compare the clinical effectiveness of local hyperthermia vs cryotherapy for the treatment of plantar warts. This multi-centre, open, 2-arm, non-randomized concurrent controlled trial included 1,027 patients, who received either cryotherapy or local hyperthermia treatment. Three months after treatment, local hyperthermia and cryotherapy achieved complete clearance rates of 50.9% and 54.3%, respectively. Recurrence rates were 0.8% and 12%, respectively. Pain scores during local hyperthermia were significantly lower than for cryotherapy. Both local hyperthermia and cryotherapy demonstrated similar efficacy for clearance of plantar warts; while local hyperthermia had a lower recurrence rate and lower pain sensation during treatment.
Key words: local hyperthermia, cryotherapy, warts, clinical trial
Plantar warts are a common skin disease, caused by human papillomavirus (HPV), which results in warty lesions on the feet. There are many treatment options, such as destructive therapy, virucidal therapy, antimitotic therapy, immunotherapy, etc. (1). Although most plantar warts disappear spontaneously without treatment over a period of years, many patients seek treatment due to discomfort or because they are prevented from performing sports and other activities of daily living. The annual cost of managing the condition has been reported to be at least £40 million in England and Wales (2).
SIGNIFICANCE
Plantar warts are a common skin disease worldwide, resulting in warty lesions on the foot. Many treatment options have been used, with varying efficacies. Cryotherapy is a conventional treatment for plantar warts; however, pain during treatment and a relatively high recurrence rate limit its use. Local hyperthermia has been used successfully in the treatment of warts, and is almost painless. This study demonstrates the efficacy and safety of local hyperthermia and cryotherapy in the treatment of plantar warts. Local hyperthermia had similar efficacy, but a lower recurrence rate and lower pain sensation, compared with cryotherapy.
Hyperthermia, with a temperature of 39–48°C, has been used in the treatment of some neoplasms (3). Local hyperthermia in the treatment of warts achieved cure rates ranging from 56% to 79.2%. There are many studies with different temperatures, from 40°C to 150°C, and different protocols, such as successive, intermittent hyperthermia, etc. (4–6). Our previous open trial in patients with plantar warts attained a 65.3% (15/23) cure rate using local hyperthermia at 45.3°C (7). The subsequent randomized, patient-blinded, sham treatment-controlled trial of local hyperthermia at a mean temperature of 44°C attained a 53.6% cure rate (15/28) in the treatment group and 11.5% (3/26) in the sham treatment group at 3-month follow-up. The adverse effects were mild, including a tolerable burning sensation, and 2 cases of heat-induced bullae (8).
The cellular immune response plays an important role in the elimination of HPV-infected keratinocytes. Subjected to heat at 44°C, Langerhans cells (LCs) migrational maturation could be promoted in both normal and HPV-infected skin compared with at 42°C. In addition, the antigen-presenting capability of LCs might be augmented by hyperthermia due to a higher percentage of migratory LCs with mature phenotype (9). Yoshioka et al. (10) further observed that the decrease in epidermal LCs lasted 1 week with hyperthermia treatment at 43°C, and the number of epidermal LCs returned to normal in approximately 2 weeks.
This study (10) used a 2-phase hyperthermia treatment protocol, with 14 days between phases, based on dynamic changes in epidermal LCs (10). In addition, it is common practice that multiple cryotherapy is usually delivered with an interval of approximately 14 days, to allow for tissue recovery from cryodamage.
A Cochrane systematic review has highlighted a lack of good-quality evidence for the treatment of cutaneous HPV infection, to support clinical decision-making. Forty-six out of 60 trials identified in the review (77%) were classified as low quality (11), heterogeneity was high between the trials, and analyses were often misleading or inappropriate. A major conclusion of the review was that a trial comparing hyperthermia with cryotherapy was urgently needed.
The aim of the current study was to conduct a prospective multi-centre non-randomized controlled clinical trial to compare the clinical effectiveness of local hyperthermia at 44°C vs cryotherapy with liquid nitrogen.
