Abstract
Purpose
Peroxisomal biogenesis disorders (PBDs) represent a spectrum of conditions that result in vision loss, sensorineural hearing loss, neurologic dysfunction, and other abnormalities resulting from aberrant peroxisomal function caused by mutations in PEX genes. With no treatments currently available, we sought to investigate the disease mechanism in a patient with a PBD caused by defects in PEX6 and to probe whether overexpression of PEX6 could restore peroxisome function and potentially offer therapeutic benefit.
Design
Laboratory-based study.
Participants
A 12-year-old boy sought treatment with hearing loss and retinopathy. After negative results in an Usher syndrome panel, targeted genetic testing revealed compound heterozygous mutations in PEX6. These included a 14-nucleotide deletion (c.802_815del: p.(Asp268Cysfs∗8)) and a milder missense variant (c.35T→C:(p.Phe12Ser)).
Methods
Patient-derived skin fibroblasts were cultured, and a PEX6 knockout cell line was developed using clustered regularly interspaced short palindromic repeats and Cas9 technology in HEK293T cells to emulate a more severe disease phenotype. Immunoblot analysis of whole cell lysates was performed to assess peroxisome number. Immunofluorescence studies used antibodies against components of the peroxisomal protein import pathway to interrogate the effects of mutations in PEX6 on protein trafficking.
Main Outcome Measures
Primary outcome measures were peroxisome abundance and matrix protein import.
Results
Peroxisome number was not significantly different between control fibroblasts and patient fibroblasts; however, fewer peroxisomes were observed in PEX6 knockout cells compared with wild-type cells (P = 0.04). Analysis by immunofluorescent microscopy showed significantly impaired peroxisomal targeting signal 1- and peroxisomal targeting signal 2-mediated matrix protein import in both patient fibroblasts and PEX6 knockout cells. Overexpressing PEX6 resulted in improved matrix protein import in PEX6 knockout cells.
Conclusions
Mutations in PEX6 were responsible for combined hearing loss and retinopathy in our patient. The primary peroxisomal defect in our patient’s skin fibroblasts was impaired peroxisomal protein import as opposed to reduction in the number of peroxisomes. Genetic strategies that introduce wild-type PEX6 into cells deficient in PEX6 protein show promise in restoring peroxisome function. Future studies of patient-specific induced pluripotent stem cell-derived retinal pigment epithelium cells may clarify the role of PEX6 in the retina and the potential for gene therapy in these patients.
Keywords: Hearing loss, Peroxisomal biogenesis disorders, Peroxisome, PEX6, Retinal degeneration, Usher syndrome
Abbreviations and Acronyms: CRISPR, clustered regularly interspaced short palindromic repeats; DTM, docking translocation module; GFP, green fluorescent protein; HEK293T, human embryonic kidney 293T; PBD, peroxisomal biogenesis disorder; PBS, phosphate-buffered saline; PTS1, peroxisomal targeting signal 1; PTS2, peroxisomal targeting signal 2; RPE, retinal pigment epithelium; WT, wild-type
Peroxisomal biogenesis disorders (PBDs) are genetically and phenotypically heterogeneous conditions caused by aberrant peroxisome function. Inherited in an autosomal recessive pattern, PBDs occur in 1 in 50 000 individuals and are characterized by a range of disabilities including severe vision loss, sensorineural hearing loss, neurologic dysfunction (leukodystrophy and developmental delay), craniofacial abnormalities, vertebral anomalies, and liver dysfunction. This spectrum of disorders is caused by defects in at least 14 different PEX genes, which encode peroxin proteins involved in peroxisome membrane assembly, matrix protein import, and peroxisomal division.1 Only symptomatic therapies exist such as hearing aids, nutritional therapy, and vision aids. A significant need exists to identify appropriate therapies because there are no disease-modifying treatments currently available.
Peroxisomal biogenesis disorders may be considered a form of syndromic inherited retinal dystrophy because most patients demonstrate a generalized retinopathy with systemic features. Peroxisomal biogenesis disorders comprise 4 clinical syndromes that differ in disease onset and severity and include, from most to least severe: Zellweger syndrome, neonatal adrenoleukodystrophy, infantile Refsum disease, and Heimler syndrome. In milder forms of disease, sensorineural hearing loss and retinal dystrophy may be the most striking initial features. As a result, many patients may be misdiagnosed with Usher syndrome.2 Several reports exist of patients with hearing loss and retinopathy who showed negative results for pathogenic variants in genes associated with Usher syndrome and later were discovered to have a PBD.2,3
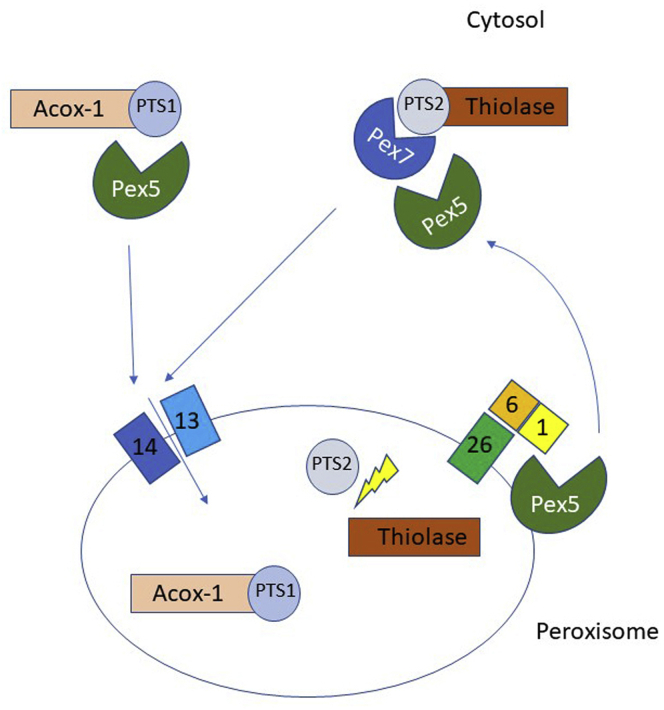
Dysfunction of a dynamic, single membrane-bound organelle called the peroxisome underlies the PBDs. Peroxisomes perform diverse functions, including β-oxidation of very long-chain fatty acids, α-oxidation of branched-chain fatty acids, bile acid and plasmalogen synthesis, and the detoxification of reactive oxygen species.4 Recent evidence even supports the role of peroxisomes in phagocytosis and the innate immune response.5 Unlike mitochondria, peroxisomes do not contain their own genome. As a result, peroxisomes rely on a transport mechanism to import nuclear-encoded protein into their matrix to allow them to perform their diverse functions (Fig 1).
Figure 1.
Illustration of a model of peroxisomal matrix protein import. Nascent polypeptides synthesized in the cytosol, destined for the peroxisomal lumen, are labeled with either a C-terminal peroxisomal targeting signal 1 (PTS1) tripeptide or an N-terminal peroxisomal targeting signal 2 (PTS2) nonapeptide. PEX5, a cytosolic shuttle, delivers PTS1-containing cargo to the docking and translocation module (DTM) comprising PEX13 and PEX14. Cargo containing the PTS2 nonapeptide is delivered to the DTM by a separate cytosolic shuttle, PEX7, in conjunction with PEX5 (long isoform). A membrane pore forms, and the protein cargo is delivered inside the peroxisome. After reaching the lumen, the PTS2, but not the PTS1, targeting signal is cleaved off. PEX5 is removed from the peroxisomal membrane by the action of the receptor and exportomer module comprising PEX1, PEX6, and PEX26. PEX1 and PEX6 use ATP to extract PEX5 from the peroxisomal membrane so that it is available for subsequent rounds of matrix protein import.
