Abstract
Objective
The Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes (Pre-DICTED) Program is a diabetes prevention trial comparing the diabetes conversion rate at 3 years between the intervention group, which receives the incentivized lifestyle intervention program with stepwise addition of metformin, and the control group, which receives the standard of care. We describe the baseline characteristics and compare Pre-DICTED participants with other diabetes prevention trials cohort.
Research design and methods
Participants were aged between 21 and 64 years, overweight (body mass index (BMI) ≥23.0 kg/m2), and had pre-diabetes (impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT)).
Results
A total of 751 participants (53.1% women) were randomized. At baseline, mean (SD) age was 52.5 (8.5) years and mean BMI (SD) was 29.0 (4.6) kg/m2. Twenty-three per cent had both IFG and IGT, 63.9% had isolated IGT, and 13.3% had isolated IFG. Ethnic Asian Indian participants were more likely to report a family history of diabetes and had a higher waist circumference, compared with Chinese and Malay participants. Women were less likely than men to meet the physical activity recommendations (≥150 min of moderate-intensity physical activity per week), and dietary intake varied with both sex and ethnicity. Compared with other Asian diabetes prevention studies, the Pre-DICTED cohort had a higher mean age and BMI.
Conclusion
The Pre-DICTED cohort represents subjects at high risk of diabetes conversion. The study will evaluate the effectiveness of a community-based incentivized lifestyle intervention program in an urban Asian context.
Keywords: Preventive Medicine, Prediabetic State
WHAT IS ALREADY KNOWN ON THIS TOPIC
Community-based diabetes prevention programs are increasingly employed in an Asian context, to counter the growing diabetes prevalence driven by urbanization and increasing prevalence of sedentary lifestyles. Baseline differences between subgroups can affect the effectiveness of a prevention program.
WHAT THIS STUDY ADDS
In a cohort of high-risk individuals (body mass index ≥23.0 kg/m2 and impaired fasting glucose/impaired glucose tolerance) recruited in Singapore, physical activity and dietary profile varied with sex: only 58.2% of women reported engaging in at least 150 min of moderate-intensity physical activity (or its equivalent) per week, compared with 68.8% of men. However, women also had lower saturated fat intake (12.9% energy vs 13.7% energy) and higher dietary fiber intake (8.5 g/1000 kcal vs 7.8 g/1000 kcal).
Dietary profile varied with ethnic group (Chinese, Malays and Asian Indians): Indian participants had the highest carbohydrate intake (52.4% vs 44.9% and 48.4% energy for Chinese and Malay participants, respectively), but also the highest dietary fiber (10.0 vs 7.9 and 8.3 g/1000 kcal) and lowest saturated fat (12.3% vs 13.4% and 13.5% energy) intake.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY.
Community-based prevention programs trade individualized lifestyle counseling for cost reduction. A lifestyle modification curriculum that considers the significant differences between men and women in lifestyle factors at baseline could recover some of the benefits of individualized counseling.
Baseline differences between diabetes prevention program cohorts and between ethnic groups partially account for differences in program effectiveness. The results presented here provide a means to ground and facilitate inter-program comparisons.
Introduction
Type 2 diabetes (T2D) is a persistent public health issue. The global prevalence of T2D has been steadily increasing in the past three decades,1 and the number of people worldwide with diabetes, of which T2D cases form an overwhelming majority, is projected to increase from 530 million in 2021 to 780 million by 2045, with an accompanying increase in prevalence from 10.5% to 12.2%.2 Population aging and improved care and survival of people with diabetes contribute to this growing prevalence, particularly in high-income countries.2 3 For low and middle-income countries, these factors are compounded by a shift towards sedentary lifestyles and high-calorie diets in both urban and rural settings.4 5 Although prevalence has been increasing, data from multiple high-income countries indicate a stable or declining trend in diabetes incidence since 2010, and diabetes prevention programs involving lifestyle modification have been highlighted as possible contributors to this favorable change.4 6 Diabetes prevention programs focus on individuals with pre-diabetes, as they are at a higher risk of developing T2D compared with individuals with normal blood glucose.7–9 Importantly, multiple large randomized controlled trials have shown that the risk of T2D is reduced in individuals with impaired glucose tolerance (IGT) after intervention with lifestyle modification or metformin.10–14
The Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes (Pre-DICTED) Program is a diabetes prevention trial in Singapore that aims to compare the diabetes conversion rate over 3 years in overweight or obese individuals with elevated risk of diabetes (impaired fasting glucose (IFG) and/or IGT) between the intervention group, which receives the community-based stepwise diabetes prevention program with added financial incentives, and the control group, which receives the current standard of care. Similar to the Diabetes Community Lifestyle Improvement Program (D-CLIP),14 the Pre-DICTED Study uses a stepwise program consisting of group-based lifestyle modification classes and subsequent addition of metformin where necessary, and sets forth weight loss (≥5% of baseline weight) and physical activity goals (moderate physical activity ≥150 min per week) for intervention participants. However, in contrast with D-CLIP, Pre-DICTED additionally has a maintenance phase where recommendations for physical activity and dietary modification are delivered to intervention group participants via monthly mobile phone text messages. The Pre-DICTED Program also includes financial incentives that are tied to (1) attendance of the lifestyle modification classes, and (2) achievement and maintenance of the weight loss goal (5% of baseline weight).
As a large pragmatic trial that enrolls urban Asian adults with pre-diabetes, the Pre-DICTED Program can help to supplement our knowledge of diabetes prevention in a real-world, contemporary urban Asian context. The present report describes the baseline characteristics of Pre-DICTED participants and compares the Pre-DICTED participants with other diabetes prevention trials cohort.
Research design and methods
Overview of study design
Pre-DICTED is a randomized open-label, parallel arms, controlled trial that compares a diabetes prevention program consisting of lifestyle interventions with stepwise addition of metformin and added financial incentives (intervention) versus the current standard of care (control) in overweight or obese individuals with IFG and/or IGT. The primary outcome is the development of diabetes, diagnosed based on an annual oral glucose tolerance test (OGTT) (2-hour plasma glucose of ≥11.1 mmol/L) or semiannual fasting glucose measurement (fasting plasma glucose (FPG) ≥7.0 mmol/L).15
Screening and recruitment
Participants were recruited through (1) referrals from healthcare professionals in polyclinics (primary care clinics), general practitioner clinics, and hospitals; (2) referrals from regional community screening programs; and (3) advertisements on an online health information and services portal (HealthHub). The screening step consisted of a 75 g OGTT, and pre-diabetes was diagnosed based on two glycemic criteria: (1) FPG 6.1–6.9 mmol/L (IFG), and/or (2) 2-hour plasma glucose after a 75 g glucose load 7.8–11.0 mmol/L (IGT). Additional inclusion criteria were age between 18 and 64 years, body mass index (BMI) ≥23.0 kg/m2, and Singapore citizenship or permanent residency. Major exclusions were health conditions that impede participation in a lifestyle modification program (eg, active cancer, recent myocardial event within 6 months), known allergic reaction to metformin, or use of medications known to alter glucose tolerance. Participants were allocated to intervention and control arms with a 1:1 ratio, using a computer-generated randomization list based on permuted block randomization.
