Abstract
Introduction:
Condomless anal intercourse (AI) confers a far greater likelihood of HIV transmission than condomless vaginal intercourse (VI). However, little is known about AI practice over the life course of women, to what extent AI practice is condom-protected, and whether it is associated with other HIV risk behaviors. We aim to describe longitudinal AI practice among HIV-seronegative women and to identify subgroups with distinct trajectories of AI practice.
Methods:
Using data from the Women’s Interagency HIV Study, an observational cohort of US women with or at risk for HIV, we described AI practice among HIV-seronegative participants. Group-based trajectory modeling was used to identify distinct AI trajectories. We used multinomial regression to examine associations between baseline characteristics and trajectory group membership.
Results:
A third of the 1,085 women in our sample reported any AI over follow-up (median follow up = 14 years). AI decreased more sharply with age compared to VI. Consistent condom use during AI was low: twice the proportion of women never reported using condoms consistently during AI compared to during VI. 5 trajectory groups were identified: AI & VI persistors (N = 75) practiced AI and VI consistently over follow-up (AI & VI desistors (N = 169) tended to practice AI and VI when young only, while VI persistors (N = 549), VI desistors (N = 167), and AI & VI inactives (N = 125) reported varying levels of VI practice, but little AI. AI & VI persistors reported multiple male partners and exchange sex at more visits than other groups. Women who identified as bisexual/lesbian (vs heterosexual), who had ever experienced physical and sexual violence (vs never), and/or who reported above the median number of lifetime male sex partners (vs median or below) had approximately twice the odds of being AI & VI persistors than being VI persistors.
Conclusions:
We identified a small subgroup of women who practice AI and report inconsistent condom use along with other risk behaviors throughout the life course; they may therefore particularly benefit from ongoing access to HIV prevention services including pre-exposure prophylaxis.
Keywords: Heterosexual, Sexual Behavior, Anal Sex, Women, Transmission, Prevention
INTRODUCTION
The likelihood of HIV transmission during condomless receptive anal intercourse (AI) may be up to 18-fold higher than that during condomless receptive vaginal intercourse (VI).1 Cross-sectional studies consistently suggest that AI is commonly practiced among US cisgender women (referred to here as women), with a systematic review on AI among young people estimating that 25% (95% confidence interval [CI]: 20–29%) of sexually active young women and girls (aged <25 years) in North America had ever practiced AI.2 A survey of sexually active women living in 20 US cities with high HIV prevalence found that 30% reported AI within the past year,3 and a mathematical modeling study based on the same survey estimated that 4 in 10 new HIV infections in that population may be attributed to condomless AI.4
While we have a fair understanding through cross-sectional studies of how commonly women may practice AI, there has, to our knowledge, been no longitudinal examination of women’s AI practice over the life course. Systematic reviews on heterosexual AI practice measured cross-sectionally among young people, female sex workers, and South Africans have found that the proportions of individuals reporting heterosexual AI were generally high and did not increase with length of recall period.2,5,6 Without a longitudinal analysis of AI practice, it is unclear whether this is due to people continuing to practice AI once initiated or to more accurate reporting of AI over shorter recall periods. In addition, it remains unclear whether AI practice increases or decreases with age, with one cross-sectional study finding that reported AI practice over the past year decreases with age among urban US women at increased risk for HIV,7 another finding that AI remains constant across ages among US women nationally,8 and yet another finding that AI increases with age among urban US women.3 A further obfuscation to our understanding of AI over the life course is that most cross-sectional studies reporting AI sample younger women, leaving little known about AI practice in later years.
This paper describes and characterizes patterns of AI practice over the life course among US HIV-seronegative women in the Women’s Interagency HIV Study (WIHS), the largest and longest prospective cohort of women with or at risk for HIV. To improve our understanding of the relationship between practicing AI and HIV acquisition, our analysis includes only HIV-seronegative women. Specifically, this paper aims to a) describe AI practice over 20 years of follow-up, b) identify groups with distinct trajectories of AI practice, c) describe AI practice within each trajectory group, and d) identify individual baseline characteristics associated with group membership, for HIV-seronegative women in the WIHS cohort. Identifying subgroups with distinct AI practice trajectories could lead to better targeting of HIV and STI prevention interventions.
METHODS
The WIHS Cohort
The WIHS is the largest, and longest, ongoing prospective cohort study of HIV among US women, comprising 3,677 women with HIV and 1,305 demographically matched women at risk for HIV. Initial recruitment occurred in 1994, with further recruitment waves in 2001–02 and 2011–12, at 6 urban sites (Bronx, NY; Washington, DC; San Francisco, CA; Los Angeles, CA; Chicago, IL; and Brooklyn, NY). In 2013, a 4th enrollment wave expanded recruitment to sites in the Southern US (Chapel Hill, NC; Atlanta, GA; Miami, FL; Birmingham, AL; and Jackson, MS). Detailed information on the cohort is provided elsewhere.9 Eligibility criteria varied slightly for each wave (Supplementary Table 1). Briefly, HIV-seronegative women were eligible for inclusion if they reported a recent high-risk sexual behavior, diagnosis of an STI, or drug use that increased their risk of acquiring HIV, with the exception of wave 1 when there were no behavioral eligibility criteria and HIV-seronegative women were demographically matched to recruited women with HIV.
Data were collected on lifetime AI practice (ever having practiced AI) at baseline. Follow-up visits, which were conducted approximately every 6 months, collected data on whether a male sex partner was reported since last visit, and if so, whether AI had been practiced. since last visit. Condom use was measured by asking whether condoms had been used “always,” “sometimes,” or “never” during AI since last visit. The equivalent data were collected on VI. Information on demographic, behavioral, structural, and psychosocial factors was also collected at both baseline and over follow-up. Data were collected in face-to-face interviews or, when necessary, by phone interview.
Data Analysis
WIHS participants who were HIV-seronegative at baseline and for whom baseline data and at least three 6-monthly follow-up visits were available (n = 1,085) from 1994 to 2014 were included in this analysis. Data visualization and descriptive statistics were used to describe AI practice over follow-up; namely the proportion of visits at which AI was reported, AI prevalence over different time frames measured from baseline (defined as reporting any AI within time frame), as well as consistent condom use during AI since last visit. VI practice over follow-up was described in the same way, to contrast and more fully understand AI patterns.
