Abstract
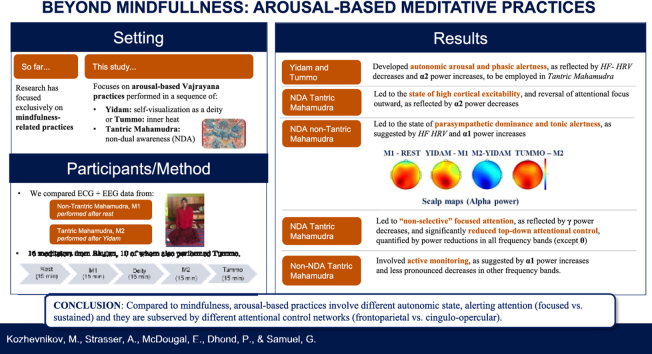
Here we report meditative techniques, which modulate attentional control by arousal-driven influences and not by monitoring continuous thought processes as during mindfulness-related practices. We focus on Vajrayana (Tantric Buddhism) practices, during which a sequence of generation (self-visualization as a deity - Yidam) or completion with sign (inner heat -Tummo) stages necessarily precedes non-dual awareness (NDA) Tantric Mahamudra. We compared the electrocardiographic and electroencephalographic correlates of Mahamudra performed after rest (non-Tantric Mahamudra) with Mahamudra performed after Yidam (Tantric Mahamudra) in 16 highly experienced Vajrayana practitioners, 10 of whom also performed Tummo. Both Yidam and Tummo developed the state of PNS withdrawal (arousal) and phasic alertness, as reflected by HF HRV decreases and Alpha2 power increases, later neurophysiologically employed in Tantric Mahamudra. The latter led to the unique state of high cortical excitability, “non-selective” focused attention, and significantly reduced attentional control, quantified by power reductions in all frequency bands, except Theta. In contrast, similar to mindfulness-related practices, non-Tantric Mahamudra was performed in a state of PNS dominance (relaxation), tonic alertness, and active monitoring, as suggested by Alpha1 power increases and less pronounced decreases in other frequency bands. A neurobiological model of meditation is proposed, differentiating arousal-based and mindfulness-related practices.
Keywords: Non-dual awareness, Autonomic nervous system, Arousal, Attentional control, Mindfulness-related meditation, Arousal-based meditation
Graphical abstract
Highlights
-
•
Arousal-based meditations involve the state of PNS withdrawal and phasic alertness.
-
•
Top-down control during arousal-based practices is modulated by arousal.
-
•
Mindfulness-based practices involve the state of PNS dominance and tonic alertness.
-
•
Top-down control during mindfulness-based practices is regulated by monitoring.
-
•
NDA practices aim at non-selectivity of attention and reduction of top-down control.
1. Introduction
Most meditation research so far has focused on “mindfulness-related practices”, broadly defined as a set of attention-based, regulatory training regimes (Lutz et al., 2015), the goal of which is to develop a particular type of attentional control targeted at maintaining attention “on purpose, in the present moment, and non-judgmentally” (Kabat-Zinn, 1994). Here we refer to attentional control (also known as top-down control or executive control) as an individual's capacity to focus attention on task-relevant information for goal-directed behavior while avoiding interference from irrelevant stimuli.
From a neurobiology perspective, the underlying feature of mindfulness-related practices is that they elicit the so-called relaxation response (Benson and Klipper, 1975), a state of parasympathetic nervous system (PNS) dominance, characterized by tonic alertness or vigilance (sustained attention of a relatively permanent character) (Aston-Jones et al., 2000; Petersen and Posner, 2012). Tonic alertness is maintained by a specific type of attentional control, subserved in the brain by the cingulo-opercular network (CON), occupying medial frontal/cingulate cortex and bilateral anterior insula, which provides stable maintenance of task parameters by preventing attention reorienting to a distractor (Dosenbach et al., 2007). Although most existing frameworks of mindfulness-related practices contrast Focused Attention (FA) vs. Open Monitoring (OM) types of meditation1 (Cahn and Polich, 2006; Dahl et al., 2015; Lutz et al., 2015), assuming to differentiate such aspects of attentional control as sustaining attention on a chosen object versus monitoring the content of experience, both FA and OM practices are shown to be associated with stress reduction and the increase in tonic alertness (Britton et al., 2014; Landry and Raz, 2016). Moreover, as both FA and OM involve monitoring one's attentional states (Lutz et al., 2008) to ensure the attention is sustained for a continuous period, they are likely to be supported by the same type of attentional control subserved by the CON.
Not easily accounted for by the existing frameworks of mindfulness-related practices and being more culturally complex, a large category of other meditative practices, which instead of relaxation demonstrate an arousal response, has largely escaped the attention of meditation researchers. These include practices employed within Vajrayana (Tantric Buddhism) (Amihai and Kozhevnikov, 2014), by Sufis of the Islamic tradition (“whirling dervishes”, Cakmak and Kozhevnikov, 2016), and probably by a number of other traditions, including Hindu Tantra (Zope and Zope, 2013) and East Asian martial arts (Krein and Ilundáin, 2014). The so-called Loving-Kindness and Compassion practices found in most Buddhist traditions also generally involve arousal (Lumma et al., 2015). Arousal is a state of the autonomic nervous system (ANS), characterized by a withdrawal from PNS activity towards an enhanced sympathetic nervous system (SNS) tone. It is accompanied by phasic alertness – selective (focused) attention increments of short endurance – occurring in response to potential threat or novelty as a result of enhanced production of noradrenaline (NE) by the brainstem nucleus locus coeruleus (LC) (Aston-Jones et al., 2000).2 The phasic aspects of attentional control are supported by a lateral prefrontal-parietal or fronto-parietal control network (FPCN), anatomically and functionally separable from the CON (Dosenbach et al., 2008), which dynamically coordinates goal-relevant information across sub-processes, keeping distributed contextual and stimulus-related information online and/or shifting between mental processes (Sadaghiani and Kleinschmidt, 2016). The FPCN is functionally linked to the locus coeruleus norepinephrine (LC-NE) system and modulated by arousal (Grueschow et al., 2020; Sara and Bouret, 2012).
As arousal-based meditative practices are associated with a different autonomic state to mindfulness-related practices (PNS withdrawal vs. PNS dominance), they are likely to recruit a different type of alerting attention (phasic vs. tonic) and corresponding attentional control (FPCN vs. CON). Yet, scientific research on meditation has not differentiated between arousal-based and mindfulness-related practices, treating all meditations as involving tonic alertness and the same type of attentional control related to monitoring the content of mental experiences (e.g., Britton et al., 2014; Lomas et al., 2015; Lutz et al., 2015 for reviews). The main goal of this study is to demonstrate that arousal-based meditations are neurophysiologically fundamentally different from traditionally studied – either FA or OM – mindfulness-related practices.
Here we focus on Vajrayana Tantric practices,3 as they have been shown reliably to involve a state of arousal (Amihai and Kozhevnikov, 2014). A fundamental issue in Vajrayana training is the sequence of generation and completion stages. Completion stage practices are divided further into completion stages with signs/characteristics and without signs/characteristics (Kongtrul, 2007, p. 69).4 The latter aim to develop a culminating mental state that directly recognizes the nature of the mind beyond subject and object, referred to within the Vajrayana tradition as Mahamudra (Kragh, 2015; Yeshe, 2003). In meditation research, these states are termed Non-Dual Awareness (NDA) (Dunne, 2011;Josipovic, 2014); however, the discussion about NDA states has mainly focused on non-Tantric types Mahamudra. These, according to some Tibetan schools, can be accessed by non-Tantric (“mindfulness-based”) techniques without preliminary engagement in generation or completion with signs practices.5 In the few neuroimaging studies on NDA meditation (Fucci et al., 2018; Josipovic, 2014; Josipovic et al., 2011), participants were instructed to commence meditating Mahamudra directly from the resting state. Within the Vajrayana context, however, Tantric Mahamudra is always performed after a generation-stage practice, such as self-visualization as a deity (Yidam), within a single session. In highly trained meditators, it follows a “completion with signs” practice, such as the legendary Tummo (“inner fire”) practice. This raises the question of whether non-Tantric and Tantric Mahamudra NDA practices involve similar neurophysiology and can be grouped into a single category of NDA practices.
In this study, we examined heart rate variability (HRV) in the electrocardiogram (ECG) and spectral dynamics of the electroencephalogram (EEG) in 16 highly experienced Vajrayana practitioners from several retreat centers in Bhutan while they performed non-Tantric Mahamudra (i.e., Mahamudra following rest) and then Tantric Mahamudra (i.e., Mahamudra following Yidam). Ten of these practitioners were Tummo experts, and they also performed Tummo practice. Our first hypothesis was that Yidam and Tummo practices develop the state of PNS withdrawal, which serves as a neurophysiological precursor for Tantric Mahamudra. We expected Tummo to generate significantly higher arousal than Yidam, as it involves not only visualization but also vigorous breathing (Kozhevnikov et al., 2013). Our second hypothesis was that Tantric Mahamudra, being arousal-based meditation, is supported by a different type of alerting attention and corresponding attentional control than non-Tantric Mahamudra, associated with the state of PNS dominance and representing a mindfulness-related practice. Therefore, although Tantric and non-Tantric types of Mahamudra involve identical meditative techniques, we expect these practices to show different ANS states and corresponding EEG markers. Finally, based on the results, we propose a novel neurobiological model of meditation, which recognizes both mindfulness-related and arousal-based practices.
2. Materials & methods
2.1. Participants
The study was conducted in Bhutan under the guidance of H.E. Gyeltshen Trulku Rinpoche, a retreat master in the Drukpa Kagyu lineage. Sixteen of his experienced retreat nuns and monks from four retreat centers (Gongtung Tokden Choling, Bikhar Retreat Centre, Largyab Retreat Centre, Tashigang Nunnery), who follow the Drukpa Kagyu lineage of Vajrayana training and Mahamudra meditation, participated in the study. These participants (5 nuns and 11 monks) had a mean age of 33.18 (±7.28) and an average of 7.75 (±2.91) years of Vajrayana meditation experience in a retreat setting. Six of these participants (1 female) completed three three-year retreats (meditation experience in retreats ≥9 years), while the other ten practitioners have completed two three-year retreats. Only 10 practitioners (all males) out of 16 had undergone Tummo training. All participants provided written, informed consent for their participation in the study. The study was approved by the National University of Singapore's review board.
2.2. Procedure
Data from the 5 nuns and 1 monk were recorded at Gyeltshen Trulku Rinpoche's Tashigang Nunnery (Eastern Bhutan), and data from the other 10 monks were collected in one of the typical houses in Thimphu - the capital city of Bhutan. Fig. 1 outlines the sequence of the practices. At the beginning of each session, participants sat for 15 min of rest, during which they were explicitly instructed not to meditate but to remain seated and simply relax (without performing any meditation). After rest, participants were asked to perform 15 min of Mahamudra meditation (M1, non-Tantric Mahamudra). Following a short break of 2–3 min, the participants performed 15 min of Yidam practice, followed by 15 min of Mahamudra meditation (M2, Tantric Mahamudra). After another 4–5-min break, 10 practitioners, who master Tummo practice, were also asked to perform 15 min of Tummo (Forceful Breath) meditation. Although Tummo is usually practiced before Tantric Mahamudra, since only 10 practitioners in our sample have experience with this practice, we asked them to perform it at the end in order to have a consistent set of data from all 16 participants on the other three types of practices (M1, Yidam, and M2). EEG and ECG data were continuously recorded throughout the study (see Fig. 2). Participants had their eyes open during the experiment as they usually perform these meditation practices with open eyes. A translator provided each participant with the instructions, translated from English to Tibetan or Dzongka (Bhutanese language), on the activities and sequence of meditative practices to be performed during the experiment.
Fig. 1.
Outline of the sequence of practices, M1 (non-Tantric Mahamudra), Yidam (Yidam self-visualization), and M1 (Tantric Mahamudra) practices.
Fig. 2.
Experimental set-up. Practitioner performs a sequence of meditative practices, while his EEG and ECG recordings are taken.
2.3. Vajrayana practices
Yidam (translated from Tibetan by the term “meditational deity”) practice, also known as Deity self-visualization practice, is a central generation-stage practice in Vajrayana (Dalai Lama and Tsongkhapa, 2017; Studstill, 2005). The deities used include peaceful, semi-wrathful, and wrathful forms (with the more wrathful forms aimed at destroying internal obstacles to Buddhahood). The practice focuses on self-identification with a deity (see Fig. 3), typically at the center of a complex array of secondary deities (mandala). The self-visualized content during Yidam is multimodal; colorful 3D images (e.g., the Deity's body, ornaments, and environment) and representations of sensorimotor body schema, feelings, and emotions of the Deity are generated, entirely replacing the practitioner's sense of self.
Fig. 3.
An example of a wrathful meditation deity (Vajrakilaya), used by Vajrayana meditators in their Yidam practice.
Tummo is widely used within Tibetan Vajrayana traditions, forming part of the Six Yogas of Nāropa and Six Yogas of Niguma practices (Evans-Wentz, 2002; Dalai Lama II Dge- Dun-Rgya-Mtsho et al., 1985; Mullin, 1996). In terms of Nyingma school of Vajrayana Buddhism, Tummo is a completion stage practice “with signs”. The practice involves working with the flow of internal breath through a network of visualized channels (nadi) and junction points (chakra) within the body. It involves a special breathing technique called “the vase”: after deep and prolonged inhalation through both nostrils, practitioners hold their breath while performing isometric contractions of both abdominal and pelvic muscles so that the abdominal wall protrudes and retracts (Evans-Wentz, 2002), followed by forceful, relatively short exhalation. Different Tummo-type practices exist, varying in the intensity of their breathing techniques, goals, and visualization content. The type of Tummo practice performed by the practitioners in this study was Forceful Breath, the goal of which is to raise the “inner fire”. It involves voluntary regulated forceful and vigorous “vase” breathing and visualization of a rising flame that starts below the navel and that rises towards the crown of the head with each breath, followed by mental imagery of “moon-fluid” drops falling from the top of the head (“crown-chakra”) down along the spine (“central-channel”), and blazing the inner fire (Evans-Wentz, 2002; Mullin, 1996).
