Abstract:
The expression of positive social (i.e., prosocial) behavior is governed by a multitude of sensory and cognitive abilities to identify and recognize key features of potential social partners, elucidate social and individual status, and maintain appropriate behaviors. Oxytocin (OT) is a neuropeptide that has been implicated as a major player in regulating prosocial behavior, and much of its role in social situations has been uncovered. As social behavior inherently comprises sequential processes related to multimodal assessments of interactive features, a comprehensive approach to understanding the functions of OT in these prosocial behavior sequences is required. Here, the author discusses recent evidence illustrating the functioning of OT neural circuits in the processing of multimodal components of social behavior, including the detection/recognition of social cues via the olfactory bulb through olfactory cortices, evaluation of social features via the circuits of the paraventricular nucleus of the hypothalamus to the medial amygdala, and maintenance of prosocial behaviors via the circuits of the ventral tegmental area to the nucleus accumbens. A review of rodent studies with an emphasis on mice and rats is also provided to investigate the effects of OT in interaction with other neurotransmitters, such as serotonin and dopamine, to characterize the neuromodulatory mechanisms that mediate the sequences of prosocial engagements. The review further highlights OT function as a temporal dynamic of specific neural circuits.
Keywords: Oxytocin, Prosocial behavior, Neural circuits, Behavioral sequence, Olfactory bulb, Amygdala, Hypothalamus, Ventral tegmental area, Raphe nucleus
Graphical abstract
Highlights
-
•
A neuromodulator oxytocin regulates neural processes of complex pprosocial behavior.
-
•
OT mediated prosocial behavior consists of sequential processes of multisensory components.
-
•
Detection of social olfactory cues is mediated by OT input from the PVN of the hypothalamus.
-
•
Investigatory social approach is regulated by the hypothalamus to medial amygdala OT pathway.
-
•
OT inputs to reward ciruits are involved in maintaining social contact with familiar partners.
1. Introduction
The peptide hormone oxytocin (OT) is a neuromodulator involved in regulating the adaptive social behaviors of all vertebrates (Nishimori et al., 1996). OT consists of nine amino acids (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly); the first six amino acids form a ring, while the remaining three form a short tail (Kimura and Tanizawa, 1992; Inoue et al., 1994). Central release of OT governs several significant biological functions, including reproduction-related processes, such as mating and nursing, as well as social processes (Landgraf, 1995; Gimpl and Fahrenholz, 2001). Over the last three decades, focused efforts have also led to considerable advances in understanding the functional role of OT in social and physiological conditions and related disorders, and much of its role in social regulation has been uncovered, including the cellular and molecular mechanisms involved in the role of OT and its target receptor (OTR) in neuronal cells. Nevertheless, a key issue that still needs more attention is their multifunctional roles in the sequential processes of interactive social behavior, which in turn involve multiple levels of coordination with other neurotransmitters and neural circuits. This review addresses the complexity of OT and OTergic neuronal circuitry regulation underlying the multimodal sequential processing of prosocial communicative behavior in rodent models.
2. Neural mechanisms of OT function
In the mammalian central nervous system, OT is synthesized mainly in the supraoptic nucleus (SON) and paraventricular nucleus (PVN), as well as in the accessory magnocellular nuclei of the hypothalamus (Sofrniew, 1983; Swanson and Sawchenko, 1983; Landgraf and Neumann, 2004). The OT peptide is released into peripheral circulation via the posterior pituitary and plays a fundamental role in reproduction-related processes such as uterine contractions, delivery, and lactation (Gimple and Fahrenholz, 2001). Central release of OT is mainly mediated by the PVN which contains parvocellular and magnocellular neurons (Swanson and Sawchenko, 1983). The magnocellular neurons of the PVN are primarily responsible for the innervation of forebrain regions (Dölen et al., 2013; Ross et al., 2009), while the parvocellular neurons likely project to distinct regions of the spinal cord and brainstem (Sawchenko and Swanson, 1982). Recently, Eliava et al. (2016) discovered a subset of hypothalamic parvocellular neurons that project onto magnocellular neurons to modulate both immediate and long-term OT release. Thus, central OT is likely transported via axonal projections of both magnocellular and parvocellular neurons in the PVN, which are distributed to various sites in the brain.
3. OTR and its distribution
The OTR is encoded by a single gene and belongs to the G protein-coupled receptor (GPCR) superfamily, in which the ligand binding structure is highly conserved across species (Gimpl and Fahrenholz, 2001; Busnelli et al., 2013). The OTR forms a small receptor sub-family with the three structurally related arginine-vasopressin (AVP) receptors (V1aR, V1bR, and V2R) (Grinevich et al., 2016). As the ligand-binding pockets in the OTR and AVP receptor subfamily are extremely conserved, they appear to have a high binding affinity for endogenous OT and AVP in mice (e.g., OT to OTR affinity value: 0.83 nM, AVP to OTR affinity value: 0.87 nM) (Busnelli et al., 2013). Therefore, it has proven problematic to develop highly selective ligands that bind only to either receptor and to detect OTR-specific immunoreactivity using anti-OTR antibodies (Yoshida et al., 2009; Manning et al., 2012).
OTRs form heterodimers with other GPCRs, and these interactions enormously expand their signal transduction repertoire. There are three biased analogues that are capable of inducing only a specific subset of OTR/G protein couplings: carbetocin, atosiban, and D-Nal-OVT (Busnelli et al., 2012; Passoni et al., 2016; Busnelli and Chini, 2018). The OT agonist carbetocin is a selective OTR/Gq analogue that is able to induce OTR/Gq coupling in the absence of OTR/Gi or OTR/Go stimulation (Passoni et al., 2016). Atosiban is an agonist that selectively activates OTR/Gi3 coupling and inhibits cell proliferation and neuronal firing (Busnelli et al., 2012; Busnelli and Chini, 2018), as does D-Nal-OVT via promoting only OTR/Gi1 coupling (Eliava et al., 2016).
As most commercially available anti-OTR antibodies are unreliable and stain both OT-positive and -negative tissues in mice (Yoshida et al., 2009), in-situ hybridization to OTR mRNA and autoradiography for detecting specific OTR ligands have been utilized to elucidate the distribution of OTRs in several species (Gimple and Fahrenholz, 2001; Jurek and Neumann, 2018). Several brain regions with moderate to high OTR density have been identified using the OTR gene promoter, including the accessory olfactory bulb, medial septal nucleus, posterior region of the complex amygdala nucleus, ventral hypothalamic areas (medial supramammillary nucleus and dorsomedial and ventromedial nuclei), subiculum area of the hippocampus, anterolateral cortical areas (entorhinal and piriform cortices), and basal forebrain areas (dorsal tegmental, vestibular, raphe, spinal trigeminal, and solitary tract subnuclei) (Gould and Zingg, 2003; Jurek and Neumann, 2018).
As OTRs are expressed throughout the brain, OT has to reach them efficiently and in sufficient amounts to activate them at various distances from the hypothalamus (e.g., the PVN), by means of long-range axonal projections. A large number of OT fibers are innervated in regions that express OTR at sufficiently high levels (Knobloch et al., 2012; Tang et al., 2020). High-density axonal projections are present in the basal ganglia area, nucleus accumbens (NAc), lateral septal nucleus, bed nucleus of the stria terminalis (BNST), medial amygdala (MeA), paraventricular thalamic nucleus, and mid hind-brain areas including the raphe nucleus and periaqueductal gray (Mitre et al., 2016; Jurek and Neumann, 2018). A few regions of the rat forebrain, including the ventral pallidum, medial preoptic area, and ventromedial hypothalamic nucleus, express moderate to high OTR levels but are unlikely to receive direct OT projections (Grinevich et al., 2016). This localization mismatch between OT axonal terminals and OTR binding sites can partially be ascribed to technical difficulties in the detection of these OT projection fibers and OTR expression.
Recently, a viral tracing method with a fluorescent marker protein under an OT gene promoter was used to visualize OT fibers projecting from hypothalamic neurons to different regions of the brain (Grinevich et al., 2016). The distribution patterns of the OTR differ between species (Campbell et al., 2009), in males and females (Dumais and Veenema, 2016), and across different developmental stages (Grinevich et al., 2015; Tabbaa and Hammock, 2020). OT secretion from the axonal terminals also varies depending on functional status in the context of socio-environmental factors and developmental stages (Grinevich et al., 2016; Grinevich and Stoop, 2018); thus, OTR expression is largely variable. This is apparent from the fact that reported OT neuronal projection and OTR localization and density vary widely across studies investigating their distribution (Jurek and Neumann, 2018). For example, the lateral septum receives dense OT fibers from the SON and PVN (Russell et al., 1992; Neumann et al., 1991; Knobloch et al., 2012), and can increase OTR expression by as much as four times in response to social stimuli or pharmacological treatment (Zoicas et al., 2014; Neumann et al., 1991). In other brain regions such as the NAc, basal fiber density and OT levels are considered undetectable (Knobloch et al., 2012; Ross et al., 2009), but increase substantially after social interactions to concentrations comparable to those after OTR activation (Ross et al., 2009). Selective viral tracing has confirmed that OT neuron axons project into the NAc, although the signal is relatively weak (Tang et al., 2020).
Recently developed genetic procedures using highly selective viral tracing have enabled the discrete identification of OT neuron axonal projections in the PVN (Tang et al., 2020). The majority of OT neurons in the PVN are magnocellular neurons (97%), while the rest are parvocellular neurons (3%) (Althammer and Grinevich, 2018). Retrograde viral tracing into the PVN illustrated that most afferent inputs between the parvocellular and magnocellular neurons are common, but the number of axonal projections varies (Tang et al., 2020). A few exceptions to these axonal afferent inputs to the PVN include the paraventricular nucleus of the thalamus, insular cortex, and habenular nucleus projecting to only parvocellular neurons, and the substantia nigra to only magnocellular neurons (Tang et al., 2020). Although parvocellular neurons can activate the magnocellular neurons within the PVN (Eliava et al., 2016), the functions of parvocellular neurons during social interaction between female rats likely differs from those of magnocellular neurons.
4. Circulatory delivery of OT and behavioral functions
Several studies have indicated that circulating OT can cross the blood-brain barrier (BBB) to a certain extent (Lim et al., 2005). Intravenous injection of OT has been shown to raise its plasma levels, but an increase in levels in the cerebrospinal fluid (CSF) is evident only when a relatively supraphysiological dose of OT (e.g., 100 ng/kg) is injected subcutaneously (Jin et al., 2007). However, the functions of the BBB differ with age, and peripheral injection of OT into neonatal mice induces significant alterations in central OT-OTR signaling and metabolism (Carter et al., 2008; Tabbaa and Hammock, 2020). It is possible that exogenous OT can induce the release of endogenous OT via binding to OTergic neurons (Lambert et al., 1994), creating a peptide feedback loop that leads to central release due to a small amount of OT crossing the BBB (Moos et al., 1989).
