Abstract
Breast cancer is the leading cause of cancer death among women worldwide. In solid tumors, the microenvironment plays a critical role in tumor development, and it has been described a communication between the different cell types that conform the stroma, including fibroblasts, pericytes, adipocytes, immune cells and cancer-associated fibroblasts. Intercellular communication is bidirectional, complex, multifactorial and is mediated by the secretion of molecules and extracellular vesicles. The extracellular vesicles are vesicles limited by two membranes that are secreted by normal and cancer cells into the extracellular space. Extracellular vesicle cargo is complex and includes proteins, miRNAs, DNA and lipids, and their composition is specific to their parent cells. Extracellular vesicles are taken up for neighboring or distant cells. Particularly, extracellular vesicles from breast cancer cells are taken up for fibroblasts and it induces the activation of fibroblasts into cancer-associated fibroblasts. Interestingly, cancer associated fibroblasts release extracellular vesicles that are taken up for breast cancer cells and promote migration, invasion, proliferation, epithelial–mesenchymal transition, changes in metabolism, chemoresistance, evasion of immune system and remodeling of extracellular matrix. In addition, the enrichment of specific cargos in extracellular vesicles of breast cancer patients has been suggested to be used as biomarkers of the disease. Here we review the current literature about the intercommunication between tumor cells and cancer associated fibroblasts through extracellular vesicles in breast cancer.
Keywords: cancer-associated fibroblasts, extracellular vesicles, breast cancer, stroma, microenvironment
Introduction
Breast cancer is one of the most prevalent diseases in the world and the rate of new cases continues increasing. According to Global Cancer Statistics (2020), it has been estimated 19.3 million new cancer cases and 10 million cancer deaths in 2020.1 Over the last decades, more resources and efforts have been directed at increasing our knowledge about the molecular and cellular processes involved in cancer. In solid tumors, the stroma plays an important role in every stage of the disease, from induction of early proliferation to the creation of a metastatic niche.2 The functions carried out by stromal cells may be considered in both tumor suppression and tumor promotion, which is consistent with the complexity of this compartment.3 The stroma consists of a variety of cell types including fibroblast, myofibroblasts, cancer-associated fibroblasts (CAFs), immune, epithelial, vascular and smooth muscle cells, which secrete a variety of molecules including cytokines and extracellular matrix (ECM) components.4 The intercellular communication between the tumor and its microenvironment is bidirectional, complex, and multifactorial.2 Extracellular vesicles (EVs) play an essential role in tumor progression, because they mediate a variety of cellular processes directly related with tumor progression including migration, invasion, and the creation of a tumor supporting stroma and an environment for the recruitment and transformation of CAFs.5–8 Here we review the current literature about the intercommunication between tumor cells and CAFs mediated by EVs in breast cancer.
Extracellular Vesicles
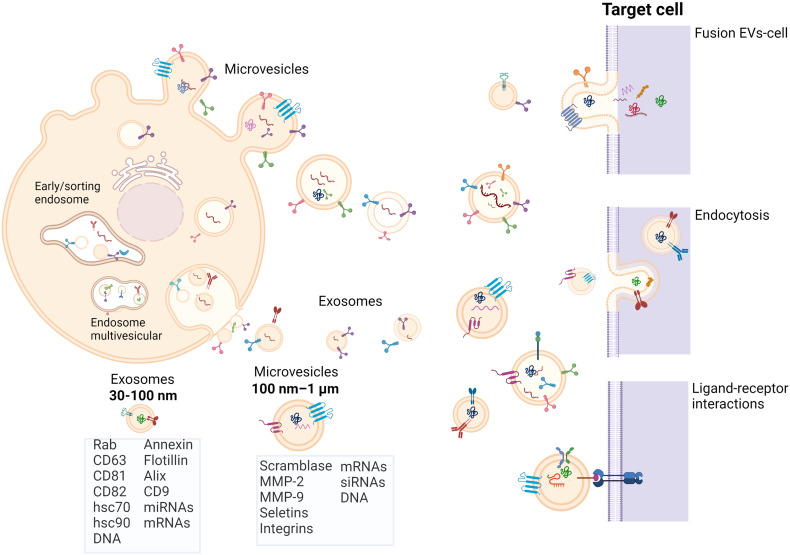
EVs are vesicles limited by two membranes that are secreted by normal and cancer cells into the extracellular space. The term “extracellular vesicle” was first used in 1971.9 However, the foundation of the International Society for Extracellular Vesicles in 2011, proposed the replacement of the terms “exosomes” and “microvesicles” with the term “extracellular vesicles,” a generic name adopted by consensus by the international scientific community.10 Although their designation remains controversial, based on size, biogenesis, or release pathways, EVs have been classified as microvesicles, exosomes, and apoptotic bodies.11–13
Exosomes
Exosomes were first reported in 1983 by Johnstone and colleagues as vesicles of endosomal origin secreted by reticulocytes during differentiation.14 Exosomes are small membrane vesicles, ranging in size from 30 to 100 nm in diameter with a cup-shaped morphology and a density ranging from 1.13 g/ml (for B cell-derived exosomes) to 1.19 g/ml (for intestinal cell-derived exosomes).15 The exosome formation process consists of four stages: initiation, endocytosis, formation of multivesicular bodies (MVBs), and exosome secretion.16 MVBs are endocytic structures created by the budding of an endosomal membrane into the lumen of the compartment. Once these structures form, they may be sorted for cargo degradation into the lysosome or released into the extracellular space as exosomes by fusing with the plasma membrane. The mechanisms underlying the sorting of the cargo into intraluminal vesicles are not fully understood, however it is suggested that exosomal sorting is mediated by both endosomal sorting complex required for transport-dependent and -independent signals.8,17 As a consequence of their endosomal origin, nearly all exosomes, independent of the cell type from which they originate, contain marker proteins involved in membrane transport and fusion (Rab GTPases, annexins, flotillin), MVBs biogenesis (Alix and TSG101), heat shock proteins (HSP70 and HSP90), integrins, and tetraspanins (CD63, CD9, CD81, and CD82). Another feature of exosomes is their enrichment in lipids present in rafts, such as cholesterol, sphingolipids, ceramide, and glycerophospolipids with long and saturated fatty-acyl chains.17–19
Microvesicles
Microvesicles bud directly from the plasma membrane and are larger than exosomes, with a size ranging from 100 nm to 1 μm.20 Shedding of microvesicles is considered a physiological phenomenon that accompanies cell activation, growth, and early apoptosis. A variety of extracellular stimuli are associated with an increase in the secretion of microvesicles including cytokines, endotoxins, hypoxia, oxidative stress, and exposure to shear stress.21–27 Moreover, a variety of intracellular factors influence microvesicle shedding, such as the increase in cytosolic calcium levels and the degradation of the membrane cytoskeleton.28–30 The composition of microvesicles is complex and includes scramblase, cholesterol, and phosphatidylserine in the outer membrane.25,31 Moreover, some microvesicles exhibit an enrichment of proteins associated with membrane lipid rafts.29,32,33
Exosomes and microvesicles interact with target cells through three mechanisms: (1) membrane fusion and subsequent transfer of their cargo; (2) endocytosis and release of cargo; and (3) ligand-receptor interactions.34–36 However, EVs also exert an effect on the ECM through the activity of matrix metalloproteinases (MMPs) embedded in their membrane, such as MMP-2 and MMP-937,38 (Figure 1).
Figure 1.
Biogenesis of EVs. Microvesicles bud directly from plasma membrane, whereas exosomes arise from multivesicular bodies. EVs interact with target cells through three mechanism: (1) EVs fusion with transfer of their cargos; (2) EVs endocytosis and release of their cargos; (3) Receptor ligand interaction with activation of signal transduction pathways. Figure created with BioRender.com (2022).
Abbreviation: EVs, extracellular vesicles.
