Abstract
In this study, we cryopreserved pig spermatozoa using carboxylated poly-L-lysine (CPLL) as the cryoprotectant to determine its efficacy. Pig spermatozoa were placed in a freezing extender containing 3% (v/v) glycerol and different CPLL concentrations. The motility indices of the spermatozoa cryopreserved with 0.25% (v/v) CPLL at 6 (59.3), 9 (53.7), and 12 (26.2) h after thawing were significantly higher (P < 0.01 or P < 0.05) than those of the spermatozoa cryopreserved without CPLL (53.7, 40.1, and 17.5 at 6, 9, and 12 h after thawing, respectively). The concentration of CPLL in the freezing extender did not affect the ability of frozen-thawed spermatozoa to fertilize oocytes in vitro. However, the blastocyst formation rate of embryos derived from spermatozoa cryopreserved with 0.25% CPLL (24.6%) was significantly higher (P < 0.01) than that of embryos derived from spermatozoa cryopreserved without CPLL (11.2%). The conception rate of the sows inseminated with spermatozoa cryopreserved with 0.25% CPLL (72.2%) was not significantly different from that of the sows inseminated with spermatozoa stored at 17°C (81.3%). However, the mean number of total piglets born to the former (10.0) was significantly lower (P < 0.05) than that of total piglets born to the latter (13.4). The results showed that CPLL in the freezing extender maintained the motility of frozen-thawed pig spermatozoa and improved the in vitro development of embryos produced by in vitro fertilization. In addition, we have demonstrated that piglets could be obtained with artificial insemination using spermatozoa cryopreserved with CPLL.
Keywords: Carboxylated poly-L-lysine, Cryopreservation, Cryoprotectant, Pig, Spermatozoa
Fresh or refrigerated mammalian spermatozoa lose viability and motility several days after ejaculation. Hence, cryopreservation of spermatozoa is the best way of preserving the genetic resources of males with superior genotypes for an extended duration of time and is an effective way of performing artificial insemination (AI). During the freezing process, spermatozoa are subjected to physical and chemical influences, such as changes in temperature and osmotic pressure, which reduce their viability, motility, fertilization capacity, and reproductive performance after AI [1, 2]. Therefore, a lot of research has been conducted regarding freezing extenders, freezing methods, and cryoprotectants to minimize the effects on spermatozoa [2]. The water in the acrosome of spermatozoa expands during freezing and damages the sperm head. Cryoprotectants prevent the disintegration of cell membranes and cytoplasm damage caused by the formation of ice crystals. Many laboratories use glycerol to freeze spermatozoa [3]. However, the freezing and thawing process damages pig spermatozoa even in the presence of glycerol, reducing their fertilization capacity and reproductive performance after AI [4]. As a result, in pigs, frozen-thawed spermatozoa are used in less than 1% of all AI performed worldwide [5]. Thus, there is an urgent need to develop a highly efficient and low toxicity cryoprotectant for pig spermatozoa.
Carboxylated poly-L-lysine (CPLL) is an ampholytic polymer compound obtained by synthesizing an aqueous solution of ε-poly-L-lysine and succinic anhydride and then converting 65 mol% of the amino groups to carboxyl groups [6]. It has been reported that CPLL exhibits higher cryopreservation efficiency and lower cytotoxicity than dimethyl sulfoxide (DMSO), which has been used for several decades as the most efficient cryoprotectant for many types of cells and tissues [6,7,8]. Nuclear magnetic resonance experiments have demonstrated that the water, sodium-ion, and polymer-chain signals in the CPLL solution broaden upon cooling, indicating increasingly restricted mobility and increased solution viscosity. In addition, strong intermolecular interactions promote the glass transition of CPLL, trapping water and salt in the gaps of the reversible matrix and preventing intracellular ice formation and osmotic shock during freezing. The reduction in the cellular stress may be responsible for cryoprotection [9]. Recent studies have shown that CPLL is also effective for cryopreservation of germ cells such as pig embryos at the pronuclear stage [10] and bovine, buffalo, rabbit, and human spermatozoa [11,12,13,14]. In the present study, we investigated the efficacy of CPLL as a cryoprotectant for pig spermatozoa.
Materials and Methods
Ethics statement
In the present study, semen collection from boars and AI of sows were outsourced to the Chiran piglet supply center, a local pig farm that routinely produces piglets using AI with spermatozoa stored at 17°C. The work was performed according to the Institutional Guidelines for Animal Experiments and in compliance with the Japanese Act on Welfare and Management of Animals (Act No. 105 and Notification No. 6).
Preparation of CPLL
To synthesize CPLL, 100 ml of 25% (w/w) ε-poly-L-lysine aqueous solution (JNC Corporation, Tokyo, Japan) and 12.7 g of succinic anhydride (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) were mixed and allowed to react at 50°C for 1 h [6].