Group-based trajectory modeling was then used to identify subgroups of women with contrasting AI practice trajectories. Group-based trajectory modeling is a semi-parametric approach used to identify subgroups (or classes) of individuals within populations that follow distinct trajectories over time, in contrast to traditional growth curve modeling which identifies a mean trajectory for the entire sample.10 Trajectory groups can be thought of as unobserved (latent) longitudinal strata where population variability is captured by the different trajectories across groups. Group-based trajectory modeling uses a finite set of unique polynomial functions corresponding to a discrete trajectory to summarize the heterogeneity of individual differences in change over time within the data.11,12
AI practice for each woman since the last study visit was trichotomized into (1) no male sex partner, (2) sexual activity with male partner but no AI, and (3) sexual activity with male partner and AI practice. Using this ordinal variable measured at each visit as the model indicator and age as a continuous covariate, trajectory group models with 2, 3, 4, and 5 groups were fitted to the data, and model fits were compared. A number of criteria were used to determine the optimal number of groups: the Bayesian Information Criterion as a measure of goodness-of-fit, average posterior probabilities of group membership as a measure of classification quality, and group size (groups comprising <5% of the sample were avoided).11,13
To identify socio-demographic, structural, and behavioral factors associated with longitudinal patterns of AI practice, bivariate and multivariable multinomial regression was used to examine associations between baseline characteristics and trajectory group membership. The trajectory group with the highest proportion of the sample was used as the reference group to maximize statistical power. Guided by a literature-based conceptual framework, covariates of interest available in the WIHS dataset were identified and selected a priori, and all entered into the multivariable model (Supplementary Figure 1).14 Given the long median follow-up time, only variables which captured longer-term exposures and behaviors were used (eg, ever having injected drugs), rather than shorter-term and possibly more transient exposures and behaviors (eg, injecting drugs in the past 6 months).
Race/ethnicity (black vs Hispanic/white/other), education (less than high school vs high school or higher), sexual orientation or identity (heterosexual vs lesbian or bisexual), ever having experienced sexual or physical violence (none vs either physical or sexual violence/both), ever having injected drugs (no vs yes), ever having traded sex for money/drugs (referred to here as practicing exchange sex, no vs yes), and number of male sex partners ever (<11 vs ≥ 11) were entered into the model. In addition to these covariates, the multivariable model controlled for recruitment wave and age (as continuous variable). Generalized estimating equations (GEEs) were used to account for possible correlation between observations within each study site at baseline using an exchangeable working correlation structure.15
6 of the 10 included variables contained missing data (sexual orientation, education, lifetime history of being, lifetime number of male sex partners, and lifetime history of exchange sex). In this context, a complete case analysis would have dropped 27% of the sample from the analysis. Missing values were therefore dealt with using multiple imputation chained equations, an iterative process that imputes multiple variables through posterior prediction distribution using a series of univariate chained equations.16 Twenty iterations were used and the datasets produced were combined following Rubin’s rule.17 As a sensitivity analysis, we also performed the analysis on the subset of complete cases.
All analyses were conducted using the R statistical software18 with the “ggplot2” package19 for producing plots, “llcm”20 to identify distinct trajectory groups, “MICE”21 for multiple imputation and “nnet” for multinomial logistic regression.22 Supplementary Table 2 provides an overview of the analysis.
Ethics
All participants provided written informed consent. Ethical approval for data collection was granted by review boards at each study site, and for this analysis, by review boards at the National Institute of Health and Imperial College London.
RESULTS
Participant Characteristics
Of 1,305 HIV-seronegative women recruited, data from at least 3 follow-up visits were available for 1,085 women (83%), comprising 23,651 visits. Table 1 summarizes baseline characteristics of the sample. Only 23 women seroconverted during follow-up and were retained in this analysis. Participants were followed up for a median of 14 years (interquartile range [IQR] = 5–18). Median age at enrollment was 35 years (IQR = 28–42), although this varied substantially by wave, ranging from 28 to 48 years in waves 2 and 3, respectively. Nearly two-thirds of women described themselves as non-Hispanic black (64%). Most (82%) identified as heterosexual. A third were employed and just over half had a household income of less than $12,000/year. A history of violence was common, with 56% and 39% of women reporting physical and sexual violence ever, respectively (excluding women with missing values). Ever having practiced AI was reported by 43% of women (excluding those with missing values), and ever practicing exchange sex was reported by 35% of women.
Table 1.
Baseline characteristics of 1,085 HIV-seronegative WIHS cohort participants
| Variable | Category | N (%) or median (IQR) |
|---|---|---|
| Years of follow-up | 14.0 (5.0–18.0) | |
| Recruitment wave | First (1994) | 445 (41.0%) |
| Second (2001–02) | 354 (32.6%) | |
| Third (2011–12) | 81 (7.5%) | |
| 5th (2013–15) | 205 (18.9%) | |
| Site | Atlanta, GA* | 76 (7.2%) |
| Birmingham, AL* | 24 (2.2%) | |
| Bronx, NY | 195 (17.9%) | |
| Brooklyn, NY | 149 (13.7%) | |
| Chapel Hill, NC* | 44 (4.1%) | |
| Chicago, IL | 111 (10.2%) | |
| Jackson, MS* | 26 (2.4%) | |
| Los Angeles, CA | 136 (12.5%) | |
| Miami, FL* | 35 (3.3%) | |
| San Francisco, CA | 161 (14.8%) | |
| Washington, DC | 129 (11.9%) | |
| Age in years | 35 (28–42) | |
| Race/ethnicity | Black† | 691 (63.7%) |
| Hispanic/Latina | 230 (21.1%) | |
| White† | 122 (11.2%) | |
| Other | 42 (3.9%) | |
| Sexual orientation | Heterosexual | 890 (82.0%) |
| Bisexual | 118 (10.9%) | |
| Lesbian | 60 (5.5%) | |
| Missing | 17 (1.6%) | |
| Education | Less than high school | 356 (32.8%) |
| High school or more | 726 (66.9%) | |
| Missing | 3 (0.3%) | |
| Marital status | Married or living with a partner | 362 (33.4%) |
| Not married or living with a partner | 722 (66.6%) | |
| Missing | 1 (0.1%) | |
| Household income | <$12,000/year | 609 (56.1%) |
| ≥$12,000/year | 435 (40.1%) | |
| Missing | 41 (3.8%) | |
| Employed | Yes | 362 (33.4%) |
| No | 620 (66.4%) | |
| Missing | 3 (0.3%) | |
| Physical violence, ever‡ | Yes | 464 (42.8%) |
| No | 370 (34.1%) | |
| Missing | 251 (23.1%) | |
| Sexual violence, ever‡ | Yes | 325 (30.0%) |
| No | 505 (46.5%) | |
| Missing | 255 (23.5%) | |
| Injection drug use, ever | Yes | 223 (20.6%) |
| No | 862 (79.4%) | |
| Number of male sex partners, ever | Median (IQR) | 12 (6–35) |
| Missing | 71 | |
| Number of female sex partners, ever | 0 | 755 (69.6%) |
| ≥1 | 328 (30.2%) | |
| Missing | 2 (0.2%) | |
| Anal intercourse, ever§ | Yes | 419 (38.6%) |
| No | 562 (51.8%) | |
| Missing | 104 (9.6%) | |
| Exchange sex, ever | Yes | 382 (35.3%) |
| No | 700 (64.5%) | |
| Missing | 3 (0.3%) |
IQR = interquartile range.
New sites were added in the 4th recruitment wave. All other sites were added during the first recruitment wave. Variables for which there is no “missing” category contain no missing values.
Black refers to non-Hispanic black women. White refers to non-Hispanic white women.