Mutations in PEX1 and PEX6 account for approximately 60% and 10% of all PBDs, respectively.6 PEX1 and PEX6 belong to a group of ATPases called the AAA ATPases (ATPases associated with diverse cellular activities). In addition to the established roles of PEX1 and PEX6 in matrix protein import, cells devoid of these proteins have been shown to have reduced peroxisome numbers.7,8 Despite this, the precise mechanism by which different mutations in these genes contribute to the spectrum of PBD severity is not fully known.
Our laboratory sought to understand how a patient’s specific mutations in a peroxisomal gene, PEX6, impair cell metabolism. In addition, because no disease-modifying treatments currently are available, we examined whether overexpressing PEX6 could restore peroxisome function, leading to a possibility of gene replacement therapy in future studies.
Methods
Participant Consent and Study Ethics
Written informed consent was obtained from each participant in this study. Institutional review board/ethics committee approval was obtained from the University of Alberta Health Research Ethics Board - Biomedical Panel (identifier, Pro00074451). This study adhered to the tenets of the Declaration of Helsinki.
Cell Culture
Human embryonic kidney 293T (HEK293T) cells (Thermo Scientific) were grown in Cytiva HyClone Dulbecco’s modified eagle medium (Thermo Scientific) with L-glutamine, sodium pyruvate, and 10% Gibco fetal bovine serum (Thermo Scientific) with penicillin 50 IU/ml and streptomycin 50 μg/ml added. Skin fibroblasts were collected from the patient and from each of his parents by performing a 3- to 4-mm superficial dermal biopsy on the underside of the upper arm under local anesthetic. Our laboratory had available a skin fibroblast cell line that was used as a control and was wild-type (WT) for PEX6. Skin fibroblasts were cultured in the same medium conditions except that 15% fetal bovine serum was used. Cells were incubated at 37° C and at 5% CO2 concentration.
Antibodies
Antibodies were purchased and used for western blot and immunofluorescence assays as indicated in Supplemental Table 1.
Cell Harvesting
Human embryonic kidney 293T cells and skin fibroblasts were grown to confluence on 6-well plates (Thermo Scientific). The growth medium was removed, and the cells were washed 3 times with Cytiva HyClone phosphate-buffered saline (PBS; Thermo Scientific). Cell lysis was performed with 100 μl of ice-cold RIPA buffer (Boston BioProducts) with Halt Protease Inhibitor Cocktail (Thermo Scientific) added to each well. The cells were harvested mechanically using cell scrapers (Falcon). The cell lysates were incubated on ice for 15 minutes. Next, the lysates were centrifuged at 16 100 g for 15 minutes at 4° C (Standard rotor FA-45-24-11; Eppendorf), and the supernatant was collected. Protein concentration was determined via a colorimetric technique using the Pierce BCA Protein Assay Kit (Thermo Scientific).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
Precast 10% and 12% polyacrylamide gels were purchased from Bio-Rad, and 30 μg of protein were loaded in each lane. Two microliters of Chameleon Duo Pre-Stained Protein Ladder (LI-COR) were used as a protein size standard in a single lane. After gel running and transfer onto nitrocellulose membrane, Intercept PBS Blocking Buffer (LI-COR) was applied for 1 hour on a tabletop shaker. Next, the blocking buffer was removed, primary antibodies (in the dilutions given in Supplemental Table 1) were added to the membrane, and the blot was left overnight to incubate at 4° C on a tabletop shaker. On the following morning, the membrane was washed with PBS with 0.05% Tween 20. Next, goat antimouse (IRDye 680CW) and goat anti-rabbit (IRDye 800CW) secondary antibodies (LI-COR) were added, and the membrane was placed on the shaker for 45 minutes at room temperature. After 3 more washes, the blots then were scanned using the Odyssey CLx imaging system (LI-COR).
Immunofluorescence Microscopy
Human embryonic kidney 293T cells and skin fibroblasts were grown on number 2 glass coverslips (22 × 22 mm; Fisher Scientific) to approximately 50% confluency. Growth medium was removed, and cells were washed twice with PBS with 0.05% Tween 20, and fixed with 2% paraformaldehyde for 20 minutes at room temperature. After 2 washes, cells were incubated for 15 minutes in a blocking buffer (1% bovine serum albumin and 0.5% Triton X-100 in PBS). A 1-hour incubation with primary antibodies diluted in blocking solution (details of antibodies used and dilutions are in Supplemental Table 1) was followed by a 1-hour incubation with secondary antibodies, with 2 washes after each step. Finally, cells were incubated for 5 minutes with 4′,6-diamidino-2-phenylindole added to a final concentration of 5 μM and washed twice before mounting onto glass microscope slides using ProLong Glass Antifade Mountant (Invitrogen) and being dried overnight. Immunofluorescence images were captured with a spinning disc confocal microscope (Quorum Technologies) at the Cell Imaging Center at the University of Alberta. Multiple cells (> 20 cells) from each slide were examined, and images were captured of representative samples.
Plasmid Preparation and Subcloning
A PEX6 (NM_000287.4) pcDNA3.1+/C-(K)-DYK expression plasmid was obtained (GenScript) and transformed DH5α-competent Escherichia coli cells (Thermo Scientific). To create a PEX6 c.35T→C version of the same expression plasmid, we used a mutagenic forward primer (5′-GATCGGATCCTTATGGCGGTCGCTGTCTTGCGGGTCCTGGAGCCCTCTCCGACCGAG -3′) that incorporated the base pair change (underlined) as well as a BamHI restriction site. The reverse primer (5′-GATCGCGGCCGCCTAGCAGGCAGCAAACTTGCGC-3′) included a NotI restriction site. After polymerase chain reaction amplification using the plasmid as a template, the product and recipient plasmid was subject to restriction digestion reaction with BamHI and NotI (Thermo Scientific) and ligated at a 3:1 molar ratio of insert to plasmid, generating the PEX6 c.35T→C pcDNA3.1+/C-(K)-DYK expression plasmid. Successful cloning was confirmed by restriction digestion and Sanger sequencing.
Commercially available peroxisomal targeting signal 1 (PTS1) expression plasmid pEGFP-C1+SKL (Addgene) was obtained for the in vitro PTS1-mediated protein import assay. The in vitro green fluorescent protein signal was assessed with the EVOS M5000 Imaging System (Thermo Scientific).
Clustered Regularly Interspaced Short Palindromic Repeats and Cas9-Mediated PEX6 Deletion
The Alt-R clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 system (IDT) with a predesigned guide RNA (crisprRNA: 5′-/AltR1/ACC GCA AAG GAG GAC ACC ACG UUU UAG AGC UAU GCU/AltR2/-3′) and fluorescently labelled transactivating crisprRNA ATTO 550 was used to generate PEX6 knockout HEK293T cells. Briefly, we combined the crisprRNA and transactivating crisprRNA to generate an RNA duplex. The ribonucleoprotein complex was created by adding the Cas9 nuclease, and this complex was transfected into 400 000 HEK293T cells per well in a 12-well plate using Lipofectamine CRISPRMAX Cas9 Transfection Reagent (Thermo Scientific) in Opti-MEM Reduced Serum Medium (Thermo Scientific). The cells were incubated for 48 hours at 37° C. Fluorescently labelled transfected cells were sorted using fluorescence-activated cell sorting and seeded as individual cells into each well of a 96-well plate. These cells were allowed to expand over 2 weeks. Ten clonal cell lines were screened by Sanger sequencing of the CRISPR target site to identify a culture with mutations disrupting both copies of PEX6. In one of the colonies, we identified a homozygous single base deletion in exon 1 of PEX6 (c.544del). The frameshift was predicted to abolish protein function. The PEX6 c.544del HEK293T cells were selected for downstream assays and are referred to as PEX6 knockout HEK293T cells for the remainder of this article.