Freezing extender
A basic freezing extender, Niwa and Sasaki Freezing extender [15] with a modification (mNSF), composed of 80% (v/v) 0.31 M trehalose anhydrous (Hayashibara Co., Ltd., Okayama, Japan), 20% (v/v) egg yolk, 0.075 mg/ml amikacin sulfate (Meiji Seika Pharma Co., Ltd., Tokyo, Japan), 0.025 mg/ml dibekacin sulfate (Meiji), and 250 U/ml penicillin G potassium (Meiji) was used. Modified NSF-1 (mNSF-1) was prepared by adding 0.74% (v/v) Equex-STM paste (Reproduction Provisions LLC, Walworth, WI, USA) to mNSF. Modified NSF-2 (mNSF-2) was prepared by adding 0.74% Equex-STM paste, 6% (v/v) glycerol (Nacalai Tesque, Kyoto, Japan), and different concentrations of CPLL to mNSF.
Freezing and thawing of spermatozoa
The sperm-rich fraction of the semen was collected from Duroc boars using the gloved-hand technique at the local pig farm. The semen was diluted 4 times with HIRO-SWINE B solution (Hiroshima Cryo-preservation Service, Hiroshima, Japan) supplemented with 0.1% (v/v) bayrocin (5% injection solution; Bayer Yakuhin, Ltd., Osaka, Japan) and transported to the laboratory at 25°C. The diluted semen was centrifuged at 1710 ×g for 15 min at 25°C to remove the seminal plasma and HIRO-SWINE B solution. The precipitated spermatozoa were gently suspended with Modena solution [16] (10.0 × 108 spermatozoa/ml) and then cooled for 2.5 h at 25°C and 1.5 h at 15°C. The sperm suspension was centrifuged at 1710 ×g for 15 min at 15°C, and the supernatant was removed. After removal, the precipitated spermatozoa were gently resuspended in mNSF-1, and the concentration of spermatozoa was adjusted to 20.0 × 108/ml. The sperm suspension was further cooled for 30 min at 15°C, for 1.5 h at 10°C, and for 1 h at 5°C. After cooling, the sperm suspension was diluted in the same volume of mNSF-2 (10.0 × 108 spermatozoa/ml). The final concentrations of CPLL were adjusted to 0, 0.125, 0.25, and 0.5% (v/v). The sperm suspension was held for 20 min at 5°C for glycerol equilibration and was subsequently transferred into 0.5 ml plastic straws. The straws were placed in liquid nitrogen vapor for 10 min and stored in liquid nitrogen.
During thawing, the straw with frozen spermatozoa was removed from liquid nitrogen and held for 8 sec at room temperature. Then, it was transferred to water maintained at 37°C for 15 sec. After thawing, the sperm suspension was diluted in 4.5 ml of HEPES (Nacalai)-buffered Tyrode solution containing lactate, pyruvate, and polyvinyl alcohol (HEPES-TLP-PVA) [17].
In vitro maturation of oocytes
The methods employed for the collection and in vitro maturation of oocytes were based on those described in previous reports [17,18,19]. Briefly, pig ovaries were collected from prepubertal gilts at a local slaughterhouse and transported at 35–38°C in saline to the laboratory. The cumulus-oocyte complexes (COCs) were recovered from follicles (2‒6 mm in diameter) and washed twice with HEPES-TLP-PVA. Only those COCs with a compact cumulus mass and evenly granulated ooplasm were selected, transferred to droplets (200 µl) of maturation medium in groups of 40 to 50 under paraffin oil (Nacalai) in a 35 mm polystyrene dish (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), and cultured for 36 to 38 h at 38.5°C in an atmosphere with 5% CO2 in air. The maturation medium was composed of 90% (v/v) TCM-199 with Earle’s salts (Gibco BRL, Grand Island, NY, USA) supplemented with 0.91 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO, USA), 3.05 mM D-glucose (FUJIFILM Wako), 0.57 mM cysteine hydrochloride hydrate (Sigma), 10 ng/ml epidermal growth factor (Sigma), 10 IU/ml eCG (Aska Pharmaceutical Co., Ltd., Tokyo, Japan), 10 IU/ml hCG (Aska), 100 µg/ml amikacin sulfate, 0.1% (w/v) PVA (Sigma), and 10% (v/v) pig follicular fluid.