Tantric Mahamudra meditation is performed following Yidam or Tummo and corresponds to a completion “without signs” (i.e., without any explicit visualization). In it, the self-generated visualization of all conceptual aspects of the preceding practices is dissolved (e.g., the deity and its entourage dissolve into emptiness) to achieve awareness devoid of any conceptualization. Mahamudra practice emphasizes evenly distributed attention directed outwards but not towards particular objects or experiences. Although various aspects of experience (e.g., thoughts, feelings, or images) may arise spontaneously, the practitioner is instructed to let them subside on their own accord, without letting the mind dwell on or analyze them (Traleg Kyabgon Rinpoche, 2004). Thus, Mahamudra is considered to be a practice with no object of meditation; it does not require noticing or watching the content of one's mind from the perspective of a non-judgmental observer (as in more familiar forms of Vipassana or Mindfulness), an activity that is associated with a dualistic mind (Tulku Urgyen Rinpoche, 2000)
2.4. ECG and EEG recordings protocol
EEG was continuously recorded at the Fp1, Fp2, F3, Fz, F4, F7, F8, T3, T4, T5, T6, C3, Cz, C4, P3, Pz, P4, O1, O2, and POz scalp regions positioned according to the standard international 10–20 channel system using a B-Alert X24 wireless EEG headset (Advanced Brain Monitoring, Inc.), as well as from 2 additional electrodes placed on the right and left mastoids. ECG was recorded via two electrodes placed on the right collarbone and below the left rib cage. EEG and ECG were sampled at 256 Hz and referenced to the average between the two mastoid electrodes.
Heart rate variability (HRV) analysis. Prior to calculating the HRV parameters, each ECG recording was visually inspected for gross artifacts and subsequently corrected for ectopic beats and non-valid RR intervals (i.e., the time between two successive R-waves of the QRS complex, representing ventricular depolarization). Frequency-domain HRV parameters were estimated with Welch's method in HRV analysis software (Pichot et al., 2016). We extracted HF and LF frequencies for the first and last 4 min at the beginning and end of each meditation period (excluding the first and last 10 s of each recording), respectively. We used normalized spectral band powers for HF (0.15–0.40 Hz) and LF (0.04–0.15 Hz) for all statistical analyses.
Although HRV is widely used as an assessment of ANS activity, particularly of cardiac vagal tone, influences of the respiratory parameters on its HF component have been well recognized (e.g., Brown et al., 1993). In this study, however, due to a close association between respiratory parameters and ANS states, which is impossible to de-couple without causing unattended effects on the experimental task parameters (Quintana and Heathers, 2014, for a review), we did not control or factor in the respiratory patterns. Indeed, voluntary breath regulation required by Tummo practice involves a shorter duration of exhalation relative to inhalation, which itself might lead to the decreases of cardiovagal nerve tonus and consequent HF HRV decrease (Telles et al., 2011). Furthermore, in experimental conditions involving spontaneous breathing at a normal rate (between 12 and 16 breaths per min), as is the case in Yidam, control of respiration parameters is discouraged since this would require additional mental concentration, which might contribute to additional decreases in HF HRV, not related to the experimental condition (Sasaki and Maruyama, 2014).
The HRV data of one Tummo expert out of 16 practitioners were excluded from the final analyses due to technical problems with the ECG recording. Two additional practitioners (both Tummo experts) had their HRV data for the first set of meditative practices (Rest, M1, Yidam, M2) removed due to artifacts however, their Tummo data were retained. The final analyses include separate HRV analyses of 13 practitioners (7 of whom are Tummo experts) for the first set of meditative practices and 9 Tummo experts for HRV changes during Tummo practice.
Since the HRV data (HF and LF) in all conditions (RestSTART, RestEND, M1END, YidamSTART, YidamEND, M2END, TummoSTART, TummoEND) were normally distributed (Kolmogorov-Smirnoff test, all ps > .05), parametric statistics were applied. Specifically, using a mixed 4X2 ANOVA with Practice (RestSTART, M1END, YidamEND, M2END) as the within-subject factor and Tummo Expertise (experts, non-experts) as between-subject factor, we contrasted HF, LF, and LF/HF in the last 4 min of each practice to the last 4 min of the preceding condition (i.e., YidamEND vs. M1END, M2END vs. YidamEND), with the only exception of choosing the first 4 min (instead of the last 4 min) in the Rest condition (RestSTART) as the baseline for the last 4 min of M1 (M1END). These baselines were chosen because practitioners performed Rest, M1, Yidam, and M2 sequentially, and we were interested in the changes in HRV parameters (i.e., HF, LF, and LF/HF) between different practices. Furthermore, although instructed to simply rest, not meditate, some practitioners reported meditating during Rest, as indicated by informal interviews after the experiment. Thus, RestSTART instead of RestEND was chosen as a baseline for M1END to avoid the confounding effect of different types of meditation performed during Rest.
To examine if meditation experience (years spent in retreats) affected practitioners' resting and meditative states, Meditation Experience was added as a covariate to the above statistical analysis. Additionally, to examine whether Yidam's wrathfulness (peaceful, semi-wrathful, and wrathful) might have affected the HRV changes achieved during this practice, we added Wrathfulness of Yidam as a covariate to repeated measures ANOVA with Time (YidamSTART, YidamEND) as the within-subject factor. Since only 7 out of 9, Tummo practitioners had a complete set of HRV data for the first set of meditative practices, the HRV analysis of their Tummo practice (N = 9) was conducted separately using TummoSTART as a baseline to TummoEND.
Spectral Power Analysis of the EEG. The EEG signal was pre-processed in EEGLAB (Delorme and Makeig, 2004). Data were pre-processed with a 0.5 Hz high-pass and 50 Hz notch-filter (to remove line-noise). For artifact removal, we used Infomax Independent Component Analysis (Infomax ICA) to reject ocular and muscular artifacts (Delorme et al., 2007). For each subject, well characterized ICA artifacts were identified and subtracted from the data. These components accounted for frontal eye artifacts and temporal muscle noise. Experimenter RD used visual inspection of component scalp topographies and power spectrum to reject these artifactual ICA components.
Power spectral distribution (PSD) was estimated with the Fast Fourier Transform (FFT) algorithm (2 s FFT window length and spectral accuracy of 10 points per Hz) in the EEGLAB plugin Darbeliai (Delorme and Makeig, 2004) for the final 4 min of each meditation period (excluding the last 10 s of each recording). Similar to our ECG analyses, in the rest condition, we also estimated the PSD for the first 4 min (excluding the first 20 s) of the recordings.
Subsequently, the mean power values at the Delta (1–4 Hz), Theta (4–8 Hz), Alpha (8–12 Hz), including Alpha 1 (8.5–10 Hz) and Alpha 2 (10.5–12 Hz), Beta (13–25 Hz), including Beta 1 (12–15 Hz), Beta 2 (16–18 Hz), and Beta 3 (20–25 Hz), and Gamma (35–45 Hz) frequencies were used in the statistical analyses. These a-priori frequency bands were selected to allow comparisons between our findings and previous meditation studies, most of which have used a similar choice of pre-defined frequency bands (Cahn and Polich, 2006; Lee et al., 2018; Lomas et al., 2015, for reviews). Averaged PSD values were topographically visualized on scalp maps in MATLAB (R2020b, The Mathworks, Inc.).
Similar to HRV analysis, we compared the EEG changes for each frequency band for the last 4 min of each of the meditative practices (M1END, YidamEND, M2END, TummoEND) to the last 4 min of the preceding condition (i.e., YidamEND vs. M1END, M2END vs. YidamEND), except choosing the first 4 min of Rest condition (RestSTART) as the baseline for the last 4 min of M1 (M1END). For all the statistical analyses below, we set the significance level to 0.05 (i.e., if the p-value is less than 0.05, we reject H0), but we report marginally significant results as well.
First, statistical analyses of the spectral power for each frequency band for the first set of the meditation practices (N = 16, all practitioners) were conducted. False discovery rate (FDR; Benjamini and Hochberg, 1995) was used to analyze how EEG power changed for each meditative practice in relation to its baseline. Additionally, since the spectral data for each electrode in all the conditions and frequency bands for all the practitioners (RestSTART, RestEND, M1END, YidamEND, M2END) showed normal distribution (Kolmogorov-Smirnoff test, all ps > .05), parametric statistics were applied. Specifically, a mixed 20 × 4 × 2 ANOVA with Electrode (Fp1, Fp2, F3, Fz, F4, F7, F8, T3, T4, T5, T6, C3, Cz, C4, P3, Pz, P4, O1, O2, POz) and Practice (RestSTART, M1END, YidamEND, M2END) as within-subject factor and Tummo Expertise (experts, non-experts) as between-subject factor was conducted. In addition, to examine if Meditation Experience (measured as years spent in retreats) affected the practitioners' EEG resting and meditative states, Meditation Experience was added as a covariate to the statistical analysis. To further examine the effects of scalp region and laterality, we also performed repeated-measures 4 × 4 × 3 ANOVA with Practice (RestSTART, M1END, YidamEND, M2END), Region (Frontal, Central, Parietal, Occipital), and Laterality (Left, Midline, Right) as within-subject factors. For that, we divided the scalp into 4 regions, each of which consisted of an average of several electrodes that were selected according to their location: Frontal (Fp1, Fp2, F3, Fz, F4), Central (C3, Cz, C4), Parietal (P3, Pz, P4), and Occipital (O1, O2). For potential effects of hemisphere (laterality), we divided the scalp into 3 areas, each of which consisted of the average of 3 electrodes that were selected according to their location: Left (Fp1, F3, F7, C3, P3, O1), Right (Fp2, F4, F8, C4, P4, O2), and Midline (Fz, Cz, Pz). For all the ANOVA post-hoc comparisons, Bonferroni correction for multiple comparisons was applied.
Second, to study the effect of practicing Tummo on meditative and resting states of the practitioners, we conducted separate statistical analyses of the spectral power for Tummo experts (N = 10) for each frequency band for all meditative practices. Parametric statistics were applied with FDR (Benjamini and Hochberg, 1995) to analyze the changes in EEG power for each meditative practice in relation to its baseline, as the spectral data for each electrode in all the conditions and frequency bands for 10 Tummo experts showed normal distribution (Kolmogorov-Smirnoff test, all ps > .05). Specifically, a 20X5 repeated measures ANOVA with Electrode and Practice (RestSTART, M1END, YidamEND, M2END, and TummoEND) as within-subject factors was conducted, followed by a 5 × 4 × 3 repeated measures ANOVA for Tummo practitioners with Practice (RestSTART, M1END, YidamEND, M2END, TummoEND), Region (Frontal, Central, Parietal, Occipital) and Laterality (Left, Midline, Right) as within-subject factors.
Relationship between EEG spectral power and HRV data. For each meditation practice, we also computed the Pearson correlation coefficient between HRV changes and corresponding changes in EEG spectral power across those scalp areas and frequency bands, which exhibited significant changes during the meditation practice.
EEG Coherence Analysis. The MATLAB function “mscohere” was adopted for the head surface EEG coherence calculation. Mean coherence for each frequency was defined as the average coherence across all bins within that frequency range. In total, 18 electrode pairs were selected to investigate changes incoherence (Fz-Cz, Cz-Pz, Fz-Pz, F3–F7, F4–F8, F3–P3, F4–P4, F7–P3, F8–P4, F3–F4, F7–F8, C3–C4, P3–P4, F3–C3, F4–C4, C3–P3, C4–P4, Fp1-Fp2), separately for Delta, Theta, Alpha, Beta, and Gamma.
To compare changes in coherence across practices, for each frequency band, for all 16 participants, we first ran pairwise comparisons for each of the electrode pairs in each meditative practice compared to its baseline, controlling for FDR with Benjamini and Hochberg (1995). Furthermore, since the coherence data for each electrode pair in all conditions and frequency bands for all 16 practitioners were normally distributed (Kolmogorov-Smirnoff test, all ps > .05), a parametric mixed 18 × 4 × 2 ANOVA with Electrode Pair and Practice (RestSTART, M1END, YidamEND, M2END) as within-subject factors and Tummo Expertise (Tummo experts, non-Tummo practitioners) as between-subject factor was used to investigate the changes in EEG coherence. Second, for the 10 Tummo experts, a 18X5 repeated measures ANOVA with Electrode Pair and Practice (RestSTART, M1END, YidamEND, M2END, TummoEND) as within-subject factors and Meditation Experience (years spent in retreats) as a covariate was conducted.
Although neuroscience research (Schoffelen and Gross, 2009) has questioned the extent of how reliably head surface EEG coherence analysis might reflect true functional connectivity between the brain regions (as activity from the same underlying source is picked up by many electrodes), the EEG coherence analysis was used in the current study to allow comparisons between our findings and previous meditation studies, which rely on the same method (Lee et al., 2018; Lomas et al., 2015, for reviews).
3. Theoretical framework
As changes in ANS activity are related to LC discharge frequency and the corresponding type of alertness (Aston-Jones and Cohen, 2005), different ANS states (PNS withdrawal vs. dominance) suggest different alerting attention and attentional control mechanisms. LC neurons might exhibit tonic or phasic modes of functioning by firing tonically (by continuous baseline activity, 1 to 6 spikes per second) or with short phasic bursts of higher frequency (10–15 spikes per second). Higher firing frequency within the tonic mode of LC activity is associated with decreased stress and task utility; it produces the state of heightened tonic alertness, characterized by high behavioral flexibility and scanning attentiveness (Aston-Jones et al., 2000). In contrast, the phasic mode of LC activity is associated with activation of the LC-NE system and elevated release of NE, which occurs concurrently with autonomic arousal, reflecting complementary cognitive (LC-NE) and physiological (arousal) contributions to the mobilization for action in response to arousing stimuli. The LC-NE activation produces the state of phasic alertness, associated with heightened selective (focused) attention,6 necessary for task performance of high utility (Aston-Jones et al., 2000; Sara and Bouret, 2012).