Apoptotic Bodies
Apoptotic bodies are secreted exclusively by apoptotic cells during the late stages of apoptosis and their size varies between 50 nm and 2μm.39 The content of apoptotic bodies includes nuclear material, cellular organelles, and membrane/cytosolic fractions. The distinctive characteristics of apoptotic bodies include phosphatidylserine in the outer leaflet, a high permeability membrane, irregular shape and densities greater than 1.23 g/ml.40–42
Cancer-associated Fibroblasts
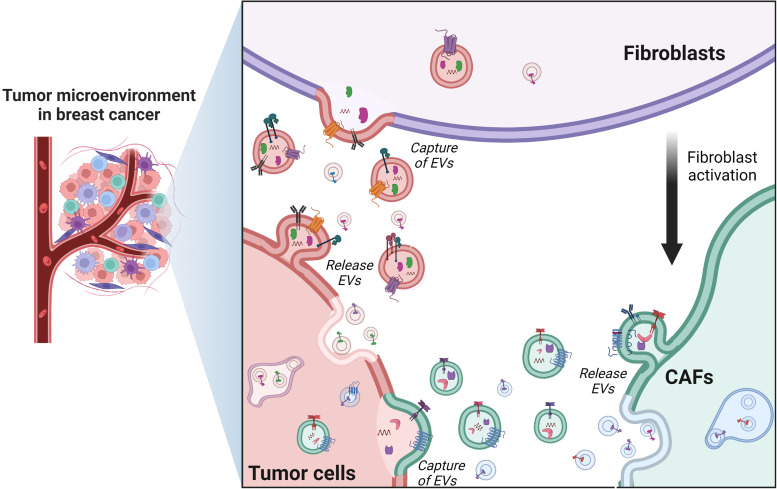
Fibroblasts are the most abundant resident cells of connective tissue, and their main function is the synthesis and maintenance of ECM components including collagen, laminin, fibronectin, and proteoglycans. Moreover, fibroblasts are a heterogeneous population that are predominantly in G0 phase, however they are found in mitosis during tissue remodeling and repair.43,44 CAFs are a group of activated fibroblasts with significant heterogeneity and plasticity in the tumor microenvironment that participate by the secretion of a variety of factors in the regulation of tumor occurrence, development, metastasis, and therapeutic resistance.44 Harold F. Dvorak stated in 1986 that “tumors are wounds that do not heal” due the similarity of some processes in the tumor microenvironment with those of wound healing, such as, proliferation, angiogenesis, survival, and migration.45,46 In agreement with Dvorak, CAFs share characteristics with myofibroblasts, a very common long-spindled cell type associated with wound healing,47,48 and constitute a significant fraction of cells in the solid tumor stroma. In breast cancer, CAFs represent around 80% of all the stromal cells,49 and are essential in the tumor microenvironment because they secrete ECM components, cytokines, EVs, growth factors, and other soluble molecules.50 The purpose of this review is to familiarize readers with aspects related with the communication between breast cancer cells and CAFs via EVs. The role of CAFs in cancer is a broad and very interesting topic, which is outside of the scope of this review. We refer to an excellent review that addresses this topic (Kalluri, 201651).
EVs from Breast Cancer Cells Promote CAFs Formation
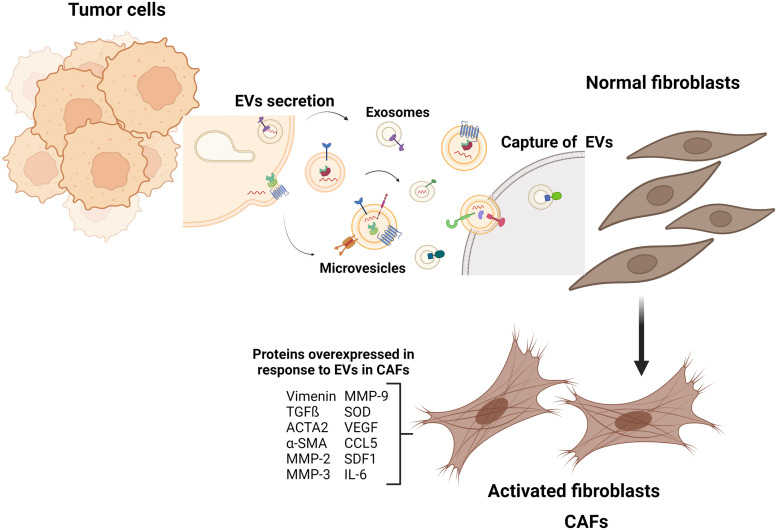
The abundance of CAFs in breast tumors is well known and the role of CAFs in cancer progression has been studied in detail, being propose that CAFs may be useful biomarkers in breast cancer.52 However, over the last decade, interest has grown with respect to the role of EVs in the development of breast cancer, because a higher number of EVs in breast cancer women compared with healthy controls has been described.53,54 CAFs from breast tumors have different origins, for instance human bone marrow mesenchymal stem cells (BM-MSCs) acquire a CAF phenotype after 30 days of exposition to tumor cell-conditioned media,55 whereas normal fibroblasts co-cultured with MDA-MB-468 breast cancer cells result in hepatocyte growth factor secretion and the enhancement of tumor growth, a behavior typical of CAFs.56 Moreover, EVs from breast cancer cells induce the acquisition of a CAFs-like phenotype in normal fibroblasts.57–60 Particularly, breast cancer cells release EVs containing miR-125b, which is a negative regulator of p53, and the transfer of miR-125b to fibroblasts results in the overexpression of markers associated with fibroblast activation, such as Acta2, MMP-2, and MMP-3, which promote a CAFs-like phenotype.57 Similarly, exosomes released from MDA-MB-231 and MCF-7 breast cancer cells, transfer miR-146a to fibroblasts, which regulates the expression of thioredoxin interacting protein (TXNIP) and activates the Wnt/beta catenin pathway, and then the induction of a CAF phenotype with their recruitment to the tumor site. In contrast, EVs released from non-tumor epithelial cells MCF10A do not induce the acquisition of a CAF phenotype58 (Figure 2).
Figure 2.
Intercommunication between CAFs and breast cancer cells. Activation of breast cancer cells induces the release of EVs that activate normal fibroblasts into CAFs. Reprinted from “Tumor microenvironment with Callout,” by BioRender.com (2022).
Abbreviations: EVs, extracellular vesicles; CAFs, cancer-associated fibroblasts.
It has been demonstrated that other cell types, different from fibroblasts, are able to acquire a CAF phenotype, such as adipocytes and adipose-derived mesenchymal stem cells (ADSCs). Particularly, EVs released from MDA-MB-231 breast cancer cells induce a CAF-like phenotype in ADSCs, which is mediated by activation of TGFβRI, TGFβRII, and SMAD2. ADSCs-like CAF phenotypes are functional and express proteins such as SDF1, VEGF, CCL5, and TGFβ, which act as factors that promote tumor development.59 In addition, breast cancer cells release exosomes that activate normal fibroblasts and convert them into myofibroblasts (also known as CAFs), which is mediated by surviving, SOD1, actin alpha 2 (ACTA2) smooth muscle and vimentin, and it promotes cancer proliferation and metastasis.60 In an in vitro model of fibroblasts, deletion of the p85 regulatory subunit of phosphoinositide 3-kinases (PI3Ks) class IA, leads to the activation of normal fibroblasts into CAFs that increase the secretion of TGFβ, SDF1, MMP-2, and MMP-9 compared with wild-type fibroblasts.61 Exosomes released from hypoxic MCF-7 cells induce CAFs phenotypes with secretion of IL-6, which is mediated by peroxisomal proliferator-activated receptor (PPARγ) and retinoid X receptor (RXR)62 (Figure 3).
Figure 3.
EVs from breast cancer cells induce the acquisition of CAF phenotype. Breast cancer cells release EVs carrying miRNAs, lipids, and proteins that activate normal fibroblasts into CAFs. The CAFs are functional and express proteins that act as factors to promote tumor development. Figure created with BioRender.com (2022).
Abbreviations: EVs, extracellular vesicles; CAFs, cancer-associated fibroblasts.