PATIENTS AND METHODS
This multi-centre, open, 2-arm non-randomized concurrent controlled trial was conducted at 11 centres across China. The trial was approved by the ethics committee of China Medical University [2012 (110)] and was performed in accordance with the principles of the Declaration of Helsinki.This clinical trial is registered at Chinese Clinical Trial Registry (registration number: ChiCTR-TRC-12002588)
Study population
Participants were recruited between January 2012 and December 2014 from outpatient clinics of 11 hospitals in 10 cities in mainland China (Shenyang, Shanghai, Xi’an, Beijing, Hefei, Naning, Guiyang, Guangzhou, Changsha, and Huhehot). The diagnosis of plantar warts was based on typical clinical manifestations.
Informed consent was obtained from all patients. Included patients were in the age range16–65 years. The patients were excluded from the study for the following reasons: a history of topical or systemic use of drugs and therapy history in the past 3 months; seropositive for HIV; those who presented with other systemic infections; patients with severe hypersensitivity diseases or severe systemic illnesses; those currently involved in a clinical trial for plantar warts; and patients who were unable to give informed consent.
Once the clinicians had explained the nature of the trial, the patients chose treatment with either cryotherapy or local hyperthermia therapy, or the clinician allocated them to a treatment, with the patient’s consent.
Cryotherapy treatment. Participants who entered cryotherapy with liquid nitrogen received a maximum of 2 treatments, given 2 weeks ±3 days apart.
Liquid nitrogen was applied with a spray or cotton bud. Debridement before treatment and masking of the surrounding area was performed. Two cycles of freeze and thaw was applied according to the protocol of general practice. In individuals with multiple warts, each lesion received cryo-treatment.
Local hyperthermia treatment. A patented hyperthermia device with an infrared emitting source was used, as reported previously (8, 9). The device output infrared heat locally onto a skin lesion without direct contact. The heated skin surface temperature (range 37–48°C) was controlled automatically and stabilized at the pre-set temperature (± 0.1°C). The surface temperature was adjusted to 44 ± 1°C, according to the patient’s tolerability. Patients received hyperthermia treatment once a day for 3 consecutive days. Each treatment session lasted 30 min. At 14 ± 3 days later, patients received similar treatments for 2 consecutive days. A single lesion was chosen for treatment, usually the largest, or the one with most load-bearing pain for patients with multiple lesions.
Four follow-up visits were scheduled, at 1, 2, 3 and 6 months after treatment. For each visit, assessment of clearance of plantar warts, recurrence, and any adverse events were documented. The final follow-up was conducted via a phone call or revisit, to document whether there were any further changes or lesion relapses.
Outcome measurements
Primary outcome. The complete disappearance of warty lesion was regarded as cure, whereas the presence of any remaining visible primary lesions was regarded as treatment failure. The primary outcome was the rate of complete clearance of all plantar warts at 3 months after the last treatment. Clearance of plantar wart was defined as the restoration to “normal appearance” of the skin. Secondary outcomes. Secondary outcomes included the demographic and clinical parameters that might influence the rate of clearance, assessment of severity of pain during the treatments, other adverse events, and recurrence rate.
Statistical analysis
Pearson’s χ2 test was used to compare the rate of clearance, recurrence, and adverse events between the 2 groups. Unconditional logistic regression models were constructed to examine relevant parameters (age, whether the plantar wart had been treated previously, the diameter of the target lesion, and the duration of warts) associated with clearance of plantar warts. Factors with p < 0.20 in the univariate analysis were included as logistic variables, and those with p < 0.05 were retained in the final multiple regression model. A modified intention-to-treat analysis (modified ITT) was used to assess the rate of clearance of plantar warts. The ITT population included all patients who received at least 1 round of treatment and had 1 or more follow-up visits after treatment. The t-test was used to analyse pain during treatment on a visual analogue scale (VAS). Analyses were conducted in SPSS (version 20.0) using 2-sided significance tests at the 5% significance level for the primary outcome measure and 1% significance level for secondary outcome measures.