The states of tonic and phasic alertness are supported by different attentional control networks, the CON and FPCN, respectively (Sadaghiani and Kleinschmidt, 2016). Arousal-related influences play an important role in modulating the neuronal activity in the FPCN (Grueschow et al., 2020; Hernaus et al., 2017; Sara and Bouret, 2012). If the current focus of attention has sufficient priority, fronto-parietal regions, under arousal, contribute to the enhanced excitation of high-priority information and inhibition of low-priority information (adaptive gain theory, Aston-Jones and Cohen, 2005; GANE model, Mather et al. (2016). Otherwise, LC-NE activation promotes a global reset of attention, consistent with the “network reset” theory, allowing large-scale brain network reconfigurations to respond appropriately to the environmental demands (Bouret and Sara, 2005), and thus serving as a mechanism for the engagement of selective attention towards more relevant stimuli (Arnsten et al., 2012; Vazey et al., 2018).
To evaluate the changes in the activity of the ANS system across four meditative practices (Yidam, M1, M2, and Tummo), we used ECG measures, shown to be reliably related to the activity of both PNS and SNS (Camm et al., 1996). Specifically, changes in HF are associated with PNS activity, while changes in LF are assumed to reflect both SNS and PNS activation (Akselrod et al., 1981;Pomeranz et al., 1985). The ratio of LF to HF (LF/HF) has been used to quantify the dynamic relationship between sympathetic and parasympathetic nerve activities (i.e., the sympatho-vagal balance) (Pagani et al., 1986), with psychological stress being associated with an increased LF/HF ratio (Sloan et al., 1994); although Billman (2013) has challenged this assumption. While HF increases generally indicate PNS activation (Pomeranz et al., 1985), HF decreases can serve as a marker of PNS withdrawal associated arousal (i.e., decreased PNS and increased SNS activation) (Chalmers et al., 2014; Toledo et al., 2003; von Rosenberg et al., 2017). Therefore, in this study, the pattern of an increasing HF index was interpreted to indicate the state of PNS dominance (relaxation), while a pattern of HF decreasing with no significant increases in LF/HF was interpreted as indicative of PNS withdrawal (arousal).
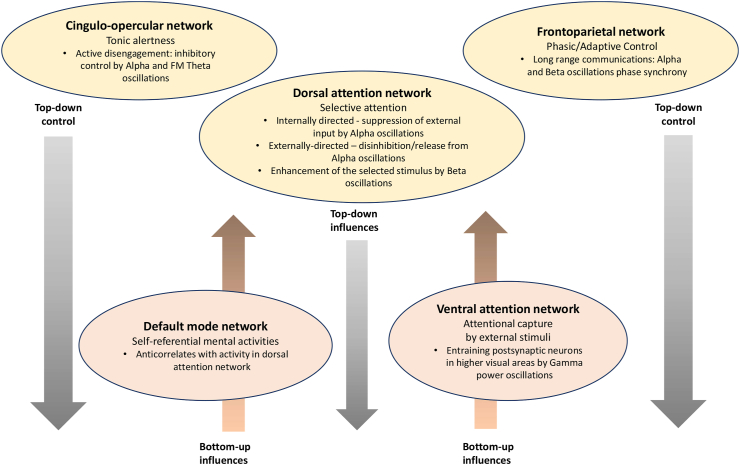
To analyze the changes in EEG spectral power and coherence with respect to the type of attentional control employed by each of the four meditative practices, we adopted a theoretical framework, detailed below (Fig. 4), which summarizes recent neuroscience research on the brain networks contributing to the control of attention in relation to top-down and bottom-up influences.
Fig. 4.
Large-scale networks, participating in attentional control (cingulo-opercular network and fronto-parietal network), attention networks (dorsal attention and ventral attention), and default mode network. The networks which exert top-down control are indicated in yellow and networks mediating bottom-up influences, are indicated in pink. EEG markers of each network are listed under the name of the network. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In addition to the FPCN that supports phasic aspects of attentional control and the CON that maintains tonic alertness (Dosenbach et al., 2007; Sadaghiani and Kleinschmidt, 2016), there are two attention networks, the dorsal attention network (DAN) and the ventral attention network (VAN), working together to support attention orienting. The DAN comprises the intraparietal sulcus (IPS), superior parietal lobule (SPL), and the frontal eye field (FEF), while the VAN is anchored in the temporoparietal junction (TPJ) and ventral frontal cortex (VFC). Similar to the FPCN, the DAN supports phasic aspects of attention (Sadaghiani and D’Esposito, 2015); it implements top-down selective attention that involves orienting towards and focusing on particular information while deprioritizing the other (Corbetta et al., 2008). The VAN mediates bottom-up influences (e.g., detection of behaviorally relevant stimuli) by serving as an alerting mechanism for the DAN when a salient stimulus, outside of attentional focus, triggers reorienting of attention. In addition, the default mode network (DMN), implicated in a variety of self-referential mental activities (Buckner et al., 2008; Raichle, 2015), may also trigger attention reorienting by sending signals to the DAN. The FPCN mediates the functional interaction between the DAN and VAN (Vincent et al., 2008). During the tasks requiring selective attention, to protect the DAN from attention reorienting, the FPCN deactivates the VAN's regions that may interfere with task performance (Shulman et al., 2007). Also, the FPCN coordinates the DAN-DMN coupling, contributing to the negative correlations often reported between these two networks (Dixon et al., 2018). During sustained attention, the CON deactivates both the VAN and DMN, preventing reorientation to unimportant information (Corbetta et al., 2008).
Research on brain oscillations provided evidence that bottom-up influences are mediated by narrowband Gamma-band oscillations (Magazzini and Singh, 2018; Michalareas et al., 2016; van Kerkoerle et al., 2014), the primary source of anatomical feedforward projections. Conversely, top-down influences are mainly mediated by low-frequency oscillations, in particular Alpha and Beta (Fries., 2015), which are the primary sources of anatomical backward projections. Delta band activity has been suggested to play a role in “interference control”, responsible for inhibiting sensory afferences interfering with task performance (Harmony, 2013). Theta rhythms, mainly frontal midline theta (FM-Theta), have been related to top-down control functions related to sustained attention (Clayton et al., 2015; Mazaheri and Picton, 2005). Alpha band oscillations are thought to facilitate the processing of a specific sensory input through functional inhibition of task-irrelevant regions (“gating-by-inhibition”) (Jensen and Mazaheri, 2010; Klimesch et al., 2007, 2012), whereas enhanced Beta band activity has been related to the dominance of top-down influences overriding the effect of potentially novel, or unexpected, external events (Buschman and Miller, 2007; Engel and Fries, 2010). In addition, there has been increasing evidence that during the states of phasic alertness, the FPCN exerts top-down control on sensory areas by means of temporal synchrony of lower frequencies, in particular Alpha and Beta (Bastos et al., 2015; Michalareas et al., 2016; Miller and Buschmann, 2013; Sauseng et al., 2005). The DAN might reduce or increase Alpha power in task-relevant regions, depending on the task's processing mode (internal vs. external). During the tasks requiring “internally directed” selective attention, increased Alpha activity reflects suppression of external input (either from the VAN or DMN) that may disturb the maintenance of working memory representations (Jensen and Mazaheri, 2010; Klimesch, 2012). During the tasks requiring “externally directed” selective attention, Alpha power activity decreases, which leads to disinhibition of task-relevant brain regions, and the state of enhanced cortical excitability (i.e., the strength of the response of cortical neurons to a given stimulation) (Jensen and Mazaheri, 2010; Lange et al., 2013; Romei et al., 2008; van Dijk et al., 2008). Conversely, during the states of tonic alertness, the CON exerts top-down control via enhanced Alpha power, often accompanied by enhanced FM-Theta power, reflecting widespread inhibition of neural activity (Sadaghiani and Kleinschmidt, 2016).
Recently, narrowband Gamma rhythms have been suggested to provide a competitive advantage to the neuronal group of lower visual areas, activated by the attended stimulus, in entraining postsynaptic neurons in higher visual areas (Fries, 2015); thus, on the one hand, facilitating conscious perception, while on the other hand mediating perceptual suppression of unattended stimuli (Fries et al., 2002; Sedley and Cunningham, 2013). The notion of bottom-up Gamma-influences, subserving propagating sensory representations in a feedforward manner across the cortical hierarchy, and Beta influences, subserving feedback (backward) communication of top-down predictions, has received further support in neuroscience research (Arnal and Giraud, 2012; Bastos et al., 2015; Bosman et al., 2012; Michalareas et al., 2016), providing the basis for a predictive coding model of visual processing7 (Bastos et al., 2012 for a review).
The theoretical framework outlined in Fig. 4 allows us to make specific predictions regarding EEG correlates of the meditative practices examined in this study. Specifically, we expect Yidam and Tummo to be performed in an aroused state and show increases in Alpha power, reflecting employment of “internally directed” selective attention. As these practices aim to strengthen top-down influences, we also expect increases in EEG coherence (particularly in fronto-parietal Alpha coherence reflecting activation of the FPCN) and increases in Beta and Gamma power due to focusing attention on selected visual images. For non-Tantric Mahamudra, which we expect to involve a state of PNS dominance, the theoretical framework predicts increases in Alpha and FM-Theta power, reflecting top-down control exerted by the CON to maintain the state of tonic alertness. In contrast, for Tantric Mahamudra (M2), which we expect to involve arousal, we predict Alpha power decreases, reflecting employment of “externally directed” selective attention and the state of enhanced cortical excitability. Furthermore, as Mahamudra practice (either Tantric or non-Tantric) aims to weaken top-down influences and instructs the practitioners not to direct attention toward objects or experiences (Traleg Kyabgon Rinpoche, 2004), we expect decreases in EEG coherence and reductions in Beta and Gamma power, respectively.
4. Results
4.1. Heart rate variability
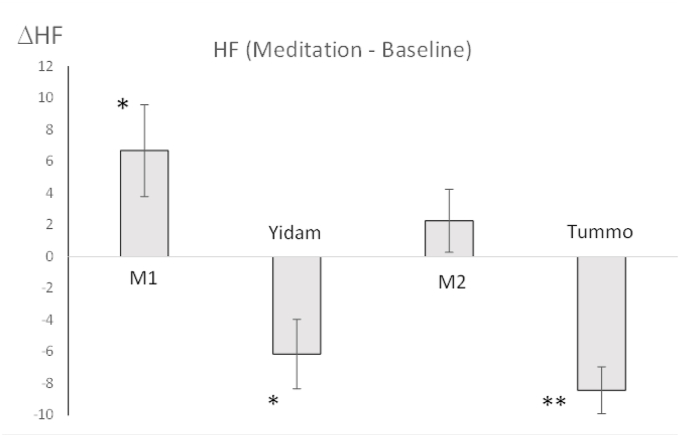
The results for the changes in normalized HF-HRV band power (ΔHF), the main marker of ANS activity in this study, for each meditative practice in relation to its baseline are presented in Fig. 5.
Fig. 5.
HF differences between meditation practices and baseline (ΔHF for M1END was computed in relation to RestSTART, YidamEND in relation to M1END, ΔHF for M2END in relation to YidamEND and ΔHF for TummoEND in relation to TummoSTART). START, first 4 min; END, last 4 min.
First Set of Meditative Practices. For HF, the main effect of Practice was significant, F(3,33) = 3.23, p = .03, ηp2 = 0.23. There was no significant effect of Tummo Expertise, F < 1, p = .81. The interaction between Tummo Expertise and Practice was also non-significant, F (3,33) = 1.01, p = 54. Based on our hypothesis that M1 will cause a state of relaxation, while Yidam, Tummo, and M2 will be performed in an aroused state, we conducted planned contrasts comparing HF between 1) M1END and RestSTART; 2) YidamEND and M1END, and 3) M2END and YidamEND. There was a significant increase in HF during M1END in comparison to RestSTART, F(1,12) = 5.36, p = .04, ηp2 = 0.33, but a significant decrease in HF during YidamEND in comparison to M1END, F(1,12) = 7.80, p = .02, ηp2 = 0.41. There was no significant change in HF during M2END compared to YidamEND, F (1,12) = 1.31, p = .28, suggesting that the level of arousal was sustained during M2 meditation. When Meditation Experience (years spent in retreats) was added as a covariate into the above ANOVA, neither its effect on HF, F(1,10) = 1.10, p = .32 nor the interaction between Meditation Experience and Practice, F < 1, p = .68, were significant.
As for LF, the main effect of Practice was not significant, F(3,33) = 1.67, p = .19, as well as the effect of Expertise, F < 1, p = .56. The effect of Practice or Expertise on LF/HF was also not significant, F(3,36) = 1.95, p = .14 and F < 1, p = .52, respectively.
Tummo practice. For those practitioners who performed Tummo practice, there was a significant decrease in HF between the last and first 4 min of the Tummo practice [mean HFEND = 10.25, SD = 4.32 and mean HFSTART = 17.98, SD = 4.62, F(1,8) = 28.62, p <. 001, ηp2 = 0.78. There was a marginally significant increase in LF, F(1,8) = 5.24, p = .051, ηp2 = 0.40, but no significant changes in LF/HF, F(1,8) = 2.95, p = .19. Although there was a significant decrease in HF between the last and first 4 min of Yidam (YidamEND in comparison with YidamSTART) as well (mean HFEND = 20.38, SD = 8.77 and mean HFSTART = 25.37, SD = 13.63, F(1, 12) = 5.29, p = .04, ηp2 = 0.31), it was only about half as large as during Tummo (20% for Yidam vs. 43%) and of smaller effect size (ηp2 = 0.31 for Yidam vs. ηp2 = 0.78 for Tummo). Furthermore, while the changes in LF during Yidam were not significant, F < 1, p = .46, for Tummo, they were marginally significant, suggesting that in addition to the withdrawal from the PNS system, as it occurs during Yidam, Tummo also leads to an increase in SNS tone.