In vitro fertilization
After in vitro maturation, the COCs were transferred into a 15 ml tube containing 2 ml of HEPES-TLP-PVA supplemented with 0.1% (w/v) hyaluronidase (Sigma) and vortexed for 90 sec to remove cumulus cells from oocytes. Denuded oocytes were transferred to droplets (80 µl) of fertilization medium in groups of 15 to 20 under paraffin oil in a 35 mm polystyrene dish. The fertilization medium was composed of 114.0 mM NaCl (Nacalai), 3.2 mM KCl (Nacalai), 6.76 mM CaCl2·2H2O (Nacalai), 0.5 mM MgCl2·6H2O (Nacalai), 0.1 mM sodium pyruvate, 10.0 mM sodium lactate (Sigma), 0.35 mM NaH2PO4·2H2O (Nacalai), 5.0 mM D-glucose, 25.0 mM NaHCO3 (Nacalai), 0.3% (w/v) bovine serum albumin (Fraction V; Sigma), 100 µg/ml amikacin sulfate, and 2.0 mM caffeine (Sigma). The frozen spermatozoa were thawed immediately before insemination, as described above. HEPES-TLP-PVA containing frozen-thawed spermatozoa was centrifuged at 760 ×g for 10 min at 38°C, and the supernatant was removed. The precipitated spermatozoa were gently suspended in the fertilization medium at a concentration of 3.5 × 107 spermatozoa/ml, and 20 µl of this sperm suspension was introduced into the 80 µl droplet that contained denuded oocytes at a final concentration of 7.0 × 106 spermatozoa/ml. The oocytes and spermatozoa were then cultured for 12 h at 38.5°C in an atmosphere with 5% CO2 in air.
Experimental designs
In Experiment 1, the effects of CPLL in the freezing extender on the motility of frozen-thawed spermatozoa were examined. After thawing, HEPES-TLP-PVA containing spermatozoa was centrifuged at 760 ×g for 10 min at 38°C, and the supernatant was removed. The precipitated spermatozoa were gently suspended in 1 ml of the fertilization medium without caffeine and incubated at 38.5°C in an atmosphere with 5% CO2 in air. After incubation for 0, 3, 6, 9, and 12 h, 20 µl of the sperm suspension was introduced into an 80 µl droplet of the fertilization medium, and the motility of spermatozoa was subjectively estimated under an inverted microscope. The motility of spermatozoa was expressed as a motility index, which was calculated using the following formula:
Motility index = (100r + 75x + 50y + 25z) / 100
where r, x, y, and z are percentages of spermatozoa with the most active forward movement, active forward movement, slow forward movement, and rotative or pendulum-like movement when considering all spermatozoa in the field of view of the microscope, respectively.
In Experiment 2, the effects of CPLL in the freezing extender on the ability of frozen-thawed spermatozoa to fertilize oocytes in vitro were examined. After culture with spermatozoa for 12 h, oocytes were mounted, fixed for 48 h in 25% (v/v) acetic acid (Nacalai) in ethanol (Nacalai) at room temperature, stained with 1% (w/v) orcein (FUJIFILM Wako) in 45% (v/v) acetic acid, and examined for sperm penetration under a Nomarski differential interference microscope. Oocytes with a first polar body and metaphase II chromosomes and those penetrated by spermatozoa were considered mature. When oocytes had one or more swollen sperm heads and/or male pronuclei and corresponding sperm tails, they were considered penetrated. Polyspermic oocytes containing at least one male pronucleus were classified as oocytes penetrated with male pronuclei.
In Experiment 3, the effects of CPLL in the freezing extender on the in vitro developmental ability of embryos derived from frozen-thawed spermatozoa were determined. After culture with spermatozoa for 12 h, oocytes were transferred to 50 µl droplets of modified PZM-3 [17] and were cultured at 38.5°C in an atmosphere with 5% CO2, 5% O2, and 90% N2. The oocytes were assessed for cleavage and blastocyst formation on second and seventh day of culture, respectively. At the end of the culture period, the blastocysts were placed on a slide with a drop of mounting medium composed of glycerol and phosphate-buffered saline (9:1) containing 100 µg/ml Hoechst 33342 (Sigma). A coverslip was placed on the blastocysts, and the nuclei were counted under ultraviolet light.
In Experiment 4, the ability of spermatozoa cryopreserved with CPLL to produce offspring after AI was examined. AI was carried out at the same pig farm where semen was collected from the boars. Sows (Landrace × Large White) were given a single injection of 1000 IU of eCG (PMS-A 1000 U; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) on the weaning day, and estrus was detected 4 to 6 days after eCG administration using back pressure reaction. The sows were artificially inseminated three times at 4‒7 h, 23‒26 h, and 28‒31 h after the confirmation of estrus using an intrauterine insemination method [20]. Spermatozoa cryopreserved in the freezing extender containing 0.25% CPLL were transported to the pig farm and thawed immediately before AI. The freezing extender containing frozen-thawed spermatozoa recovered from 10 straws was diluted in 45 ml of HIRO-SWINE B solution, and the total amount of the sperm suspension (5.0 × 109 spermatozoa/50 ml) was injected each time. Some sows were artificially inseminated using spermatozoa collected from the same boars and stored at 17°C. Collected semen was diluted in HIRO-SWINE B solution to a final concentration of 3.0 × 107 spermatozoa/ml, and 80 ml of the sperm suspension was transferred into each semen bottle. The bottles were stored at 17°C for 7 to 10 days, and the entire amount of the sperm suspension in a bottle (2.4 × 109 spermatozoa/80 ml) was injected at one time. The pregnancy of the sows was confirmed by the non-return of the estrus at 21 to 28 days after AI, and the pregnant sows were allowed to go to term.