RESULTS
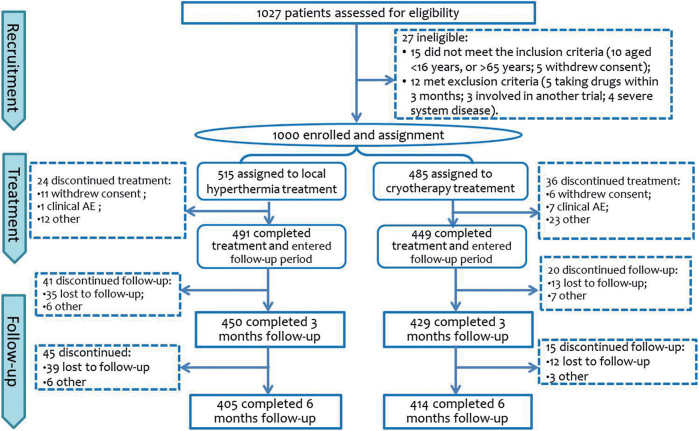
Fig. 1 shows the trial flowchart. A total of 1,027 participants were screened for eligibility, 1,000 were enrolled in the study and assigned to either the local hyperthermia arm (n = 515) or the cryotherapy arm (n = 485). Of these, 918 were included in the modified ITT analysis (484 from the local hyperthermia group, 434 from the cryotherapy group). Both groups were well matched for age and sex (p > 0.5 for all, Student’s t-test).
Fig. 1.
Trial flowchart. AE: adverse event.
Treatment responses
A total of 879 participants were eligible for evaluation 3 months after treatment. Of the 121 (12.9%) patients who dropped out of the study, 65 were from the local hyperthermia group, and 56 were from the cryotherapy group. The reasons for dropout are listed in Fig. 1.
At 3-month follow-up 50.9% of patients (229/450) in the local hyperthermia group and 54.3% (233/429) in the cryotherapy group were evaluated as cured. There was no statistically significant difference between the 2 groups (p = 0.31). The complete clearance was the number of people cured divided by the number of people who completed all the treatments and 3-month follow-up.
The rate of complete clearance of the plantar warts was assessed with a modified ITT. At 3-month follow-up, 47.31% of patients (229/484) in the local hyperthermia group and 53.69% (233/434) in the cryotherapy group were evaluated as cured. Again, there was no statistically significant difference between the 2 groups (p = 0.054), as shown in Table I.
Table I.
Treatment response of both groups at different follow-up time-points
| Follow-up time | Cured, n | Cure rate (%) | χ2 | p-value |
|---|---|---|---|---|
| 1 month after treatment | ||||
| Local hyperthermia (n = 484) | 88 | 18.2 | 65.44 | <0.001 |
| Cryotherapy (n = 434) | 185 | 42.6 | ||
| 2 months after treatment | ||||
| Local hyperthermia (n = 475) | 156 | 32.8 | 27.92 | <0.001 |
| Cryotherapy (n = 433) | 217 | 50.1 | ||
| 3 months after treatment | ||||
| Local hyperthermia (n = 450) | 229 | 50.9 | 1.03 | 0.310 |
| Cryotherapy (n = 429) | 233 | 54.3 | ||
| 6 months after treatment | ||||
| Local hyperthermia (n = 405) | 258 | 63.7 | 3.06 | 0.086 |
| Cryotherapy (n = 414) | 239 | 57.7 | ||
| Total by modified intention-to-treat | ||||
| Local hyperthermia (n = 484) | 295a | 61.0 | 1.21 | 0.271 |
| Cryotherapy (n = 434) | 249a | 57.4 | ||
Complete clearance was assessed with a modified ITT.