Wrathfulness of Yidam. While Tummo practice involves visualization of a semi-wrathful deity (“Vajravarahi”, who is one of the main semi-wrathful deities associated with the Drukpa Kagya lineage), Yidam might involve the visualization of peaceful, semi-peaceful, and fully wrathful deities. To examine whether the wrathfulness of the Yidam might have affected the level of arousal achieved during this practice, we conducted a repeated-measures ANOVA for Yidam with Time (YidamSTART, YidamEND) as the within-subject factor and wrathfulness of Yidam as a covariate. There were insignificant HF changes from YidamSTART to YidamEND, F(1,11) = 2.17, p = .16, while the interaction between Time and Wrathfulness was significant, F(1,11) = 6.16, p = .03, ηp2 = 0.35, suggesting that the more wrathful the visualized deity, the higher the level of arousal achieved. HF decreases during self-visualization as a peaceful deity was 6%, as a semi-wrathful deity – 22%, and as a wrathful - 29%.
4.2. EEG spectral power and coherence analysis
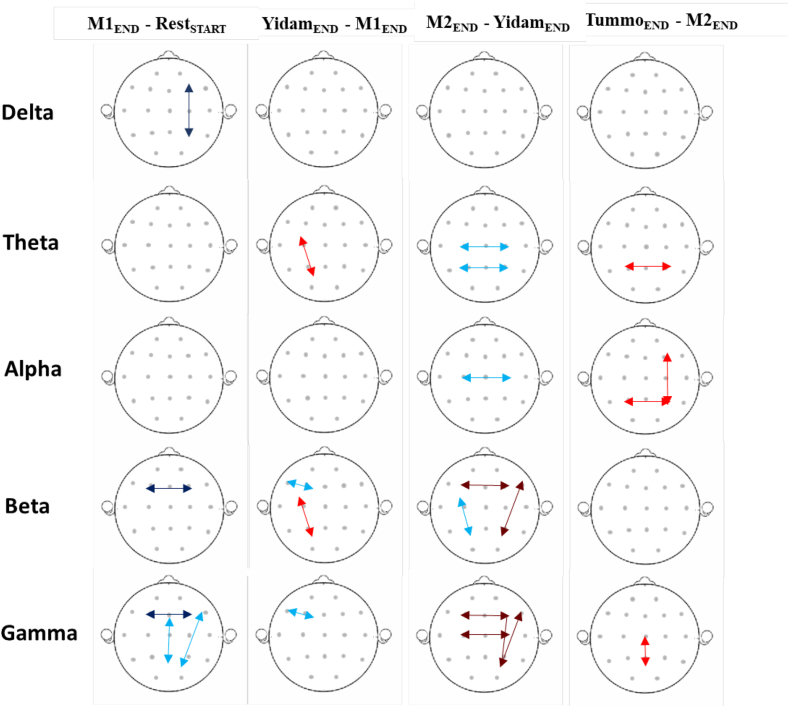
The statistical analyses for EEG spectral power for each frequency band are presented in Table 1, Table 2, Table 3, Table 4, Table 5. The scalp maps for Tummo practitioners across all meditative practices are presented in Fig. 6. The results of the EEG coherence analyses are presented in Fig. 7. The results are analyzed and discussed within the framework of attentional control, illustrated in Fig. 4.
Table 1.
Results of statistical analyses for Delta power.
| DELTA POWER |
|||
|---|---|---|---|
| Multiple comparisons with FDR correction | 20 × 4 × 2 mixed ANOVA | 4 × 4 × 3 mixed ANOVA | |
| First set of meditative practices, performed by all practitioners (N = 16) | M1: Decrease in F7 (p = .07) M2: Decreases in Fz (p < .05), F4 and Fp2 (ps = .06) Yidam: Increases in F8 and T4 (ps = .06) |
Sig effect of Practice, F(3, 42) = 2.84, p = .049, ηp2 = .17; sig decrease during M2,F(1.14) = 5.03, p = .04, ηp2 = .26 Sig effect of Electrode,F(19, 266) = 2.56, p = .000, ηp2 = .15 with higher power in Cz than in O1, O2, P3, P4 and higher power in Fz than in P3 (ps < .05) No sig effect of Tummo Expertise, F(1,14) = 3.21, p = .09. Non-sig effect of Meditation Experience, F < 1, p = .44 |
Sig effect of Region, F(3, 45) = 5.19, p = .004, ηp2 = .27, with higher power in the frontal and central regions than in occipital, ps < .05 Sig effect of Lateralization, F(2,30) = 8.40, p = .001, ηp2 = .38; midline power is higher than left and right (p < .05) Marg Practice X Lateralization, F(6,84) = 2.03, p = .07, ηp2 = .13, with the effect of Lateralization more pronounced during meditative practices than rest |
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | 5 × 4 × 3 repeated measures ANOVA | |
| All practices performed by Tummo experts (N = 10) | M2: Decreases in F3, C3, Cz, T3, P3, P4 (ps < .05), F4 and in Fp2 (ps < .07) Tummo: Increases in F3, F7, F8, Cz, C4, P3, P4, Pz, POz (p < .05) and in Fp1, C3, T3 and T4 (p < .06) |
Sig effect of Practice, F(4,36) = 4.41, p = .005, ηp2 = .33; sig decrease during M2, F(1,9) = 12.37, p = .007, ηp2 = .58; and sig increase during Tummo,F(1,9) = 17,57, p = .002, ηp2 = .66 Sig effect of Electrode, F(19,171) = 2.10, p = .007, ηp2 = .19 with power higher in Cz than in P3 and T5 (ps < .05) Sig Practice X Electrode, F(76, 684) = 2.11, p = .000, ηp2 = .19 |
No sig effect of Region, F(3, 27) = 1.89. p = .16. Sig effect of Lateralization, F(2,18) = 3.95, p = .04, ηp2 = .31 with midline power higher than left (p < .05) |
Table 2.
Results of statistical analyses for Theta power.
| THETA POWER | |||
|---|---|---|---|
| Multiple comparisons with FDR correction | 20 × 4 × 2 mixed ANOVA | 4X4X3X2 mixed ANOVA | |
| First set of meditative practices, performed by all practitioners (N = 16) | No sig changes in any electrode for any practice | No sig effect of Practice, F < 1, p = .99 Sig effect of Electrode,F (19, 266) = 6.74, p = .000, ηp2 = .33, with higher power in Cz than in C4, O2, P3, P4, Pz, T3, T5, T6 (ps < .05) and higher power in Fz than in C4, P3, P4, T3, T6, and T5 (ps < .05) Sig diff between Tummo and non-Tummo practitioners during Rest, F(1,14) = 4.93, p = .04, ηp2 = .26, but not meditation (p = .44), with Tummo experts having lower baseline power No sig effect of Meditation Experience F < 1, p = .95 |
Sig effect of Region, F(3, 45) = 7.07, p = .001, ηp2 = .32, with higher power in the frontal and central regions than in parietal (ps < .05) Sig effect of Lateralization, F(2,30) = 23.69, p = .000, ηp2 = .61; midline Theta power is higher than left and right (p < .001) Sig Region X Lateralization, F(6,84) = 2.79, p = .02, ηp2 = .16, with the highest Theta power in frontal and central midline regions |
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | 5 × 4 × 3 repeated measures ANOVA | |
| All practices performed by Tummo experts (N = 10) | M1: Increases in Fz (ps < .07) | No sig effect of Practice, F (4,36) = 2.13, p = .10 Sig effect of Electrode, F(19,171) = 5.50, p = .000, ηp2 = .38 with power higher in Cz than in C4, P3, and P4 (ps < .05) Marg Practice X Electrode, F (76, 684) = 1.31, p = .05, ηp2 = .13 |
Sig effect of Region, F(3,27) = 3.02, p = .047, ηp2 = .25, with marg higher power in the central region than in parietal (p = .07) Sig effect of Lateralization, F(2,18) = 26.21, p = .000, ηp2 = .74, with midline power higher than left (p < .001) Sig Region X Lateralization, F(6,54) = 3.71, p = .03, ηp2 = .29, with the highest Theta power in frontal and central midline regions |
Table 3.
Results of statistical analyses for Alpha power.
| ALPHA POWER |
|||||
|---|---|---|---|---|---|
| Multiple comparisons with FDR correction | 20 × 4 × 2 mixed ANOVA | 4X4X3X2 mixed ANOVA | |||
| First set of meditative practices, performed by all practitioners (N = 16) | M2: Decrease in F7 (p = .07) | No sig effect of Practice, F < 1, p = .78 Sig effect of Electrode,F (19,266) = 5.95, p = .00, ηp2 = .30, with higher power in Cz and POz than in T5 (ps < .05) No sig effect of Tummo Expertise, F < 1, p = .49, but Practice X Tummo is marg, F(3,42) = 2.81, p = .05, ηp2 = .16 No sig effect of Meditation Experience, F < 1, p = .85 |
Sig effect of Region, F(3, 42) = 3.66, p = .02, ηp2 = .21 with Alpha power in the frontal region being lower than in the central region (p < .02) Sig effect of Lateralization, F(2,30) = 5.21, p = .01, ηp2 = .27; midline Alpha power is higher than left and right (p < .02) |
||
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | 5 × 4 × 3 repeated measures ANOVA | |||
| All practices performed by Tummo experts (N = 10) | M1: Increase in P3 (p < .05) Yidam: Increase in P4 (p = .07) M2: Decreases in F7, P3, T3, Pz, (p < .05), and in Fp1, F4 (p < .06) Tummo: Increases in F3, F7, Fp1, T3, T6, P3, P4, Pz, POz (p < .05), and in F4, Cz (p < .06) |
Sig effect of Practice, F(4,36) = 6.89, p = .000, ηp2 = .43; sig decrease during M2,F(1.9) = 6.54, p = .03, ηp2 = .42 sig increase during Tummo,F(1.9) = 30.80, p = .000, ηp2 = .77 Sig effect of Electrode, F(19,171) = 10.75, p = .000, ηp2 = .38 with power higher in C4 and Cz than in F7, T3, T4 (ps < .05). |
Sig effect of Region, F(3,27) = 9.09, p = .00, ηp2 = .50, with power in the frontal region being lower than in the central (p < .03) Sig effect of Lateralization, F(2,18) = 8.33, p = .003, ηp2 = .48, with midline power higher than left (p = .004) Sig Region X Lateralization, F(6,54) = 4.09, p = .002, ηp2 = .31, with midline power being the highest in anterior regions (p < .05) and higher than left in posterior regions |
||
| ALPHA 1 POWER | ALPHA 2 POWER | ||||
| Multiple comparisons with FDR correction; 20X5 repeated measures ANOVA | Multiple comparisons with FDR correction; 20X5 repeated measures ANOVA | ||||
| All practices performed by Tummo experts (N = 10) | M1: Increases in P4, P3, O1, O2, F4 (p < .05) Tummo: Increases in T3, Fp, F7 (ps < .05) Sig effect of Practice, F(4,36) = 4.88, p = .003, ηp2 = .35; sig increases during M1,F(1.9) = 6.56, p = .03, ηp2 = .42 and Tummo,F(1.9) = 7.00, p = .027, ηp2 = .43. |
Yidam: Increases in P4 (p = .06) M2: Decreases in P3, Fp1 (p < .05) Tummo: Increases in Fp1, P3, POz, and T6 (ps < .05) Sig effect of Practice, F(4,36) = 4.86, p = .003, ηp2 = .35; marg decrease during M2,F(1.9) = 4.34, p = .06, ηp2 = .33 and sig increase during Tummo,F(1.9) = 19.50, p = .002, ηp2 = .70 |
|||
Table 4.
Results of statistical analyses for Beta power.
| BETA POWER |
|||
|---|---|---|---|
| Multiple comparisons with FDR correction | 20 × 4 × 2 mixed ANOVA | 4 × 4 × 3 mixed ANOVA | |
| First set of meditative practices, performed by all practitioners (N = 16) | M1: Decrease in T4 (p < .05) Yidam: Increase in T4 (p < .08) |
No sig effect of Practice, F < 1, p = .66 Sig effect of Electrode,F (19, 266) = 2.85, p = .000, ηp2 = .17, with higher power in F3, F4 than T6 (ps < .05) Sig diff between Tummo and non-Tummo practitioners, F(1,14) = 6.28, p = .03, ηp2 = .31, during meditation, F(1,14) = 5.33, p = .04, ηp2 = .28 and rest, F(1,14) = 5.85, p = .03, ηp2 = .30 with Tummo experts having lower Beta power. No sig effect of Meditation, F < 1, p = .45 |
No sig effect of Region, F(3, 42) = 1.91, p = .16 No sig effect of Lateralization, F < 1, p = .64 |
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | 5 × 4 × 3 repeated measures ANOVA | |
| All practices performed by Tummo experts (N = 10) | M1: Decrease in T4 (p < .07) M2: Decreases in T4 and P3 (p < .07) Tummo: Increases in C4, P3, P4, Pz, POz, O1, O2 (p < .05) |
Sig effect of Practice, F(4,36) = 2.70, p = .046, ηp2 = .23; sig increase during Tummo,F(1.9) = 9.85, p = .01, ηp2 = .52 Sig effect of Electrode, F(19,171) = 1.96, p = .01, ηp2 = .18 (no sig pairwise comparisons) |
No sig effect of Region, F < 1, p = .67 No sig effect of Lateralization, F < 1, p = .42 |
| BETA 1 POWER | BETA 2 POWER | BETA 3 POWER | |
| Multiple comparisons with FDR corr, 20X5 ANOVA | Multiple comparisons with FDR corr, 20X5 ANOVA | Multiple comparisons with FDR corr, 20X5 ANOVA | |
| All practices performed by Tummo experts (N = 10) | M1: Decrease in T4 (p < .07) M2: Decreases in F8, P3, T3 (p < .05) and T3 (ps < .07) Tummo: Increases in F3, T3 (ps < .05), P4, Pz, O2 (ps < .07) Marg effect of Practice, F(4,36) = 1.98, p = .10, ηp2 = .18; marg decrease during M2,F(1.9) = 4.05. p = .07, ηp2 = .31 and sig increase during Tummo, F(1,9) = 4.34, p = .01, ηp2 = .53 |
M1: Decrease in T4 (p < .05) M2: Decreases in F7, F8, T3 (p < .07) Tummo: Increases in T3, T6, O2 (ps < .07) Sig effect of Practice, F(4,36) = 2.87, p = .03, ηp2 = .25; and sig increase during Tummo, F(1,9) = 11.18, p = .009, ηp2 = .55 |
Yidam: Increases in P3 (p = .07) Tummo: Increases in C4, P3, P4, Pz, O1, O2, and POz (ps < .05) and Cz (ps < .07) Marg effect of Practice, F(4,36) = 2.60, p = .05, ηp2 = .22; and sig increase during Tummo, F(1,9) = 9.40, p = .01, ηp2 = .51 |
Table 5.