CAFs-Derived EVs Promote an Aggressive Phenotype in Mammary Tumor Cells
CAFs can induce growth and tumorigenesis in breast cancer.63,64 Tumor development is regulated by the induction of a variety of cellular processes including changes in the phenotype of the tumor cells through the EMT process, which leads to an increase in the aggressiveness of the tumor cells. Proteins and miRNAs carried by EVs can promote neoplastic transformation and are widely involved in the different stages of breast cancer development.55,65,66 The focal adhesion kinase (FAK) is a non-receptor tyrosine kinase and an increased FAK expression and activity are frequently associated with metastatic and poor prognosis in breast cancer.67,68 FAK deletion in in vitro and in vivo models shows an increase in miR-16 and miR-148a levels, which are tumor suppressors in breast cancer. Exosomes released from CAFs that do not express FAK induce downregulation of genes regulated by miR-16 and miR-148a, such as CCNE1, TWIST1, WNT1, and WNT10B in breast cancer cells. In addition, FAK expression in CAFs is required to promote cell migration and metastasis induced by CAFs-derived exosomes in breast tumors.69 Survivin is a member of the apoptosis inhibitor family that is expressed at high levels in breast cancer and regulates cell division and apoptosis.70,71 Breast cancer cells-activated CAFs release exosomes that promote proliferation, migration, colony formation, and EMT in MDA-MB-231 and MCF-7 breast cancer cells; however, these events are not induced by EVs from mammary non-tumorigenic epithelial cells HBL-100. Moreover, an in vivo model using BALB/c-NU mice shows that survivin induces overexpression of SOD1 in CAFs to promote proliferation and metastasis, whereas SOD1 expression is higher in breast cancer women stage II, III, and IV compared with breast cancer women stage I.60
PI3Ks are a family of enzymes involved in various cellular processes including growth, proliferation, differentiation, motility, survival, and intracellular trafficking,72,73 which are implicated in cancer.72 The expression of the regulatory subunit p85α of PI3K is downregulated in the stroma of late-stage breast cancer tumors and in patients with lymph node metastasis. In an in vivo model, depletion of p85-/- in fibroblasts, which are coinoculated with tumor cells, induces formation of large tumors and metastases to the liver, whereas wild-type fibroblasts do not induce these effects. Deletion of p85 leads to the activation of normal fibroblasts into CAFs, whereas exosomes released from CAFs p85-/- promote migration and invasion in MDA-MB-231 and 4T1 breast cancer cells, and one cargo of these exosomes is Wnt10b, which is associated with the Wnt/β catenin signaling pathway.61
During metastasis, cancer cells spread from their origin to distant parts of the body. The most common sites of breast cancer metastases are bone, brain, liver, and lung.74,75 CAFs-secreted exosomes enhance metastasis in breast cancer. Exosomes released from breast cancer cells MDA-MB-231 and MCF-7 transfer miR-146a to fibroblasts, which induces the activation into CAFs, whereas CAFs transformed by EVs promote migration and invasion in MCF-7 breast cancer cells through miR-146a, which activates the Wnt pathway. EVs released from breast cancer cells MDA-MB-231 and MCF-7 are associated with an increase in lung metastasis and proliferation in a model in vivo.58 CAFs-derived exosomes isolated from breast cancer biopsies contain high levels of miR-378 and are associated with breast cancer stages III and IV and a decrease in survival. An in vitro analysis shows that miRNAs from CAFs-derived exosomes are associated with cancer stem cells-like properties in response to the regulation of Oct3/4, Nanog, and Sox, as well as with changes in the EMT phenotype, regulation of Snail and Zeb, and anchorage-independent cell growth in breast cancer cells.76 Another study demonstrates that CAFs-derived exosomes obtained from breast cancer patients promote a series of cellular processes involved in tumor progression. Particularly, miR-500a-5p is transferred to tumor cells through CAFs-derived exosomes and promotes proliferation, migration, EMT phenotype and formation of spheroids in MDA-MB-231 and MCF-7 breast cancer cells. The mechanism is unknown but may involve the downregulation of miR-500a-5p targets, being proposed by the ubiquitin-specific peptidase 28 (USP28) gene, which encodes a deubiquitinase involved in the DNA damage response checkpoint.77 The coinoculation of CAFs overexpressing miR-500a-5p and MDA-MB-231 cells in nude mice show that high levels of miR-500a-5p are transferred to tumor cells, which promote tumor growth, metastasis, and the EMT phenotype. The miRNA-500a-5p is upregulated in tissues of breast cancer patients and is associated with lower survival, whereas the USP28 gene is downregulated in the tissues of breast cancer patients with lymph node metastasis.77
The transfer of miRNAs expressed at low levels is also involved in promotion of tumor development. Treatment of MDA-MB-231 breast cancer cells with CAFs-derived EVs expressing low levels of miR-30e improves the viability, migration, and invasion.78 Particularly, miR-30e negatively regulates the collagen triple helix repeat-containing protein 1 (CTHRC1), via the Wnt/β-catenin pathway, and the overexpression of CTHRC1 increases the migratory capacity of cells.78,79 The miR-30e downregulation and overexpression of CTHRC1 are found in breast cancer patients and in tumor-bearing mice formed with tumor cells treated with CAFs-derived EVs.78 In an orthotopic mouse model of breast cancer, coinoculation of MDA-MB-231 breast cancer cells with fibroblasts increase tumor cell metastasis, whereas in an in vitro model, the conditioned medium of fibroblasts stimulates the protrusive activity and mobility of tumor cells, and it is dependent on signaling involved with planar cell polarity (PCP). These findings demonstrate that autocrine Wnt-PCP signaling is induced by fibroblast-derived exosomes that are loaded with Wnt11 and CD81, which are transferred to MDA-MB-231 breast cancer cells to promote metastasis.80,81 Another study with CAFs-derived exosomes obtained from triple-negative breast cancer (TNBC) patients show that these exosomes express low levels of miR-4516 compared with exosomes from normal fibroblasts. An in vitro study demonstrates that low expression of miR-4516 is associated with an increase in the proliferation of TNBC cells through FOSL1, a target of miR-451, and that patients with TNBC show high expression of FOSL1, and it is associated with lower survival. These findings indicate that the transfer of CAFs-derived exosomes, expressing low levels of miR-4516, enhances FOSL1 expression in TNBC cells and promotes tumor development.82
Primary tumors promote metastasis by the formation of a supportive microenvironment at a secondary organ site, termed the pre-metastatic niche. Interestingly, the pre-metastatic changes in the pre-metastatic niche may be mediated by EVs.83,84 EVs secreted by breast cancer cells can travel to normal lung tissue cells, the metastatic niche, in an orthotopic nude mouse model of breast cancer.85 Using an in vivo mouse model, tumors formed by invasive TNBC cells SUM159 induce an increased expression of proteins associated with the pre-metastatic niche formation including fibronectin, tenascin, periostin, and MMP-9 in lung tissue, however tumors derived from non-invasive MCF-7 breast cancer cells do not increase the expression of these proteins. Furthermore, stimulation with conditioned media from lung tissue cells induces the proliferation and migration of MCF-7 and SUM159 breast cancer cells, and EVs released from TNBC cells MDA-MB-231, SUM159 and LRCP17 induce the expression of proteins involved in the pre-metastatic niche including periostin and fibronectin in lung fibroblasts. In contrast, EVs from non-invasive MCF-7 and T47D breast cancer cells do not induce the expression of periostin and fibronectin in lung fibroblasts.86 These findings strongly suggest that EVs mediate the pre-metastatic niche formation in women with TNBC.