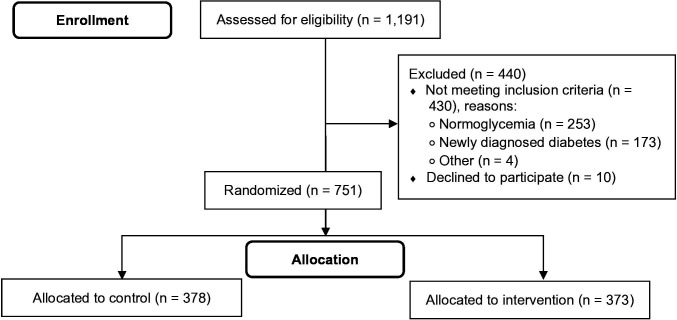
Screening and recruitment were carried out from November 2017 to February 2021. A total of 1191 individuals were referred to the program for screening. Of these, 440 (36.9%) were excluded (253 had normal blood glucose, 173 had newly diagnosed diabetes, 10 declined to participate, 4 were ineligible for other reasons), and 751 individuals were randomized to the control or intervention arm (figure 1).
Figure 1.
Flow chart showing the screening and enrollment of participants in the Pre-DICTED Program. Pre-DICTED, Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes.
Interventions
The intervention program consists of 12 group-based lifestyle modification sessions (two nutrition workshops, nine exercise sessions, one goal-setting workshop) delivered in person over 6 weeks.15 At semiannual follow-up visits, intervention arm participants at the highest risk of diabetes development ((1) IFG and glycated hemoglobin (HbA1c) ≥6.0%, or (2) IFG and IGT) are prescribed metformin at a starting dose of 250 mg two times per day for 3 months with subsequent uptitration to 500 mg two times per day.
The intervention program includes financial incentives for (1) attending lifestyle modification sessions, and (2) achieving the weight loss target (≥5% of baseline weight). Intervention arm participants receive a S$10 incentive (sports vouchers) for attending each lifestyle modification session (maximum amount for 12 sessions=S$120). If they meet the weight loss target (≥5% of baseline weight) at any of the study visits at 3, 6, 12, 18, 24, 30 and 36 months, they are eligible for additional incentives. Eligible participants can choose to receive a direct payment or a lottery ticket (1 in 10 chance of winning). For example, at month 3, a participant who meets the weight loss target can opt to receive a direct payment of S$40 (cash), or a lottery ticket with a 1 in 10 chance of winning S$400 (cash). The expected value of the weight loss incentive increases with time to encourage weight loss maintenance. With every follow-up visit, the direct payment incentive increases by S$10, while the lottery prize increases by S$100. The maximum direct payment to a participant for achieving and maintaining weight loss is S$40+S$50+S$60+S$70+S$80+S$90+S$100=S$490.
Measurements and assessments
Measurement and assessment procedures have been previously detailed.15 Anthropometric measurements such as those for height, weight, waist circumference and blood pressure were obtained by trained study coordinators following established standard operational procedures. To obtain measurements for HbA1c, FPG, 2-hour plasma glucose in OGTT and the lipid profile, participants were instructed to fast overnight for at least 10 hours, and blood samples were collected using standard phlebotomy procedures. Standardized interviewer-administered questionnaires were used to obtain self-reported data on demographic characteristics, medical history, nutrition, quality of life, and physical activity. To represent the prevalence of hypertension and lipid disorders in the Pre-DICTED cohort more accurately, we combined self-reports from two interview questions. Participants were classified as having hypertension (or a lipid disorder) only if they (1) reported a physician diagnosis of hypertension (or a lipid disorder), and (2) were taking blood pressure-lowering (or lipid-lowering) medication. Height, weight, waist circumference and blood pressure are assessed at baseline, month 3, month 6 and semiannually thereafter. After baseline, the lipid profile, FPG and HbA1c are assessed semiannually, while the 2-hour plasma glucose in OGTT is assessed annually.
Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) and its scoring protocol. The time spent doing vigorous-intensity physical activity, moderate-intensity physical activity and walking was combined into a single summary score, specified in metabolic equivalent of task-minutes, that takes into account the different levels of energy expended.16 Physical activity was categorized based on the 2010 WHO guidelines for physical activity, which recommends a minimum of 150 min of moderate-intensity physical activity (or its equivalent) per week for all adults aged above 18 years.17 The health-related quality of life was assessed with the EuroQol 5-dimension 5-level (EQ-5D-5L) questionnaire.18 The EQ-5D-5L visual analog scale records a participant’s overall health (0=worst imaginable health, 100=best imaginable health), and the EQ-5D-5L health utility score indicates a participant’s utility given their self-reported health state. The health utility score is calculated using the value set derived from the preferences of patients with heart disease in Singapore.19 Both the IPAQ and the EQ-5D-5L questionnaire are administered at baseline and semiannually thereafter.
Dietary intake over the past year was assessed using a validated semiquantitative 163-item food frequency questionnaire (FFQ) with additional subquestions on food subtypes and cooking methods.20 Participants reported consumption of one standard serving as a number of times either per day, per week, or per month. Items consumed less than once per month were coded as ‘rarely/never’. Dietary intake of food was standardised to daily frequencies and multiplied by standard serving sizes (grams). A nutrient database for the FFQ was constructed using the nationally representative 24-hour dietary recall data that were used for FFQ development.20 For dietary data analysis, we excluded those with missing FFQ (n=1) and those with invalid energy intake (ie, had extreme energy intake of ≤500 kcal/day or ≥6000 kcal/day; n=43). The FFQ is administered at baseline, month 6 and month 12.
Statistical analysis
Baseline characteristics are reported as mean (SD) for approximately normally distributed variables, and median (IQR) for skewed variables. Categorical variables are summarized with frequencies and percentages. Characteristics by sex were compared using t-tests, Mann-Whitney U test and χ² tests as appropriate. Characteristics by ethnicity were compared using analysis of variance tests, Kruskal-Wallis tests and χ² tests as appropriate. In the ethnicity-stratified analysis, only participants who identified as belonging to one of the three major ethnic groups (Chinese, Malay, Indian) were considered. Participants who identified with other ethnic groups (12 men, 13 women) were excluded from the ethnicity-stratified analysis. R (V.4.0.3) was used for all analyses.
Results
Overall baseline characteristics
Baseline characteristics for the overall cohort are shown in table 1. The mean age at baseline was 52.5 years, and 53.1% of the participants were women. Over 95% of all participants identified as belonging to one of the three major ethnic groups in Singapore: 77.4% identified as Chinese, 8.9% identified as Malay, and 10.4% identified as Indian. Over one-third of all participants reported having hypertension (43.4%) or a lipid disorder (36.5%). Mean systolic/diastolic blood pressure was 129/84 mm Hg and mean total cholesterol, high-density lipoprotein cholesterol, triglycerides and low-density lipoprotein cholesterol were 5.0, 1.3, 1.5 and 3.0 mmol/L, respectively.
Table 1.