Results of statistical analyses for Gamma power.
| NARROWBAND GAMMA POWER |
|||
|---|---|---|---|
| Multiple comparisons with FDR correction | 20 × 4 × 2 mixed ANOVA | 4X4X3X2 mixed ANOVA | |
| First set of meditative practices, performed by all practitioners (N = 16) | M1: Decrease in T4 (p < .05) during M1 Yidam: Increase in T4 (p < .08) M2: Decrease in T5 (p < .05) |
No sig effect of Practice, F < 1, p = .56 Sig effect of Electrode,F(19,266) = 3.82, p = .000, ηp2 = .21, with power in F4 marginally higher than in Fz (p = .08) Sig diff between Tummo and non-Tummo practitioners, F(1,14) = 15.92, p = .001, ηp2 = .53, during meditation, F(1,14) = 12.48, p = .004, ηp2 = .47 and rest, F(1,14) = 16.15, p = .001, ηp2 = .54 with Tummo experts having lower Gamma power No sig effect of Meditation Experience, F < 1, p = .77 |
Sig effect of Region, F(3, 42) = 2.98, p = .04, ηp2 = .18, with higher power in the central region than in parietal (p=.06) Sig effect of Lateralization, F(2,28) = 14.99, p = .000, ηp2 = .52, midline Gamma power is lower than left and right (p < .005). |
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | 5 × 4 × 3 repeated measures ANOVA | |
| All practices performed by Tummo experts (N = 10) | M1: Decrease in T5 (p < .06), Tummo: Increases in P3, P4, Pz, POz, Cz, and C4 (p < .05) and in O2 and T6 (p < .06) M2: Decreases in F4, F8 (p < .05), P3, POz p < .08) |
Sig effect of Practice, F(4,36) = 3.91, p = .01, ηp2 = .30; sig increase, F(1,9) = 10.37, p = .01, ηp2 = .54 during Tummo Sig effect of Electrode, F(19,171) = 2.49, p = .001, ηp2 = .22 |
Sig effect of Region, F(3,27) = 3.75, p = .02, ηp2 = .29, with higher power in the central region than in parietal (p = .05) Sig effect of Lateralization (2,18) = 9.44, p = .002, ηp2 = .51, with midline Gamma being the lowest (p < .05) |
| BROADBAND GAMMA POWER | |||
| Multiple comparisons with FDR correction | 20X5 repeated measures ANOVA | ||
| All practices performed by Tummo experts (N = 10) | Decrease in T4 (p < .05) during M1 Tummo: Increases in T5, F3, Fz, P3, P4, Pz, POZ, O1 and O2 (p < .05) |
Sig effect of Practice, F(4,36) = 8,39, p = .001, ηp2 = .58; sig increase F(1,9) = 21.48, p = .001, ηp2 = .71during Tummo Sig effect of Electrode, F(19,171) = 3.83, p = .001, ηp2 = .29 (no sig pairwise comparisons) |
|
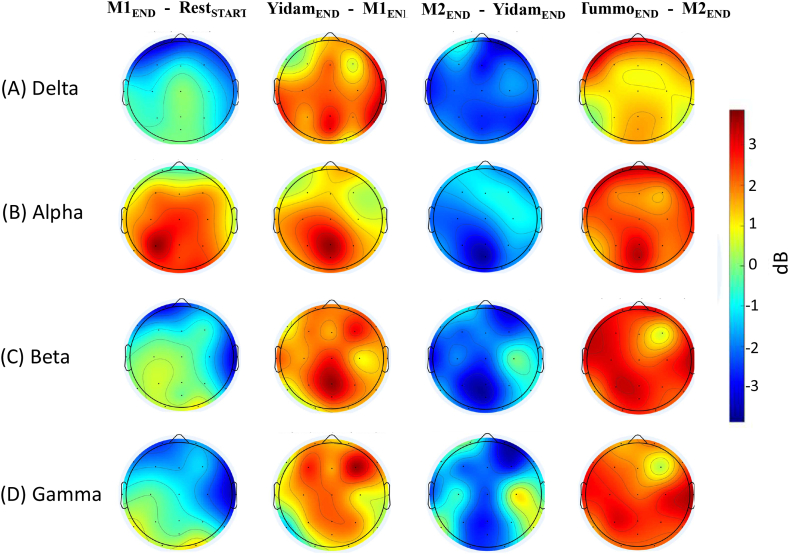
Fig. 6.
Scalp maps for Tummo experts for EEG frequency power across all meditative practices in relation to their baselines (A) Delta, (B) Alpha, (C)Beta, and (D) Gamma.
Fig. 7.
Sensor space coherence mapping. EEG coherence mapping for all meditative practices in relation to their baselines Black dots represent the locations of the recording EEG electrode. The dark blue (decrease) and dark red (increase) lines connecting 2 dots between these two sensors indicate there was significant or near significant change in coherence for all the practitioners (N = 16). Light blue (decrease) and light red (increase) represent significant or near significant changes in coherence for Tummo experts (N = 10). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.2.1. Delta (1–4 Hz)
As shown in Table 1, both Yidam and Tummo practices showed increases in Delta power. For Yidam, however, they were marginal and limited to the right frontal (F8) site, while for Tummo practice they were significant and widespread (Fig. 6A). As for M1 and M2, they both showed decreases in Delta power. Although we observed only a trend for left frontal decreases (in F7) in Delta power during M1, the decreases during M2 were significant and widespread. We did not observe a significant effect of either Tummo expertise or meditative experience on the practitioners' Delta power during their meditative states or rest.
For Delta coherence, for all practitioners (N = 16), multiple comparisons with FDR correction revealed a marginally significant decrease in F4–P4 (p < .06) for M1END in comparison with RestSTART. The effect of Practice on Delta coherence was not significant, F < 1, p = .79. There was no difference in Delta coherence between Tummo experts and other practitioners in any practice, F(1,14) = 1.82, p = .20 or during Rest, F < 1, p = .56. Furthermore, for Tummo experts (N = 10), multiple comparisons with FDR correction revealed no significant changes in Delta coherence for any electrode pairs in any meditative practice. The effect of Practice on Delta Coherence for Tummo experts was also non-significant, F < 1, p = .95. Finally, when Meditation Experience was added as a covariate to ANOVA to investigate its effect on the resting state coherence, the results yielded no significant effect, F(1,14) = 1.59, p = .03.
4.2.2. Theta (4–8 Hz)
No significant changes in Theta power during any meditation were observed (Table 2). Since previous studies reported significant increases in FM-Theta for different meditative practices (Baijal and Srinivasan, 2010; Braboszcz et al., 2017; Pasquini et al., 2015; Takahashi et al., 2005), we also conducted planned contrasts for all the practitioners (N = 16) at the Fz electrode for each of the meditative practices in comparison with its baseline yielded no significant effect (all ps > .10). For Tummo experts (N = 10), however, there was a marginally significant increase in Theta power at Fz during M1, F(1,9) = 4.54, p = .06, ηp2 = 0.34, but no significant changes at Fz for any other practice (all ps > .33). As for the resting Theta power, it was lower in Tummo experts, as compared to other practitioners.
For Theta coherence, for all practitioners (N = 16), multiple comparisons with FDR correction revealed no significant changes for any electrode pairs in any of the meditative practices (all ps > .13). The effect of Practice on Theta coherence was not significant, F < 1, p = .91. There was no difference between Tummo and non-Tummo practitioners in any of the practices, F < 1, p = .83, or during Rest, F < 1, p = .46. For Tummo experts (N = 10), multiple comparisons with FDR correction revealed 1) a marginally significant increase in C3–P3 (p < .06) for YidamEND in comparison with M1END; 2) marginally significant decreases in C3–C4 and P3–P4 for M2END in comparison with YidamEND (ps < .06); and 3) a significant increase in P3–P4 (p < .05) for TummoEND in comparison with M2END. The effect of Practice on Theta Coherence was non-significant, F < 1, p = .83. Finally, adding Meditation Experience as a covariate to the ANOVA yielded only a marginally significant effect of Meditation Experience on resting Theta coherence, F(1,14) = 3.70, p = .07, ηp2 = 0.21, so that the meditators with longer experience displayed a trend for higher Theta coherence during rest.
4.2.3. Alpha (8–12 Hz)
As shown in Table 3, only Tummo experts exhibited significant changes in Alpha power during the meditative practices (for non-Tummo practitioners, the effect of Practice was not significant, F < 1, p = .54). In particular, for M1, Tummo experts showed significant increases in the left parietal areas. During Yidam, the increases were observed as a trend in parietal areas, and during Tummo practice, they were significant and the most pronounced in posterior and frontal sites (Fig. 6B). As for M2, it was the only meditation during which Tummo experts showed Alpha power decreases, significant in the left frontal and parietal regions. There was no effect of meditation experience on Alpha power, and we did not observe any significant differences between Tummo and non-Tummo practitioners during their meditative or resting states. For Tummo experts, we conducted further analyses for Alpha 1 (8–10 Hz) and Alpha 2 (10–12 Hz) power separately, also included in Table 3. While for Yidam, Tummo, and M2, the most pronounced changes were observed for Alpha 2 power, M1 exhibited significant changes in Alpha 1 power only.
As for Alpha Coherence (Fig. 7), for all the practitioners (N = 16), multiple comparisons with FDR correction revealed no significant changes in Alpha coherence for any electrode pairs in any of the meditative practices (all ps > .17). The effect of Practice on Alpha coherence was not significant, F < 1, p = .82. There was no difference between Tummo experts and other practitioners in meditative practices, F < 1, p = .53, or during Rest, F < 1, p = .47. For Tummo experts, multiple comparisons with FDR correction revealed 1) a marginally significant decrease for M2END in comparison with YidamEND in C3–C4 (p < .06); and 2) significant increases in F4–P4 (p < .05) and P3–P4 (p < .01) for TummoEND in comparison with M2END. The effect of Practice on Alpha Coherence was non-significant, F < 1, p = .44. Finally, adding Meditation Experience as a covariate to ANOVA yielded a marginally significant effect of Meditation Experience on resting Alpha coherence, F(1,14) = 4.26, p = .058, ηp2 = 0.23, with more experienced practitioners, independently of their Tummo expertise, exhibiting higher Alpha coherence (in particular in the left fronto-parietal regions) during rest.
4.2.4. Beta (12–25 Hz)
As shown in Table 4, there were near significant decreases in Beta power during M1 and increases during Yidam in the right temporal areas (T4) for all practitioners. During M2, the decreases (in right temporal and left parietal areas) were marginally significant for Tummo experts only. Overall, Tummo experts had a lower Beta power during both meditative (M1, Yidam, and M2) and resting states compared to other practitioners. However, during Tummo practice, they exhibited significant Beta power increases, the most pronounced in the centro-parietal and occipital sites (Fig. 6C), consistent with previous studies on Tummo (Benson et al., 1990; Kozhevnikov et al., 2013). As Tummo practice, in addition to requiring active maintenance of cognitive set (e.g., visualizing attributes of a deity, flames), involves imagery of exact movements of “psychic energy” along the spine, based on the feeling of heat, Beta band activity may facilitate efficient processing of feedback (e.g., proprioceptive signals) and recalibrating the sensorimotor system (Baker, 2007), which could explain centro-parietal (Rolandic) Beta power increases during this practice. Furthermore, to separate the effect of Beta-band rhythms of varying frequencies, and in particular high Beta, linked to emotion (Symons et al., 2016), autonomic arousal, and the stress response (Abhang et al., 2016), additional analyses of Beta 1 (12–15 Hz), Beta 2 (15–18 Hz), and Beta 3 (18–25 Hz) were conducted for Tummo experts (Table 4). For both Beta 1 and Beta 2 power, there were significant and near significant decreases in specific sites during M1 and M2, with M2 exhibiting greater and more widespread decreases than M1, involving temporal and parietal (Beta 2) and fronto-parietal (Beta 1) areas. For Tummo practice, the increases were significant and widespread for Beta 1 and Beta 2 (including centro-parietal and fronto-temporal regions). For Beta 3, there were marginally significant increases in the left parietal site (P3) for Yidam as well as significant increases, localized in central-parietal regions for Tummo, suggesting the link of these two practices to emotion and arousal (see the following result section on the relationship between HRV and EEG spectral data).