Hypoxia is one of the main features of solid tumors and is associated with poor prognosis in breast cancer.87 Exosomes released from MCF-7 cells that were subjected to hypoxic conditions express high levels of ApoE, CAIX, miR27b, and miR130B, which slightly increase the formation of mammospheres. In addition, exosomes released from hypoxic MCF-7 cells induce CAFs phenotypes with secretion of IL-6, which is mediated by PPARγ/RXR. However, treatment with the PPARγ/RXR receptor agonists, PGZ and IIF, partly inhibit mammosphere formation and Notch3 expression in MCF-7 cells.62
A limited number of studies about the tumor suppressive properties of CAFs have been published. It is clear that CAFs can suppress tumor growth during the early stages of tumor progression, but they can support tumor progression at later stages in parallel with the dual role of TGF-β.88–90 However, the molecular mechanisms that decrease tumor growth are not fully understood. Exosomes released from mesenchymal stem cells (MSCs) are transferred to MDA-MB-231 breast cancer cells and downregulate the expression of VEGF and VEGFR-1 through the transfer of miR-100, which inhibit the expression of mTOR in conjunction with HIF-1α. Therefore, exosomes from MSCs induce changes in conditioned medium of MDA-MB-231 cells, and partly inhibit the migration and formation of angiogenesis-like tubules in endothelial cells.91 Moreover, miR-1-3p is downregulated in breast tumor tissue and is associated with metastasis, whereas miR-1-3p is also downregulated in CAFs-derived EVs isolated from breast cancer patients. The breast cancer cell lines MDA-MB-231, SKBR3, and MCF-7 cells express low levels of miR-1-3p. In contrast, EVs released from CAFs overexpressing miR-1-3p decrease growth and metastasis in breast tumors through inhibition of EMT and proliferation. Interestingly, miR-1-3p downregulates the expression of GLIS gene, which is a promiscuous transcription factor that regulates expression of numerous genes.92
CAFs-Derived EVs Regulate the Metabolism of Mammary Tumor Cells
Metabolic reprogramming is an important hallmark of malignant tumors, which may be regulated by the microenvironment. Changes in metabolism in the different areas of a solid tumor have been demonstrated to occur in various malignancies, including breast cancer.93 Alterations in protein expression are associated with metabolic changes in tumor cells and the intercommunication between CAFs and tumor cells is involved with these metabolic changes93,94 Particularly, exosomes from MDA-MB-231 breast cancer cells show high levels of integrin beta 4 (ITGB4), which can be transferred to CAFs with an increase in glycolysis and lactate production. Moreover, the transfer of ITGB4 promotes the expression of mitochondrial fission proteins, such as FIS1, and the phosphorylation of dynamin-like protein 1 (DBP1) in CAFs, whereas stimulation with conditioned media of CAFs, overexpressing ITGB4, increases proliferation, migration and EMT in breast cancer cells.94 A comparative analysis of proteins in exosomes from MSCs and exosomes from breast cancer cells MDA-MB-231 and MCF-7 demonstrate that MMP-2 and fibronectin are expressed in exosomes from MSCs, but they are not expressed in exosomes from MDA-MB-231 and MCF-7 cells. However, exosomes from MSCs transfer an active form of MMP-2 to MDA-MB-231 and MCF-7 cells and it promotes tumor invasion.95
CAFs release EVs that transmit miRNAs and lncRNAs into adjacent tumor cells to modulate their behavior. CAFs and breast cancer cells undergo reciprocal metabolic reprogramming through horizontal gene transfer via EVs.96 The overexpression of miR-105 in breast cancer cells enhances vascular permeability and induces metastasis, whereas the transfer of miR-105 via exosomes disrupts tight junctions and the integrity of endothelial monolayers, and it promotes breast cancer metastasis.97 MDA-MB-231 cells release EVs expressing miR-105, a miRNA induced by MYC, which is transferred to patients-derived CAFs, and it promotes changes in glucose and glutamine metabolism with production of glutamate and lactate, compounds that nourish adjacent tumor cells. Furthermore, CAFs expressing miR-105 are able to detoxify cells for the conversion of lactic acid and ammonium into energy-rich metabolites, which nourishes tumor cells, and these CAFs promote tumor growth in NOD/SCID/IL2R-null (NSG) mice. These findings demonstrate a bidirectional communication between tumor cells and CAFs, which modify the microenvironment and contribute to tumor feeding and growth.98
Glycolysis is one of the main energy production pathways in mammalian mammary cells. EVs released from CAFs expressing low levels of miR-7641 promote the formation of a stem cell phenotype and metabolic changes including an increase of glycolysis in MDA-MB-231 and SKBR3 tumor cells through activation of HIF-1α pathway.99 CAFs-derived exosomes decrease the rate of oxygen consumption, mitochondrial function, lactate levels, and increase the rate of extracellular acidification in MCF-7 and MDA-MBA-453 breast cancer cells. Moreover, inhibition of SNHG3 expression, a noncoding RNA of more than 200 nucleotides, in CAFs-derived exosomes restores lactate production in target cells by reduction of miR-330 levels, whereas CAFs-derived exosomes expressing SNHG3 promote proliferation and dysregulate mitochondrial function in breast cancer cells. The miR-330 acts on pyruvate kinase (PKM) isoforms and promotes glycolysis in MDA-MBA-453 breast cancer cells.98–100 In contrast, it has been described different metabolic regulation mediated by miR-122. EVs from MDA-MB-231 cells and breast cancer patients express high levels of miR-122, and these EVs significantly inhibit the uptake of 2-NDBG, a fluorescent glucose analog, in fibroblasts compared with EVs from healthy controls.101 Moreover, an increase of miR-122 expression in fibroblasts decreases the expression of PKM2 and the glucose transporter (GLUT1), and it decreases glucose uptake of CAFs and increase the availability of glucose for breast cancer cells. An in vivo model shows that EVs from breast cancer patients are captured by the metastasis niche.101 These findings support the proposal that cancer progression is mediated by the metabolic regulation of CAFs and breast cancer cells via EVs.
CAFs-Derived EVs Modulate the Immune Response in the Tumor Microenvironment
Many tumors develop mechanisms of immune evasion to survive, including secretion of tumor immunomodulatory proteins, reduced expression of antigen-presenting proteins and EVs secretion.102,103 Exosomes released from breast cancer cells can influence the immune system by interacting with T cells, natural killer (NK) cells, and macrophages.104–106 Cancer cells express the natural killer group 2 member D (NKG2D) receptor and its activation promotes the cytotoxic effect of NK cells, and provides a costimulatory signal on T cells. Exosomes are able to mediate immune evasion by downregulation of NKG2D receptor expression in the effector cells including breast cancer cells.107 CAFs, isolated from breast tumors and adjacent tissues, secrete exosomes expressing high levels of miR-92, a miRNA implicated with the nuclear translocation of the yes-associated protein 1 (YAP1), which is a regulator of protein programmed death ligand 1 (PD-L1).108 PD-L1 is a protein that prevents the immune cell attack to healthy cells, and its overexpression promotes T cell tolerance and escape from the host immune system during tumorigenesis.109 Exosomes from CAFs modulate the expression of PD-L1 in MCF-7 breast cancer cells and induce upregulation of PD-L1 in NK and T cells, which promote apoptosis and a decrease in proliferation in T cells.108 In summary, the intercommunication between CAFs and tumor cells modulate the immune response and contributes to tumor progression via EVs.
CAFs-Derived EVs Modulate Composition of ECM
Breast tumors have excessive production of connective tissue and up to 90% of mass may consist of stroma, which presents alterations in density and composition of ECM.45 The miR-9 promotes proliferation, invasion, migration, and EMT in breast cancer cells and is associated with poor overall survival in breast cancer patients.110,111 Moreover, miR-9 is overexpressed in CAFs from TNBC tumors, but it is not overexpressed in CAFs from luminal A and luminal B breast tumors. EVs from TNBC MDA-MB-231 cells express miR-9, which is transferred to normal fibroblasts and regulates the expression of genes that are involved in the remodeling of ECM, including EGF containing fibulin extracellular matrix protein 1 (EFEMP1), collagen type I alpha 1 (COL1A1), and MMP-1. In addition, EVs from fibroblasts containing high levels of miR-9 are captured for MDA-MB-231 cells and stimulate migration and EMT process.112 EVs depleted of FN30, a negative regulator of fibronectin expression, from Ca1a mammary mesenchymal cells promote that normal lung fibroblasts (HLF) increase fibronectin accumulation and displacement of the linear architecture of matrix, whereas these effects are not induced by EVs depleted of FN30 from Ca1h epithelial cells.113
The alterations of stiffness and degradation of ECM contribute to tumor growth, and CAFs play a pivotal role in ECM composition.114 Microvesicles released from MDA-MB-231 breast cancer cells promote fibroblast activation, proliferation, spreading, and stiffness on matrices, however these effects are only reported in a rigid matrix that mimics mammary tumor tissue. Likewise, tumor cell-derived microvesicles induce an increase in the traction force of fibroblasts on a rigid matrix that mimics mammary tumor tissue. Interestingly, the biological effects described above are primarily induced by microvesicles from invasive MDA-MB-231 breast cancer cells, however some effects have been demonstrated using microvesicles from non-invasive MCF-7 breast cancer cells, whereas microvesicles from mammary non-tumorigenic epithelial cells MCF10A are not able to induce these biological effects.115