Demographics, clinical characteristics and lifestyle risk factors by intervention status in Pre-DICTED
| Overall (n=751) |
Control (n=378) |
Intervention (n=373) |
|
| Demographic characteristics | |||
| Age, years, mean (SD) | 52.5 (8.5) | 52.3 (8.6) | 52.7 (8.4) |
| Sex, n (%) | |||
| Male | 352 (46.9) | 178 (47.1) | 174 (46.6) |
| Female | 399 (53.1) | 200 (52.9) | 199 (53.4) |
| Ethnicity, n (%) | |||
| Chinese | 581 (77.4) | 289 (76.5) | 292 (78.3) |
| Malay | 67 (8.9) | 35 (9.3) | 32 (8.6) |
| Indian | 78 (10.4) | 41 (10.8) | 37 (9.9) |
| Others | 25 (3.3) | 13 (3.4) | 12 (3.2) |
| Employment status, n (%) | |||
| Employed or student | 600 (80.0) | 294 (78.0) | 306 (82.0) |
| Homemaker | 69 (9.2) | 35 (9.3) | 34 (9.1) |
| Retired | 44 (5.9) | 29 (7.7) | 15 (4.0) |
| Unemployed | 37 (4.9) | 19 (5.0) | 18 (4.8) |
| Highest education level, n (%) | |||
| Primary | 66 (8.8) | 35 (9.3) | 31 (8.3) |
| Secondary | 225 (30.0) | 124 (32.8) | 101 (27.1) |
| Higher education including vocational | 207 (27.6) | 92 (24.3) | 115 (30.8) |
| University | 253 (33.7) | 127 (33.6) | 126 (33.8) |
| Clinical characteristics | |||
| Anthropometry | |||
| BMI, kg/m2, mean (SD) | 29.0 (4.6) | 29.2 (4.7) | 28.7 (4.5) |
| BMI categories, kg/m2, n (%) | |||
| <27.5 | 343 (45.7) | 159 (42.1) | 184 (49.3) |
| ≥27.5 | 408 (54.3) | 219 (57.9) | 189 (50.7) |
| Waist circumference, cm, mean (SD) | 95.2 (10.6) | 95.3 (10.6) | 95.0 (10.7) |
| Family history of diabetes in 1st-degree relatives, n (%) | 432 (57.5) | 228 (60.3) | 204 (54.7) |
| History of gestational diabetes mellitus, n (%) women | 60 (15.1) | 32 (16.0) | 28 (14.1) |
| Medical conditions, n (%) | |||
| Hypertension | 326 (43.4) | 167 (44.2) | 159 (42.6) |
| Lipid disorders | 274 (36.5) | 145 (38.4) | 129 (34.7) |
| Ischemic heart disease | 36 (4.8) | 18 (4.8) | 18 (4.9) |
| Stroke | 12 (1.6) | 8 (2.1) | 4 (1.1) |
| Other comorbidities | 77 (10.3) | 38 (10.1) | 39 (10.5) |
| Blood pressure, mm Hg, mean (SD) | |||
| Systolic | 129.2 (14.6) | 129.7 (14.8) | 128.7 (14.4) |
| Diastolic | 84.0 (10.7) | 84.2 (10.7) | 83.7 (10.6) |
| Lipids, mmol/L, mean (SD) | |||
| Total cholesterol | 5.0 (0.9) | 5.0 (0.9) | 5.0 (1.0) |
| HDL cholesterol | 1.3 (0.3) | 1.3 (0.3) | 1.3 (0.3) |
| Triglycerides | 1.5 (0.8) | 1.6 (0.9) | 1.5 (0.7) |
| LDL cholesterol | 3.0 (0.8) | 3.0 (0.8) | 3.0 (0.8) |
| Glycemic testing, mean (SD) | |||
| HbA1c, % | 6.0 (0.4) | 5.9 (0.4) | 6.0 (0.4) |
| FPG, mmol/L | 5.8 (0.6) | 5.8 (0.6) | 5.8 (0.6) |
| 2-hour plasma glucose, mmol/L | 8.9 (1.4) | 9.0 (1.3) | 8.9 (1.4) |
| Pre-diabetes classification, n (%) | |||
| Isolated IFG | 100 (13.3) | 38 (10.1) | 62 (16.6) |
| Isolated IGT | 480 (63.9) | 245 (64.8) | 235 (63.0) |
| IFG & IGT | 171 (22.8) | 95 (25.1) | 76 (20.4) |
| Lifestyle risk factors and quality of life | |||
| EQ-5D-5L health-related quality of life, mean (SD) | |||
| Health utility score | 0.96 (0.09) | 0.96 (0.09) | 0.95 (0.09) |
| EQ-VAS | 79.9 (15.0) | 80.1 (15.3) | 79.7 (14.7) |
| Smoking, n (%) | |||
| Ex-smoker | 52 (6.9) | 28 (7.4) | 24 (6.4) |
| No | 651 (86.7) | 327 (86.5) | 324 (86.9) |
| Yes | 48 (6.4) | 23 (6.1) | 25 (6.7) |
| Physical activity, MET-min per week, n (%) | |||
| <600* (not meeting recommendations) | 275 (36.9) | 135 (35.8) | 140 (37.9) |
| ≥600* (meets recommendations) | 471 (63.1) | 242 (64.2) | 229 (62.1) |
| Nutrient intake†, mean (SD) | |||
| Energy, kcal/day | 2560 (1143) | 2553 (1145) | 2567 (1143) |
| Carbohydrate, %E | 46.1 (8.3) | 46.3 (7.7) | 45.9 (8.8) |
| Protein, %E | 16.4 (3.0) | 16.2 (2.9) | 16.5 (3.0) |
| Total fat, %E | 36.8 (6.5) | 36.7 (6.1) | 37.0 (6.9) |
| Saturated fat, %E | 13.3 (2.5) | 13.4 (2.5) | 13.2 (2.5) |
| Dietary fiber, g/1000 kcal | 8.2 (2.0) | 8.1 (2.0) | 8.3 (2.0) |
| Food group intake†, median (IQR) | |||
| Refined grains, g/1000 kcal | 156.0 (111.5–200.1) | 160.4 (117.0–203.3) | 149.8 (107.9–196.2) |
| Whole grains, g/1000 kcal | 28.2 (9.4–57.5) | 26.4 (7.0–51.9) | 30.3 (11.6–62.0) |
| Red meat, g/1000 kcal | 21.1 (12.1–31.6) | 20.7 (11.4–32.1) | 21.1 (12.3–31.3) |
| Poultry, g/1000 kcal | 19.3 (12.1–29.2) | 18.6 (11.8–28.3) | 20.1 (12.7–30.5) |
| Fresh seafood, g/1000 kcal | 15.3 (9.5–22.9) | 14.9 (9.3–22.6) | 15.6 (9.9–23.3) |
| Dairy, g/1000 kcal | 52.8 (26.7–95.1) | 52.8 (27.7–96.2) | 53.0 (26.2–93.0) |
| Total vegetables, g/1000 kcal | 86.7 (68.8–108.3) | 87.8 (71.5–108.2) | 86.2 (66.8–108.3) |
| Fresh fruit, g/1000 kcal | 23.5 (8.1–46.0) | 21.8 (7.4–43.5) | 24.9 (9.4–47.5) |
Numbers missing: employment status (n=1); waist circumference (n=1); medical conditions–hypertension (n=2); lipid disorders (n=2); ischemic heart disease (n=2); stroke (n=3); LDL cholesterol (n=6); HbA1c (n=1); EQ-5D-5L (n=3); physical activity (n=5); dietary intake (n=1).