Violence victimization variables have many missing values as ethical approval was not granted at the Los Angeles and San Francisco study sites.
The number of missing values is high because in the first recruitment wave, women reporting no sex partners in the past 6 months were not asked whether they had ever practiced AI. In subsequent waves, all women were asked whether they had ever practiced AI.
Description of AI Practice and Other Sexual Behaviors Over Follow-Up
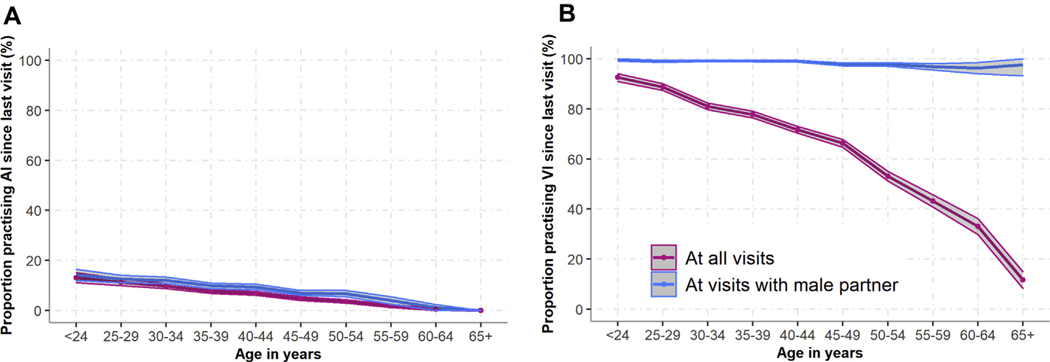
Over follow-up, almost all women (94%) reported a male sex partner and a third (33%) reported AI at any visit. Measured over different time frames from baseline onward, AI prevalence increased gradually as the time since baseline increased before plateauing from 10 years onward (Supplementary Figure 2: 6-month prevalence = 8%, 10-year prevalence = 37%, 15-year prevalence = 40%). The proportion of women reporting AI since last visit decreased with age, both among the whole sample (including women reporting no male sex partner since last visit) and among the subset reporting a male sex partner since last visit (decreasing from 23% of women aged <25 years to 0% of women aged 65+ years reporting a male sex partner) (Figure 1A). This decrease was also observed among the subset of women reporting any AI over follow-up (Supplementary Figure 3).
Figure 1.
The proportion of women reporting (A) AI practice and (B) VI practice since last visit, by age (at all visits and at visits when a male partner was reported). Shaded areas represent 95% confidence intervals. AI = anal intercourse; VI = vaginal intercourse.
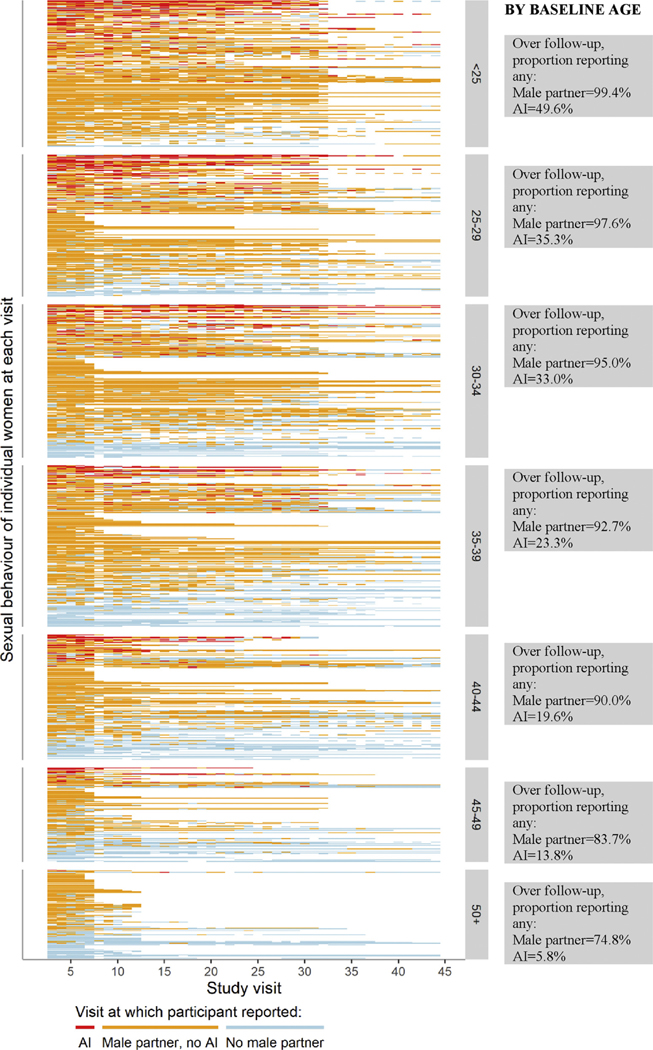
Figure 2 displays the individual trajectories of AI practice and having a male sex partner over follow-up for each of the 1,085 included women, grouped by age at baseline. It illustrates the wide variety of sexual activity patterns among women over the life course, and reinforces the observation that while reporting a male sex partner and AI practice both decrease with age, AI practice decreases more sharply.
Figure 2.
Individual trajectories of reporting AI, having a male sex partner but not reporting AI, and having no male sex partner at any time since the last study visit (typically 6 months), grouped by age group at baseline and sorted by the percentage of visits with AI, then percentage of visits with a male partner but no AI, and then percentage of visits with no male partner. White sections represent missing values or missed visits. AI = anal intercourse.
VI was practiced at all visits when AI practice was reported. AI practice was reported at 10% and 17% of visits when women reported at least one male sex partner and multiple male partners, respectively (Table 2). Among women who reported any AI over follow-up (Supplementary Figure 5B), nearly two-thirds reported it at less than a quarter of visits when a male sex partner was reported, while 13% reported it at half or more of visits when a male sex partner was reported.
Table 2.
Behavior over follow-up visits: percentage of visits in which various sexual practices since last visit were reported
| Behavior reported since last visit | Number of visits over follow-up | % (95% CI) |
|---|---|---|
| At all visits | 23,651 | |
| AI | 6.6% (6.3—6.9%) | |
| VI | 69.1% (68.5—69.8%) | |
| Any male sex partner | 70.4% (69.9—71.0%) | |
| Any female sex partner | 8.3% (8.0—8.8%) | |
| Multiple male sex partners (2+) | 17.3% (16.8—17.8%) | |
| Exchange sex | 3.7% (3.5—4.0%) | |
| At visits with male sex partner(s) reported | 16,659 | |
| AI | 9.5% (9.0—9.9%) | |
| VI | 98.8% (98.5—98.9%) | |
| At visits with AI practice reported | 1,495 | |
| Consistent condom use during AI | 25.8% (23.5—28.0%) | |
| At visits with VI practice reported | 16,101 | |
| Consistent condom use during VI | 24.1% (23.4—24.4%) | |
| At visits with multiple male sex partners reported (2+) | 4,090 | |
| AI | 17.3% (16.1—18.5%) | |
| VI | 98.5% (98.1—98.9%) | |
| At visits with AI practice and multiple male partners (2+) reported | 641 | |
| Consistent condom use during AI | 36.0% (32.3—39.8%) | |
| At visits with VI practice and multiple male partners (2+) reported | 3,931 | |
| Consistent condom use during VI | 26.2% (24.8—27.6%) |
The recall period for all sexual behaviors was “since last visit.” Most visits (93.1%) were approximately 6 months prior, 5.0% were approximately 12 months prior, and 1.9% longer than 12 months prior. Consistent condom use refers to reporting “always” using condoms during AI or VI, since last visit. VI was reported at all visits when AI was reported.