Transfection
Human embryonic kidney 293T cells were seeded at 250 000 cells per well on a 6-well plate and transfected with 4 μg of plasmid using Lipofectamine 2000 (Thermo Scientific) according to the manufacturer’s protocol.
Skin fibroblasts from the patient, his parents, and a control participant were transfected using the Human Dermal Fibroblast Nucleofector Kit (Lonza). A T75 flask containing confluent fibroblasts was trypsinized, and 500 000 cells were pipetted into a 1.5-ml Eppendorf tube and subsequently centrifuged for 5 minutes at ×400 g (Standard Rotor FA-45-24-11; Eppendorf), and the cell pellet was retained. Next, 2 μg of plasmid was combined with 110 μl of the Nucleofector Solution (containing supplement 1) to resuspend the cell pellet. This solution was placed into an aluminum cuvette and then into the Nucleofector 2b device (Lonza). After electroporation, the solution was transferred into a single well containing growth medium in a 6-well plate. The fibroblasts were incubated at 37° C and 5% CO2 concentration and the in vitro PTS1 expression plasmid green fluorescent protein (GFP) signal was examined 48 hours later.
Image Processing and Statistics
Microscopy images were processed as z-stacks using ImageJ software (National Institutes of Health). Images were enhanced in Photoshop (Adobe) using the levels feature without reaching saturation. For quantification, the number of puncta per cell was determined using the Analyze Particles feature in ImageJ after image thresholding. Western blot data were quantified using Image Studio Lite software version 5.2 (National Institutes of Health, https://imagej.nih.gov/ij/), and the results were compared by using Student t test, with α = 0.05 considered to be statistically significant. Statistical analysis was performed in Microsoft Excel 2016 (Microsoft). Figures were created using Microsoft PowerPoint 2016 (Microsoft).
Results
Clinical Case of a Patient with Sensorineural Hearing Loss and a Retinopathy
A 12-year-old child of French-Canadian, Swedish, and Welsh origin sought treatment at our ocular genetics clinic with severe congenital sensorineural hearing loss and retinopathy leading to significant vision loss. Initially, no family history of inherited ocular or systemic disease was reported. His past medical history was otherwise unremarkable.
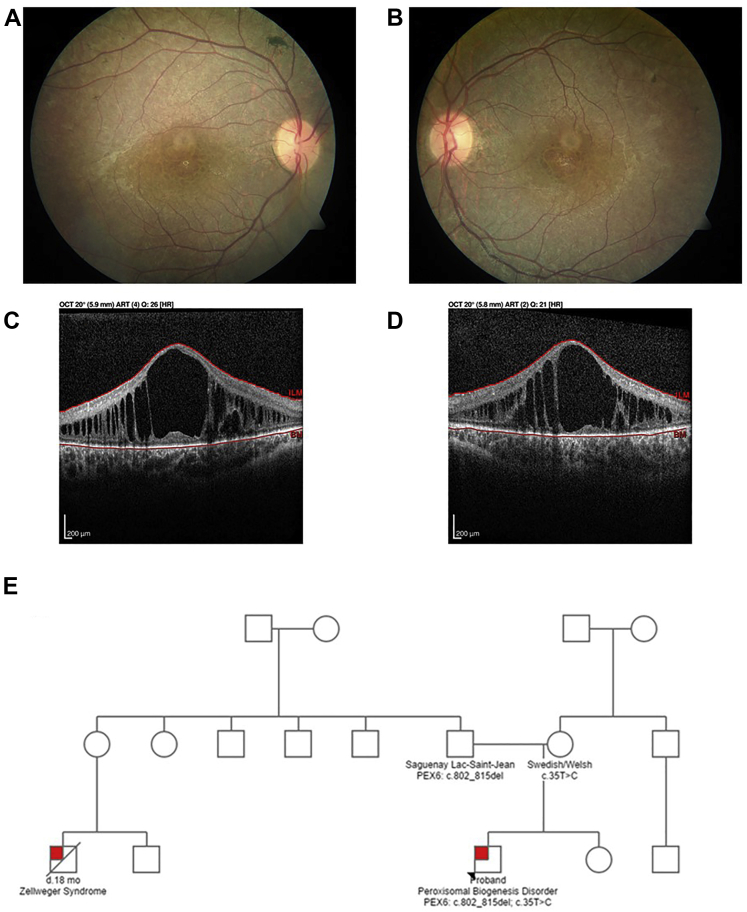
At the time of examination, best-corrected visual acuity was 20/150 in the right eye and 20/150 in the left eye. Intraocular pressures were normal. Extraocular movements were full, and no strabismus was observed. Pupils were equal and reactive to light and accommodation. Anterior segment examination was unremarkable, but posterior segment examination revealed a mottled fundus appearance with granular retinal pigment epithelium (RPE) changes in both eyes (Fig 2A, B). The optic nerves appeared grossly normal; however, the maculae demonstrated prominent cystic cavities revealed by OCT imaging (Fig 2C, D). Given the concern for an underlying retinal dystrophy, full-field electroretinography was performed and demonstrated essentially extinguished rod- and cone-driven responses, confirming an underlying retinopathy.
Figure 2.
A, B, Color fundus photographs of the (A) right and (B) left eyes of a 12-year-old patient with a peroxisomal biogenesis disorder. Both fundi demonstrate a mottled appearance to the retinal pigment epithelium with trace intraretinal pigment migration. The optic nerves appear normal, but mild retinal arteriolar attenuation appears in both eyes. C, D, OCT B-scan images obtained at the level of the fovea in the (C) right and (D) left eyes showing large cystoid cavities that significantly distort the retinal architecture in both eyes. E, Three-generation pedigree of a family with a peroxisomal biogenesis disorder (red box). The proband, indicated with an asterisk, carries compound heterozygous mutations in PEX6: c.802_815del: p.(Asp268Cysfs∗8) and c.35T→C: p.(Phe12Ser). The proband’s first cousin, who was not genotyped, died of Zellweger syndrome at 18 months of age.
Given the co-occurrence of congenital sensorineural hearing loss and retinal dystrophy, the first postulated diagnosis was Usher syndrome. Subsequently, analysis of an Usher syndrome gene panel consisting of 15 genes (Blueprint Genetics) failed to reveal pathogenic variants, and so the laboratory reflexively tested genes associated with peroxisomal disorders. Pathogenic variants in PEX genes recently were identified in other patients with a clinical diagnosis of Usher syndrome, but genetic testing was not revealing.2 Compound heterozygous mutations in PEX6 (NM_000287.4) were discovered: c.802_815del:p.(Asp268Cysfs∗8) and c.35T→C: p.(Phe12Ser). The deletion was determined to be inherited paternally and the missense mutation was determined to be inherited maternally. The 14-base pair paternally inherited deletion is a founder mutation in the Saguenay-Lac-St-Jean, a French-Canadian population, and also is found in patients diagnosed with Zellweger syndrome.7 The missense variant also was reported previously in a patient with a PBD, but the severity of the phenotype was not described.9 We later discovered that our patient’s first cousin had been diagnosed clinically with Zellweger syndrome and died at 18 months of age (Fig 2E).