All comparisons between intervention and control groups have p values above 0.01. The comparison for pre-diabetes classification has p=0.02; all other comparisons have p values above 0.05.
*600 MET-min is equivalent to 150 min of moderate-intensity physical activity.16
†Forty-three participants were excluded from analysis due to invalid daily energy intake (≤500 kcal or ≥6000 kcal).
BMI, body mass index; EQ-5D-5L, EuroQol 5-dimension 5-level; EQ-VAS, EQ-5D-5L visual analog scale; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LDL, low-density lipoprotein; MET-min, metabolic equivalent of task-minutes; Pre-DICTED, Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes.
Mean BMI and glycemic profile reflected the inclusion and exclusion criteria. Mean BMI was 29.0 kg/m2 and mean baseline FPG, 2-hour plasma glucose and HbA1c levels were 5.8 mmol/L, 8.9 mmol/L and 6.0% (42 mmol/mol), respectively. Isolated IGT was the most common pre-diabetes classification for Pre-DICTED participants: 63.9% had isolated IGT, 22.8% had both IFG and IGT, and 13.3% had isolated IFG.
The majority of participants (57.5%) reported a family history of diabetes in first-degree relatives, and 15.1% of women reported a history of gestational diabetes mellitus. Overall, few participants reported a history of smoking: 6.4% reported current smoking, and 6.9% reported former smoking. However, less than two-thirds (63.1%) met the recommendations for physical activity (at least 150 min of moderate-intensity physical activity, or its equivalent, per week). In terms of diet, the mean energy intake was 2560 kcal/day. Mean carbohydrate, protein, total fat and saturated fat intake (% energy) were 46%, 16%, 37% and 13%, respectively.
The control and intervention groups were generally similar in sociodemographic and clinical characteristics, as well as diabetes lifestyle risk factors (table 1). A slight imbalance is observed in the pre-diabetes classification: the intervention group has a higher percentage of isolated IFG participants (16.6% vs 10.1%), and a lower percentage of participants with both IFG and IGT (20.4% vs 25.1%). However, the two groups have similar means and SD for FPG, 2-hour plasma glucose and HbA1c (table 1).
Baseline characteristics by sex
We observed sex differences in several risk factors (table 2). Women were less likely to report a history of smoking (3.0% of women vs 25.0% of men), hypertension (37.8% vs 49.7%) and lipid disorders (31.7% vs 42.0%). However, women were less likely to meet the physical activity recommendations at baseline: 58.2% of women reported engaging in at least 150 min of moderate-intensity physical activity (or its equivalent) per week, compared with 68.8% of men. In terms of diet, women had lower mean energy intake (2413 kcal for women vs 2738 kcal for men) and lower saturated fat intake (12.9% energy vs 13.7% energy) (table 2). Additionally, women had a higher intake of dietary fiber (8.5 g/1000 kcal vs 7.8 g/1000 kcal). In parallel, intake of some food groups also differed between men and women. Women consumed less refined grains and red meat and more fresh fruits than men.
Table 2.
Demographic characteristics and diabetes risk factors by sex in Pre-DICTED
| Men (n=352) |
Women (n=399) |
P value | |
| Demographic characteristics | |||
| Age, years, mean (SD) | 52.8 (8.7) | 52.3 (8.3) | 0.35 |
| Employment status, n (%) | <0.01 | ||
| Employed or student | 306 (87.2) | 294 (73.7) | |
| Homemaker | 0 (0.0) | 69 (17.3) | |
| Retired | 30 (8.5) | 14 (3.5) | |
| Unemployed | 15 (4.3) | 22 (5.5) | |
| Highest education level, n (%) | <0.01 | ||
| Primary | 22 (6.2) | 44 (11.0) | |
| Secondary | 72 (20.5) | 153 (38.3) | |
| Higher education including vocational | 110 (31.2) | 97 (24.3) | |
| University | 148 (42.0) | 105 (26.3) | |
| Clinical characteristics | |||
| BMI, kg/m2, mean (SD) | 28.4 (4.1) | 29.4 (5.0) | <0.01 |
| Medical conditions, n (%) | |||
| Hypertension | 175 (49.7) | 151 (37.8) | <0.01 |
| Lipid disorders | 148 (42.0) | 126 (31.7) | <0.01 |
| Glycemic testing, mean (SD) | |||
| HbA1c, % | 5.9 (0.4) | 6.0 (0.4) | 0.58 |
| FPG, mmol/L | 5.8 (0.6) | 5.8 (0.6) | 0.97 |
| 2-hour plasma glucose, mmol/L | 9.0 (1.4) | 8.9 (1.4) | 0.34 |
| Lifestyle risk factors | |||
| Smoking, n (%) | <0.01 | ||
| Ex-smoker | 49 (13.9) | 3 (0.8) | |
| No | 264 (75.0) | 387 (97.0) | |
| Yes | 39 (11.1) | 9 (2.3) | |
| Physical activity, MET-min per week, n (%) | <0.01 | ||
| <600* (not meeting recommendations) | 109 (31.2) | 166 (41.8) | |
| ≥600* (meets recommendations) | 240 (68.8) | 231 (58.2) | |
| Nutrient intake†, mean (SD) | |||
| Energy, kcal/day | 2738 (1188) | 2413 (1084) | <0.01 |
| Carbohydrate, %E | 45.9 (7.5) | 46.2 (8.9) | 0.58 |
| Protein, %E | 16.2 (2.6) | 16.5 (3.3) | 0.13 |
| Total fat, %E | 37.0 (6.1) | 36.7 (6.9) | 0.56 |
| Saturated fat, %E | 13.7 (2.4) | 12.9 (2.5) | <0.01 |
| Dietary fiber, g/1000 kcal | 7.8 (1.8) | 8.5 (2.0) | <0.01 |
| Food group intake†, median (IQR) | |||
| Refined grains, g/1000 kcal | 161.6 (120.8–205.1) | 150.4 (103.0–197.0) | 0.02 |
| Whole grains, g/1000 kcal | 26.2 (7.2–53.5) | 29.8 (11.0–59.5) | 0.07 |
| Red meat, g/1000 kcal | 22.8 (14.7–34.1) | 19.1 (9.8–29.1) | <0.01 |
| Poultry, g/1000 kcal | 19.4 (12.8–27.8) | 19.2 (11.3–31.0) | 0.84 |
| Fresh seafood, g/1000 kcal | 14.6 (9.7–21.9) | 16.0 (9.5–24.2) | 0.11 |
| Dairy, g/1000 kcal | 49.0 (25.2–93.1) | 53.9 (29.9–97.0) | 0.13 |
| Total vegetables, g/1000 kcal | 85.4 (69.8–103.6) | 87.8 (68.5–111.7) | 0.16 |
| Fresh fruit, g/1000 kcal | 20.0 (7.1–42.0) | 27.6 (9.9–53.0) | <0.01 |
Numbers missing: employment status (n=1), HbA1c (n=1), physical activity (n=5), dietary intake (n=1).