Central release of OT has been linked to changes in cognitive and social status-related processes, including the regulation of social behavior systems and the salience of socially relevant stimuli based on olfactory and auditory cues (Richard et al., 1991; Insel and Young, 2001; Young, 2001; Insel, 2010; Churchland and Winkielman, 2012; Choe et al., 2015; Marlin et al., 2015; Oettl et al., 2016). OT has been identified as a modulator of emotional and defensive reactions, such as anxiety, depression, and escape behavior (Neumann and Landgraf, 2012; Neumann and Slattery, 2016; Olivera-Pasilio & Dabrowska, 2020), all of which are factors influencing the segmentalized processing of social behavior (Knobloch et al., 2012; Arakawa, 2020b). It is hypothesized that OT circuits play discrete roles in different sequential processes in social behavior in a context-dependent manner; therefore, we will review these neural circuits by focusing on the processing related to prosocial behavior, including olfactory detection/discrimination via the olfactory bulb to the olfactory cortex, social approach/investigation via the PVN-amygdala-raphe circuits, and maintenance of social interaction/contact via the NAc and the ventral tegmental area (VTA) circuits.
5. OT function in social behavior
Although central OT is known to be involved in several physiological functions, including those related to reproduction, the circadian rhythm, and stress regulation, it is mainly known as a modulator of social behavior (Jurek and Neumann, 2018). An increasing number of studies in humans using intranasal administration of OT have indicated a critical role for it in enhancing positive social behaviors such as trust, affiliation, and empathy (Kosfeld et al., 2005; Keech et al., 2018). Subsequent studies have also implicated the effectiveness of using intranasal OT administration to ameliorate symptoms of social dysfunction in various psychiatric conditions, including schizophrenia, autism, psychopathy, and social anxiety (Ma et al., 2016). Autism is a neurodevelopmental disorder affecting two major core behavioral symptoms, namely impaired social interactions and communication and restricted/repetitive behaviors (Baronio et al., 2015; Arakawa, 2020), and OT has garnered significant interest in the scientific community as an investigational treatment for autism.
Conversely, accumulating evidence has demonstrated that OT can also induce anti-social (negative social) effects, including increased feelings of mistrust or envy towards unknown individuals in humans (Shamay-Tsoory et al., 2009; Bartz et al., 2011; Grillon et al., 2013) and enhanced maternal aggression towards unfamiliar conspecifics or intruders in rodent models (Ferris et al., 1992; de Jong et al., 2014). Therefore, it may be argued that OT enhances attention oriented to associated social stimuli (the social salience hypothesis; Shamay-Tsoory and Abu-Akel, 2016); thus, the responses of animals would depend on the social characteristics (e.g., cooperative or threatening) they encounter (Declerck et al., 2010). Furthermore, the exact functional contribution of central OT is still unclear because of the complexities of the social processes involved in compositive human behaviors. The effect of OT is also expected to be derived from the output of complex neural processing involving interactions with other neurotransmitters, such as dopamine (DA) and serotonin (5-HT), and AVP, to orchestrate the regulation of multiple aspects of behavioral processes.
The use of rodent models based on OT mutations and OT micro-infusion has provided a solid foundation for experimental research on the importance of OT function in social behavior-related processes, including those related to cue detection and discrimination, memory, social preference, and other pro- or anti-social interactions (Carter et al., 2008; Lim et al., 2005). Across many studies, central administration of OT agonists has been shown to enhance recognition and memory for peers and partners as exhibited by altered social investigation (Ferguson et al., 2001; Young et al., 2002; Arakawa et al., 2010). Studies on transgenic mice have also shown that the loss of the capacity to respond to social cues in OT synthesis knockout mice is fully restored by micro-infusion of OT into the MeA before social encounters (Ferguson et al., 2001). Since prosocial behavior is a sequential process accompanied by multiple modulations of the cognitive subsystems involved in detection, recognition, memory, behavioral strategies, and motives, it is difficult to clarify the specific functions of OT in each sequential social process. Furthermore, several studies have provided evidence that OT functions are a consequence of interactions with other neurotransmitters (Jurek and Neumann, 2018). In the following chapters, we will address the factors, such as prosocial processing with other neuromodulators, that confound our understanding of the effects of OT. We will also illustrate a putative OT/OTR neural circuit for the sequential regulation of prosocial behavior in rodent models.
6. Sequential processing of prosocial behavior
Social interaction is well known to be a multifactorial process (Arakawa et al., 2011) (Fig. 1). The complex neural organization underlying social behavior has been extensively investigated using rodent models, allowing us to exhaustively elucidate the related neuronal mechanisms in highly social species. Working successfully with rodent models necessitates accounting for characteristic as well as species-specific differences between the constructs of interest. The most common laboratory rodents (i.e., mice and rats) are nocturnal, and thus use olfactory and tactile senses as the primary tools in adaptive behaviors and survival strategies (Arakawa and Iguchi, 2018).
Fig. 1.
A diagram of the relationships between familiarity and behavioral strategies towards social encounters. At a distance, volatile olfactory signals are detected, which activates exploratory behavior. In a proximity, risk assessment behavior is performed to gather more information regarding social features of social stimuli via non-volatile and tactile signals. When getting familiar with the social stimulus, staying in the proximity and body contact are permitted each other, and thus, time spent in proximity/contact is increased.
Olfaction is the major modality through which rodents detect and identify potential social partners based on volatile signals, particularly in the initial phase of encountering unfamiliar social cues (Brennan and Kendrick, 2006; Arakawa et al., 2011). Volatile odor cues, along with vocalization, facilitate the determination of whether individual signal recipients display approach or avoidance behavior to social stimuli for further investigation. Following approach to a range in which they can engage in physical contact with the source of the social stimuli, rodents exchange nonvolatile compounds as olfactory signals in conjunction with tactile-whisking palpation for gathering social information and achieving body contact with each other (Arakawa and Iguchi, 2018). During these assessment processes, rodents exhibit typical behaviors and postures involving back-and-forth movements and stretching of the body towards the social stimuli as a mode of risk assessment (Blanchard et al., 2011), which is a major component of novelty assessment. Moreover, when becoming familiar with social stimuli, rodents typically engage in physical contacts with each other in the form of huddling. Huddling with familiar conspecifics is common among most mammals to maintain prosocial relationships (Alberts, 2007; Arakawa et al., 2007). As with cuddle contacts in humans, empirical evidence from rodent models illustrates that this innate preference for huddling is indicative of the motivation for bodily contact with familiar conspecifics (IJzerman et al., 2015; Morrison et al., 2016).
Although humans are not highly dependent on odorant cues or the tactile sense to make decisions regarding social interactions, these multisensory modalities are essential for rodents in all aspects of social interaction, including the recognition and assessment of a would-be social partner. The multiple sensory inputs discretely activate neural pathways coupled to the expression of social behavior (Zarate, 2014; Arakawa and Iguchi, 2018). Thus, preclinical rodent models of social behavior need to involve a comprehensive analysis of variables involved in initial olfactory detection and recognition of social partners and subsequent investigatory assessment and contact reception behaviors during olfactory and tactile social engagement. Moreover, the neural mechanisms of OT circuitry are hypothesized to be involved in all aspects of the prosocial engagement.
7. Social olfactory detection regulated by the OT system
Social interactions between rodents, particularly during the initial phase of detection, rely heavily on olfactory cues (Keverne and Brennan, 1996; Arakawa et al., 2011), with the corresponding assessment of social stimuli involving two discrete olfactory processes (Brennan and Kendrick, 2006; Linster and Fontanini, 2014) (Fig. 2). Airborne volatile cues trigger the detection and discrimination of potential social stimuli and facilitate the determination of subsequent strategies for social interactions, for example, approach-withdrawal behavior for risk assessment (Blanchard et al., 2011). Approach behavior towards social stimuli leads to individuals subsequently being in range of physical contact with each other and activates sniffing and uptake of nonvolatile odorants from bodily, facial, and anogenital areas of the opponents to gather more information regarding social features (Arakawa et al., 2011).
Fig. 2.
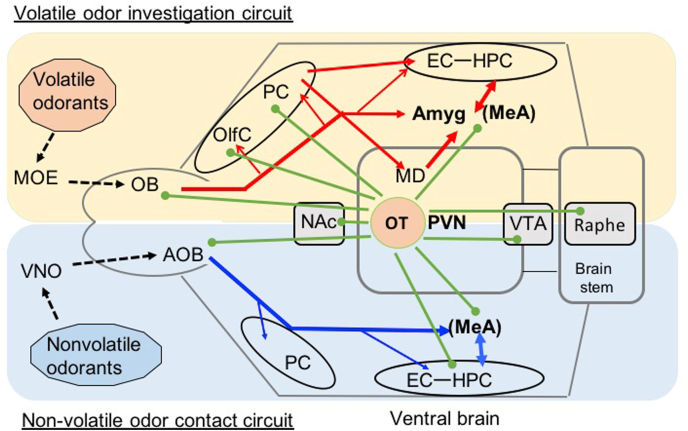
Olfactory circuity coordinated with OT neurons in mouse ventral brain. Volatile odorants as a social signal are processed via the main olfactory epithelium (MOE), olfactory bulb (OB), olfactory and piriform cortex (PC) mediodorsal thalamus (MD), amygdala (Amyg), and Entorhinal cortex (EC) and hippocampus (HPC). Nonvolatile odorants as a contact social signal are processed through the vomeronasal organ (VNO), accessary olfactory bulb (AOB), and medial amygdala (MeA). OT neurons innervate from the paraventricular nucleus of hypothalamus (PVN) to several brain sites associated with these olfactory processes.
Volatile molecules are mainly processed via the main olfactory epithelium, which projects to the main olfactory bulb (MOB) (De Castro, 2009; Courtiol and Wilson, 2015). Axons from the MOB project through the olfactory cortices, including the piriform cortex, and reach the olfactory thalamus and/or the amygdala directly (De Castro, 2009). On the other hand, nonvolatile molecules are taken up to reach the circuits projecting from the vomeronasal organ to the accessory olfactory bulb (AOB) (Mucignat-Caretta, 2010). Projection neurons from the AOB directly send their axons to the amygdala (Mucignat-Caretta, 2010).
Both the MOB and AOB (more densely projected to AOB) contain OT terminals originating from OT neurons of the PVN (Vaccari et al., 1998; Knobloch et al., 2012). The MOB contains inter-neuronal networks mediated by mitral and tufted cells connected to granule cells (Brunjes et al., 2005; Balu et al., 2007). OTRs are expressed in the granule cell-containing region of the MOB and may play a role in local computations in coordination with olfactory cortical circuits (Numan, 2006; Mitre et al., 2016). AVP receptors are involved, perhaps more intensely, in the processing of social odor discrimination processed via the MOB to the anterior olfactory nucleus pathway (Wacker et al., 2011). OT also induces long-term potentiation of excitatory input from mitral to granule cells in the AOB (Fang et al., 2008).
Essential olfactory processing does not require the OT system, as mice with genetic OT deletion can detect and recognize non-social or predatory odors normally (Kavaliers et al., 2003; Oettl et al., 2016). Social odors require specific processing of conspecific components, and OT plays a critical role in learning that is associated with the ability to detect and recognize social odors (Oettl et al., 2016). Exogenous OT enhances olfactory exploration towards social cues in rats, which is associated with increased firing rates of neurons in the anterior olfactory nucleus (Knobloch et al., 2012; Oettl et al., 2016). The anterior olfactory nucleus contains a high level of OTRs and receives innervation from OT neurons of the hypothalamic PVN (Freund-Mercier et al., 1987; Vaccari et al., 1998). Furthermore, the OT-OTR system in the olfactory bulb enhances odor coding by increasing the inhibitory tone innervated to the granule cells of the MOB, improving the signal-to-noise ratios of social odor responses (Markopoulos et al., 2012; Knobloch et al., 2012; Oettl et al., 2016). Therefore, OT also plays a regulatory role in coding social olfactory information.