Statistical analysis
Data, except for conception rates determined in Experiment 4, were analyzed by one-way ANOVA followed by Fisher’s protected least significant difference test. Percentage data were subjected to arcsine transformation before ANOVA. The homogeneity of variance was assessed using Bartlett’s test. Conception rates were analyzed using chi-squared test. A probability of P < 0.05 was considered statistically significant.
Results
Experiment 1
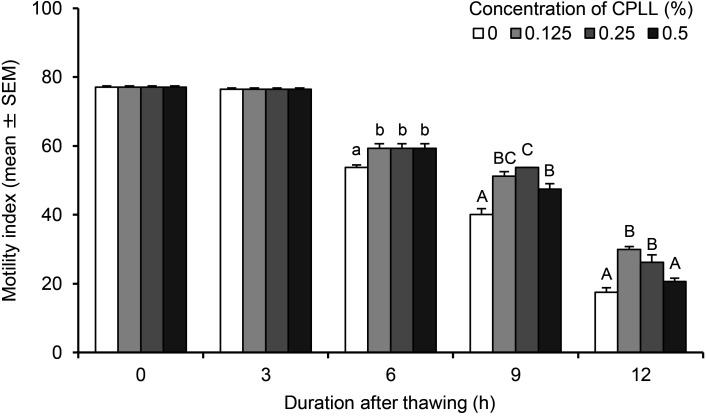
When the motility of spermatozoa was evaluated at 0 and 3 h after thawing, motility indices (77.1 ± 0.3 and 76.5 ± 0.3 at 0 and 3 h, respectively) were not observed to be affected by the different CPLL concentrations in the freezing extender (Fig. 1). However, at 6 h after thawing, the motility indices at 0.125‒0.5% CPLL (59.3 ± 1.4) were significantly higher (P < 0.05) than that at 0% CPLL (53.7 ± 0.8). Similarly, the addition of 0.125‒0.5% CPLL to the freezing extender significantly increased (P < 0.01) the motility index at 9 h after thawing compared to that at 0% CPLL (47.5 ± 1.6‒53.7 ± 0 vs. 40.1 ± 1.7). In addition, the motility index at 0.25% CPLL (53.7 ± 0) was significantly higher (P < 0.01) than that at 0.5% CPLL (47.5 ± 1.6). At 12 h after thawing, the motility indices at 0.125 and 0.25% CPLL (26.2 ± 2.2‒30.0 ± 0.8) were significantly higher (P < 0.01) than those at 0 and 0.5% CPLL (17.5 ± 1.3‒20.6 ± 1.0).
Fig. 1.
Motility index of pig spermatozoa cryopreserved with different concentrations of carboxylated poly-L-lysine (CPLL). Experiments were repeated four times. Values with different letters within each duration are significantly different (A–C: P < 0.01; a–b: P < 0.05).
Experiment 2
As shown in Table 1, the addition of 0.125‒0.5% CPLL to the freezing extender did not affect the rates of matured oocytes (75.9‒83.8%). The rates of penetrated oocytes (73.5‒77.1%) were not affected by the different CPLL concentrations. Similarly, no significant differences in the rates of oocytes with male and female pronuclei (89.6‒96.3%) and polyspermic penetration (19.4‒24.4%) were observed among the CPLL concentrations used. Consequently, the rates of normally fertilized oocytes, in which one male pronucleus and one female pronucleus were formed after monospermic penetration (68.5‒76.9%), were not affected by the different CPLL concentrations used in this study.
Table 1. Sperm penetration of pig oocytes inseminated in vitro with spermatozoa cryopreserved with different concentrations of carboxylated poly-L-lysine (CPLL) a.
| Concentration of CPLL (%) | No. of oocytes |
|||||
|---|---|---|---|---|---|---|
| Examined | Matured (mean % ± SEM) b |
Penetrated (mean % ± SEM) c |
With male and female pronuclei (mean % ± SEM) d |
Polyspermic penetrated (mean % ± SEM) d |
Monospermic with one male pronucleus and one female pronucleus (mean % ± SEM) d |
|
| 0 | 45 | 35 (77.5 ± 6.7) | 27 (77.1 ± 1.1) | 24 (89.6 ± 6.5) | 6 (20.2 ± 10.3) | 18 (69.4 ± 16.3) |
| 0.125 | 45 | 34 (75.9 ± 5.1) | 25 (73.5 ± 0.8) | 23 (91.7 ± 4.2) | 5 (20.8 ± 11.0) | 18 (70.8 ± 15.0) |
| 0.25 | 43 | 36 (83.8 ± 1.9) | 27 (75.6 ± 7.7) | 26 (96.3 ± 3.7) | 5 (19.4 ± 10.0) | 21 (76.9 ± 12.9) |
| 0.5 | 40 | 32 (79.0 ± 4.7) | 24 (74.7 ± 4.4) | 22 (93.0 ± 3.5) | 5 (24.4 ± 12.4) | 17 (68.5 ± 10.8) |
a Experiments were repeated three times. b Percentage per oocytes examined. c Percentage per oocytes matured. d Percentage per oocytes penetrated.