AI = anal intercourse; VI = vaginal intercourse; 95% CI = 95% confidence interval (calculated using the Clopper-Pearson confidence interval for a binomial proportion).
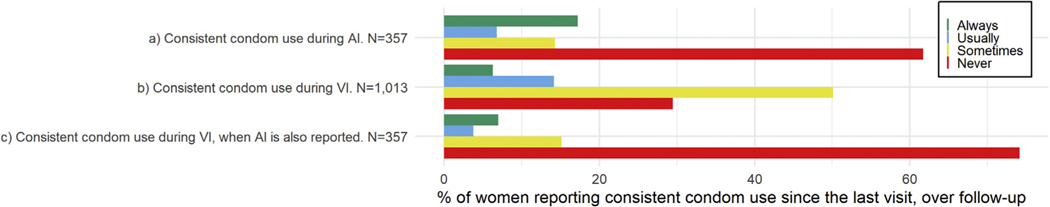
Consistent condom use during AI and VI was reported at a similar proportion of visits (~25% of visits when either act was reported), while consistent condom use during AI was reported more often when multiple partners were reported (36% of visits) than at all visits when AI was reported (26% of visits) (Table 2). The proportions of women reporting consistent condom use during AI and VI since last visit varied little by age (Supplementary Figure 4). Conversely, when examined over follow-up, the proportion of women who never reported consistent condom use was twice as high during AI than during VI (Figure 3A and b). Interestingly, the proportion of women reporting never using condoms consistently during VI was more than 2.5 times higher for the subset of visits when both VI and AI were reported than visits when VI only was reported (Figure 3B and C).
Figure 3.
Proportions of women reporting consistent condom use (A) during AI at all visits at which AI is reported, (B) during VI at all visits when VI is reported, and (C) during VI at all visits when AI is also reported. Consistent condom use is defined as reporting having always used condoms since last visit. Never = consistent condom use during AI since last visit at 0% of visits when AI was reported, sometimes = consistent condom use during AI at 1–49% of visits when AI was reported, usually = consistent condom use during AI at 50–99% of visits when AI was reported, always = consistent condom use during AI at 100% of visits when AI was reported. Equivalent measures and categorizations were used for condom use during VI. All of the 357 women who practice AI over follow-up are also included in the 1,013 women represented in plot b as there are no women who practise AI but not VI over follow-up.
Description of AI Practice and Other Sexual Behaviors Within Trajectory Groups
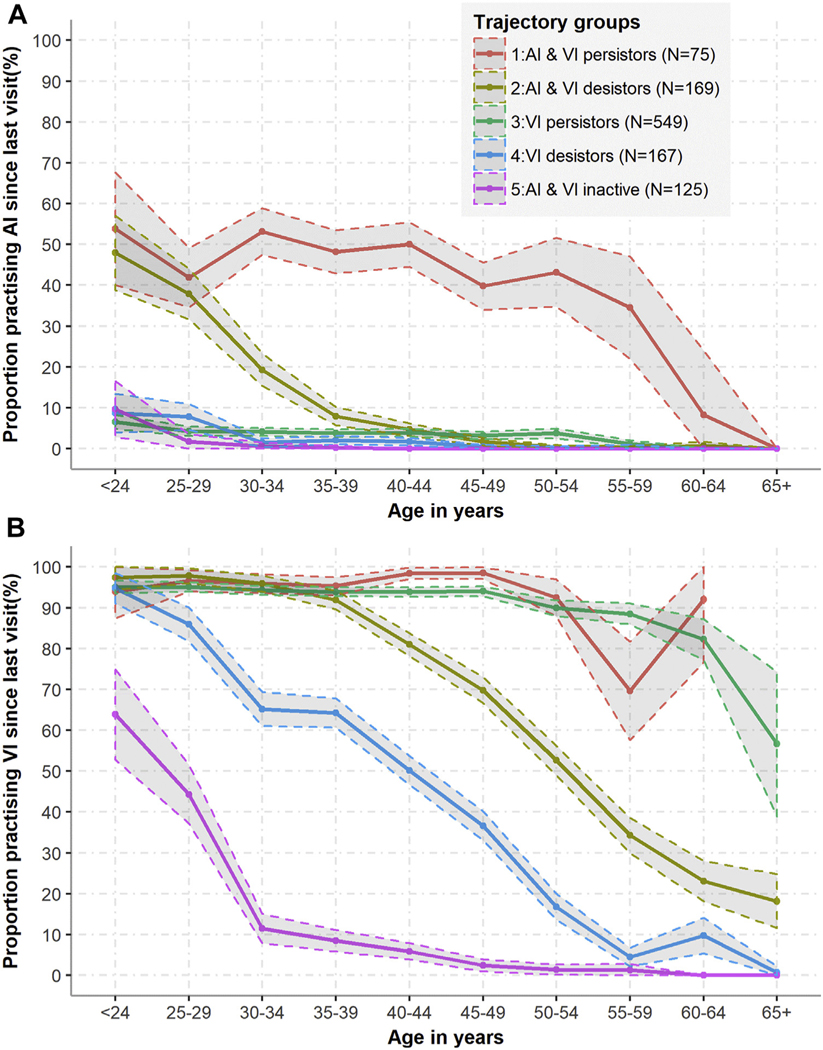
A 5-group model was chosen as best describing AI and VI practice trajectories (see footnote of Supplementary Table 3 for explanation of model choice). 3 of the groups identified (groups 3–5) reported little AI practice over follow-up and were termed VI persistors (comprising 51% of sample; practice VI at all ages), VI desistors (15% of sample; VI practice declines gradually with age), and AI & VI inactives (12% of sample; VI practice declines rapidly with age) (Figure 4). AI practice was more common among group 1 (AI & VI persistors, 7% of sample; practice AI and VI at all ages) and 2 (AI & VI desistors, 16% of sample; AI and VI practice declines with age) women (Figure 4A). While AI practice decreased slowly with age among AI & VI persistors (group 1), AI practice among AI & VI desistors (group 2) declined sharply from age 25 (Figure 4). AI practice was reported during 49% of visits when a male partner was reported among AI & VI persistors (group 1), which was substantially higher than groups 3–5, where it ranged from 4% to 6% of visits, while group 2 reported AI at 10% of visits when a male partner was reported (Table 3). Nearly all women (96%) in group 1 reported AI during at least a quarter of visits when a male sex partner was reported, and 40% reported AI during at least half of such visits. By contrast, the approximately 3-quarters of women in the other groups who reported any AI over follow-up reported it occasionally (<25% of visits) (Supplementary Figure 6).