OT signaling in the piriform cortex is required for acquiring social odor learning (Choe et al., 2015). Dense labeling of the OTR and OTergic terminals in the piriform cortex and other olfactory cortices has been documented (Illig and Haberly, 2003; Sosulski et al., 2011). OTR signaling in the piriform cortex also seems to be essential for entraining initial sensory representation of social olfactory cues (Choe et al., 2015). Selective blocking of OTR signaling in the piriform cortex disrupts the detection of neutral, non-social odors formerly paired with female mice, suggesting that OTR signaling in the piriform cortex appears to mediate social salience to an initially neutral odor (Choe et al., 2015).
Moreover, OT administration rapidly reduces GABAergic inhibition in several brain regions, including the hippocampus (Owen et al., 2013), auditory cortex, piriform cortex, and hypothalamic PVN (Marlin et al., 2015; Mitre et al., 2016). This transient disinhibition is effective in modulating and re-activating the neural mechanism underlying long-term changes in synaptic and spiking responses to social stimuli and ontogenetic social events. For example, maternal behaviors, such as a particular response to a pup's distress call, are observed only for experienced mothers, and virgin females respond poorly to pup calls (Marlin et al., 2015). OT modulation of synaptic and spiking responses to pup distress calls can trigger both rapid modulation of synaptic transmission and long-term plasticity by disinhibition of GABAergic signals (Mitre et al., 2016).
A form of OT-mediated plasticity through sensory experiences of different modalities has been identified in early development (Zheng et al., 2014). Mice were subjected to sensory deprivation by either whisker trimming at birth (i.e., tactile deprivation) or rearing in total darkness (i.e., visual deprivation). In vitro recordings in layer II/III of auditory, somatosensory, and visual pyramidal cells showed a reduction in spontaneous firing rates, as expected. Microarray screens to search for changes in gene expression showed that OT mRNA was consistently downregulated after sensory deprivation. The reduction in OT mRNA levels which would account for the reduced excitatory synaptic transmission, could be reversed by postnatal environmental enrichment (Zheng et al., 2014). These data suggest that sensory experiences can mediate OTergic gene expression in the PVN and lead to a reduction in OT activities required for synaptic transmission related to sensory processes in multiple cortical areas during early development (Grinevich and Stoop, 2018).
OT may not be involved in interactive neural processing with other neurotransmitters in the olfactory system. Along with GABAergic neurons, dopaminergic neurons and their axonal terminals richly innervate the glomerular layers in the olfactory bulb (Pignatelli and Belluzzi, 2017), but dopaminergic regulation is unlikely to be required for essential olfactory processing (O'Dell et al., 2011). For example, the administration of methamphetamine, which acts as an indirect DA agonist, does not have a significant impact on olfactory habituation to social odors as tested using the habituation/dishabituation test, although a slight impairment in olfactory discrimination is observed (O'Dell et al., 2011). Similarly, mice with genetic dilution of DA type 2 receptors exhibit normal odor discrimination (Kruzich et al., 2004). There are serotonergic projections to both the MOB and AOB, largely excitatory in the MOB and predominately inhibitory in the AOB (Huang et al., 2017), indicating the olfactory-MOB processing dominance of 5-HT. Serotonergic signals are required neither for normal olfactory processing nor social odor discrimination, since neuronal 5-HT synthesis knockout mice (Tph2−/−) demonstrated normal olfactory discrimination for social cues, but impaired social approach/contact (Mosienko et al., 2012; Huo et al., 2018), which is regulated by other brain regions as discussed below.
8. Social investigation regulated by OTergic projection from the PVN
In rodents, OT neurons in the PVN are directly projected to the MeA (Keshavarzi et al., 2014; Cadiz-Moretti et al., 2016; Cservenák et al., 2017), where the OTR mediates the effects on social signals to result in the expression of approach behavior, such as in the social recognition test (Ferguson et al., 2001; Arakawa et al., 2010) (Fig. 3). This test is used to assess the ability of animals to discriminate between social stimuli based on social memory (Engelmann et al., 1995; Arakawa and Iguchi, 2018). The test relies on the exposure of subject mice to conspecifics as stimulus animals and on monitoring the duration that subject mice spend investigating those conspecifics. It is hypothesized that mice have a motive to investigate/sniff ‘unfamiliar’ social cues, such as odors; thus, subject mice would spend more time investigating social cues if they are unfamiliar, and less if they are familiar. Subject mice are exposed over several trials to a social stimulus that is initially unfamiliar; repeated exposures to the same social stimulus induce habituation, and the investigation time in subsequent trials declines. Following habituation, a change to a novel, unfamiliar stimulus restores the investigatory reaction to initial levels if subject mice are able to detect a difference between the previous and current social stimuli, known as dishabituation.
Fig. 3.
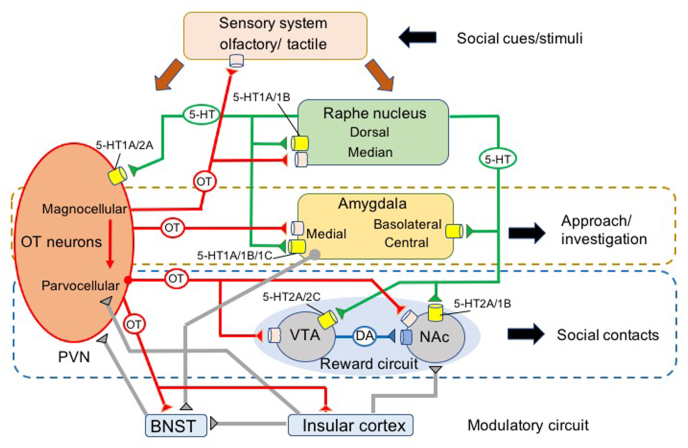
OT neural circuits regulating multiple processes of prosocial behaviors. When sensory inputs associated with social stimuli are delivered via the sensory systems, including olfactory and tactile senses, the OT neurons in the paraventricular nucleus of the hypothalamus (PVN) consist of two discrete cell types (e.g., magnocellular and parvocellular neurons) and the serotonergic neurons in the raphe nucleus are stimulated. OT neurons innervate with several brain sites, including the olfactory bulb-cortices, raphe nucleus, amygdala complex, and ventral tegmental area (VTA) and nucleus accumbens (NAc). 5-HT neurons are also projected to several brain sites associated with OT neurons, including the raphe nucleus, amygdala, and reward circuit (VTA and NAc). The 5-HT and OT in the PVN can activate the release of OT. The OT neurons from the PVN to medial amygdala play a significant role in approach and investigatory behaviors to social stimuli. OT neurons also stimulate the reward circuits of VTA-NAc via dopamine neurons to maintain contact-based social behavior such as huddling. A modulatory circuit including the insular cortex to the bed nucleus of the stria terminalis (BNST) receives OT input integrates sensory social information and sends back modulatory signals to the PVN and reward circuit, providing adaptable behavioral responses in a social situation (evaluation) dependent manner.
OT null mice did not induce the expression of c-fos, a neuronal activity marker, in the MeA when they encountered a social stimulus, and maintained a moderate level of investigation toward the same social stimulus over repeated trials in the habituation/dishabituation task; however, micro-infusion of OT into the MeA before the test restored impaired habituation to the social stimulus (Ferguson et al., 2001). A similar phenotype has been confirmed in mice with genetic OTR dilution—OTR knockout mice do not reduce investigation time when exposed to the familiar stimulus mouse in a similar habituation paradigm (Choleris et al., 2006; Lee et al., 2008). Since these mice with OT deletion exhibited normal performance in non-social or predatory odor discrimination (Kavaliers et al., 2004), the function of OT can be ascribed to the expression/regulation of approach behavior in response to social cues rather than to memory-related odor discrimination. Accordingly, OT knockout mice spent less time in social interactions with conspecifics when they could directly contact each other (Pobbe et al., 2012). However, these OTR null mice spent less time investigating a ‘non-social’ object as well (Leonzino et al., 2019), which indicates a particular behavioral role of OT in the regulation of approach behavior to both social and non-social novel stimuli.
Several lines of evidence suggest that 5-HT-related mechanisms are involved in the regulation of OT release from the PVN. Firstly, 5-HT1A receptors are distributed widely throughout the brain (Riad et al., 2000; Matias et al., 2017), and OT neurons in the PVN have been shown to be immunopositive for 5-HT1A receptors (Zhang et al., 2004). Stimulation of postsynaptic 5-HT1A/2A receptors on OT neurons in the PVN leads to downstream release of endogenous OT (Badgy & Kalogeras, 1993; Jørgensen et al., 2003; Osel-Owusu et al., 2005; Petrunich- Rutherford et al., 2018), while OT response after systemic administration of the 5-HT1A partial agonist ipsapirone was significantly reduced following lesions to the PVN (Bagdy, 1996). Secondly, two 5-HT2 receptor agonists, DOI (5-HT2A) and MK212 (5-HT2C), also stimulated OT mRNA expression in the PVN (Jørgensen et al., 2003). The amphetamine derivative 3,4-methylenedioxymethamphetamine (MDMA) is an agonist of multiple 5-HT receptors, including the 1A, 1B, 1D, 2A, and 2C receptors (Fletcher et al., 2002), and stimulates OT neurons in the PVN; moreover, this stimulation is blocked by co-administration of the 5-HT1A antagonist WAY-100635 (Hunt et al., 2011). Finally, restraint stress-induced OT release in the PVN is blocked by intracerebroventricular administration of either of the 5-HT antagonists WAY-106635 (5-HT1A) or ketanserin (5-HT2A/2C) (Jørgensen et al., 2002). Therefore, one of the serotonergic receptors, most likely the 5-HT1A or 5-HT2A receptor, both of which colocalize in the same cells as OT (Ho et al., 2007; Osei-Owusu et al., 2005), stimulates OT synthesis in the PVN (Chiodera et al., 1996), which in turn activates social approach behavior (Arakawa, 2017). Accordingly, central OT infusion as well as systemic injection of the 5-HT1A agonists 8-OH-DPAT (Tang et al., 2020) or buspirone (Arakawa, 2017) produce increased sniffing and approach to unfamiliar conspecifics in response to volatile social odors, which are blocked by central injection of a specific OTR antagonist (Arakawa et al., 2015; Arakawa, 2017).
The neural circuitry of 5-HT and OT in the PVN is likely formed during postnatal development. Perinatal administration of the 5-HT agonist 5-methoxytryptamine, upregulates plasma 5-HT levels and morphological reconstruction in OT neurons in the PVN, including decreased co-localization of OT with 5-HT2A but not 5-HT1A receptors (Edwards et al., 2018). This mutual regulation between 5-HT and OT suggests discrete mechanisms underlying OT-regulation by 5-HT neurons existing with the 5-HT1A/2A receptor balance in the PVN (Lefevre et al., 2017).
Following approach to conspecifics, mice display typical response patterns based on familiarity, such as facial investigation of the opponents by whisker palpation along with muzzle sniffing, which usually induces little avoidance (flight response) in approached mice. Anogenital investigation of unfamiliar opponents typically leads to a flight response (~40%) (Arakawa et al., 2007, 2015). Injection of either OT or buspirone, a 5-HT1A agonist, robustly decreased the flight response in the approached animals and contributed to the maintenance of approach/contact behavior (Arakawa et al., 2015, 2017). Tph2 knockout mice exhibit a higher frequency of the flight response (~80%) on being approached (Mosienko et al., 2012), which indicates that their extreme aggressiveness (i.e., rejection of social contact) is due to diminished sensitivity of 5-HT1A receptors (Peeters et al., 2019). OT infusion in the MeA restores approach behavior in female Tph2 knockout mice (Huo et al., 2018), suggesting that the 5-HT-OT signal in the PVN-MeA circuitry plays a key role in expressing approach behavior in response to social cues and maintaining social contact acceptance.