Factors influencing clearance
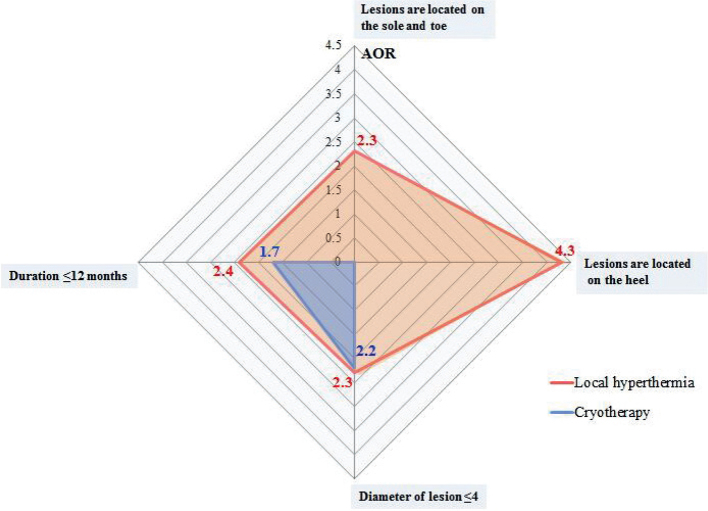
Univariate factors among baseline characteristics that were significantly associated with rates of clearance were analysed with stepwise multiple logistic regression. Three factors were kept in the final model in the local hyperthermia group that favoured the clearance of lesions, including the maximum diameter of the target lesion ≤ 4 mm (adjusted odds ratio (AOR) 2.3, 95% confidence interval (95% CI) 1.3–4.1, p = 0.003); the duration of disease ≤ 12 months (AOR 2.4, 95% CI 1.5–3.8, p = 0.001); target lesions are located on the toes (AOR 2.3, 95% CI 1.1–4.7, p = 0.024) and heels (AOR 4.3, 95% CI 1.7–11.0, p = 0.002). In the cryotherapy group, 2 factors were kept in the final model, including the maximum diameter of the target lesion ≤ 4 mm (AOR 2.2, 95% CI 1.3–3.9, p = 0.002) and the duration of disease ≤ 12 months (AOR 1.7, 95% CI 1.1–2.5, p = 0.002) (Table II and Fig. 2).
Table II.
Parameters in the local hyperthermia and cryotherapy groups analysed with stepwise multiple logistic regression
| Factors | Local hyperthermia | Cryotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Clearance n (%) | COR (95% CI) | p-value | AOR (95% CI) | p-value | N | Clearance n (%) | COR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Age | ||||||||||||
| <24 years | 166 | 119 (71.7) | 2.3 (1.4–3.5) | < 0.001 | 1.5 (0.9–2.5) | 0.097 | 137 | 86 (62.8) | 1.2 (0.8–2.0) | 0.376 | ||
| 24–30 years | 155 | 90 (58.1) | 1.2 (0.8–1.9) | 0.342 | 1.1 (0.7–1.7) | 0.729 | 158 | 83 (52.3) | 0.8 (0.5–1.3) | 0.386 | ||
| >30 years | 163 | 86 (52.8) | 1.00 | 1.00 | – | 139 | 80 (57.6) | 1.0 | ||||
| Sex, n | ||||||||||||
| Male | 225 | 140 (63.1) | 1.18 (0.8–1.7) | 0.381 | – | – | 198 | 121 (61.1) | 1.3 (0.9–2.0) | 0.141 | ||
| Female | 259 | 155 (59.4) | 1.00 | 236 | 128 (54.2) | 1.0 | ||||||
| Location of lesion | ||||||||||||
| Toes | 313 | 196 (62.6) | 1.8 (1.0–3.6) | 0.068 | 2.3 (1.1–4.7) | 0.024 | 278 | 152 (54.6) | 0.6 (0.3–1.4) | 0.241 | ||
| Heel | 57 | 44 (77.2) | 3.7 (1.6–9.0) | 0.003 | 4.3 (1.7–11.0) | 0.002 | 65 | 35 (53.8) | 0.6 (0.3–1.5) | 0.271 | ||
| Arch | 42 | 25 (59.5) | 1.6 (0.7–3.9) | 0.276 | 1.7 (0.7–4.3) | 0.270 | 37 | 26 (70.3) | 1.2 (0.4–3.4) | 0.680 | ||
| Sole | 32 | 11 (34.4) | 0.6 (0.2–1.5) | 0.263 | 0.8 (0.3–2.3) | 0.730 | 22 | 15 (68.2) | 1.1 (0.4–3.6) | 0.845 | ||
| Others | 40 | 19 (47.5) | 1.00 | 1.00 | – | 32 | 21 (65.6) | 1.0 | ||||
| Diameter of lesion | ||||||||||||
| ≤4 mm | 116 | 91 (78.4) | 3.5 (2.1–5.8) | < 0.001 | 2.3 (1.3–4.1) | 0.003 | 217 | 140 (64.5) | 2.4 (1.4–4.3) | 0.002 | 2.2 (1.3–3.9) | 0.005 |
| 4.1–7.9 mm | 144 | 89 (61.8) | 1.5 (1.0–2.3) | < 0.049 | 1.3 (0.8–2.1) | 0.223 | 149 | 80 (53.7) | 1.6 (0.9–2.8) | 0.132 | 1.5 (0.8–2.7) | 0.174 |
| ≥8 mm | 224 | 115 (57.7) | 1.00 | 1.00 | – | 68 | 29 (42.6) | 1.0 | 1.0 | |||
| Number of lesions | ||||||||||||
| 1–3 | 230 | 145 (63.0) | 1.2 (0.8–1.7) | 0.369 | – | – | 220 | 124 (56.4) | 0.9 (0.6–1.3) | 0.666 | ||
| ≥4 or mosaic | 254 | 150 (59.1) | 1.00 | 214 | 125 (58.4) | 1.0 | ||||||
| Duration | ||||||||||||
| ≤12 months | 195 | 147 (75.4) | 2.9 (2.0–4.3) | < 0.001 | 2.4 (1.5–3.8) | < 0.001 | 220 | 142 (64.5) | 1.8 (1.2–2.7) | 0.002 | 1.7 (1.1–2.5) | 0.008 |
| >12 months | 289 | 148 (51.2) | 1.00 | 1.00 | – | 214 | 107 (50.0) | 1.0 | 1.0 | |||