Chemoresistance in Tumor Cells is Modulated by CAFs-Derived EVs
EVs are important players in breast cancer chemoresistance.116 Exosomes carrying HER-2 play a pivotal role in the inhibition of trastuzumab activity,117 whereas exosomes carrying P-glycoprotein (P-gp) are proposed as another mechanism of exosomes-mediated drug resistance in breast cancer.118 Moreover, exosomes from MCF-7 breast cancer cells resistant to tamoxifen promote proliferation of wild-type MCF-7 cells in the presence of tamoxifen, which is mediated by the transfer of miR-221 and miR-222.119
The communication between tumor and stromal cells promotes resistance to hormone therapy in breast cancer.120,121 Estrogen receptor-positive tumors are metabolically dependent on oxidative phosphorylation rather than glycolysis,122 whereas mitochondrial DNA (mt-DNA) is encapsulated in EVs and it can be horizontally transferred to low metastatic cells and stromal cells in the tumor microenvironment.123 Patients with hormone therapy-resistant breast cancer show high levels of mt-DNA expressed in EVs from their plasma and the mitochondrial oxidative phosphorylation system (OXPHOS) is increased in the tumors of these patients, and when oxidative phosphorylation is inhibited in tumor cells, the cells are re-sensitized to hormonal therapy.124 An in vivo model shows that breast cancer tumors with resistance to hormone therapy have greater number of CAFs compared with tumors that do not have resistance to hormone therapy. Genomic analyses of a xenograft model (immunodeficiency mice NOD/SCID) of breast cancer demonstrates that the mitochondrial genome is expressed in murine CAFs (mCAFS)-derived EVs, including mitochondrial proteins such as ATP5A and VDAC1, whereas hormone therapy decreases mt-DNA levels, oxidative phosphorylation, and the activity of mitochondria, resulting in cells that are inactive or dormant.124
Endogenous RNAs under pathophysiological conditions, such as cancer, may act as damage-associated molecular patterns (DAMPs) to activate pattern recognition receptors (PRRs).125,126 A relationship between TNBC 1833 cells, a metastatic cell line obtained from MDA-MB-231 cells that respond to interferon-stimulated genes (ISGs), and cells of the stroma, which mediate communication via exosomes secretion, has been established. CAFs-derived exosomes transfer and increase the acquisition of stromal RNAs in 1833 cells compared with MCF-7 estrogen receptor-positive breast cancer cells that do not respond to ISGs. An analysis of genetic material expressed in exosomes demonstrates an enrichment of noncoding RNAs, including increased levels of 5'-PPP-RNAs.127 Likewise, the RN75L1, a 5'-PPP-RNA, is found at high levels in CAFs-derived exosomes and its expression is regulated by NOTCH-MYC signaling pathway. Furthermore, the transfer of RN75L1 to tumor cells via exosomes induces the activation of the RIG-1 receptor, which is a PRR. The binding of RN75L1 to RIG-1 occurs in the absence of RNA-binding proteins, which generate an unshielded RN7SL1 in stroma exosomes, and then RN7SL1 acts as a DAMP. An in vivo model of breast cancer shows that RIG-1 activation by RN75L1 promotes protection of tumor cells against DNA damage and chemoresistance, and induces growth and metastasis. Furthermore, increased levels of unshielded RN75L1 are found in exosomes from TNBC patients compared with estrogen receptor-positive breast tumors patients.127
Cancer stem cells (CSCs) play an important role in the tumor microenvironment,128 and enrichment of CAFs and CSCs is described in tumor xenografts and tissues of breast cancer patients resistant to hormone therapy. In a model of luminal breast cancer, the transfer of EVs released from CAFs containing increased levels of miR-222 and miR-221 promotes resistance to hormone therapy in MCF-7 cells, whereas this effect is not induced by EVs from normal fibroblasts. Furthermore, treatment with EVs from CAFs in combination with hormone therapy results in an increase in CSCs, a decrease in estrogen receptor expression and upregulation of Notch.129 The biogenesis of miR-221 is mediated by STAT3/IL-6 pathway and inhibition of this pathway decreases the metastasis of tumor stem cells and low formation of metastatic niches.129 In summary, the transfer of miR-222 into tumor cells through EVs from CAFs induces resistance to hormonal therapy and increase the number of CSCs, which promote the progression of the tumor (Table 1).
Table 1.
Summary of miRNAs in EVs Associated with CAFs in Breast Cancer.
| miRNA | Secreting cells | Observations | Ref. |
|---|---|---|---|
| miR-125b | Mouse 4T1 and 4TO7 breast cancer cells. MCF10CA1a (CA1a) mammary epithelial cells | EVs transfer miR-125b to resident fibroblasts and promote a CAF-like phenotype | 57 |
| miR-146a | MDA-MB-231 and MCF7 breast cancer cells CAFs transformed by breast cancer cells1101 |
EVs transfer miR-146a to fibroblasts and induce CAF
transformation Transfer of miR-16a to breast cancer cells MCF-7 induces migration and invasion. |
58 |
| miR-16 and miR-148a | FAK-null CAFs | Contribute to reduction of tumor cell activities and metastasis | 69 |
| miR-500a-5p | CAFs isolated from breast cancer patients | EVs transfer miR-500A-5p to breast cancer cells and promote proliferation, migration, EMT, and formation of spheroids. In the in vivo model it promotes tumor growth and metastasis. | 77 |
| miR-30e | CAFs | EVs with low levels of miR-30e improve the viability, migration, and invasion of MDA-MB-231 mammary tumor cells. | 78, 79 |
| miR-4516 | CAFs obtained from tissues of TNBC patients | Transfer of CAFs-derived exosomes with low levels of miR-4516 enhances FOSL1 expression in TNBC cells to promote tumor development | 82 |
| miR27b and miR130B | MCF-7 cells under hypoxic conditions CAFs transformed by breast cancer cell. |
Exosomes transferred to normal fibroblasts induce CAFs
formation. CAFs-derived exosomes carrying miR27b and miR130B slightly increase the formation of mammospheres. |
62 |
| miR-100 | Mesenchymal stem cells | Exosomes expressing miR-100 to breast cancer cells inhibit mRNA expression of mTOR with participation of HIF-1α. | 91 |
| miR-1-3p | CAFs isolated from mammary tumors | miR-1-3 is downregulated in CAFs and breast cancer cells. Transfer of EVs with overexpression of miR-1-3p to breast cancer cells promotes a decrease in growth and metastasis in an in vitro and in vivo model of breast cancer. | 92 |
| miR-105 | MDA-MB-231 breast cancer cells CAFs transformed by breast cancer cells |
miR-105 is transferred to patient-derived CAFs and induces
metabolic alterations, including changes in glucose and
glutamine metabolism. CAFs reprogrammed by miR-105 improvement tumor growth in an in vivo model. |
98 |
| miR-7641 | CAFs obtained from biopsies of patients with breast cancer | Reduction of miR-7641 levels improves the formation of a stem cell phenotype and metabolic changes in breast cancer cells. | 99 |
| miR-330 | CAFs obtained from patients with breast cancer | miR-330 participates in glycolysis regulation in breast cancer cells. | 98 |
| miR-122 | MDA-MB-231 breast cancer cells | miR-122 induces a decrease in glucose uptake by CAFs and to an increased availability of glucose for breast cancer cells. | 101 |
| miR-92 | CAFs isolated from breast tumor tissues | High levels of miR-92 in EVs from CAFs alter the function, induces apoptosis and decrease proliferation of T cells. In an in vivo model it suppresses the function of immune system. | 108 |
| miR-9 | MDA-MB-231 breast cancer cells | miR-9 downregulates genes in fibroblasts that encode for proteins involved in remodeling of ECM. | 110 |
| miR-222 | CAFs | miR-222 in CAFs-derivate EVs promotes resistance to hormone therapy in MCF-7 cells and increase of the number of cancer stem cells. It also promotes the evolution and progression of the tumor in an in vivo model. | 119 |
EVs from Breast Cancer Cells and CAFs as Biomarkers
Biomarker is a biological molecule found in blood or other fluids or tissues that can be used for screening, diagnostic and/or prognosis of a disease.130 In breast cancer, biomarkers involve a wide range of biochemical entities found in body fluids including the components of EVs from tumor cells.131 In this regard, it has been proposed that specific miRNAs or proteins expressed in EVs could be used as biomarkers of breast cancer.132 A study shows that EVs express a significant increase of phosphoproteins in breast cancer patients compared with healthy controls, and the expression of these phosphoproteins has been associated with membrane reorganization, intercellular communication and metastasis.133 Particularly, the phosphoprotein FAK is expressed in EVs from breast cancer cells or CAFs, and it represents a good candidate to be evaluated as biomarker.69 In addition, RALGAPA2, PKG1, and TJP2 are significantly elevated in EVs from breast cancer patients compared with healthy controls, and it has been suggested that these proteins are candidates for breast cancer markers.133
We previously mentioned that miRNAs can be transferred via EVs to exert their functions. Particularly, miR-9 regulates and actively participates in tumor progression.111 The miR-9 can be transferred via EVs to normal fibroblasts, which promotes their conversion into CAFs, with downregulation of genes involved in ECM remodeling, and then enhancing the motility of breast cancer cells.110,134 Therefore, miR-9 is a potential biomarker of tumorigenesis and metastasis in breast cancer. Moreover, an in vivo model of breast cancer shows that miR-105 is increased in exosomes during pre-metastasis.97 Likewise, miR-105 from MDA-MB-231 breast cancer cells is transferred via EVs to patients-derived CAFs and induces metabolic alterations including changes in glucose and glutamine metabolism.98 It is another candidate to be used as a biomarker.