Experiment 3
As shown in Table 2, the cleavage rates of oocytes (25.0‒33.2%) were not affected by the different CPLL concentrations. However, the blastocyst formation rate of oocytes at 0.25% CPLL (24.6%) was significantly higher (P < 0.01) than that at 0% CPLL (11.2%). The mean numbers of cells in the blastocysts (45.8‒61.9) were not affected by the different CPLL concentrations used in this study.
Table 2. In vitro development of pig oocytes inseminated in vitro with spermatozoa cryopreserved with different concentrations of carboxylated poly-L-lysine (CPLL) a.
| Concentration of CPLL (%) | No. of oocytes cultured | No. (mean % ± SEM) of oocytes developed to |
Mean no. ± SEM of cells in blastocysts | |
|---|---|---|---|---|
| ≥ 2-cell (2) b | Blastocyst (7) b | |||
| 0 | 158 | 40 (25.0 ± 9.3) | 18 (11.2 ± 2.7) c | 61.9 ± 5.3 |
| 0.125 | 154 | 41 (26.9 ± 7.9) | 26 (16.0 ± 4.2) cd | 55.4 ± 4.2 |
| 0.25 | 159 | 42 (26.4 ± 7.8) | 39 (24.6 ± 2.5) d | 54.0 ± 4.3 |
| 0.5 | 145 | 48 (33.2 ± 6.7) | 28 (19.6 ± 2.1) cd | 45.8 ± 3.8 |
a Experiments were repeated five times. b Numbers in parentheses indicate the time of examination (days of culture). c–d Values with different superscripts are significantly different (P < 0.01).
Experiment 4
When spermatozoa cryopreserved with 0.25% CPLL were used for AI, 72.2% of the inseminated sows became pregnant and farrowed piglets (Table 3). This conception rate was not significantly different from that of sows inseminated using spermatozoa stored at 17°C (81.3%). The mean number of total piglets born to the sows inseminated using spermatozoa cryopreserved with 0.25% CPLL (10.0) was significantly lower (P < 0.05) than that of total piglets born to the sows inseminated using spermatozoa stored at 17°C (13.4). However, the mean number of living piglets (8.7‒11.5) was not significantly different between these two groups of sows. The preservation methods used for spermatozoa did not affect the mean weight of the alive piglets (1.46‒1.58 kg).
Table 3. Conception and farrow of sows artificially inseminated with spermatozoa cryopreserved with 0.25% carboxylated poly-L-lysine (CPLL) or stored at 17°C.
| Spermatozoa | No. of sows inseminated | No. (%) of sows conceived | No. (mean no. ± SEM) of piglets |
Mean weight ± SEM of alive piglets (kg) | |
|---|---|---|---|---|---|
| Total | Alive | ||||
| Cryopreserved with 0.25% CPLL | 18 | 13 (72.2) | 130 (10.0 ± 1.0) a | 113 (8.7 ± 1.0) | 1.58 ± 0.05 |
| Stored at 17ºC | 16 | 13 (81.3) | 174 (13.4 ± 0.9) b | 149 (11.5 ± 1.0) | 1.46 ± 0.05 |
a–b Values with different superscripts are significantly different (P < 0.05).
Discussion
One of the major factors that affect the normality of cryopreserved spermatozoa is cryoprotectants. For bovine spermatozoa, for which cryopreservation technology has been established, a freezing extender containing approximately 6% glycerol has been used [21]. However, in the case of pigs, the addition of 6% glycerol to the freezing extender is highly toxic and reduces the viability of spermatozoa, and hence, about 3% glycerol is used instead [22]. Therefore, it is thought that the effect of glycerol as a cryoprotectant for pig spermatozoa has not been fully established, resulting in low survival and motility rates.
Although DMSO is widely used as a cryoprotectant for various types of cells [23,24,25,26,27], it is cytotoxic because of high osmotic pressure [28, 29]. The effectiveness of CPLL as a cryoprotectant was examined in comparison with DMSO. It was reported that CPLL has a lower osmotic pressure than DMSO because of its high molecular weight and is effective in inhibiting ice recrystallization [6]. Therefore, CPLL did not exhibit cytotoxicity in mouse L929 cells or human dermal fibroblasts, even at concentrations as high as 20‒25% [6, 7]. Furthermore, when bovine fibroblasts were cryopreserved using CPLL as a cryoprotectant, good proliferation was observed even when they were thawed and cultured without removing the added CPLL [8]. This phenomenon was not observed when using DMSO, indicating that the toxicity of CPLL is lower than that of DMSO [8]. In addition, the membrane integrity rate of cryopreserved bovine spermatozoa and conception rate after AI were improved by reducing the glycerol concentration in the freezing extender from 6.5% to 3.25% and adding 0.5% CPLL [11]. Consequently, the improvement in the motility of frozen-thawed pig spermatozoa and in vitro development of derived embryos in the present study could be attributed to the addition of CPLL to the freezing extender, which enhanced the cryoprotective effect without increasing the toxic effect. The most suitable concentration of CPLL in the presence of 3% glycerol for pig spermatozoa was 0.25%, whereas the most suitable concentration of CPLL in the presence of 3.25% glycerol for bovine spermatozoa was 0.5% [11]. Moreover, the most suitable concentrations of CPLL have been reported to be 0.75% in the presence of 5% glycerol for buffalo spermatozoa [12], 0.3% in the presence of 7% glycerol for human spermatozoa [14] and 1% in the presence of 8% DMSO for rabbit spermatozoa [13]. These results suggest that the optimal concentration of CPLL for cryopreservation of spermatozoa varies with the species.