COR: crude OR; AOR: adjusted odds ratio with p ≤ 0.2 in univariate analysis were entered in multivariate Cox regression model; 95% CI: 95% confidence interval. The population of local hyperthermia group and cryotherapy group were analyzed by modified intention-to-treat.
Fig. 2.
Factors that influence clearance of plantar warts. Univariate factors among baseline characteristics that were significantly associated with rates of clearance were analysed using stepwise multiple logistic regression. AOR: adjusted odds ratio.
Recurrence
The rate of recurrence in the local hyperthermia group was 0.8% (4/484) and in the cryotherapy group 12% (52/434). The difference in recurrence rate between the 2 groups was statistically significant (χ2=49.71, p < 0.001).
Pain score during treatment and other adverse effects
Patients who experienced pain during treatment were asked to rate their pain using a 0–10 ascending visual analogue scale (where, 0: no pain; 10: excruciating pain). The mean ± standard deviation (SD) pain sores were 0.4 ± 0.9 in the local hyperthermia group and 5.5 ± 2.3 in the cryotherapy group, respectively. There was a statistically significant difference in the severity of pain between the 2 groups (t = 43.5, p < 0.001).
Data on other adverse events were collected at each visit. All patients in both groups experienced heat/ cold-induced temporary erythema. The other adverse reactions associated with hyperthermia treatments were erythema (12 cases), oedema (4 cases), blistering (4 cases), blood blistering (1 case), scar (3 cases), itch (3 cases) and pigmentation (10 cases), while cold-induced erythema (45 cases), oedema (39 cases), blistering (56 cases), blood blistering (40 cases), scar (62 cases), itch (35 cases), and pigmentation (120 cases) were observed in the cryotherapy group. One patient died of cerebral haemorrhage during the observational period. This serious adverse event (SAE) was classes as unrelated to the trial treatment (local hyperthermia).
DISCUSSION
Most cases of plantar warts have a self-limiting clinical course of approximately 2–3 years (2); however, patients often seek treatment due to discomfort, load-bearing pain or other reasons. There are many treatment options available for plantar warts, but the levels of evidence are poor. Cryotherapy is one of the most common hospital-based therapies for plantar warts; however, pain during treatment, relatively high recurrence rates and other limitations limit its use (12). Our previous small-scale clinical trial found that local hyperthermia at 44°C was effective in the treatment of plantar warts, compared with sham treatment. The current comparative study of local hyperthermia and cryotherapy may help clinicians to choose the most appropriate method in clinical practice. The current study used the treatment protocol as reported previously for the local hyperthermia arm (8); and, for the cryotherapy arm, a maximum of 2 treatments were given 2 weeks apart for each lesion, as reported previously (12, 13). The current study showed similar cure rates between the 2 groups after 3 months. Cryotherapy was associated with a higher recurrence rate, higher pain sensation and more adverse events, while patients in the hyperthermia group had more treatment visits and longer treatment time.