These behavioral patterns related to approach and withdrawal to physical contact are frequently reciprocated during a social interaction. Such approach-withdrawal behaviors represent typical risk assessment behavior (Blanchard et al., 2011), which involve searching and assessing the environment by stretching the body back and forth. Such risk assessment behaviors of male mice in response to a predatory odor are disrupted by an intracerebroventricular injection of a specific OTR antagonist (Samuelsen and Meredith, 2011). A recent study demonstrated that the types of OT neurons in the PVN could be the key to mediating approach-contact patterns. Magnocellular OT neurons in the PVN modulate social investigation/approach without physical contact (novelty investigation), which likely switches to control by parvocellular OT neurons when rats can physically encounter peer social stimuli (Tang et al., 2020). Signals from parvocellular OT neurons associated with physical contact with peers would be sent to the NAc, which is linked to the reward circuit (Dölen and Malenka, 2014). Accordingly, one of the social deficit models of autism, Fmr1 knockout mice, is diluted a set of target genes for fragile X syndrome (Mineur et al., 2006), which require activation of parvocellular OT neurons to appropriately express prosocial reward learning (Lewis et al., 2020).
The sense of touch is a highly social component in both animals and humans (Glone et al., 2014). Whisker sensations represent the rodent tactile sense, while the bodily touch sense also plays a major role in peer social interaction (Lenschow and Brechit, 2015), as mice frequently engage in huddling with familiar conspecifics (side-by-side physical contact) (Alberts, 2007). Central infusion of OT facilitates huddling and anogenital contact behavior among unfamiliar mice (Arakawa et al., 2015) and mouse pups (Harshaw et al., 2018). Moreover, 5-HT regulates rhythmic whisking (Hattox et al., 2003) and the whisker touch sense (Arakawa et al., 2014; Gaspar and Lillesaar, 2012). Injection of the partial 5-HT1A-agonist buspirone also increased physical body contact with unfamiliar mice during social encounters (Arakawa, 2017). Taken together, these behavioral data support the idea that 5-HT and OT in the PVN play a mediatory role in the approach-withdrawal tactile contact process during social engagements.
9. Social approach modulated by OT in the amygdala
The amygdala consists of three discrete subdivisions—the basolateral portion, composed of the lateral, basolateral and basomedial nuclei; the centro-medial portion, composed of the medial and central nuclei; and the cortical portion, composed of the anterior and posterior cortical nuclei (Sah et al., 2003; McDonald, 1998). These subregions are collectively involved in the regulation of several fundamental behavioral processes, such as the determination of the emotional significance of sensory stimuli, the modulation of fear-related defensive responses (LeDoux, 2000), and the regulation of social behavior (Gothard, 2020). The OT-OTR system in the MeA plays a key role in the expression of approach behavior in response to social cues and in the maintenance of social contact with familiar partners (Arakawa et al., 2010, 2015; Arakawa, 2017). Although systemic OTR blockage does not influence social approach in male mice (Haskal de la Zerda et al., 2020), micro-infusion of an OTR antagonist into the MeA blocks the activation of social approach and contact behaviors in response to social stimuli (Arakawa et al., 2010; Samuelsen and Meredith, 2011).
Sex-specific activities of OT have been reported in response to social stimuli in the subregions of the amygdala based on human fMRI studies (Domes et al., 2007, 2010; Rilling et al., 2012, 2014). Rodent studies have also illustrated sex-specific differences in OT function in mediating male versus female social interest depending on the subregions of the amygdala. Male mice showed higher social investigation accompanied by higher OTR mRNA expression in the MeA (Arakawa et al., 2010; Murakami et al., 2011), while female mice displayed enhanced OTR mRNA expression in the central amygdala particularly in response to juvenile male approach (Dumais et al., 2016). However, the sex-specificity of OT function is not always consistent, since the MeA in female mice has also been identified as a target of OT to promote social approach, as shown by a local infusion of antisense oligonucleotides targeting OTR expression in the MeA of female mice (Choleris et al., 2007).
In addition to OT synthesis, 5-HT may be involved in the amygdalar regulation of social investigation/approach in rodents. There are two distinct ascending serotonergic systems: serotonergic fibers from the dorsal raphe nucleus extend anchored innervations to the striatum, amygdala, and ventral hippocampus, while those from the median raphe nucleus show large, round, sparse innervations to a wide range of regions including the sensory cortices, hippocampus, and hypothalamus (Hensler, 2006; Gaspar and Lillesaar, 2012). Although the MeA receives 5-HT innervation from the dorsal raphe nucleus (Azmitia and Whitaker-Azmitia, 1991), it is unclear whether 5-HT is directly involved in the regulation of social approach in the MeA. The MeA expresses several types of 5-HT receptors, in particular the 5-HT1A (Kia et al., 1996), 5-HT1B (Sari et al., 1999), and 5-HT2C receptors (Abramowski et al., 1995). The 5-HT2C receptor in the MeA may be involved in the regulation of aggressive behavior via inhibitory GABAergic interneurons (Asan et al., 2013). The activation of 5-HT1A receptors or blocking of 5-HT2 receptors in the MeA by microinjection induces anxiolytic effects (de Paula et al., 2016). While it is still unknown whether the OTR system in the MeA is coordinated with serotonergic innervation to 5-HT receptors, most serotonergic regulation appears to be via mediation by basolateral amygdala projecting neurons (Bocchio et al., 2016) or the central amygdala (Ferretti et al., 2019). It is likely that the 5-HT in the amygdala plays a regulatory role in the emotional state, rather than in social behavior directly (Ferretti et al., 2019; Bubak et al., 2020).
The emergence of approach behavior in response to peer social stimuli is also coordinatively controlled by other behavioral processes. When exposed to unfamiliar social or potentially threatening stimuli, animals display more investigatory behavioral components in order to assess the risk posed by the confronted stimuli (Blanchard and Blanchard, 1989; Blanchard et al., 2011). If the confronted stimulus is evaluated as a threat or danger, the risk assessment behavior is linked to subsequent avoidance responses (Blanchard et al., 2011), in which OT neurons innervated to the anterior BNST are involved (Steinman et al., 2019). The anteromedial portions of the BNST receive inputs from the PVN and MeA, and send back direct projections to the PVN and central amygdala (Dong and Swanson, 2006). The BNST circuits are likely a key center for stress-related avoidance responses (Lebow and Chen, 2016), as exposure to social defeat stress increases OT expression in the anterior BNST (Nasanbuyan et al., 2018). OTR activation in the anteromedial BNST induces avoidance of unfamiliar social stimuli in female California mice, whereas administration of OTR antagonist into the anterior BNST reduces the acquisition of fear-associated learning in rats (Moaddab and Dabrowska, 2017) and suppresses social avoidance following stress exposure in female California mice (Duque-Wilckens et al., 2018). Therefore, the BNST-amygdala circuits coordinate with the PVN-MeA circuits, in which OT acts to regulate investigatory behaviors toward social stimuli in a familiarity- and threat-dependent manner (Steinman et al., 2019). In addition, sex-dimorphic hormonal regulation in the BNST is clearly documented—female mice predominantly exhibit OT-dependent behavioral effects, while male mice show AVP-dependent BNST effects on behavior (Rigney et al., 2019).
10. OTergic mediation of the 5-HT system
Several lines of evidence have illustrated that OT conversely regulates the release/effects of 5-HT (Lefevre et al., 2017). For instance, an intracerebroventricular injection of OT reduced anxiety-related behavior in the open field test, which was fully blocked by systemic injection of ritanserin, an antagonist of 5-HT2A/2C receptors (Yoshida et al., 2009). The existence of mutual interactions between OT and 5-HT is endorsed by several observations of neuromodulatory effects in the NAc. OTRs are found in the presynaptic terminals of serotonergic inputs from the dorsal raphe into the NAc (Dö;len et al., 2013). OT-mediated activation in the NAc requires activation of 5HT1B receptors, which is associated with synaptic plasticity in the NAc neurons sustaining social approach behavior (Dö;len et al., 2013). In addition, recent findings have demonstrated that OTRs are co-localized with 5-HT2A receptors in several brain regions, including the hippocampus, cingulate cortex, and NAc (Chruscicka et al., 2019). Activation of the 5-HT2A signal attenuates the OTR-mediated Gαq signal in the NAc (Chruscicka et al., 2019), suggesting serotonergic mediation of neural OT activity via 5-HT1B/2A receptors in the NAc. It is conceivable that a similar regulatory mechanism also exists in the dorsal periaqueductal gray (dPAG).
OT administration in the dPAG facilitated 5-HT neurotransmission, which regulates anti-escape behaviors in social or predatory situations (Vilela-Costa et al., 2019). The 5-HT effect in the dPAG is mediated via activation of 5-HT1A or 2A receptors (De Oliveira Sergio et al., 2020; De Paula Soares and Zangrossi, 2004; Mongeau and Marsden, 1997; Nogueira and Graeff, 1995; Spiacci et al., 2016). The majority (>90%) of 5-HT2A receptor-labelled cells are colocalized with GABAergic interneurons in the PAG (Griffiths and Lovick, 2002). It is indicated that activation of 5-HT1A receptors hyperpolarizes cells and leads to inhibition of dPAG neuronal activities, while the activation of 5-HT2A receptors via OT stimulation induces depolarization of the dPAG cells through an inhibition of GABAergic interneurons (Brandã;o et al., 1991; Griffiths and Lovick, 2002). Moreover, micro-infusion of a GABAA receptor antagonist into the dPAG blocked OT/5-HT2A-induced behavioral effects (De Oliveira Sergio et al., 2020; Vilela-Costa et al., 2019).
The raphe nuclei have a high density of OTRs that are involved in the regulation of 5-HT release by OT (Pagani et al., 2015). 5-HT1A receptors are presynaptic in the raphe nucleus and postsynaptic in other brain regions (Albert et al., 2014). Presynaptic 5-HT1A receptors in the dorsal and median raphe nuclei act as autoreceptors, forming a micro-feedback loop to tightly regulate 5-HT neuronal activity within the raphe nucleus (Toth, 2003; Stiedl et al., 2015) and through interaction with OT (Stiedl et al., 2015). OT may modulate 5-HT neurotransmission by upregulating 5-HT1A receptors in the dorsal raphe nucleus in humans (Mottolese et al., 2014) and the median raphe nucleus in mice (Yoshida et al., 2009; Pagani et al., 2015). The release of 5-HT from the raphe nucleus stimulates OT neurons in the PVN, where feedback OT projections from the PVN activate 5-HT1A autoreceptors in the raphe nucleus, leading to a reduction/termination of 5-HT release from it, thus creating a long-range indirect feedback loop. Systemic injection of the 5-HT agonist 5-methoxytryptamine causes massive increase in extracellular 5-HT levels, leading to a reduction of OT release in the PVN (Kostoglou-Athanassiou and Forsling, 1998), which can be explained by an upregulation of 5-HT1A autoreceptors in the raphe nucleus, resulting in a reduction of 5-HT stimulation in OT neurons in the PVN. Accordingly, site-specific conditional knockout of the OTR on serotonergic neurons of the raphe nucleus, leading to a loss of negative feedback of 5-HT, did not increase anxiety-like behavior, but reduced intruder-aggression in male resident mice (Pagani et al., 2015). Activation of postsynaptic 5-HT1A and 1B receptors in the raphe nucleus also leads to anti-aggressive effects in rodents (de Boer and Koolhaas, 2005), while a deficit of 5-HT signals in the brain results in extreme hyper aggression in mice (Mosienko et al., 2012). Therefore, OT signals delivered in the raphe nucleus play a role in fine-tuning 5-HT signals in the nucleus via the mediation of presynaptic 5-HT1A autoreceptors and postsynaptic 5-HT1A and 1B receptors.