EV cargos are specific to their parental cells, and a growing number of studies propose that EV cargos may be used as biomarkers for breast cancer, because EVs are very stable and circulate in blood and other body fluids, Therefore, EVs in the different body fluids represent the liquid biopsies. Liquid biopsies allow the analysis of proteins and miRNAs expressed in EVs and emerge as biomarkers for early detection, diagnosis and prognosis in breast cancer.131,132 However, further studies are required to establish the EV components as biomarkers of breast cancer.
Conclusion
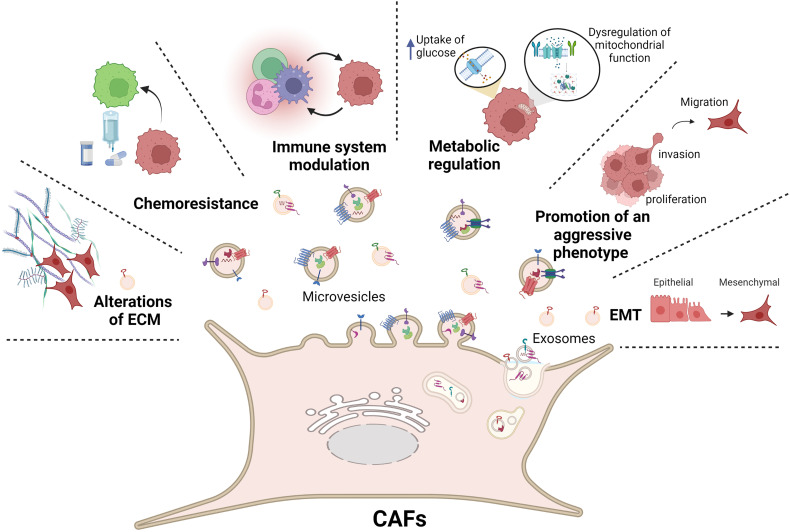
In summary, current evidence indicates that communication between breast cancer cells and CAFs via EVs represents a novel mechanism that promotes migration, invasion, immune evasion, drug resistance, EMT, and modulates the ECM composition and promotes metastasis in breast cancer (Figure 4).
Figure 4.
CAFs transfer their cargos to breast cancer cells through EVs supporting tumor growth and the acquisition of an aggressive phenotype. Tumor development is regulated by EVs through the induction of invasion, proliferation, migration and EMT. CAFs release EVs that mediate changes in metabolism, immune modulation and chemoresistance in breast cancer cells, and modulate the expression of ECM components. Figure created with BioRender.com (2022).
Abbreviations: EVs, extracellular vesicles; CAFs, cancer-associated fibroblasts; ECM, extracellular matrix.
Abbreviations
- CAFs
cancer-associated fibroblasts
- ECM
extracellular matrix
- EVs
extracellular vesicles
- MVBs
multivesicular bodies
- Rab
Ras-like small GTPase
- TSG101
tumor susceptibility gene 101
- HSP70
heat shock protein 70
- HSP90
heat shock protein 90
- MMPs
metalloproteinases
- Acta2
actin alpha 2
- TXNIP
thioredoxin interacting protein
- TGFβRI, II
transforming growth factor β receptor-I and -II
- SMAD2
SMAD family member 2
- VEGF
vascular endothelial growth factor
- CCL5
C-C motif chemokine ligand 5
- TGFβ1
transforming growth factor-beta 1
- SOD1
superoxide dismutase 1
- PI3K
phosphoinositide 3-kinase
- SDF1
stromal cell-derived factor 1
- PPARγ
peroxisome proliferator-activated receptor
- RXR
retinoid X receptor
- EMT
epithelial–mesenchymal transition
- FAK
focal adhesion kinase
- CCNE1
cyclin E1
- TWIST1
twist family BHLH transcription factor 1
- WNT1
Wnt family member 1
- WNT10B
Wnt family member 10B
- Snail
snail family transcriptional repressor 1
- Zeb
zinc finger E-box binding
- USP28
ubiquitin-specific peptidase 28
- CTHRC1
collagen triple helix repeat containing
- FOSL1
Fos-like 1
- MSC
mesenchymal stem cell
- VEGFR-1
vascular endothelial growth factor receptor-1
- GLIS
GLIS family zinc finger
- ITGB4
integrin beta 4
- FIS1
mitochondrial fission 1 protein
- DBP1
dynamin-1-like protein
- HIF-1α
hypoxia-inducible factor 1alpha
- SNHG3
small nucleolar RNA host gene 3
- PKM
pyruvate kinase
- 2-NDBG
2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl] amino)-2-deoxy-D-glucose
- GLUT1
glucose transporter 1
- PKM2
pyruvate kinase M2
- NKG2D
natural killer group 2 member D receptor
- YAP1
yes-associated protein 1
- PD -L1
programmed cell death ligand 1
- EGF
epidermal growth factor
- EFEMP1
EGF containing fibulin extracellular matrix protein 1
- COL1A1
collagen type I alpha 1 chain
- HER2/ErbB-2/neu
human epidermal growth factor receptor 2
- OXPHOS
oxidative phosphorylation
- ISGs
interferon-stimulated genes
- RIG-1
retinoic acid-inducible gene I
- CSCs
cancer stem cells
- STAT3
signal transducer and activator of transcription 3.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. Rocio Castillo-Sanchez was supported by a grant from CONACYT-Mexico.
ORCID iD: Eduardo Perez Salazar https://orcid.org/0000-0002-3041-7902
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Wernert N. The multiple roles of tumour stroma. Virchows Arch. 1997;430(6):433–443. doi: 10.1007/s004280050053 [DOI] [PubMed] [Google Scholar]
- 3.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477 [DOI] [PubMed] [Google Scholar]
- 4.Li H, Fan X, Houghton JM. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101(4):805–815. doi: 10.1002/jcb.21159 [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 6.Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci. 2016;53(2):121–131. doi: 10.3109/10408363.2015.1092496 [DOI] [PubMed] [Google Scholar]
- 7.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722 [DOI] [PubMed] [Google Scholar]
- 8.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 9.Aaronson S, Behrens U, Orner R, Haines TH. Ultrastructure of intracellular and extracellular vesicles, membranes, and myelin figures produced by Ochromonas danica. J Ultrasruct Res. 1971;35(5-6):418–430. doi: 10.1016/S0022-5320(71)80003-5 [DOI] [PubMed] [Google Scholar]
- 10.Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3(1):26913. doi: 10.3402/jev.v3.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, el Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–R134. doi: 10.1093/hmg/dds317 [DOI] [PubMed] [Google Scholar]
- 14.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- 15.Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147(3):599–610. doi: 10.1083/jcb.147.3.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- 17.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (1979). 2008;319(5867):1244–1247. doi: 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- 18.Wubbolts R, Leckie RS, Veenhuizen PTM, et al. Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–10972. doi: 10.1074/jbc.M207550200 [DOI] [PubMed] [Google Scholar]
- 19.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–212. doi: 10.1016/j.biochi.2006.10.014 [DOI] [PubMed] [Google Scholar]
- 20.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ SJJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. doi: 10.1182/blood.V94.11.3791 [DOI] [PubMed] [Google Scholar]
- 21.Barry OP, FitzGerald GA. Mechanisms of cellular activation by platelet microparticles. Thromb Haemostasis. 1999;82(8):794–800. doi: 10.1055/s-0037-1615913 [DOI] [PubMed] [Google Scholar]
- 22.Beaudoin AR, Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. BBA Rev Biomembr. 1991;1071(3):203–219. doi: 10.1016/0304-4157(91)90014-N [DOI] [PubMed] [Google Scholar]
- 23.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–421. doi: 10.1016/j.ceb.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med. 2004;53(4):210–230. doi: 10.2302/kjm.53.210 [DOI] [PubMed] [Google Scholar]
- 25.Hugel B, Martínez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2005;20(1):22–27. doi: 10.1152/physiol.00029.2004 [DOI] [PubMed] [Google Scholar]
- 26.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59(2):277–287. doi: 10.1016/S0008-6363(03)00367-5 [DOI] [PubMed] [Google Scholar]
- 27.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007. doi: 10.1111/j.1365-2141.2007.06514.x [DOI] [PubMed] [Google Scholar]
- 28.Moskovich O, Fishelson Z. Live cell imaging of outward and inward vesiculation induced by the complement C5b-9 complex. J Biol Chem. 2007;282(41):29977–29986. doi: 10.1074/jbc.M703742200 [DOI] [PubMed] [Google Scholar]
- 29.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27(3):375–387. doi: 10.1007/s00281-005-0004-1 [DOI] [PubMed] [Google Scholar]
- 30.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495. doi: 10.1038/sj.leu.2404296 [DOI] [PubMed] [Google Scholar]
- 31.Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–1132. doi: 10.1182/blood.v89.4.1121 [DOI] [PubMed] [Google Scholar]
- 32.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095 [DOI] [PubMed] [Google Scholar]
- 33.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160(2):673–680. doi: 10.1016/S0002-9440(10)64887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886 [DOI] [PubMed] [Google Scholar]
- 35.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 37.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9(1):11–21. doi: 10.1038/nrm2319 [DOI] [PubMed] [Google Scholar]
- 38.Lozito TP, Tuan RS. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. J Cell Physiol. 2012;227(2):534–549. doi: 10.1002/jcp.22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6(1):21–29. doi: 10.1038/nrrheum.2009.229 [DOI] [PubMed] [Google Scholar]
- 40.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):27–33. doi: 10.1161/ATVBAHA.110.218123 [DOI] [PubMed] [Google Scholar]