Cryotherapy, which is commonly used in the treatment of warts, is associated with pain, scarring and a long recovery time. The reported overall cure rates of cryotherapy range from 46.2% to 70.7% in several relatively large cohort observations (14, 15). The overall cure rate in the current study was 53.7% in the cryotherapy arm, a moderate cure rate compared with previous reports. Manoeuvre techniques, disease condition or population difference may contribute to the varied responses to cryotherapy. The present study found that the maximum diameter of the target lesion and the duration of disease might affect the treatment response in cryotherapy.
Patients with warts have variations in clinical course, possibly due to genetic polymorphisms or the establishment of immune response against the virus. A previous study showed that morphological characteristics and HPV genotype influence treatment response and potentially influence future treatment decisions for warts (13). The current study found that a maximum diameter of the target lesion of ≤4 mm, and a duration of disease ≤12 months had statistically higher cure rates both in the local hyperthermia group and cryotherapy group. In patients treated with local hyperthermia, those with target lesions located on the toes and heels had higher rates of clearance according to stepwise multiple logistic regression analysis, while this phenomenon was not observed in the cryotherapy group. We speculate that the underlying HPV types, and regional immunological or physiological status may contribute to the rate of clearance. Despite the fact that younger age appears to be more significantly associated with higher rates of clearance, the age of the patients is not an independent factor. Sex and the number of lesions were not associated with clearance rates in either group.
Most of the patients in the local hyperthermia group reported that their warts cleared unnoticed, whereas some patients felt itchy or noticed their lesions “bulging up” before the warts disappeared. Only 1 target lesion was chosen for treatment in patients with multiple lesions; either the largest lesion, or the one with most load-bearing pain. However, in patients with multiple warts, if the target lesion disappeared after treatment, the untreated lesions also disappeared. The establishment of a specific immune response against HPV-infected keratinocytes may explain this phenomenon.
Absorption of heat energy by tissue is dependent on temperature and length of time applied (16). More cells undergo apoptosis above 43°C, which would facilitate the establishment of a specific immune response (17, 18). Our previous study has shown that local hyperthermia at 44°C is, in general, well tolerated by patients with plantar warts without any burning sensation. A few cases in the hyperthermia group experienced transitory blistering, possibly due to extra heating due to a shift of target site during the treatment. Adverse events in the cryotherapy group were well recognized during daily practice, such as pain, blistering and scarring.
A previous study showed that loss of LCs in the skin enhances contact dermatitis (19). Local hyperthermia could promote the migration of LCs to draining lymph nodes. LCs in the epidermis could uptake and present antigens to specific T cells. We thus speculate that local hyperthermia is favourable to a specific T cell mediated immune response against HPV. In an unpublished observation we found that the decrease in epidermal LCs lasted 1 week with hyperthermia treatment at 43°C. Thus, the loss of LCs in skin by hyperthermia may increase the immune response necessary to kill virus-infected keratinocytes. Homeostasis of LCs in the epidermis would be reached in 1–2 weeks (10); hence we set a 2-week interval between treatment sessions to ensure that the second episode of treatment had a sufficient number of epidermal LCs being targeted. Since epidermal turnover time in normal skin ranges from 27 to75 days, depending on anatomical site, a 3-month period was chosen as the end-point for restoration to normal status, a time-period that conforms with most studies on this topic.
For some patients, local hyperthermia helped relieve the pain. Although the underlying mechanism remains unknown, this effect was much appreciated by patients who could not avoid a load-bearing position.
Limitations
This study has a number of limitations. Due to the competitive nature of the patients’ enrollment, the numbers of treated patients for different study sites varies (data not shown). Although this study was initially registered as a randomized trial, there was unexpected low compliance with randomization of patients into the 2 treatment arms. We thus enrolled patients according to their preference, or directly assigned the grouping, after explanation of the nature of the study. Although there was a 3-month washout time before enrollment, no details were collected regarding the medical and therapy history regarding the warts prior to enrollment, and thus there is a lack of information on the influence of this history on subsequent treatment. In addition, the study design tabulated affected sites on the toes, heel, arch and sole, which limited more detailed analysis of other sites.
Conclusion
In summary, this large-scale study found that both cryotherapy and local hyperthermia were generally safe and had similar rates of effectiveness in the treatment of plantar warts. Some clinical characteristics of the warts influenced the treatment response in both local hyperthermia and cryotherapy groups.