Figure 4.
Proportions of women reporting (A) AI practice and (B) VI practice, since last visit by age group and by trajectory group. Trajectory groups are numbered in descending order of the proportion of visits during which AI was reported. Shaded areas represent 95% confidence intervals.
Table 3.
Percentages of visits in which various sexual practices since last visit were reported, by trajectory group
| Behavior reported since last visit | Group 1: AI &VI persistors % (95% CI) | Group 2: AI & VI desistors % (95% CI) | Group 3: VI persistors % (95% CI) | Group 4: VI desistors % (95% CI) | Group 5: AI & VI inactives % (95% CI) |
|---|---|---|---|---|---|
| At all visits | Nv = 1,660 | Nv = 4,458 | Nv = 10,622 | Nv = 4,231 | Nv = 2,680 |
| AI | 46.4% (43.9–48.8) | 6.9% (6.2–7.7) | 3.8% (3.4–4.2) | 1.7% (1.3–2.1) | 0.4% (0.2–0.7) |
| VI | 93.8% (92.7–95.0) | 66.5% (65.1–67.9) | 92.2% (91.6–92.7) | 43.2% (41.7–44.7) | 8.0% (7.0–9.0) |
| Any male sex partner | 95.6% (94.6–96.6) | 68.0% (66.6–69.3) | 93.2% (92.7–93.7) | 44.9% (43.4–46.4) | 9.3% (8.2–10.4) |
| Any female sex partner | 7.7% (6.4–9.0) | 2.5% (2.0–3.0) | 3.0% (2.6–3.3) | 8.2% (7.3–9.0) | 39.8% (37.8–41.6) |
| Multiple male sex partners (2+) | 40.1% (37.8–42.5) | 15.5% (14.5–16.6) | 21.9% (21.1–22.6) | 8.1% (7.3–8.9) | 2.5% (1.9–3.1) |
| Exchange sex | 13.6% (11.9–15.2) | 3.0% (2.5–3.5) | 3.8% (3.4–4.2) | 2.1% (1.7–2.6) | 1.1% (0.7–1.5) |
| At visits with male sex partner(s) reported | Nv = 1,587 | Nv = 3,030 | Nv = 9,896 | Nv = 1,898 | Nv = 248 |
| AI | 48.5% (46.0–51.1) | 10.3% (9.2–11.4) | 4.1% (3.7–4.5) | 3.9% (3.0–4.8) | 6.1% (2.7–9.5) |
| VI | 98.2% (97.5–98.9) | 98.5% (98.1–99.0) | 99.0% (98.8–99.2) | 98.1% (97.5–98.7) | 94.2% (91.2–97.3) |
| At visits with AI practice reported | Nv = 735 | Nv = 297 | Nv = 382 | Nv = 69 | Nv = 12 |
| Consistent condom use during AI | 24.8% (21.6–27.9) | 31.3% (26.0–36.6) | 21.7% (17.6–25.9) | 36.2% (24.6–47.9) | 16.7% (0.0–41.4) |
| At visits with VI practice reported | Nv = 1,534 | Nv = 2,919 | Nv = 9,634 | Nv = 1,802 | Nv = 212 |
| Consistent condom use during VI | 15.9% (14.1–19.2) | 13.8% (9.9–17.8) | 23.2% (22.3–24.0) | 32.0% (29.9–34.2) | 32.1% (38.4–25.7) |
| At visits with multiple male sex partners reported | Nv = 666 | Nv = 693 | Nv = 2,321 | Nv = 342 | Nv = 68 |
| AI | 54.7% (50.8–58.6) | 19.2% (16.1–22.3) | 7.1% (6.0–8.2) | 7.3% (4.4–10.3) | 12.5% (2.8–22.2) |
| VI | 98.2% (97.1–99.2) | 98.8% (98.0–99.6) | 68.9% (98.4–99.3) | 97.6% (95.9–99.2) | 91.9% (85.0–98.9) |
| At visits with AI practice and multiple male partners reported | Nv = 342 | Nv = 122 | Nv = 149 | Nv = 22 | Nv = 6 |
| Consistent condom use during AI | 37.7% (32.4–42.9) | 36.9% (28.2–54.6) | 30.2% (22.7–37.7) | 50.0% (27.3–72.7) | 16.7% (0.0–59.5) |
| At visits with VI practice and multiple male partners reported | Nv = 644 | Nv = 670 | Nv = 2,239 | Nv = 321 | Nv = 57 |
| Consistent condom use during VI | 20.8% (17.7–240) | 28.1% (24.6–31.5) | 25.9% (24.1–27.8) | 34.0% (28.7–39.2) | 31.6% (19.1–44.0) |
Nv = number of visits over follow-up; AI = anal intercourse; VI = vaginal intercourse; 95% CI = 95% confidence interval (calculated using the Clopper-Pearson confidence interval for a binomial proportion). The recall period for all sexual behaviors was “since last visit,” which was typically 6 months prior. The groups identified through group-based trajectory modeling are numbered in order of declining proportion of visits in which AI was reported. Consistent condom use is defined as using condoms during every AI or VI act since last visit. Group 1 consists of 75 women, group 2 of 169, group 3 of 549, group 4 of 167, and group 5 of 125.
Although the proportions of visits at which a male partner was reported were similar in group 1 (AI & VI persistors) and 3 (VI persistors) (96% and 93%, respectively), the fraction of visits at which AI was reported was more than 10-fold greater in group 1 than group 3. The group with the smallest fraction of visits reporting a male sex partner (group 5: AI & VI inactives) had the largest fraction of visits with a female sex partner reported (40%). Group 1 (AI & VI persistors) reported exchange sex at 14% of visits and multiple male partners at 40% of visits, which was markedly more common than among other groups (Table 3).
Baseline Correlates of Trajectory Group Membership
Bivariate associations between trajectory group membership and sociodemographic and structural characteristics and behaviors are shown in Supplementary Table 4 and multivariable multinomial regression results are shown in Table 4. In multivariable models, compared to black women, Hispanic women had increased odds of being AI & VI persistors (group 1) (adjusted odds ratio [aOR] = 1.36, 95% CI: 1.02–2.60), and white women over twice the odds of being AI & VI inactives (group 5) rather than VI persistors (reference group 3). Women identifying as bisexual or lesbian, compared to heterosexual, had twice the odds (aOR = 1.98, 95% CI: 1.01–3.86) of being AI &VI persistors (group 1), and 12-fold the odds (aOR = 12.50, 95% CI: 7.45–26.37) of being AI & VI inactives (group 5) than being VI persistors.
Table 4.