*600 MET-min is equivalent to 150 min of moderate-intensity physical activity.16
†Forty-three participants were excluded from analysis due to invalid daily energy intake (≤500 kcal or ≥6000 kcal).
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET-min, metabolic equivalent of task-minutes; Pre-DICTED, Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes.
Baseline characteristics by ethnicity
Indian participants had the highest mean waist circumference (101.3 vs 94.3 and 95.6 cm for Chinese and Malay participants, respectively), while Chinese participants had the lowest mean BMI and mean waist circumference (table 3). Moreover, a higher percentage of Indian participants reported a family history of diabetes in first-degree relatives (80.8% vs 54.4% and 59.7% for Chinese and Malay participants). The proportion of participants meeting the physical activity recommendations was lowest for Indian participants (54.5%, 63.4% and 67.2% for Indian, Chinese and Malay participants, respectively), but a statistical test for difference in population proportions did not reach significance (p=0.24). Indian participants also had the marginally higher mean HbA1c and FPG concentrations, compared with Chinese and Malay participants (table 3).
Table 3.
Diabetes risk factors by ethnicity in Pre-DICTED
| Chinese (n=581) |
Malay (n=67) |
Indian (n=78) |
P value | |
| Age, years, mean (SD) | 52.8 (8.4) | 51.5 (8.9) | 52.2 (7.9) | 0.42 |
| BMI, kg/m2, mean (SD) | 28.4 (4.3) | 31.4 (4.6) | 31.2 (5.6) | <0.01 |
| Waist circumference, cm, mean (SD) | 94.3 (10.4) | 95.6 (8.6) | 101.3 (11.8) | <0.01 |
| Family history of diabetes in 1st-degree relatives, n (%) | 316 (54.4) | 40 (59.7) | 63 (80.8) | <0.01 |
| History of gestational diabetes mellitus, n (%) women | 46 (16.0) | 6 (12.8) | 7 (14.0) | 0.82 |
| Glycemic testing, mean (SD) | ||||
| HbA1c, % | 5.9 (0.5) | 6.0 (0.4) | 6.1 (0.3) | 0.02 |
| FPG, mmol/L | 5.8 (0.6) | 5.8 (0.5) | 5.9 (0.6) | 0.07 |
| 2-hour plasma glucose, mmol/L | 8.9 (1.4) | 8.9 (1.3) | 8.9 (1.3) | 0.98 |
| Physical activity, MET-min per week, n (%) | 0.24 | |||
| <600* (not meeting recommendations) | 212 (36.6) | 22 (32.8) | 35 (45.5) | |
| ≥600* (meets recommendations) | 367 (63.4) | 45 (67.2) | 42 (54.5) | |
| Nutrient intake†, mean (SD) | ||||
| Energy, kcal/day | 2586 (1149) | 2492 (1131) | 2342 (1123) | 0.21 |
| Carbohydrate, %E | 44.9 (8.1) | 48.4 (6.7) | 52.4 (8.1) | <0.01 |
| Protein, %E | 16.9 (2.8) | 15.1 (2.4) | 13.5 (2.5) | <0.01 |
| Total fat, %E | 37.4 (6.5) | 36.2 (5.6) | 33.5 (6.5) | <0.01 |
| Saturated fat, %E | 13.4 (2.4) | 13.5 (2.5) | 12.3 (2.8) | <0.01 |
| Dietary fiber, g/1000 kcal | 7.9 (1.7) | 8.3 (2.0) | 10.0 (2.4) | <0.01 |
| Food group intake†, median (IQR) | ||||
| Refined grains, g/1000 kcal | 153.0 (110.5–197.8) | 156.6 (103.8–203.9) | 171.5 (132.7–206.9) | 0.10 |
| Whole grains, g/1000 kcal | 29.8 (10.2–60.6) | 21.9 (5.7–40.3) | 24.8 (8.1–42.6) | 0.06 |
| Red meat, g/1000 kcal | 23.8 (16.4–34.4) | 11.1 (7.0–18.8) | 6.3 (2.8–10.0) | <0.01 |
| Poultry, g/1000 kcal | 21.1 (14.6–31.3) | 16.0 (8.7–20.6) | 8.2 (2.9–13.6) | <0.01 |
| Fresh seafood, g/1000 kcal | 16.0 (10.6–23.5) | 15.2 (10.6–21.2) | 9.5 (5.2–15.6) | <0.01 |
| Dairy, g/1000 kcal | 48.4 (24.9–87.0) | 56.2 (31.5–95.1) | 70.2 (45.8–123.6) | <0.01 |
| Total vegetables, g/1000 kcal | 90.9 (72.5–111.7) | 73.3 (56.8–89.2) | 78.0 (58.6–95.9) | <0.01 |
| Fresh fruit, g/1000 kcal | 23.5 (7.9–46.0) | 23.1 (10.7–40.6) | 28.7 (9.0–58.0) | 0.57 |
Numbers missing: waist circumference (n=1), HbA1c (n=1), physical activity (n=3), dietary intake (n=1).
*600 MET-min is equivalent to 150 min of moderate-intensity physical activity.16
†Forty-three participants were excluded from analysis due to invalid daily energy intake (≤500 kcal or ≥6000 kcal).
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET-min, metabolic equivalent of task-minutes; Pre-DICTED, Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes.
In terms of diet, there was no statistically significant difference in the mean energy intake between ethnic groups (table 3). Indian participants had the highest carbohydrate (52.4% vs 44.9% and 48.4% energy for Chinese and Malay participants, respectively) and dietary fiber intake (10.0 vs 7.9 and 8.3 g/1000 kcal) and the lowest protein (13.5% vs 16.9% and 15.1% energy), total fat (33.5% vs 37.4% and 36.2% energy) and saturated fat (12.3% vs 13.4% and 13.5% energy) intake. They also had the lowest intake of red meat (6.3 vs 23.8 and 11.1 g/1000 kcal for Chinese and Malay participants, respectively), poultry (8.2 vs 21.1 and 16.0 g/1000 kcal), fresh seafood (9.5 vs 16.0 and 15.2 g/1000 kcal) and the highest intake of dairy (70.2 vs 48.4 and 56.2 g/1000 kcal). In contrast, Chinese participants had the highest intake of vegetables (90.9 vs 73.3 and 78.0 g/1000 kcal for Malay and Indian participants, respectively). No significant difference was observed in the other food groups between ethnic groups.