Biochemical analysis of the patient’s serum revealed mildly elevated very long-chain fatty acids: C26:0/C22:0, 0.028 μmol/l (normal, < 0.020 μmol/l), and C24:0/C22:0, 1.05 μmol/l (normal, 0.44–1.16 μmol/l). Pristanic acid, phytanic acid, and pipecolic acid levels all were within normal limits. Brain magnetic resonance imaging demonstrated diffuse white matter changes in the thalami, brainstem, cerebellum, and periventricular regions, consistent with a peroxisomal disorder. Given these findings, the patient was diagnosed with a PBD resulting from biallelic mutations in PEX6. In addition to regular ophthalmology follow-up, the patient was referred to medical genetics for further systemic evaluation and dietary intervention.
Patient-Specific PEX6 Mutations Lead to Reduced PEX6 Protein Levels, But Not Reduced Peroxisome Levels
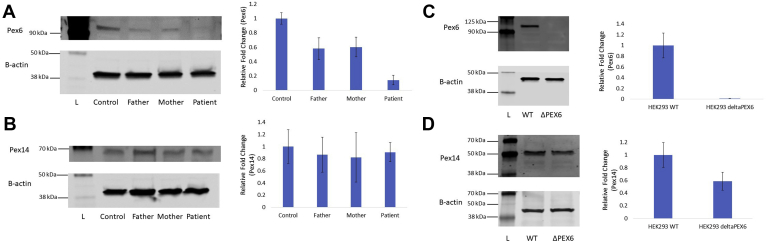
The frameshift mutation in PEX6, c.802_815del: p.(Asp268Cysfs∗8), is classified as pathogenic according to the American College of Medical Genetics and Genomics classification system, and the missense mutation, c.35T→C: p.(Phe12Ser), is classified as likely pathogenic.10 To determine the effect of the patient-specific mutations in PEX6, c.802_815del and c.35T→C, on the abundance of PEX6 protein, endogenous PEX6 protein levels in each fibroblast line were determined by western blot. PEX6 protein was reduced significantly in patient fibroblasts (PEX6 c.802_815del/c.35T→C) to 14% of control (WT/WT) levels (P = 0.0001; n = 3; Fig 3A). PEX6 abundance in the father’s (PEX6 WT/c.802_815del) and mother’s (PEX6 WT/c.35T→C) fibroblasts did not differ from one another, but were reduced significantly to approximately 60% of that detected in control fibroblast PEX6 levels (P = 0.01; n = 3; Fig 3A).
Figure 3.
A, Endogenous amounts of PEX6 protein in skin fibroblast lysates. PEX6 was reduced significantly in the father’s fibroblasts (P = 0.01), the mother’s fibroblasts (P = 0.01), and patient’s fibroblasts (P = 0.0001; n = 3 experimental replicates) compared with control fibroblasts. B, Endogenous amounts of PEX14 protein in skin fibroblast lysates. Levels of PEX14, a peroxisomal membrane protein and surrogate marker for peroxisome number, do not differ significantly among the father’s, mother’s, and patient’s fibroblasts compared with control fibroblasts (control vs. patient fibroblasts, P = 0.64; n = 3 experimental replicates). C, Endogenous amounts of PEX6 protein in human embryonic kidney 293T (HEK293T) wild-type (WT) cell lysates and PEX6 knockout cell lysates. PEX6 protein is undetectable in the PEX6 knockout cell lysates compared with WT cells (P = 0.002; n = 3 experimental replicates). D, Endogenous amounts of PEX14 protein in HEK293T WT cell lysates and PEX6 knockout cell lysates. PEX14 protein was reduced significantly in PEX6 knockout cells by approximately 40% compared with WT cells (P = 0.04; n = 3 experimental replicates). Upper and lower blots in each figure are from the same gel. L = ladder (protein size standard).
To determine whether these PEX6 mutations had an effect on the overall quantity of peroxisomes, PEX14, a peroxisomal membrane protein, was used as a surrogate marker for peroxisome number on a western blot.11 However, the findings showed no significant difference in PEX14 protein levels (and by inference, the number of peroxisomes) in the fibroblasts of the patient or his parents as compared with control fibroblasts (P = 0.64; n = 3; Fig 3B).
Characterizing a Clustered Regularly Interspaced Short Palindromic Repeats and Cas9-Derived PEX6 Knockout Cell Line
Given that the fibroblasts with compound heterozygous PEX6 mutations arose from a patient with a peroxisomal biogenesis disorder on the milder end of the spectrum, we generated a PEX6 knockout line in HEK293T cells to emulate a more severe phenotype. A CRISPR-Cas9 system generated a homozygous PEX6 c.544delG in exon 1 in HEK293T cells. Predictably, western blot showed that the PEX6 protein was absent in the PEX6 knockout, contrasting with WT HEK293T cells (Fig 3C).
To investigate further whether peroxisome number can be reduced by a lack of PEX6, we again quantified the levels of PEX14 as a marker of peroxisome number by western blot in this cell line. Unlike in patient fibroblasts with compound heterozygous mutations in PEX6, PEX14 was significantly reduced in HEK293T PEX6 knockout cells by 41% compared with WT cells (P = 0.04; n = 3; Fig 3D).
Peroxisomal Targeting Signal 1-Mediated Peroxisomal Matrix Protein Import Is Disrupted in Patient Fibroblasts and PEX6 Knockout Cells
Given that the results from patient fibroblasts did not suggest a reduced peroxisome number, we investigated whether matrix protein import, in which PEX6 plays a major role, was disrupted in the patient’s cells (Fig 1). A GFP-PTS1 expression plasmid was introduced into control and patient fibroblasts via electroporation. After 48 hours, the distribution and intensity of the GFP signal was evaluated using the EVOS cell imaging system. Peroxisomal targeting signal 1 is a tripeptide containing a serine-lysine-leucine sequence that is present on the C-terminus of nascent polypeptides synthesized in the endoplasmic reticulum and targets proteins to the peroxisome. Control fibroblasts demonstrated an intracellular punctate GFP signal, as would be expected if the GFP-PTS1 was localized successfully to the peroxisomes. However, the patient fibroblasts demonstrated a more diffuse cytosolic GFP signal, suggesting impaired PTS1-mediated peroxisomal protein import (Fig 4). Analysis of GFP-PTS1 distribution in the father’s and mother’s fibroblasts revealed a pattern similar to control fibroblasts (data not shown).
Figure 4.
Microscopic images of in vitro green fluorescent protein (GFP)-peroxisomal targeting signal 1 (PTS1) expression in fibroblasts and human embryonic kidney 293T (HEK293T) cells. Cells were transfected with GFP-labelled PTS1 to interrogate the competency of peroxisomal matrix protein import. Control fibroblasts and wild-type HEK293T cells demonstrate intracellular punctate GFP signal, suggesting appropriate PTS1-mediated targeting of proteins to peroxisomes. However, the patient fibroblasts and PEX6 knockout cells demonstrate diffuse cytosolic GFP signal, suggesting impaired PTS1-mediated peroxisomal protein import.