The results showed that CPLL in the freezing extender does not affect the ability of frozen-thawed pig spermatozoa to fertilize oocytes in vitro. Cumulus-free oocytes were inseminated with frozen-thawed spermatozoa in a caffeine-containing medium. Abeydeera and Day [30] and Tanihara et al. [31] have reported that penetration of pig spermatozoa is completed within 5‒6 h after insemination under the same conditions. Because the motility of spermatozoa at 0 and 3 h after thawing was not affected by different concentrations of CPLL in the freezing extender, spermatozoa were likely able to penetrate oocytes, regardless of the presence of CPLL.
However, CPLL in the freezing extender improved the blastocyst formation rate of embryos produced by in vitro fertilization with frozen-thawed pig spermatozoa, although it did not affect their cleavage rate. Jarrell et al. [32] have reported that a fertilized oocyte of a pig synthesizes proteins with mRNAs from the oocyte until the 4-cell stage, after which protein synthesis is conducted using the mRNAs from the embryo. Therefore, the maternal DNA of the oocyte and the paternal DNA of the spermatozoon are important for embryonic development after the 4-cell stage. In vitro fertilization experiments using gamma-irradiated spermatozoa showed that even spermatozoa with severe DNA damage were functionally intact at the membrane-, organelle-, and motility-parameter levels. The spermatozoa with DNA damage showed normal binding characteristics to the zona pellucida, and the fertilization rate of the oocytes and their cleavage rate remained normal. However, embryos obtained by in vitro fertilization using DNA-damaged spermatozoa underwent an apoptotic process and died at the 4- to 8-cell stage, possibly because of the abnormal expression of the embryonic DNA [33]. Thus, reproductive defects caused by the paternal DNA abnormalities do not appear at the fertilization level but at the beginning of the embryonic DNA expression. Freezing and thawing procedures affect the paternal DNA status in pig spermatozoa. Immediately after thawing, there is no apparent increase in DNA fragmentation [34], but after thawing and incubation at 37°C, the percentage of spermatozoa with different degrees of DNA damage increases [35, 36]. Therefore, the results of the present study suggest that CPLL might have the ability to maintain the paternal DNA of spermatozoa in a normal state during cryopreservation. This hypothesis is consistent with the lack of any effect of the presence or absence of CPLL in the freezing extender on in vitro fertilization potential of cryopreserved spermatozoa. In this context, it has recently been reported that the expression of genes related to cell adhesion and proliferation of cryopreserved human induced pluripotent stem cells is more similar to that of unfrozen human induced pluripotent stem cells in the CPLL-based freezing solution than in the DMSO-based freezing solution [37].
The results of the present study indicate that pig spermatozoa cryopreserved with CPLL could be used for offspring production via AI. In Japan, Shimada and his co-workers have led research in this field and have obtained a conception rate of 80.2% and litter size of 10.1 piglets using the freezing extender adjusted to high osmotic pressure and low glycerol concentration by adding polymyxin B and seminal plasma to the pretreatment and thawing solution, respectively [38]. The conception rate of sows and mean number of total piglets after AI using spermatozoa cryopreserved with CPLL are comparable to their results. However, this litter size is small compared to those born to sows inseminated with spermatozoa stored at 17°C. In addition, the mean number of live piglets also tended to be lower (P = 0.053). Therefore, we believe that there is a room for further improvement.
In conclusion, we have established a cryopreservation protocol using CPLL as a cryoprotectant for pig spermatozoa. This protocol can be effectively used to preserve the genetic resources of boars with superior genotypes. However, further refinement of this protocol might be needed to use cryopreserved spermatozoa instead of fresh or refrigerated spermatozoa for pig production on commercial farms.
Conflicts of interests
The authors have no conflicts of interest to declare.