- 42.Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. The Journal of Immunology. 2001;166(12):7309–7318. doi: 10.4049/jimmunol.166.12.7309 [DOI] [PubMed] [Google Scholar]
- 43.Fries KM, Blieden T, Looney RJ, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72(3):283–292. doi: 10.1006/clin.1994.1144 [DOI] [PubMed] [Google Scholar]
- 44.Gieniec KA, Butler LM, Worthley DL, Woods SL. Cancer-associated fibroblasts—heroes or villains? Br J Cancer. 2019;121(4):293–302. doi: 10.1038/s41416-019-0509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HF D. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11. doi: 10.1158/2326-6066.CIR-14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427 [DOI] [PubMed] [Google Scholar]
- 48.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. doi: 10.1007/BF02147594 [DOI] [PubMed] [Google Scholar]
- 49.Sappino A–P, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth–muscle differentiation in stromal cells of malignant and non–malignant breast tissues. Int J Cancer. 1988;41(5):707–712. doi: 10.1002/ijc.2910410512 [DOI] [PubMed] [Google Scholar]
- 50.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32(1–2):303–315. doi: 10.1007/s10555-012-9415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 52.Conklin MW, Keely PJ. Why the stroma matters in breast cancer. Cell Adh Migr. 2012;6(3):249–260. doi: 10.4161/cam.20567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, et al. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44(3):208–214. doi: 10.1016/j.arcmed.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 54.Lowry MC, Gallagher WM, O’Driscoll L. The role of exosomes in breast cancer. Clin Chem. 2015;61(12):1457–1465. doi: 10.1373/clinchem.2015.240028 [DOI] [PubMed] [Google Scholar]
- 55.Katanov C, Lerrer S, Liubomirski Y, et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 2015;6(1). doi: 10.1186/s13287-015-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyan SW, Kuo WH, Huang CK, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One. 2011;6(1):e15313. doi: 10.1371/journal.pone.0015313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu LT, Peng B, Zhang DX, et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J Extracell Vesicles. 2019;8(1):1599680. doi: 10.1080/20013078.2019.1599680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang SS, Ma S, Dou H, et al. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res. 2020;391(2):111983. doi: 10.1016/j.yexcr.2020.111983 [DOI] [PubMed] [Google Scholar]
- 59.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012. doi: 10.3892/ijo.2011.1193 [DOI] [PubMed] [Google Scholar]
- 60.Li K, Liu T, Chen J, Ni H, Li W. Survivin in breast cancer-derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J Biol Chem. 2020;295(40):13737–13752. doi: 10.1074/jbc.RA120.013805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W. Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene. 2017;36(33):4692–4705. doi: 10.1038/onc.2017.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papi A, Storci G, Guarnieri T, et al. Peroxisome proliferator activated receptor-α/hypoxia inducible factor-1α interplay sustains carbonic anhydrase IX and apoliprotein E expression in breast cancer stem cells. PLoS One. 2013;8(1):e54968. doi: 10.1371/journal.pone.0054968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang M, Li Y, Zhang H, Nan F. Breast cancer stromal fibroblasts promote the generation of CD44 + CD24-cells through SDF-1/CXCR4 interaction. J Exp Clin Cancer Res. 2010;29(1). doi: 10.1186/1756-9966-29-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamura Y, Asai N, Enomoto A, et al. Akt-girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015;75(5):813–823. doi: 10.1158/0008-5472.CAN-14-1317 [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Li Z, Li X, Xia J. Intercellular transfer of messenger RNAs in multiorgan tumorigenesis by tumor cell-derived exosomes. Mol Med Rep. 2015;11(6):4657–4663. doi: 10.3892/mmr.2015.3312 [DOI] [PubMed] [Google Scholar]
- 66.Zhang ZJ, Ma SL. miRNAs in breast cancer tumorigenesis (review). Oncol Rep. 2012;27(4):903–910. doi: 10.3892/or.2011.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yom CK, Noh DY, Kim WH, Kim HS. Clinical significance of high focal adhesion kinase gene copy number and overexpression in invasive breast cancer. Breast Cancer Res Treat. 2011;128(3):647–655. doi: 10.1007/s10549-010-1150-2 [DOI] [PubMed] [Google Scholar]
- 68.Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37(1):9–15. doi: 10.1016/j.humpath.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 69.Wu HJ, Hao M, Yeo SK, Guan JL. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene. 2020;39(12):2539–2549. doi: 10.1038/s41388-020-1162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan S, Yuan Y, Valenzuela MM, et al. Early diagnostic value of survivin and its alternative splice variants in breast cancers. J Clin Oncol. 2012;30(27):35–35. doi: 10.1200/jco.2012.30.27_suppl.35 [DOI] [Google Scholar]
- 71.Khan S, Jutzy JMS, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinase: implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17(1):615–675. doi: 10.1146/annurev.cellbio.17.1.615 [DOI] [PubMed] [Google Scholar]
- 73.Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 2019;20(9):515–534. doi: 10.1038/s41580-019-0129-z [DOI] [PubMed] [Google Scholar]
- 74.Yeeravalli R, Das A. Molecular mediators of breast cancer metastasis. Hematol/Oncol Stem Cell Ther. 2021;14(4):275–289. doi: 10.1016/j.hemonc.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 75.Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13(2):88–94. doi: 10.1016/j.clbc.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 76.Donnarumma E, Fiore D, Nappa M, et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8(12):19592–19608. doi: 10.18632/oncotarget.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B, Sang Y, Song X, et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11(8):3932–3947. doi: 10.7150/THNO.53412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xi C, Wang J, Sun H, Zhang X, Kang H. Loss of microRNA-30e induced by extracellular vesicles from cancer-associated fibroblasts promotes breast cancer progression by binding to CTHRC1. Exp Mol Pathol. 2021;118:104586. doi: 10.1016/j.yexmp.2020.104586 [DOI] [PubMed] [Google Scholar]
- 79.Pyagay P, Heroult M, Wang Q, et al. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96(2):261–268. doi: 10.1161/01.RES.0000154262.07264.12 [DOI] [PubMed] [Google Scholar]
- 80.Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 81.Luga V, Wrana JL. Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73(23):6843–6847. doi: 10.1158/0008-5472.CAN-13-1791 [DOI] [PubMed] [Google Scholar]
- 82.Kim JE, Kim BG, Jang Y, Kang S, Lee JH, Cho NH. The stromal loss of miR-4516 promotes the FOSL1-dependent proliferation and malignancy of triple negative breast cancer. Cancer Lett. 2020;469:256–265. doi: 10.1016/j.canlet.2019.10.039 [DOI] [PubMed] [Google Scholar]
- 83.Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65(3):383–390. doi: 10.1016/j.addr.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 86.Medeiros B, Goodale D, Postenka C, et al. Triple-negative primary breast tumors induce supportive premetastatic changes in the extracellular matrix and soluble components of the lung microenvironment. Cancers (Basel). 2020;12(1):172. doi: 10.3390/cancers12010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jögi A, Ehinger A, Hartman L, Alkner S. Expression of HIF-1α is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer. PLoS One. 2019;14(12):e0226150. doi: 10.1371/journal.pone.0226150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engle SJ, Hoying JB, Boivin GP, Ormsby I, Gartside PS, Doetschman T. Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59(14):3379–3386. http://www.ncbi.nlm.nih.gov/pubmed/10416598 [PubMed] [Google Scholar]
- 89.Flaberg E, Markasz L, Petranyi G, et al. High-throughput live-cell imaging reveals differential inhibition of tumor cell proliferation by human fibroblasts. Int J Cancer. 2011;128(12):2793–2802. doi: 10.1002/ijc.25612 [DOI] [PubMed] [Google Scholar]