To evaluate the PTS1 protein import pathway in vitro in the CRISPR-Cas9–mediated PEX6 knockout HEK293T cells, the same GFP-PTS1 expression plasmid was transfected into control and PEX6 knockout cells using a Lipofectamine reagent. Similar to control fibroblasts, WT HEK293T cells demonstrated intracellular punctate GFP signal, suggesting appropriate PTS1-mediated targeting of proteins to peroxisomes. However, the PEX6 knockout cells demonstrated a more diffuse cytosolic GFP signal, like the patient fibroblasts (Fig 4). In both cases, defects in PEX6 appeared to cause impaired PTS1-mediated peroxisomal protein import.
Interrogating Peroxisomal Targeting Signal 2-Mediated Peroxisomal Protein Import by Immunofluorescence
Because the in vitro study of GFP-PTS1 distribution suggested that the PTS1 protein import pathway was defective in patient fibroblasts and PEX6 knockout cells, we evaluated protein import via the peroxisomal targeting signal 2 (PTS2)-mediated pathway (Fig 1). Although most peroxisomal matrix proteins are imported via the PTS1 pathway, the PTS2 pathway is required for the import of several proteins, including thiolase, an enzyme involved in the peroxisomal β-oxidation pathway.12
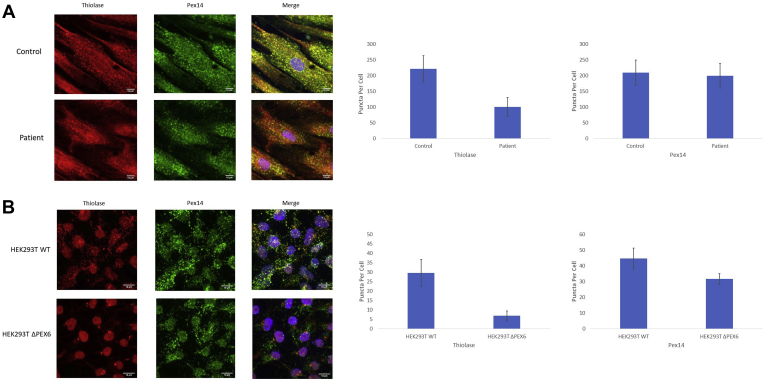
In control fibroblasts, thiolase demonstrated an intracellular punctate distribution that colocalized with PEX14, the peroxisomal membrane marker that was used in the previous western blot assays. This result suggested that the control fibroblasts had appropriate PTS2-mediated protein import. However, the patient fibroblasts demonstrated a more diffuse, cytosolic, thiolase pattern with fewer intracellular puncta present per cell compared with controls (P < 0.001; n = 10 cells). The diffuse thiolase pattern evident in the patient fibroblasts suggested impaired PTS2-mediated peroxisomal protein import (Fig 5A). Notably, the patient fibroblasts showed a PEX14 distribution consistent with the control fibroblasts on immunofluorescence. No difference was found in the number of PEX14 puncta per cell when comparing patient fibroblasts with control fibroblasts (P < 0.58; n = 10 cells), consistent with the similar PEX14 abundance demonstrated on western blot (Fig 3B).
Figure 5.
A, Immunofluorescence images of skin fibroblasts from control and patient sources. Thiolase, a protein imported via a peroxisomal targeting signal 2 (PTS2)-mediated process, shows intracellular punctate localization that overlaps with PEX14, a peroxisomal membrane protein, suggesting appropriate PTS2-mediated targeting of proteins to peroxisomes in control fibroblasts. However, the patient fibroblasts demonstrate a more diffuse cytosolic thiolase localization with few puncta, despite a normal distribution of PEX14, suggesting impaired PTS2-mediated peroxisomal protein import. Fewer thiolase-labelled puncta per cell are present in the patient fibroblasts compared with the control fibroblasts (P < 0.001; n = 10 cells), but no difference in the number of PEX14-labelled puncta per cell is seen (P = 0.58; n = 10 cells). B, Immunofluorescence images of human embryonic kidney 293T (HEK293T) wild-type cells and PEX6 knockout cells. Similar to control fibroblasts, thiolase shows intracellular punctate localization that overlaps with PEX14 in wild-type cells, suggesting appropriate PTS2-mediated targeting of proteins to peroxisomes. However, the PEX6 knockout cells demonstrate a more diffuse cytosolic thiolase localization with few puncta localized in a peculiar perinuclear distribution, suggesting impaired PTS2-mediated peroxisomal protein import. Fewer thiolase-labelled puncta per cell are present (P < 0.0001; n = 40 cells) and also fewer PEX14-labelled puncta are present in the knockout cells (P < 0.0001; n = 40 cells), consistent with reduced peroxisome abundance.
To evaluate the PTS2-mediated protein import pathway in CRISPR-Cas9–mediated PEX6 knockout HEK293T cells, a similar immunofluorescence assay was performed and the cellular distribution of thiolase and PEX14 was determined in HEK293T cells. In WT cells, thiolase demonstrated a punctate intracellular pattern that colocalized with PEX14, consistent with the pattern evident in control fibroblasts, suggesting an intact PTS2-mediated protein import pathway. However, the PEX6 knockout cells demonstrated a more diffuse, cytosolic, thiolase distribution similar to the pattern present in the patient fibroblasts, suggesting impaired PTS2 protein import. Consistent with the fibroblast results, we observed fewer thiolase-labelled puncta per cell in the PEX6 knockout cells compared with WT cells (P < 0.0001; n = 40 cells). In the PEX6 knockout cells, unlike the patient fibroblasts, fewer PEX14-labelled puncta were present compared with WT cells (P < 0.0001; n = 40 cells), and the few puncta that were present clustered around the cell nucleus (Fig 5B). This reduction in PEX14-labelled puncta in the PEX6 knockout cells is consistent with reduced PEX14 abundance demonstrated on western blot (Fig 3D).
The Processing of Peroxisomal Targeting Signal 2-Thiolase Confirmed Defects in Protein Import in Patient Fibroblasts and PEX6 Knockout Cells
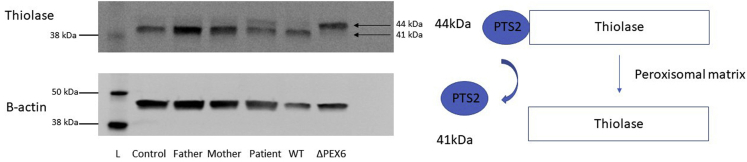
Thiolase exists as a 44-kDa protein with an N-terminal nonapeptide PTS2 sequence. Thiolase is recognized by PEX7, a protein crucial in targeting PTS2-containing proteins to the peroxisome. Together, this complex is shuttled to the peroxisomal membrane aided by cytosolic PEX5.13 Functional PEX6 is required to release PEX5 from the membrane for subsequent rounds of PTS2-mediated protein import. As soon as thiolase reaches its destination in the peroxisomal lumen, the N-terminal PTS2 is cleaved enzymatically, leaving behind a 41-kDa thiolase protein (Fig 1).
In normal fibroblasts, as well as those of both carrier parents, most thiolase is detected as a 41-kDa band on a western blot, suggesting that PTS2 signal has been cleaved off, and thus appropriate peroxisomal localization results. However, in the patient fibroblasts, an additional band visible at 44 kDa is present, reflecting the presence of some uncleaved, and thus mislocalized, thiolase (Fig 6).
Figure 6.