As for Beta Coherence (Fig. 7), for all the practitioners (N = 16), multiple comparisons with FDR correction revealed 1) a significant decrease in Beta coherence in F3–F4 (p < .05) for M1END in comparison with RestSTART; and 2) significant increases in F3–F4 and F8–P4 (p < .05) for M2END in comparison with YidamEND. Overall, the effect of Practice on Beta coherence was not significant, F(3,42) = 2.35, p = .09. There was no difference between Tummo and non-Tummo practitioners during Rest, F(1,14) = 1.57, p = .23. There was, however, a significant difference in Beta coherence between Tummo and non-Tummo practitioners during meditative practices, F(1,14) = 5.31, p = .037, ηp2 = 28, particularly during M2END, F(1,14) = 11.06, p = .005, ηp2 = 0.44, with Tummo practitioners having significantly lower Beta coherence. For Tummo experts, multiple comparisons with FDR correction revealed: 1) a marginally significant increase in C3–P3 (p < .07), but a significant decrease in F3–F7 (p = .009) for YidamEND in comparison with M1END; and 2) a marginally significant decrease in C3–P3 (p = .06) for M2END in comparison with YidamEND. Furthermore, in contrast to non-Tummo practitioners who exhibited increased fronto-parietal beta coherence during M2, no such pattern was observed for Tummo experts. No changes in Beta coherence were observed during Tummo practice. The overall effect of Practice on Beta Coherence was non-significant, F < 1, p = .72. Finally, adding Meditation Experience as a covariate to ANOVA yielded no significant effect of Meditation Experience on resting Beta coherence, F(1,14) = 2.16, p = .16.
4.2.5. Gamma (35–45 Hz)
As shown in Table 5, for all the practitioners, we observed significant Gamma power decreases during M1 and M2 in the temporal sites and marginal increases in T4 during Yidam. In addition, Tummo experts exhibited significant Gamma power decreases during M2 in the right frontal (F4 and F8) sites, and marginally significant posterior Gamma decreases. The only significant increases in Gamma power (particularly in centro-parietal sites) were observed during Tummo practice (Fig. 6D), consistent with previous research (Benson et al., 1990; Kozhevnikov et al., 2013). While there was no significant effect of Tummo Expertise or Meditation Experience on Gamma power, Tummo experts had a significantly lower Gamma power during both meditative and resting states than other practitioners. Furthermore, for Tummo experts, additional analyses were conducted to investigate the effect of broadband or “high Gamma” (45–80 Hz), known to be mainly associated with affective perception, related to either positive or negative emotional states (Yang et al., 2020). The increases in broadband Gamma during Tummo practice were significant and extended from centro-parietal sites to the frontal areas, possibly indicating the involvement of affective states (e.g., perception of oneself being on fire or feeling bliss) associated with this practice.
As for Gamma Coherence (Fig. 7), for all practitioners (N = 16), multiple comparisons with FDR correction revealed 1) a significant decrease in Gamma coherence in F3–F4 (p < .05) for M1END in comparison with RestSTART; 2) significant increases in C4–P4 and F8–P4 (ps < .05), and marginal increases in F4–P4 and F3–F4 (p < .06) for M2END in comparison with YidamEND. Overall, the effect of Practice on Gamma coherence was marginally significant, F(3,42) = 2.46, p = .08, ηp2 = 0.15. Further repeated measures ANOVAs demonstrated that there was a significant overall increase in Gamma coherence for M2END compared with YidamEND, F(1,14) = 7.24, p = .02, ηp2 = 0.34. However, the interaction between Practice and Tummo was marginally significant, F(1,14) = 3.78, p = .07, ηp2 = 0.21, so that while non-Tummo practitioners exhibited significant increase in Gamma coherence for M2END in comparison with YidamEND, F(1, 5) = 10.33, p = .046, ηp2 = 0.67, Tummo experts did not, F < 1, p = .57 (Fig. 7). For Tummo experts, multiple comparisons with FDR correction revealed 1) a significant decrease in Fz-Pz (p < .05) and marginally significant decreases in F3–F4 and F8–P4 (p < .07) for M1END in comparison with RestSTART; 2) a significant decrease in F3–F7 (p < .05) for YidamEND in comparison with M1END; and 3) a significant increase in Cz-Pz (p < .01) for TummoEND in comparison with M2END. Thus, during M1, in addition to significant decreases in left frontal Gamma coherence, exhibited by all the practitioners, Tummo experts showed additional decreases in central and right fronto-parietal Gamma coherence. During M2, Tummo experts did not show any significant changes in Gamma coherence. Overall, Gamma coherence (particularly fronto-parietal) in Tummo experts was significantly lower than that of non-Tummo practitioners, F(1,14) = 4.76, p = .047, ηp2 = 0.25, not only during M2, F(1,14) = 7.73, p = .015, ηp2 = 0.36, but also during Yidam, F(1,14) = 3.55, p = .08, ηp2 = 0.20. The significant increases in Gamma coherence (in midline centro-parietal regions) were exhibited by Tummo experts during Tummo practice only. Finally, adding Meditation Experience as a covariate to ANOVA yielded no significant effect of Meditation Experience on resting Gamma coherence, F(1,14) = 1.07, p = .32.
4.3. Relationship between EEG spectral power and HRV parameters
We examined the relationship between EEG spectral power (dynamics) in different frequency bands, across scalp surface areas that exhibited significant changes during specific meditation practices, and HRV parameter dynamics occurring during these practices. We did so for each of the meditation practices (M1, Yidam, and Tummo) separately, except for M2, which did not show any significant changes in HRV (i.e., the HF level, developed as a result of Yidam, was sustained during M2, as well as no significant changes were observed during M2 in either LF or LF/HF). None of the correlations was significant (all ps > .12), except the correlation between centro-parietal and occipital Beta 3 and ΔLF for Tummo practice (r = 0.67, p = .049). The changes (increases) in LF are indicative of SNS activity (Kim et al., 2018; Reyes del Paso et al., 2013), and the significant correlation between Beta 3 activity and LF increases supports previous research suggesting the link between high Beta oscillations and autonomic arousal (Abhang et al., 2016; Hall et al., 2007), and further validate our HRV findings indicating a shift towards SNS activity during Tummo practice.
5. Discussion
Supporting our first hypothesis, the results of HRV analysis demonstrate that Vajrayana practices are structured such that they provoke increasingly higher levels of arousal to be employed in the culminating practice of Tantric Mahamudra. With accessing higher arousal levels, neuroendocrine profiles diverge depending on practitioners' expertise and immediate cognitive resources (Epel et al., 1998). The “control” case is mediated by the sympatho-adrenal system and characterized by NE increases, in contrast to the “loss of control” case, mediated by the cortico-adrenal system and characterized by increases in cortisol and decreases in testosterone (Cardinali, 2018). Using systematic approaches to produce increasingly higher arousal, Vajrayana practitioners thus seem to be in control of their sympatho-adrenal system. Self-identifying oneself with a powerful deity, wearing garlands of skulls, and stepping on corpses, leads to arousal, with higher levels of arousal achieved by visualizing oneself as a more wrathful deity. Moreover, Tummo practice, which employs focused visual imagery and a vigorous breathing technique characterized by long deep inhalation and relatively short and forceful exhalation, allows the practitioner to achieve arousal of double intensity, accompanied by heightened SNS tone, within 15 min only as compared to arousal achieved by performing 15 min of Yidam. Furthermore, according to our results, Tantric Mahamudra does not produce arousal by itself but is performed in the state of arousal achieved by Yidam. Indeed, after 15 min of M2 practice, the practitioners still exhibited a state of PNS withdrawal compared to baseline, although it was already in its dissipation mode, not as high as immediately after the completion of Yidam.8 Conversely, non-Tantric Mahamudra generated the state of PNS dominance, suggesting its similarity to mindfulness-related practices (Ditto et al., 2006; Krygier et al., 2013; Tang et al., 2009).
Supporting our second hypothesis, our EEG findings are largely consistent with our predictions. The most evident difference between the Tantric and non-Tantric types of Mahamudra was in the direction of Alpha power changes exhibited by Tummo experts, who showed significant increases in Alpha power during M1, similar to previous research on OM meditations (Britton et al., 2014; Lomas et al., 2015 for reviews), but displayed Alpha power decreases during M2. As M1 was performed in a state of PNS dominance, the observed Alpha power increases, especially pronounced in the Alpha1 band, along with marginally significant increases in FM Theta, suggest the use of a general inhibitory filter (Klimesch et al., 1999), engaged by the CON, to monitor the state of tonic alertness (sustained attention) during the practice. During this monitoring process, the CON deactivates both the VAN and DMN, thus facilitating the disengagement from all brain processes, irrelevant to the monitoring process itself (Sadaghiani and Kleinschmidt, 2016). As the process is common to all mindfulness-related practices, it is not surprising that the increases in Alpha and Theta power have been consistently reported in previous research on mindfulness-related meditation (Lomas et al., 2015 for a review).
As for M2, performed in a state of PNS withdrawal and phasic alertness, posterior Alpha power decreases (especially evident in the Alpha2 band) suggest disinhibition of previously suppressed visual areas, reorienting selective visual attention outward, and a corresponding state of enhanced cortical excitability (Jensen and Mazaheri, 2010; Sadaghiani and Kleinschmidt, 2016). As Alpha power decreases can only be effective relative to a background of high Alpha power (Sadaghiani and Kleinschmidt, 2016), the practice of Tantric Mahamudra seems possible only when the state of heightened arousal and elevated Alpha power is already achieved by preceding practices, thus supporting the need for a multistage practice system in Vajrayana training. Indeed, although observed in Tummo experts only, both Yidam and Tummo practices led to increases in Alpha power (similar to M2, the most pronounced in the Alpha2 band), suggesting the use of filters, which support the maintenance of mental images, as required by these practices, by inhibiting any interfering input. According to arousal theories (Mather et al., 2016), the neuronal activity of the FPCN, modulated by arousal, leads to further enhancement of “internally-directed” selective attention during these practices by amplifying the attentional focus on the mental images and suppressing any other signals mediated by the VAN or DMN pathways. As soon as sufficiently high arousal is achieved, the associated LC-NE activity acts as a “network reset signal” (Bouret and Sara, 2005) to release the VAN from the inhibitory Alpha activity and drive the DAN to reverse the attentional focus outward, as instructed by Mahamudra practice. The observed patterns of Alpha power increases during Yidam and Tummo and decreases during M2 suggest the engagement of focused attention, in a state of heightened phasic alertness, on the internal imagery during Yidam or Tummo, and external visual field during M2.
In addition to Alpha power increases, both Yidam and Tummo produced EEG power increases in all other frequency bands, except Theta. As Theta activity is related to the changes in attentional control during sustained attention (Clayton et al., 2015), not surprisingly, we did not observe such changes during Yidam, Tummo, or M2, involving focused attention. As for other EEG frequency bands, while the power increases were marginal during Yidam, they were significant and widespread during Tummo. Similarly, Tummo experts exhibited significant increases in centro-parietal Theta and Beta coherence during Yidam and fronto-parietal Alpha and central and/or parietal Alpha, Theta, and Gamma coherence during Tummo. Overall, the increases in EEG power and coherence during Yidam and Tummo point to strengthening top-down attentional control (Fig. 4), with Tummo evoking this process to a greater extent.
Conversely, both types of Mahamudra exhibited more decreases than increases in EEG power and coherence. Consistent with previous research that reported Delta and Beta power decreases during OM meditations (Faber et al., 2015; Lehmann et al., 2012), we observed significant decreases in frontal Delta and temporal Beta power during M1, as well as widespread and significant decreases for both Delta and Beta (in particular Beta 1) bands, exhibited by Tummo experts during M2. Tummo experts also showed significant decreases in fronto-parietal Alpha coherence during M1 and central and/or parietal Theta, Alpha, and Beta coherence during M2. Interestingly, the decreased Beta coherence in the C3–P3 electrode pair (Rolandic Beta, associated with the sensorimotor system) during M2 was the opposite of the increased Beta coherence in the same electrode pair during Yidam that preceded M2, which might reflect the act of strengthening the body schema (by imagining replacing one's body with the body of a deity), following the feeling of its dissolution during M2. Overall, the above patterns suggest a reduction of top-down control mechanisms responsible for “interference” control (Delta) or maintenance of the current brain state (Beta) during both types of Mahamudra, with a significantly greater reduction exhibited by Tummo experts during M2 compared to M1.
Although one must be careful when interpreting Gamma power in EEG, as some researchers believe it to be an artifact generated by miniature eye movements (Yuval-Greenberg et al., 2008), our results provide important insights into the nature of NDA meditations. In contrast to mindfulness-related OM meditations, consistently associated with increased posterior Gamma-band activity (Braboszcz et al., 2017; Cahn et al., 2010), we observed marginal decreases in temporal Gamma power exhibited by all the practitioners during M1 and M2 and significant frontal and marginal occipital Gamma power decreases exhibited by Tummo experts during M2. According to our predictions, we observed reduced Gamma activity during M1 and M2, along with Beta power decreases, suggesting a reduced communication between higher and lower sensory areas, preventing “attentional selection” of any particular object. In contrast to OM meditations, NDA practices do not stress the need to notice and monitor a distractor but distribute attention across the entire visual field (Traleg Kyabgon Rinpoche, 2004). The reduced Beta and Gamma power, also reported during other NDA practices such as Tibetan Dzogchen-Rigpa (Amihai and Kozhevnikov, 2014) and Hindu Thoughtless Emptiness (Hinterberger et al., 2014), might represent a unique neural signature of NDA meditations, either non-Tantric or Tantric, suggesting non-selectivity of attention (i.e., reduced attention orienting processes), weakening of predictive coding states, and consequently a greater reduction of top-down control during these practices, compared to OM meditations.