Acknowledgments
The authors express their gratitude toward the staff at the Kagoshima City Meat Inspection Office and Meat Center Kagoshima (Kagoshima, Japan) for supplying pig ovaries, and the staff at the Chiran Piglet Supply Center (Kagoshima, Japan) for their technical assistance. The authors would also like to thank Editage (www.editage.com) for English language editing. The present study was supported by Grants for Livestock Promotion from the Japan Racing Association and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 20K06472 to Kazuchika Miyoshi). This study is a part of a dissertation submitted by the first author in partial fulfillment of the Ph.D. degree; all authors have provided their consent for this.
References
- 1.Purdy PH. A review on goat sperm cryo-preservation. Small Rumin Res 2006; 63: 215–225. [Google Scholar]
- 2.Yeste M. Recent advances in boar sperm cryopreservation: state of the art and current perspectives. Reprod Domest Anim 2015; 50(Suppl 2): 71–79. [DOI] [PubMed] [Google Scholar]
- 3.Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett 2004; 25: 375–388. [PubMed] [Google Scholar]
- 4.Johnson LA, Aalbers JG, Arts JAM. Use of boar spermatozoa for artificial insemination. II. Fertilizing capacity of fresh and frozen spermatozoa in gilts inseminated either at a fixed time or according to Walsmeta readings. J Anim Sci 1982; 54: 126–131. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Gil JE, Estrada E. Artificial insemination in boar reproduction. In: Bonet S, Casas I, Holt WV, Yeste M (eds.), Boar Reproduction. Berlin: Springer; 2013: 589–608. [Google Scholar]
- 6.Matsumura K, Hyon SH. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials 2009; 30: 4842–4849. [DOI] [PubMed] [Google Scholar]
- 7.Jin OS, Lee JH, Shin YC, Lee EJ, Lee JJ, Matsumura K, Hyon SH, Han DW. Cryoprotection of fibroblasts by carboxylated poly-L-lysine upon repeated freeze/thaw cycles. Cryo Lett 2013; 34: 396–403. [PubMed] [Google Scholar]
- 8.Fujikawa T, Ando T, Gen Y, Hyon SH, Kubota C. Cryopreservation of bovine somatic cells using antifreeze polyamino-acid (carboxylated poly-L-lysine). Cryobiology 2017; 76: 140–145. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura K, Hayashi F, Nagashima T, Rajan R, Hyon SH. Molecular mechanisms of cell cryopreservation with polyampholytes studied by solid-state NMR. Commun Mater 2021; 2: 15. [Google Scholar]
- 10.Kamoshita M, Kato T, Fujiwara K, Namiki T, Matsumura K, Hyon SH, Ito J, Kashiwazaki N. Successful vitrification of pronuclear-stage pig embryos with a novel cryoprotective agent, carboxylated ε-poly-L-lysine. PLoS One 2017; 12: e0176711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujikawa T, Imamura S, Tokumaru M, Ando T, Gen Y, Hyon SH, Kubota C. Cryoprotective effect of antifreeze polyamino-acid (Carboxylated Poly-l-Lysine) on bovine sperm: A technical note. Cryobiology 2018; 82: 159–162. [DOI] [PubMed] [Google Scholar]
- 12.Tariq A, Ahmad M, Iqbal S, Riaz MI, Tahir MZ, Ghafoor A, Riaz A. Effect of carboxylated poly l-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen. Theriogenology 2020; 144: 8–15. [DOI] [PubMed] [Google Scholar]
- 13.Küçük N, Raza S, Matsumura K, Uçan U, Serin İ, Ceylan A, Aksoy M. Effect of different carboxylated poly l-lysine and dimethyl sulfoxide combinations on post thaw rabbit sperm functionality and fertility. Cryobiology 2021; 102: 127–132. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi H, Nishioka M, Maezawa T, Kitano Y, Terada-Yoshikawa K, Tachibana R, Kato M, Hyon SH, Gen Y, Tanaka K, Toriyabe K, Nii M, Kondo E, Ikeda T. Carboxylated poly-L-lysine as a macromolecular cryoprotective agent enables the development of defined and xeno-free human sperm cryopreservation reagents. Cells 2021; 10: 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi K, Kashiwazaki N, Nagai T, Noguchi J, Shimada A, Takahashi R, Hirabayashi M, Shino M, Ueda M, Kaneko H. Reproduction in pig using frozen-thawed spermatozoa from epididymis stored at 4 C. J Reprod Dev 1999; 45: 345–350. [Google Scholar]
- 16.Funahashi H, Sano T. Select antioxidants improve the function of extended boar semen stored at 10 °C. Theriogenology 2005; 63: 1605–1616. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Yoshida M, Miyoshi K. Utility of ultrasound stimulation for activation of pig oocytes matured in vitro. Mol Reprod Dev 2005; 72: 396–403. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi K, Inoue S, Himaki T, Mikawa S, Yoshida M. Birth of cloned miniature pigs derived from somatic cell nuclear transferred embryos activated by ultrasound treatment. Mol Reprod Dev 2007; 74: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi K, Kawaguchi H, Maeda K, Sato M, Akioka K, Noguchi M, Horiuchi M, Tanimoto A. Birth of cloned microminipigs derived from somatic cell nuclear transfer embryos that have been transiently treated with valproic acid. Cell Reprogram 2016; 18: 390–400. [DOI] [PubMed] [Google Scholar]