Multivariable analysis of baseline characteristics associated with trajectory group membership among HIV-seronegative women in the WIHS cohort, using group 3 (VI persistors) as reference group
| Group 1: AI & VI persistors (N = 75) |
Group 2: AI & VI desistors (N = 169) |
Group 4: VI desistors (N = 167) |
Group 5: AI & VI inactives (N = 125) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Category | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI |
| Sociodemographic and structural variables | |||||||||
| Age in years | Continuous | 1.00 | 0.96–1.03 | 1.07 | 1.04–1.10 | 1.04 | 1.01–1.06 | 1.02 | 1.01–1.05 |
| Race/ethnicity | Black | Ref | Ref | Ref | Ref | ||||
| Hispanic | 1.36 | 1.02–2.60 | 1.04 | 0.62–1.96 | 0.98 | 0.62–1.57 | 0.89 | 0.45–1.92 | |
| White* | 1.51 | 0.77–4.67 | 1.08 | 0.61–2.05 | 1.68 | 0.92–4.30 | 2.39 | 1.20–4.76 | |
| Other | 2.29 | 0.55–6.45 | 1.38 | 0.57–3.39 | 0.91 | 0.35–2.55 | 1.44 | 0.27–3.71 | |
| Sexual orientation | Heterosexual | Ref | Ref | Ref | Ref | ||||
| Lesbian/bisexual | 1.98 | 1.01–3.86 | 1.47 | 0.83–2.59 | 1.94 | 1.12–3.37 | 12.50 | 7.43–26.37 | |
| Education | <High school | Ref | Ref | Ref | Ref | ||||
| ≥High school | 1.35 | 0.76–2.40 | 1.01 | 0.67–1.49 | 1.43 | 0.95–2.15 | 1.12 | 0.70–1.80 | |
| Violence victimization Ever† | None | Ref | Ref | Ref | Ref | ||||
| Either physical or sexual violence | 1.34 | 0.85–2.82 | 1.08 | 0.64–1.84 | 1.86 | 0.69–2.04 | 1.31 | 0.75–2.27 | |
| Both physical and sexual violence | 2.22 | 1.09–4.81 | 1.88 | 1.13–3.12 | 0.91 | 0.51–1.63 | 1.28 | 0.70–2.37 | |
| Behavioral variables | |||||||||
| Injection drug use ever | No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.02 | 0.51–2.01 | 0.81 | 0.55–1.74 | 1.26 | 0.78–2.05 | 1.25 | 0.72–2.19 | |
| Exchange sex ever‡ | No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.05 | 0.56–1.93 | 1.09 | 0.65–1.77 | 1.03 | 0.40–1.24 | 0.95 | 0.54–1.67 | |
| Number of male sex partners ever | Below median (<11) | Ref | Ref | Ref | Ref | ||||
| Median or higher (≥11) | 2.08 | 1.09–3.88 | 0.87 | 0.57–1.32 | 0.68 | 0.45–1.05 | 0.26 | 0.12–0.46 | |
aOR = adjusted odds ratio; CI = confidence interval; Ref = reference group.
The model is also adjusted for recruitment wave. The largest trajectory group was used as the reference group: group 3: VI persistors. Trajectory groups are numbered in descending order of the proportion of visits during which AI practice was reported. Bold denotes odds ratios with CIs not including 1. Missing values were imputed. The variables recruitment wave, age, race, and injection drug use ever had no missing values, sexual orientation had 17, education status had 3, sexual violence had 255, physical violence had 251, exchange sex ever had 3, and lifetime number of male sex partners had 10. Violence victimization questions have many missing values as ethical approval was not granted at the Los Angeles and San Francisco study sites.
Non-Hispanic white.
Physical and sexual violence victimization were highly correlated with one another. The 2 variables were therefore combined into one to reduce multi-collinearity in the models.
Ever exchanged sex for drugs or money.
Compared to women with no history of violence victimization, women who reported physical and sexual violence ever had around twice the odds of being AI & VI persistors (group 1) and AI & VI desistors (group 2), than being VI persistors. Women with a history of exchange sex, compared to women with no such history, had nearly twice the odds of being AI & VI persistors (group 1) in bivariate analysis, but there was no association after multivariable adjustment. Compared to women reporting median or lower number of lifetime male partners, women who reported above the median had twice the odds (aOR = 2.08, 95% CI: 1.09–3.88) of being AI & VI persistors and quarter the odds (aOR = 0.26, 95% CI: 0.12–0.46) of being AI & VI inactives, than being VI persistors.
Results were similar when the analysis was restricted to the complete cases only (Supplementary Table 5). Multiple imputation was generally more efficient as can be seen by the narrower CIs.
DISCUSSION
This analysis offers the first detailed longitudinal examination of heterosexual AI practice. Overall, AI practice was fairly common among HIV-seronegative women in the WIHS cohort, with a third reporting AI at least once over a median 14 years of follow-up. AI practice decreased more sharply with age compared to VI. For most who practiced AI, it was an episodic rather than regular sexual practice, with nearly two-thirds reporting AI practice at fewer than a quarter of visits. Compared to when VI only was practiced, we found periods of AI practice to be disproportionately accompanied by reporting multiple male sex partners and inconsistent condom use, particularly for the small group of women who continued to practice AI throughout their lives (AI and VI persistors), who were also more likely to report practicing exchange sex.
Our multinominal regression analysis of baseline characteristics found that Hispanic women (vs black women), women who identify as bisexual or lesbian (vs heterosexuals), women who had ever experienced both sexual and physical violence(vs no violence), and women who reported above median number of male sex partners (vs median or below) were more likely to be in the AI and VI persistors group than in the VI persistors group. These associations with longitudinal AI practice are similar to associations identified in cross-sectional studies, where AI practice has been reported as more common among Hispanic than black women,23–28 among white compared to black women,3,29,30 among women who have sex with both men and women,3,25,31,32 among survivors of sexual23,33–38 and physical violence,33,39–41 and among women with multiple male sex partners.3,8,42,43
Overall, AI was reported at a tenth of visits at which a male sex partner was reported, and by 12% of women within the first year of follow-up. This is similar to the 11% of women in a nationally representative 2015 survey who reported AI in the past year,44 but less common than the 30% reporting AI over the same recall period in the National HIV Behavioral Surveillance System (NHBS-HET), which sampled sexually active women of low socio-economic status living in US cities with high HIV prevalence.3 Despite recruiting from an ostensibly similar population using similar eligibility criteria, women in the NHBS-HET sample displayed higher risk sexual behavior overall, with a third reporting transactional sex in the past year compared to 8% in the WIHS cohort, so it is not surprising that AI prevalence was also higher in that study. Using data on AI prevalence and frequency from the NHBS-HET sample, a mathematical modeling study estimated that 4 in 10 new HIV infections in this population could be attributed to condomless AI practice,4 highlighting that condomless AI is a key risk factor for HIV acquisition among women which should not be ignored.