- 90.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi: 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pakravan K, Babashah S, Sadeghizadeh M, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017;40(5):457–470. doi: 10.1007/s13402-017-0335-7 [DOI] [PubMed] [Google Scholar]
- 92.Tao S, Li H, Ma X, et al. Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Ther. 2021;28(6):634–648. doi: 10.1038/s41417-020-00244-x [DOI] [PubMed] [Google Scholar]
- 93.Santos JM, Hussain F. Higher glucose enhances breast cancer cell aggressiveness. Nutr Cancer. 2020;72(5):734–746. doi: 10.1080/01635581.2019.1654527 [DOI] [PubMed] [Google Scholar]
- 94.Sung JS, Kang CW, Kang S, et al. ITGB4-mediated Metabolic reprogramming of cancer-associated fibroblasts. Oncogene. 2020;39(3):664–676. doi: 10.1038/s41388-019-1014-0 [DOI] [PubMed] [Google Scholar]
- 95.Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015;47(1):244–252. doi: 10.3892/ijo.2015.3001 [DOI] [PubMed] [Google Scholar]
- 96.Alamoudi AA, Alnoury A, Gad H. miRNA in tumour metabolism and why could it be the preferred pathway for energy reprograming. Brief Funct Genomics. 2018;17(3):157–169. doi: 10.1093/bfgp/elx023 [DOI] [PubMed] [Google Scholar]
- 97.Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan W, Wu X, Zhou W, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5):597–609. doi: 10.1038/s41556-018-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Hua F, Zhan Y, et al. Carcinoma associated fibroblasts small extracellular vesicles with low miR-7641 promotes breast cancer stemness and glycolysis by HIF-1α. Cell Death Discov. 2021;7(1). doi: 10.1038/s41420-021-00524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, Zhao Z, Liu W, Li X. SNHG3 Functions as miRNA sponge to promote breast cancer cells growth through the metabolic reprogramming. Appl Biochem Biotechnol. 2020;191(3):1084–1099. doi: 10.1007/s12010-020-03244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9 [DOI] [PubMed] [Google Scholar]
- 103.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213(1):131–145. doi: 10.1111/j.1600-065X.2006.00442.x [DOI] [PubMed] [Google Scholar]
- 104.Yang Y, Li CW, Chan LC, et al. Exosomal PD-L1 harbors active defense function to suppress t cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–864. doi: 10.1038/s41422-018-0060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wen SW, Sceneay J, Lima LG, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–6827. doi: 10.1158/0008-5472.CAN-16-0868 [DOI] [PubMed] [Google Scholar]
- 106.Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M, Moon WK. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9(7):7398–7410. doi: 10.18632/oncotarget.23238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34(3):206–213. doi: 10.1016/j.bcmd.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 108.Dou D, Ren X, Han M, et al. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front Immunol. 2020;11. doi: 10.3389/fimmu.2020.02026 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol. 2018;71(3):189–194. doi: 10.1136/jclinpath-2017-204853 [DOI] [PubMed] [Google Scholar]
- 110.Liu DZ, Chang B, Li XD, Zhang QH, Zou YH. MicroRNA-9 promotes the proliferation, migration, and invasion of breast cancer cells via down-regulating FOXO1. Clinical and Translational Oncology. 2017;19(3):1133–1140. doi: 10.1007/s12094-017-1650-1 [DOI] [PubMed] [Google Scholar]
- 111.Li X, Zeng Z, Wang J, et al. MicroRNA-9 and breast cancer. Biomed Pharmacother. 2020;122:109687. doi: 10.1016/j.biopha.2019.109687 [DOI] [PubMed] [Google Scholar]
- 112.Baroni S, Romero-Cordoba S, Plantamura I, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7(7):e2312–e2312. doi: 10.1038/cddis.2016.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Libring S, Shinde A, Chanda MK, et al. The dynamic relationship of breast cancer cells and fibroblasts in fibronectin accumulation at primary and metastatic tumor sites. Cancers (Basel). 2020;12(5):1270. doi: 10.3390/cancers12051270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120(3):2782–2790. doi: 10.1002/jcb.27681 [DOI] [PubMed] [Google Scholar]
- 115.Schwager SC, Bordeleau F, Zhang J, Antonyak MA, Cerione RA, Reinhart-King CA. Matrix stiffness regulates microvesicle-induced fibroblast activation. Am J Physiol Cell Physiol. 2019;317(1):C82–C92. doi: 10.1152/ajpcell.00418.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimarães JE, Vasconcelos MH. The role of extracellular vesicles in the hallmarks of cancer and drug resistance. Cells. 2020;9(5):1141. doi: 10.3390/cells9051141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ciravolo V, Huber V, Ghedini GC, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–667. doi: 10.1002/jcp.22773 [DOI] [PubMed] [Google Scholar]
- 118.Lv MM, Zhu XY, Chen WX, et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumor Biol. 2014;35(11):10773–10779. doi: 10.1007/s13277-014-2377-z [DOI] [PubMed] [Google Scholar]
- 119.Wei Y, Lai X, Yu S, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147(2):423–431. doi: 10.1007/s10549-014-3037-0 [DOI] [PubMed] [Google Scholar]
- 120.Holton SE, Bergamaschi A, Katzenellenbogen BS, Bhargava R. Integration of molecular profiling and chemical imaging to elucidate fibroblast-microenvironment impact on cancer cell phenotype and endocrine resistance in breast cancer. PLoS One. 2014;9(5):e96878. doi: 10.1371/journal.pone.0096878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mercier I, Casimiro MC, Wang C, et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7(8):1212–1225. doi: 10.4161/cbt.7.8.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Mahony F, Razandi M, Pedram A, Harvey BJ, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26(12):2058–2070. doi: 10.1210/me.2012-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takenaga K, Koshikawa N, Nagase H. Intercellular transfer of mitochondrial DNA carrying metastasis-enhancing pathogenic mutations from high- to low-metastatic tumor cells and stromal cells via extracellular vesicles. BMC Mol Cell Biol. 2021;22(1). doi: 10.1186/s12860-021-00391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sansone P, Savini C, Kurelac I, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114(43). doi: 10.1073/pnas.1704862114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roulois D, Loo Yau H, Singhania R, et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell. 2015;162(5):961–973. doi: 10.1016/j.cell.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi: 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 127.Nabet BY, Qiu Y, Shabason JE, et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell. 2017;170(2):352–366.e13. doi: 10.1016/j.cell.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caldas-Lopes E, Gomez-Arteaga A, Guzman ML. Approaches to targeting cancer stem cells in solid tumors. Curr Stem Cell Res Ther. 2019;14(4):421–427. doi: 10.2174/1574888x14666190222164429 [DOI] [PubMed] [Google Scholar]
- 129.Sansone P, Berishaj M, Rajasekhar VK, et al. Evolution of cancer stem-like cells in endocrine-resistant metastatic breast cancers is mediated by stromal microvesicles. Cancer Res. 2017;77(8):1927–1941. doi: 10.1158/0008-5472.CAN-16-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Srivastava A, Creek DJ. Discovery and validation of clinical biomarkers of cancer: a review combining metabolomics and proteomics. Proteomics. 2019;19(10):1700448. doi: 10.1002/pmic.201700448 [DOI] [PubMed] [Google Scholar]
- 131.Sadovska L, Eglitis J, Line A. Extracellular vesicles as biomarkers and therapeutic targets in breast cancer. Anticancer Res. 2015;35(12):6379–6390. [PubMed] [Google Scholar]
- 132.Urabe F, Kosaka N, Ito K, Kimura T, Egawa S, Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318(1):C29–C39. doi: 10.1152/ajpcell.00280.2019 [DOI] [PubMed] [Google Scholar]
- 133.Chen IH, Xue L, Hsu CC, et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A. 2017;114(12):3175–3180. doi: 10.1073/pnas.1618088114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kia V, Paryan M, Mortazavi Y, Biglari A, Mohammadi-Yeganeh S. Evaluation of exosomal miR-9 and miR-155 targeting PTEN and DUSP14 in highly metastatic breast cancer and their effect on low metastatic cells. J Cell Biochem. 2019;120(4):5666–5676. doi: 10.1002/jcb.27850 [DOI] [PubMed] [Google Scholar]