11. Reward systems sustaining social contact behavior
Social interaction with familiar conspecifics has reward properties which motivate animals to engage in physical contact involving familiar social stimuli, such as huddling, and maintain certain relationships with partners (Young and Barrett, 2015). There is a direct relationship between the reward value of social interactions and the frequency of seeking those interactions (Borland et al., 2018; Netser et al., 2020), similar to the reward value of drugs and their seeking (Veeneman et al., 2012; Doherty et al., 2013). OT acts on the DA circuit in the NAc, which receives projections from the VTA (Buffington et al., 2016; Song et al., 2016) and is a key node of the reward circuit in mice (Dö;len et al., 2013). OTR-expressing VTA neurons also project to the NAc and other forebrain regions containing several different types of neurons, including DA neurons (less than 10%), glutamate neurons (almost 50%), and GABA neurons (Dobi et al., 2010; Peris et al., 2017). Glutamate and GABA neurons expressing OTR in the VTA may play a modulatory role in the DA-reward circuit (Xiao et al., 2018). OT fibers from mainly the parvocellular neurons in the PVN projecting to the VTA (Hung et al., 2017; Xiao et al., 2017) are in close apposition to DA neurons in the VTA that project to the NAc (Melis et al., 2007). OTR is expressed in DA-containing neurons in the VTA (Hung et al., 2017; Peris et al., 2017) and activation of OTRs in the caudal VTA leads to DA efflux in the NAc (Melis et al., 2007; Shahrokh et al., 2010).
Maintaining social interactions in mice is associated with the activation of NAc-projecting VTA DA neurons (Gunaydin et al., 2014). Moreover, the OT neurons in the PVN that project to the VTA tightly mediate the extent of social interaction between mice (Hung et al., 2017; Borland et al., 2018). Selective inhibition of OTRs in the NAc (Dolen et al., 2013) or the OT neurons projecting into the VTA (Hung et al., 2017) induces a deficit in social contact/preference in the conditioned social place preference test and the social interaction test in male mice. It has been hypothesized that OT facilitates social contact and attachment by stimulating the transition from novelty investigation to contact preference with increasing familiarity (Tops et al., 2013; Beier et al., 2015). Accordingly, it has been indicated (Xiao et al., 2017; Charlet and Grinevich, 2017) that parvocellular OT neurons in the PVN projecting to the VTA facilitate the behavioral transition from investigatory behavior to partner-directed (contact) behavior by inhibiting the parvocellular OT-projecting DA circuits in the substantia nigra. The DA circuits of the VTA-NAc are stimulated by OT input, mediating the balance of excitation and inhibition in the neurons (Hung et al., 2017) via presynaptic endocannabinoid-dependent mechanisms (Xiao et al., 2018).
NAc neurons are also mediated by axonal projection from the insular cortex (Wright and Groenewegen, 1996), by which sensory and social information is integrated and reaches the reward system to regulate social approach behavior. The insular cortex receives inputs from almost all brain regions, including the sensory cortices, thalamus, and amygdala, and sends back projections to a wide range of brain sites, such as the amygdala, PAG, BNST, and NAc (Gehrlach et al., 2020). The insular cortex contains dense OTR binding (Dumais et al., 2013) that receives OT inputs from the parvocellular neurons of the PVN (Knobloch et al., 2012; Tang et al., 2020). Thus, a blockage of OTR in the insular cortex disrupts approach behavior to stress-related social stimuli depending on the age of the opponents (Rogers-Carter et al., 2018). Chemogenetic inhibition of NAc-projecting neurons in the posterior insular cortex blocks social approach behavior towards stressed juvenile conspecifics in rats. Furthermore, inactivation of the posterior insular cortex reduces the freezing response of rats to predatory odor (Rodriguez et al., 2020), while inactivation of the anterior insular cortex attenuates the acquisition of the contextual fear response in rats (Alves et al., 2013). The insular cortex may act via OTR neurons projecting to the NAc as a mediator of stress-related defense behavior in a context-dependent manner. Sensory signal integration generated in the insular cortex interconnected with stress-related neural circuits, including those in the BNST and amygdala, may induce a modification of reward-relevant prosocial behaviors via the insular cortex-NAc pathway. These circuits would allow animals to regulate flexible approach-contact responses depending on the social reward characteristics of the opponents (Steinman et al., 2019).
There are some sex-specific differences in the effects of the interaction between OT and DA on the expression of social behaviors (Gillies et al., 2014). Studies in both male and female rodents have established a critical role associated with social reward effects for OTRs in the VTA in activating DA-containing neurons (Xiao et al., 2017; Hung et al., 2017). However, same-sex social interactions are more rewarding in females than in males in mouse models. For example, basal extracellular levels of DA in the NAc are higher in female rats than in males (Virdee et al., 2014), and females display a higher rate of DA uptake and release than males (Walker et al., 2000). The effect of exogenous OT on the amount of social interaction is also sex-specific--the effect of OT infusion in the VTA is induced by smaller amounts in females than in males (Borland, 2019). Despite these sex-specific differences in the response to OT in the VTA, no such differences in terms of the number of OT-containing neurons activated by same-sex social interactions were observed in either the PVN or SON (Borland, 2019).
5-HT neurons projecting to the VTA might play a role in sustaining social contact behavior. Serotoninergic neurons, including those in the dorsal and median raphe nuclei, project to the mesolimbic structures of the NAc and VTA (Mylecharane, 1996). Serotonergic input from the dorsal raphe to the VTA is involved in vulnerability to social stress (Zou et al., 2020). Selective inhibition of serotonergic neurons from the dorsal raphe into the VTA enhanced social avoidance to social aversive (defeated) stimuli (Zou et al., 2020). GABAergic signals in the VTA mediate social avoidance behavior, which can be ameliorated by 5-HT2A receptor agonists (Kimmey et al., 2019). 5-HT2C receptors are also involved in DA firing activities in the VTA (Bubar et al., 2007). The stimulation of 5-HT2C receptors induced inhibition of DA firing in the VTA and release of DA in the NAc (Di Giovanni et al., 2001; Theile et al., 2009), which is mediated by GABAergic neurons in the VTA (Bubar et al., 2011). 5-HT2A suppresses but 5-HT2C upregulates GABA neurons in the VTA, which underlies neural plasticity in behavioral responses. These 5-HT microcircuits in the VTA indirectly sustain social approach/contact by inhibiting withdrawal/avoidance responses. Thus, 5-HT is a major player interacting with OT in terms of functional links between the raphe nucleus and the PVN-amygdala-VTA in the sequential processes of behavioral regulation.
12. Conclusion
In this review, we propose a model for a better understanding of the multifactorial processing of OT underlying prosocial communicative behavior in rodent models. Social interaction between conspecifics is a dynamic sequence of behavioral patterns associated with multimodal regulation, and the role and effect of OT in this process changes from moment to moment to accommodate temporal behavioral expressions. Moreover, OT also functionally cooperates with other neuromodulators such as 5-HT and DA. Significant efforts need to be undertaken to elucidate the temporal dynamics of OT function in the regulation of prosocial processing. Prosocial behaviors in rodents are multi-modal (e.g., olfactory and tactile), distance-based processes characterized by the detection and discrimination of social cues, followed by approach/evaluation of familiarity, and ultimately leading to contact/huddling with each other as a form of prosocial behavior. Central OT action plays a fundamental role in these sequential processes. It facilitates the fine-tuning of social olfactory signals from a potential social partner via various processes through the olfactory bulb to the olfactory cortices. OT also coordinates with 5-HT to stimulate approach/investigatory behaviors in response to the social stimuli via the hypothalamic PVN to MeA circuits. Finally, OT-DA processes in the VTA to NAc circuits maintain social contact-based prosocial relationships with social partners, with modifications by insular cortex-BNST circuits. Recent technical advances, such as viral-targeted manipulation of specific neuronal circuits and real-time in vivo monitoring of targeted neuronal activities, allow researchers to illustrate the temporal sequential maps of neural processes during complex prosocial interactions. Incorporating behavioral analysis of the sequential processing of prosocial interactions promises a more precise understanding of the temporal neuroregulatory mechanisms involving OT and other related factors. The strategy delineates the circuit dynamics in the sequence of behaviors by adding a new motif to the complex picture of OT circuit interactions that critically regulate prosocial behaviors.
Funding
This study was supported by JSPS KAKENHI Grant Number JP19K24681, Japan.
Credit Author Statement
Hiroyuki Arakawa is fully responsible for the content of the article and contributed to the submitted version of the manuscript, entitled “Dynamic regulation of oxytocin neuronal circuits in the sequential processes of prosocial behavior in rodent models”.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
A Peer Review Overview and (sometimes) Supplementary data associated with this article: https://doi.org/10.1016/j.crneur.2021.100011.