One of the limitations of our study is the use of face-to-face interviews, which may have affected the accuracy of reporting due to social desirability bias and in particular may have led to underreporting of the oft-stigmatized AI practice.2 Given the small number of women who seroconverted over follow-up (N =23), we were unable to examine how AI practice affected subsequent HIV acquisition. As a number of serial cross-sectional studies indicate that heterosexual AI may be becoming more common over time,45–47 it would have been beneficial to conduct an age-period cohort analysis to examine whether patterns of AI practice over the life course have changed over time. However, the varying eligibility criteria from wave to wave precluded this analysis. However, in providing a thorough analysis of women’s AI practice over the life course, our study addresses an important gap in our understanding of women’s STI and HIV vulnerabilities. A major strength of our analysis is that we not only described AI practice across the whole sample, but that we also identified and described variability in AI practice among subgroups of women. As such, AI practice over the life course is now much better understood.
CONCLUSIONS
Our findings indicate that while AI is likely a regular and long-term part of sexual practice for a small subgroup of women at risk of HIV acquisition in the United States, a substantial fraction of women experience AI transiently at some point in their life and that condom use during AI is largely inconsistent. Clinicians should therefore include questions on AI practice when assessing patients’ HIV and STI risk. In addition, to detect anal STIs, women should be offered both rectal and vaginal tests, rather than solely vaginal tests, as is currently the norm in routine STI screening.48
AI persistors, those who practice AI regularly over the life course and use condoms inconsistently, are likely particularly vulnerable to HIV acquisition. Of note, women in this subgroup more often reported physical and sexual violence, multiple male partners, and exchange sex. Given the clustering of HIV vulnerabilities in this subgroup, they may particularly benefit from PrEP, and other ongoing HIV prevention strategies. However, AI practice may often be unplanned, rendering adherence to oral PrEP difficult. In which case, injectable PrEP, recently found to be more efficacious than oral PrEP among men, may offer the promise of effective longer-term prevention if found to also be efficacious among women.49 Public health messaging emphasizing the importance of condom use would likely be most effective when coupled with interventions to reduce gender-based violence, as our findings indicate that AI often occurs in the context of violence, when women are unlikely able to negotiate condom use.
Supplementary Material
ACKNOWLEDGMENTS
Acknowledgements data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (principal investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV).
Funding:
The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and Other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR). Rebecca F. Baggaley is supported by a Wellcome Trust Institutional Strategic Support Fund Fellowship (204801/Z/16/Z). Mathieu Maheu-Giroux’s research program is funded by a career award from the Fonds de Recherche du Québec - Santé. Tonya Taylor’s work is supported by HRSA-19-008 (Cohen and Reinhardt, mPI 7/1/19-6/30/24 and 1R21NR018348-01 (Wilson) 7/1/18-6/30/20. Dominika Seidman’s work is supported by grant 5K12HD001262-18 from the National Institutes of Health. We acknowledge joint-centre funding from the UK Medical Research Council and Department for International Development (Grant reference MR/R015600/1). This award is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. We thank the HIV Prevention Trials Network (HPTN) Modelling Centre (NIH UM1-AI-068617) and the HPTN Leadership and Operations Center (UM1AI068619) for support. Support for the HPTN is provided by the NIAID of the National Institutes of Health (ceNIH).
Footnotes
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Branwen Nia Owen; Marie-Claude Boily; Rebecca F. Baggaley; Mathieu Maheu-Giroux; Jocelyn Elmes; Catalina Ramirez; Adaora A. Adimora; Andrew Edmonds
(b) Acquisition of Data
Adaora A. Adimora; Kemi Sosanya; Tonya Taylor; Michael Plankey; Jocelyn Elmes; Dominika Seidman; Kathleen M. Weber; Elizabeth T. Golub; Anandi N. Sheth; Hector Bolivar; Deborah Konkle-Parker
(c) Analysis and Interpretation of Data
Branwen Nia Owen
Category 2
(a) Drafting the Article
Branwen Nia Owen; Rebecca F. Baggaley; Mathieu Maheu-Giroux; Jocelyn Elmes; Adaora A. Adimora; Catalina Ramirez; Andrew Edmonds; Kemi Sosanya; Tonya Taylor; Michael Plankey; Julie Cederbaum; Dominika Seidman; Kathleen M. Weber; Elizabeth T. Golub; Anandi N. Sheth; Hector Bolivar; Deborah Konkle-Parker; Marie-Claude Boily
(b) Revising It for Intellectual Content
Branwen Nia Owen; Rebecca F. Baggaley; Mathieu Maheu-Giroux; Jocelyn Elmes; Adaora A. Adimora; Catalina Ramirez; Andrew Edmonds; Kemi Sosanya; Tonya Taylor; Michael Plankey; Julie Cederbaum; Dominika Seidman; Kathleen M. Weber; Elizabeth T. Golub; Anandi N. Sheth; Hector Bolivar; Deborah Konkle-Parker; Marie-Claude Boily
Category 3
(a) Final Approval of the Completed Article
Branwen Nia Owen; Rebecca F. Baggaley; Mathieu Maheu-Giroux; Jocelyn Elmes; Adaora A. Adimora; Catalina Ramirez; Andrew Edmonds; Kemi Sosanya; Tonya Taylor; Michael Plankey; Julie Cederbaum; Dominika Seidman; Kathleen M. Weber; Elizabeth T. Golub; Anandi N. Sheth; Hector Bolivar; Deborah Konkle-Parker; Marie-Claude Boily
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jsxm.2020.06.007.