ACKNOWLEDGEMENTS
The authors thank Shi Wei from the University of Alabama for his critical reading of the manuscript. This study was funded by the Public Welfare Research Fund for Healthcare, Ministry of Health (201202013) and Clinical Research Center for skin diseases, Liaoning Province.
This clinical trial was registered at Chinese Clinical Trial Registry (registration number: ChiCTR-TRC-12002588)
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lipke MM. An armamentarium of wart treatments. Clin Med Res 2006; 4: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al. Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model. Health Technol Assess 2006; 10: iii, ix–87. [DOI] [PubMed] [Google Scholar]
- 3.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497. [DOI] [PubMed] [Google Scholar]
- 4.Ghonemy S. Treatment of recalcitrant plantar warts with long-pulsed Nd: YAG laser versus cantharidin-podophylline resin-salicylic acid. J Cosmet Laser Ther 2017; 19: 347–352. [DOI] [PubMed] [Google Scholar]
- 5.Hsu VM, Aldahan AS, Tsatalis JP, Perper M, Nouri K. Efficacy of Nd: YAG laser therapy for the treatment of verrucae: a literature review. Lasers Med Sci 2017; 32: 1207–1211. [DOI] [PubMed] [Google Scholar]
- 6.Izadi Firouzabadi L, Khamesipour A, Ghandi N, Hosseini H, Teymourpour A, Firooz A. Comparison of clinical efficacy and safety of thermotherapy versus cryotherapy in treatment of skin warts: a randomized controlled trial. Dermatol Ther 2018; 31: e12564. [DOI] [PubMed] [Google Scholar]
- 7.Gao XH, Gao D, Sun XP, Huo W, Hong YX, Li XD, et al. Non-ablative controlled local hyperthermia for common warts. Chin Med J (Engl) 2009; 122: 2061–2063. [PubMed] [Google Scholar]
- 8.Huo W, Gao XH, Sun XP, Qi RQ, Hong Y, McHepange UO, et al. Local hyperthermia at 44 degrees C for the treatment of plantar warts: a randomized, patient-blinded, placebo-controlled trial. J Infect Dis 2010; 201: 1169–1172. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Gao XH, Jin L, Wang Y, Hong Y, McHepange UO, et al. Local hyperthermia could induce migrational maturation of Langerhans cells in condyloma acuminatum. J Dermatol Sci 2009; 54: 121–123. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka A, Miyachi Y, Imamura S, Hiraoka M, Jo S, Abe M. Suppression of contact sensitivity by local hyperthermia treatment due to reduced Langerhans cell population in mice. Br J Dermatol 1989; 120: 493–501. [DOI] [PubMed] [Google Scholar]
- 11.Cockayne S, Hewitt C, Hicks K, Jayakody S, Kang’ombe AR, Stamuli E, et al. Cryotherapy versus salicylic acid for the treatment of plantar warts (verrucae): a randomised controlled trial. BMJ 2011; 342: d3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield-Jones SE. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol 2014; 171: 696–712. [DOI] [PubMed] [Google Scholar]
- 13.Hogendoorn GK, Bruggink SC, de Koning MNC, Eekhof JAH, Hermans KE, Rissmann R, et al. Morphological characteristics and human papillomavirus genotype predict the treatment response in cutaneous warts. Br J Dermatol 2018; 178: 253–260. [DOI] [PubMed] [Google Scholar]
- 14.Bruggink SC, Gussekloo J, Egberts PF, Bavinck JNB, de Waal MWM, Assendelft WJJ, et al. Monochloroacetic acid application is an effective alternative to cryotherapy for common and plantar warts in primary care: a randomized controlled trial. J Invest Dermatol 2015; 135: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 15.Walczuk I, Eertmans F, Rossel B, Cegielska A, Stockfleth E, Antunes A, et al. Efficacy and safety of three cryotherapy devices for wart treatment: a randomized, controlled, investigator-blinded, comparative study. Dermatol Ther (Heidelb) 2018; 8: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Gao XH, Li X, Hong Y, Qi R, Chen HD, et al. Local hyperthermia induces apoptosis of keratinocytes in both normal skin and condyloma acuminata via different pathways. Apoptosis 2009; 14: 721–728. [DOI] [PubMed] [Google Scholar]
- 18.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86–89. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 2005; 23: 611–620. [DOI] [PubMed] [Google Scholar]