Peer Review Overview and Supplementary data. Supplementary data
A Peer Review Overview and (sometimes) Supplementary data associated with this article:
References
- Abramowski D., Rigo M., Duc D., Hoyer D., Staufenbiel M. Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34:1635e1645. doi: 10.1016/0028-3908(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Albert P.R., Vahid-Ansari F., Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front. Behav. Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts J.R. Huddling by rat pups: ontogeny of individual and group behavior. Dev. Psychobiol. 2007;49(1):22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- Althammer F., Jirikowski G., Grinevich V. The oxytocin system of mice and men- similarities and discrepancies of oxytocinergic modulation in rodents and primates. Peptides. 2018;109:1–8. doi: 10.1016/j.peptides.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Blanchard D.C., Blanchard R.J. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav. Brain Res. 2007;176(1):27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Arakawa K., Deak T. Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience. 2010;171(4):1141–1151. doi: 10.1016/j.neuroscience.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Cruz S., Deak T. From models to mechanisms: odorant communication as a key determinant of social behavior in rodents during illness-associated states. Neurosci. Biobehav. Rev. 2011;35(9):1916–1928. doi: 10.1016/j.neubiorev.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Suzuki A., Zhao S., Tsytsarev V., Lo F.S., Hayashi Y., Itohara S., Iwasato T., Erzurumlu R.S. Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors. J. Neurosci. 2014;34(36):12001–12014. doi: 10.1523/JNEUROSCI.1663-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Blanchard D.C., Blanchard R.J. Central oxytocin regulates social familiarity and scent marking behavior that involves amicable odor signals between male mice. Physiol. Behav. 2015;146:36–46. doi: 10.1016/j.physbeh.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Alves F.H.F., Gomes F.V., Reis D.G., Crestani C.C., Corrêa F.M.A., Guimarães F.S., Resstel L.B.M. Involvement of the insular cortex in the consolidation and expression of contextual fear conditioning. Eur. J. Neurosci. 2013;38(2):2300–2307. doi: 10.1111/ejn.12210. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Involvement of serotonin and oxytocin in neural mechanism regulating amicable social signal in male mice: implication for impaired recognition of amicable cues in BALB/c strain. Behav. Neurosci. 2017;131(2):176–191. doi: 10.1037/bne0000191. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Iguchi Y. Ethological and multi-behavioral analysis of learning and memory performance in laboratory rodent models. Neurosci. Res. 2018;135:1–12. doi: 10.1016/j.neures.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Arakawa H. From multisensory assessment to functional interpretation of social behavioral phenotype in transgenic mouse models of autism spectrum disorders. Front. Psychiatr. 2020;11:592408. doi: 10.3389/fpsyt.2020.592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E., Steinke M., Lesch K.-P. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histol. Cell Biol. 2013;139:785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- Azmitia E.C., Whitaker-Azmitia P.M. Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system. J. Clin. Psychiatr. 1991;52 Supple:4–16. [PubMed] [Google Scholar]
- Badgy G., Kalogeras K.T. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain Res. 1993;611(2):330–332. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin, and ACTH/corticosterone responses. Behav. Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Balu R., Pressler R.T., Strowbridge B.W. Multiple modes of synaptic excitation of olfactory bulb granule cells. J. Neurosci. 2007;27(21):5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronio D., Castro K., Gonchoroski T., Mueller de Melo G., Della Flora Nunes G., et al. Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PloS One. 2015;10(1):e0116363. doi: 10.1371/journal.pone.0116363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J., Simeon D., Hamilton H., Kim S., Crystal S., Braun A., et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc. Cognit. Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier K.T., Steinberg E.E., DeLoach K.E., Xie S., Miyamichi K., Schwarz L., Gao X.J., Kremer E.J., Malenka R.C., Luo L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162(3):622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R.J., Blanchard D.C. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard D.C., Griebel G., Pobbe R., Blanchard R.J. Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 2011;35(4):991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Bocchio M., McHugh S.B., Bannerman D.M., Sharp T., Capogna M. Serotonin, amygdala and fear: assembling the puzzle. Front. Neural Circ. 2016;10:24. doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology. 2019;44:785–792. doi: 10.1038/s41386-018-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland J.M., Grantham K.N., Aiani L.M., Frantz K.J., Alberts H.E. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology. 2018;95:128–137. doi: 10.1016/j.psyneuen.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão M.L., Lopez-Garcia J.A., Graeff F.G., Roberts M.H. Electrophysiological evidence for excitatory 5-HT2 and depressant 5-HT1A receptors on neurones of the rat midbrain tectum. Brain Res. 1991;556(2):259–266. doi: 10.1016/0006-8993(91)90313-k. [DOI] [PubMed] [Google Scholar]
- Brennan P.A., Kendrick K.M. Mammalian social odours: attraction and individual recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1476):2061–2078. doi: 10.1098/rstb.2006/1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunjes P.C., Illig K.R., Meyer E.A. A field guide to the anterior olfactory nucleus (cortex) Brain Res. Rev. 2005;50(2):305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bubak A.N., Watt M.J., Yaeger J.D.W., Renner K.J., Swallow J.G. The stalk-eyed fly as a model for aggression - is there a conserved role for 5-HT between vertebrates and invertebrates? J. Exp. Biol. 2020;223(pt 1) doi: 10.1242/jeb.132159. jeb132159. [DOI] [PubMed] [Google Scholar]
- Bubar M.J., Cuningham K.A. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146 doi: 10.1016/j.neuroscience.2006.12.07. 286–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar M.J., Stutz S.J., Cummingham K.A. 5-HT2C receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PloS One. 2011;6(6):e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M., Saulière A., Manning M., Bouvier M., Galés C., Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J. Biol. Chem. 2012;287(6):3617–3629. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M., Bulgheroni E., Manning M., Kleinau G., Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J. Pharmacol. Exp. Therapeut. 2013;346:318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M., Chini B. Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr. Top. Behav. Neurosci. 2018;35:3–29. doi: 10.1007/7854_2017_6. [DOI] [PubMed] [Google Scholar]
- Cadiz-Moretti B., Otero-Garcia M., Martinez-Garcia F., Lanuza E. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct. Funct. 2016;221:1033–1065. doi: 10.1007/s00429-014-0954-y. PMID: 25503449. [DOI] [PubMed] [Google Scholar]
- Campbell P., Ophir A.G., Phelps S.M. Central vasopressin and oxytocin receptor distributions in two species of signing mice. J. Comp. Neurol. 2009;516:321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Grippo A.J., Pournajafi-Nazarloo H., Ruscio M.G., Porges S.W. Oxytocin, vasopressin and sociality. Prog. Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Charlet A., Grinevich V. Oxytocin mobilizes midbrain dopamine toward sociality. Neuron. 2017;95:235–237. doi: 10.1016/j.neuron.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Chiodera P., Volpi R., Capretti L., Caffarri G., Magotti M.G., Coiro V. Different effects of the serotonergic agonists buspirone and sumatriptan on the posterior pituitary hormonal responses to hypoglycemia in humans. Neuropeptides. 1996;30(2):187–192. doi: 10.1016/s0143-4179(96)90086-4. PMID: 8771561. [DOI] [PubMed] [Google Scholar]
- Choe H.K., Reed M.D., Benavidez N., Montgomery D., Soares N., Yim Y.S., Choi G.B. Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron. 2015;87(1):152–163. doi: 10.1016/j.neuron.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E., Ogawa S., Kavaliers M., Gustafsson J.A., Korach K.S., Muglia L.J., Pfaff D.W. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Gene Brain Behav. 2006;5(7):528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Choleris E., Little S.R., Mong J.A., Puram S.V., Langer R., Pfaff D.W. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104(11):4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chruscicka B., Wallace Fitzsimons S.E., Borroto-Escuela D.O., Druelle C., Stamou P., Nally K., et al. Attenuation of oxytocin and serotonin 2A receptor signaling through novel heteroreceptor formation. ACS Chem. Neurosci. 2019;10(7):3225–3240. doi: 10.1021/acschemneuro.8b00665. [DOI] [PubMed] [Google Scholar]
- Churchland P.S., Winkielman P. Modulating social behavior with oxytocin: how does it work? what does it mean? Horm. Behav. 2012;61(3):392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol E., Wilson D.A. The olfactory thalamus: unanswered questions about the role of the mediodorsal thalamic nucleus in olfaction. Front. Neural Circ. 2015;9:49. doi: 10.3389/fncir.2015.00049. PMID: 26441548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenák M., Keller D., Kis V., Fazekas E.A., Öllös H., Lékó A.H., Szabó É.R., Renner É., Usdin T.B., Palkovits M., Dobolyi Á. A thalamo-hypothalamic pathway that activates oxytocin neurons in social contexts in female rats. Endocrinology. 2017;158(2):335–348. doi: 10.1210/en.2016-1645. PMID: 27841935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer S.F., Koolhaas J.M. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur. J. Pharmacol. 2005;526(1–3):125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- De Castro F. Wiring olfaction: the cellular and molecular mechanisms that guide the development of synaptic connections from the nose to the cortex. Front. Neurosci. 2009;3:52. doi: 10.3389/neuro.22.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck C.H., Boone C., Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 2010;57:368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- de Jong T.R., Beiderbeck D.I., Neumann I.D. Measuring virgin female aggression in the female intruder test (FIT): effects of oxytocin, estrous cycle, and anxiety. PloS One. 2014;9:e91701. doi: 10.1371/journal.pone.0091701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira Sergio T., Tercino Frias A., Helena Vilela-Costa H., Cg De Oliveira D., Zuardi A.W., Zangrossi H., Jr. Serotonin mediates the panicolytic-like effect of oxytocin in the dorsal periaqueductal gray. J. Psychopharmacol. 2020;34(4):383–390. doi: 10.1177/0269881120907960. [DOI] [PubMed] [Google Scholar]
- de Paula Soares V., Zangrossi H., Jr. Involvement of 5-HT1A and 5-HT2 receptors of the dorsal periaqueductal gray in the regulation of the defensive behaviors generated by the elevated T-maze. Brain Res. Bull. 2004;64(2):181–188. doi: 10.1016/j.brainresbull.2004.06.007. [DOI] [PubMed] [Google Scholar]
- de Paula B.B., Andrade Leite-Panissi C.R. Distinct effect of 5-HT1A and 5-HT2A receptors in the medial nucleus of the amygdala on tonic immobility behavior. Brain Res. 2016;1643:152–158. doi: 10.1016/j.brainres.2016.04.073. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G., Di Matteo V., La Grutta V., Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience. 2001;103:111–116. doi: 10.1016/s0306-4522(00)00561-3. [DOI] [PubMed] [Google Scholar]
- Dobi A., Margolis E.B., Wang H.L., Harvey B.K., Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J. Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J.M., Cooke B.M., Frantz K.J. A role for the prefrontal cortex in heroin- seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacology: Off. Publ. Am. Coll. Neuropsychopharmacol. 2013;38:446–454. doi: 10.1038/npp.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G., Malenka R.C. The emerging role of nucleus accumbens oxytocin in social cognition. Biol. Psychiatr. 2014;76:354–355. doi: 10.1016/j.biopsych.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., Büchel C., Braus D.F., Herpertz S.C. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatr. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G., Lischke A., Berger C., Grossmann A., Hauenstein K., Heinrichs M., Herpertz S.C. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais K.M., Bredewold R., Mayer T.E., Veenema A.H. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dumais K.M., Veenema A.H. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N., Steinman M.Q., Busnelli M., Chini B., Yokoyama S., Pham M., Laredo S.A., et al. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol. Psychiatr. 2018;83(3):203–213. doi: 10.1016/j.biopsych.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.A., Madden A.M.K., Zup S.L. Serotonin receptor regulation as a potential mechanism for sexually dimorphic oxytocin dysregulation in a model of autism. Brain Res. 2018;1701:85–92. doi: 10.1016/j.brainres.2018.07.020. [DOI] [PubMed] [Google Scholar]