Furthermore, according to our results, Tantric Mahamudra led to a greater reduction in top-down control than non-Tantric Mahmadura, as reflected by Alpha, Beta, and Gamma power decreases during the former vs. Alpha power increases and less significant Delta, Beta, and Gamma power decreases during the latter. Although both NDA practices aim to achieve non-selectivity of attention, non-Tantric Mahamudra represents non-selective disengagement from internal or external signals (non-selective sustained attention), while Tantric Mahamudra can be described as non-selective engagement in all sensory experiences (non-selective focused attention). The former relies on top-down filters “blocking” perception from interfering stimuli (as reflected by Alpha power increases during M1), thus rendering it impossible to significantly reduce top-down control or reverse the attentional focus outward. The latter, under the influence of arousal, might lead to the unique perceptual state, “unbiased” from top-down and bottom-up influences, thus resonating with the goal of the practice to destroy “habitual dualistic modes of perception” (Dowman and Downs, 2010, p. 315). Previously, a similar state, termed “virtual” salience, was reported only in animal studies, achieved as a result of LC photoactivation, during which LC neurons continued to exhibit phasic modes of functioning by firing with phasic bursts of high frequency in the absence of any salient stimuli to which the attention was directed (Vazey et al., 2018).
In our study, only Tummo experts have consistently shown the EEG patterns, which could be associated with the state of Tantric Mahamudra (decreased Alpha, Beta, and Gamma power)9. In addition, only Tummo experts did not show fronto-parietal Beta coherence increases during M2. Overall, they had lower Beta and Gamma power during both meditation and rest, compared to other practitioners, suggesting their greater flexibility in attentional control processes. Due to the neurophysiological coupling of the respiratory cycle and the LC-NE system (Melnychuk et al., 2018), vigorous breathing during Tummo is likely to lead to higher LC-NE activation and greater release of NE compared to imagery alone. During the years of practice, Tummo experts become highly proficient in reaching and controlling states of heightened arousal and related LC-NE activity. Thus, they can develop these states quickly and efficiently and flexibly regulate their top-down control processes.
In conclusion, our findings provide evidence for the existence of arousal-based practices, which, similar to mindfulness-related meditations, can be described as “attention-based, regulatory training regimes”; however, they involve a different ANS state (PNS withdrawal vs. dominance), alerting attention (focused vs. sustained), and type of attentional control (arousal-modulated FPCN vs. CON). Importantly, during arousal-based practices, attentional control is modulated by arousal-driven influences and not directed at monitoring ongoing content of thought as during mindfulness-related practices. Therefore, we propose that the ANS state is a critical dimension to consider while proposing a scientific taxonomy of meditation, as arousal-based and mindfulness-related practices cannot be grouped into a single category of meditation in terms of their physiology and neurocognitive processes. Indeed, recent attempts to include Loving-Kindness meditation, shown to lead to HF decreases (Lumma et al., 2015), into the existing taxonomies of mindfulness-related practices, mainly resulted in adding additional descriptive dimensions (“empathic”) to the FA/OM taxonomy (Dahl et al., 2015; Lippelt et al., 2014). Similarly, Tantric and non-Tantric Mahamudra cannot be grouped into a single category of NDA practices, as the former represents an arousal-based meditation, while the latter belongs to the mindfulness-related category of practices.
Additionally, our findings suggest that the level of attentional control should be considered as an additional dimension in meditation taxonomies. While Yidam and Tummo aim to strengthen top-down control, Mahamudra (either Tantric or non-Tantric Mahamudra)10 aims to reduce it. EEG markers of Yidam and Tummo are quite different from those of Mahamudra, even though all three Tantric practices are characterized by the same ANS state (arousal). Therefore, to generate reliable predictions about EEG markers of any given meditation, both dimensions, ANS state (arousal vs. relaxation) and level of attentional control (strengthening vs. weakening), should be considered. Mindfulness-related meditations, characterized by the state of PNS dominance, can also be differentiated into the practices aiming to strengthen attentional control (FA) vs. the practices aiming to reduce it (OM), with a large body of research focusing on identifying the difference in neural correlates between FA and OM (Manna et al., 2010; Lee et al., 2018for a review). Although the FA/OM taxonomy allows differentiation in relation to the level of attentional control (Malinowski, 2013), it applies only to mindfulness-related meditations.
Limitations affecting the generalizability of our findings are the small sample size due to the challenges in accessing experienced Vajrayana practitioners, particularly Tummo experts. Due to these methodological difficulties, Tummo practice in this research was performed outside of the regular sequence (after, instead of before, Tantric Mahamudra). Despite these limitations, we delineated the differences in the neurobiology of arousal-based and mindfulness-related meditations. Furthermore, this study proposes a theoretical framework which generates testable predictions about any given meditation at physiological and neurocognitive levels. Further investigation of arousal-based meditations can potentially contribute to future studies on the neurobiology of arousal in humans, as most current research on the topic is limited to animal studies manipulating the level of arousal and phasic alertness by optogenetics or pharmacological means. It can also help delineate how sympatho-adrenal system can be regulated, which is currently poorly understood, opening up a wide range of possible medical and behavioral interventions, such as optimization of attentional control concerning environmental challenges (Grueschow et al., 2020), prevention of cognitive decline (Knight et al., 2020), and the attenuation of the innate immune response (Kox et al., 2014).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by Ministry of Education, Singapore, grant #FY2016-FRC3-015 to MK. We thank H.E. Gyeltshen Trulku Rinpoche for his help with recruiting experienced Vajrayana practitioners and providing their support in conducting the study, Michael Sheehy for comments regarding Vajrayana practices, and Ido Amihai for his help with EEG data analyses.
Footnotes
The contrast between FA and OM types of meditation was introduced on the basis of a shamatha/vipashyana distinction, which could be found across many Buddhist traditions. The Pali term vipassana corresponds historically to Sanskrit vipashyana, but in contemporary Western usage, Vipassana is frequently used to refer to a specific group of modern meditation techniques popularized in the West under that name from the 1960s onwards, of which the best known is that taught by S.M. Goenka.
Autonomic PNS withdrawal-associated arousal accompanied by phasic alertness is a different state from what has been referred to as “tonic arousal” (Howells et al., 2010; Van Olst et al., 1967). Tonic arousal is a state of PNS dominance (relaxation), accompanied by heightened tonic alertness.
Vajrayana is one of the three branches of Buddhism (Theravada, Mahayana, and Vajrayana) still practiced today. Vajrayana practices form the most distinctive and characteristic part of the Tibetan and Himalayan Buddhist tradition.
Here “sign” or “characteristic” implies conceptualization, reification or mental labelling, which might constitute a part of the practice and rely on visualization of the channels, chakras, or flow of energy.
The contrast between FA and OM types of meditation was introduced on the basis of a shamatha/vipashyana distinction, which could be found across many Buddhist traditions. The Pali term vipassana corresponds historically to Sanskrit vipashyana, but in contemporary Western usage, Vipassana is frequently used to refer to a specific group of modern meditation techniques popularized in the West under that name from the 1960s onwards, of which the best known is that taught by S.M. Goenka.
While the terms “selective” and “focused” attention are related, and often used interchangeably, in this paper we refer to “selective (focused) attention” as involving two dissociable processes, such as selection of an appropriate stimuli (orienting) and then focusing on the stimuli (Albonico et al., 2018), and to “focused attention” as a latter process of focusing on the stimuli.
Predictive coding (Friston, 2005, 2010; Rao and Ballard, 1999) states that the brain the brain encodes top-down models of the world to predict and suppress sensory inputs from lower levels. A predictive model is created in higher cortical areas and communicated through feedback (backward) connections to lower sensory areas, whereas feedforward connections process and project an error signal, indicating the mismatch between the predicted and the actual sensory input. If the error signal is larger than the level of expected statistical noise, it will cause the model to update so that it better predicts sensory input in the future.
As any state, mental or physical, once achieved, arousal does not end instantaneously but persists for a certain period. Although the length of the period depends on the length and intensity of the preceding meditation, among other factors (e.g., age, experience in meditation, heart condition), previous research has shown that it takes about 15–20 min after a 20-min Yidam practice, for arousal to dissipate (Kozhevnikov et al., 2009).
As top-down attentional control is based on the factors, which are internal to the observer (Awh et al., 2012), such as one's goals, prior knowledge, and experience, its reduction in some sense resonates with the idea of “ego dissolution” that most meditative traditions are striving to achieve. However, one needs to learn how to regulate top-down control, including its strengthening, before it can be reduced (e.g., to learn FA before mastering OM meditation), so that meditation traditions usually offer a range of practices, targeted at achieving different levels of attentional control.
Although Tummo practice is viewed in the west as exceptionally rare and “exotic”, in many Vajrayana schools it is an essential prerequisite for practicing NDA meditation (personal communications, Gyeltshen Trulku Rinpoche, personal communication, March 14, 2018), with full-scale Tantric Mahamudra being taught only after practitioners had at least several years of Tummo experience.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100053.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abhang P.A., Gawali B.W., Mehrotra S.C. In: Introduction to EEG- and Speech-Based Emotion Recognition. Abhang P.A., Gawali B.W., Mehrotra S.C., editors. Academic Press; 2016. Chapter 1 - introduction to emotion, electroencephalography, and speech processing; pp. 1–17. [DOI] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Albonico A., Martelli M., Bricolo E., Frasson E., Daini R. Focusing and orienting spatial attention differently modulate crowding in central and peripheral vision. J. Vis. 2018;18:1–17. doi: 10.1167/18.3.4. [DOI] [PubMed] [Google Scholar]
- Amihai I., Kozhevnikov M. Arousal vs. relaxation: a comparison of the neurophysiological and cognitive correlates of Vajrayana and Theravada meditative practices. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal L.H., Giraud A.-L. Cortical oscillations and sensory predictions. Trends Cognit. Sci. 2012;16(7):390–398. doi: 10.1016/j.tics.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T., Wang M.J., Paspalas C.D. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Rajkowski J., Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Awh E., Belopolsky A.V., Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cognit. Sci. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baijal S, Srinivasan N. Theta activity and meditative states: Spectral changes during concentrative meditation. Cogn. Process. 2010;11(1):31–38. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol. 2007;17(6):649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.M., Usrey W.M., Adams R.A., Mangun G.R., Fries P., Friston K.J. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.M., Vezoli J., Bosman C.A., Schoffelen J.-M., Oostenveld R., Dowdall J.R., De Weerd P., Kennedy H., Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological). 1995;57:289–300. [Google Scholar]
- Benson H., Klipper M.Z. Morrow; New York: 1975. The Relaxation Response. [Google Scholar]
- Benson H., Malhotra M.S., Goldman R.F., Jacobs G.D., Hopkins P.J. Three case reports of the metabolic and electroencephalographic changes during advanced Buddhist meditation techniques. Behav. Med. 1990;16(2):90–95. doi: 10.1080/08964289.1990.9934596. [DOI] [PubMed] [Google Scholar]
- Billman G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013;4:1. doi: 10.3389/fphys.2013.00026. –5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman C.A., Schoffelen J.-M., Brunet N., Oostenveld R., Bastos A.M., Womelsdorf T., Rubehn B., Stieglitz T., De Weerd P., Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S., Sara S.J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Braboszcz C., Cahn B.R., Levy J., Fernandez M., Delorme A. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W.B., Lindahl J.R., Cahn B.R., Davis J.H., Goldman R.E. Awakening is not a metaphor: the effects of Buddhist meditation practices on basic wakefulness. Ann. N. Y. Acad. Sci. 2014;1307:64–81. doi: 10.1111/nyas.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.E., Beightol L.A., Koh J., Eckberg D.L. Important influence of respiration on human R-R interval power spectra is largely ignored. J. Appl. Physiol. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. https://doi: 10.1152/jappl.1993.75.5.2310 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buschman T.J., Miller E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cahn B.R., Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cahn B.R., Delorme A., Polich J. Occipital gamma activation during Vipassana meditation. Cognit. Process. 2010;11:39–56. doi: 10.1007/s10339-009-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak Y.O., Kozhevnikov M. 2016. Sympathetic System Activation Sufi Whirling Dervishes during Spinning Episodes. Unpublished manuscript. [Google Scholar]
- Camm A.J., Malik M., Bigger J.T., Breithardt G., Cerutti S., Cohen R.J., Coumel P., Fallen E.L., Kennedy H.L., Kleiger R.E. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology; 1996. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. [Google Scholar]
- Cardinali D.P. In: Autonomic Nervous System: Basic and Clinical Aspects. Cardinali D.P., editor. Springer International Publishing; Cham: 2018. Third level: the hypothalamus; pp. 175–244. [DOI] [Google Scholar]
- Chalmers J.A., Quintana D.S., Abbott M.J.-A., Kemp A.H. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatr. 2014;5 doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton M.S., Yeung N., Cohen Kadosh R. The roles of cortical oscillations in sustained attention. Trends Cognit. Sci. 2015;19:188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C.J., Lutz A., Davidson R.J. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cognit. Sci. 2015;19:515–523. doi: 10.1016/j.tics.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalai Lama II Dge- Dun-Rgya-Mtsho, Glenn M, Zasep Rinpoche . Selected Works of the Dalai Lama II: Tantric Yogas of Sister Niguma (Teachings of the Dalai Lamas) Snow Lion; Ithaca, NY: 1985. [Google Scholar]