Comparison with other diabetes prevention and pre-diabetes management programs
The study design and baseline characteristics of Pre-DICTED are compared with other diabetes prevention and pre-diabetes management programs in table 4. The baseline glycemic profile (mean FPG, 2-hour plasma glucose, HbA1c) in Pre-DICTED is similar to those in the landmark diabetes prevention trials (US Diabetes Prevention Program (DPP),10 21 Finnish Diabetes Prevention Study (DPS),11 22 Da Qing IGT and Diabetes Study13). Compared with other Asian diabetes prevention and pre-diabetes management programs, Pre-DICTED participants had a similar or higher mean 2-hour plasma glucose concentration. Due to differences in inclusion criteria, the Pre-DICTED cohort had a lower percentage of isolated IFG participants (13.3%), compared with D-CLIP (30.1%)14 and the Zensharen Study for Prevention of Lifestyle Diseases (59.1%).23 Whereas Pre-DICTED used the WHO criteria to identify participants with IFG (FPG 6.1–6.9 mmol/L),24 D-CLIP and the Zensharen Study used the more permissive American Diabetes Association criteria (FPG 5.6–6.9 mmol/L).14 23 25
Table 4.
Key baseline characteristics of Pre-DICTED versus other large pre-diabetes trials involving lifestyle intervention
| Pre-DICTED | Diabetes Community Lifestyle Improvement Program | Indian Diabetes Prevention Programme* | Kerala Diabetes Prevention Programme | Da Qing IGT and Diabetes Study | Beijing Prediabetes Reversion Program | Zensharen Study for Prevention of Lifestyle Diseases* | US Diabetes Prevention Program | Finnish Diabetes Prevention Study* | |
| No of participants | 751 | 576 | 531 | 1007 | 530 | 1945 | 641 | 3234 | 522 |
| Year of recruitment (country) | 2017–2021 (Singapore) | 2009–2012 (Chennai, India) | 2001–2002 (India) | 2013 (Kerala, India) | 1986 (Da Qing, China) | 2007–2011 (Beijing, China) | 2003 (Japan) | 1996–1999 (USA) | 1993–1998 (Finland) |
| Study design | RCT, 2 arms: (1) usual care, (2) group-based LI+stepwise addition of metformin +incentives |
RCT, 2 arms: (1) usual care, (2) group-based LI+stepwise addition of metformin |
RCT, 4 arms: (1) usual care, (2) LI, (3) metformin, (4) LI+metformin |
Cluster RCT, 2 arms: (1) health education booklet on lifestyle modification, (2) group-based LI |
Cluster RCT, 4 arms: (1) usual care, (2) diet, (3) exercise, (4) diet+exercise |
RCT, 4 arms: (1) LI+placebo, (2) LI+pioglitazone, (3) intensive LI+placebo, (4) intensive LI+pioglitazone |
RCT, 2 arms: (1) infrequent LI (4 sessions over 3 years), (2) frequent LI (9–11 sessions over 3 years) |
RCT, 3 arms: (1) placebo, (2) metformin, (3) LI |
RCT, 2 arms: (1) usual care, (2) LI |
| Age, years, mean | 52.5 | 44.4 | 45.2 | 46.0 | 45.0 | 53 | 48 | 50.6 | 55 |
| Women, % | 53 | 37 | 21 | 47 | 47 | 58 | 29 | 68 | 67 |
| BMI, kg/m2, mean | 29.0 | 27.9 | 26.3 | 24.9 | 25.8 | 26† | 27.1 | 34.0 | 31.0 |
| Waist circumference, cm, mean | 95.2 | 94.8 | 89.8 | 88.3 | NR | 89† | 91.9 | 105.1 | 100.5 |
| Family history of diabetes, % | 58 | 57 | 50 | 48 | NR | NR | NR | 69 | 64 |
| Glycemic testing, mean | |||||||||
| FPG, mmol/L | 5.8 | 5.7 | 5.5 | 5.8 | 5.6 | 6.0† | 5.9 | 5.9 | 6.2 |
| 2-hour plasma glucose, mmol/L | 8.9 | 8.3 | 8.6 | 5.9 | 9.0 | 8.9† | 7.4 | 9.1 | 8.9 |
| HbA1c, % | 6.0 | 6.0 | 6.2 | 5.6 | NR | 5.8† | 5.4 | 5.9 | 5.6 |
| Lipids, mmol/L, mean | |||||||||
| Total cholesterol | 5.0 | NR | 5.1 | 5.7 | NR | 4.9† | 5.5 | 5.3 | 5.6 |
| HDL cholesterol | 1.3 | NR | NR | 1.3 | NR | 1.2† | 1.4 | 1.1 | 1.2 |
| Triglycerides | 1.5 | NR | 1.9 | 1.1† | NR | 1.5† | 1.4† | 1.8 | 1.7 |
| LDL cholesterol | 3.0 | NR | NR | 3.8 | NR | 3.2† | NR | 3.2 | NR |
| Blood pressure, mm Hg, mean | |||||||||
| Systolic | 129 | NR | 124 | 123 | NR | 120† | 131 | 124 | 136 |
| Diastolic | 84 | NR | 76 | 75 | NR | 78† | 81 | 78 | 86 |
*Values for continuous variables are based on the respective control groups; values for the overall cohort were not reported.
†Values are medians.
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IGT, impaired glucose tolerance; LDL, low-density lipoprotein; LI, lifestyle intervention; NR, not reported; Pre-DICTED, Pre-Diabetes Interventions and Continued Tracking to Ease-out Diabetes; RCT, randomized controlled trial.
At baseline, the mean age in the Pre-DICTED cohort (52.5 years) was similar to the median age in the Beijing Prediabetes Reversion Program (53 years),26 but higher compared with D-CLIP, Kerala DPP, Indian DPP and the Da Qing IGT Study.12–14 27 Moreover, the mean BMI in Pre-DICTED was higher compared with all other Asian studies under consideration (table 4). This is partly due to differences in the BMI inclusion criteria (D-CLIP: BMI ≥23 kg/m2, or waist circumference ≥90 cm for men and ≥80 cm for women in D-CLIP; Beijing Prediabetes Reversion Program: 22 kg/m2≤BMI<35 kg/m2; no BMI inclusion criteria for the Da Qing Study, Indian DPP and Kerala DPP), and may additionally reflect demographic differences as well as the growing prevalence of overweight and obesity in Asia.
Compared with the US DPP and Finnish DPS, Pre-DICTED participants had lower mean BMI. However, despite the lower BMI, the Pre-DICTED and US DPP cohorts had similar mean lipid profile and systolic/diastolic blood pressure (table 4), and Pre-DICTED participants were more likely to report a history of hypertension and lipid disorders (43.4% and 36.5%, respectively, in Pre-DICTED; 34.6% and 26.9% with hypertension and high cholesterol, respectively, in the US DPP).21 This is consistent with earlier studies indicating that cardiovascular disease risk factors occurred in Asian populations at a lower BMI compared with European populations.28
Conclusion
The Pre-DICTED trial is a large randomized clinical trial that evaluates the effectiveness of an incentivized community-based stepwise program (lifestyle modification, with addition of metformin where needed) at lowering diabetes risk in overweight adults with pre-diabetes in Singapore. Baseline results indicate that the control and intervention groups had similar sociodemographic characteristics and diabetes risk. Most participants (63.9%) had isolated IGT, 22.8% had both IFG and IGT, and only 13.3% had isolated IFG. The mean age of the cohort was 52.5 years and the mean BMI was 29.0 kg/m2. Compared with cohorts from other Asian diabetes prevention studies, the Pre-DICTED cohort showed a higher risk profile: participants had higher mean age and BMI.