- 20.Watson PF, Behan JR. Intrauterine insemination of sows with reduced sperm numbers: results of a commercially based field trial. Theriogenology 2002; 57: 1683–1693. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer RG, Berndtson WE, Ennen BD, Pickett BW. Influence of glycerol concentration and freezing rate on post-thaw motility of bovine spermatozoa in Continental straws. J Dairy Sci 1976; 59: 2134–2137. [DOI] [PubMed] [Google Scholar]
- 22.Corcuera BD, Marigorta P, Sagüés A, Saiz-Cidoncha F, Pérez-Gutiérrez JF. Effect of lactose and glycerol on the motility, normal apical ridge, chromatin condensation and chromatin stability of frozen boar spermatozoa. Theriogenology 2007; 67: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 23.Chang Q, Cheng CC, Jing H, Sheng CJ, Wang TY. Cryoprotective effect and optimal concentration of trehalose on aortic valve homografts. J Heart Valve Dis 2015; 24: 74–82. [PubMed] [Google Scholar]
- 24.Kuliková B, Di Iorio M, Kubovicova E, Kuzelova L, Iaffaldano N, Chrenek P. The cryoprotective effect of Ficoll on the rabbit spermatozoa quality. Zygote 2015; 23: 785–794. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura Y, Ujihira M, Nogawa S, Kimura K, Ichikawa H, Mabuchi K. Investigation of the influence of cell density of human fibroblasts cryopreserved inside collagen sponges at various cooling rates. Cryo Lett 2007; 28: 337–346. [PubMed] [Google Scholar]
- 26.Naaldijk Y, Staude M, Fedorova V, Stolzing A. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol 2012; 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu ZW, Quinn PJ. Dimethyl sulphoxide: a review of its applications in cell biology. Biosci Rep 1994; 14: 259–281. [DOI] [PubMed] [Google Scholar]
- 28.Jiang G, Bi K, Tang T, Wang J, Zhang Y, Zhang W, Ren H, Bai H, Wang Y. Down-regulation of TRRAP-dependent hTERT and TRRAP-independent CAD activation by Myc/Max contributes to the differentiation of HL60 cells after exposure to DMSO. Int Immunopharmacol 2006; 6: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 29.Young DA, Gavrilov S, Pennington CJ, Nuttall RK, Edwards DR, Kitsis RN, Clark IM. Expression of metalloproteinases and inhibitors in the differentiation of P19CL6 cells into cardiac myocytes. Biochem Biophys Res Commun 2004; 322: 759–765. [DOI] [PubMed] [Google Scholar]
- 30.Abeydeera LR, Day BN. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod 1997; 57: 729–734. [DOI] [PubMed] [Google Scholar]
- 31.Tanihara F, Nakai M, Kaneko H, Noguchi J, Otoi T, Kikuchi K. Evaluation of zona pellucida function for sperm penetration during in vitro fertilization in pigs. J Reprod Dev 2013; 59: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarrell VL, Day BN, Prather RS. The transition from maternal to zygotic control of development occurs during the 4-cell stage in the domestic pig, Sus scrofa: quantitative and qualitative aspects of protein synthesis. Biol Reprod 1991; 44: 62–68. [DOI] [PubMed] [Google Scholar]
- 33.Silva PFN, Gadella BM. Detection of damage in mammalian sperm cells. Theriogenology 2006; 65: 958–978. [DOI] [PubMed] [Google Scholar]
- 34.Flores E, Ramió-Lluch L, Bucci D, Fernández-Novell JM, Peña A, Rodríguez-Gil JE. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 2011; 76: 1450–1464. [DOI] [PubMed] [Google Scholar]
- 35.Alkmin DV, Martinez-Alborcia MJ, Parrilla I, Vazquez JM, Martinez EA, Roca J. The nuclear DNA longevity in cryopreserved boar spermatozoa assessed using the Sperm-Sus-Halomax. Theriogenology 2013; 79: 1294–1300. [DOI] [PubMed] [Google Scholar]
- 36.Yeste M, Estrada E, Casas I, Bonet S, Rodríguez-Gil JE. Good and bad freezability boar ejaculates differ in the integrity of nucleoprotein structure after freeze-thawing but not in ROS levels. Theriogenology 2013; 79: 929–939. [DOI] [PubMed] [Google Scholar]
- 37.Ota A, Hyon SH, Sumi S, Matsumura K. Gene expression analysis of human induced pluripotent stem cells cryopreserved by vitrification using StemCell Keep. Biochem Biophys Rep 2021; 28: 101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki T, Shimada M. New strategies of boar sperm cryopreservation: development of novel freezing and thawing methods with a focus on the roles of seminal plasma. Anim Sci J 2012; 83: 623–629. [DOI] [PubMed] [Google Scholar]