- Dalai Lama, Tsongkhapa . In: The Great Exposition of Secret Mantra, Volume Two: Deity Yoga. Hopkins J., editor. Snow Lion; Ithaca, NY: 2017. [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open-source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A., Sejnowski T., Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. https://doi: 10.1016/j.neuroimage.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditto B., Eclache M., Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann. Behav. Med. 2006;32:227–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- Dixon M.L., Vega A., Mills C., Andrews-Hanna J., Spreng R.N., Cole M.W., Christoff K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci. USA. 2018;115:1598–1607. doi: 10.1073/pnas.1715766115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. https://doi: 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cognit. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowman K., Downs H.R. State University of New York Press; New York: 2010. Masters of Mahamudra: Songs and Histories of the Eighty-Four Buddhist Siddhas. [Google Scholar]
- Dunne J. Toward an understanding of non-dual mindfulness. Contemp. Buddhism. 2011;12(1):71–88. doi: 10.1080/14639947.2011.564820. [DOI] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Epel E.S., McEwen B.S., Ickovics J.R. Embodying psychological thriving: physical thriving in response to stress. J. Soc. Issues. 1998;54:301–322. doi: 10.1111/j.1540-4560.1998.tb01220.x. [DOI] [Google Scholar]
- Evans-Wentz W.Y. Pilgrims Publishing; India: 2002. Tibetan Yoga and Secret Doctrines: or Seven Books of Wisdom of the Great Path. [Google Scholar]
- Faber P.L., Lehmann D., Gianotti L.R.R., Milz P., Pascual-Marqui R.D., Held M., Kochi K. Zazen meditation and no-task resting EEG compared with LORETA intracortical source localization. Cognit. Process. 2015;16:87–96. doi: 10.1007/s10339-014-0637-x. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P., Schröder J.-H., Roelfsema P.R., Singer W., Engel A.K. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J. Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Fucci E., Abdoun O., Caclin A., Francis A., Dunne J.D., Ricard M., Davidson R.J., Lutz A. Differential effects of non-dual and focused attention meditations on the formation of automatic perceptual habits in expert practitioners. Neuropsychologia. 2018;119:92–100. doi: 10.1016/j.neuropsychologia.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueschow M., Kleim B., Ruff C.C. Role of the locus coeruleus arousal system in cognitive control. J. Neuroendocrinol. 2020;32 doi: 10.1111/jne.12890. n/a. [DOI] [PubMed] [Google Scholar]
- Hall M., Thayer J.F., Germain A., Moul D., Vasko R., Puhl M., Miewald J., Buysse D.J. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav. Sleep Med. 2007;5:178–193. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- Harmony T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013;7:83. doi: 10.3389/fnint.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaus D., Casales Santa M.M., Offermann J.S., Van Amelsvoort T. Noradrenaline transporter blockade increases fronto-parietal functional connectivity relevant for working memory. Eur. Neuropsychopharmacol. 2017;27:399–410. doi: 10.1016/j.euroneuro.2017.02.004. https://doi:10.1016/j.euroneuro.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Hinterberger T., Schmidt S., Kamei T., Walach H. Decreased electrophysiological activity represents the conscious state of emptiness in meditation. Front. Psychol. 2014;5:99. doi: 10.3389/fpsyg.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells F.M., Stein D.J., Russell V.A. Perceived mental effort correlates with changes in tonic arousal during attentional tasks. Behav. Brain Funct. 2010;6:39. doi: 10.1186/1744-9081-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z., Dinstein I., Weber J., Heeger D.J. Influence of meditation on anti-correlated networks in the brain. Front. Hum. Neurosci. 2011;5:183. doi: 10.3389/fnhum.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z. Neural correlates of nondual awareness in meditation. Ann. N. Y. Acad. Sci. 2014:9–18. doi: 10.1111/nyas.12261. 1307. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Neural correlates of nondual awareness in meditation. Ann. N. Y. Acad. Sci. 2014:9–18. doi: 10.1111/nyas.12261. 1307. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Hyperion; New York, NY: 1994. Wherever You Go There You Are: Mindfulness Meditation in Everyday Life. [Google Scholar]
- Kim H.-G., Cheon E.-J., Bai D.-S., Lee Y.H., Koo B.H. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15:235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cognit. Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Knight E.L., Giuliano R.J., Shank S.W., Clarke M.M., Almeida D.M. Parasympathetic and sympathetic nervous systems interactively predict change in cognitive functioning in midlife adults. Psychophysiology. 2020;57 doi: 10.1111/psyp.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongtrul J. A Detailed Presentation of the Process of Meditation in Vajrayāna. Translated and Annotated by Sarah Harding (Kalu Rinpoché Translation Group) Snow Lion; Ithaca, NY: 2007. The treasury of knowledge. Book eight, Part Four. Esoteric instructions. [Google Scholar]
- Kox M., Eijk L.T.G.J. v, Zwaag J., Wildenberg J. v d, Sweep C.G.J., Hoeven J.G. v d, Pickkers P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. U.S.A. 2014;111:7379–7384. doi: 10.1073/pnas.1322174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov M., Elliott J., Shephard J., Gramann K. Neurocognitive and somatic components of temperature increases during g-tummo meditation: legend and reality. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov M, Louchakova O, Josipovic Z, Motes MA. The enhancement of visuospatial processing efficiency through Buddhist Deity meditation. Psychological Science. 2009;20(5):645–653. doi: 10.1111/j.1467-9280.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Kragh U.T. International Institute for Buddhist Studies of the International College for Postgraduate Buddhist Studies; 2015. Tibetan Yoga and Mysticism: A Textual Study of the Yogas of Nāropa and MAHĀMUDRĀ Meditation in the Medieval Tradition of Dags Po. [Google Scholar]
- Krein K., Ilundáin J. Philosophy and the Martial Arts. Routledge; 2014. Mushin and flow: an east–west comparative analysis. [Google Scholar]
- Krygier J.R., Heathers J.A.J., Shahrestani S., Abbott M., Gross J.J., Kemp A.H. Mindfulness meditation, well-being, and heart rate variability: a preliminary investigation into the impact of intensive vipassana meditation. Int. J. Psychophysiol. 2013;89:305–313. doi: 10.1016/j.ijpsycho.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Landry M., Raz A. Heightened states of attention: from mental performance to altered states of consciousness and contemplative practices. Intellectica. Revue de l’Association Pour La Recherche Cognitive. 2016;66:139–159. doi: 10.3406/intel.2016.1822. [DOI] [Google Scholar]
- Lange J., Oostenveld R., Fries P. Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J. Neurosci. 2013;33:3212–3220. doi: 10.1523/JNEUROSCI.3755-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.J., Kulubya E., Goldin P., Goodarzi A., Girgis F. Review of the neural oscillations underlying meditation. Front. Neurosci. 2018 doi: 10.3389/fnins.2018.00178. https://doi: 10.3389/fnins.2018.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D., Faber P.L., Tei S., Pascual-Marqui R.D., Milz P., Kochi K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. Neuroimage. 2012;60(2):1574–1586. doi: 10.1016/j.neuroimage.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Lippelt D.P., Hommel B., Colzato L.S. Focused attention, open monitoring and loving kindness meditation: effects on attention, conflict monitoring, and creativity - a review. Front. Psychol. 2014;5:1083. doi: 10.3389/fpsyg.2014.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas T., Ivtzan I., Fu C.H.Y. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci. Biobehav. Rev. 2015;57:401–410. doi: 10.1016/j.neubiorev.2015.09.018. https://doi: 10.1016/j.neubiorev.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Lumma A.-L., Kok B.E., Singer T. Is meditation always relaxing? Investigating heart rate, heart rate variability, experienced effort and likeability during training of three types of meditation. Int. J. Psychophysiol. 2015;97:38–45. doi: 10.1016/j.ijpsycho.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Lutz A., Slagter H.A., Dunne J.D., Davidson R.J. Attention regulation and monitoring in meditation. Trends Cognit. Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Jha A.P., Dunne J.D., Saron C.D. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 2015;70:632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzini L., Singh K.D. Spatial attention modulates visual gamma oscillations across the human ventral stream. Neuroimage. 2018;166:219–229. doi: 10.1016/j.neuroimage.2017.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski P. Neural mechanisms of attentional control in mindfulness meditation. Front. Neurosci. 2013;7:8. doi: 10.3389/fphys.2016.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A., Raffone A., Perrucci M.G., Nardo D., Ferretti A., Tartaro A., et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010;82:46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley SW. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci. 2016;39:e200. doi: 10.1017/S0140525X15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A., Picton T.W. EEG spectral dynamics during discrimination of auditory and visual targets. Cognit. Brain Res. 2005;241:81–96. doi: 10.1016/j.cogbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Melnychuk M.C., Dockree P.M., O'Connell R.G., Murphy P.R., Balsters J.H., Robertson I.H. Coupling of respiration and attention via the locus coeruleus: effects of meditation and pranayama. Psychophysiology. 2018;55 doi: 10.1111/psyp.13091. [DOI] [PubMed] [Google Scholar]
- Michalareas G., Vezoli J., van Pelt S., Schoffelen J.M., Kennedy H., Fries P. Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron. 2016;89:384–397. doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Buschman T.J. Cortical circuits for the control of attention. Curr. Opin. Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin G.H. Snow Lion Publications; 1996. Tsongkhapa's Six Yogas of Naropa. [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ. Res. 1986;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pasquini HA, Tanaka GK, Basile LF.H, Velasques B, Lozano MD, Ribeiro P. Electrophysiological Correlates of Long-Term Soto Zen Meditation. BioMed. Res. Int. 2015;e598496 doi: 10.1155/2015/598496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot V., Roche F., Celle S., Barthélémy J.-C., Chouchou F. HRVanalysis: a free software for analyzing cardiac autonomic activity. Front. Physiol. 2016;7 doi: 10.3389/fphys.2016.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Macaulay R.J., Caudill M.A., Kutz I., Adam D., Gordon D., Kilborn K.M., Barger A.C., Shannon D.C., Cohen R.J. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 1985;248(1 Pt 2):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Quintana D.S., Heathers J.A.J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. https://doi: 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Rao R.P.N., Ballard D.H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999;2(1):79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso G.A., Langewitz W., Mulder L.J.M., van Roon A., Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Romei V., Brodbeck V., Michel C., Amedi A., Pascual-Leone A., Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cerebr. Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., D'Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebr. Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Kleinschmidt A. Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cognit. Sci. 2016;20:805–817. doi: 10.1016/j.tics.2016.09.0. [DOI] [PubMed] [Google Scholar]
- Sara S.J., Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Maruyama R. Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. Tohoku J. Exp. Med. 2014;233:155–163. doi: 10.1620/tjem.233.155. https://doi: 10.1620/tjem.233.155 [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Doppelmayr M., Pecherstorfer T., Freunberger R., Hanslmayr S. EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum. Brain Mapp. 2005;26:148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen J.M., Gross J. Source connectivity analysis with MEG and EEG. Hum. Brain Mapp. 2009;30:1857–1865. doi: 10.1002/hbm.20745. https://doi: 10.1002/hbm.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley W., Cunningham M.O. Do cortical gamma oscillations promote or suppress perception? An under-asked question with an over-assumed answer. Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Astafiev S.V., McAvoy M.P., d'Avossa G., Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cerebr. Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Sloan R.P., Shapiro P.A., Bagiella E., Boni S.M., Paik M., Bigger J.T., Jr., et al. Effect of mental stress throughout the day on cardiac autonomic control. Biol. Psychol. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Studstill R. The unity of mystical traditions: the transformation of consciousness in Tibetan and German mysticism. Stud. Hist. Relig. 2005;107 Brill Academic Pub. [Google Scholar]
- Symons AE, El-Deredy W, Schwartze M, Kotz SA. The functional role of neural oscillations in non-verbal emotional communication. Front. Hum. Neurosci. 2016;10(239) doi: 10.3389/fnhum.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Murata T, Hamada T, Omori M, Kosaka H, Kikuchi M, Yoshida H, Wada Y. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int. J. Psychophysiol. 2005;55(2):199–207. doi: 10.1016/j.ijpsycho.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Tang Y., Ma Y., Fan Y., Feng H., Wang J., Feng S., Lu Q., Hu B., Lin Y., Li J., Zhang Y., Wang Y., Zhou L., Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telles S., Singh N., Balkrishna A. Heart rate variability changes during high frequency yoga breathing and breath awareness. Biopsychosoc Medicine. 2011;5 doi: 10.1186/1751-0759-5-4. https://doi: 10.1186/1751-0759-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E., Gurevitz O., Hod H., Eldar M., Akselrod S. Wavelet analysis of instantaneous heart rate: a study of autonomic control during thrombolysis. Am. J. Physiol. 2003;284:1079–1091. doi: 10.1152/ajpregu.00287.2002. [DOI] [PubMed] [Google Scholar]
- Traleg Kyabgon Rinpoche . Shambhala Publications; 2004. Mind at Ease: Self-Liberation through Mahamudra Meditation. [Google Scholar]
- Tulku Urgyen Rinpoche, in M.B. Schmidt (Ed). As It Is. Vol. II. 2nd. Rangjung Yeshe Publications; 2000. [Google Scholar]
- van Dijk H., Schoffelen J.M., Oostenveld R., Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkoerle T., Self M.W., Dagnino B., Gariel-Mathis M.-A., Poort J., van der Togt C., Roelfsema P.R. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl. Acad. Sci. U.S.A. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Olst E.H., Orlebeke J.F., Fokkema S.D. Skin conductance as a measure of tonic and phasic arousal. Acta Psychol. 1967;27:262. doi: 10.1016/0001-6918(67)90067-4. [DOI] [PubMed] [Google Scholar]
- Vazey E.M., Moorman D.E., Aston-Jones G. Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E9439–E9448. doi: 10.1073/pnas.1803716115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rosenberg W., Chanwimalueang T., Adjei T., Jaffer U., Goverdovsky V., Mandic D.P. Resolving ambiguities in the LF/HF ratio: LF-HF scatter plots for the categorization of mental and physical stress from HRV. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Tong L., Shu J., Zhuang N., Yan B., Zeng Y. High gamma band EEG closely related to emotion: evidence from functional network. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshe T. Simon and Schuster; 2003. Becoming the Compassion Buddha: Tantric Mahamudra for Everyday Life. [Google Scholar]
- Yuval-Greenberg S., Tomer O., Keren A.S., Nelken I., Deouell L.Y. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. https://doi: 10.1016/j.neuron.2008.03.027 [DOI] [PubMed] [Google Scholar]
- Zope S.A., Zope R.A. Sudarshan kriya yoga: breathing for health. Int. J. Yoga. 2013;6:4–10. doi: 10.4103/0973-6131.105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.