The Pre-DICTED cohort showed a higher risk profile compared with the resident population of Singapore. Cardiometabolic risk factors were more prevalent in Pre-DICTED. All Pre-DICTED participants have elevated BMI (BMI ≥23.0 kg/m2) by trial design, and about half (54.3%) had high-risk BMI (BMI ≥27.5 kg/m2). In contrast, only 21% of all individuals and 35% of elevated-BMI individuals had high-risk BMI in the Singapore National Population Health Survey (NPHS) 2020.29 In addition, the Pre-DICTED cohort was less likely to meet physical activity and dietary recommendations. Sixty-three per cent of the Pre-DICTED cohort met the WHO physical activity recommendations, compared with 76% of NPHS 2020 respondents.29 Mean total daily energy (2560 vs 2470 kcal) and total fat intake (37% vs 35% energy) were higher in the Pre-DICTED cohort compared with respondents of the 2018 National Nutrition Survey,30 and mean saturated fat intake (13%) was above the recommended level (10% of total energy intake).
The baseline results indicate that there is heterogeneity in lifestyle risk factors between men and women, as well as between Chinese, Malay and Indian participants. Women were less likely to meet physical activity recommendations compared with men, but they had higher intake of dietary fiber and fresh fruits and lower intake of refined grains, red meat and saturated fat. In the comparison between ethnic groups, Indian participants were the least likely to meet physical activity recommendations, but had higher dietary fiber intake, lower saturated fat intake and lower red meat, poultry and seafood intake than Chinese and Malay participants. These baseline differences in physical activity and dietary intake are generally consistent with prior studies on dietary intake and their cultural determinants,29 31–36 and may suggest a need for a sex/ethnicity-sensitive approach to conducting lifestyle intervention programs.
As a pragmatic trial that aims to replicate the benefits of landmark efficacy trials while conserving healthcare resources, Pre-DICTED offers a common lifestyle modification curriculum for all intervention participants. Whereas landmark explanatory trials such as the US DPP,10 the Finnish DPS,11 22 and the Da Qing IGT and Diabetes Study13 provided lifestyle counseling sessions on an individual basis, the Pre-DICTED Program consists of group-based classes. Although the group-based classes do not provide recommendations tailored to an individual’s risk profile, a variety of meal plans and physical activities are provided as examples during the lifestyle modification sessions. Moreover, intervention participants are guided to set their own individual goals during the goal-setting session, and there is flexibility as to how the participants choose to work towards the 5% weight loss goal.
The intervention program of Pre-DICTED differs from most existing DPPs in the stepwise addition of metformin: metformin is offered to intervention participants at follow-up visits if they remain at a high risk of developing diabetes despite the lifestyle modification classes.15 In that regard, Pre-DICTED is most similar to D-CLIP: both are community-based stepwise programs that supplement lifestyle intervention with metformin where needed. The key difference in contrast to D-CLIP is that the Pre-DICTED Program includes financial incentives that encourage participants to (1) attend the lifestyle modification classes, and (2) achieve and maintain a weight loss of at least 5%. Poor program attendance limits the effectiveness of community-based programs, and extrinsic incentives are a potential solution: randomized trials with Medicaid participants with pre-diabetes in the USA have shown that adding session-based financial incentives to a community DPP improves session attendance.37–39 Given the importance of weight loss for diabetes risk reduction,40–42 the Pre-DICTED Program also offers incentives for weight loss. Financial incentives have been shown to improve weight loss and weight loss maintenance when combined with an evidence-based weight loss program.43–45
The baseline characteristics indicate some limitations of the Pre-DICTED trial. The Pre-DICTED cohort is more highly educated, compared with the general population: 91.2% of the cohort had at least secondary education, compared with 75.5% of the general population.46 This is partly due to the upper age limit of 64 years in the inclusion criteria, and marked increases in educational attainment with decreasing cohort age in Singapore.46 Nonetheless, after taking demographic trends into account, individuals with only primary education are still under-represented in Pre-DICTED. The Pre-DICTED cohort also shows a slight under-representation of Malays. Malay, Indian and Chinese individuals formed 8.9%, 10.4% and 77.4%, respectively, of the Pre-DICTED cohort, compared with 13.5%, 9.0% and 74.3% of the Singapore resident population.46 Since participants were referred to Pre-DICTED by physicians in primary care clinics, self-referral or community screening programs—similar to what would occur in a real-world prevention program—the representation discrepancies can be seen as indicating education-based or ethnic-based preferences in prevention program participation. If so, this may suggest a need for more measures to enable and encourage health-seeking behaviors in under-represented groups. Lastly, while future subgroup analyses by sex and ethnicity could give an indication of the intervention effect in these groups, the trial was not powered for such analyses.15
Overall, the baseline data indicate that Pre-DICTED had recruited an appropriate high-risk cohort to study the effect of a stepwise intervention on diabetes prevention. Pre-DICTED participants had high BMI, low levels of physical activity and high levels of calorie intake, relative to the Singapore general population and national recommendations. These factors place them at a higher risk of developing T2D, are modifiable, and are targeted by the intervention program. The inclusion of a maintenance phase and financial incentives to promote sustained lifestyle modification marks a departure from the landmark diabetes prevention trials as well as the pragmatic contemporary diabetes prevention programs designed for high-risk Asian adults. The results from the Pre-DICTED trial will provide further insight on diabetes prevention in an urban Asian context and serve to inform Singapore’s health policy and strategy.
Acknowledgments
The authors thank all the clinical research coordinators for their valuable help in running the Pre-DICTED Program.
Footnotes
Contributors: YMB conceived the study. K-FY and YQL analyzed the data. K-FY, YQL and YMB wrote the manuscript. All authors critically reviewed the manuscript and approved the submitted manuscript. YMB is the guarantor of this work; YMB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This trial is funded by the Ministry of Health, Singapore, and sponsored by Singapore Health Services (SingHealth).
Disclaimer: The funder and sponsor do not have any role in study design; collection, management analysis, and interpretation of data; writing of the report; and the decision to summit the report for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics approval
This study involves human participants and was approved by SingHealth Centralised Institutional Review Board (CIRB) (reference number: 2017/2699). Participants gave informed consent to participate in the study before taking part.