Processing of thiolase in skin fibroblasts and human embryonic kidney 293T (HEK293T) cells on immunoblot. Thiolase, a protein imported into the peroxisomal matrix via a peroxisomal targeting signal 2 (PTS2)-mediated pathway, exists as a 44-kDa protein with its nonapeptide N-terminal PTS2 sequence. After being localized to the peroxisomal matrix, the PTS2 signal is cleaved, leaving behind a 41-kDa thiolase protein. In control fibroblasts, the father’s fibroblasts, and the mother’s fibroblasts, most of the thiolase exists in its cleaved state, suggesting appropriate peroxisomal localization. In the patient fibroblasts, a visible thiolase band appears at the 44-kDa level, suggesting the presence of some uncleaved thiolase as a result of mislocalization. Wild-type (WT) HEK293T cells have the majority of thiolase existing in the cleaved state, compared with PEX6 knockout cells, where most thiolase is uncleaved, suggesting mislocalization. Upper and lower blots are from the same gel. L = ladder (protein size standard).
Similar to control fibroblasts, in WT HEK293T cells, we detected only the cleaved thiolase protein as a single band at 41 kDa. In contrast, the PEX6 knockout cells do not show any thiolase at 41 kDa; the detected protein is at 44 kDa on a western blot, suggesting mislocalization of thiolase and retention of the N-terminal PTS2 signal (Fig 6).
PEX6 Replacement Rescues Some Peroxisomal Targeting Signal 2 Protein Import in PEX6 Knockout Cells
To determine whether the protein import defect in PEX6-deficient cells could be rescued by introducing WT PEX6, the knockout cells were transfected with an expression plasmid containing WT FLAG-tagged PEX6. The HEK293T cells were selected over the fibroblasts for the initial assay because they are considerably easier to transfect and because the difference in thiolase processing between WT and PEX6 knockout cells is greater compared with control and patient fibroblasts.
Human embryonic kidney 293T PEX6 knockout cells were transfected with a FLAG-tagged PEX6 expression plasmid. After 72 hours, thiolase processing was evaluated on a western blot as described above. PEX6 overexpression in the PEX6 knockout cells resulted in the appearance of a 41-kDa thiolase band, suggesting that some thiolase reached the peroxisomal matrix. The ratio of processed to unprocessed thiolase was increased significantly after PEX6 overexpression; it was determined to be 29% of that of WT HEK293T cells (P = 0.01; n = 2; Fig 7).
Figure 7.
PEX6 overexpression in human embryonic kidney 293T (HEK293T) wild-type (WT) and PEX6 knockout cells. A FLAG-tagged PEX6 expression plasmid was transfected into HEK293T cells, resulting in an approximately 40-fold increase in PEX6 levels. Both WT PEX6 (6WT) and PEX6 c.35T→C (6M) overexpression resulted in some thiolase cleavage compared with PEX6 knockout cells receiving the empty vector (EV), suggesting the rescue of some peroxisomal targeting signal 2 (PTS2)-mediated peroxisomal protein import with PEX6 overexpression (HEK293 ΔPEX6 EV vs. 6WT; P = 0.01; n = 2 experimental replicates). Both PEX6 WT and PEX6 c.35T→C overexpression increased the ratio of processed to unprocessed thiolase to nearly 30% of WT levels. Upper and lower blots are from the same gel. L = ladder (protein size standard).
Our patient with a peroxisomal biogenesis disorder showed compound heterozygous changes in PEX6 (c.802_815del/c.35T→C). The 14-base pair deletion leads to a frameshift and subsequent nonsense-mediated mRNA decay, confirmed by quantitative polymerase chain reaction analysis (data not shown). The effect of the missense change, p.(Phe12Ser), on peroxisome function is unknown; however, the mother who carried this mutation showed reduced PEX6 protein, suggesting it may lead to protein degradation (Fig 3A). To investigate how the missense variant affects protein import, a FLAG-tagged PEX6 c.35T→C expression plasmid was generated by site-directed mutagenesis and transfected into HEK293T PEX6 knockout cells. Similar to WT PEX6 overexpression, PEX6 c.35T→C overexpression resulted in a 41-kDa thiolase band on a western blot, suggesting that some PTS2-mediated peroxisomal protein import was restored. The ratio of processed to unprocessed thiolase was increased significantly after transfection with the PEX6 missense variant compared with cells receiving an empty vector (P = 0.01; n = 3). The processing of thiolase was determined to be 27% of WT HEK293T cells, which was not significantly different from WT PEX6 overexpression (Fig 7).
Discussion
Our patient demonstrated severe congenital sensorineural hearing loss and an early-onset retinal dystrophy. In the absence of other characteristic phenotypic findings, it would be difficult to make a clinical diagnosis of a PBD. Based on these findings alone, the patient had been considered to have a variant of Usher syndrome, the most common cause of combined deafness and blindness. Fortuitously, given that molecular genetic testing was available, biallelic mutations in PEX6 were identified and he subsequently was diagnosed with a PBD. This led to an additional workup, including biochemical testing and a brain magnetic resonance imaging, that demonstrated a leukodystrophy characteristic of a peroxisomal disorder. When considering the PBD disease spectrum, he likely falls within the infantile Refsum disease category because his overall presentation is milder. Additionally, the presence of neurologic changes and the absence of enamel changes and nail disease make a diagnosis of Heimler syndrome less likely.14
Given our patient’s overall milder phenotypic manifestations, his age and his ability to participate in additional clinical testing provided an opportunity to study his peroxisomal function. Although the PEX6 c.802_815del mutation has been shown previously to produce negligible PEX6 protein,7 we determined that the PEX6 c.35T→C mutation also reduced PEX6 protein levels by approximately 40% compared with a control (Fig 3A). Although the patient’s skin fibroblasts showed significantly reduced endogenous levels of PEX6 protein on western blot (Fig 3A), we did not detect a difference in peroxisome number using a surrogate marker of peroxisome abundance, PEX14 (Fig 3B). In addition, the relative amount and distribution of PEX14 by immunofluorescence did not differ remarkably between control fibroblasts and patient fibroblasts, in keeping with the western blot data (Fig 5A).
In PEX6 knockout HEK293T cells, created as a model for the more severe Zellweger syndrome-like peroxisomal phenotype, no PEX6 protein was detectable on western blot (Fig 3C). In contrast to the patient fibroblasts, a significant reduction in PEX14 was observed on western blot and via immunofluorescence, suggesting reduced peroxisome number in the knockout cells (Figs 3D and 5B). In PEX6 knockout cells, PEX14 levels were 59% of PEX14 levels in WT HEK293T cells as determined by western blot.
Law et al8 demonstrated that a knock-down of PEX1 and PEX26, the other 2 components of the peroxisome exportomer complex with PEX6, led to significantly reduced peroxisome number. In a series of experiments, the authors concluded that the primary defect was one of excessive peroxisomal degradation as opposed to reduced matrix protein import.8 Although we did not evaluate whether the reduced peroxisome number resulted from excessive peroxisomal degradation in the PEX6 knockout cells, this idea is plausible and would support the findings from the above study.8 We believe that excessive peroxisomal degradation may be a feature of more severe mutations (such as early stop codons, frameshifts, or large deletions) in PEX1, PEX6, and PEX26 and contribute to a more severe disease phenotype.