Conflict of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Baggaley RF, Owen BN, Silhol R, et al. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am J Reprod Immunol 2018;80:e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen BN, Brock PM, Butler AR, et al. Prevalence and Frequency of Heterosexual Anal Intercourse Among Young People: A Systematic Review and Meta-analysis. AIDS Behav 2015;19:1338–1360. [DOI] [PubMed] [Google Scholar]
- 3.Hess KL, DiNenno E, Sionean C, et al. ; NHBS Study Group. Prevalence and Correlates of Heterosexual Anal Intercourse Among Men and Women, 20 U.S. Cities. AIDS Behav 2016; 20:2966–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmes J, Silhol R, Hess KL, et al. Receptive anal sex contributes substantially to heterosexually-acquired HIV infections among at-risk women in Twenty US Cities: results from a modelling analysis. Am J Reprod Immunol 2020:e13263. [DOI] [PMC free article] [PubMed]
- 5.Owen BN, Elmes J, Silhol R, et al. How common and frequent is heterosexual anal intercourse among South Africans? A systematic review and meta-analysis. J Int AIDS Soc 2017;19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen BN, Baggaley RF, Elmes J, et al. What Proportion of Female Sex Workers Practise anal Intercourse and How Frequently? A Systematic Review and Meta-analysis. AIDS Behav 2020;24:697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross M, Holte SE, Marmor M, et al. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIV-NET Vaccine Preparedness Study 2 Protocol Team. J Acquir Immune Defic Syndr 2000;24:393–398. [DOI] [PubMed] [Google Scholar]
- 8.Benson LS, Martins SL, Whitaker AK. Correlates of Heterosexual Anal Intercourse among Women in the 2006–2010 National Survey of Family Growth. J Sex Med 2015;12:1746–1752. [DOI] [PubMed] [Google Scholar]
- 9.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018;47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab 2014;65:205–210. [DOI] [PubMed] [Google Scholar]
- 11.Andruff H, Carraro N, Thompson A, et al. Latent Class Growth Modelling: A Tutorial. Tutor Quant Methods Psychol 2009; 5:11–24. [Google Scholar]
- 12.Nagin DS. Group-based modelling of development. Cambridge, MA: Harvard University; 2005. [Google Scholar]
- 13.Garrett ES, Zeger SL. Latent class model diagnosis. Biometrics 2000;56:1055–1067. [DOI] [PubMed] [Google Scholar]
- 14.Owen BN. A neglected risk factor in HIV prevention and implications for prevention. Imperial College; London; 2019. [Google Scholar]
- 15.Hanley JA, Negassa A, Edwardes MD deB, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–375. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple Imputation after 18+ Years. J Am Stat Assoc 1996;91:473–489. [Google Scholar]
- 17.Rubin D Multiple Imputation for Nonresponse in Surveys. Hoboken, New Jersey: John Wiley & Sons; 1987. [Google Scholar]
- 18.Team RCR. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018; ISBN 3–900051-07–0. [Google Scholar]
- 19.Wickham H ggplot2: Elegant Graphics for Data Analysis. New York: Sringer; 2009. [Google Scholar]
- 20.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. J Stat Softw 2017;78:1–56. [Google Scholar]
- 21.van Buuren S Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 22.Venables W, Ripley B. Modern Applied Statistics with S. New York: Springer; 2002. [Google Scholar]
- 23.Champion JD, Roye CF. Toward an understanding of the context of anal sex behavior in ethnic minority adolescent women. Issues Ment Health Nurs 2014;35:509–516. [DOI] [PubMed] [Google Scholar]
- 24.Carter M, Henry-Moss D, Hock-Long L, et al. Heterosexual anal sex experiences among Puerto Rican and black young adults. Perspect Sex Reprod Health 2010;42:267–274. [DOI] [PubMed] [Google Scholar]
- 25.Roye CF, Krauss BJ, Silverman PL. Prevalence and correlates of heterosexual anal intercourse among Black and Latina female adolescents. J Assoc Nurses AIDS Care 2010;21:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quadagno D, Sly DF, Harrison DF, et al. Ethnic differences in sexual decisions and sexual behavior. Arch Sex Behav 1998; 27:57–75. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds GL, Fisher DG, Napper LE, et al. Heterosexual Anal Sex Reported by Women Receiving HIV Prevention Services in Los Angeles County. Women’s Heal Issues 2010;20:414–419. [DOI] [PubMed] [Google Scholar]
- 28.McLellan-Lemal E, O’Daniels CM, Marks G, et al. Sexual risk behaviors among African-American and Hispanic women in five counties in the Southeastern United States: 2008–2009. Womens Health Issues 2012;22:e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javanbakht M, Guerry S, Gorbach PM, et al. Prevalence and correlates of heterosexual anal intercourse among clients attending public sexually transmitted disease clinics in Los Angeles County. Sex Transm Dis 2010;37:369–376. [PubMed] [Google Scholar]
- 30.Leichliter JS, Chandra A, Liddon N, et al. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. J Infect Dis 2007;196:1852–1859. [DOI] [PubMed] [Google Scholar]
- 31.Foxman B, Aral SO, Holmes KK. Interrelationships among douching practices, risky sexual practices, and history of self-reported sexually transmitted diseases in an urban population. Sex Transm Dis 1998;25:90–99. [DOI] [PubMed] [Google Scholar]
- 32.Mackesy-Amiti ME, McKirnan DJ, Ouellet LJ. Relationship characteristics associated with anal sex among female drug users. Sex Transm Dis 2010;37:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hess KL, Javanbakht M, Brown JM, et al. Intimate partner violence and anal intercourse in young adult heterosexual relationships. Perspect Sex Reprod Health 2013;45:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lescano CM, Houck CD, Brown LK, et al. Correlates of heterosexual anal intercourse among at-risk adolescents and young adults. Am J Public Health 2009;99:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahs B, Swank E, Clevenger L. Troubling Anal Sex: Gender, Power, and Sexual Compliance in Heterosexual Experiences of Anal Intercourse. Gend Issues 2015;32:19–38. [Google Scholar]
- 36.Champion JD. Context of sexual risk behaviour among abused ethnic minority adolescent women. Int Nurs Rev 2011;58:61–67. [DOI] [PubMed] [Google Scholar]
- 37.Champion JD, Shain RN, Piper J, et al. Sexual Abuse and Sexual Risk Behaviors of Minority Women with Sexually Transmitted Diseases. West J Nurs Res 2001;23:241–254. [DOI] [PubMed] [Google Scholar]
- 38.Wingood GM, DiClemente RJ. Child sexual abuse, HIV sexual risk, and gender relations of African-American women. Am J Prev Med 1997;13:380–384. [PubMed] [Google Scholar]
- 39.El-Bassel N, Gilbert L, Wu E, et al. HIV and intimate partner violence among methadone-maintained women in New York City. Soc Sci Med 2005;61:171–183. [DOI] [PubMed] [Google Scholar]
- 40.Koblin BA, Hoover DR, Xu G, et al. Correlates of anal intercourse vary by partner type among substance-using women: baseline data from the UNITY study. AIDS Behav 2010;14:132–140. [DOI] [PubMed] [Google Scholar]
- 41.Raj A, Santana MC, La Marche A, et al. Perpetration of intimate partner violence associated with sexual risk behaviors among young adult men. Am J Public Health 2006;96:1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorbach PM, Manhart LE, Hess KL, et al. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex Transm Dis 2009; 36:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calsyn DA, Hatch-Maillette MA, Meade CS, et al. Gender differences in heterosexual anal sex practices among women and men in substance abuse treatment. AIDS Behav 2013;17:2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbenick D, Bowling J, Fu T-C (Jane), et al. Sexual diversity in the United States: Results from a nationally representative probability sample of adult women and men. Xu J, editor. PLoS One 2017;12:e0181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brody S, Weiss P. Heterosexual anal intercourse: increasing prevalence, and association with sexual dysfunction, bisexual behavior, and venereal disease history. J Sex Marital Ther 2011;37:298–306. [DOI] [PubMed] [Google Scholar]
- 46.CDC. Key Statistics from the National Survey of Family Growth[Internet]. National Centre for Health Statistics. 2018. Available at: https://www.cdc.gov/nchs/nsfg/key_statistics/s.htm#analsex. Accessed July 20, 2020. [Google Scholar]
- 47.Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet (London, England). 2013;382:1781–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao G, Hoover KW, Nye MB, et al. Infrequent Testing of Women for Rectal Chlamydia and Gonorrhea in the United States. Clin Infect Dis 2018;66:570–575. [DOI] [PubMed] [Google Scholar]
- 49.HIV Prevention Trials Network. Long-acting injectable cabotegravir is highly effective for the prevention of HIV infection in cisgender men and transgender women who have sex with men.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.