- Eliava M., Melchior M., Knobloch-Bollmann H.S., Wahis J., da Silva Gouveia M., Tang Y., et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89(6):1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M., Watjak C.T., Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 1995;58(2):315–321. doi: 10.1016/0031-9384(95)00053-L. [DOI] [PubMed] [Google Scholar]
- Fang L.-Y., Quan R.-D., Kaba H. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci. Lett. 2008;438(2):133–137. doi: 10.1016/j.neulet.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Ferguson J.N., Aldag J.M., Insel T.R., Young L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V., Maltese F., Contarini G., Nigro M., Bonavia A., Huang H., Gigliucci V., et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol. 2019;29:1938–1953. doi: 10.1016/j.cub.2019.04.070. [DOI] [PubMed] [Google Scholar]
- Ferris C.F., Foote K.B., Meltser H.M., Plenby M.G., Smith K.L., Insel T.R. Oxytocin in the amygdala facilitates maternal aggression. Ann. N. Y. Acad. Sci. 1992;652:456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x. [DOI] [PubMed] [Google Scholar]
- Fletcher P.J., Korth K.M., Robinson S.R., Baker G.B. Multiple 5-HT receptors are involved in the effects of acute MDMA treatment: studies on locomotor activity and responding for conditioned reinforcement. Psychopharmacology (Berl.) 2002;162(3):282–291. doi: 10.1007/s00213-002-1104-4. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M.J., Stoeckel M.E., Palacios J.M., Pazos A., Reichhart J.M., Porte A., Richard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20(2):599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Lillesaar C. Probing the diversity of serotonin neurons. Phil. Trans. R. Soc. B. 2012;367:2382–2394. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrlach D.A., Weiand C., Gaitanos T.N., Cho E., Klein A.S., Hennrich A.A., et al. A whole-brain connectivity map of mouse insular cortex. eLife. 2020;9:e55585. doi: 10.7554/eLife.55585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G.E., Virdee K., McArthur S., Dalley J.W. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience. 2014;282:69–85. doi: 10.1016/j.neuroscience.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. PMID: 11274341. [DOI] [PubMed] [Google Scholar]
- Glone F., Wessberg J., Olausson H. Discriminative and affective tough: sensing and feeling. Neuron. 2014;82:737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Gould B.R., Zingg H.H. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Gothard K.M. Multidimensional processing in the amygdala. Nat. Rev. Neurosci. 2020;21(10):565–575. doi: 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Krimsky M., Charney D.R., Vytal K., Ernst M., Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol. Psychiatr. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J.L., Lovick T.A. Co-localization of 5-HT2A-receptor- and GABA-immunoreactivity in neurones in the periaqueductal grey matter of the rat. Neurosci. Lett. 2002;326(3):151–154. doi: 10.1016/s0304-3940(02)00182-9. [DOI] [PubMed] [Google Scholar]
- Grinevich C., Desarménien M.G., Chini B., Tauber M., Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front. Neuroanat. 2015;8:164. doi: 10.3389/fnana.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V., Knobloch-Bollmann H.S., Eliava M., Busnelli M., Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatr. 2016;79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Grinevich V., Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio-emotional behaviors. Neuron. 2018;99(5):887–904. doi: 10.1016/j.neuron.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Gunaydin L.A., Grosenick L., Finkelstein J.C., Kauvar I.V., Fenno L.E., Adhikari A., Lammel S., Mirzabekov J.J., Airan R.D., Zalocusky K.A., Tye K.M., Anikeeva P., Malenka R.C., Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C., Leffel J.K., Alberts J.R. 2018. Oxytocin and the Warm Outer Glow: Thermoregulatory Deficits Cause Huddling Abnormalities in Oxytocin-Deficits Mouse Pups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskal de la Zerda S., Netser S., Magalnik H., Wagner S. Impaired sex preference, but not social and social novelty preferences, following systemic blockade of oxytocin receptors in adult male mice. Psychoneuroendocrinology. 2020;116:104676. doi: 10.1016/j.psyneurn.2020.104676. [DOI] [PubMed] [Google Scholar]
- Hattox A., Li Y., Keller A. Serotonin regulates rhythmic whisking. Neuron. 2003;39(2):343–352. doi: 10.1016/s0896-6273(03)00391-x. PMID: 12873389. [DOI] [PubMed] [Google Scholar]
- Hensler J.G. Serotonergic modulation of the limbic system. Neurosci. Biobehav. Rev. 2006;30(2):203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ho S.S., Chow B.K., Yung W.H. Serotonin increases the excitability of the hypothalamic paraventricular nucleus magnocellular neurons. Eur. J. Neurosci. 2007;25(10):2991–3000. doi: 10.1111/j.1460-9568.2007.05547.x. PMID: 17561813. [DOI] [PubMed] [Google Scholar]
- Huang Z., Thiebaud N., Fadool D.A. Differential serotonergic modulation across the main and accessory olfactory bulbs. J. Physiol. 2017;595(11):3515–3533. doi: 10.1113/JP273945. PMID: 28229459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L.W., Neuner S., Polepalli J.S., Beier K.T., Wright M., et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–1411. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.E., McGregor I.S., Cornish J.L., Callaghan P.D. MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res. Bull. 2011;86(1–2):65–73. doi: 10.1016/j.brainresbull.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Huo Y., Zhang Y., Guo H., Liu Y., Fang Q., Zhang J. Tph2-/- female mice restore socio-sexual recognition through upregulating ERalpha and OTR genes in the amygdala. PloS One. 2018;13(2) doi: 10.1371/journal.pone.0193395. e0193395. PMID: 29470551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig K.R., Haberly L.B. Odor-evoked activity is spatially distributed in piriform cortex. J. Comp. Neurol. 2003;457(4):361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- IJzerman H., Coan J., Wagemans F., Missler M., van Beest I., Lindenberg S., et al. A theory of social thermoregulation in human primates. Front. Psychol. 2015;6:464. doi: 10.3389/fpsyg.2015.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Timura T., Azuma C., Inazawa J., Takemura M., Kikuchi T., et al. Structural organization of the human oxytocin receptor gene. J. Biol. Chem. 1994;269(51):32451–32456. [PubMed] [Google Scholar]
- Insel T.R., Young L.J. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Liu H.-X., Hirai H., Torashima T., Nagai T., Lopatina P., Shnayder N.A., Yamada K., et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446(7131):41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Jørgensen H., Knigge U., Kjaer A., Warberg J. Serotonergic involvement in stress-induced vasopressin and oxytocin secretion. Eur. J. Endocrinol. 2002;147(6):815–824. doi: 10.1530/eje.0.1470815. [DOI] [PubMed] [Google Scholar]
- Jorgensen H., Kjaer A., Knigge U., Mollers M., Warberg J. Serotonin stimulates hypothalamic mRNA expression and local release of neurohypophysial peptides. J. Neuroendocrinol. 2003;15:564–571. doi: 10.1046/j.1365-2826.2003.01032.x. [DOI] [PubMed] [Google Scholar]
- Jurek B., Neumann I.D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Colwell D.D., Choleris E., Agmo A., Muglia L.J., Ogawa S., Pfaff D.W. Impaired discrimination of and aversion to parasited male odors by female oxytocin knockout mice. Genes Brain Behav. 2003;2:220–230. doi: 10.1034/j.1601-183x.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Choleris E., Agmo A., Pfaff D.W. Olfactory-mediated parasite recognition and avoidance: linking genes to behavior. Horm. Behav. 2004;46(3):272–283. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Keech B., Crowe S., Hocking D.R. Intranasal oxytocin, social cognition and neurodevelopmental disorders: a meta-analysis. Psychoneuroendocrinology. 2018;87:9–19. doi: 10.1016/j.psyneuen.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Keshavarzi S., Sullivan R.K., Ianno D.J., Sah P. Functional properties and projections of neurons in the medial amygdala. J. Neurosci. 2014;34(26):8699–8715. doi: 10.1523/JNEUROSCI.1176-14.2014. PMID: 24966371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne E.B., Brennan P.A. Olfactory recognition memory. J. Physiol. Paris. 1996;90(5–6):399–401. doi: 10.1016/s0928-4257(97)87929-6. [DOI] [PubMed] [Google Scholar]
- Kia H.K., Miquel M.C., Brisorgueil M.J., Daval G., Riad M., El Mestikawy S., Hamon M., Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289e305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kimmey B.A., Ostroumov A., Dani J.A. 5-HT2A receptor activation normalizes stress-induced dysregulation of GABAergic signaling in the ventral tegmental area. Proc. Natl. Acad. Sci. U.S.A. 2019;116(52):27028–27034. doi: 10.1073/pnas.1911446116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M.J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kostoglou-Athanassiou I., Forsling M.L. Effect of 5-hydroxytryptamine and pineal metabolites on the secretion of neurohypophysial hormones. Brain Res. Bull. 1998;46(5):417–422. doi: 10.1016/s0361-9230(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Kruzich P.J., Grandy D.K. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R.C., Dayanithi G., Moos F.C., Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J. Physiol. 1994;478(Pt2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R. Mortyn Jones memorial lecture. Intracerebrally released vasopressin and oxytocin: measurement, mechanisms and behavioural consequences. J. Neuroendocrinol. 1995;7(4):243–253. doi: 10.1111/j.1365-2826.1995.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lebow M.A., Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatr. 2016;21(4):450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H.-J., Caldwell H.K., Macbeth A.H., Tolu S.G., Young W.S., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149(7):3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A., Richard N., Jazayeri M., Beuriat P.A., Fieux S., Zimmer L., Duhamel J.R., Sirigu A. Oxytocin and serotonin brain mechanisms in the nonhuman primate. J. Neurosci. 2017;37(28):6741–6750. doi: 10.1523/JNEUROSCI.0659-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow C., Brecht M. Barrel cortex membrane potential dynamics in social touch. Neuron. 2015;85(4):718–725. doi: 10.1016/j.neuron.2014.12.059. [DOI] [PubMed] [Google Scholar]
- Leonzino M., Ponzoni L., Braida D., Gigliucci V., Busnelli M., et al. Impaired approach to novelty and striatal alterations in the oxytocin receptor deficient mouse model of autism. Horm. Behav. 2019;114:104543. doi: 10.1016/j.yhbeh.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Lewis E.M., Stein-O'Brien G.L., Patino A.V., Nardou R., Grossman C.D., Brown M., Bangamwabo B., et al. Parallel social information processing circuits are differentially impacted in autism. Neuron. 2020;108:659–675. doi: 10.1016/j.neuron.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.M., Bielsky I.F., Young L.J. Neuropeptides and the social brain: potential rodent models of autism. Int. J. Dev. Neurosci. 2005;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Linster C., Fontanini A. Functional neuromodulation of chemosensation in vertebrates. Curr. Opin. Neurobiol. 2014;29:82–87. doi: 10.1016/j.conb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Shamay-Tsoory S., Han S., Zink C.F. Oxytocin and social adaptation: insights from neuroimaging studies of healthy and clinical populations. Trends Cognit. Sci. 2016;20(2):133–145. doi: 10.1016/j.tics.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Manning M., Misicka A., Olma A., Bankowski K., Stoev S., Chini B., Durroux T., Mouillac B., Corbani M., Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulos F., Rokni D., Gire D.H., Murthy V.N. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin B.J., Mitre M., D'amour J.A., Chao M.V., Froemke R.C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548):499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias S., Lottem E., Dugue G.P., Mainen Z.F. Activity patterns of serotonin neurons underlying cognitive flexibility. Elife. 2017;6:e20552. doi: 10.7554/eLife.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Melis T., Cocco C., Succu S., Sanna F., Pillolla G., et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extra- cellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 2007;2007(26):1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Mineur Y.S., Huynh L.X., Crusio W.E. Social behavior deficits in the Fmr1 mutant mouse. Behav. Brain Res. 2006;168(1):172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mitre M., Marlin B.J., Schiavo J.K., Morina E., Norden S.E., Hackett T.A., et al. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci. 2016;36∗8:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab M., Dabrowska J. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology. 2017;121:130–139. doi: 10.1016/j.neuropharm.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R., Marsden C.A. Effect of imipramine treatments on the 5-HT1A-receptor-mediated inhibition of panic-like behaviours in rats. Psychopharmacology (Berl.) 1997;131(4):321–328. doi: 10.1007/s002130050299. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J. Physiol. 1989;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. Keep calm and cuddle on: social touch as a stress buffer. Adapt. Hum. Behav. Physiol. 2016;2:344–362. doi: 10.1007/s40750-016-0052-x. [DOI] [Google Scholar]