Because peroxisome number was not altered detectably in the patient’s fibroblasts, we evaluated whether the cells were deficient in peroxisomal matrix protein import, one of the other main roles of PEX6.13 We separately evaluated the 2 pathways of peroxisomal matrix protein import: the PTS1 and PTS2 pathways (Fig 1). We found that patient fibroblasts, as well as PEX6 knockout HEK293T cells, transfected with GFP-PTS1 displayed a more diffuse, cytosolic GFP signal. This result differed from control fibroblasts and WT cells, in which punctate GFP-labelled structures were evident (Fig 4). These findings from both cell types support the assertion that PEX6 mutation leads to impaired PTS1-mediated peroxisomal matrix protein import. Although a complete absence of PEX6 may reduce peroxisome number, our experiments in patient fibroblasts as well as a knockout cell line additionally implicate PEX6 in PTS1-mediated protein import, where defects may contribute to peroxisome dysfunction.
When we interrogated PTS2-mediated peroxisomal protein import by immunofluorescence in patient fibroblasts and PEX6 knockout HEK293T cells, we identified fewer thiolase-labelled puncta that colocalized with PEX14 when compared with control fibroblasts and WT cells, suggesting impaired PTS2-mediated protein import (Fig 5). In addition, fewer PEX14-labelled puncta per cell were present in the PEX6 knockout cells, consistent with the reduced peroxisomal number noted on western blot (Fig 3D). These results, together with the findings in the GFP-PTS1 transfected cells, point to a generalized defect in peroxisomal protein import resulting from mutations in PEX6.
As further evidence of impaired peroxisomal matrix protein import, we evaluated the processing of thiolase on a western blot. After being imported into the peroxisomal matrix, the enzyme Tysnd1 cleaves the N-terminal PTS2 signal from thiolase.15 The difference in molecular size between processed or cleaved and unprocessed or uncleaved thiolase can be resolved on a western blot, thus distinguishing the two forms. Patient cells contained thiolase in both forms—cleaved and uncleaved—suggesting that thiolase was not reaching the peroxisomal matrix (Fig 6). Although it was difficult to assess the degree of residual peroxisomal protein import function by the cell imaging assays, the presence of some cleaved thiolase in the patient fibroblasts supports the existence of residual protein import and explains the milder phenotype in our patient. Both carrier parents showed a pattern of thiolase processing that was the same as that of control fibroblasts (Fig 6). Our cell model of a more severe peroxisomal phenotype, the PEX6 knockout HEK293T cells, demonstrated no cleaved thiolase (Fig 6). Given that these cells lacked PEX6 protein, this finding further supports that the presence of at least some PEX6 is critical for matrix protein import.13
Taken together, our results highlight that the type of mutation in PEX6 can influence the peroxisomal phenotype evident on a cellular level, which in turn is reflected by the observed disease severity in a patient. We hypothesize that hypomorphic mutations and a genotype where functional PEX6 is reduced, but not completely absent, will lead to defective matrix protein import, but not a reduction in peroxisome number. Our data corroborated previous reports in which severe mutations that abrogated PEX6 function led to reduced peroxisome number.7 We suggest that an accompanying decrease in matrix protein import is an additional relevant mechanism of pathogenesis in such cases as well. This combination of defects in peroxisome biogenesis and matrix protein import results in a more severe disease phenotype.
This distinction may have important therapeutic implications. For example, the chemical chaperone arginine has been shown to improve matrix protein import in fibroblasts from a patient with a mild PBD resulting from a PEX6 mutation.16 Although we did not assess the effect of temperature on peroxisome protein import in our patient’s fibroblasts, milder PEX6 PBDs have been found to have reduced protein import when cells are exposed to higher temperatures.17 This finding has been attributed to the destabilizing effect of missense mutations on the PEX6 protein.17 As a result, chemical chaperones such as arginine may stabilize PEX6 and rescue some import activity in mild PBDs. Consistent with this suggestion, a patient with PEX12 mutations was treated with L-arginine and demonstrated improved serum biochemical parameters and improved hearing.18 However, chemical chaperones may not be expected to result in significant improvement in patients with more severe disease that features both impaired protein import and a reduced peroxisome number.
To investigate whether genetic approaches could improve impaired peroxisome protein import, we evaluated whether overexpressing WT PEX6 in PEX6 knockout cells might rescue peroxisome function by assessing thiolase processing on western blot. In tandem, we transfected PEX6 knockout cells with a plasmid containing the PEX6 c.35T→C variant to evaluate whether this patient-specific mutation could promote matrix protein import. Overexpression of both WT PEX6 and PEX6 c.35T→C each resulted in a similar improvement in the processing of thiolase in PEX6 knockout cells, from negligible to nearly 30% that of PEX6 WT (Fig 7). This experiment highlights a potential role for PEX6 overexpression in recovering peroxisome function. In addition, this assay also provides evidence to show that PEX6 c.35T→C has protein import capacity. We hypothesize that PEX6 c.35T→C instead may destabilize PEX6 protein; we plan to evaluate this with a cycloheximide chase assay. Finally, we plan to evaluate thiolase localization via immunofluorescence after WT PEX6 overexpression in PEX6 knockout cells. In keeping with the western blot data, we expect matrix protein import to be restored in this experiment.
One of the limitations of our study is that we evaluated the effect of patient-specific PEX6 mutations in skin fibroblasts. Numerous other studies similarly have evaluated PBDs in skin fibroblasts17,19, 20, 21; however, cutaneous manifestations of disease are not typical of PBDs.22 Skin fibroblasts are obtained readily via a superficial dermal punch biopsy. As a result, the convenience of establishing a primary cell line in culture with patient-specific mutations makes the use of skin fibroblasts attractive. However, significant heterogeneity exists in the gene expression profile of different tissue types; 50% of all genes demonstrate tissue-specific expression.23 Since peroxisomes are ubiquitous organelles, skin fibroblasts may be an acceptable model to evaluate peroxisome function in general. However, to evaluate tissue-specific effects of peroxisome dysfunction, such as in the retina, it would be important to use a model such as induced pluripotent stem cell-derived RPE cells that demonstrate similar membrane potential, ion transport, and gene expression profiles as native RPE24 or an in vivo animal model.
In addition, our study is limited by the analysis of a single patient with PBD. Although peroxisomal disorders reportedly are rare,25 their actual prevalence may be higher. More widespread availability of genetic testing could solve molecularly PBD cases that overlap considerably with other disorders, such as Usher syndrome.26 Although conclusions made in a single patient cannot necessarily be generalized to all patients with peroxisomal disorders, our findings of disrupted protein import and normal peroxisome number in our patient’s fibroblasts demonstrate that specific mutations can have different effects on peroxisome metabolism. Moreover, the consequences of these mutations may require different therapeutic approaches.
In summary, we demonstrated that skin fibroblasts from a patient with compound heterozygous mutations in PEX6, c.35T→C: p.(Phe12Ser) and c.802_815del: p.(Asp268Cysfs∗8), showed abnormal peroxisomal matrix protein import, but not abnormal peroxisomal number. This finding contrasts with the PEX6 knockout HEK293T cell line, which exhibited both a severe defect in matrix protein import and a significant reduction in peroxisome number. We hypothesize that the reduction in peroxisome number is a feature of the more severe phenotypes along the PBD spectrum and is likely the result of frameshift or nonsense mutations in PEX6 that result in little protein product. These differences become important when therapeutic approaches are considered. Finally, we provide evidence that genetic strategies, which restore WT PEX6 into cells devoid of PEX6 protein, can restore peroxisome function. Future research with patient-specific induced pluripotent stem cell-derived RPE cells may clarify further the potential role of gene therapy as a treatment approach in patients with PBDs.