References
- 1.Khan MAB, Hashim MJ, King JK, et al. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health 2020;10:107–11. 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 2019;366:l5003. 10.1136/bmj.l5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation . IDF diabetes atlas. 10th edition, 2021. https://diabetesatlas.org/atlas/tenth-edition/ [Google Scholar]
- 5.Ranasinghe P, Jayawardena R, Gamage N, et al. Prevalence and trends of the diabetes epidemic in urban and rural India: a pooled systematic review and meta-analysis of 1.7 million adults. Ann Epidemiol 2021;58:128–48. 10.1016/j.annepidem.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 6.Magliano DJ, Chen L, Islam RM, et al. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol 2021;9:203–11. 10.1016/S2213-8587(20)30402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–12. 10.1016/j.diabres.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Man REK, Charumathi S, Gan ATL, et al. Cumulative incidence and risk factors of prediabetes and type 2 diabetes in a Singaporean Malay cohort. Diabetes Res Clin Pract 2017;127:163–71. 10.1016/j.diabres.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Wong M-S, Gu K, Heng D, et al. The Singapore impaired glucose tolerance follow-up study: does the ticking clock go backward as well as forward? Diabetes Care 2003;26:3024–30. 10.2337/diacare.26.11.3024 [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran A, Snehalatha C, Mary S, et al. The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97. 10.1007/s00125-005-0097-z [DOI] [PubMed] [Google Scholar]
- 13.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The dA Qing IGT and diabetes study. Diabetes Care 1997;20:537–44. 10.2337/diacare.20.4.537 [DOI] [PubMed] [Google Scholar]
- 14.Weber MB, Ranjani H, Staimez LR, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care 2016;39:1760–7. 10.2337/dc16-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung K-F, Gandhi M, Lam AYR, et al. The pre-diabetes interventions and continued tracking to Ease-out diabetes (Pre-DICTED) program: study protocol for a randomized controlled trial. Trials 2021;22:522. 10.1186/s13063-021-05500-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IPAQ Research Committee . Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms, 2005. Available: https://sites.google.com/site/theipaq/scoring-protocol [Accessed 24 January 2022].
- 17.World Health Organization . Global recommendations on physical activity for health. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 18.Feng Y-S, Kohlmann T, Janssen MF, et al. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res 2021;30:647–73. 10.1007/s11136-020-02688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M, Tan RS, Lim SL, et al. Investigating 5-Level EQ-5D (EQ-5D-5L) values based on preferences of patients with heart disease. Value Health 2022;25:451–60. 10.1016/j.jval.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Whitton C, JC H, Tay Z, et al. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary intakes in a multi-ethnic Asian population using 24-h dietary recalls and biomarkers. Nutrients 2017;9:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Prevention Program Research Group . The diabetes prevention program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindström J, Louheranta A, Mannelin M, et al. The Finnish diabetes prevention study (Dps): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–6. 10.2337/diacare.26.12.3230 [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Watanabe M, Nishida J, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–60. 10.1001/archinternmed.2011.275 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation, 2006. Available: https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf
- 25.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34 Suppl 1:S62–9. 10.2337/dc11-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Paul SK, Zhou X, et al. Rationale, design, and baseline characteristics of Beijing prediabetes reversion program: a randomized controlled clinical trial to evaluate the efficacy of lifestyle intervention and/or pioglitazone in reversion to normal glucose tolerance in prediabetes. J Diabetes Res 2017;2017:7602408. 10.1155/2017/7602408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathish T, Oldenburg B, Tapp RJ, et al. Baseline characteristics of participants in the Kerala diabetes prevention program: a cluster randomized controlled trial of lifestyle intervention in Asian Indians. Diabet Med 2017;34:647–53. 10.1111/dme.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. WHO Expert Consultation, WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 29.Ministry of Health, Singapore . National population health survey, 2020. Available: https://www.moh.gov.sg/docs/librariesprovider5/default-document-library/nphs-2020-survey-report.pdf
- 30.Health Promotion Board, Singapore . National Nutrition Survey 2018 Shows Gradual Improvements in Singaporeans’ Dietary Habits, 2018. Available: https://www.hpb.gov.sg/article/national-nutrition-survey-2018-shows-gradual-improvements-in-singaporeans-dietary-habits
- 31.Bärebring L, Palmqvist M, Winkvist A, et al. Gender differences in perceived food healthiness and food avoidance in a Swedish population-based survey: a cross sectional study. Nutr J 2020;19:140. 10.1186/s12937-020-00659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westenhoefer J. Age and gender dependent profile of food choice. Forum Nutr 2005;57:44–51. 10.1159/000083753 [DOI] [PubMed] [Google Scholar]
- 33.Wardle J, Haase AM, Steptoe A, et al. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med 2004;27:107–16. 10.1207/s15324796abm2702_5 [DOI] [PubMed] [Google Scholar]
- 34.Grzymisławska M, Puch EA, Zawada A, et al. Do nutritional behaviors depend on biological sex and cultural gender? Adv Clin Exp Med 2020;29:165–72. 10.17219/acem/111817 [DOI] [PubMed] [Google Scholar]
- 35.Arganini C, Saba A, Comitato R, et al. Gender differences in food choice and dietary intake in modern western societies. In: Maddock J, ed. Public health – social and behavioral health. Rijeka (Croatia): InTech, 2012. https://www.intechopen.com/chapters/36935 [Google Scholar]
- 36.RY N, Wong YS, Yeo JY, et al. The associations between dietary practices and dietary quality, biological health indicators, perceived stress, religiosity, culture, and gender in multicultural Singapore. J Ethn Foods 2018;5:220–7. [Google Scholar]
- 37.Alva ML, Romaire M, Acquah J. Impact of financial incentives on diabetes prevention class attendance and program completion: evidence from Minnesota, montana, and new York. Am J Health Promot 2019;33:601–5. 10.1177/0890117118794087 [DOI] [PubMed] [Google Scholar]
- 38.Desai JR, Vazquez-Benitez G, Taylor G, et al. The effects of financial incentives on diabetes prevention program attendance and weight loss among low-income patients: the we can prevent diabetes cluster-randomized controlled trial. BMC Public Health 2020;20:1587. 10.1186/s12889-020-09683-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanEpps EM, Troxel AB, Villamil E, et al. Effect of Process- and Outcome-Based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot 2019;33:372–80. 10.1177/0890117118783594 [DOI] [PubMed] [Google Scholar]
- 40.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–7. 10.2337/dc06-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish diabetes prevention study. Lancet 2006;368:1673–9. 10.1016/S0140-6736(06)69701-8 [DOI] [PubMed] [Google Scholar]
- 42.Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care 2014;37:943–9. 10.2337/dc13-1954 [DOI] [PubMed] [Google Scholar]
- 43.West DS, Krukowski RA, Finkelstein EA, et al. Adding financial incentives to online group-based behavioral weight control: an RCT. Am J Prev Med 2020;59:237–46. 10.1016/j.amepre.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpp KG, John LK, Troxel AB, et al. A randomized controlled trial of financial incentives for weight loss. JAMA 2008;300:2631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelstein EA, Tham K-W, Haaland BA, et al. Applying economic incentives to increase effectiveness of an outpatient weight loss program (TRIO) - A randomized controlled trial. Soc Sci Med 2017;185:63–70. 10.1016/j.socscimed.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 46.Department of Statistics, Ministry of Trade & Industry, Singapore . Census of population 2020 statistical release 1: demographic characteristics, education, language and religion, 2021. Available: https://www.singstat.gov.sg/publications/reference/cop2020/cop2020-sr1/census20_stat_release1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.