- Mosienko V., Bert B., Beis D., Matthes S., Fink H., Bader M., Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottolese R., Redouté J., Costes N., Le Bars D., Sirigu A. Switching brain serotonin with oxytocin. Proc. Natl. Acad. Sci. U.S.A. 2014;111(23):8637–8642. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucignat-Caretta C. The rodent accessory olfactory system. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2010;196(10):767–777. doi: 10.1007/s00359-010-0555-z. PMID: 20607541. [DOI] [PubMed] [Google Scholar]
- Murakami G., Hunter R.G., Fontaine C., Ribeiro A., Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur. J. Neurosci. 2011;34(3):469–477. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- Mylecharane E.J. Ventral tegmental area 5-HT receptors: mesolimbic dopamine release and behavioural studies. Behav. Brain Res. 1996;73(1–2):1–5. doi: 10.1016/0166-4328(96)00061-7. [DOI] [PubMed] [Google Scholar]
- Nasanbuyan N., Yoshida M., Takayanagi Y., Inutsuka A., Nishimori K., Yamanaka A., Onaka T. Oxytocin-oxytocin receptors systems facilitate social defeat posture in male mice. Endocrinology. 2018;159:763–775. doi: 10.1210/en.2017-00606. [DOI] [PubMed] [Google Scholar]
- Neumann I., Russell J.A., Wolff B., Landgraf R. Naloxone increases the release of oxytocin, but not vasopressin, within limbic brain areas of conscious parturient rats: a push-pull perfusion study. Neuroendocrinology. 1991;54(6):545–551. doi: 10.1159/000125958. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Slattery D.A. Oxytocin in general anxiety and social fear: a translational approach. Biol. Psychiatr. 2016;79(3):213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Netser S., Meyer A., Magalnik H., Zylbertal A., Haskal de la Zerda S., Briller M., et al. Distinct dynamics of social motivation drive differential social behavior in laboratory rat and mouse strains. Nat. Commun. 2020;11(1):5908. doi: 10.1038/s41467-020-19569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K., Young L.J., Guo Q., Wang Z., Insel T.R., Matzuk M.M. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira R.L., Graeff F.G. Role of 5-HT receptor subtypes in the modulation of dorsal periaqueductal gray generated aversion. Pharmacol. Biochem. Behav. 1995;52(1):1–6. doi: 10.1016/0091-3057(94)00402-5. [DOI] [PubMed] [Google Scholar]
- Numan M., Fleming A.S., Lévy F. In: Knobil and Neill's Physiology of Reproduction. third ed. Neill J.D., editor. Elsevier; San Diego: 2006. Maternal behavior; pp. 1921–1993. [Google Scholar]
- O'Dell S.J., Feinberg L.M., Marshall J.F. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav. Brain Res. 2011;216:196–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Oettl L.-L., Ravi N., Schneider M., Scheller M.F., Schneider P., Mitre M., et al. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016;90(3):609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Pasilio V., Dabrowska J. Oxytocin promotes accurate fear discrimination and adaptive defensive behaviors. Front Neurosci. 2020;14:583878. doi: 10.3389/fnins.2020.583878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Owusu P., James A., Crane J., Scrogin K.E. 5-hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J. Pharmacol. Exp. Therapeut. 2005;313(3):1324–1330. doi: 10.1124/jpet.104.082073. [DOI] [PubMed] [Google Scholar]
- Owen S.F., Tuncdemir S.N., Bader P.K., Tirko N.N., Fishell G., Tsien R.W. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500(7463):458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani J.H., Williams Avram S.K., Cui Z., Song J., É Mezey, Senerth J.M., Baumann M.H., Young W.S. Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Gene Brain Behav. 2015;14:167–176. doi: 10.1111/gbb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passoni I., Leonzino M., Gigliucci V., Chini B., Busnelli M. Carbetocin isa functional selective Gq agonist that does not promote oxytocin receptor recycling after inducing ß-arrestin-independent internalisation. J. Neuroendocrinol. 2016;28(4) doi: 10.1111/jne.12363. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters D.G.A., de Boer S.F., Terneusen A., Newman-Tancredi A., Varney M.A., Verkes R.-J., Homberg J.R. Enhanced aggressive phenotype of Tph2 knockout rats is associated with diminished 5-HT1A receptor sensitivity. Neuropharmacology. 2019;153:134–141. doi: 10.1016/j.neuropharm.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Peris J., MacFadyen K., Smith J., de Kloet A.D., Wang L., Krause E.G. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 2017;525(5):1094–1108. doi: 10.1002/cne.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrunich-Rutherford M.L., Garcia F., Battaglia G. 5-HT1A receptor-mediated activation of neuroendocrine responses and multiple protein kinase pathways in the peripubertal rat hypothalamus. Neuropharmacology. 2018;139:173–181. doi: 10.1016/j.neuropharm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Pignatelli A., Belluzzi O. Dopaminergic neurons in the main olfactory bulbs: an overview from an electrophysiological perspective. Front. Neuroanat. 2017;11:7. doi: 10.3389/fnana.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe R.L.H., Pearson B.L., Blanchard D.C., Blanchard R.J. Oxytocin receptor and Mecp2 308/Y knockout mice exhibit altered expression of autism-related social behaviors. Physiol. Behav. 2012;107(5):641–648. doi: 10.1016/j.physbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M., Garcia S., Watkins K.C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417(2):181–194. [PubMed] [Google Scholar]
- Richard P., Moos F., Freund-Mercier M.J. Central effects of oxytocin. Physiol. Rev. 1991;71(2):331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Rigney N., Whylings J., Mieda M., de Vries G.J., Petrulis A. Sexually dimorphic vasopressin cells modulate social investigation and communication in sex-specific ways. eNeuro. 2019;6(1):e0415–e0418. doi: 10.1523/ENEURO.0415-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., Thompson R., Ditzen B., Patel R., Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., Chen X., Gautam P., Stair S., Haroon E., Thompson R., Ditzen B., Patel R., Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Ceric F., Murgas P., Harland B., Torrealba F., Contreras M. Interoceptive insular cortex mediates both innate fear and contextual threat conditioning to predator odor. Front. Behav. Neurosci. 2020;13:283. doi: 10.3389/fnbeh.2019.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter M.M., Varela J.A., Gribbons K.B., Pierce A.F., McGoey M.T., Ritchey M, Christianson J.P. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci. 2018;21(3):404–414. doi: 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Young L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Neumann I., Landgraf R. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push-pull perfusion study. J. Neurosci. 1992;12(3) doi: 10.1523/JNEUROSCI.12-03-01024. L 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samuelsen C.L., Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y., Miquel M.C., Brisorgueil M.J., Ruiz G., Doucet E., Hamon M., Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88(3):899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Sawchenko P.E., Swanson L.W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 1982;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Shahrokh D.K., Zhang T.Y., Diorio J., Gratton A., Meaney M.J. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Fischer M., Dvash J., Harari H., Perach-Bloom N., Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol. Psychiatr. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Abu-Akel A. The social salience hypothesis of oxytocin. Biol. Psychiatr. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog. Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Song Z., Borland J.M., Larkin T.E., O'Malley M., Albers H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–172. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski D., Bloom M.L., Cutforth T., Axel R., Datta S.R. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472(7342):213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiacci A., Jr., Pobbe R.L.H., Matthiesen M., Zangrossi H., Jr. 5-HT1A receptors of the rat dorsal raphe lateral wings and dorsomedial subnuclei differentially control anxiety- and panic-related defensive responses. Neurpharmacology. 2016;107:471–479. doi: 10.1016/j.neuropharm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Steinman M.Q., Duque-Wilckens N., Trainor B.C. Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biol. Psychiatr. 2019;85(10):792–801. doi: 10.1016/j.biopsych.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O., Pappa E., Konradsson-Geuken A., Ogren S.O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015;6:162. doi: 10.3389/fphar.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L.W., Sawchenko P.E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tabbaa M., Hammock E.A.D. Orally administered oxytocin alters brain activation and behaviors of pre-weaning mice. Horm. Behav. 2020;118:104613. doi: 10.1016/j.yhbeh.2019.104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Benusiglio D., Lefeve A., Hilfiger L., Althammer F., Bludau A., et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020;23(9):1125–1137. doi: 10.1038/s41593-020-0674-y. [DOI] [PubMed] [Google Scholar]
- Theile J.W., Morikawa H., Gonzales R.A., Morrisett R.A. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J. Pharmacol. Exp. Therapeut. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M., Huffmeijer R., Linting M., Grewen K.M., Light K.C., Koole S.L., Bakermans-Kranenburg M.J., van Ijzendoorn M.H. The role of oxytocin in familiarization-habituation responses to social novelty. Front. Psychol. 2013;4:761. doi: 10.3389/fpsyg.2013.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur. J. Pharmacol. 2003;463(1–3):177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- Vaccari C., Lolait S.J., Ostrowski N.L. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139(12):5015–5033. doi: 10.1210/endo.139.6382. [DOI] [PubMed] [Google Scholar]
- Veeneman M.M., Broekhoven M.H., Damsteegt R., Vanderschuren L.J. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology: Off. Publ. Am. Coll. Neuropsychopharmacol. 2012;37:487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela-Costa H.H., Spiacci A., Jr., Bissolli I.G., Zangrossi H., Jr. A shift in the activation of serotonergic and non-serotonergic neurons in the dorsal raphe lateral wings subnucleus underlies the panicolytic-like effect of fluoxetine in rats. Mol. Neurobiol. 2019;56(9):6487–6500. doi: 10.1007/s12035-019-1536-z. [DOI] [PubMed] [Google Scholar]
- Virdee K., McArthur S., Brischoux F., Caprioli D., Ungless M.A., Robbins T.W., et al. Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology: Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:339–350. doi: 10.1038/npp.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D.W., Engelmann M., Tobin V.A., Meddle S.L., Ludwig M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Ann. N. Y. Acad. Sci. 2011;1220:106–116. doi: 10.1111/j.1749-6632.2010.05885.x. [DOI] [PubMed] [Google Scholar]
- Walker Q.D., Rooney M.B., Wightman R.M., Kuhn C.M. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Wright C.I., Groenewegen H.J. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73(2):359–373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]
- Xiao L., Priest M.F., Kozorvitskiy Y. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. Elife. 2018;7:e33892. doi: 10.7554/eLife.33892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Priest M.F., Nasenbeny J., Lu T., Kozorovitskiy Y. Biased oxytocinergic modulation of midbrain dopamine systems. Neuron. 2017;95:368–384. doi: 10.1016/j.neuron.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J., Barrett C.E. Neuroscience. Can oxytocin treat autism? Science. 2015;347:825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J., Lim M.M., Gingrich B., Insel T.R. Cellular mechanisms of social attachment. Horm. Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young L.J., Pitkow L.J., Ferguson J.N. Neuropeptides and social behavior: animal models relevant to autism. Mol. Psychiatr. 2002;7(Suppl. 2):S38–S39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takayamagi Y., Inoue K., Kimura T., Young L.J., Onaka T., Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate J.M. Oxytocin for all senses. Nat. Neurosci. 2014;17(3):340. doi: 10.1038/nn0314-340. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gray T.S., D'Souza D.N., Carrasco G.A., Damjanoska K.J., Dudas B., Garcia F., Zaineli G.M., et al. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exp. Therapeut. 2004;210(1):59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]
- Zheng J.-J., Li S.-J., Zhang X.-D., Miao W.-Y., Zhang D., Yao H., Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci. 2014;17(3):391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]
- Zoicas I., Slattery D.A., Neumann I.D. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39(13):3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W.-J., Song Y.-L., Wu M.-Y., Chen X.-T., You Q.-L., et al. A discrete serotonergic circuit regulates vulnerability to social stress. Nat. Commun. 2020;11:4218. doi: 10.1038/s41467-020-18010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.