Manuscript no. D-21-00008.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by a Clinician-Scientist Emerging Leader Award from Fighting Blindness Canada (2018–2020; M.D.B.). The funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. Institutional Review Board / Ethics Committee approval was obtained from the University of Alberta Health Research Ethics Board - Biomedical Panel (No. Pro00074451). All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Benson, MacDonald
Analysis and interpretation: Benson, Papp, Casey, Radziwon, St. Laurent, Doucette, MacDonald
Data collection: Benson, Papp, Casey, Radziwon, St. Laurent, Doucette, MacDonald
Obtained funding: Study was performed as part of regular employment duties at the University of Alberta
Overall responsibility: Benson, MacDonald
Supplementary Data
References
- 1.Fujiki Y., Abe Y., Imoto Y., et al. Recent insights into peroxisome biogenesis and associated diseases. J Cell Sci. 2020;133(9):jcs236943. doi: 10.1242/jcs.236943. [DOI] [PubMed] [Google Scholar]
- 2.Barillari M.R., Karali M., Di Iorio V., et al. Mild form of Zellweger spectrum disorders (ZSD) due to variants in PEX1: detailed clinical investigation in a 9-years-old female. Mol Genet Metab Rep. 2020;24:100615. doi: 10.1016/j.ymgmr.2020.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventura M.J., Wheaton D., Xu M., et al. Diagnosis of a mild peroxisomal phenotype with next-generation sequencing. Mol Genet Metab Rep. 2016;9:75–78. doi: 10.1016/j.ymgmr.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mast F.D., Fagarasanu A., Knoblach B., Rachubinski R.A. Peroxisome biogenesis: something old, something new, something borrowed. Physiology (Bethesda) 2010;25(6):347–356. doi: 10.1152/physiol.00025.2010. [DOI] [PubMed] [Google Scholar]
- 5.Di Cara F., Sheshachalam A., Braverman N.E., et al. Peroxisome-mediated metabolism is required for immune response to microbial infection. Immunity. 2017;47(1):93–106. doi: 10.1016/j.immuni.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Tan A.P., Gonçalves F.G., Almehdar A., Soares B.P. Clinical and neuroimaging spectrum of peroxisomal disorders. Top Magn Reson Imaging. 2018;27(4):241–257. doi: 10.1097/RMR.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 7.Levesque S., Morin C., Guay S.P., et al. A founder mutation in the PEX6 gene is responsible for increased incidence of Zellweger syndrome in a French Canadian population. BMC Med Genet. 2012;13:72. doi: 10.1186/1471-2350-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law K.B., Bronte-Tinkew D., Di Pietro E., et al. The peroxisomal AAA ATPase complex prevents pexophagy and development of peroxisome biogenesis disorders. Autophagy. 2017;13(5):868–884. doi: 10.1080/15548627.2017.1291470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg S., Chen L., Wei L., et al. The PEX Gene Screen: molecular diagnosis of peroxisome biogenesis disorders in the Zellweger syndrome spectrum. Mol Genet Metab. 2004;83(3):252–263. doi: 10.1016/j.ymgme.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Richards S., Aziz N., Bale S., et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant P., Ahlemeyer B., Karnati S., et al. The biogenesis protein PEX14 is an optimal marker for the identification and localization of peroxisomes in different cell types, tissues, and species in morphological studies. Histochem Cell Biol. 2013;140(4):423–442. doi: 10.1007/s00418-013-1133-6. [DOI] [PubMed] [Google Scholar]
- 12.Kunze M., Malkani N., Maurer-Stroh S., et al. Mechanistic insights into PTS2-mediated peroxisomal protein import: the co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J Biol Chem. 2015;290(8):4928–4940. doi: 10.1074/jbc.M114.601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrosa A.G., Francisco T., Ferreira M.J., et al. A mechanistic perspective on PEX1 and PEX6, two AAA+ proteins of the peroxisomal protein import machinery. Int J Mol Sci. 2019;20(21):5246. doi: 10.3390/ijms20215246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daich Varela M., Jani P., Zein W.M., et al. The peroxisomal disorder spectrum and Heimler syndrome: deep phenotyping and review of the literature. Am J Med Genet C Semin Med Genet. 2020;184(3):618–630. doi: 10.1002/ajmg.c.31823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurochkin I.V., Mizuno Y., Konagaya A., et al. Novel peroxisomal protease Tysnd1 processes PTS1- and PTS2-containing enzymes involved in beta-oxidation of fatty acids. EMBO J. 2007;26(3):835–845. doi: 10.1038/sj.emboj.7601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berendse K., Ebberink M.S., Ijlst L., et al. Arginine improves peroxisome functioning in cells from patients with a mild peroxisome biogenesis disorder. Orphanet J Rare Dis. 2013;8:138. doi: 10.1186/1750-1172-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratbi I., Falkenberg K.D., Sommen M., et al. Heimler syndrome is caused by hypomorphic mutations in the peroxisome-biogenesis genes PEX1 and PEX6. Am J Hum Genet. 2015;97(4):535–545. doi: 10.1016/j.ajhg.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorlin A., Briand G., Cheillan D., et al. Effect of 1-arginine in one patient with peroxisome biogenesis disorder due to PEX12 deficiency. Neuropediatrics. 2016;47(3):179–181. doi: 10.1055/s-0036-1578798. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka A.J., Okumoto K., Tamura S., et al. A newly identified mutation in the PEX26 gene is associated with a milder form of Zellweger spectrum disorder. Cold Spring Harb Mol Case Stud. 2019;5(1):a003483. doi: 10.1101/mcs.a003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran C., Hewson S., Steinberg S.J., Mercimek-Mahmutoglu S. Late-onset Zellweger spectrum disorder caused by PEX6 mutations mimicking X-linked adrenoleukodystrophy. Pediatr Neurol. 2014;51(2):262–265. doi: 10.1016/j.pediatrneurol.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell M.A., Leane P.B., Paton B.C., Crane D.I. Novel PEX1 coding mutations and 5’ UTR regulatory polymorphisms. Hum Mutat. 2005;26(3):279. doi: 10.1002/humu.9356. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg S.J., Raymond G.V., Braverman N.E., et al. In: GeneReviews. Adam M.P., Ardinger H.H., Pagon R.A., et al., editors. University of Washington; Seattle: 1993–2020. Zellweger spectrum disorder. December 12, 2003 (updated December 21, 2017).https://www.ncbi.nlm.nih.gov/books/NBK1448/ Available at: Accessed June 4, 2021. [Google Scholar]
- 23.Yang RY, Quan J, Sondaei R, et al. A systematic survey of human tissue-specific gene expression and splicing reveals new opportunities for therapeutic target identification and evaluation. bioRxiv. 311563. 10.1101/311563. Availabe at: https://www.biorxiv.org/content/10.1101/311563v1. Accessed June 4, 2021. [DOI]
- 24.Kokkinaki M., Sahibzada N., Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29(5):825–835. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasiljevic E., Ye Z., Pavelec D.M., et al. Carrier frequency estimation of Zellweger spectrum disorder using ExAC database and bioinformatics tools. Genet Med. 2019;21(9):1969–1976. doi: 10.1038/s41436-019-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raas-Rothschild A., Wanders R.J., Mooijer P.A., et al. A PEX6-defective peroxisomal biogenesis disorder with severe phenotype in an infant, versus mild phenotype resembling Usher syndrome in the affected parents. Am J Hum Genet. 2002;70(4):1062–1068. doi: 10.1086/339766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.